Abstract
Aim:
This research aimed to evaluate the effect of gastroesophageal reflux disease (GERD) on pulmonary volumes, airflows, and airway resistance in the patients without respiratory symptoms and compare them with the healthy subjects.
Background:
GERD is the return of gastric content into the esophagus and beyond. GERD may play an essential role in the extraesophageal diseases, including chest pain, asthma, laryngitis, chronic cough, and sinusitis. The relation between GERD and airway involvement in asthma and also bronchoconstrictor effects of GERD are well recognized, but its impact on lung parameters in the patients with GERD without respiratory symptoms is unclear.
Methods:
In a case-control study, 78 GERD patients without pulmonary symptoms and 93 healthy subjects as control group were enrolled. The impulse oscillometry examined airway resistance. The body plethysmograph measured the pulmonary volumes and airflows.
Results:
The mean age of GERD patients and the healthy subjects were 37.30±9.76 and 34.74±11.10, respectively. A total of 53.8% of patients and 67.7% of healthy subjects were male. The lung volumes measured by the body plethysmography were normal in both patients and healthy subjects. However, there was a significant difference between the groups in forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) (P=0.01) and maximal mid expiratory flow (MMEF) (P=0.008). Airway resistance at R5Hz was significantly higher in the case group than the control group (P=0.001).
Conclusion:
The results of the current study demonstrated that GERD patients have small airway disease even in the absence of respiratory symptoms.
Key Words: Gastroesophageal reflux, Lung function, Plethysmography, Airway resistance, Oscillometry
Introduction
Gastroesophageal reflux disease (GERD) is a common gastroesophageal problem that is caused by retrograde bolus movement of gastric acidic content into the esophagus and may reach higher levels up to laryngopharynx (1). The prevalence of GERD is 8.8%-25.9% in European countries and 8.7%-33.1% in the Middle East (2). The prevalence of weekly and monthly symptoms of GERD in the North-West of Iran is about 26.8% and 34.1%, respectively (3).
The mechanism responsible for the GERD pathogenesis includes the increase in the duration of lower esophageal sphincter relaxation, decrease in the pressure of lower esophageal sphincter, esophageal acid clearance interruption, hiatal hernia, slow gastric passage and increased gastric acid secretion (4). GERD has a vital role in some problems such as chest pain (5), asthma (6), laryngitis (7), chronic cough (8), sinusitis (9), increased upper gastrointestinal bleeding risk (10), esophageal stricture (11), intestinal metaplasia (12), Barrett’s esophagus (13) and esophageal adenocarcinoma (14). The role of the esophageal diseases in airway disorders has not been yet completely understood. The airway hyper-responsiveness caused by the direct mucosa damage increased vagal tone and bronchial spasm (15). Also, neuro-inflammatory reflexes may have a role in the airway responsiveness by releasing tachykinins peptides, including P, and A neuro-quinine (15). The combination of these mechanisms can cause the afferent vagus impulses and finally, airway stimulation (15). The previous studies have shown the effects of GERD bronchoconstrictor on asthma (16, 17).
The atypical manifestation or extra-esophageal syndromes of GERD has received considerable attention in recent years. Based on the previous findings in the correlation between GERD and lung diseases and the possible effects of GERD with different mechanisms on the tracheobronchial tree; this study was designed to determine the lung volumes, airflows and airway resistance in the patients with GERD without respiratory symptoms in comparison with healthy subjects.
Methods
After Institutional Review Board (IRB) approval, in a prospective case-control study, the volumes, flow rates, and airway resistance values were measured in the patients with GERD without respiratory symptoms according to the American Thoracic Society (ATS) guideline (18). Then, the measures were compared with age- and gender-matched healthy subjects. The validated Persian version of Gastro-esophageal Reflux Questionnaire (GERQ) was used for the GERD diagnosis (19). The patients and healthy subjects were selected among the individuals who visited the Gastroenterology Clinic of a tertiary referral Hospital of Tabriz University of Medical Sciences, during January 2015 and December 2016.
Inclusion criteria were patients with GERD, aged over 15 years with heartburn and acid regurgitation at least two times a week with no history of treatment which was confirmed by an expert gastroenterologist and GERQ. The patients with any signs and symptoms of lung diseases or any gastrointestinal tract diseases, smoking and alcohol consumption, body mass index (BMI) over 30Kg/m2, pregnancy, congestive heart failure, acute coronary syndrome, use of medications that can affect the tests (i.e. corticosteroids, theophylline, and non-steroidal anti-inflammatory drugs) and patients who were unable to thoroughly perform the lung function tests were excluded from the study. All patients underwent a complete interview and physical examination to exclude associated conditions.
Impulse oscillometry (IOS; Jaeger, Würzburg, Germany) was performed for determining the airway resistance. The lung volume and airflow were measured by body plethysmography (Jaeger, Würzburg, Germany). Also, to increase the accuracy of the measurement, the devices were calibrated daily.
The informed consent was obtained from all patients after full explanation of the study protocol. The patients were also assured about the confidentiality of their information. Patients could withdraw from the study at any time. No additional costs were imposed on the participants.
All data were analyzed using the Statistical Package for the Social Sciences version 16 (SPSS Inc., Chicago, IL, USA). The chi-square test was used to compare the qualitative variables. The quantitative variables were analyzed using the independent t-test. The Kolmogorov-Smirnov statistical test and histogram were used for the analysis of data distribution normality. P-value≤0.05 was considered statistically significant.
Results
Among 107 patients with GERD, 19 were excluded because they had at least one of the exclusion criteria. In the present study, we studied 78 patients in the patient group (53.8% male) and 93 healthy subjects in the control group (67.7% males). The groups were matched for age (P=0.30) and gender (P=0.06). The mean ages of the patients in the case and control groups were 37.30±9.76 and 34.74±11.10 years, respectively (P=0.11). The mean BMI of the patients and healthy subjects were 25.98±4.22 and 25.48±3.14 Kg/m2, respectively (P=0.37).
Table 1 highlights the plethysmography and IOS findings in the two groups. There was a significant difference between groups in forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) (P=0.01) as well as maximal mid expiratory flow (MMEF) (P=0.008). Moreover, FVC (L), FVC (%), FEV1 (%), residual volume (RV), and RV/total lung capacity (TLC) were not statistically significant (P>0.05).
Table 1.
Plethysmography and impulse-oscillometry findings in groups
| Variables | Case (mean±SD) | Control (mean±SD) | P |
|---|---|---|---|
| FVC (L) | 4.31 ± 1.07 | 4.42 ± 0.97 | 0.22 |
| FVC (%) | 106.93 ± 13.34 | 105.31 ± 14.80 | 0.45 |
| FEV1 (L) | 3.53 ± 0.70 | 3.48 ± 0.72 | 0.64 |
| FEV1 (%) | 104.79 ± 8.71 | 106.51 ± 11.87 | 0.28 |
| FEV1/FVC | 83.04 ± 6.47 | 85.29 ± 5.68 | 0.01 |
| RV (L) | 1.51 ± 0.69 | 1.54 ± 0.55 | 0.72 |
| RV/TLC | 25.56 ± 8.33 | 26.04 ± 9.86 | 0.73 |
| MMEF (L/S) | 3.58 ± 0.74 | 3.88 ± 0.72 | 0.008 |
| R5Hz (KPa/L/s) | 3.37 ± 0.81 | 3.04 ± 0.42 | 0.001 |
| R5Hz (%) | 121.65 ± 47.17 | 113.52 ± 37.78 | 0.21 |
FVC, Forced vital capacity; FEV1, First expiratory volume in 1 second; RV, residual volume; TLC, total lung capacity; MMEF, mid-maximal expiratory flow; SD, Standard deviation
*The American Thoracic Society criterion has been used to compute the prediction reference values.
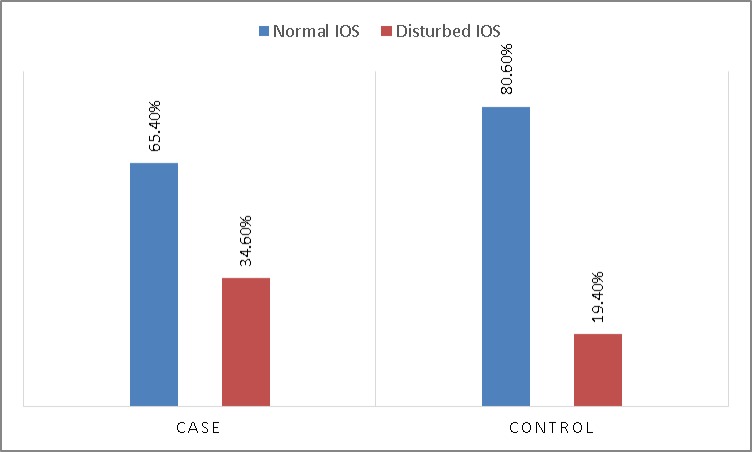
Figure 1 shows the results of IOS in the groups. The IOS results between two groups showed that the airway resistance (R5Hz) was significantly higher in the patients (P=0.02) and there was a negative correlation between R5Hz and FEV/FVC percentage (R=-0.25) (P=0.001) and MMEF (R=-0.31) (P<0.001).
Figure 1.
Results of impulse-oscillometry between two groups
IOS, impulse-oscillometry
Comparison of the plethysmography and IOS findings revealed a significant negative correlation between R5Hz and FEV1 (R=-0.2) (P=0.008), FEV1/FVC percentage (R=-0.18) (P=0.01), MMEF (R=-0.35) (P<0.001). Moreover, there was a positive correlation between R5Hz and RV/TLC (R=0.16) (P=0.03). Finally, a negative correlation was found between R5Hz percentage and RV/TLC percentage (R=-0.15) (P=0.04), and MMEF (R=-0.34) (P<0.001).
Discussion
GERD is a common gastroesophageal disorder that is caused by the return of the stomach contents into the esophagus which impairs the quality of life (20). Since the esophagus and lung have the same embryonic origin, the contribution of esophageal disorders in cough, asthma, and other respiratory tract problems is possible. Although vagal reflexes and micro aspirations are postulated as possible mechanisms for the respiratory effects of GERD, immunological causes can play a role (21).
The relationship between reflux and respiratory symptoms is a two-way correlation. In one hand, respiratory symptoms such as cough, dyspnea, and wheezing are seen in the patients with GERD. On the other hand, GERD is common in patients with lung diseases. Among the associated disorders of GERD, most of the studies were done on bronchial asthma (22). The treatment of GERD was effective in the treatment of asthma (16).
Despite the numerous studies about the GERD and pulmonary function impairment in patients with known pulmonary diseases, there were only a few studies about pulmonary function in asymptomatic patients (23, 24).
In the present study, we showed an obstructive pattern by IOS. Our findings were in line with Manjunath et al. (25) study results that reported predominantly obstructive ventilatory impairment in the asymptomatic GERD patients. They also showed a restrictive impairment in these patients.
We measured the large and small airway resistance in this study with IOS, besides the measurement of the lung volumes. Our findings showed that in patients with GERD, even with no clinical manifestation of pulmonary dysfunction, there was a small airway disease characterized by a reduction in FEV1/FVC ratio, MMEF, and increased R5Hz. These findings were consistent with the results of previous studies (26, 27).
Although these studies showed evidence of parenchymal involvement based on the decreased FVC and impaired diffusing capacity of the lungs for carbon monoxide (DLCO), in our research, FVC and TLC were not significantly different between the patients and controls (P˃0.05).
The main difference of this study from the previous studies was the application of IOS for the direct measurement of the airway resistance, which showed higher in the patients compared to the controls (P=0.001). The reduction in FEV1/FVC and MMEF is secondary to the increased airway resistance, and direct measurement of this resistance may be more sensitive in detecting the early small airway diseases.
Another important distinction between this study and others is that we did not evaluate respiratory function in GERD patients with a known pulmonary disease such as bronchial asthma and other obstructive pulmonary diseases, which was the subject of almost all the studies performed previously.
The main question is on the significance of these abnormalities in small airways. Also, it is unknown that if these abnormalities left untreated, they will progress toward an established pulmonary airway disease or not. For the lack of sufficient evidence, precise answer to this question requires further investigation, but it seems that the nature of the involvement of the airways in GERD is similar to bronchial asthma and these patients may benefit from anti-reflux treatment.
The present study had some limitations. The patients did not follow up for determining the GERD effects on the long-term respiratory function. Considering the significant impact of GERD on pulmonary function, cohort studies are necessary to investigate the susceptibility to pulmonary disease in the GERD.
The results of this study showed that patients with GERD suffer from small airway involvement even when there is no symptom related to the respiratory system.
Acknowledgment
The results of this study showed that patients with GERD suffer from small airway involvement even when there is no symptom related to the respiratory system.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Kahrilas PJ. Clinical practice Gastroesophageal reflux disease. New Engl J Med. 2008;359:1700–7. doi: 10.1056/NEJMcp0804684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871–80. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somi M FS, Nasseri-Moghaddam S, Jazayeri E, Mirinezhad S, Godrati S, Golchin M. Prevalence and risk factors of gastroesophageal reflux disease in Tabriz, Iran. Iran J Public Health. 2008;37:85–0. [Google Scholar]
- 4.Najafimehr H, Ashtari S, Mohaghegh Shalmani H, Fazeli Z, Yadegari H, Taherinejad H, et al. Influence of working in auto factory on gastroesophageal reflux disease. Gastroenterol Hepatol Bed Bench. 2018;11:S1–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Liuzzo JP, Ambrose JA. Chest pain from gastroesophageal reflux disease in patients with coronary artery disease. Cardiol Rev. 2005;13:167–73. doi: 10.1097/01.crd.0000148844.13702.ce. [DOI] [PubMed] [Google Scholar]
- 6.Sharifi A, Ansarin K. Effect of gastroesophageal reflux disease on disease severity and characteristics of lung functional changes in patients with asthma. J Cardiovascular Thoracic Res. 2014;6:223–8. doi: 10.15171/jcvtr.2014.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva CE, Niedermeier BT, Portinho F. Reflux Laryngitis: Correlation between the Symptoms Findings and Indirect Laryngoscopy. Int Archive Otorhinolaryngol. 2015;19:234–7. doi: 10.1055/s-0034-1399794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis DO. Chronic Cough and Gastroesophageal Reflux Disease. Gastroenterol Hepatol. 2016;12:64–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Lin YH, Chang TS, Yao YC, Li YC. Increased Risk of Chronic Sinusitis in Adults With Gastroesophgeal Reflux Disease: A Nationwide Population-Based Cohort Study. Med. 2015;94:e1642. doi: 10.1097/MD.0000000000001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chait MM. Gastroesophageal reflux disease: Important considerations for the older patients. World J Gastrointest Endosc. 2010;2:388–96. doi: 10.4253/wjge.v2.i12.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukaya M, Abe T, Nagino M. Rapid progressive long esophageal stricture caused by gastroesophageal reflux disease after pylorus-preserving pancreatoduodenectomy. BMC Surg. 2016;16:19. doi: 10.1186/s12893-016-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietz J, Meurer L, Maffazzoni DR, Furtado AD, Prolla JC. Intestinal metaplasia in the distal esophagus and correlation with symptoms of gastroesophageal reflux disease. Dis Esophagus. 2003;16:29–32. doi: 10.1046/j.1442-2050.2003.00288.x. [DOI] [PubMed] [Google Scholar]
- 13.Mikolasevic I, Bokun T, Filipec Kanizaj T. Gastroesophageal reflux disease, Barrett esophagus, and esophageal adenocarcinoma - where do we stand? Croatian Med J. 2018;59:97–9. doi: 10.3325/cmj.2018.59.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook MB, Corley DA, Murray LJ, Liao LM, Kamangar F, Ye W, et al. Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: a pooled analysis from the Barrett's and Esophageal Adenocarcinoma Consortium (BEACON) PLoS ONE. 2014;9:e103508. doi: 10.1371/journal.pone.0103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein MR. Possible mechanisms of influence of esophageal acid on airway hyperresponsiveness. Am J Med. 2003;115:55S–9. doi: 10.1016/s0002-9343(03)00194-3. [DOI] [PubMed] [Google Scholar]
- 16.Mastronarde JG. Is There a Relationship Between GERD and Asthma? Gastroenterol Hepatol. 2012;8:401–3. [PMC free article] [PubMed] [Google Scholar]
- 17.Ates F, Vaezi MF. Insight Into the Relationship Between Gastroesophageal Reflux Disease and Asthma. Gastroenterol Hepatol. 2014;10:729–36. [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–61. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 19.Nasseri-Moghaddam S, Razjouyan H, Habibi R, Rafaat-Zand K, Ahrari B, Nouraie M, et al. Reliability, validity, and feasibility of the Mayo gastroesophageal reflux questionnaire (GERQ) in a Persian-speaking population”. Iran J Public Health. 2008;37:64–74. [Google Scholar]
- 20.Maleki I, Masoudzadeh A, Khalilian A, Daheshpour E. Quality of life in patients with gastroesophageal reflux disease in an Iranian population. Gastroenterol Hepatol Bed Bench. 2013;6:96–100. [PMC free article] [PubMed] [Google Scholar]
- 21.Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. 2009;137:1776–84. doi: 10.1053/j.gastro.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 22.Broers C, Tack J, Pauwels A. Review article: gastro-oesophageal reflux disease in asthma and chronic obstructive pulmonary disease. Aliment Pharmacol Ther. 2018;47:176–91. doi: 10.1111/apt.14416. [DOI] [PubMed] [Google Scholar]
- 23.Bonacin D, Fabijanic D, Radic M, Puljiz Z, Trgo G, Bratanic A, et al. Gastroesophageal reflux disease and pulmonary function: a potential role of the dead space extension. Int Med J Exp Clin Res. 2012;18:CR271–5. doi: 10.12659/MSM.882731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morehead RS. Gastro-oesophageal reflux disease and non-asthma lung disease. Eur Respir Rev. 2009;18:233–43. doi: 10.1183/09059180.00002509. [DOI] [PubMed] [Google Scholar]
- 25.Manjunath H, Venkatesh D, Jalihal U, Kumar MP. An Altered Pulmonary Function–A Cause or Consequence of Gastro Esophageal Reflux Disease (GERD) Al Ameen J Med Sci. 2011;4:391–5. [Google Scholar]
- 26.Mise K, Capkun V, Jurcev-Savicevic A, Sundov Z, Bradaric A, Mladinov S. The influence of gastroesophageal reflux in the lung: a case-control study. Respirolology. 2010;15:837–42. doi: 10.1111/j.1440-1843.2010.01777.x. [DOI] [PubMed] [Google Scholar]
- 27.de la Hoz RE, Christie J, Teamer JA, Bienenfeld LA, Afilaka AA, Crane M, et al. Reflux symptoms and disorders and pulmonary disease in former World Trade Center rescue and recovery workers and volunteers. J Occup Environ Med. 2008;50:1351–4. doi: 10.1097/JOM.0b013e3181845f9b. [DOI] [PubMed] [Google Scholar]