Abstract
Background
Platelet-endothelial aggregation receptor 1 (PEAR-1) is a transmembrane receptor involved in platelet activation and megakaryopoiesis whose expression is driven by DNA methylation. PEAR1 variants were associated with differential platelet response to activation and cardiovascular outcomes. We aimed at investigating the link between PEAR1 methylation and platelet and leukocyte function markers in a family-based population.
Results
We measured PEAR1 methylation in 605 Moli-family participants with available blood counts, plasma P-selectin and C-reactive protein, whole blood platelet P-selectin, and platelet-leukocyte mixed conjugate measurements. We performed principal component analysis (PCA) to identify groups of highly correlated CpG sites. We used linear mixed regression models (using age, gender, BMI, smoking, alcohol drinking, being a proband for family recruitment, being a member of myocardial infarction (MI) family as fixed effects, and family as a random effect) to evaluate associations between PEAR1 methylation and phenotypes. PEAR1 methylation Factor2, characterized by the previously identified megakaryocyte-specific CpG sites, was inversely associated with platelet-monocyte conjugates, P-selectin, and WBC counts, while positively associated with the platelet distribution width (PDW) and with leukocyte CD11b and L-selectin. Moreover, PEAR1 Factor2 methylation was negatively associated with INFLAscore, a low-grade inflammation score. The latter was partially mediated by the PEAR1 methylation effect on platelet variables. PEAR1 methylation association with WBC measurements and INFLAscore was confirmed in the independent cohort FLEMENGHO.
Conclusions
We report a significant link between epigenetic signatures in a platelet functional gene and inflammation-dependent platelet function variability measured in two independent cohorts.
Introduction
Platelet-endothelial aggregation receptor 1 (PEAR-1) is a membrane receptor involved in cell-cell interactions, particularly expressed in platelets, megakaryocytes, and endothelial cells. PEAR-1 sustains activation of the platelet integrin αIIbβ3 through its src family kinase (c-Src)-dependent phosphorylation that stabilizes platelet aggregate formation [1]. The direct activation of PEAR-1 not only by its pentameric ligand, the FcεR1α chain, but also by anti-PEAR-1 antibodies, dextran sulfate, synthetic glycopolymers, and natural fucoidans triggers potent platelet aggregation [1–4]. Numerous large studies have identified PEAR1 genetic variants as determinants of platelet response/function variability, both in the general population and in cohorts with cardiovascular outcomes [5–29], suggesting that PEAR-1 may be a signaling component, capable of modulating several functional platelet pathways in physiological conditions, but also in the context of anti-platelet therapy and cardiovascular disease. This seems to be the case in particular for rs12041331 and rs12566888, 2 PEAR1 variants in linkage disequilibrium (LD) located in intron 1 of the PEAR1 gene locus [30]. In particular, PEAR1 rs12041331 G/A substitution leads to lower platelet PEAR1 expression [6] and reduces endothelial cell migration in carriers of the A allele [31], while a negative association of rs12566888 with WBC, neutrophil, and monocyte numbers in a large-scale Exomechip analysis has been reported by Eicher and colleagues [25]. The latter opened up the possibility for a pleiotropic role of PEAR1 in influencing not only platelet function variability but also hematopoiesis at large. Indeed, PEAR1 expression increases during megakaryocyte (MK) differentiation and PEAR1 knock-down CD34+ cells show higher proliferation of immature MKs, whereas terminal MK maturation (proplatelet formation) is not affected in the absence of PEAR-1 [32]. In addition, expression profiling on normal human bone marrow sections also showed transient PEAR1 positivity in myeloid precursors, yet absent in mature granulocytes [32].
We have previously identified a region within the first untranslated exon of the PEAR1 gene that, towards the later stages of MK specification, undergoes a significant increase of DNA methylation level in parallel to PEAR1 expression [30]. We found the same region to be differentially methylated between megakaryocyte and endothelial cells and to be part of a superenhancer that coordinates expression of multiple genes involved in cell cycle and cell proliferation through long-range chromosome interactions [33]. This type of epigenetic regulation contributes to the fine-tuning of PEAR1 expression, but it remains unclear, at the population level, whether PEAR1 epigenetic variability would contribute to explain variability of platelet function and would also have an impact on hematopoiesis and leukocyte function.
In this study, we investigated PEAR1 methylation as a marker of platelet and leukocyte formation, their activation and cross-talk, using DNA samples from a family-based cohort study (the Moli-family study) [34–36], characterized by a large set of hematological activation markers. Our major results were replicated in a second independent population-based cohort (the FLEMENGHO study) [37–39].
Results
Demographics of the population studied are shown in Table 1. Blood cell counts, platelet, and leukocyte activation markers are reported in Table 2.
Table 1.
General characteristics of Moli-family participants
| Characteristic | Moli-family |
|---|---|
| Number (%) of participants | 605 |
| Women | 330 (54.5) |
| Current smoking | 165 (27.4) |
| Drinking alcohola | 122 (21.8) |
| Hypertension | 206 (34.2) |
| Diabetes | 36 (6.0) |
| Mean (± SD) characteristic | |
| Age, years | 42.3 ± 18.8 |
| Body mass index, kg/m2 | 26.5 ± 5.4 |
| Blood pressure, mm Hg | |
| Systolic | 130.2 ± 20.6 |
| Diastolic | 77.1 ± 11.6 |
| Total serum cholesterol, mmol/L | 5.06 ± 1.10 |
| HDL cholesterol, mmol/L | 1.49 ± 0.38 |
| Total-to-HDL cholesterol ratio | 3.56 ± 1.03 |
HDL indicates high-density lipoprotein. Hypertension was a blood pressure of ≥ 140 mmHg systolic or ≥ 90 mmHg diastolic or use of antihypertensive drugs. Diabetes mellitus was a fasting plasma glucose level of ≥ 7.0 mmol/L or use of antidiabetic agents
aDefined as drinking at least 15 g of alcohol daily
Table 2.
Platelet and WBC variable distribution in the Moli-family cohort
| Variable | Moli-family (n = 605) |
|---|---|
| Platelet measurements | |
| Platelet count, 109/L | 249.4 ± 60,6 |
| Mean platelet volume, fL | 8.59 ± 0.98 |
| Plateletcrit, % | 0.21 ± 0.05 |
| Platelet distribution width, % | 16.4 ± 0.6 |
| Soluble P-selectin, μg/L | 82.7 ± 38.0 |
| Platelet P-selectin, % | 2.76 ± 3.65 |
| Platelet/monocytes aggregates, % | 7.24 ± 8.62 |
| Platelet/PMN aggregates, % | 4.32 ± 5.02 |
| Inflammation measurements | |
| White blood cell count, 109/L | 6.19 ± 1.52 |
| Neutrophils, % | 61.0 ± 8.0 |
| Lymphocyte, % | 32.0 ± 7.0 |
| Neutrophil/lymphocyte ratio | 2.08 ± 0.83 |
| Monocyte, % | 7.0 ± 2.0 |
| Red blood cell, 1012/L | 4.89 ± 0.5 |
| Platelet/lymphocyte ratio | 135.9 ± 47.6 |
| INFLAscore* | − 0.23 ± 5.59 |
| C-reactive protein, mg/mL | 1.7 ± 1.74 |
| Monocyte L-selectin, % | 10.4 ± 7.2 |
| PMN L-selectin, % | 25.7 ± 21.3 |
| Monocyte CD11b, % | 51.5 ± 24.1 |
| PMN CD11b, % | 44.0 ± 25.1 |
*INFLAscore is calculated as follows: 10 tiles of each biomarker levels (CRP, WBC, platelets, G/L ratio) were generated. For all four components, being in the highest deciles (7 to 10) gave a score which increased from 1 to 4, while being in the lowest deciles (1 to 4) was negatively scored from − 4 to − 1. Being in the deciles 5 or 6 got zero points. In such a way, the INFLA-score ranges from − 16 to 16 and comes up as the sum of the four biomarkers. An increase in the score represented an increase in low-grade inflammation intensity
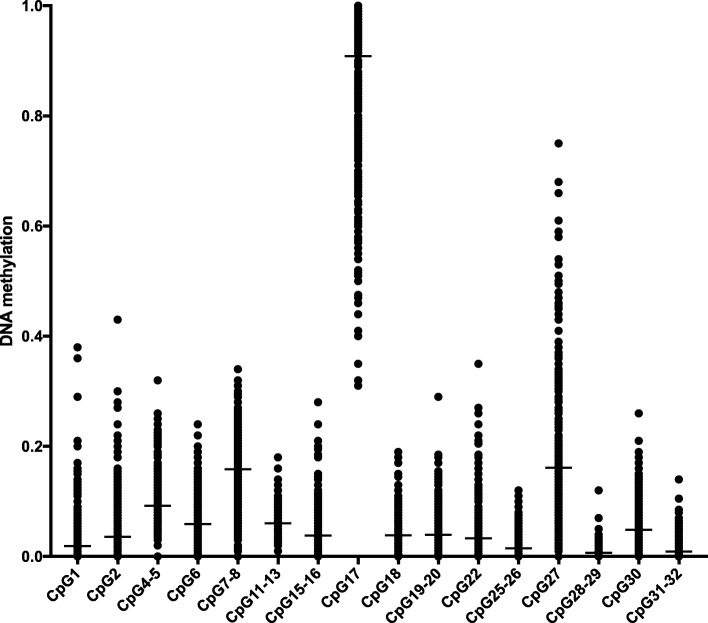
After removing duplicate units and units with standard deviation (SD) between replicates higher than 5% as described [30, 40, 41], we obtained a total of PEAR1 16 CpG units for further analysis. These PEAR1 CpG sites, identified for the Moli-family cohort, include the previously identified megakaryocyte-specific CpG sites [30]. The exact genomic location of each CpG site in PEAR1 is reported in the Additional file 1: Table S1. For each of these sites, Fig. 1 shows the distribution of the fractional degree of methylation, uncovering a wide variation in methylation within and between different sites.
Fig. 1.
PEAR1 CGI1 DNA methylation distribution in the Moli-family cohort. Dot-plot representation of PEAR1 CpG unit (depicted on the Y axis) methylation distribution across the Moli-family participants (N = 605). Mean is shown for each unit as a black bar
After exclusion of failing samples, a total of 605 Moli-family participants were included for further analyses. Mean and SD of PEAR1 CpG sites methylation ranged from 0.6 ± 1.0% for CpG28–29 up to 90.8 ± 14.1% for CpG17. Only CpG7–8, 17, and 27 had methylation averages across the population higher than 10%, while all the other 13 CpG units appeared less methylated (0 to 10%) (Fig. 1 and Additional file 1: Table S1). Several of the examined CpGs showed strong inter-correlations (Additional file 1: Table S2).
Because PEAR1 CpG site methylation showed significant inter-correlations, we used principal component analysis (PCA) with the aim of identifying common underlying factors (patterns) that explain the largest variation in methylation. For this analysis, 370 individuals were included having all the PEAR1 CpG units successfully measured. Three-, four-, and five-factor solutions were discarded on the basis of the scree plot. Two main methylation factors finally emerged with PCA. Factor1 is characterized by high positive loadings of CpGs 4–5, 6, 11–13, 17, 18, 27, and 30, and Factor2 by that of CpGs 7–8, 15–16, 19–20, 22, 25–26, and 31–32 (Additional file 1: Table S3). Three more CpG units (CpG1, CpG2, and CpG28–29) did not appear to contribute to any of the identified methylation factors; therefore, they were included in further analyses as separate methylation variables (Table 4 and Additional file 1: Table S4).
Table 4.
Platelets do not influence the association between Factor2 PEAR1 methylation and WBC counts in the Moli-family cohort
| Model A | Model B | |||||||
|---|---|---|---|---|---|---|---|---|
| Beta | SE | p value | R-square | Beta | SE | p value | R-square | |
| WBC | − 0.123 | 0.058 | 0.036 | 0.020 | − 0.150 | 0.054 | 0.006 | 0.025 |
| Lymphocytes | 0.210 | 0.055 | 0.0002 | 0.009 | 0.224 | 0.056 | < 0.0001 | 0.051 |
| Monocytes | 0.239 | 0.055 | < 0.0001 | 0.023 | 0.268 | 0.057 | < 0.0001 | 0.069 |
| Granulocytes | − 0.275 | 0.055 | < 0.0001 | 0.046 | − 0.286 | 0.056 | < 0.0001 | 0.079 |
Model A adjusted by age, gender, BMI, smoking (being an ever smoker and pack-years), drinking (a drinker was defined as drinking daily more than 15 g of alcohol), being a proband for family recruitment, being a member of MI family as fixed effects, and family as a random effect. Model B is Model A +adjustment by platelets, PDW, platelet P-selectin, platelet/monocyte, and platelet/PMN. R-squares were calculated using a linear regression model including covariates used as fixed effect in the mixed models. Standardized values of phenotypes and methylation are reported. Significant p values are reported in italics
PEAR1 methylation is associated with ex vivo platelet-monocyte mixed aggregates
Linear mixed regression analysis was performed to assess the relation between PEAR1 methylation patterns (Factor1, Factor2, CpG1, CpG2 and CpG28–29) and platelet activation phenotypes, considering whole blood baseline platelet P-selectin and platelet-monocyte or platelet-leukocyte mixed conjugates already measured in the cohort [34]. We found that platelet-monocyte, but not platelet-polymorphonuclear cell (PMN), mixed conjugates were consistently negatively associated with PEAR1 Factor2 methylation (Additional file 1: Table S4) with the association explaining 3.5% of platelet-monocyte aggregate variability (Additional file 1: Table S4). The association between PEAR1 Factor2 methylation and platelet P-selectin almost reached significance with a p value of 0.08. PEAR1 Factor1, CpG1, CpG2, and CpG28–29 methylation was not associated with any of the platelet-specific variables measured (Additional file 1: Table S4).
In a previous study, focused on the methylation of the same PEAR1 region, we identified some specific CpG sites that significantly changed their methylation status according to PEAR1 expression during differentiation of megakaryocyte precursors (CpG4–5, 7–8, 11–13, 15–16, 19–20, 22) [30]. Based on that finding, and because PEAR1 Factor2 mainly depends on these MK-specific CpG sites (Additional file 1: Table S3), we also performed a specific CpG methylation analysis considering the 16 PEAR1 CpG units as single measures in the Moli-family cohort. By using this type of approach, we identified CpG sites 7–8 and 19–20 as the most significantly associated PEAR1 sites with platelet P-selectin and platelet-PMN aggregates (Additional file 1: Table S5).
PEAR1 methylation is associated with white blood cell measurements
We investigated the possible association of PEAR1 methylation with blood cell counts in the Moli-family cohort. While no significant association was found with platelet count, mean platelet volume (MPV), or plateletcrit (Pct), PEAR1 Factor2 methylation was positively associated with platelet distribution width (PDW) (Table 3), a measure of platelet heterogeneity and indirect estimate of megakaryopoiesis variation. This association explained 2.6% of PDW variability (Table 3).
Table 3.
PEAR1 methylation is significantly associated with PDW, WBC, lymphocyte, monocyte, and neutrophil counts in the Moli-family cohort
| Factor1 | Factor2 | P1 | P2 | P28_29 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | p value | R-square | Beta | SE | p value | R-square | Beta | SE | p value | R-square | Beta | SE | p value | R-square | Beta | SE | p value | R-square | |
| Platelets | − 0.088 | 0.048 | 0.071 | 0.006 | 0.083 | 0.052 | 0.109 | 0.007 | 0.009 | 0.038 | 0.800 | 0.000 | − 0.011 | 0.042 | 0.788 | 0.000 | − 0.025 | 0.038 | 0.500 | 0.001 |
| MPV | − 0.033 | 0.053 | 0.530 | 0.001 | 0.001 | 0.058 | 0.993 | 0.000 | 0.017 | 0.040 | 0.679 | 0.000 | − 0.004 | 0.044 | 0.928 | 0.000 | 0.048 | 0.041 | 0.233 | 0.004 |
| Pct | − 0.105 | 0.049 | 0.034 | 0.008 | 0.100 | 0.053 | 0.058 | 0.007 | 0.014 | 0.038 | 0.790 | 0.000 | − 0.001 | 0.042 | 0.979 | 0.000 | − 0.005 | 0.038 | 0.888 | 0.000 |
| PDW | 0.023 | 0.056 | 0.677 | 0.002 | 0.145 | 0.058 | 0.013 | 0.026 | 0.052 | 0.043 | 0.223 | 0.002 | − 0.052 | 0.045 | 0.249 | 0.000 | − 0.010 | 0.041 | 0.800 | 0.000 |
| WBC | − 0.047 | 0.056 | 0.397 | 0.001 | − 0.123 | 0.058 | 0.036 | 0.020 | − 0.018 | 0.041 | 0.657 | 0.000 | − 0.066 | 0.045 | 0.143 | 0.008 | 0.001 | 0.041 | 0.989 | 0.000 |
| Lymphocytes | 0.126 | 0.053 | 0.019 | 0.002 | 0.210 | 0.055 | 0.0002 | 0.009 | 0.027 | 0.041 | 0.521 | 0.000 | 0.097 | 0.044 | 0.028 | 0.001 | 0.006 | 0.041 | 0.887 | 0.000 |
| Monocytes | − 0.085 | 0.052 | 0.105 | 0.005 | 0.239 | 0.055 | < 0.0001 | 0.023 | 0.075 | 0.037 | 0.045 | 0.005 | 0.040 | 0.041 | 0.327 | 0.001 | 0;015 | 0.037 | 0.691 | 0.000 |
| Neutrophils | − 0.105 | 0.055 | 0.081 | 0.003 | − 0.275 | 0.055 | < 0.0001 | 0.046 | − 0.049 | 0.041 | 0.228 | 0.001 | − 0.104 | 0.044 | 0.019 | 0.009 | − 0.008 | 0.040 | 0.851 | 0.000 |
Model adjusted by age, gender, BMI, smoking (being an ever smoker and pack-years), drinking (a drinker was defined as drinking daily more than 15 g of alcohol), being a proband for family recruitment, being a member of MI family as fixed effects, and family as a random effect. R-squares were calculated using a linear regression model including covariates used as fixed effect in the mixed models. Standardized values of phenotypes and methylation are reported. Significant p values are reported in italics
In addition to that, we identified an inverse relationship between both PEAR1 Factor2 and total white blood cell (WBC) counts and neutrophil percentage and a positive one with both lymphocyte and monocyte percentages (Table 3). PEAR1 methylation could explain 4.6% of neutrophil percentage variability (Table 3).
When adjusting the analysis for platelet variables (platelet count, PDW, platelet P-selectin, platelet/monocyte, and platelet/PMN mixed aggregates), all WBC measurements still remained significantly associated with Factor2 with increased significance and effect of the associations (Table 4 Model B). When removing the platelet influence on this association, PEAR1 Factor2 methylation could explain 5.1, 6.9, and 7.9% of lymphocyte, monocyte, and granulocyte variability, respectively (Table 4 Model B), suggesting that the association between PEAR1 methylation and these cells was independent of thrombopoiesis or platelet activity.
PEAR1 methylation is associated with markers of inflammation
Because PEAR1 methylation appeared to be involved at a different level in platelet and WBC variability, and because both platelets and leukocytes are mediators of cellular inflammation, we have further investigated whether PEAR1 methylation would be linked to inflammation markers, available in the Moli-family cohort. For this purpose, we studied the association between PEAR1 methylation and markers of platelet and leukocyte activation and C-reactive protein (CRP) levels.
PEAR1 Factor2 methylation was strongly and significantly associated with soluble P-selectin levels following an inverse relationship (Table 5 Model A). Interestingly, this association could explain 10% of plasma P-selectin variability (Table 5 Model A). Soluble P-selectin not only results from platelet activation, but can also be of endothelial cell origin; therefore, we corrected this association for platelet-specific covariates (Table 5, Model B). We observed that, while almost no change occurred considering the effect (ß values) of PEAR1 methylation on plasma P-selectin levels, the variability of the same marker explained by the methylation dropped from 10 to 5% (Table 5, Model B).
Table 5.
PEAR1 Factor2 methylation association with markers of cell and plasmatic inflammation partially depends upon platelet activation n the Moli-family cohort
| Model A | Model B | |||||||
|---|---|---|---|---|---|---|---|---|
| Beta | SE | p value | R-square | Beta | SE | p value | R-square | |
| Soluble P-selectin | − 0.197 | 0.057 | < 0.0001 | 0.100 | − 0.207 | 0.054 | 0.0002 | 0.051 |
| Monocyte CD11b | 0.034 | 0.057 | 0.549 | 0.005 | 0.021 | 0.057 | 0.719 | 0.0004 |
| PMN CD11b | 0.122 | 0.058 | 0.035 | 0.043 | 0.144 | 0.058 | 0.014 | 0.020 |
| Monocyte L-selectin | 0.155 | 0.065 | 0.019 | 0.021 | 0.127 | 0.066 | 0.056 | 0.013 |
| PMN L-selectin | 0.151 | 0.061 | 0.015 | 0.059 | 0.183 | 0.063 | 0.004 | 0.030 |
| CRP | − 0.078 | 0.053 | 0.145 | 0.016 | − 0.087 | 0.054 | 0.112 | 0.009 |
| INFLAscorea | − 0.143 | 0.058 | 0.014 | 0.026 | − 0.111 | 0.059 | 0.057 | 0.012 |
Model A adjusted by age, gender, BMI, smoking (being an ever smoker and pack-years), drinking (a drinker was defined as drinking daily more than 15 g of alcohol), being a proband for family recruitment, being a member of MI family as fixed effects, and family as a random effect. Model B is Model A +adjustment by platelets, PDW, platelet P-selectin, platelet/monocyte, and platelet/PMN. aINFLAscore was adjusted only for PDW, soluble P-selectin, platelet P-selectin, platelet-monocyte aggregates, and platelet-PMN aggregates, but not platelet number, as this variable is accounted in its formula. R-squares were calculated using a linear regression model including covariates used as fixed effect in the mixed models. Standardized values of phenotypes and methylation are reported. Significant p values are reported in italics
PEAR1 Factor2 methylation had a significant positive relation with expression of CD11b on PMNs and of L-selectin on both monocytes and PMNs (Table 5). However, when the model was adjusted for platelet-dependent covariates (Table 5 Model B), these associations partially decreased in significance, their effect, and the percentage of variability, despite residual associations with PEAR1 methylation, indicative of a relation between PEAR1 methylation and platelet-dependent but also platelet-independent inflammation.
We did not find any association between PEAR1 Factor2 and CRP level (Table 5, Model A), but we observed an inverse association with INFLAscore, a validated composite marker of low grade inflammation status [42, 43] calculated from the combination of plasmatic and cellular biomarkers (CRP levels, platelet and total WBC counts, and granulocyte/lymphocyte (G/L) ratio, Table 1). This association that explained the 2.6% of total INFLAscore variability in the Moli-family cohort, also disappeared when the model was adjusted for platelet function variables (PDW, soluble P-selectin, platelet P-selectin, platelet/monocyte and platelet/PMN) (Table 5, Model B). On the contrary, the association of PEAR1 Factor2 methylation with platelet-monocyte mixed aggregates was still significant and of similar effect when adjusting the multivariate model for CRP (Additional file 1: Table S6, Model B).
Replication study in the FLEMENGHO cohort
To replicate the Moli-family findings, we have studied PEAR1 methylation in 1002 participants belonging to the FLEMENGHO cohort [37–39]. General characteristics and blood cell count variables of FLEMENGHO participants are reported in Additional file 1: Table S7. Because PEAR1 methylation distribution slightly differs among the two studies, possibly due to underlying population differences, PCA analysis gave rise to different factors in FLEMENGHO compared to Moli-family. Therefore, we have used single CpG analysis to replicate the Moli-family findings. Interestingly, methylation at CpG4–5, CpG7–8, CpG11–13, and CpG 19–20 in the FLEMENGHO cohort was significantly associated with the percentage of neutrophils and lymphocytes in the same fashion as in the Moli-family cohort (Additional file 1: Table S8). Measurements of L-selectin expression on monocytes and PMN were not available for FLEMENGHO participants; however, circulating L-selectin was measured [44, 45]. Using the same model, circulating L-selectin levels in FLEMENGHO were consistently and inversely associated with methylation at CpG7–8 and CpG19–20 in accordance with Moli-family data (Additional file 1: Table S8). In addition, INFLAscore was also negatively associated with methylation estimates at CpG7.8 and CpG11.13 in FLEMENGHO, confirming Moli-family results.
Discussion
DNA methylation estimates at the PEAR1 CpG island (CGI) 1 region are associated with variability of a series of platelet and WBC parameters ranging from function/activation (P-selectin, L-selectin, CD11b) to cell heterogeneity (PDW) and number (WBC, monocytes, and granulocytes).
PEAR1 was first identified as a genetic determinant of platelet function variability mostly in in vitro assays and after platelet stimulation [5–12, 14–16, 25–27, 29]. PEAR-1 in platelets supports activation of the αIIbβ3 integrin [1] and influences MK precursor proliferation through control of the PI3K-PTEN pathway [32]. We have already shown that PEAR1 methylation controls PEAR1 expression during megakaryopoiesis [30, 46] and is lower in carriers of rs12041331, a CpG-SNP whose presence alters the methylation pattern of the PEAR1 intron1 region causing differential binding of nuclear proteins [30].
We report now, for the first time, that DNA methylation at the same locus is associated with platelet activation variability, at baseline in a well-defined population. We observed an inverse relation between PEAR1 methylation and platelet-monocyte mixed cell conjugates and with platelet P-selectin at baseline conditions (Additional file 1: Tables S4 and S5). Interestingly, we found the CpG sites mostly involved in this association to be among the ones described in the MK-specific experiments [30]. In line with our findings, Wurtz and colleagues [14] reported rs12041331 (associated with lower methylation) to be linked to lower platelet aggregation but increased P-selectin expression. Circulating monocyte-platelet aggregates have been demonstrated to be a sensitive marker of in vivo platelet activation (together with platelet surface P-selectin) [47]. Platelet-leukocyte conjugates measured in the Moli-family cohort, as used in the present analysis, are mainly formed upon platelet but not leukocyte activation [34]. In addition, PEAR1 methylation-dependent platelet activation (platelet-monocyte aggregates) is only very mildly affected by CRP levels (Additional file 1: Table S6), while yet having a role in influencing inflammation (Table 5). Indeed, platelet-leukocyte conjugates have served as a specific marker of platelet activation/function in several thrombo-inflammatory conditions [48–54].
We did not find any association between PEAR1 (methylation) and platelet counts; however, we did observe a significant positive relation with PDW, a measure of platelet size heterogeneity, with possible consequences on platelet formation and function [55, 56]. In our study, we could not clarify whether PEAR-1 plays a role in controlling the PDW, because this is not demonstrated by our analysis; however, we have previously observed that individuals carrying the A-allele of rs12041331, therefore showing lower methylation levels at CGI1, also had a lower number of big MK colony forming units (CFU) compared to GG homozygotes [30].
Very recently, a possible role of PEAR-1 in controlling hematopoiesis at large has been suggested by the study of Eicher and colleagues [25]. In this meta-analysis, rs12566888, a PEAR1 variant in LD with rs12041331, was significantly associated with a reduced number of total WBC, monocytes, and neutrophils. PEAR-1 (JEDI) was already described to have a role in the fine regulation of the early stages of hematopoietic differentiation, presumably through the Notch pathway, beside its role as phagocytic receptor involved in clearing dead sensory neurons [57]. Our earlier evidence demonstrated transient PEAR1 expression in myeloid precursors [32]. Further supporting these data, we have recently shown that the PEAR1 CGI1 region is part of a larger enhancer that in hematopoietic precursors mediates interactions with genes that are very active in cell differentiation because they regulate protein synthesis, cell cycle, and cell proliferation, possibly via DNA methylation changes [33]. All these data point to a possible role of PEAR-1 in driving variability of formation of hematopoietic cells and are in line with the association of PEAR1 methylation with white blood cell counts in both Moli-family and FLEMENGHO cohorts, a link that seems to be independent from platelets.
In accordance with the identified link between PEAR1 methylation, platelet, and WBC measurements, Factor2 methylation was also associated with other inflammatory markers. PEAR1 Factor2 methylation can explain about 10% of soluble P-selectin variability, which is remarkable for a platelet surface molecule, considered to have a modulating role in platelet activation. Yet, the P-selectin association may also reflect PEAR1 methylation-mediated effects in vascular endothelial cells that abundantly express both PEAR1 and P-selectin [1, 58, 59]. Indeed, when correcting the association for platelet-specific variables, the variability of plasma P-selectin levels explained by PEAR1 methylation was half-sized, suggesting a possible alternative (endothelial) cell origin of plasma P-selectin. In accordance with the latter, the rs12041331 variant was also described as a determinant of endothelial cell function [31] and DNA methylation at PEAR1 is associated with differential PEAR1 expression in different endothelial cell types [33]. We also found in the Moli-family cohort a significant association with the leukocyte specific activation markers CD11b and L-selectin and INFLAscore, a composite score integrating both cell and plasmatic inflammation parameters (Table 5). Adjustment for platelet variables, however, partially affected these associations, suggesting that platelet activation determined by PEAR1 methylation variability can have an impact on inflammation, in agreement with the role of platelets as an inflammatory mediator [60, 61]. Together with P- and E-selectin, L-selectin mediates the first adhesive step during inflammation [59, 62] and is rapidly shed from the cell surface upon leukocyte activation in order to promote cell migration after extravasation [63]. This is in line with the negative association of PEAR1 methylation with circulating L-selectin levels measured in the replication cohort FLEMENGHO. Together, this evidence suggests that the observed association could in part result from platelet-dependent P-selectin-PSGL-1 interactions on leukocytes, subsequently enhancing leukocyte activation and L-selectin shedding [64, 65].
In conclusion, we report for the first time that PEAR1 methylation is a marker of platelet activation and WBC count variability, in turn regulating the underlying inflammatory status of an individual. By analyzing two independent cohorts, we were able to provide the first link between epigenetic gene regulation and platelet biology, and function variability and hitherto platelet-dependent inflammatory processes. Future studies should investigate the role of PEAR-1 in hematopoietic specification, proliferation, and cell function, in relation to its importance for immunity and inflammation regulation and possible thrombo-inflammatory clinical outcomes.
Materials and methods
Study population
The Moli-family cohort [35, 36] includes 754 white subjects (≥ 15 years old) from 54 extended pedigrees (23 families with personal or familial history of early myocardial infarction (MI)—MI family—and 31 families without) recruited in the Southern Molise region in Italy. All participants were relatives of index subjects enrolled in the population-based Moli-sani study [66, 67]. In all subjects, a complete medical history and information about smoking and alcohol-drinking habits were obtained via a structured questionnaire. Height, body weight, and blood pressure were measured as described [36, 66]. Blood samples were obtained between 07:00 and 09:00 from participants who had fasted overnight and had refrained from smoking for at least 6 h. Enrollment and data collection were performed as previously described [68]. Of the 754 Moli-family participants, 623 had good quality DNA samples to perform the methylation analysis.
The FLEMENGHO cohort includes 3343 White European participants living in a geographically defined area of Northern Belgium [37, 39, 69]. The follow-up cycles between 2005 and 2015 included 1447 participants, among which 786 and 661 participants had one and two examinations, respectively. Among 2108 examinations, 1266 DNA or blood samples of 1118 subjects had a good quality for methylation measurement. For the analysis, we further excluded subjects with any missing information of blood count (n = 37), or CRP (n = 37), or soluble L-selectin (n = 12). Finally, 1002 FLEMENGHO participants were included in the current analysis.
In both cohorts, individuals with overt inflammatory conditions were excluded from the analysis (CRP levels > 10 mg/dl).
Flow cytometry and biochemical measurements
Moli-family
Mixed platelet-leukocyte conjugates and markers of platelet or leukocyte activation were measured in the Moli-family participants as described [34]. Briefly, venous blood was collected in 3.8% trisodium citrate vacutainer tubes and processed between 10 and 20 min after collection. Whole blood was then either immediately fixed using a commercially available fixative (ThromboFix™, Beckman Coulter Inc.) or stimulated in vitro using both platelet and leukocyte agonists, namely ADP and collagen or fMLP, or LTB4, respectively. Details of the protocol were reported before [34]. Platelet-leukocyte conjugates, platelet P-selectin, leukocyte CD11b, and L-selectin expression were measured in whole blood as described [34]. Platelets, including platelet aggregates, were defined by morphological characteristics and by CD42b positivity. PMN and monocyte populations were defined on the basis of the side scatter (SS) characteristics within the CD45+ population [34].
Biochemical analyses were performed in the centralized Moli-sani laboratory. All hematological cytometric analyses were performed by the same cell counter (Coulter HMX, Beckman Coulter, IL Milan, Italy), within 1 h from venipuncture. Soluble P-selectin was measured in stored plasma via the Human P-selectin Platinum Enzyme-linked Immunosorbent Assay (ELISA) kit (Affimetrix, eBioscience). High-sensitivity (hs) CRP was measured in serum as described [43, 67].
FLEMENGHO
Hematological cytometric analyses were performed by the Centrum voor Medische Analysen (Herentals, Belgium). Randox Laboratories Ltd. (County Antrim, Northern Ireland, UK) blindly measured CRP and soluble L-selectin using Biochip Array Technology according to the manufacturer’s instructions (Adhesion Molecule and Cerebral II arrays) and a sandwich assay format [44, 45].
INFLAscore in Moli-family and FLEMENGHO
INFLAscore, which had been previously used within the Moli-sani cohort [42, 43] to evaluate the possible synergistic effect of both plasma and cellular biomarker of inflammation, was calculated in both Moli-family and FLEMENGHO as described: 10 tiles of each biomarker levels (CRP, WBC, platelets, granulocyte/lymphocyte (G/L) ratio) were generated. For all four components, being in the highest deciles (7 to 10) gave a score which increased from 1 to 4, while being in the lowest deciles (1 to 4) was negatively scored from − 4 to − 1. Being in the deciles 5 or 6 got zero points. In such a way, the INFLAscore ranges from − 16 to 16 and comes up as the sum of the four biomarkers. An increase in the score represented an increase in low-grade inflammation intensity.
DNA methylation analysis
PEAR1 (CGI1) methylation was evaluated using the Sequenom EpiTYPER MassARRAY (Agena) platform as described [30, 40, 41] on white blood cell DNA from 605 Moli-family and 1002 FLEMENGHO participants.
Bisulfite treatment was conducted on 1 μg of genomic DNA using the MethylDetector kit (Active Motif) according to the manufacturer’s instructions, except for the incubation protocol during the conversion, performed for a total of 16 h as described [70]. The amplicon to study PEAR1 methylation was designed using the Sequenom EpiDesigner software (http://www.epidesigner.com/) [30]. All PCR amplifications were performed in duplicate. For the CpG-specific analysis, data were discarded when the duplicate measurements had a SD equal to or greater than 5% [30, 40, 41]. Sequenom peaks with reference intensity above 2 and overlapping units were excluded from the analysis [30, 40, 41]. To exclude possible intra-plate differences, a sample of K562 DNA, with known PEAR1 methylation profile (around 90%), was carried on in each plate.
Statistical analysis
All analyses were performed using SAS/STAT software (Version 9.4 for Windows©2009. SAS Institute Inc. and SAS are registered trademarks of SAS Institute Inc., Cary, NC, USA).
We used principal component analysis (PCA) on the correlation matrix of the PEAR1 16 CpG sites to identify PEAR1 methylation patterns in the Moli-family cohort [71]. We characterized the factors using the PEAR1 methylation sites with an absolute factor loading greater than 0.25. Criteria for number of factor selection were eigenvalue > 1.0 as revealed by the scree test. Each subject received a factor score, calculated by summing the observed methylation site values, each weighted by factor loadings. Linear mixed regression models were used to evaluate associations between PEAR1 methylation factors or single CpG sites and phenotypes. Age, gender, BMI, smoking (being an ever smoker and pack-years), alcohol drinking (a drinker was defined as drinking daily more than 15 g of alcohol), being a proband for family recruitment, and being a member of MI family were treated as fixed effects, and family stratification as a random effect. R-squares were calculated using a linear regression model including covariates used as fixed effect in the mixed models. Platelet parameters or CRP were used where appropriate as indicated below to verify their potential role as confounders on the effect of PEAR1 methylation on white blood cell or platelet parameters.
To replicate the Moli-family findings in FLEMENGHO, we used a multivariate regression model with age, gender, BMI, smoking (being an ever smoker and pack-years), and drinking (a drinker was defined as drinking daily more than 15 g of alcohol) as covariates.
A false discovery rate (FDR) method (Benjamini-Hochberg) were used to adjust p values for multiple testing with a p value (pFDR) < 0.05 considered as statistically significant.
Supplementary information
Acknowledgements
Moli-Family Study Collaborators:
Principal Investigator: Licia Iacoviello (IRCCS Neuromed, Pozzilli, and University of Insubria, Varese, Italy)
Study coordination: Branislav Vohnout (Slovak Medical University, Bratislava, Slovakia)
Scientific Committee: Marcello Arca (“Sapienza” University of Rome, Rome, Italy); Chiara Cerletti, Maria Benedetta Donati, Giovanni de Gaetano (IRCCS Neuromed, Pozzilli, Italy); Roberto Lorenzet^ (Catholic University, Campobasso, Italy)
Data management and analysis: Augusto di Castelnuovo (Mediterranea Cardiocentro, Napoli, Italy), Simona Costanzo (IRCCS Neuromed, Pozzilli, Italy); Francesco Gianfagna (Mediterranea Cardiocentro, Napoli, Italy, and University of Insubria, Varese, Italy); Romina di Giuseppe (Christian-Albrechts University of Kiel, Kiel, Germany); Branislav Vohnout (Slovak Medical University, Bratislava, Slovakia)
Recruitment: Branislav Vohnout (Slovak Medical University, Bratislava, Slovakia); Antonella Cutrone (Catholic University, Campobasso, Italy)
Biobank and centralized laboratory: Amalia De Curtis (IRCCS Neuromed, Pozzilli, Italy); Sara Magnacca (Mediterranea Cardiocentro, Napoli, Italy)
Studies on platelets and leukocytes: Chiara Cerletti, Benedetta Izzi (IRCCS Neuromed, Pozzilli, Italy); Marilena Crescente (Queen Mary University of London, London, United Kingdom); Agnieszka Pampuch (Catholic University, Campobasso, Italy), Chiara Tamburrelli (Catholic University, Campobasso, Italy)
Studies on blood coagulation: Roberto Lorenzet^, Antonella Cutrone, Emanuela Napoleone, Filomena Zurlo (Catholic University, Campobasso, Italy)
Genetics laboratory: Marcello Arca, Luisa Nanni (“Sapienza” University of Rome, Rome, Italy)
^Deceased
Abbreviations
- CFU
Colony forming units
- CGI
CpG island
- CRP
C-reactive protein
- c-Src
src family kinase
- FDR
False discovery rate
- G/L
Granulocyte/lymphocyte
- Hs
High sensitivity
- LD
Linkage disequilibrium
- MI
Myocardial infarction
- MK
Megakaryocyte
- MPV
Mean platelet volume
- PCA
Principal component analysis
- Pct
Plateletcrit
- PDW
Platelet distribution width
- PEAR-1
Platelet-endothelial aggregation receptor 1
- PMN
Polymoprphonuclear cell
- SD
Standard deviation
- WBC
White blood cell
Authors’ contributions
BI designed and performed the experiments on both Moli-family and FLEMENGHO samples, analyzed the data, and wrote the manuscript; FG designed and performed the statistical analysis for the Moli-family cohort and reviewed the manuscript; WY performed the replication statistical analysis in the FLEMENGHO cohort and reviewed the manuscript; KC performed the experiments; ADeC (Amalia De Curtis) performed the biochemical measurements for the Moli-family study; ADiC (Augusto Di Castelnuovo) gave advice on statistical analysis; CC, MBD, GdG, LI, and JAS originally inspired the Moli-sani and FLEMENGHO study, respectively; PV, CC, MBD, GdG, and JS reviewed the manuscript; MFH and LI designed the experiments and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the “Fonds voor Wetenschappelijk Onderzoek (FWO) Vlaanderen” Research Grant 1508715N and the “Programma financiering KU Leuven (PF/10/014)”. The Moli-Family study was supported by research grants from Telethon foundation (grant GGP04198, L.I.) and the Italian Ministry of University and Research (MIUR) (Decreto no 1588-19/11/2004). The FLEMENGHO study was supported by the European Union (HEALTH-F7-305507 HOMAGE), the European Research Council (Advanced Researcher Grant 2011-294713-EPLORE and Proof-of-Concept Grant 713601-uPROPHET), and the European Research Area Net for Cardiovascular Diseases (JTC2017-046-PROACT). B.I. was a FWO Post-doctoral Fellow (12M2715N) and a Postdoctoral Fellow of the Fondazione Umberto Veronesi (years 2017 and 2018), Milan Italy. F.G. was supported by the Italian Ministry of Health 2011 (Young Investigator Grant n. 167/GR-2011-02351736).
Availability of data and materials
All data analyzed in this study are included in this published article. Raw data used for the analysis are availbale upon request.
Ethics approval and consent to participate
The Moli-family and FLEMENGHO studies were approved by the Ethical Committee of the Catholic University of Rome (Italy) and of the University of Leuven (Belgium), respectively. All subjects provided written informed consent, except children for whom the relative caregivers did.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Benedetta Izzi, Phone: +39 0823 1548845, Email: izzi.epigenomica@gmail.com, Email: benedetta.izzi@moli-sani.org.
Francesco Gianfagna, Email: francesco.gianfagna@uninsubria.it.
Wen-Yi Yang, Email: wenyi.yang@kuleuven.be.
Katrien Cludts, Email: katrien.cludts@kuleuven.be.
Amalia De Curtis, Email: amalia.decurtis@moli-sani.org.
Peter Verhamme, Email: peter.verhamme@uzleuven.be.
Augusto Di Castelnuovo, Email: dicastel@ngi.it.
Chiara Cerletti, Email: chiara.cerletti@moli-sani.org.
Maria Benedetta Donati, Email: mbdonati@moli-sani.org.
Giovanni de Gaetano, Email: giovanni.degaetano@moli-sani.org.
Jan A. Staessen, Email: jan.staessen@kuleuven.be
Marc F. Hoylaerts, Email: marc.hoylaerts@kuleuven.be
Licia Iacoviello, Email: licia.iacoviello@moli-sani.org.
on behalf of Moli-family Investigators:
Licia Iacoviello, Branislav Vohnout, Marcello Arca, Chiara Cerletti, Maria Benedetta Donati, Giovanni de Gaetano, Roberto Lorenzet, Augusto di Castelnuovo, Simona Costanzo, Francesco Gianfagna, Romina di Giuseppe, Antonella Cutrone, Amalia De Curtis, Sara Magnacca, Benedetta Izzi, Marilena Crescente, Agnieszka Pampuch, Chiara Tamburrelli, Emanuela Napoleone, Filomena Zurlo, and Luisa Nanni
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13148-019-0744-8.
References
- 1.Kauskot A, Di Michele M, Loyen S, Freson K, Verhamme P, Hoylaerts MF. A novel mechanism of sustained platelet alphaIIbbeta3 activation via PEAR1. Blood. 2012;119(17):4056–4065. doi: 10.1182/blood-2011-11-392787. [DOI] [PubMed] [Google Scholar]
- 2.Vandenbriele C, Sun Y, Criel M, Cludts K, Van Kerckhoven S, Izzi B, et al. Dextran sulfate triggers platelet aggregation via direct activation of PEAR1. Platelets. 2016;27(4):365–372. doi: 10.3109/09537104.2015.1111321. [DOI] [PubMed] [Google Scholar]
- 3.Kardeby C, Falker K, Haining EJ, Criel M, Lindkvist M, Barroso R, et al. Synthetic glycopolymers and natural fucoidans cause human platelet aggregation via PEAR1 and GPIbalpha. Blood Adv. 2019;3(3):275–287. doi: 10.1182/bloodadvances.2018024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y, Vandenbriele C, Kauskot A, Verhamme P, Hoylaerts MF, Wright GJ. A human platelet receptor protein microarray identifies the high affinity immunoglobulin E receptor subunit alpha (FcepsilonR1alpha) as an activating platelet endothelium aggregation receptor 1 (PEAR1) ligand. Mol Cell Proteomics. 2015;14(5):1265–1274. doi: 10.1074/mcp.M114.046946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eicher JD, Xue L, Ben-Shlomo Y, Beswick AD, Johnson AD. Replication and hematological characterization of human platelet reactivity genetic associations in men from the Caerphilly Prospective Study (CaPS) J Thromb Thrombolysis. 2016;41(2):343–350. doi: 10.1007/s11239-015-1290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faraday N, Yanek LR, Yang XP, Mathias R, Herrera-Galeano JE, Suktitipat B, et al. Identification of a specific intronic PEAR1 gene variant associated with greater platelet aggregability and protein expression. Blood. 2011;118(12):3367–3375. doi: 10.1182/blood-2010-11-320788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrera-Galeano JE, Becker DM, Wilson AF, Yanek LR, Bray P, Vaidya D, et al. A novel variant in the platelet endothelial aggregation receptor-1 gene is associated with increased platelet aggregability. Arterioscler Thromb Vasc Biol. 2008;28(8):1484–1490. doi: 10.1161/ATVBAHA.108.168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson AD, Yanek LR, Chen MH, Faraday N, Larson MG, Tofler G, et al. Genome-wide meta-analyses identifies seven loci associated with platelet aggregation in response to agonists. Nat Genet. 2010;42(7):608–613. doi: 10.1038/ng.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones CI, Bray S, Garner SF, Stephens J, de Bono B, Angenent WG, et al. A functional genomics approach reveals novel quantitative trait loci associated with platelet signaling pathways. Blood. 2009;114(7):1405–1416. doi: 10.1182/blood-2009-02-202614. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Suktitipat B, Yanek LR, Faraday N, Wilson AF, Becker DM, et al. Targeted deep resequencing identifies coding variants in the PEAR1 gene that play a role in platelet aggregation. PLoS One. 2013;8(5):e64179. doi: 10.1371/journal.pone.0064179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis JP, Ryan K, O'Connell JR, Horenstein RB, Damcott CM, Gibson Q, et al. Genetic variation in PEAR1 is associated with platelet aggregation and cardiovascular outcomes. Circ Cardiovasc Genet. 2013;6(2):184–192. doi: 10.1161/CIRCGENETICS.111.964627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qayyum R, Becker LC, Becker DM, Faraday N, Yanek LR, Leal SM, et al. Genome-wide association study of platelet aggregation in African Americans. BMC Genet. 2015;16:58. doi: 10.1186/s12863-015-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voora D, Horton J, Shah SH, Shaw LK, Newby LK. Polymorphisms associated with in vitro aspirin resistance are not associated with clinical outcomes in patients with coronary artery disease who report regular aspirin use. Am Heart J. 2011;162(1):166–172. doi: 10.1016/j.ahj.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Wurtz M, Nissen PH, Grove EL, Kristensen SD, Hvas AM. Genetic determinants of on-aspirin platelet reactivity: focus on the influence of PEAR1. PLoS One. 2014;9(10):e111816. doi: 10.1371/journal.pone.0111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang Q, Cui Y, Zhao X, Zhao N. Identification of PEAR1 SNPs and their influences on the variation in prasugrel pharmacodynamics. Pharmacogenomics. 2013;14(10):1179–1189. doi: 10.2217/pgs.13.108. [DOI] [PubMed] [Google Scholar]
- 16.Yao Y, Tang XF, Zhang JH, He C, Ma YL, Xu JJ, et al. Association of PEAR1 genetic variants with platelet reactivity in response to dual antiplatelet therapy with aspirin and clopidogrel in the Chinese patient population after percutaneous coronary intervention. Thromb Res. 2016;141:28–34. doi: 10.1016/j.thromres.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Xiang Q, Zhou S, Lewis JP, Shuldiner AR, Ren G, Cui Y. Genetic variants of PEAR1 are associated with platelet function and antiplatelet drug efficacy: a systematic review and meta-analysis. Curr Pharm Des. 2017;23(44):6815–6827. doi: 10.2174/1381612823666170817122043. [DOI] [PubMed] [Google Scholar]
- 18.Yao Y, Tang XF, He C, Song Y, Xu JJ, Meng XM, et al. Effect of PEAR1 genetic variants on 1-year outcomes in Chinese patients with acute myocardial infarction after percutaneous coronary intervention. J Atheroscler Thromb. 2018;25(5):454–459. doi: 10.5551/jat.39982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nie XY, Li JL, Qin SB, Fu Y, Liang GK, Shi LW, et al. Genetic mutations in PEAR1 associated with cardiovascular outcomes in Chinese patients with acute coronary syndrome. Thromb Res. 2018;163:77–82. doi: 10.1016/j.thromres.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Keramati AR, Yanek LR, Iyer K, Taub MA, Ruczinski I, Becker DM, et al. Targeted deep sequencing of the PEAR1 locus for platelet aggregation in European and African American families. Platelets. 2019;30(3):380-6. [DOI] [PMC free article] [PubMed]
- 21.Zhang S, Zhu J, Li H, Wang L, Niu J, Zhu B, et al. Study of the association of PEAR1, P2Y12, and UGT2A1 polymorphisms with platelet reactivity in response to dual antiplatelet therapy in Chinese patients. Cardiology. 2018;140(1):21–29. doi: 10.1159/000488101. [DOI] [PubMed] [Google Scholar]
- 22.Sokol J, Skerenova M, Ivankova J, Simurda T, Stasko J. Association of genetic variability in selected genes in patients with deep vein thrombosis and platelet hyperaggregability. Clin Appl Thromb Hemost. 2018;24(7):1027–1032. doi: 10.1177/1076029618779136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stimpfle F, Bauer M, Rath D, Schaeffeler E, Schwab M, Gawaz M, et al. Variants of PEAR1 are associated with outcome in patients with ACS and stable CAD undergoing PCI. Front Pharmacol. 2018;9:490. doi: 10.3389/fphar.2018.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pi L, Xu Y, Fu L, Zhang L, Liu Y, Zhou H, et al. A PEAR1 polymorphism (rs12041331) is associated with risk of coronary artery aneurysm in Kawasaki disease. Ann Hum Genet. 2019;83(1):54-62. [DOI] [PubMed]
- 25.Eicher JD, Chami N, Kacprowski T, Nomura A, Chen MH, Yanek LR, et al. Platelet-related variants identified by exomechip meta-analysis in 157,293 individuals. Am J Hum Genet. 2016;99(1):40–55. doi: 10.1016/j.ajhg.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Hu Y, Wen Z, Li H, Hu X, Zhang Y, et al. Association of PEAR1 rs12041331 polymorphism and pharmacodynamics of ticagrelor in healthy Chinese volunteers. Xenobiotica. 2017;47(12):1130-8. [DOI] [PubMed]
- 27.Peng LL, Zhao YQ, Zhou ZY, Jin J, Zhao M, Chen XM, et al. Associations of MDR1, TBXA2R, PLA2G7, and PEAR1 genetic polymorphisms with the platelet activity in Chinese ischemic stroke patients receiving aspirin therapy. Acta Pharmacol Sin. 2016;37(11):1442–1448. doi: 10.1038/aps.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y, Sun S, Liang J, Liu S, Jiang Y, Xu L, et al. PEAR1 gene polymorphism in a Chinese pedigree with pulmonary thromboembolism. Medicine (Baltimore) 2016;95(51):e5687. doi: 10.1097/MD.0000000000005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Backman JD, Yerges-Armstrong LM, Horenstein RB, Newcomer S, Shaub S, Morrisey M, et al. Prospective evaluation of genetic variation in platelet endothelial aggregation receptor 1 reveals aspirin-dependent effects on platelet aggregation pathways. Clin Transl Sci. 2017;10(2):102-9. [DOI] [PMC free article] [PubMed]
- 30.Izzi B, Pistoni M, Cludts K, Akkor P, Lambrechts D, Verfaillie C, et al. Allele-specific DNA methylation reinforces PEAR1 enhancer activity. Blood. 2016;128(7):1003–1012. doi: 10.1182/blood-2015-11-682153. [DOI] [PubMed] [Google Scholar]
- 31.Fisch AS, Yerges-Armstrong LM, Backman JD, Wang H, Donnelly P, Ryan KA, et al. Genetic variation in the platelet endothelial aggregation receptor 1 gene results in endothelial dysfunction. PLoS One. 2015;10(9):e0138795. doi: 10.1371/journal.pone.0138795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kauskot A, Vandenbriele C, Louwette S, Gijsbers R, Tousseyn T, Freson K, et al. PEAR1 attenuates megakaryopoiesis via control of the PI3K/PTEN pathway. Blood. 2013;121(26):5208–5217. doi: 10.1182/blood-2012-10-462887. [DOI] [PubMed] [Google Scholar]
- 33.Izzi B, Noro F, Cludts K, Freson K, Hoylaerts MF. Cell-specific PEAR1 methylation studies reveal a locus that coordinates expression of multiple genes. Int J Mol Sci. 2018;19(4):E1069. [DOI] [PMC free article] [PubMed]
- 34.Izzi B, Pampuch A, Costanzo S, Vohnout B, Iacoviello L, Cerletti C, et al. Determinants of platelet conjugate formation with polymorphonuclear leukocytes or monocytes in whole blood. Thromb Haemost. 2007;98(6):1276–1284. [PubMed] [Google Scholar]
- 35.Vohnout B, Gianfagna F, Lorenzet R, Cerletti C, de Gaetano G, Donati MB, et al. Genetic regulation of inflammation-mediated activation of haemostasis: family-based approaches in population studies. Nutr Metab Cardiovasc Dis. 2011;21(11):857–861. doi: 10.1016/j.numecd.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Gianfagna F, Tamburrelli C, Vohnout B, Crescente M, Izzi B, Pampuch A, et al. Heritability, genetic correlation and linkage to the 9p21.3 region of mixed platelet-leukocyte conjugates in families with and without early myocardial infarction. Nutr Metab Cardiovasc Dis. 2013;23(7):684–692. doi: 10.1016/j.numecd.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Staessen JA, Wang JG, Brand E, Barlassina C, Birkenhager WH, Herrmann SM, et al. Effects of three candidate genes on prevalence and incidence of hypertension in a Caucasian population. J Hypertens. 2001;19(8):1349–1358. doi: 10.1097/00004872-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Zagato L, Kuznetsova T, Tripodi G, Zerbini G, Richart T, et al. Angiotensin-converting enzyme I/D and alpha-adducin Gly460Trp polymorphisms: from angiotensin-converting enzyme activity to cardiovascular outcome. Hypertension. 2007;49(6):1291–1297. doi: 10.1161/HYPERTENSIONAHA.106.085498. [DOI] [PubMed] [Google Scholar]
- 39.Yang WY, Petit T, Thijs L, Zhang ZY, Jacobs L, Hara A, et al. Coronary risk in relation to genetic variation in MEOX2 and TCF15 in a Flemish population. BMC Genet. 2015;16:116. doi: 10.1186/s12863-015-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izzi B, Francois I, Labarque V, Thys C, Wittevrongel C, Devriendt K, et al. Methylation defect in imprinted genes detected in patients with an Albright's hereditary osteodystrophy like phenotype and platelet Gs hypofunction. PLoS One. 2012;7(6):e38579. doi: 10.1371/journal.pone.0038579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izzi B, Decallonne B, Devriendt K, Bouillon R, Vanderschueren D, Levtchenko E, et al. A new approach to imprinting mutation detection in GNAS by Sequenom EpiTYPER system. Clin Chim Acta. 2010;411(23–24):2033–2039. doi: 10.1016/j.cca.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 42.Pounis G, Bonaccio M, Di Castelnuovo A, Costanzo S, de Curtis A, Persichillo M, et al. Polyphenol intake is associated with low-grade inflammation, using a novel data analysis from the Moli-sani study. Thromb Haemost. 2016;115(2):344–352. doi: 10.1160/th15-06-0487. [DOI] [PubMed] [Google Scholar]
- 43.Bonaccio M, Di Castelnuovo A, Pounis G, De Curtis A, Costanzo S, Persichillo M, et al. A score of low-grade inflammation and risk of mortality: prospective findings from the Moli-sani study. Haematologica. 2016;101(11):1434–1441. doi: 10.3324/haematol.2016.144055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng YM, Thijs L, Zhang ZY, Yang WY, Huang QF, Wei FF, et al. Glomerular function in relation to circulating adhesion molecules and inflammation markers in a general population. Nephrol Dial Transplant. 2018;33(3):426–435. doi: 10.1093/ndt/gfx256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang WY, Zhang ZY, Thijs L, Bijnens EM, Janssen BG, Vanpoucke C, et al. Left ventricular function in relation to chronic residential air pollution in a general population. Eur J Prev Cardiol. 2017;24(13):1416–1428. doi: 10.1177/2047487317715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson AD. Pairing megakaryopoiesis methylation with PEAR1. Blood. 2016;128(7):890–892. doi: 10.1182/blood-2016-06-723940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104(13):1533–1537. doi: 10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- 48.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116(12):3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bunescu A, Seideman P, Lenkei R, Levin K, Egberg N. Enhanced Fcgamma receptor I, alphaMbeta2 integrin receptor expression by monocytes and neutrophils in rheumatoid arthritis: interaction with platelets. J Rheumatol. 2004;31(12):2347–2355. [PubMed] [Google Scholar]
- 50.Irving PM, Macey MG, Feakins RM, Knowles CH, Frye JN, Liyanage SH, et al. Platelet-leucocyte aggregates form in the mesenteric vasculature in patients with ulcerative colitis. Eur J Gastroenterol Hepatol. 2008;20(4):283–289. doi: 10.1097/MEG.0b013e3282f246c2. [DOI] [PubMed] [Google Scholar]
- 51.Pamuk GE, Vural O, Turgut B, Demir M, Umit H, Tezel A. Increased circulating platelet-neutrophil, platelet-monocyte complexes, and platelet activation in patients with ulcerative colitis: a comparative study. Am J Hematol. 2006;81(10):753–759. doi: 10.1002/ajh.20655. [DOI] [PubMed] [Google Scholar]
- 52.Setianto BY, Hartopo AB, Gharini PP, Anggrahini DW, Irawan B. Circulating soluble CD40 ligand mediates the interaction between neutrophils and platelets in acute coronary syndrome. Heart Vessel. 2010;25(4):282–287. doi: 10.1007/s00380-009-1199-1. [DOI] [PubMed] [Google Scholar]
- 53.Zachem CR, Alpers CE, Way W, Shankland SJ, Couser WG, Johnson RJ. A role for P-selectin in neutrophil and platelet infiltration in immune complex glomerulonephritis. J Am Soc Nephrol. 1997;8(12):1838–1844. doi: 10.1681/ASN.V8121838. [DOI] [PubMed] [Google Scholar]
- 54.Kuligowski MP, Kitching AR, Hickey MJ. Leukocyte recruitment to the inflamed glomerulus: a critical role for platelet-derived P-selectin in the absence of rolling. J Immunol. 2006;176(11):6991–6999. doi: 10.4049/jimmunol.176.11.6991. [DOI] [PubMed] [Google Scholar]
- 55.Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14(1):28–32. [PMC free article] [PubMed] [Google Scholar]
- 56.Wiwanitkit V. Plateletcrit, mean platelet volume, platelet distribution width: its expected values and correlation with parallel red blood cell parameters. Clin Appl Thromb Hemost. 2004;10(2):175–178. doi: 10.1177/107602960401000208. [DOI] [PubMed] [Google Scholar]
- 57.Wu HH, Bellmunt E, Scheib JL, Venegas V, Burkert C, Reichardt LF, et al. Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat Neurosci. 2009;12(12):1534–1541. doi: 10.1038/nn.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vandenbriele C, Kauskot A, Vandersmissen I, Criel M, Geenens R, Craps S, et al. Platelet endothelial aggregation receptor-1: a novel modifier of neoangiogenesis. Cardiovasc Res. 2015;108(1):124-38. [DOI] [PubMed]
- 59.McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107(3):331–339. doi: 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theilmeier G, Lenaerts T, Remacle C, Collen D, Vermylen J, Hoylaerts MF. Circulating activated platelets assist THP-1 monocytoid/endothelial cell interaction under shear stress. Blood. 1999;94(8):2725–2734. doi: 10.1182/blood.V94.8.2725.420k18_2725_2734. [DOI] [PubMed] [Google Scholar]
- 61.Theilmeier G, Michiels C, Spaepen E, Vreys I, Collen D, Vermylen J, et al. Endothelial von Willebrand factor recruits platelets to atherosclerosis-prone sites in response to hypercholesterolemia. Blood. 2002;99(12):4486–4493. doi: 10.1182/blood.V99.12.4486. [DOI] [PubMed] [Google Scholar]
- 62.McEver RP, Zhu C. Rolling cell adhesion. Annu Rev Cell Dev Biol. 2010;26:363–396. doi: 10.1146/annurev.cellbio.042308.113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venturi GM, Tu L, Kadono T, Khan AI, Fujimoto Y, Oshel P, et al. Leukocyte migration is regulated by L-selectin endoproteolytic release. Immunity. 2003;19(5):713–724. doi: 10.1016/S1074-7613(03)00295-4. [DOI] [PubMed] [Google Scholar]
- 64.Cerletti C, Evangelista V, de Gaetano G. P-selectin-beta 2-integrin cross-talk: a molecular mechanism for polymorphonuclear leukocyte recruitment at the site of vascular damage. Thromb Haemost. 1999;82(2):787–793. [PubMed] [Google Scholar]
- 65.Totani L, Evangelista V. Platelet-leukocyte interactions in cardiovascular disease and beyond. Arterioscler Thromb Vasc Biol. 2010;30(12):2357–2361. doi: 10.1161/ATVBAHA.110.207480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Centritto F, Iacoviello L, di Giuseppe R, De Curtis A, Costanzo S, Zito F, et al. Dietary patterns, cardiovascular risk factors and C-reactive protein in a healthy Italian population. Nutr Metab Cardiovasc Dis. 2009;19(10):697–706. doi: 10.1016/j.numecd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 67.Di Castelnuovo A, de Curtis A, Costanzo S, Persichillo M, Olivieri M, Zito F, et al. Association of D-dimer levels with all-cause mortality in a healthy adult population: findings from the MOLI-SANI study. Haematologica. 2013;98(9):1476–1480. doi: 10.3324/haematol.2012.083410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.di Giuseppe R, Bonanni A, Olivieri M, Di Castelnuovo A, Donati MB, de Gaetano G, et al. Adherence to Mediterranean diet and anthropometric and metabolic parameters in an observational study in the ‘Alto Molise’ region: the MOLI-SAL project. Nutr Metab Cardiovasc Dis. 2008;18(6):415–421. doi: 10.1016/j.numecd.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 69.Yang WY, Petit T, Cauwenberghs N, Zhang ZY, Sheng CS, Thijs L, et al. PEAR1 is not a major susceptibility gene for cardiovascular disease in a Flemish population. BMC Med Genet. 2017;18(1):45. doi: 10.1186/s12881-017-0411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Izzi B, Binder AM, Michels KB. Pyrosequencing evaluation of widely available bisulfite conversion methods: considerations for application. Med epigenetics. 2014;2(1):28–36. doi: 10.1159/000358882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim JO, Mueller CW. Factor analysis: statistical method and practical issues. Thousand Oaks: Sage Publications; 1978. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed in this study are included in this published article. Raw data used for the analysis are availbale upon request.