Abstract
Background and Aims
The development of Arundo donax as a biomass crop for use on drought-prone marginal lands in areas with warm to hot climates is constrained by the lack of variation within this species. We investigated the effect of morphological and physiological variation on growth and tolerance to drought under field conditions in three ecotypes of A. donax collected from habitats representing a climate gradient: a pre-desert in Morocco, a semi-arid Mediterranean climate in southern Italy and a warm sub-humid region of central Italy.
Methods
The three A. donax ecotypes were grown under irrigated and rain-fed conditions in a common garden field trial in a region with a semi-arid Mediterranean climate. Physiological and morphological characteristics, and carbohydrate metabolism of the ecotypes were recorded to establish which traits were associated with yield and/or drought tolerance.
Key Results
Variation was observed between the A. donax ecotypes. The ecotype from the most arid habitat produced the highest biomass yield. Stem height and the retention of photosynthetic capacity later in the year were key traits associated with differences in biomass yield. The downregulation of photosynthetic capacity was not associated with changes in foliar concentrations of sugars or starch. Rain-fed plants maintained photosynthesis and growth later in the year compared with irrigated plants that began to senescence earlier, thus minimizing the difference in yield. Effective stomatal control prevented excessive water loss, and the emission of isoprene stabilized photosynthetic membranes under drought and heat stress in A. donax plants grown under rain-fed conditions without supplementary irrigation.
Conclusions
Arundo donax is well adapted to cultivation in drought-prone areas with warm to hot climates. None of the A. donax ecotypes exhibited all of the desired traits consistent with an ‘ideotype’. Breeding or genetic (identification of quantitative trait loci) improvement of A. donax should select ecotypes on the basis of stem morphology and the retention of photosynthetic capacity.
Keywords: Photosynthesis, bioenergy, drought, biomass, chlorophyll fluorescence, common garden
INTRODUCTION
The rapid growth of biomass crops is associated with high rates of photosynthesis (PN) and transpirative water loss (Karp and Shield, 2008; Angelini et al., 2009). Supplying the large volumes of water needed to sustain growth is a major challenge for the cultivation of biomass crops in drought-prone marginal lands in areas with warm to hot climates (Cosentino et al., 2006; Tuck et al., 2006). Field trials in the Mediterranean have shown that the giant reed (Arundo donax) outperforms other biomass crops in terms of primary metabolism, growth and biomass yield under both irrigated and rain-fed conditions (Angelini et al., 2009; Mantineo et al., 2009). The clonal reproduction of A. donax may have impaired its development as a biomass crop due to a lack of genetic diversity that can be exploited in breeding more productive and/or drought-tolerant varieties (Pilu et al., 2014; Valli et al., 2017). However, previous studies have reported variation in the biomass yield of A. donax ecotypes collected from different habitats (Cosentino et al., 2006; Sánchez et al., 2015; Haworth et al., 2017c). We established a common garden field trial in a semi-arid region with a strong Mediterranean climate involving three ecotypes of A. donax that have demonstrated contrasting responses to water availability in order to investigate whether these ecotypic differences in yield might be associated with variation in physiological and/or morphological traits.
The three ecotypes of A. donax were collected from habitats representing a climate gradient: a warm-humid sub-Mediterranean region of Central Italy [the ‘Italian’ ecotype from Haworth et al. (2017b), hereafter referred to as the ‘Tuscan’ ecotype]; a semi-arid Mediterranean region of Sicily, southern Italy [‘ecotype 6’ from the slopes of Mount Etna (Cosentino et al., 2006), hereafter referred to as the ‘Sicilian’ ecotype]; and a strongly arid pre-desert area of Marrakech, Morocco (hereafter referred to as the ‘Moroccan’ ecotype). Arundo donax is frequently found adjacent to water bodies growing in soils with high water availability (Danin, 2004; Watts and Moore, 2011), suggesting that the ‘yield penalty’ imposed by drought may be too severe for a fast-growing species such as A. donax to be successfully cultivated in drought-prone marginal lands. Species or varieties with lower rates of PN and stomatal conductance (Gs) generally show smaller proportional reductions in yield after drought stress than their faster growing counterparts with greater rates of photosynthetic gas exchange (Lauteri et al., 2014). Arundo donax does show pronounced reduced biomass gain in the short term during severe drought (Haworth et al., 2017b). However, while rain-fed plants do produce lower biomass yield than irrigated plants over the whole growing season, these reductions are often not statistically significant (Cosentino et al., 2014; Haworth et al., 2017c; Curt et al., 2018). Maintenance of the productive performance of A. donax under drought-stressed conditions may be related to an extensive root system enabling access to water deep within the soil profile (Mann et al., 2013); adjustment of stomatal physiological behaviour to optimize water use efficiency (WUE) under a range of growth conditions (Haworth et al., 2018a, c); and modification of the emission of protective antioxidants such as volatile organic compounds (Ahrar et al., 2015; Haworth et al., 2017a).
Previous studies have observed remarkably little variation between A. donax ecotypes in gas exchange measurements of PN and Gs under well-watered and drought-stressed conditions (Sánchez et al., 2015; Haworth et al., 2017b, c; Zegada-Lizarazu et al., 2018) consistent with evidence indicating a lack of genetic variability within the species (Khudamrongsawat et al., 2004; Ahmad et al., 2008; Mariani et al., 2010; Pilu et al., 2014). However, to the best of our knowledge, more in-depth analyses of the photosynthetic capacities of contrasting A. donax ecotypes have not been reported in the literature. Physiological differences in the emission of isoprene have been observed between the Tuscan and Moroccan ecotypes under moderate drought stress. The increased emission of isoprene in the Moroccan ecotype may reflect the selective pressures induced by multigenerational growth in an environment characterized by high temperatures and the rapid onset of persistent drought events (Haworth et al., 2017a), due to the role played by isoprene as a mobile antioxidant in stabilizing photosynthetic membranes (Velikova and Loreto, 2005).
The biomass yield of A. donax is associated with the density, weight and height of stems (Cosentino et al., 2006; Angelini et al., 2009). Indeed, the greater yield of the Sicilian ecotype in trials was related to increased stem height (Haworth et al., 2017c). Observations of the Moroccan and Tuscan ecotypes grown in pots found differences in the morphology of xylem vessels (but not stem height), with the Moroccan ecotype possessing larger vessels and increasing the proportion of larger vessels under drought stress. In contrast, the Tuscan ecotype reduced xylem vessel diameter, thus lowering hydraulic conductivity and potentially increasing resistance to xylem embolism during episodes of reduced water availability or high evapotranspirative demand (Haworth et al., 2017b). Stem morphological parameters have been shown to be heritable traits in A. donax (Pilu et al., 2014), suggesting that selection based upon stem morphology may enable the development of A. donax varieties on the basis of yield and drought tolerance characteristics.
We conducted a common garden experiment in Catania, Sicily, Italy, to assess the morphological and physiological characteristics of the Moroccan, Sicilian and Tuscan A. donax ecotypes and whether any variation in traits could be utilized in the development of more productive and/or drought-tolerant genotypes (a so-called ‘ideotype’: Donald, 1968). The aims of this study were to: (1) monitor leaf gas exchange, isoprene emission and photosynthetic capacity in the three ecotypes under rain-fed and irrigated conditions at key intervals related to soil water availability throughout the growing season; (2) investigate whether any variation in morphological traits influenced biomass yield in irrigated and rain-fed plots of the three A. donax ecotypes; and (3) assess whether any physiological and/or morphological characteristics of the A. donax ecotypes may be desirable in terms of enhanced growth and biomass yield and/or drought tolerance in the context of the development of A. donax as a viable biomass crop for cultivation in drought-prone rain-fed marginal lands.
MATERIALS AND METHODS
Plant material and field site
Rhizomes were collected from single clonal stands in Marrakesh, Morocco (30 °C mean summer temperature, approx. 200 mm precipitation per annum), the slopes of Mount Etna in Sicily, Italy (26 °C mean summer temperature, approx. 400 mm precipitation) and Sesto Fiorentino, Florence, Italy (mean summer temperature of 23 °C, approx. 800 mm precipitation), during March 2015. After collection, the rhizomes were cut into portions 20 cm in length (approx. 200 g in weight) with at least one visible growing bud per rhizome. The rhizomes were planted in six 4.0 × 3.0 m sized plots for each A. donax ecotype at a distance of 50 cm between plants within rows 0.8 m apart with six plants per row (a total of 30 plants per plot at a density of 2.5 plants m–2) at the agricultural research station of the University of Catania (37°25’N, 15°03’E; 10 m a.s.l.). To minimize edge effects, a 2.4 m thick border of three rows of A. donax was planted around the experimental field. To allow the plants to establish, irrigation equivalent to 100 % of evapotranspiration (ETC) was applied to all plants in 2015. In February 2016, on day 45 of the study (day 1 being 1 January 2016) the stems were cut at a height of 10 cm and new stems were allowed to develop for the 2016 growing season. In the 2016 growing season, irrigation of half of the experimental field was stopped, while the remainder of the field continued to receive supplementary irrigation (i.e. three irrigated plots and three rain-fed plots for each ecotype) equivalent to 100 % of ETc determined as:
where Eo is the evaporation of water from a class-A pan (mm); Kp is the pan coefficient; and Kc is the crop growth stage (ranging from 0.5 to 1.6). Daily rainfall was subtracted from the daily calculation of water to be supplied as irrigation (Allen et al., 1998). Stem height, the total number of stems per rhizome and the number of green leaves on five stems per plot were recorded on days 96, 119, 152, 181, 211, 255, 305 and 415. To be consistent with standard commercial farming practices used in the cultivation of A. donax, the final harvest of the A. donax plants was conducted on day 415 (February 2017) as this is the period of the year when the moisture content of the stems is lowest and drying of the plants would require the least energy (Cosentino et al., 2006). Yield as dry matter was calculated by collecting 1 m2 sub-samples of the stems and leaves from the plots of each ecotype and treatment, and then oven drying them at 105 °C until their weight remained constant. Soil samples were collected from a depth of 0–90 cm, and the soil water content was determined gravimetrically by drying the soil in a ventilated oven at 105 °C (Killi et al., 2014). The soil at the site is a clay-rich xerofluvent soil. Full details of the location are provided in Cosentino et al. (2006).
Leaf gas exchange measurements
Leaf gas exchange analyses were performed in four measurement periods during the growing season: first (days 123–134), second (days 200–211), third (days 249–260) and fourth (days 298–09) (Fig. 1). All measurements were performed in situ using a LiCor Li6400XT plant photosynthesis system connected to a 6400-40 leaf chamber fluorimeter cuvette (LiCor, Lincoln, NE, USA). Simultaneous point measurements of PN, Gs, the internal sub-stomatal concentration of CO2 (Ci) and the actual quantum efficiency of PSII (ΦPSII) (Genty et al., 1989) were performed on the second uppermost fully expanded leaf on two plants from the centre of each plot. The following environmental conditions were set in the cuvette: 2000 μmol m–2 s–1 photosynthetically active radiation (PAR; 10 % blue and 90 % red light), 400 ppm [CO2], leaf temperature set to 30 °C (tight control of leaf temperature under field conditions is difficult: leaf temperature varied from 25.3 to 36.1 °C with a mean value of 29.9 ºC and s.d. of 2.3 ºC) and a relative humidity (RH) of 60 %. To reduce diffusive leaks through the chamber gasket, a supplementary gasket was added and the IRGA (infrared gas analyser) exhaust air was fed into the space between the chamber and the supplementary external gasket. To determine ΦPSII, the multiphase fluorescence setting was used with an initial saturating pulse of 8000 μmol m–2 s–1. However, it was not possible to estimate mesophyll conductance (Gm) of the A. donax plants using the variable J method (Harley et al., 1992), possibly due to underestimation of maximal fluorescence under light-adapted conditions (Fm’) (Loriaux et al., 2013) or limitation of the approach associated with species with high Gs and Gm where the difference between the internal sub-stomatal concentration of [CO2] and that within the chloroplast envelope is less apparent (Loreto et al., 1992).
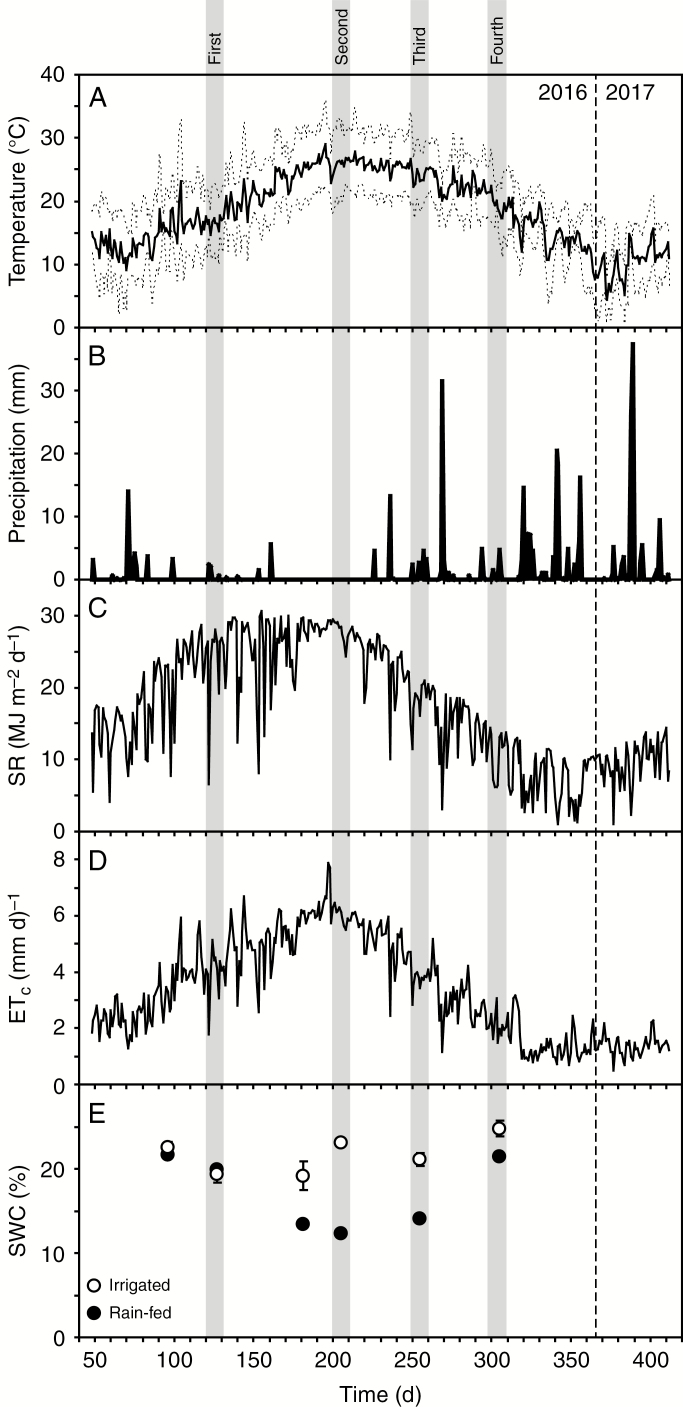
Fig. 1.
Growth conditions during the study period: (A) daily temperature at the site – the upper dashed line shows the maximum daily temperature, the solid line shows the mean daily temperature and the lower dashed line shows the minimum daily temperature; (B) daily precipitation; (C) daily solar radiation; (D) evapotranspiration (ETc); and (E) gravimetric measurements of soil water content taken at the mid-point of each measurement period from the irrigated and rain-fed portions of the experimental field. The four measurement periods are marked by horizontal grey shading. Day 0 was considered to be 1 January 2016. The previous year’s (2015 growing season) A. donax was harvested on day 45. The dashed vertical line at day 366 marks the end of the 2016 calendar year.
The same leaves used for point measurements of gas exchange and chlorophyll fluorescence were also used in determination of the response of PN to increasing Ci. The [CO2] within the leaf cuvette was lowered to 50 μmol mol–1 for 30 min to open stomata fully and remove any diffusive limitations to PN before [CO2] was increased in stages every 3–4 min after PN had stabilized ([CO2] steps: 50, 100, 200, 300, 400, 600, 800, 1000, 1200, 1400, 1600, 1800 and 2000 μmol mol–1) (Centritto et al., 2003). Leaf temperature remained at 30 °C and RH at 60 % throughout the PN–Ci response curve. The maximum carboxylation rate of ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) (Vcmax), the maximum rate of electron transport for regeneration of ribulose-1,5-bisphosphate (RuBP) (Jmax), triose phosphate utilization (TPU) and mesophyll conductance (Gm) were calculated from the PN–Ci curve corrected to a standard temperature of 25 °C following Ethier and Livingston (2004). Due to the extremely rapid stomatal closure (Haworth et al., 2018a) observed in drought-stressed A. donax, it was not possible to complete the PN–Ci response curve above a [CO2] of 400 μmol mol–1; stomatal limitations effectively prevented assessment of the biochemical assimilation of [CO2] (e.g. Lovelli and Perniola, 2014). Therefore it was only possible to calculate Vcmax from the PN–Ci response in the rain-fed plants under conditions of low soil water content during the second and third measurement period.
Isoprene emission
The emission of isoprene was measured in the field from the same leaves of A. donax used for gas exchange analysis under the same environmental settings, but using a LiCor Li6400 fitted with a 6 cm2 cuvette and LED light unit. When monitoring isoprene emission, air from the cuvette with the enclosed leaf passed through a biphasic adsorbent trap containing 30 mg of Tenax and 20 mg of Carboxen (Gerstel, Mülheim an der Ruhr, Germany). A pump (Elite 5, A. P. Buck, Orlando, FL, USA) was used to pass 2 L of air through each trap at a rate of 200 mL min–1. Measurements of the concentration of isoprene in the ambient air (blanks) were performed using an empty leaf cuvette before and after each measurement. The traps were then stored at 4 °C prior to analysis in the laboratory. Isoprene was first desorbed from traps at high temperature and then measured using a gas chromatograph–mass spectrometer (GC-MS; Agilent 7890A, Agilent Technologies, Santa Clara, CA, USA) connected to a thermal desorption unit (Gerstel) with an Agilent HP-INNOWAX (30 m × 0.32 mm × 0.15 μm) GC column. A 5977A mass selective detector with electron ionization operating at 70 eV was used for analysis. Isoprene was identified through comparison of the retention time and mass spectrum with an isoprene analytical standard (Sigma Aldrich, St. Louis, MO, USA) injected into the GC-MS at different concentrations. The isoprene analytical standard was also used to construct a calibration curve by injecting known concentrations of isoprene into the GC-MS. The data were analysed using Agilent MassHunter Workstation software.
Leaf sampling, determination of foliar relative water content and analysis of starch and sugars
Leaf samples were collected at the end of each measurement period after completion of the leaf gas exchange and chlorophyll fluorescence measurements. The first fully most expanded leaf was collected from the same two plants in each plot adjacent to the leaf that had been used for physiological analysis (n = 6 for each ecotype and treatment). The lower 4–5 cm of each leaf was removed to be used for determination of foliar relative water content (RWC) following the protocol of Diaz-Pérez et al. (1995). The remainder of the leaf was flash-frozen in liquid nitrogen before being stored at –80 °C prior to analysis of starch and sugar content. To identify and quantify soluble carbohydrates, 3 × 5 mL of a solution of EtOH/H2O (75/25) was used to extract carbohydrates from 300 mg of fresh leaf tissue. The ethanol was then reduced under a vacuum at 30 °C with a Büchi P12 Multivapor unit equipped with a Büchi V-855 vacuum controller (Buchi, Flawil, Switzerland), and the resulting pellet was then rinsed with 2 mL of Milli-Q water. The aqueous extract was then purified by solid–liquid extraction through -CHX and -SAX pre-packed Bond-Elute cartridges (Varian, Harbor City, CA, USA), and the eluate was completely reduced under a vacuum. The samples were then rinsed with ultrapure water and injected in a Series 250 LC binary pump equipped with an LC 30-RI detector (Perkin-Elmer). Soluble carbohydrates were then separated using a 7.7 × 300 mm Hi-Plex Ca column (Agilent Technologies, CA, USA) maintained at 85 ± 1 °C; the eluent used was ultra-pure water at a flow rate of 0.8 mL min–1 during a 20 min run. Individual carbohydrates were identified by comparison of retention times with those of carbohydrate standards (Sigma Aldrich). Starch was quantified following Chow and Landhäusser (2004) on the pellet of material that was produced during ethanol extraction for the analysis of soluble carbohydrates. Starch was digested into glucose using a mixture of enzymes consisting of 1000 U of α-amylase and 5 U of amyloglucosidase. The resultant glucose was then quantified using peroxidase–glucose oxidase/o-dianisidine reagent (Sigma-Aldrich), reading the absorbance at 525 nm after the addition of sulphuric acid.
Statistical analyses
Statistical analyses were performed using SPSS 24 (IBM, Armonk, NY, USA). One-way analysis of variance (ANOVA) with a least significant difference (l.s.d.) post-hoc test was used to assess differences in variance between samples associated with either ecotype or treatment effects, with homogenous groups denoted in figures by the use of identical letters. A multiple regression was used to identify which factors were significantly associated with the yield of the A. donax ecotypes.
RESULTS
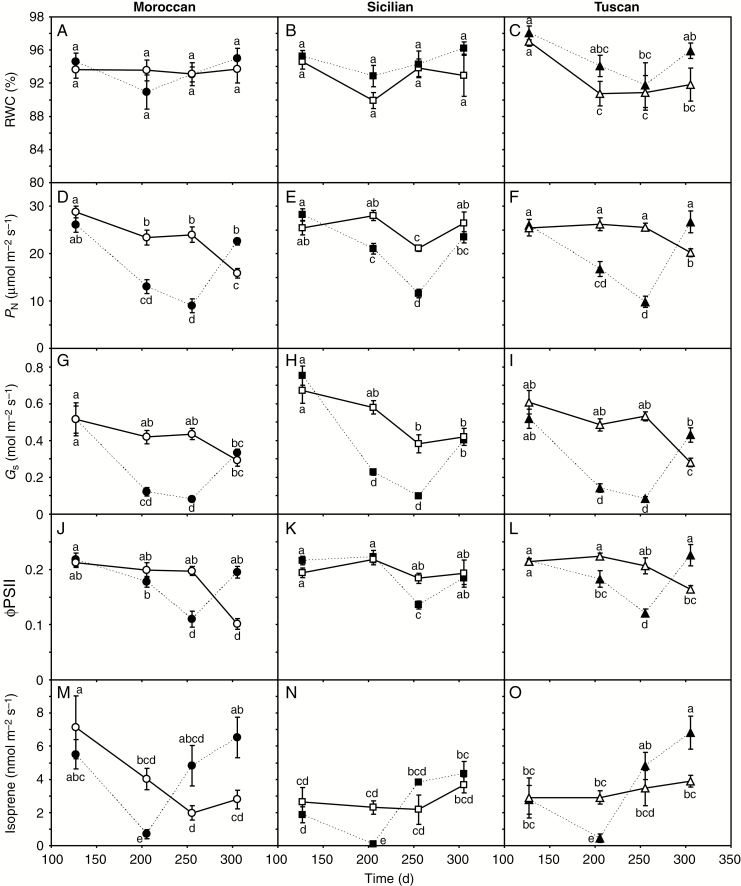
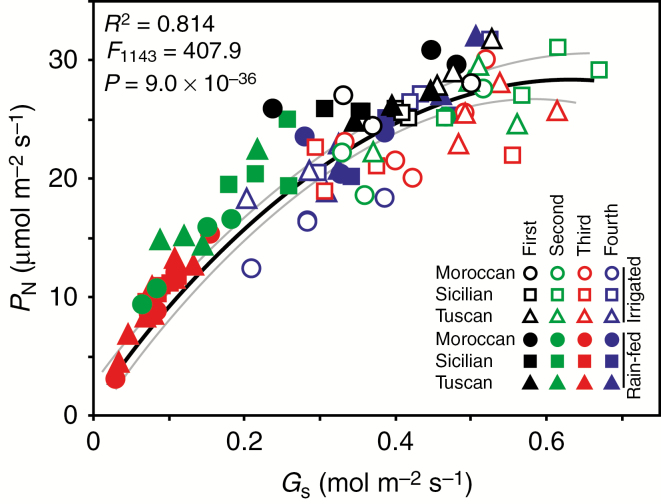
Over the course of the growing season as temperature (Fig. 1A) and ETC (Fig. 1D) rose and levels of precipitation fell, soil water content (SWC) in the upper 90 cm soil layer declined from about 23 % to a low of 11 % (Fig. 1E). This reduction in soil water availability corresponded to declines in PN and Gs in all three A. donax ecotypes, but foliar RWC was unaffected by SWC (Fig. 2A–I). When water availability did not limit gas exchange early in the growing season, the A. donax ecotypes exhibited similar high rates of PN of 25–30 μmol m–2 s–1 (Figs 2D–F and 3). Rates of Gs were highest in the Sicilian ecotype during the first measurement period (0.6–0.8 mol m–2 s–1) (one-way ANOVA: F1,34 = 6.688; P = 0.015). However, the Gs values of the irrigated Sicilian plants declined by 38 % over the growing season (Fig. 2H). In contrast, the Gs values of the irrigated Moroccan and Tuscan ecotypes remained constant during the first three measurement periods. Notably, in the fourth measurement period after the recovery of soil water levels, the rain-fed Moroccan and Tuscan ecotypes exhibited higher Gs, PN and ΦPSII than their irrigated counterparts (although Gs was higher in the rain-fed than in the irrigated Moroccan ecotype, this was not significant). In contrast, the irrigated and rain-fed Sicilian plants exhibited similar Gs, PN and ΦPSII values during the final measurement period (Fig. 2D–H). The rain-fed Moroccan and Tuscan plants showed an increase in isoprene emission beginning in the third period and continuing into the fourth, where they were statistically greater than the emission rates of their irrigated counterparts (one-way ANOVA: F1,22 = 10.283; P = 0.018). In contrast, the rain-fed Sicilian ecotype, while showing a similar pattern of increased isoprene emission, generally exhibited lower rates of isoprene emission in the third and fourth measurement periods that did not differ from values observed in irrigated plants (Fig. 2M–O) (one-way ANOVA: F1,10 = 3.851; P = 0.097). Photosynthesis was strongly related to Gs in the A. donax ecotypes at all of the measurement points throughout the growing season (Fig. 3) (linear regression: R2 = 0.814; F1,143 = 407.9; P = 9.0 × 10–36).
Fig. 2.
Foliar relative water content (RWC) (A–C), photosynthesis (PN) (D–F), stomatal conductance to water vapour (Gs) (G–I), the actual quantum efficiency of photosystem II (ΦPSII) (J–L) and isoprene emission (M–O) of Moroccan, Sicilian and Tuscan ecotypes of A. donax grown under irrigated (solid line, open symbols) and rain-fed (dashed line, filled symbols) conditions. Error bars indicate 1 s.e. standard error either side of the mean. Letters indicate homogenous groups for each parameter using a one-way ANOVA with an l.s.d. post-hoc test.
Fig. 3.
The relationship of photosynthesis (PN) to stomatal conductance (Gs) in Moroccan, Sicilian and Tuscan ecotypes of A. donax grown under irrigated and rain-fed conditions during the four measurement periods. Non-linear regression was used to assess the relationship. The solid black line represents the line of best fit and the grey lines either side of the linear regression indicate 95 % confidence intervals of the regression line.
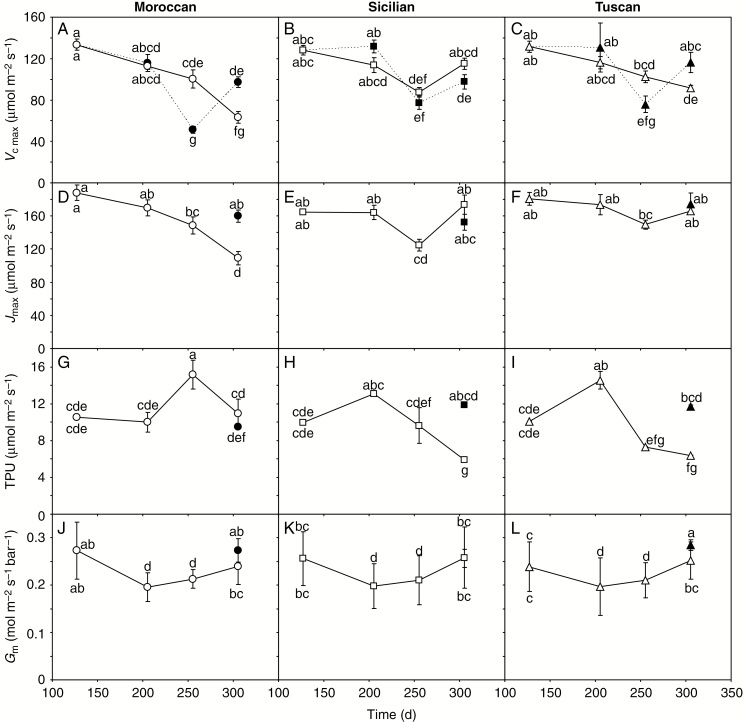
Early in the growing season when SWC was high (Fig. 1E), the photosynthetic capacity of the A. donax ecotypes was remarkably similar when assessed using PN–Ci response curves (Fig. 4A–D). Drought stress did not strongly affect Vcmax, with the exception of the third measurement period (Fig. 4A–C), which may be considered the maximum extent of stress, where the Vcmax values of the rain-fed plants were significantly lower than their irrigated counterparts in the Moroccan (one-way ANOVA: F1,10 = 27.793; P = 0.0004) and Tuscan (one-way ANOVA: F1,10 = 21.723; P = 0.0034) ecotypes. However, Vcmax values of the rain-fed Moroccan and Tuscan plants were 53 and 27 % greater, respectively, than those of their irrigated counterparts during the fourth measurement period (Fig. 5A–C). Due to the rapid stomatal closure of A. donax, it was not possible to complete the PN–Ci curves of the rain-fed plants during the second and third periods where SWC was at its lowest (Fig. 1E). The Jmax of irrigated Moroccan plants showed a progressive decline throughout the growing season, and by the final measurement period Jmax values of the rain-fed plants were 46 % higher than in the irrigated A. donax (Fig. 4D) (one-way ANOVA: F1,10 = 14.889; P = 0.012). In contrast, levels of Jmax in the irrigated Sicilian and Tuscan ecotypes were relatively constant throughout the growing season, with non-statistically significant differences between irrigated and rain-fed plants during the final fourth measurements (Fig. 4E, F). The TPU of irrigated plants was greatest in the Moroccan ecotype during the third measurement period, while the highest TPU values occurred in the second measurement period in the Sicilian and Tuscan ecotypes. Rain-fed Sicilian and Tuscan plants showed 102 and 83 % higher TPU values, respectively, than irrigated plants in the fourth period, whereas there was no difference in TPU values of the rain-fed and irrigated Moroccan plants (Fig. 4G–I) (one-way ANOVA: Sicilian, F1,10 = 46.115; P = 0.001; Tuscan, F1,10 = 13.168; P = 0.015; Moroccan, F1,10 = 0.004; P = 0.954). Mesophyll conductance of the irrigated plants ranged from 0.196 to 0.273 mol m–2 s–1 bar–1 during the growing season. Rates of Gm were higher, but not significantly, in the rain-fed Tuscan and Moroccan ecotypes during the final measurement period (Fig. 4J–L).
Fig. 4.
Parameters calculated from the PN–Ci response curves of Moroccan, Sicilian and Tuscan ecotypes of A. donax grown under irrigated (solid line, open symbols) and rain-fed (dashed line, filled symbols) conditions: (A–C) the maximum carboxylation rate of ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) (Vcmax); (D–F) the maximum rate of electron transport for regeneration of ribulose-1,5-bisphosphate (RuBP) (Jmax); (G–I) triose phosphate utilization (TPU); and (J–L) mesophyll conductance to CO2 (Gm). The PN–Ci response curves are available in Supplementary Data Fig. S1. Error bars indicate 1 s.e. either side of the mean. Letters indicate homogenous groups for each parameter using a one-way ANOVA with an l.s.d. post-hoc test.
Fig. 5.
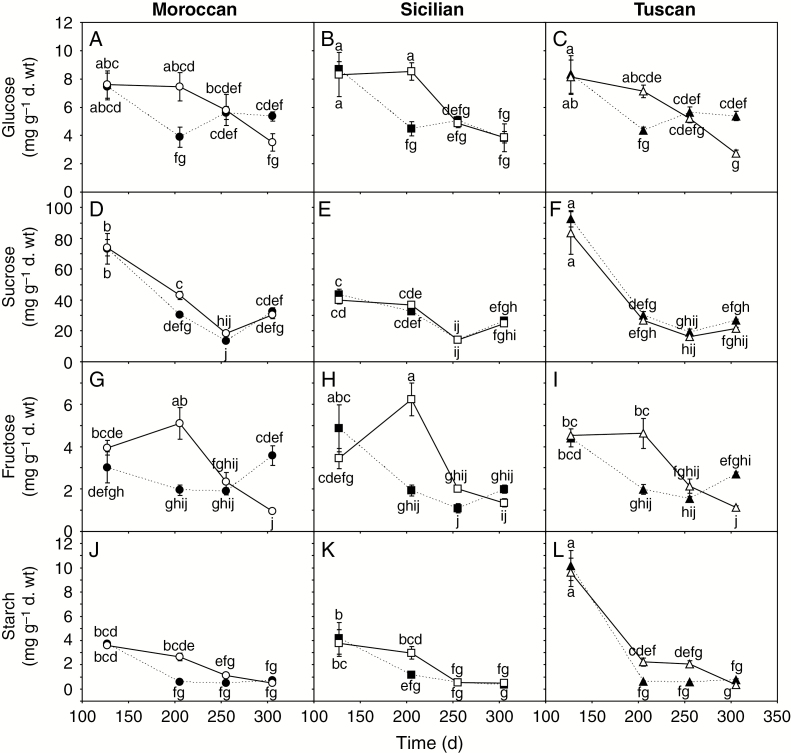
Concentrations of glucose (A–C), sucrose (D–F), fructose (G–I) and starch (J–L) in the leaves of the Moroccan, Sicilian and Tuscan ecotypes of A. donax grown under irrigated (solid line, open symbols) and rain-fed (dashed line, filled symbols) conditions. Error bars indicate 1 s.e. either side of the mean. Letters indicate homogenous groups for each parameter using a one-way ANOVA with an l.s.d. post-hoc test.
Analysis of foliar levels of carbohydrates suggested a general trend of declining concentrations of starch and sugars during the growing season in the irrigated A. donax plants (Fig. 5). The concentration of starch in the leaves of the rain-fed A. donax declined more rapidly than that of their irrigated counterparts, but all were statistically identical by the fourth measurement period (Fig. 5J–L). No differences were observed in the concentration of sucrose between leaves of irrigated and rain-fed A. donax plants (Fig. 5D–F). In rain-fed plants, levels of fructose generally declined over the first three measurement periods before increasing in the fourth period (Fig. 5G–I). The concentration of glucose also fell from the first to the second period before rising in the third and fourth measurement periods in the rain-fed A. donax ecotypes (Fig. 5A–C).
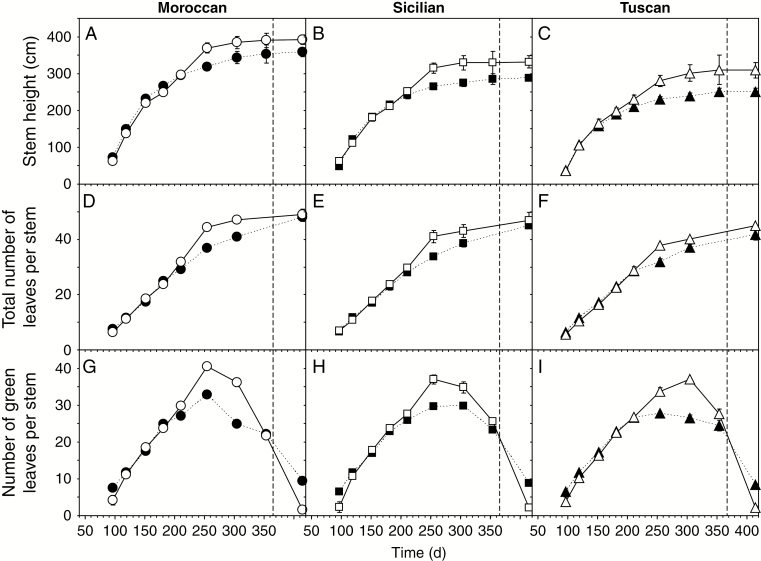
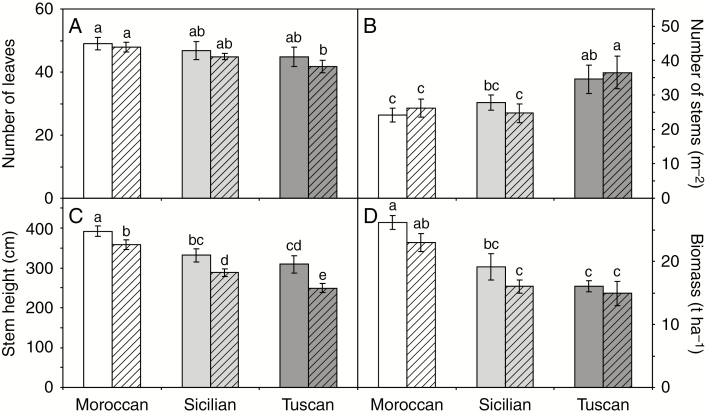
Stem height (Fig. 6A–C), the total number of leaves (Fig. 6D–F) and the number of green leaves (Fig. 6G–I) of the irrigated and rain-fed plants were broadly consistent from day 96 to 181 in the three A. donax ecotypes. After day 181, the irrigated plants showed increased growth relative to their rain-fed counterparts. However, it is noteworthy that from day 255 to 415, the greatest increases in stem height (39.3 cm) and leaf number (11.2) occurred in the rain-fed Moroccan plants, while growth had slowed in the irrigated A. donax (Fig. 6A). At the end of the growing season, rain-fed plants from all three ecotypes had green leaves, while the leaves of their irrigated counterparts were entirely senesced (Fig. 6G–I). At the final harvest, there was no significant difference in the number of leaves (Fig. 7A) or stem height (Fig. 7C) between irrigated and rain-fed plants of the same ecotype. Irrigation did not significantly affect density of stems; the Tuscan ecotype exhibited the highest density of stems and the Moroccan the lowest (Fig. 7B). Stem height was greatest in the Moroccan ecotype and lowest in the Tuscan (Fig. 7C), and these ecotypic differences corresponded to biomass yield, with the Moroccan A. donax being the most productive (Fig. 7D). Irrigation induced increased yield in all three ecotypes. However, this stimulation of growth was non-significant in all three ecotypes, ranging from 7.7 to 19.4 %. A multiple regression was used to predict biomass yield of A. donax from ecotype, irrigation regime, morphological characteristics (shown in Fig. 7), ΦPSII and gas exchange parameters (shown in Figs 2 and 4). Yield was significantly predicted (R2 = 0.991; F4,26 = 34.638; P = 0.002) by irrigation (P = 0.006), stem height (P = 0.033), PN in the third measurement period (P = 0.032), and both PN (P = 0.011) and Gs (P = 0.018) during the fourth period.
Fig. 6.
Stem height (A–C), the total number of leaves per stem (D–F) and the number of green leaves per stem (G–I) of the Moroccan, Sicilian and Tuscan ecotypes of A. donax grown under irrigated (solid line, open symbols) and rain-fed (dashed line, filled symbols) conditions during the 2016 growing season. Error bars indicate 1 s.e. either side of the mean. The dashed vertical line at day 366 marks the end of the 2016 calendar year.
Fig. 7.
Morphological parameters of the Moroccan, Sicilian and Tuscan ecotypes of A. donax grown under irrigated (open fill) and rain-fed (hatched fill) conditions at the final harvest on day 415: (A) number of leaves; (B) density of stems; (C) stem height; and (D) biomass. Error bars indicate 1 s.e. either side of the mean. Letters indicate homogenous groups for each parameter using a one-way ANOVA with an l.s.d. post-hoc test.
DISCUSSION
Low genetic variability in A. donax has constrained efforts to characterize ecotypic differences that may be utilized in the development of commercially viable productive and/or drought-tolerant ideotype varieties (Valli et al., 2017). This study has confirmed previous observations of remarkable similarity in photosynthetic gas exchange parameters between ecotypes (e.g. Sánchez et al., 2015; Haworth et al., 2017c; Zegada-Lizarazu et al., 2018) and identified differences in the pattern of changes in photosynthetic capacity throughout the growing season. Morphological variation that may account for the differences in yield observed between A. donax ecotypes in this (Fig. 7D) and other studies (e.g. Cosentino et al., 2006; Sánchez et al., 2015; Haworth et al., 2017c) was identified.
The three A. donax ecotypes examined in this study showed high rates of PN and Gs similar to previous gas exchange measurements on field-grown (e.g. Cosentino et al., 2016; Haworth et al., 2017a) and naturally occurring (Rossa et al., 1998) stands of A. donax. Early in the growing season, when soil water availability was not limiting to growth, the A. donax ecotypes showed very similar levels of PN, Gs and ΦPSII when determined on a leaf area basis (Fig. 2). This lack of variability in gas exchange and chlorophyll fluorescence parameters confirms the findings of previous analyses of the same (Cosentino et al., 2016; Haworth et al., 2017b, c; Zegada-Lizarazu et al., 2018) and different (Sánchez et al., 2015) A. donax ecotypes. The photosynthetic capacity observed in the three A. donax ecotypes during the same initial measurement period (Fig. 4) was very similar, accounting for the similarity in PN rates under the same growing conditions (Fig. 2D–F) and indicative of the lack of genetic variability found in the species (Khudamrongsawat et al., 2004; Mariani et al., 2010; Pilu et al., 2014). Notably, after the first measurements on irrigated A. donax, we observed variation between the ecotypes in photosynthetic capacity (Fig. 4) which corresponded to point measurements of PN (Fig. 2D–F). The retention of photosynthetic capacity throughout the growing season, as observed in the Sicilian ecotype (Figs 2E and 4B), may be a feature to be exploited in achieving higher PN and growth in A. donax. The decline in values of Vcmax and Jmax throughout the year in the irrigated Moroccan A. donax are indicative of the downregulation of photosynthetic capacity (e.g. Van Oosten et al., 1994; Wingler and Roitsch, 2008). However, foliar concentrations of sugars and starch in the Moroccan A. donax also fell over the course of the growing season, and similar patterns were observed in the carbohydrate metabolism of the Sicilian and Tuscan ecotypes. The stability of PN values at high [CO2] in the PN–Ci response curves indicated the absence of any feedback limitation to PN (Sharkey et al., 2007). Furthermore, in the irrigated A. donax plants, TPU did not follow variations in Vcmax and Jmax (Fig. 4) (cf. Yang et al., 2016). These findings suggest that downregulation of photosynthetic capacity in the leaves of the A. donax ecotypes was not associated with the availability of phosphorylated compounds (Sharkey and Vanderveer, 1989) or with the inhibition of the expression of photosynthetic enzymes (Sheen, 1990; Van Oosten et al., 1994) related to increased abundance of sugars (e.g. Wingler and Roitsch, 2008). One possible explanation for the decline in sugar and starch concentrations, despite high PN, in the leaves of A. donax over the course of the year (Fig. 5) may be the high availability of sinks for photosynthate (e.g. Paul and Driscoll, 1997) in the form of rapid stem elongation (Fig. 6) (Cosentino et al., 2016) or storage within the rhizome (Proietti et al., 2017). The higher levels of foliar starch found earlier in the year may reflect the impact of carbohydrates translocated from the rhizome to the stem to fuel rapid growth alongside high PN leading to a build-up of carbohydrates stored as starch (e.g. Asaeda et al., 2006). Future work should focus on the concentration of sugars in leaves along the stem to allow development of a more representative analysis of photosynthetic capacity and carbohydrate metabolism in A. donax.
Drought stress imposed by growth under rain-fed conditions induced statistically similar declines in both PN and Gs in the three A. donax ecotypes (Fig. 2D–I). Reduced PN under rain-fed conditions appeared to be largely the result of diffusive constraints associated with stomatal closure (Fig. 3) (Romero-Munar et al., 2017), illustrating the highly functional and effective stomatal control found in A. donax (Haworth et al., 2018a). This stomatal control probably accounted for the ‘isohydric’ behaviour of the rain-fed plants (Sade et al., 2012), as foliar RWC did not decline as drought progressed (Fig. 2A–C). Drought stress did not significantly affect the Vcmax of the rain-fed plants in the second measurement period, despite the lowest SWC values being observed during this interval (Fig. 1E). The maximum rate of carboxylation of RubisCO did significantly decline in the rain-fed Moroccan and Tuscan ecotypes by the third measurement period; moreover, Vcmax in both the rain-fed and irrigated Sicilian plants was lower in this period (Fig. 4A–C). This suggests that higher temperature (the third measurement period occurred after approx. 50 d of sustained high temperatures: Fig. 1A) was a contributory factor in reduced Vcmax due to lower specificity of RubisCO for CO2 (Jordan and Ogren, 1984), reduced activity of RubisCO activase (Feller et al., 1998) and lower solubility of CO2 (Berry and Björkman, 1980). Nonetheless, a combination of drought and high temperatures may have also negatively affected other processes. The thylakoid membranes within the chloroplast envelope are sensitive to damage induced by heat stress (Crafts-Brandner and Salvucci, 2002) and the excess energy associated with drought as photochemistry declines (Kalaji et al., 2016). The reduction in ΦPSII values of the rain-fed plants at the third measurement period may be consistent with reduced PSII performance due to thermal and photo-oxidative damage to the thylakoid membranes associated with high temperatures and drought (Flexas and Medrano, 2002; Killi et al., 2016; Haworth et al., 2018b). This fall in ΦPSII coincided with a rise in the emission of isoprene (Fig. 2M–O), possibly related to the function of isoprene as a mobile antioxidant stabilizing and protecting photosynthetic membranes (Velikova and Loreto, 2005). Analysis of A. donax suggests that the methylerythritol phosphate (MEP) pathway regulating isoprene synthesis is co-ordinated with the methionine pathway under drought stress, with increased formation of compounds such as dimethylsulphoniopropionate (Haworth et al., 2017a) which also serves as a protective antioxidant (Sunda et al., 2002; Husband and Kiene, 2007; Husband et al., 2012). The rain-fed Sicilian A. donax exhibited a less pronounced reduction in ΦPSII (Fig. 2K) and lower isoprene emission than the Moroccan and Tuscan ecotypes. This may suggest that the Sicilian ecotype possessed enhanced capacity in terms of protective heat-shock proteins (e.g. Heckathorn et al., 1998) and/or antioxidants (e.g. Sekmen et al., 2014) to mitigate the impact of heat and drought stress (e.g. Killi et al., 2016). The Moroccan A. donax has also been shown to emit more isoprene, but dissipate less energy as heat via non-photochemical quenching, than the Tuscan ecotype under less severe moderate drought stress (Haworth et al., 2017a). An inverse relationship between isoprene emission and non-photochemical quenching is found in many stressed and non-stressed plant species (Pollastri et al., 2014), suggesting that isoprene effectively regulates the electron transport flow in the photosynthetic membranes. This may suggest that further analysis of the protective physiologies of different A. donax ecotypes may enable identification of attributes to develop increased tolerance to drought and heat stress.
An inverse relationship was observed between stem height and density in the three A. donax ecotypes (Fig. 7). The largest biomass yield was observed in the Moroccan ecotype which exhibited the tallest but also the lowest density of stems. Moreover, stem height was the only morphological parameter that significantly contributed to yield and, as one of the few heritable traits found in A. donax (Pilu et al., 2014), may represent a key attribute for exploitation in attempts to increase biomass yield. The Moroccan ecotype has been shown to have more xylem vessels with a higher diameter than the Tuscan A. donax under both well-watered and drought-stressed conditions (Haworth et al., 2017b). The greater cross-sectional area of the xylem vessels in the Moroccan ecotype may reflect the influence of selective pressures induced by growth in an arid environment where water is available for growth for relatively short periods. In fact, the higher hydraulic conductivity of the Moroccan A. donax (Haworth et al., 2017b) may favour rapid growth (Fan et al., 2012) to exploit the brief intervals where conditions are suitable for growth (Grime, 1977). The density of wood is inversely related to the diameter of xylem vessels (Preston et al., 2006). Indeed, the Moroccan stems have been shown to possess a lower structural density than their Tuscan counterpart (Haworth et al., 2017b). Moreover, the differences in water transport structures may also relate to the chemical composition of the stems in terms of the proportions of lignin, cellulose and hemicellulose (e.g. Novaes et al., 2010), a consideration for future work if the A. donax material is to be used as a source for bio-compounds (Yoshida et al., 2008; Saikia et al., 2015). It is noteworthy that while irrigation increased biomass (Fig. 7D) and the rate of growth (Fig. 6) of the A. donax ecotypes over the course of the growing season, irrigation did not result in a statistically significant increase in overall biomass production. The use of supplementary irrigation in the cultivation of A. donax is therefore difficult to justify. A key finding of this study is that in the irrigated A. donax, rates of PN began to decline (Fig. 2D–F) as downregulation of photosynthetic capacity (Fig. 4) occurred, and the plants no longer increased in height (Fig. 6). In contrast, after drought stress as soil water availability increased, the rain-fed A. donax recovered PN with photosynthetic capacity, and extended their growth later into the year, allowing the rain-fed plants to minimize the growth benefit of irrigation. This is particularly notable in terms of the total number of leaves and the number of photosynthetically active green leaves in the rain-fed plants (Fig. 6D–I), allowing increased potential for ‘whole-plant’ light harvesting and growth later in the year (e.g. Koyama and Kikuzawa, 2009). This study replicates the harvesting regime in late February to early March that would most probably be utilized in commercial cultivation of A. donax for biomass purposes (Cosentino et al., 2006; Monti et al., 2015); this would suggest that supplementary irrigation of A. donax is unnecessary and that A. donax is suitable for growth in drought-prone rain-fed marginal lands in regions with a warm to hot climate.
We observed an average above-ground biomass yield of 22.3 t ha–1. This is consistent with values reported for A. donax established for 2 years (Hidalgo and Fernandez, 2001; Cosentino et al., 2006; Angelini et al., 2009), although lower than yield values of 38–40 t ha−1 observed in more established plots (>4 years old) (Angelini et al., 2009; Dragoni et al., 2015). The results of this study have shown that while the A. donax ecotypes from contrasting habitats are largely similar in terms of maximum photosynthetic capacity (Figs 2 and 4) and have highly effective stomatal control (Figs 2 and 3), it is possible to unravel physiological and morphological variation between the ecotypes that may enable the development of more productive and/or drought-tolerant commercial varieties of A. donax. Stem height and maintenance of PN rates through the retention of photosynthetic capacity late into the growing season appear to be key attributes towards more productive A. donax. None of the ecotypes examined exemplified such an ideotype for A. donax (an ideotype being an ideal plant form in line with the concept proposed by Donald, 1968). While the Moroccan ecotype produced the longest stems and largest yield, it also showed the most pronounced downregulation of photosynthetic capacity (Fig. 4) and reduction in leaf gas exchange (Fig. 2D) later in the year. The Sicilian ecotype retained photosynthetic capacity (Fig. 4H) and PN (Fig. 2E) later in the year, and also emitted less previously fixed carbon as isoprene (Fig. 2A) (e.g. Brilli et al., 2007), but developed shorter stems than its Moroccan counterpart. Stem elongation in A. donax, as in many grasses, continues until flowering (Pollock and Cairns, 1991; Cosentino et al., 2007). Given that the flowers of A. donax are sterile (Boland, 2006), selection on the basis of delayed or absence of flowering (variation in flowering was observed in Cosentino et al., 2006) may be a viable strategy to increase stem height by prolonging growth later into the year (Clifton-Brown and Lewandowski, 2002). Genetic (Fu et al., 2016) and mutagenic (Valli et al., 2017) approaches utilizing the transcriptome of A. donax may offer the best way forward in terms of the development of a highly productive and drought-tolerant A. donax ideotype for cultivation in drought-prone rain-fed marginal lands.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of Figure S1: response curves of photosynthesis to the sub-stomatal concentration of [CO2] (Ci) of Moroccan, Sicilian and Tuscan ecotypes of Arundo donax.
ACKNOWLEDGEMENTS
We thank Said Wahbi (Université Cadi Ayyad, Marrakech) for providing rhizomes of the Moroccan ecotype. We are grateful for the assistance of the staff of the Di3A Experimental Field (University of Catania), Alessandra Pellegrino and Salvatore La Rosa (CNR-IVALSA). This work was funded by the EU FP7 project WATBIO (Development of improved perennial non-food biomass and bioproduct crops for water stressed environments – no.311929).
LITERATURE CITED
- Ahmad R, Liow P-S, Spencer DF, Jasieniuk M. 2008. Molecular evidence for a single genetic clone of invasive Arundo donax in the United States. Aquatic Botany 88: 113–120. [Google Scholar]
- Ahrar M, Doneva D, Koleva D, et al. 2015. Isoprene emission in the monocot Arundineae tribe in relation to functional and structural organization of the photosynthetic apparatus. Environmental and Experimental Botany 119: 87–95. [Google Scholar]
- Allen RG, Pereira LS, Raes D, Smith M. 1998. Crop evapotranspiration – Guidelines for computing crop water requirements. FAO irrigation and drainage paper 56. Rome: FAO. [Google Scholar]
- Angelini LG, Ceccarini L, Di Nassa NNo, Bonari E. 2009. Comparison of Arundo donax L. and Miscanthus×giganteus in a long-term field experiment in Central Italy: analysis of productive characteristics and energy balance. Biomass and Bioenergy 33: 635–643. [Google Scholar]
- Asaeda T, Manatunge J, Roberts J, Hai DN. 2006. Seasonal dynamics of resource translocation between the aboveground organs and age-specific rhizome segments of Phragmites australis. Environmental and Experimental Botany 57: 9–18. [Google Scholar]
- Berry J, Björkman O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology 31: 491–543. [Google Scholar]
- Boland JM. 2006. The importance of layering in the rapid spread of Arundo donax (giant reed). Madrono 53: 303–312. [Google Scholar]
- Brilli F, Barta C, Fortunati A, Lerdau M, Loreto F, Centritto M. 2007. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytologist 175: 244–254. [DOI] [PubMed] [Google Scholar]
- Centritto M, Loreto F, Chartzoulakis K. 2003. The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant, Cell and Environment 26: 585–594. [Google Scholar]
- Chow PS, Landhäusser SM. 2004. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiology 24: 1129–1136. [DOI] [PubMed] [Google Scholar]
- Clifton-Brown JC, Lewandowski I. 2002. Screening Miscanthus genotypes in field trials to optimise biomass yield and quality in Southern Germany. European Journal of Agronomy 16: 97–110. [Google Scholar]
- Cosentino SL, Copani V, D’Agosta GM, Sanzone E, Mantineo M. 2006. First results on evaluation of Arundo donax L. clones collected in Southern Italy. Industrial Crops and Products 23: 212–222. [Google Scholar]
- Cosentino SL, Patane C, Sanzone E, Copani V, Foti S. 2007. Effects of soil water content and nitrogen supply on the productivity of Miscanthus × giganteus Greef et Deu. in a Mediterranean environment. Industrial Crops and Products 25: 75–88. [Google Scholar]
- Cosentino SL, Scordia D, Sanzone E, Testa G, Copani V. 2014. Response of giant reed (Arundo donax L.) to nitrogen fertilization and soil water availability in semi-arid Mediterranean environment. European Journal of Agronomy 60: 22–32. [Google Scholar]
- Cosentino SL, Patanè C, Sanzone E, Testa G, Scordia D. 2016. Leaf gas exchange, water status and radiation use efficiency of giant reed (Arundo donax L.) in a changing soil nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. European Journal of Agronomy 72: 56–69. [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME. 2002. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiology 129: 1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curt MD, Sanz M, Mauri PV, et al. 2018. Effect of water regime change in a mature Arundo donax crop under a xeric Mediterranean climate. Biomass and Bioenergy 115: 203–209. [Google Scholar]
- Danin A. 2004. Arundo (Gramineae) in the Mediterranean reconsidered. Willdenowia 34: 361–369. [Google Scholar]
- Diaz-Pérez JC, Shackel KA, Sutter EG. 1995. Relative water content and water potential of tissue 1. Journal of Experimental Botany 46: 111–118. [Google Scholar]
- Donald CM. 1968. The breeding of crop ideotypes. Euphytica 17: 385–403. [Google Scholar]
- Dragoni F, Di Nasso NN, Tozzini C, Bonari E, Ragaglini G. 2015. Aboveground yield and biomass quality of giant reed (Arundo donax L.) as affected by harvest time and frequency. BioEnergy Research 8: 1321–1331. [Google Scholar]
- Ethier GJ, Livingston NJ. 2004. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant, Cell and Environment 27: 137–153. [Google Scholar]
- Fan ZX, Zhang SB, Hao GY, Ferry Slik J, Cao KF. 2012. Hydraulic conductivity traits predict growth rates and adult stature of 40 Asian tropical tree species better than wood density. Journal of Ecology 100: 732–741. [Google Scholar]
- Feller U, Crafts-Brandner SJ, Salvucci ME. 1998. Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) activase-mediated activation of rubisco. Plant Physiology 116: 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J, Medrano H. 2002. Energy dissipation in C-3 plants under drought. Functional Plant Biology 29: 1209–1215. [DOI] [PubMed] [Google Scholar]
- Fu Y, Poli M, Sablok G, et al. 2016. Dissection of early transcriptional responses to water stress in Arundo donax L. by unigene-based RNA-seq. Biotechnology for Biofuels 9: 54. doi: 10.1186/s13068-016-0471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- Grime J. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. The American Naturalist 111: 1169–1194. [Google Scholar]
- Harley PC, Loreto F, Dimarco G, Sharkey TD. 1992. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiology 98: 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth M, Catola S, Marino G, et al. 2017. a Moderate drought stress induces increased foliar dimethylsulphoniopropionate (DMSP) concentration and isoprene emission in two contrasting ecotypes of Arundo donax. Frontiers in Plant Science 8: 1016 oi: 10.3389/fpls.2017.01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth M, Centritto M, Giovannelli A, et al. 2017. b Xylem morphology determines the drought response of two Arundo donax ecotypes from contrasting habitats. GCB Bioenergy 9: 119–131. [Google Scholar]
- Haworth M, Cosentino SL, Marino G, et al. 2017. c Physiological responses of Arundo donax ecotypes to drought: a common garden study. GCB Bioenergy 9: 132–143. [Google Scholar]
- Haworth M, Cosentino SL, Marino G, et al. 2018. a Increased free abscisic acid during drought enhances stomatal sensitivity and modifies stomatal behaviour in fast growing giant reed (Arundo donax L.). Environmental and Experimental Botany 147: 116–124. [Google Scholar]
- Haworth M, Marino G, Brunetti C, Killi D, De Carlo A, Centritto M. 2018b The impact of heat stress and water deficit on the photosynthetic and stomatal physiology of olive (Olea europaea L.) – a case study of the 2017 heat wave. Plants 7: pii: E76. doi: 10.3390/plants7040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth M, Scutt CP, Douthe C, et al. 2018. c Allocation of the epidermis to stomata relates to stomatal physiological control: stomatal factors involved in the diversification of the angiosperms and development of amphistomaty. Environmental and Experimental Botany 151: 55–63. [Google Scholar]
- Heckathorn SA, Downs CA, Sharkey TD, Coleman JS. 1998. The small, methionine-rich chloroplast heat-shock protein protects photosystem II electron transport during heat stress. Plant Physiology 116: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M, Fernandez J. 2001. Biomass production of ten populations of giant reed (Arundo donax L.) under the environmental conditions of Madrid (Spain). In: World conference on biomass for energy and industry. Spain: Sevilla, 1881–1884. [Google Scholar]
- Husband JD, Kiene RP. 2007. Occurrence of dimethylsulfoxide in leaves, stems, and roots of Spartina alterniflora. Wetlands 27: 224–229. [Google Scholar]
- Husband JD, Kiene RP, Sherman TD. 2012. Oxidation of dimethylsulfoniopropionate (DMSP) in response to oxidative stress in Spartina alterniflora and protection of a non-DMSP producing grass by exogenous DMSP plus acrylate. Environmental and Experimental Botany 79: 44–48. [Google Scholar]
- Jordan DB, Ogren WL. 1984. The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase: dependence on ribulosebisphosphate concentration, pH and temperature. Planta 161: 308–313. [DOI] [PubMed] [Google Scholar]
- Kalaji HM, Jajoo A, Oukarroum A, et al. 2016. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiologiae Plantarum 38: 1–11. [Google Scholar]
- Karp A, Shield I. 2008. Bioenergy from plants and the sustainable yield challenge. New Phytologist 179: 15–32. [DOI] [PubMed] [Google Scholar]
- Khudamrongsawat J, Tayyar R, Holt JS. 2004. Genetic diversity of giant reed (Arundo donax) in the Santa Ana River, California. Weed Science 52: 395–405. [Google Scholar]
- Killi D, Anlauf R, Kavdir Y, Haworth M. 2014. Assessing the impact of agro-industrial olive wastes in soil water retention: implications for remediation of degraded soils and water availability for plant growth. International Biodeterioration and Biodegradation 94: 48–56. [Google Scholar]
- Killi D, Bussotti F, Raschi A, Haworth M. 2016. Adaptation to high temperature mitigates the impact of water deficit during combined heat and drought stress in C3 sunflower and C4 maize varieties with contrasting drought tolerance. Physiologia Plantarum 159: 130–147. [DOI] [PubMed] [Google Scholar]
- Koyama K, Kikuzawa K. 2009. Is whole‐plant photosynthetic rate proportional to leaf area? A test of scalings and a logistic equation by leaf demography census. The American Naturalist 173: 640–649. [DOI] [PubMed] [Google Scholar]
- Lauteri M, Haworth M, Serraj R, Monteverdi MC, Centritto M. 2014. Photosynthetic diffusional constraints affect yield in drought stressed rice cultivars during flowering. PLoS One 9: e109054. doi: 10.1371/journal.pone.0109054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Harley PC, Dimarco G, Sharkey TD. 1992. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiology 98: 1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loriaux S, Avenson T, Welles J, et al. 2013. Closing in on maximum yield of chlorophyll fluorescence using a single multiphase flash of sub‐saturating intensity. Plant, Cell and Environment 36: 1755–1770. [DOI] [PubMed] [Google Scholar]
- Lovelli S, Perniola M. 2014. Low CO2 does not remove diffusional limitation to photosynthesis in salt stressed tomato during osmotic phase. Acta Physiologiae Plantarum 36: 1953–1956. [Google Scholar]
- Mann JJ, Barney JN, Kyser GB, Di Tomaso JM. 2013. Miscanthus × giganteus and Arundo donax shoot and rhizome tolerance of extreme moisture stress. Global Change Biology Bioenergy 5: 693–700. [Google Scholar]
- Mantineo M, D’Agosta GM, Copani V, Patanè C, Cosentino SL. 2009. Biomass yield and energy balance of three perennial crops for energy use in the semi-arid Mediterranean environment. Field Crops Research 114: 204–213. [Google Scholar]
- Mariani C, Cabrini R, Danin A, et al. 2010. Origin, diffusion and reproduction of the giant reed (Arundo donax L.): a promising weedy energy crop. Annals of Applied Biology 157: 191–202. [Google Scholar]
- Monti A, Zanetti F, Scordia D, Testa G, Cosentino SL. 2015. What to harvest when? Autumn, winter, annual and biennial harvesting of giant reed, miscanthus and switchgrass in northern and southern Mediterranean area. Industrial Crops and Products 75: 129–134. [Google Scholar]
- Novaes E, Kirst M, Chiang V, Winter-Sederoff H, Sederoff R. 2010. Lignin and biomass: a negative correlation for wood formation and lignin content in trees. Plant Physiology 154: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M, Driscoll S. 1997. Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant, Cell and Environment 20: 110–116. [Google Scholar]
- Pilu R, Cassani E, Landoni M, et al. 2014. Genetic characterization of an Italian giant reed (Arundo donax L.) clones collection: exploiting clonal selection. Euphytica 196: 169–181. [Google Scholar]
- Pollastri S, Tsonev T, Loreto F. 2014. Isoprene improves photochemical efficiency and enhances heat dissipation in plants at physiological temperatures. Journal of Experimental Botany 65: 1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock C, Cairns A. 1991. Fructan metabolism in grasses and cereals. Annual Review of Plant Biology 42: 77–101. [Google Scholar]
- Preston KA, Cornwell WK, DeNoyer JL. 2006. Wood density and vessel traits as distinct correlates of ecological strategy in 51 California coast range angiosperms. New Phytologist 170: 807–818. [DOI] [PubMed] [Google Scholar]
- Proietti S, Moscatello S, Fagnano M, Fiorentino N, Impagliazzo A, Battistelli A. 2017. Chemical composition and yield of rhizome biomass of Arundo donax L. grown for biorefinery in the Mediterranean environment. Biomass and Bioenergy 107: 191–197. [Google Scholar]
- Romero-Munar A, Baraza E, Cifre J, Achir C, Gulías J. 2017. Leaf plasticity and stomatal regulation determines the ability of Arundo donax plantlets to cope with water stress. Photosynthetica 56: 698–706. [Google Scholar]
- Rossa B, Tüffers A, Naidoo G, Willert D. 1998. Arundo donax L. (Poaceae) – a C3 species with unusually high photosynthetic capacity. Botanica Acta 111: 216–221. [Google Scholar]
- Sade N, Gebremedhin A, Moshelion M. 2012. Risk-taking plants: anisohydric behavior as a stress-resistance trait. Plant Signaling and Behavior 7: 767–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia R, Chutia RS, Kataki R, Pant KK. 2015. Perennial grass (Arundo donax L.) as a feedstock for thermo-chemical conversion to energy and materials. Bioresource Technology 188: 265–272. [DOI] [PubMed] [Google Scholar]
- Sánchez E, Scordia D, Lino G, Arias C, Cosentino SL, Nogués S. 2015. Salinity and water stress effects on biomass production in different Arundo donax L. clones. BioEnergy Research 8: 1461–1479. [Google Scholar]
- Sekmen AH, Ozgur R, Uzilday B, Turkan I. 2014. Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environmental and Experimental Botany 99: 141–149. [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. 2007. Fitting photosynthetic carbon dioxide response curves for C-3 leaves. Plant, Cell and Environment 30: 1035–1040. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Vanderveer PJ. 1989. Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiology 91: 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. 1990. Metabolic repression of transcription in higher plants. The Plant Cell 2: 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunda W, Kieber D, Kiene R, Huntsman S. 2002. An antioxidant function for DMSP and DMS in marine algae. Nature 418: 317–320. [DOI] [PubMed] [Google Scholar]
- Tuck G, Glendining MJ, Smith P, House JI, Wattenbach M. 2006. The potential distribution of bioenergy crops in Europe under present and future climate. Biomass and Bioenergy 30: 183–197. [Google Scholar]
- Valli F, Trebbi D, Zegada-Lizarazu W, Monti A, Tuberosa R, Salvi S. 2017. In vitro physical mutagenesis of giant reed (Arundo donax L.). GCB Bioenergy 9: 1380–1389. [Google Scholar]
- Van Oosten JJ, Wilkins D, Besford RT. 1994. Regulation of the expression of photosynthetic nuclear genes by CO2 is mimicked by regulation by carbohydrates – a mechanism for the acclimation of photosynthesis to high CO2. Plant, Cell and Environment 17: 913–923. [Google Scholar]
- Velikova V, Loreto F. 2005. On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell and Environment 28: 318–327. [Google Scholar]
- Watts DA, Moore GW. 2011. Water-use dynamics of an invasive reed, Arundo donax, from leaf to stand. Wetlands 31: 725–734. [Google Scholar]
- Wingler A, Roitsch T. 2008. Metabolic regulation of leaf senescence: interactions of sugar signalling with biotic and abiotic stress responses. Plant Biology 10: 50–62. [DOI] [PubMed] [Google Scholar]
- Yang JT, Preiser AL, Li Z, Weise SE, Sharkey TD. 2016. Triose phosphate use limitation of photosynthesis: short-term and long-term effects. Planta 243: 687–698. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Liu Y, Uchida S, et al. 2008. Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinensis to monosaccharides. Bioscience, Biotechnology, and Biochemistry 72: 805–810. [DOI] [PubMed] [Google Scholar]
- Zegada-Lizarazu W, Della Rocca G, Centritto M, Parenti A, Monti A. 2018. Giant reed genotypes from temperate and arid environments show different response mechanisms to drought. Physiologia Plantarum 163: 490–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.