Abstract
Background
The population effects of influenza vaccination in children have not been extensively studied, especially in tropical, developing countries. In rural Senegal, we assessed the total (primary objective) and indirect effectiveness of a trivalent inactivated influenza vaccine (IIV3).
Methods
In this double-blind, cluster-randomized trial, villages were randomly allocated (1:1) for the high-coverage vaccination of children aged 6 months through 10 years with either the 2008–09 northern hemisphere IIV3 or an inactivated polio vaccine (IPV). Vaccinees were monitored for serious adverse events. All village residents, vaccinated and unvaccinated, were monitored for signs and symptoms of influenza illness using weekly home visits and surveillance in designated clinics. The primary outcome was all laboratory-confirmed symptomatic influenza.
Results
Between 23 May and 11 July 2009, 20 villages were randomized, and 66.5% of age-eligible children were enrolled (3918 in IIV3 villages and 3848 in IPV villages). Follow-up continued until 28 May 2010. There were 4 unrelated serious adverse events identified. Among vaccinees, the total effectiveness against illness caused by the seasonal influenza virus (presumed to all be drifted A/H3N2, based on antigenic characterization data) circulating at high rates among children was 43.6% (95% confidence interval [CI] 18.6–60.9%). The indirect effectiveness against seasonal A/H3N2 was 15.4% (95% CI -22.0 to 41.3%). The total effectiveness against illness caused by the pandemic influenza virus (A/H1N1pdm09) was -52.1% (95% CI -177.2 to 16.6%).
Conclusions
IIV3 provided statistically significant, moderate protection to children in Senegal against circulating, pre-2010 seasonal influenza strains, but not against A/H1N1pdm09, which was not included in the vaccine. No indirect effects were measured. Further study in low-resource populations is warranted.
Clinical Trials Registration
Keywords: randomized controlled trial, human influenza, influenza vaccines, developing countries, herd immunity
A cluster-randomized trial among Senegalese children showed 43.6% total effectiveness of the inactivated influenza vaccine against drifted H3N2 seasonal influenza, but no effect against pandemic H1N1 2009, a new strain absent from the vaccine. No significant indirect effects were measured.
Influenza, an acute, highly communicable viral respiratory disease, affects persons of all ages. In low- and middle-income countries, the prevention and control of influenza may be particularly challenging, because circulation may not conform to the traditional northern and southern hemisphere seasons for which the vaccine is formulated [1–4] and because funding mechanisms are lacking [5, 6].
In low- and middle-income countries, focusing influenza vaccination efforts on children may provide individual protection to children and decrease the chances of the children spreading influenza to close contacts, thereby reducing the overall burden of influenza in the community [7, 8]. To evaluate the effectiveness of vaccinating children in a low-resource, tropical setting, we initiated this Phase IV, cluster-randomized trial of the trivalent inactivated influenza vaccine (IIV3) in Senegal, where there were no national recommendations for routine influenza vaccination. The primary objective was to estimate the total (direct plus indirect) effectiveness of IIV3 in reducing the rate of laboratory-confirmed symptomatic influenza among vaccinated children in villages with IIV3 campaigns, compared to control-vaccinated children in comparator villages [9]. An important secondary objective, which determined the study design, was to evaluate the indirect effects in the community after vaccinating children.
METHODS
Study Design
This was a double-blind, cluster-randomized trial with a control vaccine (inactivated polio vaccine [IPV]). The trial was conducted in 20 geographically contiguous villages in the Niakhar Demographic Surveillance System (DSS) [10]. Vaccinations of children were planned for June, prior to the anticipated influenza season [11]. Participants were then monitored for the occurrence of signs and symptoms of influenza, using active and enhanced, passive surveillance both from July through May of the following year.
Ethics approval was obtained from the National Ethics Committee for Health Research (Senegal Ministry of Health and Social Welfare) and Western Institutional Review Board. The study was conducted in accordance with the principles of the Declaration of Helsinki (2008) and in compliance with Good Clinical Practice guidelines.
Participants
Healthy children 6 months through 10 years of age were eligible for study vaccination if a parent’s primary residence was the Niakhar DSS, the child’s family was not expecting to migrate out of the area during the study period, and a parent was willing to provide written informed consent [12]. The exclusion criteria included hypersensitivity to any component in either IIV3 or IPV, hypersensitivity after previous administration of any influenza or polio vaccine, and fever (≥38°C axillary) [13].
Because secondary objectives (eg, overall and indirect effectiveness) required assessing outcomes among the entire population in the 20 villages in the trial, regardless of vaccination status and age, informed consent for the collection of clinical data and respiratory specimens for any person identified with signs and symptoms of influenza was obtained using a separate informed consent process.
Randomization and Masking
Villages were randomly allocated (1:1) to receive IIV3 or IPV campaigns. A lack of data on influenza attack rates in the population meant village randomization could not be based on such data. Randomization was stratified, first according to which side of a flood-prone zone each village was located, and then according to the presence of a weekly market. All possible enumerations of allocation of 10 villages to 1 arm and 10 to the other were computer-generated by blinded biostatisticians. The 5600 possible randomization schemes were then constrained [14] to only those 495 where the total population, mean inter-compound distance, and mean inter-village distance for the intervention groups were within 5%. A random number generator was then used to choose 1 scheme and to assign 1 arm to the IIV3 campaign and 1 arm to the IPV campaign.
Vaccines were provided in identical, prefilled syringes with commercial labels, but the labels were masked in Dakar using pre-printed, coded stickers before delivery to the Niakhar Field Station. Blinded nurses were hired from Dakar to conduct the vaccinations and only worked on the study during the vaccination period. Except as noted above, other study personnel conducting follow-up activities (ie, clinical data collection, specimen collection and testing, monitoring, data management, and statistical analyses) remained blinded throughout the study.
Procedures
The study products were IIV3 of the 2008–2009 northern hemisphere formulation (Vaxigrip, Sanofi Pasteur, Lyon, France; lots D5813 and D9672), containing A/Brisbane/59/2007 (H1N1)-like, A/Brisbane/10/2007 (H3N2)-like, and B/Florida/04/2006 (Yamagata lineage)-like strains, and IPV (IMOVAX Polio, Sanofi Pasteur, Lyon, France; lot B0283).
Children 6 months through 8 years of age received 2 doses of IIV3 or IPV intramuscularly, with 1 dose at enrollment and another dose 1 month later. Children 9 and 10 years of age received 1 dose of IIV3 or IPV at enrollment only.
Upon the completion of the vaccinations, surveillance was initiated among the entire population and standardized criteria were used to identify participants with signs and symptoms of influenza. Trained field workers visited every village compound weekly to query all residents for the recent onset of symptoms (active surveillance). Study physicians staffing the 3 health posts within the Niakhar DSS monitored patients for the recent onset of illness (enhanced, passive surveillance). For each ill subject, trained medical technicians collected a pooled nasal and throat swab specimen. Serious adverse events (SAEs) occurring among vaccinees within 1 month of each dose were documented by field workers and study physicians. The sponsor provided funds to cover the costs of basic medical care for acute respiratory infections in all residents.
Outcomes
The primary outcome was laboratory-confirmed symptomatic influenza caused by any influenza type/subtype contained in IIV3, regardless of match. Symptomatic influenza was defined as follows: (1) among children under 2 years of age, the sudden onset of fever (>37.5°C axillary) or subjective (parent-reported) feverishness, plus at least 1 other symptom (cough, sore throat, nasal congestion, rhinorrhea, or difficulty breathing), and (2) among individuals 2 years and older, the sudden onset of fever (>37.5°C axillary) or subjective (parent- or participant-reported) feverishness, plus either a cough or sore throat. Laboratory confirmation was defined as the detection of the influenza virus (type and subtype identified) in a swab specimen collected during the clinical episode. Specimens were tested at Senegal’s National Influenza Center for the presence of the influenza virus by real-time reverse transcription polymerase chain reaction (rRT-PCR). The antigenic characterization of a subset of influenza positive specimens was conducted at the Centers for Disease Control and Prevention.
Safety endpoints included SAEs occurring during the first month after each vaccination. All SAE reports were reviewed by sponsor physicians and an Independent Safety Monitor.
Statistical Analysis
During our initial planning, we estimated that approximately 24 000 persons would reside in the 20 villages, with approximately 8000 age-eligible children for participation in the vaccination campaigns and 16 000 either too young or too old to participate in the campaigns. For this cluster-randomized trial, a design effect (DE) was required to account for the correlation of participant outcomes within the village clusters. However, since the intra-cluster correlation coefficient for laboratory-confirmed symptomatic influenza in this population was unknown, we assumed a DE of 2. Under this DE, and assuming a 10% attack rate of our outcome of interest, a total of 1896 children would need to be enrolled in the vaccination campaigns to detect a total vaccine effectiveness of 50% with a minimal study power of 80% (at a 2-sided Type I error rate of no more than 5%). To evaluate the indirect effects of high-coverage vaccination, we planned to enroll up to 8000 children in the vaccination campaigns (near 100% participation), leaving approximately 16 000 age-ineligible, unvaccinated residents. Under a DE of 2 and assuming a 6% attack rate (a 10% baseline attack rate among unvaccinated infants and children, comprising an estimated 20% of the analysis population, and a 5% baseline attack rate among unvaccinated adolescents and adults, comprising an estimated 80% of the analysis population), a total of 14 404 unvaccinated residents would need to be under surveillance to detect an indirect effectiveness of 25% with a minimal study power of 80%.
Among vaccinees, the total effectiveness (and its 95% confidence interval [CI]) was calculated as 1 minus the odds ratio times 100%. This odds ratio was estimated as the exponentiated coefficient for the village-level treatment assignment (dummy variable for village IIV3 allocation) from a logistic regression model fit to the individual-level data via generalized estimating equations, assuming exchangeable correlation matrices to account for within-village correlations (clustering) [15]. There were 2 dummy variables included to account for stratified randomization. Analyses were implemented using Stata, version 11 (StataCorp LP, College Station, TX), and R, version 3.1.1 [16]. Generalized estimating equations regressions were fitted using the geepack R library, with standard error and CI model coefficients estimated using the package’s jackknife routine. Secondary objectives included the estimation, using an analytic approach similar to that described above, of indirect effectiveness among age-ineligible residents and overall effectiveness among the entire population.
To investigate whether indirect effectiveness estimates might be confounded by village-level differences in study vaccine coverage among those age-eligible children in each village, post hoc exploratory analyses were also conducted. Given that vaccine coverage, randomization stratum, and treatment assignment were all village-level characteristics, for these unplanned analyses, a 2-stage method for conducting analyses was used, based upon cluster-level summaries [17]. Indirect effectiveness estimates among those residents who were age-ineligible for study vaccination were produced using this method, both not adjusting and adjusting for the variability of vaccine coverage at the village level. Confidence intervals were estimated using the t-distribution–based method, with degrees of freedom altered when adjusting for cluster-level covariates [17].
Primary total effectiveness analyses were performed on a modified intention-to-treat (mITT) basis. In this village-randomized trial, the strict intention-to-treat analysis would be at the village level, but total effectiveness must be analyzed at the individual level. Therefore, intention-to-treat was modified by shifting it to the individual level, to require that the child consented for participation and was enrolled, regardless of their subsequent receipt of the study vaccines. The total effectiveness was also analyzed on a per protocol basis and included all children who met eligibility criteria, received the protocol-specified number of vaccine doses, and contributed at least 1 day of person-time of study follow-up. Indirect effectiveness was also analyzed as the mITT. Overall effectiveness was analyzed using all residents of the study area.
The study is registered with ClinicalTrials.gov, number NCT00893906.
RESULTS
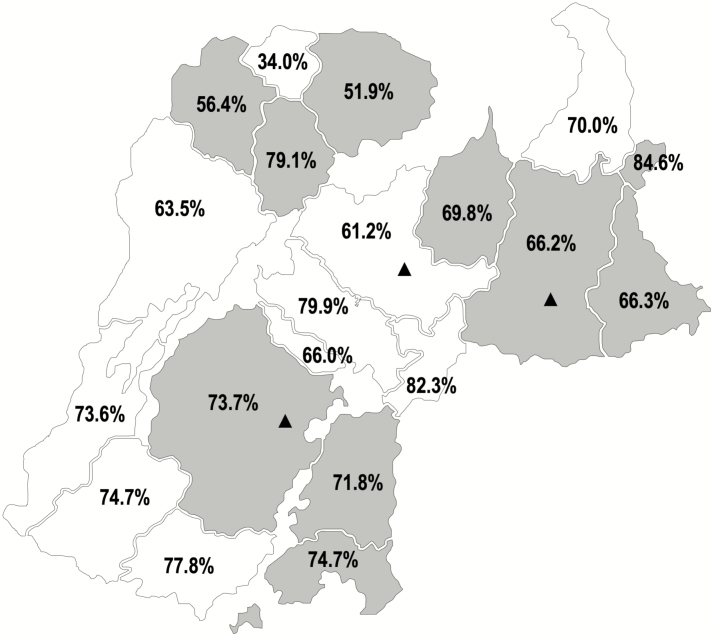
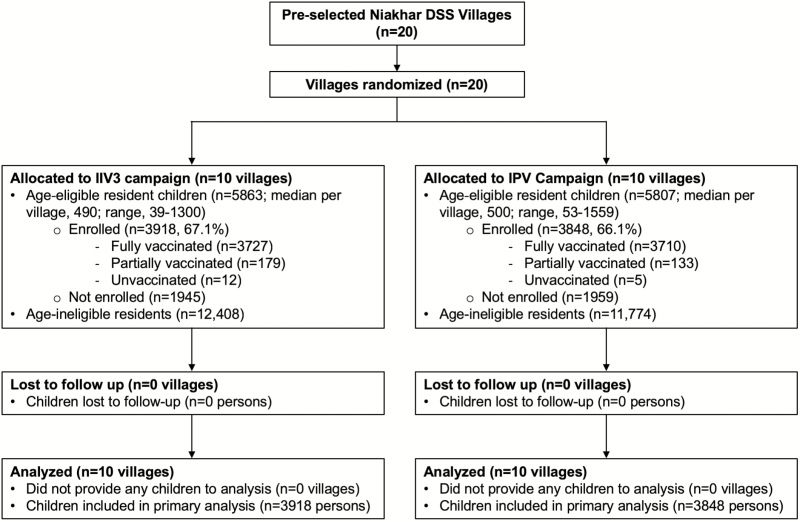
All 20 villages pre-selected for inclusion in the trial were randomized (Figure 1). There were 11 670 children who were age-eligible for study vaccination (Figure 2). Vaccination occurred between 23 May and 11 July 2009. Table 1 shows the baseline characteristics of randomized villages and the children living in those villages. While there were modestly more children in IIV3 villages, the characteristics were generally similar between the study arms. Approximately two-thirds of age-eligible children received Dose 1. Coverage ranged from 34.0% to 84.6% (Figure 1).
Figure 1.
Geographic distribution of villages randomized to IIV3 and IPV campaigns and achieved village-level vaccination coverage among age-eligible children for the campaigns during the trial, from the Niakhar Demographic Surveillance System. The IIV3 campaigns were conducted in the 10 villages that are shaded gray and the IPV campaigns were conducted in the 10 villages that are shaded white. Coverage is shown as the percent of age-eligible children—6 months through 10 years of age—receiving at least 1 dose. Enhanced, passive surveillance was conducted in the 3 health posts marked with triangles. Abbreviations: IIV3, trivalent inactivated influenza vaccine; IPV, inactivated polio vaccine.
Figure 2.
Study profile. The profile is designed for the primary objective of total effectiveness. Abbreviations: DSS, Demographic Surveillance System; IIV3, trivalent inactivated influenza vaccine; IPV, inactivated polio vaccine.
Table 1.
Baseline Demographic Characteristics and Vaccination Data for Each Study Group
| IIV3 Arm (n = 10 villages) |
IPV Arm (n = 10 villages) |
|
|---|---|---|
| Cluster-level characteristics | ||
| Mean village population size (SD) | 1827 (1189) | 1758 (1328) |
| Residents per compound (SD) | 13.6 (15.7) | 14.1 (17.2) |
| Individual-level characteristics | ||
| Total population (all ages) | 18 271 | 17 581 |
| Sex | ||
| Male (%) | 8926 (48.9) | 8606 (49.0) |
| Female (%) | 9305 (50.9) | 8927 (50.8) |
| Unknown (%) | 40 (0.2) | 48 (0.3) |
| Mean age of population years (SD) | 22.3 (19.2) | 22.5 (19.5) |
| Number (%) of age-eligible children | 5863 | 5807 |
| 6 through 35 months | 1734 (29.6) | 1688 (29.1) |
| 3 through 5 years | 1719 (29.3) | 1689 (29.1) |
| 6 through 8 years | 1538 (26.2) | 1564 (26.9) |
| 9 through 10 years | 872 (14.9) | 866 (14.9) |
| Information on enrollment and vaccination | ||
| Number of age-eligible children enrolled (% of all age-eligible children) | 3918 (66.8) | 3848 (66.3) |
| 6 through 35 months | 1017 (58.7) | 1000 (59.2) |
| 3 through 5 years | 1314 (76.4) | 1262 (74.7) |
| 6 through 8 years | 1208 (78.5) | 1238 (79.2) |
| 9 through 10 years | 379 (43.5) | 348 (40.2) |
| Number receiving Dose 1 (% of those enrolled) | 3906 (99.7) | 3843 (99.9) |
| 6 through 35 months | 1017 (100.0) | 1000 (100.0) |
| 3 through 5 years | 1313 (99.9) | 1260 (99.8) |
| 6 through 8 years | 1206 (99.8) | 1238 (100.0) |
| 9 through 10 years | 370 (97.6) | 345 (99.1) |
| Number receiving Dose 2 (% of those receiving Dose 1) | 3357 (95.4) | 3365 (96.2) |
| 6 through 35 months | 956 (94.0) | 955 (95.5) |
| 3 through 5 years | 1261 (96.0) | 1224 (97.1) |
| 6 through 8 years | 1140 (94.5) | 1186 (95.8) |
Abbreviations: IIV3, trivalent inactivated influenza vaccine; IPV, inactivated poliovirus vaccine; SD, standard deviation.
There were 4 unrelated SAEs identified among vaccinees during the first month after study vaccination (only 1 SAE, fracture of the right humerus, occurred in an IIV3 recipient).
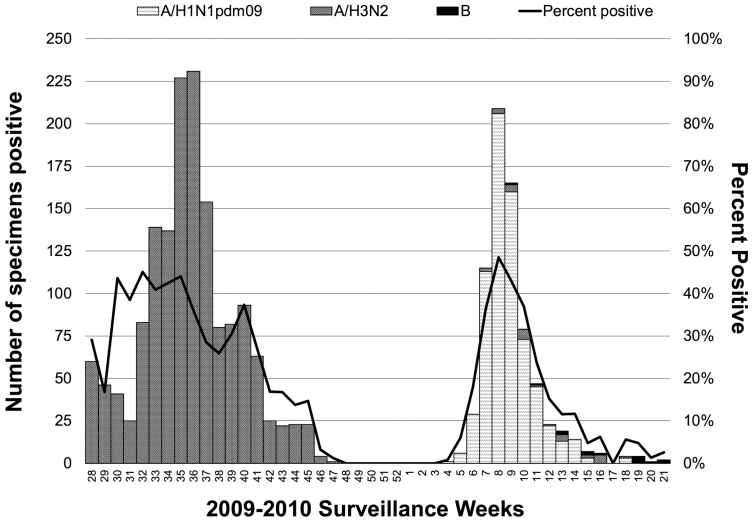
Influenza A/H3N2 virus was circulating in the population when surveillance was initiated the week following Dose 2 vaccinations (Figure 3). During 2009, 5475 swab specimens were collected from Niakhar residents with signs and symptoms of influenza, with 1559 being rRT-PCR positive for A/H3N2. Only the A/H3N2 virus was detected through December 2009. Of 588 A/H3N2–positive specimens collected in July and August, 40 were sent to the Centers for Disease Control and Prevention for antigenic characterization. Of these, 30 were successfully characterized as A/Perth/16/2009 (H3N2)-like virus, a strain not included in the study IIV3. In late January 2010, pandemic influenza A/H1N1 (2009) virus (A/H1N1pdm09) appeared in Senegal and widely circulated in the Niakhar population until early May. While no A/H1N1pdm09 viruses were antigenically characterized, all rRT-PCR detections were made using protocols and primer-probe sets designed for the detection of this new pandemic virus [18]. During 2010, A/H3N2 and B influenza viruses were only sporadically detected.
Figure 3.
Influenza detection, by type and subtype, from Week 28 of 2009 to Week 21 of 2010, Niakhar Demographic Surveillance System. The graph is a stacked column chart where numbers of real-time reverse transcription polymerase chain reaction–positive detections for each strain, each week, are stacked and can be visually summed. Of the 1559 A/H3N2 detections, 61 were from residents of the 10 Niakhar villages not randomized to study vaccines who presented to health posts. Additionally, 99 detections from residents of the 20 Niakhar villages randomized to study vaccines were excluded from the effectiveness analyses (82 were from residents whose signs and symptoms did not meet the clinical case definition and 17 were from residents with a previous A/H3N2 detection that season). Determination of B lineage was not routine practice for the National Influenza Centers in 2009–2010.
In the analysis of total vaccine effectiveness against laboratory-confirmed, symptomatic, seasonal influenza caused by A/H3N2, 300 outcomes occurred among children in IIV3 villages (cumulative incidence of 7.7 cases per 100 children) and 481 occurred among children in IPV villages (cumulative incidence of 12.5 per 100 children), for a total effectiveness of 43.6% (95% CI 18.6–60.9%; Table 2). The per protocol analysis of total effectiveness against A/H3N2 seasonal influenza was similar. The total effectiveness against A/H3N2 influenza was moderate among older children (3 through 10 years of age; ~60%), but lower and not statistically significant among infants and young children (6 through 35 months of age).
Table 2.
Total Effectiveness of Trivalent Inactivated Influenza Vaccine in Preventing Laboratory-confirmed Symptomatic Influenza Among Vaccinees, by Type/Subtype and Age Group
| IIV3 Villages | IPV Villages | ||||||
|---|---|---|---|---|---|---|---|
| Analysis | Cases (n) | N | Cumulative Incidencea | Cases (n) | N | Cumulative Incidencea | Adjusted VETb % (95% CI) |
| VE T (mITT) | |||||||
| Any type A or Bc | 489d | 3918 | 12.48 | 585d | 3848 | 15.20 | 25.2 (−5.7 to 47.0) |
| Seasonal strains | |||||||
| A/H3N2 | 300 | 3918 | 7.66 | 481 | 3848 | 12.50 | 43.6 (18.6 to 60.9) |
| B | 2 | 3918 | 0.05 | 3 | 3848 | 0.08 | 23.0 (−361.3 to 87.1) |
| Pandemic strain | |||||||
| A/H1N1pdm09 | 204 | 3918 | 5.21 | 115 | 3848 | 2.99 | −52.1 (−177.2 to 16.6) |
| VE T (PP) | |||||||
| A/H3N2 | 283 | 3727 | 7.59 | 463 | 3710 | 12.48 | 43.7 (19.0 to 60.9) |
| 6 through 35 months | 155 | 956 | 16.21 | 183 | 955 | 19.16 | 20.6 (−16.3 to 45.8) |
| 3 through 5 years | 79 | 1261 | 6.26 | 157 | 1224 | 12.83 | 57.7 (34.7 to 72.7) |
| 6 through 8 years | 37 | 1140 | 3.25 | 102 | 1186 | 8.60 | 63.6 (37.5 to 78.8) |
| 9 through 10 years | 12 | 370 | 3.24 | 21 | 345 | 6.09 | 53.1 (−5.8 to 79.2) |
| A/H1N1pdm09 | 198 | 3727 | 5.31 | 109 | 3710 | 2.94 | −53.9 (−180.4 to 15.5) |
| 6 through 35 months | 44 | 956 | 4.60 | 34 | 955 | 3.56 | −30.8 (−128.3 to 25.0) |
| 3 through 5 years | 60 | 1261 | 4.76 | 32 | 1224 | 2.61 | −56.2 (−238.2 to 27.8) |
| 6 through 8 years | 71 | 1140 | 6.23 | 32 | 1186 | 2.70 | −101.5 (−328.2 to 5.2) |
| 9 through 10 years | 23 | 370 | 6.22 | 11 | 345 | 3.19 | −88.7 (−383.5 to 26.4) |
Abbreviations: CI, confidence interval; IIV3, trivalent inactivated influenza vaccine; IPV, inactivated poliovirus vaccine; mITT, modified intention-to-treat; n, number of cases; N, number of children followed; PP, per protocol; VET, total vaccine effectiveness.
aPer 100 persons through the entire surveillance period: 15 July 2009 through 28 May 2010.
bEstimated using a logistic regression model fit, using generalized estimating equations, assuming an exchangeable correlation matrix to account for within-village correlation of participant observations.
cAny influenza type/subtype contained in IIV3, regardless of match.
dOnly the first episode is counted for those infected by more than 1 strain.
Among the entire population, both vaccinated and unvaccinated, the overall effectiveness of the IIV3 vaccination campaign in preventing laboratory-confirmed, symptomatic, seasonal influenza caused by A/H3N2 was estimated at 31.7% (95% CI 6.0–50.3%). Among the population that was age-ineligible for the vaccination campaigns (those <6 months or ≥11 years of age), the indirect effectiveness of the IIV3 vaccination campaign in preventing laboratory-confirmed, symptomatic, seasonal influenza caused by A/H3N2 was estimated at 15.4% (95% CI -22.0 to 41.3%). Age group–specific, indirect effectiveness estimates among age-ineligible infants, adolescents, and adults and among age-eligible children not consenting for study vaccination are shown in Table 3. No age group–specific estimate of indirect effectiveness was statistically significant. Exploratory analyses of indirect effectiveness against A/H3N2 among those who were age-ineligible for study vaccination, using alternative methods, gave estimates that were similar and also not statistically significant, adjusting for randomization stratum effects (21.8%, 95% CI -27.6 to 52.1%) or adjusting for randomization stratum effects and for village-level coverage among age-eligible children (21.4%, 95% CI -30.0 to 52.5%).
Table 3.
Indirect Effectiveness of Trivalent Inactivated Influenza Vaccine in Preventing Laboratory-confirmed Symptomatic H3N2 Influenza Among Non-vaccinees, by Age Group
| IIV3 Villages | IPV Villages | ||||||
|---|---|---|---|---|---|---|---|
| Age Groupa | Cases, n | N | Cumulative Incidenceb | Cases, n | N | Cumulative Incidenceb | Adjusted VEIc % (95% CI) |
| <6 months | 49 | 1082 | 4.53 | 49 | 1064 | 4.61 | 7.0 (−59.2 to 45.6) |
| 6 months through 10 years | 114 | 1945 | 5.86 | 120 | 1959 | 6.13 | 7.6 (−29.2 to 33.9) |
| 11 through 17 years | 45 | 2773 | 1.62 | 68 | 2672 | 2.54 | 45.1 (−1.6 to 70.4) |
| 18 through 49 years | 53 | 6065 | 0.87 | 57 | 5667 | 1.01 | 0.3 (−74.6 to 43.1) |
| 50 through 64 years | 30 | 1750 | 1.71 | 19 | 1686 | 1.13 | −35.5 (−135.1 to 21.9) |
| >64 years | 8 | 728 | 1.10 | 6 | 677 | 0.89 | … |
Abbreviations: CI, confidence interval; IIV3, trivalent inactivated influenza vaccine; IPV, inactivated poliovirus vaccine; n, number of cases; N, number of children followed; VEI, indirect effectiveness.
aThe age was unable to be determined for 10 unvaccinated residents of IIV3 villages and 8 unvaccinated residents of IPV villages.
bPer 100 persons through the entire surveillance period: 15 July 2009 through 28 May 2010.
cEstimated using a logistic regression model fit, using generalized estimating equations, assuming an exchangeable correlation matrix to account for within-village correlation of participant observations.
Although the primary mITT analysis of total vaccine effectiveness was designed to include any influenza type/subtype contained in IIV3, regardless of match, a lower primary estimate of 25.2% (95% CI -5.7 to 47.0%) reflects the fact that seasonal IIV3 did not protect against the novel A/H1N1pdm09 (Table 2). In fact, in the analysis of total effectiveness against laboratory-confirmed, symptomatic influenza caused by A/H1N1pdm09 in this population, the effectiveness was negative (-52.1%, 95% CI -177.2 to 16.6%), although not statistically significant (Table 2).
DISCUSSION
With this cluster-randomized influenza vaccine trial in Senegal, our goal was to generate data (disease burden, total effects of vaccine among children, and indirect effects experienced by a community when children are vaccinated) to inform decisions on the use of influenza vaccines in low-resource settings. We confirmed that the circulation of seasonal type A influenza occurs predominantly during the rainy season and that influenza is a frequent cause of clinical illness. Among children receiving IPV in control clusters, the attack rates of laboratory-confirmed clinical influenza for the predominating seasonal strain (A/H3N2) were high, ranging from 6% among 9 and 10 years olds to 19% among children younger than 3 years of age. In comparison, IIV3 had a moderate effectiveness in reducing the attack rates of seasonal influenza illness among all children 6 months through 10 years of age. Notably, the low effectiveness of 21% against this seasonal strain among children younger than 3 years was not statistically significant, while effectiveness for children older than 3 years was approximately 60%. The A/Perth/16/2009 (H3N2)-like virus was a new antigenic variant, and the phylogenetic analysis and antigenic characterization of the hemagglutinin gene suggested that the efficacy of IIV3 based on the A/Brisbane/10/2007 (H3N2)-like virus would have been low [19, 20]. Even in years of a high antigenic match between the vaccine strains and a circulating virus, efficacy estimates of unadjuvanted, inactivated influenza vaccines in children vary by season, population, and study design [21]. Recent, adequately powered, randomized, controlled trials reported efficacy estimates against any severity of influenza in the 43–60% range, with higher estimates reported for older children and against more severe instances of the disease [22–24].
On average, approximately two-thirds of all children 6 months through 10 years of age, who represented approximately 22% of the total population, received a vaccine as part of this trial. Despite the significant effectiveness among vaccinated children, we demonstrated no significant, indirect effects among the more than 24 000 children and adults who did not receive IIV3 in the same communities. In a cluster-randomized trial in 2008–09 in Canada among just over 3000 persons in Hutterite communities, the indirect effectiveness was measured at 60% (95% CI 8–83%) among non-recipients in clusters where the IIV3 vaccine coverage among children 36 months through 15 years reached 83%, which translated to 40% of the entire population [25]. The lack of indirect effects in our study may be attributable to the higher contact rates, where large, extended families live in close quarters in densely grouped compounds [26]. Additionally, the lack of indirect effects may be due to lower vaccination coverage, with an IIV3 that had a suboptimal match with the circulating strain (and hence possibly suboptimal direct vaccine effectiveness) or differing social patterns that affect influenza transmission (eg, intense contact between unvaccinated persons, even in villages where influenza vaccination was conducted among children, or the movement of people between villages or study area gathering places, such as markets).
As the trial was beginning, the first cases of influenza caused by A/H1N1pdm09 were being reported in the United States, and by 11 June 2009, when we had administered the first doses to all children, the World Health Organization had already declared a global pandemic (on 25 April 2009) [27]. Thus, this trial is unique in that we collected data on not only seasonal influenza, but also on pandemic influenza occurring in closely monitored, randomized cohorts of vaccinated children and their communities. Our active surveillance detected the A/H1N1pdm09 virus in the Niakhar population in late January 2010, later than many parts of the world [28]. Overall, the A/H1N1pdm09 attack rates among vaccinated children were lower than those seen with the seasonal A/H3N2 virus. In contrast to A/H3N2, rates of A/H1N1pdm09 virus infection were higher among children in villages receiving IIV3 as compared to IPV, although the difference did not reach statistical significance. A number of studies, particularly observational ones, in 2009 identified that the receipt of the seasonal IIV3 was associated with higher rates of A/H1N1pdm09 influenza infection, as compared to unvaccinated persons. A small trial in Hong Kong children found higher rates of A/H1N1pdm09 influenza infection, but not clinical disease, among recipients of seasonal IIV3, as compared to placebo recipients [29]. In Canada, 4 observational studies linked the previous receipt of northern hemisphere seasonal IIV3 with an increased risk of A/H1N1pdm09 influenza illness, although there was no observed increase in the severity of illness [30]. As the Canadian studies were observational, biases and confounding may have affected estimates. In this large-scale, randomized, controlled, and blinded trial, with less inherent bias, our results paralleled those of the Canadian studies. Whether prior vaccination with a homologous subtype of influenza could increase the risk of A/H1N1pdm09 infection is unknown, and the mechanism is disputed.
In this trial, during a single season in tropical, developing Senegal, symptomatic influenza was common among children. Influenza vaccination campaigns were feasible and the vaccine was well tolerated. While the total vaccine effects against seasonal influenza were moderate among children 3 years and older, low effectiveness among younger children, in particular, emphasizes the continued need for better influenza vaccines for young children. Interestingly, we also found that vaccinating two-thirds of children 6 months through 10 years of age did not induce measurable indirect effects. A systematic review found a low level of evidence in the literature for influenza vaccination providing indirect effects, indicating that the topic is complicated and more studies are needed [31]. Finally, among IIV3 recipients, our finding of negative effectiveness against H1N1pdm09, though not statistically significant, reminds us of the complexity of vaccine-induced immunity and supports further study of the interaction between immune responses to influenza antigens in vaccines and subsequent exposure to homologous but shifted (and drifted) wild-type influenza virus. Such data will be particularly relevant to informing future universal vaccine development and deployment.
Notes
Author contributions. K. M. N. and J. C. V. conceived of the study. A. D., O. M. D., J. C. V., K. M. N., J. R. O., J. D. S., M. E. H., K. E. L., and M.-A. W. designed the trial. A. D., O. M. D., M. N. N., D. D., J. C. V., J. R. O., J. D. S., C. S., S. L. E., K. E. L., and M.-A. W. developed the study methods and data collection instruments. J. D. S. and M. E. H. designed the randomization and J. D. S. and J. C. V. performed the randomization. A. D., D. D., and B. D. collected the data and biological specimens. E. A. F., D.G., and M. N. N. performed the laboratory assays. J. R. O. and K. M. N. served as medical monitors for PATH. C. S. and M.-A. W. designed and coordinated the data management. J. D. S., S. Z. Z., M. E. H., and J. C. V. designed the statistical analyses. J. D. S. and S. Z. Z. performed the statistical analyses and M. E. H. and J. C. V. verified their accuracy. A. D. served as the study’s Principal Investigator in Senegal and led the team at the Institut de Recherche Pour le Développement, which administers the Niakhar Demographic Surveillance System. O. M. D. led the team at the Institut Pasteur de Dakar, which houses Senegal’s National Influenza Center. J. C. V. served as the Primary Investigator for the cooperative agreement between PATH and the Centers for Disease Control and Prevention, and M.-A. W. served as its Program Officer. J. C. V., K. M. N., J. R. O., and J. D. S. drafted the manuscript. A. D., O. M. D., M. N. N., J. D. S., J. R. O., K. D. C. L., S. Z. Z., K. E. L., M. E. H., M.-A. W., K. M. N., and J. C. V. critically revised the manuscript. All authors had full access to the study data, had the opportunity to review drafts, and approved the final version submitted for publication.
Acknowledgments. The authors thank all the families who participated in this trial and the full research teams at the Institut de Recherche Pour le Développement and the Institut Pasteur de Dakar in Senegal. They thank Sanofi Pasteur for donating the study vaccines. They thank Dr Kathryn Edwards for serving as the study’s Independent Safety Monitor and Dr Xiyan Xu of the US Centers for Disease Control and Prevention for the antigenic characterization of submitted influenza-positive specimens. This study was a collaboration of PATH (Seattle, Washington, and Dakar, Senegal), the Institut de Recherche Pour le Développement (Dakar, Senegal), the Institut Pasteur de Dakar (Dakar, Senegal), and the US Centers for Disease Control and Prevention (Atlanta, Georgia). Supporting PATH in fulfilling its sponsor obligations, the Agence Africaine De Recherche en Sante Humaine conducted site monitoring and the Fred Hutchinson Cancer Research Center conducted statistical analyses for vaccine effectiveness.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of any collaborating institution.
Financial support. This work was supported by a cooperative agreement from the Centers for Disease Control and Prevention (U01IP000174) to PATH. Sanofi Pasteur donated the study vaccines, but had no other role in the study.
Potential conflicts of interest. J. D. S., S. Z. Z., and M. E. H. were partially funded by National Institutes of Health (grant R37 AI032042). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Newman LP, Bhat N, Fleming JA, Neuzil KM. Global influenza seasonality to inform country-level vaccine programs: an analysis of WHO FluNet influenza surveillance data between 2011 and 2016. PLOS One 2018; 13:e0193263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hirve S, Newman LP, Paget J, et al. Influenza seasonality in the tropics and subtropics - when to vaccinate? PLOS One 2016; 11:e0153003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steinhoff MC, Katz J, Englund JA, et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis 2017; 17:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tapia MD, Sow SO, Tamboura B, et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis 2016; 16:1026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lambach P, Alvarez AM, Hirve S, et al. Considerations of strategies to provide influenza vaccine year round. Vaccine 2015; 33:6493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ott JJ, Klein Breteler J, Tam JS, Hutubessy RC, Jit M, de Boer MR. Influenza vaccines in low and middle income countries: a systematic review of economic evaluations. Hum Vaccin Immunother 2013; 9:1500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Longini IM Jr, Halloran ME. Strategy for distribution of influenza vaccine to high-risk groups and children. Am J Epidemiol 2005; 161:303–6. [DOI] [PubMed] [Google Scholar]
- 8. Jordan R, Connock M, Albon E, et al. Universal vaccination of children against influenza: are there indirect benefits to the community? A systematic review of the evidence. Vaccine 2006; 24:1047–62. [DOI] [PubMed] [Google Scholar]
- 9. Halloran ME, Struchiner CJ. Study designs for dependent happenings. Epidemiology 1991; 2:331–8. [DOI] [PubMed] [Google Scholar]
- 10. Delaunay V, Douillot L, Diallo A, et al. Profile: the Niakhar Health and Demographic Surveillance System. Int J Epidemiol 2013; 42:1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dosseh A, Ndiaye K, Spiegel A, Sagna M, Mathiot C. Epidemiological and virological influenza survey in Dakar, Senegal: 1996-1998. Am J Trop Med Hyg 2000; 62:639–43. [DOI] [PubMed] [Google Scholar]
- 12. Victor JC, Lewis KD, Diallo A, et al. Efficacy of a Russian-backbone live attenuated influenza vaccine among children in Senegal: a randomised, double-blind, placebo-controlled trial. Lancet Glob Health 2016; 4:e955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Regional Office for Africa. Mid-Level Management Course for EPI Managers: Block III: Logistics: Module 9: Immunization safety. World Health Organization. Regional Office for Africa, 2017. Avaialble at: http://www.who.int/iris/handle/10665/260490 [Google Scholar]
- 14. Moulton LH. Covariate-based constrained randomization of group-randomized trials. Clin Trials 2004; 1:297–305. [DOI] [PubMed] [Google Scholar]
- 15. McCullagh P, Nelder JA.. Generalized linear models. 2nd ed. London, United Kingdom: Chapman and Hall, 1989. [Google Scholar]
- 16. The R Foundation. The R project for statistical computing. Available at: https://www.r-project.org. Accessed 28 March 2018. [Google Scholar]
- 17. Hayes RJ, Moulton LH.. Cluster randomised trials. Baco Raton, Florida: Chapman & Hall/CRC, Taylor & Francis Group, LLC, 2009:163–94. [Google Scholar]
- 18. World Health Organization. CDC protocol of realtime RTPCR for influenza A (H1N1), 2009. Available at: http://www.who.int/csr/resources/publications/swineflu/realtimeptpcr/en/. Accessed 28 September 2018. [Google Scholar]
- 19. Madhi SA, Dittmer S, Kuwanda L, et al. Efficacy and immunogenicity of influenza vaccine in HIV-infected children: a randomized, double-blind, placebo controlled trial. AIDS 2013; 27:369–79. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2010 influenza season (southern hemisphere winter). Wkly Epidemiol Rec 2009; 84:421–31. [PubMed] [Google Scholar]
- 21. Rotrosen ET, Neuzil KM. Influenza: a global perspective. Pediatr Clin North Am 2017; 64:911–36. [DOI] [PubMed] [Google Scholar]
- 22. Claeys C, Zaman K, Dbaibo G, et al. ; Flu4VEC Study Group Prevention of vaccine-matched and mismatched influenza in children aged 6-35 months: a multinational randomised trial across five influenza seasons. Lancet Child Adolesc Health 2018; 2:338–49. [DOI] [PubMed] [Google Scholar]
- 23. Vesikari T, Knuf M, Wutzler P, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med 2011; 365:1406–16. [DOI] [PubMed] [Google Scholar]
- 24. Jain VK, Rivera L, Zaman K, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med 2013; 369:2481–91. [DOI] [PubMed] [Google Scholar]
- 25. Loeb M, Russell ML, Moss L, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA 2010; 303:943–50. [DOI] [PubMed] [Google Scholar]
- 26. Munier A, Diallo A, Marra A, et al. Evolution of malaria mortality and morbidity after the emergence of chloroquine resistance in Niakhar, Senegal. Malar J 2009; 8:270 Available at: https://www.ncbi.nlm.nih.gov/pubmed/19943921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. Swine influenza; statement by WHO Director-General, Dr Margaret Chan. Available at: http://www.who.int/mediacentre/news/statements/2009/h1n1_20090425/en/. Accessed 28 September 2018. [Google Scholar]
- 28. Nzussouo NT, Michalove J, Diop OM, et al. Delayed 2009 pandemic influenza A virus subtype H1N1 circulation in West Africa, May 2009-April 2010. J Infect Dis 2012; 206(Suppl 1):S101–7. [DOI] [PubMed] [Google Scholar]
- 29. Cowling BJ, Ng S, Ma ES, et al. Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin Infect Dis 2010; 51:1370–9. [DOI] [PubMed] [Google Scholar]
- 30. Skowronski DM, De Serres G, Crowcroft NS, et al. Association between the 2008–09 seasonal influenza vaccine and pandemic H1N1 illness during spring-summer 2009: four observational studies from Canada. PLOS Med 2010; 7:e1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mertz D, Fadel SA, Lam PP, et al. Herd effect from influenza vaccination in non-healthcare settings: a systematic review of randomised controlled trials and observational studies. Euro Surveill 2016; 21:30378 Available at: https://www.ncbi.nlm.nih.gov/pubmed/27784531 [DOI] [PMC free article] [PubMed] [Google Scholar]