Abstract
Background
Ibrutinib is an approved treatment for relapsed or refractory chronic lymphocytic leukemia (cll) and small lymphocytic lymphoma (sll). The effect of ibrutinib dose reduction compared with discontinuation in a population-based setting is unclear.
Methods
To examine the patterns of ibrutinib use in a Canadian population-based setting, we analyzed a retrospective cohort of patients with relapsed or refractory cll or sll treated with ibrutinib.
Results
The 64 patients diagnosed with cll or sll had a median age of 76.5 years. Most had unmutated ighv (immunoglobulin variable heavy chain). A hematologic response occurred in 39 patients regardless of the ibrutinib dose. The most common toxicities were infection, bruising or bleeding, and musculoskeletal problems, with a median time to first toxicity of 14 days. More than half the cohort experienced a dose reduction, with musculoskeletal problems, cytopenias, and infection being the leading causes; surgery was the most frequent indication for holding treatment. Only 26 of the 64 patients (40.6%) stayed on the recommended maximal dose of ibrutinib. No differences in reported toxicities or hematologic response rates were evident between the patients receiving maximal and submaximal therapy. At the end of the study period, 53 patients from the initial cohort remained on ibrutinib.
Conclusions
More than half the study patients received ibrutinib therapy at a submaximal dose without evidence of increased frequency of toxicities or disease progression. The rate of ibrutinib discontinuation was lower in our cohort than has been reported in other settings. Submaximal ibrutinib dosing will have to be further systematically evaluated.
Keywords: Ibrutinib, chronic lymphocytic leukemia, small lymphocytic lymphoma, toxicity, clinical response
INTRODUCTION
Chronic lymphocytic leukemia (cll) is the most common adult leukemia in North America1. Within Manitoba, the incidence of newly diagnosed cases is approximately 100 per year2. Small lymphocytic lymphoma (sll), which is classified together with cll by the World Health Organization, has clinical and pathologic characteristics similar to those in cll, but notably, sll lacks a leukemic phase and is usually associated with lymphadenopathy or splenomegaly (or both)3.
Treatment options for cll depend on the patient’s performance status, comorbidities, and cytogenetics4. Despite recent advances in treatment, no therapy is curative in older patients; currently, goals of treatment focus on attainment of durable remissions. Among patients who are considered young and fit [defined by an age less than 65 years and a cumulative illness rating score (cirs)5 of 6 or less], a regimen of fludarabine, cyclophosphamide, and rituximab for 6 cycles is often the first-line standard of care6. For those who have a cirs score greater than 6 and who are eligible for chemotherapy, obinutuzumab and chlorambucil are offered7 in the first-line setting.
Ibrutinib, an inhibitor of Bruton tyrosine kinase, a critical enzyme in the B cell receptor–signalling pathway8, has demonstrated efficacy in the treatment of several B cell malignancies. At a daily dose of 420 mg, ibrutinib received accelerated approval for the treatment of relapsed or refractory (r/r) cll, and more recently, as initial treatment for patients with 17p deletion9–11. In the initial phase iii trial for r/r cll, ibrutinib (compared with ofatumumab) was associated with superior progression-free survival (pfs), overall response rate, and overall survival at 8 months10. In an updated analysis, median pfs was not reached in the ibrutinib group, with 74% of patients being alive at 24 months, regardless of the presence of high-risk cytogenetics12. Within the trial setting, sustained adherence to continuous once-daily maximal dosing has been demonstrated to be an important contributor to improved pfs. However, patients in the trials had a discontinuation rate exceeding 20%, partly because of the requirement to discontinue therapy on study, with no options for dose reduction11,13. Toxicities accounted for 7% of the discontinuations10,13. Outside of clinical trials, management of toxicities might necessitate dose reductions or interruptions in therapy. The implications of such practices in a population-based setting are not entirely clear. However, recently emerging data suggest that maximal-dose therapy might not be necessary for long-term disease control14–17.
To date, the population-based experience with ibrutinib in the setting of r/r cll or sll has not been evaluated in Manitoba. In view of that situation and of the uncertainty about the implications of submaximal ibrutinib therapy for patient outcomes, we conducted a retrospective cohort study of patients with r/r cll or sll treated with ibrutinib. We examined patient demographics and patterns of ibrutinib use, including dose intensity, frequency of toxicities associated with treatment, and hematologic response. In particular, the effects of various dosing schedules on the number and type of reported toxicities and on patient response were investigated.
METHODS
Study Design and Participants
All patients with r/r cll or sll receiving ibrutinib in the Canadian province of Manitoba were identified for study inclusion. Data from the electronic charting system, the provincial oncology drug program, the Manitoba Cancer Registry, the Manitoba Tumour Bank, and the cll caisis database (http://www.caisis.org/) were used. Patients starting treatment up to March 2017 were included for data extraction and analysis.
Data Collection and Measurement
Retrospectively, using electronic chart review, basic demographic data for the patients were extracted [age, sex, Rai stage, cirs, fluorescence in situ hybridization (fish) status, ighv mutational status, CD38 status, and zap-70 status at various stages of treatment]. The number and type of previous therapies; duration of ibrutinib treatment; ibrutinib dose history, including number and reasons for dose reductions; and treatment discontinuations and interruptions were collected based on electronic medical record documentation. Complete blood counts before and throughout ibrutinib treatment were obtained. All toxicities experienced by patients while receiving ibrutinib, as reflected in the electronic medical record, were captured. Because of the retrospective nature of the data collection, documented toxicities were not amenable to grading. Hematologic response was determined by normalization of blood counts based on International Workshop on Chronic Lymphocytic Leukemia criteria during the study period18.
On review of ibrutinib treatment receipt for the patient cohort, 2 dosing schedule patterns emerged, which were then grouped into two categories: full dose and submaximal dose. Patients were considered to have received a full dose if they started treatment with a full dose and remained at that dose level; if they started treatment at a lower dose and were subsequently titrated up to a full dose; or if they started with a full dose, were moved to a reduced dose, but subsequently returned to a full dose. Patients were considered to be submaximally dosed if they began treatment with a full dose, but were moved to a reduced dose and remained at a reduced dose; if they started treatment at a lower dose, were increased to a full dose, but were moved to a reduced dose; or if they started at a lower dose and stayed at a lower dose.
Statistical Analysis
Patient demographics and clinical features are described using frequency tables, medians, and interquartile ranges, and visually using graphics. Differences between patient subgroups were investigated using the chi-square and Fisher exact tests for categorical data and Wilcoxon Mann–Whitney tests for continuous data. Values of p ≤ 0.05 were considered statistically significant. Time to response is presented using cumulative incidence curves, with differences between groups deemed significant when the p values, as described by Gray19, are 0.05 or less.
RESULTS
Patient Demographics
Our study cohort included 64 patients (25 women, 39 men; median age at the start of ibrutinib treatment: 76.5 years). Table I summarizes the demographic and cll or sll characteristics of the cohort by dose schedule; Table II summarizes hematologic characteristics in the cohort. At the time of ibrutinib treatment, 13 patients had early Rai stage cll (0, i); 46 had late Rai stage cll (ii–iv); and 5 had sll. Of the 52 patients with a known fish status, 12 had low-risk (del 13q), 19 had intermediate-risk (trisomy 12 or normal), and 21 had high-risk (del 11q or 17p) fish abnormalities. Patient ighv status was unmutated in 36 and mutated in 17; 32 patients were zap-70–positive; and 26 patients were CD38-positive. Of the 21 patients in the high-risk fish group, 13 also had unmutated ighv. Patients had received 1–6 prior treatments: 16 patients had received 1 prior treatment; 18 patients, 2 prior treatments; 21 patients, 3 prior treatments; 6 patients, 4 prior treatments; and 3 patients, 5 or more prior treatments. The median cirs score in this cohort was 6 at the time of ibrutinib treatment, with a range of 2–16 (Table II).
TABLE I.
Descriptive characteristics for 64 patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in Manitoba approved for treatment with ibrutinib between August 2014 and March 2017
| Characteristic | Dose group [n (%)] | p Valuec | |
|---|---|---|---|
|
| |||
| Fulla | Submaximalb | ||
| Patients | 26 | 38 | |
|
| |||
| Sex | |||
| Women | 7 (26.92) | 18 (47.37) | 0.0997 |
| Men | 19 (73.08) | 20 (52.63) | |
|
| |||
| Diagnosis | |||
| CLL | 26 (100.00) | 26 (68.42) | 0.0010 |
| SLL | 0 (0.00) | 12 (31.58) | |
|
| |||
| RAI stage at ibrutinib start | |||
| Early (0–I) | 6 (23.08) | 7 (18.42) | 0.1717d |
| Late (II–IV) | 20 (76.92) | 26 (68.42) | |
| SLL | 0 (0.00) | 5 (13.16) | |
|
| |||
| FISH statuse | |||
| Normal | 2 (7.69) | 8 (21.05) | 0.2576d |
| Low | 8 (30.77) | 4 (10.53) | |
| Intermediate | 4 (15.38) | 5 (13.16) | |
| High | 8 (30.77) | 13 (34.21) | |
| Not done | 4 (15.38) | 8 (21.05) | |
|
| |||
| IgHV mutation status | |||
| Mutated | 6 (23.08) | 11 (28.95) | 0.1489 |
| Unmutated | 18 (69.23) | 18 (47.37) | |
| Missing/unknown | 2 (7.69) | 9 (23.68) | |
|
| |||
| ZAP-70 positivity (>20%) | |||
| Positive | 12 (46.15) | 20 (52.63) | 0.7703d |
| Negative | 14 (53.85) | 17 (44.74) | |
| Missing/unknown | 0 (0.00) | 1 (2.63) | |
|
| |||
| CD38 positivity (>20%) | |||
| Positive | 7 (26.92) | 19 (50.00) | 0.1551d |
| Negative | 17 (65.38) | 15 (39.47) | |
| Test not done | 2 (7.69) | 4 (10.53) | |
|
| |||
| Total treatments before ibrutinib | |||
| 1 | 4 (16.00) | 12 (31.58) | 0.4150d |
| 2 | 9 (36.00) | 9 (23.68) | |
| 3 | 8 (32.00) | 13 (34.21) | |
| 4 | 4 (16.00) | 2 (5.26) | |
| 5 | 0 (0.00) | 1 (2.63) | |
| 6 | 1 (3.85) | 1 (2.63) | |
Those who started treatment with a full dose and stayed at the full dose; those who started at a lower dose that was subsequently titrated up to a full dose; and those who started at a full dose, were moved to a reduced dose, but subsequently returned to a full dose.
Those who started treatment with a full dose, but who were subsequently moved to a reduced dose; those who started treatment at a lower dose, increased to a full dose, but were subsequently moved to a reduced dose; and those who started at a lower dose and stayed at a lower dose.
By chi-square test unless otherwise specified.
By Fisher exact test.
Normal = patients without detectable FISH abnormalities; Low = patients with 13q FISH abnormalities; Intermediate = patients with trisomy 12 FISH abnormalities; High = patients with 11q, 14q, or 17p FISH abnormalities.
FISH = fluorescence in situ hybridization.
TABLE II.
Hematologic characteristics at the time of ibrutinib start for 64 patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in Manitoba approved for treatment with ibrutinib between August 2014 and March 2017
| Characteristic | Dose group | Pts (n) | Median | IQR | Range | p Valuec |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| Fulla | 26 | 74 | 8 | 59–87 | 0.14 | |
| Submaximalb | 38 | 77.5 | 10 | 58–87 | ||
| Overall | 64 | 76.5 | 11 | 58–87 | ||
|
| ||||||
| CIRS | ||||||
| Fulla | 25 | 6 | 3 | 2–11 | 0.60 | |
| Submaximalb | 37 | 6 | 3 | 3–16 | ||
| Overall | 62 | 6 | 3 | 2–16 | ||
|
| ||||||
| WBC count (×109/L) | ||||||
| Fulla | 25 | 22 | 54.8 | 0.6–278 | 0.09 | |
| Submaximalb | 38 | 9.3 | 17.5 | 2–257.4 | ||
| Overall | 63 | 12.3 | 36.7 | 0.6–278 | ||
|
| ||||||
| Hemoglobin (g/L) | ||||||
| Fulla | 25 | 124 | 31 | 12–145 | 0.94 | |
| Submaximalb | 38 | 115.5 | 24 | 12–165 | ||
| Overall | 63 | 118 | 30 | 12–165 | ||
|
| ||||||
| Platelets (×109/L) | ||||||
| Fulla | 25 | 106 | 88 | 13–227 | 0.88 | |
| Submaximalb | 38 | 112 | 72 | 10–369 | ||
| Overall | 63 | 108 | 82 | 10–369 | ||
|
| ||||||
| Lymphocytes (×109/L) | ||||||
| Fulla | 25 | 16.3 | 47.6 | 0–285 | 0.11 | |
| Submaximalb | 38 | 5.8 | 19.4 | 0.5–245.4 | ||
| Overall | 63 | 7 | 37.1 | 0–285 | ||
|
| ||||||
| Creatinine (μmol/L) | ||||||
| Fulla | 25 | 92 | 37 | 59–191 | 0.65 | |
| Submaximalb | 38 | 86.5 | 26 | 21–160 | ||
| Overall | 63 | 88 | 35 | 21–191 | ||
|
| ||||||
| β2-Microglobulin (mg/L) | ||||||
| Fulla | 18 | 4.6 | 3.5 | 2.5–12.3 | 0.45 | |
| Submaximalb | 30 | 4.2 | 3.3 | 2–13.7 | ||
| Overall | 48 | 4.2 | 3.2 | 2–13.7 | ||
Those who started treatment with a full dose and stayed at the full dose; those who started at a lower dose that was subsequently titrated up to a full dose; and those who started at a full dose, were moved to a reduced dose, but subsequently returned to a full dose.
Those who started treatment with a full dose, but who were subsequently moved to a reduced dose; those who started treatment at a lower dose, increased to a full dose, but were subsequently moved to a reduced dose; and those who started at a lower dose and stayed at a lower dose.
By Wilcoxon Mann–Whitney test.
IQR = interquartile range; CIRS = cumulative illness rating score; WBC = white blood cell.
Full-dose ibrutinib (420 mg daily) was given in 26 patients (40.6%); the remaining 38 patients either started on a submaximal dose (<420 mg daily) or experienced a dose reduction because of toxicity. Of the 38 patients who received submaximal doses, 10 (26%) started with, and remained on, a submaximal dose. Women accounted for only 27% of the patients who remained on a full dose. All sll patients fell into the submaximal dose group. No further differences in clinical or prognostic factors—including age, cirs score, Rai stage, fish status, ighv mutation status, zap-70 or CD38 positivity, and number of prior treatments—were different between the dose groups.
Dose Adherence and Toxicities
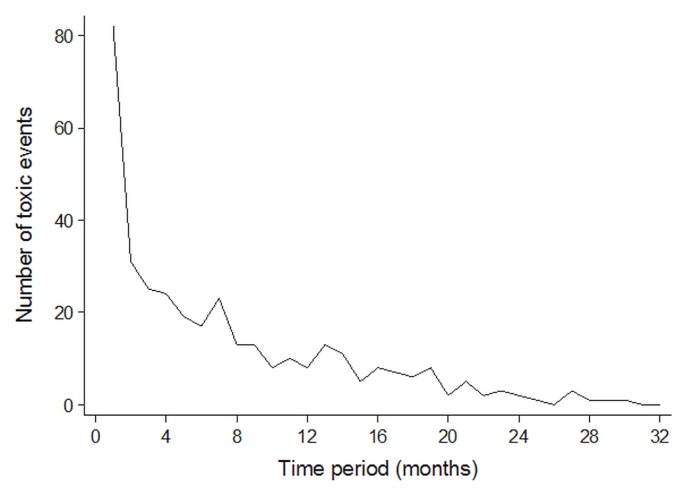
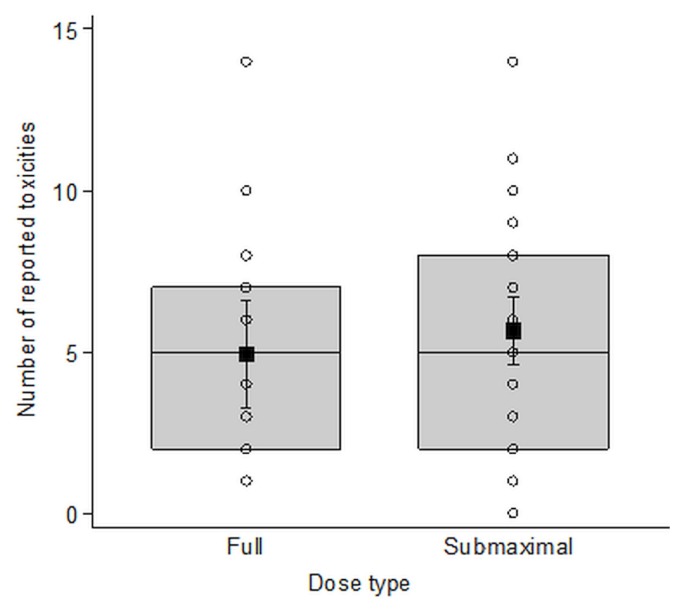
The median time to ibrutinib from the last treatment was 4.41 years [interquartile range (iqr): 3.86; range: 0.33–15.61 years]. The most commonly recorded toxicities (Table III) were infections (predominantly upper and lower respiratory, skin and soft tissue, and urinary tract), bleeding and bruising, and musculoskeletal problems (predominantly arthralgia and myalgia). Toxicities were reported more frequently in the first 4 months of treatment (Figure 1), with a median time to first toxicity of 14 days (95% confidence interval: 11 days to 19 days). Although not the most common, gastrointestinal effects, chiefly diarrhea, were seen earlier after ibrutinib start than other toxicities were. Rates of toxicity did not differ significantly between the patients receiving full and submaximal doses (Figure 2).
TABLE III.
All reported toxicities for 64 patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in Manitoba approved for treatment with ibrutinib between August 2014 and March 2017
| Toxicity | Patients | |
|---|---|---|
| (n) | (%) | |
| Infection | 177 | 44.14 |
| Bruising and bleeding | 58 | 14.46 |
| Musculoskeletal problem | 45 | 11.22 |
| Gastrointestinal effects | 32 | 7.98 |
| Cardiac effects | 27 | 6.73 |
| Hair and nail effects | 21 | 5.24 |
| Skin and mucous membranesa | 18 | 4.49 |
| Malignancy | 11 | 2.74 |
| Neurologic effects | 6 | 1.50 |
| Biochemical abnormality | 3 | 0.75 |
| None | 3 | 0.75 |
Non-infectious changes.
FIGURE 1.
Toxicity pattern. Number of toxicities reported over the course of 32 months’ follow-up after treatment with ibrutinib in 64 patients with relapsed or refractory chronic lymphocytic leukemia in Manitoba. Median time to first toxicity was 14 days (95% confidence interval: 11 days to 19 days).
FIGURE 2.
No difference in reported toxicities by dose intensity. The number of reported toxicities by dose intensity (full or submaximal) in 64 patients treated with ibrutinib for relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma in Manitoba. Patients receiving a full dose included those who started treatment with a full dose and stayed at the full dose; those who started at a lower dose that was subsequently titrated up to a full dose; and those who started at a full dose, were moved to a reduced dose, but subsequently returned to a full dose. Patients receiving a submaximal dose were those who started treatment with a full dose, but who were subsequently moved to a reduced dose; those who started treatment at a lower dose, increased to a full dose, but were subsequently moved to a reduced dose; and those who started at a lower dose and stayed at a lower dose.
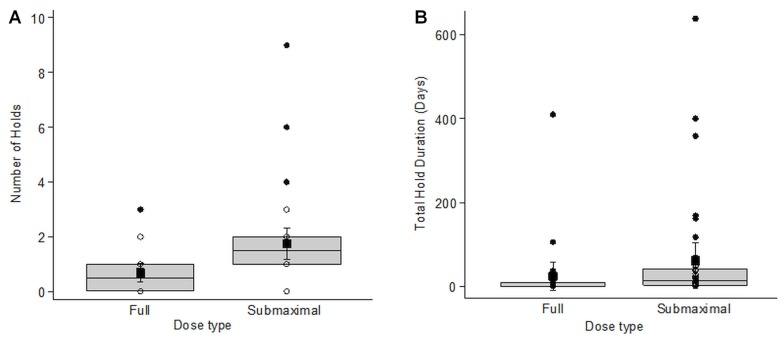
More than half the study cohort experienced at least 1 dose reduction during the study period. The 59 dose reduction events that occurred had a mean duration of 145.9 days (Table IV). The most common indications for dose reductions were musculoskeletal problems (28%; median duration: 161 days; iqr: 224 days; range: 0–617 days), cytopenias (18.6%; median duration: 65 days; iqr: 224 days; range: 4–373 days), and infections (15%; median duration: 112 days; iqr: 78 days; range: 1–599 days). Similarly, about two thirds of the cohort (67.2%) had at least 1 dose interruption or hold during the study period, with 84 holds occurring overall. The most common indication was surgery (34.5%), and as expected, because of the bleeding risk associated with ibrutinib, the associated median duration was short (3.5 days; iqr: 6 days; range: 0–41 days). Infection and cytopenias were the next most common indications. When comparing the frequency of holds between the dose groups, the submaximal dose group had more frequent holds [median: 1.5 vs. 0.5 in the full dose group; p = 0.0037; Figure 3(A), Table V]. Holds within the submaximal dose group were also longer [median duration: 13 days (iqr: 38 days) vs. 1 day (iqr: 9 days) in the full dose group; p = 0.0039; Figure 3(B)]. However, after the exclusion of several outliers in the submaximal dose group, that difference was no longer present.
TABLE IV.
Reasons for all reported dose reductions in 33 patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in Manitoba approved for treatment with ibrutinib between August 2014 and March 2017
| Reason | Patients affected [n (%)] | Duration (days) | ||
|---|---|---|---|---|
| Median | IQR | Range | ||
| Musculoskeletal problem | 17 (28.81) | 161.00 | 224.00 | 0–617 |
| Cytopenia | 11 (18.64) | 65.00 | 224.00 | 4–373 |
| Infection | 9 (15.5) | 112.00 | 78.00 | 1–599 |
| Cardiac effects | 6 (10.17) | 39.50 | 98.00 | 3–599 |
| Gastrointestinal effects | 6 (10.17) | 63.00 | 86.00 | 22–206 |
| Bruising and bleeding | 3 (5.08) | 13.00 | 589.00 | 10–599 |
| Medication interaction | 3 (5.08) | 7.00 | 1.00 | 6–7 |
| Neurologic effects | 3 (5.08) | 123.00 | 288.00 | 45–333 |
| Hair and nail effects | 1 (1.69) | 266.00 | 0 | 266–266 |
IQR = interquartile range.
FIGURE 3.
Hold data for 64 patients treated with ibrutinib for relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma in Manitoba. (A) Number of holds by dose intensity. Kruskal–Wallis p = 0.0037 with outliers. Without outliers, p = 0.1797. (B) Total duration of holds in days, by dose intensity. Kruskal–Wallis p = 0.0039 with outliers. Without outliers, p = 0.3017. Patients receiving a full dose included those who started treatment with a full dose and stayed at the full dose; those who started at a lower dose that was subsequently titrated up to a full dose; and those who started at a full dose, were moved to a reduced dose, but subsequently returned to a full dose. Patients receiving a submaximal dose were those who started treatment with a full dose, but who were subsequently moved to a reduced dose; those who started treatment at a lower dose, increased to a full dose, but were subsequently moved to a reduced dose; and those who started at a lower dose and stayed at a lower dose.
TABLE V.
Reasons for all reported holds in 43 patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in Manitoba approved for treatment with ibrutinib between August 2014 and March 2017
| Reason | Patients affected [n (%)] | Duration (days) | ||
|---|---|---|---|---|
| Median | IQR | Range | ||
| Surgery | 29 (34.52) | 3.50 | 6.00 | 0–41 |
| Infection | 15 (17.86) | 7.00 | 18.00 | 1–403 |
| Cytopenia | 7 (8.33) | 99.00 | 301.00 | 2–357 |
| Gastrointestinal effects | 7 (8.33) | 11.00 | 32.00 | 1–83 |
| Musculoskeletal problem | 7 (8.33) | 11.00 | 302.00 | 2–308 |
| Cardiac effects | 5 (5.95) | 8.00 | 52.00 | 4–92 |
| Bruising and bleeding | 4 (4.76) | 9.00 | 15.00 | 2–24 |
| Medication interaction | 3 (3.57) | 6.00 | 9.300 | 3–12 |
| Rash | 3 (3.57) | 6.00 | 6.00 | 3–9 |
| Neurologic effects | 2 (2.38) | 32.50 | 47.00 | 9–56 |
| Radiation treatment | 1 (1.19) | 17.00 | 0 | 17–17 |
| Transformation | 1 (1.19) | 56.00 | 0 | 56–56 |
IQR = interquartile range.
Time to Hematologic Response
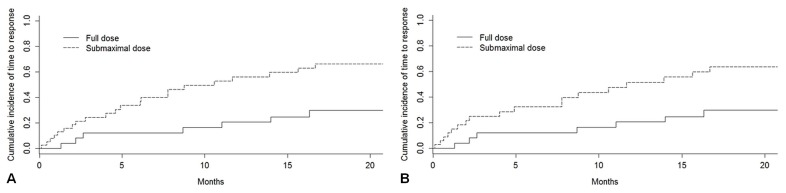
Of the 64 study patients, 39 achieved a complete hematologic response. Patients in the submaximal dose group had a significantly shorter time to achievement of hematologic response [Figure 4(A), p = 0.01]. On further review, that group had a comparatively lower burden of white blood cells and thus achieved a normalized lymphocyte count faster. Furthermore, that group included all of the sll patients in the cohort. When the analysis omitted the sll patients, no difference in time to hematologic response was observed between the dose groups [p = 0.06, Figure 4(B)].
FIGURE 4.
Time to response for 64 patients treated with ibrutinib for relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in Manitoba. (A) Time to response, by dose intensity, patients with SLL or CLL, p = 0.01. (B) Time to response, by dose intensity, excluding patients with SLL, p = 0.06. Patients receiving a full dose included those who started treatment with a full dose and stayed at the full dose; those who started at a lower dose that was subsequently titrated up to a full dose; and those who started at a full dose, were moved to a reduced dose, but subsequently returned to a full dose. Patients receiving a submaximal dose were those who started treatment with a full dose, but who were subsequently moved to a reduced dose; those who started treatment at a lower dose, increased to a full dose, but were subsequently moved to a reduced dose; and those who started at a lower dose and stayed at a lower dose.
Clinical Outcomes
Of the 64 study patients, only 11 (17%) discontinued ibrutinib during the study period. Of those 11 patients, 6 had been receiving a submaximal dose. The reasons for discontinuation were toxicity (1 diarrhea, 1 ventricular tachycardia20), progression (2 transformations to lymphoma, 1 cll progression), and functional decline unrelated to cll progression or the toxicity of ibrutinib (n = 6).
DISCUSSION
Within our cohort of cll and sll patients receiving ibrutinib for r/r disease, more than half are no longer on a full dose, and no clear difference in reported toxicities have been evident. Despite a tendency toward dose reduction in this cohort, only 3 patients were observed to experience disease progression, common in a highly relapsed population setting. A hematologic response was noted in more than half the patients, suggesting that a submaximal dose of ibrutinib might still result in effective disease control. An unexpected finding was that adjustment of ibrutinib dosing, a frequent practice in response to intolerance and toxicities, did not result in differences in the observed toxicities. The standard dose of ibrutinib has been listed as 420 mg once daily until progression, based on both toxicity profile and kinase inhibition in early clinical trials for r/r cll21. It is clear in clinical practice that patients have more frequently experienced toxicities, and adjustment of dose intensity is a commonly used strategy to mitigate those toxicities22.
As is often observed in the non-clinical-trial setting10, our patients were slightly older and might have been slightly lower risk (lower percentage of high-risk disease by fish), but they had an overrepresentation of unmutated ighv. When ibrutinib initially came into practice, it was assumed that lowering the dose or stopping treatment would be associated with rapid relapse and possibly transformation into an aggressive diffuse large B cell lymphoma with an extremely poor prognosis. That perception later proved to be associated with participants in the initial trials who represented a highly treated relapsed population rather than a population in whom ibrutinib had been stopped10. That observation is reflected in our results: the hematologic response rates exceeded 50%, and of 3 progressions, 2 occurred in patients receiving a maximal dose, and 1 occurred in the most heavily pretreated individual in the submaximal dose group.
It is important to emphasize that our study cohort had no options for a clinical trial or another agent, and thus maintenance on some form of treatment was essential for disease control. In addition, it must be stated that, because of the dose reductions, our cohort’s drug discontinuation rates were also lower than those reported in the clinical trial setting (17% vs. 26%), because dose reductions were not allowed in the clinical trial. Compared with a large retrospective cohort of patients with cll treated with ibrutinib in the front-line setting, in which 8% of patients were started on ibrutinib at a submaximal dose and 17% of patients required a reduction in the ibrutinib dose while on treatment, our cohort’s discontinuation rates remained lower compared with the 24% of patients in that cohort who discontinued ibrutinib therapy after a median of 6.5 months23.
Within the clinical trial population, data suggest that adherence to ibrutinib at the maximal dose is related to duration of response, with an interruption in therapy of 8 days or more being associated with shorter median pfs 11. In contrast, in the clinical setting, several observational studies24–26 have demonstrated good tolerance of therapy at the submaximal dose, with no evidence of reduced pfs or overall survival. Biologic plausibility to support those findings stems from observations in a recent pilot study that occupancy of Bruton tyrosine kinase, and downstream signalling, are reduced after initial high-dose ibrutinib and that ongoing molecular inhibition of Bruton tyrosine kinase might not require the same dose14. With respect to our findings, no differences in the frequency of toxicities were observed between the dose-intensity groups or with time on treatment. A potential limiting factor is that the lack of a difference reflects our study’s inability to obtain toxicity grades.
Despite the submaximal therapy received by most patients, no differences in hematologic response rates or clinical outcomes were observed when only the patients with cll were compared. Patients with sll were all in the submaximal dose group, and when they were analyzed together with the cll patients, a difference in the submaximal dose group was observed, suggesting that a submaximal dose might have achieved a quicker hematologic response. Given that our study used the original International Workshop on Chronic Lymphocytic Leukemia criteria, normalization of lymphocyte count was included in the hematologic response criteria. What we observed was that patients with sll did not achieve similar increases in their lymphocyte counts, and thus normalization of the white blood cell count occurred earlier for them, skewing the results. No appreciable differences in patient- or cancer-specific characteristics apart from sex could explain the durability of response regardless of dose, given that the submaximal dose group had a larger fraction of female participants. Recent reports have suggested sex differences in drug dosing, with both pharmacodynamics and drug interactions leading to increased susceptibility to drug toxicities27. An additional consideration is that our patient cohort was older and likely had additional comorbidities and took additional medications. It is possible that drug interactions or liver or kidney function might have led to decreased drug clearance. Those parameters are currently being explored by an examination of Manitoba’s health services databases rather than patient reports and physician and health records. It should be noted that patient adherence to prescribed ibrutinib treatment was assumed throughout the study period. However, despite provincial drug coverage, without a patient-associated drug cost, complete adherence has been demonstrated not to be the case, with adherence rates of 63% being reported in the Canadian setting28. In addition, with practice tending toward treatment-free periods and multiple agents in combination, the proper doses will also have to be worked out.
Given that the median pfs for patients receiving ibrutinib in the trial setting was not reached in an interim analysis after 24 months of follow-up11, we acknowledge that a longer duration of follow-up in our cohort will be needed to assess for noninferiority of the submaximal dose compared with the recommended (maximal) dose. Reassuringly, however, the submaximal dose did not appear to be harmful.
One of the issues not addressed by our study, but clearly observed, was the initiation of patients on ibrutinib at a submaximal dose. Whether that approach reflects physician choice or patient factors could not be assessed in this retrospective study.
CONCLUSIONS
Given the rapidly changing treatment environment in cll, with ibrutinib emerging as a therapeutic intervention with longevity, results from our cohort study suggest that dose reductions and interruptions for toxicities instead of switching lines of treatment without evidence of progression or effect on hematologic response might be an option. Such an approach would thus reserve a therapy for progression until the future of drug discontinuation is better understood. Certainly, the clinical efficacy of lower doses will have to be further systematically evaluated.
ACKNOWLEDGMENTS
The Health Research Ethics Board at the University of Manitoba (HS19018) approved this study. Funding for the study was provided by the CancerCare Manitoba Foundation and a Research Manitoba CLL Cluster Grant.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: DD has attended advisory boards for AstraZeneca and Merck, and has received an honorarium from Boehringer Ingelheim; VB has attended advisory boards for Janssen, AbbVie, and Astra Zeneca; VB and JBJ have received research funding from Janssen and Gilead; and VB has received research funding from Research Manitoba CLLuster, a New investigator Grant, and the CancerCare Manitoba Foundation. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Matasar MJ, Ritchie EK, Consedine N, Magai C, Neugut AI. Incidence rates of the major leukemia subtypes among US Hispanics, Blacks, and non-Hispanic Whites. Leuk Lymphoma. 2006;47:2365–70. doi: 10.1080/10428190600799888. [DOI] [PubMed] [Google Scholar]
- 2.Seftel MD, Demers AA, Banerji V, et al. High incidence of chronic lymphocytic leukemia (cll) diagnosed by immunophenotyping: a population-based Canadian cohort. Leuk Res. 2009;33:1463–8. doi: 10.1016/j.leukres.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain N, O’Brien S. Initial treatment of cll: integrating biology and functional status. Blood. 2015;126:463–70. doi: 10.1182/blood-2015-04-585067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–6. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 6.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 7.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with cll and coexisting conditions. N Engl J Med. 2014;370:1101–10. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 8.Niemann CU, Wiestner A. B-Cell receptor signaling as a driver of lymphoma development and evolution. Semin Cancer Biol. 2013;23:410–21. doi: 10.1016/j.semcancer.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen Inc. Imbruvica: Ibrutinib Capsules [product monograph] Toronto, ON: Janssen; 2019. [Google Scholar]
- 10.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–37. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown JR, Hillmen P, O’Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 resonate study in patients with previously treated cll/sll. Leukemia. 2018;32:83–91. doi: 10.1038/leu.2017.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barr PM, Brown JR, Hillmen P, et al. Impact of ibrutinib dose adherence on therapeutic efficacy in patients with previously treated cll/sll. Blood. 2017;129:2612–15. doi: 10.1182/blood-2016-12-737346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen LS, Bose P, Cruz ND, et al. A pilot study of lower doses of ibrutinib in patients with chronic lymphocytic leukemia. Blood. 2018;132:2249–59. doi: 10.1182/blood-2018-06-860593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mato A, Nabhan C, Kay NE, et al. Real-world clinical experience in the Connect chronic lymphocytic leukaemia registry: a prospective cohort study of 1494 patients across 199 US centres. Br J Haematol. 2016;175:892–903. doi: 10.1111/bjh.14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mato AR, Samp JC, Gauthier G, Terasawa E, Brander DM. Drivers of treatment patterns in patients with chronic lymphocytic leukemia stopping ibrutinib or idelalisib therapies. Cancer Biol Ther. 2018;19:636–43. doi: 10.1080/15384047.2018.1449616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghia P, Cuneo A. Ibrutinib in the real world patient: many lights and some shades. Haematologica. 2016;101:1448–50. doi: 10.3324/haematol.2016.155986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of cll. Blood. 2018;131:2745–60. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 19.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. doi: 10.1214/aos/1176350951. [DOI] [Google Scholar]
- 20.Lampson BL, Yu L, Glynn RJ, et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129:2581–4. doi: 10.1182/blood-2016-10-742437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes J, Mato A, Sharman JP. Monitoring and management of toxicities of novel B cell signaling agents. Curr Oncol Rep. 2018;20:49. doi: 10.1007/s11912-018-0694-x. [DOI] [PubMed] [Google Scholar]
- 23.Mato AR, Roeker LE, Allan JN, et al. Outcomes of front-line ibrutinib treated cll patients excluded from landmark clinical trial. Am J Hematol. 2018;93:1394–401. doi: 10.1002/ajh.25261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mato AR, Timlin C, Ujjani C, et al. Comparable outcomes in chronic lymphocytic leukaemia (cll) patients treated with reduced-dose ibrutinib: results from a multi-centre study. Br J Haematol. 2018;181:259–61. doi: 10.1111/bjh.14540. [DOI] [PubMed] [Google Scholar]
- 25.U.K. CLL Forum. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101:1563–72. doi: 10.3324/haematol.2016.147900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winqvist M, Asklid A, Andersson PO, et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: data from 95 consecutive patients treated in a compassionate use program. A study from the Swedish Chronic Lymphocytic Leukemia Group. Haematologica. 2016;101:1573–80. doi: 10.3324/haematol.2016.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soldin OP, Mattison DR. Sex differences in pharmaco-kinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48:143–57. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters A, Wiernikowski J, Barker S, Mahler M, Luker S, Lodick A. Ibrutinib for chronic lymphocytic leukemia: impact of the Canadian You&I patient support program on treatment adherence [abstract E1022] EHA Library. 2017. 180798. [Available online at: https://library.ehaweb.org/eha/2017/22nd/180798/anthea.peters.ibrutinib.for.chronic.lymphocytic.leukemia.impact.of.the.html; cited 14 August 2019]