Abstract
This study aimed to describe the prevalence and extent of periodontal diseases among adults in the southern region of the Lisbon Metropolitan Area. This population-based cross-sectional study included 1,064 randomized participants (aged 18 to 95 years, 617 females/447 males). Sociodemographic, behaviours and medical information were recorded. Periodontal conditions were assessed with a full-mouth circumferential periodontal examination. It was used the American Association of Periodontology/European Federation of Periodontology 2017 case definitions. A logistic regression analysis was applied to ascertain hypothetical risk factors towards periodontitis. The prevalence of periodontitis was 59.9%, with 24.0% and 22.2% of the participants exhibiting severe and moderate periodontitis, respectively. The risk of periodontitis significantly increased with age (OR = 1.05, 95% CI: 1.04–1.06), for active and former smokers (OR = 3.76 and OR = 2.11, respectively), with lower education levels (OR = 2.08, OR = 1.86, for middle and elementary education, respectively) and with diabetes mellitus (OR = 1.53). This study confirms a high burden of periodontitis in the target (Portuguese) sub-population. The findings provide a comprehensive understanding that will empower appropriate national public oral health programmes and population-based preventive actions.
Subject terms: Risk factors, Dental diseases, Chronic inflammation
Introduction
Prevalence of periodontal diseases endures a substantial epidemiological challenge, while estimates presented in recent years have been very dissimilar, even in countries with alike socio-economically standards1–3. Thus, these have contributed to the lack of comprehensive understanding of the periodontal status worldwide. In addition, periodontitis has a large socioeconomic impact and it is estimated that is responsible for 54 billion USD/year in lost productivity and a major portion of the 442 billion USD/year cost for oral diseases4. Also, these polymicrobial inflammatory diseases are extremely impacting on other systemic conditions, such as diabetes mellitus (DM)5, stroke6,7, rheumatoid arthritis8, inflammatory bowel disease9, or preterm birth10.
Over the last decades, periodontitis case definitions have undergone paradigmatic changes evolving from a diagnosis based in terms of clinical attachment loss (CAL) and probing depth (PD), as proposed by the CDC Working Group11 and revised accordingly12, to a diagnosis proposed in the new American Association of Periodontology (AAP)/European Federation of Periodontology (EFP) based mainly upon CAL and considering the interproximal space as an adjacent common zone13. In fact, all efforts made to improve these diagnostic criteria focused on the prevention of underestimation of periodontitis and to reveal the natural history of periodontitis, especially in older subjects.
To date, very few data have provided a comprehensive assessment of the periodontal status of the Portuguese population14–16. A single national epidemiological study was conducted, in 2015, by the Portuguese Health General Directorate15 using the Community Periodontal Index of Treatment Needs (CPITN). The obtained results estimated a prevalence of 10.8% and 15.3% of periodontal diseases in adults and elderly, respectively15. These results contrast, specifically, and due to geographical proximity, with the last national Spanish periodontal survey where 38.4% of subjects had periodontal pockets17, as well with other developed countries studies where found prevalence ranged from 51.0 to 88.3% in the USA, Italy (Turin), Norway (Troms) or Germany (Pomerania)12,18–21 and World Health Organization (WHO) global reports3.
Due to the recent disclosure of the new periodontal stage consensus13, there is still limited data coming from epidemiological studies employing these diagnostic criteria in Europe. Also, the available Portuguese national epidemiologic data relies on CPITN methodology which is inadequate to describe the periodontal status of populations22. Consequently, it is essential to carry out studies using the new case definitions which will allow a comprehensive understanding of the current periodontal status in the Portuguese population and the assessment of associated risk factors, to allow future international comparability. Therefore, it is mandatory to investigate the burden of periodontal disease of a Portuguese target population and its associated risk factors, to serve as a foundation for future national public health strategies.
Therefore, this study was aimed to investigate the distribution of periodontal diseases using a population-based stratified sample of adults from the southern region of the Lisbon Metropolitan Area. The prime purposes of this study were: (1) to comprehensively describe the prevalence and extent of periodontal diseases according to the Workshop in 201713, (2) to evaluate potential periodontal diseases risk indicators.
Results
Study sample
The characteristics of the 1,064 subjects included in the study, according to the periodontal diagnosis, are shown in Table 1. The mean age of participants was 60.9 (±16.3) years, 58.0% were women, 63.3% reported having an elementary education level and 52.2% were retired. The prevalence of moderate and severe periodontitis increased with age. Moreover, the majority of the population (81.9%) report not knowing what periodontal disease is, 37.6% brush their teeth once or less daily, and 70.2% of subjects with severe periodontitis have never performed interproximal cleaning.
Table 1.
Sociodemographic characteristics, behaviors, attitudes towards oral health and medical information (diabetes and comorbidity) of the included participants, presented as n (%), according to the severity of periodontal status (N = 1,064).
| No Disease n (%) | Gingivitis n (%) | Mild n (%) | Moderate n (%) | Severe n (%) | Total n (%) | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 117 (34.2) | 23 (27.1) | 64 (43.8) | 111 (47.0) | 132 (51.8) | 447 (42.0) |
| Female | 225 (65.8) | 62 (72.9) | 82 (56.2) | 125 (53.0) | 123 (48.2) | 617 (58.0) |
| Age (years) | ||||||
| 18–30 | 34 (9.9) | 17 (20.0) | 10 (6.8) | 1 (0.4) | 0 (0.0) | 62 (5.8) |
| 31–40 | 42 (12.3) | 7 (8.2) | 11 (7.5) | 10 (4.2) | 5 (2.0) | 75 (7.1) |
| 41–50 | 62 (18.1) | 11 (12.9) | 21 (14.4) | 23 (9.8) | 19 (7.4) | 136 (12.8) |
| 51–60 | 50 (14.6) | 5 (5.9) | 15 (10.3) | 32 (13.6) | 35 (13.7) | 137 (12.9) |
| 61–70 | 82 (24.0) | 26 (30.6) | 45 (30.8) | 81 (34.3) | 94 (36.9) | 328 (30.8) |
| 71–80 | 58 (17.0) | 16 (18.8) | 38 (26.0) | 57 (24.2) | 75 (29.4) | 244 (22.9) |
| >80 | 14 (4.1) | 3 (3.5) | 6 (4.1) | 32 (13.6) | 27 (10.6) | 82 (7.7) |
| Educational level | ||||||
| No education | 8 (2.3) | 3 (3.5) | 6 (4.1) | 11 (4.7) | 14 (5.5) | 42 (4.0) |
| Elementary | 176 (51.5) | 50 (58.8) | 94 (64.4) | 173 (73.3) | 180 (70.6) | 673 (63.3) |
| Middle | 94 (27.5) | 23 (27.1) | 35 (24.0) | 38 (16.1) | 43 (16.9) | 233 (21.9) |
| Higher | 64 (18.7) | 9 (10.6) | 11 (7.5) | 14 (5.9) | 18 (7.1) | 116 (10.9) |
| Marital status | ||||||
| Single | 81 (23.7) | 23 (27.1) | 22 (15.1) | 22 (9.3) | 22 (8.6) | 170 (16.0) |
| Married/Union of fact | 213 (62.3) | 49 (57.7) | 92 (63.0) | 158 (66.9) | 172 (67.5) | 684 (64.3) |
| Divorced | 25 (7.3) | 8 (9.4) | 20 (13.7) | 25 (10.6) | 25 (9.8) | 103 (9.7) |
| Widowed | 23 (6.7) | 5 (5.9) | 12 (8.2) | 31 (13.1) | 36 (14.1) | 107 (10.1) |
| Occupation | ||||||
| Student | 11 (3.2) | 7 (8.2) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 19 (1.8) |
| Employed | 138 (40.4) | 27 (31.8) | 53 (36.3) | 52 (22.0) | 57 (22.3) | 327 (30.7) |
| Unemployed | 63 (18.4) | 16 (18.8) | 13 (8.9) | 35 (14.8) | 36 (14.1) | 163 (15.3) |
| Retired | 130 (38.0) | 35 (41.2) | 79 (54.1) | 149 (63.1) | 162 (63.5) | 555 (52.2) |
| Smoking status | ||||||
| Non-smoker | 238 (69.6) | 58 (68.2) | 88 (60.3) | 122 (51.7) | 120 (47.1) | 626 (58.8) |
| Former smoker | 71 (6.7) | 14 (16.5) | 38 (26.0) | 76 (32.2) | 94 (36.9) | 293 (27.5) |
| Current Smoker | 33 (3.1) | 13 (15.3) | 20 (13.7) | 38 (16.1) | 41 (16.1) | 145 (13.6) |
| Family income (monthly, €) | ||||||
| <=600 | 74 (22.0) | 24 (30.0) | 37 (25.5) | 61 (26.2) | 74 (29.3) | 270 (25.8) |
| 601–1,500 | 194 (57.7) | 46 (57.5) | 92 (63.4) | 137 (58.8) | 143 (56.5) | 612 (58.4) |
| >1,500 | 68 (20.2) | 10 (12.5) | 16 (11.0) | 35 (15.0) | 36 (14.2) | 165 (15.8) |
| Periodontal diseases awareness | ||||||
| Yes | 75 (21.9) | 12 (14.1) | 26 (17.8) | 36 (15.2) | 44 (17.3) | 193 (18.1) |
| No | 267 (78.1) | 73 (85.9) | 120 (82.2) | 200 (84.8) | 211 (82.7) | 871 (81.9) |
| Brushing frequency (daily) | ||||||
| 3+ | 65 (19.0) | 16 (18.8) | 24 (16.4) | 39 (16.5) | 27 (10.6) | 171 (16.1) |
| 2 | 196 (57.3) | 36 (42.3) | 74 (50.7) | 122 (51.7) | 132 (51.8) | 560 (52.6) |
| 1 | 78 (22.8) | 29 (34.1) | 46 (31.5) | 65 (27.5) | 84 (32.9) | 302 (28.4) |
| 0 | 3 (0.9) | 4 (4.7) | 2 (1.4) | 10 (4.2) | 12 (4.7) | 31 (2.9) |
| Interproximal cleaning | ||||||
| Yes | 82 (24.0) | 10 (11.8) | 31 (21.2) | 36 (15.3) | 26 (10.2) | 185 (17.4) |
| Occasionally | 71 (20.8) | 10 (11.8) | 25 (17.1) | 27 (11.4) | 28 (11.0) | 161 (15.1) |
| No | 189 (55.3) | 65 (76.5) | 90 (61.6) | 173 (73.3) | 201 (78.8) | 718 (67.5) |
| Diabetes Mellitus | ||||||
| Yes | 44 (12.9) | 9 (10.6) | 31 (21.2) | 46 (19.5) | 74 (29.0) | 204 (19.2) |
| No | 298 (87.1) | 76 (89.4) | 115 (78.8) | 190 (80.5) | 181 (71.0) | 860 (80.8) |
| Comorbidity (by Aimetti 2015) | ||||||
| Yes | 260 (76.0) | 63 (74.1) | 123 (84.2) | 206 (87.3) | 227 (89.0) | 879 (82.6) |
| No | 82 (24.0) | 22 (25.9) | 23 (15.8) | 30 (12.7) | 28 (11.0) | 185 (17.4) |
| Total | 342 (32.1) | 85 (8.0) | 146 (13.7) | 236 (22.2) | 255 (24.0) | 1064 (100.0) |
Prevalence and severity of periodontal disease
The prevalence of periodontitis (mild, moderate and severe stages) was 59.9% (95% CI 56.9–62.8), with 22.2% of moderate stage (95% CI 19.7–24.8%) and 24.0% of severe stage (95% CI 21.4–26.6%), respectively. The prevalence and correspondent estimates of localized and generalized periodontitis amounted to 23.2% (95% CI: 20.7–25.9%) and 36.7% (95% CI: 33.8–39.6%) respectively (Table 2). Further, periodontal health is a well distributed status, whereas periodontal diseases exhibited a distinct scattering (Fig. 1).
Table 2.
Prevalence of localized and generalized periodontitis, stratified by age and gender.
| Females | Males | Total | ||||
|---|---|---|---|---|---|---|
| n | Prev. (95% CI) (%) | n | Prev. (95% CI) (%) | n | Prev. (95% CI) (%) | |
| Localized | 139 | 56.3 (50.1–62.5) | 108 | 43.7 (37.5–49.9) | 247 | 23.2 (20.7–25.9) |
| 18–30 | 5 | 3.6 (0.5–6.7) | 6 | 5.6 (1.2–9.9) | 11 | 4.5 (1.9–7.0) |
| 31–40 | 14 | 10.1 (5.1–15.1) | 5 | 4.6 (0.7–8.6) | 19 | 7.7 (4.4–11.0) |
| 41–50 | 20 | 14.4 (8.6–20.2) | 10 | 9.3 (3.8–14.7) | 30 | 12.1 (8.1–16.2) |
| 51–60 | 20 | 14.4 (8.6–20.2) | 13 | 12.0 (5.9–18.2) | 33 | 13.4 (9.1–17.6) |
| 61–70 | 51 | 36.7 (28.7–44.7) | 34 | 31.5 (22.7–40.2) | 85 | 34.4 (28.5–40.3) |
| 71–80 | 25 | 18.0 (11.6–24.4) | 28 | 25.9 (17.7–34.2) | 53 | 21.5 (16.3–26.6) |
| 80+ | 4 | 2.9 (0.1–5.7) | 12 | 11.1 (5.2–17) | 16 | 6.5 (3.4–9.5) |
| Generalized | 191 | 49.0 (44.0–54.0) | 199 | 51.0 (46.0–56.0) | 390 | 36.7 (33.8–39.6) |
| 18–30 | 0 | 0.0 (0.0–0.0) | 0 | 0.0 (0.0–0.0 | 0 | 0.0 (0.0–0.0 |
| 31–40 | 5 | 3.6 (1–6.2) | 2 | 1.9 (0.0–3.7) | 7 | 1.8 (0.5–3.1) |
| 41–50 | 13 | 9.4 (5.2–13.5) | 20 | 18.5 (13.1–23.9) | 33 | 8.5 (5.7–11.2) |
| 51–60 | 23 | 16.5 (11.3–21.8) | 26 | 24.1 (18.1–30.0) | 49 | 12.6 (9.3–15.9) |
| 61–70 | 71 | 51.1 (44–58.2) | 64 | 59.3 (52.4–66.1) | 135 | 34.6 (29.9–39.3) |
| 71–80 | 57 | 41. (34–48) | 60 | 55.6 (48.7–62.5) | 117 | 30.0 (25.5–34.5) |
| 80+ | 22 | 15.8 (10.7–21) | 27 | 25. (19–31.0) | 49 | 12.6 (9.3–15.9) |
| Molar–Incisor Pattern | 330 | — | 307 | — | 637 | — |
| No | 311 | 94.2 (91.7–96.8) | 295 | 96.1 (93.9–98.3) | 606 | 95.1 (93.5–96.8) |
| Yes | 19 | 5.8 (3.2–8.3) | 12 | 3.9 (1.7–6.1) | 31 | 4.9 (3.2–6.5) |
Figure 1.
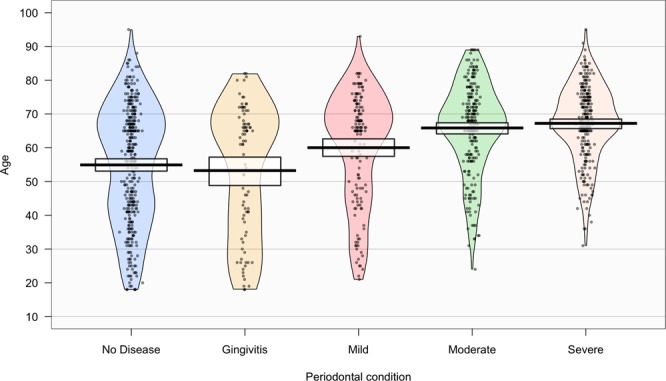
Density plot exhibiting the distribution of periodontal conditions over the age range.
Clinical attachment loss (CAL) and probing depth (PD)
The mean values of PD, CAL, recession (REC), missing teeth and teeth with mobility as well the prevalence and extent of CAL and PD by selected threshold are presented in Table 3. Mean PD and the number of sites with PD ≥4 mm and ≥6 mm remained similar across all age groups. The average CAL and number of sites with CAL ≥4 mm and ≥6 mm were unequally distributed in the population for all age groups, increasing with age, while exhibiting a moderate significant correlation. The number of missing teeth is also related to the mean CAL across age groups, that is, the higher the number of missing teeth the greater the CAL average but for PD this is not so evident (Fig. 2). Mean REC, missing teeth and teeth with mobility also increased with age increase.
Table 3.
PD, CAL, REC, missing teeth and teeth with mobility (presented as mean, standard deviation and 95% CI for mean), stratified by CAL and PD thresholds (%) (≥4 and ≥6 mm) and age group.
| Measures | 18–30 | 31–40 | 41–50 | 51–60 | 61–70 | 71–80 | >80 | rho* (p-value) | Total |
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) [95% CI] | Mean (SD) [95% CI] | Mean (SD) [95% CI] | Mean (SD) [95% CI] | Mean (SD) [95% CI] | Mean (SD) [95% CI] | Mean (SD) [95% CI] | Mean (SD) [95% CI] | ||
| Mean PD (mm) |
1.8 (0.4) [1.7–1.9] |
1.9 (0.6) [1.7–2.0] |
1.9 (0.7) [1.8–2.1] |
2.0 (0.8) [1.9–2.1] |
2.0 (0.9) [1.9–2.1] |
1.9 (0.7) [1.8–2.0] |
1.9 (0.7) [1.7–2.0] |
−0.027 (0.382) |
1.9 (0.8) [1.9–2.0] |
| PD ≥ 4 mm (%) |
3.8 (5.4) [1.4–4.1] |
6.3 (13.6) [3.2–9.5] |
7.9 (13.8) [5.5–10.2] |
9.5 (15.5) [6.9–12.1] |
9.5 (17.4) [7.7–11.4] |
7.6 (14.3) [5.8–9.4] |
6.4 (13.6) [3.4–9.4] |
0.015 (0.614) |
8.0 (15.0) [7.1–8.9] |
| PD ≥ 6 mm (%) |
0.0 (0.2) [0.0–0.1] |
1.0 (4.2) [0.1–2.0] |
1.3 (4.4) [0.5–2.0] |
1.6 (3.9) [1.0–2.3] |
2.2 (6.5) [1.5–2.9] |
1.8 (6.1) [1.1–2.6] |
0.9 (3.1) [0.3–1.6] |
0.047 (0.126) |
1.6 (5.3) [1.3–1.9] |
| Mean CAL (mm) |
1.8 (0.4) [1.7–1.9] |
2.0 (0.8) [1.8–2.2] |
2.2 (1.0) [2.1–2.4] |
2.6 (1.5) [2.4–2.9] |
2.9 (1.6) [2.8–3.1] |
2.9 (1.4) [2.8–3.1] |
3.4 (1.5) [3.1–3.7] |
0.349 (<0.001) |
2.7 (1.4) [2.6–2.8] |
| CAL ≥ 4 mm (%) |
3.1 (5.5) [1.7–4.5] |
7.9 (15.9) [4.2–11.5] |
14.8 (21.0) [11.2–18.4] |
22.4 (26.7) [17.9–26.9] |
27.9 (28.7) [24.8–31.0] |
30.1 (27.3) [26.6–33.5] |
40.4 (30.8) [33.7–47.2] |
0.416 (<0.001) |
24.1 (27.4) [22.5–25.8] |
| CAL ≥ 6 mm (%) |
0.1 (0.2) [0.0–0.1] |
2.1 (9.5) [0.0–4.2] |
4.0 (10.7) [2.2–5.8] |
8.9 (18.0) [5.9–12.0] |
12.1 (22.0) [9.7–13.5] |
10.9 (18.4) [8.6–13.2] |
15.0 (20.7) [10.4–19.5] |
0.336 (<0.001) |
9.2 (18.4) [8.1–10.3] |
| Mean REC (mm) |
0.0 (0.0) [0.0–0.0] |
0.1 (0.3) [0.0–0.2] |
0.3 (0.5) [0.2–0.4] |
0.7 (0.9) [0.5–0.8] |
1.0 (1.1) [0.8–1.1] |
1.1 (1.1) [0.9–1.2] |
1.6 (1.2) [1.3–1.8] |
0.562 (<0.001) |
0.8 (1.0) [0.7.0.8] |
| Missing Teeth (n) |
0.9 (1.2) [0.6–1.2] |
2.3 (3.3) [1.5–3.0] |
5.1 (5.1) [4.2–6.0] |
8.2 (5.5) [7.2–9.1] |
10.8 (6.5) [10.0–11.5] |
12.0 (6.6) [11.2–12.8] |
14.0 (7.5) [12.3–15.6] |
0.544 (<0.001) |
9.1 (7.0) [8.6–9.5] |
| Teeth with mobility (n) |
0.1 (0.4) [0.0–0.2] |
0.3 (1.4) [0.0–0.6] |
0.6 (1.5) [0.3–0.8] |
1.3 (2.5) [0.9–1.8] |
1.4 (2.6) [1.1–1.7] |
1.1 (1.9) [0.9–1.4] |
1.3 (2.2) [0.8–1.8] |
0.197 (<0.001) |
1.1 (2.2) [0.9–1.2] |
*Overall trend across age groups assessed by Spearman’s rank correlation coefficient (rho). Significant correlations identified in bold (p < 0.05).
Figure 2.
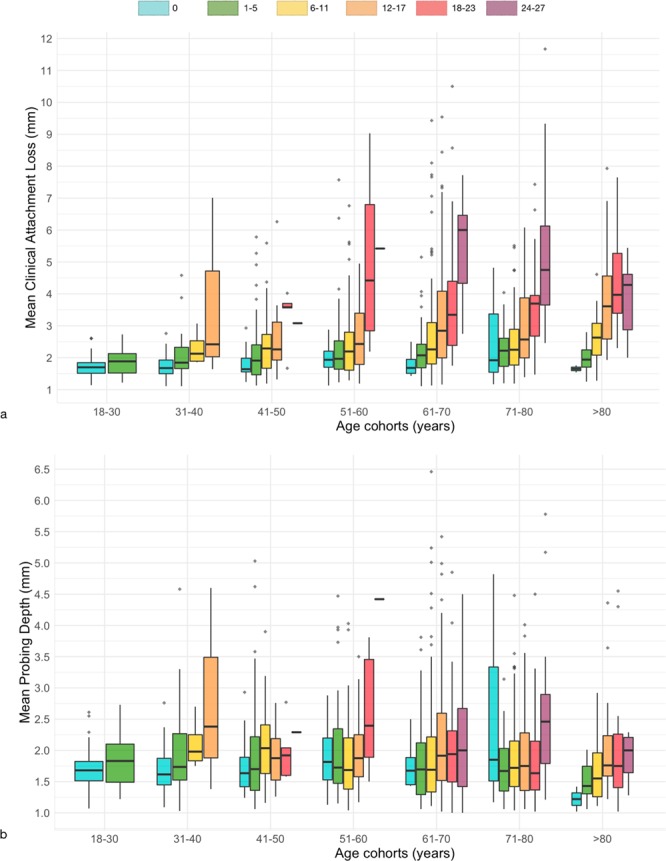
Distribution of tooth loss (coloring) as a function of mean clinical attachment loss (a) or mean probing depth (b), according to age cohorts (x-axis).
Bleeding on probing (BoP) and plaque index (PI)
The mean values of BoP and PI, stratified by periodontitis severity and age group, are presented in Table 4. BoP was equally distributed in the population for all age groups, and increased with level of severity of periodontitis, with a mean of 5.7% for persons with no periodontitis, 15.9% for persons with non-severe periodontitis and 28.5% for persons with severe periodontitis. Similarly, the average PI was 23.2%, and increased with the severity of periodontitis and age.
Table 4.
Mean Bleeding on Probing (BoP) and Plaque Index (PI) (%) (presented as mean, standard deviation and 95% CI for mean), stratified by periodontitis severity and age group.
| Measures | 18–30 | 31–40 | 41–50 | 51–60 | 61–70 | 71–80 | >80 | rho* (p-value) | Total |
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) [95% CI] | Mean (SD) [95% CI] | Mean (SD) [95% CI] | Mean (SD) [95% CI] | Mean (SD) [95% CI] | Mean (SD) [95% CI] | Mean (SD) [95% CI] | Mean (SD) [95% CI] | ||
| Mean BoP (%) |
13.6 (17.1) [9.3–18.9] |
12.5 (16.8) [8.7–16.4] |
15.1 (20.0) [11.7–18.5] |
15.7 (22.5) [11.9–19.5] |
15.2 (21.3) [12.9–17.5] |
14.9 (20.9) [12.2–17.5] |
14.3 (20.1) [9.9–18.8] |
−0.021 (0.502) |
14.8 (20.6) [13.6.16.1] |
| No Periodontitis |
8.3 (9.8) [5.6–11.1] |
5.6 (8.3) [3.2–8.0] |
5.4 (9.6) [3.1–7.6] |
2.9 (4.5) [1.7–4.1] |
6.1 (10.8) [4.0–8.1] |
5.5 (8.3) [3.6–7.5] |
5.8 (13.5) [–1.2-12.7] |
−0.096 (0.048) |
5.7 (9.3) [4.8–6.5] |
| Non-severe Periodontitis |
38.1 (22.4) [23.1–53.1] |
23.5 (19.7) [14.5–32.5] |
20.2 (16.8) [15.1–25.3] |
17.8 (17.9) [12.6–23.1] |
13.5 (18.8) [10.1–16.8] |
13.8 (20.9) [9.6–18.1] |
11.3 (17.5) [5.5–17.0] |
−0.287 (<0.001) |
15.9 (19.6) [13.9–17.9] |
| Severe Periodontitis | — |
34.2 (25.0) [3.1–65.3] |
40.5 (28.5) [26.8–54.3] |
33.1 (31.4) [22.3–43.9] |
27.9 (26.7) [22.4–33.4] |
25.4 (24.8) [19.7–31.1] |
24.0 (23.5) [14.7–33.3] |
0.127 (0.043) |
28.5 (26.8) [25.2–31.9] |
| Total Periodontitis |
38.1 (22.4) [23.1–53.1] |
25.6 (20.7) [17.2–33.9] |
26.3 (22.8) [20.6–32.1] |
24.3 (25.5) [18.7–30.0] |
19.6 (23.6) [16.5–22.8] |
18.9 (23.4) [15.4–22.5] |
16.6 (13.5) [11.4–21.8] |
−0.181 (<0.001) |
21.0 (23.5) [19.1–22.8] |
| Mean PI (%) |
11.0 (15.9) [7.0–15.0] |
10.6 (21.0) [5.7–15.4] |
11.4 (19.8) [8.1–14.8] |
20.3 (24.4) [15.3–25.3] |
23.7 (29.9) [20.4–26.9] |
31.1 (32.9) [27.0–35.3] |
42.6 (37.6) [34.3–50.8] |
0.296 (<0.001) |
23.2 (30.3) [21.4–25.0] |
| No Periodontitis |
9.5 (14.8) [5.4–13.7] |
7.3 (15.0) [3.0–11.6] |
6.4 (14.2) [3.0–9.7] |
7.7 (13.9) [3.9–11.4] |
13.6 (22.7) [9.3–17.9] |
20.9 (26.2) [14.9–27.0] |
29.3 (36.7) [10.4–48.2] |
0.220 (<0.001) |
12.3 (21.1) [10.3–14.3] |
| Non-severe Periodontitis |
17.9 (20.0) [4.5–31.3] |
17.7 (31.3) [3.4–32.0] |
16.4 (23.1) [9.3–23.4] |
17.0 (22.5) [10.4–23.5] |
24.2 (29.1) [19.1–29.3] |
33.5 (34.8) [26.4–40.5] |
42.1 (38.0) [29.6–54.6] |
0.231 (<0.001) |
26.0 (31.1) [22.8–29.1] |
| Severe Periodontitis | — |
12.7 (13.8) [−4.4–29.7] |
19.3 (25.2) [7.2–31.5] |
44.7 (39.8) [31.0–58.4] |
34.6 (34.2) [27.6–41.6] |
38.2 (34.2) [30.3–46.0] |
51.7 (36.1) [37.4–65.9] |
0.191 (0.002) |
37.3 (35.1) [33.0–41.6] |
| Total Periodontitis |
17.9 (20.0) [4.5–31.3] |
16.7 (28.6) [5.2–28.3] |
17.3 (23.6) [11.4–23.2] |
28.8 (33.8) [21.4–36.2] |
28.7 (31.7) [24.4–32.9] |
35.5 (34.5) [30.3–40.8] |
46.1 (37.3) [36.8–55.3] |
0.231 (<0.001) |
30.5 (33.2) [27.9–33.1] |
BoP – Bleeding on Probing, PI – Plaque Index, SD – Standard Deviation.
*Overall trend across age groups assessed by Spearman’s rank correlation coefficient (rho). Significant correlations identified in bold (p < 0.05).
Risk factors for periodontitis
Crude and adjusted Odds Ratio (OR) for putative risk factors towards periodontitis were determined and are presented in Table S2 (Supplemental) and Table 5, respectively. Within the final reduced model obtained by a multivariate logistic regression procedure, age (OR = 1.05, 95% CI: 1.04–1.06), educational level (OR = 2.08, 95% CI: 1.32–3.27, OR = 1.86, 95% CI: 1.13–3.05, for middle and elementary education, respectively), smoking status (OR = 3.76, 95% CI: 2.44–5.80 and OR = 2.11, 95% CI: 1.52–2.91, for current smoker and former smoker, respectively) and DM (OR = 1.53, 95% CI: 1.06–2.21) were the significantly risk indicators that were identified towards periodontitis.
Table 5.
Multivariate logistic regression analysis (final reduced model) (*) on potential risk factors towards periodontitis.
| Predictor variables | Odds Ratio (OR) | OR (95% CI) | p-value |
|---|---|---|---|
| Age | 1.05 | 1.04–1.06 | <0.001 |
| Education | |||
| Higher | 1 | — | — |
| Middle | 2.08 | 1.32–3.27 | 0.002 |
| Elementary | 1.86 | 1.13–3.05 | 0.015 |
| No education | 2.08 | 0.88–4.90 | 0.095 |
| Smoking status | — | — | <0.001 |
| Non-smoker | 1 | — | — |
| Current Smoker | 3.76 | 2.44–5.80 | <0.001 |
| Former smoker | 2.11 | 1.52–2.91 | <0.001 |
| Diabetes Mellitus | |||
| No | 1 | — | — |
| Yes | 1.53 | 1.06–2.21 | 0.023 |
*The model was statistically significant, χ2(7) = 174.786, p < 0.001, explained 20.5% (Nagelkerke R2) of the variance and correctly classified 68.7% of cases.
Discussion
This is the first periodontal population-based representative study carried out in Portugal and one of the very first to use the new periodontitis and gingivitis case definitions13,23. The results of this epidemiological study indicate that seven out of ten adults in the target population had some type of periodontal disease, and six out of ten had periodontitis. Moreover, almost half of the population exhibited moderate and severe periodontitis.
In particular, this study sample was low educated, with the majority being below secondary education, and a largest share were under a situation of work inactivity. Also, they self-reported good brushing frequency, poor interproximal cleaning habits and low periodontal disease awareness, being equivalent to the national average24. Regarding the systemic state, a very high percentage presented comorbidities. The prevalence of smokers and former smokers were 13.6% and 27.5%, respectively. The DM prevalence was slightly above the national average, however it is explained by the greater percentage of elderly among the included sample25.
In the Portuguese panorama, oral health care is mostly provided in private (liberal) practices26. Further, there is a lack of dental services in public hospitals and health centres from the National Health Service, and the absence of domiciliary dental care26. Besides, the Portuguese Public Oral Health Programme (PPOHP) has only started in 2005 and, in 2008, limited “dental vouchers” were created focusing children, adolescents and vulnerable groups (pregnant, Human Immunodeficiency Virus patients and elders with low-socioeconomic status)27. Besides, the serious financial constraints from the 2007–2008 financial crisis have limited access to private dental services. Thus, these reasons might have contributed to the high prevalence of periodontal disease reported in the present study, and support the necessity of a comprehensive national oral program, greater availability of dental services and increased awareness for these diseases.
In Portugal, to date, there is only one national epidemiological study on the prevalence of periodontal disease, though it can not be compared with the present study because it used CPITN methodology15. Oppositely, the present findings indicated a higher severity of periodontal destruction. In fact, the use of partial recording protocols underestimate the periodontal prevalence and extent by almost 50%22,28.
Few periodontal epidemiological studies provide comprehensive and comparable information in Europe. Furthermore, due to the novelty of the AAP/EFP consensus, the number of studies using this case definition is still scarce. When compared to other European population-based representative studies, the Tromstannen–Oral Health in Northern Norway (TOHNN) study reported an overall prevalence of 9.1% of severe periodontitis21, and in the Periodontitis and Its Relation to Coronary Artery Disease (PAROKRANK) in Sweden the prevalence of severe periodontitis was of 6.2%29. The Study of Health in Pomerania (SHIP) revealed a prevalence of 17.6% of severe periodontitis and 25.3% of moderate periodontitis30, while for the Turin regional survey these prevalence were of 39.9% and 40.8% for severe and moderate periodontitis respectively18. In the USA, the National Health and Nutrition Examination Survey (NHANES) 2009–2012 estimates of severe and moderate periodontitis were of 8.9% and 30.9%, respectively12.
Drawing parallels with the findings of the present investigation, the prevalence of severe periodontitis was only surpassed by the Turin study, whereas for moderate periodontitis the estimates ranked lower18. Notwithstanding, the aforementioned studies used the CDC/AAP case definition as already mentioned, and it is not known what is the difference magnitude between these two classifications.
Undoubtedly, the prevalence of moderate and severe periodontitis peaked in the age of 61–70 years old (34.3% and 36.9%, respectively), having subsequently reduced. Another important aspect to be addressed is the relevantly high prevalence of both localized (34.4%) and generalized (34.6%) periodontitis in the same age interval. Similar results have been found in other articles18,21,30.
Further, the multivariate logistic regression analysis performed in this study revealed age, education, smoking status and diabetes mellitus as significantly potential risk factors towards periodontitis.
Similarly to previous literature, periodontal complications was linked to aging within this population12,18,21,31,32. Moreover, the clinical periodontal hallmarks (CAL and PD), tooth loss and teeth with mobility were age-related. However, previous data and this survey suggest that intact supporting periodontal tissues prevail in patients of all age ranges, suggesting pathological CAL is not an aging consequence per se32,33.
Concerning the smoking status, being an active smoker was strongly associated with periodontitis (adjusted OR = 3.76), while past smoking history revealed a lower but also significant association (adjusted OR = 2.11). These results are in accordance with previous studies whose OR ranged between 2 and 614,18,34–36 and is widely accepted that smoking has a harmful effect on the onset and progression of periodontitis along with other risk factors for periodontitis13,37,38. Likewise, it is also very important to highlight the influence of a past history of smoking activity and the repercussions of bad behaviours on the periodontal status and on tooth loss in the long-term39.
Regarding the education level, low educated people had a higher risk of having periodontitis being in line with previous reports12,19,21,31,40. In this population, the number of low educated participants is substantial and can be explained by the high number of elderly population and represent a generation that had little educational access. As a risk factor, low educational attainment has been linked to a greater loss of periodontal support and is more prominent when evaluated together with other sociocultural determinants1,41,42.
As well, DM was a risk factor towards periodontitis in this population in the same way as established in the literature5,43,44. Diabetes increases the risk for periodontitis (particularly if poorly controlled) and evidence suggests that advanced periodontitis also compromises glycaemic control. The new consensus has established DM as a grading modifier for the progression of periodontitis through the glycated hemoglobin (HbA1c) levels. Though, we have recorded HbA1c of our DM patients, as part of the standard clinical follow-up in FHUs, most non-DM patients have never been analyzed for, and to prevent bias in the multivariate analysis we will address this in a future focused study.
Strengths and limitations
This survey has numerous strengths, including the representativeness and global geographic coverage based on the FHUs where the study was carried out, the sample size calculation stratified for each FHU, the strict methodology followed and the employment of the new AAP/EFP case definition enabling future comparability across studies.
Nevertheless, there are some shortcomings to mention. Due to the peculiarly low periodontitis prevalence previously reported and that based sample size calculation, more than half of the participants had ≥61 years old, which might have overestimated the prevalence of periodontitis. Also, the target population’s sociodemographic characteristics and oral hygiene behaviours must be carefully considered when extrapolating the present findings to other European populations, particularly the elderly subset that had low education and economic constraints. Lastly, people were directly invited to participate in the study, which can bias the population coverage for sampling, however also increases the probability of having a more accurate representation of the participant’s oral situation.
These findings provide new knowledge that will empower appropriate public oral health programmes and population-based preventive actions. Our results support that a comprehensive national oral program with higher accessibility is imperative. A national study is urgently needed to confirm the data reported in our investigation. Next, it will be essential to follow risk groups highlighted by the model developed in this study, namely the elderly, diabetics, smokers and those with low education. Finally, it is necessary to increase the population awareness to periodontitis signs, symptoms and consequences to the general health.
Conclusions
This study reveals a high burden of periodontitis in the adult population of the southern region of the Lisbon Metropolitan Area, in Portugal. Age, education level, smoking status and diabetes mellitus were identified as significantly potential risk factors towards periodontitis.
Methods
This study was approved by the Research Ethics Committee of the Regional Health Administration of Lisbon and Tagus Valley, IP (Portugal) (Approval numbers: 3525/CES/2018 and 8696/CES/2018) and in accordance with the Declaration of Helsinki, as revised in 2013. Following examination, each participant was informed of their periodontal status and signed informed consent prior to enrollment. Patients with diagnosed periodontal diseases were referred to the Egas Moniz Dental Clinic (EMDC) for treatment without additional costs. This survey followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines45.
Study design and sampling procedure
The Study of Periodontal Health in Almada-Seixal (SoPHiAS) was designed as a population-based cross-sectional representative study, geographically stratified, with a target population of subjects over 18 years of age (adults and elderly), living in the municipalities of Almada and Seixal, in Portugal. Almada and Seixal, are two of the largest municipalities located in the southern part of the Lisbon Metropolitan Area, a NUTS II region (PT17). This region, with over 2.8 million inhabitants, includes 18 municipalities and is the most populated Portuguese Metropolitan Area and the second most populated NUTS II region of the country. In Portugal, all residents are covered by the National Health System and assigned to a General Practitioner of a public Family Health Unit (FHU). FHUs are grouped in Health Centers grouping (ACES), depending on the geographic region. For this study, the ACES Almada-Seixal was defined as the study group. All twenty-two ACES Almada-Seixal FHUs were included to ensure a global geographic and socioeconomic coverage of the Almada and Seixal territory. In September 2018, according to the institutional data provided, the two municipalities had 386,168 inhabitants in the selected age groups (adults and elderly). To achieve an estimate of the periodontitis prevalence in the population, with a margin of error of 3.0%, for a 95% confidence level, a minimum of 962 individuals were needed to be examined, based on the previously reported national prevalence data of 10.8% and 15.3%, for adults and elderly, respectively15. The required sample was stratified according to the number of adult (age group from 18–64 years) and elderly (65 years or older) subjects assigned to each FHU, based on the information provided by ACES Almada-Seixal. The invitation to participate in the survey was made by direct contact at the waiting room of the FHU, explaining the purpose of the study and including a description of the clinical examination. After a detailed explanation with the information sheet delivery to the patient, individuals who agreed to participate signed the informed consent form. A questionnaire was completed by each subject and collected before the periodontal examination.
Gingivitis and periodontitis case definitions
Gingivitis and periodontitis cases were defined according to the new AAP/EFP consensus13,23. A gingivitis case was defined if total score of bleeding on probing (BoP) ≥10%23. A participant was a periodontitis case if: interdental CAL ≥2 non‐adjacent teeth, or Buccal or Oral CAL ≥3 mm with PD >3 mm is detectable at ≥2 teeth. Then, periodontitis staging was defined according to severity and extent13. For the severity, interdental CAL at site of greatest loss of 1–2 mm, 3–4 and ≥5 was considered as mild (stage 1), moderate (stage 2), and severe (stage 3 and stage 4), respectively13. Additionally, patients were defined for the extent, wherein a case was described as localized (<30% of teeth involved), generalized (≥30% of teeth involved) or molar/incisor pattern13.
Clinical periodontal examination
Two calibrated investigators (VM and JB) performed a full-mouth periodontal examination, on an average of 30 minutes. Each clinical examination was performed under proper lighting with the individuals seated on a regular adjustable stretcher in the FHU’s medical office. No radiographic examination was made.
All fully erupted teeth, excluding third molars, implants and retained roots, were examined by means of a daily sterilized dental mirror and a manual periodontal North Carolina probe (Hu-Friedy; Chicago, Illinois, USA). The number of missing teeth was recorded. Further, dichotomous plaque index (PI), gingival recession (REC), PD, and BoP were circumferentially recorded at six sites per tooth (mesiobuccal, buccal, distobuccal, mesiolingual, lingual, and distolingual). PD was measured as the distance from the free gingival margin to the bottom of the pocket and REC as the distance from the cementoenamel junction (CEJ) to the free gingival margin, and this assessment was assigned a negative sign if the gingival margin was located coronally to the CEJ. CAL was calculated as the algebraic sum of REC and PD measurements for each site. The measurements were rounded to the lowest whole millimeter. Furcation involvement (FI) was assessed using a 2 N probe (Hu-Friedy; Chicago, Illinois, USA) following46 in molars, and upper first premolars if applicable, and tooth mobility was appraised following.
Sociodemographic and medical questionnaires
Information on sociodemographic characteristics and behaviors was collected by self-reported questionnaire. The questionnaire covered questions on the following items: (1) gender, age, marital status, educational level, occupation; (2) monthly family gross income; (3) smoking habits; (4) oral hygiene-related behaviors (tooth brushing frequency, interproximal cleaning, etc.); (5) attitudes and awareness towards oral health; (6) diabetes mellitus (DM) and comorbidities18.
Education was categorized according to the 2011 International Standard Classification of Education (ISCED-2011)47: No education (ISCED 0 level), Elementary (ISCED 1–2 levels), Middle (ISCED 3–4 levels), Higher (ISCED 5–8 levels). Occupation status of each participant was classified as: student, employed, unemployed or retired. Marital status was defined as: married/union of fact, divorced, single or widowed. Smoking status was defined as non-smoker, current smoker or former smoker. Family gross income was categorised in three levels: less or equal to 600, 601 to 1500 and higher than 1500 euros per month.
Measurement reliability and reproducibility
Two examiners (VM and JB) were trained under the supervision of an experienced senior periodontist (RA), prior to data collection. For the purpose of measurement reliability and reproducibility, a total of 10 volunteers seeking care at EMDC were randomly selected and evaluated. These patients were not further involved in the study. Volunteers were examined by the senior periodontist, the ‘reference examiner’, and the two field clinicians. Measurements were repeated one week later in the same volunteers. Measurement reliability and reproducibility were assessed by the intra-class correlation coefficient (ICC). Obtained ICC inter-examiner values were 0.98 and 0.99, for CAL and PD, respectively. The intra-examiner ICC ranged from 0.97 to 0.99, for both PD and CAL.
Data analysis
Data analysis was performed using SPSS Statistics version 25.0 for Windows (IBM; Armonk, New York, USA). Descriptive and inferential statistics methodologies were applied. Spearman’s rank correlation coefficient (rho) was used to assess correlations between periodontal clinical data and age. Binomial logistic regression analysis was used to model the relationship between periodontitis and several potential risk factors. Preliminary analyses were performed using univariate models. Next, a multivariate model was constructed for periodontal disease estimation. Only variables showing a significance p ≤ 0.25 in the univariate model were included in the multivariate stepwise procedure. The contribution of each variable to the model was evaluated by Wald statistics. Interactions were also analyzed for all tested variables. The final reduced model was obtained with the following predictor variable categories: age, education, smoking status and diabetes. Odds ratio (OR) and 95% confidence intervals (95% CI) were calculated for both univariate and multivariate analyses. The level of statistical significance was set at 5% in all inferential analyses.
Supplementary information
Acknowledgements
Egas Moniz - Cooperativa de Ensino Superior, CRL is acknowledged for financial, scientific and logistical support. Health Centers grouping (ACES) Almada-Seixal from the Regional Health Administration of Lisbon and Tagus Valley, IP is kindly acknowledged for organizational and logistical support. Instituto de Ciências Biomédicas Abel Salazar is acknowledged for scientific support. We also acknowledge Joana Lopes, Mariana Patrão, Débora Ferreira, Patrick Lopes, Daniel Cruz, Inês Magalhães and Madalena Eraclides for invaluable help during fieldwork. As part of their interaction with the community and social responsibility, Egas Moniz - Cooperativa de Ensino Superior, CRL granted a triage appointment, orthopantomography, a dental cleaning visit, and, in the case of diagnosis of a periodontal disease, the treatment until the first revaluation.
Author contributions
The project was conceived by J.B., V.M., J.J.M., L.P., L.A., M.A.C. and R.C.A. The clinical material was collected by J.B. and V.M. All field work was conducted by J.B. and V.M. with the help of J.J.M., L.P. and L.A. Bioinformatics analysis was conducted by L.P., J.B. & V.M. J.B., V.M., J.J.M., L.P., L.A., M.A.C. and R.C.A. interpreted the data and wrote the manuscript. All authors read and approved the final manuscript.
Data availability
Due to legal arrangements with governmental institutions, we are not authorized to disclose data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: João Botelho and Vanessa Machado.
Supplementary information
is available for this paper at 10.1038/s41598-019-52116-6.
References
- 1.Albandar JM. Global risk factors and risk indicators for periodontal diseases. Periodontol. 2000. 2002;29:177–206. doi: 10.1034/j.1600-0757.2002.290109.x. [DOI] [PubMed] [Google Scholar]
- 2.Dye BA. Global periodontal disease epidemiology. Periodontol. 2000. 2012;58:10–25. doi: 10.1111/j.1600-0757.2011.00413.x. [DOI] [PubMed] [Google Scholar]
- 3.Petersen PE, Ogawa H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontol. 2000. 2012;60:15–39. doi: 10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- 4.Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017;44:456–462. doi: 10.1111/jcpe.12732. [DOI] [PubMed] [Google Scholar]
- 5.Preshaw PM, et al. Periodontitis and diabetes: a two-way relationship Matrix metalloproteinase NHANES National Health and Nutrition Examination Survey. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lafon A, et al. Periodontal disease and stroke: A meta-analysis of cohort studies. Eur. J. Neurol. 2014;21:1155–1161. doi: 10.1111/ene.12415. [DOI] [PubMed] [Google Scholar]
- 7.Leira Y, et al. Association between periodontitis and ischemic stroke: a systematic review and meta-analysis. Eur. J. Epidemiol. 2017;32:43–53. doi: 10.1007/s10654-016-0170-6. [DOI] [PubMed] [Google Scholar]
- 8.Fuggle NR, Smith TO, Kaul A, Sofat N. Hand to mouth: A systematic review and meta-analysis of the association between rheumatoid arthritis and periodontitis. Front. Immunol. 2016;7:1–10. doi: 10.3389/fimmu.2016.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papageorgiou SN, et al. Inflammatory bowel disease and oral health: systematic review and a meta-analysis. J. Clin. Periodontol. 2017;44:382–393. doi: 10.1111/jcpe.12698. [DOI] [PubMed] [Google Scholar]
- 10.Manrique-Corredor EJ, et al. Maternal periodontitis and preterm birth: Systematic review and meta-analysis. Community Dent. Oral Epidemiol. 2019;47:243–251. doi: 10.1111/cdoe.12450. [DOI] [PubMed] [Google Scholar]
- 11.Page RC, Eke PI. Case Definitions for Use in Population-Based Surveillance of Periodontitis. J. Periodontol. 2007;78:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 12.Eke, P. I. et al. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 - 2012. J. Periodontol. 1–18, 10.1902/jop.2015.140520 (2015). [DOI] [PMC free article] [PubMed]
- 13.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018;45:S149–S161. doi: 10.1111/jcpe.12945. [DOI] [PubMed] [Google Scholar]
- 14.Machado V, et al. Prevalence and extent of chronic periodontitis and its risk factors in a Portuguese subpopulation: a retrospective cross-sectional study and analysis of Clinical Attachment Loss. PeerJ. 2018;6:e5258. doi: 10.7717/peerj.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DGS. III Estudo de Prevalência das Doenças Orais. (2015).
- 16.Araújo MD, Maló P. Prevalence of periodontitis, dental caries, and peri-implant pathology and their relation with systemic status and smoking habits: Results of an open- cohort study with 22009 patients in a private rehabilitation center. J. Dent. 2017;67:36–42. doi: 10.1016/j.jdent.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Carasol M, et al. Periodontal conditions among employed adults in Spain. J. Clin. Periodontol. 2016;43:548–556. doi: 10.1111/jcpe.12558. [DOI] [PubMed] [Google Scholar]
- 18.Aimetti, M. et al. Prevalence of periodontitis in an adult population from an urban area in North Italy: findings from a cross-sectional epidemiological survey. J. Clin. Periodontol. 622–631, 10.1111/jcpe.12420 (2015). [DOI] [PubMed]
- 19.Krustrup U, Erik Petersen P. Periodontal conditions in 35–44 and 65–74-year-old adults in Denmark. Acta Odontol. Scand. 2006;64:65–73. doi: 10.1080/00016350500377859. [DOI] [PubMed] [Google Scholar]
- 20.Schutzhold S, et al. Changes in prevalence of periodontitis in two German population-based studies. J. Clin. Periodontol. 2015;42:121–130. doi: 10.1111/jcpe.12352. [DOI] [PubMed] [Google Scholar]
- 21.Holde, G. E., Oscarson, N., Trovik, T. A., Tillberg, A. & Jönsson, B. Periodontitis Prevalence and Severity in Adults: A Cross-Sectional Study in Norwegian Circumpolar Communities. J. Periodontol. 1–17, 10.1902/jop.2017.170164 (2017). [DOI] [PubMed]
- 22.Machado V, et al. Partial recording protocols performance on the assessment of periodontitis severity and extent: Bias magnitudes, sensibility, and specificity. Rev. Port. Estomatol. Med. Dent. e Cir. Maxilofac. 2018;59:145–153. [Google Scholar]
- 23.Trombelli L, Farina R, Silva CO, Tatakis DN. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Periodont. 2018;89:S46–S73. doi: 10.1002/JPER.17-0576. [DOI] [PubMed] [Google Scholar]
- 24.Santos J, et al. Oral hygiene habits in Portugal: results from the first Health Examination Survey (INSEF 2015) Acta Odontol. Scand. 2019;77:334–339. doi: 10.1080/00016357.2018.1564839. [DOI] [PubMed] [Google Scholar]
- 25.Direção-Geral-da-Saúde, M.-S. Programa Nacional para a diabetes (2017).
- 26.Kravitz, A., Bullock, A., Cowpe, J. & Barnes, E. EU Manual of Dental Practice 2015 (Edition 5.1). Council of European Dentints, 287–296 (2015).
- 27.Simões J, et al. Ten years since the 2008 introduction of dental vouchers in the Portuguese NHS. Health Policy. 2018;122:803–807. doi: 10.1016/j.healthpol.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Beltrán-Aguilar ED, Eke PI, Thornton-Evans G, Petersen PE. Recording and surveillance systems for periodontal diseases. Periodontol. 2000. 2012;60:40–53. doi: 10.1111/j.1600-0757.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naimi-akbar A, et al. Attitudes and lifestyle factors in relation to oral health and dental care in Sweden: a cross- sectional study. Acta Odontol. Scand. 2019;77:282–289. doi: 10.1080/00016357.2018.1539238. [DOI] [PubMed] [Google Scholar]
- 30.Holtfreter B, Ch S, Biffar R, Th K. Epidemiology of periodontal diseases in the study of health in Pomerania. J. Clin. Periodontol. 2009;36:114–123. doi: 10.1111/j.1600-051X.2008.01361.x. [DOI] [PubMed] [Google Scholar]
- 31.Bourgeois D, Bouchard P, Epidemiology MC. Epidemiology of periodontal status in dentate adults. J. Periodontal Res. 2007;42:219–227. doi: 10.1111/j.1600-0765.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 32.Suvan JE, Finer N, D’Aiuto F. Periodontal complications with obesity. Periodontol. 2000. 2018;78:98–128. doi: 10.1111/prd.12239. [DOI] [PubMed] [Google Scholar]
- 33.Papapanou PN, Lindhe J. Preservation of probing attachment and alveolar bone levels in 2 random population samples. J. Clin. Periodontol. 1992;19:583–588. doi: 10.1111/j.1600-051X.1992.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 34.Bergström J, Preber H. Tobacco Use as a Risk Factor. J. Periodontol. 1994;65:545–550. doi: 10.1902/jop.1994.65.5s.545. [DOI] [PubMed] [Google Scholar]
- 35.Bergström J. Periodontitis and Smoking: An Evidence-Based Appraisal. J. Evid. Based. Dent. Pract. 2006;6:33–41. doi: 10.1016/j.jebdp.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat. Rev. Dis. Prim. 2017;3:17038. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 37.Leite FRM, Nascimento GG, Scheutz F, López R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. American Journal of Preventive Medicine. 2018;54:831–841. doi: 10.1016/j.amepre.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Papapanou PN, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018;45:S162–S170. doi: 10.1111/jcpe.12946. [DOI] [PubMed] [Google Scholar]
- 39.Chambrone L, Chambrone D, Lima LA, Chambrone LA. Predictors of tooth loss during long-term periodontal maintenance: A systematic review of observational studies. J. Clin. Periodontol. 2010;37:675–684. doi: 10.1111/j.1600-051X.2010.01587.x. [DOI] [PubMed] [Google Scholar]
- 40.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J. Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 41.Geyer S, Schneller T, Micheelis W. Social gradients and cumulative effects of income and education on dental health in the fourth German oral health study. Community Dent. Oral Epidemiol. 2010;38:120–128. doi: 10.1111/j.1600-0528.2009.00520.x. [DOI] [PubMed] [Google Scholar]
- 42.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 43.Casanova L, Hughes FJ, Preshaw PM. Diabetes and periodontal disease: A two-way relationship. Br. Dent. J. 2014;217:433–437. doi: 10.1038/sj.bdj.2014.907. [DOI] [PubMed] [Google Scholar]
- 44.D’Aiuto F, et al. Evidence summary: The relationship between oral diseases and diabetes. Br. Dent. J. 2017;222:944–948. doi: 10.1038/sj.bdj.2017.544. [DOI] [PubMed] [Google Scholar]
- 45.Von Elm E, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4:1623–1627. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamp S-E, Nyman S, Lindhe J. Periodontal treatment of multirooted teeth - results after 5 years. J. Clin. Periodontol. 1975;2:126–135. doi: 10.1111/j.1600-051X.1975.tb01734.x. [DOI] [PubMed] [Google Scholar]
- 47.UNESCO-Institute-for-Statistics. International Standard Classification of Education ISCED 2011 (2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to legal arrangements with governmental institutions, we are not authorized to disclose data.


