Abstract
The genetic basis of inflammatory bowel disease remains to be elucidated completely. Here we report on a patient with inflammatory bowel disease who has mosaic tetrasomy of the short arm of chromosome 9, a genomic region that harbours the type I interferon gene cluster. We show that increased interferon activation is present in peripheral blood and intestinal tissue from this patient, similar to previous reports of autoinflammatory organ damage driven by interferon activation in other patients with this chromosomal abnormality. To our knowledge, this is the first case of tetrasomy 9p-associated interferonopathy driving intestinal inflammation and highlights the role that type-I interferon pathways can play in the pathogenesis of intestinal inflammation.
Keywords: Tetrasomy 9p, inflammatory bowel disease, interferonopathy
1. Introduction
Tetrasomy 9p [T9p] is a rare aneuploidy that results from a supernumerary chromosome generated from two copies of the short arm of chromosome 9. It typically occurs in mosaic form and has variable phenotypic expression but is frequently associated with intellectual disability, low-set ears and ophthalmological abnormalities; occasionally, cardiac or genitourinary alterations are also seen.1 Rarely, no obvious phenotype is observed.2–4 Interestingly, the short arm of chromosome 9 hosts 17 type I interferon [IFN] genes [type I IFN gene cluster]. Two reports have linked copy number variations of this gene cluster with IFN-driven pathology, including systemic lupus erythematosus [SLE] and inflammatory myositis, suggesting a gene dosage effect as a pathogenic mechanism driving interferonopathy and clinical presentation.5,6 However, no association exists between this karyotypic abnormality and intestinal inflammation.
2. Case report
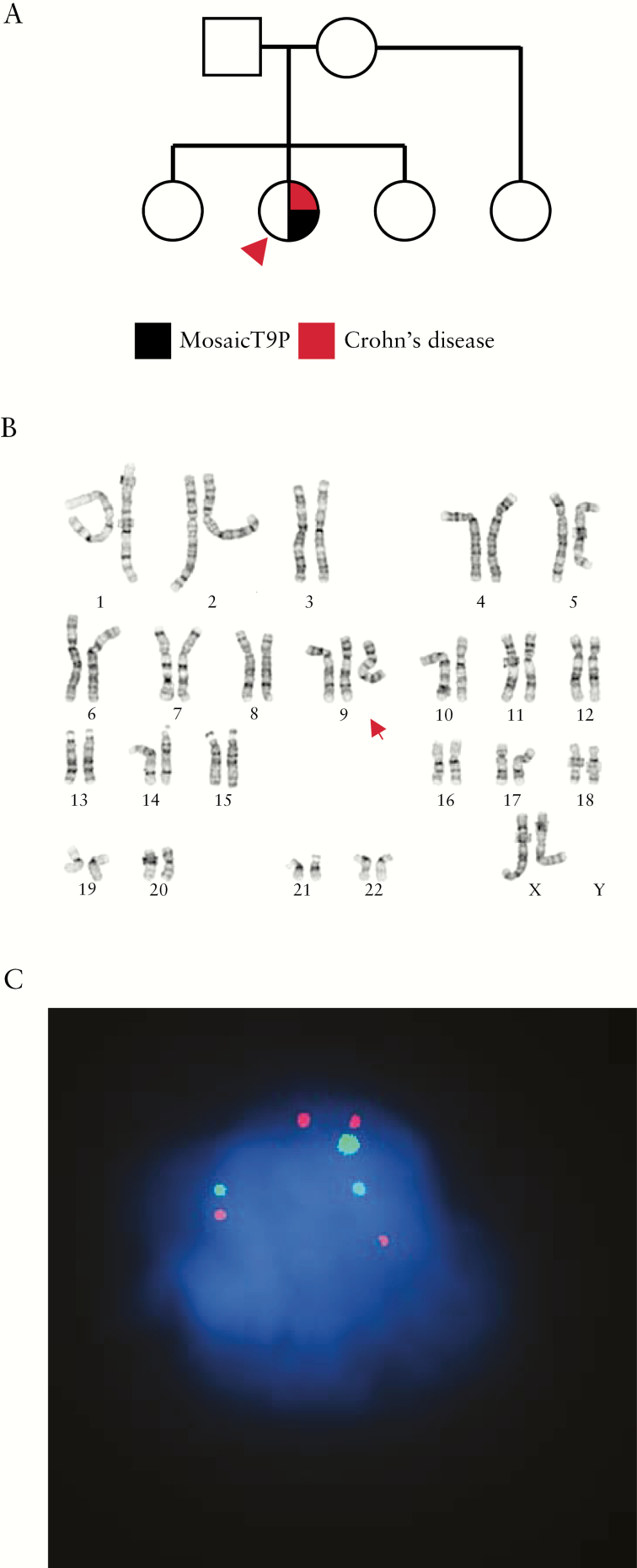
We report on a previously healthy 12-year-old Caucasian female with no significant family history [Figure 1A] who presented with multiple symptoms suggestive of autoinflammation, including inflammatory bowel disease [IBD]. She presented at age 8 years with a 2-week history of persistent fever, diffuse abdominal pain, lower extremity arthralgias and bilateral lower extremity rash. She had no dysmorphic features, and normal psychomotor and intellectual development. Laboratory investigation was remarkable for elevated inflammatory markers (C-reactive protein [CRP] 18.3 mg/dL, erythrocyte sedimentation rate 67 mm/hr), hypoalbuminaemia [2.4 g/dL] and leukocytosis [20.2 × 103/mm]. These features triggered an immunological evaluation, which was largely unrevealing [Supplementary Table 1]. Abdominal computed tomography imaging revealed thickening of the gastric wall, prompting upper endoscopic evaluation. Findings were significant for thickened gastric folds and mucosal changes suggestive of gastritis, as well as duodenitis. Biopsy specimens demonstrated chronic active gastritis with marked foveolar hyperplasia and significant active duodenal inflammation. Bone marrow and later peripheral blood karyotype indicated that the patient has mosaic tetrasomy of the short arm of chromosome 9 [Figure 1B], with generation of a pseudo-dicentric chromosome [mos 47,XX,+psu-idic (9)(q13)/46,XX]. These cytogenetic findings were confirmed by buccal swab fluorescence in situ hybridization staining using a 9p-specific probe [Figure 1C]. The level of mosaicism ranged from 65–70% in circulating leukocytes and bone marrow cells to 51% in buccal swab.
Figure 1.
General clinical characteristics of the patient. [A] Family pedigree. Proband with mosaic tetrasomy 9p [T9p] is shown [arrowhead]. [B] Abnormal mosaic female chromosome analysis of peripheral blood with one cell line containing a pseudo dicentric chromosome 9 [red arrow] in 13 of 20 cells examined. [C] Buccal swab p16 fluorescence in situ hybridization analysis showing three loci corresponding to the centromeric region of chromosome 9 [green] and four signals corresponding to its short arm [red] in 50% of cells examined [n = 167].
Although vasculitis was not proven histologically, treatment with steroids was initiated for presumed atypical Henoch-Schönlein purpura. She was also given proton pump inhibitors. Her symptoms improved moderately for a brief period but she was readmitted 6 months later for persistent abdominal pain and arthralgias. Although there were no obvious small bowel changes on magnetic resonance enterography, endoscopic evaluation revealed acute ileitis and persistent gastritis. Laboratory testing at the time was consistent with active intestinal inflammation [stool calprotectin 744 µg/g, CRP 7.12 mg/dL], and her symptoms improved on intravenous steroids.
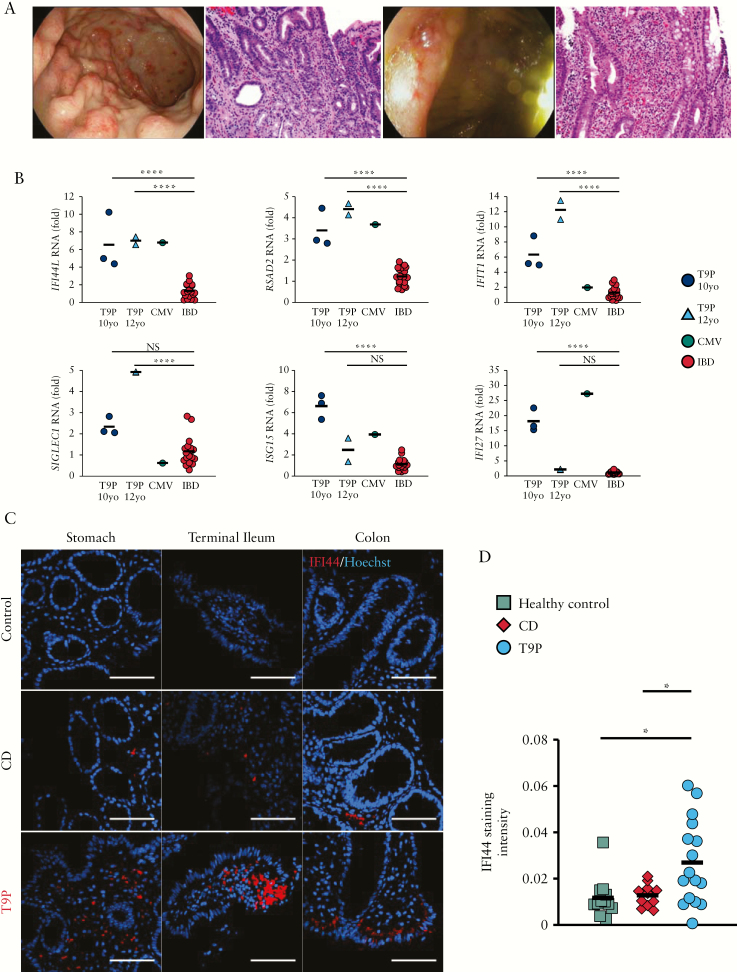
She was discharged on a tapered oral steroid regimen, but her symptoms showed steroid dependency and she was then started on azathioprine [2 mg/kg]. She was readmitted 8 months later for worsening abdominal pain and significant elevation in inflammatory markers [CRP 26.2 mg/dL]. Endoscopic evaluation showed persistent diffuse gastric erythema and scattered superficial ulcerations in the colon and terminal ileum. Histological findings of chronic active gastritis and foveolar hyperplasia involving the body of the stomach, active ileitis and colitis with cryptitis and crypt abscesses [but no architectural distortion] were demonstrated [Figure 2A]. The constellation of symptoms and findings prompted a diagnosis of early-onset IBD, with features resembling Crohn’s disease [CD]. Adjustment of the azathioprine dose based on therapeutic drug monitoring eventually resulted in symptom control.
Figure 2.
General findings. [A] Endoscopic images and corresponding histology. Stomach [left], ileocecal valve [right]. The gastric biopsy shows severe active chronic gastritis of the antral mucosa with increased lamina propria chronic inflammation [negative cytomegalovirus immunohistochemistry, not shown]. The ileal biopsy shows moderate active ileitis, including moderate cryptitis, crypt abscess, mild villous blunting and increased lamina propria chronic inflammation [histology, 200× magnification]. [B] Interferon stimulated gene expression levels measured by quantitative reverse transcriptase PCR in whole blood-derived RNA from proband [n = 1] at two time points (10 and 12 years old [yo]), cytomegalovirus [CMV] colitis [n = 1] and inflammatory bowel [IBD] disease controls (ulcerative colitis [UC] = 6, Crohn’s disease [CD] = 3). All IBD patients had active disease at the time of sample collection. Fold rates of transcript abundance are displayed compared to the same reference control samples. ****p < 0.0001 [unpaired Student’s t-test, comparisons are Bonferroni corrected]. [C] IFI44 [red] immunofluorescence staining in intestinal sections from tetrasomy 9p [T9p] proband, a healthy control and a patient with active ilieocolonic CD [scale bar = 100 µm]. [D] Aggregate quantification of the IFI44 staining intensity from stomach, terminal ileum and colon shown in C. *p < 0.05 [unpaired Student’s t-test].
Because the short arm of chromosome 9 harbours the type I IFN gene cluster, we examined whether the patient had evidence of increased expression of IFN-stimulated genes [ISGs] in peripheral leukocytes. Expression of six ISGs in circulating leukocytes showed significant elevations [p < 0.0001] in all transcripts when compared against those seen in paediatric patients with active IBD receiving immunosuppressive therapy and comparable to those seen in intestinal inflammation secondary to viral infection – a known inducer of IFN stimulation [Figure 2B]. This gene signature has been previously validated as the most sensitive biomarker for type I IFN induction.7–9 These results were consistent over time [at 10 and 12 years of age] and persisted in spite of azathioprine treatment at the later age tested. This agrees with previously observed cases with T9p and SLE.5,6 To examine whether the patient’s intestinal inflammation was associated with increased ISG expression in the affected tissues, we performed immunofluorescence staining for IFI44, an ISG whose expression is significantly induced by known triggers of type I IFN.10 Biopsy samples demonstrated IFI44-positive cells throughout the examined gastrointestinal tract [Figure 2C] and quantification of the IFI44 signal in our patient was increased compared to a healthy control without active intestinal inflammation and a patient with active ileocolonic CD (Figure 2D; statistical significance [p < 0.05] was achieved after aggregating the data at all three anatomical sites). Thus, gastrointestinal inflammation in this patient was associated with increased local expression of IFN-responsive genes, suggesting that type I IFN expression is probably responsible for the enteropathy in this case.
3. Discussion
Type I interferonopathies are a group of monogenic disorders that result in overactivation of type I IFN signalling.11 Inappropriate activation of this antiviral response results in systemic signs of autoinflammation and varying degrees of autoimmunity. While there is ample phenotypic heterogeneity within this group of disorders, all of them share a hallmark ISG signature,12 as shown also in this case. The most studied clinical entity in this category, Aicardi-Goutieres syndrome, is characterized by severe brain inflammation in infancy, resulting in brain calcifications, serious neurological deficits and seizures. Serological features that overlap with SLE are well known in these patients. However, other syndromes in this broad disease category can include diverse clinical manifestations, including pulmonary involvement. Enteropathy, including Crohn’s-like jejunal involvement and protein-losing enteropathy, has been described in X-linked pigmentary reticulate disorder, an interferonopathy syndrome caused by mutations in POLA1.8
The development of an interferonopathy as a result of T9p has been previously reported,6 and the clinical presentation included myositis and features of SLE. It is thought that this is the result of a gene dosage effect, whereby the tetrasomy leads to a doubling of the number of type I IFN genes. The involvement of type I IFN in the pathogenesis of autoinflammatory manifestations in patients with T9p raises the possibility that targeted therapy with JAK inhibitors, such as tofacitinib, could provide clinical benefit. Indeed, this agent has already shown effectiveness in other IFN-mediated diseases, such as rheumatoid arthritis,13 as well as monogenic interferonopathies.14,15 The mechanism by which azathioprine may be playing a role in controlling this patient’s disease is unclear, but we speculate that the reported effects of this drug on RAC1 may impair IFN production by natural killer cells,16 as has been suggested in CD patients,17 and also as seen in individuals with DOCK2 deficiency.18 Interestingly, while certain interferonopathies have been associated with gastrointestinal inflammation as mentioned above, its occurrence in T9p has not previously been reported. While we speculate that the increased type-I IFN gene dosage that results from the tetrasomy is responsible for this phenotype, we cannot rule out that additional genes contained in the tetrasomy region may play a role in the clinical manifestations observed in this patient. In conclusion, this report supports the idea that pathological activation of the type-I IFN pathway can result in gastrointestinal inflammation in defined genetic disorders and is in line with the identification in a genome-wide association study of risk alleles for IBD that map to this pathway.19
Funding
This work was supported by the NIH: R01DK073639, R56AI113274 to E.B. and 5 K12 HD-068369-05 to L.S-D. This work was also supported by Children’s Health Clinical Research Advisory Committee: CCRAC [195] to L.S-D.
Conflict of Interest
The authors declare no personal, professional or financial conflicts of interest.
Supplementary Material
Acknowledgments
We thank our patients and their families for agreeing to participate in this study. We also thank John Shelton and the staff of the UT Southwestern Molecular Pathology Core, as well as the histology department at Dallas Children’s Medical Center for their assistance with the immunostaining analysis.
Author Contributions
L.S-D. gathered clinical information, and performed most of the molecular and immunofluorescence experiments; P.S. and J.W. performed expression analyses. B.G. gathered clinical data; P.K. performed all genetic analyses. J.Y.P. performed histological analyses. E.B. oversaw molecular and biochemical studies. L.S-D. and E.B. wrote the manuscript.
References
- 1. El Khattabi L, Jaillard S, Andrieux J, et al. Clinical and molecular delineation of tetrasomy 9p syndrome: report of 12 new cases and literature review. Am J Med Genet A 2015;167:1252–61. [DOI] [PubMed] [Google Scholar]
- 2. Baronchelli S, Conconi D, Panzeri E, et al. Cytogenetics of premature ovarian failure: an investigation on 269 affected women. J Biomed Biotechnol 2011;2011:370195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McAuliffe F, Winsor EJ, Chitayat D. Tetrasomy 9p mosaicism associated with a normal phenotype. Fetal Diagn Ther 2005;20:219–22. [DOI] [PubMed] [Google Scholar]
- 4. Papoulidis I, Kontodiou M, Tzimina M, et al. Tetrasomy 9p mosaicism associated with a normal phenotype in two cases. Cytogenet Genome Res 2012;136:237–41. [DOI] [PubMed] [Google Scholar]
- 5. Zhuang H, Kosboth M, Lee P, et al. Lupus-like disease and high interferon levels corresponding to trisomy of the type I interferon cluster on chromosome 9p. Arthritis Rheum 2006;54:1573–9. [DOI] [PubMed] [Google Scholar]
- 6. Frémond ML, Gitiaux C, Bonnet D, et al. Mosaic tetrasomy 9p: a mendelian condition associated with pediatric-onset overlap myositis. Pediatrics 2015;136:e544–7. [DOI] [PubMed] [Google Scholar]
- 7. Rice GI, Kasher PR, Forte GM, et al. Mutations in ADAR1 cause Aicardi-Goutières syndrome associated with a type I interferon signature. Nat Genet 2012;44:1243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Starokadomskyy P, Gemelli T, Rios JJ, et al. DNA polymerase-α regulates the activation of type I interferons through cytosolic RNA:DNA synthesis. Nat Immunol 2016;17:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rice GI, Melki I, Frémond ML, et al. Assessment of type I interferon signaling in pediatric inflammatory disease. J Clin Immunol 2017;37:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kitamura A, Takahashi K, Okajima A, Kitamura N. Induction of the human gene for p44, a hepatitis-C-associated microtubular aggregate protein, by interferon-alpha/beta. Eur J Biochem 1994;224:877–83. [DOI] [PubMed] [Google Scholar]
- 11. Crow YJ. Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci 2011;1238:91–8. [DOI] [PubMed] [Google Scholar]
- 12. Crow YJ, Chase DS, Lowenstein Schmidt J, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A 2015;167A:296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fleischmann R, Kremer J, Cush J, et al. ; ORAL Solo Investigators Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 14. Frémond ML, Rodero MP, Jeremiah N, et al. Efficacy of the Janus kinase ½ inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in 3 children. J Allergy Clin Immunol 2016;138:1752–5. [DOI] [PubMed] [Google Scholar]
- 15. König N, Fiehn C, Wolf C, et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann Rheum Dis 2017;76:468–72. [DOI] [PubMed] [Google Scholar]
- 16. Shin JY, Wey M, Umutesi HG, Sun X, Simecka J, Heo J. Thiopurine prodrugs mediate immunosuppressive effects by interfering with Rac1 protein function. J Biol Chem 2016;291:13699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yusung S, McGovern D, Lin L, Hommes D, Lagishetty V, Braun J. NK cells are biologic and biochemical targets of 6-mercaptopurine in Crohn’s disease patients. Clin Immunol 2017;175:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dobbs K, Domínguez Conde C, Zhang SY, et al. Inherited DOCK2 deficiency in patients with early-onset invasive infections. N Engl J Med 2015;372:2409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jostins L, Ripke S, Weersma RK, et al. ; International IBD Genetics Consortium (IIBDGC) Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.