Abstract
Background
Programmatic data on the baseline risk of tuberculosis in people living with HIV (PLHIV) are needed to evaluate long-term effectiveness of the ongoing isoniazid preventive therapy (IPT) roll-out in India.
Methods
We estimated the incidence rate and risk factors of tuberculosis disease in adult PLHIV initiating first- and second-line anti-retroviral therapy (ART) prior to widespread IPT in a public ART center in Pune, India.
Results
4067 participants contributing 5205.7 person-years of follow-up on first-line ART and 871 participants contributing 1031.7 person-years of follow-up on second-line ART were included in the analysis. The incidence rate of tuberculosis was 4.39 cases (95%CI 3.86–5.00) per 100 person-years on first-line ART and 1.64 cases (95%CI 1.01–2.63) per 100 person-years on second-line ART (p < 0.001). After adjusting for competing risks, male sex (aSHR = 1.33, 95%CI 1.02–1.74, p = 0.03), urban residence (aSHR = 1.53, 95%CI 1.13–2.07, p = 0.006) and CD4+ counts < 350 cells/mm3 (aSHR = 3.06 vs CD4 > 350 cells/mm3, 95%CI 1.58–5.94, p < 0.001) at ART initiation were associated with higher risk of tuberculosis independent of ART regimen.
Conclusion
Risk of tuberculosis was lower in PLHIV receiving second-line ART compared to first-line ART. Prioritizing IPT in PLHIV with low CD4+ counts, urban residence and in males may further mitigate the risk of tuberculosis during ART.
Keywords: HIV, Tuberculosis, Second-line ART, Competing risks, India
Background
Tuberculosis disease (TB) has surpassed HIV as the leading infectious cause of mortality globally. An estimated one-third of the world’s population is infected with Mycobacterium tuberculosis, over 10 million developed TB and 1.5 million died in 2017. People living with HIV (PLHIV) are at high risk of developing TB and account for nearly 9% of new TB cases and nearly 300,000 TB-related deaths globally [1].
Widespread anti-retroviral therapy (ART) has significantly reduced the incidence of TB in PLHIV. However, the burden of TB remains high in this population with prevalence ranging from 3 to 72% in high burden settings [2]. While first-line ART regimens containing a non-nucleotide reverse transcriptase inhibitor (NNRTI) have significantly reduced the burden of AIDS and non-AIDS defining events, treatment failure can occur in nearly 20% of patients, requiring a switch to a second-line protease inhibitor (PI)-based regimen [3]. PLHIV receiving second-line regimens represent a growing and potentially high-risk population for TB.
To further reduce the burden of TB, the World Health Organization (WHO) recommends isoniazid prevention therapy (IPT) for all PLHIV in high TB-burden settings [4]. India has the third highest HIV burden and the highest TB burden globally with nearly one million new TB cases among PLHIV each year [5]. India’s National AIDS Control Organization (NACO) provides free first- and second-line ART, and has recommended IPT in all PLHIV since December 2016 [6]. However, programmatic data on the risk of TB in PLHIV receiving first- and second-line ART in India are limited. Documenting the burden and risk factors of TB in this population, especially prior to widespread IPT implementation, will provide a baseline estimate to compare the long-term effectiveness of IPT and, inform prioritized phasing in of IPT to reduce the burden of TB among PLHIV in India.
Therefore, we conducted a retrospective cohort study using programmatic data from one of India’s largest public sector ART delivery programs in Pune, Maharashtra. Our study aimed to estimate the incidence rate and risk factors of TB among HIV-infected adults receiving first- and second-line ART in the absence of widespread IPT.
Methods
Study design and setting
We conducted a retrospective cohort study of HIV-infected adults registered at the Byramjee-Jeejeebhoy Government Medical College-Sassoon General Hospitals (BJGMC-SGH) ART center in Pune, India. BJGMC-SGH is a public tertiary referral hospital and one of the largest NACO supported ART centers in India serving the population of Pune city and its surrounding semi-urban and rural region, with over 37,000 registered HIV-infected patients and nearly 1200 patients on second-line PI-based ART regimens. All patients registered at the BJGMC-SGH ART center received free clinical care and treatment as per national program guidelines [7]. Prior to June 2016, ART was initiated in HIV-infected adults with a CD4+ cell count below 350 cells/mm3 regardless of symptoms, or in the event of an opportunistic infection, including TB, regardless of CD4+ cell counts. These guidelines changed in June 2016 and ART was initiated in HIV-infected adults with a CD4+ cell count below 500 cells/mm3, or in the event of an opportunistic infection regardless of CD4+ cell counts thereafter. During the study period, standard first-line ART included lamivudine, zidovudine or stavudine, and nevirapine or efavirenz based regimens. Tenofavir was included on a case-by-case basis. All patients underwent monthly clinical evaluations. The primary criterion for initiating second-line ART was a viral load (VL) above 5000 copies/mL; however, TB was also considered a criterion at the discretion of the treating physician. Standard second-line ART included a boosted atazanavir-based PI regimen, except in the case of known toxicity or adverse events where a boosted lopinavir-based PI regimen was prescribed. Nucleoside reverse transcriptase inhibitors were selected on a case-by-case basis and depended on the patient’s first-line regimen. National guidelines recommending IPT for all PLHIV were implemented at the BJGMC-SGH ART center starting April 2017.
Study procedures
Data for this analysis were extracted from existing BJGMC-SGH ART center databases. We identified adults (≥18 years) who initiated first- or second-line ART from January 2010 to December 2016 at the BJGMC-SGH ART center for inclusion in our study. We excluded participants with prevalent TB, defined as a clinical (symptom and/or chest radiograph evaluation suggestive of TB) or microbiological (Acid Fast Bacilli [AFB] on smear microscopy) diagnosis of TB or receiving TB therapy at first- or second-line ART initiation. We additionally excluded participants who were transferred to another ART center for treatment within one day of ART initiation as these participants would not be available for follow-up at the BJGMC-SGH ART clinic. Socio-demographic and clinical characteristics, and CD4+ cell counts closest to first- or second-line ART initiation were extracted for analysis. Participants with unavailable data at ART initiation were excluded. Urban residence was defined as having a residential address within the Pune city zonal limits.
All participants at the ART center underwent screening for TB at each clinic visit using a WHO recommended questionnaire to assess symptoms of current cough, night sweats, weight loss and fever. Participants testing positive on the symptom screening questionnaire underwent chest radiography and AFB smear microscopy on at least two clinical samples for confirmation of suspected TB. The final diagnosis of TB recorded in the ART center database was extracted for analysis. Participants lost to follow-up, defined according to NACO guidelines as missing three consecutive monthly clinic visits, were identified from the ART center database. Similarly, data on all-cause mortality was extracted from the ART center databases.
The Institutional Review Boards of Johns Hopkins University and BJGMC-SGH approved the project.
Statistical analysis
The primary outcome of our study was incident TB disease in participants on first- or second-line ART. The date of TB treatment initiation entered in the participant medical record was used as a proxy for the date of TB diagnosis. Person-time at risk on first- or second-line ART was calculated from the date of respective ART initiation until the occurrence of the first mutually exclusive event of incident TB, death, transfer to another ART center, loss to follow-up, administrative censoring on 31st December 2016 or date of second-line ART initiation among participants who failed first-line ART. Participants initially receiving first-line ART who subsequently received second-line ART did not contribute person-time for second-line ART analysis. Participants who developed TB and died were assigned an outcome of incident TB. Incidence rate (IR) was calculated as the number of incident TB cases divided by the person-time at risk and expressed as events per 100 person-years with accompanying Poisson exact 95% confidence intervals (CI). Kaplan-Meier survival analysis was used to estimate the proportion of participants on first- and second-line ART who remained free of TB after stratifying by CD4+ cell counts at initiation of first- and second-line ART respectively. We identified risk factors for incident TB in participants on first- and second-line ART using all-cause death and loss to follow-up (LTFU) as competing risks to address a potential bias induced by differential outcome ascertainment commonly seen in programmatic data. Sub-distribution hazard ratios (SHR) were estimated using univariable and multivariable competing risks regression previously described by Fine and Gray [8]. Multivariable regression models included baseline participant characteristics such as age, sex, CD4+ cell counts at respective ART initiation and urban residence. Additionally, we included duration on first-line ART prior to second-line ART initiation. Categorical data were summarized as proportions and compared using Fisher’s exact test. Continuous data were summarized as medians with accompanying interquartile range (IQR) and compared using the Wilcoxon rank-sum or Kruskal-Wallis test. P-values less than 0.05 were considered statistically significant. Data were analyzed in Stata 15 (StataCorp, Texas).
Results
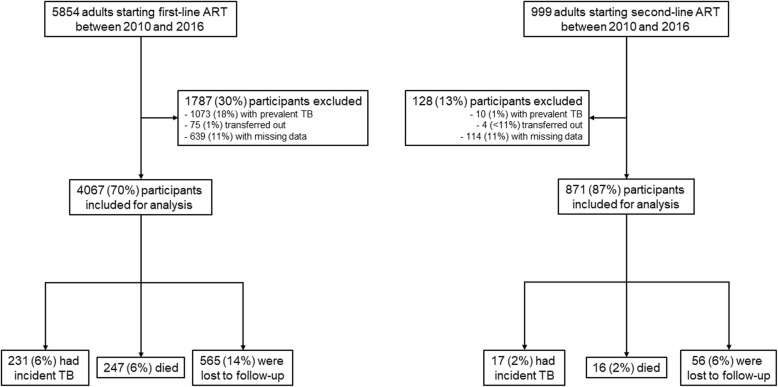
We identified 5854 and 999 adults initiating first- and second-line ART between January 2010 and December 2016, respectively (Fig. 1). We excluded 1073 (18%) and 10 (1%) participants on first- and second-line ART with prevalent TB, respectively. An additional 79 (2%) participants who were transferred to another ART center and 753 (13%) participants with unavailable socio-demographic or CD4+ data at their respective ART initiation were excluded. Overall, 4067 participants contributing 5205.7 person-years of follow-up on first-line ART and 871 participants contributing 1031.7 person-years of follow-up on second-line ART were included in the analysis. The median (IQR) follow-up time was 12 (4–25) and 15 (11–18) months on first- and second-line ART, respectively (Table 1).
Fig. 1.
Consort diagram of participant enrollment. ART – antiretroviral therapy, TB – tuberculosis
Table 1.
Participant characteristics by first- and second-line ART receipt
| Characteristics | Overall (n = 4938) | First-line ART (n = 4067) | Second-line ART (n = 871) | p-value first- vs second-line ART |
|---|---|---|---|---|
| Age, median (IQR) | 36 (30–43) | 36 (30–42) | 40 (34–45) | < 0.001 |
| Male, n (%) | 2587 (52) | 2018 (50) | 569 (65) | < 0.001 |
| CD4+ count/mm3 at respective ART initiation, median (IQR) | 201 (107–298) | 184 (97–265) | 313 (182–506) | < 0.001 |
| Urban residence, n (%) | 3408 (69) | 2874 (71) | 534 (62) | < 0.001 |
| Follow-up duration (months), median (IQR) | 13 (5–23) | 12 (4–25) | 15 (11–18) | < 0.001 |
| Prior TB | 53 (1) | 27 (1) | 26 (3) | < 0.001 |
| Incident TB, n (%) | 248 (5) | 231 (6) | 17 (2) | < 0.001 |
| Died, n (%) | 288 (6) | 270 (7) | 18 (2) | < 0.001 |
| Lost to follow-up, n (%) | 621 (13) | 565 (14) | 56 (6) | < 0.001 |
ART Antiretroviral therapy, IQR Interquartile range, n – frequency
Among participants on first-line ART, 231 (6%) developed TB, 247 (6%) died and 565 (14%) were lost to follow-up. Among participants on second-line ART, 17 (2%) developed TB, 16 (2%) died and 56 (6%) were lost to follow-up (Fig. 1). Compared to participants on first-line ART, those receiving second-line ART were older (median [IRQ] age 36 [30–42] vs 40 [34–45] years), more likely to be male (50% vs 65%), less like to live in an urban setting (71% vs 62%), more likely to have had TB in the past (1% vs 3%) and had higher CD4+ cell counts (median [IQR] CD4+ 184 [97–265] vs 313 [182–506] cells/mm3) at their respective ART initiation (p < 0.001 for all comparisons) (Table 1).
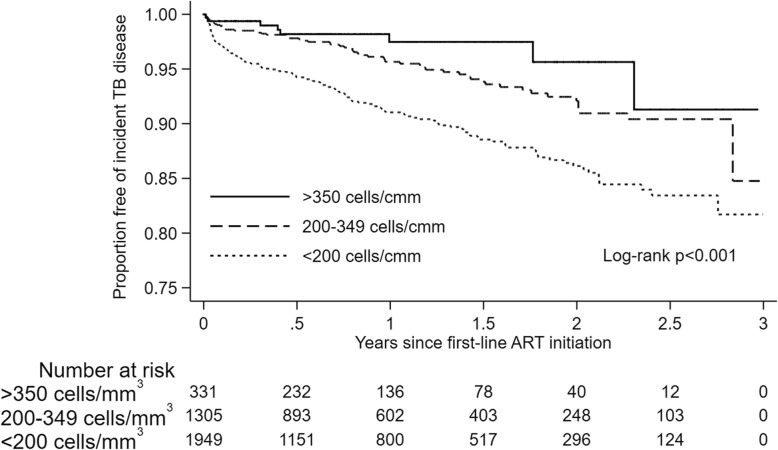
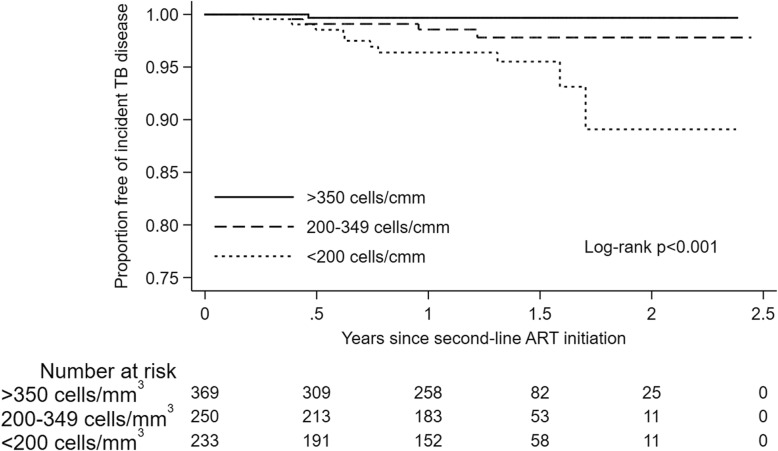
The overall incidence rate of TB in our study was 3.94 cases (95%CI 3.48–4.46) per 100 person-years. The incidence rate of TB among participants on first-line ART was 4.39 cases (95%CI 3.86–5.00) per 100 person-years, significantly higher than 1.64 cases (95%CI 1.01–2.63) per 100 person-years among participants on second-line ART (p < 0.001). The incidence rate of TB was highest during the first 3 months of first- or second-line ART initiation (Table 2). Participants on second-line ART had lower incidence rates of TB compared to those on first-line ART after stratifying by age, sex, CD4+ cell count at their respective ART initiation, time since their respective ART initiation and type of residence (Table 2). Overall, 119 of 231 (52%) and 5 of 17 (29%) TB cases occurred within 6 months of initiating first- and second-line ART, respectively. Earlier occurrence of TB was more common in participants with CD4+ counts below 350 cells/mm3 (p < 0.001) (Figs. 2 and 3).
Table 2.
Incidence rate of TB disease per 100 person-years in participants receiving first- and second-line ART
| Characteristics | Overall | First-line ART | Second-line ART | p-value first- vs second-line ART | |||
|---|---|---|---|---|---|---|---|
| IR | 95%CI | IR | 95%CI | IR | 95%CI | ||
| Overall | 3.94 | 3.48–4.46 | 4.39 | 3.86–5.00 | 1.64 | 1.01–2.63 | < 0.001 |
| Age | |||||||
| 18–29 | 3.67 | 2.75–4.88 | 3.82 | 2.83–5.16 | 2.55 | 0.95–6.79 | 0.44 |
| 30–39 | 4.10 | 3.39–4.96 | 4.39 | 3.61–5.35 | 2.25 | 1.12–4.51 | 0.07 |
| 40–49 | 3.92 | 3.11–4.95 | 4.72 | 3.71–6.01 | 1.21 | 0.50–2.92 | 0.004 |
| > 50 | 3.85 | 2.56–5.80 | 4.78 | 3.17–7.19 | 0 | – | – |
| Sex | |||||||
| Female | 3.26 | 2.69–3.95 | 3.46 | 2.84–4.22 | 1.67 | 0.75–3.72 | 0.08 |
| Male | 4.63 | 3.94–5.46 | 5.48 | 4.62–6.49 | 1.62 | 0.89–2.92 | < 0.001 |
| CD4+ count at respective ART initiation (cells/mm3) | |||||||
| > 350 | 1.14 | 0.61–2.12 | 1.94 | 0.97–3.89 | 0.42 | 0.10–1.71 | 0.06 |
| S200–349 | 2.86 | 2.25–3.64 | 3.05 | 2.37–3.91 | 1.64 | 0.68–3.96 | 0.18 |
| < 200 | 5.55 | 4.78–6.45 | 5.72 | 4.90–6.68 | 3.74 | 2.01–6.95 | 0.18 |
| Time since respective ART initiation | |||||||
| < 3 months | 112.77 | 92.23–137.89 | 122.22 | 99.85–149.60 | 13.63 | 1.92–96.82 | 0.02 |
| 3 to 6 months | 17.23 | 11.97–24.79 | 17.49 | 11.81–25.88 | 15.78 | 5.92–42.04 | 0.84 |
| > 6 months | 2.05 | 1.72–2.44 | 2.22 | 1.84–2.67 | 1.19 | 0.67–2.10 | 0.04 |
| Residence | |||||||
| Urban | 4.31 | 3.74–4.96 | 4.63 | 4.00–5.36 | 2.37 | 1.43–3.93 | 0.01 |
| Semi-urban or rural | 3.01 | 2.31–3.94 | 3.74 | 2.85–4.91 | 0.50 | 0.12–2.00 | 0.006 |
ART Antiretroviral therapy, IR Incidence rate, CI Confidence interval
Fig. 2.
Proportion of participants on first-line ART surviving free of incident TB disease stratified by CD4+ cell counts. Cmm – cubic millimeter, TB – tuberculosis, ART – antiretroviral therapy
Fig. 3.
Proportion of participants on second-line ART surviving free of incident TB disease stratified by CD4+ cell counts. Cmm – cubic millimeter, TB – tuberculosis, ART – antiretroviral therapy
Multivariable regression analysis with death and loss to follow-up as competing risks identified male sex (aSHR = 1.35, 95%CI 1.03–1.77, p = 0.02), CD4+ count less than 200 cells/mm3 at ART initiation (aSHR = 3.03 vs CD4+ count > 350 cells/mm3, 95%CI 1.48–6.17, p = 0.002) and urban residence (aSHR = 1.42 vs non-urban residence, 95%CI 1.04–1.94, p = 0.02) as independent risk factors for TB among participants on first-line ART. Similarly, CD4+ count less than 200 cells/mm3 at ART initiation (aSHR = 9.95 vs CD4+ count > 350 cells/mm3, 95%CI 2.38–41.60, p = 0.002) and urban residence (aSHR = 4.60 vs non-urban residence, 95%CI 0.96–21.86, p = 0.05) were independently associated with higher risk of TB among participants on second-line ART (Table 3).
Table 3.
Risk factors of incident TB disease in participants receiving first-line ART with death and loss to follow-up as competing risks
| Characteristics | Overall | First-line ART | Second-line ART | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | |||||||
| SHR (95%CI) | p-value | SHR (95%CI) | p-value | SHR (95%CI) | p-value | SHR (95%CI) | p-value | SHR (95%CI) | p-value | SHR (95%CI) | p-value | |
| Age | ||||||||||||
| 18–29 | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| 30–39 | 1.09 (0.78–1.54) | 0.58 | 1.00 (0.70–1.41) | 0.99 | 1.15 (0.80–1.64) | 0.44 | 1.02 (0.71–1.47) | 0.90 | 0.92 (0.27–3.08) | 0.89 | 0.95 (0.27–3.34) | 0.94 |
| 40–49 | 1.00 (0.69–1.45) | 0.96 | 0.91 (0.62–1.33) | 0.63 | 1.17 (0.80–1.72) | 0.40 | 0.96 (0.65–1.43) | 0.87 | 0.39 (0.10–1.44) | 0.15 | 0.41 (0.08–1.90) | 0.25 |
| > 50 | 0.94 (0.57–1.54) | 0.80 | 0.84 (0.50–1.41) | 0.51 | 1.12 (0.67–1.86) | 0.64 | 0.94 (0.55–1.59) | 0.83 | – | – | – | – |
| Sex | ||||||||||||
| Female | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Male | 1.31 (1.02–1.68) | 0.03 | 1.33 (1.02–1.74) | 0.03 | 1.44 (1.11–1.87) | 0.006 | 1.35 (1.03–1.77) | 0.02 | 0.96 (0.36–2.57) | 0.94 | 1.14 (0.35–3.69) | 0.81 |
| CD4+ count at respective ART initiation (cells/mm3) | ||||||||||||
| > 350 | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| 200–349 | 2.74 (1.41–5.33) | 0.003 | 2.18 (1.09–4.36) | 0.02 | 1.76 (0.84–3.68) | 0.12 | 1.80 (0.86–3.76) | 0.11 | 3.98 (0.81–19.51) | 0.08 | 4.22 (0.89–19.94) | 0.07 |
| < 200 | 4.90 (2.58–9.30) | < 0.001 | 3.75 (1.91–7.37) | < 0.001 | 3.04 (1.49–6.18) | 0.002 | 3.03 (1.48–6.17) | 0.002 | 8.80 (2.05–37-71) | 0.003 | 9.95 (2.38–41.60) | 0.002 |
| Residence | ||||||||||||
| Semi-urban or rural | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Urban | 1.56 (1.15–2.11) | 0.004 | 1.53 (1.13–2.07) | 0.006 | 1.38 (1.01–1.88) | 0.03 | 1.42 (1.04–1.94) | 0.02 | 4.52 (1.03–19.70) | 0.04 | 4.60 (0.96–21.86) | 0.05 |
| ART | ||||||||||||
| First-line | Ref | Ref | – | – | – | – | – | – | – | – | ||
| Second-line | 0.34 (0.21–0.56) | < 0.001 | 0.48 (0.28–0.82) | 0.008 | – | – | – | – | – | – | – | – |
| Duration on first-line ART prior to second-line ART initiation | ||||||||||||
| Per-year increase | – | – | – | – | – | – | – | – | 1.00 (0.99–1.01) | 0.10 | 1.00 (0.99–1.00) | 0.88 |
SHR sub distribution hazard ratio, CI Confidence interval, Ref Reference group. All multivariable analyses included age, sex, CD4+ cell counts and residence. The overall multivariable analysis additionally included type of ART
A subset of 150 (17%) participants receiving second-line ART had data available on CD4+ cell counts at the time of their first-line ART initiation. The median (IQR) CD4+ count at first-line ART initiation among participants receiving second-line ART was 382 (218–571) cells/mm3. Higher CD4+ cell counts at first-line ART initiation were associated with lower risk of TB during second-line ART receipt (SHR = 0.97 per unit higher CD4+ cell count, 95%CI 0.95–0.99, p = 0.008). Kaplan-Meier survival curves for the proportion of these participants who remained free of TB after stratifying by CD4+ cell counts at initiation of first-line ART are depicted in Additional file 1: Fig. S1.
Discussion
To our knowledge, our report is the largest programmatic study to estimate the incidence rate of TB among adult PLHIV receiving first- and second-line ART prior to widespread IPT implementation in India. We estimated an incidence rate of 4.39 TB cases per 100 person-years among participants on first-line ART, which was significantly higher than the estimated incidence rate of 1.64 TB cases per 100 person-years among participants on second-line ART. We additionally found an association between low CD4+ cell counts at ART initiation, male sex and urban residence, and higher risk of TB in participants on first- and second-line ART.
While the risk of TB on first-line ART has been reported in many settings [9], few studies have evaluated TB risk during second-line ART and none have estimated the incidence rate of TB during second-line ART in India [10]. Our study addresses this knowledge-gap by estimating age, sex and CD4+ cell count stratified incidence rates of TB in PLHIV receiving PI based second-line ART regimens under programmatic conditions in western India. We found a lower risk of TB among participants receiving second-line ART compared to those on first-line ART, independent of age, sex, residence, CD4+ cell counts and competing risks of LTFU and death. While our study results are consistent with prior reports of a switch to PI based regimens reducing the incidence of opportunistic infections and mortality in PLHIV with virologic failure [11–13], a survivor bias likely to be present in those who initiated second-line ART after failing prior regimens, or a delay in switching to second-line ART may have contributed to our study findings. Our estimated incidence rate of TB on first-line ART is comparable to that reported from studies in South Africa [14–16] and one study in western India [17], but is lower than the incidence rate previously reported from other Indian cohorts [18, 19]. Undiagnosed TB in participants on first-line ART who died in our study and, lower CD4+ cell counts at ART initiation, oversampling of male participants and the inclusion of prevalent TB in estimating disease burden in prior studies may explain some of these differences.
Immune suppression has long been associated with TB and prior studies in HIV-infected individuals have shown a dose-response relationship between CD4+ cell counts, particularly at ART initiation, and risk of TB [20–23]. While we did not evaluate time-updated CD4+ cell counts, we found a similar association between low CD4+ cell counts at ART initiation, particularly CD4+ under 200 cells/mm3, and higher risk of TB during follow-up. Further, we found a higher risk of TB during second-line ART in participants with lower CD4+ cell counts at their first-line ART initiation. Overall, 50% of all TB cases in our study occurred within 6 months of first- or second-line ART initiation and the incidence rate of TB was highest during the first 3 months of ART. Unmasking of subclinical TB among participants with low CD4+ cell counts may partly explain our study findings. Subclinical TB is difficult to diagnose and a recent clinical trial did not find a significant health benefit of empiric TB therapy over IPT in advanced HIV disease [24]. Consistent with prior recommendations, our results suggest systematic screening for TB and prioritization of IPT in HIV-infected adults with low CD4+ cell counts.
Male participants had higher risk of TB compared to their female counterparts during first-line ART. This may partly be attributed to males having more advanced immunosuppression at ART initiation compared to females. Our finding may also reflect the global epidemiology of TB where inherent biological or social differences may account for a higher burden of TB in males compared to females [25, 26]. Furthermore, we did not evaluate exposure to alcohol and tobacco smoke, important risk factors of TB over-represented in males, which may have accounted for a higher risk of TB compared to females. Finally, participants residing in an urban region had higher risk of TB compared to those with a semi-urban or rural residence. This finding may be indicative of the underlying transmission dynamics of TB due to overcrowding and social networks which may differ between urban and non-urban regions.
Our study has limitations. The median follow-up time in our cohort was 13 months and nearly 26% of participants were transferred to another ART center during follow-up. While these participants did not have TB at the time of transfer, those with a rural or semi-urban residence were more likely to be transferred leading to shorter observed follow-up time and possible underestimation of TB incidence in this group. Similarly, nearly 13% of participants were lost to follow-up which was more likely to occur in males and those with CD4+ counts less than 200 cells/mm3. While we partially addressed this limitation by accounting for loss to follow-up and death as competing risks, a differential outcome ascertainment may have underestimated the incidence rate of TB in our study. Furthermore, information on CD4+ cell counts at follow-up visits, VL at the time of TB diagnosis or second-line ART initiation, M.tuberculosis infection and contact with a known TB case, and smoking were unavailable, limiting the assessment of key risk factors for TB in our study population. Finally, prevalent and incident TB disease was diagnosed according to standard programmatic guidelines which rely on symptom screening followed by smear microscopy. Culture confirmation of TB disease was lacking and we may have underestimated the incidence of TB disease in our study.
Conclusion
Our study leverages programmatic data from a large sample size of HIV-infected adults to estimate the incidence rate of TB during first- and second-line ART prior to widespread IPT. In doing so, we have provided an estimate of TB risk in a real-world setting to serve as a reference for evaluating the effectiveness of the ongoing IPT rollout in ART clinics in India. Additionally, we used competing risks regression to account for some of the limitations of retrospective analysis of cohort data where loss to follow-up and death may prevent the accurate ascertainment of TB during first- and second-line ART; thereby identifying high-risk individuals for IPT prioritization. Risk of tuberculosis was lower in PLHIV receiving second-line ART compared to first-line ART. Prioritizing IPT in PLHIV with low CD4+ counts, urban residence and in males may further mitigate the risk of tuberculosis during ART.
Supplementary information
Additional file 1: Fig. S1. Proportion of participants on second-line ART surviving free of incident TB disease stratified by CD4+ cell counts at their first-line ART initiation. Cmm – cubic millimeter, TB – tuberculosis, ART – antiretroviral therapy.
Acknowledgements
We would like to thank NACO, the staff at the ART centers of BJGMC-SGH and Rohini Kamble for collecting the data.
Availability of data and material
All data on which the conclusions of the paper rely are already shown in the tables, figures and results section. The raw data used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Consent to publish
Not applicable as details, images, or videos relating to an individual person has not been shared in the manuscript.
Abbreviations
- AFB
Acid fast bacilli
- ART
Antiretroviral therapy
- BJGMC
Byramjee-Jeejeebhoy Government Medical College
- CI
Confidence interval
- IPT
Isoniazid preventive therapy
- IQR
Interquartile range
- IR
Incidence rate
- LTFU
Loss to follow-up
- NACO
National AIDS Control Organization
- NNRTI
Non-nucleotide reverse transcriptase inhibitor
- PI
Protease inhibitor
- PLHIV
People living with HIV
- SGH
Sassoon General Hospitals
- SHR
Sub-distribution hazard ratios
- TB
Tuberculosis
- VL
Viral load
- WHO
World Health Organization
Authors’ contributions
VM, DK and AG1 conceived the study. DK, SS1, BR and SS2 contributed to data collection. SN and IM coordinated the study and provided oversight. AC, NG and AG1 analyzed the data. AG1, VM, IM, JG and AG2 wrote the manuscript. All authors critically reviewed the manuscript for intellectual content and approved the final version.
Funding
This study was supported through a grant from TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health (NIH)‘s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, and the National Institute on Drug Abuse, as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA) [U01 AI069907] and the National Institutes of Health funded Johns Hopkins Baltimore-Washington-India Clinical Trials Unit for NIAID Networks [UM1 AI069497]. AG1 was supported by NIH Research Training Grant # D43 TW009340 funded by the NIH Fogarty International Center, NINDS, NIMH, NHBLI and NIEHS. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Ethics approval and consent to participate
The Institutional Review Boards of Johns Hopkins University (reference number FWA00005752) and the Ethics Committee of Byramjee-Jeejeebhoy Government Medical College (reference number FWA00005797) approved the project. Individual participant consent was exempt because we are reporting on a retrospective analysis of programmatic data collected as part of standard-of-care from the public ART center in Pune, India. Administrative permission was not required to access the raw data for this manuscript.
Competing interests
All authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dileep Kadam, Ivan Marbaniang and Vidya Mave contributed equally to this work.
Contributor Information
Akshay N. Gupte, Email: agupte1@jhmi.edu
Dileep Kadam, Email: deelipkadam@gmail.com.
Shashikala Sangle, Email: shashisangle@yahoo.com.
Bharat B. Rewari, Email: bbrewari@hotmail.com
Sonali Salvi, Email: sonalionly@gmail.com.
Amol Chavan, Email: amolc.jhu@gmail.com.
Smita Nimkar, Email: nsmita13@gmail.com.
Jonathan Golub, Email: jgolub@jhmi.edu.
Nikhil Gupte, Email: nikhil_jhumit@yahoo.com.
Amita Gupta, Email: agupta25@jhmi.edu.
Ivan Marbaniang, Email: ivanmarb@gmail.com.
Vidya Mave, Email: vidyamave@gmail.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12879-019-4569-z.
References
- 1.Global tuberculosis report. Geneva: World Health Organization;2018.
- 2.Gao J, Zheng P, Fu H. Prevalence of TB/HIV co-infection in countries except China: a systematic review and meta-analysis. PLoS One. 2013;8(5):e64915. doi: 10.1371/journal.pone.0064915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell TB, Smeaton LM, Kumarasamy N, et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med. 2012;9(8):e1001290. doi: 10.1371/journal.pmed.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva: World Health Organization;2011.
- 5.India TB Report. New Delhi: Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare;2018.
- 6.Guidelines on Prevention and Management of TB in PLHIV at ART Centers. New Delhi: National AIDS Control Organization, Ministry of Health and Family Welfare;2016.
- 7.Antiretroviral Therapy Guidelines for HIV-Infected Adults and Adolescents Inculuding Post-exposure Prophylaxis. National AIDS Control Organisation, Ministry of Health and Family Welfare, Government of India;2007.
- 8.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 9.Suthar AB, Lawn SD, del Amo J, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001270. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarty J, Sundar S, Chourasia A, et al. Outcome of patients on second line antiretroviral therapy under programmatic condition in India. BMC Infect Dis. 2015;15:517. doi: 10.1186/s12879-015-1270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gsponer T, Petersen M, Egger M, et al. The causal effect of switching to second-line ART in programmes without access to routine viral load monitoring. AIDS. 2012;26(1):57–65. doi: 10.1097/QAD.0b013e32834e1b5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thao VP, Quang VM, Wolbers M, et al. Second-line HIV therapy outcomes and determinants of mortality at the largest HIV referral Center in Southern Vietnam. Medicine (Baltimore) 2015;94(43):e1715. doi: 10.1097/MD.0000000000001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramadhani Habib O., Bartlett John A., Thielman Nathan M., Pence Brian W., Kimani Stephen M., Maro Venance P., Mwako Mtumwa S., Masaki Lazaro J., Mmbando Calvin E., Minja Mary G., Lirhunde Eileen S., Miller William C. The Effect of Switching to Second-Line Antiretroviral Therapy on the Risk of Opportunistic Infections Among Patients Infected With Human Immunodeficiency Virus in Northern Tanzania. Open Forum Infectious Diseases. 2016;3(1):ofw018. doi: 10.1093/ofid/ofw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermans SM, Kiragga AN, Schaefer P, Kambugu A, Hoepelman AI, Manabe YC. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PLoS One. 2010;5(5):e10527. doi: 10.1371/journal.pone.0010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a south African cohort. AIDS. 2005;19(18):2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 16.Liu E, Makubi A, Drain P, et al. Tuberculosis incidence rate and risk factors among HIV-infected adults with access to antiretroviral therapy. AIDS. 2015;29(11):1391–1399. doi: 10.1097/QAD.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hira SK, Shroff HJ, Lanjewar DN, Dholkia YN, Bhatia VP, Dupont HL. The natural history of human immunodeficiency virus infection among adults in Mumbai. Natl Med J India. 2003;16(3):126–131. [PubMed] [Google Scholar]
- 18.Alvarez-Uria G, Pakam R, Midde M, Naik PK. Incidence and mortality of tuberculosis before and after initiation of antiretroviral therapy: an HIV cohort study in India. J Int AIDS Soc. 2014;17:19251. doi: 10.7448/IAS.17.1.19251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghate M, Deshpande S, Tripathy S, et al. Incidence of common opportunistic infections in HIV-infected individuals in Pune, India: analysis by stages of immunosuppression represented by CD4 counts. Int J Infect Dis. 2009;13(1):e1–e8. doi: 10.1016/j.ijid.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 20.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23(13):1717–1725. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bock P, Jennings K, Vermaak R, et al. Incidence of tuberculosis among HIV-positive individuals initiating antiretroviral treatment at higher CD4 counts in the HPTN 071 (PopART) trial in South Africa. J Acquir Immune Defic Syndr. 2018;77(1):93–101. doi: 10.1097/QAI.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Group TAS. Danel C, Moh R, et al. A trial of early Antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 23.Group ISS. Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseinipour MC, Bisson GP, Miyahara S, et al. Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): a multicountry open-label randomised controlled trial. Lancet. 2016;387(10024):1198–1209. doi: 10.1016/S0140-6736(16)00546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nhamoyebonde S, Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis. 2014;209(Suppl 3):S100–S106. doi: 10.1093/infdis/jiu147. [DOI] [PubMed] [Google Scholar]
- 26.Borgdorff MW, Nagelkerke NJ, Dye C, Nunn P. Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. Int J Tuberc Lung Dis. 2000;4(2):123–132. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Proportion of participants on second-line ART surviving free of incident TB disease stratified by CD4+ cell counts at their first-line ART initiation. Cmm – cubic millimeter, TB – tuberculosis, ART – antiretroviral therapy.
Data Availability Statement
All data on which the conclusions of the paper rely are already shown in the tables, figures and results section. The raw data used and/or analyzed in the current study are available from the corresponding author on reasonable request.