Abstract
Introduction
Nonadherence to pharmacotherapies complicates studies of comparative pharmacotherapy effectiveness. Modeling adherence and abstinence simultaneously may facilitate analysis of both treatment acceptability and effectiveness.
Methods
Secondary analyses of a three-arm randomized comparative trial of nicotine patch, varenicline, and combination nicotine patch and lozenge among adult daily smokers (N = 1086) were conducted. Adherence rates collected via interactive voice response systems during the first 27 days of quitting were compared across treatment conditions. Repeated measures latent class analyses of adherence and abstinence in 3-day parcels through 27 days of a quit attempt were conducted with treatment, demographic, and smoking history covariates.
Results
Adherence varied across treatments and was lowest for nicotine lozenge use in combination nicotine replacement therapy (NRT). Five latent classes that differed significantly in 6-month abstinence rates were retained, including three subgroups of adherent participants varying in treatment response and two nonadherent groups varying in abstinence probabilities. Nonadherence was more likely among those receiving varenicline and combination NRT, relative to patch monotherapy. Varenicline and combination NRT did not promote abstinence among adherent latent classes but did promote abstinence among those partially adherent, relative to patch alone. Combination therapy attenuated increased risk of treatment disengagement with more years smoking. Minority smokers, those high in dependence, and those with shorter past abstinence were at increased risk for low-adherence and low-abstinence latent classes.
Conclusions
Varenicline and combination nicotine patch and lozenge are less likely to be used as directed and may not increase first-month abstinence better than patch alone when taken adherently.
Implications
This secondary analysis of adherence and abstinence in a comparative effectiveness trial shows that adherence is highest for the nicotine patch, next highest for varenicline, and lowest for combination nicotine patch and lozenge therapy due to low lozenge use. Distinct latent classes were found that varied in both first-month abstinence and adherence. Varenicline and combination NRT may not enhance abstinence over patch alone among smokers who take medication adherently. Adherent use of medication especially benefits those who are low in dependence and have positive quitting histories; it is less beneficial to at-risk smokers and members of racial minorities.
Introduction
Most smokers who attempt to quit resume smoking, even with pharmacotherapy and counseling.1 Widespread underuse of pharmacotherapy2–7 may suppress quit rates.3,8,9 Abstinence rates are higher among more adherent individuals2,3,10 and continued medication use after lapsing may prevent progression to full relapse.11 Causal relationships between adherence and smoking cessation outcome are not entirely clear, however,10 as nonadherence may prompt cessation failure or cessation failure may decrease adherence. In addition, adherence is self-selected (eg, subject to third-variable influences such as conscientiousness, marital support, and stress).10 To disentangle these alternatives, it is essential to consider abstinence and adherence together. A person-centered approach may be useful in identifying individual differences associated with particular patterns of medication use and abstinence, as we do not yet know which smokers are likely to succeed without using pharmacotherapy adherently and which are at risk of failing to benefit even when adherent. Comparison of medication responders and nonresponders might separate person factors that affect adherence (acceptability) from those that affect medication response (effectiveness), and thus inform future treatment-matching algorithms that may enhance overall treatment impacts.
This study examined data on medication use and abstinence from a randomized comparative effectiveness trial (N = 1086) of nicotine patch monotherapy, varenicline, and combination nicotine patch and lozenge therapy,12 pharmacotherapies whose efficacy is supported by rigorous meta-analyses.1,13–15 Analyses focused on the first month of quitting, when most relapses begin and when smoking cessation pharmacotherapy effects are most pronounced.16,17 Analyses of medication use and cessation outcomes for combination nicotine replacement therapy (NRT) and varenicline are important because head-to-head adherence data are lacking for these best-performing pharmacotherapies.2,3,18,19 As such, we do not know the extent to which their clinical effects are differentially related to adherence. Simultaneous analysis of adherence and abstinence will speak to the degree to which treatments differ in adherence patterns and correlates, and the extent to which the treatments support abstinence among people who use medication regularly.
Patch, pill, and lozenge adherence data and daily smoking status in the first 27 days of a quit attempt were examined. This study addressed the following research questions: (1) How adherent are smokers to nicotine and varenicline pharmacotherapy regimens in the first 27 days of cessation, and does this differ by regimen? (2) What distinct patterns or classes of abstinence and adherence emerge in the first 27 days of quitting in repeated measures latent class analyses (RMLCAs),20 and how do these relate to pharmacotherapy regimen? (3) What individual characteristics are associated with latent abstinence–adherence class membership? (4) Do latent abstinence–adherence classes differ in longer-term abstinence rates of public health significance? These questions are particularly important to address using the current data drawn from a trial that showed a surprising lack of benefit of either varenicline or combination NRT over patch monotherapy in abstinence rates beyond 1 week postquit,12 in contrast to previous trials and meta-analyses showing that both varenicline and combination NRT significantly increase abstinence rates relative to patch therapy.13–15 The current analyses helped to determine whether this unexpected lack of benefit may be due to differential adherence or equivalent treatment responsivity and used a person-centered approach to identify correlates of these outcomes of clinical importance that may be useful in future treatment matching.
Methods
Data for this study were drawn from an open-label randomized clinical trial of 12-week nicotine patch therapy (n = 241), varenicline (n = 424), and combination nicotine patch and lozenge (n = 421), all offered with cessation counseling.12
Participants
Participants were recruited in Madison and Milwaukee, Wisconsin. To be eligible, individuals must have been at least 18 years old, smoking at least 5 cigarettes/day (with a carbon monoxide level of at least 4 ppm), literate in English, reachable by telephone, and motivated to quit. Exclusion criteria included current use of bupropion or other smoking cessation treatment, use of other tobacco products more than 2 days weekly, use of e-cigarettes, end-stage renal disease, uncontrolled hypertension, suicidal behavior in the past 5 years, current suicidal ideation, psychosis, moderate or severe depressive symptoms, serious medical conditions, and pregnancy or unwillingness to prevent pregnancy. Of the 1086 individuals enrolled, 1045 (96.2%) provided sufficient data to be included in these analyses. Summary demographic and smoking history data for this sample is shown in Table 1.
Table 1.
Summary of Demographics and Smoking History Among the Sample Retained for Analysis (N = 1045)
| Continuous covariate | Mean | SD |
|---|---|---|
| Age (years) | 48.26 | 11.65 |
| Cigarettes/day | 17.01 | 8.34 |
| Years smoked | 28.74 | 12.11 |
| Number of past quit attempts | 3.92 | 6.09 |
| Longest period abstinent (days) | 127.69 | 143.81 |
| Fagerström Test for Cigarette Dependence total score | 4.80 | 2.11 |
| Categorical covariate | N | % |
| Smokes menthol cigarettes | 521 | 49.9 |
| Race (split into minority and Caucasian for analyses) | ||
| Caucasian | 704 | 67.4 |
| African American | 293 | 28.0 |
| Other minority | 48 | 4.6 |
| Gender (female) | 543 | 52.0 |
| Annual household income (split at $25 000 for analyses) | ||
| Under $10 000 | 196 | 18.8 |
| $10 000–$24 999 | 208 | 19.9 |
| $25 000–$49 999 | 272 | 26.1 |
| Greater than $50 000 | 318 | 31.7 |
| Time to first cigarette after waking (split at 30 min for analyses) | ||
| Within 5 min | 352 | 33.7 |
| 6–30 min | 459 | 43.9 |
| 31–60 min | 150 | 14.4 |
| After 60 min | 80 | 7.7 |
Procedures
Study procedures were approved by institutional review boards at the University of Wisconsin and Aurora Health Care. Screening was completed over the phone, and in physical examination and physiological assessment visits. Randomization was not blinded in this open-label trial but took place after screening and immediately before treatment. Participants completed three screening visits, five office visits, and one treatment call through 12 weeks postquit. Participants received nightly automated interactive voice response system survey calls assessing medication use daily from 1 week prequit to 2 weeks postquit and every other day in weeks 3 and 4 postquit. Follow-up calls were conducted 6- and 12-month postquit, with carbon monoxide verification of claimed abstinence at 6 months.
Treatments
Treatment began 10 days prequit for those randomized to varenicline, with 0.5 mg once daily for 3 days, 0.5 mg twice daily for 4 days, and 1 mg twice daily from 3 days prequit to 11 weeks postquit. NRTs began on the target quit day and extended to 12 weeks postquit. Individuals who smoked more than 10 cigarettes at baseline were advised to use 21 mg patches for 8 weeks, 14 mg patches for 2 weeks, and 7 mg patches for 2 weeks. Lighter smokers (5–10 cigarettes/day) received 10 weeks of 14 mg patches and 2 weeks of 7 mg patches. Individuals who smoked within 30 minutes of waking received 4 mg lozenges; those who smoked later received 2-mg lozenges. Individuals were advised to use at least 5 lozenges per day. In all conditions, bachelor-level counselors delivered six 10- to 20-minute sessions of US Public Health Service–based counseling from 1 week prequit to 12 weeks postquit.
Measures
The interactive voice response system prompted nightly reports of the number of pills taken, the number of lozenges used, and/or patch use (according to condition). Adherence was coded as binary. Individuals in the patch-only condition were coded as adherent if they reported wearing a patch that day. Those in the combination condition were coded as adherent if they reported wearing a patch and using at least 4 lozenges per day (80% of the recommended minimum level of lozenge use). Individuals in the varenicline condition were coded as adherent only if they reported taking both pills that day.
Through the end of treatment 12 weeks postquit, participants completed timeline follow-back assessments21 of smoking each day since the last visit. Daily self-reported abstinence status (any smoking = 0, abstinence = 1) for each of the first 27 days of the quit attempt indicated abstinence in RMLCAs. A binary indicator was used in favor of smoking heaviness because total abstinence was common and models with multinomial indicators could not be estimated with the number of repeats modeled. Because adherence data were not available daily in the latter half of the assessment period, and because models with too many indicators did not converge, adherence and abstinence data were aggregated into 3-day parcels for RMLCA (eg, days 1–3, 4–6, and 7–9). Participants were coded as abstinent only if no smoking was reported in the 3-day parcel. Participants were coded as adherent only if the mean of their daily binary adherence ratings was 1 (indicating perfect adherence on days when data were available) for a given parcel. Multiple imputation was not used, as RMLCA does not yet integrate results across multiple imputed datasets. Missing data were not imputed as nonadherent or smoking, as these methods of imputation can distort findings.22–24 Instead, missing RMLCA indicator data were addressed with maximum likelihood estimation. Six-month smoking status was intent-to-treat (19.4% missing) 7-day point-prevalence abstinence based on self-reported total abstinence from tobacco in the past 7 days confirmed with a carbon monoxide level below 6 ppm.25
Covariates were assessed via questionnaire at baseline and included demographics (sex, age, racial/ethnic minority status, and education), smoking history (quit attempts and duration, years smoked, smoking heaviness, and living with a smoker), and cigarette dependence measured by the Fagerström Test for Cigarette Dependence, a 6-item measure of physiological dependence where higher scores (range 0–10) indicate greater dependence.26,27 Secondary covariates used as control variables to assess the robustness of relations between the primary covariates and latent class included annual household income, marital status, and menthol use.
Data Analysis
Analysis of variance compared mean interactive voice response adherence rates across pharmacotherapy regimens in SPSS 22.0 (IBM Corporation, Armonk, NY). All RMLCA models were estimated in Mplus version 7.328 using maximum likelihood estimation with 1000 random starts. The latent class solution was determined first in an unconditional model and then in a conditional model containing treatment indicators and other covariates. Treatment, smoking history, and demographics were entered as covariates of latent class membership in conditional models built by first screening each covariate in a model that also contained dummy coded treatment variables, and then adding those that were significant at p < .05 to a multivariate model. Covariates that were not significantly related to class at p < .05 in the multivariate model were pruned. An alpha correction for multiple comparisons was not applied in the model building process but was applied to the final model results using the Benjamini–Hochberg approach.29 The degree to which covariate relations with latent classes changed when control variables (income, marital status, and menthol use) were entered was assessed as well. The results presented below compared varenicline and combination NRT to the patch monotherapy condition; supplementary analyses compared varenicline versus combination NRT. Marginal 6-month abstinence rates were estimated for each RMLCA latent class using the Lanza method for categorical distal outcomes.30 This unbiased, Bayesian method31 estimates pairwise differences in marginal mean 6-month abstinence rates among the latent classes.
Results
Descriptive Analyses
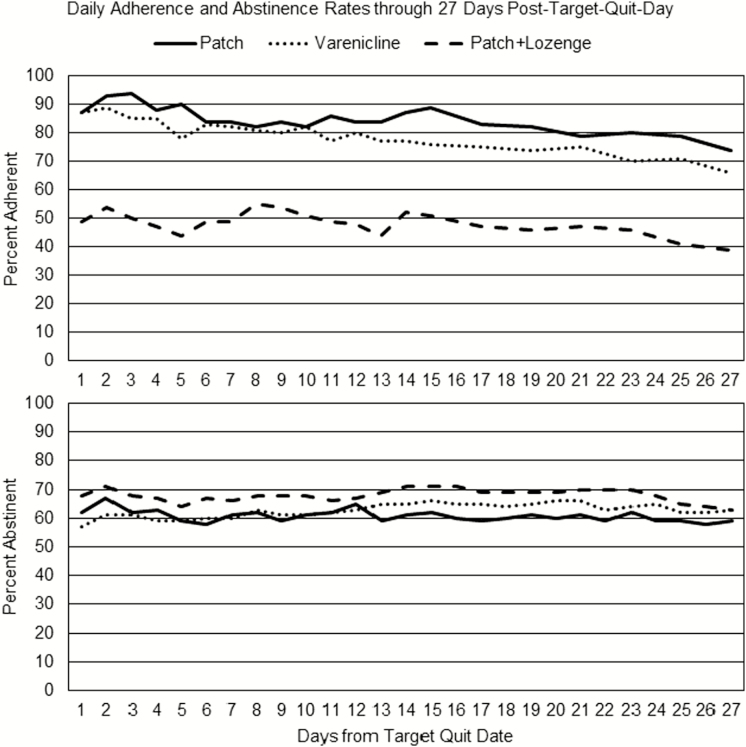
At the report level, adherence data were reported on a mean of 73.1% (SD = 23.1%) of scheduled interactive voice response bedtime calls among 1042 participants (95.9% of the sample). Report- and person-level rates of adherence are displayed by condition in Table 2. Daily abstinence and adherence status are plotted by condition for the first 27 days of the quit attempt in Figure 1.
Table 2.
Descriptive Adherence and Abstinence Data by Treatment Condition (N = 1045)
| Adherence measures | Patch (n = 231) | Varenicline (n = 398) | Patch + lozenge (n = 416) | |
|---|---|---|---|---|
| Evening report-level analyses | n = 193 | n = 344 | n = 349 | |
| Number of evening adherent reports completed | 2688 | 4716 | 5009 | |
| Adherent (1 patch, 2 pills, patch + ≥4 lozenges) | 84.4% | 77.7% | 54.4% | |
| Partially adherent (1 pill, patch or ≥1 lozenge) | 14.6% | 90.6% | ||
| Used patch | 84.3% | |||
| Used 1–3 lozenges | 27.9% | |||
| Did not use any study medication | 16.6% | 7.7% | 9.4% | |
| Person-level analyses | n = 193 | n = 344 | n = 349 | p |
| Mean (SD) % observed days adherent (1 patch, 2 pills, patch + ≥4 lozenges) | 82.5%a (25.5%) | 75.2%b (31.6%) | 47.1%c (36.3%) | <.001 |
| Intent-to-treat mean (SD) % days adherent (missing = nonadherent) | 60.5%a (39.7%) | 43.0%b (33.8%) | 26.0% c (29.3%) | <.001 |
| Person-level smoking calendar analyses | n = 231 | n = 398 | N = 416 | |
| Mean (SD) % observed days abstinent | 61.1% (48.8%) | 62.5% (48.4%) | 65.6% (46.7%) | .07 |
| Intent-to-treat mean (SD) % days abstinent (missing = smoking) | 58.7%a (49.2%) | 61.5%a,b (48.6%) | 64.1%b (47.2%) | .047 |
Superscripts a, b, c indicate that adherence or abstinence rates differed significantly among groups with differing superscripts in an analysis of variance.
Figure 1.
The top panel shows the mean adherence rate across persons by day and treatment condition through 27 days after the target quit date. The bottom panel shows the mean abstinence rate by condition in the same period. Adherence was defined as reported full adherence. For the patch-only condition, this meant reporting patch use. For the varenicline condition, this meant reporting taking two pills. For the combination nicotine replacement therapy (NRT) condition, this meant reporting patch use and using at least 4 lozenges per day. In all conditions, abstinence was defined as no reported smoking on that calendar day.
Repeated Measures Latent Class Analysis of Parceled Adherence and Abstinence
Unconditional models with up to eight latent classes were tested (see Supplementary Table 1). Model fit indices (Bayesian Information Criterion [BIC] and sample-size adjusted BIC [aBIC]) continued to improve, and the Bootstrap Likelihood Ratio Test (BLRT) was significant for additional classes up to eight latent classes (six classes for the BIC), but there were problems with identification of the six-class model and the improvement in model fit over the five-class model was modest, so the five-class model was selected. The final model had acceptable entropy (0.84), a measure of the accuracy of participant classification in latent classes.20,32
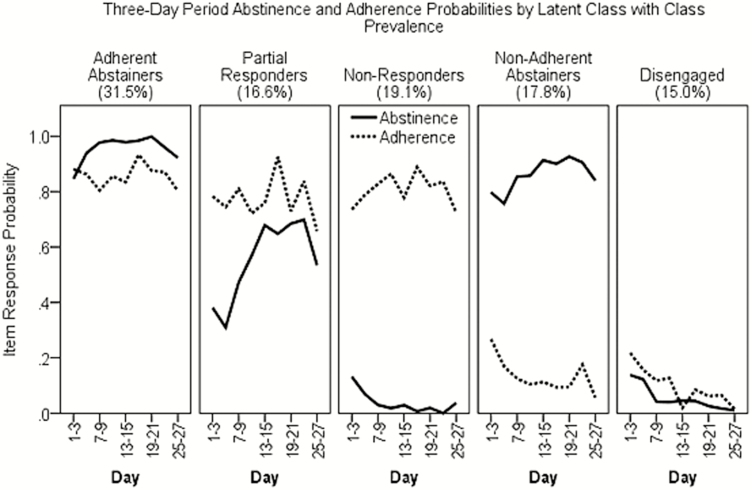
In the final unconditional model (Figure 2), three classes had high probabilities of adherence but varying probabilities of abstinence: the largest class, Adherent Abstainers (31.5% prevalence), had high probabilities of abstinence through 27 days postquit; Partial Responders (16.6% prevalence) had intermediate abstinence probabilities (near 50%); and Nonresponders (19.1%) had near-zero abstinence probabilities. The remaining two latent classes were both low in adherence but varied in abstinence; Nonadherent Abstainers (17.8%) had high abstinence probabilities whereas Disengaged (15.0%) smokers had near-zero abstinence probabilities. This latent class structure persisted with the introduction of covariates (which were not treated as auxiliary variables and could have altered the latent class structure), with only minor changes in item response probabilities and latent class prevalence.
Figure 2.
Latent class prevalence and the estimated probability of abstinence (solid line) and medication adherence (dashed line) in 3-day parcels in the first 27 days postquit (N = 1045) for the unconditional five-class repeated measures latent class analyses (RMLCAs) solution.
Treatment was significantly related to latent classes in conditional models (Supplementary Table 2). Receiving combination NRT rather than patch alone increased the odds of membership in low-adherence classes, but was not related to abstinence among the adherent classes (ie, effectiveness). Those receiving combination NRT were more likely than those receiving only patch to be Disengaged than Adherent Abstainers (odds ratio [OR] = 8.95), Partial Responders (OR = 6.67), or Nonresponders (OR = 16.67), and were significantly more likely to be Nonadherent Abstainers than Adherent Abstainers (OR = 23.70), Partial Responders (OR = 17.58), or Nonresponders (OR = 41.08). Although the pattern was similar for varenicline, the only varenicline effect (vs. patch alone) to reach Benjamini–Hochberg corrected significance was increased likelihood of being a Nonadherent Abstainer rather than Nonresponder (OR = 4.69).
Treatments did not differentiate among adherent classes (Adherent Abstainers, Partial Responders, and Nonresponders). As such, treatment response rates did not differ among regimens taken with at least moderate adherence. In supplemental analyses comparing varenicline and combination NRT (not shown), combination NRT reduced adherence without increasing abstinence among those taking the medication regularly, relative to varenicline. The odds of membership in the two nonadherent classes were greater in those receiving combination NRT (vs. varenicline) relative to all three adherent classes, and membership among the adherent latent classes did not differ between varenicline and combination NRT.
The final model (Supplementary Table 2) contained the following covariates associated with latent class at p < .05: years smoking, number of past quit attempts, longest period of past abstinence, cigarette dependence as measured by the Fagerström Test for Cigarette Dependence, and minority status (identifying as a member of a racial or ethnic minority group). Only years smoking interacted with treatment. The risk of Disengagement was greater (at p < .05) with more years smoking in the patch-only condition (OR = 2.06 vs. Adherent Abstainers, OR = 2.17 vs. Nonadherent Abstainers, OR = 2.08 vs. Nonresponders, and OR = 2.13 vs. Partial Responders), but this relation was attenuated by combination NRT (OR = 0.41 vs. Adherent Abstainers, OR = 0.45 vs. Nonadherent Abstainers, OR = 0.34 vs. Nonresponders, and 0.41 vs. Partial Responders). Although the relation between years smoked and latent class was not significant at the Benjamini–Hochberg critical p value, the interaction between years smoking and combination NRT was, with combination NRT reducing the risk of disengagement among veteran smokers (OR = 0.34) versus patch alone. Varenicline had a similar interaction effect (OR = 0.41), but it was not significant at the corrected p value. No other treatment by covariate interaction was significant.
Greater cigarette dependence was associated with significantly increased risk of treatment Nonresponse (OR = 1.52 vs. Partial Response) and decreased likelihood of being able to quit without medication, that is, be a Nonadherent Abstainer rather than Disengaged, OR = 0.54, even with Benjamini–Hochberg correction. Higher Fagerström Test for Cigarette Dependence scores were associated with greater risk of Nonresponse than Adherent Abstinence (OR = 1.35), lower likelihood of Nonadherent Abstinence than Adherent Abstinence (OR = 0.70), and lower likelihood of Partial Response than Disengagement (OR = 0.69), but these relations were not significant with Benjamini–Hochberg correction.
Minority status was associated with greater risk of smoking, such that members of minority groups were more likely to be Disengaged than Adherent Abstainers (OR = 1.84), Nonadherent Abstainers (OR = 1.85), or Partial Responders (OR = 1.79), and were more likely to be Nonresponders than Partial Responders (OR = 1.47) or Adherent Abstainers (OR = 1.50).
Quitting history was associated with latent class, such that more quit attempts were associated with increased odds of treatment response (being an Adherent Abstainer rather than Nonresponder, OR = 1.45), but this association was not significant after Benjamini–Hochberg correction. Longer quit durations in days were associated with significantly increased odds of Adherent Abstinence than Partial Response (OR = 1.52) after Benjamini–Hochberg correction. Other associations with past abstinence duration, increased odds of Adherent Abstinence versus Nonresponse (OR = 1.32) and Disengagement (OR = 1.32) and increased odds of Nonadherent Abstinence versus Partial (OR = 1.49), were not significant at corrected p values. Gender was unrelated to latent class membership. Age was related but was dropped from the model due to collinearity with years smoking. In secondary models, menthol use, income, and marital status were also included; this did not alter the pattern of results, so the simpler model is displayed in Supplementary Table 2.
Latent classes differed significantly in terms of biochemically confirmed 6-month abstinence (see Supplementary Figure 1). Adherent Abstainers achieved a 62.0% abstinence rate at 6-month follow-up, significantly higher than the abstinence rates in the other four latent classes. Nonadherent Abstainers’ abstinence rate of 42.7% was significantly lower than that of Adherent Abstainers, but higher than all other classes. Partial Responders attained a 16.7% abstinence rate. Nonresponders and the Disengaged quit at similarly low rates (5%).
Discussion
Results indicated that nonadherence was prevalent and related to pharmacotherapy regimen, individual differences, and 6-month confirmed abstinence rates. The following discussion addresses the research questions that motivated this research.
How Adherent Are Smokers to Pharmacotherapy Regimens in the First 27 Days of Cessation Efforts, and Does This Differ by Regimen?
Self-reported adherence to nicotine patch therapy exceeded 80%. Varenicline adherence was significantly lower, but still above 75%. Use of a patch and at least 4 lozenges per day was below 50%, due to low lozenge use. Although these mean adherence rates seem strong for patch and varenicline, these self-reported (not imputed) data are likely upper-bound estimates and do not capture declines in adherence over time.2,3 Bimodal distribution of adherence rates, with modes near 0% and 100%, and the fact that the most prevalent latent class was characterized by consistent adherence and abstinence also suggest that nonadherence occurs in a subpopulation of smokers that might be profitably targeted in future research. Although roughly one-third of participants were nonadherent, the remaining two-thirds took medication as directed more days than not.
What Distinct Patterns or Classes of Abstinence and Adherence Emerge in the First 27 Days of Quitting, and How Do These Relate to Pharmacotherapy Regimen?
Three latent classes with high levels of adherence varied in quitting success (indicated by high-, moderate-, or low-abstinence probabilities across time). Two nonadherent classes similarly differed in abstinence (those who were engaged in quitting, but not fully adherent to treatment, vs. those who were disengaged from both treatment and quitting). Results showed that abstinence rates did not differ by regimen when medications were taken adherently. Combination NRT increased (vs. patch) the odds of classification in the Nonadherent Abstainer class, relative to the adherent Partial and Nonresponder classes, perhaps due to partial adherence. Varenicline increased odds of Nonadherent Abstinence versus Nonresponse. Perhaps full adherence to these regimens is particularly challenging among those most sensitive to its side effects, and this promotes abstinence while suppressing adherence. The enhanced treatments may be harder to take consistently (eg, due to side effects or inconvenience/burden), but still promote abstinence when taken less often than directed. Varenicline has greater side effects (eg, nausea or sleep disturbance), at least in some smokers, than patch alone,12,33,34 and combination NRT introduces side effects of lozenge use (eg, indigestion, hiccups, or mouth problems), which occurred significantly more often in the combination NRT than the patch condition in this study.12 These results suggest that greater investigation of use patterns is needed to understand the causes of the differential effectiveness of the three agents observed in other clinical trials,13–15,35 but not replicated in this trial.12
What Individual Characteristics Are Associated With Abstinence–Adherence Class Membership?
In support of the validity of the latent class solution, smoking history and cigarette dependence were associated with latent class membership. Combination NRT mitigated relations between years smoking and greater risk of being disengaged from quitting. This interaction suggests that enhanced treatment may have greater benefits among smokers with more extensive smoking histories. This was independent of number of past quit attempts, which was a covariate in the final model. Similarly, longer past abstinence was associated with Adherent Abstinence versus Partial Response. Other studies show that more quit attempts and longer prior abstinence are associated with greater quitting success,36,37 perhaps through learning, motivation, or environmental support. In addition, greater cigarette dependence was associated with smoking (greater risk of Nonresponse and Disengagement, reduced odds of Nonadherent Abstinence relative to select classes), consistent with research linking Fagerström Test for Cigarette Dependence scores with greater difficulty quitting.37,38
Minority status was associated with latent classes, such that members of minority groups were more likely to be Disengaged than in any other class except Nonresponders, and were more likely to be Nonresponders than Partial Responders, independent of menthol use, income, and marital status. Thus, members of minority groups were both less likely to use medications adherently and less likely to abstain while using them. Research into malleable factors that may suppress minority group members’ use of (eg, low health literacy39) and response to pharmacotherapies is needed. In sum, these results were consistent with research linking covariates to difficulty quitting,36–41 and helped differentiate treatment response from treatment engagement (eg, showing that cigarette dependence and minority status are associated with continued smoking even in adherent latent classes).
Do Abstinence–Adherence Classes Differ in Terms of Longer-Term Abstinence Rates of Public Health Significance?
Markedly and significantly different confirmed 6-month abstinence rates among the classes supported their clinical significance. Consistently abstinent latent classes had impressive 6-month abstinence rates, but those who were also consistently adherent did significantly better (60% abstinent) than those who abstained without adhering (43%). These high rates of abstinence among highly adherent individuals may reflect medication effects, or motivation, perceived treatment efficacy, or similar third variables that promote full adherence and prevent relapse. The fact that Nonresponders who took medication consistently during the first month, but continued smoking, did not fare better at 6 months (5% abstinent) than those who were Disengaged suggests that the factors that drive adherence in Nonresponders are insufficient for long-term quitting, however. Both Partial (17%) and Nonresponders (5%) who were highly adherent had significantly lower 6-month abstinence rates than the two Abstainer classes (regardless of adherence). Thus, adherence was related to 6-month abstinence in the context of abstinence, but not in the context of intermittent or continued smoking.
Limitations
These data were derived from an open-label comparative effectiveness trial of three agents without placebo control that showed no differences in treatment effectiveness after 1 week postquit.12 As such, it is not possible to determine the extent to which regimen intensity (vs. side effects, expectancies, etc.) is the key dimension associated with differential adherence across regimens and the findings may not generalize to other contexts in which treatments differ in sustained abstinence rates.13–15 This was also an analysis of abstinence status rather than smoking heaviness and does not fully capture change. Arbitrary time units were used (eg, 3-day parcels), with some loss of temporal resolution and missing data. Analysis of 6-month abstinence rates was intent-to-treat with all missing values treated as smoking, as it is not yet possible to incorporate multiple imputation in RMLCA. In addition, these data do not disentangle reciprocal or third-variable (eg, motivation, self-efficacy) influences on abstinence and adherence.10 It is possible that self-selection factors that influence latent class membership differ across the treatment arms of the study, and thus, we cannot infer that treatments caused differences in latent class membership. As such, interpreting treatment relations with latent class is not as straightforward as in analysis of randomized interventions on outcomes, given potential differences in latent class determinants across conditions. It is also premature to conclude that membership in favorable latent classes mediates treatment effects on 6-month abstinence, as mediation analyses were not conducted due to the probabilistic nature of assignment of individuals to classes. In addition, the sample enrolled may not represent the broader population of smokers fully. Results may not generalize to self-quitters, those with severe mental illness, and racial and ethnic groups not well represented in this sample. Finally, RMLCA power and sample size estimation require extensive simulation42; this was not done a priori in this secondary analysis. Power may be low to detect sizeable differences in covariates among classes, as this depends on the size of the latent classes. As such, some class comparisons may be underpowered in this study and replication of covariate relations with latent classes is needed.
Conclusions
Combination NRT and varenicline are less likely to be used as directed than patch monotherapy, which may undercut their effectiveness. Among highly adherent participants, combination NRT and varenicline did not appear to enhance first-month abstinence probabilities. Partial adherence to varenicline and combination NRT may still promote abstinence and mitigate risks that accrue with years smoking, however. Person-focused analyses generated new understanding of treatment use and its effects, and identified individual differences associated with membership in high-risk latent classes. This approach may aid development of interventions to mitigate such risks.
Funding
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (grant number R01HL109031 to Timothy B. Baker) and by the National Institute on Drug Abuse at the National Institutes of Health (grant number R01DA033303 to Danielle E. McCarthy and Saul Shiffman). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The parent clinical trial is registered at ClinicalTrials.gov (NCT01553084).
Declaration of Interests
The authors have no competing interests to declare. Both authors attest that they had full access to all of the secondary data used in the study and take responsibility for the accuracy of the data analysis.
Supplementary Material
Acknowledgments
We thank the staff of the Center for Tobacco Research and Intervention at the School of Medicine and Public Health, University of Wisconsin–Madison for sharing these data and Wendy Theobald for her assistance in preparing the manuscript.
References
- 1. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services; Public Health Service; 2008. [Google Scholar]
- 2. Balmford J, Borland R, Hammond D, Cummings KM. Adherence to and reasons for premature discontinuation from stop-smoking medications: data from the ITC Four-Country Survey. Nicotine Tob Res. 2011;13(2):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Catz SL, Jack LM, McClure JB, et al. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine Tob Res. 2011;13(5):361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burns EK, Levinson AH. Discontinuation of nicotine replacement therapy among smoking-cessation attempters. Am J Prev Med. 2008;34(3):212–215. [DOI] [PubMed] [Google Scholar]
- 5. Smith SS, Keller PA, Kobinsky KH, et al. Enhancing tobacco quitline effectiveness: identifying a superior pharmacotherapy adjuvant. Nicotine Tob Res. 2013;15(3):718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swan GE, McClure JB, Jack LM, et al. Behavioral counseling and varenicline treatment for smoking cessation. Am J Prev Med. 2010;38(5):482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159(17):2033–2038. [DOI] [PubMed] [Google Scholar]
- 8. Hollands GJ, McDermott MS, Lindson-Hawley N, Vogt F, Farley A, Aveyard P. Interventions to increase adherence to medications for tobacco dependence. Cochrane Database Syst Rev. 2015;2:CD009164. [DOI] [PubMed] [Google Scholar]
- 9. Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther. 2008;30(10):1852–1858. [DOI] [PubMed] [Google Scholar]
- 10. Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction. 2007;102(5):809–814. [DOI] [PubMed] [Google Scholar]
- 11. Ferguson SG, Gitchell JG, Shiffman S. Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction. 2012;107(7):1349–1353. [DOI] [PubMed] [Google Scholar]
- 12. Baker TB, Piper ME, Stein JH, et al. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: a randomized clinical trial. JAMA. 2016;315(4):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018;5:CD000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2016;5:CD006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97(9):1093–1108. [DOI] [PubMed] [Google Scholar]
- 17. Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–284. [DOI] [PubMed] [Google Scholar]
- 18. Liberman JN, Lichtenfeld MJ, Galaznik A, et al. Adherence to varenicline and associated smoking cessation in a community-based patient setting. J Manag Care Pharm. 2013;19(2):125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah SD, Wilken LA, Winkler SR, Lin SJ. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc (2003). 2008;48(5):659–665. [DOI] [PubMed] [Google Scholar]
- 20. Collins LM, Lanza ST. RMLCA and LTA. In: Collins LM, Lanza ST, eds. Latent Class and Latent Transition Analysis: With Applications in the Social, Behavioral, and Health Sciences. Hoboken, NJ: Wiley; 2010:181–224. [Google Scholar]
- 21. Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12:101–112. [Google Scholar]
- 22. Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102(10):1564–1573. [DOI] [PubMed] [Google Scholar]
- 23. Blankers M, Smit ES, van der Pol P, de Vries H, Hoving C, van Laar M. The missing=smoking assumption: a fallacy in internet-based smoking cessation trials?Nicotine Tob Res. 2016;18(1):25–33. [DOI] [PubMed] [Google Scholar]
- 24. Salim A, Mackinnon A, Christensen H, Griffiths K. Comparison of data analysis strategies for intent-to-treat analysis in pre-test-post-test designs with substantial dropout rates. Psychiatry Res. 2008;160(3):335–345. [DOI] [PubMed] [Google Scholar]
- 25. Marrone GF, Shakleya DM, Scheidweiler KB, Singleton EG, Huestis MA, Heishman SJ. Relative performance of common biochemical indicators in detecting cigarette smoking. Addiction. 2011;106(7):1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 27. Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom test for cigarette dependence. Nicotine Tob Res. 2012;14(1):75–78. [DOI] [PubMed] [Google Scholar]
- 28. Muthén LK, Muthén BO.. Mplus User’s Guide. 8th ed. Los Angeles, CA: Muthén & Muthén; (1998–2017). [Google Scholar]
- 29. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Statist Soc. 1995;57(1):289–300. [Google Scholar]
- 30. Lanza ST, Tan X, Bray BC. Latent class analysis with distal outcomes: a flexible model-based approach. Struct Equ Modeling. 2013;20(1):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: three-step approaches using Mplus. Struct Equ Modeling. 2014;21(3):329–341. [Google Scholar]
- 32. Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. J Classification. 1996;13(2):195–212. [Google Scholar]
- 33. Aubin HJ, Bobak A, Britton JR, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax. 2008;63(8):717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lerman C, Schnoll RA, Hawk LW Jr, et al. ; PGRN-PNAT Research Group. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koegelenberg CF, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA. 2014;312(2):155–161. [DOI] [PubMed] [Google Scholar]
- 36. Hyland A, Borland R, Li Q, et al. Individual-level predictors of cessation behaviours among participants in the International Tobacco Control (ITC) four country survey. Tob Control. 2006;15(suppl 3):iii83–iii94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, West R. Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study. Addict Behav. 2009;34(4):365–373. [DOI] [PubMed] [Google Scholar]
- 38. Sweitzer MM, Denlinger RL, Donny EC. Dependence and withdrawal-induced craving predict abstinence in an incentive-based model of smoking relapse. Nicotine Tob Res. 2013;15(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Osborn CY, Cavanaugh K, Wallston KA, et al. Health literacy explains racial disparities in diabetes medication adherence. J Health Commun. 2011;16(suppl 3):268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trinidad DR, Pérez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101(4):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fu SS, Sherman SE, Yano EM, van Ryn M, Lanto AB, Joseph AM. Ethnic disparities in the use of nicotine replacement therapy for smoking cessation in an equal access health care system. Am J Health Promot. 2005;20(2):108–116. [DOI] [PubMed] [Google Scholar]
- 42. Dziak JJ, Lanza ST, Tan X. Effect size, statistical power and sample size requirements for the bootstrap likelihood ratio test in latent class analysis. Struct Equ Modeling. 2014;21(4):534–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.