Abstract
Background
People with Parkinson disease (PD) frequently experience low back pain (LBP), yet the impact of LBP on functional mobility, physical activity, and quality of life (QOL) has not been described in PD.
Objective
The objectives of this study were to describe body positions and functional activities associated with LBP and to determine the relationships between LBP-related disability and PD motor sign severity, physical activity level, and QOL.
Design
The study was a cross-sectional study.
Methods
Thirty participants with idiopathic PD (mean age = 64.6 years [SD = 10.3]; 15 women) completed the Revised Oswestry Disability Questionnaire (RODQ), a measure of LBP-related disability. PD motor symptom severity was measured using the Movement Disorder Society-Unified Parkinson Disease Rating Scale Part III (MDS-UPRDS III). The Physical Activity Scale for the Elderly (PASE) was used to measure self-reported physical activity. The Parkinson Disease Questionnaire-39 (PDQ-39) was used to measure QOL. Descriptive statistics were used to characterize LBP intensity and LBP-related disability. Spearman correlations were used to determine relationships between the RODQ and the MDS-UPDRS III, PASE, and PDQ-39.
Results
LBP was reported to be of at least moderate intensity by 63.3% of participants. LBP most frequently impaired standing, sleeping, lifting, and walking. The RODQ was significantly related to the MDS-UPDRS III (r = 0.38), PASE (r = −0.37), PDQ-39 summary index (r = 0.55), PDQ-39 mobility subdomain (r = 0.54), and PDQ-39 bodily pain subdomain (r = 0.44).
Limitations
Limitations included a small sample of people with mild to moderate PD severity, the fact that RODQ is a less frequently used measure of LBP-related disability, and the lack of a non-PD control group.
Conclusions
LBP affected walking, sleeping, standing, and lifting in this small sample of people with mild to moderate PD. Greater LBP-related disability was associated with greater motor sign severity, lower physical activity level, and lower QOL in people with PD.
Parkinson disease (PD) is associated with resting tremor, bradykinesia, rigidity, and postural instability.1 These cardinal signs are easily recognized and regularly treated by neurologists. However, secondary conditions are underrecognized and subsequently undertreated. Musculoskeletal pain is commonly reported by people with PD and relates to increasing impairment in quality of life (QOL).2,3 The low back is the most common site for musculoskeletal pain in PD.4 Low back pain (LBP) affects between 43% and 74% of people with PD, making it the most common secondary condition in this population.2,5,6 The prevalence of LBP in PD significantly exceeds that of age-matched controls.5,6
LBP affects a large part of the general population. The Global Burden of Disease Study reported that back pain (low back and neck) ranked highest in terms of disability.7 Approximately 77% of older adults seeking care for back pain experience continued symptoms and disability 12 months later.8 Cedraschi et al noted that older adults with LBP were 2.66 times more likely to report impaired mobility-related QOL than older adults without LBP.9 In 2006, investigators reported that LBP-related expenses in the Medicare population approached $1 billion.10 Further, health care spending on low back and neck pain had the third highest increase from 1996 to 2013, behind treatment for hyperlipidemia and treatment for septicemia.11
Investigations into factors related to LBP in PD are limited. Rigidity relates to pain frequency in PD, but this was not specific to pain in the low back.12 Lumbar spinal alignment weakly relates to subjective pain rating and mobility-related QOL in PD.3 It is unclear if and how LBP affects functional mobility (eg, standing, sitting, walking) in people with PD. Further, the relationship between LBP-related disability and PD motor symptom severity, physical activity level, and QOL in people with PD has not been examined. As such, the purposes of this study were twofold: to describe the effects of LBP on functional mobility in PD and to determine the relationships between LBP-related disability and PD motor signs, physical activity level, and QOL in PD. We hypothesized that in people with PD LBP would most commonly affect standing,13,14 and LBP-related disability would significantly relate to PD motor symptom severity, physical activity level, and QOL.
Methods
Data for this study were collected from baseline evaluations of participants who consented to take part in either a yoga or a gait-cueing study at Washington University in St Louis, Missouri. Participants were included if they had idiopathic PD, determined on the basis of UK Brain Bank criteria.15 Participants were excluded if they had any of the following: a history of neurological deficits aside from PD, deep brain stimulation, a Mini-Mental State Examination16 score of < 24, or an inability to walk 3 m with or without an assistive device. For the purposes of this analysis, participants with a history of spinal surgery were excluded. A power analysis was not conducted for this study because it was an exploratory investigation. All participants provided written informed consent for their respective study in accordance with the Human Research Protection Office at Washington University.
To characterize LBP-related disability in PD, participants completed the Revised Oswestry Disability Questionnaire (RODQ).17 This questionnaire includes 10 questions related to the intensity of, positions and activities associated with, and current status of LBP. The functional activities assessed in the RODQ are sitting, standing, walking, lifting, sleeping, and traveling. Scores on the RODQ range from 0% to 100%, with 0% indicating no LBP-related disability and 100% indicating LBP that severely affects functional mobility. The RODQ has high test-retest reliability.17
Motor sign severity was measured using the Movement Disorder Society-Unified Parkinson Disease Rating Scale Part III (MDS-UPDRS III).18 The MDS-UPDRS III total score ranges from 0 to 132 points, with higher scores indicating greater severity of PD motor manifestations. Within the MDS-UPDRS III, we were interested in the impact of posture and rigidity on LBP-related disability given previous reports of associations between these constructs and pain in PD.3,12 Posture was measured using item 3.13, with a score of 0 indicating no postural impairment and a score of 4 indicating severe postural abnormality. Rigidity (item 3.3) was measured by totaling the appendicular (ie, right and left upper and lower extremities) and axial (ie, neck) scores to produce an aggregate rigidity score, which ranged from 0 (no rigidity) to 20 (severe appendicular and axial rigidity). We also separated appendicular and axial rigidity given previous reports of low back stiffness among older adults with LBP.19 The MDS-UPDRS III was administered by a trained rater while participants were on anti-PD medication, defined as approximately 45 to 90 minutes since their last dose.
Physical activity level was measured using the Physical Activity Scale for the Elderly (PASE).20 The PASE is a reliable and valid measure of physical activity in older adults.20 Via self-report, participants detail their participation in light, moderate, and vigorous physical activities during the previous 7 days. Higher scores indicate greater levels of physical activity.
QOL was measured using the Parkinson Disease Questionnaire-39 (PDQ-39).21 The PDQ-39 consists of the summary index score and 8 subdomain scores to describe QOL. For the purposes of the study, we were interested in the summary index score and the following subdomain scores: activities of daily living, bodily pain, and mobility. The PDQ-39 is a valid measure of QOL in PD.21
Descriptive statistics characterized the sample as well as participant responses on RODQ items related to LBP intensity and the effect of LBP on functional positions and activities. Because all data were ordinal, Spearman correlations were calculated to index the relationships between the RODQ and the MDS-UPDRS III, PASE, and PDQ-39 (α = .05). All statistical analyses were conducted with SPSS version 24.0 (IBM SPSS, Chicago, IL, USA).
Role of the Funding Source
This study was funded by the Central Nervous System Recovery Restoration Grant–Washington University Department of Neurology and HealthSouth, National Institutes of Health (NIH) (NICHD K12 HD055931), and the Washington University Institute of Clinical and Translational Sciences (NIH UL1 TR002345) from the National Center for Advancing Translational Sciences of NIH, American Parkinson Disease Association (APDA) Advanced Research Center for Parkinson Disease at Washington University, Greater St Louis Chapter of the APDA, and Barnes-Jewish Hospital Foundation (Elliot Stein Family Fund and Parkinson Disease Research Fund). The funding sources had no role in designing the study, data collection, analyzing or interpreting results, or disseminating the results. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Results
Fifty-six participants completed baseline testing sessions for their respective studies. Of these, 40 participants reported some degree of LBP during functional activities. Of the 40, there were 10 participants excluded for the following reasons: (1) missing items on 1 or more questionnaires (n = 8), (2) previous lumbar surgery (n = 1), and (3) ambiguity as to whether the participant actually had LBP (n = 1). The final data set included 30 participants (Tab. 1).
Table 1.
Demographic Characteristics of 30 People With PD and Low Back Paina
| Characteristic | Value |
|---|---|
| Age, y | 64.6 (10.3) |
| Sex (no. of women) | 15 |
| BMI | 24.7 (3.6) |
| Years with PD diagnosis | 4.9 (3.8) |
| MDS-UPDRS III | 26.7 (10.9) |
| Hoehn and Yahr stage (no. of participants) | |
| II | 28 |
| III | 2 |
| LEDD (mg) | 824.0 (543.2) |
| RODQ (%)b | 18 (22.5) |
| MDS-UPDRS III postureb | 0 (2) |
| MDS-UPDRS III total rigidityb | 5 (4) |
| MDS-UPDRS III axial rigidityb | 0 (2) |
| PASE | 143.4 (77.1) |
| PDQ-39 summary index | 18.1 (13.5) |
| PDQ-39 mobility subdomain | 14.8 (17.1) |
| PDQ-39 ADL | 14.3 (16.1) |
| PDQ-39 bodily pain subdomain | 34.2 (21.6) |
aValues are reported as mean (SD) unless otherwise indicated. ADL = activities of daily living; BMI = body mass index; LEDD = levodopa equivalent daily dose; MDS-UPDRS III = Movement Disorder Society-Unified Parkinson Disease Rating Scale Part III; PASE = Physical Activity Scale for the Elderly; PD = Parkinson Disease; PDQ-39 = Parkinson Disease Questionnaire-39; RODQ = Revised Oswestry Disability Questionnaire.
bReported as median (interquartile range).
The median RODQ score for the group with LBP was 18% (interquartile range = 22.5). Nineteen of the 30 participants with LBP (63.3%) indicated the pain severity to be of at least moderate intensity, as indicated by a score of at least 2 on section 1 of the RODQ. Key findings from the RODQ are detailed in Table 2.
Table 2.
Key RODQ Findings From 30 People With PD and LBPa
| Activity | Finding |
|---|---|
| Standing | 23 participants (76.7%) indicated LBP with standing |
| 11 of 23 participants (47.8%) indicated they could not stand for >1 h because of LBP | |
| Sitting | Only 6 participants (20%) indicated LBP prevented them from sitting for ≥1 h |
| Walking | 15 participants (50%) indicated some degree of LBP with walking |
| 4 of 15 participants (26.7%) indicated LBP prevented them from walking >1.6 km (>1 mile) | |
| Lifting | 16 participants (53.3%) indicated LBP affected their ability to lift heavy weights off floor |
| Sleeping | 17 participants (56.7%) indicated some LBP with sleeping |
| 7 of 17 participants (41.2%) indicated some reduction in sleep time because of LBP | |
| Traveling | 20 participants (66.7%) indicated LBP affected their travel |
| Only 2 of 20 participants (10%) were compelled to seek other forms of travel because of LBP |
aLBP = low back pain; PD = Parkinson Disease; RODQ = Revised Oswestry Disability Questionnaire.
RODQ correlated with the MDS-UPDRS III, indicating that participants with greater LBP-related disability had greater motor sign severity (Fig. 1A). Further, LBP-related disability increased as standing posture impairments increased (Fig. 1B). Although the MDS-UPDRS III total rigidity score was not significantly associated with LBP-related disability (Fig. 1C), LBP-related disability increased as axial rigidity increased (Fig. 1D). With respect to the relationship between LBP-related disability and physical activity, the RODQ score significantly increased as PASE decreased. These data indicated that participants with greater LBP-related disability had lower levels of self-reported physical activity (Fig. 2). With respect to the relationship between LBP-related disability and QOL, the PDQ-39 summary index, PDQ-39 mobility subdomain, and PDQ-39 bodily pain subdomain were significantly related to the RODQ (Fig. 3). These relationships indicated that as LBP-related disability increased, overall QOL, mobility-related QOL, and bodily-pain–related QOL worsened. The RODQ score was not significantly related to PDQ-39 activities of daily living (Fig. 3).
Figure 1.
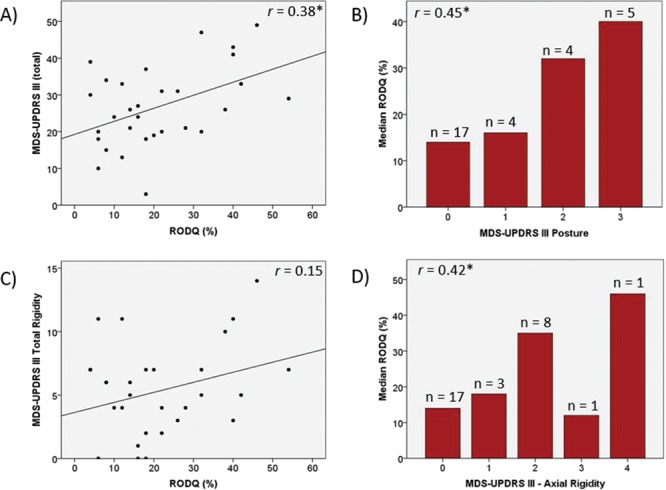
Relationships between Revised Oswestry Disability Questionnaire (RODQ) and Movement Disorder Society-Unified Parkinson Disease Rating Scale Part III (MDS-UPDRS III) (A), MDS-UPDRS III posture (B), MDS-UPDRS III total rigidity (C), and MDS-UPDRS III axial rigidity (D). Single asterisk (*) indicates P < .05.
Figure 2.
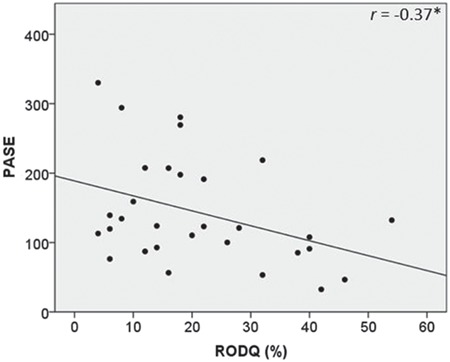
Relationship between Revised Oswestry Disability Questionnaire (RODQ) and Physical Activity Scale for the Elderly (PASE). Single asterisk indicates P < .05.
Figure 3.
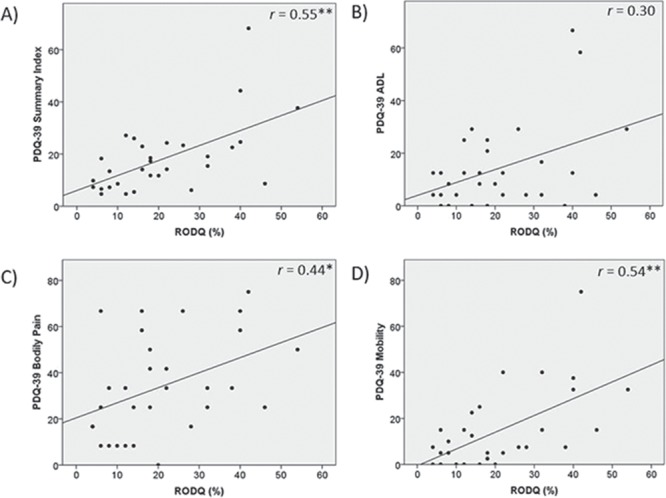
Relationships between Revised Oswestry Disability Questionnaire (RODQ) and Parkinson Disease Questionnaire-39 (PDQ-39) summary index (A), PDQ-39 activities of daily living (ADL) subdomain (B), PDQ-39 bodily pain subdomain (C), and PDQ-39 mobility subdomain (D). *P < .05; **P < .01.
Discussion
There were several key findings in this investigation. First, people with mild to moderate PD motor symptom severity who had concomitant LBP reported low to moderate LBP-related disability. Second, LBP frequently impaired activities such as standing, lifting, and sleeping. Finally, as LBP-related disability increased, people with PD and LBP had greater PD motor symptom severity and reported lower self-reported physical activity levels and QOL.
Given the high prevalence of LBP in people with PD, the relatively low level of self-reported LBP-related disability in our sample was unexpected. Though we did not have a control group in our study, the RODQ disability score in our sample with PD was approximately one-half that reported by Coyle et al for a sample of older adults with LBP but not PD (33.9% on the Modified Low Back Pain Disability Questionnaire).22 Reduced sensory threshold for pain and possibly reduced mechanical perception of pain in people with PD may account for this finding.23 It also should be noted that our sample with PD had a lower average age and body mass index compared with the older adults,22 which limits the ability to accurately compare groups. Further, this sample with PD was very active and had relatively low MDS-UPDRS III scores, reflecting mild PD severity. Further research is needed to directly compare older adults and people with PD to determine whether and how these populations differ in terms of LBP intensity and related disability.
The RODQ scores indicated that LBP negatively affected standing, sleeping, and lifting in this sample. In older adults without PD, LBP relates to transitional movements (eg, sit-to-stand) and functional performance (eg, Timed “Up & Go” Test), suggesting that functional performance worsens as LBP increases.22 These tasks were not measured in the present study and warrant investigation in people with PD and LBP. Our findings are consistent with previous studies in middle-aged adults that suggest standing, sleeping, and lifting are commonly associated with LBP.24–27 Whether the mechanisms underlying the relationships between LBP and these specific functional activities are shared or different between middle-aged adults and people with PD remains unclear. Future work should examine static postures and dynamic movement patterns in people with and without PD with LBP to determine if and how abnormalities contribute to LBP in this population.
This investigation extends previous findings by demonstrating associations between 2 common PD symptoms (ie, postural deficits and axial rigidity) and LBP-related disability. The increase in LBP-related disability as postural deficits increased was expected given previous reports of a relationship between LBP intensity and lumbar posture in people with PD and LBP.3 Our findings demonstrated that postural deficits in people with PD may relate to LBP that impairs performance of daily activities. We also noted that axial rigidity relates to LBP-related disability in PD. Allen et al reported total rigidity (ie, appendicular plus axial) related to pain (but not specifically LBP) frequency and pain that interfered with work.12 Sions and Hicks reported that back stiffness was related to physical health and LBP-related disability in older adults.19 In agreement with this report, our findings suggest that axial rigidity may contribute to LBP-related disability in people with PD. However, because we used the neck rigidity rating on the MDS-UPDRS III as our measure of axial rigidity and did not directly measure low back stiffness, the results should be interpreted with caution.
LBP-related disability significantly related to MDS-UPDRS III scores such that people with greater motor sign severity had a greater degree of LBP-related disability. This is not surprising given that rigidity and postural deficits relate to pain in PD3,12 and tend to worsen over time. Interestingly, our participants had relatively mild motor symptoms yet still reported a low to moderate degree of LBP-related disability, as indicated by the RODQ scores in this study. These data suggest that neurologists and health care professionals should inquire about LBP in patients with PD because it may have a greater effect on function than PD symptoms, particularly early in the course of the disease. We suggest that greater LBP-related disability relates to reduced physical activity levels. This is important for 2 reasons. First, reduced physical activity implies greater time spent in sedentary positions, which may increase the risk of LBP.28 Additionally, reduced physical activity in PD may worsen gait difficulty, postural instability, and QOL.29 Furthermore, increasing leisure-time physical activity may reduce LBP.30
Finally, greater LBP-related disability related to reduced overall QOL in people with PD and LBP. More specifically, lower mobility-related and bodily pain-related QOL was associated with greater LBP-related disability. Watanabe et al reported a significant relationship between LBP intensity and mobility-related QOL.3 It is possible that the relationship between LBP-related disability and bodily pain-related QOL was not as strong because 1 of the 3 questions in the bodily pain subdomain of the PDQ-39 inquires about unpleasant hot or cold feelings. These feelings may relate more to central pain (ie, poorly localized pain, not limited to dermatome or neural distribution31) rather than pain of musculoskeletal origin. However, this claim requires confirmation in future studies because we did not categorize participants by pain type (eg, musculoskeletal vs dystonic vs central).
This study has several limitations. First, the sample size was small and included mainly people with mild PD motor signs. This limits the generalizability of the study findings and suggests caution in interpretation of the correlation coefficients. Investigators should attempt to reproduce the study findings in a larger sample of people with PD motor symptom severity that ranges from mild to severe. Second, because participants consented to take part in an exercise study, people with more severe LBP may have been unwilling or unable to participate, thereby biasing the sample. A cross-sectional design with the specific purpose of examining LBP-related disability in PD should be utilized to recruit a sample with a wide range of PD and LBP severity. Third, the use of the RODQ is less common than the Modified Low Back Pain Disability Questionnaire32,33 (formerly known as the Modified Oswestry Low Back Pain Disability Questionnaire) and not widely validated. However, given the preliminary nature of this study, the RODQ was likely sufficient to determine LBP-related disability in PD. Investigators should validate use of a LBP-specific questionnaire in PD to allow for standardization in future studies. Fourth, we did not measure LBP duration, and LBP intensity was measured by the first question of the RODQ rather than more commonly used scales such as a visual analog scale or a numeric rating scale. Finally, because this was a cross-sectional study, we are unable to suggest factors that may cause LBP in people with PD. Longitudinal studies of people with PD and without LBP are necessary to investigate factors predictive of the subsequent development of LBP.
Conclusion
People with mild PD reported low to moderate LBP-related disability. LBP-related disability increased as axial rigidity and postural deficits worsened. LBP-related disability was associated with increased PD motor symptom severity, decreased self-reported physical activity, and reduced QOL in people with PD. Given the potential negative impact of LBP in this population, it is imperative that health care professionals identify and treat LBP in people with PD.
Author Contributions and Acknowledgments
Concept/idea/research design: R.P. Duncan, L.R. Van Dillen, J.M. Garbutt, J.S. Perlmutter
Writing: R.P. Duncan, L.R. Van Dillen, J.M. Garbutt, G.M. Earhart, J.S. Perlmutter
Data analysis: R.P. Duncan, L.R. Van Dillen, J.S. Perlmutter
Fund procurement: R.P. Duncan, G.M. Earhart
Providing participants: J.S. Perlmutter
Providing facilities/equipment: G.M. Earhart
Consultation (including review of manuscript before submitting): L.R. Van Dillen, J.M. Garbutt, G.M. Earhart, J.S. Perlmutter
The authors thank Peter Myers, Elinor Harrison, Adam Horin, Marie McNeely, and Ellen Sutter for their assistance with data collection.
Ethics Approval
All participants provided written informed consent for their respective study in accordance with the Human Research Protection Office at Washington University.
Funding
This study was funded by the Central Nervous System Recovery Restoration Grant–Washington University Department of Neurology and HealthSouth, National Institutes of Health (NIH) (NICHD K12 HD055931), and the Washington University Institute of Clinical and Translational Sciences (NIH UL1 TR002345) from the National Center for Advancing Translational Sciences of NIH, American Parkinson Disease Association (APDA) Advanced Research Center for Parkinson Disease at Washington University, Greater St Louis Chapter of the APDA, and Barnes-Jewish Hospital Foundation (Elliot Stein Family Fund and Parkinson Disease Research Fund). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Disclosures and Presentations
The authors completed the ICJME Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
A power analysis was not conducted for this study because this was an exploratory investigation.
These data were presented as part of a platform presentation for the Academy of Neurologic Physical Therapy at the Combined Sections Meeting of the American Physical Therapy Association; February 21–24, 2018; New Orleans, Louisiana.
References
- 1. Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. [DOI] [PubMed] [Google Scholar]
- 2. Kim YE, Jeon BS. Musculoskeletal problems in Parkinson's disease. J Neural Transm (Vienna). 2013;120:537–542. [DOI] [PubMed] [Google Scholar]
- 3. Watanabe K, Hirano T, Katsumi K, et al. Characteristics and exacerbating factors of chronic low back pain in Parkinson's disease. Int Orthop. 2015;39:2433–2438. [DOI] [PubMed] [Google Scholar]
- 4. Young Blood MR, Ferro MM, Munhoz RP, Teive HA, Camargo CH. Classification and characteristics of pain associated with Parkinson's disease. Parkinsons Dis. 2016;2016:6067132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Etchepare F, Rozenberg S, Mirault T, et al. Back problems in Parkinson's disease: an underestimated problem. Joint Bone Spine. 2006;73:298–302. [DOI] [PubMed] [Google Scholar]
- 6. Broetz D, Eichner M, Gasser T, Weller M, Steinbach JP. Radicular and nonradicular back pain in Parkinson's disease: a controlled study. Mov Disord. 2007;22:853–856. [DOI] [PubMed] [Google Scholar]
- 7. Global Burden of Disease Study C Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet .2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rundell SD, Sherman KJ, Heagerty PJ, Mock CN, Jarvik JG. The clinical course of pain and function in older adults with a new primary care visit for back pain. J Am Geriatr Soc. 2015;63:524–530. [DOI] [PubMed] [Google Scholar]
- 9. Cedraschi C, Luthy C, Allaz AF, Herrmann FR, Ludwig C.. Low back pain and health-related quality of life in community-dwelling older adults. Eur Spine J. 2016;25:2822–2832. [DOI] [PubMed] [Google Scholar]
- 10. Weiner DK, Kim YS, Bonino P, Wang T. Low back pain in older adults: are we utilizing healthcare resources wisely? Pain Med. 2006;7:143–150. [DOI] [PubMed] [Google Scholar]
- 11. Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996-2013. JAMA. 2016;316:2627–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allen NE, Wong CM, Canning CG, Moloney N. The association between Parkinson's disease motor impairments and pain. Pain Med. 2016;17:456–462. [DOI] [PubMed] [Google Scholar]
- 13. Gregory DE, Callaghan JP. Prolonged standing as a precursor for the development of low back discomfort: an investigation of possible mechanisms. Gait Posture. 2008;28:86–92. [DOI] [PubMed] [Google Scholar]
- 14. Nelson-Wong E, Callaghan JP. Transient low back pain development during standing predicts future clinical low back pain in previously asymptomatic individuals. Spine (Phila Pa 1976). 2014;39:E379–383. [DOI] [PubMed] [Google Scholar]
- 15. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Folstein MF, Robins LN, Helzer JE. The mini-mental state examination. Arch Gen Psychiatry. 1983;40:812. [DOI] [PubMed] [Google Scholar]
- 17. Hudson-Cook N, Tomes-Nicholson K, Breen A. A. Revised Oswestry Disability Questionnaire In: Roland MO, Jenner JR, eds. Back Pain: New Approaches to Rehabilitation and Education .New York, NY: Manchester University Press; 1989. [Google Scholar]
- 18. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 19. Sions JM, Hicks GE. Back stiffness is associated with physical health and low Back pain-related disability in community-dwelling older adults. Pain Med. 2017;18:866–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. [DOI] [PubMed] [Google Scholar]
- 21. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson's disease questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing. 1997;26:353–357. [DOI] [PubMed] [Google Scholar]
- 22. Coyle PC, Velasco T, Sions JM, Hicks GE. Lumbar mobility and performance-based function: an investigation in older adults with and without chronic low back pain. Pain Med. 2017;18:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nolano M, Provitera V, Estraneo A, et al. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain. 2008;131:1903–1911. [DOI] [PubMed] [Google Scholar]
- 24. Andersen JH, Haahr JP, Frost P. Risk factors for more severe regional musculoskeletal symptoms: a two-year prospective study of a general working population. Arthritis Rheum. 2007;56:1355–1364. [DOI] [PubMed] [Google Scholar]
- 25. Sorensen CJ, Johnson MB, Callaghan JP, George SZ, Van Dillen LR. Validity of a paradigm for low back pain symptom development during prolonged standing. Clin J Pain. 2015;31:652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kelly GA, Blake C, Power CK, O'Keeffe D, Fullen BM. The association between chronic low back pain and sleep: a systematic review. Clin J Pain. 2011;27:169–181. [DOI] [PubMed] [Google Scholar]
- 27. Coenen P, Gouttebarge V, Burght AS, et al. The effect of lifting during work on low back pain: a health impact assessment based on a meta-analysis. Occup Environ Med. 2014;71:871–877. [DOI] [PubMed] [Google Scholar]
- 28. Heneweer H, Vanhees L, Picavet HS. Physical activity and low back pain: a U-shaped relation? Pain. 2009;143:21–25. [DOI] [PubMed] [Google Scholar]
- 29. Bloem BR, Steijns JA, Smits-Engelsman BC. An update on falls. Curr Opin Neurol. 2003;16:15–26. [DOI] [PubMed] [Google Scholar]
- 30. Shiri R, Falah-Hassani K. Does leisure time physical activity protect against low back pain? Systematic review and meta-analysis of 36 prospective cohort studies. Br J Sports Med. 2017;51:1410–1418. [DOI] [PubMed] [Google Scholar]
- 31. Beiske AG, Loge JH, Ronningen A, Svensson E.. Pain in Parkinson's disease: prevalence and characteristics. Pain. 2009;141:173–177. [DOI] [PubMed] [Google Scholar]
- 32. Fritz JM, Irrgang JJ. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys Ther .2001;81:776–788, erratum 2008;88:138–139. [DOI] [PubMed] [Google Scholar]
- 33. Fritz JM, Irrgang JJ. Correction: a comparison of a modified Oswestry low back pain disability questionnaire and the Quebec Back Pain Disability Scale. Phys Ther. 2001;81:776–788. Phys Ther.2008;88: 138–9. [DOI] [PubMed] [Google Scholar]


