Abstract
Context
Nearly one-third of all thyroid cancers are ≤1 cm.
Objective
To determine diagnostic pathways for microcarcinomas vs larger cancers.
Design/Setting/Participants
Patients from Georgia and Los Angeles Surveillance, Epidemiology, and End Results (SEER) registries with differentiated thyroid cancer diagnosed in 2014 or 2015 were surveyed. Survey data were linked to SEER data on tumor and treatment characteristics. Multivariable logistic regression analysis was performed.
Main Outcome Measures
Method of nodule discovery; reason for thyroid surgery.
Results
Of patients who underwent surgery, 975 (38.2%) had cancers ≤1 cm, and 1588 cancers (61.8%) were >1 cm. The reported method of nodule discovery differed significantly between patients with cancers ≤1 cm and those with cancers >1 cm (P < 0.001). Cancer ≤1 cm was associated with nodule discovery on thyroid ultrasound (compared with other imaging, OR, 1.59; 95% CI, 1.21 to 2.10), older patient age (45 to 54 years vs ≤44, OR, 1.45; 95% CI, 1.16 to 1.82), and female sex (OR, 1.51; 95% CI, 1.22 to 1.87). Hispanic ethnicity (OR, 0.71; 95% CI, 0.57 to 0.89) and Asian race (OR, 0.67; 95% CI, 0.49 to 0.92) were negative correlates. Cancers ≤1 cm were associated with lower likelihood of surgery for a nodule suspicious or consistent with cancer (OR, 0.48; 95% CI, 0.40 to 0.57).
Conclusion
Thyroid microcarcinomas are more likely to be detected by ultrasound and less likely to be associated with surgery scheduled for known thyroid cancer. Understanding diagnostic pathways allows for targeted interventions to decrease overdiagnosis and overtreatment.
Linking SEER data to patient surveys revealed thyroid microcarcinomas are more likely to be detected by thyroid ultrasound and patients were less likely to have surgery for known cancer.
The number of newly diagnosed thyroid cancers has doubled during the last three decades (1–3), with overall incidence increasing by 3% annually (4). Despite the US Preventive Services Task Force (USPSTF) recommending since 1996 against thyroid cancer screening in asymptomatic individuals by either physical examination or ultrasound (5, 6), the increasing incidence of thyroid cancer in the United States is predominantly due to the increased detection of small papillary cancers; with tumor size ≤1 cm defined as thyroid microcarcinoma (7–9). These microcarcinomas typically have an indolent course without increased risk for death, suggesting cancer overdiagnosis (1, 10). The 2009 American Thyroid Association (ATA) guidelines on differentiated thyroid cancer recommended that in the overwhelming majority of scenarios, only nodules >1 cm should undergo evaluation and work-up with fine needle aspiration (FNA) biopsy (11). This was reinforced by the more recent 2015 guidelines, which further stated that FNA biopsy is recommended for nodules ≥1 cm with high- and intermediate-suspicion sonographic patterns, nodules ≥1.5 cm with a low-suspicion sonographic pattern and nodules ≥2 cm with very-low-suspicion sonographic pattern (12).
Because screening and evaluations of thyroid nodules ≤1 cm are not recommended, it is unclear why so many thyroid microcarcinomas are being identified. Although a portion of these nodules may be discovered by performing thyroid ultrasound for screening despite recommendation against screening by USPSTF, some of these nodules may be uncovered during other neck imaging studies unrelated to the thyroid gland. Once a small thyroid nodule is detected, regardless the method of detection, some clinicians would perform an FNA biopsy despite the recent ATA guidelines recommendation against performing biopsies of small thyroid nodules (12). In a recent survey study, 67% of the responders said they would perform an FNA in a thyroid nodule <1 cm with suspicious sonographic pattern (13). It is also possible that these small cancers are incidental findings after thyroidectomy for other reasons, including treatment of indeterminate thyroid nodules, goiter, hyperthyroidism, or thyroid nodules with compressive symptoms.
With the known increase in diagnosis of small thyroid cancers, it is important to understand how thyroid microcarcinomas vs thyroid cancers >1 cm are being diagnosed in the United States. Therefore, we collaborated with the Surveillance, Epidemiology, and End Results (SEER) registries Georgia and Los Angeles County, which enabled us to study a diverse, population-based cohort. We linked patient surveys to SEER data, which include details on initial pathology and treatment. We hypothesized that the patient-reported diagnostic pathway for cancers ≤1 cm would differ from that of larger cancers and that thyroid cancers ≤1 cm would more likely be an incidental finding on imaging studies or an incidental pathologic finding after thyroidectomy for another indication.
Methods
Study population and data collection
We identified patients with newly diagnosed differentiated thyroid cancer (International Classification of Diseases for Oncology histology codes 8050, 8260,8290,8330, 8331,8332, 8335, 8337,8340, 8341, 8342, 8343,8344,8350, and 8450), from ages 18 to 79 years who were ascertained by the SEER registries of Georgia and Los Angeles County between 1 January 2014, and 31 December 2015. Patients were mailed surveys from February 2017 through October 2018. We used a modified Dillman survey method, which included a $20 cash incentive, telephone follow-up calls, tracing, postcards, and second mailings to improve response rate (14). All materials were sent in English and Spanish to those with Spanish surnames and interviewers were bilingual. Responses to the survey were then merged with clinical data from SEER for each of the completed surveys. This study was approved by the University of Michigan Institutional Review Board as well as the institutional review boards of the participating SEER registries, including Emory University, University of Southern California, the Committee for the Protection of Human Subjects (the California institutional review board), and the California Cancer Registry.
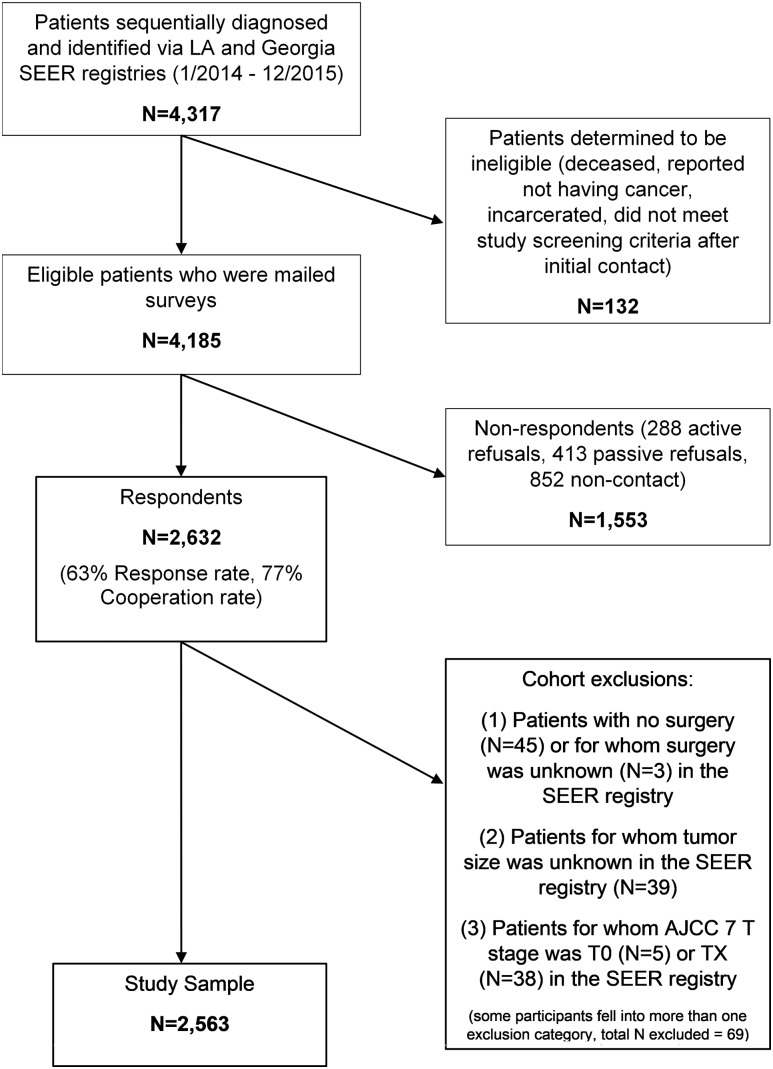
As shown in Fig. 1, of the 4317 patients who met the sample selection criteria, 132 patients were ineligible because they were deceased, incarcerated, reported they did not have a cancer diagnosis, or did not meet screening eligibility criteria after the initial contact was made. Of the remaining 4185 eligible patients who were mailed a survey, 2632 responded, resulting in a 63% response rate and a 77% cooperation rate among those who could be located (15). Those with no surgery or unknown surgery, T0 or TX, or unknown tumor size per SEER database (n = 69) were excluded from this analysis, which was focused on patients who underwent thyroid surgery (i.e., lobectomy, total thyroidectomy, or total thyroidectomy with neck dissection) and had known tumor size. The final cohort size was 2563 patients.
Figure 1.
Flow diagram illustrating cohort selection. AJCC, American Joint Committee on Cancer; LA, Los Angeles
Measures
The questionnaire content was developed on the basis of a conceptual framework and our research questions and hypotheses. We used standard techniques to assess content validity, including review by design experts and pilot studies in selected clinic populations.
Pathways to diagnosis of thyroid nodule or thyroid cancer
Patients were asked about the circumstances of their thyroid nodule discovery, whether they had undergone FNA biopsy of their thyroid nodule, and reasons for thyroid surgery. Specifically, respondents were asked about the discovery of their thyroid nodule, with the following options: didn’t know about nodule, felt it myself, doctor felt it, had a thyroid ultrasound, found on imaging test other than thyroid ultrasound, such as CT scan. They were also asked about history of FNA biopsy of their nodule prior to thyroid surgery, with yes or no answer options. Finally, patients were also asked why they had thyroid surgery, with options including the following: nodule the doctor thought may be cancer, nodule the doctor thought wasn’t cancer, nodule that had unclear or indeterminate risk for cancer, large thyroid or goiter, symptoms such as difficulty swallowing or difficulty breathing while lying flat, overactive thyroid or hyperthyroidism, and other. Patients could select more than one option. Answers were prioritized and categorized in the following order: nodule suspicious or consistent with cancer, nodule indeterminate, goiter or compressive symptoms, other.
Covariates
Patient-reported demographic covariates in the survey included sex, race/ethnicity (specifically, white, Hispanic, black, Asian, other/unknown), and insurance status (i.e., private vs other including Medicare, Medicaid, or none). Thyroid cancer demographic variables and clinical characteristics abstracted from hospital medical records and reported to the SEER registries included age at diagnosis, tumor size at diagnosis (≤1 cm and >1cm), tumor histology (i.e., papillary thyroid cancer, and follicular thyroid cancer/Hürthle cell thyroid cancer), and SEER stage (i.e., localized, and regional/distant).
Additional variables
We also asked patients to provide information about the number of first-degree relatives (i.e., parents, sibling, or child) who had thyroid cancer (later categorized as 0, 1, ≥2), and we asked about personal history of radiation exposure (yes or no). Radiation exposure was defined as radiation treatment of another cancer or radiation from a catastrophic event such as Chernobyl and did not include radiation due to x-rays or CT scan of neck, chest, or brain. We also inquired about the specialty of the physician responsible for informing the patient of their thyroid cancer diagnosis (i.e., surgeon, endocrinologist, primary care provider, other).
Statistical analyses
Descriptive statistics were generated for the study sample and nonweighted frequencies were reported. All statistical analyses incorporated weights to account for differential nonresponse and reduce potential nonresponse bias. The weight computation included the use of design weights to account for differential probability of sample selection and nonresponse weights to account for disproportionate nonresponse rates across different patient subgroups.
χ 2 test was used to compare demographic and clinical characteristics of nodules ≤1 cm vs >1 cm. We also used χ2 tests to compare the distributions of the method of nodule discovery and the reasons for undergoing thyroid surgery in patients with cancer size ≤1 cm vs >1 cm. The reported P values correspond to the Pearson χ2 with a Rao and Scott correction.
Multivariable logistic regression analysis was used to determine factors associated with detection of thyroid cancer ≤1 cm vs >1 cm and then to determine characteristics associated with nodule discovery with thyroid ultrasound. In addition, multivariable logistic regression analysis was used to determine correlates of the indication for surgery for a nodule suspicious for or consistent with cancer. Missing data were <4.2% per survey item and only patients with no missing data on all variables were used in each model. All analyses were performed using R, version 3.5.2 (R Project, https://cran.r-project.org/bin/windows/base/old/3.5.2/), and Stata, version 15.1 (StataCorp, College Station, TX). P < 0.05 was considered statistically significant.
Results
As shown in Table 1, characteristics were compared between patients with differentiated thyroid cancer with tumor size ≤1 cm and those with tumor size >1 cm. Tumor size was ≤1 cm in 38% of patients; thus, 62% had tumor size >1 cm. Multiple factors were significantly associated with a tumor size ≤1 cm, including age, sex, race/ethnicity, cancer type, SEER stage, FNA biopsy, type of surgery, and physician specialty. There was no significant difference between cancers ≤1 cm and >1 cm in regard to insurance, family history of thyroid cancer, or personal history of radiation exposure.
Table 1.
Demographics and Clinical Characteristics (n = 2563)
| Tumor ≤1 cm (n = 975) | Tumor >1 cm (n = 1588) | χ 2 P Value | |
|---|---|---|---|
| Age, y | <0.001 | ||
| ≤ 44 | 270 (30.8) | 609 (41.6) | |
| 45-54 | 252 (24.8) | 367 (22.3) | |
| 55-64 | 264 (25.8) | 338 (19.8) | |
| ≥ 65 | 189 (18.6) | 274 (16.3) | |
| Sex | 0.002 | ||
| Female | 789 (81.2) | 1200 (76.0) | |
| Male | 186 (18.8) | 388 (24.0) | |
| Race/ethnicity | <0.001 | ||
| White | 593 (56.9) | 877 (50.3) | |
| Hispanic | 162 (18.2) | 342 (23.8) | |
| Black | 121 (14.4) | 164 (11.7) | |
| Asian | 72 (8.9) | 162 (12.0) | |
| Other | 15 (1.6) | 34 (2.2) | |
| Insurance | 0.056 | ||
| Private | 673 (71.0) | 1047 (67.3) | |
| Other | 275 (29.0) | 496 (32.7) | |
| Cancer type | <0.001 | ||
| Papillary | 964 (98.9) | 1426 (89.8) | |
| Follicular and Hürthle cell | 11 (1.1) | 162 (10.2) | |
| SEER stage | <0.001 | ||
| Localized | 826 (85.0) | 953 (59.1) | |
| Regional and distant | 149 (15.0) | 635 (40.9) | |
| FNA performed | <0.001 | ||
| Yes | 801 (83.7) | 1463 (93.0) | |
| No | 158 (16.3) | 109 (7.0) | |
| Type of surgery | <0.001 | ||
| Lobectomy | 179 (18.6) | 137 (8.9) | |
| Total thyroidectomy | 420 (44.3) | 560 (36.2) | |
| Total thyroidectomy with LND | 376 (37.1) | 891 (54.9) | |
| Physician specialty responsible for informing patient of cancer diagnosis | <0.001 | ||
| Surgeon | 447 (47.6) | 573 (37.2) | |
| Endocrinologist | 344 (37.5) | 613 (40.3) | |
| Primary care provider | 83 (9.1) | 206 (13.6) | |
| Other | 56 (5.8) | 134 (8.9) | |
| History of thyroid cancer in first-degree relativea | 0.320 | ||
| 0 | 826 (86.1) | 1383 (88.2) | |
| 1 | 101 (10.6) | 139 (8.8) | |
| ≥2 | 31 (3.3) | 45 (3.0) | |
| Personal history of radiation exposureb | 0.740 | ||
| No | 877 (91.2) | 1424 (91.6) | |
| Yes | 86 (8.8) | 138 (8.4) |
Data reported as no. (%) unless otherwise indicated.
Abbreviation: LND, lymph node dissection.
Patients with family history of thyroid cancer in first-degree relative are categorized as follows: 0, no family history of thyroid cancer; 1, one family member with thyroid cancer; ≥2, two or more family members with thyroid cancer.
Patients were asked about exposure to radiation, not including x-rays or CT scans, prior to thyroid cancer diagnosis. This could include radiation treatment of another cancer or radiation from a catastrophic event such as Chernobyl. Of these patients with reported exposure to radiation, 18 of 77 (23.3%) and 20 of 130 (15.8%) reported radiation to the neck as treatment of another cancer in patients with cancers ≤1 cm and > 1cm, respectively.
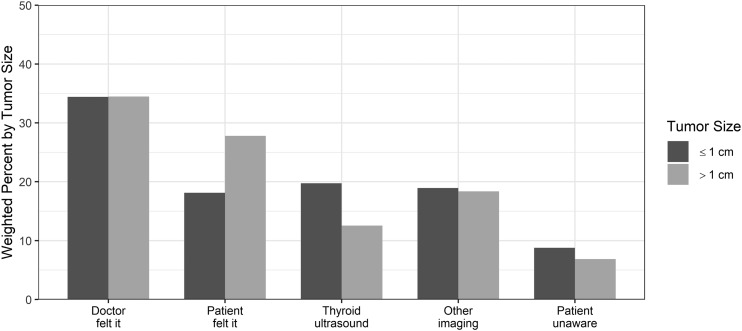
As shown in Fig. 2, the proportion of patients reporting that their doctor felt the nodule or the nodule was detected with another imaging test was similar for patients with cancers ≤1 cm and those with cancers >1 cm. In contrast, patients were less likely to report feeling their own nodule if their cancer was ≤1 cm, compared with patients with larger cancers. Patients with cancers ≤1 cm were more likely to report their nodule was initially detected with thyroid ultrasound (P < 0.001).
Figure 2.
Patient-reported method of initial nodule discovery. χ2P < 0.001. Of the patients who reported having their nodule found with “other imaging,” 159 of 188 patients (86.6%) with cancers ≤1 cm and 254 of 303 patients (85.2%) with cancers >1 cm reported that this imaging test was ordered for another, unrelated medical problem.
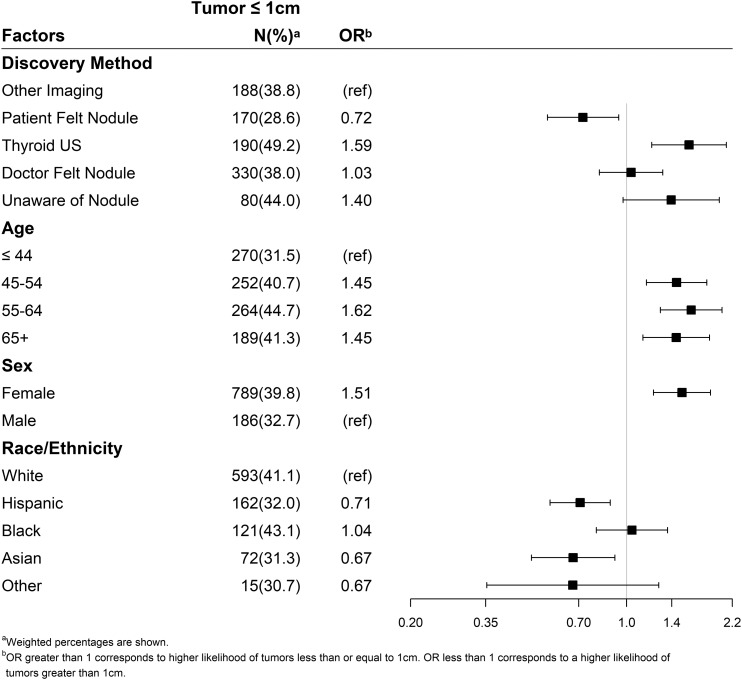
By multivariable logistic regression analysis, the detection of tumors ≤1 cm was associated with having had a thyroid ultrasound (OR, 1.59; 95% CI, 1.21 to 2.10), female sex (OR, 1.51; 95% CI, 1.22 to 1.87), and age 45 to 54 years, 55 to 64 years, and ≥65 years (e.g., age 55 to 64 years: OR 1.62; 95% CI, 1.29 to 2.04 compared with ≤44 years; Fig. 3). In contrast, Asian (OR, 0.67; 95% CI, 0.49 to 0.92) and Hispanic (OR, 0.71; 95% CI, 0.57 to 0.89) ethnicity as well as report of patient-palpated nodule (OR, 0.72; 95% CI, 0.55 to 0.94) were less likely to be associated with cancers ≤1 cm.
Figure 3.
Multivariable analysis comparing factors associated with diagnosing microcarcinomas as compared with larger cancers. ref, reference; US, ultrasound.
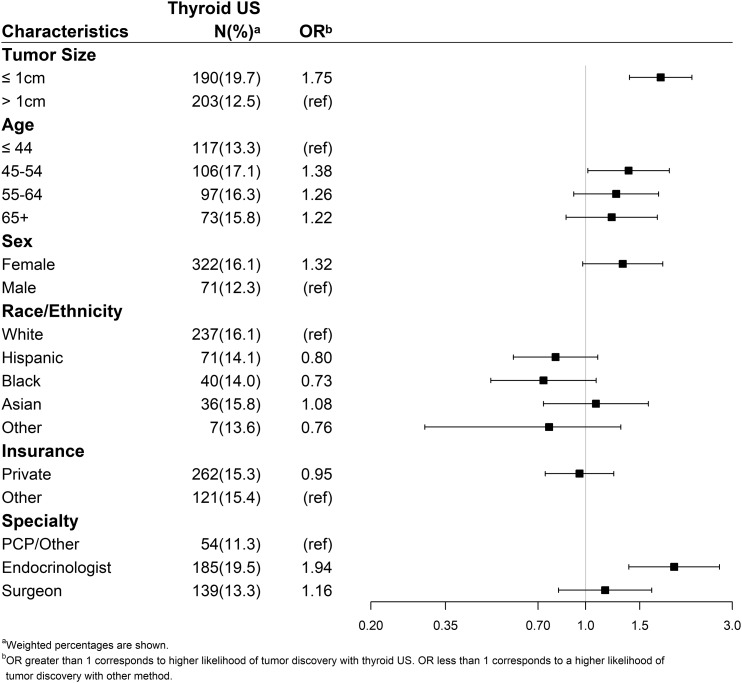
Factors associated with identification of the nodule with thyroid ultrasound vs any other method were assessed using a multivariable model (Fig. 4). Patient age (45 to 54 years vs ≤44 years), tumor size (≤1 cm vs >1 cm), and notification of cancer diagnosis by an endocrinologist vs other specialty were all significantly associated with initial detection of the nodule by thyroid ultrasound.
Figure 4.
Multivariable analysis evaluating the characteristics associated with patient report of thyroid ultrasound leading to their nodule discovery. PCP, primary care physician; ref, reference; US, ultrasound.
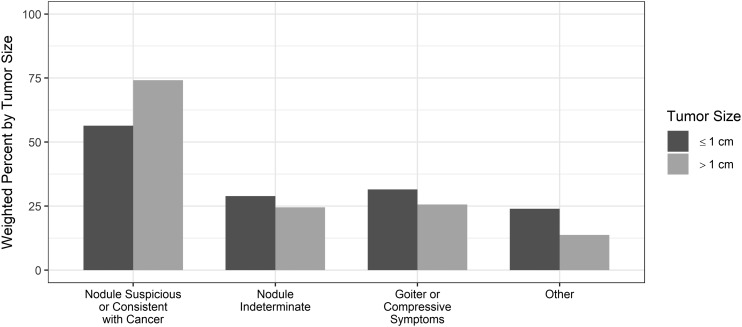
As demonstrated in Fig. 5, among all patients undergoing thyroid surgery, those with larger tumors were more likely to do so because of a nodule suspicious or consistent with cancer compared with those with microcarcinomas. In contrast, patient report of surgery being scheduled for indeterminate nodules, goiter, and other reasons was more likely with microcarcinomas.
Figure 5.
Patient-reported reason for thyroid surgery. The categories are not exclusive. χ2P values are < 0.001, = 0.016, = 0.002, < 0.001, as respectively shown in the figure. These tests were significant after adjusting for multiple testing.
In multivariable analysis controlling for age, race, and health insurance, scheduling surgery for a nodule suspicious or consistent with cancer was less likely for female patients (OR, 0.65; 95% CI, 0.52 to 0.81) and for patients with cancer size ≤1 cm (OR, 0.48; 95% CI, 0.40 to 0.57; data not shown).
Discussion
In our study, patients with differentiated thyroid cancer with tumor size ≤1 cm had a different diagnostic pathway than those with tumor size >1 cm. Thyroid microcarcinomas were more likely than larger cancers to be detected with thyroid ultrasound. Some of these microcarcinomas may be incidental postoperative findings; they also are less likely to be associated with a surgery planned for a known or suspected thyroid cancer. Factors associated with diagnosis of a microcarcinoma included female sex and older age, whereas Asians and Hispanics were less likely to have a microcarcinoma diagnosis compared with those in other racial or ethnic groups.
Thyroid nodule management has dramatically changed over the past two decades. The use of high-resolution thyroid ultrasound has become standard of care in the treatment of patients with thyroid nodules, and the recent use of molecular testing of thyroid nodules provides additional information about the nature of undetermined thyroid nodules (16, 17). A recent publication using SEER-Medicare and Medicare data showed that an increase in the area-level use of thyroid ultrasound as initial imaging in adults ≥65 years of age was associated with diagnosis of thyroid cancer over time, including cancers ≤1 cm (16). In a web-based, large, contemporary, international survey of clinical endocrinologists on the management of thyroid nodules, the percentage of respondents obtaining baseline ultrasound and using ultrasound guidance for FNA (98%) has increased significantly in comparison with earlier surveys (13).
Prior studies attempted to determine pathways to thyroid cancer diagnosis and used methods such as a qualitative interview of 25 patients (17); a questionnaire complemented by pathology report in 452 patients in New South Wales (18); medical record linkage of residents in Olmsted County, Minnesota (19); structured telephone interviews of 1007 patients in Queensland, Australia (20); and a retrospective study of >2000 patients from a single, large academic tertiary referral center (21). Findings from these studies suggested the increased incidence of thyroid cancer has been influenced by the use of diagnostic neck imaging (17–19, 21). Our large, population-based study, which includes a depth of clinical detail complemented by patient report, supports prior findings and provides additional details on the different pathways associated with the diagnosis and treatment of thyroid microcarcinomas compared with larger cancers. Similar to prior studies, we found that women and older patients are at risk for diagnosis of microcarcinomas. Because of our diverse cohort, we were also able to show that after controlling for other factors, Asians and Hispanics were less likely to be diagnosed with microcarcinomas.
A unique aspect of our study is that this cohort was linked to SEER data, a reputable source of cancer statistics, and therefore includes additional clinical details such as cancer type, tumor size, SEER stage, and surgical treatment (22). Second, this study includes a large cohort (n = 2563) providing patient-reported data about the circumstances of their thyroid nodule and thyroid cancer diagnosis. Third, this study includes a population-based cohort with expected distribution of cancer size but a more diverse patient population compared with other survey studies, with adequate representation of black and Hispanic patients.
Similar to other survey studies relying on patient report, a limitation of this study is the risk of recall bias; however, for cancer care in particular, patient self-report has been shown to be reliable (23–25). In addition, even though 84% of patients reported having undergone FNA biopsy of their thyroid nodules, we do not know if the index nodule biopsied was the cancer or if there were other suspicious findings noted with ultrasound, such as worrisome lymph nodes, that led to FNA and subsequent cancer diagnosis. Therefore, we cannot say most thyroid nodules ≤1 cm underwent FNA biopsy. Per patient report, thyroid nodule palpation by physician and patient was the most common pathway leading to microcarcinomas diagnosis, followed by thyroid ultrasound. However, concomitant with experiences in clinical practice, “palpating” a thyroid nodule may be misleading, because all palpable masses do not always correspond to thyroid nodules.
There has been an increase in the incidence of thyroid cancer over the last 30 years, especially in small thyroid cancers (1). Because almost three-fourths of all thyroid cancer diagnoses occur in women, the increase in thyroid cancer incidence has disproportionately impacted women. Despite USPSTF (5, 6) recommending against screening for thyroid cancer in asymptomatic adults, and 2015 ATA thyroid nodules and thyroid cancer guidelines recommending against FNA biopsy of thyroid nodule ≤1 cm (12), the incidence of small thyroid cancers remains high. The overwhelming majority of these small cancers have an excellent prognosis and thus serve as an illustration of cancer overdiagnosis. Overdiagnosis of thyroid cancer has led to overtreatment (26), with many patients undergoing surgery and often receiving radioactive iodine for cancers that may never cause them harm (27–30).
By evaluating this large, diverse cohort of patients with differentiated thyroid cancer, our study highlights the different pathways to cancer diagnosis for thyroid microcarcinomas vs larger thyroid cancers and identifies at-risk patients. Our findings emphasize the need to curb unnecessary diagnosis and treatment of thyroid microcarcinomas. Recently, there has been a campaign to avoid unnecessary treatment in patients with small thyroid cancer and to establish programs for active surveillance (31–35). Although not all have been supportive of active surveillance in the management of thyroid cancer, there is a need for practice change in the management of thyroid nodules in the United States. Neck or thyroid ultrasound should not be ordered in patients without symptoms or signs of thyroid enlargement. Evaluation of thyroid nodules ≤1 cm should be according to 2015 ATA guidelines on thyroid nodule and thyroid cancer; furthermore, FNA biopsy of these small thyroid nodules should be avoided (12). Until change in nodule detection and management is implemented, decreasing small thyroid cancer incidence will be challenging, and female patients will remain uniquely susceptible to overdiagnosis and potential overtreatment.
Acknowledgments
Financial Support: This work is supported by the National Cancer Institute (NCI; Grant R01 CA201198 to M.R.H.). Dr. Haymart is also suupported by the Agency for Healthcare Research and Quality (Grant R01 HS024512 to M.R.H.). The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885, Centers for Disease Control and Prevention (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344 and the NCI’s SEER Program, under contract HHSN261201800015I awarded to the University of Southern California (USC). The collection of cancer incidence data in Georgia was supported by contract HHSN261201800003I, Task Order HHSN26100001, from the NCI and cooperative agreement 5NU58DP003875-04 from the CDC.
The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California and State of Georgia Departments of Public Health, the NCI, and the CDC or their contractors and subcontractors is not intended nor should be inferred.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Glossary
Abbreviations:
- ATA
American Thyroid Association
- FNA
fine needle aspiration
- SEER
Surveillance
- Epidemiology
and End Results
- USPSTF
US Preventive Services Task Force
References and Notes
- 1. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164–2167. [DOI] [PubMed] [Google Scholar]
- 2. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–617. [DOI] [PubMed] [Google Scholar]
- 3. Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. 2015;25(10):1127–1136. [DOI] [PubMed] [Google Scholar]
- 4. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bibbins-Domingo K, Grossman DC, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Siu AL, Tseng CW; US Preventive Services Task Force. Screening for thyroid cancer. US Preventive Services Task Force recommendation statement. JAMA. 2017;317(18):1882–1887. [DOI] [PubMed] [Google Scholar]
- 6. US Preventive Services Task Force. Baltimore, MD: Williams & Wilkins; 1996. [Google Scholar]
- 7. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–322. [DOI] [PubMed] [Google Scholar]
- 8. Davies L, Morris LG, Haymart M, Chen AY, Goldenberg D, Morris J, Ogilvie JB, Terris DJ, Netterville J, Wong RJ, Randolph G; AACE Endocrine Surgery Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: the increasing incidence of thyroid cancer. Endocr Pract. 2015;21(6):686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 2011;21(3):231–236. [DOI] [PubMed] [Google Scholar]
- 10. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–613. [DOI] [PubMed] [Google Scholar]
- 11. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer [published corrections appear Thyroid. 2010;20(8):942 and 2010;20(6):674–675]. Thyroid. 2009;19(11):1167–1214. [DOI] [PubMed] [Google Scholar]
- 12. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burch HB, Burman KD, Cooper DS, Hennessey JV, Vietor NOA. A 2015 survey of clinical practice patterns in the management of thyroid nodules. J Clin Endocrinol Metab. 2016;101(7):2853–2862. [DOI] [PubMed] [Google Scholar]
- 14. Dillman DA. Mail and Internet Surveys: The Tailored Design Method, 2nd ed.New York, NY: Wiley; 2007. [Google Scholar]
- 15. American Association for Public Opinion Research. Response rates. An overview. Available at: https://www.aapor.org/Education-Resources/For-Researchers/Poll-Survey-FAQ/Response-Rates-An-Overview.aspx. Accessed 2 March 2018.
- 16. Haymart MR, Banerjee M, Reyes-Gastelum D, Caoili E, Norton EC. Thyroid ultrasound and the increase in diagnosis of low-risk thyroid cancer. J Clin Endocrinol Metab. 2019;104(3):785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nickel B, Brito JP, Moynihan R, Barratt A, Jordan S, McCaffery K. Patients’ experiences of diagnosis and management of papillary thyroid microcarcinoma: a qualitative study. BMC Cancer. 2018;18(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kahn C, Simonella L, Sywak M, Boyages S, Ung O, O’Connell D. Pathways to the diagnosis of thyroid cancer in New South Wales: a population-based cross-sectional study. Cancer Causes Control. 2012;23(1):35–44. [DOI] [PubMed] [Google Scholar]
- 19. Brito JP, Al Nofal A, Montori VM, Hay ID, Morris JC. The impact of subclinical disease and mechanism of detection on the rise in thyroid cancer incidence: a population-based study in Olmsted County, Minnesota during 1935 through 2012. Thyroid. 2015;25(9):999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rahman ST, McLeod DSA, Pandeya N, Neale RE, Bain CJ, Baade P, Youl PH, Jordan SJ. Understanding pathways to the diagnosis of thyroid cancer: are there ways we can reduce over-diagnosis? Thyroid. 2019;29(3):341–348. [DOI] [PubMed] [Google Scholar]
- 21. Bahl M, Sosa JA, Nelson RC, Esclamado RM, Choudhury KR, Hoang JK. Trends in incidentally identified thyroid cancers over a decade: a retrospective analysis of 2,090 surgical patients. World J Surg. 2014;38(6):1312–1317. [DOI] [PubMed] [Google Scholar]
- 22. Surveillance, Epidemiology, and End Results Program (SEER). SEER is an authoritative source for cancer statistics in the United States. Available at: www.seer.cancer.gov. Accessed 4 September 2018.
- 23. Clegg LX, Potosky AL, Harlan LC, Hankey BF, Hoffman RM, Stanford JL, Hamilton AS. Comparison of self-reported initial treatment with medical records: results from the Prostate Cancer Outcomes Study. Am J Epidemiol. 2001;154(6):582–587. [DOI] [PubMed] [Google Scholar]
- 24. Schootman M, Jeffe DB, West MM, Aft R. Self-report by elderly breast cancer patients was an acceptable alternative to Surveillance, Epidemiology, and End Results (SEER) abstract data. J Clin Epidemiol. 2005;58(12):1316–1319. [DOI] [PubMed] [Google Scholar]
- 25. Phillips KA, Milne RL, Buys S, Friedlander ML, Ward JH, McCredie MR, Giles GG, Hopper JL. Agreement between self-reported breast cancer treatment and medical records in a population-based breast cancer family registry. J Clin Oncol. 2005;23(21):4679–4686. [DOI] [PubMed] [Google Scholar]
- 26. Welch HG, Doherty GM. Saving thyroids - overtreatment of small papillary cancers. N Engl J Med. 2018;379(4):310–312. [DOI] [PubMed] [Google Scholar]
- 27. Haymart MR, Esfandiari NH, Stang MT, Sosa JA. Controversies in the management of low-risk differentiated thyroid cancer. Endocr Rev. 2017;38(4):351–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haymart MR, Muenz DG, Stewart AK, Griggs JJ, Banerjee M. Disease severity and radioactive iodine use for thyroid cancer. J Clin Endocrinol Metab. 2013;98(2):678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haymart MR, Banerjee M, Stewart AK, Koenig RJ, Birkmeyer JD, Griggs JJ. Use of radioactive iodine for thyroid cancer. JAMA. 2011;306(7):721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American Thyroid Association. American Thyroid Association statement: epidemic or thyroid cancer overdiagnosis? 2016. Available at: http://www.thyroid.org/epidemic-thyroid-diagnosis/. Accessed 12 September 2018.
- 31. Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, Tomoda C, Takamura Y, Kobayashi K, Miya A. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34(1):28–35. [DOI] [PubMed] [Google Scholar]
- 32. Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34(6):1222–1231. [DOI] [PubMed] [Google Scholar]
- 33. Haser GC, Tuttle RM, Urken ML. Challenges of active surveillance protocols for low-risk papillary thyroid microcarcinoma in the United States. Thyroid. 2016;26(7):989–990. [DOI] [PubMed] [Google Scholar]
- 34. Leboulleux S, Tuttle RM, Pacini F, Schlumberger M. Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance? Lancet Diabetes Endocrinol. 2016;4(11):933–942. [DOI] [PubMed] [Google Scholar]
- 35. Castro MR, Morris JC, Ryder M, Brito JP, Hay ID. Most patients with a small papillary thyroid carcinoma enjoy an excellent prognosis and may be managed with minimally invasive therapy or active surveillance. Cancer. 2015;121(18):3364–3365. [DOI] [PubMed] [Google Scholar]