Abstract
Background
We examined the impact of vancomycin-resistant Enterococcus (VRE) bloodstream infection (BSI) on outcomes of allogeneic hematopoietic cell transplantation (HCT) utilizing the Center for International Blood and Marrow Transplant Research database.
Methods
Adult and pediatric patients (N = 7128) who underwent first HCT for acute leukemia or myelodysplastic syndrome from 2008 through 2012 were analyzed as 3 groups—VRE BSI, non-VRE BSI, without BSI—according to BSI status at 100 days (D100) after allogeneic HCT. Multivariable models examined the effect of VRE BSI for overall survival (OS) and nonrelapse mortality (NRM) at 1 year.
Results
Of 7128 patients, 258 (3.2%) had VRE BSI, 2398 (33.6%) had non-VRE BSI, and 4472 (63%) had no BSI. The median time to VRE BSI and non-VRE BSI were D11 and D15, respectively. Compared with non-VRE BSI patients, VRE BSI patients were older, had advanced-stage acute leukemia, and received umbilical cord blood (UCB) allografts. In multivariable models, VRE BSI was associated with lower OS (relative risk [RR], 2.9;(99% confidence interval [CI], 2.2–3.7) and increased NRM (RR, 4.7; 99% CI, 3.6–6.2) (P < .0001) for both. Other predictors for worse OS and increased NRM were non-VRE BSI, older age, advanced disease stage, UCB allograft, – mismatch, comorbidity index ≥3, and cytomegalovirus seropositivity (P < .001 for all variables).
Conclusions
VRE BSI is associated with lowest OS and highest NRM compared with patients without BSI or non-VRE BSI. Novel interventions that address the pathophysiology of VRE BSI have the potential of improving survival after HCT.
Keywords: vancomycin-resistant Enterococcus (VRE), bacteremia, hematopoietic stem cell transplantation, mortality
In this registry study, vancomycin-resistant Enterococcus (VRE) bloodstream infection (BSI) by day 100 after hematopoietic stem cell transplantation was associated with decreased 1-year survival (P < .0001). VRE BSI increased risk of nonrelapse mortality by 4.7 fold and 2.7 fold compared with no BSI or non-VRE BSI, respectively.
The incidence of vancomycin-resistant Enterococcus (VRE) bloodstream infection (BSI) after allogeneic hematopoietic cell transplantation (HCT) ranges from 3.6% to 22% [1–8]. VRE colonization, mucosal barrier injury, neutropenia, antibiotic use, and renal insufficiency are known risk factors for VRE BSI [9–12]. VRE BSI, a marker of intestinal dysbiosis, is associated with poor transplant outcomes [13, 14], longer hospitalization compared with non-VRE BSI [3, 15], and increased mortality [16–18]. Mortality associated with VRE BSI ranges from 4% to 70% in single-center studies [1]. There are no consortium-level data related to the impact of VRE BSI on long-term survival post HCT. We conducted a retrospective analysis utilizing the Center for International Blood and Marrow Transplant Research (CIBMTR) database to investigate the impact of VRE BSI within the first 100 days post HCT on overall survival (OS), nonrelapse mortality (NRM), and relapse at 1 year following HCT.
METHODS
Data Source
Data were obtained from the CIBMTR, a voluntary working group of more than 450 transplantation centers worldwide that contribute data for consecutive allogeneic and autologous HCT procedures to a statistical center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis (Supplementary Material).
Patients
The study population included patients who received their first allogeneic HCT for acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), or myelodysplastic syndrome (MDS) between January 2008 and December 2012 contained in the CIBMTR database. Patients received bone marrow (BM), peripheral blood stem cells (PBSCs), or umbilical cord blood (UCB) allografts from either matched related or unrelated donors. Transplants utilizing haploidentical donors and T-cell–depleted allografts were included in the analyses.
Definitions
Centers report infection data to the CIBMTR with an organism code, date of onset, and site of infection. These data do not include information regarding method of diagnosis, severity of infection, or treatment. BSI was defined as a bacterial organism reported from the blood/buffy coat obtained either from peripheral blood or a central venous catheter. VRE BSI was defined as a VRE isolate in ≥1 blood cultures between 10 days before (D10) and 100 days after HCT (D100) as reported to CIBMTR. Non-VRE BSI was defined as ≥1 positive blood cultures by any bacterium(a) except VRE and without BSI as patients without a bacterial BSI between D10 and D100.
Conditioning intensity was defined as myeloablative or reduced intensity/nonmyeloablative using defined criteria [19]. Performance status at the time of HCT was defined according to the Karnofsky performance scale (KPS) for patients aged ≥16 years or the Lansky scale for those aged <16 years. Disease status was categorized as early (AML/ALL in CR1 [first complete remission] or MDS with refractory anemia with or without ringed sideroblasts, refractory cytopenias with multilineage dysplasia with/without ringed sideroblasts, and MDS with 5q-syndrome); intermediate (AML/ALL in ≥CR2); or advanced (AML/ALL untreated, primary induction failure, or relapse or MDS including refractory anemia with excess blasts). human leucocyte antigen (HLA)-match grade categories for unrelated donors were categorized as previously published [20].
Outcomes and Endpoints
OS and NRM, relapse, and disease-free survival (DFS) were assessed at 180 days and 1 year following HCT. OS was defined as the time from HCT to death from any cause. Patients were censored at time of last follow-up. NRM, defined as death without evidence of relapse or progression of underlying malignancy, was estimated using the cumulative incidence function with relapse/progression as the competing risk. Progressive disease or recurrence of disease was estimated using the cumulative incidence function with death as the competing risk. Chronic graft versus host disease (GVHD) was scored using the National Institutes of Health consensus criteria [21]. Patients who survived without recurrence or progressive disease were censored at the date of last contact. Primary causes of death were reported by the centers. Centers report cause of death information to the CIBMTR following the instructions in the CIBMTR Forms Instruction Manual for primary and contributory causes of death [22].
Statistical Analyses
To assess the impact of early VRE BSI on the outcomes of interest, patients were categorized into 3 mutually exclusive groups based upon bacterial BSI status by D100: VRE BSI, patients with BSI caused by VRE; non-VRE BSI, patients with BSI caused by any bacteria except VRE; and without BSI, patients without any bacterial BSI.
The χ2 test for categorical variables and the Wilcoxon 2-sample test for continuous variables compared patient-, disease-, and transplant-related factors present at the time of transplant. Time-dependent variables of neutrophil engraftment and development of acute GVHD are descriptive only as these events occurred after HCT but developed simultaneously with the bacterial BSI (or not) that created the cohorts for the analysis. The Kaplan-Meier estimator determined probability of OS and DFS, whereas cumulative incidence estimates accounting for competing risk provided values for other endpoints. Because the cohorts are determined by an event occurring (or not) by D100, we performed univariate analyses for the entire population from the time of transplant (D0) and a landmark analysis for only those patients alive at D100. Using this approach, the trends and statistical significance for all events were similar; thus, we report the results for the entire population unless specified otherwise.
A Cox model for the entire population was fit to assess the impact of VRE BSI on the outcomes of OS, DFS, NRM, and relapse. The proportional hazards assumption was checked and, if violated, the variable was included as time-dependent covariate. Examination for interaction between the main effect and significant covariates occurred. Variables examined in the multivariable models included age (≤20 years vs 21–40 years vs 41–50 years vs >60 years); KPS (≥90% vs <90%); disease stage (AML/ALL early [CR1] vs AML/ALL intermediate [≥CR2] vs AML/ALL advanced [relapsed/refractory], vs MDS early [refractory anemia (RA), refractory anemia with ringed sideroblasts (RS), refractory cytopenia with multilineage dysplasia (RCMD), RCMD/RS] vs MDS advanced [RAEB1, RAEB2]); HCT-comorbidity index (HCT-CI) (0 vs 1–2 vs ≥3); donor (D)/recipient (R) cytomegalovirus (CMV) serostatus (both negative vs any positive); conditioning intensity (myeloablative vs nonmyeloablative/reduced intensity); donor/HLA-match (HLA-identical sibling vs 8/8 unrelated vs mismatched unrelated vs UCB vs other [mismatched related/haploidentical or unrelated with HLA missing]); antithymocyte globulin/alemtuzumab use (no vs yes); GVHD prophylaxis (calcineurin inhibitor [CNI] + methotrexate others vs CNI + mycophenolate mofetil ± others vs T-cell depletion [in vivo or ex vivo] vs other); and year of HCT (2008–2009 vs 2010–2012). Cox models incorporating acute GVHD at any time after transplant, only prior to BSI, or excluding acute GVHD were constructed. Relative risks and significant covariates were unchanged across these 3 models; therefore, models shown do not include acute GVHD. The score test of Commenges and Andersen examined for center effect [23]. Multivariable analyses for OS, DFS, relapse, and NRM all demonstrated a center effect.
RESULTS
Patient Population
From 2008–2012, 7861 patients who received first allogeneic HCT for AL and MDS were reported to CIBMTR. To minimize reporting biases, centers that reported 0 or 100% patients with coagulase-negative staphylococci (CoNS) BSI and centers that reported 100% patients with BSI were excluded (269 patients from 50 centers). In addition, 464 patients were excluded for missing consent (n = 281) or missing data (n = 183). After excluding 733 patients, 7128 patients from 181 centers (135 US centers) were included in the final analyses. The median follow-up of survivors for the entire cohort was 59 months (95% confidence interval [CI], 3–87); VRE BSI, 65 months (95% CI, 24–74); non-VRE BSI 60, months (95% CI, 3–87); and control 59, months (95% CI, 3–87)]; although all events are examined at 1 year following transplantation.
Incidence of VRE BSI
A total of 258 (3.6%) patients had VRE BSI, 2398 (33.6%) had non-VRE BSI, and 4472 (63%) had no BSI during the study period. VRE accounted for 9.7% of all BSI (258/2656). Supplementary Figure S1 shows the distribution of BSI organisms. Gram-positive organisms comprised 56% of all BSI: VRE, 9.7%; vancomycin-sensitive Enterococci (VSE), 4%; viridans group streptococci (VGS); 8%; coNS, 27%; and staphylococci other than coNS, 7%.
The median time to VRE BSI was D11 (<1–99 days) prior to neutrophil engraftment, which occurred at a median of D15 (<1–111) for the population. The majority of VRE BSI (n = 155, 60%) occurred between the start of conditioning and 14 days after neutrophil engraftment. While 111 (43%) patients with VRE BSI had VRE as the sole pathogen of BSI, the remaining 147 patients (57%) had polymicrobial BSI with CoNS (20%) and Enterobacteriaceae (11%) being the most frequent copathogens. Among the various stem cell sources, VRE BSI was highest in UCB recipients (n = 103, 5.6%) compared to BM (n = 110, 4.2%) and PBSC recipients (n = 45, 2.5%).
Comparison of Patients With VRE BSI With Non-VRE BSI or Without BSI
Table 1 shows a comparison of baseline and clinical characteristics for the 3 patient groups. Compared with non-VRE BSI, VRE BSI patients were older (>60 years); more likely to have advanced stage AML/ALL; received UCB, a myeloablative conditioning regimen, and high-dose total body irradiation (≥1200 Gy); and were CMV donor seronegative (D–) and recipient seropositive (R+). Median time (range) from diagnosis to HCT was 7 months (1–224), 7 months (<1–313), and 6 months (<1–291) for patients with VRE BSI, non-VRE BSI, and without BSI, respectively (P = .005).
Table 1.
Baseline Characteristics of Patients With Vancomycin-resistant Enterococcus Bloodstream Infection (VRE BSI), Non-VRE BSI, and Without BSI
| VRE BSI | Non-VRE BSI | Without BSI | ||
|---|---|---|---|---|
| Characteristic | N (%) | N (%) | N (%) | P Valuea |
| Patients | 258 | 2398 | 4472 | … |
| Number of centers | 76 | 174 | 177 | … |
| Patient related | ||||
| Age (y), median (range) | 49 (1–73) | 42 (<1–79) | 49 (<1–78) | <.001 |
| Disease-related | ||||
| Disease stage at transplant | … | … | … | <.001 |
| AML/ALL early | 100 (39) | 953 (40) | 1908 (43) | … |
| AML/ALL intermediate | 45 (17) | 613 (26) | 884 (20) | … |
| AML/ALL advanced | 62 (24) | 364 (15) | 613 (14) | … |
| MDS early | 16 (6) | 175 (7) | 415 (9) | … |
| MDS advanced | 34 (13) | 275 (11) | 630 (14) | … |
| Missing | 1 (<1) | 18 (<1) | 22 (<1) | … |
| Hematopoietic cell transplantation comorbidity index | … | … | … | .023 |
| 0 | 87 (34) | 1056 (44) | 1927 (43) | … |
| 1 | 50 (19) | 343 (14) | 649 (15) | … |
| 2 | 31 (12) | 263 (11) | 536 (12) | … |
| ≥ 3 | 89 (34) | 692 (29) | 1263 (28) | … |
| Missing | 1 (<1) | 44 (2) | 97 (2) | … |
| White blood cell count prior to preparative regimen, median (range), 109/L | 3.6 (0.0–26.2) | 3.9 (0.0–28.9) | 3.9 (0.0–28.9) | .036 |
| Missing | 6 (2) | 26 (1) | 44 (<1) | … |
| Transplant-related | ||||
| Conditioning regimen intensity | … | … | … | <.001 |
| Myeloablative | 206 (80) | 1894 (79) | 3149 (70) | … |
| Reduced intensity conditioning/nonmyeloablative | 52 (20) | 504 (21) | 1323 (30) | … |
| TBI dose | … | … | … | <.001 |
| No TBI | 119 (46) | 1131 (47) | 2480 (55) | … |
| <1200 cGy | 78 (30) | 751 (31) | 1297 (29) | … |
| ≥1200 cGy | 61 (24) | 516 (22) | 695 (16) | … |
| Donor/Recipient cytomegalovirus status | … | … | … | <.001 |
| +/+ | 48 (19) | 499 (21) | 1043 (23) | … |
| +/– | 15 (6) | 159 (7) | 418 (9) | … |
| –/+ | 128 (50) | 988 (41) | 1627 (36) | … |
| –/– | 64 (25) | 718 (30) | 1307 (29) | … |
| Missing | 3 (1) | 34 (1) | 77 (2) | … |
| Graft type | … | … | … | <.001 |
| Bone marrow | 45 (17) | 360 (15) | 662 (15) | … |
| Peripheral blood | 110 (43) | 1225 (51) | 2892 (65) | … |
| Cord blood | 103 (40) | 813 (34) | 918 (21) | … |
| Graft versus host disease prophylaxis | … | … | … | <.001 |
| CSA/TAC + MTX ± othersb | 115 (45) | 1114 (46) | 2348 (53) | … |
| CSA/TAC + MMF ± othersb | 100 (39) | 866 (36) | 1393 (31) | … |
| CSA/TAC ± othersb | 18 (7) | 326 (14) | 537 (12) | … |
| Othersb | 25 (10) | 92 (4) | 194 (4) | … |
| Granulocyte-colony stimulating factor, granulocyte-macrophage- colony stimulating factor (day 3 to day 15) | 170 (66) | 1434 (60) | 2379 (53) | <.001 |
| Supplemental intravenous immunoglobulin | 122 (47) | 1170 (49) | 1766 (39) | <.001 |
| Year of transplant | … | … | … | <.001 |
| 2008 | 76 (29) | 741 (31) | 1194 (27) | … |
| 2009 | 75 (29) | 672 (28) | 1073 (24) | … |
| 2010 | 47 (18) | 451 (19) | 837 (19) | … |
| 2011 | 34 (13) | 283 (12) | 642 (14) | … |
| 2012 | 26 (10) | 251 (10) | 726 (16) | … |
The following variables were not significant: fungal infection within 3 months prior to hematopoietic cell transplantation (HCT), neutrophil counts prior to preparative regimen, lymphocyte count prior to preparative regimen, sex, Karnofsky performance scale score at HCT. Donor and recipient gender match, antithymocyte globulin/Campath as conditioning for graft versus host disease prophylaxis.
Abbreviations: ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; CSA, cyclosporine; MDS, myelodysplastic syndrome; MMF: mycophenolate mofetil; MTX, methotrexate; TAC: tacrolimus; TBI, total body irradiation.
a P value: comparisons are made across the 3 groups.
bOther includes ex vivo T-cell depletion, CD34+ selection, cyclophosphamide, antithymocyte globulin/Campath, sirolimus +other (not TAC or CSA) or other.
Table 2 describes the frequency and time to event for neutrophil engraftment, platelet engraftment, and acute GVHD within the 3 groups. Because these events occurred prior to the defining event of BSI (or not) by D100 and both the defining event and the analyzed event are time dependent, univariate comparisons are not statistically appropriate.
Table 2.
Time-dependent Variables of Patients With Vancomycin-resistant Enterococcus Bloodstream Infection (VRE BSI), Non-VRE BSI, and Without BSI
| Variable | VRE BSI | Non-VRE BSI | Without BSI |
|---|---|---|---|
| Patients | 258 | 2398 | 4472 |
| Time from transplant to BSI, median (range) days |
11 (<1–99) | 11 (<1–100) | … |
| ANC >500+, n (%) | |||
| Yes | 214 (83) | 2249 (94) | 4321 (97) |
| No | 44 (17) | 144 (6) | 138 (3) |
| Missing | 0 | 5 (<1) | 13 (<1) |
| Time to ANC >500, days from HCT | 18 (1–89) | 16 (<1–99) | 15 (<1–111) |
| Platelet >20 × 109/L,a n (%) | |||
| Yes | 143 (55) | 1958 (82) | 4049 (91) |
| No | 112 (43) | 409 (17) | 395 (9) |
| Missing | 3 (1) | 31 (1) | 28 (<1) |
| Time to platelet >20 × 109/L, median (range) days from HCT |
27 (1–640) | 24 (<1–1180) | 19 (<1–753) |
| Acute GVHD grade II–IV, n (%) | |||
| Yes | 104 (40) | 1070 (45) | 1673 (37) |
| No | 150 (58) | 1307 (55) | 2761 (62) |
| Missing | 4 (2) | 21 (<1) | 38 (<1) |
| Time to acute GVHD, median (range) days | 26 (7–158) | 27 (7–176) | 29 (7–178) |
Abbreviations: ANC, absolute neutrophil count; BSI, bloodstream infection; GVHD, graft versus host disease; HCT, hematopoietic cell transplantation.
aNumber of patients who achieved an ANC >500 mm3 or platelet count >20 × 109/L after HCT.
Univariate Analyses of HCT Outcomes
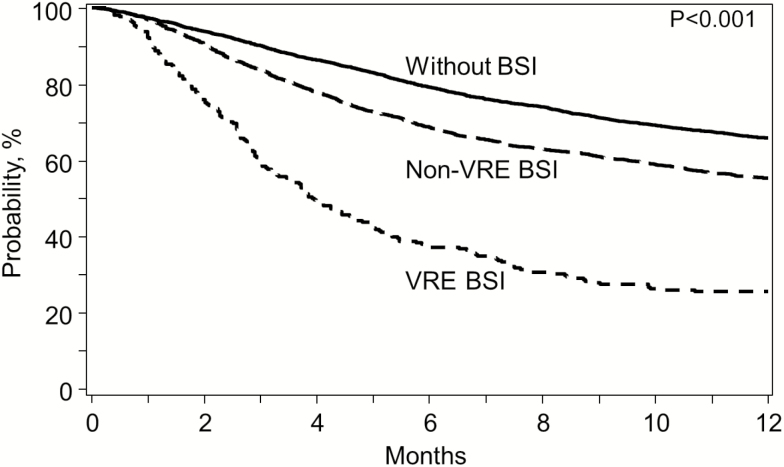
Table 3 shows the probabilities of OS, NRM, DFS, and relapse for VRE BSI, non-VRE BSI, and without BSI. Patients with VRE BSI had the lowest OS and DFS and the highest NRM at 1 year post HCT (P < .0001 for all). In contrast, the probability of relapse was similar across the groups. To assess the immediate impact of VRE, we compared survival at 30 days from BSI onset between 258 patients with VRE BSI and 2398 patients with non-VRE BSI. VRE BSI was associated with worse 30-day survival, 78% (95% CI, 72%–82%) and 90% (95% CI, 89%–91%), respectively (P < .001). Table 4 describes the cause of death for patients by 1 year.
Table 3.
Univariate Outcomes of Overall Survival, Disease-free Survival, Nonrelapse Mortality, and Relapse by 1 Year for Patients With Vancomycin-resistant Enterococcus Bloodstream Infection (VRE BSI), Non-VRE BSI, and Without BSI
| Outcome | VRE BSI, % (99% CI) |
Non-VRE BSI, % (99% CI) |
Without BSI, % (99% CI) |
P Value |
|---|---|---|---|---|
| Overall survival | 26 (20–31) | 56 (54–58) | 66 (65–67) | <.0001 |
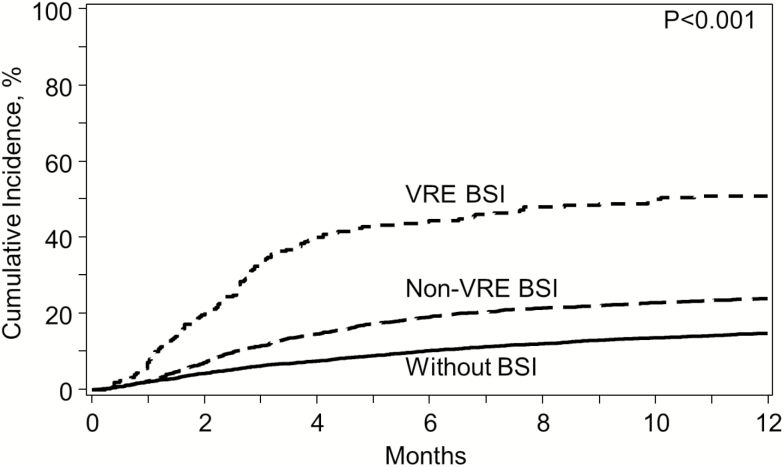
| Nonrelapse mortality | 51 (44–57) | 24 (22–26) | 15 (14–16) | <.0001 |
| Disease-free survival | 23 (18–29) | 48 (46–50) | 44 (53–56) | <.0001 |
| Relapse | 26 (21–32) | 28 (26–30) | 30 (29–32) | .0656 |
Abbreviation: CI, confidence interval.
Table 4.
Primary Cause of Death by 1 Year Post–Hematopoietic Cell Transplantation of Patients With Vancomycin-resistant Enterococcus Bloodstream Infection (VRE BSI), non-VRE BSI, and Without BSI by Day 100
| Variable | VRE BSI, N (%) |
Non-VRE BSI, N (%) |
Without BSI, N (%) |
|---|---|---|---|
| Patients who died by 1 year post hematopoietic cell transplantation | 192 (74) | 1063 (44) | 1519 (34) |
| Relapse (recurrent/persistent disease) | 47 (24) | 440 (41) | 807 (53) |
| Organ failure | 49 (26) | 166 (16) | 223 (15) |
| Infection | 29 (15) | 195 (18) | 182 (12) |
| Graft versus host disease | 28 (15) | 125 (12) | 145 (10) |
| Other cause | 19 (10) | 48 (5) | 65 (4) |
| Hemorrhage | 8 (4) | 27 (3) | 26 (2) |
| Idiopathic pneumonitis | 5 (3) | 19 (2) | 22 (1) |
| Graft rejection | 5 (3) | 23 (2) | 21 (1) |
| Unknown | 2 (1) | 12 (1) | 18 (1) |
| Secondary malignancy | 0 | 8 (<1) | 10 (<1) |
Infection was a secondary/contributing cause of death: VRE BSI = 45 (23%), non-VRE BSI = 187 (18%), without BSI = 215 (14%).
Multivariate Analyses of HCT Outcomes
Overall Survival
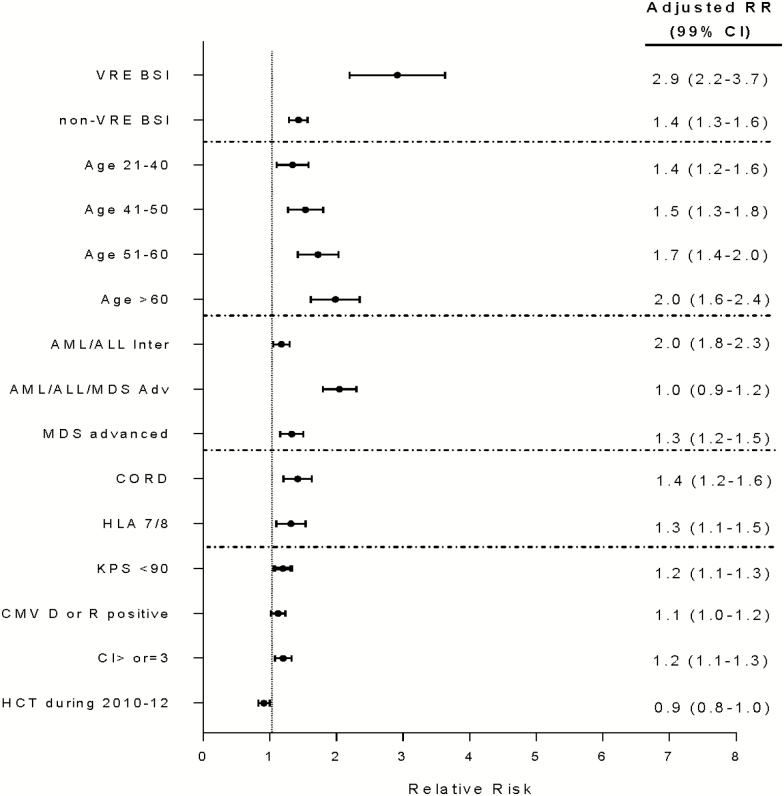
By 1 year post HCT, 26% (95% CI, 20%–31%) of patients with VRE BSI were alive (Figure 1). After adjusting for covariates, VRE BSI was associated with worse OS (Figure 2). VRE BSI patients had a 2.9-fold increased risk of death compared with patients without BSI. In addition, older age (>60 years), advanced disease stage, KPS <90, HCT-CI ≥3 and receipt of an unrelated ≤7/8 HLA matched, or cord blood allograft and CMV R and/or D seropositivity were independent predictors of mortality (P value <.0001 for all). In contrast, undergoing HCT between 2010 and 2012 was associated with improved OS (0.0067).
Figure 1.
Kaplan-Meier estimates of overall survival for patients with VRE BSI (dotted), non-VRE BSI (dashed), and without BSI (solid) by day 100. Abbreviations: BSI, bloodstream infection; VRE, vancomycin-resistant Enterococcus.
Figure 2.
Multivariate analysis of risk factors for overall survival. x-axis, adjusted relative risk; dots, adjusted relative risk; whiskers, 99% confidence intervals. Abbreviations: ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; BSI, bloodstream infection; CI, confidence interval; CMV, cytomegalovirus; CORD, cord blood allograft; HCT, hematopoietic cell transplantation; HLA, human leucocyte antigen; KPS, Karnofsky performance scale; MDS, myelodysplastic syndrome; RR, risk ratio; VRE, vancomycin-resistant Enterococcus.
Nonrelapse Mortality
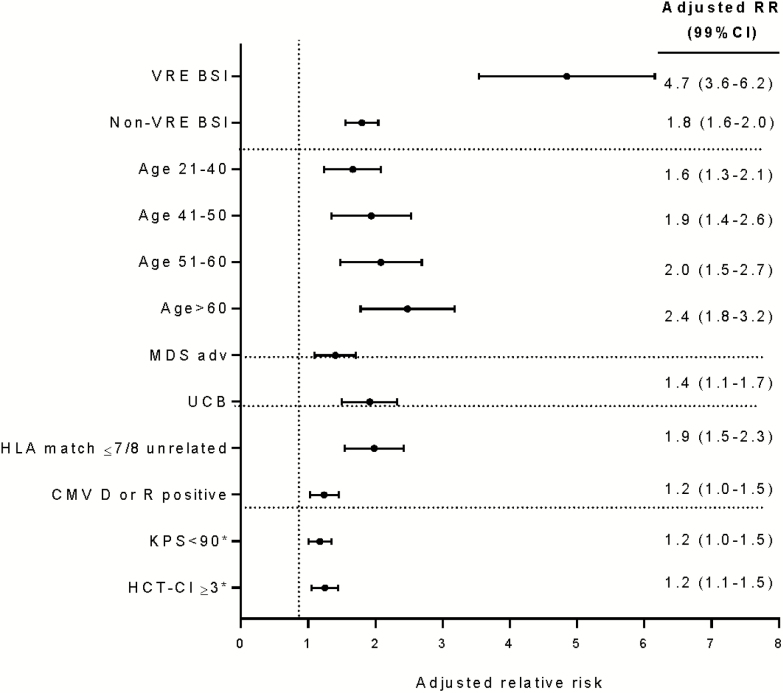
NRM for VRE BSI was 51% (95% CI, 44%–57%) at 1 year (Figure 3). After adjusting for covariates, VRE BSI patients had 4.7-fold increased NRM compared with patients without BSI (P < .001). Other factors associated with increased NRM were older age, KPS ≤90, HCT-CI ≥3, CMV R and/or D seropositivity, and receipt of allograft from other than matched related donor (Figure 4).
Figure 3.
Cumulative incidence of nonrelapse mortality for patients with VRE BSI (dotted), non-VRE BSI (dashed), and without BSI (solid) by day 100. Abbreviations: BSI, bloodstream infection; VRE, vancomycin-resistant Enterococcus.
Figure 4.
Multivariate analysis of risk factors for nonrelapse mortality. x-axis, adjusted relative risk; dots, adjusted relative risk; whiskers, 99% confidence intervals. Abbreviations: BSI, bloodstream infection; CI, confidence interval; CMV, cytomegalovirus; HCT, hematopoietic cell transplantation; HLA, human leucocyte antigen; KPS, Karnofsky performance scale; MDS, myelodysplastic syndrome; RR, risk ratio; UCB, umbilical cord blood; VRE, vancomycin-resistant Enterococcus.
Contrast Analyses
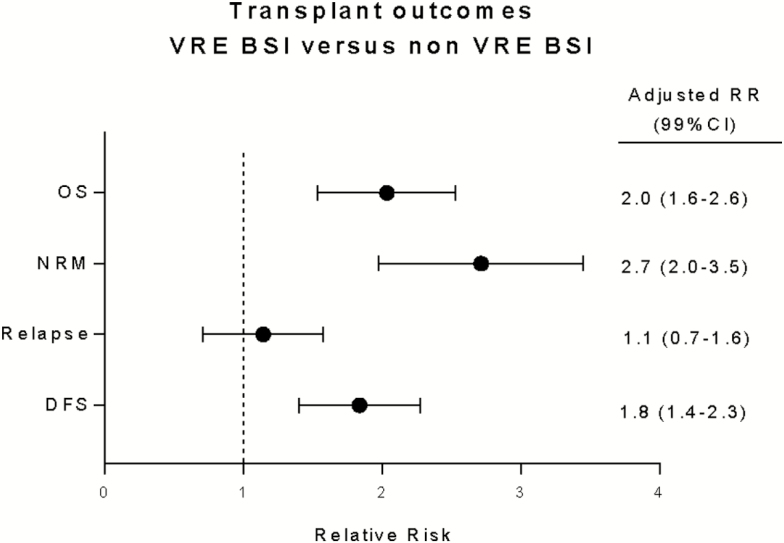
To assess the impact of VRE BSI compared with non-VRE BSI on OS and NRM, we performed contrast analyses (Supplementary Table S1). After adjusting for covariates, VRE BSI conferred approximately 2-times higher risk of death and 2.7-times higher risk of NRM. In contrast, risk of relapse was similar for VRE and non-VRE BSI (Figure 5).
Figure 5.
Impact of vancomycin-resistant Enterococcus bloodstream infection (VRE BSI) on OS, NRM, and DFS compared with non-VRE BSI, adjusted for covariates. x-axis, adjusted relative risk; dots, adjusted relative risk; whiskers, 99% confidence intervals. Abbreviations: CI, confidence interval; DFS, disease-free survival; NRM, nonrelapse mortality; OS, overall survival; RR, risk ratio.
In our cohort, a wide range of bacteria caused BSI with differences in pathophysiology and outcomes. In addition, >50% of VRE BSI had concomitant pathogens. To further assess the impact of VRE as the sole BSI pathogen, we performed univariate analyses of OS and NRM comparing pairwise VRE BSI (as single pathogen n = 111) with VRE BSI (with concomitant pathogens n = 147), VSE BSI (n = 89), viridians group streptococci BSI (n = 192), and Enterobacteriaceae BSI (n = 256) (Supplementary Figure S2 shows the univariate analyses for OS). OS and NRM at 1 year were similar between VRE BSI as single pathogen or VRE BSI with concomitant pathogens. In contrast, patients with VRE BSI had lower OS and higher NRM compared with VSE, VGS, or gram negative rods (GNR). The small numbers for each BSI category precluded multivariable models.
DISCUSSION
Results presented here show that VRE BSI that occurred by D100 was associated with decreased OS and increased NRM in the first year post HCT after adjusting for patient and transplant characteristics. To our knowledge, we are the first to compare the impact of VRE BSI to non-VRE BSI on long-term HCT outcomes in a large, multiinstitutional cohort. Despite the availability of antibiotics against VRE [24, 25], patients with VRE BSI had 4.7- and 2.7-fold increased risk of 1-year NRM compared with patients without BSI or non-VRE BSI, respectively.
Previous studies with shorter follow-up report an increased risk of death with VRE BSI [5, 6, 8, 16, 26]. Post-engraftment VRE BSI has been associated with increased mortality, possibly due to an association of VRE BSI with GVHD [3, 12]. Due to the interplay of infection and GVHD as well as the cohort determination by events occurring by D100, univariate comparisons of GVHD frequency is not statistically appropriate. To assess for the impact of acute GVHD, multivariable models that incorporate acute GVHD prior to infection or without acute GVHD were performed. The results were similar for outcomes of OS, DFS, NRM, and relapse. Therefore, in order to focus on the infection impact, the final model does not include acute GVHD as a time-dependent covariate.
Reported rates for primary cause of death due to GVHD or infection were similar between patients with VRE BSI and non-VRE BSI. In contrast, organ failure was the cause of death in 26% and 16% of patients with VRE BSI and non-VRE BSI, respectively. No data were collected regarding the type or causes of organ failure that led to death. VRE sepsis and/or preexisting comorbidities in patients with VRE BSI may partially account for the higher proportion of deaths due to organ failure among VRE patients. Disruption of normal microbiota by antibiotics in the context of VRE colonization is postulated to lead to intestinal domination of VRE and subsequent VRE BSI during neutropenia [10]. Thus, VRE BSI may be a surrogate marker of microbial dysbiosis that has been associated with poor transplant outcomes [12, 27].
Our multivariate models demonstrated a center effect. In designing the study, consideration for potential center reporting bias occurred such that patients coming from centers that reported 0 or 100% of their patients with CoNS BSI and centers that reported 100% of their patients having BSI were excluded. Furthermore, the analysis included the test for center effect, and the results reported are adjusted accordingly. However, the presence of this center effect highlights the need for large multicenter database evaluations of VRE BSI, as well as single-center case-control cohort analyses with detailed matching schemata that are not possible using large registry databases. Recently developed integrative scoring models predict the risk of VRE BSI post HCT [12]. Given the strong negative association of VRE with survival, factoring the risk of VRE BSI in addition to other variables may more accurately predict risk of mortality following allogeneic transplantation. Further studies to examine this are warranted.
The strengths of our study include a multicenter, large, and diverse population and detailed and uniform data collection post HCT. Adjustment for the center effect corrects for center-specific practices. The limitations of our study are expected in a transplant-focused rather than infection-focused database. First, there are no data on antibiotic exposure, including infection prophylaxis and treatment data. During the study period, the National Comprehensive Cancer Network guidelines endorsed fluoroquinolone-based prophylaxis [28, 29]. The choice of antibiotics for empiric treatment of fever and neutropenia or treatment of specific BSI may vary across centers. An association between exposure to antibiotics with anaerobic activity and subsequent VRE BSI or death has been recognized [30, 31]. Second, the status of VRE colonization is unknown. VRE colonization is a strong predictor for VRE BSI and a surrogate marker of loss of microbial diversity [10]. Finally, the CIBMTR does not capture severity of mucosal injury. The lack of all of these data preclude a meaningful assessment of risk factors for VRE BSI. Furthermore, our study was not designed to assess any causality between VRE BSI and survival. Despite these limitations, our study includes more than 7000 patients who received transplants during 2008–2012, allowing important assessment of the clear negative impact of VRE BSI on survival. Despite a better understanding of the pathophysiology of VRE BSI, clinical practice regarding VRE has not substantially changed [1]; thus, our findings remain clinically relevant. Future studies are encouraged as broader changes in HCT practices may alter VRE BSI risks and outcomes due to this complication.
In summary, early VRE BSI was an independent predictor of OS and NRM at 1 year post HCT. Given NRM of 51% among patients with VRE BSI, our study provides a strong impetus for well-designed interventional studies to modify factors that contribute to this life-threatening complication and potentially improve survival.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Contributors. The following members of the Center for International Blood and Marrow Transplant Research (CIBMTR®) Infection and Immune Reconstitution Working Committee contributed to this work: Hisham Abdel-Azim, Division of Hematology, Oncology and Blood & Marrow Transplantation, Children’s Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, CA; Ibrahim A. Ahmed, Department of Hematology Oncology and Bone Marrow Transplantation, the Children’s Mercy Hospitals and Clinics, Kansas City, MO; Mahmoud Aljurf, Department of Oncology, King Faisal Specialist Hospital Center & Research, Riyadh, Saudi Arabia; Joseph Antin, Division of Hematologic Malignancies, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA; Karen Kuhn Ballen, Division of Hematology/Oncology, University of Virginia Health System, Charlottesville, VA; Amer Beitinjaneh, University of Miami, Miami, FL; Valerie I. Brown, Division of Pediatric Oncology/Hematology, Department of Pediatrics, Penn State Hershey Children’s Hospital and College of Medicine, Hershey, PA; Jan Cerny, UMass Memorial Medical Center, Worcester, MA; Richard Champlin, Department of Stem Cell Transplantation and Cellular Therapy, Division of Cancer Medicine, the University of Texas, MD Anderson Cancer Center, Houston, TX; Nelson Chao, Division of Cell Therapy and Hematological Malignancies, Department of Medicine, Duke University Medical Center, Durham, NC; Saurabh Chhabra, Division of Hematology and Oncology, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI; Parastoo B. Dahi, Department of Medicine, Adult Bone Marrow Transplant Service, Memorial Sloan Kettering Cancer Center, New York, NY; Andrew Daly, Tom Baker Cancer Centre, Calgary, AB, Canada; Christopher Dandoy, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Christopher C. Dvorak, Department of Pediatrics, University of California San Francisco, Benioff Children’s Hospital, San Francisco, CA; Stephen Forman, City of Hope, Duarte, CA; Cesar Freytes, Texas Transplant Institute, San Antonio, TX; Siddhartha Ganguly, Division of Hematological Malignancy and Cellular Therapeutics, University of Kansas Health System, Kansas City, KS; Shahrukh K. Hashmi, Department of Oncology, King Faisal Specialist Hospital Center & Research, Riyadh, Saudi Arabia and Department of Internal Medicine, Mayo Clinic, Rochester, MN; Mohamed A. Kharfan-Dabaja, Division of Hematology-Oncology, Blood and Marrow Transplantation Program, Mayo Clinic, Jacksonville, FL; Hillard Lazarus, Seidman Cancer Center, University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH; Per Ljungman, Department of Cellular Therapy and Allogeneic Stem Cell Transplantation, Karolinska University Hospital, Stockholm, Sweden; Adriana K. Malone, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY; Guru Murthy, Division of Hematology and Oncology, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI; Taiga Nishihori, Department of Blood and Marrow Transplantation, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; Kristin Page, Division of Pediatric Blood and Marrow Transplantation, Duke University Medical Center, Durham, NC; Ravi (Sai Ravi) Pingali, Department of Stem Cell Transplantation and Cellular Therapy, Division of Cancer Medicine, the University of Texas; Vijay Reddy, Department of Internal Medicine, University of Central Florida College of Medicine, Orlando, FL; Ayman Saad, Division of Hematology/Oncology, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL; Bipin N Savani, Division of Hematology/Oncology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN; Matthew Seftel, Department of Medical Oncology and Hematology, Cancer Care Manitoba, Winnipeg, Canada; Jeffrey Szer, Department Clinical Haematology and Bone Marrow Transplantation, Royal Melbourne Hospital, Victoria, Australia; Ravi Vij, Division of Hematology and Oncology, Washington University School of Medicine, St. Louis, MO; Daniel Weisdorf, Division of Hematology, Oncology and Transplantation, Department of Medicine, University of Minnesota Medical Center, Minneapolis, MN; Basem M. William, Ohio State Medical Center, James Cancer Center, Columbus, OH; Kirsten Williams, Experimental Transplantation and Immunology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD; Baldeep Wirk, Division of Bone Marrow Transplant, Seattle Cancer Care Alliance, Seattle, WA; Jean Yared, Blood & Marrow Transplantation Program, Division of Hematology/Oncology, Department of Medicine, Greenebaum Cancer Center, University of Maryland, Baltimore, MD; and Lolie C. Yu, Division of Hematology/Oncology & Hematopoietic Stem Cell Transplant, the Center for Cancer and Blood Disorders, Children’s Hospital/Louisiana State University Medical Center, New Orleans, LA. In Memoria: Oliver Press, Fred Hutchinson Cancer Research Center, Seattle, WA.
Disclaimer. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA), or any other agency of the US government.
Financial support. This work was supported by the National Institutes of Health (grant numbers P30 CA008748 and P01 CA023766). The CIBMTR® is supported primarily by a Public Health Service grant/cooperative agreement (grant number 5U24CA076518) from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases; a grant/cooperative agreement (grant number 1U24HL138660) from NHLBI and NCI; a contract (number HHSH250201700006C) with Health Resources and Services Administration/Department of Health and Human Services; 2 grants (grant numbers N00014-17-1-2388, N00014-17-1-2850, and N00014-18-1-2045) from the Office of Naval Research; and grants from the following: Adaptive Biotechnologies; *Amgen, Inc.; an anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; *CytoSen Therapeutics, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Karius, Inc.; Karyopharm Therapeutics, Inc.; *Kite Pharma, Inc.; Medac, GmbH; *Mediware; the Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; Mundipharma EDO; National Marrow Donor Program; Novartis Pharmaceuticals Corporation; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; Swedish Orphan Biovitrum, Inc.; *Takeda Oncology; and University of Minnesota. The asterisks indicate corporate membership. G. A. P. was partially funded by the NCI Cancer Center (support grant number P30 CA008748).
Potential conflicts of interest.J. J. A. has served as a medical consultant for MoreHealth. C. A. L. University Medical Center Utrecht. C. A. L. has compensation from Chimerix in Europe as a medical advisor for Brincidofovir studies from 2015– present. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
CIBMTR® Infection and Immune Reconstitution Working Committee:
Hisham Abdel-Azim, Ibrahim A Ahmed, Mahmoud Aljurf, Joseph Antin, Karen Kuhn Ballen, Amer Beitinjaneh, Valerie I Brown, Jan Cerny, Richard Champlin, Nelson Chao, Saurabh Chhabra, Parastoo B Dahi, Andrew Daly, Christopher Dandoy, Christopher C Dvorak, Stephen Forman, Siddhartha Ganguly, Shahrukh K Hashmi, Mohamed A Kharfan-Dabaja, Hillard Lazarus, Per Ljungman, Adriana K Malone, Guru Murthy, Taiga Nishihori, Kristin Page, Ravi (Sai Ravi) Pingali, Vijay Reddy, Ayman Saad, Bipin N Savani, Matthew Seftel, Jeffrey Szer, Ravi Vij, Daniel Weisdorf, Basem M William, Kirsten Williams, Baldeep Wirk, and Jean Yared
References
- 1. Benamu E, Deresinski S. Vancomycin-resistant enterococcus infection in the hematopoietic stem cell transplant recipient: an overview of epidemiology, management, and prevention. F1000Res 2018; 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seo SK, Xiao K, Huang YT, et al. . Impact of peri-transplant vancomycin and fluoroquinolone administration on rates of bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients: a 12-year single institution study. J Infect 2014; 69:341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ford CD, Gazdik MA, Lopansri BK, et al. . Vancomycin-resistant enterococcus colonization and bacteremia and hematopoietic stem cell transplantation outcomes. Biol Blood Marrow Transplant 2017; 23:340–6. [DOI] [PubMed] [Google Scholar]
- 4. Almyroudis NG, Fuller A, Jakubowski A, et al. . Pre- and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis 2005; 7:11–7. [DOI] [PubMed] [Google Scholar]
- 5. Tavadze M, Rybicki L, Mossad S, et al. . Risk factors for vancomycin-resistant enterococcus bacteremia and its influence on survival after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2014; 49:1310–6. [DOI] [PubMed] [Google Scholar]
- 6. Avery R, Kalaycio M, Pohlman B, et al. . Early vancomycin-resistant enterococcus (VRE) bacteremia after allogeneic bone marrow transplantation is associated with a rapidly deteriorating clinical course. Bone Marrow Transplant 2005; 35:497–9. [DOI] [PubMed] [Google Scholar]
- 7. Kamboj M, Chung D, Seo SK, et al. . The changing epidemiology of vancomycin-resistant Enterococcus (VRE) bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Biol Blood Marrow Transplant 2010; 16:1576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vydra J, Shanley RM, George I, et al. . Enterococcal bacteremia is associated with increased risk of mortality in recipients of allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012; 55:764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ubeda C, Taur Y, Jenq RR, et al. . Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 2010; 120:4332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taur Y, Xavier JB, Lipuma L, et al. . Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012; 55:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang Y, Vicente M, Parsad S, et al. . Evaluation of risk factors for vancomycin-resistant Enterococcus bacteremia among previously colonized hematopoietic stem cell transplant patients. Transpl Infect Dis 2013; 15:466–73. [DOI] [PubMed] [Google Scholar]
- 12. Webb BJ, Healy R, Majers J, et al. . Prediction of bloodstream infection due to vancomycin-resistant enterococcus in patients undergoing leukemia induction or hematopoietic stem-cell transplantation. Clin Infect Dis 2017; 64:1753–9. [DOI] [PubMed] [Google Scholar]
- 13. Dubberke ER, Hollands JM, Georgantopoulos P, et al. . Vancomycin-resistant enterococcal bloodstream infections on a hematopoietic stem cell transplant unit: are the sick getting sicker? Bone Marrow Transplant 2006; 38:813–9. [DOI] [PubMed] [Google Scholar]
- 14. Taur Y, Jenq RR, Perales MA, et al. . The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014; 124:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ray AJ, Hoyen CK, Taub TF, Eckstein EC, Donskey CJ. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA 2002; 287:1400–1. [DOI] [PubMed] [Google Scholar]
- 16. Weinstock DM, Conlon M, Iovino C, et al. . Colonization, bloodstream infection, and mortality caused by vancomycin-resistant enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant 2007; 13:615–21. [DOI] [PubMed] [Google Scholar]
- 17. Zirakzadeh A, Gastineau DA, Mandrekar JN, Burke JP, Johnston PB, Patel R. Vancomycin-resistant enterococcal colonization appears associated with increased mortality among allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2008; 41:385–92. [DOI] [PubMed] [Google Scholar]
- 18. Poutsiaka DD, Skiffington S, Miller KB, Hadley S, Snydman DR. Daptomycin in the treatment of vancomycin-resistant Enterococcus faecium bacteremia in neutropenic patients. J Infect 2007; 54:567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bacigalupo A, Ballen K, Rizzo D, et al. . Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15:1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weisdorf D, Spellman S, Haagenson M, et al. . Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant 2008; 14:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Filipovich AH, Weisdorf D, Pavletic S, et al. . National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11:945–56. [DOI] [PubMed] [Google Scholar]
- 22. CIBMTR Forms Instruction Manual. Available at: https://www.cibmtr.org/manuals/fim/1/en/topic/q1-4-recipient-death-data [Google Scholar]
- 23. Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal 1995; 1:145–56; discussion 157–9. [DOI] [PubMed] [Google Scholar]
- 24. Twilla JD, Finch CK, Usery JB, Gelfand MS, Hudson JQ, Broyles JE. Vancomycin-resistant Enterococcus bacteremia: an evaluation of treatment with linezolid or daptomycin. J Hosp Med 2012; 7:243–8. [DOI] [PubMed] [Google Scholar]
- 25. McKinnell JA, Patel M, Shirley RM, Kunz DF, Moser SA, Baddley JW. Observational study of the epidemiology and outcomes of vancomycin-resistant Enterococcus bacteraemia treated with newer antimicrobial agents. Epidemiol Infect 2011; 139:1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cho SY, Lee DG, Choi SM, et al. . Impact of vancomycin resistance on mortality in neutropenic patients with enterococcal bloodstream infection: a retrospective study. BMC Infect Dis 2013; 13:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shono Y, Docampo MD, Peled JU, et al. . Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 2016; 8:339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tomblyn M, Chiller T, Einsele H, et al. ; Center for International Blood and Marrow Research; National Marrow Donor Program; European Blood and Marrow Transplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Disease Canada; Centers for Disease Control and Prevention Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baden LR, Swaminathan S, Angarone M, et al. . Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14:882–913. [DOI] [PubMed] [Google Scholar]
- 30. Edmond MB, Ober JF, Weinbaum DL, et al. . Vancomycin-resistant Enterococcus faecium bacteremia: risk factors for infection. Clin Infect Dis 1995; 20:1126–33. [DOI] [PubMed] [Google Scholar]
- 31. Lucas GM, Lechtzin N, Puryear DW, Yau LL, Flexner CW, Moore RD. Vancomycin-resistant and vancomycin-susceptible enterococcal bacteremia: comparison of clinical features and outcomes. Clin Infect Dis 1998; 26:1127–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.