Abstract
OBJECTIVE:
To evaluate health care provider adherence to the surgical protocol endorsed by the National Cogmprehensive Cancer Network and the American College of Obstetricians and Gynecologists at the time of risk-reducing salpingo-oophorectomy and compare adherence between gynecologic oncologists and obstetrician-gynecologists (ob-gyns).
METHODS:
In this multicenter retrospective cohort study, women were included if they had a pathogenic BRCA mutation and underwent risk-reducing salpingo-oophorectomy between 2011 and 2017. Adherence was defined as completing all of the following: collection of washings, complete resection of the fallopian tube, and performing the Sectioning and Extensively Examining the Fimbriated End (SEE-FIM) pathologic protocol.
RESULTS:
Of 290 patients who met inclusion criteria, 160 patients were treated by 18 gynecologic oncologists and 130 patients by 75 ob-gyns. Surgery was performed at 10 different hospitals throughout a single metropolitan area. Demographic and clinical characteristics were similar between groups. Overall, 199 cases (69%) were adherent to the surgical protocol. Gynecologic oncologists were more than twice as likely to fully adhere to the full surgical protocol as ob-gyns (91% vs 41%, P<01). Specifically, gynecologic oncologists were more likely to resect the entire tube (99% vs 95%, P=.03), to have followed the SEE-FIM protocol (98% vs 82%, P<01), and collect washings (94% vs 49%, P<01). Complication rates did not differ between groups. Occult neoplasia was diagnosed in 11 patients (3.8%). The incidence of occult neoplasia was 6.3% in gynecologic oncology patients and 0.8% in obstetrics and gynecology patients (P=.03).
CONCLUSION:
Despite clear surgical guidelines, only two thirds of all health care providers were fully adherent to guidelines. Gynecologic oncologists were more likely to follow surgical guidelines compared with general ob-gyns and more likely to diagnose occult neoplasia despite similar patient populations. Rates of risk-reducing surgery will likely continue to increase as genetic testing becomes more widespread, highlighting the importance of health care provider education for this procedure. Centralized care or referral to subspecialists for risk-reducing salpingo-oophorectomy may be warranted.
The lifetime risk of developing ovarian cancer is approximately 40% for women with a pathogenic germline BRCA1 mutation and approximately 20% for those with a pathogenic germline BRCA2 mutation.1,2 Risk-reducing salpingo-oophorectomy decreases the risk of ovarian and fallopian tube cancer by 80–90%, decreases breast cancer risk, and provides an overall mortality benefit.3–6 For women with a pathogenic BRCA mutation, the National Comprehensive Cancer Network (NCCN)7 and the American College of Obstetricians and Gynecologists (ACOG)6 endorse a set of surgical guidelines to provide the optimal risk reduction for these patients. Earlier age at surgery confers greater risk reduction, therefore the recommended age to undergo risk-reducing salpingo-oophorectomy is between the ages of 35–40 years, or when child bearing is complete. This can be delayed until age 45 for women with a BRCA2 mutation, as the typical age of onset is higher in these patients.6,7 The surgical protocol requires the following: complete resection of the fallopian tube, collection of pelvic washings, ligation of the ovarian vessels 2–3 cm proximal to the ovary, and survey of the entire abdomen with biopsies as indicated.6,7 Importantly, a detailed pathologic review that involves serial sectioning and microscopic examination of the entire specimen is also included as a recommendation. These surgical recommendations were initially published by ACOG in the “Hereditary Breast and Ovarian Cancer Syndrome” Practice Bulletin in 2009 and were reaffirmed in 2017.6
These guidelines are based on an improved understanding of carcinogenesis and the natural history of ovarian and fallopian tube cancers. The majority of high grade serous epithelial ovarian cancers originate from the fallopian tube,8,9 and fallopian tube carcinoma can shed malignant cells into the peritoneal cavity even in early stage disease, which is why removing the entire tube is warranted. Serial sectioning and microscopic examination of the entire specimen for risk-reducing salpingo-oophorectomy is a well-established, evidence-based practice. This is done using the Sectioning and Extensively Examining the Fimbriated End (SEE-FIM) protocol, which involves the pathologic review of the entire specimen in 2–3 mm sections as well as longitudinal sectioning of the fimbria.10 This protocol is important because the risk of occult malignancy in these patients is between 2% and 10% at the time of risk-reducing salpingo-oophorectomy and lesions are often microscopic.11,12 Following a standardized protocol is associated with higher rates of diagnosis.5,9,11
As awareness and accessibility of genetic testing increases, more patients will seek care for risk-reduction counseling and surgery. Understanding adherence patterns to evidence-based guidelines is imperative for ensuring quality patient care and identifying areas in need of improvement. The objective of this study was to report the compliance to the NCCN-recommended surgical protocol and compare adherence rates between gynecologic oncologists and general obstetrician-gynecologists (ob-gyns).
METHODS
The Institutional Review Board at all participating sites (Fairview Health System and HealthPartners) approved this study. The participating sites are part of two large health care systems that encompass multiple hospitals within a single metropolitan area. The sites employ both academic and private practice gynecologic oncologists, ob-gyns, and pathologists.
Patient records were identified through International Classification of Diseases, 9th Revision and 10th Revision codes and cross-referenced with a regional genetic counseling database. Patients were included in the analysis if they met the following criteria: 1) had a pathogenic germline BRCA1 or 2 mutation; 2) underwent risk-reducing salpingo-oophorectomy between January 1, 2011, and December 31, 2017; and 3) both operative report and full pathology report were available for review. Patients with a variant of unknown significance, suspected cancer, previous salpingo-oophorectomy, or who opted out of research participation were excluded.
The primary objective was to compare health care provider adherence to the NCCN-and ACOG-recommended surgical protocol between gynecologic oncologists and ob-gyns within two large health care systems. The secondary objective was to determine the rate of serous tubal intraepithelial carcinoma and invasive ovarian or tubal malignancy, hereafter referred to as occult neoplasm, present in this patient population at the time of surgery.
Three measures defined adherence to the recommended surgical protocol: collection of pelvic washings, resection of the entire fallopian tube, and completion of the SEE-FIM protocol. Review of cytology reports determined if pelvic washings had been collected. For the fallopian tube to be counted as “entirely resected,” tubal length needed to be more than 5 cm on pathology review (including the fimbriated end). If it did not measure more than 5 cm, resection was considered incomplete unless otherwise mentioned in the operative report or the patient had previously undergone bilateral tubal ligation. We chose this measurement based on the lower limit of normal tubal length (7 cm).13 We then accounted for 1.5 cm of unresectable cornual tube and another 0.5 cm as a margin of measuring error. To determine-whether the SEE-FIM protocol was followed, the pathology report had to note that the specimen was submitted in its entirety and the fimbria was longitudinally sectioned. Any reports that were ambiguous were reviewed by a gynecologic pathologist, and the original slides were requested as needed. To ensure that pathology reports accurately reflected that the SEE-flM protocol was being followed and the reports were interpreted correctly, one half of the original specimens were reviewed by a gynecologic pathologist and compared with the pathology report.
Demographic and clinical characteristics were collected for all patients that met inclusion criteria. Surgical data and surgeon specialty were also collected. Surgeon specialty was determined either from the electronic medical record or through hospital or clinic websites. Characteristics of all women in the study, within and across study sites, were summarized and compared by health care provider type using Wilcoxon rank sum tests and χ2 or Fisher exact tests as appropriate. Generalized linear models were used to model outcomes accounting for correlation within health care provider. Odds ratios and 95% CIs were reported. All analyses were conducted using SAS 9.4, and P=. 05 was considered statistically significant.
RESULTS
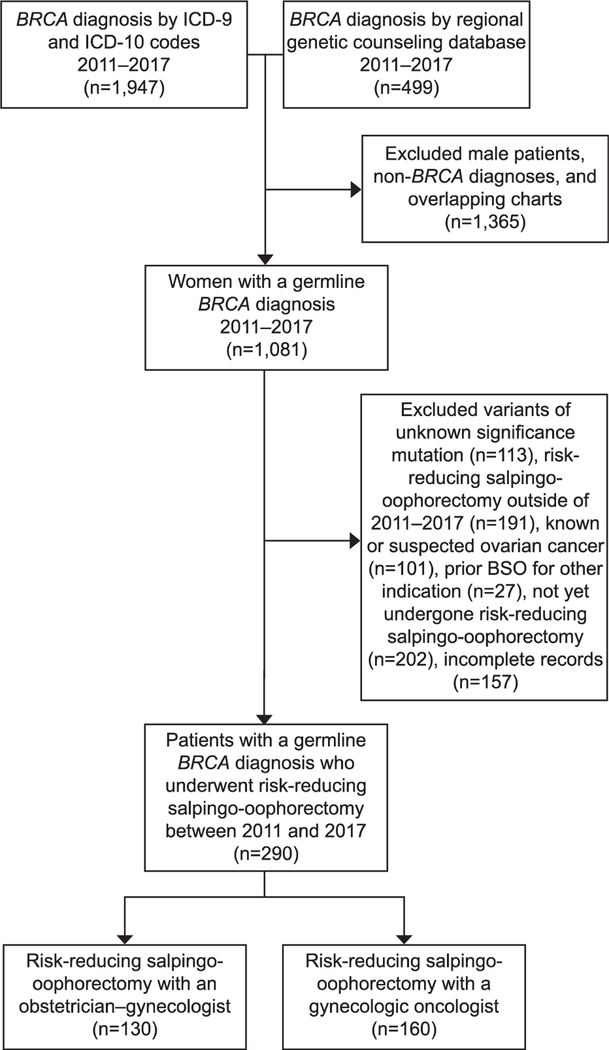
A total of 290 women met inclusion criteria, of which 130 patients were treated by 75 ob-gyns and 160 patients were treated by 18 gynecologic oncologists (Fig. 1). Surgery was performed at 10 different hospitals throughout the metropolitan area. Patients receiving care from gynecologic oncologists and ob-gyns were similar in age, body mass index, parity, personal or family history of breast cancer, and race-ethnicity (Table 1). In total, 149 (51%) had BRCA1 mutations and 141 (49%) had BRCA2 mutations. The percentage of BRCA1 mutation carriers was higher in the gynecologic oncologist group (56% vs 44%, P=.04). Mean time from diagnosis to surgery was shorter in gynecologic oncologists compared with ob-gyns, but this did not reach statistical significance (389 days vs 512, P=.07). Surgical complication rate and type of surgery did not differ between groups (Table 2). Route of surgery and rate of concomitant hysterectomy was also similar.
Fig. 1.
Flow diagram describing patient inclusion. ICD, International Classification of Diseases; BSO, bilateral sal-pingo-oophorectomy.
Wilhite. Adherence to Risk-Reducing Salpingo-oophorectomy Guidelines. Obstet Gynecol 2019.
Table 1.
Demographics and Clinical Characteristics of the Study Population
| Patient Demographic | Obstetrician-Gynecologist (n=130) |
Gynecologic Oncologist (n=160) |
Overall (N=290) |
P* |
|---|---|---|---|---|
| Mean age at surgery (y) | 49±10 | 49±10 | 49±10 | .78 |
| Mean BMI at surgery (kg/m2) | 29±8 | 28±7 | 28±8 | .33 |
| Mean parity | 2.0±1 | 2.0±2 | 2.0±2 | .93 |
| Family history breast or ovarian cancer | 93 (72) | 116 (73) | 209 (72) | .57 |
| Mutation type | .04 | |||
| BRCA1 | 57 (44) | 90 (56) | 149 (51) | |
| BRCA2 | 73 (56) | 70 (44) | 141 (49) | |
| Race-ethnicity | .69 | |||
| White | 116 (90) | 145 (91) | 261 (90) | |
| Black | 4 (3.0) | 5 (3.1) | 9 (3.1) | |
| Asian | 4 (3.0) | 3 (1.9) | 7 (2.4) | |
| Hispanic | 1 (0.8) | 1 (0.6) | 2 (0.7) | |
| Ashkenazi | 3 (2.3) | 3 (1.9) | 6 (2.0) | |
| Personal history of breast cancer | 70 (54) | 91 (57) | 161 (56) | .53 |
BMI, body mass index.
Data are mean±SD or n (%) unless otherwise specified.
Wilcoxon rank-sum for continuous variables; Pearson’s χ2 for categorical variables. P for race-ethnicity was calculated as white compared with non-white.
Table 2.
Surgical Characteristics
| Surgical Characteristics | Obstetrician-Gynecologist (n=130) |
Gynecologic Oncologist (n=160) |
Overall (N=290) |
P* |
|---|---|---|---|---|
| Mean days from BRCA diagnosis to surgery† | 512±746 | 389±637 | 444±689 | .07 |
| Type of surgery | .22 | |||
| Laparoscopic | 99 (76) | 114 (71) | 213 (73) | |
| Robotic | 25 (19) | 43 (27) | 68 (23) | |
| Open | 5 (3.8) | 3 (1.9) | 8 (2.8) | |
| Vaginal | 1 (0.8) | 0 | 1 (0.3) | |
| Prior hysterectomy | 7 (5.4) | 19 (12) | 26 (8.9) | .06 |
| RRSO with hysterectomy | 56 (43) | 80 (50) | 136 (59) | .21 |
| Complications | 5 (3.8) | 3 (1.9) | 8 (2.8) | .31 |
| BRCA documented in pathology report‡ | 89 (74) | 148 (94) | 237 (85) | <.01 |
RRSO, risk-reducing salpingo-oophorectomy.
Data are mean±SD or n (%) unless otherwise specified.
Pearson’s χ2 for categorical variables. Mean days from BRCA diagnosis and Complications are from a generalized linear model.
Data available from 263 health care providers.
Data available for 278 health care providers.
Overall, 199 cases (69%) were compliant with NCCN and ACOG recommendations. Specifically, rates of adherence to complete resection of the fallopian tube, SEE-FIM protocol, and pelvic washings were 98%, 91%, and 74%, respectively. Gynecologic oncologists were more than twice as likely to adhere to all recommendations as ob-gyns (91% vs 41%, P<.01) (Table 3). Specifically, surgery performed by a gynecologic oncologist was more likely to involve resection of the whole fallopian tube (99% vs 95%, P=.03), adherence to the SEE-FIM protocol (98% vs 82%, P<.01), and collection of pelvic washings (94% vs 49%, P<.01 (Table 3). The diagnosis of BRCA was clearly stated in the pathology report more frequently in cases performed by gynecologic oncologists compared with ob-gyns (94% vs 74%, P<.01).
Table 3.
Adherence to National Comprehensive Cancer Network and American College of Obstetrician Gynecologists Surgical Protocol
| Obstetrician-Gynecologist (n=130) |
Gynecologic Oncologist (n=160) |
Overall (N=290) |
OR (95% Cl) |
P* | |
|---|---|---|---|---|---|
| Total adherence | 53 (41) | 146 (91) | 199 (68.6) | 2.2 (1.7–3.0) | <.01 |
| Complete resection | 124 (95) | 159 (99) | 283 (97) | 1.1 (1.0–1.1) | .03 |
| SEE-FIM protocol followed | 107 (82) | 156 (98) | 263 (91) | 1.2 (1.1–1.3) | <.01 |
| Cytology performed | 64 (49) | 151 (94) | 215 (74) | 1.9 (1.5–2.4) | <.01 |
OR, odds ratio; SEE-FIM, Sectioning and Extensively Examining the Fimbriated End.
Data are n (%) unless otherwise specified.
From generalized linear models.
In total, 11 patients (3.8%) had invasive or preinvasive tubal or ovarian disease at the time of surgery (Table 4). Two patients had metastatic breast cancer to the tube or ovary found at the time of pathology review, but these patients were not included in the occult neoplasm analysis. Five patients (1.8%) had isolated serous tubal intraepithelial carcinoma, four had invasive carcinoma (1.3%), and two had serous tubal intraepithelial carcinoma as well as evidence of invasive cancer (0.7%). The SEE-FIM protocol was followed in all 11 of these cases. When broken down by specialty, gynecologic oncology patients were more likely to be diagnosed with occult neoplasm than obstetrics and gynecology patients (6.3% vs 0.8%, P=.03). The mean age of patients diagnosed with occult malignancy was 53.6 years (SD±8.9). Six patients with occult neoplasm had a BRCA1 mutation (4.0%) and five patients had a BRCA2 mutation (3.5%). All pelvic washings were negative for malignancy. Median follow up was 32.4 months (interquartile range 16.8–52.8). There have been no cases of primary peritoneal cancer to date.
Table 4.
Occult Neoplasm Diagnosis
| Occult Neoplasm | Obstetrician–Gynecologist (n=130) | Gynecologic Oncologist (n=160) | Overall (N=290) | P* |
|---|---|---|---|---|
| Occult neoplasm | 1 (0.8) | 10 (6.3) | 11 (3.8) | .03 |
| STIC only | 1 (0.8) | 4 (2.6) | 5 (1.8) | |
| Invasive cancer only | 0 | 4 (2.6) | 4 (1.3) | |
| Both | 2 (1.3) | 2 (0.7) | ||
STIC, serous tubal intraepithelial carcinoma
Data are n (%) unless otherwise specified.
Fisher exact test.
Six of the ob-gyn (4.6%) surgeries and 82 (51%) of the gynecologic oncology surgeries were performed at the university hospital (P<.01). The SEE-FIM protocol was followed in 87 (99%) of cases performed at the university hospital and 176 cases (87%) performed at nonuniversity hospitals (P<.01). Six of the 11 cases of occult neoplasm (55%) were diagnosed at the university hospital (P=. 10).
DISCUSSION
In this study, adherence to a risk-reducing surgical protocol was examined, and rates of adherence and occult neoplasm were compared between health care provider type. Despite evidence-based guidelines, only two thirds of BRCA mutation carriers who underwent risk-reducing salpingo-oophorectomy received complete guideline-based care. Gynecologic oncologists were more likely to adhere to guidelines compared with ob-gyns, and occult neoplasm was identified more frequently in the gynecologic oncologist group.
The rate of occult neoplasm at the time of risk-reducing salpingo-oophorectomy varies based on study populations, definition of diagnosis, and adherence to pathologic protocols.5,13–15 Pathologic data from the Gynecologic Oncology Group trial GOG-0199 reported that the incidence of occult malignancy in BRCA1 mutation carriers was 4.5% and BRCA2 carriers was 3.5%, This compares favorably with our results, which demonstrate rates of 4.0% and 3.5%, respectively.14 Age has been consistently reported as a primary risk factor for the diagnosis of occult neo-plasm in BRCA mutation carriers undergoing risk-reducing surgery.11,12‘14–16 Powell et al reported a 4.3% rate of neoplasm in women under 50 years of age, compared with 17% in women older than 50.11 Supporting this, in a more recent multicenter study looking at predictive and protective factors associated with serous tubal intraepithelial lesions, serous tubal intraepithelial carcinoma, and invasive carcinoma in BRCA patients, age was again found to be the only associated factor for occult malignancy.16 Given the mean age in both cohorts was 49, we would have expected to see a similar incidence of occult neoplasm between groups. Therefore, it is clinically significant that despite similar ages, there were high rates of detection in patients undergoing surgery by gynecologic oncologists.
Current literature suggests that following a strict pathology review correlates with higher rates of diagnoses.5,9,11 Viswanathan et al report the rate of occult malignancy (including serous tubal intraepithelial lesions, serous tubal intraepithelial carcinoma, and invasive carcinoma) nearly doubled from 6.3% with conventional microsectioning to 11.9% with additional sampling of the fallopian tube and review by multiple pathologists, suggesting the risk of occult malignancy is even higher than previously thought.
The lower rates of comprehensive pathology review in the ob-gyn group could at least partially explain the discrepancy in rates of diagnosis.
There are multiple factors that could contribute to these findings. We do acknowledge that the pathologists are ultimately responsible for executing the comprehensive pathology review of the specimen. It is possible that the pathologist may associate a specimen received from an oncologist as having a higher potential for malignancy, leading to an inherent bias to look more closely for malignancy. Importantly, the surgeon has a responsibility to ensure the pathologist is aware of the patient’s diagnosis and reason for surgery. In cases performed by gynecologic oncologists, the clinical history reflected in the pathology report more frequently mentioned “BRCA” as a diagnosis, which may have contributed to higher SEE-FIM protocol compliance rates. These differences suggest that proper communication between the surgeon and pathologist is imperative. Multiple opportunities exist to improve communication. For example, pathologic requests could be discussed during the preoperative time-out, the surgeon themselves could note that the patient carries a BRCA mutation and request SEE-FIM protocol on the requisition form, or they could be in direct communication with the pathologist following the procedure. Specifically stating the patient has a BRCA mutation on the pathology report is preferable to “family history of breast cancer” or another diagnosis that may be present at the time of surgery. When a health care provider notices that the final pathology report omits reference to the SEE-FIM protocol in a patient with a BRCA mutation, they could render the appropriate feedback to trigger a corrective action on the laboratory’s part. Finally, we speculate that gynecologic oncologists and their operating room staff may be more accustomed to collecting washings, which could lead to higher compliance rates in obtaining peritoneal cytology.
The strengths of this study include multicenter data collection, a large sample size, and a variety of health care providers in both academic and nonacademic settings. Although these were advantages and allow the results to be more generalizable, the hybrid nature of both health systems poses a challenge for analyzing rates of protocol adherence by academic status.
This study is limited by the use of retrospective data, though the goal of measuring health care provider compliance would be difficult to accomplish prospectively. Additionally, owing to the retrospective nature of this study, we felt that it was most important to include data that was as objective as possible to fairly assess compliance. Collection of washings, the SEE-FIM protocol, and length of fallopian tube are all objective measures that are detailed in a pathology report.
Ideally, we would like to know whether health care provider type confers a survival benefit, but this is not feasible given the long survival after risk-reducing salpingo-oophorectomy. However, we do speculate that, if occult malignancy is missed, patients will not receive indicated treatment and may have higher risk of recurrence or progression in the future.7,11
In conclusion, our data suggest that there is a discrepancy in adherence to guideline-based care between ob-gyns and gynecologic oncologists. This lack of adherence could contribute to the difference in rates of occult neoplasm. This study emphasizes the need for health care provider education and the importance of communication with the pathology team responsible for submitting and reviewing the specimen. Rates of risk-reducing surgery will likely continue to increase as genetic testing becomes more widespread. Referral to a subspecialist or a hospital that has health care providers who routinely do these procedures may be warranted to ensure patients are receiving more complete, guideline-based care.
Supplementary Material
Acknowledgments
Research reported in this publication was supported to BKE by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number K12HD055887. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors thank the William C. Bernstein MD Family Cancer Registry for allowing them access to the registry, and Buvana Reddy, MD for serving as Principle Investigator for the Health Partners sites.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Kuchenbaeker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017;317:2402–16. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 2007;25:1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van’t Veer L, Garber JE, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 2002;346:1616–22. [DOI] [PubMed] [Google Scholar]
- 4.Kauff ND, Domcheck SM, Friebe TM, Robson ME, Lee J, Garber JE, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1 and BRCA2 associated breast and genealogical cancer: a multi center, prospective study. J Clin Oncol 2008;26:1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finch AP, Lubinski J, Moller P, Singer CF, Karlan B, Senter L, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol 2014;32:1547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hereditary breast and ovarian cancer syndrome. ACOG Practice Bulletin No. 182. American College of Obstetricians and Gynecologists. Obstetrics Gynecol 2017;130:el 10–26. [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN). Genetic/familial high-risk assessment: breast and ovarian (version 3.2019). Available at: http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Retrieved March 11, 2019.
- 8.Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol 2013;209:409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, et al. The tubal fimbria is a preferred site for early adeno-carcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol 2006;30:230–6. [DOI] [PubMed] [Google Scholar]
- 10.College of American Pathologists (CAP). Protocol for the examination of specimens with carcinoma of the ovary. 2009. Available at: https://www.cap.org/apps/docs/committees/can-cer/cancer_protocols/2009/0vary_09protocol.pdf. Accessed May 2019.
- 11.Powell CB, Chen LM, McLennan J, Crawford B, Zaloudek C, Rabban JT, et al. Risk-reducing salpingo-oophorectomy (RRSO) in BRCA mutation carriers: experience with a consecutive series of 111 patients using a standardized surgical-pathological protocol. Int J Gynecol Cancer 2011;21:846–51. [DOI] [PubMed] [Google Scholar]
- 12.Domchek SM, Friebel TM, Garber JE, Isaacs C, Matloff E, Eeles R, et al. Occult ovarian cancers identified at risk-reducing salpingo-oophorectomy in a prospective cohort of BRCA1/2 mutation carriers. Breast Cancer Res Treat 2010; 124:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langer J Normal anatomy of the female pelvis and transvaginal sonography In: Norton ME, Scoutt LM, Feldstein VA, editors. Callen’s ultrasonography in obstetrics and gynecology. 6th ed. Philadelphia (PA): Elsevier; 2017:804–34. [Google Scholar]
- 14.Sherman ME, Piedmonte M, Phuong LM, Ioffe OB, Ronnett BM Van Le L, et al. Pathologic findings at risk-reducing salpingo-oophorectomy: primary results from Gynecologic Oncology Group trial GOG-0199. J Clin Oncol 2014;32:3275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamb JD, Garcia RL, Goff BA Paley PJ, Swisher EM. Predictors of occult neoplasia in women undergoing risk-reducing salpingo-oophorectomy. AmJ Obstet Gynecol 2006;194:1702–9. [DOI] [PubMed] [Google Scholar]
- 16.Visvanathan K, Shaw P, May BJ, Bahadirli-Talbott A, Kaushiva A, Risch H, et al. Fallopian tube lesions in women at high risk for ovarian cancer: a multicenter study. Cancer Prev Res (Phila) 2018;11:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.