Abstract
Background
Pre‐eclampsia is associated with deficient intravascular production of prostacyclin, a vasodilator, and excessive production of thromboxane, a vasoconstrictor and stimulant of platelet aggregation. These observations led to the hypotheses that antiplatelet agents, low‐dose aspirin in particular, might prevent or delay development of pre‐eclampsia.
Objectives
To assess the effectiveness and safety of antiplatelet agents, such as aspirin and dipyridamole, when given to women at risk of developing pre‐eclampsia.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (30 March 2018), and reference lists of retrieved studies. We updated the search in September 2019 and added the results to the awaiting classification section of the review.
Selection criteria
All randomised trials comparing antiplatelet agents with either placebo or no antiplatelet agent were included. Studies only published in abstract format were eligible for inclusion if sufficient information was available. We would have included cluster‐randomised trials in the analyses along with individually‐randomised trials, if any had been identified in our search strategy. Quasi‐random studies were excluded. Participants were pregnant women at risk of developing pre‐eclampsia. Interventions were administration of an antiplatelet agent (such as low‐dose aspirin or dipyridamole), comparisons were either placebo or no antiplatelet.
Data collection and analysis
Two review authors assessed trials for inclusion and extracted data independently. For binary outcomes, we calculated risk ratio (RR) and its 95% confidence interval (CI), on an intention‐to‐treat basis. For this update we incorporated individual participant data (IPD) from trials with this available, alongside aggregate data (AD) from trials where it was not, in order to enable reliable subgroup analyses and inclusion of two key new outcomes. We assessed risk of bias for included studies and created a 'Summary of findings' table using GRADE.
Main results
Seventy‐seven trials (40,249 women, and their babies) were included, although three trials (relating to 233 women) did not contribute data to the meta‐analysis. Nine of the trials contributing data were large (> 1000 women recruited), accounting for 80% of women recruited. Although the trials took place in a wide range of countries, all of the nine large trials involved only women in high‐income and/or upper middle‐income countries. IPD were available for 36 trials (34,514 women), including all but one of the large trials. Low‐dose aspirin alone was the intervention in all the large trials, and most trials overall. Dose in the large trials was 50 mg (1 trial, 1106 women), 60 mg (5 trials, 22,322 women), 75mg (1 trial, 3697 women) 100 mg (1 trial, 3294 women) and 150 mg (1 trial, 1776 women). Most studies were either low risk of bias or unclear risk of bias; and the large trials were all low risk of bas.
Antiplatelet agents versus placebo/no treatment
The use of antiplatelet agents reduced the risk of proteinuric pre‐eclampsia by 18% (36,716 women, 60 trials, RR 0.82, 95% CI 0.77 to 0.88; high‐quality evidence), number needed to treat for one women to benefit (NNTB) 61 (95% CI 45 to 92). There was a small (9%) reduction in the RR for preterm birth <37 weeks (35,212 women, 47 trials; RR 0.91, 95% CI 0.87 to 0.95, high‐quality evidence), NNTB 61 (95% CI 42 to 114), and a 14% reduction infetal deaths, neonatal deaths or death before hospital discharge (35,391 babies, 52 trials; RR 0.85, 95% CI 0.76 to 0.95; high‐quality evidence), NNTB 197 (95% CI 115 to 681). Antiplatelet agents slightly reduced the risk of small‐for‐gestational age babies (35,761 babies, 50 trials; RR 0.84, 95% CI 0.76 to 0.92; high‐quality evidence), NNTB 146 (95% CI 90 to 386), and pregnancies with serious adverse outcome (a composite outcome including maternal death, baby death, pre‐eclampsia, small‐for‐gestational age, and preterm birth) (RR 0.90, 95% CI 0.85 to 0.96; 17,382 women; 13 trials, high‐quality evidence), NNTB 54 (95% CI 34 to 132). Antiplatelet agents probably slightly increase postpartum haemorrhage > 500 mL (23,769 women, 19 trials; RR 1.06, 95% CI 1.00 to 1.12; moderate‐quality evidence due to clinical heterogeneity), and they probably marginally increase the risk of placental abruption, although for this outcome the evidence was downgraded due to a wide confidence interval including the possibility of no effect (30,775 women; 29 trials; RR 1.21, 95% CI 0.95 to 1.54; moderate‐quality evidence).
Data from two large trials which assessed children at aged 18 months (including results from over 5000 children), did not identify clear differences in development between the two groups.
Authors' conclusions
Administering low‐dose aspirin to pregnant women led to small‐to‐moderate benefits, including reductions in pre‐eclampsia (16 fewer per 1000 women treated), preterm birth (16 fewer per 1000 treated), the baby being born small‐for‐gestational age (seven fewer per 1000 treated) and fetal or neonatal death (five fewer per 1000 treated). Overall, administering antiplatelet agents to 1000 women led to 20 fewer pregnancies with serious adverse outcomes. The quality of evidence for all these outcomes was high. Aspirin probably slightly increased the risk of postpartum haemorrhage of more than 500 mL, however, the quality of evidence for this outcome was downgraded to moderate, due to concerns of clinical heterogeneity in measurements of blood loss. Antiplatelet agents probably marginally increase placental abruption, but the quality of the evidence was downgraded to moderate due to low event numbers and thus wide 95% CI.
Overall, antiplatelet agents improved outcomes, and at these doses appear to be safe. Identifying women who are most likely to respond to low‐dose aspirin would improve targeting of treatment. As almost all the women in this review were recruited to the trials after 12 weeks' gestation, it is unclear whether starting treatment before 12 weeks’ would have additional benefits without any increase in adverse effects. While there was some indication that higher doses of aspirin would be more effective, further studies would be warranted to examine this.
Plain language summary
Antiplatelet agents for preventing pre‐eclampsia and its complications
We set out to assess the ability of antiplatelet agents, such as aspirin and dipyridamole, to prevent women from developing pre‐eclampsia during pregnancy and to improve health outcomes for them and their babies. We also wanted to find out whether these medicines had any undesirable effects for the mother or baby.
What is the question?
Do low doses of aspirin help to prevent pre‐eclampsia, and reduce the number of preterm births before 37 weeks, small‐for‐gestational‐age babies, infant deaths and other unwanted effects?
Why is this important?
Pre‐eclampsia is a condition experienced by some women during pregnancy and is evident as high blood pressure and protein in the urine. This condition can lead to serious complications for the mother and her baby (in fact, it is one of the leading causes of illness and death in pregnancy). The mother’s placenta may not be functioning properly, which limits the blood supply to the unborn baby so that it is at risk of poor growth and being born early as a result of preterm labour, or needing to be delivered early. Pre‐eclampsia affects the platelets in the women’s blood so that they are more ready to clump and cause the blood to clot. Antiplatelet drugs like aspirin prevent blood clotting and have a role in preventing pre‐eclampsia and its complications.
What evidence did we find?
We searched for randomised controlled trials in March 2018. Our review includes 77 trials, involving 40,249 women and their babies, although it wasn't possible to include results form three of these trials (233 women) . We included information about the results for women and babies in two different formats: 36 trials (34,514 women) reported 'individual participant data' (IPD), where we received information about each of the individuals involved; all the other trials reported 'aggregate data' (AD), where each study reports the average information about the individuals involved in the study. By using IPD, we could conduct very thorough and accurate analyses; and by combining both the AD and the IPD, we could include all the available information on this question.
Nine of the trials included more than 1000 women, and all of these large trials were at low risk of bias. Low‐dose aspirin alone was the intervention in all the large trials, and most trials overall. Almost all the women were recruited to the trials after 12 weeks' gestation. Most women were at risk of developing pre‐eclampsia, and the trials included women with normal blood pressure, existing long‐term high blood pressure or pregnancy‐induced high blood pressure. High‐quality evidence showed that the use of antiplatelet agents reduced the risk of pre‐eclampsia by 18%, or less than one sixth (36,716 women, 60 trials). This meant that 61 women had to be treated with an antiplatelet drug for one woman to benefit by avoiding pre‐eclampsia. The risk of preterm birth was reduced by 9% (35,212 women, 47 trials) and the number of infant deaths before or around the time of birth was reduced by 15% (35,391 women, 52 trials). Antiplatelet agents reduced the risk of small‐for‐gestational‐age babies (35,761 mothers, 50 trials) and pregnancies with serious adverse outcomes (17,382 mothers; 13 trials). Moderate‐quality evidence showed that only slightly more women lost more than 500 mL of blood immediately after birth, termed postpartum haemorrhage (23,769 mothers, 19 trials), indicating that aspirin is safe. Doses of aspirin less than 75 mg appear to be safe. Higher doses might be better, but we do not know whether they increase adverse effects.
What does this mean?
Low doses of aspirin slightly reduce the risk of pre‐eclampsia and its complications. As most women in this review were in trials evaluating low‐dose aspirin, the reassurance about the safety of aspirin may not apply to higher doses or other antiplatelet agents. Further research should aim to identify women who are most likely to respond to low‐dose aspirin treatment. While it is possible that higher doses of aspirin may be more effective, further studies are needed to determine whether higher doses are both more effective and safe for women and babies.
Summary of findings
for the main comparison.
| Antiplatelet agents compared with no antiplatelet agents/placebo for pre‐eclampsia prevention | ||||||
|
Patient or population: pregnant women considered to be at risk of developing pre‐eclampsia Settings: maternity hospitals, recruitment usually in ante natal clinics Intervention:any antiplatelet agent (such as low‐dose aspirin or dipyridamole) Comparison:no treatment with antiplatelet agents/placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no antiplatelet | Antiplatelet agents | |||||
| Proteinuric pre‐eclampsia | 92 per 1000 |
16 fewer per 1000 (22 fewer to 11 fewer) |
RR 0.82 (0.77 to 0.88) |
36,716 (60 trials) | ⊕⊕⊕⊕ high | |
| Any reported infant death (fetal, neonatal, or before hospital discharge | 33 per 1000 |
5 fewer per 1000 (9 fewer to 1 fewer) |
RR 0.85 (0.76 to 0.95) |
35,391 (52 trials) |
⊕⊕⊕⊕ high | |
|
Preterm birth (before 37 weeks' gestation) |
175 per 1000 |
16 fewer per 1000 (23 fewer to 9 fewer) |
RR 0.91 (0.87 to 0.95) |
35,212 (47 trials) | ⊕⊕⊕⊕ high | |
| Small‐for‐gestational age | 47 per 1000 |
7 fewer per 1000 (11 fewer to 3 fewer) |
RR 0.84 (0.76 to 0.92) |
35,761 (50 trials) | ⊕⊕⊕⊕ high | |
| Pregnancy with serious adverse outcome (composite including maternal death, baby death, pre‐eclampsia, small‐for‐gestational age, preterm birth) ‐ only trials with individual participant data | 197 per 1000 |
20 fewer per 1000 (30 fewer to 8 fewer) |
RR 0.90 (0.85 to 0.96) |
17,382 (13 trials) |
⊕⊕⊕⊕ high | |
| Postpartum haemorrhage > 500 mL | 143 per 1000 |
9 more per 1000 (0 fewer to 19 more) |
RR 1.06 (1.00 to 1.12) |
23,769 (19 trials) |
⊕⊕⊕⊝ moderatea | |
| Placental abruption | 7 per 1000 |
2 more per 1000 (0 fewer to 4 more) |
RR 1.21 (0.95 to 1.54) |
30,775 (29 trials) |
⊕⊕⊕⊝ moderateb | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. Although this effect estimate did not meet our threshold for statistical heterogeneity (threshold i2 = 40%), we downgraded due to concerns about clinical heterogeneity between trials in methods for measurement of blood loss (‐1).
b. Wide confidence interval including appreciable harm for antiplatelet agents and crossing line of no effect (‐1).
Background
Description of the condition
Pre‐eclampsia is defined as high blood pressure (hypertension) associated with proteinuria (protein in the urine) (Gifford 2000). It occurs in the second half of pregnancy and complicates between 2% to 8% of pregnancies (Ananth 2013; Auger 2016; Thornton 2013; WHO 1988). Pre‐eclampsia can also affect other maternal organs, leading to problems in liver, kidneys and brain, and to abnormalities of the clotting system (Mol 2016). As the placenta is also involved, there are increased risks for the baby (Mol 2016). The most common are poor growth due to inadequate blood supply through the damaged placenta, and the problems of prematurity (related either to the spontaneous onset of preterm labour or to early delivery to protect the mother or the fetus) (Saigal 2008). Pre‐eclampsia is discussed in more detail in the generic protocol of interventions for preventing pre‐eclampsia (Meher 2005).
High blood pressure is common during pregnancy, and around 10% of women will have their blood pressure recorded as above normal at some point before delivery (NICE 2010). For women who develop raised blood pressure but have no proteinuria or any other complication, pregnancy outcome is similar to that for women who have normal blood pressure. Raised blood pressure alone occurring for the first time during pregnancy is known as gestational hypertension, or pregnancy‐induced hypertension (Tranquilli 2014). One of the difficulties in caring for women with gestational hypertension is that it is so common, and there is no reliable way of predicting who will progress to more severe disease. Therefore, very large numbers of these women are admitted to hospital or to day‐care units for assessment, or receive antenatal care designed for high‐risk women. Women with gestational hypertension or mild pre‐eclampsia usually feel well. It is only when blood pressure is very high (greater than 160 mmHg systolic or greater than 110 mmHg diastolic) or they develop symptoms of severe pre‐eclampsia, such as headache, epigastric pain or visual disturbances, that they may feel unwell.
Although the outcome following pre‐eclampsia or eclampsia (the rare occurrence of seizures superimposed on pre‐eclampsia) is good for most women, particularly in high‐income settings, these conditions remain major causes of maternal mortality (Say 2014). A quarter of a million women die each year of pregnancy‐related causes, and 99% of these deaths occur in low‐ and middle‐income countries (Mahler 1987; Rosenfield 1985; WHO 2014). An estimated 9% to 26% of the maternal deaths in low‐ and middle‐income countries are associated with hypertensive disorders of pregnancy (Duley 1992a; WHO 2006), as are 18% of the direct obstetric deaths in the UK (CMACE UK 2011). Perinatal mortality is also increased (Ananth 1995; Dept of Health 1996). There is little good quality information about morbidity for either mother or baby, but it is likely that this too is high.
Description of the intervention
Antiplatelet drugs act by decreasing platelet aggregation and inhibiting thrombus formation. The most common antiplatelet agent is aspirin, which is also known as acetylsalicylic acid. It is widely available without prescription, usually as 300 mg tablets, and used as an anti‐inflammatory drug for minor aches and pains, and to reduce fever. Aspirin has its antiplatelet effect by inhibiting the production of thromboxane, which under normal circumstances binds platelet molecules together to create a patch over damage to the wall of blood vessels (ACOG 2018). Low‐dose aspirin (usually 75 mg) is used for long‐term therapy to help prevent heart attacks, strokes and blood clots (thrombosis) in people at high risk. It is also used after heart attacks, to prevent another happening.
Aspirin does have side effects. When taken at higher doses and for prolonged periods (in doses up to 300 mg, for more than five years), it can lead to gastrointestinal and cerebral bleeding (De Berardis 2012). However, as aspirin for prevention of pre‐eclampsia is prescribed at the lower end of this range of doses, and for limited duration, these more serious problems are unlikely.
How the intervention might work
Pre‐eclampsia is a complex condition, and the initial cause is faulty implantation of the placenta early in pregnancy (Uzan 2011). The primary problem is thought to be deficient trophoblast invasion of the spiral arteries in the uterus during the second trimester, leading to underperfusion of the circulation between uterus and placenta, with consequent reduction in blood flow through the placenta (placental ischaemia) (Redman 1991; Uzan 2011). The resulting placental damage is thought to lead to release of factors into the maternal circulation, which are responsible for the maternal syndrome (Roberts 2009). Activation of platelets and the clotting system may occur early in the course of the disease, before clinical symptoms develop (Janes 1995; Redman 1978). Deficient intravascular production of prostacyclin, a vasodilator, with excessive production of thromboxane, a platelet‐derived vasoconstrictor and stimulant of platelet aggregation (Bussolino 1980) have also been demonstrated to occur in pre‐eclampsia.
These observations led to the hypotheses that antiplatelet agents, and low‐dose aspirin in particular, might prevent or delay the development of pre‐eclampsia and that, for women who already have the disorder, the risk of adverse events might be reduced (Dekker 1993). A further hypothesis is that the effect of antiplatelets may be different if treatment is started before placental implantation is complete (CLASP 1994). If this hypothesis were correct, the greatest benefit should be seen in women who started treatment before 16 weeks' gestation, with the effect attenuating with later onset of treatment (Askie 2007; Bujold 2010; PARIS 2005). Similarly, it remains unclear as to the most appropriate dose of antiplatelet therapy for the prevention of pre‐eclampsia in order to maximise benefits whilst minimising harms (Uzan 1998). Many of the large trials have evaluated 60 mg or 75 mg, whilst some trials have used the higher dose of 100 mg or 150 mg. It has been suggested that low doses of aspirin (< 75 mg) may selectively inhibit the cyclo‐oxygenase pathway in platelet production but not in vessel wall endothelium, thereby diminishing the synthesis of thromboxane but not of prostacyclin. A higher dose may inhibit both thromboxane and prostacyclin, thereby neutralising the effect of the intervention (Masotti 1979). However, there is also limited evidence from randomised trials that a higher dose of aspirin (75 mg or higher) may effect a greater reduction in the risk of pre‐eclampsia (Duley 2007).
Why it is important to do this review
The hypothesis that antiplatelet drugs, low‐dose aspirin in particular, might prevent pre‐eclampsia and its complications was first tested in several small randomised trials which reported striking benefits in terms of reducing the risk of hypertension and proteinuria (Dekker 1993). The trials were too small to provide reliable information about other more substantive outcomes, such as perinatal mortality, although there were anecdotal reports of women exposed to aspirin which suggested promising benefits. In addition, there was no information about the potential hazards of this therapy, such as a possible increased risk of bleeding for both the woman and her baby, and possible adverse effects on infant and child development. The promising results of these early trials of low‐dose aspirin led to several large trials being conducted in various parts of the world. Before these results became available, however, the use of low‐dose aspirin had already become relatively widespread for women considered to be at increased risk of pre‐eclampsia. When the large trials were published, they led to widespread disappointment that low‐dose aspirin was not effective for prevention of pre‐eclampsia (Barth 1998; Beilin 1994; Darling 1998). It was only when all trials were combined in a systematic review that it became clear that there were indeed modest reductions in the risk of pre‐eclampsia and some of its consequences associated with the use of antiplatelet agents (Duley 2001; Knight 2000a). None of the large trials had been powered to detect such modest effects, and so the differences only achieved statistical significance when combined within the meta‐analysis.
Over 35,000 pregnant women have been entered into randomised trials evaluating low‐dose aspirin. In the past, several systematic reviews have attempted to summarise these results (Collins 1995; Duley 2001; Imperiale 1991; Leitich 1997; Rey 1996; Sanchez‐Ramos 1994; Sharts‐Engel 1992), although none of these remain up to date. The previous update of this review concluded antiplatelet agents, largely low‐dose aspirin, have moderate benefits when used for prevention of pre‐eclampsia and its consequences; and that further information is required to assess which women are most likely to benefit, when treatment is best started, and at what dose (Duley 2007).
Recent systematic reviews published elsewhere have focused on specific subgroups of women, for example based either on risk factors for development of pre‐eclampsia (Coomarasamy 2001; Coomarasamy 2003; Rossi 2011; Ruano 2005; Trivedi 2011) or gestation at randomisation when antiplatelet therapy is started (Bujold 2010; Roberge 2013). Nevertheless, there is reasonable agreement that low‐dose aspirin appears to be safe, at least in the short term, and that it reduces the risk of pre‐eclampsia for a broad range of women. However, as the risk of pre‐eclampsia reduces the number of women who need to be treated to prevent one case of pre‐eclampsia grows. So whilst the public health benefits may be clear, it is less certain whether there is worthwhile benefit for individual women at low‐to‐moderate risk of developing pre‐eclampsia (Askie 2007; Roberts 2007). Several issues remain controversial. These include whether dose, type of preparation, or starting treatment early in pregnancy are factors that substantially influence effectiveness. Also, there is concern about potential for publication bias, due to enthusiasm for the use of low‐dose aspirin, which may have led to speedy publication of small positive trials in high‐profile journals, with small negative trials taking far longer to appear, and then doing so only in more obscure publications (Broughton Pipkin 1996; Knight 2000a).
Since the last update of this Cochrane Review, the PARIS individual participant data (IPD) meta‐analysis of trials evaluating antiplatelet drugs for prevention of pre‐eclampsia and its complications has been published (Askie 2007). Overall, 63 trials were identified as potentially eligible (with 38,026 women). Data were obtained from 36 studies (34,288 women, 90% of randomised women). Of these, 31 trials recruited women for primary prevention of pre‐eclampsia (32,217 women and 32,819 babies). IPD from these 31 studies have been incorporated into this update. The methods used for the PARIS review (PARIS 2005) have been added to the methods for this updated Cochrane Review.
A wide variety of other interventions have been suggested for possible prevention of pre‐eclampsia. Other Cochrane Reviews cover calcium supplementation (Hofmeyr 2018), magnesium supplementation (Makrides 2014), protein intake and nutritional advice (Ota 2015), salt intake (Duley 1999b), omega‐3 fatty acid additions (Middleton 2018 and antioxidants (Rumbold 2008). Although some of these interventions are promising, to date only aspirin and calcium have been shown to have clinically worthwhile benefits in prevention of pre‐eclampsia and its complications (Meads 2008).
The aims of this review are (i) to identify as many of both the published and unpublished antiplatelet trials as possible and (ii) to estimate the benefits and hazards of antiplatelet agents when used for the prevention of pre‐eclampsia.
Objectives
To assess the effectiveness and safety of antiplatelet agents, such as aspirin and dipyridamole, when given to women at risk of developing pre‐eclampsia.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials comparing antiplatelet agents with either placebo or no antiplatelet agent during pregnancy. Studies only published in abstract format were eligible for inclusion if sufficient information was available. Cluster‐randomised controlled trials were eligible (but none were identified). Quasi‐random studies were excluded.
Types of participants
Pregnant women considered to be at risk of developing pre‐eclampsia. This included women with normal blood pressure and those with chronic hypertension, as well as women with pregnancy‐induced or gestational hypertension.
For studies where IPD were available, individual women were classified into risk categories based on the criteria in the PARIS protocol (Askie 2007). They were classified as low risk if they had any one of the following risk factors: primiparity, family history of pre‐eclampsia, age greater than 40 years, or multiple pregnancy. Women were classified as moderate risk if they had any two of the previous risk factors. Women were classified as high risk if they had any one of the following risk factors: diabetes, chronic hypertension, renal disease, autoimmune disease, gestational hypertension, positive uterine artery Doppler, previous pre‐eclampsia or previous fetal/neonatal death associated with pre‐eclampsia. Women with no identifiable risk factor in the available IPD were grouped with the low‐risk women. Hence for studies where IPD were available, data from one trial could appear in more than one 'maternal risk' category and in more than one 'gestational age at randomisation' category, as individual women within each trial could be classified into different risk and gestational age subgroups.
For studies where aggregate data (AD) only were available, inclusion criteria were used to group women into low, moderate, high or unclassified risk according to the same criteria outlined above, so that categorisation was consistent with IPD analyses. Women in a particular trial could be placed in one category only (unless appropriate subgroup data were available from the trial publication), as IPD were not available.
Types of interventions
Comparisons of any antiplatelet agent (such as low‐dose aspirin or dipyridamole) with either placebo or no antiplatelet agent. This was regardless of dose and duration of therapy or mode of administration, and irrespective of whether in combination with another agent.
Types of outcome measures
For trials with IPD, outcomes were defined using the PARIS 2005 definitions. For trials with only AD available, the trialists' own outcome definitions were used.
Primary outcomes
For the women
Pre‐eclampsia (as reported by the trialist)
For the children
Death (fetal, neonatal, or before hospital discharge)
Preterm birth (all birth before 37 weeks)
Small‐for‐gestational age (preferably using below the third centile of birthweight for gestational age, but otherwise the most extreme centile available)
Secondary outcomes
For the women
Gestational hypertension (new hypertension with onset after 20 weeks' gestation)
Death (secondary outcome as likely to be a rare event in the trials)
Elective delivery (induction of labour or elective caesarean section)
Caesarean section (emergency plus elective)
Bleeding episodes
Abruption of the placenta
Antepartum haemorrhage
Postpartum haemorrhage
Complications of epidural anaesthesia
Need for blood transfusion
Measures of serious maternal morbidity
Eclampsia
Liver failure
Renal failure
Disseminated intravascular coagulation
Rare adverse events
Temporary blindness
Major psychiatric disorders
For the children
Bleeding episodes (such as intraventricular haemorrhage)
Infant and child development (such as cerebral palsy, cognitive delay, deafness, and blindness, as defined by the trialist)
For the pregnancy (as defined in the PARIS protocol)
Serious adverse outcome (pregnancy where the woman dies or develops pre‐eclampsia, or the baby is preterm, small‐for‐gestational age, or dies before discharge from hospital)
Use of health service resources
For the woman
Antenatal hospital admission
Visits to day care units
Use of intensive care
Ventilation
Dialysis
For the children
Admission to special care/intensive care nursery
Duration of mechanical ventilation
Length of stay in hospital
Development and special needs after discharge
Search methods for identification of studies
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (30 March 2018). We carried out an updated search in September 2019 and added 27 new trial reports to Studies awaiting classification.
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (30 March 2018) using the search methods detailed in Appendix 1.
Searching other resources
We asked trialists in the PARIS Collaboratio if they knew of any further studies.
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, see Duley 2007.
For this update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
At least two review authors independently assessed for inclusion each of the potential studies that we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
For this update we combined aggregate data (AD) with individual participant data (IPD). Meta‐analyses based on IPD allow more powerful and uniformly consistent analyses, whilst allowing better characterisation of subgroups and outcomes, compared to those based on AD (Riley 2010; Tudur Smith 2016). We included both AD and IPD in this update in order to combine all available information. The use of IPD supported more detailed and accurate analyses. It enabled us to classify participants by specific characteristics, supporting analyses subgrouped by maternal risk of developing pre‐eclampsia, and also provided new data on key outcomes. Combining IPD with AD meant that it was possible to bring together all eligible trials even where IPD was unavailable.
IPD from trials within the PARIS Collaboration were collected according to the methods described in the Askie 2007 paper as follows. Data to be collected were agreed after extensive consultation within the PARIS Collaborative Group. Anonymised data for each of the pre‐specified variables were requested for each woman randomised. Data were supplied in a variety of formats, re‐coded as necessary, and were checked for internal consistency, consistency with published reports, and for missing items. Information about the trials, e.g. randomisation method and antiplatelet dose, were cross‐checked with published reports, trial protocols, and data collection sheets. Quality and integrity of the randomisation processes were assessed by reviewing the chronological randomisation sequence and pattern of assignment, as well as the balance of baseline characteristics across treatment groups (taking into account stratification factors). Inconsistencies or missing data were discussed with relevant trialists and corrected when necessary. Finalised data for each study were verified with the relevant trialists before being combined into the final PARIS dataset.
Where IPD were available from the PARIS dataset, these were extracted for use in the review, using the subgroup classifications and outcome definitions defined in this review.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
During the collection of IPD for the PARIS Collaboration, additional information was sought from the authors of the studies included in this Collaboration to further clarify the 'Risk of bias' assessments. Additional information gained during this process (that was not available from the published data alone) is referred to as the ‘PARIS assessment’ in the text and tables of this review.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding of participants, personnel and outcome assessment (checking for possible performance and detection bias)
We described for each included study the methods used, if any, to blind study participants, personnel and outcome assessors from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update, we evaluated the quality of the evidence comparing antiplatelet agents with placebo/no antiplatelet for the outcomes proteinuric pre‐eclampsia, fetal or neonatal death, preterm birth, small‐for‐gestational‐age, pregnancy with serious adverse outcome, postpartum haemorrhage, and placental abruption using the GRADE approach as outlined in the GRADE handbook. The GRADE approach uses five considerations (trial limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for specific outcomes. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, inconsistency, imprecision of effect estimates or publication bias.
'Summary of findings' table
We used GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create a 'Summary of findings’ table for the maternal and child outcomes listed above that were evaluated with the GRADE approach. The 'Summary of findings' table presents summaries of the intervention effect and measures of quality according to the GRADE approach.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference as outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We would have included cluster‐randomised trials in the analyses along with individually‐randomised trials, if any had been identified in our search strategy. If we had found any we would have adjusted their sample sizes using the methods described in the Handbook [Section 16.3.4] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we had used ICCs from other sources, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. If we had identified cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. We would have considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and an interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
We would also have acknowledged heterogeneity in the randomisation unit and performed a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials and other unit of analysis issues
As a cross‐over design is not a valid study design for this review, we planned to exclude these studies. If we identified trials with more than two treatment groups, we planned to include them. For studies comparing two different antiplatelet regimens with placebo or no treatment, we planned to combine data for the two antiplatelet groups. For trials with three or more arms, we planned to combine data where possible, but split the arms by subgroup where necessary (e.g. trials administering different doses to different arms were split when analysing subgroups by dose). Each intervention arm was then compared to the full control group within its subgroup. This was the case for two trials with a total of 367 women (EPREDA 1991; S Africa 1988).
The mother was the unit of analysis with an event being any baby having an event (e.g. any baby being small‐for‐gestational age) in case of multiple pregnancies.
Dealing with missing data
For included studies, we noted levels of attrition. Where more than 20% of participants were lost to follow‐up, the study was excluded. For individual outcomes where more than 20% of data were missing, the trials were excluded from the meta‐analysis for that particular outcome. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. We included data for long‐term follow‐up of women and children where losses to follow‐up were greater than 20%, providing that substantive bias between the groups was unlikely. We documented completeness of follow‐up for these studies alongside any results.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T2, I² and Chi² statistics. We regarded heterogeneity as substantial if an I2 was greater than 40% and either a T2 was greater than zero or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
For outcomes with 10 or more studies contributing events in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate it, including exploration of any apparent differences between results from IPD and AD in the funnel plots.
Data synthesis
Data were synthesised using a two‐stage approach which involved calculating treatment effect estimates for both the individual participant and aggregate data separately, and then combining these for an overall effect estimate when appropriate. Individual participant and aggregate data were further subgrouped according to maternal risk, gestational age at trial entry, aspirin dosage and use of placebo (see section below). When the definition of the outcomes varied too strongly across types of data (individual participant versus aggregate), they were analysed separately. For some outcomes, only IPD were available, while for others, only AD were available.
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
In future updates, If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses:
maternal risk of pre‐eclampsia at trial entry (as defined under types of participant): low risk; moderate risk; or high risk;
gestation at trial entry: before 20 weeks' gestation; at or after 20 weeks' gestation; unclear or unspecified;
type of antiplatelet agent: low‐dose aspirin alone; all other types of antiplatelet agent and low‐dose aspirin combined with other antiplatelet agents;
by dose of aspirin: less than 75 mg; greater than or equal to 75 mg; dose not known;
by type of control group intervention: placebo; no placebo.
Subgroup analyses were restricted to the primary outcomes.
Since we had IPD and AD for most outcomes that were entered as separate subgroups, interaction tests available with RevMan (RevMan 2014) were not meaningful for the subgroups and were therefore not reported and interpreted. Instead, we visually inspected the forest plots to assess differences between subgroups. Furthermore, we conducted additional analyses for the most relevant outcomes (proteinuric pre‐eclampsia, fetal or neonatal death, preterm birth, small‐for‐gestational age), in which we combined IPD and AD to perform interaction tests available with RevMan (RevMan 2014) to test for maternal risk subgroup differences (Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8). Results of these subgroup analyses were reported quoting the Chi2 statistic and P value, and the interaction test I² value.
1.5. Analysis.
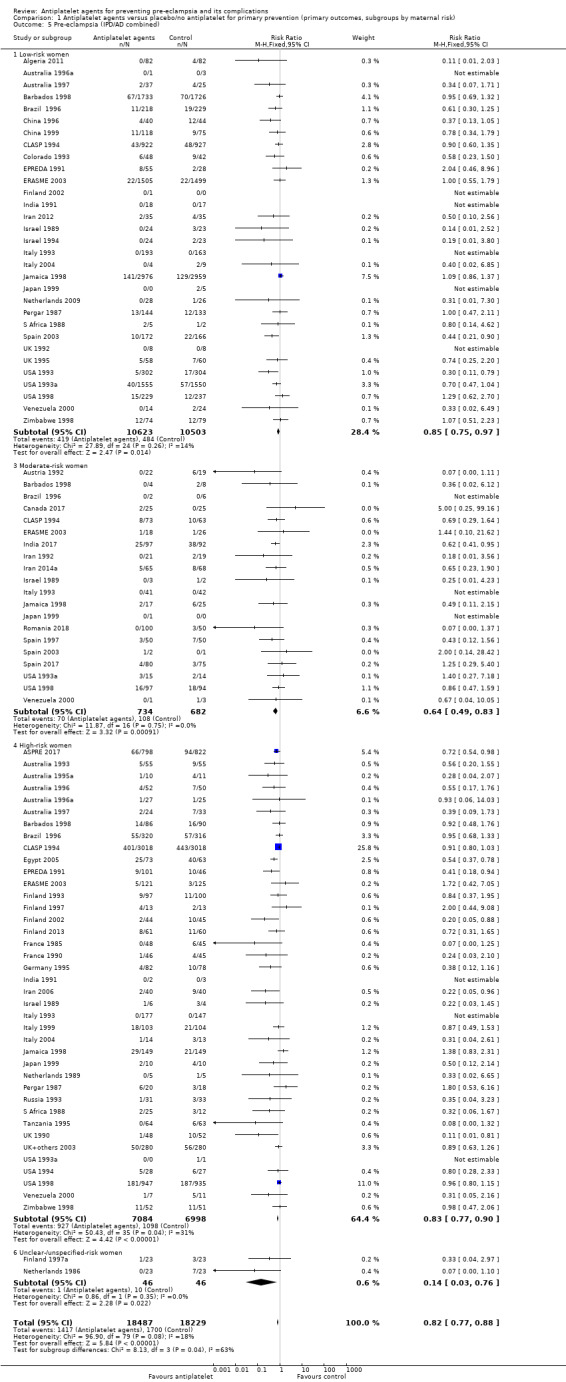
Comparison 1 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by maternal risk), Outcome 5 Pre‐eclampsia (IPD/AD combined).
1.6. Analysis.
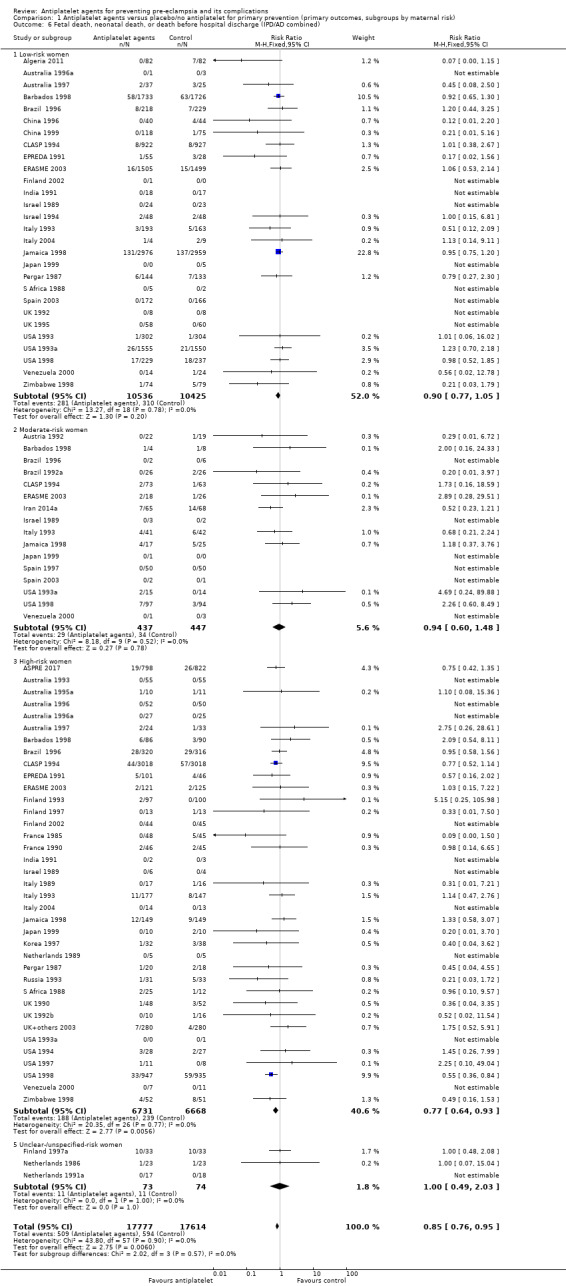
Comparison 1 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by maternal risk), Outcome 6 Fetal death, neonatal death, or death before hospital discharge (IPD/AD combined).
1.7. Analysis.
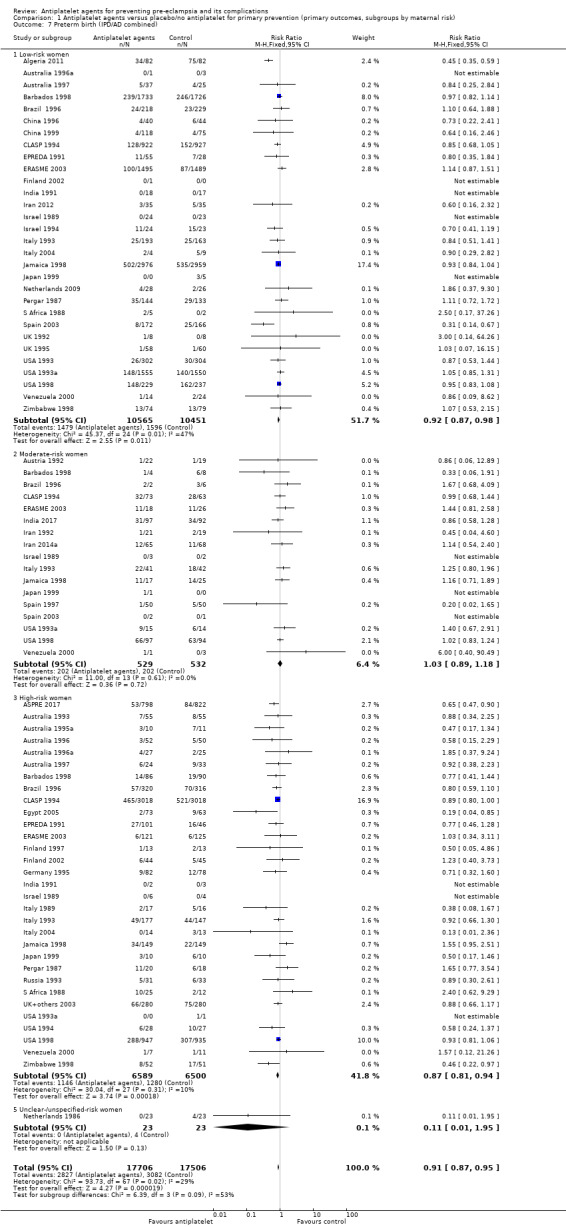
Comparison 1 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by maternal risk), Outcome 7 Preterm birth (IPD/AD combined).
1.8. Analysis.
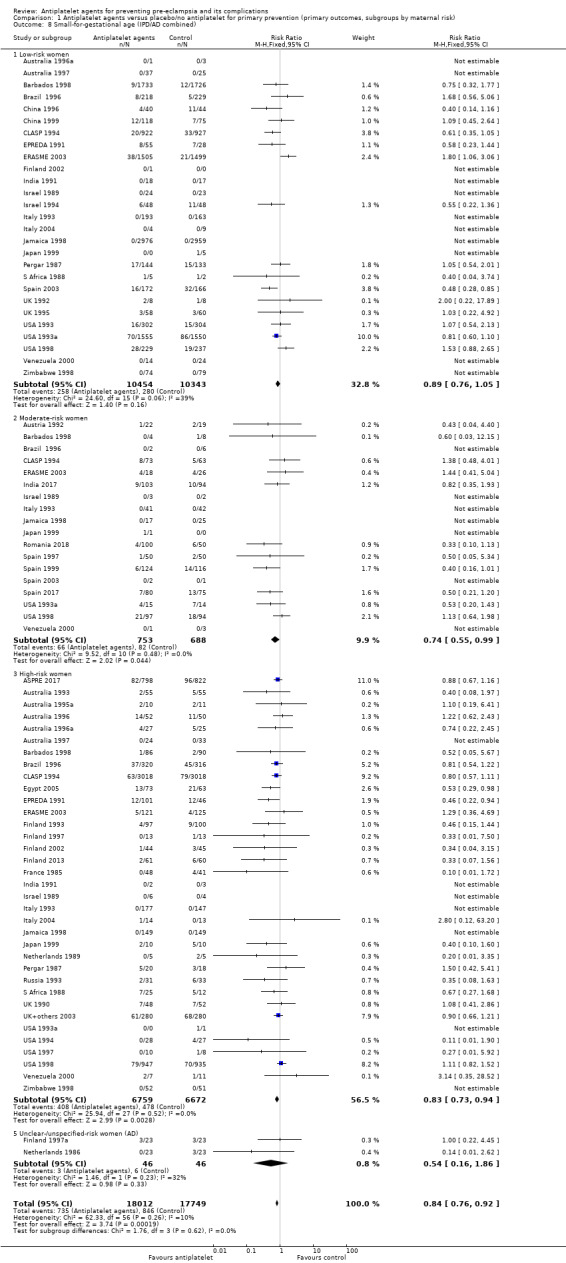
Comparison 1 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by maternal risk), Outcome 8 Small‐for‐gestational age (IPD/AD combined).
Sensitivity analysis
We planned sensitivity analyses (restricted to the primary outcomes) excluding studies with high risk of bias in three or more 'Risk of bias' domains. Since this was not the case for any of the studies, no sensitivity analyses were performed.
Results
Description of studies
Results of the search
See: Figure 1.
1.
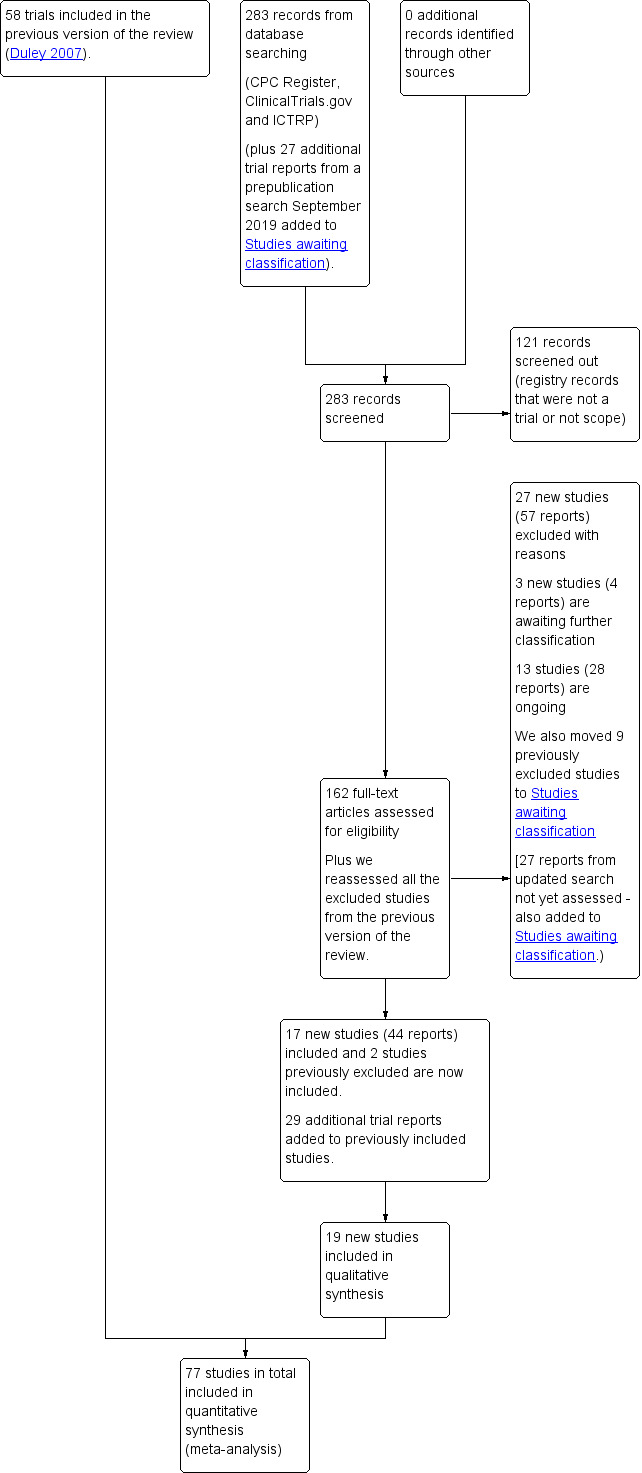
Study flow diagram.
We assessed 160 new trial reports (146 from the new search and 16 that were awaiting classification in the previous version of the review (Duley 2007)). We also reassessed the previously excluded studies. We included two previously excluded studies (India 1991; Pergar 1987), and moved nine studies previously excluded to Studies awaiting classification as if further information became available they could potentially be included (Netherlands 1991; Netherlands/UK 1994; Slovenia 1992; Slovenia 1994; South Africa 1986; Switzerland 2000; Uganda 1992; USA 1988a; USA 1990).
Of the 160 reports, we included 17 new studies (42 reports) and excluded 27 studies (57 reports), A further 29 reports were additional publications for studies already included in the review. We added three new studies (four reports) to Studies awaiting classification and 13 studies (28 reports) are ongoing.
We carried out an updated search in September 2019 and added 27 new trial reports to Studies awaiting classification for consideration at the next update.
Included studies
There were 77 randomised trials involving 40,586 women included in this review.
For the PARIS IPD review, the search was last updated in December 2005. At that time 63 trials (with 38,026 women) were eligible for inclusion (Askie 2007). Of these, we were unable to trace the investigators for seven trials, one trialist declined to participate, data were confirmed as lost or non‐retrievable for 17 trials, and although available, were not supplied for two small trials. Ultimately, data were therefore available from 36 trials and 34,288 women (90% of randomised women at that time). Of these, 31 trials recruited women in a primary prevention setting (total 32,217 women and 32,819 babies).
Details for each trial are in the Characteristics of included studies table.
Methods
All included studies were randomised trials. Overall, methodological quality of these trials was good, with around half the trials having low risk of bias and relatively few having high risk of bias for any assessment (Figure 2). The nine large trials (over 1000 women) were all high quality (Figure 3). For sequence generation, all trials were low or unclear risk of bias (Figure 4). Similarily, for concealment of allocation almost all studies were either low or unclear risk of bias, with all the large trials low risk, and only two small trials (India 2017, Tanzania 1995) high risk. High risk of bias was most common for blinding (performance and detection bias), which was usually associated with the trials with no placebo.
2.
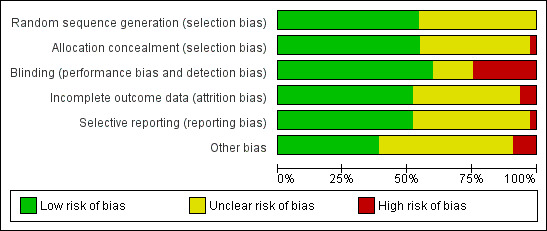
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
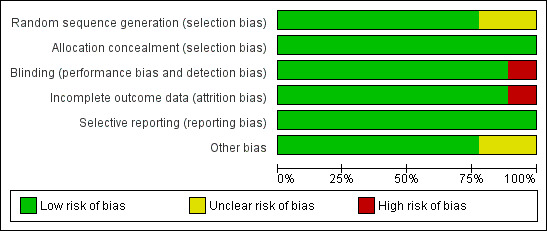
'Risk of bias' graph for the nine large trials: review authors' judgements about each risk of bias item presented as percentages across all studies.
4.
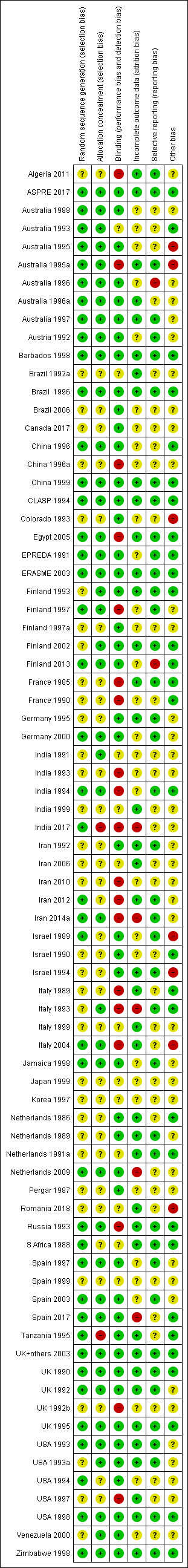
Risk of bias, summary: review authors' judgements about each risk of bias item for each included study.
Sample size
Data were reported for less than 50 women in 19 trials, 50 to 99 women in 17 trials, for 100 to 999 women in 32 trials, and over 1000 women in nine trials. Sample size for the nine large trials (> 1000 women) ranged from 1009 (Brazil 1996) to 9364 women (CLASP 1994). The total sample size for these nine studies was 32,195 women, hence 79% of the women randomised to the trials included in this review were recruited to these nine studies (ASPRE 2017; Barbados 1998; Brazil 1996; CLASP 1994; ERASME 2003; Italy 1993; Jamaica 1998; USA 1993a; USA 1998).
Settings
These trials were largely conducted at maternity hospitals, or maternity units within hospitals. Recruitment was usually at antenatal clinics. Trials were conducted in 27 countries. While the majority (66 trials) were conducted in one country, there were five international trials that recruited in more than one country. Of these, two were large trials including over 1000 women (ASPRE 2017, CLASP 1994). Most trials were conducted in high‐resource settings, but some single‐centre and multicentre trials recruited women in middle‐ and low‐resource settings. There were nine large trials in total: six of these took place in high‐income countries (ASPRE 2017; Barbados 1998; ERASME 2003; Italy 1993; USA 1993a; USA 1998); two took place in upper‐middle income countries (Brazil 1996; Jamaica 1998), and one took place in centres in both high‐ and upper‐middle income countries (CLASP 1994) (economies defined as per World Bank 2018).
The most common recruitment countries were Australia (eight trials), the USA (seven trials), the UK (seven trials), France (five trials), the Netherlands (five trials), Israel (five trials), Italy (five trials), India (five trials), Finland (five trials), Iran (five trials) and Spain (four trials).
Eight trials were published between 1985 and 1989, 48 between 1990 and 1999, 11 between 2000 and 2009, and 10 between 2010 and 2018.
Participants
There was a wide range in incidence of pre‐eclampsia between women in different trials (2% to 60% in the placebo arm) and, in many studies, between low‐risk and high‐risk women in the same trial. Most trials were primary prevention studies, and so recruited women without gestational hypertension who were at risk of developing pre‐eclampsia. Early small trials published in the 1980s and early 1990s recruited women at high risk of pre‐eclampsia, The subsequent large trials primarily recruited women across the range of low, moderate or high risk of pre‐eclampsia. IPD data were available for eight of these nine large studies, allowing individual women in each trial to be analysed in the appropriate risk category. In three large trials almost all the women were at low or moderate risk, with just one high‐risk woman in USA 1993a, 176 (5% of 3,697) in Barbados 1998, and 246 (7% of 3294) in ERASME 2003. In Italy 1993 just over one quarter (324/1106, 29%) of women were high risk, and in Jamaica 1998 just under a half. Two thirds of women were high risk in Brazil 1996 (636/1009, 63%) and CLASP 1994,(6036/9364, 64%), as were three quarters of those in USA 1998 (1882/2539, 74%). For one recently published large trial, IPD data were not available (ASPRE 2017). This trial aimed to recruit women at high risk for preterm pre‐eclampsia (which they defined as delivery with pre‐eclampsia before 37 weeks' gestation), and risk status for the women at trial entry was assessed using an algorithm that combined maternal factors, mean arterial pressure, uterine artery pulsatility index and maternal serum pregnancy‐associated plasma protein‐A and placental growth factor.
A small number of trials were secondary prevention studies, and so recruited women who already had gestational hypertension and/or intrauterine growth restriction at trial entry (Australia 1988; Australia 1995; Germany 2000; India 1993; India 1994; India 1999; Israel 1990). Four of the early trials were both primary and secondary prevention studies, and so recruited women with or without gestation hypertension and/or intrauterine growth restriction (Brazil 1996; CLASP 1994; India 1991; Italy 1993).
Interventions and comparisons
Interventions varied as to dose of aspirin, gestation at commencement, and use of other treatments. In most trials, aspirin alone was compared with placebo or no treatment. Aspirin alone was the intervention for all nine large trials. The dose was 50 mg in one trial (Italy 1993), 60 mg in five (Brazil 1996; CLASP 1994; Jamaica 1998; USA 1993a; USA 1998), 100 mg in one (ERASME 2003) and 150 mg in one (ASPRE 2017) Eight of these large trials used placebo for the control group, and for one (Italy 1993) no treatment was the control intervention. Of the smaller trials using an intervention that was not aspirin alone, five used a combination of aspirin and dipyridamole or dipyridamole alone versus control (EPREDA 1991; France 1985; France 1990; Russia 1993; S Africa 1988), one small trial used heparin and dipyridamole versus control (Australia 1995a), another combined aspirin with vitamins C and E and fish oil (Venezuela 2000), another compared ozagrel hydrochloride with placebo (Japan 1999) ,and one compared trapidil with placebo (Germany 1995). Most trials used some form of placebo. One small trial compared different duration of aspirin treatment (to 32 weeks or 36 weeks) with placebo (Romania 2018); the two intervention groups are combined in this review.
Outcomes
Most trials reported data for the main outcomes pre‐eclampsia, preterm delivery, perinatal death and the infant being small‐for‐gestational age. Our secondary outcomes were often not reported by the included studies.
Excluded studies
Overall, 77 studies were excluded from the review. The reasons for exclusion for each trial are listed in the Characteristics of excluded studies table.
Reasons for exclusion were: 31 studies were excluded because they were not randomised trials, or because allocation was quasi‐random or unclear (Argentina 1994; Brazil 1992; Brazil 1995; China 2016; China 2017; East Germany 1988; Egypt 1998; India 1986; India 1997; India 1998; India 2002; India 2002a; Iran 2014; Italy 1990; Italy 1990a; Italy 1991; Italy 1994; Italy 2002; Japan 1989; Libya 2000; Pakistan 1994; Poland 1996; Poland 1999; Slovenia 1998; Thailand 1996; Trinidad 1998; Tunisia 1989; Turkey 1994; USA 1990; Vietnam 2017; West Germany 1977). One study was a cross‐over design (Egypt 1991). For 10 studies, the trial was not addressing the question of prevention of pre‐eclampsia (for example studies were of prevention of miscarriage, women were recruited before pregnancy, or women were not at risk of pre‐eclampsia at recruitment) (Egypt 2017; ERASME 2003a; Finland 2007; France 2001; Iran 2016; Iran 2017; Italy 2009; Sweden 2017; USA 1989; USA 2013). Ten studies were not trials evaluating antiplatelet drugs versus placebo or no treatment (Canada 2015; India 2011; Ireland 1995; Israel 2006; Italy 2005; Italy 2006; Panama 2014; Russia 1997; Tunisia 1990; USA 1993b). Twelve studies did not report any relevant clinical outcomes (Australia 1989a; China 1991; East Germany 1986; Equador 1998; Finland 1993a; Germany 1986; Ireland 2014; New Zealand 1990; UK 1992a; UK 1993; USA 1993c; USA 1996). For another 13 studies, there were other concerns about risk of bias (Australia 1989; Brazil 1996a; Colombia 1996; India 2001; Iran 2002; Iran 2013; Italy 1988; New Zealand 1998; New Zealand 2000; Spain + others 2000; UK 1994; UK 2000; USA 2012).
Risk of bias in included studies
Details for each trial are in the Characteristics of included studies table. There is variation in study quality, although overall most studies were either low risk of bias or unclear risk of bias (Figure 2; Figure 4). The studies with unclear risk of bias are mostly the smaller trials, with the nine large studies (more than 1000 women) having low risk of bias (Figure 3; Figure 5). Of the nine large trials, six were low risk of bias for all domains (Figure 5). One study (USA 1993a), was deemed at unclear risk of selection bias because there was no information about the method of randomisation, and also at unclear risk of 'other bias' due to concerns that the baseline characteristics of the enrolled population may have been unrepresentative of the general population; another (Italy 1993) did not use a placebo, and was also assessed as high risk of bias for blinding of the assessment of outcome and for attrition bias (there was higher loss to follow‐up in the no treatment arm); the third (Jamaica 1998) was unclear risk of 'other' bias, as significantly fewer women taking aspirin complied with their treatment than those taking placebo (63% versus 68%).
5.

'Risk of bias' summary for the nine large trials: review authors' judgements about each risk of bias item for each study
Overall, the nine large (more than 1000 women) trials were low risk of bias and together recruited 32,195 women (80% of women in the review). Most women in the review were therefore recruited to studies with low risk of bias. The AD studies had smaller samples sizes, and many had unclear risk of bias.
Allocation
Seven of the nine large studies (> 1000 women) were low risk for both selection bias domains (Figure 5). For two of the large studies, risk of bias for sequence generation was unclear; in one (Italy 1993), the same randomisation sheets were used in error for several centres in the early stages of the trial, and for the other (USA 1993a), the method for sequence generation is not stated. No included studies were high risk of bias for sequence generation. All nine large trials were low risk of bias for concealment of allocation. Only two studies were high risk for concealment of allocation (India 2017; Tanzania 1995). For the smaller studies, it was unclear whether sequence generation was adequate for 33 of them, and whether concealment of the allocation at trial entry was adequate for 33 of them.
Blinding
In the majority of trials,including eight of the nine large trials, participants, clinicians and research personnel were blinded to the treatment, and the risk of bias in this domain was low. Only one of the larger trials (Italy 1993) and 18 of the smaller trials did not blind adequately. In most cases this was because they did not use a placebo, and none of the trials without a placebo attempted to blind assessment of the outcome; these trials were high risk for both performance bias and detection bias.
Incomplete outcome data
Overall, 40 trials were low risk of attrition bias, whilst for 32 trials risk of this bias was unclear, and five were at high risk of attrition bias. Of the nine large trials, eight were low risk (Figure 5). For one large trial risk of attrition bias was high (Italy 1993), as there was a higher loss to follow‐up in the no treatment arm in this study (which did not use a placebo). For four small trials risk of bias for incomplete outcome data was also high (India 2017; Iran 2014a; Netherlands 2009; Spain 2017), largely due to imbalanced losses to follow‐up between groups.
Selective reporting
Overall, 40 trials were low risk of bias for selective reporting, and for 35 this risk was unclear. Risk of reporting bias was low for all nine large trials (Figure 5). Risk of reporting bias was high for two trials; Finland 2013 as reported different outcomes in the registration document than in the paper, and Australia 1996 did not report data on the primary outcome of perinatal death. Risk of reporting bias was unclear for 35 small trials where the expected outcomes were not reported, outcomes additional to those pre‐specified were reported, or there was insufficient information to judge in the absence of a protocol.
Other potential sources of bias
In total, 30 trials were low risk of other potential sources of bias, and for 40 this risk was unclear. Of the large trials, seven were at low risk of other potential biases, and two were at unclear risk: for one (Jamaica 1998), this was due to slight imbalances in two baseline characteristics and the fact that fewer women taking aspirin complied with their treatment than those taking placebo; for the other (USA 1993a), there was an imbalance in systolic blood pressure at trial entry, and concern that the trial population was not representative of the general population of women (Figure 5). There were seven small trials that had high risk of other potential sources of bias. Five trials stopped early, three due to results from interim analyses (Australia 1995; Australia 1995a; Israel 1989), and two because of slow recruitment (Colorado 1993; Italy 2004). Two trials were high risk of bias because of differences in baseline characteristics (Israel 1994; Romania 2018).
Effects of interventions
See: Table 1
Overall, 77 trials involving 40,249 women and their babies are included in this review. Of these trials, 31 individual patient data (IPD) trials plus 38 aggregate data (AD trials) included primary prevention (prevention of pre‐eclampsia for women without gestational hypertension or intrauterine growth restriction at trial entry) only; and five IPD trials plus three AD trials included secondary prevention (prevention of pre‐eclampsia treatment for women who already have gestational hypertension, and/or intrauterine growth restriction at trial entry) only; and four trials, all IPD (Brazil 1996; CLASP 1994; India 1991; Italy 1993), included both primary prevention and secondary prevention arms. Where possible, data have been presented in the appropriate comparison. AD trials were largely for primary prevention; a small proportion included secondary prevention women who could not be separated out, therefore data for all women have been included under primary prevention.
Antiplatelet agents versus placebo or no treatment for the primary prevention of pre‐eclampsia and its complications
Primary outcomes
Proteinuric pre‐eclampsia
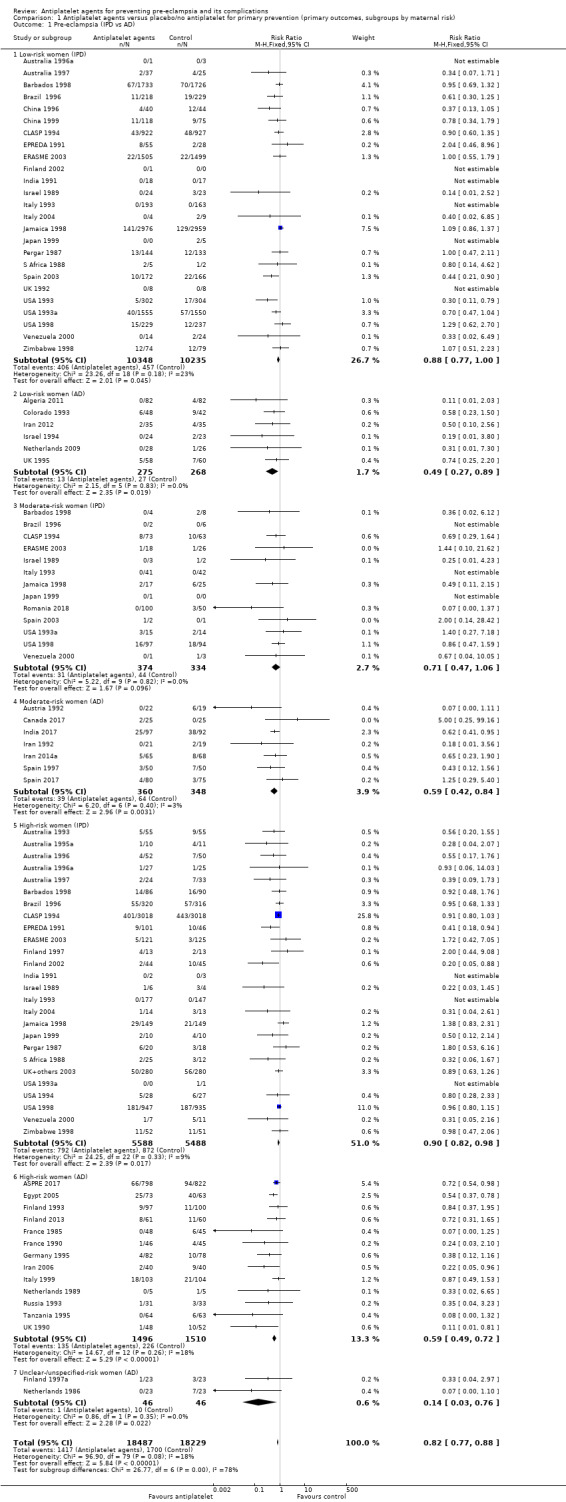
Overall there was an 18% reduction in the risk of pre‐eclampsia associated with the use of antiplatelet agents (36,716 women, 60 trials; risk ratio (RR) 0.82, 95% confidence interval (CI) 0.77 to 0.88; assumed risk with placebo/no treatment 92 per 1000 women; corresponding risk with antiplatelet agents 16 fewer per 1000, 95% CI 22 fewer to 11 fewer; high‐quality evidence) (Table 1 and Analysis 1.1; Figure 6). The risk difference (RD) was ‐1.66% (95% CI ‐2.23% to ‐1.09%), with the number needed to treat for one woman to benefit by avoiding pre‐eclampsia (NNTB) being 61 women (95% CI 45 to 92).
1.1. Analysis.
Comparison 1 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by maternal risk), Outcome 1 Pre‐eclampsia (IPD vs AD).
6.
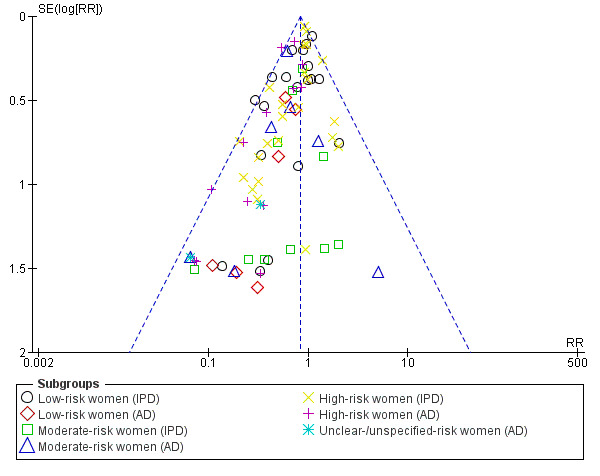
Funnel plot of comparison: 1 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by maternal risk), outcome: 1.1 Pre‐eclampsia (IPD vs AD).
Subgroups by maternal risk
There appear to be no significant differences between the subgroups when participants were subgrouped by maternal risk of pre‐eclampsia at trial entry (Chi² = 8.13, df = 3 (P = 0.04), I² = 63.1%; Analysis 1.5). However, the subgroups were very imbalanced in size making possible subgroup differences hard to assess. For women at low risk, in the 25 trials reporting IPD there was a 12% reduction in pre‐eclampsia (20,583 women, 25 trials; RR 0.88, 95% CI 0.77 to 1.00), and in the six trials with AD, although the point estimate suggests a larger reduction, the CI is wide (543 women, 6 trials; RR 0.49, 95% CI 0.27 to 0.89; Analysis 1.1.2). For the small number of moderate‐risk women in trials with IPD, the overall CI was wide and includes 1.00 (708 women, 13 trials; RR 0.71, 95% CI 0.47 to 1.06), whilst in the seven trials with only AD available the reduction in RR appears to more clearly indicate a reduction (708 women, 7 trials; RR 0.59, 95% CI 0.42 to 0.84). For high‐risk women, in studies with IPD available there was a reduction in RR (11,076 women, 26 trials; RR 0.90, 95% CI 0.82 to 0.98) that was consistent with the overall effect, whilst for the small studies with only AD available there appears to be a larger risk reduction (3006 women, 13 trials; RR 0.59, 95% CI 0.49 to 0.72).
Subgroups by gestation at randomisation
For women randomised before 20 weeks' gestation, there was a reduction in risk of pre‐eclampsia associated with the use of antiplatelet agents in studies with IPD available (18,950 women, 27 trials; RR 0.86, 95% CI 0.78 to 0.95; Analysis 2.1.1). For women randomised at or after 20 weeks' gestation with IPD available, the results did not clearly indicate a risk reduction (13,173 women, 26 trials; RR 0.93, 95% CI 0.84 to 1.04). However, the CIs overlap, so we are uncertain whether this is suggestive of real differences between the two subgroups. In trials with only AD available, the risk reduction appears to be larger both for women randomised before 20 weeks' gestation (3560 women, 19 trials; RR 0.61, 95% CI 0.51 to 0.73) and for those randomised at or after 20 weeks (7 trials, 515 women; RR 0.26, 95% CI 0.14 to 0.49), when compared with the IPD subgroups. For the AD trials, the CIs do not overlap, suggesting a possible subgroup difference by gestation at randomisation, with the effect being greater for women randomised at or after 20 weeks', However, the AD should be interpreted with particular caution, as the numbers in each subgroup are relatively small and individual women are not necessarily in the correct subgroup.
2.1. Analysis.
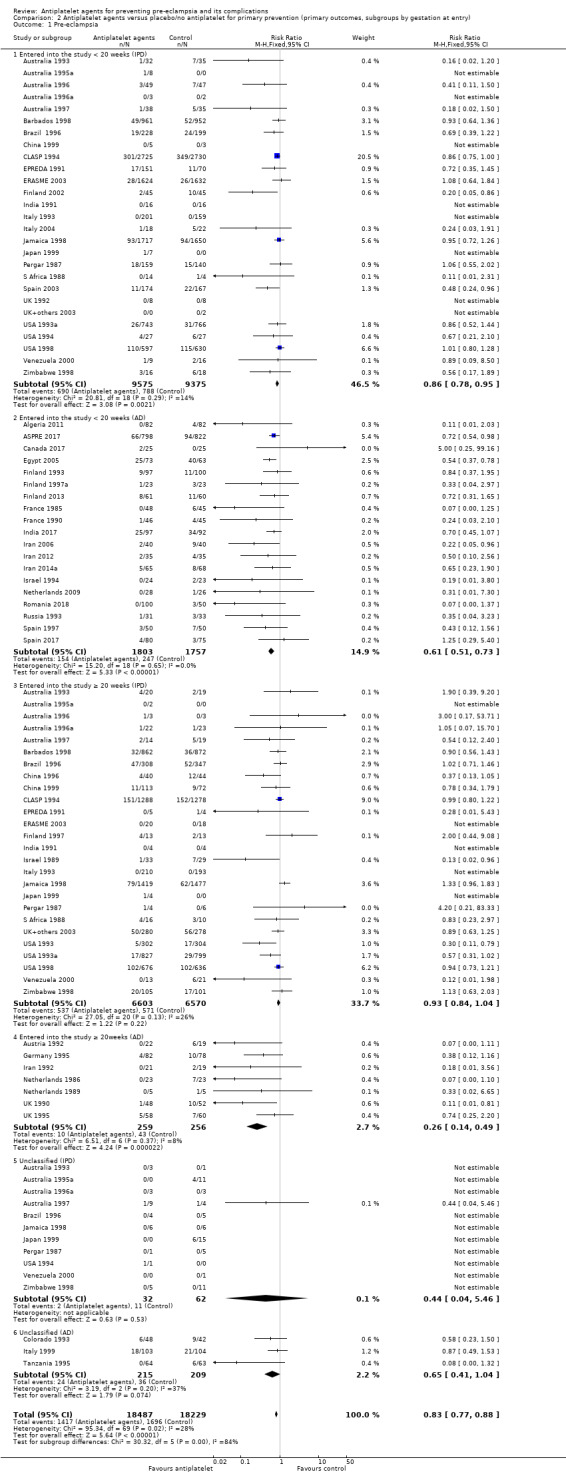
Comparison 2 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by gestation at entry), Outcome 1 Pre‐eclampsia.
The PARIS review protocol included a planned subgroup analysis based on gestation at trial entry: less than 16 weeks, 16 to 19 completed weeks, 20 to 23 completed weeks, 24 to 27 completed weeks, and 28 weeks or more (PARIS 2005). Data for this subgroup analysis are presented here (Figure 7). There are no clear differences between the subgroups. Due to recent interest in whether earlier administration of antiplatelets before 16 weeks' gestation might be beneficial, the IPD for trial entry before 16 weeks and at or after 16 weeks are presented in Figure 8; there is no clear difference between the subgroups (RR 0.90, 95% CI 0.78 to 1.03 and RR 0.90, 95% CI 0.83 to 0.99, respectively).
7.
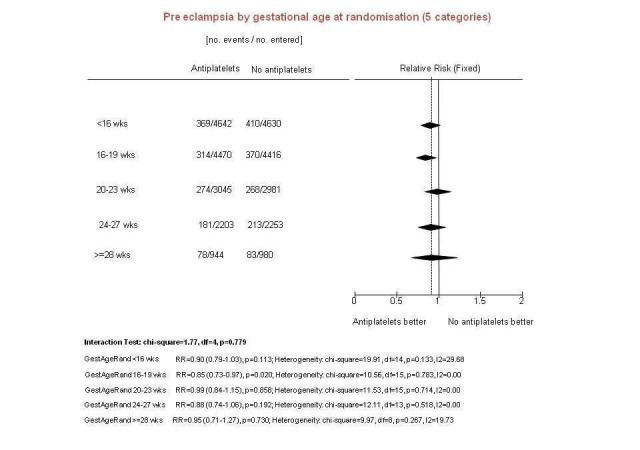
Pre‐specified subgroup analysis using PARIS IPD
8.
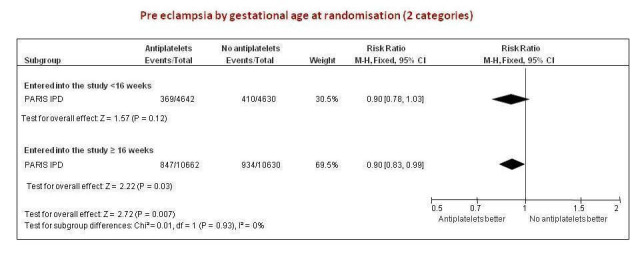
Condensed version of Figure 4 (2 subgroups only) using PARIS IPD
Subgroups by use of placebo
We did not detect likely differences between the subgroups by use of placebo. When assessing subgroup differences by use of placebo, the subgroups were very imbalanced in size for both IPD and AD trials, because in both cases many more women were given a placebo than not. The reduction in risk of pre‐eclampsia was similar regardless of whether women were recruited to placebo‐controlled trials or not, both in studies with IPD (albeit there was no significant reduction in the fewer and smaller IPD trials that were not placebo controlled) and those with AD (Analysis 3.1).
3.1. Analysis.
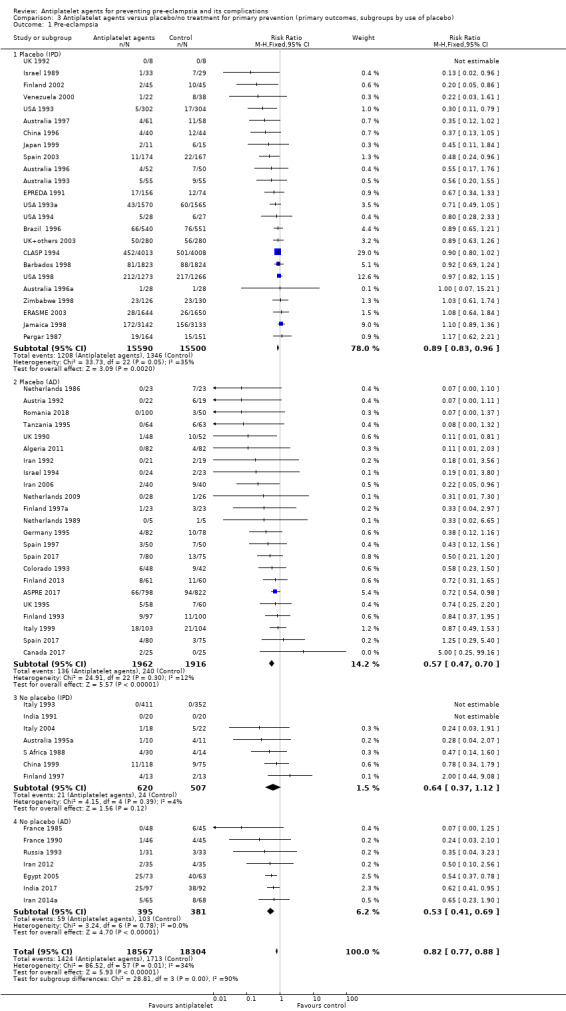
Comparison 3 Antiplatelet agents versus placebo/no treatment for primary prevention (primary outcomes, subgroups by use of placebo), Outcome 1 Pre‐eclampsia.
Subgroups by dose of aspirin
There was a reduction in the risk of pre‐eclampsia for women allocated 75 mg aspirin or more in the IPD trials (9107 women, 16 trials; RR 0.78, 95% CI 0.66 to 0.92), and the point estimate suggested a larger reduction in AD trials (3505 women, 19 trials; RR 0.58, 95% CI 0.49 to 0.70; Analysis 4.1). For women allocated aspirin < 75 mg, there was a slight reduction in studies with IPD, however the 95% CI includes the possibility of no effect (11 trials, 22,618 women; RR 0.92, 95% CI 0.85 to 1.00), whilst there appeared to be a more marked reduction in studies with only AD (586 women, 6 trials; RR 0.59, 95% CI 0.39 to 0.89).
4.1. Analysis.
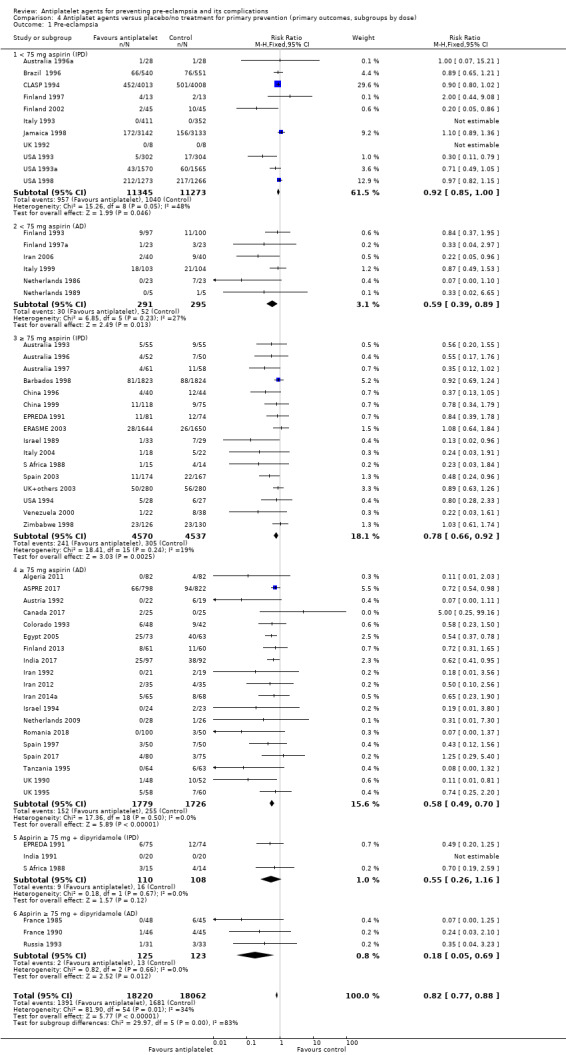
Comparison 4 Antiplatet agents versus placebo/no treatment for primary prevention (primary outcomes, subgroups by dose), Outcome 1 Pre‐eclampsia.
A few small trials combined aspirin with dipyridamole. In view of the small numbers, we are uncertain about the effect of aspirin with dipyridamole on pre‐eclampsia for both IPD studies (218 women, 3 trials; RR 0.55, 95% CI 0.26 to 1.16), and AD studies (248 women, 3 trials; RR 0.18, 95% CI 0.05 to 0.69; Analysis 4.1).
Any reported death: fetal, neonatal or before hospital discharge
Fifty‐two trials (35,391 babies) reported fetal deaths, neonatal deaths or infant deaths. When any reported deaths were analysed together, regardless of when the death occurred, there was a 15% reduction in the risk of a baby death before hospital discharge associated with the use of antiplatelet agents (35,391 women, 52 trials; RR 0.85, 95% CI 0.76 to 0.95; assumed risk with placebo/no treatment 33 per 1000 babies; corresponding risk with antiplatelet agents 5 fewer per 1000, 95% CI 9 fewer to 1 fewer; high‐quality evidence) (Table 1 and Analysis 1.2; Figure 9). The risk difference is ‐0.51% (95% CI ‐0.87% to ‐0.15%), and NNTB 197 (115 to 681).
1.2. Analysis.
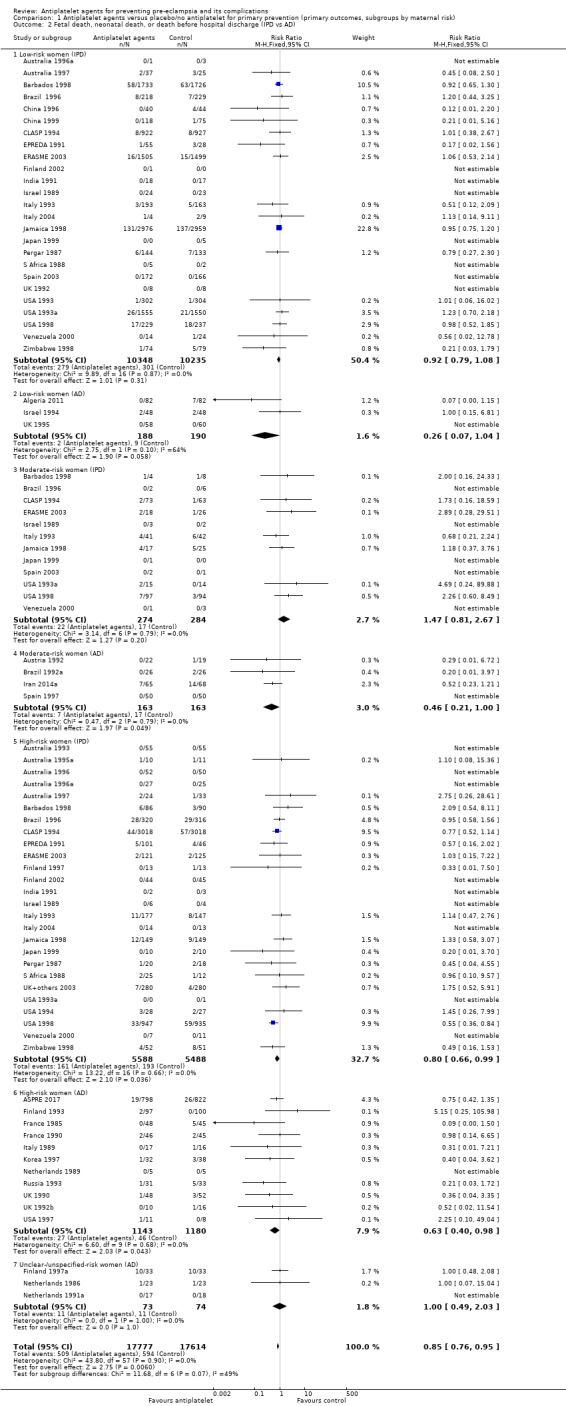
Comparison 1 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by maternal risk), Outcome 2 Fetal death, neonatal death, or death before hospital discharge (IPD vs AD).
9.
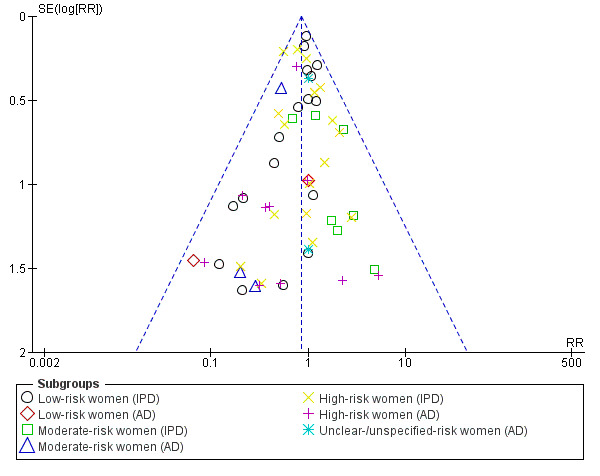
Funnel plot of comparison: 1 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (subgrouped by maternal risk), outcome: 1.4 Fetal and neonatal deaths.
Subgroups by maternal risk
This reduction in the RR for baby death was consistent across the subgroups by maternal risk at trial entry (Chi² = 2.02, df = 3 (P = 0.57), I² = 0%; Analysis 1.6).
Subgroups by gestation at randomisation, use of placebo, and dose of aspirin
For women randomised before 20 weeks' gestation, aspirin resulted in a reduction in fetal, neonatal, or infant death in both the IPD trials (18,950 babies, 27 trials; RR 0.87, 95% CI 0.75 to 1.02), and the AD trials (2657 babies, 11 trials; RR 0.62, 95% CI 0.44 to 0.88). For those randomised at or after 20 weeks, there was little or no difference between antiplatelet agents and placebo/no treatment for IPD trials (13,173 babies, 26 trials; RR 0.93, 95% CI 0.76 to 1.15), and the findings were unclear for AD trials, probably due to small sample size and few events; (350 babies; 6 trials; RR 0.46, 95% CI 0.11 to 1.98; Analysis 2.2).
2.2. Analysis.
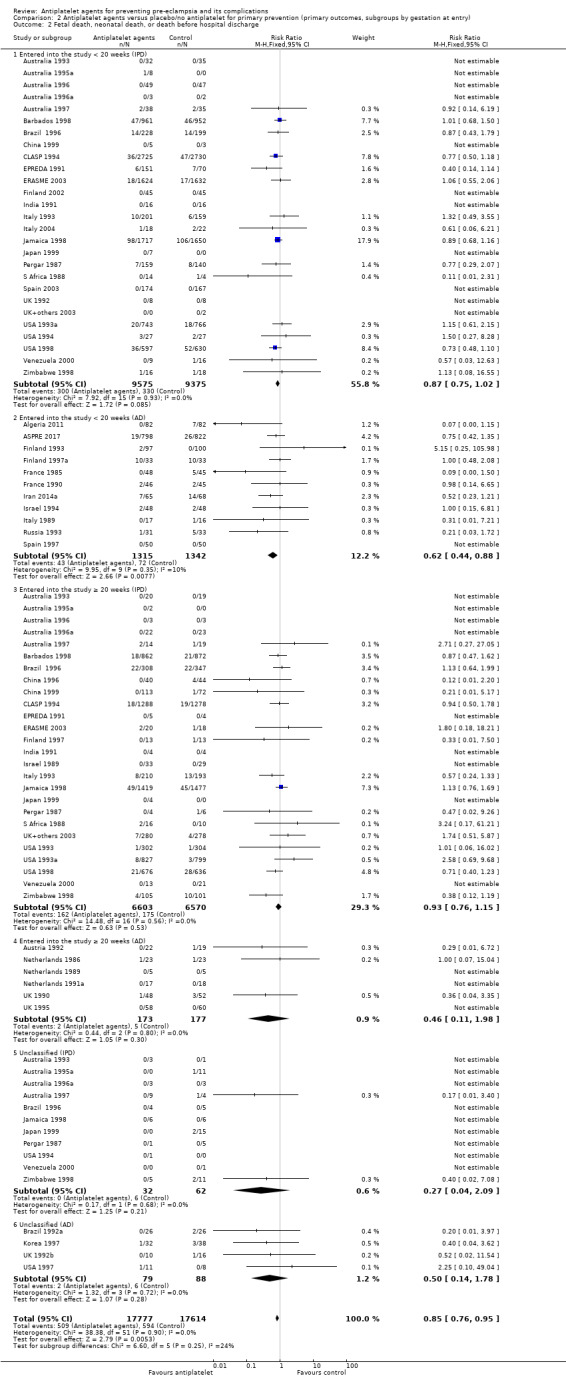
Comparison 2 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by gestation at entry), Outcome 2 Fetal death, neonatal death, or death before hospital discharge.
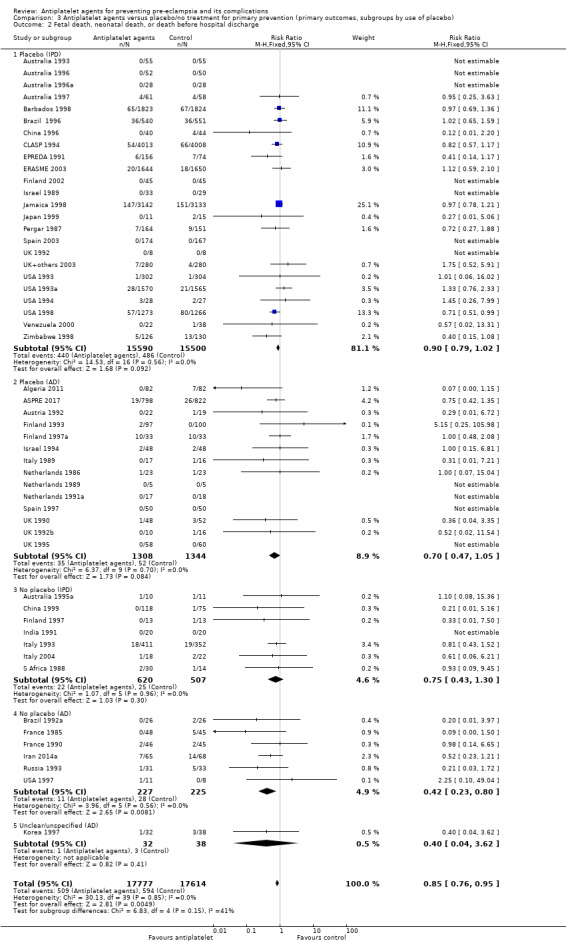
When subgrouped by use of placebo, similar effects were seen in trials with and without placebo control for both IPD and AD. However, the 95% CIs for most subgroups included no effect, probably due to smaller sample sizes and thus wider CIs (Analysis 3.2).
3.2. Analysis.
Comparison 3 Antiplatelet agents versus placebo/no treatment for primary prevention (primary outcomes, subgroups by use of placebo), Outcome 2 Fetal death, neonatal death, or death before hospital discharge.
The results for < 75 mg aspirin alone were consistent across analyses using IPD and AD. For studies using less than 75 mg of aspirin there is probably a small reduction associated with antiplatelet agents, in the IPD trials (27,025 babies, 16 trials; RR 0.89, 0.78, 1.01), and in the AD trials a similar point estimate but wider CI due to the smaller number of babies (720 babies, 10 trials; RR 0.81, 95% CI 0.45 to 1.45). The effect of antiplatelet agents compared with placebo/no treatment where women were given 75 mg or more of aspirin was somewhat unclear for IPD trials (4733 babies, 12 trials; RR 0.94, 95% CI 0.61 to 1.45), while evidence from trials with AD giving women more than 75 mg aspirin suggested a large reduction in fetal or neonatal death for this subgroup (2165 babies, 6 trials; RR 0.51, 95% CI 0.34 to 0.78). In the small studies using 75 mg or more aspirin plus dipyridamole, we are uncertain about the effect on fetal or neonatal death, because of the small sample sizes and low event rates for both IPD (218 babies, 3 trials; RR 0.66, 95% CI 0.21 to 1.80), and AD (256 babies 4 trials; RR 0.90, 95% CI 0.23 to 3.51; Analysis 4.2).
4.2. Analysis.
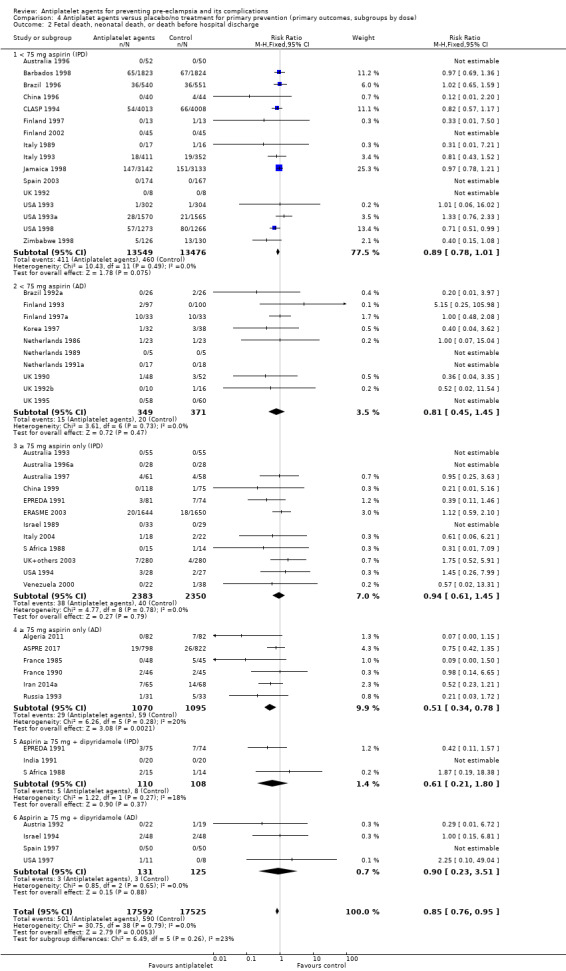
Comparison 4 Antiplatet agents versus placebo/no treatment for primary prevention (primary outcomes, subgroups by dose), Outcome 2 Fetal death, neonatal death, or death before hospital discharge.
Classifying deaths by the time of death (fetal, neonatal, infant or childhood death), there appear to be no differences in the risk of death in any of the categories (Analysis 5.12; Figure 10).
5.12. Analysis.
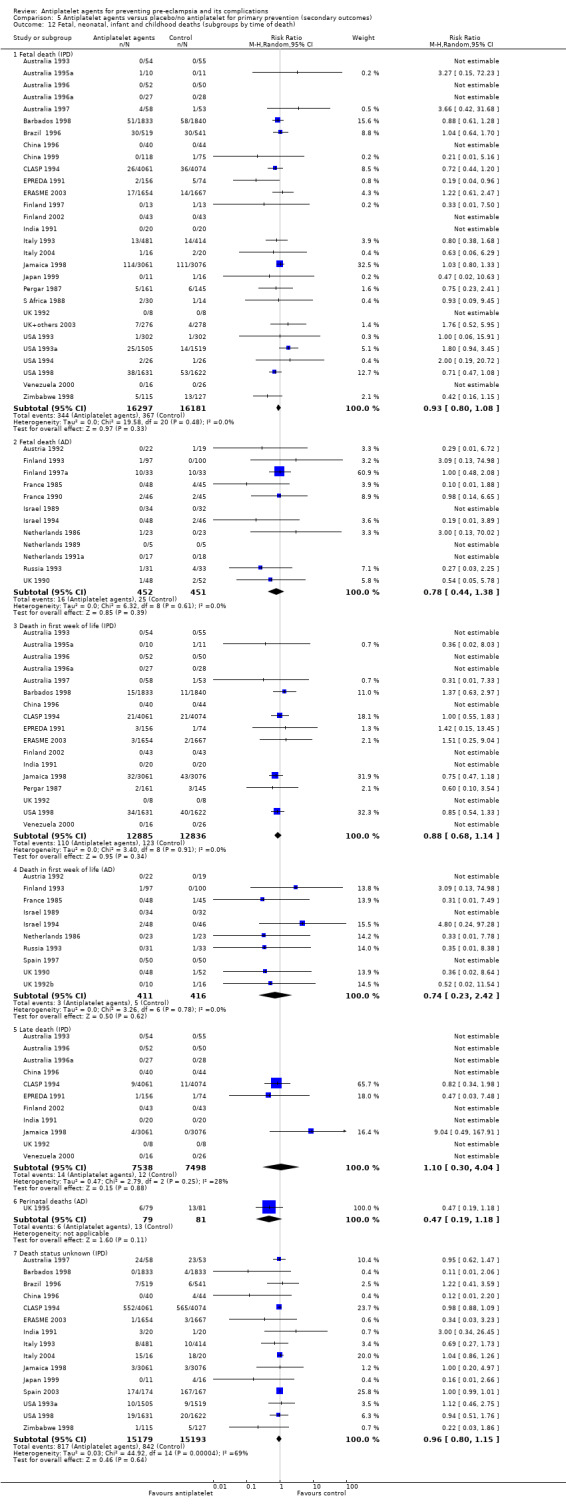
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 12 Fetal, neonatal, infant and childhood deaths (subgroups by time of death).
10.
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.12 Fetal, neonatal, infant and childhood deaths (subgroups by time of death).
Preterm birth
Overall, in the 47 trials reporting this outcome (35,212 women), there was a small (9%) reduction in the RR for birth before 37 completed weeks (35,212 women, 47 trials; RR 0.91, 95% CI 0.87 to 0.95; assumed risk with placebo/no treatment 175 per 1000 women; corresponding risk with antiplatelet agents 16 fewer per 1000, 95% CI 23 fewer to 9 fewer; high‐quality evidence) (Table 1 and Analysis 1.3; Figure 11), with a risk difference of ‐1.64% (95% CI ‐2.38% to ‐0.88%); NNTB 61 (42 to 114).
1.3. Analysis.
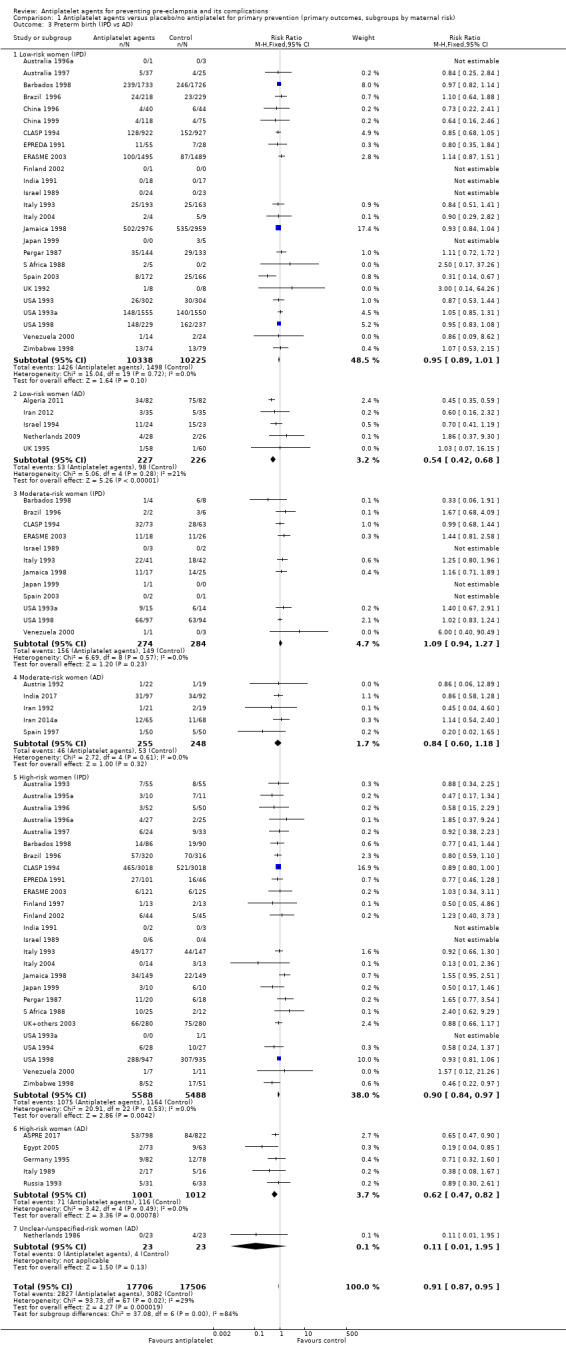
Comparison 1 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by maternal risk), Outcome 3 Preterm birth (IPD vs AD).
11.
Funnel plot of comparison: 1 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by maternal risk), outcome: 1.3 Preterm birth (IPD vs AD).
There was evidence to suggest a slight reduction in preterm birth before 34 weeks' gestation amongst women given antiplatelet agents compared with those given placebo/no treatment (32,253 women, 31 trials; RR 0.90, 95% CI 0.83 to 0.97; Analysis 5.15; Figure 12). The effects were less clear for preterm births before 32 weeks (32,319 women, 31 trials; RR 0.92, 95% CI 0.83 to 1.02; Analysis 5.16; Figure 13), and before 28 weeks' gestation (32,135 women, 30 trials; RR 0.87, 95% CI 0.74 to 1.01; Analysis 5.17; Figure 14), both of which included a slight reduction but the CI crossed the line of no effect. We conducted further analysis of evidence on the effects of antiplatelet agents on preterm birth provided by IPD trials only, analysed in terms of mutually exclusive subgroups by time of preterm birth. However, looking at preterm birth before 28 weeks (32,155 women, 30 trials; RR 0.87, 95% CI 0.74 to 1.01), from 28 to 31 weeks (32,155 women, 30 trials; RR 0.98, 95% CI 0.85 to 1.13), from 32 to 33 weeks (32,155 women, 30 trials; RR 0.84, 95% CI 0.73 to 0.97), and from 34 to 36 weeks' gestation (32,155 women, 30 trials; RR 0.93, 95% CI 0.87 to 1.00), there were no clear differences between these subgroups (Analysis 5.18; Figure 15).
5.15. Analysis.
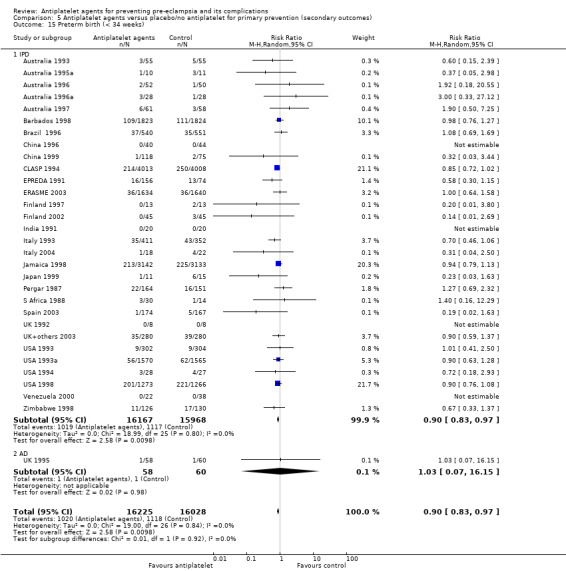
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 15 Preterm birth (< 34 weeks).
12.
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.15 Preterm birth (< 34 weeks).
5.16. Analysis.
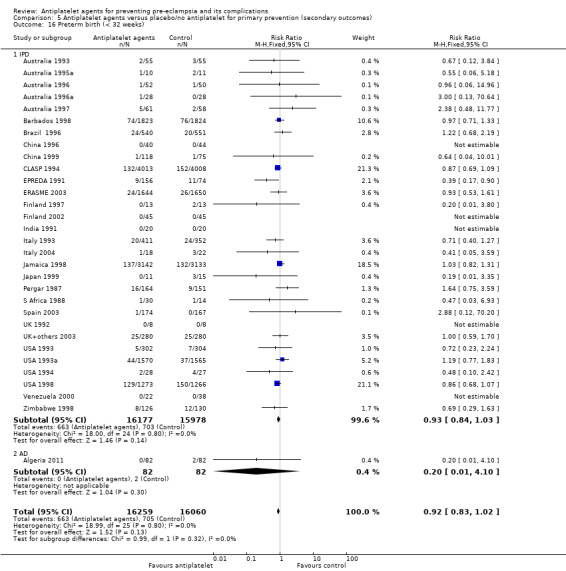
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 16 Preterm birth (< 32 weeks).
13.
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.16 Preterm birth (< 32 weeks).
5.17. Analysis.
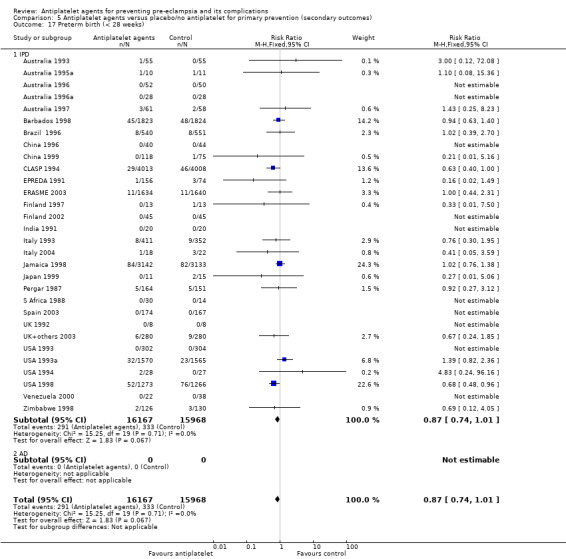
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 17 Preterm birth (< 28 weeks).
14.
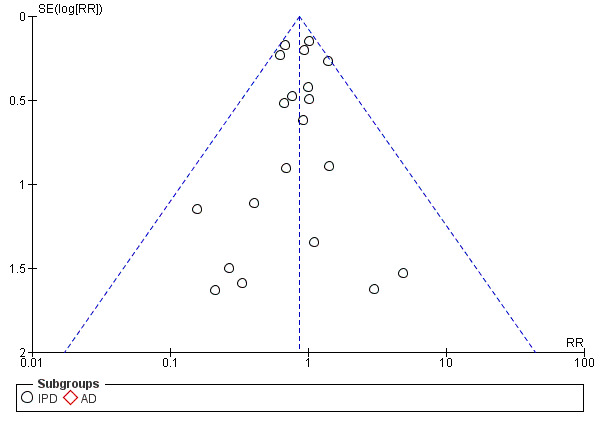
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.17 Preterm birth (< 28 weeks).
5.18. Analysis.
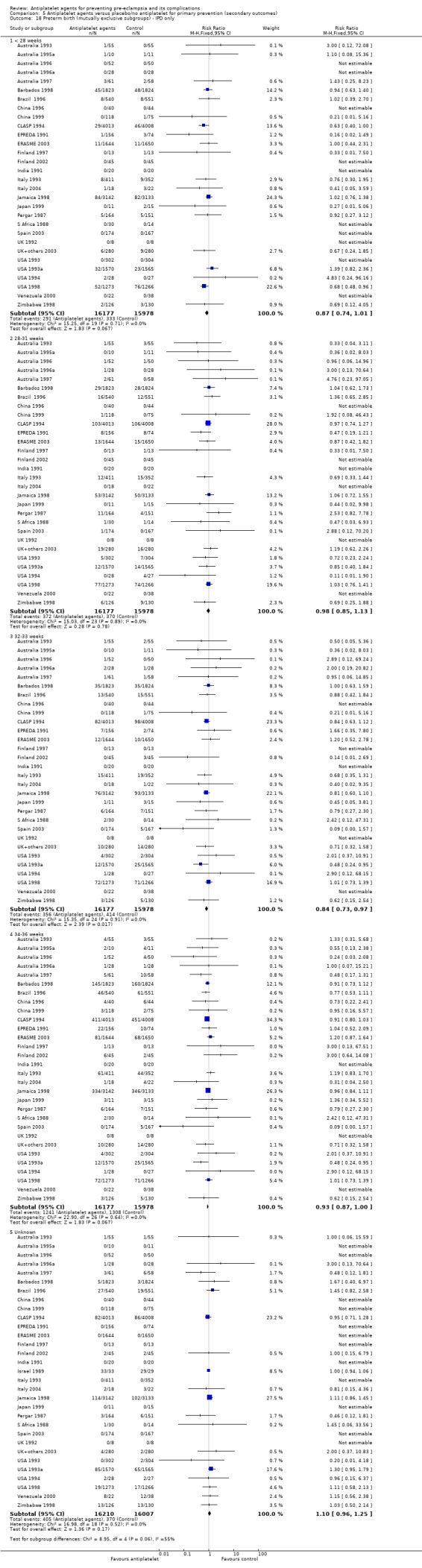
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 18 Preterm birth (mutually exclusive subgroups) ‐ IPD only.
15.
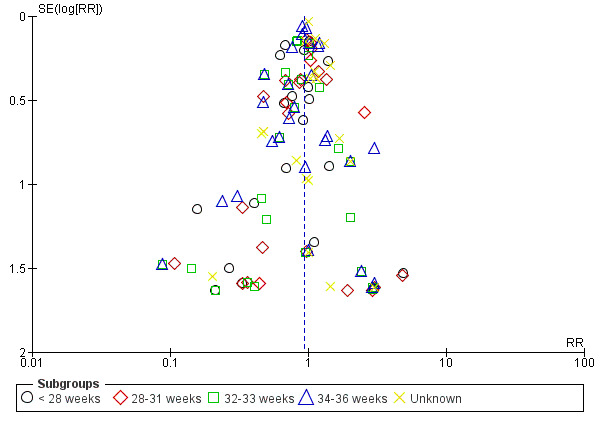
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.18 Preterm birth (mutually exclusive subgroups) ‐ IPD only.
Subgroups by maternal risk
There appeared to be no significant heterogeneity between the subgroups by maternal risk, but again the subgroups are imbalanced in size (Chi² = 6.39, df = 3 (P = 0.09), I² = 53.0%; Analysis 1.7). While the CIs largely overlap, there was no clear reduction in preterm birth in the low‐risk group for trials where IPD were available (20,563 women, 25 trials; RR 0.95, 95% CI 0.89 to 1.01), but a 10% reduction in the high‐risk group for IPD (11,076 women, 26 trials; RR 0.90 95%, CI 0.84 to 0.97). In the small subgroup of women at moderate risk of pre‐eclampsia in IPD trials, the evidence suggested there was probably little or no difference in preterm birth between women receiving antiplatelet agents and those receiving placebo/no treatment (558 women, 12 trials; RR 1.09, 95% CI 0.94 to 1.27), but we note that this subgroup is substantially smaller than the others for IPD, so this result probably reflects the play of chance in a small subgroup. The results from the small trials with AD suggested a larger reduction in preterm birth for women at low risk of pre‐eclampsia (453 women, 5 trials; RR 0.54, 95% CI 0.42 to 0.68) and also for those at high‐risk (2013 women, 5 trials; RR 0.62, 95% CI 0.47 to 0.82). For AD trials, the effects for women at moderate‐risk of pre‐eclampsia were unclear (503 women, 5 trials; RR 0.84, 95% CI 0.60 to 1.18; Analysis 1.3.4).
Subgroups by gestation at randomisation
Most subgroups suggested a consistent small reduction in preterm birth, regardless of whether recruitment was before or after 20 weeks' gestation, although again the subgroups varied considerably in size (Analysis 2.3). For women randomised before 20 weeks' gestation, IPD trials suggested a slight reduction in preterm birth amongst women receiving antiplatelet agents beginning before 20 weeks' gestation, although the CI touched on no effect (18,930 women, 27 trials; RR 0.94, 95% CI 0.88 to 1.00); whereas AD trials suggested a more substantial benefit (2610 women, 11 trials; RR 0.63, 95% CI 0.53 to 0.74). For women randomised at or after 20 weeks' gestation, trials with IPD suggested a similar effect to women beginning the drugs earlier (13,173 women, 26 trials; RR 0.91, 95% CI 0.85 to 0.98), whereas the effects were unclear in the AD trials (287 women, 4 trials; RR 0.56, 95% CI 0.28 to 1.12).
2.3. Analysis.
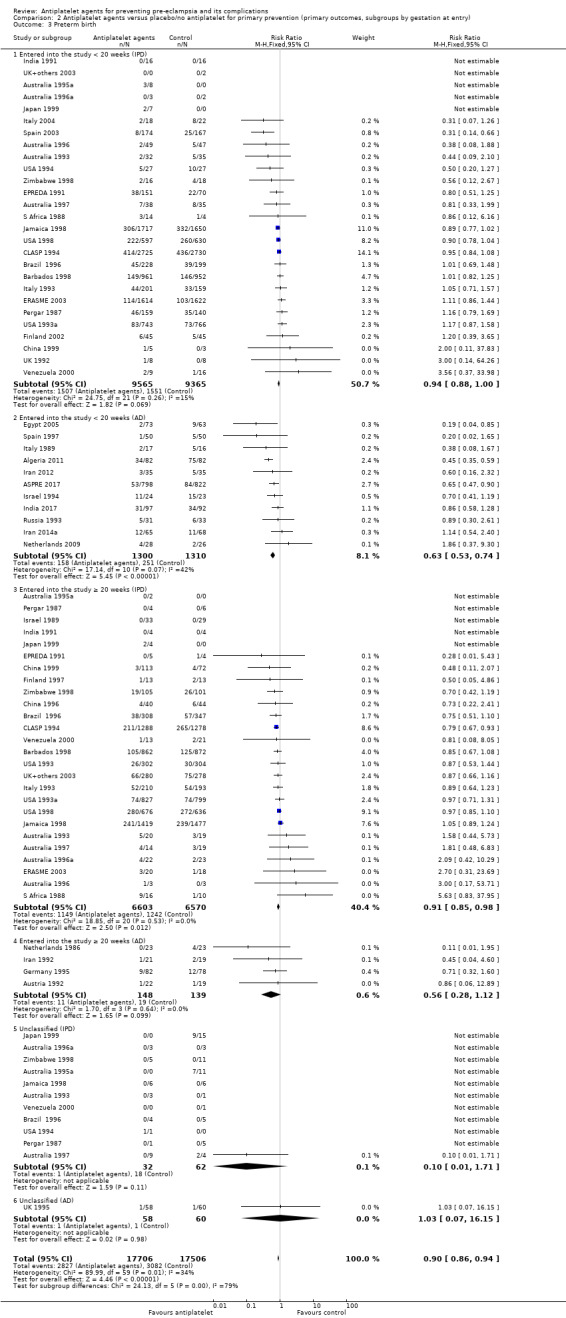
Comparison 2 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by gestation at entry), Outcome 3 Preterm birth.
Subgroups by use of placebo
There was no indication of clear subgroup differences by use of placebo for this outcome, however there were some differences between the IPD and the AD effect estimates. The findings from IPD trials where women received placebo (31,070 women, 24 trials; RR 0.93, 95% CI 0.89 to 0.98) were not substantially different from those without placebo, although the 95% CI without placebo crossed the line of no effect (1127 women, 7 trials; RR 0.90, 95% CI 0.72 to 1.14) . In AD trials the pattern was similar ‐ with the results for no placebo being unclear ‐ although the point estimates were greater both with placebo (2423 women, 11 trials; RR 0.57, 95% CI 0.47 to 0.69) and without (592 women, 5 trials; RR 0.79; 95% CI 0.58, 1.08; Analysis 3.3).
3.3. Analysis.
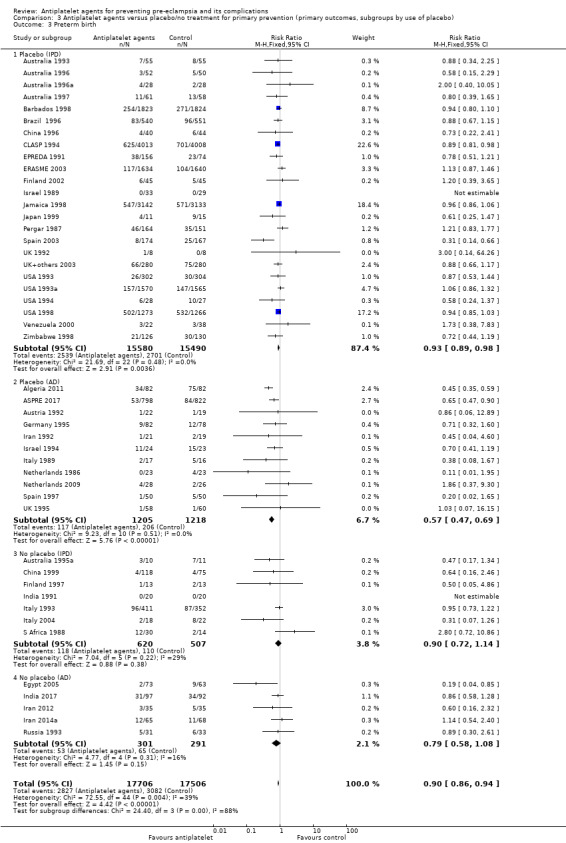
Comparison 3 Antiplatelet agents versus placebo/no treatment for primary prevention (primary outcomes, subgroups by use of placebo), Outcome 3 Preterm birth.
Subgroups by dose of aspirin
The evidence did not suggest different rates of preterm birth by aspirin dosing. In IPD trials, the findings when women received less than 75 mg (22,618 women, 11 trials; RR 0.93, 95% CI 0.89 to 0.98) overlapped substantially with the findings from women who received 75 mg or more (9087 women, 16 trials; RR 0.86, 95% CI 0.73 to 1.01). For AD trials, the findings were unclear when women received less than 75 mg aspirin (79 women, 2 trials; RR 0.29, 95% CI 0.08 to 1.09), while the effect seemed to indicate a more marked reduction than the IPD data when women received 75 mg or more (2712 women, 12 trials; RR 0.65, 95% CI 0.50 to 0.84). Again, in view of the small number of women involved in trials combining aspirin with dipyridamole, we are uncertain about the effect of aspirin with dipyridamole on preterm birth for both IPD (218 women, 3 trials; RR 1.37, 95% CI 0.34 to 5.51) and AD studies (64 women, 1 trial; RR 0.89, 95% CI 0.30 to 2.61; Analysis 4.3).
4.3. Analysis.
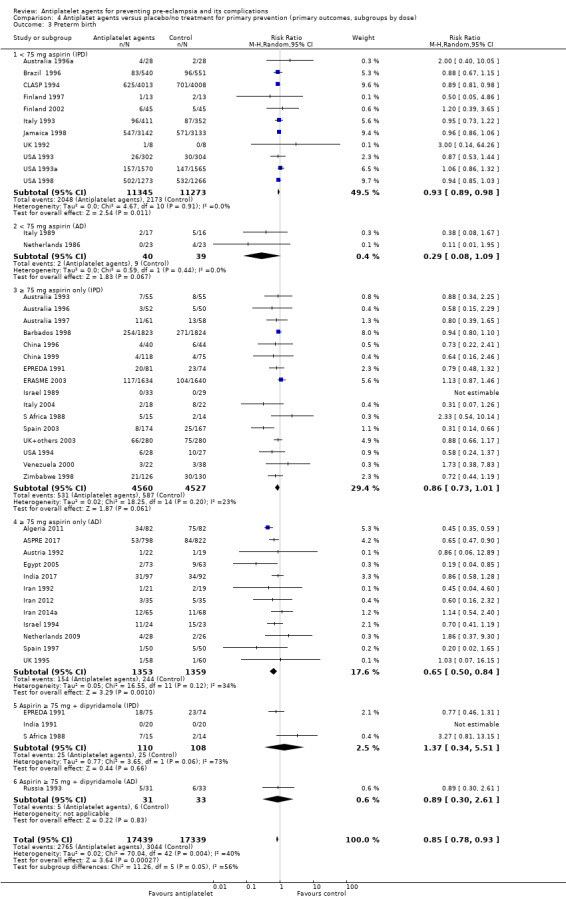
Comparison 4 Antiplatet agents versus placebo/no treatment for primary prevention (primary outcomes, subgroups by dose), Outcome 3 Preterm birth.
Small‐for‐gestational‐age baby
There was a 16% reduction in the risk of the baby being born small‐for‐gestational‐age when women were given antiplatelet agents compared with placebo/no treatment (35,761 babies, 50 trials; RR 0.84, 95% CI 0.76 to 0.92; assumed risk with placebo 47 per 1000 women; corresponding risk with antiplatelet agents 7 fewer per 1000, 95% CI 11 fewer to 3 fewer; high‐quality evidence) (Table 1 and Analysis 1.4; Figure 16). The risk difference was ‐0.69%, 95% CI ‐1.11% to ‐0.26%; NNTB 146, 95% CI 90 to 386. Results for this outcome are also analysed by severerity for AD (Analysis 5.19; Figure 17) and IPD (Analysis 5.20; Figure 18).
1.4. Analysis.
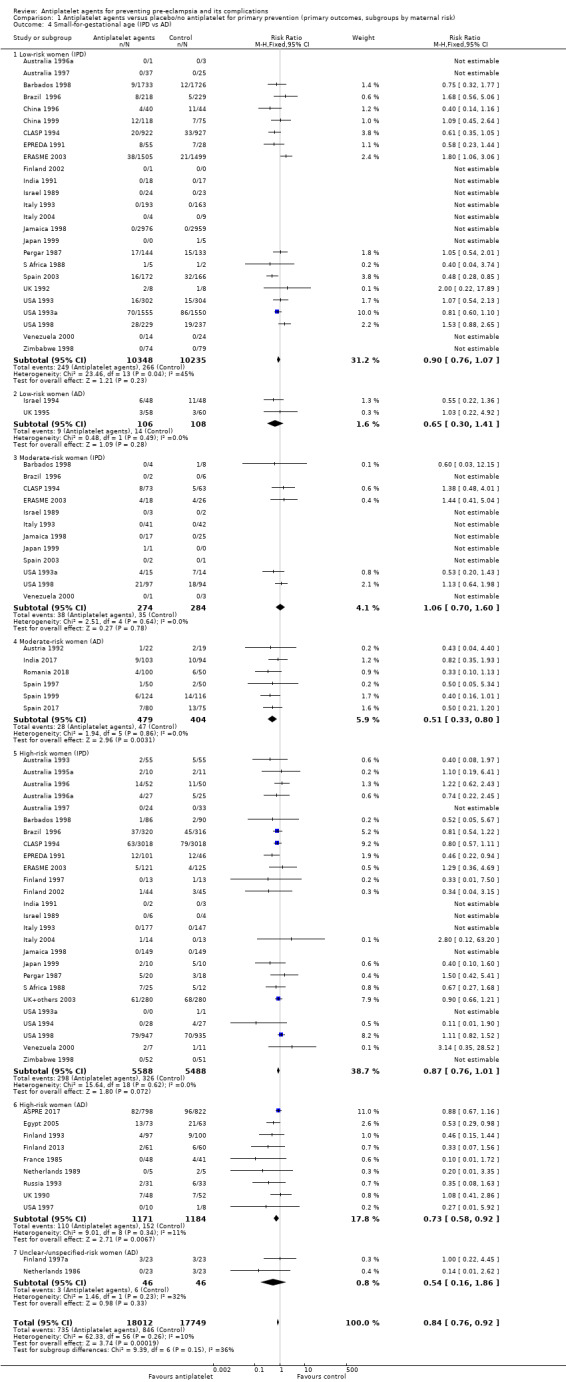
Comparison 1 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by maternal risk), Outcome 4 Small‐for‐gestational age (IPD vs AD).
16.
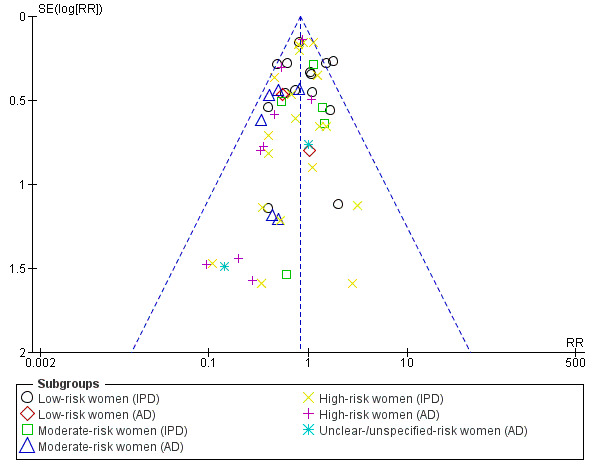
Funnel plot of comparison: 1 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by maternal risk), outcome: 1.4 Small‐for‐gestational age (IPD vs AD).
5.19. Analysis.
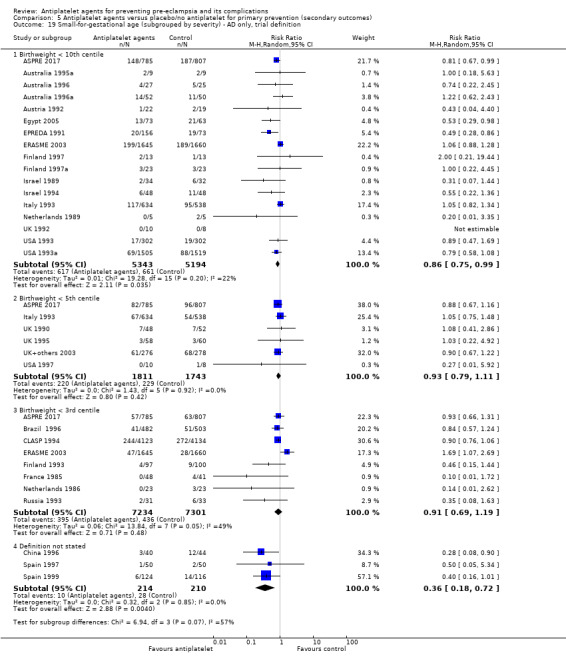
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 19 Small‐for‐gestational age (subgrouped by severity) ‐ AD only, trial definition.
17.
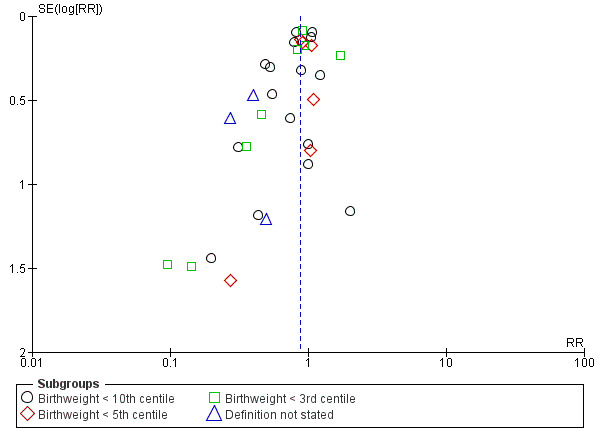
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.19 Small‐for‐gestational age (subgrouped by severity) ‐ AD only, trial definition.
5.20. Analysis.
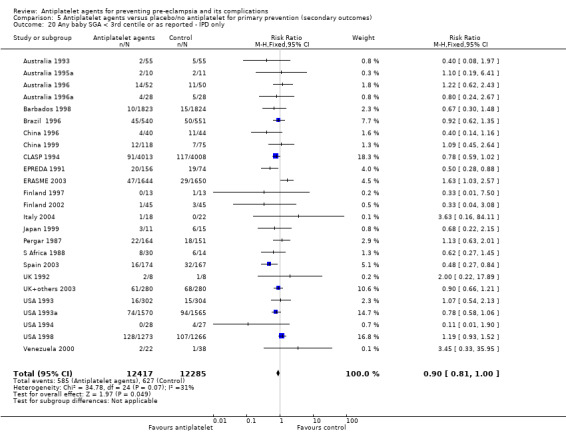
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 20 Any baby SGA < 3rd centile or as reported ‐ IPD only.
18.
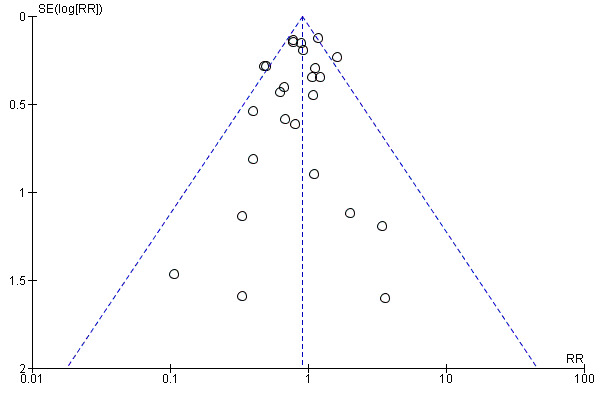
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.20 Any baby SGA < 3rd centile or as reported ‐ IPD only.
Subgroups by maternal risk
This result was consistent across the subgroups by risk status at trial entry (Chi² = 1.76, df = 3 (P = 0.62), I² = 0%; Analysis 1.8).
Subgroups by gestation at randomisation
There was a reduction the risk of the baby being born small‐for‐gestational‐age if the mother was randomised before 20 weeks' gestation (IPD trials: 18,950 babies, 27 trials: RR 0.82, 95% CI 0.70 to 0.95; AD trials: 3211 babies, 13 trials: RR 0.67, 95% CI 0.55 to 0.82), but antiplatelet agents made little or no difference in IPD trials for women randomised at 20 weeks or after (IPD 13,173 women, 26 trials: RR 0.99 95% CI 0.85 to 1.16), and for AD trials the effect was unclear (AD trials 315 women, 5 trials: RR 0.69, 95% CI 0.35 to 1.37; Analysis 2.4). Again, although the effects appeared to differ slightly across these subgroups, all the subgroups are imbalanced in size, so it is not clear whether there are likely to be any real differences by gestation at randomisation for this outcome.
2.4. Analysis.
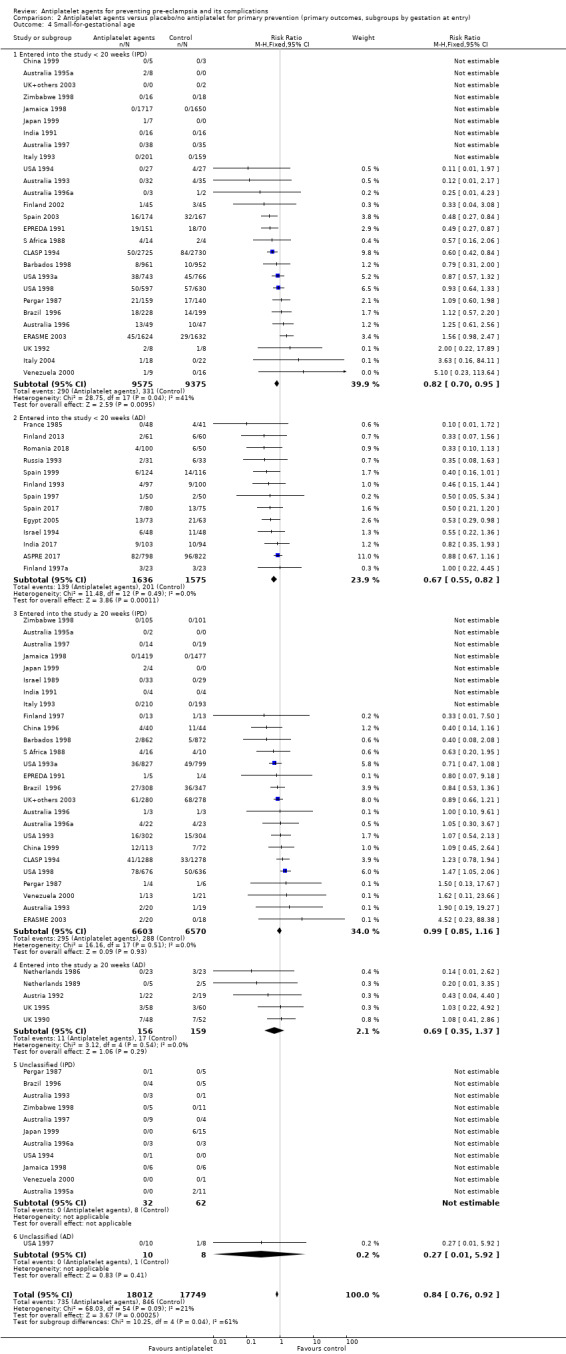
Comparison 2 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by gestation at entry), Outcome 4 Small‐for‐gestational age.
Subgroups by use of placebo
Subgroup analyses do not suggest differences by use of placebo, however the results from IPD trials do not completely align with those from AD. In trials with both IPD and AD that were controlled by placebo, antiplatelet agents appeared to reduce the risk of the baby being small‐for‐gestational‐age, although the CI touched on no effect in IPD trials (31,090 women, 24 trials; RR 0.90 95% CI 0.81 to 1.00) while the reduction appeared clearer in AD trials (2885 women, 13 trials; RR 0.72, 95% CI 0.58 to 0.90). When no placebo was given to women in the control group, the effect was unclear in the IPD trials (1127 women, 7 trials; RR 0.91, 95% CI 0.52 to 1.58), whereas AD suggested a clear reduction (504 women, 5 trials; RR 0.52, 95% CI 0.33 to 0.82). For AD trials both with and without placebo, the reduction in risk appeared greater than in IPD trials (Analysis 3.4).
3.4. Analysis.
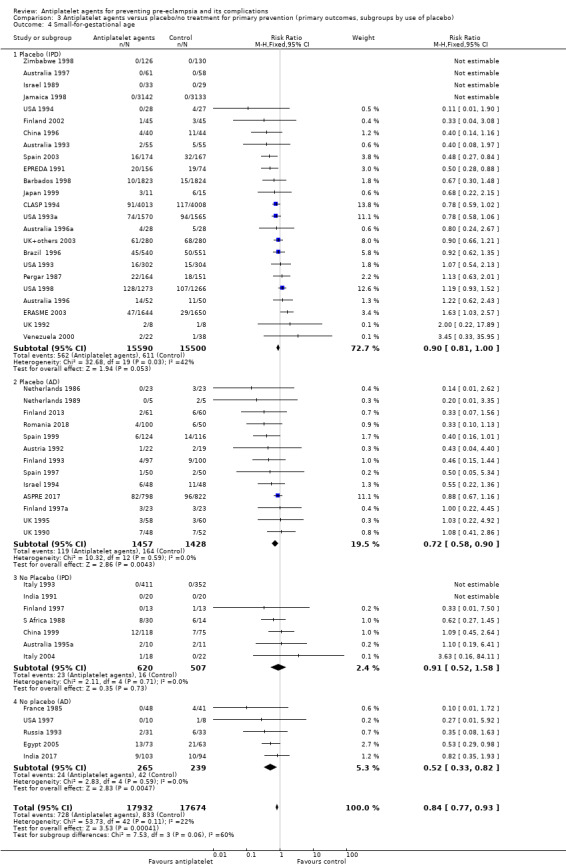
Comparison 3 Antiplatelet agents versus placebo/no treatment for primary prevention (primary outcomes, subgroups by use of placebo), Outcome 4 Small‐for‐gestational age.
Subgroups by dose of aspirin
There was no clear indication of subgroup differences by dose of aspirin for this outcome. For IPD trials, the effects appeared to be similar whether women were given less than 75 mg aspirin (22,618 women, 11 trials; RR 0.92, 95% CI 0.79 to 1.07), or greater than or equal to 75 mg (9107 women, 16 trials; RR 0.76, 95% CI 0.53 to 1.08). For trials with AD giving women less than 75 mg aspirin, the effect was unclear (299 women, 4 trials; RR 0.49, 95% CI 0.22 to 1.13), whereas there seemed to be a marked reduction for women given 75 mg or more (3092 women, 13 trials; RR 0.72, 95% CI 0.58 to 0.88). Where trials gave aspirin with dipyridamole, the sample sizes were too small to yield precise effect estimates so the effects are unclear (Analysis 4.4).
4.4. Analysis.
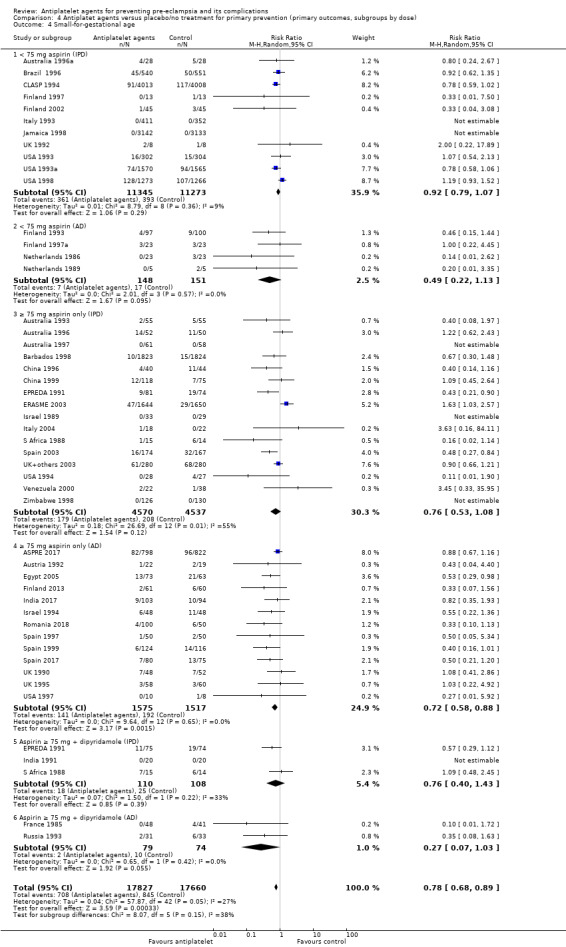
Comparison 4 Antiplatet agents versus placebo/no treatment for primary prevention (primary outcomes, subgroups by dose), Outcome 4 Small‐for‐gestational age.
Secondary outcomes
Gestational hypertension
There was only evidence available from IPD trials for this outcome. Findings using the best available definition of gestational hypertension suggested that compared with placebo or no treatment, antiplatelet agents make little or no difference to whether women developed gestational hypertension after trial entry (27,834 women; 25 studies; RR 0.95, 95% CI 0.90 to 1.01; Analysis 5.8; Figure 19). The findings using triallists' own definition of gestational hypertension (27,834 women, 25 studies; RR 0.95, 95% CI 0.89 to 1.01; Analysis 5.9; Figure 20) and using the PARIS definition (27,519 women, 24 studies; RR 0.95, 95% CI 0.90 to 1.02; Analysis 5.10; Figure 21) were very similar.
5.8. Analysis.
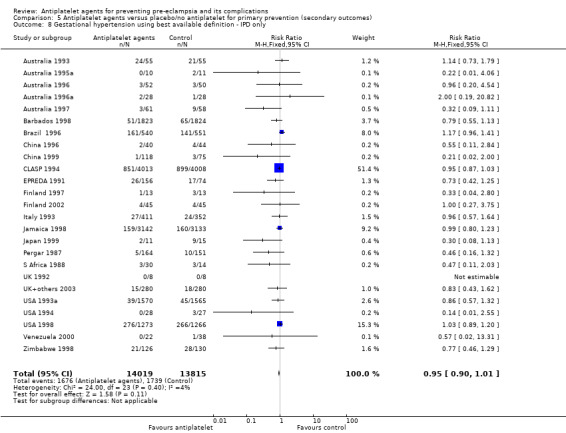
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 8 Gestational hypertension using best available definition ‐ IPD only.
19.
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.8 Gestational hypertension using best available definition ‐ IPD only.
5.9. Analysis.
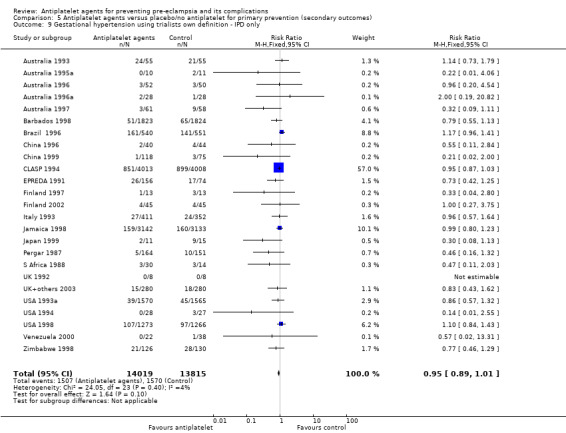
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 9 Gestational hypertension using trialists own definition ‐ IPD only.
20.
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.9 Gestational hypertension using trialists own definition ‐ IPD only.
5.10. Analysis.
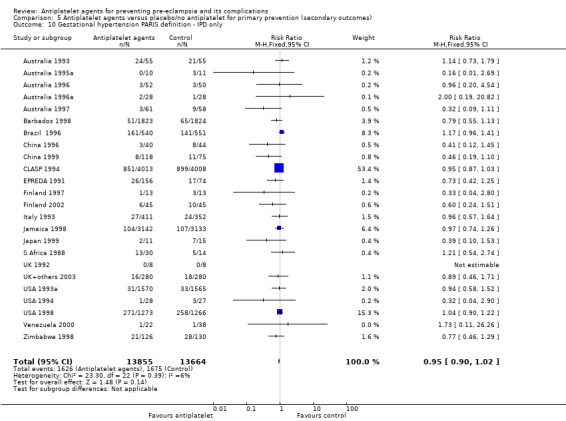
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 10 Gestational hypertension PARIS definition ‐ IPD only.
21.
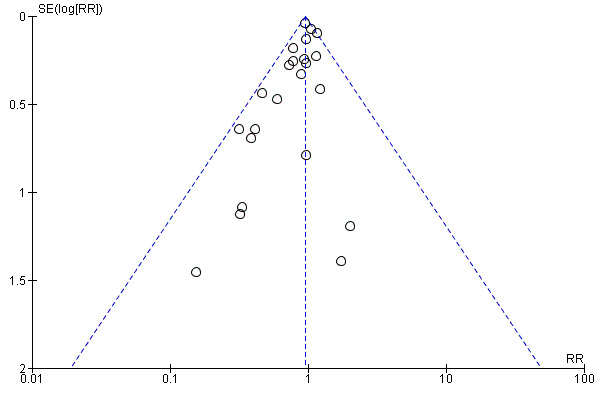
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.10 Gestational hypertension PARIS definition ‐ IPD only.
Postpartum haemorrhage > 500 mL
Data on postpartum haemorrhage were not included in previous versions of this Cochrane Review, but are included in this update as this outcome was part of the dataset collected for the PARIS IPD review. We used the trialist‐defined outcome of 'postpartum haemorrhage', which was usually defined as more than 500 mL. Evidence from 19 trials with reliable available data (16 IPD trials, three AD trials) suggested that antiplatelet agents probably slightly increase the risk of postpartum haemorrhage, although the 95% CI touches on the line of no effect (23,769 women, 19 trials; RR 1.06, 95% CI 1.00 to 1.12; assumed risk with placebo/no treatment 143 per 1000; corresponding risk with antiplatelet agents 9 more per 1000, 95% CI 0 more to 19 more; moderate‐quality evidence; Analysis 5.27; Figure 22).
5.27. Analysis.
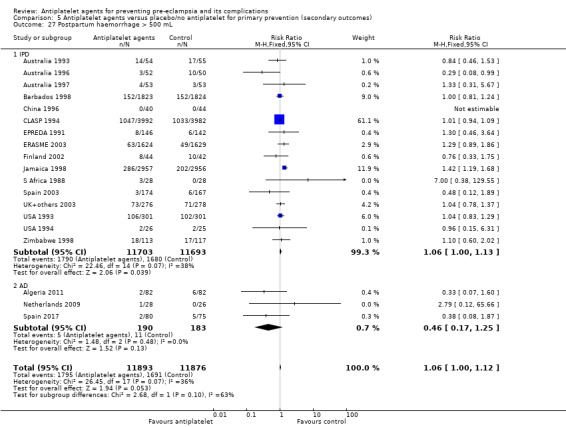
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 27 Postpartum haemorrhage > 500 mL.
22.
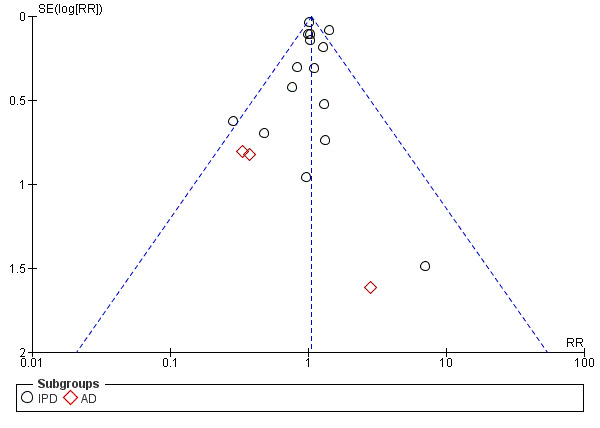
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.27 Postpartum haemorrhage > 500 mL.
Pregnancy with serious adverse outcome
The availability of IPD from the PARIS Collaboration enabled the analysis of a composite outcome of 'pregnancy with serious adverse outcome'. This was defined as a pregnancy where the mother dies or develops pre‐eclampsia, or where any baby is preterm, small‐for‐gestational age, or does not survive to discharge from hospital. Analysis of these data suggest that antiplatelets reduce the incidence of this composite of adverse outcomes (17,382 women, 13 trials; average RR 0.90, 95% CI 0.85 to 0.96; assumed risk with placebo/no treatment 197 per 1000 women; corresponding risk with antiplatelet agents 20 fewer per 1000, 95% CI 30 fewer to 8 fewer; high‐quality evidence; Analysis 5.22; Figure 23).
5.22. Analysis.
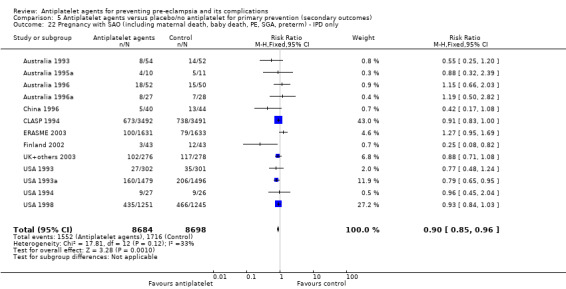
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 22 Pregnancy with SAO (including maternal death, baby death, PE, SGA, preterm) ‐ IPD only.
23.
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.22 Pregnancy with SAO (including maternal death, baby death, PE, SGA, preterm) ‐ IPD only.
Placental abruption
Antiplatelet agents probably marginally increase the risk of placental abruption, however the 95% CI included both the line of no effect and a substantial increase in this outcome. This imprecision in the effect estimate is likely to be due to the rarity of this outcome 30,775 women, 29 trials; RR 1.21, 95% CI 0.95 to 1.54; assumed risk with placebo/no treatment 7 per 1000 women; corresponding risk with antiplatelet agents 2 more per 1000, 95% CI 0 more to 4 more; moderate‐quality evidence; Analysis 5.26; Figure 24).
5.26. Analysis.
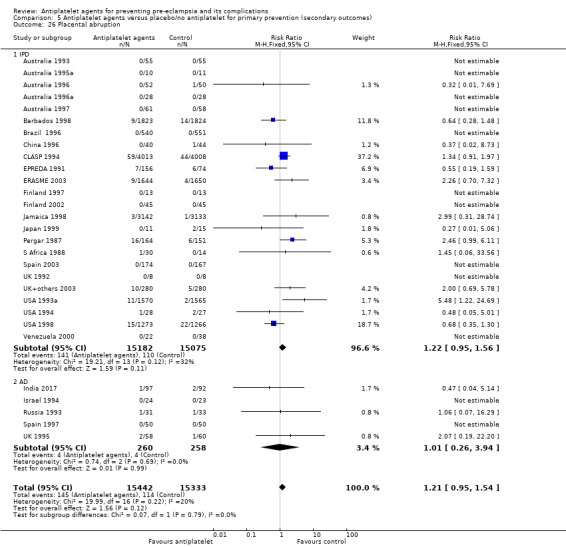
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 26 Placental abruption.
24.
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.26 Placental abruption.
Long‐term follow‐up for the children
Two trials assessed the children in early childhood (CLASP 1994; Italy 1993) . CLASP 1994 suggested no difference between treatment and control groups in any of the measures of health and development at 12 to 18 months (Analysis 5.31; Analysis 5.32; Analysis 5.34). (Italy 1993 suggested that antiplatelet agents may lower the risk of gross or fine motor problems at 18 months compared with control (15/427 versus 26/361, RR 0.49, 95% CI 0.26 to 0.91; Analysis 5.31), but suggested no difference in any of the other reported outcomes at 18 months (Analysis 5.31; Analysis 5.33; Analysis 5.34). This result should be interpreted with caution, however, as the trial was not placebo controlled and so assessment was unblinded, and 27% of children were lost to follow‐up
5.31. Analysis.
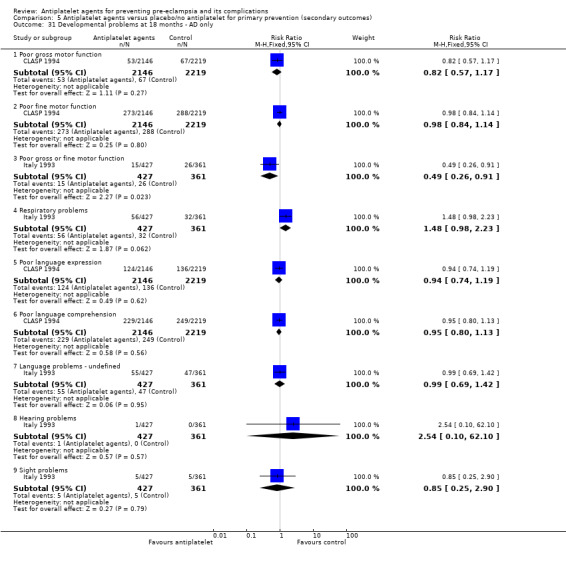
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 31 Developmental problems at 18 months ‐ AD only.
5.32. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 32 Behaviour problems at 18 months ‐ AD only.
5.34. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 34 Growth at 18 months ‐ AD only.
5.33. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 33 Malformations at 18 months ‐ AD only.
Other secondary outcomes
There was a slight reduction for the treatment group in birthweight less than 2500g (31,522 babies, 32 trials; RR 0.92, 95% CI 0.88 to 0.97; Analysis 5.21; Figure 25), and also in the risk of any baby requiring assisted ventilation (RR 0.80, 95% CI 0.66 to 0.97; 7383 mothers; 15 trials; Analysis 5.28; Figure 26).
5.21. Analysis.
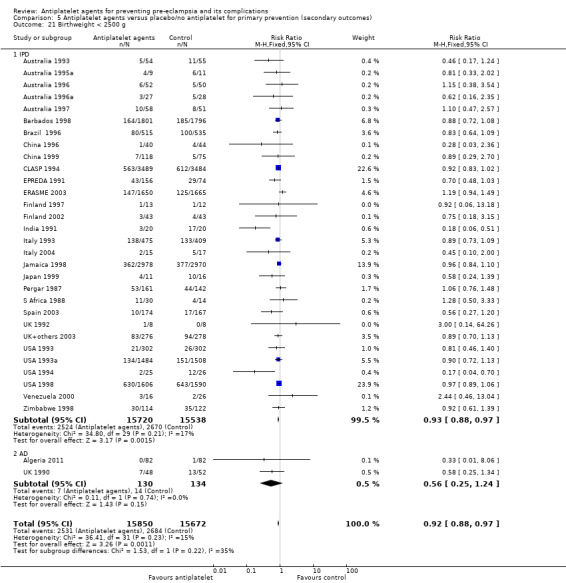
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 21 Birthweight < 2500 g.
25.
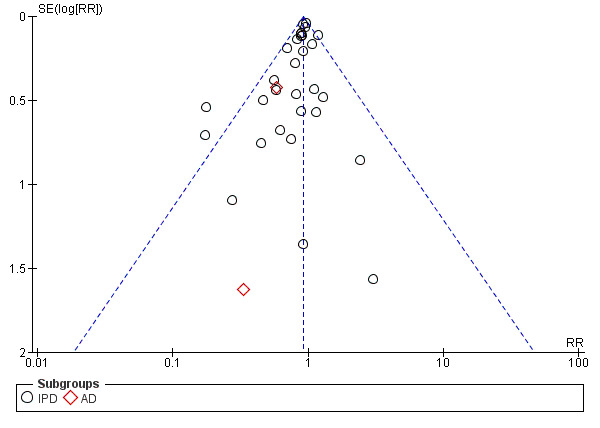
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.21 Birthweight < 2500 g.
5.28. Analysis.
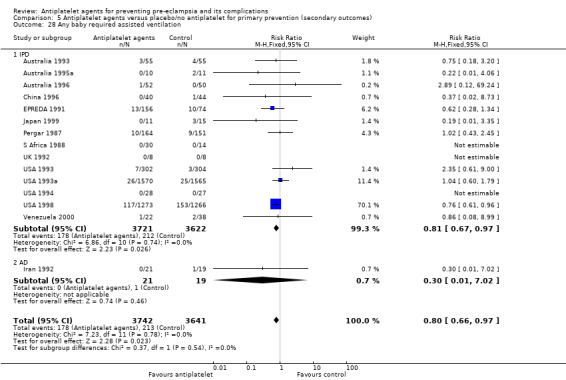
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 28 Any baby required assisted ventilation.
26.
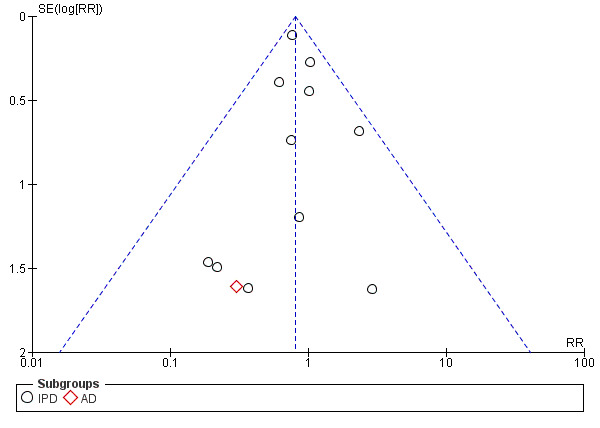
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.28 Any baby required assisted ventilation.
The evidence suggested that antiplatelet agents make little or no difference to hospital admission for women during pregnancy (AD trials only; 12,964 women, 3 trials; RR 1.03, 95% CI 0.97 to 1.09; Analysis 5.23), non‐spontaneous labour (induced labour or pre‐labour caesarean) (IPD trials only; 29,838 women, 24 trials; RR 1.02, 95% CI 0.98 to 1.05; Analysis 5.24; Figure 27), gestation at onset proteinuria (IPD trials only; 3442 women; 14 trials; mean difference (MD) ‐0.42 weeks, 95% CI ‐1.40 to 0.56; Analysis 5.11; Figure 28), gestation at birth (MD 0.02 weeks, 95% CI ‐0.04 to 0.09; 31,669 mothers; 34 trials; Analysis 5.14; Figure 29), antepartum haemorrhage (IPD trials only; RR 1.04, 95% CI 0.92 to 1.17; 30,513 mothers, 25 trials; Analysis 5.25; Figure 30), or caesarean section (32,698 women, 36 trials; RR 1.03, 95% CI 0.99 to 1.07; Analysis 5.40; Figure 31), Antiplatelet agents probably make little or no difference to the risk of HELLP syndrome (20,130 women, 16 trials; RR 0.77, 95% CI 0.44 to 1.36; Analysis 5.2) and severe maternal morbidity (28,065 women; 15 trials; RR 1.00, 95% CI 0.72 to 1.39; Analysis 5.1; Figure 32), although for these outcome the 95% CIs are wide, possibly due to the sample size being underpowered given the rarity of these events. In terms of outcomes for the baby, there was probably little or no difference in the risk of intraventricular haemorrhage (32,224 babies, 20 trials; RR 0.99, 95% CI 0.72 to 1.36; Analysis 5.29; Figure 33), other neonatal bleeding (30,715 babies, 20 trials; RR 0.90, 95% CI 0.75 to 1.08; Analysis 5.30; Figure 34), or in admission to special care baby unit (32,808 babies, 29 trials; RR 0.95, 95% CI 0.90 to 1.00; Analysis 5.35; Figure 35).
5.23. Analysis.
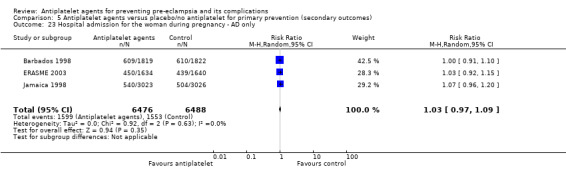
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 23 Hospital admission for the woman during pregnancy ‐ AD only.
5.24. Analysis.
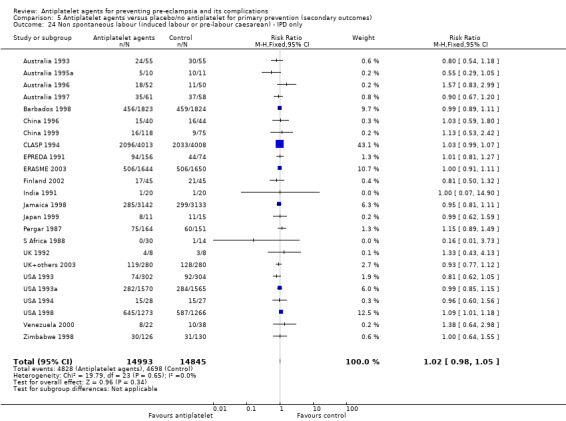
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 24 Non spontaneous labour (induced labour or pre‐labour caesarean) ‐ IPD only.
27.
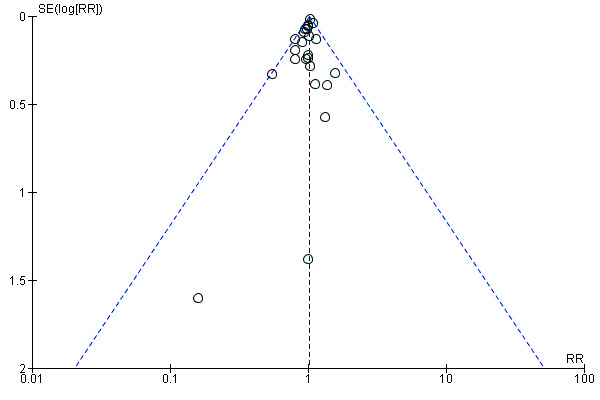
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.24 Non spontaneous labour (induced labour or pre‐labour caesarean) ‐ IPD only.
5.11. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 11 Gestation at onset proteinuria by best definition ‐ IPD only.
28.
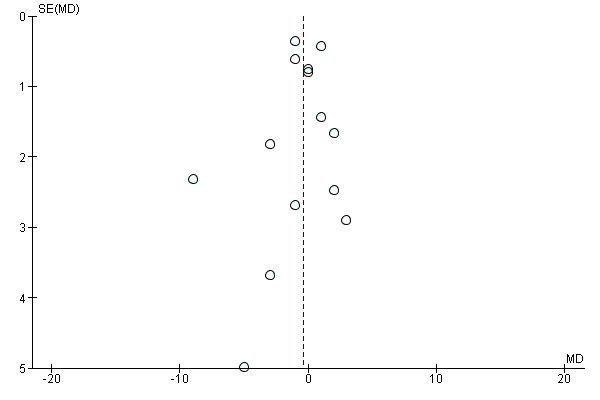
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.11 Gestation at onset proteinuria by best definition ‐ IPD only.
5.14. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 14 Gestation at birth (mean, weeks).
29.
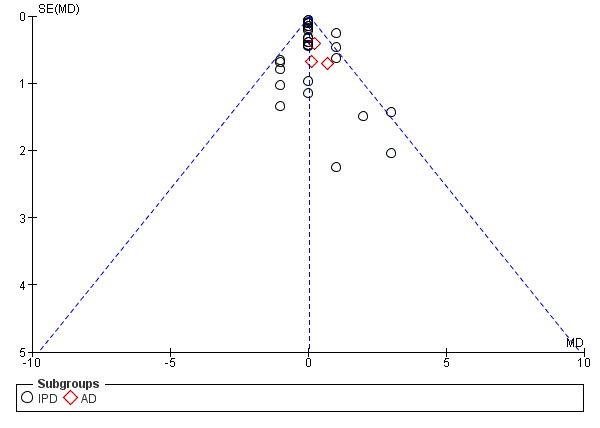
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.14 Gestation at birth (mean, weeks).
5.25. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 25 Antepartum haemorrhage ‐ IPD only.
30.
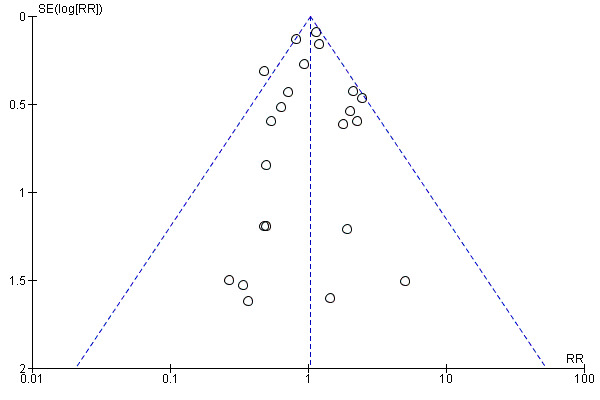
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.25 Antepartum haemorrhage ‐ IPD only.
5.40. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 40 Caesarean section.
31.
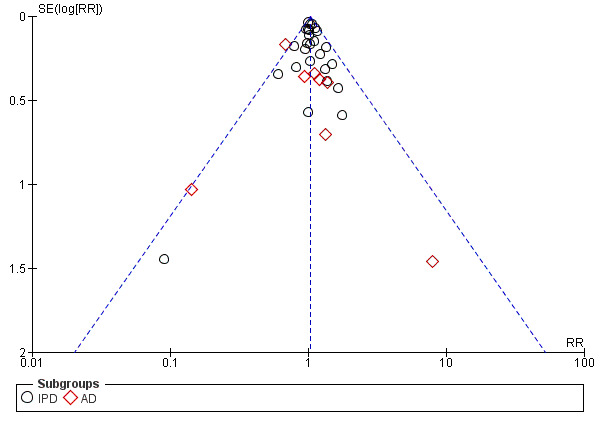
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.40 Caesarean section.
5.2. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 2 HELLP syndrome.
5.1. Analysis.
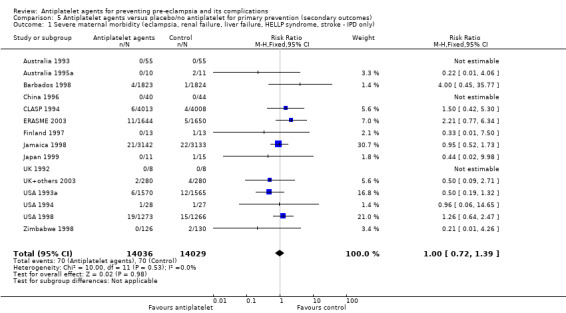
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 1 Severe maternal morbidity (eclampsia, renal failure, liver failure, HELLP syndrome, stroke ‐ IPD only).
32.
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.1 Severe maternal morbidity (eclampsia, renal failure, liver failure, HELLP syndrome, stroke ‐ IPD only).
5.29. Analysis.
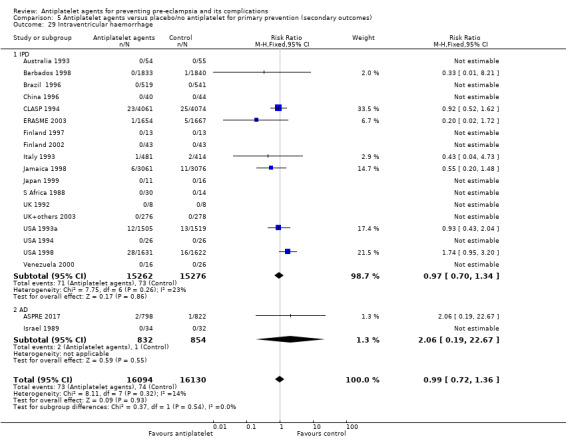
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 29 Intraventricular haemorrhage.
33.
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.29 Intraventricular haemorrhage.
5.30. Analysis.
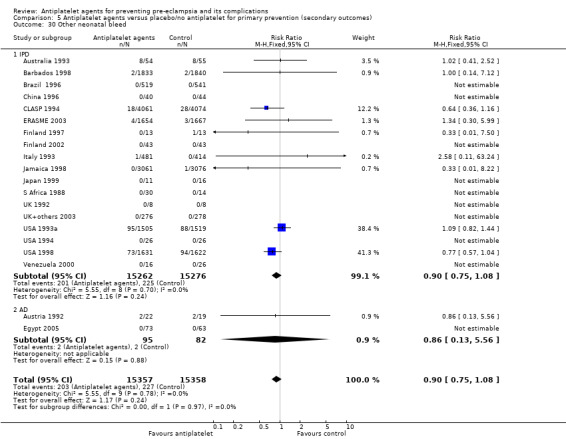
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 30 Other neonatal bleed.
34.
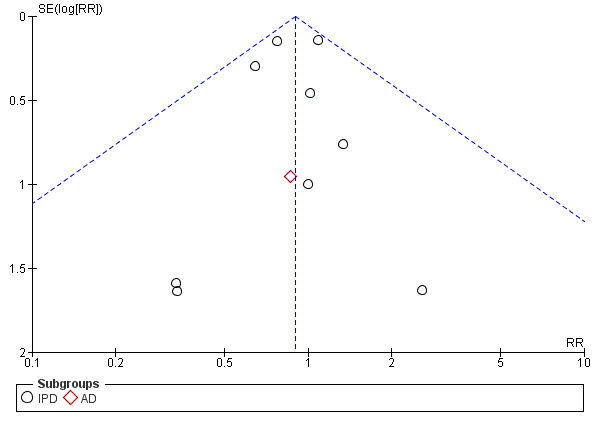
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.30 Other neonatal bleed.
5.35. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 35 Admission to a special care baby unit.
35.
Funnel plot of comparison: 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), outcome: 5.35 Admission to a special care baby unit.
It was unclear whether there was a difference between treatment and control groups in the risk for the mother of disseminated intravascular coagulation (10,828 women; 9 trials; RR 0.32, 95% CI 0.01 to 7.57; Analysis 5.3), stroke (IPD trials only; 10,828 mothers; 9 trials; RR 2.99, 95% CI 0.12 to 73.40; Analysis 5.4), renal failure (IPD trials only; 16,502 mothers; 11 trials; RR 1.29, 95% CI 0.35 to 4.79; Analysis 5.5), liver failure (IPD trials only; 10838 women, 9 trials; not estimable due to no reported events; Analysis 5.6), pulmonary oedema (IPD trials only; 16,732 mothers, 12 trials; RR 0.84, 95% CI 0.37 to 1.89; Analysis 5.7), eclampsia (24,947 women, 17 trials; RR 1.03, 95% CI 0.66 to 1.60; Analysis 5.38), or maternal death (IPD trials only; 28,675 women, 18 trials; RR 1.75, 95% CI 0.51 to 5.96; Analysis 5.39).
5.3. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 3 Disseminated intravascular coagulation ‐ IPD only.
5.4. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 4 Stroke ‐ IPD only.
5.5. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 5 Renal failure ‐ IPD only.
5.6. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 6 Liver failure ‐ IPD only.
5.7. Analysis.
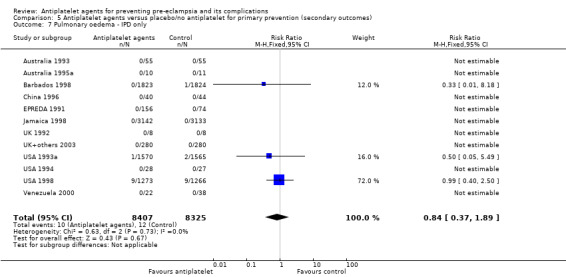
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 7 Pulmonary oedema ‐ IPD only.
5.38. Analysis.
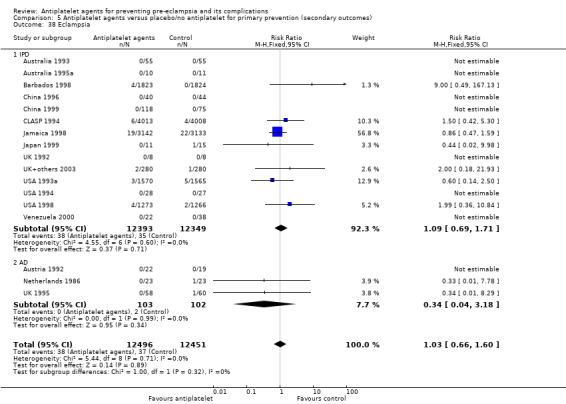
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 38 Eclampsia.
5.39. Analysis.
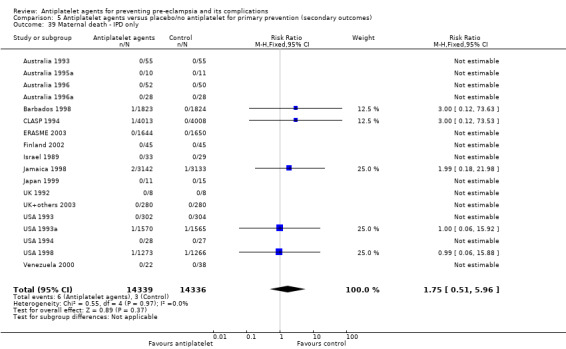
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 39 Maternal death ‐ IPD only.
Two trials assessed the children in early childhood (CLASP 1994; Italy 1993). In one, (CLASP 1994), no difference was apparent between treatment and control groups in any measure of health and development at 12 to 18 months. The other (Italy 1993) reported a higher risk of gross and fine motor problems at 18 months in treatment compared with control children (15/427 versus 26/361, RR 0.49, 95% CI 0.26 to 0.91), and there was also the possibility of a higher risk of respiratory problems in children of mothers who had received aspirin (56/427 versus 32/361; RR 1.48, 95% CI 0.98 to 2.23) (Analysis 5.31). These results should be interpreted with caution, however, as the trial was not placebo controlled and so assessment was unblinded, and 27% of children were lost to follow‐up.
2. Antiplatelet agents versus placebo or no treatment for secondary prevention of pre‐eclampsia and its complications in women with gestational hypertension
Primary outcomes
Proteinuric pre‐eclampsia
Combined evidence from IPD and AD trials suggested that antiplatelet agents reduced the risk of pre‐eclampsia when administered to women who already have gestational hypertension (secondary prevention), compared with administration of placebo/no treatment (1813 women, 7 trials; average RR 0.67, 95% CI 0.47 to 0.95; Analysis 6.2).
6.2. Analysis.
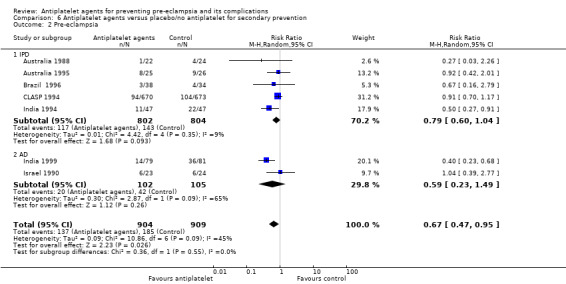
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 2 Pre‐eclampsia.
Any reported death: fetal, neonatal or before hospital discharge
In the seven trials with IPD available, there was evidence that antiplatelet agents may make little or no difference to fetal or neonatal death where mothers were treated who already had gestational hypertension (1950 women, 7 trials; RR 1.00, 95% CI 0.68 to 1.47; Analysis 6.9). In the two small AD trials, there appears to have been a reduction in the relative risk of any reported death (fetal, neonatal or infant) for the treatment group (260 women, 2 trials; RR 0.36, 95% CI 0.15 to 0.84; Analysis 6.8).
6.9. Analysis.
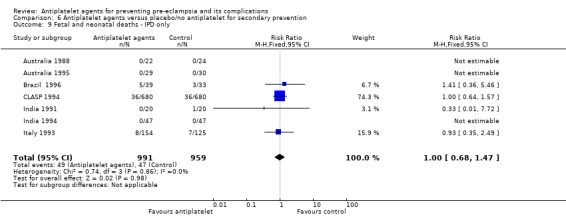
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 9 Fetal and neonatal deaths ‐ IPD only.
6.8. Analysis.
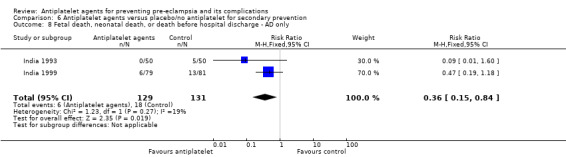
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 8 Fetal death, neonatal death, or death before hospital discharge ‐ AD only.
Preterm birth
Administration of antiplatelet agents for secondary prevention appeared to slightly reduce the incidence of preterm birth, but the 95% just crossed the line of no effect (< 37 weeks) (IPD and AD trials; 2070 women, 9 trials; RR 0.89, 95% CI 0.78 to 1.01; Analysis 6.10).
6.10. Analysis.
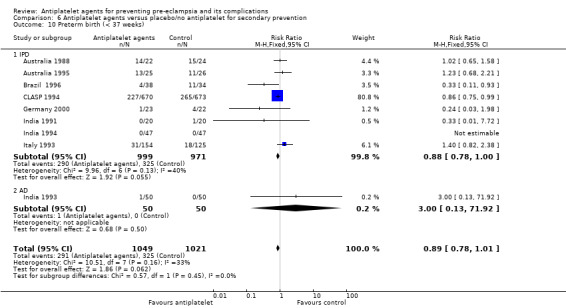
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 10 Preterm birth (< 37 weeks).
Small‐for‐gestational age
It is unclear whether antiplatelet agents reduce the risk of babies being born small‐for‐gestational age when given to women for secondary prevention (1791 babies; 6 trials; average RR 0.76, 95% CI 0.54 to 1.09; Analysis 6.11).
6.11. Analysis.
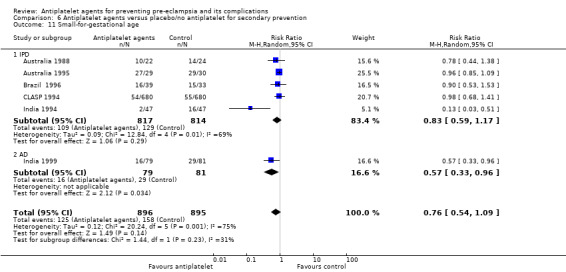
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 11 Small‐for‐gestational age.
Secondary outcomes
For secondary prevention, antiplatelet agents appeared to make little or no difference to the risk of: caesarean section (2327 women, 9 trials; RR 1.04, 95% CI 0.94 to 1.16; Analysis 6.12); non‐spontaneous labour (IPD trials only: RR 0.96, 95% CI 0.77 to 1.18; 1540 women; 5 trials; Analysis 6.13); severe hypertension using trialists' definition (IPD trials only; RR 0.94, 95% CI 0.83 to 1.07; 1834 mothers; 5 trials; Analysis 6.4); pregnancy with severe adverse outcome (IPD trials only; 1970 mothers, 8 trials; RR 0.95, 95% CI 0.84 to 1.06; Analysis 6.19); and they also appeared to make little difference to gestation at birth (IPD trials only: 1927 mothers, 8 trials; MD 0.15 weeks, 95% CI ‐0.12 to 0.41; Analysis 6.20).
6.12. Analysis.
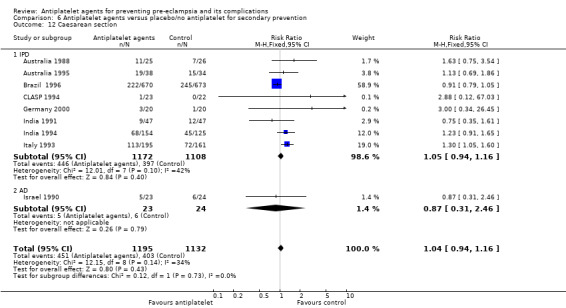
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 12 Caesarean section.
6.13. Analysis.
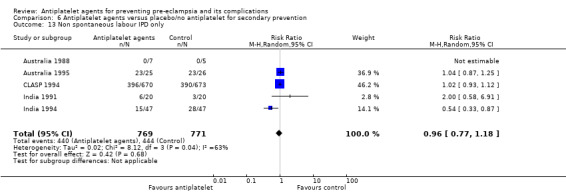
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 13 Non spontaneous labour IPD only.
6.4. Analysis.
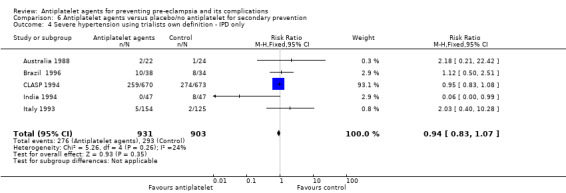
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 4 Severe hypertension using trialists own definition ‐ IPD only.
6.19. Analysis.
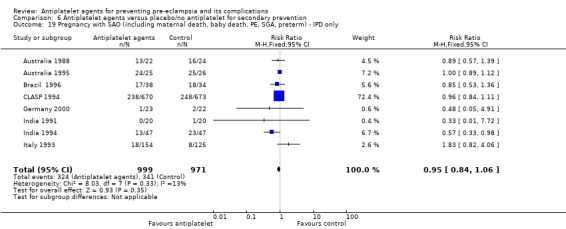
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 19 Pregnancy with SAO (including maternal death, baby death, PE, SGA, preterm) ‐ IPD only.
6.20. Analysis.
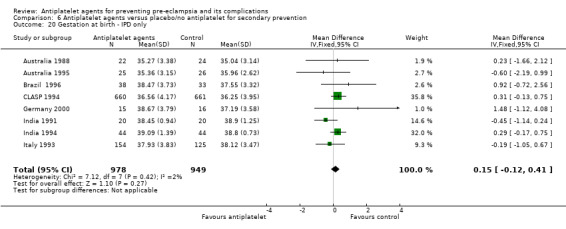
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 20 Gestation at birth ‐ IPD only.
In terms of outcomes for the baby, the results suggested that antiplatelet agents are associated with a reduction in the risk of babies having birthweight less than 2500g (IPD and AD trials; 1966 babies, 9 trials; average RR 0.76, 95% CI 0.59 to 0.97; Analysis 6.21). However they make little or no difference to admission to a special care baby unit (IPD trials only; RR 0.97, 95% CI 0.86 to 1.10; 1910 babies, 6 trials; Analysis 6.22), while it is unclear whether they have an impact on the need for assisted ventilation (IPD trials only: 542 babies, 5 trials; RR 0.50, 95% CI 0.20 to 1.22; Analysis 6.23).
6.21. Analysis.
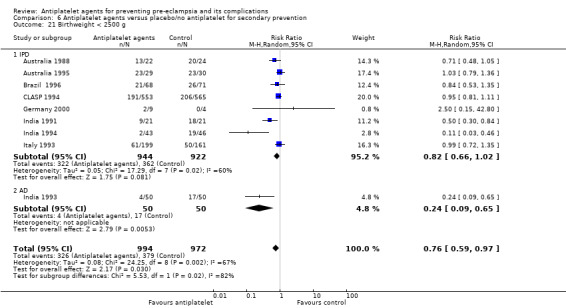
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 21 Birthweight < 2500 g.
6.22. Analysis.
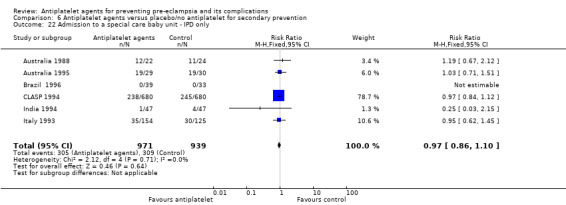
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 22 Admission to a special care baby unit ‐ IPD only.
6.23. Analysis.
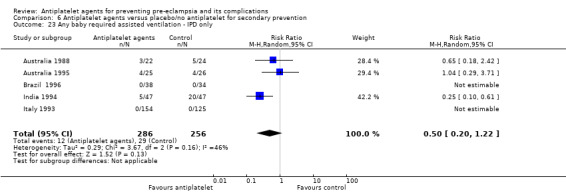
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 23 Any baby required assisted ventilation ‐ IPD only.
It was unclear whether antiplatelet agents make a difference to secondary prevention of all the other secondary outcomes. In these cases the point estimates suggested a clinically important effect, but were accompanied by wide confidence intervals substantially crossing the line of no effect: severe pre‐eclampsia (1509 women; 3 trials; RR 0.78, 95% CI 0.48 to 1.26; Analysis 6.1), gestation at onset proteinuria (IPD trials only; 410 women, 2 trials; MD ‐1.05 weeks, 95% CI ‐3.70 to 1.59; Analysis 6.7), antepartum haemorrhage (IPD trials only; RR 1.11, 95% CI 0.75 to 1.64; 1606 women, 5 trials; Analysis 6.14), postpartum haemorrhage > 500 mL (IPD trials only; 1573 women; 5 trials; RR 1.09, 95% CI 0.85 to 1.40; Analysis 6.16), HELLP syndrome (IPD trials only, not estimable due to no reported events; Analysis 6.17), severe maternal morbidity (IPD trials only: RR 0.72, 95% CI 0.14 to 3.61; 1483 women; 3 trials; Analysis 6.18) . The effects were also unclear for eclampsia (1743 women, 5 trials; RR 0.47, 95% CI 0.13 to 1.67; Analysis 6.3), and placental abruption (IPD trials only; 1606 women, 5 trials; RR 1.39, 95% CI 0.63 to 3.05; Analysis 6.15), probably due to there being very few events for both outcomes.
6.1. Analysis.
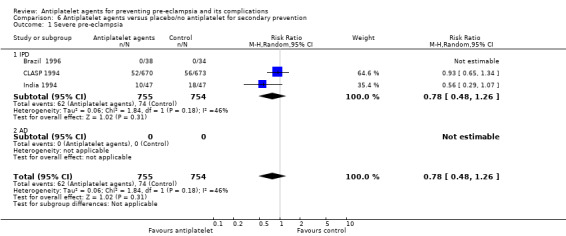
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 1 Severe pre‐eclampsia.
6.7. Analysis.
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 7 Gestation at onset proteinuria by best definition ‐ IPD only.
6.14. Analysis.
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 14 Antepartum haemorrhage ‐ IPD only.
6.16. Analysis.
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 16 Postpartum haemorrhage > 500 mL ‐ IPD only.
6.17. Analysis.
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 17 HELLP Syndrome ‐ IPD only.
6.18. Analysis.
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 18 Severe maternal morbidity ‐ IPD only.
6.3. Analysis.
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 3 Eclampsia.
6.15. Analysis.
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 15 Placental abruption ‐ IPD only.
Discussion
Summary of main results
This review of randomised trials evaluating antiplatelet agents for prevention of pre‐eclampsia and its complications demonstrates that these agents, in particular low‐dose aspirin, have small‐to‐moderate benefits. For the first time, this update incorporates the individual participant data (IPD) analysis published by the PARIS collaborative group (Askie 2007). Methods for the two reviews have now been merged, and this Cochrane Review includes IPD wherever these are available. IPD are available from 36 trials including 34,514 women (80% of the total number of women included in this review).
We found high‐quality evidence that the use of antiplatelet agents reduces the risk of pre‐eclampsia (where most women in the included trials were given low‐dose aspirin). This reduction in pre‐eclampsia is reflected in modest improvement in outcomes for the baby: antiplatelet agents slightly reduce the risk for preterm birth before 37 completed weeks, and they reduce fetal or neonatal deaths, and small‐for‐gestational‐age babies. For this Cochrane Review the outcome included any preterm birth before 37 weeks' gestation. A separate analysis using IPD for the 17 trials (28,797 women) that supplied data on whether onset of labour was spontaneous or indicated (induced or elective caesarean) in the PARIS dataset concluded that antiplatelet agents are associated with a reduction in spontaneous preterm birth (van Vliet 2017). The effect size of a 7% reduction was similar to that report here.
There was a slight increase in postpartum blood loss of > 500 mL associated with the use of antiplatelet agents. This result should be interpreted cautiously, however, given the well‐known difficulties of accurately estimating blood loss after birth, and because the outcome does not take into account mode of birth (for caesarean births, clinically important postpartum haemorrhage is usually considered to be more than 1000 mL). If true, this slight increase would lead to 9 more women having blood loss > 500 mL per 1000 women giving birth, relative to an assumed baseline risk of 143 women per 1000. It should be noted that although the 95% confidence interval is narrow, it does include the possibility of no effect (1.00 to 1.12). Blood loss > 500 mL is the traditional definition of postpartum haemorrhage, but in terms of clinical importance postpartum haemorrhage of 500 mL to 1000 mL is considered relatively minor, with 1001 mL to 2000 mL considered moderate loss and > 2000 mL major postpartum haemorrhage. Hence the clinical relevance of this slight increase in minor postpartum haemorrhage is unclear.
Exploratory analyses in the PARIS analysis found the overall results to be sensitive to even small changes in the cut‐off used to define postpartum blood loss. When the definition was changed from ‘greater than' to 'greater than or equal to' 500 mL, the change in definition had up to a four‐fold effect on the estimated effect size, and influenced statistical significance (Askie 2007). This underlines further the importance of interpreting the results for this outcome with caution. Data presented here are based on the pre‐specified definition for this Cochrane Review, which was ‘postpartum haemorrhage as defined in the trial’.
Antiplatelet agents probably marginally increase the risk of placental abruption, whilst they make little or no difference to gestational hypertension. There was a 10% reduction in the overall risk of having a pregnancy with a serious adverse outcome (defined as pregnancy where the mother dies or develops pre‐eclampsia or if any baby is preterm, small‐for‐gestational age, or does not survive to discharge from hospital).
The results for secondary prevention were less clear. Fewer trials involving a much smaller number of women reported on secondary prevention, compared with primary prevention. While the analyses suggested that antiplatelet agents probably lower the risk of proteinuric pre‐eclampsia for women with gestational hypertension, for other primary outcomes the results suggested either that antiplatelet agents may make little or no difference (preterm birth), or were too imprecise to support firm conclusions (fetal and neonatal death; small‐for‐gestational age). Overall, for secondary prevention, the available evidence did not consistently indicate that antiplatelet agents confer a benefit for women and their babies.
The overall results of the review have not changed significantly after incorporation of IPD. Nevertheless, for several main outcomes IPD show more conservative estimates of the treatment effect than AD. It is not plausible that treatment effects really are bigger if IPD were not available. There are several possible explanations for this systematic difference between results based on IPD and AD. The first is that this is related to lower methodological quality, for trials which were eligible for the PARIS analysis, but for which IPD were not available (i.e. availability of IPD may be a marker for higher methodological quality). For example, a significant proportion of studies with only AD available were of uncertain risk of bias. Another possibility is publication bias; as shown in Figure 6, smaller AD studies tended to report larger effects of antiplatelet agents for pre‐eclampsia, leading to a skewed funnel plot. However, the effects remain stable (whilst slightly more conservative) when only including IPD, with no indication of publication bias. Other possible explanations are that the difference in treatment effect is due to differences in the populations in the trials with IPD and those with AD, or a difference in dose of the intervention. For example, two thirds of the women in trials with AD were in trials evaluating the higher does (75 mg or more) of aspirin, compared with less that one third of the women in trials with IPD. As discussed below, trials with the higher dose tended to report a bigger effect.
Incorporating IPD has allowed more accurate classification of risk for individual women included in many of the trials. This improves the precision of the subgroup analyses. For a small number of trials only AD are available, and so for these studies the subgroup analysis by baseline risk is classified at trial level. The differences between the subgroups by maternal risk were not significant, so any individual subgroup result should be interpreted with caution. Because of the different baseline levels, and slightly different risk reduction rate (albeit not significant), the results suggest seven fewer cases of pre‐eclampsia per 1000 low‐risk women, with a range from 12 fewer to one fewer, and 27 fewer cases per 1000 high‐risk women, with a range from 36 fewer to 16 fewer.
It is possible that starting antiplatelet agents before implantation and trophoblast invasion are complete may increase the reduction in relative risk of pre‐eclampsia. In this review, data are presented by before and after 20 weeks' gestation, and we have added figures with the pre‐specified analyses by gestation at trial entry in the PARIS IPD dataset. There is no clear evidence that antiplatelet agents are more effective if given early in pregnancy (Meher 2017). This differs from analysis based on AD only (Bujold 2010; Roberge 2017; Xu 2015), as gestation at trial entry is based on data from individual women, rather than being determined at trial level and power is greater as far greater numbers of women are included in the analysis (Tong 2017).
There is some evidence that a higher dose of aspirin (75 mg or more) may be more effective than a low dose (less than 75 mg), but this requires further evaluation (Seidler 2018), ideally in trials comparing alternative dose regimens. Higher doses may also have higher risk, and the current reassurance about safety applies only to lower doses. There was no significant difference between trials with placebo and without placebo.
Although the subgroups presented in this review were all prespecified, their results should be interpreted with caution. As with all subgroup analyses, comparisons between subgroups are observational and so prone to all the limitations of observational analyses. Emphasis on the differences between subgroups is potentially misleading; provided the direction of effect is likely to be the same across subgroups, the best guide for clinical practice is the overall effect across all subgroups.
Overall completeness and applicability of evidence
It has been suggested that the promising early systematic reviews of antiplatelet therapy may have reflected publication bias (Broughton Pipkin 1996). Graphs (called funnel plots) of the effect size against sample size for each trial have been consistently asymmetric, suggesting that small negative trials may be missing. In this review, most of the small positive trials were published in the 1980s and early 1990s. It remains possible that small negative trials conducted at that time have still not been published. Interestingly, the recently conducted small studies added in this update are also largely positive. The funnel plot of the data for pre‐eclampsia therefore continues to be asymmetric (Figure 6). However, as discussed above, this asymmetry largely disappears (while the effect remains stable) when only trials with IPD are included. The funnel plot for data on fetal deaths and neonatal deaths is more symmetric (Figure 9). Publication bias is not the only cause of funnel plot asymmetry, and in this review it may also be due to differences in maternal characteristics between the small studies and the large trials.
Quality of the evidence
As summarised in the Table 1, we assessed the quality of the evidence for the seven key outcomes using GRADE. Overall, the results for pre‐eclampsia, fetal or neonatal death, preterm birth, small‐for‐gestational age, and pregnancy with serious adverse outcome (composite including maternal death, baby death, pre‐eclampsia, small‐for‐gestational age, preterm birth) were rated as high‐quality evidence, while postpartum haemorrhage (< 500 mL) and placental abruption were rated as moderate‐quality evidence.
We did not downgrade any of the outcomes for risk of bias, since the majority of studies were of low risk of bias, and in particular, the large trials that had the largest influence on the results were low risk of bias for most domains. There was substantial clinical heterogeneity in the measurement of postpartum haemorrhage, due to the well‐known difficulties of accurately estimating blood loss after birth, we thus downgraded the quality of the evidence by one level for inconsistency. There was little heterogeneity for any of the other outcomes. Since the populations, settings, and treatments were representative for the question of interest for all rated outcomes, none of the outcomes were downgraded for indirectness. Generally, the results for our primary outcomes were precise due to the high number of included trials and women, whereas many trials did not report on our secondary outcomes so many of those results were imprecise. Of our primary outcomes, the only outcome that had to be downgraded for imprecision was placental abruption, where the confidence interval was wide due to the low number of events.
For some of our secondary outcomes and for the subgroup analyses, future evidence may still change our conclusions. Similarly, the quality of the evidence for secondary prevention settings (i.e. women with gestational hypertension at trial entry) was not sufficient to derive final conclusions, and future research may change the conclusions drawn from this moderate‐ to‐low‐quality evidence. Also, the large number of subgroups and outcomes means that at least a few of the statistically significant results may merely reflect the play of chance (with a P value of 0.05, one in 20 can be expected to be positive, purely by chance). Where only a proportion of eligible trials reported a particular outcome and large numbers of women are missing, there is also the potential to be misled by bias.
Potential biases in the review process
This review is based on an extensive search strategy. It includes studies from a wide range of countries, and those published in languages other than English. Nevertheless, it remains possible that studies have been missed, but should that be the case they will be included in future updates once they become available.
Agreements and disagreements with other studies or reviews
Systematic reviews evaluating antiplatelet agents for prevention of pre‐eclampsia published elsewhere have focused on specific subgroups of women, for example, based on specific risk factors for developing pre‐eclampsia (Coomarasamy 2001; Coomarasamy 2003; Rossi 2011; Ruano 2005; Trivedi 2011), or gestation at randomisation (Bujold 2010; Roberge 2013; Xu 2015), or dose and gestation (Roberge 2017). Most systematic reviews evaluating low‐dose aspirin for high‐risk and low‐risk women (mainly based on medical and obstetric history, obstetric factors, and ultrasound) show similar small‐to‐moderate benefits for prevention of pre‐eclampsia (Meher 2013) as this Cochrane Review. Using IPD, the Paris Collaborative Group found no clear evidence that antiplatelet agents are any more or less effective in reducing the relative risk of pre‐eclampsia and its consequences for any particular subgroup of women (Askie 2007). Current research is focusing on biomarkers to identify women at risk of pre‐eclampsia (Akolekar 2013). Whether this will help identify women most likely to benefit from aspirin remains to be seen.
Early and late administration of low‐dose aspirin has been compared in several systematic reviews (Bujold 2010; Roberge 2013; Xu 2015). All defined early administration as 16 weeks or earlier, and late as 16 weeks or after. The first review concluded that early administration of aspirin is associated with a reduction in pre‐eclampsia and intrauterine growth restriction, whereas late administration is not (Bujold 2010). The second review concluded that early administration was associated with a reduction in perinatal death and other adverse perinatal outcomes (Roberge 2013). The third review concluded that early administration was associated with a greater reduction in pre‐eclampsia, perinatal death, fetal growth restriction, and preterm birth (Xu 2015) In these trials women were not randomised to whether they had early or late administration of low‐dose aspirin. A fourth review looked at dose and gestation, and concluded there is a dose‐response effect (Roberge 2017). As with all subgroup analyses, any comparison is therefore observational. Even if there is a statistically significant difference between subgroups, this may be due to confounding, rather than any real difference in effect. For example, in these reviews, the control event rate for pre‐eclampsia was high for women having early administration of low‐dose aspirin (8.1% in Bujold 2010 and 8.4% in Roberge 2013). Also, all three reviews were based on AD and so some women in the analyses will have been incorrectly classified. Our IPD analysis demonstrates no clear difference in the relative risk of pre‐eclampsia based on whether gestation at trial entry was before or after 16 weeks' gestation (Meher 2017).
Authors' conclusions
Implications for practice.
Administration of antiplatelet agents leads to a 17% reduction in the relative risk of developing pre‐eclampsia. For primary prevention of pre‐eclampsia, for every 64 women treated, one case of pre‐eclampsia will be prevented. For women who are at high risk, the results suggest that one case of pre‐eclampsia will be prevented for every 39 treated. There are also smaller risk reductions associated with the use of antiplatelet agents of preterm birth (9%), with 61 women needing to be treated to prevent one case, and of fetal or neonatal death (14%) with 213 women needing to be treated to prevent one case. Adverse effects appear to be low although data were not reported by all trials. The results suggest a slight increase in postpartum haemorrhage of > 500 mL. Overall, there was a 10% reduction in pregnancies with serious adverse outcomes.
As most women in this review were in trials evaluating low‐dose aspirin, the reassurance about safety may not apply to higher doses or other agents. However, there was some indication that higher doses may be more effective in preventing pre‐eclampsia. The evidence presented in this review may be summarised and made available to women at risk of pre‐eclampsia. The decision about whether to take aspirin during pregnancy may then be made in consultation between the woman and her doctor. As the reductions in risk are small to moderate, relatively large numbers of women will need to be treated to prevent pre‐eclampsia and its consequences. However, from a public health perspective these moderate benefits are likely to be worthwhile, and low‐dose aspirin may be worth considering for more widespread use.
Implications for research.
Identifying women who are most likely to respond to low‐dose aspirin would allow improved targeting of treatment. As almost all the women in this review were recruited to the trials after 12 weeks' gestation, it is unclear whether starting treatment before 12 weeks would have additional benefits without any increase in adverse effects. While there was some indication that higher doses of aspirin would be more effective, further studies would be warranted to examine whether a higher dose would be more effective and safe.
Feedback
Coomarasamy, February 2001
Summary
[Aspirin has clinically significant benefit in high risk groups ‐ Summary NNT can mislead clinicians and women]
Editor ‐ The systematic review (1;2) of antiplatelet drugs for prevention of pre‐eclampsia found statistically significant reduction in pre‐eclampsia and other outcomes such as fetal or neonatal death. The authors concluded that the benefit was 'small to moderate' and the implication for practice was that 'relatively large numbers of women will need to be treated to prevent a single adverse outcome'. With the numbers of women needed to be treated to prevent one case of pre‐eclampsia reported as 100 (95% CI 59 to 167), clinicians (and women) might not think treatment worthwhile.
However, calculating numbers needed to treat from pooled meta‐analysis data may be inappropriate, if it is possible to identify subgroups of patients with substantially differing baseline risks(3). In women with high levels of baseline risk, and assuming constant relative risk from treatment, numbers needed to treat are smaller (4), and both clinicians and women may be much more likely to wish to use aspirin to prevent pre‐eclampsia. It has been suggested(1;2) that meta‐analysis of individual patient data would be useful both in identifying high‐risk subgroups, and estimating the benefit they derive from antiplatelet treatment. However, such meta‐analyses generally take a long time to complete(5). What should clinicians do in the mean time?
We can see no reason for thinking that the reduction in relative risk for various risk levels will be substantially different. If high‐risk (or low risk) women can be identified, by any means, specific numbers needed to treat can then be generated by using pooled relative risk estimates from reviews of effectiveness(4), making the decision to treat (or not) more appropriate and, in this particular case, probably more clear‐cut for most women.
We systematically reviewed the accuracy of uterine artery Doppler in early pregnancy for predicting pre‐eclampsia(6). In clinically high‐risk women, a positive Doppler result (abnormal flow velocimetry ratio or the presence diastolic notch) meant a 23.5% (95% CI 18.6 to 29.2) risk of developing pre‐eclampsia. With baseline risk elevated to this level and assuming the global estimated relative risk of 0.85 (1), we estimate that 31 (95% CI 18 to 55) patients will be needed to be treated with aspirin to prevent one case of pre‐eclampsia. We would thus expect most women with abnormal uterine artery Dopplers, when advised by their clinicians, to request antiplatelet treatment.
1. Duley L, Henderson‐Smart DJ, Knight M, King JF. Anteplatelet drugs for prevention of pre‐eclampsia and its consequences: systematic review. BMJ 2001;322: 329‐333.
2. Knight M, Duley L, Henderson‐Smart DJ, King JF. Antiplatelet agents for preventing and treating pre‐eclampsia. Cochrane Database.Syst.Rev. 2000;CD000492.
3. Smeeth L, Haines A, Ebrahim S. Numbers needed to treat derived from meta‐analyses‐‐sometimes informative, usually misleading. BMJ 1999;318:1548‐51.
4. Guyatt GH, Sackett DL, Sinclair JC, Hayward R, Cook DJ, Cook RJ. Users' guides to the medical literature. IX. A method for grading health care recommendations. Evidence‐Based Medicine Working Group. JAMA 1995;274:1800‐4.
5. Stewart LA,.Clarke MJ. Practical methodology of meta‐analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat.Med. 1995;14:2057‐79.
6. Chien PF, Arnott N, Gordon A, Owen P, Khan KS. How useful is uterine artery Doppler flow velocimetry in the prediction of pre‐eclampsia, intrauterine growth retardation and perinatal death? An overview. BJOG. 2000;107:196‐208.
Reply
The main aim of our review was to summarise the evidence. We agree the number needed to treat will be more favourable for women at higher risk, nevertheless, women at moderate/low risk do also seem to benefit. The public health benefit of a 15% reduction in pre‐eclampsia and a 14% reduction in stillbirth and neonatal death is difficult to quantify, but is likely to be important. Aspirin is the best we have to offer for prevention of pre‐eclampsia, with the added advantages of being low cost and reasonable reassurance about safety.
As indicated in our review, we are addressing the issue of potential variations in effect size for women with different baseline risk by undertaking a review based on data from individual women.
[reply from the review team, September 2002]
Contributors
Comments by:
A Coomarasamy Research Fellow in Obstetrics Education Resource Centre Birmingham Women's Hospital Metchley Park Road, Birmingham B15 2TG arricoomar@hotmail.com
Harry Gee Consultant Obstetrician Birmingham Women's Hospital, B15 2TG
Khalid S Khan Consultant Obstetrician and Gynaecologist Birmingham Women's Hospital, B15 2TG
David Braunholtz Senior Research Fellow Department of Public Health & Epidemiology Public Health Building University of Birmingham, B15 2TT
What's new
| Date | Event | Description |
|---|---|---|
| 4 October 2018 | New citation required but conclusions have not changed | Updating this review did not change the conclusion of the previous version of this review that antiplatelet agents reduce pre‐eclampsia, preterm birth, and neonatal death. |
| 4 October 2018 | New search has been performed | Search updated. Updated previously included studies with available individual participant data (IPD), added IPD from two not previously included studies (Pergar 1987; Spain 2003), and added aggregate data (AD) from 17 new studies (Algeria 2011; ASPRE 2017; Brazil 2006; Canada 2017, Finland 2013; Germany 1995; India 1991; India 2017Iran 1992; Iran 2006; Iran 2010; Iran 2012; Iran 2014a, Korea 1997; Netherlands 2009, Romania 2018, Spain 2017). The use of IPD enabled us to classify participants by risk of developing pre‐eclampsia and analyse the effect of antiplatelet agents for different risk groups. Including the new studies and IPD in the updated review allowed for the novel inclusion of the two key outcomes, postpartum haemorrhage and pregnancy with serious adverse outcome (composite including maternal death, baby death, pre‐eclampsia, small‐for‐gestational age, preterm birth). Antiplatelet agents reduced the risk of pregnancy with serious adverse outcome, but probably slightly increased the risk of postpartum haemorrhage (> 500 mL). A prepublication search in September identified 27 new trial reports. These have been added to the Studies awaiting classification section for consideration at the next update. |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 31 May 2010 | Amended | Search updated. Sixteen reports added to Studies awaiting classification. |
| 1 September 2008 | Amended | Converted to new review format. |
| 7 February 2007 | New citation required but conclusions have not changed | This version updates two previously published reviews in The Cochrane Library (Knight 2000a and Duley 2003). |
| 30 July 2006 | New search has been performed | Seven additional trials have been included (Brazil 1992a; China 1996a; Egypt 2005; Finland 1997a; Italy 2004;UK+others 2003; USA 1997), and one study which had previously been excluded (Russia 1993) is now included after additional clarification from trialists. Fifteen additional trials have been assessed and excluded (Brazil 1996a; Egypt 1991; Egypt 1998a; Equador 1998; India 1986; India 2001; India 2002; India 2002a; Italy 1990; Italy 2002; Italy 2005; Japan 1989; Russia 1997; Tunisia 1990a; Tunisia 1994a). These changes have not made a substantive difference to the overall conclusions of this review. Trials previously classified as 'treatment' rather than 'prevention' (India 1993; India 1994; Israel 1990; subgroup of UK 1992; subgroup of CLASP 1994; subgroup of Italy 1993) are now included with this review, but under a different comparison for secondary prevention of pre‐eclampsia. The review 'Antiplatelet agents for preventing and treating pre‐eclampsia' (Knight 2000a) has therefore been withdrawn from The Cochrane Library. |
| 25 September 2002 | Feedback has been incorporated | Feedback and reply from authors added. |
Acknowledgements
Our thanks to Colin Bagent, Caroline Crowther, Michael de Swiet, Adrian Grant, Hein Odendaal, Chris Redman, Jeffrey Robinson, Isabel Walker and Henk Wallenburg for helpful comments on the original protocol for this review, and to Rory Collins, James King and Marian Knight for their work on earlier versions of the review. Thanks also to Robyn North (Australia 1996a) and John Anthony (S Africa 1988) who have provided additional unpublished data; to Arri Coomarasamy who kindly provided an English translation of Russia 1993; to Karla Soares who helped with translation and data extraction of Brazil 1992a; and to members of the PARIS Collaborative Group who have provided additional unpublished data about randomisation and concealment of allocation for some studies and contributed the individual participant data.
We also wish to acknowledge the major contribution of Lesley Stewart to the PARIS Collaboration as a member of the Steering Group and IPD methodology expert. Her contribution ensured the PARIS IPD dataset was of the highest possible quality.
David Henderson‐Smart was a review author from the first version of this Cochrane Review, and he was a leading and enthusiastic contributor to the PARIS Collaborative Group. David died in February 2013, and we dedicate this update to his memory.
Parts of this review were generated using RevMan HAL v 4.2. You can find more information about RevMan HAL here.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Kathleen Brookfield, MD, PhD, Assistant Professor, Obstetrics and Gynecology, Oregon Health and Science University, Portland, OR, USA; Maged Costantine, MD, Professor, Obstetrics and Gynecology, Division Director Maternal Fetal Medicine, The Ohio State University, Department of Obstetrics and Gynecology, Columbus, OH, USA.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search Methods
ICTRP (30 March 2018)
(each line was run separately)
aspirin AND pregnancy
aspirin AND pre‐eclampsia
aspirin AND preeclampsia
antiplatelet(s) AND pregnancy
hypertension AND pregnancy AND aspirin
ClinicalTrials.gov (30 March 2018)
Advanced search
Interventional studies | hypertension in pregnancy | aspirin
Interventional studies | pre‐eclampsia | aspirin
Interventional studies | pregnancy | antiplatelet agents
Interventional studies | pregnancy | antiplatelet drugs
Interventional studies | pre‐eclampsia | antiplatelet agents
Interventional studies | pre‐eclampsia | antiplatelet drugs
Interventional studies | hypertension in pregnancy | antiplatelet agents
Interventional studies | hypertension in pregnancy | antiplatelet drugs
Data and analyses
Comparison 1. Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by maternal risk).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia (IPD vs AD) | 60 | 36716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.77, 0.88] |
| 1.1 Low‐risk women (IPD) | 25 | 20583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.00] |
| 1.2 Low‐risk women (AD) | 6 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.27, 0.89] |
| 1.3 Moderate‐risk women (IPD) | 13 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.47, 1.06] |
| 1.4 Moderate‐risk women (AD) | 7 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.42, 0.84] |
| 1.5 High‐risk women (IPD) | 26 | 11076 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.82, 0.98] |
| 1.6 High‐risk women (AD) | 13 | 3006 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.49, 0.72] |
| 1.7 Unclear‐/unspecified‐risk women (AD) | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.76] |
| 2 Fetal death, neonatal death, or death before hospital discharge (IPD vs AD) | 52 | 35391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.76, 0.95] |
| 2.1 Low‐risk women (IPD) | 25 | 20583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.79, 1.08] |
| 2.2 Low‐risk women (AD) | 3 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.07, 1.04] |
| 2.3 Moderate‐risk women (IPD) | 12 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.81, 2.67] |
| 2.4 Moderate‐risk women (AD) | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.21, 1.00] |
| 2.5 High‐risk women (IPD) | 26 | 11076 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.66, 0.99] |
| 2.6 High‐risk women (AD) | 11 | 2323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.40, 0.98] |
| 2.7 Unclear‐/unspecified‐risk women (AD) | 3 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.49, 2.03] |
| 3 Preterm birth (IPD vs AD) | 47 | 35212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.87, 0.95] |
| 3.1 Low‐risk women (IPD) | 25 | 20563 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.89, 1.01] |
| 3.2 Low‐risk women (AD) | 5 | 453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.42, 0.68] |
| 3.3 Moderate‐risk women (IPD) | 12 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.94, 1.27] |
| 3.4 Moderate‐risk women (AD) | 5 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.60, 1.18] |
| 3.5 High‐risk women (IPD) | 26 | 11076 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.97] |
| 3.6 High‐risk women (AD) | 5 | 2013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.47, 0.82] |
| 3.7 Unclear‐/unspecified‐risk women (AD) | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.95] |
| 4 Small‐for‐gestational age (IPD vs AD) | 50 | 35761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.76, 0.92] |
| 4.1 Low‐risk women (IPD) | 25 | 20583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.76, 1.07] |
| 4.2 Low‐risk women (AD) | 2 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.41] |
| 4.3 Moderate‐risk women (IPD) | 12 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.70, 1.60] |
| 4.4 Moderate‐risk women (AD) | 6 | 883 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.33, 0.80] |
| 4.5 High‐risk women (IPD) | 26 | 11076 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.76, 1.01] |
| 4.6 High‐risk women (AD) | 9 | 2355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.58, 0.92] |
| 4.7 Unclear‐/unspecified‐risk women (AD) | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.16, 1.86] |
| 5 Pre‐eclampsia (IPD/AD combined) | 60 | 36716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.77, 0.88] |
| 5.1 Low‐risk women | 31 | 21126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.97] |
| 5.3 Moderate‐risk women | 20 | 1416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.49, 0.83] |
| 5.4 High‐risk women | 39 | 14082 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.77, 0.90] |
| 5.6 Unclear‐/unspecified‐risk women | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.76] |
| 6 Fetal death, neonatal death, or death before hospital discharge (IPD/AD combined) | 52 | 35391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.76, 0.95] |
| 6.1 Low‐risk women | 28 | 20961 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.05] |
| 6.2 Moderate‐risk women | 16 | 884 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.48] |
| 6.3 High‐risk women | 37 | 13399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.93] |
| 6.5 Unclear‐/unspecified‐risk women | 3 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.49, 2.03] |
| 7 Preterm birth (IPD/AD combined) | 47 | 35212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.87, 0.95] |
| 7.1 Low‐risk women | 30 | 21016 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.87, 0.98] |
| 7.2 Moderate‐risk women | 17 | 1061 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.89, 1.18] |
| 7.3 High‐risk women | 31 | 13089 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 7.5 Unclear‐/unspecified‐risk women | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.95] |
| 8 Small‐for‐gestational age (IPD/AD combined) | 50 | 35761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.76, 0.92] |
| 8.1 Low‐risk women | 27 | 20797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.05] |
| 8.2 Moderate‐risk women | 18 | 1441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.55, 0.99] |
| 8.3 High‐risk women | 35 | 13431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.73, 0.94] |
| 8.5 Unclear‐/unspecified‐risk women (AD) | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.16, 1.86] |
Comparison 2. Antiplatelet agents versus placebo/no antiplatelet for primary prevention (primary outcomes, subgroups by gestation at entry).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 60 | 36716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.77, 0.88] |
| 1.1 Entered into the study < 20 weeks (IPD) | 27 | 18950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.95] |
| 1.2 Entered into the study < 20 weeks (AD) | 19 | 3560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.51, 0.73] |
| 1.3 Entered into the study ≥ 20 weeks (IPD) | 26 | 13173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.04] |
| 1.4 Entered into the study ≥ 20weeks (AD) | 7 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.49] |
| 1.5 Unclassified (IPD) | 11 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.04, 5.46] |
| 1.6 Unclassified (AD) | 3 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.41, 1.04] |
| 2 Fetal death, neonatal death, or death before hospital discharge | 52 | 35391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.76, 0.95] |
| 2.1 Entered into the study < 20 weeks (IPD) | 27 | 18950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.75, 1.02] |
| 2.2 Entered into the study < 20 weeks (AD) | 11 | 2657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.44, 0.88] |
| 2.3 Entered into the study ≥ 20 weeks (IPD) | 26 | 13173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.76, 1.15] |
| 2.4 Entered into the study ≥ 20 weeks (AD) | 6 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.11, 1.98] |
| 2.5 Unclassified (IPD) | 11 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.04, 2.09] |
| 2.6 Unclassified (AD) | 4 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.14, 1.78] |
| 3 Preterm birth | 47 | 35212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.86, 0.94] |
| 3.1 Entered into the study < 20 weeks (IPD) | 27 | 18930 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.00] |
| 3.2 Entered into the study < 20 weeks (AD) | 11 | 2610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.53, 0.74] |
| 3.3 Entered into the study ≥ 20 weeks (IPD) | 26 | 13173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.85, 0.98] |
| 3.4 Entered into the study ≥ 20 weeks (AD) | 4 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.28, 1.12] |
| 3.5 Unclassified (IPD) | 11 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.1 [0.01, 1.71] |
| 3.6 Unclassified (AD) | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 16.15] |
| 4 Small‐for‐gestational age | 50 | 35761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.76, 0.92] |
| 4.1 Entered into the study < 20 weeks (IPD) | 27 | 18950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.70, 0.95] |
| 4.2 Entered into the study < 20 weeks (AD) | 13 | 3211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.55, 0.82] |
| 4.3 Entered into the study ≥ 20 weeks (IPD) | 26 | 13173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.85, 1.16] |
| 4.4 Entered into the study ≥ 20 weeks (AD) | 5 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.35, 1.37] |
| 4.5 Unclassified (IPD) | 11 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Unclassified (AD) | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.01, 5.92] |
Comparison 3. Antiplatelet agents versus placebo/no treatment for primary prevention (primary outcomes, subgroups by use of placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 60 | 36871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.77, 0.88] |
| 1.1 Placebo (IPD) | 24 | 31090 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.83, 0.96] |
| 1.2 Placebo (AD) | 22 | 3878 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.47, 0.70] |
| 1.3 No placebo (IPD) | 7 | 1127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.37, 1.12] |
| 1.4 No placebo (AD) | 7 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.41, 0.69] |
| 2 Fetal death, neonatal death, or death before hospital discharge | 52 | 35391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.76, 0.95] |
| 2.1 Placebo (IPD) | 24 | 31090 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.02] |
| 2.2 Placebo (AD) | 14 | 2652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.47, 1.05] |
| 2.3 No placebo (IPD) | 7 | 1127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.43, 1.30] |
| 2.4 No placebo (AD) | 6 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.23, 0.80] |
| 2.5 Unclear/unspecified (AD) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.04, 3.62] |
| 3 Preterm birth | 47 | 35212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.86, 0.94] |
| 3.1 Placebo (IPD) | 24 | 31070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.89, 0.98] |
| 3.2 Placebo (AD) | 11 | 2423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.47, 0.69] |
| 3.3 No placebo (IPD) | 7 | 1127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.14] |
| 3.4 No placebo (AD) | 5 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.08] |
| 4 Small‐for‐gestational age | 49 | 35606 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.77, 0.93] |
| 4.1 Placebo (IPD) | 24 | 31090 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 1.00] |
| 4.2 Placebo (AD) | 13 | 2885 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.58, 0.90] |
| 4.3 No Placebo (IPD) | 7 | 1127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.52, 1.58] |
| 4.4 No placebo (AD) | 5 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.82] |
Comparison 4. Antiplatet agents versus placebo/no treatment for primary prevention (primary outcomes, subgroups by dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 56 | 36282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.77, 0.88] |
| 1.1 < 75 mg aspirin (IPD) | 11 | 22618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 1.2 < 75 mg aspirin (AD) | 6 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.39, 0.89] |
| 1.3 ≥ 75 mg aspirin (IPD) | 16 | 9107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.66, 0.92] |
| 1.4 ≥ 75 mg aspirin (AD) | 19 | 3505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.49, 0.70] |
| 1.5 Aspirin ≥ 75 mg + dipyridamole (IPD) | 3 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.26, 1.16] |
| 1.6 Aspirin ≥ 75 mg + dipyridamole (AD) | 3 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.69] |
| 2 Fetal death, neonatal death, or death before hospital discharge | 49 | 35117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.76, 0.95] |
| 2.1 < 75 mg aspirin (IPD) | 16 | 27025 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| 2.2 < 75 mg aspirin (AD) | 10 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.45, 1.45] |
| 2.3 ≥ 75 mg aspirin only (IPD) | 12 | 4733 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.61, 1.45] |
| 2.4 ≥ 75 mg aspirin only (AD) | 6 | 2165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.34, 0.78] |
| 2.5 Aspirin ≥ 75 mg + dipyridamole (IPD) | 3 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.21, 1.80] |
| 2.6 Aspirin ≥ 75 mg + dipyridamole (AD) | 4 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.23, 3.51] |
| 3 Preterm birth | 43 | 34778 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.78, 0.93] |
| 3.1 < 75 mg aspirin (IPD) | 11 | 22618 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.89, 0.98] |
| 3.2 < 75 mg aspirin (AD) | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.08, 1.09] |
| 3.3 ≥ 75 mg aspirin only (IPD) | 16 | 9087 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.73, 1.01] |
| 3.4 ≥ 75 mg aspirin only (AD) | 12 | 2712 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.50, 0.84] |
| 3.5 Aspirin ≥ 75 mg + dipyridamole (IPD) | 3 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.34, 5.51] |
| 3.6 Aspirin ≥ 75 mg + dipyridamole (AD) | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.30, 2.61] |
| 4 Small‐for‐gestational age | 47 | 35487 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.68, 0.89] |
| 4.1 < 75 mg aspirin (IPD) | 11 | 22618 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.79, 1.07] |
| 4.2 < 75 mg aspirin (AD) | 4 | 299 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.13] |
| 4.3 ≥ 75 mg aspirin only (IPD) | 16 | 9107 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.53, 1.08] |
| 4.4 ≥ 75 mg aspirin only (AD) | 13 | 3092 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.88] |
| 4.5 Aspirin ≥ 75 mg + dipyridamole (IPD) | 3 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.40, 1.43] |
| 4.6 Aspirin ≥ 75 mg + dipyridamole (AD) | 2 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.07, 1.03] |
Comparison 5. Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe maternal morbidity (eclampsia, renal failure, liver failure, HELLP syndrome, stroke ‐ IPD only) | 15 | 28065 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| 2 HELLP syndrome | 16 | 20130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.44, 1.36] |
| 2.1 IPD | 14 | 19912 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.53, 1.87] |
| 2.2 AD | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.03, 1.10] |
| 3 Disseminated intravascular coagulation ‐ IPD only | 9 | 10828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.57] |
| 4 Stroke ‐ IPD only | 9 | 10828 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.99 [0.12, 73.40] |
| 5 Renal failure ‐ IPD only | 11 | 16502 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.35, 4.79] |
| 6 Liver failure ‐ IPD only | 9 | 10828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Pulmonary oedema ‐ IPD only | 12 | 16732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.37, 1.89] |
| 8 Gestational hypertension using best available definition ‐ IPD only | 25 | 27834 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.01] |
| 9 Gestational hypertension using trialists own definition ‐ IPD only | 25 | 27834 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.89, 1.01] |
| 10 Gestational hypertension PARIS definition ‐ IPD only | 24 | 27519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.02] |
| 11 Gestation at onset proteinuria by best definition ‐ IPD only | 14 | 3442 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.40, 0.56] |
| 12 Fetal, neonatal, infant and childhood deaths (subgroups by time of death) | 45 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Fetal death (IPD) | 29 | 32478 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.80, 1.08] |
| 12.2 Fetal death (AD) | 12 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.44, 1.38] |
| 12.3 Death in first week of life (IPD) | 17 | 25721 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.68, 1.14] |
| 12.4 Death in first week of life (AD) | 10 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.23, 2.42] |
| 12.5 Late death (IPD) | 11 | 15036 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.30, 4.04] |
| 12.6 Perinatal deaths (AD) | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.19, 1.18] |
| 12.7 Death status unknown (IPD) | 15 | 30372 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.80, 1.15] |
| 13 Baby deaths after discharge from hospital ‐ AD only | 2 | 5886 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.21, 1.34] |
| 13.1 Deaths 8 days to 18 months | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.15, 18.57] |
| 13.2 Hospital discharge ‐ 12 months | 1 | 4688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.99] |
| 13.3 Hospital discharge ‐ 18 months | 1 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.20, 23.66] |
| 14 Gestation at birth (mean, weeks) | 34 | 31669 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.04, 0.09] |
| 14.1 IPD | 30 | 31438 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.05, 0.09] |
| 14.2 AD | 4 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.33, 0.88] |
| 15 Preterm birth (< 34 weeks) | 31 | 32253 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.83, 0.97] |
| 15.1 IPD | 30 | 32135 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.83, 0.97] |
| 15.2 AD | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.07, 16.15] |
| 16 Preterm birth (< 32 weeks) | 31 | 32319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.02] |
| 16.1 IPD | 30 | 32155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 16.2 AD | 1 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.10] |
| 17 Preterm birth (< 28 weeks) | 30 | 32135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.01] |
| 17.1 IPD | 30 | 32135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.01] |
| 17.2 AD | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Preterm birth (mutually exclusive subgroups) ‐ IPD only | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 < 28 weeks | 30 | 32155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.01] |
| 18.2 28‐31 weeks | 30 | 32155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.13] |
| 18.3 32‐33 weeks | 30 | 32155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.73, 0.97] |
| 18.4 34‐36 weeks | 30 | 32155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| 18.5 Unknown | 31 | 32217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.96, 1.25] |
| 19 Small‐for‐gestational age (subgrouped by severity) ‐ AD only, trial definition | 30 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 Birthweight < 10th centile | 17 | 10537 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 0.99] |
| 19.2 Birthweight < 5th centile | 6 | 3554 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.79, 1.11] |
| 19.3 Birthweight < 3rd centile | 8 | 14535 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.69, 1.19] |
| 19.4 Definition not stated | 3 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.18, 0.72] |
| 20 Any baby SGA < 3rd centile or as reported ‐ IPD only | 25 | 24702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 1.00] |
| 21 Birthweight < 2500 g | 32 | 31522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.88, 0.97] |
| 21.1 IPD | 30 | 31258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.88, 0.97] |
| 21.2 AD | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.25, 1.24] |
| 22 Pregnancy with SAO (including maternal death, baby death, PE, SGA, preterm) ‐ IPD only | 13 | 17382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.85, 0.96] |
| 23 Hospital admission for the woman during pregnancy ‐ AD only | 3 | 12964 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.97, 1.09] |
| 24 Non spontaneous labour (induced labour or pre‐labour caesarean) ‐ IPD only | 24 | 29838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.98, 1.05] |
| 25 Antepartum haemorrhage ‐ IPD only | 25 | 30513 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.17] |
| 26 Placental abruption | 29 | 30775 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.95, 1.54] |
| 26.1 IPD | 24 | 30257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.95, 1.56] |
| 26.2 AD | 5 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.26, 3.94] |
| 27 Postpartum haemorrhage > 500 mL | 19 | 23769 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.00, 1.12] |
| 27.1 IPD | 16 | 23396 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.00, 1.13] |
| 27.2 AD | 3 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.17, 1.25] |
| 28 Any baby required assisted ventilation | 15 | 7383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.66, 0.97] |
| 28.1 IPD | 14 | 7343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.67, 0.97] |
| 28.2 AD | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.02] |
| 29 Intraventricular haemorrhage | 20 | 32224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.36] |
| 29.1 IPD | 18 | 30538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.70, 1.34] |
| 29.2 AD | 2 | 1686 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.19, 22.67] |
| 30 Other neonatal bleed | 20 | 30715 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.08] |
| 30.1 IPD | 18 | 30538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.08] |
| 30.2 AD | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.13, 5.56] |
| 31 Developmental problems at 18 months ‐ AD only | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 31.1 Poor gross motor function | 1 | 4365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.17] |
| 31.2 Poor fine motor function | 1 | 4365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.84, 1.14] |
| 31.3 Poor gross or fine motor function | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.26, 0.91] |
| 31.4 Respiratory problems | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.98, 2.23] |
| 31.5 Poor language expression | 1 | 4365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.74, 1.19] |
| 31.6 Poor language comprehension | 1 | 4365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 31.7 Language problems ‐ undefined | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.42] |
| 31.8 Hearing problems | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [0.10, 62.10] |
| 31.9 Sight problems | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.25, 2.90] |
| 32 Behaviour problems at 18 months ‐ AD only | 1 | 4365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.75, 1.01] |
| 32.1 Poor sleep pattern | 1 | 4365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.75, 1.01] |
| 33 Malformations at 18 months ‐ AD only | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.27, 2.02] |
| 34 Growth at 18 months ‐ AD only | 2 | 10306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.84, 1.07] |
| 34.1 Height < 3rd or 10th centile | 2 | 5153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.09] |
| 34.2 Weight < 3rd or 10th centile | 2 | 5153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.79, 1.16] |
| 35 Admission to a special care baby unit | 29 | 32808 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.91, 1.00] |
| 35.1 IPD | 23 | 30519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.91, 1.01] |
| 35.2 AD | 6 | 2289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.09] |
| 36 Child admitted to hospital ‐ AD only | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 36.1 At 12 months | 1 | 4168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.08] |
| 36.2 At 18 months | 1 | 4365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.89, 1.11] |
| 37 Non‐routine GP consultation for child ‐ AD only | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 37.1 At 12 months | 1 | 4168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.99, 1.02] |
| 37.2 At 18 months | 1 | 4365 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.96, 1.04] |
| 38 Eclampsia | 17 | 24947 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.66, 1.60] |
| 38.1 IPD | 14 | 24742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.69, 1.71] |
| 38.2 AD | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.18] |
| 39 Maternal death ‐ IPD only | 18 | 28675 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.51, 5.96] |
| 40 Caesarean section | 36 | 32698 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.99, 1.07] |
| 40.1 IPD | 28 | 32071 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.99, 1.07] |
| 40.2 AD | 8 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.72, 1.18] |
5.13. Analysis.
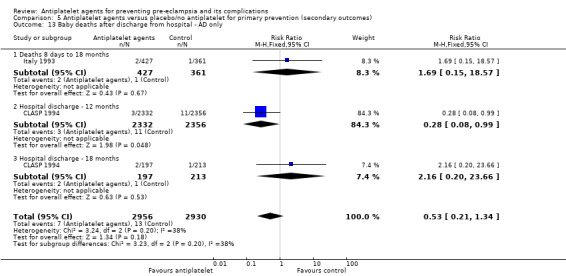
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 13 Baby deaths after discharge from hospital ‐ AD only.
5.36. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 36 Child admitted to hospital ‐ AD only.
5.37. Analysis.
Comparison 5 Antiplatelet agents versus placebo/no antiplatelet for primary prevention (secondary outcomes), Outcome 37 Non‐routine GP consultation for child ‐ AD only.
Comparison 6. Antiplatelet agents versus placebo/no antiplatelet for secondary prevention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe pre‐eclampsia | 3 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.48, 1.26] |
| 1.1 IPD | 3 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.48, 1.26] |
| 1.2 AD | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Pre‐eclampsia | 7 | 1813 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.47, 0.95] |
| 2.1 IPD | 5 | 1606 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.60, 1.04] |
| 2.2 AD | 2 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.23, 1.49] |
| 3 Eclampsia | 5 | 1743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.67] |
| 3.1 IPD | 3 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.14, 3.61] |
| 3.2 AD | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.24] |
| 4 Severe hypertension using trialists own definition ‐ IPD only | 5 | 1834 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 5 Severe hypertension PARIS definition ‐ IPD only | 5 | 1834 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.51, 1.47] |
| 6 Severe hypertension using best available def ‐ IPD only | 5 | 1834 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 7 Gestation at onset proteinuria by best definition ‐ IPD only | 2 | 410 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐3.70, 1.59] |
| 8 Fetal death, neonatal death, or death before hospital discharge ‐ AD only | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.15, 0.84] |
| 9 Fetal and neonatal deaths ‐ IPD only | 7 | 1950 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.68, 1.47] |
| 10 Preterm birth (< 37 weeks) | 9 | 2070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| 10.1 IPD | 8 | 1970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 1.00] |
| 10.2 AD | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 11 Small‐for‐gestational age | 6 | 1791 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.09] |
| 11.1 IPD | 5 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
| 11.2 AD | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.33, 0.96] |
| 12 Caesarean section | 9 | 2327 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.16] |
| 12.1 IPD | 8 | 2280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.94, 1.16] |
| 12.2 AD | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.31, 2.46] |
| 13 Non spontaneous labour IPD only | 5 | 1540 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.77, 1.18] |
| 14 Antepartum haemorrhage ‐ IPD only | 5 | 1606 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.75, 1.64] |
| 15 Placental abruption ‐ IPD only | 5 | 1606 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.63, 3.05] |
| 16 Postpartum haemorrhage > 500 mL ‐ IPD only | 5 | 1573 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.85, 1.40] |
| 17 HELLP Syndrome ‐ IPD only | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Severe maternal morbidity ‐ IPD only | 3 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.14, 3.61] |
| 19 Pregnancy with SAO (including maternal death, baby death, PE, SGA, preterm) ‐ IPD only | 8 | 1970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.06] |
| 20 Gestation at birth ‐ IPD only | 8 | 1927 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.12, 0.41] |
| 21 Birthweight < 2500 g | 9 | 1966 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.59, 0.97] |
| 21.1 IPD | 8 | 1866 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.66, 1.02] |
| 21.2 AD | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.09, 0.65] |
| 22 Admission to a special care baby unit ‐ IPD only | 6 | 1910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.10] |
| 23 Any baby required assisted ventilation ‐ IPD only | 5 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.20, 1.22] |
6.5. Analysis.
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 5 Severe hypertension PARIS definition ‐ IPD only.
6.6. Analysis.
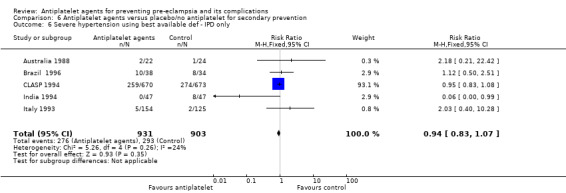
Comparison 6 Antiplatelet agents versus placebo/no antiplatelet for secondary prevention, Outcome 6 Severe hypertension using best available def ‐ IPD only.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Algeria 2011.
| Methods | Randomised controlled trial. | |
| Participants | 164 primiparous women consulting before the 10th week of amenorrhoea without previous vasculo‐renal pathology. Excluded: counter‐indication to use of aspirin, chronic arterial hypertension before pregnancy, chronic nephropathy, known auto‐immune disorder, twin pregnancies, diabetes. |
|
| Interventions | Exp: aspirin 100 mg/day, taken in the evening from 8‐10 weeks' gestation to 36 weeks' gestation Control: unclear ‐ refer to the non‐treated group most of the time, but refer to placebo group once in paper. |
|
| Outcomes | Women: maternal complications (gestation hypertensive disorders). Babies: gestation length, newborn weight, fetal complications. |
|
| Notes | Trial conducted in Blida Hospital, Algeria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomization for treatment (using anonymous sealed envelopes)" Insufficient information about the sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Quote:"anonymous sealed envelopes". No information about whether opaque or numbered. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote:"The patient and the study promoter were not blinded to the conditions of treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no missing outcome data, i.e. number randomised = number reported in outcomes. |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all expected outcomes are reported. |
| Other bias | Unclear risk | Baseline characteristics: Quote:"The control and treated groups were not statistically different with respect to age, body mass index, gestity and parity (Table 1)." However, did not report baseline BP measures. |
ASPRE 2017.
| Methods | Randomised controlled trial. Randomisation using web‐based system (sealed envelope). Stratification by site. | |
| Participants | 1776 women age of 18 years or more, singleton pregnancy, live fetus at the time that scanning was performed at 11 to 13 weeks of gestation, and a high risk (> 1 in 100) for preterm pre‐eclampsia according to a screening algorithm Exclusion criteria: unconscious or severely ill status, learning difficulties or serious mental illness, major fetal abnormality identified at the time that scanning was performed at 11 to 13 weeks of gestation, regular treatment with aspirin within 28 days before screening, bleeding disorder such as von Willebrand’s disease, peptic ulceration, hypersensitivity to aspirin, long‐term use of nonsteroidal antiinflammatory medication, and participation in another drug trial within 28 days before screening |
|
| Interventions | Intervention: aspirin at a dose of 150 mg per day that was administered from 11 to 14 weeks of gestation until 36 weeks of gestation Control: placebo |
|
| Outcomes | Primary outcome: delivery with pre‐eclampsia before 37 weeks of gestation Secondary outcomes: adverse outcomes of pregnancy before 34 weeks of gestation, before 37 weeks of gestation, and at or after 37 weeks of gestation; stillbirth or neonatal death; death and neonatal complications; neonatal therapy; and poor fetal growth (birth weight below the 3rd, 5th, or 10th percentile) |
|
| Notes | Multicentre trial at 13 maternity hospitals in the UK, Spain, Italy, Belgium, Greece and Israel. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“randomly assigned, in a 1:1 ratio, with the use of a Web‐based system (Sealed Envelope)”; stratified by participating centre |
| Allocation concealment (selection bias) | Low risk | Quote:“When randomised, participants will be assigned a randomisation code. The randomisation codes will determine who receives placebo or aspirin 150 mg. The investigational medicinal product (IMP) supplier (Mawdsley Brooks and Co) will keep and store the randomisation code list.” Aspirin and placebo tablets were identical and were packaged, labelled, stored, and distributed by Mawdsley‐Brooks. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:“double‐blind, placebo‐controlled” Quote:“The placebo tablets were identical to the aspirin tablets with respect to variables such as size, thickness, physical properties, and appearance.” Quote:“Mawdsley Brooks and Co will provide labelling (for all cartons and blister sheets) ensuring complete blinding of the IMP to all investigators and participants in the study, which includes the principal investigator, participating research doctors, pharmacists at the local clinical trial pharmacy, project managers and others involved in the trial. They are all blinded to the IMP allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 1776 women who were randomised, 152 (8.6%) later withdrew consent (78 from aspirin group and 74 from placebo group) and 4 were lost to follow‐up (2 from each group). Quote:"Statistical analyses were performed on an intention‐to‐treat basis” |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the published study protocol are reported in the results publication. |
| Other bias | Low risk | Quote:“There were no significant differences between the aspirin group and the placebo group with regard to the characteristics of the participants at baseline” |
Australia 1988.
| Methods | Women given an identification number at trial entry, with randomisation in the hospital pharmacy using a random‐number sequence linked to this number. | |
| Participants | 46 women with singleton pregnancy at 28‐36 weeks and concern about fetal welfare, in whom umbilical artery velocity waveform systolic/diastolic ratio > 95th centile. Excluded if DBP > 110 mmHg or > 90 mmHg with proteinuria, and if maternal condition likely to lead to delivery. | |
| Interventions | Exp: aspirin 150 mg daily. Control: placebo. | |
| Outcomes | Women: caesarean section; induction; placental weight. Babies: stillbirth; neonatal death; ventilation; admission to SCBU; cerebroventricular haemorrhage; birthweight; gestation at delivery; head circumference; Apgar scores. | |
| Notes | Women divided into 2 groups: high umbilical artery systolic/diastolic ratio (> 95th but < 99.5th centile) and extreme umbilical artery systolic/diastolic ratio (> 99.5th centile). Data incomplete for second group, so only included if available for all women. Continuous data only presented for some outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women given an identification number at trial entry, with randomisation in the hospital pharmacy using a random‐number sequence linked to this number. |
| Allocation concealment (selection bias) | Low risk | Patients were allocated by series of random numbers to receive aspirin or placebo tablets, which were supplied by company Faulding & Co. Quote:"Capsules were in numbered envelopes and people handing out the capsules were blinded to their contents." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"The patient, fetal welfare laboratory staff and attending obstetrician were all blind to the treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attritions/exclusions to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information ‐ protocol not available. Maternal outcomes not reported, e.g. pre‐eclampsia. |
| Other bias | Unclear risk | Baseline characteristics: Quote:"The patients in the two groups were comparable in terms of maternal age, parity, and principal reason for study" (But level of significance not specified). Placebo group had 5/24 (21%) vs treatment group 2/22 (9%) women with poor obstetric history. |
Australia 1993.
| Methods | Randomised. capsules dispensed by pharmacy. | |
| Participants | 110 women at 12‐24 weeks with either DBP >/= 90 or SBP >/= 140, or a history of PE. | |
| Interventions | Exp: aspirin 100 mg/day. Control: placebo. | |
| Outcomes | Women: PE. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables used. |
| Allocation concealment (selection bias) | Low risk | Dispensed by pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Authors confirmed identical placebo used during PARIS risk of bias assessment, however no further information available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attritions/exclusions to permit judgement ‐ number randomised not stated, details of any missing data not provided. |
| Selective reporting (reporting bias) | Unclear risk | The 3 primary outcomes pre‐specified in the protocol are reported BUT key outcome reported in paper NOT pre‐specified, i.e. mean days spent in hospital. |
| Other bias | Low risk | Article text and Table 1 indicate that there were no significant differences between either group in terms of baseline maternal details. |
Australia 1995.
| Methods | Instructions about the tablets in numbered, sealed opaque envelopes. Women shown 5 envelopes and asked to choose 1. | |
| Participants | 51 women at 28‐36 weeks with ultrasound diagnosis of restricted fetal growth, umbilical artery. Doppler systolic/diastolic ratio > 95 centile. No previous aspirin during pregnancy. | |
| Interventions | Exp: 100 mg aspirin. Control: starch tablets. | |
| Outcomes | Women: none. Babies: mean gestation at birth; birthweight (< 3 and 10 centile); Apgar 5 minutes; admission SCBU; IVH. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables used. |
| Allocation concealment (selection bias) | Low risk | Instructions about the tablets in numbered, sealed opaque envelopes. Women shown 5 envelopes and asked to choose 1. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Both the women and the staff were blinded to the contents of the tablets". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attritions/exclusions to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information ‐ protocol not available. Maternal outcomes not reported, e.g. pre‐eclampsia. |
| Other bias | High risk | Interim analysis performed ‐ DMC stopped the trial at that time because the findings did not support continuation. Table 1 of article indicates that there were no significant differences in baseline characteristics. |
Australia 1995a.
| Methods | Randomised by quote:"envelope method". 1/21 women (5%) excluded as miscarriage at 20 weeks. | |
| Participants | 21 women with renal disease. 20 had previous early onset PE. Commenced therapy at 14 weeks' gestation. | |
| Interventions | Exp: dipyridamole 75‐100 mg x 4/day + subcutaneous heparin 7500 units x 2/day. Control: no treatment. | |
| Outcomes | Women: hypertension; proteinuria; 'complications'; caesarean section. Babies: neonatal death; premature birth (< 37 weeks); IUGR (< 10th centile). | |
| Notes | Trial stopped early on advice of 'ad hoc' committee, due to complications in control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly sorted envelopes with equal treatment and control cards inside, then numbered envelopes. |
| Allocation concealment (selection bias) | Low risk | PARIS assessment based on unpublished information: opaque, sequentially numbered, sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Although not stated explicitly, it seems reasonable to assume that the treatment was not blinded due to the daily administering of aspirin to the test group and no treatment to the control group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 out of the 21 women (5%) was excluded from the analysis when she miscarried at 20 weeks due to cervical incompetence. |
| Selective reporting (reporting bias) | Low risk | Data collection sheet available. Appears that all outcomes were reported. |
| Other bias | High risk | Trial was terminated in view of the high complication rate in the control group. Article: Quote:"The two groups were well matched in terms of previous pregnancy complications (p>0.40) and outcome and on the basis of underlying renal disease". |
Australia 1996.
| Methods | Quote:'"Double‐blind randomised trial". | |
| Participants | 52 primigravid women with abnormal uterine artery waveforms on doppler examination at 22‐24 weeks. | |
| Interventions | Exp: aspirin 60 mg/day. Control: placebo. | |
| Outcomes | Women: GH; PE; caesarean section; abruption. Babies: death; preterm birth (< 37 weeks); IUGR (< 10 centile); admission SCBU. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Drug company supplied sequentially numbered packs. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. Quote:"Outcomes were collected and categorized before the randomization code was broken" PARIS assessment noted that placebo was dispensed in identical blister packs. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information available to assess, no information regarding missing outcome data reported in paper. |
| Selective reporting (reporting bias) | High risk | Did not report on primary outcome perinatal death. |
| Other bias | Unclear risk | Baseline characteristics: Quote:"There were no significant differences in maternal age or booking BP. Although there was a statistically significant difference in the GA at enrollment, (18.8 vs 18.3 weeks) this was of no clinical relevance". |
Australia 1996a.
| Methods | Randomisation by taking the next in a series of number identical blister packs. 2 women withdrew, 1 from each group. | |
| Participants | 104 primiparous women with abnormal uterine doppler flow at 18 weeks (systolic/diastolic ratio > 3.3 or S/D > 3 and early diastolic notch). Selected from 955 women screened, of whom 186 had abnormal waveforms. | |
| Interventions | Exp: aspirin 100 mg/day. Control: placebo. | |
| Outcomes | Women: GH; PE; eclampsia; APH. Babies: preterm birth; SGA. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Randomisation by taking the next in a series of numbered identical blister packs. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Authors noted during PARIS risk of bias assessment that tablets were prepared in a central pharmacy to ensure double blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women withdrew, 1 from each group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information ‐ only conference abstract available. |
| Other bias | Unclear risk | Insufficient information ‐ only conference abstract available. |
Australia 1997.
| Methods | Allocated by a series of random numbers. 10% (12/120) of women were excluded as they withdrew before starting treatment. | |
| Participants | 120 women at high risk of PE because of 1 of the following: pre‐existing hypertension (BP greater >/= 140/90 prior to pregnancy on at least 2 occasions, or on antihypertensive therapy), renal disease, previous early severe PE. Excluded if aspirin allergy, aspirin‐sensitive asthma, pre‐existing bleeding diathesis or multiple pregnancy. | |
| Interventions | Exp: aspirin 100 mg modified release daily from 17‐19 weeks until delivery. Control: placebo. | |
| Outcomes | Women: proteinuria; duration of pregnancy; indications for and mode of delivery; maximum antenatal BP; 'complications'. Babies: perinatal death; birthweight; Apgar scores. | |
| Notes | . | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated by a series of random numbers. |
| Allocation concealment (selection bias) | Low risk | Women were allocated by series of random numbers to receive aspirin or placebo tablets, which were supplied by company Faulding & Co. Article: Quote:"Capsules were in numbered envelopes and people handing out the capsules were blinded to their contents". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"double blinded." Authors noted during PARIS risk of bias assessment that the control arm received capsules containing sucrose. Not known whether active and placebo tablets were identical. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote:"Twelve subjects who withdrew prior to commencement of therapy are excluded from analysis, leaving 108 subjects (aspirin = 58, placebo = 50)." Analysis by ITT. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section of the paper are reported. However, no access to protocol. |
| Other bias | Unclear risk | No imbalance in baseline characteristics. Aspirin group ‐ 4 intrauterine deaths (none in placebo group). Birthweights calculated after removal of values for the 4 fetuses who died in utero in the aspirin‐treated group (and whose true weights could not be accurately assessed), means that quote:"results presented here are likely to be skewed in favour of the aspirin‐treated group". |
Austria 1992.
| Methods | Randomised to coded packages of medication; assessment of primary outcome blinded. | |
| Participants | 41 primigravid women with positive roll‐over test (increase of 20 mmHg in DBP) at 28‐32 weeks. Exclusions: existing hypertension, renal gut lung or heart disease, IUGR, impending preterm birth. | |
| Interventions | Exp: aspirin 80 mg/day until 37 weeks. Control: placebo. | |
| Outcomes | Women: GH; PE; caesarean section; preterm birth (37 weeks). Babies: stillbirths; neonatal death; SGA (< 10th centile); neonatal bleeding; admission to SCBU. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised‐generated randomisation lists. |
| Allocation concealment (selection bias) | Low risk | Pharmacy organised, coded packets of medication. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"double‐blind." Placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information regarding missing outcome data reported in paper. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes specified in the methods section of the paper are reported. |
| Other bias | Unclear risk | Baseline characteristics: mean age well correlated (24.7 years aspirin; 24.3 yrs placebo); no multiple pregnancies; but no information about other baseline characteristics. |
Barbados 1998.
| Methods | Single‐centre, treatment packs randomly numbered by computer in clinic and dispensed by pharmacist. 55/3697 women (1.5%) excluded after randomisation: 42 because of pack labelling errors, 8 not pregnant and 6 lost to follow‐up. | |
| Participants | 3697 women at 12‐32 weeks' gestation. Excluded if: increased risk of bleeding, aspirin allergy, high likelihood of immediate delivery, or previous placental abruption. | |
| Interventions | Exp: aspirin 75 mg controlled release daily until delivery. Control: placebo. | |
| Outcomes | Women: PE; APH; PPH; caesarean section; duration of pregnancy; use of antihypertensives and anticonvulsants. Babies: stillbirth; death before hospital discharge; days in SCBU; bleeding problems; birthweight. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list with minimisation. |
| Allocation concealment (selection bias) | Low risk | Study pharmacist dispensed treatment packs. Code for randomisation kept in Oxford. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"All staff in Barbados were blind to the treatment allocation, the randomisation code being kept in Oxford." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 55/3697 women (1.5%) excluded after randomisation: 42 because of pack labelling errors, 8 not pregnant and 6 lost to follow‐up. Quote:"All but three women from each group were followed up successfully." Quote:"Analyses were to be by intention‐to‐treat for all women randomised appropriately." |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes in protocol are reported in the paper. |
| Other bias | Low risk | Table 1 of article shows baseline characteristics were well balanced between groups. |
Brazil 1996.
| Methods | Central telephone randomisation; 39/1009 women (4%) lost to follow‐up. | |
| Participants | 1009 women at 12‐32 weeks' gestation (mean 22, 41% = or < 20 weeks) quote:"who the obstetrician thought were at risk" of PE ‐ generally low/moderate risk (primiparous 47%, chronic hypertension 47%, diabetes 6%). Excluded if bleeding risk, asthma, allergy to aspirin, gastric ulcer, placenta praevia. | |
| Interventions | Exp: aspirin 60 mg/day. Control: placebo. | |
| Outcomes | Women: PE; caesarean section; APH. Babies: SGA; perinatal death; preterm birth; neonatal bleeding. | |
| Notes | Conducted in 12 university teaching hospitals and 182 obstetric offices. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Computer‐generated randomisation lists prepared by the Clinical Trials Service Unit, Oxford University." |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation: Quote:"Entry to the study was attained by telephoning central 24 h service at Escola Paulista de Medicina in Sao Paolo". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"double‐blind", placebo used; Quote:"The actual contents of the allocated study treatment were not revealed, even after delivery, unless there was a clear medical reason for the treatment to be made known". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 39/1009 women (4%) lost to follow‐up: follow‐up was obtained for 96% (476 allocated aspirin and 494 allocated placebo); quote:"after randomisation, no woman was excluded from the trial"; ITT analyses. Secondary outcome bleeding: quote:"All bleeds after delivery were not explicitly recorded and were incompletely reported (overall rate of 0.9% compared with 26% in CLASP), but maternal transfusions were systematically sought". |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section of the paper are reported. However, no access to protocol. |
| Other bias | Low risk | No imbalance in baseline characteristics: quote:"good balance between the treatment groups for the main pre‐randomisation characteristics". |
Brazil 1992a.
| Methods | Women randomly divided into 2 groups. No further information available. Blinding not reported. Outcome not reported for 4/56 women (7%). | |
| Participants | 56 women in 2nd or 3rd quarter of pregnancy who were young primigravidas, or had chronic HT, diabetes, previous PIH, twin pregnancy, or a family history of HT. | |
| Interventions | Exp: acetylsalicylic acid 60 mg/day in a solution of 50% D‐lisine. Control: no intervention. | |
| Outcomes | Women: GH. Babies: death, birthweight (mean). | |
| Notes | Original article published in Portuguese. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women randomly divided into 2 groups. No further information available. |
| Allocation concealment (selection bias) | Unclear risk | Women randomly divided into 2 groups. No further information available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome not reported for 4/56 women (7%); ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement ‐ no protocol available. |
| Other bias | Unclear risk | Insufficient information to permit judgement. |
Brazil 2006.
| Methods | Quote:"randomised, double‐blind placebo‐controlled study." | |
| Participants | 49 pregnant women with chronic hypertension, abnormal uterine artery Doppler ultrasound, high resistance index and/or diastolic notch, 20‐28 weeks' gestation. | |
| Interventions | Exp: aspirin 100 mg/day and calcium 2 g/day. Control: placebo. |
|
| Outcomes | Women: pre‐eclampsia, duration of pregnancy. Babies: prematurity, IUGR, birthweight. |
|
| Notes | Abstracts only ‐ no outcome data available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation. No further information. |
| Allocation concealment (selection bias) | Unclear risk | Quote:"randomised, double‐blind placebo‐controlled study". No further information. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind and placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Completeness of follow‐up unclear. Abstracts only available ‐ do not report attritions/exclusions. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement ‐ abstracts only available. |
| Other bias | Unclear risk | Insufficient information to permit judgement ‐ abstracts only available. |
Canada 2017.
| Methods | Randomised | |
| Participants | Women with a twin pregnancy between 10 and 14 weeks' gestation | |
| Interventions | Aspirin 81 mg at bedtime vs placebo | |
| Outcomes | Birthweight of live infants; very low birthweight; pre‐eclampsia; platelet aggregation test | |
| Notes | Published as an abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as 'quadruple' blind (Participant, Care Provider, Investigator, Outcomes Assessor) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Published as abstract only |
China 1996.
| Methods | Quote:"Prospective randomised double‐blind study." | |
| Participants | 84 women with a singleton pregnancy at high risk of IUGR, and 28‐34 weeks' gestation. | |
| Interventions | Exp: 75 mg aspirin, from 28‐34 weeks for 6‐8 weeks. Control: placebo. | |
| Outcomes | Women: PIH; caesarean section; preterm delivery. Babies: neonatal death; IUGR; IVH. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Opaque, sequentially numbered, sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"double‐blind" and placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attritions/exclusions to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. All outcomes specified in methods section appear to be reported except pre‐eclampsia ‐ simply say aspirin quote:"may decrease the risk of feto‐placental circulation, raise the infant birthweight and prevent IUGR or preeclampsia" BUT results not shown in table. |
| Other bias | Low risk | No imbalance in baseline characteristics: quote:"The patients were principally nulliparous; and their age and gravida, and the distribution of high risk factors of IUGR were not significantly different between the two groups". |
China 1996a.
| Methods | Random allocation. No further information. Not blinded and no information on completeness of follow‐up. | |
| Participants | 104 women at 20 to 38 weeks' gestation. Inclusion criteria: older primiparous; multiparous with history of severe PIH; obesity; MAP > 12 kPa; Hb < 8; PCV > 0.37; family history of HT or PIH in mother or sister. | |
| Interventions | Exp: aspirin 50 mg/day for 3 to 5 weeks. Control: no intervention. | |
| Outcomes | Women: GH; oligohydramnios; mode of delivery (numbers not reported); PPH (numbers not reported) biochemical markers. Babies: mean birthweight. | |
| Notes | Single‐centre. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation. No further information. |
| Allocation concealment (selection bias) | Unclear risk | Random allocation. No further information. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on completeness of follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement ‐ no protocol available. |
| Other bias | Unclear risk | Insufficient information to permit judgement. |
China 1999.
| Methods | Randomisation by offering participant 5 sealed envelopes (2 aspirin, 2 calcium, 1 placebo). 132 women allocated aspirin, 154 calcium and 83 control (total 369). Women allocated calcium excluded from this review. 22 women lost to follow‐up (14 aspirin, 8 control). | |
| Participants | 215 primigravid women with MAP > 80 and < 106 early in 2nd trimester and MAP > 60 at 22‐24 weeks. | |
| Interventions | Exp: aspirin 80 mg/day until delivery. Control: unclear, no placebo mentioned. | |
| Outcomes | Women: GH; PE; eclampsia; caesarean section. Babies: gestation at delivery (mean); birthweight; Apgar scores. | |
| Notes | Authors provided additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple, i.e. coin toss. |
| Allocation concealment (selection bias) | Low risk | Opaque, sequentially numbered, sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"All randomization, data collection and data entry were undertaken by the same research assistant with the exception of outcome data, which were entered by the first two authors. The research assistant was therefore blinded to the outcome group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 women lost to follow‐up (14 aspirin, 8 control). 50 of the original 500 patients (105) eventually delivered in other hospitals and were therefore not subjected to analysis, as they could not reliably be classified into the 3 outcome groups. Quote:"Although there was no significant difference in the number of dropouts in the different randomization groups, the eventual distribution of cases was far from the 2:2:1 expected. The distribution of MAP and gestational age in the three randomized groups was therefore tested to ensure no systemic bias had occurred." |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section of the paper are reported. However, no access to protocol. |
| Other bias | Low risk | No imbalance in baseline characteristics: Table 1 shows no significant differences in maternal characteristics at time of randomisation. Quote:"The unequal numbers of patients in the two treatment groups suggests that even after allowing for the differential drop‐out rates, something went wrong with the randomization process; possibly a tendency among patients to select envelopes from 1 particular part of the pile offered to them. Nevertheless, the Kruskal‐Wallis analysis of variance showed no significant differences between groups for left lateral MAP or for gestational age at testing, suggesting that no systematic bias was introduced by the randomization procedure." |
CLASP 1994.
| Methods | By telephoning a central computerised randomisation service. 0.6% (55/9364) lost to follow‐up. International multicentre study conducted at 213 hospitals in 16 countries. Follow‐up of surviving children with GP letter at 12 months in the UK (4688 with 4675 alive at 12 months) and parental questionnaire at 18 months in the UK and Canada (410 with 407 alive at 18 months). For GP letter, 89% response rate, for parental questionnaire 86% responded. | |
| Participants | 9364 women at 12‐32 weeks' gestation at risk of PE or IUGR, or women with established PE or IUGR. | |
| Interventions | Exp: aspirin 60 mg daily until delivery. Control: placebo. | |
| Outcomes | Women: death; eclampsia; PE; bleeding complications; caesarean section; induction; problems with epidural analgesia; PPH; transfusion; use of antihypertensives or anticonvulsants; compliance. Babies: stillbirth; neonatal death; mortality at 1 year; birthweight (mean) and centile (< 3rd); gestation at delivery; admission to SCBU; IVH; other neonatal bleeding. Follow‐up at 12‐18 months: developmental delay; congenital malformations; respiratory problems; hospital admissions. | |
| Notes | Compliance: 96% started treatment, 88% took it for at least 80% of the time from entry to delivery. For some outcomes data not presented separately for prophylaxis and treatment. Follow‐up data only available for centres in the UK and Ottawa, Canada. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation; computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Quote:"by clinical staff telephoning a central 24‐hour service at the Clinical Trial Service Unit in Oxford" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"study treatment was not revealed, even after delivery, unless there was a clear medical or personal reason for the treatment to be made known" Quote:"Two senior investigators, unaware of the treatment groups of the babies' mothers, classified all deaths" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0.6% (55/9364) lost to follow‐up. Quote:"Post‐delivery follow‐up forms were obtained for 9309 (99.4%; 4659 aspirin‐allocated and 4650 placebo‐allocated) randomised women" Quote:"After randomisation, no woman was excluded from the trial"; ITT analyses. |
| Selective reporting (reporting bias) | Low risk | Protocol available. All pre‐specified primary and secondary outcomes are reported in the paper. |
| Other bias | Low risk | No imbalance in baseline characteristics: Quote:"A minimisation algorithm was used to limit differences between the treatment groups for certain prognostic baseline variables" Quote:"9364 women were randomised, with good balance between the treatment groups for the main prerandomisation prognostic factors recorded" |
Colorado 1993.
| Methods | Quote:"Randomised" ‐ no further information; completeness of follow‐up unclear. | |
| Participants | 100 nulliparous women with multiple pregnancy in quote:"early pregnancy". | |
| Interventions | Exp: aspirin 81 mg/day. Control: placebo. | |
| Outcomes | Women: GH; PE. Babies: none reported. | |
| Notes | Multicentre trial, stopped early due to slow recruitment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Randomised" ‐ no further information. |
| Allocation concealment (selection bias) | Unclear risk | Quote:"A randomised controlled double‐blind trial" ‐ no further information. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"double‐blind"; placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Completeness of follow‐up unclear. Letter only available ‐ does not report attritions/exclusions. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement ‐ letter only available. |
| Other bias | High risk | Stopped early due to slow recruitment. |
Egypt 2005.
| Methods | Computer‐generated list of random numbers. Numbers then placed in sealed envelopes, and women asked to choose 1 envelope. Randomisation, drug prescription, and allocation key kept by 1 author with no role in participant follow‐up or outcome assessment. 3 women (2%) lost to follow‐up (1 aspirin, 2 control). | |
| Participants | 139 women at 14‐16 weeks' gestation with abnormal uterine artery doppler (diastolic notch or resistance index > 90th percentile) and other risk factors for PE (previous history of PE/IUGR, essential HT, positive family history, underlying vascular disease, age < 20 years or > 40 years or gestational diabetes). Excluded: allergy to aspirin, peptic ulcer, other hepatic, renal, cardiovascular or thyroid disorder. | |
| Interventions | Exp: aspirin 75 mg/day. Control: no treatment. | |
| Outcomes | Women: PE (BP >/= 140/90 plus proteinuria > 300 mg/day); PE onset < 37 weeks; severe PE (BP > 160/110, proteinuria > 2 g, urine output < 500 mL/day, platelets < 100000/cmm, elevated liver enzymes); maternal bleeding. Babies: SGA (< 10th percentile); preterm birth; birthweight; neonatal bleeding; Apgar score at 1 and 5 minutes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Randomization was done using random number generated through a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote:"Computer‐generated list of random numbers. Numbers then placed in sealed envelopes, and women asked to choose 1 envelope. Randomisation, drug prescription, and allocation key kept by 1 author with no role in participant follow up or outcome assessment". |
| Blinding (performance bias and detection bias) All outcomes | High risk | Although not stated explicitly, it seems reasonable to assume that the treatment was not blinded due to the daily administering of aspirin to the test group and no treatment to the control group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 women (2%) lost to follow‐up (1 aspirin, 2 control). Quote:"136 cases succeeded to complete the follow‐up period, whereas 3 cases were lost. All patients were analysed in the group to which they were allocated (intention‐to‐treat analysis) and lost patients were analysed, using either the last follow‐up report or applying the worst patient scenario (patients given aspirin had the worst outcomes and those with no treatment had the best outcomes). Only 1 patient was lost from the aspirin group, whereas the other 2 patients were from the control group. For all patients, the follow‐up was lost after 37 weeks." |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section of the paper are reported. However, no access to protocol. |
| Other bias | Low risk | No significant differences in baseline characteristics. |
EPREDA 1991.
| Methods | Randomised by centre with stratification for 1 or 2 previous poor outcomes. 1 woman excluded after randomisation. | |
| Participants | 323 women at 15‐18 weeks' gestation with poor outcome during previous 2 pregnancies, at least 1 being IUGR, or IUGR in 1 previous pregnancy. Excluded: twins, uterine malformation, renal disease, secondary hypertension, diabetes, cardiac disease. | |
| Interventions | Study 1: exp: aspirin 150 mg daily, or aspirin 150 mg plus dipyridamole 225 mg daily. Control: placebo. Study 2: exp: aspirin 150 mg and dipyridamole 225 mg daily. Control: aspirin 150 mg daily. | |
| Outcomes | Women: death; DBP > 90 mmHg; proteinuria; abruption; caesarean section < 34 weeks; quote:"poor outcome". Babies: stillbirth; neonatal death; ventilation; transfer to intensive care; birthweight < 10th centile; duration of hospital stay (mean). | |
| Notes | Two separate comparisons within the one study. Only data for study 1 ('Trial A' in report) included in the review, relating to 230 women. Study 1 has 3 arms. Data for 2 antiplatelet arms combined versus control. For comparison of subgroup analysis based on dose, data presented under aspirin > 75 mg plus dipyridamole. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted blocks; quote:"randomisation by centre allowed the ratio between treated and placebo patients to be the same in each centre, and precluded a bias introduced by centre differences". |
| Allocation concealment (selection bias) | Low risk | Opaque, sequentially‐numbered, sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"double‐blind" and placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 woman excluded after randomisation. Quote:"All patients for whom the endpoint was known were included in the analysis, even if treatment was withdrawn" "The endpoint is not known for 5 patients (3 spontaneous abortions before 20 weeks, 1 therapeutic abortion because of ultrasonographically diagnosed malformations, and 1 lost to follow‐up)", and it is unclear which groups they belonged to. Treatment was also withdrawn from a further 22 women, and it is not clear whether these were balanced across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section of the paper are reported. We have access to the protocol, however it is in French. |
| Other bias | Low risk | No imbalance in baseline characteristics: quote:"The study groups were comparable for ethnic origin, social and professional status, parity (average 2.2 to 2.4), and other characteristics (table 1). A detailed analysis of the number and nature of previous poor outcomes revealed no differences between the groups" |
ERASME 2003.
| Methods | Multicentre, 28 centres in France and 1 in Belgium. Computer‐generated randomisation codes, stratified by centre in blocks of 8. Allocation via online 24 hour computer. | |
| Participants | 3294 primiparous women at 14‐20 weeks' gestation. Singleton or multiple pregnancy. Excluded: known HT, indication or contraindication to aspirin. |
|
| Interventions | Exp: aspirin 100 mg to 34 weeks. Control: placebo. | |
| Outcomes | Women: PIH; PE; placental abruption; caesarean section; induction; HELLP; PPH; hospital admission; side effects. Babies: stillbirth; neonatal death; SGA (< 10th and < 3rd centile); neonatal IVH; other bleeding; admission SCBU. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation codes, stratified by centre in blocks of 8. Quote:"A randomisation list was computer‐generated by the manufacturer before the study began. To limit the difference in group size within each centre, this list was specific for each centre and balanced in blocks of eight. Futhermore, the box numbers were thereafter randomly mixed within the blocks of eight (with a random number table, independently generated at a server site at the Department of Pharmacology, Medical School of Lille), so that the numbers were not consecutive" |
| Allocation concealment (selection bias) | Low risk | Allocation via online 24‐hour computer. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"double‐blinded placebo‐controlled"; Quote:"the excipient and vanilla aroma were identical in both powders, making it impossible for patients to identify their treatment"; "The treatment was totally double‐blinded to co‐ordinators, investigators and patients. Except in the case of clear medical need, the treatment actually taken by the patients was not to be revealed until the blind was lifted, after the completion of the study ‐ including complete data entry" Quote:"Unblinding during pregnancy or in the immediate postpartum period became necessary for 82 patients (2.5%). Most often, this was related to the need for epidural analgesia or an endouterine procedure or the need to begin aspirin treatment. The distribution of these cases was significantly different between the groups (p = 0.01), because both bleeding (n = 12 vs n = 3) and allergic effects (n = 4 vs n = 0) were observed more often in the aspirin group" Quote:"Two independent examiners, blinded to the mother's treatment allocation, looked at the files of perinatal deaths" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote:"Analylsis was by intention‐to‐treat"; Quote:"Twenty patients (0.6%) were lost to follow up, 10 in each group. The inclusion characteristics of the patients lost to follow up and those studied did not differ significantly. Pregnancy outcome was known for 3274 patients" Quote:"Pregnancy outcome was finally analysed for 1645 children in the aspirin group and 1660 in the placebo group" |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section of the paper are reported. We have access to the protocol, however it is in French. |
| Other bias | Low risk | No imbalance in baseline characteristics: quote:"The general characteristics of the patients and the clinical prognostic factors collected at inclusion were comparable between the two groups for all criteria (Table 1). In particular, there was no difference between the groups for any factor known to be associated with the risk of pre‐eclampsia (maternal age, smoking, body mass index, blood pressure readings at inclusion)." |
Finland 1993.
| Methods | Sealed envelopes. Double‐blind. 5.3% (11/208) women excluded. 6 from aspirin group (1 miscarriage, 1 termination for anencephaly, 4 discontinued due to urticaria, raised AST, or prolonged bleeding time), 5 from placebo group (1 miscarriage, 3 discontinued due to raised AST or prolonged bleeding time, 1 lost to follow‐up). | |
| Participants | 208 women with pre‐existing hypertension (BP > 140/90 before pregnancy) or previous severe PE (in immediately preceding pregnancy), and 12‐18 weeks' gestation. Excluded: women proteinuric before pregnancy. | |
| Interventions | Exp: aspirin 50 mg daily. Control: placebo. | |
| Outcomes | Women: exacerbation of hypertension +/‐ proteinuria; caesarean section; blood loss at delivery (mean); hospitalisation during pregnancy; bleeding time and DBP at 36 weeks (mean). Babies: perinatal death; admission to SCBU; birthweight (mean); SGA; gestation at delivery. | |
| Notes | 3 centres. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"randomly allocated to the experimental (ASA) or placebo (PLA) groups by sealed envelopes"; insufficient information. |
| Allocation concealment (selection bias) | Low risk | PARIS assessment noted that opaque, sequentially numbered sealed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo used; quote:"The code was broken when all the data, including the results of laboratory analyses, were available" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5.3%(11/208) women excluded. 6 from aspirin group (1 miscarriage, 1 termination for anencephaly, 4 discontinued due to urticaria, raised AST, or prolonged bleeding time), 5 from placebo group (1 miscarriage, 3 discontinued due to raised AST or prolonged bleeding time, 1 lost to follow up) ‐ reasons similar and attrition balanced between groups. Quote:"Thus the end points could be assessed in 97 women taking ASA and 100 women taking placebo" |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section of the paper are reported. However, no access to protocol. |
| Other bias | Low risk | Baseline characteristics: quote:"The clinical characteristics of ASA and PLA groups before the treatment were comparable" (however levels of significance not stated). |
Finland 1997.
| Methods | Randomised | |
| Participants | 26 high‐risk women with uterine artery bilateral notches on doppler, at 22‐24 weeks. | |
| Interventions | Exp: aspirin 50 mg. Control: no treatment. | |
| Outcomes | Women: GH; PE; placental abruption; delivery < 37 weeks. Babies: stillbirth; IUGR (< 10th centile); IVH on ultrasound; gestation at delivery (mean); birthweight (mean). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Opaque sequentially numbered envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Although not stated explicitly, it seems reasonable to assume that the treatment was not blinded due to the daily administering of aspirin to the test group and no treatment to the control group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attritions/exclusions to permit judgement. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section of the paper are reported. However, no access to protocol. |
| Other bias | Unclear risk | No imbalance in baseline characteristics, but unable to assess some. Small sample size (26) |
Finland 1997a.
| Methods | 'Randomly allocated', code of medication broken once all data available. No further information. | |
| Participants | 66 women at around 5 weeks' gestation with a history of recurrent spontaneous miscarriage. | |
| Interventions | Exp: aspirin 50 mg/day, started as soon as pregnancy test positive. Control: placebo. | |
| Outcomes | Women: PE (BP >/= 140/90 plus proteinuria > 0.3 g/day); minor bleeding; abruption; mode of delivery; length of pregnancy; glucose tolerance. Baby: early fetal loss; SGA (< 10th percentile); birthweight. | |
| Notes | Almost one third of women had a miscarriage or ectopic pregnancy (10/33 aspirin vs 10/33 placebo). Therefore denominators for other pregnancy outcomes are based on women whose pregnancy continued beyond 20 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"randomly allocated"; no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Coded medication; no other information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo used. Quote:"The code of medication was broken when all clinical and biochemical data were available" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attritions/exclusions to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are not clearly stated in the methods section of the paper (or abstract/introduction); protocol not available. |
| Other bias | Unclear risk | Baseline characteristics: insufficient information to judge if these were balanced across both groups. |
Finland 2002.
| Methods | Randomisation in pharmacy. Code broken when last woman delivered. 4 women lost to follow‐up, 2 each group. | |
| Participants | 90 women at risk of PE or IUGR with abnormal uterine doppler. 12‐14 weeks' gestation. | |
| Interventions | Exp: aspirin 0.5 mg/kg/day. Control: placebo. | |
| Outcomes | Women: GH; PE; caesarean section. Babies: death; gestation at delivery (mean); birthweight < 2500 g; admission SCBU; IVH. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pharmacy organised randomisation; no other information provided. |
| Allocation concealment (selection bias) | Low risk | Pharmacy organised randomisation, code broken when last woman delivered. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo used. Quote:"The [randomisation] code was not broken until after the delivery of the last woman randomised" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 women lost to follow‐up, 2 each group. 43/45 of women on acetylsalicylic acid and 43/45 on placebo were successfully followed up: quote:"Two women in the acetylsalicylic acid group discontinued before second visit; 1 had urticaria and the other had itching in her throat. In the placebo group 1 woman moved to another town after the second visit and a second gave no reason for dropping out after the first visit" |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section of the paper are reported. However, no access to protocol. |
| Other bias | Low risk | No imbalance in baseline characteristics: quote:"No significant differences were found between the acetylsalicylic acid and placebo groups with regard to age, weight, parity, diastolic or systolic blood pressure, multiple pregnancy, previous IUGR, previous fetus mortus, chronic hypertension, diabetes, previous PIH or previous PE". |
Finland 2013.
| Methods | ||
| Participants | 152 women with risk factors for pre‐eclampsia and abnormal uterine artery Doppler velocimetry. Excluded: allergy to aspirin; tobacco smoking; multiple pregnancy; and a history of asthma, peptic ulcer, placental ablation, inflammatory bowel diseases, rheumatoid arthritis, haemophilia or thrombophilia. |
|
| Interventions | Exp: aspirin 100 mg/day from 12 + 0 to 13 + 6 weeks + days of gestation Control: placebo |
|
| Outcomes | Women: pre‐eclampsia, gestational hypertension, early‐onset pre‐eclampsia, severe pre‐eclampsia, preterm pre‐eclampsia, length of gestation. Babies: birthweight, small for gestational age |
|
| Notes | Trial conducted in maternity clinics in ten Finnish hospitals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"randomisation was made in blocks of tens by the pharmacists not otherwise involved in the study" |
| Allocation concealment (selection bias) | Low risk | Quote:"Tampere University Hospital Pharmacy performed the randomisation"; "aspirin and placebo tablets were prepared by a pharmaceutical company to appear identical"; Quote:"The randomisation code of each participant was sealed in an envelope and was opened after the outcome diagnoses of all participants has been set by the jury" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"double‐blinded"; identical placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 121/152 women completed the trial, i.e. 31 (20%) were left out: 4 of these women had a miscarriage (3 aspirin group; 1 placebo group); 11 women lost to follow‐up or discontinued for various non medical reasons (7 aspirin group, 4 placebo group); 5 women discontinued because of a medical condition (3 placebo group, 2 aspirin group); 11 participants additionally excluded from analysis because of noncompliance with the study protocol; Quote:"The results of our intention‐to‐treat analysis do not differ from the results of the analysis made without these excluded women" |
| Selective reporting (reporting bias) | High risk | In the trial registration document (ISRCTN14030412) over 40 primary outcomes are listed. The paper reports 3 primary outcomes, only 1 of which was also listed in the trial registration document. |
| Other bias | Low risk | Baseline characteristics: outlined in paper (Table 2) and appear similar |
France 1985.
| Methods | Quote:"Randomly allocated to group A or B", no other information available. 8.8% (9/102) excluded from analysis (2 controls lost to follow‐up, 4 treatment and 3 controls had a miscarriage before 16 weeks). | |
| Participants | 102 women at high risk of PE or IUGR; for example, if several previous complicated pregnancies or vascular risk factors such as essential hypertension (BP > 160/95) or a family history of hypertension. Excluded: women with secondary hypertension or known or suspected renal disease. | |
| Interventions | Exp: aspirin 150 mg and dipyridamole 300 mg daily, from 3 months until delivery. Control: no antiplatelet agent. | |
| Outcomes | Women: PIH (BP at least 140/85 mmHg; PE; caesarean section; abnormal bleeding during delivery or caesarean section; abruption; headache. Babies: stillbirth; neonatal death; fetal malformation; birthweight < 10th and < 3rd centile (livebirths only); haemorrhagic complication (undefined). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Randomly allocated to group A or B"; no other information available. |
| Allocation concealment (selection bias) | Unclear risk | Quote:"Randomly allocated to group A or B"; no other information available. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote:"For ethical reasons the study was not double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.8% (9/102) excluded from analysis (2 controls lost to follow‐up, 4 treatment and 3 controls had a miscarriage before 16 weeks); attrition balanced across groups. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported. However, no access to protocol. |
| Other bias | Low risk | Quote:"Our empirical selection of patients did not lead to a very homogenous population, but the heterogeneity was distributed evenly between the groups (table 1), so it is unlikely to bias results." |
France 1990.
| Methods | Quote:"Randomised study", no other information given. | |
| Participants | 91 women at high risk of PIH because of previous early onset PE, severe IUGR or fetal death due to placental insufficiency; at 16 weeks' gestation. | |
| Interventions | Exp: aspirin 100 mg and dipyridamole 300 mg daily until delivery. Control: no treatment. | |
| Outcomes | Women: GH +/‐; duration of pregnancy (mean). Babies: fetal death; birthweight (mean). | |
| Notes | Published in abstract form only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Randomised study", no other information given. |
| Allocation concealment (selection bias) | Unclear risk | Quote:"Randomised study", no other information given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of allocation not mentioned in article; Although not stated explicitly, it seems reasonable to assume that the treatment was not blinded due to the daily administering of aspirin + dipyridamole to the test group and no treatment to the control group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attritions/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement ‐ conference abstract only available. |
| Other bias | Low risk | Baseline characteristics: quote:"Women in these two groups were similar in age, parity and previous obstetrical complications". |
Germany 1995.
| Methods | prospective, randomised, double‐blind, placebo‐control study | |
| Participants | 160 pregnant women at 24 to 38 weeks' gestation at risk of developing PE (+7 patients with manifest PE or PIH excluded from this analysis) | |
| Interventions | Exp: 3 x 100 mg Trapidil per day by mouth Control: placebo |
|
| Outcomes | Women: pre‐eclampsia, pre‐term delivery, 6‐keto‐PGF, TXB Babies: IUGR |
|
| Notes | Translated from German to English. Trapidil is an antiplatelet, generic name: triazolopyrimidine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 167 women enrolled in the trial (including the 7 women with manifest pre‐eclampsia or pregnancy‐induced hypertension excluded from this analysis): quote:"9 women from the Trapidil group and 7 from the placebo group dropped out of the treatment early for various reasons. The reasons given in the two groups were comparable and non‐specific, so a subjective component to tolerance of pharmaceuticals during pregnancy can be assumed." |
| Selective reporting (reporting bias) | Low risk | Protocol not available. However, all expected outcomes appear to be reported. |
| Other bias | Unclear risk | Average age of patients in the Trapidil group was 26.1 years (19‐42 years) and the average age in the placebo group was 25.9 years (18‐39 years). Unclear whether there were imbalances in any other baseline characteristics. |
Germany 2000.
| Methods | Computer‐generated random sequence. Blister packs, and the code held separately from person doing randomisation. | |
| Participants | 43 women with singleton pregnancy, < 20 weeks' gestation with early IUGR, impaired uteroplacental flow, chronic HT, or history of IUGR, stillbirth, or PE. Excluded: diabetes, pre‐existing HT or proteinuria, fetal malformation. | |
| Interventions | Exp: aspirin 100 mg/day. Control: placebo. | |
| Outcomes | Women: PE. Babies: gestation at birth (mean); birthweight (mean). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated self written Fortran program from separate university department |
| Allocation concealment (selection bias) | Low risk | Randomisation carried out by separate university department, i.e. the Department of Biometry and Medical Documentation, University of Ulm. Opaque sequentially‐numbered sealed envelopes and Identical coded blister packs, code only known to packer and statistician. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"double blind"; placebo (identical blister packs) used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis. Insufficient reporting of attritions/exclusions to permit judgement. |
| Selective reporting (reporting bias) | Low risk | Protocol not available. Outcomes not clearly specified in methods section or elsewhere although key outcomes appear to be reported. |
| Other bias | Unclear risk | No imbalance in baseline characteristics, but unable to assess some. Quote:"The PI [Pulsatility Index] did not differ below 38 weeks and the only significant finding at 38 weeks may be spurious, considering the large number of significance tests performed" |
India 1991.
| Methods | Quote: "randomized, controlled prospective trial" | |
| Participants | 200 women at risk of IUGR based on historical factors (Group I), or have evidence of IUGR in current pregnancy on the basis of clinical and/or sonological measurements (Group II). Excluded: women with IUGR with fetal abnormality, Rh‐iso‐immunisation, multiple pregnancy with hydramnios, patients with a history of haemorrhagic disease or thromboembolism, history of APH in present pregnancy |
|
| Interventions | Exp: dipyridamole 75 mg/three times per day and aspirin 1.5 mg/kg/day from 16 to 36 weeks' gestation (Group I) or from 30 weeks' onwards (Group II). Control: ?no treatment |
|
| Outcomes | Women: maternal adverse effects Babies: IUGR, adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized, controlled prospective trial"; no other information provided |
| Allocation concealment (selection bias) | Low risk | Opaque, sequentially‐numbered, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned and unclear whether control group receives no treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attritions/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information to permit judgement. Protocol is available but extremely difficult to read due to poor copying quality |
| Other bias | Unclear risk | No imbalance in baseline characteristics. Insufficient information to judge other potential sources of bias. |
India 1993.
| Methods | Method of randomisation not specified; assessment of outcome not blinded. | |
| Participants | 100 women with PIH at 24‐36 weeks' gestation. | |
| Interventions | Exp: aspirin 60 mg/day. Control: 'standard treatments only'. | |
| Outcomes | Women: severe GH (proteinuria not specified); eclampsia; preterm (gestation not specified). Babies: stillbirths; neonatal deaths; SGA. | |
| Notes | Unclear whether aspirin group also had 'standard treatments'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated"; method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | No information regarding allocation concealment provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assessment of outcome not blinded. Although not stated explicitly, it seems reasonable to assume that the treatment was not blinded due to the daily administering of aspirin to the test group and standard treatment only to the control group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attritions/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Primary/secondary outcomes not specified in methods section. |
| Other bias | Unclear risk | Baseline characteristics: quote: "On statistical analysis it was found that both groups were well matched in relation to previous obstetric complications so the results were unbiased (Table I)." Age was also similar but 30% in aspirin group were multigravada vs 12% in control group |
India 1994.
| Methods | Quote: "Randomly allocated", no other information given. | |
| Participants | 94 nulliparous women with PIH in the 3rd trimester (SBP at least 140 mmHg, or DBP at least 90 mmHg, or both, on 2 occasions more than 6 hours but less than 24 hours apart). | |
| Interventions | Exp: aspirin 75 mg daily, until 10 days before EDD. Control: no antiplatelet agent. | |
| Outcomes | Women: development of PE; eclampsia or abruption; mean fall in BP; rise in BP. Babies: neonatal death; admission to SCBU; gestational age at delivery (mean); birthweight (mean); Apgar at 1 minute; macroscopic haematuria. | |
| Notes | Exclusion criteria not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple (e.g. coin toss) method used |
| Allocation concealment (selection bias) | Low risk | Authors noted during PARIS risk of bias assessment that opaque, sequentially numbered sealed envelopes were used to ensure allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not mentioned but unlikely as intervention group receives aspirin and control receives no treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attritions/exclusions to permit judgement |
| Selective reporting (reporting bias) | Low risk | Protocol not available. Primary/secondary outcomes not specified in methods section although all expected outcomes appear to be reported. |
| Other bias | Low risk | No imbalance in baseline characteristics: quote: "No significant difference was found in these two groups regarding age, height, mean gestational age at trial entry, mean BP ‐ systolic and diastolic, incidence of proteinuric and non‐proteinuric cases, cases with established PIH" |
India 1999.
| Methods | Randomised trial, no further details. 2 women allocated aspirin lost to follow‐up, 1 allocated placebo. | |
| Participants | 163 women with PIH at 20‐32 weeks. | |
| Interventions | Exp: aspirin 60 mg daily.
Control: placebo. Treatment continued to 38 weeks. |
|
| Outcomes | Women: PE; eclampsia. Babies: perinatal death; IUGR < 10th centile. | |
| Notes | Available as an abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial; no further details. |
| Allocation concealment (selection bias) | Unclear risk | Randomised trial; no further details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Control group described in abstract as "placebo" so assume some level of blinding was attempted, however no further information available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 163 participants: 2 women allocated aspirin lost to follow‐up, 1 allocated placebo; attrition balanced across groups. ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Abstract only available. Insufficient information to judge. |
| Other bias | Unclear risk | "Baseline characteristics were similar". Abstract only available ‐ insufficient information to judge if other sources of bias exist. |
India 2017.
| Methods | Quote: "Simple computerised random number allocation" | |
| Participants | 210 women at high risk of PE, based on the criteria age > 34 years, chronic hypertension, multiple pregnancy, gestational diabetes, or high uterine artery pulsatility index. Gestation 12 to 20 weeks. | |
| Interventions | Aspirin 75 mg to 34 weeks vs no treatment | |
| Outcomes | PE; preterm birth; placental abruption; PPH, IUGR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple computerised random number allocation" |
| Allocation concealment (selection bias) | High risk | No information about concealment of allocation, but the text quote: "Simple computerised random number allocation" implies there was none. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding is not discussed, but as there was no placebo it is likely that the women and their clinicians were aware of the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21/210 (10%) women randomised were excluded from the analysis. Loss to follow‐up was higher in the control group (8/105 aspirin vs 13/105 control), and in the aspirin group for 5/8 this was because women discontinued treatment |
| Selective reporting (reporting bias) | Unclear risk | PE is the only outcome specified in methids.Data for other outcomes are reported. Protocol not available, so unclear whether all outcomes collected have been reported. |
| Other bias | Unclear risk | Groups balanced at trial entry. |
Iran 1992.
| Methods | Prospective, randomised, double‐blind study | |
| Participants | 40 pregnant women with high risk for PIH (nulliparity, twin pregnancy or a history of previous PE) Excluded: history of chronic hypertension, long‐term treatment with non‐steroidal anti‐inflammatory drugs (at least during the last 6 weeks), PIH or proteinuria |
|
| Interventions | Exp: aspirin 100 mg/day after lunch during the third trimester of pregnancy up to 1 week before delivery Control: placebo |
|
| Outcomes | Women: PIH (non‐proteinuric/proteinuric), caesarean section, length of hospitalisation during pregnancy or parturition Babies: birthweight, gestational age at birth, Apgar score, stillbirth, prematurity (< 37 weeks gestational age), ICU admission, ventilator support, macroscopic haematuria, cephalhematoma, neonatal death |
|
| Notes | Trial conducted at Hafez Hospital in Shiraz, Iran | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided into two groups" ‐ no other information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly divided into two groups" ‐ no other information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind study" and placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All medical data of each woman, including details of pregnancy and perinatal outcome collected." |
| Selective reporting (reporting bias) | Low risk | Protocol not available and outcomes not clearly specified in methods section. However, all expected outcomes appear to be reported. |
| Other bias | Unclear risk | Baseline characteristics: quote: "There is no significant difference among all groups with regard to age, gravidity, rate of twin pregnancy, previous preeclampsia and family history of essential hypertension or toxemia of pregnancy." (Table I) However, baseline blood pressures not reported. |
Iran 2006.
| Methods | Randomised controlled trial | |
| Participants | 90 pregnant women with bilateral notches in the uterine arteries; rated in Doppler velocimetry waveform analysis to be at high risk of PE. Women at 12 to 14 weeks' gestation. | |
| Interventions | Exp: aspirin 0.5 mg/kg/day Control: placebo |
|
| Outcomes | Women: PIH, PE, bleeding Babies: fetal bleeding |
|
| Notes | Abstract only. No raw data available. Unable to find contact email address for authors or any related publications. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised"; no other information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomised"; no other information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Placebo used; no other information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40/45 women on aspirin and 40/45 on placebo were successfully followed up; attrition balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only ‐ insufficient information to permit judgement |
| Other bias | Unclear risk | Abstract only available. |
Iran 2010.
| Methods | Randomised; single‐blind; parallel assignment | |
| Participants | 80 pregnant women with risk of PE (e.g. vascular disease, chronic hypertension, diabetes, age under 20 and more than 40 years old). Excluded: women with heart disease, liver disease, thyroid disease, chronic renal disease and peptic ulcer | |
| Interventions | Exp: 100 mg aspirin daily Control: no treatment |
|
| Outcomes | Women: PE, preterm labour, type of delivery Babies: IUGR, neonatal APGAR score, neonatal weight |
|
| Notes | Registration record only available. Contacted author who advised by email that they have published in Persian but not yet in English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to assess. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Single blind", and no placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Registration record only. No outcome data available yet. |
| Selective reporting (reporting bias) | Unclear risk | Registration record only. No outcome data available yet. |
| Other bias | Unclear risk | Registration record only ‐ insufficient information to judge. |
Iran 2012.
| Methods | Three‐arm, randomised single‐blind controlled trial | |
| Participants | 70 women included who had previous diagnosis of PCOS, age 18‐40 years, gestational age 6‐12 weeks, a single viable fetus Excluded: history of diabetes or hypertension; and 35 women who were in metformin treatment arm |
|
| Interventions | Exp: aspirin 80 mg/day Control: no treatment |
|
| Outcomes | Women: uteroplacental circulation (uterine artery pulsatility index ‐ PI), PE gestational diabetes, any adverse outcome Babies: preterm delivery, IUGR, infant birthweight, any adverse outcome |
|
| Notes | Conducted at Shariati Hospital, Tehran, Iran. 105 women were randomised to 3 groups of 35 each. However, 1 group that received metformin is excluded from this analysis, thus the number of women of interest here is 70. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "by a computer program" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "hidden in sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind. Quote: "The study was a single blind, controlled trial and the examiner was blinded to group status during the ultrasound examinations." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients dropped out or were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes included in the registration record were reported. |
| Other bias | Unclear risk | No baseline imbalance, but some not reported, e.g. BP. Quote: "groups were homogenous according to the mean of age, BMI, gravidity" |
Iran 2014a.
| Methods | Randomised single‐blind trial | |
| Participants | 140 singleton pregnant women, who had unexplained elevated alpha‐fetoprotein (> 2.5 multiple of median) and gestational age between 15 and 18 weeks' gestation | |
| Interventions | Aspirin 80 mg/day taken in the evening vs control | |
| Outcomes | Primary outcome was 'adverse pregnancy outcome', which was any of PE, fetal loss (miscarriage at 20‐24 weeks or intrauterine death after 24 weeks), preterm delivery, IUGR, oligohydramnios, placental abruption. NICU admission, neonatal mortality | |
| Notes | 'Control' intervention was not specified, but as the study is described as single‐blind and trial registration states no placebo was used, it is likely to have been no treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Low risk | Sequentially‐labelled opaque sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | The obstetrician was not told the woman's allocation. A nurse educator gave instructions to the women about taking the intervention. 'Control' intervention was not specified, but as the study is described as single‐blind and trial registration states no placebo was used, it is likely to have been no treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/140 (6%) women excluded from the analysis (6 allocated aspirin, 2 control). Aspirin group: 2 did not return for follow‐up, 3 had 'abortion' < 20 weeks and 1 had a fetal anomaly diagnosed after randomisation. Control group: 1 did not return for follow‐up, 1 had 'abortion' < 20 weeks. |
| Selective reporting (reporting bias) | Low risk | Protocol not available. Primary outcomes specified and reported. Secondary outcomes not stated in methods section. All expected outcomes appear to be reported |
| Other bias | Unclear risk | The abstract states that the Quote: "[t]wo groups were comparable regarding the maternal characteristics", including age, gravidity, previous 'abortion', BMI, and weight. However, differences in baseline GA (range 15‐18 weeks) not reported. |
Israel 1989.
| Methods | Coded packages of 100 pills allocated according to a computer‐generated randomisation list. | |
| Participants | 65 women with either twin pregnancy, a history of PE or in first pregnancy, and a positive roll‐over test at 28‐29 weeks' gestation. | |
| Interventions | Exp: aspirin 100 mg daily. Control: placebo. | |
| Outcomes | Women: GH +/‐ proteinuria (BP > 140/90 on at least 2 occasions within 24 hours; proteinuria > 1 g/24 hours); caesarean section; length of hospitalisation (mean). Babies: stillbirth; neonatal death; gestation at birth (mean); born < 37 weeks; birthweight < 10th centile; Apgar scores; ventilation; admission to SCBU; IVH; haematuria; cephalhematoma; sepsis workup. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Coded packages of 100 tablets |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled" Quote: "neither the subject nor the physician in charge of the study knew which pill was being taken" Quote: "data pertaining to the number of patients in whom PIH occurred were evaluated by an independent mathematician" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attritions/exclusions to permit judgement |
| Selective reporting (reporting bias) | Low risk | Protocol not available. Primary outcomes specified and reported. Secondary outcomes not stated in methods section. All expected outcomes appear to be reported. |
| Other bias | High risk | No imbalance in baseline characteristics: quote: "No significant differences were found among the groups with regard to average age, gravidity, rate of twin gestation, or family history of essential hypertension" Study was discontinued after the second 6‐month data analysis on the recommendation of an independent mathematician aware of medication coding. |
Israel 1990.
| Methods | Quote: "Divided randomly into 2 groups", no other information given. | |
| Participants | 47 nulliparous at 30‐36 weeks with mild PIH (BP > 140/90 but < 165/110), no signs of PE, normal platelets and proteinuria < 500 mg/24 hours. Excluded if aspirin sensitivity, chronic hypertension, renal disease or antihypertensive drugs. | |
| Interventions | Exp: aspirin 100 mg until 5 days before EDD. Control: placebo. | |
| Outcomes | Women: PE (BP > 165/110 with low platelet count or proteinuria > 500 mg/24 hours, or both); caesarean section. Babies: gestation at delivery; birthweight (mean); Apgar score at 5 minutes (mean). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Divided randomly into 2 groups", no other information given. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Divided randomly into 2 groups", no other information given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind"; placebo used. Quote: "Neither the patients nor the medical staff knew which pill was being taken". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attritions/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Primary outcomes specified and reported, however some other expected outcomes (e.g. neonatal and maternal bleeding) not reported. |
| Other bias | Low risk | Baseline characteristics: Table 1 shows no significant differences between the 2 groups with regard to age, mean gravidity, mean GA, mean fetal weight percentile, and the number of growth‐retarded fetuses according to ultrasound examination on admission. |
Israel 1994.
| Methods | Allocated to a coded package according to randomisation list. 1 woman withdrawn from placebo group because of thrombocytopenia ‐ outcomes included where possible. | |
| Participants | 48 women with twin pregnancies at about 18 weeks. | |
| Interventions | Exp: aspirin 100 mg/day. Control: placebo. | |
| Outcomes | Women: GH; PE; caesarean section; IUGR. Babies: preterm birth; perinatal mortality; birthweight discordancy (15%). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Coded packages |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "placebo controlled, double‐blind" Quote: "Neither the subject nor her physician knew which pill contained aspirin until the code was broken at the end of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman withdrawn from placebo group after 16 weeks of therapy at 32 weeks' gestation because of thrombocytopenia ‐ outcomes included where possible. |
| Selective reporting (reporting bias) | Low risk | Protocol not available. Outcomes not clearly specified in methods section or elsewhere although key outcomes appear to be reported. |
| Other bias | High risk | Baseline characteristics: quote: "The placebo and aspirin group were similar in age, weight gain, zygosity, gravidity, parity and obstetrical antecedents" Quote: "Both groups were similar in all respects except for PIH, which occurred in previous pregnancies in three and one cases in the placebo and aspirin groups, respectively" Quote: "We are aware of the fact that the twin population participating the study was heterogenous. For example, three cases of PIH in previous pregnancies in the placebo group and only one in the aspirin group may represent different backgrounds and different risks of developing PIH in the ensuing pregnancy, although only one of those three developed PIH in our study. In balance, the fact that 10 primipara were enrolled in the aspirin group compared with seven in the placebo probably increases the risk of developing PIH in the aspirin group" |
Italy 1989.
| Methods | Quote: "Randomly assigned", no other information given. | |
| Participants | 33 women at risk of hypertension because of essential hypertension or a significant previous obstetric history (placental insufficiency causing fetal death, severe IUGR or PE < 32 weeks). Excluded: if antiphospholipid antibodies present. | |
| Interventions | Exp: aspirin 60 mg daily from 12 weeks until delivery. Control: placebo. | |
| Outcomes | Women: GH (BP > 140/90 and BP previously normal); gestation at delivery (mean). Babies: perinatal death; assisted ventilation; haemorrhagic complications; birthweight < 10th centile for gestational age; born < 37 weeks' gestation; Apgar scores (mean) RDS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned", no other information given. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomly assigned", no other information given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The study was single blind. Only the evaluator of clinical outcomes knew the treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attritions/exclusions not reported although data suggest no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Most expected outcomes appear to be reported except proteinuria and PE |
| Other bias | Low risk | Baseline characteristics: 2 groups were comparable in age, body weight, diastolic BP and previous pregnancy complications. |
Italy 1993.
| Methods | Allocation by a telephone call to 1 of 2 randomisation centres. 5.8% (64/1106) of women lost to follow‐up (18/523 aspirin, 46/583 control). Follow‐up: postal questionnaire to parents for 1083 children at 18 months (excludes 41 born before follow‐up started). 1 reminder and up to 3 telephone calls for non‐responders. Data for 427 aspirin (72%) and 361 no treatment (73%). | |
| Participants | 1106 women at 16‐32 weeks' gestation. Prophylactic: age < 18 or > 40 years, mild‐moderate chronic hypertension, nephropathy with normal renal function and BP, PIH or IUGR in previous pregnancy, twin pregnancy). Therapeutic: PIH (DBP 90‐110 mmHg) or early IUGR (fetal abdominal circumference >/= 2 SD below mean for gestational age). Excluded: chronic disease, allergy to aspirin, fetal malformation. | |
| Interventions | Exp: aspirin 50 mg daily. Control: no treatment. | |
| Outcomes | Women: PIH +/‐ proteinuria; abruption; induced or spontaneous abortion; caesarean section. Babies: perinatal mortality; gestation at delivery; birthweight < 10th or < 5th centile; admission to SCBU; IVH; gastric bleed. At 18 months: death; malformations height and weight < 10th centile, and respiratory; motor; sight; hearing or language problems. | |
| Notes | Data not presented separately for prophylaxis and treatment, and so all women included in prophylaxis for this review. For follow‐up, no difference between responders and non‐responders in baseline characteristics and outcome at discharge from hospital. Also, no differences in information collected by post or by telephone. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation by a telephone call to 1 of 2 randomisation centres. Quote: "523 women were assigned to no treatment and 583 aspirin. The higher number of women in the aspirin group was due to the erroneous use of the same randomisation sheets for several centres in early stages of the trial. Although we recognised this error during the study, we decided that no bias could have been introduced, so no measure was taken to balance the number of subjects" |
| Allocation concealment (selection bias) | Low risk | Central telephone |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial with no placebo. Article: quote: "The frequency of caesarean section was higher, but not significantly, in aspirin‐treated women than in controls. This finding is difficult to explain, but a potential bias due to the knowledge of treatment‐group allocation cannot be excluded." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Article: quote: "Of the 1106 subjects, 46 (8.8%) in the no‐treatment group and 18 (3.1%) in the aspirin group were lost to follow‐up, so data were available for analysis in 1042 (94%) subjects (477 no‐treatment, 565 aspirin)" Quote: "The overall percentage of randomised women lost to follow‐up was low, though it was higher in the no‐treatment than in the aspirin group. This difference suggests that clinicians followed women randomised to the active group more carefully. The effect of awareness of treatment by physicians on the results of a trial is difficult to define and quantify. This potential bias may act in favour of aspirin or of no treatment and could even have varied among the various participating centres." ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but primary outcomes stated in methods section are reported. |
| Other bias | Low risk | No imbalance in baseline characteristics: "The aspirin and no‐treatment groups were similar in terms of baseline characteristics; the mean ages at randomisation were 30.7 (SD 6.4) and 30.5 (6.7) years, the mean weights 62.3 (13.1) and 61.9 (12.8) kg, mean BPs 129 (17)/81 (11) and 128 (19)/81 (13) mm Hg, and mean durations of gestation 21.4 (5.3) and 21.5 (5.3) weeks. The 2 groups also had similar proportions of nulliparae (155 [27.4%] vs 155 [32.5%]) and of women reporting one or more previous reproductive failures (138 [24.4%] vs 96 [20.1%])." |
Italy 1999.
| Methods | Quote: "Randomised". 9 women stopped treatment early, 4 aspirin and 5 control. | |
| Participants | 216 women aged 18‐36 with pre‐existing HT or history of severe PE, at 12‐26 weeks. | |
| Interventions | Exp: 50 mg aspirin/day. Control: placebo. | |
| Outcomes | Women: PE. | |
| Notes | Article is in Italian. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised". |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Article is in Italian, thus unable to assess this criteria |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "9 women stopped treatment early, 4 aspirin and 5 control." Attrition balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Article is in Italian, thus unable to assess this criteria. |
| Other bias | Unclear risk | Article is in Italian, thus unable to assess this criteria |
Italy 2004.
| Methods | Telephone randomisation using computer‐generated randomisation list (separate list for each centre). 5 women (12%) lost to follow‐up (2 aspirin, 3 control) | |
| Participants | 40 women at < 14 weeks' gestation with chronic HT +/‐ nephropathy or history of severe PE or eclampsia or IUGR or stillbirth. Excluded: allergy to aspirin, fetal malformation, current twin pregnancy, chronic disease except renal/HT/DM without hypertensive nephropathy. | |
| Interventions | Exp: 100 mg aspirin/day until delivery. Control: no treatment. | |
| Outcomes | Women: GH (SBP >/= 140 or DBP >/= 90) or PE (as above + proteinuria > 300 mg/24 hours or >/= 1+). Babies: miscarriage, mean gestation at birth, mean birthweight, birthweight < 2500 g. | |
| Notes | Trial recruitment 1998‐2000, in 2 centres in Italy. Planned sample size 160 women, but trial stopped due to slow recruitment. Data not presented separately for outcomes PIH and PE. Data for the combined outcome are therefore reported under the outcome PIH. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Central telephone: quote: "Group allocation was done by telephone calls to one randomisation centre. Separate randomisation lists for each participating centre were used." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Neither patients nor clinicians were blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 women (12%) lost to follow‐up (2 aspirin, 3 control). 19/22 women in no treatment group and 16/18 ASA group were successfully followed up. ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Key outcomes not clearly specified in methods section. |
| Other bias | High risk | There was an imbalance in baseline characteristics: quote: "Women in the ASA group were older, and the group contained fewer nulliparae than in the no‐treatment group." Trial stopped early: quote: "In August 2000, in consideration of the low rate of recruitment due to the restrictive criteria at entry (as i.e. early week of gestation at randomisation) and the decreased interest from participant centres, we stopped the trial entry." |
Jamaica 1998.
| Methods | Women given sequential numbers on admission which identified a bottle containing either aspirin or placebo. 179/6275 (3%) lost to follow‐up. 50 women with multiple pregnancy excluded. Some women entered twice and given aspirin and placebo excluded, but numbers not given. | |
| Participants | 6275 primiparous women 12‐32 weeks and no contraindication to aspirin. 144 aspirin women and 161 placebo randomised after 32 weeks, but included in analysis. | |
| Interventions | Exp: aspirin 60 mg daily until delivery. Control: placebo. | |
| Outcomes | Women: hypertension (DBP >/= 90 mmHg or SBP >/= 140 mmHg or rise of 25 mmHg DBP or 40 mmHg SBP); PE; eclampsia; caesarean section; antenatal admission; PPH. Baby: perinatal mortality; preterm delivery; birthweight < 2500 g; admission to SCBU; 5‐minute Apgar < 5; IVH; other neonatal bleeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered bottles. Identification numbers were pasted on top of the lot number which would destroy lot number if an attempt was made to remove ID number. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double‐blind"; "Both drug and placebo were yellow sugar‐coated pills"; Quote: "The scientific officer maintained the list that linked identification number to treatment group, but no data collector or clinician was able to determine the identity of the treatment for any individual. Revelation of the treatment allocations only occurred at data analysis." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 179/6275 (3%) lost to follow‐up, unclear whether balanced between groups and reasons for attrition unclear. 50 women with multiple pregnancy excluded. Some women entered twice and given aspirin and placebo excluded, but numbers not given. |
| Selective reporting (reporting bias) | Low risk | Protocol available. All outcomes are reported in the paper. |
| Other bias | Unclear risk | No imbalance in baseline characteristics: quote: "The two groups of women were similar as regards maternal age, marital status, maternal employment status, identify of major wage earner of the household, household composition and housing facilities (e.g. toilet type and source of water supply), but the mothers assigned aspirin were slightly more likely to have higher educational status, and were slightly less likely to share a toilet with other households (Table 1). There was no difference in the blood pressures at enrolment." Quote: "significantly fewer women taking aspirin complied with their treatment (aspirin 1904/3023 (63%), placebo 2070/3026 (68%), P< 0.001" |
Japan 1999.
| Methods | Quote: "Enrolled randomly", no further information. | |
| Participants | 40 women with severe PE in previous pregnancy. Enrolled at 6‐18 weeks, treatment started at 20 weeks. | |
| Interventions | Exp: ozagrel hydrochloride, 400 mg/day from 20 weeks ‐ delivery. Control: placebo. | |
| Outcomes | Women: PE. Babies: preterm delivery; delivery < 32 weeks; SGA. | |
| Notes | Ozagrel is a thromboxane synthetase inhibitor. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Enrolled randomly", no further information. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Enrolled randomly", no further information. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding in article, but placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attritions/exclusions not reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Key outcomes not clearly specified in methods section. Most expected outcomes are reported, although some are not, e.g. mortality. |
| Other bias | Unclear risk | No imbalance in baseline characteristics, but unable to assess some: quote: "There were no differences in maternal age, type, severity, and onset of preeclampsia in the previous pregnancy between the control and treatment groups" |
Korea 1997.
| Methods | Randomly assigned | |
| Participants | 70 women with chronic hypertension or a history of severe PE in their previous pregnancy. Gestational age at trial entry unclear (main text in Korean). | |
| Interventions | Exp: aspirin 60 mg/day Control: unclear |
|
| Outcomes | pregnancy‐induced hypertension, rates of emergency caesarean section for fetal distress, NICU admission, fetal growth retardation, perinatal death, GA, birth weight, Apgar score at 5 minutes | |
| Notes | Abstract only is available in English. Full article is in Korean ‐ translation may provide additional helpful information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | insufficient information; need to translate article |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | insufficient information; need to translate article |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | insufficient information; need to translate article |
| Selective reporting (reporting bias) | Unclear risk | insufficient information; need to translate article |
| Other bias | Unclear risk | insufficient information; need to translate article |
Netherlands 1986.
| Methods | Coded packages, allocated according to a randomisation list. Two women in treatment group excluded because of non‐compliance, but data for some clinical outcomes reported. | |
| Participants | 46 angiotensin II sensitive primigravid women at 28 weeks' gestation with uncomplicated pregnancies, no history of hypertension, cardiovascular or renal disease, DBP < 80 mmHg and taking no drugs except iron. | |
| Interventions | Exp: aspirin 60 mg daily. Control: placebo. | |
| Outcomes | Women: eclampsia; GH (DBP at least 95 mmHg on 2 or more occasions 6 hours apart); PE (hypertension as above plus proteinuria > 0.5 g/L); preterm delivery (< 37 weeks); caesarean section. Babies: stillbirth; neonatal death; RDS; birthweight for GA < 10th or < 3rd centiles. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation list; no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Coded packs of tablets, no further information. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "placebo‐controlled, double‐blind trial"; Quote: "The investigators were unaware of the results until the code of the study was broken." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two women in treatment group excluded because of non‐compliance, but data for some clinical outcomes reported. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "only blood pressure and proteinuria were taken as end‐points". However, proteinuria was not reported in and of itself ‐ PIH and pre‐eclampsia were reported. |
| Other bias | Low risk | Baseline characteristics: quote: "At the beginning of the study the two groups were comparable with respect to age, body weight, diastolic blood pressure, and effective pressor dose." All participants are primigravidae (so parity, previous pregnancy complications, etc. not relevant here) |
Netherlands 1989.
| Methods | Coded packages containing trial drug allocated according to a randomisation list. | |
| Participants | 10 primigravid women with chronic hypertension and a positive angiotensin II sensitivity test at 26 weeks' gestation. No proteinuria, BP < 90 mmHg diastolic, serum creatinine < 70 umol/L and an adequately grown fetus. | |
| Interventions | Exp: aspirin 60 mg. Control: placebo. | |
| Outcomes | Women: GH (rise in DBP of 20 mmHg or more); PE (hypertension as before plus proteinuria >/= 500 mg/L); caesarean section. Babies: birthweight < 10th centile. | |
| Notes | All women had methyl dopa. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Coded packs of tablets, no further information available. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "placebo‐controlled double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that all 10 patients were successfully followed up. |
| Selective reporting (reporting bias) | Low risk | Protocol not available, however it appears that all expected outcomes were reported. |
| Other bias | Unclear risk | Information on baseline characteristics not presented and compared for all participants. Small sample size. |
Netherlands 1991a.
| Methods | Coded packages allocated according to a randomisation sheet. Code broken at 34 weeks, some women then started aspirin. | |
| Participants | 36 women with a positive angiotensin II sensitivity test at 28 weeks. | |
| Interventions | Exp: aspirin 60 mg daily from 28‐32 weeks. Control: placebo. | |
| Outcomes | Women: hypertension at 34 weeks. Babies: stillbirths. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Coded packs of tablets, no further information. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "placebo‐controlled double‐blind" Quote: "Code broken at 34 weeks, some women then started aspirin" Authors confirmed via PARIS assessment that a matching placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One woman in the aspirin group withdrew from the study immediately after randomization. The angiotensin II sensitivity test was not repeated in three women in the placebo group, because they had become hypertensive before 34 weeks' gestation; in the aspirin group all women were normotensive at 34 weeks." |
| Selective reporting (reporting bias) | Low risk | Quote: "Since some of the women had been taking a low dose of aspirin after 34 weeks' gestation and others had not, the outcome of pregnancy was not analysed." Only reported key endpoint of BP response to angiotensin II. |
| Other bias | Low risk | No imbalance in baseline characteristics: quote: "A the beginning of the study the two groups were comparable with regard to age, body weight, baseline blood pressure, and heart rate" All participants normotensive and primigravid. |
Netherlands 2009.
| Methods | Prospective, randomised, double‐blind, placebo‐controlled trial. | |
| Participants | 54 previous IVF/ICSI patients with ongoing pregnancy. | |
| Interventions | Exp: aspirin 100 mg/day. Started simultaneously with the start of the oral contraceptive pill and continued until the day of the pregnancy test. Pregnant patients continued the study medication until 12 weeks of GA. | |
| Outcomes | Incidence of pregnancy complications: including first trimester blood loss,PPH, hypertensive complication (PIH, PIH with PE, PIH with HELLP), premature contractions/premature rupture of membranes, premature delivery, growth restriction/growth difference (twins), no complications | |
| Notes | 169 patients randomised in original trial (not pregnant). Follow‐up study included 54 patients (ongoing pregnancy) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using computerized tables" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by an independent pharmacist of the hospital pharmacy" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Randomization numbers corresponded with the numbers on the medication boxes containing 80 identical‐looking tablets of either 100 mg aspirin or 100 mg placebo. These medication boxes were filled by an independent person at the hospital pharmacy, assuring that both patient and investigator were blinded to the randomization and the content of the tablets" "Follow‐up medication: contained the same medication to which the patient had initially been randomized" Concealment maintained |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "All 54 patients received a questionnaire after delivery and 49 patients (90.7%) completed and returned the questionnaire. We retrieved full records of one non‐responder whereas four were lost to follow‐up." All four patients lost to follow‐up were in the aspirin group. |
| Selective reporting (reporting bias) | Unclear risk | Three outcomes (1 primary and 2 secondary) were included in the registration record. These were all reported in the published papers. However, a number of additional outcomes that were not pre‐specified were also reported. |
| Other bias | Unclear risk | Baseline characteristics: Table I shows no signIficant differences in age, FSH on cycle day 3, BMI, primary infertility, twin pregnancy. Baseline BP not reported. |
Pergar 1987.
| Methods | Double‐blind placebo‐controlled trial | |
| Participants | 300 women with history of idiopathic fetal growth. Fetal growth retardation must be <10 percentile of Lubchenco et al. Therapy began at 15 to 17 weeks' gestation. Excluded: Presence of chronic disease, allergy to dipyridamole, uterine or malformation, secondary hypertension, early fetal death in a previous pregnancy, current use of anti inflammatory drugs or anticoagulants |
|
| Interventions | Exp: dipyridamole 225mg/day Control: placebo |
|
| Outcomes | Babies: birth weight related to GA | |
| Notes | Conducted by doctors from 50 hospitals throughout France and Belgium. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study appears to be randomised; however, French translation needed to confirm this |
| Allocation concealment (selection bias) | Unclear risk | French translation needed to determine if allocation was concealed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Trial is double‐blind placebo‐controlled. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | French translation needed to judge this. |
| Selective reporting (reporting bias) | Unclear risk | French translation needed to judge this. |
| Other bias | Unclear risk | No imbalance in baseline characteristics. French translation needed to determine if there may be any other sources of bias. |
Romania 2018.
| Methods | Randomised, using sealed envelopes | |
| Participants | Women with a singleton pregnancy at risk of pre‐eclampsia, based on a positive early pregnancy screening test (combining medical history, serum pregnancy‐associated plasma protein A, mean arterial pressure, and uterine artery pulsatility index). | |
| Interventions | 1. Aspirin 150 mg to 32 weeks; 2. aspirin 150 mg to 36 weeks; 3. placebo | |
| Outcomes | PE; fetal growth restriction | |
| Notes | Data for the 2 aspirin groups combined for analysis of antiplatelets vs no antiplatelets | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation to group described as women quote: "chose a sealed envelope from a pile and opened it". No further information. |
| Allocation concealment (selection bias) | Unclear risk | Allocation described as women quote: "chose a sealed envelope from a pile and opened it". No further information. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Women were aware of their treatment allocation. Ultrasound scans were conducted blind to the allocation. Not stated whether the clinicians were aware of the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There do not appear to be any losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The outcomes to be assessed are not described, and the protocol is not available |
| Other bias | High risk | Quote: "The mean GA at enrollment was similar for all groups", however there were potentially important differences in other characteristics, including smoking (1. 8/50, 2. 3/50, 3.3/50) and nulliparity (1. 30/50, 2.24/50, 3. 36/50). |
Russia 1993.
| Methods | Stratified blocked randomisation using sealed opaque numbered envelopes. Blinding not reported. Data not available for 12 women (16%). | |
| Participants | 76 women with chronic glomerulonephritis or essential hypertension. | |
| Interventions | Exp: aspirin 125 mg plus dipyridamole 150‐225 mg/day from 12‐19 weeks' gestation. Control: no treatment. | |
| Outcomes | Women: PE, abruption. Babies: early (up to 15 weeks) and late (15 to 27 weeks) fetal deaths and perinatal death (28 weeks to 1st week of life); preterm birth (birth between 28 to 36 weeks); SGA (body mass < 2 SD for GA). | |
| Notes | Abstract published in 1994 reports 76 women recruited, Russian paper published in 1993 reports 64 women recruited. Authors confirmed additional women recruited after publication of the 1993 paper, but data not available. Data for the initial 64 women only included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not reported. Control group received no treatment (rather than placebo) so likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Abstract published in 1994 reports 76 women recruited, Russian paper published in 1993 reports 64 women recruited. Authors confirmed additional women recruited after publication of the 1993 paper, but data not available. Data for the initial 64 women only included in this review. |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but all expected outcomes appear to be reported. |
| Other bias | Low risk | Baseline characteristics (from translated document): quote: "There were no essential differences in age, in the number of first pregnancies, or in basic clinical differences (AD, proteinuria, serum level in the creatine) between the two groups. There were also no essential differences between the groups in the frequency of previous gynaecological illnesses of complications in previous childbirth." |
S Africa 1988.
| Methods | By computer‐generated random numbers, no other information. 1 woman lost to follow‐up. | |
| Participants | 44 women with elevated mid‐trimester BP, 12‐28 weeks' gestation, DBP 80‐105 mmHg, and otherwise normal. | |
| Interventions | Exp1: aspirin 81 mg daily. Exp2: aspirin 81 mg + dipyridamole 200 mg daily. Control: no antiplatelet agent. | |
| Outcomes | Women: PE. Babies: stillbirth. | |
| Notes | Published only as an abstract. Three‐arm study. Data for 2 antiplatelet arms combined for analysis versus control but presented separately in comparison of subgroup analysis based on dose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Computer‐generated random numbers, no other information. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned. Unclear whether a placebo was used ‐ they say control group received no treatment, but then refer to them as the placebo group in the paper. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 44 participants; 1 woman lost to follow‐up. Pregnancy outcome was known in 43/44 patients. |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but all expected outcomes appear to be reported. |
| Other bias | Low risk | No imbalance in baseline characteristics: quote: "The three groups were comparable for age, parity, family and personal history of hypertension and for gestational age and blood pressure at commencement of the study." |
Spain 1997.
| Methods | Computer‐generated random numbers used to prepare a table for the sequence of allocation. Tablets in identical blister packs. Allocated to 6 groups, according to treatment and timing of administration. 7 women excluded, because poor compliance or incomplete blood pressure assessments. |
|
| Participants | 107 women aged 18‐40 years at < 16 weeks' gestation and at moderate risk of PE. For example, family or own history of PIH, PE, chronic HT, cardiovascular or endocrine problem, bleeding or endocrine disease. Excluded: multiple pregnancy. |
|
| Interventions | Exp: 100 mg aspirin.
Control: placebo. Each treatment group could also be allocated to 3 different times of the day. |
|
| Outcomes | Women: GH; PE; caesarean section; abruption. Baby: death; preterm birth (< 37 weeks); IUGR. | |
| Notes | Testing the hypothesis that aspirin effects are time‐dependent, being greater in the evening. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Coded sequentially number packs of tablets: Placebo and ASA quote: "were prepared in an identical manner and provided monthly to the volunteers in a box containing three blister packs, each with 10 tablets. The boxes, grouped in packs of seven (to cover medication for the duration of pregnancy) and labeled with the randomisation number, were assigned to each patient at the time of her recruitment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" and placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "7 subjects who did not comply with all requirements set a priori for this clinical trial were eliminated from the study. Reasons for elimination included the use of additional medication during the trial, noncompliance with the assigned medication (missing more than six tablets during any given month), and the impossibility of providing all required BP profiles. Subjects providing fewer than five profiles of ambulatory BP monitoring (ABPM) were eliminated from the study." The distribution of exclusions/withdrawals between groups was not reported. |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but all expected outcomes appear to be reported. |
| Other bias | Unclear risk | Quote: "Baseline characteristics related to age, weight, height, and 24‐hour mean BP values obtained from the first profile of ABPM (before treatment started) were similar for all six groups." 70/100 women were primipara, but distribution across groups not reported? |
Spain 1999.
| Methods | Randomised. Tablets in identical blister packs. Allocated to 6 groups, according to treatment and timing of administration. 15 women excluded, because poor compliance or incomplete BP assessments. |
|
| Participants | 255 women aged 18‐40 years at < 16 weeks' gestation and at moderate risk of PE. For example, family or own history of PIH, PE, chronic HT, cardiovascular or endocrine problem, bleeding or endocrine disease. Excluded: multiple pregnancy. |
|
| Interventions | Exp: 100 mg aspirin.
Control: placebo. Each treatment group could also be allocated to 3 different times of the day. |
|
| Outcomes | Women: mean 24‐hour BP. Baby: IUGR. | |
| Notes | Testing the hypothesis that aspirin effects are time‐dependent, being greater in the evening. Data entered into the review from the main publication. Data for a total of 341 women have been presented, but in abstract only and incomplete. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | insufficient information available to make an assessment of this criteria |
| Allocation concealment (selection bias) | Unclear risk | Placebo used, and tablets in identical blister packs, but insufficient information on allocation to make assessment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Placebo used, but insufficient information available to make an assessment of this criteria. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 women excluded, because poor compliance or incomplete blood pressure assessments. |
| Selective reporting (reporting bias) | Unclear risk | insufficient information available to make an assessment of this criteria |
| Other bias | Unclear risk | insufficient information available to make an assessment of this criteria |
Spain 2003.
| Methods | Double‐blind, randomised, controlled trial | |
| Participants | 341 pregnant women at high risk for gestational hypertension or PE, including familial or own history of gestational hypertension or PE; cardiovascular; endocrine, bleeding or metabolic disease; a history of spontaneous abortion; obesity; <18 or > 35 years old; nulliparous Excluded: multiple pregnancy, chronic hypertension, chronic liver disease, any disease requiring antiinflammatory medication, diabetes or other endocrine disease and intolerance to ABPM device |
|
| Interventions | Exp: aspirin 100 mg/day from 12‐16 weeks' gestation until delivery Control: placebo Each treatment group could be allocated to 3 different times of day when tablets were to be taken. |
|
| Outcomes | Women:BP | |
| Notes | Conducted at the Obstetric Physiopathology Service (high‐risk unit) of the Hospital Clinico Universitario, Santiago de Compostela, Spain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables generated random sequence of 6 groups in blocks of 60 |
| Allocation concealment (selection bias) | Low risk | Coded sequentially numbered packs of tablets: Placebo and ASA quote: "were prepared in identical presentation and provided monthly to the volunteers in a box containing 3 blister packs, each with 10 tablets. The boxes, grouped in packs of 7 (to cover medication for the duration of pregnancy) and labeled with the randomization number, were assigned to each women at the time of her recruitment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind and placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Apart from the 341 women providing all required information, 12 subjects who provided < 4 profiles of ABPM (5 who had spontaneous abortions and 7 who withdrew from the trial after the first visit) and 4 women who missed > 6 tablets during any given month were eliminated from the study." The distribution of exclusions/ withdrawals between groups was not reported. |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but all expected outcomes appear to be reported. |
| Other bias | Unclear risk | No imbalance in baseline characteristics:quote: "Baseline characteristics (given in the Table) related to age, weight, height, and 24‐hour mean BP values obtained from the first profile of ABPM (sampled before treatment started) were similar for all 6 groups of treatment." 181/341 women were primipara, but distribution across groups not reported. |
Spain 2017.
| Methods | Randomised | |
| Participants | 186 women aged 18 years or older with a singleton pregnancy, crown rump length 45 mm to 84 mm, an abnormal Doppler at 11 to 14 weeks and no other risk factors for PE | |
| Interventions | Aspirin 150 mg extended release vs placebo, until 28 weeks' gestation | |
| Outcomes | Pulsatility index at 28 weeks; PE; early onset PE (delivery before 34 weeks); severe PE; SGA, neonatal metabolic acidosis at birth. | |
| Notes | Conducted at three university hospitals in Spain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation, stratified by site |
| Allocation concealment (selection bias) | Low risk | Sites contacted a central office for randomisation, which then sent them the allocation by fax. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Women, caregivers and investigator described as blind to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 31/186 (17%) women excluded from analysis: lost to follow‐up (7 aspirin vs 6 placebo)' voluntary withdrawal (6 vs 5), preterm birth < 28 weeks 1 vs 1), congenital malformation (1 vs 1), miscarriage, (2 vs 0), asthma attack 0 vs 1). |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Outcome reported for those listed in methods, with some additional outcome data reported that are not in methods. |
| Other bias | Low risk | Baseline characteristics balanced between groups (see table 1). |
Tanzania 1995.
| Methods | Coded packages A and B. No other information. | |
| Participants | 127 women with a positive roll‐over test at 20 weeks, at which point therapy began. Excluded if history of HT, long‐term treatment with or use within last six weeks of nonsteroidal anti‐inflamatory drugs, or increased BP before screening or proteinuria > 300 mg. | |
| Interventions | Exp: 80 mg aspirin daily.
Control: placebo. Both taken until 10 days before the expected date of delivery |
|
| Outcomes | Women: GH; PE. Babies: none. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple method e.g. coin toss |
| Allocation concealment (selection bias) | High risk | Pharmacist coded packages labelled A or B. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" and identical placebo used. Quote: "Neither the subject nor the physician knew which tablet was being taken by the particular woman." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One dropout from the aspirin group due to severe gastritis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. No neonatal outcomes reported (only maternal BP and proteinuria). |
| Other bias | Low risk | Baseline characteristics (age, diastolic BP, GA, protein and nitrites in urine, Hb) appear comparable (although significance level not provided). |
UK 1990.
| Methods | Computer‐generated randomisation list. Serially‐numbered bottles dispensed by pharmacist. 5.7% (6/106) excluded after randomisation (5 women moved house, 1 withdrew after 3 weeks). | |
| Participants | 106 primigravid women with persistently abnormal doppler waveform studies at 24 weeks' gestation. Excluded: aspirin allergy, diabetes, bleeding disorders, peptic ulceration, SLE. | |
| Interventions | Exp: aspirin 75 mg daily. Control: placebo. | |
| Outcomes | Women: GH; proteinuria; hypertension < 37 weeks' gestation; caesarean section for complications of hypertension. Babies: perinatal death; birthweight < 5th centile. | |
| Notes | Lancet contacted to confirm this study has not been retracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Pharmacist dispensed tablets in serially numbered bottles |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Patients attended for routine antenatal care but the clinicians were unaware of their treatment." Placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5.7% (6/106) excluded after randomisation (5 women moved house, 1 withdrew after 3 weeks). 4 of these were from the aspirin group; 2 from the placebo group. |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but all expected outcomes appear to be reported. |
| Other bias | Low risk | Baseline characteristics: quote: "The demographic features of the groups were similar" (although significance levels not given). |
UK 1992.
| Methods | Quote: "Simply randomised with block size 4". | |
| Participants | (a) 18 normal primigravidae, 16 weeks' gestation, and (b) 16 primigravidae with GH but no proteinuria at > 20 weeks. | |
| Interventions | Exp: aspirin 60 mg daily until delivery. Control: placebo. | |
| Outcomes | Women: duration of labour; blood loss at delivery. Babies: < 36 weeks at delivery; birthweight < 10th centile; minor bruising of newborn. | |
| Notes | Continuous data only presented for some outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simply randomised with block size of 4". Small block randomisation however trial is blinded. |
| Allocation concealment (selection bias) | Low risk | Quote: "Simply randomised with block size of 4". Small block randomisation however trial is blinded. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind", placebo used, Meyer pharmaceuticals supplied identical tablets. Quote: "A paediatrician who was unaware of the treatment allocation examined the infants 1 or 2 days after birth." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attritions/exclusions not explicitly reported but appears there were none. |
| Selective reporting (reporting bias) | Low risk | Quote: "This study was designed to measure changes in platelets and prostaglandins, rather than clinical outcomes." All expected outcomes are reported. |
| Other bias | Unclear risk | No imbalance in baseline characteristics: quote: "The aspirin and placebo groups of both the normal primigravidae and the patients with GH were well matched for age, weight and blood pressure (Table 1). In the GH group, the patients who received aspirin were recruited at a significantly more advanced gestational age than those who received placebo (P<0.05, Table 1)." "Despite the randomization procedure, the group of normal primigravidae randomized to receive aspirin had a lower initial arachidonic acid induced platelet aggregation at 16 weeks (median 62%, interquartile range 34, 79) than those randomized to receive placebo (median 92%, interquartile range 48, 94) and a lower release reaction (median 11%, interquartile range 2, 33) compared with those randomised to receive placebo (median 27%, interquartile range 0, 34). Although neither of these differences were statistically significant (P>0.05 in each case), it was felt that it was of sufficient magnitude to render direct comparison of the two groups invalid. Thus, for this aggregating agent, comparisons were drawn between the change in platelet aggregation from pre‐treatment to post‐treatment values." |
UK 1992b.
| Methods | Quote: "Randomly allocated", no other information given. | |
| Participants | 26 women with history of recurrent miscarriage or connective tissue disorder, and positive anticardiolipin antibodies. Gestational age not described. | |
| Interventions | Exp: aspirin 75 mg daily. Control: no treatment. | |
| Outcomes | Women: miscarriage. Babies: neonatal death. | |
| Notes | Abstract only available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated", no other information given. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomly allocated", no other information given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Control was no treatment therefore likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attritions/exclusions not reported in abstract. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available and insufficient information provided in abstract. |
| Other bias | Unclear risk | Insufficient information provided in abstract to judge other sources of bias. |
UK 1995.
| Methods | Computer‐generated randomisation list used to produce sealed envelopes. 4/122 women (3%) withdrew after randomisation. | |
| Participants | 122 women with no previous pregnancy proceeding beyond 12 weeks, Hb > 13.2 g/dL at 12‐19 weeks' gestation, DBP < 90 mmHg and no proteinuria. Excluded if multiple pregnancy, diabetes, recurrent miscarriage or contraindication to aspirin. | |
| Interventions | Exp: aspirin 75 mg from 18 weeks until delivery. Control: placebo. | |
| Outcomes | Women: GH; PE; eclampsia; abruption; caesarean section; induction of labour; side effects. Babies: perinatal mortality; delivery < 34 weeks' gestation; admission to SCBU; birthweight < 5th centile. | |
| Notes | Trial conducted 1989‐1992. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes" PARIS assessment confirmed that allocation concealment was achieved via opaque, sequentially numbered, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled" PARIS assessment confirmed that matching placebo was used to ensure blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/122 women (3%) withdrew from the study soon after randomisation, so that 58 women were in the low‐dose aspirin group and 60 women in the placebo group. No cases were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all expected outcomes appear to be reported. |
| Other bias | Low risk | Quote: "There were no significant differences in demographic characteristics between the groups." (includes age, weight, DBP, Hb, % Caucasian, % smokers). |
UK+others 2003.
| Methods | Computer‐generated random number lists, in blocks of 10, created by pharmaceutical company. 'Appropriately numbered drug' dispensed by each hospital pharmacy. 6 women (1%) lost to follow‐up (4 aspirin, 2 control). | |
| Participants | 560 women with singleton pregnancy at 22‐24 weeks and doppler pulsatility index > 1.6 (95th percentile). Excluded: pre‐existing hypertensive, renal or cardiovascular disease, DM, bleeding disorders, SLE, peptic ulcers, hypersensitivity to aspirin, fetal abnormality or growth restriction at 23 week scan. | |
| Interventions | Exp: aspirin 150 mg/day. Control: placebo (identical tablets containing lactose). | |
| Outcomes | Women: PE; early PE < 34 weeks; placental abruption; PPH; blood transfusion. Babies: death (stillbirth, perinatal death); preterm birth < 37 weeks; very preterm birth < 34 weeks; SGA (< 5th percentile); admission to SCBU. | |
| Notes | Trial recruitment 2001‐2002. Multicentre: 7 centres in UK, 1 in Brazil, 1 in Chile, and 1 in South Africa. Compliance: 95% in both groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number lists, in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Quote: "created by Penn Pharmaceuticals Ltd and the appropriately numbered drug was dispensed by each hospital pharmacy." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All investigators, participants and clinicians were unaware of the treatment groups." "All outcomes were determined before the randomization code of the trial was broken." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 women (1%) lost to follow‐up (4 aspirin, 2 control). 24 women in aspirin group discontinued intervention, vs 26 in placebo group. 4 women excluded from analysis in aspirin group. 2 excluded from analysis in placebo group. ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol available and all pre‐specified outcomes are reported. |
| Other bias | Low risk | No imbalance in baseline characteristics: quote: "There were no systematic differences in the baseline characteristics between the two groups" |
USA 1993.
| Methods | Efforts were made to conceal randomisation; placebo controlled; < 1% loss; blind assessment of outcome. | |
| Participants | 604 primiparous women at 24 weeks, in single antenatal clinic. Exclusions: renal or collagen disease, diabetes, essential hypertension, multiple pregnancy. | |
| Interventions | Exp: aspirin 60 mg/day, from 22 weeks. Control: placebo. | |
| Outcomes | Women: GH; PE; eclampsia; APH; caesarean section; preterm delivery (< 37, < 34, < 32 weeks). Babies: perinatal death; SGA. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "blocked randomization scheme with randomly chosen block sizes." |
| Allocation concealment (selection bias) | Low risk | Efforts were made to conceal randomisation: quote: "The University of Alabama at Birmingham Investigational Drug Service of the Drug Resource Centre generated a blocked randomization scheme." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blinded"; "identical appearing" placebo used Blind assessment of outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 1% loss: quote: "Follow‐up was maintained on 99% of the patients." Quote: "Analysis was by intention‐to‐treat." "One patient from each group was lost to follow‐up, leaving 302 patients in each group for analysis." |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all expected outcomes appear to be reported. |
| Other bias | Unclear risk | Baseline characteristics: quote: "The patients in each of these groups were of similar age ...and of similar race.... Both groups had a similar number of antepartum visits". Baseline BP, weight, height, etc. not reported. |
USA 1993a.
| Methods | Quote: "Assigned randomly". 150/3135 (4.8%) lost to follow‐up: 85 from aspirin group and 65 from placebo. | |
| Participants | 3135 nulliparous women at 13‐25 weeks with BP < 135/85 and no proteinuria; out of the 4241 entered into a run‐in compliance phase. Exclusions: chronic hypertension, diabetes, renal disease, other medical illness. | |
| Interventions | Exp: aspirin 60 mg/day. Control: placebo. | |
| Outcomes | Women: GH; PE; eclampsia; caesarean section; abruption; preterm delivery; PPH. Babies: stillbirths; neonatal deaths; SGA < 10th centile; bleeding. | |
| Notes | Mean gestation at trial entry 19.8 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Assigned randomly"; stratified by enrolment centre |
| Allocation concealment (selection bias) | Low risk | Numbered bottles containing allocated tablets Quote: "an independent data‐coordinating centre (the George Washington University Biostatistics Center) was responsible for developing the treatment allocation code" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind placebo‐controlled study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 150/3135 (4.8%) lost to follow‐up: 85 from aspirin group and 65 from placebo. ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol seems to be available and all expected outcomes appear to be reported. |
| Other bias | Unclear risk | No imbalance in baseline characteristics: quote: "The demographic and clinical characteristics of the women in the two groups were similar except that more women in the placebo group had a systolic blood pressure of 120 to 134 mm Hg (297 [19.0%] vs 245 women [15.6%] in the aspirin group; P = 0.01), although the mean systolic blood pressures in the groups were similar." Editorial: "A point of concern... is the skewed population of the study group; the study subjects were healthy, nulliparous pregnant women. Along with the reduction in the incidence of pre‐eclampsia among the women treated with aspirin, there was a marked increase in abruptio placentae (0.7% vs 0.1% in the placebo group). The incidence of abruptio placentae among unselected pregnant women is about 1%. Hence, this study group did not represent the patient population encountered by the average clinician in the US. The increase in the frequency of abruptio placentae, even though the incidence of this complication was very low, may nullify the potential benefit of low‐dose aspirin in pregnant women" |
USA 1994.
| Methods | Quote: "Randomised"; 5/54 (9%) women lost to follow‐up. | |
| Participants | 54 women with chronic hypertension or previous severe PE, enrolled at 13‐15 weeks. | |
| Interventions | Exp: aspirin 100 mg sustained release/day until 37 weeks. Control: placebo. | |
| Outcomes | Women: PE. Babies: stillbirth; SGA. | |
| Notes | Published as abstract only. Ethics form also available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised via simple method e.g. coin toss |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind, no further information available. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind"; placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/54 (9%) women lost to follow‐up. 49/54 completed study; distribution across groups not specified. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available (although ethics form is). Only abstract shows summary results ‐ therefore insufficient information to judge. |
| Other bias | Unclear risk | Insufficient information to judge |
USA 1997.
| Methods | Quote: "Randomly assigned", no further information. | |
| Participants | 19 women with antiphospholipid antibodies and </= 2 previous miscarriages with no other antiphospholipid antibody‐related complications. Gestational age not described. Excluded: previous thrombosis, early onset PE, thrombocytopenia. |
|
| Interventions | Exp: aspirin 81 mg/day throughout pregnancy. Control: usual care. | |
| Outcomes | Babies: fetal death; SGA (< 5th percentile); fetal distress at term (not defined). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned", no further information. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomly assigned", no further information. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not mentioned but aspirin was compared to usual care (not placebo) so likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attritions/exclusions not explicitly reported in article but data in Table I indicates that there were none. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available and outcomes not clearly specified in methods section or elsewhere. Therefore, insufficient information to judge. |
| Other bias | Unclear risk | Baseline characteristics: quote: "The patients' obstetric histories… and antiphospholipid antibody test results are summarised in Table I. None of the differences between groups was statistically significant." However, demographic details of groups (e.g. age, weight, etc) not provided. |
USA 1998.
| Methods | Packets prepared using computer‐generated random numbers. Opened consecutively in each centre. 36/2539 women (1%) lost to follow‐up. | |
| Participants | 2539 women 13‐26 weeks' gestation with insulin‐treated diabetes, chronic hypertension, multiple pregnancy or PE in a previous pregnancy. Women with multiple pregnancy excluded if also diabetes, chronic hypertension or proteinuria. | |
| Interventions | Exp: aspirin 60 mg daily. Control: placebo. | |
| Outcomes | Women: GH; PE; abruption; preterm delivery; PPH. Baby: death; IUGR (< 10th centile); IVH; other neonatal bleeding. | |
| Notes | Additional data provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated permuted‐block randomization sequence stratified according to clinical center and risk group." |
| Allocation concealment (selection bias) | Low risk | Quote: "The aspirin and placebo packets were prepared and labeled at a central location…each woman receiving the next labeled packet" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind, randomized, placebo‐controlled trial" Quote: "To ensure consistency in the diagnosis of preeclampsia, the records of all the women with apparent preeclampsia, worsening hypertension, new‐onset proteinuria, or proteinuria at base line of 1+ or more were reviewed independently by three physicians unaware of the treatment‐group assignments." PARIS assessment confirmed that a placebo, identical in appearance to the aspirin tablet, was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 36/2539 women (1%) lost to follow‐up: quote: "Outcome data could not be obtained on 19 women in the aspirin group and on 17 in the placebo group." Intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all expected outcomes are reported. |
| Other bias | Low risk | No imbalance in baseline characteristics: quote: "Within each risk group, there were no significant differences between the aspirin and placebo groups. |
Venezuela 2000.
| Methods | Quote: "Randomised" no further information. | |
| Participants | 127 nulliparous women < 29 weeks' gestation. At risk of PE because previous PE, obesity, HT, diabetes, nephropathy, MAP > 85, positive roll‐over test, family history PE, multiple pregnancy or < 20 years. | |
| Interventions | Exp: aspirin 100 mg x 3/week + vitamin C 500 mg/day + vitamin E 400 IU/day fish oil x 3/day. | |
| Outcomes | Women: PE. | |
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Multicentre randomised triple‐blind in blocks of 10, but no further information on method randomisation. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed boxes in blocks of 10 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "triple‐blind placebo‐controlled" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only available ‐ attritions/exclusions not reported. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only available ‐ insufficient information to judge. |
| Other bias | Unclear risk | Abstract only available ‐ insufficient information to judge. |
Zimbabwe 1998.
| Methods | Randomisation list used to determine the sequence of numbered containers. 20/250 (8%) women lost to follow‐up. | |
| Participants | 250 women at 20‐28 weeks with a history of PE in a previous pregnancy, especially if at < 32 weeks, or chronic hypertension. Excluded if hypersensitivity to aspirin, PE this pregnancy, bleeding or peptic disorder. | |
| Interventions | Exp: aspirin 75 mg/day. Control: placebo. | |
| Outcomes | Women: PE; antihypertensive drug; preterm delivery; PPH; caesarean section. Baby: death; IUGR; admission SCBU. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Identical numbered containers organised by a statistician not involved in the trial |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" and placebo used; Quote: "The clinical investigators and the subjects were blinded to the type of drug in the containers." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20/250 (8%) women lost to follow‐up: quote: "Out of the 250 subjects recruited, only 230 women (ASA, n = 113; PLA, n = 117) completed the trial. Twenty subjects (8%) were lost to follow up in the study. 12 belonged to the aspirin group, while eight belonged to the placebo group. 1 subject voluntarily withdrew from the trial. She did not develop pre‐eclampsia and had been allocated to the placebo group. The other 19 stopped attending the antenatal clinic and information regarding the progress and outcome of the pregnancy was not available." |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all expected outcomes are reported. |
| Other bias | Low risk | No imbalance in baseline characteristics: quote: "Table 1 shows the baseline characteristics between the two groups was comparable." |
ABPM: Ambulatory blood pressure monitoring APH: antepartum haemorrhage AST: aspartate aminotransferase BMI: body mass index BP: blood pressure DBP: diastolic blood pressure DM: diabetes mellitus EDD: estimated date of delivery Exp: experimental group FSH: follicle‐stimulating hormone GA: gestational age GH: gestational hypertension Hb: haemoglobin HELLP: haemolysis elevated liver enzymes and low platelets HT: hypertension ICSI: intra‐cytoplasmic‐sperm‐injection ICU: intensive care unit ITT: intention‐to‐treat IU: international unit IUGR: intrauterine growth restriction IVF: in vitro fertilisation IVH: intraventricular haemorrhage MAP: mean arterial pressure NICU: neonatal intensive care unit PCV: packed cell volume PE: pre‐eclampsia PIH: pregnancy‐induced hypertension PPH: postpartum haemorrhage RDS: respiratory distress syndrome RH: Rhesus SBP: systolic blood pressure SCBU: special care baby unit SD: standard deviation SGA: small‐for‐gestational age SLE: systemic lupus erythematosus vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Argentina 1994 | Abstract only, no clinical outcomes reported. Randomisation unclear. |
| Australia 1989 | 41% of participants (9/16) excluded post randomisation as refused to take treatment. Trial abandoned. Intervention: aspirin vs placebo. |
| Australia 1989a | No relevant outcomes reported. Study design: quote: "randomly treated", no other information given. Participants: 27 women with uncomplicated twin pregnancies at 28‐30 weeks' gestation. Interventions: aspirin 100 mg daily vs placebo. Outcomes: mean placental weight, mean gestation at delivery, mean birthweight. |
| Brazil 1992 | Method of allocation to treatment group not stated. No clinical outcomes reported. Available as an abstract only. Participants: 67 high‐risk women with abnormal doppler at 26 weeks. Interventions: 60 mg aspirin daily vs placebo. |
| Brazil 1995 | Women with pre‐eclampsia, no relevant outcomes reported, randomisation unclear. |
| Brazil 1996a | Study discontinued prematurely due to local problems. A few women recruited but no outcome data available. Study design: not known. Participants: women at 12‐26 weeks with chronic hypertension. Intervention: aspirin 100 mg daily vs placebo. Outcomes: pre‐eclampsia; prematurity; IUGR. |
| Canada 2015 | Comparision of different doses of aspirin |
| China 1991 | Abstract only available in English, no clinical outcomes. Participants: women at risk of PIH. Interventions: aspirin 50 mg vs placebo. |
| China 2016 | Quasi‐randomised study. Allocation 'according to the order of admission to hospital' |
| China 2017 | Quasi‐randomised study. Allocation 'according to the order of hospitalisation' |
| Colombia 1996 | 200 women included in the study, data only presented for 97 who completed the protocol. Study design: randomised trial, no other information. Participants: 200 high‐risk women: primigravidae, with antecedents of PIH or chronic hypertension. Intervention: 100 mg aspirin vs placebo. |
| East Germany 1986 | No clinical outcomes, available as abstract only. Groups not balanced Study design: quote: "prospective randomised study". Participants: 142 women in the 3rd trimester. Interventions: aspirin (96 women) vs no antiplatelet agent (46 women). |
| East Germany 1988 | Method of allocation not stated, described as quote: "double‐blind" but 2 very different interventions. No outcomes reported. Participants: 100 primigravidae with quote: "normal pregnancies". Interventions: aspirin vs magnesium sulphate vs placebo. |
| Egypt 2017 | Not women at risk of pre‐eclampsia. Randomised 60 women with asymmetric IUGR and abnormal Doppler at 28‐32 weeks. |
| Egypt 1991 | Cross‐over study, women with established pre‐eclampsia at trial entry, no relevant clinical outcomes reported. Study design: 'allocated at random', no further information. Participants: 20 primigravid women in the 3rd trimester with SBP >/= 160 mmHg, DBP >/= 110 mmHg, lower limb oedema 2+, and proteinuria 3+ or 4+. Interventions: aspirin 75 mg vs conventional therapy (oral methyldopa, diazepam, and 25% glucose infusion). Outcomes: changes in BP and albuminuria; lower limb oedema; and urine volume. |
| Egypt 1998 | Unclear if randomised trial. Study design: women 'put' into 3 groups (30, 30 and 13). No further information. Imbalance suggests not a randomised trial Participants: 73 women with abnormal uterine artery flow on doppler ultrasound. Intervention: group 1 received aspirin 75 mg/day, group 2 received allylestrenol 5 mg twice daily, and group 3 was control. Outcomes: uterine artery blood flow; pregnancy outcome. No data on clinical outcomes reported. |
| Equador 1998 | No data on clinical outcomes reported. Available as abstract only. Study design: 'randomised'. No further information. Participants: pregnant women who met all inclusion criteria. Intervention: aspirin 100 mg/day vs placebo. |
| ERASME 2003a | Comparison of doppler vs no doppler estimation of uterine artery flow velocities. Study design: randomised trial (2 treatment: 1 control). Participants: 1870 nulliparous women at 14‐20 weeks' gestation. Intervention: doppler at 22 to 24 weeks. If abnormal doppler given 100 mg aspirin until 36 weeks. |
| Finland 1993a | No clinical outcomes reported. Study design: randomised with sealed numbered opaque envelopes. Participants: 14 women with systemic lupus erythematosus. Intervention: 50 mg aspirin vs placebo. |
| Finland 2007 | Aspirin/placebo began prior to pregnancy, for women have invitro fertilisation. |
| France 2001 | Comparison of doppler with no doppler. Study design: multicentre randomised trial. Numbered sealed envelopes. 184 (6%) lost to follow‐up. Participants: 3317 women in routine antenatal clinic. Intervention: doppler, with aspirin if results abnormal, vs no doppler. |
| Germany 1986 | Abstract only, no clinical outcomes reported. |
| India 1986 | Unclear whether randomised trial. Likely that participants include women with established pre‐eclampsia at trial entry. No relevant clinical outcomes reported. Study design: 'double blind'. Participants: 68 women with IUGR and mild‐moderate pre‐eclampsia at 28 weeks' gestation. Intervention: dipyridamole 100 mg x 3/day vs placebo. Outcomes: fetal weight. |
| India 1997 | Quasi‐random study, consecutive women allocated treatment or control. 21/71 women (29%) excluded from the analysis. Participants: 71 women with a positive roll‐over test at 28‐32 weeks, and previous history of essential hypertension or PIH. Interventions: 50 mg aspirin, not stated whether placebo. Outcomes: PIH, gestation at delivery, birthweight. |
| India 1998 | Not a randomised trial. 22 neonates born to women who took aspirin during pregnancy compared to matched controls. Women allocated to aspirin consecutively. |
| India 2001 | 10 out of 50 women (20%) excluded from analysis as a result of loss to follow‐up. Study design: women divided into 2 groups based on random‐number sequence obtained from standard tables. Participants: 50 women at 16‐32 weeks with history of IUGR or PIH +/‐ proteinuria in previous pregnancy after 32 weeks or current twin pregnancy. Intervention: aspirin 1 mg/kg/day vs placebo. Outcomes: hypertension, pre‐eclampsia; maternal mortality; mode of delivery; perinatal death; prematurity; severe IUGR. |
| India 2002 | Quasi‐randomised study. Study design: every alternate woman given aspirin and the others served as controls. Participants: 215 women at 15‐16 weeks with history of either recurrent missed abortion or IUGR or unexplained stillbirth or pre‐eclampsia/eclampsia remote from term or DVT or chorea gravidarum (B‐HCG > 2 multiples of the mean for that gestation). Intervention: aspirin 1.2 mg/kg/day vs no treatment. Outcomes: early onset pre‐eclampsia, miscarriage, preterm birth, IUGR, birthweight. |
| India 2002a | Unclear whether randomised trial. Study design: not reported. 2 groups: 1 group was given aspirin and the other was given placebo. Participants: 76 high‐risk women (not defined). Intervention: aspirin 50 mg/day vs placebo, until 36 weeks. Outcomes: hypertension; mild and severe PIH; eclampsia; duration of pregnancy; duration of labour; mode of delivery; APH; PPH; fetal death; birthweight; Apgar score; neonatal complications. |
| India 2011 | Compares aspirin with or without antioxidant ‐ Excluded because both groups get aspirin. Study design: prospective randomised comparative study Participants: 60 primigravida women with mild PIH Intervention: licopene 2 g + 75 mg aspirin vs 75 mg aspirin alone Outcomes: pre‐eclampsia, mode of delivery, fetal outcome |
| Iran 2002 | Likely inadequate concealment of allocation. Random‐number tables for allocation, without any mention of blinding, and imbalances between the groups at trial entry. Study design: allocation using random number tables, no further information. Participants: 990 nulliparous women, < 20 weeks. Intervention: aspirin 75 g vs 500 mg calcium vs no treatment. |
| Iran 2013 | There are concerns about high risk of bias. Unclear whether exclusions were pre‐ or post‐randomisation Study design: randomised, double‐blind, placebo‐controlled trial with parallel assignment Participants: 64 pregnant women at higher risk for gestational hypertension and pre‐eclampsia Intervention:100 mg/day aspirin at awakening vs 100 mg/day aspirin at bed time vs placebo at awakening or bed time Outcomes: BP, pre‐eclampsia, maternal complications, fetal complications |
| Iran 2014 | Quasi‐random study ‐ allocation by odd and even dates for referral |
| Iran 2016 | Women with pre‐eclampsia recruited, not a trial of prevention of pre‐eclampsia |
| Iran 2017 | Not prevention of pre‐eclampsia. Study recruited women with history of recurrent miscarriage when they were not pregnant |
| Ireland 1995 | Comparison of 2 different aspirin preparations. No clinical outcomes. Study design: quote: "randomly assigned in a double‐blind fashion". Participants: 18 normotensive women and 18 women with pre‐eclampsia. Intervention: aspirin 75 mg/day vs controlled‐release aspirin 75 mg/day. Outcomes: no clinical outcomes reported. |
| Ireland 2014 | Pilot trial to assess feasibility of a large trial. Clinical outcomes not reported |
| Israel 2006 | The comparator is an anticoagulant (enoxaparin), not an antiplatelet. As anticoagulants are not used for the prevention of pre‐eclampsia, this study is not eligible. Study design: multicentre randomised comparative cohort study Participants:104 women with unexplained recurrent miscarriages Interventions: aspirin 100 mg/day vs enoxaparin 40 mg/day Outcomes: uterine blood flow, umbilical blood flow, incidence of haemorrhage, thrombocytopenia, allergic reactions, live births, birth weight, incidence of major or minor clinically significant haemorrhages, presence of congenital malformations |
| Italy 1988 | Inadequate allocation concealment. Study design: randomised trial using open random‐number tables. Participants: 34 high‐risk women. Interventions: heparin 15,000 IU/day sc and dipyridamole 300 mg/day, compared with untreated controls. |
| Italy 1990 | Does not seem to have been randomised. Described as 'random selection' of women with PIH, but control group did not have PIH. Participants: 20 women with PIH at < 36 weeks' gestation. Interventions: picotamide vs no treatment. Outcomes: no data reported on clinical outcomes. |
| Italy 1990a | Quote: "Random selection" of women with PIH but control group did not have PIH. Participants: 63 pregnant women at risk of pre‐eclampsia Intervention: heparin calcium 15,000 IU/day sc and dipyridamole 225 mg/day by mouth vs no treatment Outcomes: pre‐eclampsia, perinatal death, IUGR, gestational age at birth, emergency caesarean section |
| Italy 1991 | Does not appear to be a randomised trial. |
| Italy 1994 | Doesn't appear to be an RCT Participants: at risk patients identified through a ‘double gate’ selection method based on parity, obstetric history, increased vascular resistance in the uteroplacental districts Intervention: low dose aspirin (100 mg) vs placebo Outcomes: gestational hypertension, proteinuria, IUGR, fetal growth retardation |
| Italy 2002 | Not a randomised trial. Study design was a retrospective case‐control study. Participants: 52 women at 12 weeks with chronic hypertension. Intervention: aspirin 100 mg/day plus antihypertensive treatment vs antihypertensive treatment alone. Outcomes: PIH, pre‐eclampsia, severe SGA, gestation at delivery, preterm delivery < 34 weeks, birthweight. |
| Italy 2005 | Comparison of anticoagulant (low molecular weight heparin) with no treatment. Study design: computer‐generated random‐number sequence. Participants: 85 women with history of pre‐eclampsia and DD genotype for angiotensin‐converting enzyme. Intervention: low molecular weight heparin 5000 IU/day vs no treatment. Outcomes: pre‐eclampsia; IUGR; gestation at delivery; birthweight. |
| Italy 2006 | The comparator is an anticoagulant (enoxaparin), not an antiplatelet. As anticoagulants are not used for the prevention of pre‐eclampsia, this study is not eligible. Study design: randomised Participants: women with 2 or more unexpected pregnancy loss Interventions: 100 mg/day aspirin vs 40 mg/day enoxaparin vs 100 mg/day aspirin + 40 mg/day enoxaparin Outcomes: pregnancy losses, live births |
| Italy 2009 | Not prevention of pre‐eclampsia. Women with recurrent miscarriage |
| Japan 1989 | Quasi‐random allocation on the basis of odd and even record numbers, women with established pre‐eclampsia at trial entry, and no clinical outcomes reported. Participants: 40 women with pre‐eclampsia. Interventions: antithrombin III concentrate vs no treatment. |
| Libya 2000 | Abstract only. Large imbalance between the groups in an open study (538 vs 372), no information about concealment of allocation. Study design:quote: "randomised". Participants: 910 primigravid women. Interventions: aspirin 150 mg/day from 20 weeks vs no aspirin. |
| New Zealand 1990 | No outcomes reported, this was a feasibility study and the trial was abandoned due to poor recruitment. Study design: quote: "randomised trial" no other information. Participants: 4 nulliparous women < 16 weeks. Interventions: aspirin 100 mg vs placebo. |
| New Zealand 1998 | Secondary prevention trial > 20% of recruited women excluded. 34/99 (34%) women excluded as <14 days on trial treatment. Study design: randomised trial. Participants: 99 women with normal anatomy scan < 20 weeks and ultrasound diagnosis of IUGR at 24‐36 weeks, plus abnormal umbilical doppler. Interventions: 100 mg aspirin daily vs placebo. Outcomes: caesarean section, birthweight, baby deaths, days in hospital for the baby. |
| New Zealand 2000 | 10 women (20%) excluded because antibody levels had not met eligibility criteria, or second test normal. Study design: computer‐generated sequence, sealed numbered opaque envelopes. Participants: 50 women with antiphospholipid syndrome, 3 or more miscarriages and 1 or more antibodies increased. Interventions: aspirin 75 mg vs placebo. Outcomes: PIH, pre‐eclampsia, caesarean section, preterm delivery, SGA. |
| Pakistan 1994 | Comparison of aspirin with antihypertensive drugs. Method of allocation unclear, but the description implies quasi‐randomisation. Study design: consecutive women randomly divided into 2 treatment groups. Participants: 200 women with either a previous history of pre‐eclampsia or eclampsia, or BP 140/90 x 2 15 days apart or mild essential hypertension. Intervention: 75 mg aspirin x 2/day vs routine antihypertensive drugs if BP > 100 mmHg. |
| Panama 2014 | Treatment of women with mild‐moderate chronic hypertension, with aspirin used for the control group Study design: randomisation using computer‐generated codes in blocks of 6. Concealment of allocation using sealed envelopes. Participants: women with mild‐moderate chronic hypertension before 20 weeks. Inverventions: furosemide, amlodipine, aspirin. |
| Poland 1996 | Not a randomised trial. Participants: 165 pregnant women who were either healthy or with EPH gestosis Intervention: aspirin 150 mg/day vs no treatment Outcomes: mean BP, plasma rennin activity, and plasma concentration of aldosterone, ANP and vasopressin |
| Poland 1999 | Comparison of aspirin with another therapy. Abstract only. Large imbalance between groups (22 vs 9) in an open study with no information about concealment of allocation. Study design: quote: "randomly assigned" in 2 to 1 ratio. No further details. Participants: 31 women with IUGR. Interventions: aspirin (1.5 mg/kg) versus 'standard treatment' with Sadamin, Partusisten, infusion of amino acids and glucose. Outcomes: gestational age at delivery, birthweight, IUGR. |
| Russia 1997 | Comparison of aspirin plus dipyridamole with glyceryl trinitrate. Randomisation: 'blinded randomisation' stratified by essential hypertension or chronic glomerulonephritis. No further information. Participants: 76 women at 16‐20 weeks with mild‐moderate hypertension, with either essential hypertension or CGN. Intervention: glyceryl trinitrate skin patch 5 mg, increasing to 20 mg if tolerated for 12 hours/day vs aspirin 125 mg/day and dipyridamole 150 to 225 mg/day. Outcomes: progressive gestational hypertension; increase in proteinuria; development or progression of renal failure; abruption; pregnancy complications; side‐effects; miscarriage or stillbirth; preterm birth; IUGR. |
| Slovenia 1998 | Quasi‐random study. Study design: quote: "randomly allocated" into 3 groups with odd and even numbers. Participants: 48 women at high risk for gestational hypertension. Interventions: aspirin 100 mg/day vs n‐3 fatty acids 300 mg/day vs no treatment. |
| Spain + others 2000 | 172/768 (22%) women lost to follow‐up. Abstract only. Study design: randomisation by sealed opaque numbered envelopes. Multicentre randomised trial in Spain, Portugal, Ecuador, Argentina and Brazil. Participants: women at 12‐16 weeks' gestation, BP < 135/85 and no proteinuria. Interventions: aspirin 100 mg vs placebo. |
| Sweden 2017 | Not women at risk of pre‐eclampsia. Trial to prevent miscarriage |
| Thailand 1996 | PARIS assessment noted that this is a quasi‐randomised study. Study design: randomisation by odd and even numbers based on last 2 digits of MRN. PARIS assessment also revealed that the trial had 32% loss to follow‐up with complete data available on only 1020 of 1500 women enrolled. |
| Trinidad 1998 | Not a randomised comparison of aspirin with placebo. Study design: alternate allocation to supplemented or control group, and the supplemented group randomised using random‐number tables to 3 intervention groups. Participants: 510 women, primigravid or with previous pre‐eclampsia. Interventions: 1200 mg calcium versus 60 mg calcium + 80 mg aspirin vs 80 mg aspirin vs control. |
| Tunisia 1989 | Not a randomised trial. Concurrent controls. Participants: 60 women with previous hypertension in pregnancy. Interventions: aspirin 250 mg 2nd daily and dipyridamole 300 mg daily if < 12 weeks' gestation or standard treatment if 12‐20 weeks. |
| Tunisia 1990 | Comparison of aspirin alone with aspirin plus dipyridamole |
| Turkey 1994 | Unclear whether randomised trial, and unable to get in touch with authors. Participants: 80 women between 8 and 32 weeks pregnant, having a history of at least 1 previous pregnancy complicated by 1 of the following, for which there was no known cause: spontaneous abortion, intrauterine fetal demise, pre‐eclampsia, or primipara age in current pregnancy Intervention: 60 mg/day aspirin vs no treatment Outcomes: mode of birth, spontaneous abortion, prematurity, pre‐eclampsia, IUGR, Apgar, weight |
| UK 1992a | No clinical outcomes reported. Study design: quote: "randomised trial" no further information. Participants: 52 high‐risk women > 24 weeks' gestation. Interventions: aspirin 75 mg/day vs placebo. |
| UK 1993 | Study of bleeding times in a subgroup of a larger trial evaluting the effect of low‐dose aspirin on pregnancy‐induced hypertension. No clinical outcomes reported. The full trial report does not appear to have been published. Participants: 30 women. Intervention: aspirin vs placebo. |
| UK 1994 | Paper retracted by journal editors, suspected fraud. |
| UK 2000 | Concern about potential for major bias: concealment of allocation not adequate, no placebo, active group had different care to control group (see below). Study design: sealed envelopes, no further details. Participants: 116 women at 19‐21 weeks with abnormal uterine artery doppler. Intervention: aspirin 100 mg. Doppler repeated at 24 weeks, if normal aspirin stopped. If persistent notching aspirin continued and further scans every 4 weeks. Control group, no aspirin, no routine doppler. Usual antenatal care. |
| USA 1989 | Participants were 40 normal pregnant women in the 3rd trimester, not women with pre‐eclampsia or considered to be at risk of pre‐eclampsia. Study design: allocation by sealed opaque numbered envelopes. Interventions: aspirin 20 mg, or 60 mg or 80 mg daily vs placebo (4 groups). Outcomes: APH, PPH, stillbirth, mean birthweight, Apgar scores. |
| USA 1990 | Interim report of 20 women from a study with a planned sample size of 160. Study design described as quote: “prospective placebo‐controlled, double‐blind study”, with no other information given. Not stated whether randomised study. Participants: primiparous women, with ultrasound confirmation of dates < 20 weeks’ gestation. Intervention: aspirin 80 mg daily vs placebo. Percentages only reported with no denominators. Published as abstract only. |
| USA 1993b | Comparison of prednisone + aspirin with aspirin alone. Study design: sequential opaque envelopes. Participants: 39 antiphospholipid antibody positive women. |
| USA 1993c | No clinical outcomes reported. Study design: quote: "randomised double‐blind crossover". Participants: 24 women with hypertension or other complications. Intervention: acetaminophen vs indomethacin versus control. |
| USA 1996 | Women with uncomplicated pregnancy. No relevant outcomes. Study design: randomised, no other information. Participants: 12 women with uncomplicated pregnancy at 28‐34 weeks. Intervention: 4 groups. Aspirin 20 mg vs 40 mg vs 80 mg vs placebo. Outcomes: haematological measures only. |
| USA 2012 | Data reported for 30/53 (57%) women, with 43% lost to follow‐up. |
| USA 2013 | Aspirin was administered pre‐conception. |
| Vietnam 2017 | Abstract only, but does not appear to be a randomised trial. Women who received aspirin appear to be compared to all pregnancies. |
| West Germany 1977 | Unclear if randomised trial. No clinical outcomes reported, published in abstract only. Study design: quote: "double‐blind trial". Participants: 40 women with suspected early IUGR, 30‐33 weeks. Intervention: dipyridamole vs placebo. |
ANP: atrial natriuretic peptide APH: antepartum haemorrhage BP: blood pressure CGN: chronic glomerulonephritis DPB: diastolic blood pressure DVT: deep vein thrombosis EPH: edema, proteinuria, hypertension HCG: human chorionic gonadotrophin IU: international unit IUGR: intrauterine growth restriction MRN: medical record number PIH: pregnancy‐induced hypertension PPH: postpartum haemorrhage RCT: randomised controlled trial SBP: systolic blood pressure sc: subcutaneous SGA: small‐for‐gestational age vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
Abdi 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Agrawala 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Amro 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Benavides 2016.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
China 2012.
| Methods | 'Randomly allocated' |
| Participants | Women at high risk of pre‐eclampsia between 13 and 16 weeks' gestation |
| Interventions | Aspirin 75 mg at bedtime vs placebo |
| Outcomes | Pre‐eclampsia; birthweight; preterm birth; perinatal death; placental abruption |
| Notes | Published in Chinese, abstract only in English. |
Cobaleda 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Finneran 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Finneran 2019a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
India 1993a.
| Methods | Randomised |
| Participants | Women with previous fetal loss > 20 weeks or IUGR in previous pregnancy |
| Interventions | Aspirin vs placebo |
| Outcomes | |
| Notes | Trial in progress in 1993, no longer recruiting. No data available. Further information requested from trialists |
Iran 2017a.
| Methods | Randomised |
| Participants | Singleton pregnancy at 11 to 14 weeks with abnormal uterine artery Doppler |
| Interventions | Aspirin 80 mg vs placebo |
| Outcomes | Pre‐eclampsia; preterm birth; intrauterine growth restriction; birthweight |
| Notes |
Lin 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Matthews 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Meher 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Mirzamoradi 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Mone 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Mone 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Mulcahy 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
NCT03574909 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
NCT03674606 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Netherlands 1991.
| Methods | Study design: quote: "randomly allocated", no other information. |
| Participants | 41 women with 2 or more previous pregnancies complicated by severe IUGR and placental infarction, no other complications. |
| Interventions | Aspirin 1 mg/kg daily and dipyridamole 75 mg x 3 daily vs aspirin alone. From 12 to 34 weeks. |
| Outcomes | No outcomes reported. |
| Notes | Available as an abstract only. Currently, this intervention is not included in the analyses for the review, but may be included in future updates. |
Netherlands/UK 1994.
| Methods | Study design: quote: "double‐blind randomised". |
| Participants | 193 primiparous women with resistance index 0.58, or more, in 1 or both arcuate arteries at 24 weeks. |
| Interventions | Allylestrenol 25 mg vs aspirin 60 mg/day vs placebo. |
| Outcomes | Pre‐eclampsia, fetal growth restriction |
| Notes | No clinical data available. Published as abstract only. |
O'Brien 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Poon 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Poon 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Qi 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Rood 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Sallam 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Sallam 2018a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Slovenia 1992.
| Methods | Study design: quote: "randomly allocated", no other information. |
| Participants | 43 women at risk of pre‐eclampsia on the basis of their obstetric history. |
| Interventions | Aspirin 150 mg + dipyridamole 225 mg daily from 16 weeks until delivery vs no treatment. |
| Outcomes | Pre‐eclampsia, mode of delivery, birthweight |
| Notes | Allocation 18 women to antiplatelets and 25 to control. No outcomes reported. Available as an abstract only. |
Slovenia 1994.
| Methods | Study design: "randomly allocated". |
| Participants | 20 women at high risk for gestational hypertension on the basis of obstetric history |
| Interventions | Aspirin 100 mg/day vs n‐3 fatty acids 3 g/day, both from 16 weeks' gestation until delivery |
| Outcomes | Not stated |
| Notes | Abstract only with no data reported. |
South Africa 1986.
| Methods | Study design: sealed numbered envelopes, no other information provided. |
| Participants | 152 primigravid women with normal blood pressure and at first antenatal hospital visit. Recruitment was largely in the second and third trimester |
| Interventions | 75 mg aspirin daily vs placebo |
| Outcomes | Pregnancy‐induced hypertension, neonatal outcome |
| Notes | Published in abstract only, no data available. |
Sutton 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Switzerland 2000.
| Methods | Study design: randomly assigned, no other information. |
| Participants | Women with a high‐risk pregnancy |
| Interventions | Aspirin 100 mg/day vs aspirin plus low molecular weight heparin. |
| Outcomes | Gestational hypertension, pre‐eclampsia, perinatal death, preterm birth, birthweight |
| Notes | Interim report of 24 women randomised ‐ of whom 2 had a miscarriage and 16 had completed the trial. Available as an abstract only. |
Uganda 1992.
| Methods | Trial planned in 1992, no further information |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
USA 1988a.
| Methods | Trial registered as planned in 1988, but no data published. Study design described as 'coded drugs'. |
| Participants | Women with a history of previous stillbirth or IUGR but negative for systemic lupus and lupus anticoagulant. |
| Interventions | Aspirin vs placebo. |
| Outcomes | |
| Notes | No clinical data available. |
USA 1990a.
| Methods | Registered as a planned trial in 1990. Recruitment due to start in November 1990, but no further information available. |
| Participants | Multiparous women with a multiple pregnancy. |
| Interventions | Aspirin vs placebo. |
| Outcomes | |
| Notes |
Wright 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Wright 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
Zhang 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in September 2019 prepublication search. Not yet assessed. |
IUGR: intrauterine growth restriction vs: versus
Characteristics of ongoing studies [ordered by study ID]
ASPIRIN trial 2017.
| Trial name or title | Aspirin supplementation for pregnancy indicated risk reduction In nulliparas (ASPIRIN) study |
| Methods | Computer‐generated randomised sequence |
| Participants | Nulliparous women between the ages of 14 and 40, with a singleton pregnancy between 6 0/7 weeks and 13 6/7 weeks gestational age (GA) confirmed by ultrasound prior to enrolment, no more than 2 previous first trimester pregnancy losses, and no contraindications to aspirin |
| Interventions | Daily administration of low‐dose (81 mg) aspirin, initiated between 6 0/7 weeks and 13 6/7 weeks GA and continued to 36 0/7 weeks GA, compared to an identical appearing placebo. |
| Outcomes | Primary outcome: incidence of PTB (birth prior to 37 0/7 weeks GA). Secondary outcomes: incidence of pre‐eclampsia/eclampsia, SGA and perinatal mortality. |
| Starting date | March 2016 |
| Contact information | |
| Notes | Being conducted in 7 low‐ and middle‐income countries |
China 2016a.
| Trial name or title | Low dose aspirin in the prevention of preeclampsia in China (APPEC) |
| Methods | Randomised |
| Participants | Women at 12 to 20 weeks' gestation with risk factors for pre‐eclampsia |
| Interventions | Aspirin 100 mg at night until 34 weeks vs placebo |
| Outcomes | Pre‐eclampsia, fetal growth restriction, PTB, placental abruption, maternal haemorrhage, neonatal intracranial haemorrhage |
| Starting date | November 2016 |
| Contact information | |
| Notes |
Egypt 2015.
| Trial name or title | Comparison between the roles of low dose aspirin and folic acid in preventing preeclampsia among high risk women screened by uterine artery Doppler at 22‐24 weeks of gestation: a randomised controlled trial |
| Methods | Randomised, using computer‐generated random numbers and sealed opaque envelopes |
| Participants | Women with high uterine artery resistance (Doppler resistance index exceeds 0.68 with or without an early diastolic notch) at 22‐ 24 weeks' gestation and high risk for developing pre‐eclampsia based on having any of the following risk factors: chronic hypertension, pre‐gestational diabetes mellitus, body mass index (BMI) > 30 kg/m² or past history of pre‐eclampsia |
| Interventions | 75 mg aspirin and 1 g folic acid per day, throughout pregnancy vs no treatment |
| Outcomes | Pre‐eclampsia, early onset pre‐eclampsia (before 34 weeks), severe pre‐eclampsia |
| Starting date | 07/05/2013 |
| Contact information | AbdelGany Hassan email: abdelgany2@gmail.com |
| Notes | Sponsor: Cairo University. Planned sample size: 210 |
France 2012.
| Trial name or title | Prevention of pre‐eclampsia and SGA by low‐dose aspirin in nulliparous women with abnormal first‐trimester uterine artery dopplers (PERASTUN) |
| Methods | Randomised |
| Participants | Nulliparous pregnant women before 16 seeks gestation selected as "high‐risk" by the presence of a bilateral uterine artery notch and/or bilateral uterine artery PI ≥ 1.7 during the first trimester ultrasound scan (11‐13 + 6 weeks), |
| Interventions | Aspirin 160 mg at bedtime vs placebo |
| Outcomes | Pre‐eclampsia, SGA, perinatal death, adverse effects, mode of delivery |
| Starting date | June 2012 |
| Contact information | |
| Notes | Multicentre trial |
Ghana 2016.
| Trial name or title | Prospects for the prevention of pregnancy‐induced hypertension and preeclampsia (4P) ‐ a randomised, placebo‐controlled, double‐blind clinical trial |
| Methods | Randomised trial |
| Participants | Women at risk of pre‐eclampsia, < 16 weeks' gestation |
| Interventions | 1. Combined aspirin and multinutrient supplement. Single capsule with 80 mg low‐dose aspirin, 1.2 g calcium, 600 IU vitamin D, 5 mg folic acid and 1000 ug vitamin B12 mixed. 2. placebo |
| Outcomes | Pregnancy‐induced hypertension, pre‐eclampsia, maternal death, eclampsia, haemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome, haemorrhage, caesarean section, other complications during pregnancy or delivery. For the baby: preterm birth, Intrauterine death, stillbirth, neonatal mortality, congenital abnormality, NICU admission or paediatrician referral, birthweight, SGA, Apgar scores, other adverse effects. Infant outcomes: weight and height, health, occurrence of disease and general health status |
| Starting date | January 2018 |
| Contact information | Joyce L. Browne, UMC Utrecht |
| Notes |
India 2018.
| Trial name or title | Pilot interventional study by different low doses of aspirin versus placebo for prevention of preeclampsia ‐ A double blind randomized control trial |
| Methods | Randomised. Sequentially numbered, sealed, opaque envelopes |
| Participants | Women with a history of pre‐eclampsia or eclampsia, with mean arterial pressure of more than 90th centile and uterine artery raised pulsatility index of more than 90th centile |
| Interventions | 3 groups: aspirin 150 mg or 75 mg, and placebo |
| Outcomes | Pre‐eclampsia, eclampsia, placental abruption, intrauterine growth restriction, stillbirth |
| Starting date | September 2016 |
| Contact information | |
| Notes |
Iran 2013.
| Trial name or title | Using of aspirin in the prevention of preeclampsia |
| Methods | Randomised, no other information |
| Participants | Women with a singleton pregnancy at 11‐14 weeks with risk factors for pre‐eclampsia, such as; nulliparity |
| Interventions | Aspirin 80 mg daily after lunch vs no treatment |
| Outcomes | Pre‐eclampsia, intrauterine growth restriction, preterm birth, stillbirth, transfusion for the mother |
| Starting date | |
| Contact information | |
| Notes |
Iran 2015.
| Trial name or title | Effect of low dose aspirin in prevention of adverse pregnancy outcomes in women with unexplained elevated alpha‐protein in second trimester screening |
| Methods | Randomised |
| Participants | Pregnant women with second‐trimester positive screening test for neural tube defect and alpha‐fetoprotein ≥ 2.5 MOM |
| Interventions | Aspirin 80 mg to 34 weeks, vs no treatment |
| Outcomes | Pre‐eclampsia, preterm birth, IUGR, admission to neonatal unit, gestation at birth, fetal death |
| Starting date | |
| Contact information | Bahadori Fatemeh: fbahadory27@yahoo.com |
| Notes |
Iran 2015a.
| Trial name or title | Evaluation of the effect of aspirin in pregnancy outcomes in women with abnormal down syndrome biochemical tests and with normal karyotype |
| Methods | Randomised |
| Participants | Pregnant women with abnormal biochemical tests in second trimester which have normal karyotype |
| Interventions | Aspirin 80 mg to 34 weeks' gestation vs no treatment |
| Outcomes | Preterm birth, fetal death, pre‐eclampsia, IUGR placental abruption |
| Starting date | April 2014 |
| Contact information | |
| Notes |
Ireland 2016.
| Trial name or title | An open‐label randomized‐controlled trial of low dose aspirin with an early screening test for pre‐eclampsia and growth restriction (TEST) |
| Methods | Randomised |
| Participants | Nulliparous low‐risk women between 11 and 14 weeks' gestation |
| Interventions | 3 arms: aspirin 75 mg, no aspirin, and aspirin depending on results of a screening test for pre‐eclampsia |
| Outcomes | Acceptablity and compliance; pre‐eclampsia, preterm birth, admission to neonatal unit, placental abruption, intra uterine growth restriction, baby death |
| Starting date | |
| Contact information | |
| Notes | Stduy completed, but trial report not yet available. |
Netherlands 2017.
| Trial name or title | Low dose aspirin in the prevention of recurrent spontaneous preterm labour ‐ the April study: a multicenter randomized placebo controlled trial. |
| Methods | Web‐based randomisation |
| Participants | Women with singleton pregnancy at 8 to 16 weeks' gestation with previous spontaneous preterm birth in a singleton pregnancy |
| Interventions | Aspirin 80 mg vs placebo |
| Outcomes | Spontaneous preterm birth, adverse neonatal and maternal outcomes |
| Starting date | Jan 2016 |
| Contact information | |
| Notes | Multicentre trial |
Spain 2012.
| Trial name or title | Prevention of preeclampsia with aspirin in recipients of donated oocytes. (PROVAS) |
| Methods | Randomised |
| Participants | Women who are between 5 and 10 weeks' gestation following oocyte donation |
| Interventions | Aspirin 100 mg vs placebo until 36 weeks' gestation |
| Outcomes | Pre‐eclampsia, preterm birth |
| Starting date | May 2014 |
| Contact information | |
| Notes |
Thailand 2017.
| Trial name or title | Combined therapy with low dose aspirin and calcium supplements during second trimester to reduce the risk of superimposed preeclampsia in pregnant women with chronic hypertension: a randomized‐controlled trial |
| Methods | Randomised |
| Participants | Women with chronic hypertension at 13‐16 weeks' gestation |
| Interventions | 1. 81 mg aspirin and calcium carbonate 1500 mg 2. placebo |
| Outcomes | Pre‐eclampsia |
| Starting date | July 2017 |
| Contact information | Sukanya Chaiyarach: sukanyatanoorat@hotmail.com |
| Notes |
IU: international units IUGR: intrauterine growth restriction MOM: multiples of the median NICU: neonatal intensive care unit PTB: preterm birth SGA: small‐for‐gestational age vs: versus
Differences between protocol and review
Objective
The previous version of this review included a second objective ("If antiplatelets were effective, our second objective was to determine which of these agents was best and to compare antiplatelet agents with other interventions"). For this update, we removed this second objective from the scope of the review. Because the review is now so large, this objective would be better covered by a separate companion review to this one.
Search methods
For this update, we added in a search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP). In addition, we removed the text for additional searches of the Cochrane Central Register of Controlled Trials (the Cochrane Library 2014, Issue 1) and Embase (1994 to January 2013), as these searches have not been updated since 2014.
Methods
Types of studies ‐ we have clarified that studies only published in abstract format were eligible for inclusion if sufficient information was available. Cluster‐randomised controlled trials were eligible (but none were identified).
Types of participants
In the previous version
Pregnant women considered to be at risk of developing pre‐eclampsia. This included women with normal blood pressure and those with chronic hypertension, as well as women with pregnancy‐induced or gestational hypertension.
Women who were either normotensive, or had chronic hypertension without superimposed pre‐eclampsia at trial entry were classified as being at high risk if they had one or more of the following: previous severe pre‐eclampsia, diabetes, chronic hypertension, renal disease, or autoimmune disease. Moderate risk was defined as any other risk factors, in particular first pregnancy, a mild rise in blood pressure and no proteinuria, abnormal uterine artery doppler scan, positive roll‐over test, multiple pregnancies, a family history of severe pre‐eclampsia, and being a teenager. When risk was unclear or unspecified, women were classified as moderate‐low risk.
In the current version
Types of participants
Pregnant women considered to be at risk of developing pre‐eclampsia. This included women with normal blood pressure and those with chronic hypertension, as well as women with pregnancy‐induced or gestational hypertension.
For studies where IPD were available, individual women were classified into risk categories based on the criteria in the PARIS protocol (Askie 2007). They were classified as low risk if they had any one of the following risk factors: primiparity, family history of pre‐eclampsia, age greater than 40 years, or multiple pregnancy. Women were classified as moderate risk if they had any two of the previous risk factors. Women were classified as high risk if they had any one of the following risk factors: diabetes, chronic hypertension, renal disease, autoimmune disease, gestational hypertension, positive uterine artery Doppler, previous pre‐eclampsia or previous fetal/neonatal death associated with pre‐eclampsia. Women with no identifiable risk factor in the available IPD were grouped with the low‐risk women. Hence for studies where IPD were available, data from one trial could appear in more than one 'maternal risk' category and in more than one 'gestational age at randomisation' category, as individual women within each trial could be classified into different risk and gestational age subgroups.
For studies where aggregate data (AD) only were available, inclusion criteria were used to group women into low, moderate, high or unclassified risk according to the same criteria outlined above, so that categorisation was consistent with IPD analyses. Women in a particular trial could be placed in one category only (unless appropriate subgroup data were available from the trial publication), as IPD were not available.
Types of outcomes
We have added that, for trials with IPD, outcomes were defined using the PARIS 2005 definitions. For trials with only AD available, the trialists' own outcome definitions were used.
We have also separated out our list of outcomes into primary and secondary outcomes.
Data collection and analysis
We have updated our methods to incorporate the standard methods used by Cochrane Pregnancy and Childbirth.
Subgroup analysis and investigation of heterogeneity
We have changed our subgroup analysis by 'type of antiplatelet':
from "type of antiplatelet agent: aspirin, or all other types"
to "low‐dose aspirin alone; all other types of antiplatelet agent and low‐dose aspirin combined with other antiplatelet agents".
GRADE methods
We have added methods for the use of GRADE and inclusion of a 'Summary of findings' table.
Contributions of authors
Marian Knight wrote the first draft of the protocol, which was then modified in discussion with David Henderson‐Smart and James King, and again following comments from others. Marian Knight, Lelia Duley and David Henderson‐Smart did the searches for previous versions of the review, with help from the Cochrane Pregnancy and Childbirth Group, and decided on potentially eligible studies. Searches for this update were done by the Cochrane Pregnancy and Childbirth Group. All the authors decided on potentially eligible studies, and helped with data extraction. Data for previous versions of the review were entered by Marian Knight, Lelia Duley, and David Henderson‐Smart.
For the 2019 update: Lisa Askie and Kylie Hunter added the IPD from the PARIS analysis. Studies were assessed for inclusion by Kylie Hunter and Anna Lene Seidler, who also extracted and entered the data . All authors contributed to checking the data. All authors have contributed to preparing the final report for this update, which was originally drafted by Lelia Duley.
Sources of support
Internal sources
NSW Centre for Perinatal Health Services Research, School of Public Health, University of Sydney, Australia.
Department of Neonatal Medicine, Royal Prince Alfred Hospital, Sydney, Australia.
Department of Perinatal Medicine, Royal Womens Hospital, Melbourne, Australia.
Medical Research Council, UK.
The University of Liverpool, UK.
The University of Nottingham, UK.
NHMRC Clinical Trials Centre, University of Sydney, Australia.
External sources
Health Technology Assessment, UK.
Declarations of interest
Lelia Duley LD was a member of the steering committee for Barbados 1998 and a co‐author of the report, and a member of the PARIS Collaborative Group. She was awarded an NIHR grant for applied research for a programme of work on care at very preterm birth
Lisa Askie received a postdoctoral fellowship (Australian NHMRC 2003‐2006) to conduct the PARIS Collaboration.
Kylie Hunter: none known.
Shireen Meher: none known.
Anna Lene Seidler: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Algeria 2011 {published data only}
- Bakhti A, Vaiman D. Prevention of gravidic endothelial hypertension by aspirin treatment administered from the 8th week of gestation. Hypertension Research ‐ Clinical & Experimental 2011;34(10):1116–20. [DOI] [PubMed] [Google Scholar]
ASPRE 2017 {published data only}
- NCT02301780. Combined multi‐marker screening and treatment with aspirin for pre‐eclampsia prevention. clinicaltrials.gov/ct2/show/record/NCT02301780 (first received 26 November 2014).
- Nicolaides K, ISRCTN13633058. Combined multi‐marker screening and randomised patient treatment with ASpirin for evidence‐based PRE‐eclampsia prevention. isrctn.com/ISRCTN13633058 (first received 28 October 2010).
- O'Gorman N, Wright D, Rolnik DL, Nicolaides KH, Poon LC. Study protocol for the randomised controlled trial: combined multimarker screening and randomised patient treatment with ASpirin for evidence‐based PREeclampsia prevention (ASPRE). BMJ Open 2016;6(6):e011801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon LC, Wright D, Rolnik DL, Syngelaki A, Delgado JL, Tsokaki T, et al. Aspirin for Evidence‐Based Preeclampsia Prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. American Journal of Obstetrics and Gynecology 2017;217(5):585.e1‐585.e5. [DOI] [PubMed] [Google Scholar]
- Poon LC, Wright D, Rolnik DL, Syngelaki A, Delgado JL, Tsokaki T, et al. Aspre trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. American Journal of Obstetrics and Gynecology 2017 [Epub ahead of print]. [DOI] [PubMed]
- Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. New England Journal of Medicine 2017; Vol. 377, issue 7:613‐22. [DOI] [PubMed]
- Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia: a multicentre, double‐blind, placebo‐controlled trial. Ultrasound in Obstetrics and Gynecology 2017;50(Suppl 1):2. [Google Scholar]
- Rolnik DL, Wright D, Poon LC, Syngelaki A, O'Gorman N, Paco Matallana C, et al. ASPRE trial: performance of screening for preterm pre‐eclampsia. Ultrasound in Obstetrics & Gynecology 2017;50(4):492‐5. [DOI] [PubMed] [Google Scholar]
- Senthilhes L, Azria E, Schmitz T. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. New England Journal of Medicine 2017;377(24):2399‐400. [DOI] [PubMed] [Google Scholar]
- Wright D, Poon LC, Rolnik DL, Syngelaki A, Delgado JL, Vojtassakova D, et al. Aspre trial: influence of compliance on beneficial effect of aspirin in prevention of preterm preeclampsia. American Journal of Obstetrics and Gynecology 2017;217(6):685.e1‐5. [DOI] [PubMed] [Google Scholar]
- Wright D, Rolnik DL, Syngelaki A, Paco Matallana C, Machuca M, Alvarado M, et al. Aspirin for evidence‐based preeclampsia prevention trial: effect of aspirin on length of stay in the neonatal intensive care unit. American Journal of Obstetrics and Gynecology 2018;218(6):612.e1‐612.e6. [DOI] [PubMed] [Google Scholar]
Australia 1988 {published data only}
- Trudinger BJ, Cook CM, Connelly AJ, Giles WB, Thompson RS. Aspirin improves fetal weight in placental insufficiency. Proceedings of 8th Annual Meeting of the Society of Perinatal Obstetricians; 1988 February 3‐6; Las Vegas, Nevada, USA. 1988:10.
- Trudinger BJ, Cook CM, Thompson RS, Giles WB, Connelly AJ. Low‐dose aspirin improves fetal weight in umbilical placental insufficiency. Lancet 1988;2:214‐5. [DOI] [PubMed] [Google Scholar]
- Trudinger BJ, Cook CM, Thompson RS, Giles WB, Connelly AJ. Low‐dose aspirin therapy improves fetal weight in umbilical placental insufficiency. American Journal of Obstetrics and Gynecology 1988;159:681‐5. [DOI] [PubMed] [Google Scholar]
Australia 1993 {published and unpublished data}
- Michael CA, Seville P, Wadeisha P, Walters BN. Randomised double blind placebo controlled trial of aspirin in the prevention of pre‐eclampsia. Proceedings of 7th World Congress of Hypertension in Pregnancy; 1990 October; Perugia, Italy. 1990:73.
- Michael CA, Walters BN. Low‐dose aspirin in the prevention of pre‐eclampsia: current evaluation. In: Teoh ES, Ratnam SS, Macnaughton MC editor(s). Maternal Physiology and Pathology. The Current Status of Gynaecology and Obstetrics Series. Carnforth: Parthenon Publishing Group Limited, 1992:183‐9. [Google Scholar]
Australia 1995 {published data only}
- Newnham JP, Godfrey M, Walters BJ, Philips J, Evans SF. Low dose aspirin for the treatment of fetal growth restriction: a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 1995;35:370‐4. [DOI] [PubMed] [Google Scholar]
Australia 1995a {published data only}
- Kincaid Smith P, North RA, Fairley KF, Kloss M, Ihle BU. Prevention of pre‐eclampsia in high risk women with renal disease: a prospective randomized trial of heparin and dipyridamole. Nephrology 1995;1:297‐300. [Google Scholar]
- North RA, Fairley KF, Kloss M, Ihle B, Kincaid‐Smith P. Prevention of pre‐eclampsia in women with renal disease. Proceedings of 7th World Congress of Hypertension in Pregnancy; 1990 October; Perugia, Italy. 1990:75.
- North RA, Fairley KF, Kloss M, Ihle B, Kincaid‐Smith P. Prevention of preeclampsia in women with renal disease [abstract]. 26th Annual Meeting of the Australasian Society of Nephrology; 1990 March 14‐16; Brisbane, Australia. 1990:9.
- North RA, Fairley KF, Kloss M, Ihle B, Oats J, Kincaid‐Smith P. Prevention of pre‐eclampsia in women with renal disease. Proceedings of 6th International Congress of the International Society for the study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:193.
Australia 1996 {published and unpublished data}
- Ferrier C, North R, Kincaid‐Smith P. Low dose aspirin delays the onset of pre‐eclampsia in pregnancies with abnormal uteroplacental circulation. Proceedings of the 10th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Seattle, Washington, USA. 1996:151.
- Kincaid‐Smith P. [personal communication] Trial to evaluate the role of aspirin (60mg) in the prevention of idiopathic intrauterine growth retardation and pregnancy induced hypertension in primigravid women with abnormal uterine artery waveforms on Doppler examination at 22‐24 weeks gestation. Letter to: Cochrane Pregnancy and Childbirth Group, Oxford UK 30 October 1991.
Australia 1996a {published data only}
- Morris JM, Fay RA, Ellwood DA, Cook C, Devonald KJ. A randomized controlled trial of aspirin in patients with abnormal uterine artery blood flow. Obstetrics & Gynecology 1996;87:74‐8. [DOI] [PubMed] [Google Scholar]
Australia 1997 {published data only}
- Gallery ED, Hawkins M, Ross M, Gyory AZ, Leslie G. Low‐dose aspirin in high risk pregnancy. Proceedings of 9th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney, Australia. 1994:71.
- Gallery ED, Ross MR, Hawkins M, Leslie GI, Gyory AZ. Low‐dose aspirin in high‐risk pregnancy. Hypertension in Pregnancy 1997;16:229‐38. [Google Scholar]
- Leslie GI, Gallery ED, Arnold JD, Ross MR, Gyory AZ. Neonatal outcome in a randomized controlled trial of low‐dose aspirin in high‐risk pregnancies. Journal of Paediatrics and Child Health 1995;31:549‐52. [DOI] [PubMed] [Google Scholar]
Austria 1992 {published data only}
- Schrocksnadel H, Sitte B, Alge A, Stechel‐Berger G, Schwegel P, Pastner E, et al. Low‐dose aspirin in primigravidae with positive roll‐over test. Gynecologic and Obstetric Investigation 1992;34:146‐50. [DOI] [PubMed] [Google Scholar]
- Schrocksnadel H, Sitte B, Alge A, Steckel‐Berger G, Daxenbichler G, Dapunt O. Low‐dose aspirin in prevention and therapy of hypertension in pregnancy [Low‐dose Aspirin in prophylaxe und therapie des schwangerschaftshochdrucks]. Gynakologisch Geburtshilfliche Rundschau 1992;32:90‐1. [DOI] [PubMed] [Google Scholar]
Barbados 1998 {published data only}
- Cruickshank J, Rotchell Y. The Barbados low‐dose aspirin study in pregnancy (BLASP). 8th World Congress on Hypertension in Pregnancy; 1992 November 8‐12; Buenos Aires, Argentina. 1992:49.
- Elder MG, Swiet M, Sullivan M. A randomised trial of low dose aspirin for primiparae in pregnancy (golding)/barbados low dose aspirin study in pregnancy (blasp) (rotchell et al.) [Letter; comment]. British Journal of Obstetrics and Gynaecology 1999;106(2):180. [DOI] [PubMed] [Google Scholar]
- Griffiths J, Rotchell YE, Cruickshank JK, Philips‐Gay M, Stewart A, Farrell B, et al. The Barbados Low‐dose Aspirin Study in Pregnancy (BLASP): a population‐based trial in a developing country with excess pre‐eclampsia and perinatal mortality [abstract]. West Indian Medical Journal 1996;45(2 Suppl):26‐7. [Google Scholar]
- Rotchell YE, Cruickshank JK, Griffiths J, Phillips G, Stuart A, Ayers S, et al. Results of the Barbados low dose aspirin study in pregnancy (BLASP): a population‐based trial in a developing country with excess pre‐eclampsia (PE) and perinatal mortality. 27th British Congress of Obstetrics & Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:31.
- Rotchell YE, Cruickshank JK, Phillips Gay M, Griffiths J, Stuart A, Farrell B, et al. Barbados low dose aspirin study in pregnancy (BLASP): a randomized controlled trial for the prevention of pre‐eclampsia and its complications. British Journal of Obstetrics and Gynaecology 1998;105:286‐92. [DOI] [PubMed] [Google Scholar]
Brazil 1992a {published data only}
- Essinger S. The use of low dose acetylsalicylic acid in prevention of pregnancy‐induced hypertension [Uso do acido acetilsalicilico em baixa dosagem na prevencao da doenca hipertensiva especifica da gestacao (DHEG)]. Revista do Colegio Brasileiro de Cirurgioes 1992;19(2):58‐62. [Google Scholar]
Brazil 1996 {published data only}
- Anonymous. ECPPA: randomised trial of low dose aspirin for the prevention of maternal and fetal complications in high risk pregnancies. British Journal of Obstetrics and Gynaecology 1996;103:39‐47. [DOI] [PubMed] [Google Scholar]
- Atallah AN. ECPPA: randomized trial of low dose aspirin for the prevention of maternal and fetal complications in high risk pregnant women [abstract]. Journal of Clinical Epidemiology 1997;50 Suppl 1:3S. [Google Scholar]
- Atallah AN, Taborda WC. ECPPA: the Brazilian low‐dose aspirin study in high risk pregnant women. Hypertension in Pregnancy 1997; Vol. 16, issue 1:139.
Brazil 2006 {published data only}
- Souza EV, Torloni MR, Atallah AN, Dos Santos GM, Kulay L Jr, Sass N. Aspirin plus calcium supplementation to prevent superimposed preeclampsia: a randomized trial. Brazilian Journal of Medical and Biological Research 2014;47(5):419‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza EV. Aspirin and calcium to prevent preeclampsia in chronic hypertensive women with abnormal uterine artery Doppler ultrasound [abstract] [Acido acetilalicilico associado ao calcio na prevencao da pre‐eclampsia em gestantes hipertensas cronicas selecionadas pela dopplervelocimetria das arterias uterinas]. Revista Brasileira de Ginecologia y Obstetricia 2006;28(2):136. [Google Scholar]
- Souza EV, Sass N, Atallah AN, Kular L Jr. Aspirin and calcium to prevent pre eclampsia in chronic hypertension women with abnormal uterine artery doppler ultrasound [abstract]. Hypertension in Pregnancy 2006;25(Suppl 1):152. [Google Scholar]
Canada 2017 {published data only}
- Bujold E, NCT02280031. Effect of low dose aspirin on birthweight in twins: the GAP trial. clinicaltrials.gov/ct2/show/NCT02280031 (31 October 2014).
- Carpentier C, Bujold E, Camire B, Tapp S, Boutin A, Demers S. P08.03: Low‐dose aspirin for prevention of fetal growth restriction and pre‐eclampsia in twins: the GAP pilot randomised trial. Ultrasound in Obstetrics and Gynecology 2017;50(Suppl 1):178. [Google Scholar]
China 1996 {published data only}
- Wang Z, Li W. A prospective randomized placebo‐controlled trial of low dose aspirin for prevention of intra uterine growth retardation. Chinese Medical Journal 1996;109:238‐42. [PubMed] [Google Scholar]
- Wang Z, Li W. Prevention of growth retardation by low dose aspirin. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics and Gynecology] 1993;28:492‐5. [PubMed] [Google Scholar]
China 1996a {published data only}
- Wu J, Yang WW, Shen WH, He Y. Small dosage aspirin in the prevention of hypertension of pregnancy. Acta Academiae Medicinae Suzhou 1996;16:551‐3. [Google Scholar]
China 1999 {published and unpublished data}
- Rogers MS, Fung HY, Hung CY. Calcium and low‐dose aspirin prophylaxis in women at high risk of pregnancy‐induced hypertension. Hypertension in Pregnancy 1999;18:165‐72. [DOI] [PubMed] [Google Scholar]
- Rogers MS, Hung C, Arumanayagam M. Platelet angiotensin II receptor status during pregnancy in Chinese women at high risk of developing pregnancy‐induced hypertension. Gynecologic and Obstetric Investigation 1996;42:88‐94. [DOI] [PubMed] [Google Scholar]
CLASP 1994 {published data only}
- Bar J, Hod M, Pardo J, Fisch B, Rabinerson D, Kaplan B, et al. Effect on fetal circulation of low‐dose aspirin for prevention and treatment of pre‐eclampsia and intrauterine growth restriction: Doppler flow study. Ultrasound in Obstetrics & Gynaecology 1997;9:262‐5. [DOI] [PubMed] [Google Scholar]
- Bar J, Padoa A, Hod M, Sullivan MH, Kaplan B, Kidron D. Decreased pathological placental findings in aspirin‐treated pregnant women at risk of hypertensive complications. Hypertension in Pregnancy 1997;16:435‐44. [Google Scholar]
- Bower SJ, Harrington KF, Schuchter K, McGirr C, Campbell S. Prediction of pre‐eclampsia by abnormal uterine Doppler ultrasound and modification by aspirin. British Journal of Obstetrics and Gynaecology 1996;103:625‐9. [DOI] [PubMed] [Google Scholar]
- CLASP (Collaborative Low‐dose Aspirin Study in Pregnancy) Collaborative Group. CLASP: a randomised trial of low‐dose aspirin for the prevention and treatment of pre‐eclampsia among 9364 pregnant women. Lancet 1994;343:619‐29. [PubMed] [Google Scholar]
- CLASP Collaborative Group. Low dose aspirin in pregnancy and early childhood development: follow up of the collaborative low dose aspirin study in pregnancy. British Journal of Obstetrics and Gynaecology 1994;102:861‐8. [DOI] [PubMed] [Google Scholar]
- Kyle PM, Buckley D, Kissane J, Swiet M, Redman CW. The angiotensin sensitivity test and low‐dose aspirin are ineffective methods to predict and prevent hypertensive disorders in nulliparous pregnancy. American Journal of Obstetrics and Gynecology 1995;173:865‐72. [DOI] [PubMed] [Google Scholar]
- Mathai M. Aspirin for pre‐eclampsia. National Medical Journal of India 1995;8:23‐4. [PubMed] [Google Scholar]
- Vikhlyaeva K, Asymbekova GU, Zerdev VP. Aspirin in profilaxis and treatment of placental insufficiency: lessons from Russian aspirin experience. Hypertension in Pregnancy 2000;19(Suppl 1):68. [Google Scholar]
Colorado 1993 {published data only}
- Porreco RP, Hickok DE, Williams MA, Krenning C. Low‐dose aspirin and hypertension in pregnancy. Lancet 1993;341:312. [DOI] [PubMed] [Google Scholar]
Egypt 2005 {published data only}
- Ebrashy A, Ibrahim M, Marzook A, Yousef D. Usefulness of aspirin therapy in high‐risk pregnant women with abnormal uterine artery doppler ultrasound at 14‐16 weeks pregnancy: randomized controlled clinical trial. Croatian Medical Journal 2005;46(5):826‐31. [PubMed] [Google Scholar]
EPREDA 1991 {published data only}
- Beaufils M, Uzan S, Breart G, Bazin B, Paris J. Prevention of preeclampsia with low‐dose aspirin: results of the epreda trial. XIth International Congress of Nephrology; 1990 July 15‐20; Tokyo, Japan. 1990:319A.
- Sureau C. Prevention of perinatal consequences of pre‐eclampsia with low‐dose aspirin: results of the EPREDA trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 1991;41:71‐3. [DOI] [PubMed] [Google Scholar]
- Uzan M, Beaufils M, Breart G, Bazin B, Capitant C, Paris J, et al. Prevention of pre‐eclampsia with low‐dose aspirin: results of the EPREDA trial. Journal of Perinatal Medicine 1990;18 Suppl:116. [Google Scholar]
- Uzan S, Beaufils M, Breart G, Bazin B, Capitant C, Paris J. Prevention of fetal growth retardation with low‐dose aspirin: findings of the EPREDA trial. Lancet 1991;337:1427‐31. [DOI] [PubMed] [Google Scholar]
- Uzan S, Beaufils M, Breart G, Bazin B, Capitant C, Paris J. Prevention of pre‐eclampsia with low‐dose aspirin: results of the EPREDA trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 1992;44:12. [Google Scholar]
- Uzan S, Beaufils M, Breart G, Bazin B, Paris J. Prevention of pre‐eclampsia with low‐dose aspirin: results of the EPREDA trial. Proceedings of 7th World Congress of Hypertension in Pregnancy; 1990 October; Perugia, Italy. 1990:34.
- Uzan S, Beaufils M, Breart G, Uzan M, Paris J. Prevention of intrauterine growth retardation and pre‐eclampsia by small doses of aspirin. Results of the French multicenter trial EPREDA and comparison with data in the literature; value of uterine Doppler [Prevention du retard de croissance intra‐uterin et de la preeclampsie par des petites doses d'Aspirine: resultats de l'essai multicentrique francais EPREDA et comparison avec les donnees de la litterature; interet du Doppler uterin]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction (Paris) 1992;21(3):315‐8. [PubMed] [Google Scholar]
ERASME 2003 {published data only}
- ERASME collaborative group. Failure of systematic low dose aspirin to prevent preeclampsia in primigravidae. Hypertension in Pregnancy 2000;19(Suppl 1):34. [Google Scholar]
- Subtil D, Goeusse P, Puech F, Lequien P, Biausque S, Breart G, et al. Aspirin (100mg) used for prevention of pre‐eclampsia in nulliparous women: the Essai Regional Aspirine Mere‐Enfant study (Part 1). BJOG: an international journal of obstetrics and gynaecology 2003;110:475‐84. [DOI] [PubMed] [Google Scholar]
- Subtil D, Quandalle F, Houfflin‐Debarge V, Malek Y, Truffert P, Breart G, et al. Low dose aspirin to prevent preeclampsia: what do we learn from a new "negative" trial? [abstract]. European Journal Obstetrics & Gynecology and Reproductive Biology 2002;104:179. [Google Scholar]
Finland 1993 {published data only}
- Viinikka L, Hartikainen‐Sorri AL, Lumme R, Hiilesmaa V, Ylikorkala O. Low dose aspirin in hypertensive pregnant women: effect on pregnancy outcome and prostacyclin‐thromboxane balance in mother and newborn. British Journal of Obstetrics and Gynaecology 1993;100:809‐15. [DOI] [PubMed] [Google Scholar]
- Viinikka L, Hartikainen‐Sorri AL, Lumme R, Hiilesmaa V, Ylikorkala O. The effect of 50mg of ASA daily on pregnancy outcome and maternal and newborn prostacyclin and thromboxane. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:93.
Finland 1997 {published data only}
- Zimmermann P, Eirio V, Koskinen J, Niemi K, Nyman R, Kujansuu E, et al. Effect of low dose aspirin treatment on vascular resistance in the uterine, uteroplacental, renal and umbilical arteries ‐ a prospective longitudinal study on a high risk population with persistent notch in the uterine arteries. European Journal of Ultrasound 1997;5:17‐30. [Google Scholar]
Finland 1997a {published data only}
- Tulppala M, Marttunen M, Soderstrom‐Anttila V, Ailus K, Palosuo T, Ylikorkala O. Low dose aspirin in prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2. Human Reproduction 1997;12(1):191. [DOI] [PubMed] [Google Scholar]
- Tulppala M, Marttunen M, Soderstrom‐Anttila V, Foudila T, Ailus K, Palosuo T, et al. Low‐dose aspirin in prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2. Human Reproduction 1997;12(7):1567‐72. [DOI] [PubMed] [Google Scholar]
Finland 2002 {published data only}
- Vainio M, Kujansuu E, Iso‐Mustajarvi M, Maenpaa J. Low dose acetylsalicylic acid in prevention of pregnancy‐induced hypertension and intrauterine growth retardation in women with bilateral uterine artery notches. BJOG: an international journal of obstetrics and gynaecology 2002;109:161‐7. [DOI] [PubMed] [Google Scholar]
- Vainio M, Kujansuu E, Riutta A, Maenpaa J. Effect of ASA on the risk of gestational hypertension and prostanoid synthesis in pregnant women screened by Doppler ultrasound. XXXIV Congress of Nordic Federation of Societies of Obstetrics and Gynecology; 2004 June 12‐15; Helsinki, Finland. 2004:21.
- Vainio M, Riutta A, Koivisto AM, Maenpaa J. 9 alpha,11 beta‐prostaglandin f2 in pregnancies at high risk for hypertensive disorders of pregnancy, and the effect of acetylsalicylic acid. Prostaglandins Leukotrienes & Essential Fatty Acids 2003;69(1):79‐83. [DOI] [PubMed] [Google Scholar]
- Vainio M, Riutta A, Koivisto AM, Maenpaa J. Prostacyclin, thromboxane a2 and the effect of low‐dose asa in pregnancies at high risk of hypertensive disorders. Acta Obstetricia et Gynecologica Scandinavica 2004;83:1119‐23. [DOI] [PubMed] [Google Scholar]
Finland 2013 {published data only}
- Kajantie E, Taipale P, Murtoniemi K, Pesonen A‐K, Raikkonen K, Laivuori H, et al. The effect of low‐dose aspirin on placental growth factor concentration in a high‐risk predo cohort. Reproductive Sciences 2018;25(1 Suppl):100A. [DOI] [PubMed] [Google Scholar]
- Taipale P, ISRCTN14030412. Prediction and prevention of preeclampsia, intrauterine growth restriction, prenatal stress and fetal programming of child's psychological development (ongoing trial). isrctn.com/ISRCTN14030412 (first received 28 February 2007).
- Villa P, Kajantie E, Raikkonen K, Pesonen AK, Hamalainen E, Vainio M, et al. Aspirin in the prevention of pre‐eclampsia in high‐risk women: a randomised placebo‐controlled PREDO Trial and a meta‐analysis of randomised trials. BJOG: an International Journal of Obstetrics and Gynaecology 2013;120(1):64‐74. [DOI] [PubMed] [Google Scholar]
- Villa P, Taipale P, Raikkonen K, Hamalainen E, Pesonen A, Kajantie, E, et al. PREDO trial ‐ acetylsalicylic acid in preventing pre‐eclampsia in high‐risk women. Pregnancy Hypertension 2010;1 Suppl 1:S20. [Google Scholar]
France 1985 {published data only}
- Beaufils M, Uzan S, Donsimoni R, Colau JC. A controlled trial of prophylactic treatment of preeclampsia: preliminary results. Archives des Maladies du Coeur 1984;77:1226‐8. [PubMed] [Google Scholar]
- Beaufils M, Uzan S, Donsimoni R, Colau JC. Prevention of pre‐eclampsia by early antiplatelet therapy. Lancet 1985;1:840‐2. [DOI] [PubMed] [Google Scholar]
France 1990 {published data only}
- Azar R, Turpin D. Effect of antiplatelet therapy in women at high risk for pregnancy‐induced hypertension. Proceedings of 7th World Congress of Hypertension in Pregnancy; 1990 October; Perugia, Italy. 1990:257.
Germany 1995 {published data only}
- Nieder J, Claus P, Augustin W. Prevention of pre‐eclampsia and fetal growth retardation by trapidil. Zentralblatt fur Gynakologie 1995;117:23‐8. [PubMed] [Google Scholar]
Germany 2000 {published data only}
- Erdmann M, Paulus WE, Flock F, Herget I, Terinde R, Grab D. Utero and fetoplacental hemodynamic measurements with low dose aspirin [Utero‐und fetoplazentae haemodynamische Messiungen unter low‐dose aspirin]. Zeitschrift fur Gerburtshilfe und Neonatologie 1999;203(1):18‐23. [PubMed] [Google Scholar]
- Grab D, Erdmann M, Paulus W, Oberhoffer R, Lang D. Effects of low dose aspirin on uterine and fetal blood flow during pregnancy: a randomized placebo controlled double blind trial. Acta Obstetricia et Gynecologica Scandinavica 1997;76:38. [DOI] [PubMed] [Google Scholar]
- Grab D, Paulus WE, Erdmann M, Terinde R, Oberhoffer R, Lang D, et al. Effects of low‐dose aspirin on uterine and fetal blood flow during pregnancy: results of a randomized, placebo‐controlled, double‐blind trial. Ultrasound in Obstetrics & Gynecology 2000;15:19‐27. [DOI] [PubMed] [Google Scholar]
India 1991 {published data only}
- Grover V, Sachdeva S, Kumari S. Evaluation of dipyridamole and aspirin in prevention and management of intrauterine growth retardation. Journal of Perinatal Medicine 1991;19(2):104. [Google Scholar]
India 1993 {published data only}
- Rai U, Chakravorty M, Juneja Y. Role of low dose aspirin in PIH. Journal of Obstetrics and Gynaecology of India 1993:883‐6.
India 1994 {published data only}
- Roy UK, Pan S. A study of low dose aspirin in prevention of pregnancy induced hypertension. Journal of the Indian Medical Association 1994;92:188‐91. [PubMed] [Google Scholar]
India 1999 {published data only}
- Shenoy S, Chandrika D, Pisharody R. RCT of low dose aspirin to prevent the progression of pregnancy induced hypertension grade A to B. Journal of Clinical Epidemiology 1999;52 Suppl 1:28S. [Google Scholar]
India 2017 {published data only}
- Sharma N, Srinivasan KJ, Nadhamuni K, Srinivasan S. Role of aspirin in high pulsatility index of uterine artery: a consort study. Journal of Obstetrics and Gynecology of India 2017 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
Iran 1992 {published data only}
- Omrani GH, Karimi MM, Zareh F. Prevention of pregnancy‐induced hypertension by low dose aspirin. Iranian Journal of Medical Sciences 1992;17(3‐4):131‐6. [Google Scholar]
Iran 2006 {published data only}
- Vaseie M. The effect of low‐dose acetylsalicylic acid (ASA) on control of hypertensive in pregnancy [abstract]. Hypertension in Pregnancy 2006;25(Suppl 1):148. [Google Scholar]
Iran 2010 {published data only}
- Kalahroudi MA. The effect of aspirin in prevention of pre eclampsia and its complication in women with abnormal uterine artery doppler ultrasound. https://en.irct.ir/trial/2718 (first received 24 April 2010).
Iran 2012 {published data only}
- Jamal A, IRCT138812123485N1. The effect of metformine on the uteroplacental circulation in comparison with aspirin in pregnant women with poly cystic ovary syndrome. http://en.irct.ir/trial/3595 (first received 6 April 2010).
- Jamal A, IRCT201011295272N1. The effect of metformine on the uteroplacental circulation in comparison with Aspirin in pregnant women with poly cystic ovary syndrome. http://en.irct.ir/trial/5621 (first received 29 December 2012).
- Jamal A, Milani F, Al‐Yasin A. Evaluation of the effect of metformin and aspirin on utero placental circulation of pregnant women with PCOS. Iranian Journal of Reproductive Medicine 2012;10(3):265‐70. [PMC free article] [PubMed] [Google Scholar]
- Jamal A, Milani F, Shabani P, Aleyasin A. The effect of metformin on the uteroplacental circulation in comparison with aspirin in pregnant women with polycystic ovary syndrome. Ultrasound in Obstetrics and Gynecology 2010;36(Suppl 1):1. [Google Scholar]
Iran 2014a {published data only}
- Khazardoost S, IRCT201203018954N1. Aspirin for prevention of adverse pregnancy outcome in women with elevated alfa‐feto protein in second trimester screening,a randomised clinical trial. http://en.irct.ir/trial/9411 (first received 5 June 2012).
- Khazardoost S, Mousavi S, Borna S, Hantoushzadeh S, Alavi A, Khezerlou N. Effect of aspirin in prevention of adverse pregnancy outcome in women with elevated alpha‐fetoprotein. Journal of Maternal‐Fetal and Neonatal Medicine 2014; Vol. 27, issue 6:561‐5. [DOI] [PubMed]
- Mousavi S. Effect of aspirin in prevention of adverse pregnancy outcome in women with elevated alpha‐fetoprotein. Journal of Perinatal Medicine 2015;43(Suppl 1):Abstract no: P‐0743. [DOI] [PubMed] [Google Scholar]
Israel 1989 {published data only}
- Schiff E, Peleg E, Goldenberg M, Rosenthal T, Ruppin E, Tamarkin M, et al. The use of aspirin to prevent pregnancy‐induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high risk pregnancies. New England Journal of Medicine 1989;321:351‐6. [DOI] [PubMed] [Google Scholar]
Israel 1990 {published data only}
- Schiff E, Barkai G, Ben‐Baruch G, Mashiach S. Low‐dose aspirin does not influence the clinical course of women with mild pregnancy‐induced hypertension. Obstetrics & Gynecology 1990;76:742‐4. [DOI] [PubMed] [Google Scholar]
Israel 1994 {published data only}
- Caspi E, Raziel A, Sherman D, Arieli S, Bukovski I, Weintraub Z. Prevention of pregnancy induced hypertension in twins by early administration of low‐dose aspirin: a preliminary report. American Journal of Reproductive Immunology 1994;31:19‐24. [DOI] [PubMed] [Google Scholar]
Italy 1989 {published data only}
- Ballerini S, Valcamonico A, Greorini G, Frusca T, Benigni A, Orisio S, et al. Low dose aspirin (ASA) given to prevent preeclampsia only partially inhibits platelet cyclooxygenase activity. Proceedings of 6th World Congress of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:234.
- Benigni A, Gregorini G, Frusca T, Chiabrando C, Ballerini S, Valcamonico A, et al. Effect of low‐dose aspirin on fetal and maternal generation of thromboxane by platelets in women at risk for pregnancy‐induced hypertension. New England Journal of Medicine 1989;321:357‐62. [DOI] [PubMed] [Google Scholar]
- Frusca T, Gregorini G, Ballerini S, Marchesi D, Bruni M. Low dose aspirin in preventing preeclampsia and IUGR. Proceedings of 6th World Congress of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:232.
Italy 1993 {published data only}
- Italian Study of Aspirin in Pregnancy. Low‐dose aspirin in prevention and treatment of intrauterine growth retardation and pregnancy‐induced hypertension. Lancet 1993;341:396‐400. [PubMed] [Google Scholar]
- Parazzini DF. Low‐dose aspirin in prevention and treatment of intrauterine growth retardation and pregnancy‐induced hypertension. Geburtshilfe und Frauenheilkunde 1993;53:822. [Google Scholar]
- Parazzini F. Multicenter randomized Italian study of low‐dose aspirin for the prevention of pre‐eclampsia and intrauterine growth retardation. Journal of Nephrology 1991;4:127‐9. [Google Scholar]
- Parazzini F, Benedetto C, Frusca T, Guaschino S, Romero M. The Italian study on low‐dose aspirin for the prevention of pre‐eclampsia and intrauterine growth retardation. Proceedings of 7th World Congress of Hypertension in Pregnancy; 1990 October; Perugia, Italy. 1990:32.
- Parazzini F, Bortolus R, Chatenoud L, Restelli S, Benedetto C. Follow‐up of children in the Italian Study of Aspirin in Pregnancy. Lancet 1994;343:1235. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Bortolus R, Chatenoud L, Restelli S, Ricci E, Marozio L, et al. Risk factors for pregnancy‐induced hypertension in women at high risk for the condition. Epidemiology 1996;7(3):306‐8. [DOI] [PubMed] [Google Scholar]
Italy 1999 {published data only}
- Volpicelli T, D'Anto V, Faticato A, Galante L, Civitillo RM, Rappa C, et al. [Trial prospettico sull'uso profilattico dell'aspirina in donne gravide ad alto rischio di preeclampsia]. Gestosi '99. 1999:159‐60.
Italy 2004 {published data only}
- Chiaffarino F, Parazzini F, Paladini D, Acaia B, Ossola W, Marozio L, et al. A small randomised trial of low‐dose aspirin in women at high risk of pre‐eclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;112:142‐4. [DOI] [PubMed] [Google Scholar]
Jamaica 1998 {unpublished data only}
- Elder MG, Swiet M, Sullivan M. A randomised trial of low dose aspirin for primiparae in pregnancy (golding)/barbados low dose aspirin study in pregnancy (blasp) (rotchell et al.) [Letter; comment]. British Journal of Obstetrics & Gynaecology 1999;106(2):180. [DOI] [PubMed] [Google Scholar]
- Forrester TE, Jones D, McCaw‐Binns A, Palmer‐Levy M, Wilks R, Bernard GW, et al. Low‐dose aspirin in pregnancy ‐ are there any benefits? [abstract]. West Indian Medical Journal 1996;45(2 Suppl):26. [Google Scholar]
- Golding J. A randomised trial of low dose aspirin for primiparae in pregnancy. British Journal of Obstetrics and Gynaecology 1998;105:293‐9. [DOI] [PubMed] [Google Scholar]
- Kahwa EK, Sargeant LA, McCaw‐Binns A, McFarlane‐Anderson N, Smikle M, Forrester T, et al. Anticardiolipin antibodies in jamaican primiparae. Journal of Obstetrics and Gynaecology 2006;26(2):122‐6. [DOI] [PubMed] [Google Scholar]
- McCaw‐Binns A, Ashley D, Hawkins N, MacGillivray I, Golding J. International variation in the incidence of hypertension in pregnancy among primiparae: the jamaican experience. 10th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Seattle, Washington, USA. 1996:168.
- McCaw‐Binns A, Jamaica Low‐Dose Aspirin Group. Low dose aspirin in pregnancy ‐ are there any benefits?. 10th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Seattle, Washington, USA. 1996:76.
Japan 1999 {published data only}
- Seki H, Kuromaki K, Takeda S, Kinoshita K, Satoh K. Trial of prophylactic administration of TXA2 synthetase inhibitor, ozagrel hydrochloride, for preeclampsia. Hypertension in Pregnancy 1999;18:157‐64. [DOI] [PubMed] [Google Scholar]
Korea 1997 {published data only}
- Kim HS, Kim KS, Kim TY, Cho JS, Park YW, Song CH. Clinical efficacy of doppler ultrasound for low dose aspirin therapy in high risk pregnancy. Korean Journal of Obstetrics and Gynecology 1997;40(1):71‐7. [Google Scholar]
Netherlands 1986 {published data only}
- Dekker GA. Low dose aspirin prevents pregnancy‐induced hypertensive disease in angiotensin‐sensitive primigravidae. Prediction and Prevention of Pregnancy‐Induced Hypertensive Disorders: a Clinical and Pathophysiologic Study. [MD thesis]. Rotterdam, The Netherlands: University Medical School, 1989:91‐102. [Google Scholar]
- Wallenburg HC, Dekker GA, Makovitz JW, Rotmans P. Low‐dose aspirin prevents pregnancy‐induced hypertension and pre‐eclampsia in angiotensin‐sensitive primigravidae. Lancet 1986;1:1‐3. [DOI] [PubMed] [Google Scholar]
Netherlands 1989 {published data only}
- Dekker GA. Prediction and prevention of pregnancy‐induced hypertensive disorders: a clinical and pathophysiologic study [MD thesis]. Rotterdam, The Netherlands: University Medical School, 1989. [Google Scholar]
Netherlands 1991a {published data only}
- Wallenburg HC, Dekker GA, Makowitz JW. Reversal of elevated vascular angiotensin II responsiveness in pregnancy by low‐dose aspirin. Proceedings of 34th Annual Meeting of the Society for Gynecologic Investigation; 1987 March 18‐21; Georgia, USA. 1987:22.
- Wallenburg HCS, Dekker GA, Makovitz JW, Rotmans N. Effect of low‐dose aspirin on vascular refractoriness in angiotensin‐sensitive primigravid women. American Journal of Obstetrics and Gynecology 1991;164:1169‐73. [DOI] [PubMed] [Google Scholar]
Netherlands 2009 {published data only}
- Lambers MJ, Groeneveld E, Hoozemans DA, Schats R, Homburg R, Lambalk CB, et al. Lower incidence of hypertensive complications during pregnancy in patients treated with low‐dose aspirin during in vitro fertilization and early pregnancy. Human Reproduction 2009;24(10):2447‐50. [DOI] [PubMed] [Google Scholar]
- Lambers MJ, Hoozemans DA, Schats R, Homburg R, Lambalk Cornelis B, Hompes PG. Low‐dose aspirin in non‐tubal IVF patients with previous failed conception: a prospective randomized double‐blind placebo‐controlled trial. Fertility and Sterility 2009;92(3):923‐9. [DOI] [PubMed] [Google Scholar]
Pergar 1987 {unpublished data only}
- Uzan S, Beaufils M, Bazin B, Danays T. Idiopathic recurrent fetal growth retardation and aspirin‐dipyridamole therapy [letter]. American Journal of Obstetrics and Gynecology 1989;160:763‐4. [DOI] [PubMed] [Google Scholar]
Romania 2018 {published data only}
- Stanescu AD, Banica R, Sima RM, Ples L. Low dose aspirin for preventing fetal growth restriction: a randomised trial. Journal of Perinatal Medicine 2015;43(Suppl 1):Abstract no: O‐0040. [DOI] [PubMed] [Google Scholar]
- Stanescu AD, Banica R, Sima RM, Ples L. Low dose aspirin for preventing fetal growth restriction: a randomised trial. Journal of Perinatal Medicine 2018 [epub ahead of print]. [DOI] [PubMed]
Russia 1993 {published and unpublished data}
- Rogov V, Tareeva I, Sidorova S, Androsova S. Prevention of pregnancy complications with acetylsalicylic acid (ASA) and dipyridamol (DP) in women with chronic glomerulonephritis (CGN) and essential hypertension (EH). Proceedings of 9th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney, Australia. 1994:280.
- Rogov VA, Tareeva I, Sidorova S, Androsova SO, Katamadze KT, Nikiforova OV. Prevention of pregnancy complications in glomerulonephritis and hypertension with acetylsalicylic acid and curantyl. Terapevticheskii Arkhiv 1993;65:65‐8. [PubMed] [Google Scholar]
S Africa 1988 {published and unpublished data}
- Railton A, Davey A. Aspirin and dipyridamole in the prevention of pre‐eclampsia: effect on plasma prostanoids 6 keto PG1a and TXB2 and clinical outcome of pregnancy. Proceedings of the 6th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:60.
- Railton A, Davey DA. Aspirin and dipyridamole in the prevention of pre‐eclampsia: effect on plasma 6 keto PGF1alpha and TxB2 and clinical outcome of pregnancy. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:48.
Spain 1997 {published data only}
- Hermida RC, Ayala DE, Iglesias M, Mojon A, Silva I, Ucieda R, et al. Time‐dependant effects of low dose aspirin administration on blood pressure in pregnant women. Hypertension 1997;30:589‐95. [DOI] [PubMed] [Google Scholar]
Spain 1999 {published data only}
- Hermida R, Ayala D, Mojon A, Fernandez J, Alonso I, Silva I, et al. Time dependant effects of low dose aspirin administration on blood pressure in high risk pregnancy. Hypertension in Pregnancy 2000;19(Suppl 1):81. [Google Scholar]
- Hermida RC, Ayala DE, Fernandez JR, Mojon A, Alonso I, Silva I, et al. Administration time‐dependent effects of aspirin in women at differing risk for preeclampsia. Hypertension 1999;33(4):1079. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Fernandez JR, Mojon A, Alonso I, Silva I, et al. Administration time‐dependent effects of aspirin in women at differing risk for preeclampsia. Hypertension 1999;34(4 Pt 2):1016‐23. [DOI] [PubMed] [Google Scholar]
Spain 2003 {published data only}
- Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low‐dose aspirin for prevention of complications in pregnancy. Chronobiology International 2013;30(1‐2):260‐79. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Fernandez JR, Mojon A, Alonso I, Ucieda R, et al. Administration‐time dependent effects of aspirin in women at high risk for preeclampsia. [abstract]. Hypertension in Pregnancy 2000;19(Suppl 1):35. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Iglesias M. Administration time‐dependant influence of aspirin on blood pressure in pregnant women. Hypertension 2003;41:651‐6. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Iglesias M. Administration‐time dependent effects of low‐dose aspirin on blood pressure in women at high risk for preeclampsia [abstract]. Hypertension in Pregnancy 2002;21(Suppl 1):47. [Google Scholar]
- Hermida RC, Ayala DE, Iglesias M. Administration‐time dependent effects of low‐dose aspirin on the incidence of complications in women at high risk for preeclampsia [abstract]. Hypertension in Pregnancy 2002;21(Suppl 1):20. [Google Scholar]
Spain 2017 {published data only}
- EudraCT Number: 2012‐000622‐22. Aspirin for the enhancement of trophoblastic invasion in women with abnormal uterine artery Doppler at 11‐14 weeks of gestation. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2012‐000622‐22 (first received 10 May 2012).
- Scazzocchio E, Oros D, Diaz D, Ramirez JC, Ricart M, Meler E, et al. Impact of aspirin on trophoblastic invasion in women with abnormal uterine artery doppler at 11 ‐ 14 weeks: a randomized controlled study (asap). Ultrasound in Obstetrics & Gynecology 2017;49:435‐41. [DOI] [PubMed] [Google Scholar]
Tanzania 1995 {published data only}
- Ramaiya C, Mgaya H. Low dose aspirin in prevention of pregnancy‐induced hypertension in primigravidae at the Muhimbili Medical Centre, Dar Es Salaam. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167):1. [PubMed] [Google Scholar]
- Ramaiya C, Mgaya HN. Low dose aspirin in prevention of pregnancy‐induced hypertension in primigravidae at the Muhimbili Medical Centre, Dar es Salaam. East African Medical Journal 1995;72:690‐3. [PubMed] [Google Scholar]
- Ramaiya CP. Low dosage of aspirin in prevention of pregnancy induced hypertension (PIH) in primigravidae at Muhimbili Medical Centre, Dar es Salaam [MD thesis]. Ethiopia: University of Dar es Salaam, 1992. [Google Scholar]
UK+others 2003 {published data only}
- Yu CK, Papageorghiou AT, Parra M, Palma Dias R, Nicolaides KH. Randomized controlled trial using low‐dose aspirin in the prevention of pre‐eclampsia in women with abnormal uterine Doppler at 23 weeks gestation. Ultrasound in Obstetrics & Gynecology 2003;22:233‐9. [DOI] [PubMed] [Google Scholar]
UK 1990 {published data only}
- McParland PJ, Pearce JM. Low dose aspirin prevents proteinuric hypertension in women with abnormal uteroplacental waveforms. Proceedings of Silver Jubilee Congress of Obstetrics and Gynaecology; 1989 July 4‐7; London, UK. 1989:8.
- McParland, Pearce JM, Chamberlain GVP. Doppler ultrasound and aspirin in recognition and prevention of pregnancy‐induced hypertension. Lancet 1990;335:1552‐5. [DOI] [PubMed] [Google Scholar]
UK 1992 {published data only}
- Louden KA, Broughton PF, Heptinsall S, Mitchell JR, Symonds EM. Maternal low‐dose aspirin spares the neonate. Proceedings of Silver Jubilee Congress of Obstetrics and Gynaecology; 1989 July 4‐7; London, UK. 1989:9.
- Louden KA, Broughton PF, Symonds EM, Tuohy P, O'Callaghan C, Heptinstall S, et al. A randomized placebo‐controlled study of the effect of low dose aspirin on platelet reactivity and serum thromboxane B2 production in non‐pregnant women, in normal pregnancy and in gestational hypertension. International Journal of Gynecology & Obstetrics 1993;40:186. [DOI] [PubMed] [Google Scholar]
- Louden KA, Broughton Pipkin F, Heptinstall S, Mitchell JR, Symonds EM. Studies of the effect of low‐dose aspirin on thromboxane production and platelet reactivity in normal pregnancy, pregnancy‐induced hypertension and neonates. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:30.
- Louden KA, Broughton Pipkin F, Symonds EM, Tuohy P, O'Callaghan C, Heptinstall S, et al. A randomized placebo‐controlled study of the effect of low dose aspirin on platelet reactivity and serum thromboxane B2 production in non‐pregnant women, in normal pregnancy, and in gestational hypertension. British Journal of Obstetrics and Gynaecology 1992;99:371‐6. [DOI] [PubMed] [Google Scholar]
- Louden KA, Broughton‐Pipkin F, Heptinstall S, Fox SC, Tuohy P, O'Callaghan C, et al. Neonatal platelet reactivity and serum thromboxane B2 production in whole blood: the effect of maternal low dose aspirin. British Journal of Obstetrics and Gynaecology 1994;101:203‐8. [DOI] [PubMed] [Google Scholar]
- Louden KA, Broughton‐Pipkin F, Heptinstall S, Mitchell RA, Symonds EM. Maternal low‐dose aspirin spares neonatal platelets. British Journal of Obstetrics and Gynaecology 1990;97:1162. [DOI] [PubMed] [Google Scholar]
UK 1992b {published data only}
- Quenby S, Farquharson R, Ramsden G. The obstetric outcome of patients with positive anticardiolipin antibodies: aspirin vs no treatment. Proceedings of 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992:443.
UK 1995 {published data only}
- Davies NJ. A study of low dose aspirin for the prevention of hypertensive disorders of pregnancy in primiparous women. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:183.
- Davies NJ, Farquharson RG, Walkinshaw SA. Low‐dose aspirin and nulliparae. Lancet 1991;338:324. [DOI] [PubMed] [Google Scholar]
- Davies NJ, Gazvani MR, Farquharson RG, Walkinshaw SA. Low‐dose aspirin in the prevention of hypertensive disorders of pregnancy in relatively low‐risk nulliparous women. Hypertension in Pregnancy 1995;14:49‐55. [Google Scholar]
USA 1993 {published and unpublished data}
- Copper R, Hauth J, Cutter G, DuBard M, Goldenberg R. Prerandomization compliance testing may predict pregnancy outcome in a randomized clinical trial. American Journal of Obstetrics and Gynecology 1993;168:414. [Google Scholar]
- Goldenberg RL, Hauth JC, Copper RL, DuBard MB, Cutter GR. The effect of low dose aspirin on fetal growth in low risk primiparas. American Journal of Obstetrics and Gynecology 1993;168:383. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, DuBard MB, Copper RL, Cutter GR. Fetal growth in women using low‐dose aspirin for the prevention of preeclampsia: effect of maternal size. Journal of Maternal Fetal Medicine 1995;4:218‐24. [Google Scholar]
- Hauth J, Goldenberg R, Parker C, DuBard M, Copper R, Cutter G. Maternal serum thromboxane B2 reduction vs pregnancy outcome in a low‐dose aspirin trial. American Journal of Obstetrics and Gynecology 1993;168:316. [DOI] [PubMed] [Google Scholar]
- Hauth J, Goldenberg R, Philips J, Copper R, DuBard M, Cutter G. Low‐dose aspirin therapy to prevent preeclampsia: safety considerations. American Journal of Obstetrics and Gynecology 1993;168:389. [DOI] [PubMed] [Google Scholar]
- Hauth JC, Goldenberg RL, DuBard MB, Copper RL, Parker CR. Low‐dose asprin does not alter longitudinal maternal blood pressures. American Journal of Obstetrics and Gynecology 1995;172:377. [Google Scholar]
- Hauth JC, Goldenberg RL, Parker CR Jr, Philips JB 3rd, Copper RL, DuBard MB, et al. Low‐dose aspirin therapy to prevent preeclampsia. American Journal of Obstetrics and Gynecology 1993;168:1083‐93. [DOI] [PubMed] [Google Scholar]
- Hauth JC, Goldenberg RL, Parker CR, Copper RL, Cutter GR. Maternal serum thromboxane B2 reduction vs pregnancy outcome in a low‐dose aspirin trial. American Journal of Obstetrics and Gynecology 1995;173:578‐84. [DOI] [PubMed] [Google Scholar]
- Hauth JC, Goldenberg RL, Parker R, Philips JB, Copper RL, DuBard MB, et al. Low‐dose aspirin therapy to prevent preeclampsia. International Journal of Gynecology & Obstetrics 1994;44:97. [DOI] [PubMed] [Google Scholar]
- Maher J, Owen J, Hauth J, Goldenberg R, Parker C. Low dose aspirin: effect on fetal urine output. American Journal of Obstetrics and Gynecology 1993;168:332. [DOI] [PubMed] [Google Scholar]
- Maher JE, Owen J, Hauth J, Goldenberg R, Parker CR Jr, Copper RL. The effect of low‐dose aspirin on fetal urine output and amniotic fluid volume. American Journal of Obstetrics and Gynecology 1993;169:885‐8. [DOI] [PubMed] [Google Scholar]
- Owen J, Maher J, Hauth J, Goldenberg R, Parker C. The effect of low dose aspirin on umbilical artery Doppler measurements. American Journal of Obstetrics and Gynecology 1993;168:357. [DOI] [PubMed] [Google Scholar]
- Owen J, Maher JE, Hauth JC, Goldenberg RL, Parker CR Jr. The effect of low‐dose aspirin on umbilical artery Doppler measurements [published erratum appears in Am J Obstet Gynecol 1994 Jun;170(6):1841]. American Journal of Obstetrics and Gynecology 1993;169:907‐11. [DOI] [PubMed] [Google Scholar]
- Wenstrom KD, Hauth JC, Goldenberg RL, DuBard M, Lea C. The effect of low‐dose aspirin on the development of preeclampsia in women with elevated hCG. American Journal of Obstetrics and Gynecology 1995;172:376. [DOI] [PubMed] [Google Scholar]
- Wenstrom KD, Hauth JC, Goldenberg RL, DuBard MB, Lea C. The effect of low‐dose aspirin on pregnancies complicated by elevated human chorionic gonadotropin levels. American Journal of Obstetrics and Gynecology 1995;173:1292‐6. [DOI] [PubMed] [Google Scholar]
USA 1993a {published data only}
- Abramovici A, Jauk V, Wetta L, Edwards R, Biggio J, Tita A. Analysis of low‐dose aspirin by smoking status for select perinatal outcomes. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl 1):S261. [Google Scholar]
- Andrikopoulou M, Purisch SE, Handal‐Orefice R, Gyamfi‐Bannerman C. Beyond preeclampsia: low dose aspirin reduces spontaneous preterm birth. American Journal of Obstetrics and Gynecology 2018;218(1):S10. [DOI] [PubMed] [Google Scholar]
- Caritis S, Sibai B, Thorn E, McLaughlin S, NICHD‐MFM Network. Pregnancy effects of non‐proteinuric gestational hypertension (GH). American Journal of Obstetrics and Gynecology 1995;172:376. [Google Scholar]
- Connealy B, Carreno C, Kase B, Hart L, Blackwell S, Sibai B. A history of prior preeclampsia is a major risk factor for preterm birth. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl 1):S264. [DOI] [PubMed] [Google Scholar]
- Sessa TG, Moretti ML, Khoury A, Pulliam DA, Arheart KL, Sibai BM. Cardiac function in fetuses and newborns exposed to low‐dose aspirin during pregnancy. American Journal of Obstetrics and Gynecology 1994;171:892‐900. [DOI] [PubMed] [Google Scholar]
- Fratto VM, Ananth CV, Gyamfi‐Bannerman C. Late preterm neonatal morbidity in hypertensive versus normotensive women. Hypertension in Pregnancy 2016;35(2):242‐9. [DOI] [PubMed] [Google Scholar]
- Ohno M, Girsen A, O'Malley K, Blumenfeld Y, Lyell D, Butwick A, et al. Does low‐dose aspirin for preeclampsia prevention increase the risk of antepartum bleeding or placental abruption?. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S189. [Google Scholar]
- Sibai B. Low‐dose aspirin (60 mg/d) in pregnancy for the prevention of preeclampsia. 8th World Congress on Hypertension in Pregnancy; 1992 November 8‐12; Buenos Aires, Argentina 1992. 1992:50.
- Sibai B, Caritis S, Phillips E, Klebanoff M, McNellis D, Rocco L. Prevention of preeclampsia: low‐dose aspirin in nulliparous women: a double‐blind, placebo controlled trial. American Journal of Obstetrics and Gynecology 1993;168:286. [Google Scholar]
- Sibai B, Caritis S, Phillips E, Klebanoff M, Paul R, Witter F, et al. Safety of low‐dose aspirin in healthy nulliparous women: a double‐blind, placebo‐controlled trial. Proceedings of 40th Annual Meeting Society for Gynecologic Investigation; 1993 March; Toronto, Canada. 1993:S102.
- Sibai B, Caritis S, Thom E, Shaw K, McNellis D, NICHD‐MFM Network. Low‐dose aspirin in nulliparous women: safety of epidural and correlation between bleeding time and maternal‐neonatal bleeding complications. American Journal of Obstetrics and Gynecology 1994;170:293. [DOI] [PubMed] [Google Scholar]
- Sibai B, Gordon T, Phillips E, McNellis D, Caritis S, Romero R, et al. Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. Proceedings of 41st Annual Clinical Meeting of The American College of Obstetricians and Gynecologists; 1993 May 3‐6; Washington DC, USA. 1993:2.
- Sibai BM, Caritis SN, Thom E, Klebanoff M, McNellis D, Rocco L, et al. Prevention of preeclampsia with low‐dose aspirin in healthy, nulliparous pregnant women. New England Journal of Medicine 1993;329:1213‐8. [DOI] [PubMed] [Google Scholar]
- Sibai BM, Caritis SN, Thom E, Shaw K, McNellis D, NICHD‐MFM Network. Low dose aspirin in nulliparous women: safety of continuous epidural block and correlation between bleeding time and maternal‐neonatal bleeding complications. American Journal of Obstetrics and Gynecology 1995;172:1533‐7. [DOI] [PubMed] [Google Scholar]
- Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D, et al. Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. American Journal of Obstetrics and Gynecology 1995;172:642‐8. [DOI] [PubMed] [Google Scholar]
- Tolcher MC, Sangi‐Haghpeykar H, Aagaard KM. Race and ethnicity varies both efficacy and attributable risk of low‐dose aspirin (asa) for preeclampsia prevention among low‐risk women. American Journal of Obstetrics and Gynecology 2018;218(1 Suppl):S203. [DOI] [PubMed] [Google Scholar]
- Witten FR. [Personal communication] Double‐blind, placebo‐controlled trial of low‐dose aspirin to prevent pre‐eclampsia. Letter to: Cochrane Pregnancy and Childbirth Group, Oxford, UK 6 September 1991.
- Yaffe S. [Personal communication] Clinical trial of low dose aspirin (65mg) as a preventative of pre‐eclampsia. Letter to: Cochrane Pregnancy and Childbirth Group, Oxford, UK 3 December 1991.
USA 1994 {published data only}
- August P, Helseth G, Edersheim TG, Hutson JM, Druzin M. Sustained release, low‐dose aspirin ameliorates but does not prevent preeclampsia (PE) in a high risk population. Proceedings of 9th International Congress, International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney, Australia. 1994:72.
USA 1997 {published data only}
- Cowchock S, Reece EA. Do low‐risk pregnant women with antiphospholipid antibodies need to be treated? Organizing group of the antiphospholipid antibody treatment trial. American Journal of Obstetrics and Gynecology 1997;176(5):1099‐100. [DOI] [PubMed] [Google Scholar]
USA 1998 {published and unpublished data}
- Abramovici A, Jauk V, Wetta L, Cantu J, Edwards R, Biggio J, et al. Low‐dose aspirin, smoking status, and the risk of spontaneous preterm birth. American Journal of Perinatology 2015;32(5):445‐50. [DOI] [PubMed] [Google Scholar]
- Abramovici A, Jauk V, Wetta L, Edwards R, Biggio J, Tita A. Low‐dose aspirin is associated with a reduction in spontaneous preterm birth among non‐smokers. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl 1):S260‐S261. [Google Scholar]
- Adkins K, Allshouse AA, Metz TD, Heyborne KD. Impact of aspirin on fetal growth in diabetic pregnancies according to white classification. American Journal of Obstetrics and Gynecology 2017;217(4):465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins K, Metz T, Heyborne K, Allshouse A. Impact of low‐dose aspirin on fetal growth in diabetic pregnancies: the importance of white classification. American Journal of Obstetrics and Gynecology 2017;216(1 Suppl 1):S292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshouse AA, Jessel RH, Heyborne KD. The impact of low‐dose aspirin on preterm birth: secondary analysis of a randomized controlled trial. Journal of Perinatology 2016;36(6):427‐31. [DOI] [PubMed] [Google Scholar]
- Cantu J, Biggio J, Jauk V, Edwards R, Owen J, Tita A. Timing of initiation of low‐dose aspirin therapy and preeclampsia prevention in high‐risk women. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl 1):S266. [Google Scholar]
- Cantu JA, Jauk VR, Owen J, Biggio JR, Abramovici AR, Edwards RK, et al. Is low‐dose aspirin therapy to prevent preeclampsia more efficacious in non‐obese women or when initiated early in pregnancy?. Journal of Maternal‐Fetal & Neonatal Medicine 2015 Jan;8:1‐5. [DOI] [PubMed] [Google Scholar]
- Cantu JA, Jauk VR, Owen J, Biggio JR, Abramovici AR, Edwards RK, et al. Is low‐dose aspirin therapy to prevent preeclampsia more efficacious in non‐obese women or when initiated early in pregnancy?. Journal of Maternal‐Fetal & Neonatal Medicine 2015;28(10):1128‐32. [DOI] [PubMed] [Google Scholar]
- Caritis NS, NICHD‐MFMU Network. Impact of preeclampsia on a subsequent pregnancy. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S120. [Google Scholar]
- Caritis S. Low dose aspirin does not prevent preeclampsia in high risk women. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S3. [Google Scholar]
- Caritis S, Sibai B, Hauth J, Lindheimer M, Klebanoff M, Thom E, et al. Low‐dose aspirin to prevent preeclampsia in women at high risk. New England Journal of Medicine 1998;338:701‐5. [DOI] [PubMed] [Google Scholar]
- Costantine M, Wu Z, Han Y, Saade G, Blackwell S. Population norms underestimate rates of small for gestational age neonates in high risk pregnancies compared to customized growth norms. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S303. [Google Scholar]
- Costantine M, Wu Z, Han Y, Saade G, Blackwell S. Relationship between fetal growth evaluated by a customized versus population approach and adverse pregnancy outcomes. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S303. [Google Scholar]
- Dixon CL, Marrs C Costantine MM, Pacheco LD, Saade GR, Chiossi G. Effect of low‐dose aspirin on the time of onset of preeclampsia and time of delivery. American Journal of Perinatology 2017; Vol. 34, issue 12:1219‐26. [DOI] [PubMed]
- Euser AG, Metz TD, Allshouse AA, Heyborne K. Low‐dose aspirin reduces the incidence of preeclampsia in women with multiple gestations and elevated human chorionic gonadotropin. Reproductive Sciences 2015;22(Suppl 1):70A‐71A. [Google Scholar]
- Euser AG, Metz TD, Allshouse AA, Heyborne KD. Low‐dose aspirin for pre‐eclampsia prevention in twins with elevated human chorionic gonadotropin. Journal of Perinatology 2016;36(8):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratto VM, Ananth CV, Gyamfi‐Bannerman C. Late preterm neonatal morbidity in hypertensive versus normotensive women. Hypertension in Pregnancy 2016;35(2):242‐9. [DOI] [PubMed] [Google Scholar]
- Handal‐Orefice R, Purisch S, Miller R, Gyamfi‐Bannerman C, Andrikopoulou M. Aspirin: a potential for reducing intrauterine growth restriction in low‐risk women. Reproductive Sciences 2018;25(1):100A. [Google Scholar]
- Hauth J, Sibai B, Caritis S, VanDorsten P, Lindheimer M, Klebanoff M, et al. Maternal serum thromboxane B2 concentrations do not predict improved outcomes in high‐risk pregnancies in a low‐dose aspirin trial. American Journal of Obstetrics and Gynecology 1998;179:1193‐9. [DOI] [PubMed] [Google Scholar]
- Hauth JC, NICHD MFMU Network. Adverse maternal, fetal and neonatal outcomes in a low dose aspirin trial who developed pre‐eclampsia. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S110. [Google Scholar]
- Hauth JC, NICHD MFMU Network. Maternal serum thromboxane B2 (TxB2) reduction did not predict improved pregnancy outcome in high risk women in a low dose aspirin trial. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S110. [DOI] [PubMed] [Google Scholar]
- Hauth JC, NICHD MFMU Network. Safety of low dose aspirin in high risk patients. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S111. [Google Scholar]
- Jessel R, Allshouse A, Heyborne K. Does low‐dose aspirin prevent preterm birth?. American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S342. [Google Scholar]
- Leon MG, Moussa HN, Longo M, Pedroza C, Haidar ZA, Mendez‐Figueroa H, et al. Rate of gestational diabetes mellitus and pregnancy outcomes in patients with chronic hypertension. American Journal of Perinatology 2016;33(8):745‐50. [DOI] [PubMed] [Google Scholar]
- Moore G, Allshouse A, Post A, Galan H, Heyborne K. Early aspirin decreases late‐onset preeclampsia (>34 weeks), particularly in women with chronic hypertension. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S144‐5. [Google Scholar]
- Moore GS, Allshouse AA, Post AL, Galan HL, Heyborne KD. Early initiation of low‐dose aspirin for reduction in preeclampsia risk in high‐risk women: a secondary analysis of the MFMU High Risk Aspirin Study. Journal of Perinatology 2015;35:328‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GS, Allshouse AA, Winn VD, Galan HL, Heyborne KD. Baseline placental growth factor levels for the prediction of benefit from early aspirin prophylaxis for preeclampsia prevention. Pregnancy Hypertension 2015;5(4):280‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Girsen A, O'Malley K, Blumenfeld Y, Lyell D, Butwick A, et al. Does low‐dose aspirin for preeclampsia prevention increase the risk of antepartum bleeding or placental abruption?. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S189. [Google Scholar]
- Sibai B. Low‐dose aspirin (60 mg/d) in pregnancy for the prevention of preeclampsia. 8th World Congress on Hypertension in Pregnancy; 1992 November 8‐12; Buenos Aires, Argentina. 1992:50.
- Tolcher MC, Sangi‐Haghpeykar H, Aagaard KM. Low‐dose aspirin (asa) for preeclampsia prevention among high‐risk women by ethnicity and race. American Journal of Obstetrics and Gynecology 2018;218(1 Suppl):S564. [DOI] [PubMed] [Google Scholar]
Venezuela 2000 {published data only}
- Rivas‐Echeverria CA, Echeverria Y, Molina L, Novoa D. Synergic use of aspirin, fish oil and vitamins C and E for the prevention of preeclampsia. Hypertension in Pregnancy 2000;19:30. [Google Scholar]
Zimbabwe 1998 {published data only}
- Byaruhanga RN, Chipato T, Rusakaniko S. A randomized controlled trial of low‐dose aspirin in women at risk from pre‐eclampsia. International Journal of Gynecology & Obstetrics 1998;60:129‐35. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Argentina 1994 {published data only}
- Fraire JC, Lopez J. Cyclooxygenase and lipoxygenase inhibition in gestational hypertension. Journal of the American Society of Nephrology 1994;5(3):561. [Google Scholar]
Australia 1989 {unpublished data only}
- Robinson JS. [Personal communication] Can the genetic risk of pre‐eclampsia be reduced by low dose aspirin?. Letter to: Cochrane Pregnancy and Childbirth Group, Oxford, UK 25 March 1993.
Australia 1989a {published data only}
- Trudinger BJ, Cook CM, Giles WB, Connelly AJ, Thompson RS. Low‐dose aspirin and twin pregnancy. Lancet 1989;334(8673):1214. [DOI] [PubMed] [Google Scholar]
Brazil 1992 {published data only}
- Montenegro CA. The effect of aspirin therapy on the uterine circulation in preeclampsia. Proceedings of the 8th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1992 November 8‐12; Buenos Aires, Argentina. 1992:66.
Brazil 1995 {published data only}
- Camara Franca BE, Silva LG, Netto HC, Rezende Filho J, Mello LI, Montenegro CA. The role of acetylsalicylic acid in prostaglandins syntesis in mild preeclampsia. Jornal Brasileiro de Ginecologia 1995;105(6):249‐68. [Google Scholar]
Brazil 1996a {published and unpublished data}
- Sass N, Rocha NS, Callegari AH, Lima PC, Camano L. The low dose aspirin study in pregnancy with chronic hypertension (AASHAC). Proceedings of the 10th World Congress on Hypertension in Pregnancy; 1996 August 4‐8; Seattle, USA. 1996:152.
Canada 2015 {published data only}
- Bujold E, NCT01352234. Evaluation of dose‐response effect of acetylsalicylic acid on placental development, preterm birth, fetal growth and hypertension in pregnancy in women with previous history of preeclampsia. clinicaltrials.gov/ct2/show/NCT01352234 (first received 11 May 2011).
- Tapp S, Demers S, Giguere Y, Leclair G, Nicolaides K, Cote S, et al. Comparison of 80 versus 160 mg of aspirin in pregnant women with previous history of pre‐eclampsia: a randomised controlled trial. Ultrasound in Obstetrics & Gynecology 2015;46(Suppl 1):S4. [Google Scholar]
China 1991 {published data only}
- Cheng WW, Zhang ZJ. Low‐dose aspirin preventing pregnancy induced hypertension. Chung Hua Fu Chan Ko Tsa Chih 1991;26(6):342‐5. [PubMed] [Google Scholar]
China 2016 {published data only}
- Liu FM, Zhao M, Wang M, Yang HL, Li L. Effect of regular oral intake of aspirin during pregnancy on pregnancy outcome of high‐risk pregnancy‐induced hypertension syndrome patients. European Review for Medical and Pharmacological Sciences 2016;20(23):5013‐6. [PubMed] [Google Scholar]
China 2017 {published data only}
- Liu F, Yang H, Zou K, Chen Y, Li G. Effect of a small dose of aspirin on quantitative test of 24‐h urinary protein in patients with hypertension in pregnancy. Experimental and Therapeutic Medicine 2017;13(1):37‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colombia 1996 {published data only}
- Hernandez F, Martinez MF, Camero A, Pinzon JA. Low dose aspirin as prophylactic therapy of pregnancy induced hypertension. Revista Colombiana de Obstetricia y Ginecologia 1996;47:197‐201. [Google Scholar]
East Germany 1986 {published data only}
- Peterseim H, Hofmann KD, Wagner F, Peterseim S, Meier P. Inhibition of prostaglandin synthetase by low‐dose acetylsalicylic acid ‐ effects on severity of pregnancy induced hypertension and fetal outcome. Proceedings of 10th European Congress of Perinatal Medicine; 1986 August 12‐16; Leipzig, Germany. 1986:290.
East Germany 1988 {published data only}
- Heinrich J. Prophylactic management of pregnancy induced hypertension (PIH). Proceedings of 11th European Congress of Perinatal Medicine; 1988; Rome, Italy. 1988:274.
Egypt 1991 {published data only}
- Toppozada M, Darwish EA, Osman YF, Abd‐Rabbo MS. Low dose acetyl salicylic acid in severe pre‐eclampsia. International Journal of Gynecology & Obstetrics 1991;35:311‐7. [DOI] [PubMed] [Google Scholar]
Egypt 1998 {published data only}
- Shalan H, Kurjak A, Lakkany N. The effect of low dose aspirin versus allylestrinol on uterine artery blood flow. Prenatal and Neonatal Medicine 1998;3 Suppl 1:141. [Google Scholar]
Egypt 2017 {published data only}
- Ali MK, Abbas AM, Yosef AH, Bahloul M. The effect of low‐dose aspirin on fetal weight of idiopathic asymmetrically intrauterine growth restricted fetuses with abnormal umbilical artery doppler indices: a randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2017 [Epub ahead of print]. [DOI] [PubMed]
- Ali MK, NCT03038607. Effect of low‐dose aspirin on fetal weight of idiopathic asymmetrically intrauterine growth restricted fetuses with abnormal umbilical doppler indices. clinicaltrials.gov/ct2/show/record/NCT03038607 (first received 31 January 2017). [DOI] [PubMed]
Equador 1998 {published data only}
- Armando CA, Martha CC, Galo EV. Prevention of the development of preeclampsia by means of the administration of low doses of aspirin. Quito 1998:80.
ERASME 2003a {published data only}
- Subtil D, Goeusse P, Houfflin‐Debarge V, Puech F, Lequien P, Breart G, et al. Aspirin (100mg) used for prevention of pre‐eclampsia in nulliparous women: the Essai Regional Aspirine Mere‐Enfant study (Part 2). BJOG: an international journal of obstetrics and gynaecology 2003;110:485‐91. [DOI] [PubMed] [Google Scholar]
Finland 1993a {published and unpublished data}
- Kaaja R, Julkunen H, Viinikka L, Ylikorkala O. Production of prostacyclin and thromboxane in lupus pregnancies: effect of small dose of aspirin. Obstetrics & Gynecology 1993;81:327‐31. [PubMed] [Google Scholar]
Finland 2007 {published data only}
- Haapsamo M, Martikainen H, Tinkanen H, Heinonen S, Nuojua‐Huttunen S, Rasanen J. Low‐dose aspirin therapy and hypertensive pregnancy complications in unselected IVF and ICSI patients: a randomized, placebo‐controlled, double‐blind study. Human Reproduction 2010;25(12):2972‐7. [DOI] [PubMed] [Google Scholar]
- Haapsamo M, Rasanen J. Low‐dose aspirin reduces uteroplacental vascular impedance during the first half of pregnancy in IVF/ICSI patients: a randomized placebo‐controlled study. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S31, Abstract no: 70. [DOI] [PubMed] [Google Scholar]
- Haapsamo M, Rasanen J, Gravett M, Nagalla S. Low‐dose aspirin influences early pregnancy placentation in IVF/ICSI patients: evidenced by a distinct maternal serum proteome profile. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S83, Abstract no: 256. [Google Scholar]
France 2001 {published data only}
- Goffinet F. Aboulker D, Paris‐Llado J, Bucourt M, Uzan M, Papiernik E, et al. Screening with a uterine Doppler in low risk pregnant women followed by low dose aspirin in women with abnormal results: a multicenter randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2001;108:510‐8. [DOI] [PubMed] [Google Scholar]
- Subtil D, Truffert P, Goeusse P, Dufour P, Uzan S, Breart G, et al. Value of systematic doppler +/‐ low dose aspirin to prevent vascular complications in primigravidae. Hypertension in Pregnancy 2000;19(Suppl 1):9. [Google Scholar]
Germany 1986 {published data only}
- Niedner W, Beller FK. The influence of ASA on pre‐eclampsia. Proceedings of the 5th International Congress of the Society for the Study of Hypertension in Pregnancy; 1986 July 7‐10; Nottingham, UK. 1986:101.
India 1986 {published data only}
- Bhattacharya N, Chaudhuri N, Ghosh S, Pradhan P, Ghosh CR. Effect of platelet aggregation inhibitor on fetal outcome in EPH gestosis with IUGR. Proceedings of the 5th Congress of the International Society for the Study of Hypertension in Pregnancy; 1986 July 7‐10; Nottingham, UK. 1986:82.
India 1997 {published data only}
- Tewari S, Kaushish R, Sharma S, Gulati N. Role of low dose aspirin in prevention of pregnancy induced hypertension. Journal of the Indian Medical Association 1997;95:43‐5, 47. [PubMed] [Google Scholar]
India 1998 {published and unpublished data}
- Dasari R, Narang A, Vasishta K, Garewal G. Effect of maternal low dose aspirin on neonatal platelet function. Indian Pediatrics 1998;35:507‐11. [PubMed] [Google Scholar]
India 2001 {published data only}
- Sehgal R, Sood M. Role of low dose aspirin for prevention of pregnancy induced hypertension. Journal of Obstetrics and Gynecology of India 2001;51(5):81‐4. [Google Scholar]
India 2002 {published data only}
- Desai P, Rao S. Role of low dose aspirin in mothers registering high serum HCG levels at mid trimester. Journal of Obstetrics and Gynecology of India 2002;52(5):30‐2. [Google Scholar]
India 2002a {published data only}
- Khanna A, Prabhakar S. Maternal and foetal outcome with low dose aspirin in pregnancy induced hypertension. Journal of Obstetrics and Gynecology of India 2002;52(3):62‐4. [Google Scholar]
India 2011 {published data only}
- Papalkar J, Rani D, Shrivastava. Comparison of lycopene and low dose aspirin with only low dose aspirin pre‐eclampsia. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:310.
Iran 2002 {published data only}
- Taherian A, Taherian A, Shirvani A. Prevention of preeclampsia with low‐dose aspirin or calcium supplementation. Archives of Iranian Medicine 2002;5:151‐6. [Google Scholar]
Iran 2013 {published data only}
- Abdali K, IRCT138803211548N5. The effect of taking aspirin in two different diurnal times (morning and night) on mean of 24 hours blood pressure in women who are at preeclampsia risk. http://en.irct.ir/trial/5621 (first received 20 February 2010).
- Abdali K, Taghizadeh R, Amoei S, Tabatabai SH. Comparison between aspirin and placebo on the mean of 24 hour blood pressure in pregnant women at preeclampsia risk, a double blind randomized controlled clinical trial. International Journal of Community Based Nursing and Midwifery 2013;1:283‐9. [Google Scholar]
Iran 2014 {published data only}
- Mesdaghinia E, Talari H, Abedzadeh‐Kalahroudi M. Effect of aspirin for prevention of preeclampsia in women with abnormal ultrasonic findings in uterine artery. Journal of Kashan University of Medical Sciences 2011;15(2):98‐104. [Google Scholar]
- Talari H, Mesdaghinia E, Abedzadeh Kalahroudi M. Aspirin and preeclampsia prevention in patients with abnormal uterine artery blood flow. Iranian Red Crescent Medical Journal 2014;16(8):e17175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iran 2016 {published data only}
- Hashemi M, Heshmat‐Ghahdarijani K, Bahrani S, Baktash F, Zarean E. Evaluation the effect of low‐dose aspirin on endothelial dysfunction in preeclamptic patients. Journal of Research in Medical Sciences 2016;21:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshmat K, IRCT2016040527135N2. Evaluation of the effect of low dose aspirin on endothelial dysfunction in preeclamptic patients. http://en.irct.ir/trial/22305 (first received 4 June 2016). [DOI] [PMC free article] [PubMed]
Iran 2017 {published data only}
- Ebrahimi BM, IRCT2016022020035N2. Study of effect of vitamin E and low‐dose aspirin on uterine artery blood flow velocity in women with recurrent pregnancy loss due to impaired uterine perfusion: clinical trial. http://en.irct.ir/trial/17776 (first received 10 May 2016).
- Mesdaghinia E, Mohammad‐Ebrahimi B, Foroozanfard F, Banafshe HR. The effect of vitamin e and aspirin on the uterine artery blood flow in women with recurrent abortion: a single‐blind randomized controlled trial. International Journal of Reproductive Biomedicine (Yazd, Iran) 2017;15(10):635‐40. [PMC free article] [PubMed] [Google Scholar]
Ireland 1995 {published data only}
- Regan CL, McAdam BF, McParland P, Boylan PC, FitzGerald GA, Fitzgerald DJ. Reduced fetal exposure to aspirin using a novel controlled release preparation in normotensive and hypertensive pregnancies. British Journal of Obstetrics and Gynaecology 1998;105:732‐8. [DOI] [PubMed] [Google Scholar]
- Regan CL, McAdam BV, McParland P, Boylan P, FitzGerald GA, Fitzgerald DJ. Pharmacology of low dose aspirin in normotensive and hypertensive pregnancies. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:301. [DOI] [PubMed]
Ireland 2014 {published data only}
- Breathnach F, ISRCTN13355246. Early aspirin to improve pregnancy outcome in diabetes. isrctn.com/ISRCTN13355246 (first received 12 February 2014).
- Subieh HA, McHugh A, Corcoran S, Backley S, Tully E, McDermott R, et al. Aspirin for optimising pregnancy outcome in pregestational diabetes: the value of objective testing of study participant compliance. Pilot for the IRELAND study (investigating the role of early low‐dose aspirin in pre‐existing diabetes). BJOG: an international journal of obstetrics and gynaecology 2017; Vol. 124:142‐3.
Israel 2006 {published data only}
- Dolitzky M, Inbal A, Segal Y, Weiss A, Brenner B, Carp H. A randomized study of thromboprophylaxis in women with unexplained consecutive recurrent miscarriages. Fertility and Sterility 2006;86(2):362‐6. [DOI] [PubMed] [Google Scholar]
Italy 1988 {published and unpublished data}
- Airoldi ML, Capetta P, Tasca A, Bertulessi C, Rossi E, Polvani F. Role of early prevention with heparin and dipyridamole in the prevention of pre‐eclampsia and placental insufficiency. Proceedings of the 6th International Congress of the International Society for the study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:233.
- Capetta P, Airoldi ML, Tasca A, Bertulessi C, Rossi E, Polvani F. Prevention of pre‐eclampsia and placental insufficiency. Lancet 1986;1:919. [DOI] [PubMed] [Google Scholar]
Italy 1990 {published data only}
- Iorio R, Horvath S, Marinoni E, Manzari G, Martinico E, Bresadola M. Use of a thromboxane receptor inhibitor in pregnancy‐induced hypertension. Proceedings of 7th World Congress of Hypertension in Pregnancy; 1990 October; Perugia, Italy. 1990:75.
Italy 1990a {published data only}
- Airoldi ML, Bertulessi C, Bosco P, Ossola MW, Barbieri M, Polvani F. Effectiveness of Heparin Calcium and Dipyridamole in preventing pregnancy hypertension. Proceedings of the 22nd International Congress on Pathophysiology of Pregnancy, June 21‐23, Budapest, Hungary. Department of Obstetrics and Gynecology Albert Szent‐Györgyi Medical University, 1990:17.
- Capetta P, Airoldi L, Iurlaro F, Rossi E, Polvani F. Prevention of pre‐eclampsia. New Trends in Gestational and Perinatal Hypertension 1990;1(3/4):265‐70. [Google Scholar]
Italy 1991 {published data only}
- Airoldi ML, Bertulessi C, Bosco P, Capetta P, Ossola MW, Barbieri M, et al. Prevention of pregnancy hypertension and fetal damage in patients with LLAC and ANA. Clinical and Experimental Hypertension 1991;B10(1 & 2):180. [Google Scholar]
Italy 1994 {published data only}
- Valensise H, Romanini C. Uterine Doppler in the identification of patients at risk for hypertension and IUGR. Journal of Perinatal Medicine 1994;22 Suppl 1:69‐72. [DOI] [PubMed] [Google Scholar]
Italy 2002 {published and unpublished data}
- Frusca T, Lojacono A, Fratelli N, Platto C, Valcamonico A, Bianchi UA. Can low dose aspirin improve perinatal outcome in chronic hypertensive patients [abstract]. Hypertension in Pregnancy 2002;21(Suppl 1):44. [Google Scholar]
Italy 2005 {published data only}
- Mello G, Parretti E, Fatini C, Riviello C, Gensini F, Marchionni M, et al. Efficacy of LMWH in lowering the recurrence rate of preeclampsia and in restoring the physiological vascular changes in ACE DD genotype women [abstract]. Hypertension in Pregnancy 2004;23(Suppl 1):162. [Google Scholar]
- Mello G, Parretti E, Fatini C, Riviello C, Gensini F, Marchionni M, et al. Low‐molecular‐weight heparin lowers the recurrence rate of preeclampsia and restores the physiological vascular changes in angiotensin‐converting enzyme DD women. Hypertension 2005;45(1):86‐91. [DOI] [PubMed] [Google Scholar]
Italy 2006 {published data only}
- Chistolini A, Torelli F, Giancotti A, Pignoloni P, Muto B, Cosimo C, et al. Recurrent fetal loss: prospective evaluation of the efficacy of three different thromboprophylaxis regimens: aspirin versus low molecular weight heparin versus low molecular weight heparin plus aspirin [abstract]. Hematology Journal 2006;91(Suppl 1):146. [Google Scholar]
- Giancotti A, Torre RL, Spagnuolo A, D'Ambrosio V, Cerekja A, Piazze J, et al. Efficacy of three different antithrombotic regimens on pregnancy outcome in pregnant women affected by recurrent pregnancy loss. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(7):1191‐4. [DOI] [PubMed] [Google Scholar]
Italy 2009 {published data only}
- Lazzarin N, Vaquero E, Exacoustos C, Bertonotti E, Romanini ME, Arduini D. Low‐dose aspirin and omega‐3 fatty acids improve uterine artery blood flow velocity in women with recurrent miscarriage due to impaired uterine perfusion. Fertility and Sterility 2009;92(1):296‐300. [DOI] [PubMed] [Google Scholar]
Japan 1989 {published data only}
- Terao T, Kobayashi T, Imai N, Oda H, Karasawa T. Pathological state of the coagulatory and fibrinolytic system in preeclampsia and the the possibility of its treatment with AT III concentrate. Asia‐Oceania Journal of Obstetrics and Gynaecology 1989;15:25‐32. [DOI] [PubMed] [Google Scholar]
Libya 2000 {published data only}
- Elmahaishi. The uses of low dose aspirin (150mg/day) in primigravida reduces the severity and complications of pregnancy induced hypertension. XVI FIGO World Congress of Obstetrics and Gynaecology; 2000 Sept 3‐8; Washington DC, USA (Book 1). 2000:98.
New Zealand 1990 {published data only}
- Hutton JD, Wilkinson AM. Poor participation of nulliparous women in a low dose aspirin study to prevent preeclampsia. New Zealand Medical Journal 1990;103:511‐2. [PubMed] [Google Scholar]
New Zealand 1998 {published data only}
- McCowan L, Harding J, Ford C, Barker S, Roberts A, Townend K. Prenatal treatment with low dose aspirin in small for gestational age fetuses with abnormal umbilical doppler: a randomised controlled trial. 2nd Annual Congress of the Perinatal Society of Australia and New Zealand; 1998 March 30‐April 4; Alice Springs, Australia. 1998:135.
- McCowan LM, Harding J, Roberts A, Barker S, Ford C, Stewart A. Administration of low dose aspirin to mothers with small for gestational age fetuses and abnormal umbilical Doppler studies to increase birthweight: a randomised double‐blind controlled trial. British Journal of Obstetrics and Gynaecology 1999;106:647‐51. [DOI] [PubMed] [Google Scholar]
New Zealand 2000 {published data only}
- Pattison N, Chamley L, Birdsall M, Zanderigo A, Liddell H, McDougall J. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. American Journal of Obstetrics and Gynecology 2000;183:1008‐12. [DOI] [PubMed] [Google Scholar]
Pakistan 1994 {published data only}
- Gilani A, Khan Z. Role of aspirin in management of pregnancy induced hypertension. A study in Pakistani population. Specialist 1994;10:323‐5. [Google Scholar]
Panama 2014 {published data only}
- Vigil‐De Gracia P, Dominguez L, Solis A. Management of chronic hypertension during pregnancy with furosemide, amlodipine or aspirin: a pilot clinical trial. Journal of Maternal‐Fetal and Neonatal Medicine 2014;27(13):1291‐4. [DOI] [PubMed] [Google Scholar]
Poland 1996 {published data only}
- Ciszek V, Kokot F, Wiecek A. Influence of acetylsalicylic acid on the hormonal profile of pregnant women with EPH gestosis [abstract]. Nephrology, Dialysis, Transplantation 1996;11:1419. [Google Scholar]
Poland 1999 {published and unpublished data}
- Kalinka J, Sieroszewski P, Hanke W, Laudanski T, Suzin J. Evaluation of the effectiveness of a low dose aspirin in the treatment of intrauterine growth retardation (IUGR). Ginekologia Polska 1999;70:126‐34. [PubMed] [Google Scholar]
Russia 1997 {published data only}
- Zozulia OV, Rogov VA, Piatakova NV, Tareeva IE. Nitric oxide: its role in the development of pregnancy complications and in their prevention in women with hypertension and chronic glomerulonephritis. Terapevticheskii Arkhiv 1997;69(6):17‐20. [PubMed] [Google Scholar]
Slovenia 1998 {published and unpublished data}
- Sajina‐Stritar B. The effect of acetylsalicylic acid and n‐3 fatty acids in prevention of complications of hypertension diseases in pregnancy. Zdravstveni Vestnik 1998;67:489‐93. [Google Scholar]
Spain + others 2000 {published and unpublished data}
- Cabero L, Bellart J, Bertini A, Althabe O, Bichou C, Bucheli R. Aspirin in early pregnancy to reduce the incidence and/or severity of pregnancy induced hypertension and intrauterine growth retardation in healthy pregnant women. Hypertension in Pregnancy 2000;19(1):98. [Google Scholar]
Sweden 2017 {published data only}
- Blomqvist L, Hellgren M, Strandell A. Acetylsalicylic acid for prevention of pregnancy loss: a randomized trial. Human Reproduction 2017;32:i11‐i12. [Google Scholar]
Thailand 1996 {published and unpublished data}
- Herabutya Y, Jetsawangsri T, Saropala N. The use of low‐dose aspirin to prevent preeclampsia. International Journal of Gynecology & Obstetrics 1996;54:177‐8. [DOI] [PubMed] [Google Scholar]
- Jetsawangsri T, Herabutya Y, Saropala N. The use of low‐dose aspirin to prevent pre‐eclampsia. Abstracts of 9th Congress of the Federation of the Asia and Oceania Perinatal Societies; 1996 November 10‐14; Singapore. 1996:129.
Trinidad 1998 {published data only}
- Bassaw B, Roopnarinesingh S, Roopnarinesingh A, Homer H. Prevention of hypertensive disorders of pregnancy. Journal of Obstetrics and Gynaecology 1998;18(2):123‐6. [DOI] [PubMed] [Google Scholar]
Tunisia 1989 {published data only}
- Hachicha J, Ammous A, Damak J, Hammami M, Ghorbel A, Rekik S, et al. Prevention of complications of hypertension in pregnancy with antiplatelets. Presse Medicale 1989;18:767‐9. [PubMed] [Google Scholar]
- Hachicha J, Ammous A, Midassi H, Dammak J, Ghorbel A, Chaabouni MN, et al. Place of antiplatelet therapy (APT) in vasculo‐renal accidents's (VRA) preventive treatment during pregnancy. 6th International Congress, International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:185.
- Rekik S, Midassi H, Bayoudh H, Mezghani M, Abid R, Ghorbel A. Place of antiplatelet therapy (APT) in vasculorenal accidents (VRA) preventive treatment in pregnancy. World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988:185.
Tunisia 1990 {published data only}
- Hachicha J, Bellaj A, Ghorbel L, Abid R, Rekik S, Jarraya A. Aspirin or aspirin and dipyridamole to prevent vasculo‐renal accidents in pregnancy. Proceedings of 7th World Congress of Hypertension in Pregnancy; 1990 October; Perugia, Italy. 1990:256.
- Hachicha J, Ben Hmida M, Kharrat M, Jarraya F, Rekik S, Jarraya A. Aspirin or aspirin and dipyridamole to prevent pregnancy‐induced hypertension [abstract]. Nephrology, Dialysis, Transplantation 1994;9:926. [Google Scholar]
Turkey 1994 {published data only}
- Okten S, Polat G, Ayata D, Albayrak U. Low‐dose aspirin prophylaxis in high‐risk pregnancies [Riskli gebeliklerde proflaktik dusuk doz aspirin]. Jinekoloji Ve Obstetrik Dergisi 1994;8(4):150‐6. [Google Scholar]
UK 1992a {published and unpublished data}
- McParland P, Pearce JM. Effect of low dose aspirin on maternal and fetal flow velocity waveforms. American Journal of Obstetrics and Gynecology 1992;166:438. [Google Scholar]
UK 1993 {published data only}
- Williams HD, Howard R, O'Donnell N, Findley I. The effect of low dose aspirin on bleeding times. Anaesthesia 1993;48:331‐3. [DOI] [PubMed] [Google Scholar]
UK 1994 {published data only}
- Hamid R, Robson M, Pearce JM. Low dose aspirin in women with raised maternal serum alpha‐fetoprotein and abnormal Doppler waveform patterns from the uteroplacental bed. British Journal of Obstetrics and Gynaecology 1994;101:481‐4. [DOI] [PubMed] [Google Scholar]
- Hamid R, Robson M, Pearce JM. Low dose aspirin in women with raised maternal serum alpha‐fetoprotein and abnormal Doppler waveform patterns from the uteroplacental circulation. International Journal of Gynecology & Obstetrics 1995;48:348‐9. [DOI] [PubMed] [Google Scholar]
UK 2000 {published data only}
- Harrington K, Kurdi W, Aquilina J, England P, Campbell S. A prospective management study of slow‐release aspirin in the palliation of uteroplacental insufficiency predicted by uterine artery Doppler at 20 weeks. Ultrasound in Obstetrics & Gynecology 2000;15:13‐8. [DOI] [PubMed] [Google Scholar]
- Kurdi W, Fayyad A, Thakur V, Harrington K. Delayed normalisation of the uterine artery Doppler waveforms is not a benign phenomenon. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;117(1):20‐3. [DOI] [PubMed] [Google Scholar]
USA 1989 {published and unpublished data}
- Mirro R, Sibai BM, Leffler CW, Chesney CM. Low dose aspirin during pregnancy. Pediatric Research 1988;23:419A. [Google Scholar]
- Sibai BM, Mirro R, Chesney CM, Leffler C. Low‐dose aspirin in pregnancy. Obstetrics & Gynecology 1989;74:551‐7. [PubMed] [Google Scholar]
USA 1990 {published data only}
- Roberts CS, Beeson JH. Low dose aspirin in the prevention of preeclampsia in nulliparas. Preliminary results of a prospective, placebo‐controlled, double blind study. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 January 23‐27; Houston, Texas, USA. 1990:89.
USA 1993b {published data only}
- Silver RK, MacGregor SN, Sholl JS, Hobart JM, Neerhof MG, Ragin A. Comparative trial of prednisone plus aspirin vs aspirin alone in the treatment of anticardiolipin antibody‐positive obstetric patients. American Journal of Obstetrics and Gynecology 1993;169:1411‐7. [DOI] [PubMed] [Google Scholar]
- Silver RK, Sholl JS, MacGregor SN, Hobart JH, Neerhof MG, Hickman AH. Prospective evaluation of single (low‐dose aspirin) vs combined (aspirin plus prednisone) therapy in the treatment of the antiphospholipid syndrome. Proceedings of 39th Annual Meeting of the Society for Gynecologic Investigation; 1992 March 18‐21; San Antonio, Texas, USA. 1992:125.
USA 1993c {published data only}
- O'Brien WF, Krammer J, O'Leary TD, Mastrogiannis DS. The effect of acetaminophen on prostacyclin production in pregnant women. American Journal of Obstetrics and Gynecology 1993;168:1164‐9. [DOI] [PubMed] [Google Scholar]
USA 1996 {published data only}
- Martin C, Varner MW, Branch DW, Rodgers G, Mitchell MD. Dose‐related effects of low dose aspirin on hemostasis parameters and prostacyclin/thromboxane ratios in late pregnancy. Prostaglandins 1996;51:321‐30. [DOI] [PubMed] [Google Scholar]
USA 2012 {published data only}
- Odibo A, NCT01547390. Early prediction and aspirin for prevention of preeclampsia. clinicaltrials.gov/ct2/show/record/NCT01547390 (first received 7 March 2012).
- Odibo AO, Goetzinger KR, Odibo L, Tuuli MG. Early Prediction and Aspirin for Prevention of Pre‐eclampsia study (EPAPP study): a randomized controlled trial. Ultrasound in Obstetrics & Gynecology 2015;46:414‐8. [DOI] [PubMed] [Google Scholar]
- Papatheodorou SI. Re: early prediction and aspirin for prevention of pre‐eclampsia (epapp) study: a randomized controlled trial. A. O. Odibo, K. R. Goetzinger, l. Odibo and M. G. Tuuli. Ultrasound Obstet Gynecol 2015; 46: 414‐8. Ultrasound in Obstetrics & Gynecology 2015;46(4):389. [DOI] [PubMed] [Google Scholar]
USA 2013 {published data only}
- Ahrens KA, Silver RM, Mumford SL, Sjaarda LA, Perkins NJ, Wactawski‐Wende J, et al. Complications and safety of preconception low‐dose aspirin among women with prior pregnancy losses. Obstetrics & Gynecology 2016;127(4):689‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell MT, Sjaarda LA, Radin RG, Kuhr D, Mumford SL, Plowden TC, et al. The effects of aspirin in gestation and reproduction (eager) trial: a story of discovery. Seminars in Reproductive Medicine 2017;35(4):344‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbins KJ, Branch DW, Silver RM, Sjaarda LA, Mumford SL, Perkins NJ, et al. Recurrent pregnancy loss in women with and without antiphospholipid antibodies. American Journal of Obstetrics & Gynecology 2016;214(1 Suppl):S199. [Google Scholar]
- Lesher LL, Matyas RA, Sjaarda LA, Newman SL, Silver RM, Galai N, et al. Recruitment for longitudinal, randomised pregnancy trials initiated preconception: lessons from the Effects of Aspirin in Gestation and Reproduction trial. Paediatric and Perinatal Epidemiology 2015;29(2):162‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone F, McAuliffe FM. Preconception low‐dose aspirin and pregnancy outcomes: results from the EAGeR randomized trial. Irish Medical Journal 2015;108(1):5. [PubMed] [Google Scholar]
- Mumford SL, Silver RM, Sjaarda LA, Wactawski‐Wende J, Townsend JM, Lynch AM, et al. Expanded findings from a randomized controlled trial of preconception low‐dose aspirin and pregnancy loss. Human Reproduction 2016;31(3):657‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh SJ, Schisterman EF, Browne RW, Lynch AM, Mumford SL, Perkins NJ, et al. Preconception maternal lipoprotein levels in relation to fecundability. Human Reproduction 2017;32(5):1055‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin RG, Mumford SL, Silver RM, Lesher LL, Galai N, Faraggi D, et al. Sex ratio following preconception low‐dose aspirin in women with prior pregnancy loss. Journal of Clinical Investigation 2015;125(9):3619‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin RG, Sjaarda LA, Perkins NJ, Silver RM, Chen Z, Lesher LL, et al. Low‐dose aspirin and sporadic anovulation in the EAGeR randomized trial. Journal of Clinical Endocrinology and Metabolism 2017;102(1):86‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman E, Silver R, Lesher L, Faraggi D, Wactawski‐Wende J, Townsend J, et al. Randomized clinical trial of preconception low dose aspirin use to improve pregnancy outcomes: EAGeR (Effects of Aspirin in Gestation and Reproduction) trial. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl 1):S352. [Google Scholar]
- Schisterman EF. Erratum. Paediatric and Perinatal Epidemiology 2014;28(3):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Mumford SL, Schliep KC, Sjaarda LA, Stanford JB, Lesher LL, et al. Preconception low dose aspirin and time to pregnancy: findings from the EAGER (Effects of Aspirin in Gestation And Reproduction) randomized trial. Journal of Clinical Endocrinology and Metabolism 2015;100(5):1785‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, NCT00467363. The effects of aspirin in gestation and reproduction: a multi‐center, controlled, double‐blind randomized trial. clinicaltrials.gov/ct2/show/record/NCT00467363 (first received 30 April 2007).
- Schisterman EF, Silver RM, Lesher LL, Faraggi D, Wactawski‐Wende J, Townsend JM, et al. Preconception low‐dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet 2014;384(9937):29‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Silver RM, Mumford SL, Galai N, Wactawski‐Wende J, Stanford JB. Randomized clinical trial of preconception low dose aspirin use and time‐to‐pregnancy: The EAGeR trial. Fertility and Sterility 2013;100(3 Suppl 1):S100. [Google Scholar]
- Schisterman EF, Silver RM, Perkins NJ, Mumford SL, Whitcomb BW, Stanford JB, et al. A randomised trial to evaluate the effects of low‐dose aspirin in gestation and reproduction: design and baseline characteristics.[Erratum appears in Paediatr Perinat Epidemiol. 2014 May;28(3):275]. Paediatric and Perinatal Epidemiology 2013;27(6):598‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RM, Ahrens K, Wong LF, Perkins NJ, Galai N, Lesher LL, et al. Low‐dose aspirin and preterm birth: a randomized controlled trial. Obstetrics and Gynecology 2015;125(4):876‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjaarda L, Mitchell E, Mumford SL, Radin R, Perkins NJ, Galai N, et al. Preconception low dose aspirin treatment improves clinical pregnancy and live birth in women with higher systemic inflammation. Fertility and Sterility 2015;104(3 Suppl 1):e349. [Google Scholar]
- Sjaarda LA, Radin RG, Silver RM, Mitchell E, Mumford SL, Wilcox B, et al. Preconception low‐dose aspirin restores diminished pregnancy and live birth rates in women with low grade inflammation: a secondary analysis of a randomized trial. Journal of Clinical Endocrinology and Metabolism 2017;102(5):1495‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vietnam 2017 {published data only}
- Cao NT, Vu QH, Truong QV, Vo VD, Tran ML. Effectiveness of low‐dose aspirin for the prevention of pre‐eclampsia. Journal of Obstetrics and Gynaecology Research 2017;43:69. [Google Scholar]
West Germany 1977 {published data only}
- Wolfrum R, Bordasch C, Holweg J, Schulz G. The therapy of chronic nutritional placenta disorders ‐ preliminary results of a double blind study. Archives of Gynecology 1977;224:114. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abdi 2019 {published data only}
- Abdi N, IRCT20181218042033N1. Evaluating the effect of aspirin on the occurrence of PLP, IUGR and preeclampsia in pregnant women with a history of preeclampsia in a previous pregnancies in Khalije fars hospital Bandar abbas,Iran in 2016‐2017. http://en.irct.ir/trial/36064 (first received 20 January 2019).
Agrawala 2019 {published data only}
- Agrawala S, Sjaarda LA, Omosigho UR, Perkins NJ, Silver RM, Mumford SL, et al. Effect of preconception low dose aspirin on pregnancy and live birth according to socioeconomic status: a secondary analysis of a randomized clinical trial. Plos One 2019; Vol. 14, issue 4:e0200533. [DOI] [PMC free article] [PubMed]
Amro 2019 {published data only}
- Amro F, NCT03961360. Effectiveness of higher aspirin dosing for prevention of preeclampsia in high risk obese gravida: an open label, comparative effectiveness, randomized controlled trial (aspreo trial). https://clinicaltrials.gov/ct2/show/NCT03961360 (first received 23 May 2019).
Benavides 2016 {published data only}
- Benavides LG, NCT02838030. Efficacy of the combination of acetylsalicylic acid and l‐arginine to prevent preeclampsia in pregnant high risk. https://clinicaltrials.gov/ct2/show/NCT02838030. NCT02838030 (20 July 2016) 2016.
China 2012 {published data only}
- Zhao YM, Xiao LP, Hu H, Yang XN, Xu YQ, Guo LM. Low‐dose aspirin prescribed at bed time for the prevention of pre‐eclampsia in high‐risk pregnant women. Reproduction and Contraception 2012;32:355–9. [Google Scholar]
Cobaleda 2018 {published data only}
- Cobaleda M, NCT03741179. A phase iii, multicentric, randomized, open‐label, parallel‐group clinical trial to detect false positives from first‐trimester preeclampsia screening (stoppre) at the second‐trimester of pregnancy. https://clinicaltrials.gov/ct2/show/NCT03741179 (first received 14 November 2018).
Finneran 2019 {published data only}
- Finneran MM, Gonzalez‐Brown VM, Smith DD, Landon MB, Rood K. 48: effect of obesity on platelet inhibition in high‐risk pregnant women treated with low dose aspirin. American Journal of Obstetrics and Gynecology 2019;220(1):S38‐9. [DOI] [PubMed] [Google Scholar]
Finneran 2019a {published data only}
- Finneran MM, Gonzalez‐Brown VM, Smith DD, Landon MB, Rood KM. Obesity and laboratory aspirin resistance in high risk pregnant women treated with low dose aspirin. American Journal of Obstetrics and Gynecology 2019;220(4):385.e1‐385.e6.. [DOI] [PubMed] [Google Scholar]
India 1993a {unpublished data only}
- Regi A. [Personal communication] Randomised controlled trial of low dose aspirin in high risk pregnancy. Letter to: Cochrane Pregnancy and Childbirth Group, Oxford, Uk 1993.
Iran 2017a {published data only}
- Movahed F, IRCT201505103025N5. The effect of aspirin administration for prevention of preeclampsia in pregnant women with abnormal findings in uterine artery doppler sonograph. http://en.irct.ir/trial/2988 (first received 8 June 2015).
- Movahed F, Lalooha F, Dabbaghi Ghale T, Rezaee Majd Z, Moinodin R, Yazdi Z. The effect of aspirin in the prevention of preeclampsia in women with abnormal uterine artery doppler ultrasonography findings. Journal of Zanjan University of Medical Sciences and Health Services 2017;25(108):11‐9. [Google Scholar]
Lin 2018 {published data only}
- Lin L, Zhu Y, Li B, Yang H. Low‐dose aspirin in the prevention of pre‐eclampsia in China (APPEC study): protocol for a multicentre randomized controlled trial. Trials 2018;19(1):608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthews 2019 {published data only}
- Matthews KC, NCT04070573. A randomized controlled trial comparing low doses of aspirin in the prevention of preeclampsia (asapp). https://clinicaltrials.gov/ct2/show/NCT04070573 (first received 27 August 2019).
Meher 2019 {published data only}
- Meher R, CTRI/2019/05/019232. Low dose aspirin in prevention of preeclampsia. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=33792 (first received 20 May 2019).
Mirzamoradi 2018 {published data only}
- Mirzamoradi M, IRCT20120918010876N6. Comparison of two various aspirin dosage (80mg and 120mg daily) on prevention of pre‐eclampsia in high risk pregnant women. http://en.irct.ir/trial/35557 (first received 16 December 2018).
Mone 2018 {published data only}
- Mone F, Mulcahy C, McParland P, Breathnach F, Downey P, McCormack D, et al. Trial of feasibility and acceptability of routine low‐dose aspirin versus early screening test indicated aspirin for pre‐eclampsia prevention (test study): a multicentre randomised controlled trial. Bmj Open 2018;8(7):e022056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mone 2019 {published data only}
- Mone F, Mulcahy C, McParland P, Downey P, Culliton M, Maguire OC, et al. Evaluation of the effect of low‐dose aspirin on biochemical and biophysical biomarkers for placental disease in low‐risk pregnancy: secondary analysis of a multicenter rct. American Journal of Perinatology 2019 [ahead of print]. [DOI] [PubMed]
Mulcahy 2019 {published data only}
NCT03574909 2018 {published data only}
- NCT03574909. Investigating the role of early low‐dose aspirin in diabetes: a phase iii multicentre double‐blinded placebo‐controlled randomised trial of low‐dose aspirin initiated in the first trimester of diabetes pregnancy. https://clinicaltrials.gov/ct2/show/NCT03574909 (first received 2 July 2018). [DOI] [PMC free article] [PubMed]
NCT03674606 2018 {published data only}
- NCT03674606. An open‐label randomized‐controlled trial of early screening test for pre‐eclampsia and growth restriction. https://clinicaltrials.gov/ct2/show/NCT03674606 (first received 17 September 2018). [DOI] [PubMed]
Netherlands/UK 1994 {published data only}
- Kraavenbrink A, Gans R, Geijn H, Dekker G. Insulin resistance, vasoactive mediators and preeclampsia. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S26. [DOI] [PubMed] [Google Scholar]
- Kraayenbrink AA, Robson M, Dekker GA, Pearce JM, Geijn HP. Prevention of preeclampsia and fetal growth retardation; allylestrenol vs aspirin, a two centered study. Proceedings of 9th International Congress, International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney, Australia. 1994:125.
Netherlands 1991 {published data only}
- Noort WA, Rotmans N, Keirse MJ, Wallenburg HC. Effect of low‐dose aspirin with and without dipyridamole on prostanoid biosynthesis in pregnancy. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:182.
- Wallenburg HC, Rotmans N, Noort WA, Keirse MJ. Effect of low‐dose aspirin with and without dipyridamole on prevention of recurrent idiopathic fetal growth retardation. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands 1991:92.
O'Brien 2019 {published data only}
- O'Brien J, NCT03893630. Role of aspirin in maternal endothelial dysfunction and uterine artery blood flow in women at risk for preeclampsia. https://clinicaltrials.gov/ct2/show/NCT03893630 (first received 28 March 2019).
Poon 2018 {published data only}
- Poon L. Prevention of preeclampsia. International Journal of Gynecology and Obstetrics 2018; Vol. 143:87.
Poon 2019 {published data only}
- Poon L, NCT03941886. Implementation of first‐trimester screening and prevention of preeclampsia: a stepped wedge cluster‐randomized trial in asia. https://clinicaltrials.gov/ct2/show/NCT03941886 (first received 8 May 2019). [DOI] [PMC free article] [PubMed]
Qi 2019 {published data only}
- Qi H, NCT04051567. Low‐dose aspirin for prevention of adverse pregnancy outcomes in twin pregnancies‐‐a multicenter, prospective, open, randomized, controlled clinical trial. https://clinicaltrials.gov/ct2/show/NCT04051567 (first received 9 August 2019).
Rood 2018 {published data only}
- Rood K, NCT03735433. The effect of 81mg vs 162mg asa for preeclampsia prevention in obese women at high risk for developing preeclampsia. https://clinicaltrials.gov/ct2/show/NCT03735433 (first received 8 November 2018).
Sallam 2018 {published data only}
- Sallam HF, NCT03725891. Comparison of two doses (81 mg versus 162 mg) of aspirin for the prevention of preeclampsia in healthy, nulliparous obese and overweight pregnant women: a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT03725891 (first received 31 October 2018).
Sallam 2018a {published data only}
- Sallam HF, NCT03726177. Comparison of two doses (81 mg versus 162mg) of aspirin for the prevention of preeclampsia in high‐risk pregnant women: a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT03726177 (first received 31 October 2018).
Slovenia 1992 {published data only}
- Sajina‐Stritar B, Novak‐Antolic Z. Antiaggregational therapy in preventing EPH gestosis and its complications. Journal of Perinatal Medicine 1992;20(Suppl 1):73. [Google Scholar]
- Sajina‐Stritar B, Novak‐Antolic Z. Antiaggregational therapy in preventing EPH gestosis and its complications. Proceedings of 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992:454.
Slovenia 1994 {published data only}
- Sajina‐Stritar B. Prevention of gestational hypertension and its complications with ASA and N‐3 fatty acids (comparative study). Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:182.
South Africa 1986 {published data only}
- Richards A, Moodley J, Norman R. The use of low dose aspirin in pregnancy induced hypertension. 23rd South African Congress of Obstetrics and Gynaecology; 1986 September 23‐26; South Africa. 1986:16.
Sutton 2018 {published data only}
- Sutton EF, Hauspurg A, Caritis SN, Powers RW, Catov JM. Maternal outcomes associated with lower range stage 1 hypertension. Obstetrics and Gynecology 2018;132(4):843‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Switzerland 2000 {published data only}
- Ferrier C, Koeferl U, Duerig P, Schneider H. Effects of LMW‐heparin and low‐dose aspirin on renal uric acid handling in high‐risk pregnancies. Hypertension in Pregnancy 2000;19(Suppl 1):O68. [Google Scholar]
- Ferrier C, Koferl U, Durig P, Schneider H. LMW heparin and low dose aspirin for prevention of preeclampsia: preliminary data of a randomised prospective study [abstract]. Hypertension in Pregnancy 2000;19(1):82. [Google Scholar]
Uganda 1992 {published data only}
- Lule J. [Personal communication] Trial of aspirin and methyldopa for moderate hypertension in pregnancy. Letter to: Cochrane Pregnancy and Childbirth Group, Oxford, UK 3 June 1993.
USA 1988a {published data only}
- O'Grady JP Wilson B. [Personal communication] Effects of low‐dose aspirin in improving fetal outcome in high risk pregnancies for intrauterine fetal growth retardation and/or preterm delivery. Letter to: Cochrane Pregnancy and Childbirth Group, Oxford, UK 19 March 1991.
USA 1990a {published data only}
- Carlson NJ. [Personal communication] Trial to evaluate the effectiveness of low dose aspirin versus placebo in the prevention of pregnancy induced hypertension in multiparous patients pregnant with multiple gestations. Letter to: Cochrane Pregnancy and Childbirth Group, Oxford, UK 6 September 1991.
Wright 2018 {published data only}
- Wright D, Rolnik DL, Syngelaki A, Paco Matallana C, Machuca M, Alvarado M, et al. Aspirin for evidence‐based preeclampsia prevention trial: effect of aspirin on length of stay in the neonatal intensive care unit. American Journal of Obstetrics and Gynecology 2018; Vol. 218, issue 6:612.e1‐6. [DOI] [PubMed]
Wright 2019 {published data only}
- Wright D, Nicolaides KH. Aspirin delays the development of preeclampsia. American Journal of Obstetrics and Gynecology 2019;220(6):580.e1‐580.e6. [DOI] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang J, ChiCTR1800015301. Aspirin for preventing placenta‐mediated pregnancy complications: A community‐based multicenter randomized controlled study. http://www.chictr.org.cn/showproj.aspx?proj=24982 (first received 22 March 2018).
References to ongoing studies
ASPIRIN trial 2017 {published data only}
- Goudar SS, CTRI/2016/05/006970. Aspirin supplementation for pregnancy indicated risk reduction in nulliparas (ASPIRIN) ‐ ASPIRIN. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=14886 (first received 31 May 2016).
- Hoffman M, NCT02409680. Aspirin supplementation for pregnancy indicated risk reduction in nulliparas (ASPIRIN). clinicaltrials.gov/ct2/show/NCT02409680 (first received 16 December 2016).
- Hoffman MK, Goudar SS, Kodkany BS, Goco N, Koso‐Thomas M, Miodovnik M, et al. A description of the methods of the aspirin supplementation for pregnancy indicated risk reduction in nulliparas (ASPIRIN) study. BMC Pregnancy and Childbirth 2017;17(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes OA, NCT01890005. Low dose aspirin between 13 and 16 weeks of pregnancy for the prevention of preeclampsia. double blind, randomized, controlled trial. clinicaltrials.gov/ct2/show/record/NCT01890005 (first received 1 July 2013).
China 2016a {published data only}
- Yang H, NCT02797249. Low dose aspirin in the prevention of preeclampsia in Chinese pregnant women. clinicaltrials.gov/ct2/show/record/NCT02797249 (first received 13 June 2016).
Egypt 2015 {published data only}
- Hassan A, PACTR201503001054393. Comparison between the roles of low dose aspirin and folic acid in preventing preeclampsia among high risk women screened by uterine artery Doppler at 22‐24 weeks of gestation: a randomised controlled trial. http://www.pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201503001054393 (first received 4 March 2015).
France 2012 {published data only}
- Perrotin F, NCT01729468. Prevention of pre‐eclampsia and sga by low‐dose aspirin in nulliparous women with abnormal first‐trimester uterine artery dopplers. clinicaltrials.gov/ct2/show/NCT01729468 (first received 20 November 2012).
Ghana 2016 {published data only}
- Browne JL, NCT02007837. Prospects for the prevention of pregnancy‐induced hypertension and preeclampsia (4p) ‐ a randomised, placebo‐controlled, double‐blind clinical trial. clinicaltrials.gov/ct2/show/NCT02007837 (first received 25 November 2016).
India 2018 {published data only}
- Kumar N, CTRI/2018/01/011155. Pilot interventional study by different low doses of aspirin versus placebo for prevention of preeclampsia ‐ a double blind randomized control trial. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=15679 (first received 5 January 2018).
Iran 2013 {published data only}
- Atarod Z, IRCT2013072914198N1. Using of low dose aspirin in the prevention of preeclampsia in pregnant women with abnormal uterin Doppler at 11‐14 weeks gestation. http://en.irct.ir/trial/13849 (first received 14 September 2013).
Iran 2015 {published data only}
- Fatemeh B, IRCT2014042017365N1. Effect of low dose aspirin in prevention of adverse pregnancy outcomes in women with unexplained elevated alpha‐protein in second trimester screening. http://en.irct.ir/trial/15984 (first received 5 May 2015).
Iran 2015a {published data only}
- Mirzai F, IRCT2014120620218N1. Evaluation of the effect of aspirin in pregnancy outcomes in patients with abnormal down syndrome biochemical tests and with normal karyotype. http://en.irct.ir/trial/17909 (first received 3 January 2015).
Ireland 2016 {published data only}
- Hussain R, EudraCT Number: 2013‐004241‐17. An open‐label randomized‐controlled trial of early screening test for pre‐eclampsia and growth restriction : a pilot study (TEST Study) ‐ TEST. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2013‐004241‐17 (first received 6 January 2014).
- Mone F, Mulcahy C, McParland P, Breathnach F, Morrison J, Daly S, et al. Performance of the fetal medicine foundation preeclampsia screening test in nulliparous women: results of the TEST Multicenter RCT. Reproductive sciences. Conference: 64th annual scientific meeting of the society for gynecologic investigation, SGI 2017. United states 2017; Vol. 24, issue 1 Supplement 1:286A‐7A.
- Mone F, Mulcahy C, McParland P, Breathnach F, Morrison JJ, Daly S, et al. Performance of the fetal medicine foundation preeclampsia screening test in nulliparous women: results of the TEST multicenter RCT. BJOG: an international journal of obstetrics and gynaecology 2017; Vol. 124:10.
- Mone F, Mulcahy C, McParland P, Culliton M, Downey P, Maguire O, et al. The impact of low dose aspirin on markers of placental disease‐results of the test multicenter RCT. BJOG: an international journal of obstetrics and gynaecology 2017; Vol. 124:11.
- Mone F, Mulcahy C, McParland P, Culliton M, Downey P, Maguire O, et al. The impact of low dose aspirin on markers of placental disease‐results of the test multicentre RCT. Reproductive Sciences 2017;24(Suppl 1):157A, Abstract no: T‐169. [Google Scholar]
- Mone F, Mulcahy C, McParland P, McAuliffe FM, Downey P, Culliton M, et al. The impact of low dose aspirin on preeclampsia biomarkers and fetal growth in low risk women. American Journal of Obstetrics and Gynecology 2018;218(1):S303. [Google Scholar]
- Mone F, Mulcahy C, McParland P, O'Mahony J, Tyrell E, Breathnach F, et al. A randomised controlled trial and cost effectiveness analysis of low dose aspirin with an early screening test for pre‐eclampsia in low risk women. BJOG: an international journal of obstetrics and gynaecology 2017; Vol. 124:10‐1.
- Mone F, Mulcahy C, McParland P, O'Mahony J, Tyrell E, Breathnach F, et al. A randomised controlled trial and cost‐effectiveness analysis of low dose aspirin with an early screening test for preeclampsia in low risk women. Reproductive sciences. Conference: 64th annual scientific meeting of the society for gynecologic investigation, SGI 2017. United states 2017; Vol. 24, issue 1 Supplement 1:68A.
- Mone F, Mulcahy C, McParland P, Stanton A, Culliton M, Downey P, et al. An open‐label randomized‐controlled trial of low dose aspirin with an early screening test for pre‐eclampsia and growth restriction (TEST): Trial protocol. Contemporary Clinical Trials 2016;49:143‐8. [DOI] [PubMed] [Google Scholar]
- Mulcahy C, McParland P, Mone F, McAuliffe FM, Cody F, Breathnach F, et al. Performance of routine first trimester sonographic evaluation of fetal anatomy: results of the multicentre test rct. American Journal of Obstetrics and Gynecology 2017;216(1 Suppl):S83, Abstract no: 117. [Google Scholar]
- Mulcahy C, Mone F, McParland P, Breathnach F, Cody F, Morrison JJ, et al. The impact of aspirin on 3D placental volumes and vascular flow indices in the first and second trimesters of pregnancy and correlation with uterine artery doppler: results of the test multicentre RCT. American Journal of Obstetrics and Gynecology 2017;216(1 Suppl):S84, Abstract no: 118. [Google Scholar]
Netherlands 2017 {published data only}
- Visser L, Boer MA, Groot CJ, Nijman TA, Hemels MA, Bloemenkamp KW, et al. Low dose aspirin in the prevention of recurrent spontaneous preterm labour ‐ the april study: a multicenter randomized placebo controlled trial. BMC Pregnancy and Childbirth 2017;17(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer M, NTR5675. Low dose aspirin in the Prevention of Recurrent Spontaneous Preterm Labour – the APRIL study. trialregister.nl/trialreg/admin/rctview.asp?TC=5675 (first received 22 January 2016).
Spain 2012 {published data only}
- EudraCT Number: 2011‐005979‐16. Prevention of preeclampsia in oocyte donation women by the administration of aspirin in early pregnancy. clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2011‐005979‐16 (14 September 2012).
- Perales A, NCT02174328. Prevention of preeclampsia with aspirin administered from the beginning of pregnancy in recipients of donated oocytes. clinicaltrials.gov/ct2/show/NCT02174328 (first received 25 June 2014).
Thailand 2017 {published data only}
- Chaiarach S, TCTR20170629006. Combined therapy with low dose aspirin and calcium supplements during second trimester to reduce the risk of superimposed preeclampsia in pregnant women with chronic hypertension: a randomized‐controlled trial. clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=2628 (first received 21 June 2017).
Additional references
ACOG 2018
- Committee on obstetric practice, Society for Maternal‐Fetal Medicine. Low‐dose aspirin use during pregnancy. Obstetrics & Gynaecology 2018;132(1):e44‐52. [Google Scholar]
Akolekar 2013
- Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagnosis and Therapy 2013;33(1):8‐15. [DOI] [PubMed] [Google Scholar]
Ananth 1995
- Ananth CV, Savitz DA, Bowes WA. Hypertensive disorders of pregnancy and stillbirth in North Carolina, 1988‐1991. Acta Obstetricia et Gynecologica Scandinavica 1995;74:788‐93. [DOI] [PubMed] [Google Scholar]
Ananth 2013
- Ananth CV, Keyes KM, Wapner RJ. Pre‐eclampsia rates in the United States, 1980–2010:age‐period‐cohort analysis. BMJ 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Askie 2007
- Askie LM, Duley L, Henderson‐Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre‐eclampsia: a meta‐analysis of individual patient data. Lancet 2007;369:1791‐8. [DOI] [PubMed] [Google Scholar]
Auger 2016
- Auger N, Luo ZC, Nuyt AM, Kaufman JS, Naimi AI, Platt RW, et al. Secular Trends in Preeclampsia Incidence and Outcomes in a Large Canada Database: A Longitudinal Study Over 24 Years. Canadian Journal of Cardiology 2016;32:987.e15‐23. [DOI] [PubMed] [Google Scholar]
Barth 1998
- Barth W Jr. Low‐dose aspirin for preeclampsia‐‐the unresolved question. New England Journal of Medicine 1998;338(11):756‐7. [DOI] [PubMed] [Google Scholar]
Beilin 1994
- Beilin L. Aspirin and pre‐eclampsia. BMJ 1994;308:1250‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Broughton Pipkin 1996
- Broughton Pipkin F, Crowther C, Swiet M, Duley L, Judd A, Lilford RJ, et al. Where next for prophylaxis against pre‐eclampsia?. British Journal of Obstetrics and Gynaecology 1996;103:603‐7. [DOI] [PubMed] [Google Scholar]
Bujold 2010
- Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta‐analysis. Obstetrics and Gynecology 2010;116:402‐14. [DOI] [PubMed] [Google Scholar]
Bussolino 1980
- Bussolino F, Benedetto C, Massobrio M, Camussi G. Maternal vascular prostacyclin activity in pre‐eclampsia. Lancet 1980;ii:702. [DOI] [PubMed] [Google Scholar]
CMACE UK 2011
- Centre for Maternal and Child Enquiries (CMACE). Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer: 2006–08. The Eighth Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG: an international journal of obstetrics and gynaecology 2011;118(Suppl):1‐203. [DOI] [PubMed] [Google Scholar]
Coomarasamy 2001
- Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for the prevention of preeclampsia in women with abnormal uterine artery Doppler: a meta‐analysis. Obstetrics and Gynecology 2001;98:861‐6. [DOI] [PubMed] [Google Scholar]
Coomarasamy 2003
- Coomarasamy A, Honest H, Papaioannou S, Gee H, Khan K. Aspirin for prevention of preeclampsia in women with historical risk factors : a systematic review. Obstetrics and Gynecology 2003;101(6):1319–332. [DOI] [PubMed] [Google Scholar]
Darling 1998
- Darling M. Low‐dose aspirin not for pre‐eclampsia. Lancet 1998;352(9125):342. [DOI] [PubMed] [Google Scholar]
De Berardis 2012
- Berardis G, Lucisanon G, D'Ettorre A, Pellegrini F, Lepore V, Tognoni G, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA 2012;307(21):2286‐94. [DOI] [PubMed] [Google Scholar]
Dekker 1993
- Dekker GA, Sibai Baha M. Low‐dose aspirin in the prevention of preeclampsia and fetal growth retardation: rationale, mechanisms, and clinical trials. American Journal of Obstetrics and Gynecology 1993;168:214‐27. [DOI] [PubMed] [Google Scholar]
Dept of Health 1996
- Department of Health. Confidential Enquiry into Stillbirths and Deaths in Infancy: 3rd Annual Report. London: Department of Health, 1996. [Google Scholar]
Duley 1992a
- Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. British Journal of Obstetrics and Gynaecology 1992;99:547‐53. [DOI] [PubMed] [Google Scholar]
Duley 1999b
- Duley L, Henderson‐Smart D. Reduced salt intake compared to normal dietary salt, or high intake, in pregnancy. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001687] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gifford 2000
- Gifford RW Jr, August PA, Cunningham G, Green LA, Lindhemier MD, McNellis D, et al. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. American Journal of Obstetrics and Gynecology 2000;183(Suppl):1‐22. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2018
- Hofmeyr GJ, Lawrie TA, Atallah ÁN, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 2018, Issue 10. [DOI: 10.1002/14651858.CD001059.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imperiale 1991
- Imperiale TF, Petrulis AS. A meta‐analysis of low‐dose aspirin for the prevention of pregnancy‐induced hypertensive disease. JAMA 1991;266:260‐4. [PubMed] [Google Scholar]
Janes 1995
- Janes S, Kyle P, Redman C, Goodall A. Flow cytometric detection of activated platelets in pregnant women prior to the development of pre‐eclampsia. Thrombosis and Haemostasis 1995;74:1059‐63. [PubMed] [Google Scholar]
Leitich 1997
- Leitich H, Egarter C, Husslein P, Kaider A, Schemper M. A meta‐analysis of low dose aspirin for prevention of intrauterine growth retardation. British Journal of Obstetrics and Gynaecology 1997;104:450‐9. [DOI] [PubMed] [Google Scholar]
Mahler 1987
- Mahler H. The safe motherhood initiative: a call to action. Lancet 1987;i:668‐70. [DOI] [PubMed] [Google Scholar]
Makrides 2014
- Makrides M, Crosby DD, Shepherd E, Crowther CA. Magnesium supplementation in pregnancy. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD000937.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masotti 1979
- Masotti G, Galanti G, Poggesi L, Abbate R, Neri Serneri GG. Differentialinhibition of prostacyclin production and platelet aggregation by aspirin. Lanct 1979;2:1213‐7. [DOI] [PubMed] [Google Scholar]
Meads 2008
- Meads CA, Cnossen JS, Meher S, Juarez‐Garcia A, ter Riet G, Duley L, et al. Methods of prediction and prevention of pre‐eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technology Assessment 2008;12(6):iii‐iv, 1‐270. [PUBMED: 18331705] [DOI] [PubMed] [Google Scholar]
Meher 2005
- Meher S, Duley L, Prevention of Pre‐eclampsia Cochrane Review Authors. Interventions for preventing pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005301] [DOI] [Google Scholar]
Meher 2013
- Meher S, Alfirevic Z. Aspirin for pre‐eclampsia: beware of subgroup meta‐analysis. Ultrasound in Obstetrics and Gynecology 2013;41(5):479‐85. [DOI] [PubMed] [Google Scholar]
Meher 2017
- Meher S, Duley L, Hunter K, Askie L. Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: an individual participant data meta‐analysis. American Journal of Obstetrics and Gynecology 2017;216:121‐8.e2. [DOI] [PubMed] [Google Scholar]
Middleton 2018
- Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega‐3 fatty acid addition during pregnancy. Cochrane Database of Systematic Reviews 2018, Issue 11. [DOI: 10.1002/14651858.CD003402.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mol 2016
- Mol BW, Roberts CT, Thangaratinam S, Magee LA, Groot CJM, Hofmeyr GJ. Pre‐eclampsia. Lancet 2016;387(10022):999‐1011. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Collaborating Centre for Women's and Children's Health (UK). Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. London: RCOG Press, 2010. [PubMed] [Google Scholar]
Ota 2015
- Ota E, Hori H, Mori R, Tobe‐Gai R, Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD000032.pub3] [DOI] [PubMed] [Google Scholar]
PARIS 2005
- Perinatal Antiplatelet Review of International Studies (PARIS) Collaboration Steering Group on behalf of the PARIS collaboration. Antiplatelet agents for prevention of pre‐eclampsia and its consequences: a systematic review and individual patient data meta‐analysis. BMC Pregnancy and Childbirth 2005;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redman 1978
- Redman C, Bonnar J, Beilin L. Early platelet consumption in pre‐eclampsia. BMJ 1978;1:467‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redman 1991
- Redman C. Current topic: pre‐eclampsia and the placenta. Placenta 1991;12:301‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rey 1996
- Rey E, Derderian F. [Efficacite de l'aspirine a faible dose au cours de al grossesse en fonction des facteurs de risque maternels et foetaux]. SOGC: journal of the Society of Obstetricians and Gynaecologists of Canada 1996;18(10):51‐60. [Google Scholar]
Riley 2010
- Riley RD, Lambert PC, Abo‐Zaid G. Meta‐analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340:c221. [DOI: 10.1136/bmj.c221] [DOI] [PubMed] [Google Scholar]
Roberge 2013
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low‐dose aspirin: a meta‐analysis. Ultrasound in Obstetrics and Gynecology 2013;41(5):491‐9. [DOI] [PubMed] [Google Scholar]
Roberge 2017
- Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta‐analysis. American Journal of Obstetrics and Gynecology 2017;216:110‐20.e6. [DOI] [PubMed] [Google Scholar]
Roberts 2007
- Roberts JM, Catov JM. Aspirin for pre‐eclampsia: compelling data on benefit and risk. Lancet 2007;369:1765‐6. [DOI] [PubMed] [Google Scholar]
Roberts 2009
- Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta 2009;30(Suppl 1):32‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenfield 1985
- Rosenfield A, Maine D. Maternal mortality ‐ a neglected tragedy. Lancet 1985;ii:83‐5. [DOI] [PubMed] [Google Scholar]
Rossi 2011
- Rossi AC, Mullin PM. Prevention of pre‐eclampsia with low‐dose aspirin or vitamins C and E in women at high or low risk: a systematic review with meta‐analysis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2011;158:9‐16. [DOI] [PubMed] [Google Scholar]
Ruano 2005
- Ruano R, Fontes RS, Zugaib M. Prevention of preeclampsia with low‐dose aspirin ‐‐ a systematic review and meta‐analysis of the main randomized controlled trials. Clinics 2005;60:407–14. [DOI] [PubMed] [Google Scholar]
Rumbold 2008
- Rumbold A, Duley L, Crowther CA, Haslam RR. Antioxidants for preventing pre‐eclampsia. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004227.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saigal 2008
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371(9608):261‐9. [DOI] [PubMed] [Google Scholar]
Sanchez‐Ramos 1994
- Sanchez‐Ramos L, Wears R, Valle GO, Gaudier FL, Adair D. Low dose aspirin for the prevention of pregnancy‐induced hypertension: a meta‐analysis. American Journal of Obstetrics and Gynecology 1994;170:408. [Google Scholar]
Say 2014
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014;2(6):e323‐e333. [DOI] [PubMed] [Google Scholar]
Seidler 2018
- Seidler AL, Ray J, Askie L. Optimal aspirin dosing for preeclampsia prevention. American Journal of Obstetrics and Gynecology 2018;219(1):117‐8. [DOI] [PubMed] [Google Scholar]
Sharts‐Engel 1992
- Sharts‐Engel NC. Aspirin for prevention of pregnancy‐induced hypertension. MCN. The American Journal of Maternal Child Nursing 1992;17(3):168. [DOI] [PubMed] [Google Scholar]
Thornton 2013
- Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population‐linked datasets: 2000‐2008. American Journal of Obstetrics and Gynecology 2013;208:476.e1‐5. [DOI] [PubMed] [Google Scholar]
Tong 2017
- Tong S, Mol BW, Walker SP. Preventing preeclampsia with aspirin: does dose or timing matter?. American Journal of Obstetrics and Gynecology 2017;216:95‐7. [DOI] [PubMed] [Google Scholar]
Tranquilli 2014
- Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregancy Hypertension 2014;4:97‐104. [DOI] [PubMed] [Google Scholar]
Trivedi 2011
- Trivedi NA. A meta‐analysis of low‐dose aspirin for prevention of preeclampsia. Journal of Postgraduate Medicine 2011;57:91‐5. [DOI] [PubMed] [Google Scholar]
Tudur Smith 2016
- Tudur Smith C, Marcucci M, Nolan SJ, Iorio A, Sudell M, Riley R, et al. Individual participant data meta‐analyses compared with meta‐analyses based on aggregate data. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.MR000007.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uzan 1998
- Uzan S, Beaufils M, Dumont A, Merviel P, Challier JC, Breart G. Does aspirin still have a role in pregnancy?. Fetal Diagnosis and Therapy 1998;13:131‐2. [DOI] [PubMed] [Google Scholar]
Uzan 2011
- Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi J‐M. Pre‐eclampsia: pathophysiology, diagnosis, and management. Vascular Health and Risk Management 2011;7:467‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Vliet 2017
- Vliet EO, Askie LA, Mol BW, Oudijk MA. Antiplatelet agents and the prevention of spontaneous preterm birth: a systematic review and meta‐analysis. Obstetrics & Gynecology 2017;129(2):327‐36. [DOI] [PubMed] [Google Scholar]
WHO 1988
- World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. American Journal of Obstetrics and Gynecology 1988;158:80‐3. [PubMed] [Google Scholar]
WHO 2006
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367(6516):1066‐74. [DOI] [PubMed] [Google Scholar]
WHO 2014
- World Health Organization. World Health Statistics 2014. Millennium Development Goals (MDGs). http://www.who.int/mediacentre/factsheets/fs290/en/ (accessed 11th September, 2014).
World Bank 2018
- World Bank. World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 (accessed 9th May 2019).
Xu 2015
- Xu TT, Zhou F, Deng CY, Huang GQ, Li JK, Wang XU. Low‐dose aspirin for preventing preeclampsia and its complications: a meta‐analysis. Journal of Clinical Hypertension 2015;17(7):567‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Collins 1995
- Collins R. Antiplatelet agents for IUGR and pre‐eclampsia. [revised 04 May 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Duley 1999
- Duley L. Aspirin for preventing and treating pre‐eclampsia. BMJ 1999;318(7186):751‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2001
- Duley L, Henderson‐Smart D, Knight M, King J. Antiplatelet drugs for prevention of pre‐eclampsia and its consequences: systematic review. BMJ 2001;322:329‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2003
- Duley L, Henderson‐Smart DJ, Knight M, King JF. Antiplatelet agents for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004659] [DOI] [PubMed] [Google Scholar]
Duley 2007
- Duley L, Henderson‐Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004659.pub2] [DOI] [PubMed] [Google Scholar]
Knight 2000a
- Knight M, Duley L, Henderson‐Smart DJ, King JF. Antiplatelet agents for preventing and treating pre‐eclampsia. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000492] [DOI] [PubMed] [Google Scholar]
Knight 2000b
- Knight M, Duley L, Henderson‐Smart D, King J. Anitplatelets for prevention of pre‐eclampsia and its consequences: a systematic review. Hypertension in Pregnancy 2000;19(Suppl 1):33. [Google Scholar]


