Abstract
Context
The age of pubertal onset is influenced by many variables in young girls. Previous studies have not examined sex hormones longitudinally around the time of breast development and their relationship to pubertal onset.
Objective
We sought to use an unbiased statistical approach to identify phenotypes of sex hormones in young girls and examine their relationship with pubertal milestones.
Design and Setting
Longitudinal observational study.
Participants and Main Outcome Measures
In 269 girls, serum concentrations of steroid sex hormones [estradiol (E2), estrone, testosterone, and dehydroepiandrosterone sulfate] were measured by HPLC-mass spectrometry at time points before, at, and after thelarche. Girls were classified into four hormone phenotypes using objective principal components and cluster analyses of longitudinal hormone data. The association between the identified phenotypes and age of pubertal milestones was estimated using Cox proportional hazards modeling.
Results
Mean ages at thelarche, pubarche, and menarche were 9.02, 9.85, and 12.30 years, respectively. Girls with low levels of all four hormones, phenotype 3b, were youngest at thelarche (8.67 years); those in phenotype 2, with the highest E2 levels and E2 surge 6 months after thelarche, were youngest at menarche (11.87 years) with shortest pubertal tempo. When controlling for race, maternal age of menarche, caregiver education, and body mass, different phenotypes were associated with the age of pubertal events.
Conclusions
Hormone phenotypic clustering can identify clinically relevant subgroups with differing ages of thelarche, pubarche, and menarche. These findings may enhance the understanding of timing of pubertal milestones and risk of adult disease.
Longitudinal sex hormone phenotypes were identified in young girls using principal component and cluster analysis. The age of pubertal milestones differed by the phenotypes.
Young girls vary greatly in their ages at thelarche, pubarche, and menarche (pubertal milestones). Girls can enter puberty (thelarche defined as breast development) between the ages of 7 and 11 years, and the interval from thelarche to menarche (pubertal tempo) is different for every girl. Many factors, including obesity and race, have been linked to alterations in the ages of attaining pubertal milestones (1–4). More importantly, earlier age of menarche has been identified as a risk factor for breast cancer (5–8) and other adverse health outcomes in later life (9–17). Earlier puberty, therefore, is associated with an increased lifetime burden of adverse health events.
Although female sex hormones change dramatically throughout puberty, previous studies have not been able to assess patterns of changes considering multiple hormones in a longitudinal cohort. Most studies examining hormone values have been cross-sectional in nature, reporting as chronologic age regardless of pubertal status or as relative to having achieved a specific pubertal milestone (e.g., having reached pubarche vs those who have not). We have described hormone levels longitudinally at time periods relative to thelarche (breast maturation), examining each hormone separately (18). Previous studies have not been able to determine if the pattern of pubertal hormone changes within girls is heterogeneous or homogenous.
The limited number of studies examining sex hormones longitudinally, coupled with the wide variability in ages of pubertal milestones among girls, has led to an interest in better understanding longitudinal hormone changes in young girls. The objective of this study was to identify groups of girls with similar patterns of changes in multiple hormones over multiple time periods during puberty. This approach differs from the cross-sectional examination of hormone values at different time points in a longitudinal cohort (18). An agnostic approach was used to examine only hormone values through principal component analysis (PCA) and cluster analysis (CA), without any attempt to associate the hormones with age of pubertal milestones or characterize them further by variables, such as race or body mass. PCA-CA has been a validated statistical approach in identifying subgroups or phenotypes of patients with different health outcomes, including cardiovascular risk (19), chronic obstructive pulmonary disease (20–22), asthma (23–25), and sleep apnea (26, 27). The clinical relevance of these phenotypes was validated using survival analyses to examine if the phenotypes were related to different ages of pubertal milestones. The identification of hormone phenotypes of girls and the quantification of their risk of earlier pubertal timing may also produce a better understanding of girls at risk for adult onset diseases, such as polycystic ovarian syndrome (PCOS) or breast cancer.
Materials and Methods
Study population
All participants were members of the Cincinnati site of the Puberty Cohort Study of Breast Cancer and the Environment Research Program. Study aims have been published elsewhere (28). In brief, this longitudinal prospective cohort was comprised of girls from the Greater Cincinnati area, recruited at age 6 to 7 years. The girls (n = 379) were recruited and seen for clinical study visits every 6 months between 2004 and 2010 and yearly until 2015. Approximately 85% were recruited through schools, with the remaining girls recruited through the Breast Cancer Registry of Greater Cincinnati to enrich the cohort with girls with a family history of breast cancer. The study visit window was ±4 weeks. The Institutional Review Board from Cincinnati Children’s Hospital Medical Center approved this study, and informed consent was given by each participant’s parent or caregiver. Girls were excluded if they presented with an underlying hormone condition or were taking oral contraceptives.
Sex and adrenal hormones
Early-morning serum samples were drawn from each study participant at each study visit and banked in freezers at −80°C. Samples later identified as those obtained at time points around thelarche were sent to the laboratory and analyzed for concentrations of estradiol (E2), estrone (E1), testosterone (T), and dehydroepiandrosterone sulfate (DHEA-S). Missing sex hormones were a result of a participant refusing to have blood drawn or insufficient blood serum for a measurement.
E2, E1, and T were measured by high-performance liquid chromatography with tandem mass spectrometry (HPLC-MS), a method for hormone analysis with greater sensitivity than past methods. An initial batch of DHEA-S was measured by radioimmunoassay for 253 girls, and later, a second batch for 205 girls was measured by HPLC-MS. The correlation between the two approaches (radioimmunoassay and HPLC-MS) for DHEA-S was 0.948. Both batches of DHEA-S assays were used in this analysis.
The laboratory, LabCorp, performing the hormone assay, was certified by the Centers for Disease Control and Prevention for testosterone and estradiol and has an average bias estimation from proficiency accuracy studies <2%. Validations to bioanalytical validation standards quantified hormone assay performance. Low, medium, and high control percent correlation coefficients of variation for interassay precision from the samples are as follows: E2, 4.4%, 3.5%, 3.3%; E1, 4.9%, 4.6%, 4.7%; DHEA-S, 6.5%, 8.4%, 7.3%; T, 9.9%, 7.9%, 5.0%. These percentages are much lower than the standard expectation of interassay precision of <15%, which indicates a low level of dispersion in the measurement of these data. Further details of hormone assay, along with lower limits of quantification, have been previously detailed (18).
Clinical characteristics
Height and weight were obtained at each study visit (28). Race (ethnicity), caregiver’s education, and mother’s age of menarche were obtained from the caregivers at the initial study visit. Family history of breast cancer was obtained from questionnaires or interviews at semiannual study visits.
Maturation staging
The primary endpoints of age at pubertal milestone, measured in months from birth, include thelarche, pubarche, and menarche. Trained and certified female staff assessed pubertal maturation. Both thelarche and pubarche were based on reaching Tanner sexual maturation stage 2 or greater. Staff observed and palpated to determine breast stage. To be assigned to a stage, all criteria had to have been met for that stage; girls with inconsistent breast staging over time were considered at the lower stage until consistently at the higher stage. Pubarche was determined using accessory light sources to assess the presence or absence of terminal hair in the pubic area. Age of menarche was self-reported from the study participants’ and/or mothers’ answers to questions regarding first menstrual cycle, asked each year, beginning when girls were 11 or 12 years old (29). Further details regarding staging have been previously published (28). Tempo was defined as the interval in months between thelarche and menarche. Pubertal pathway described the sequence of initiation of secondary sex characteristics and was categorized as pubarche before thelarche or thelarche before pubarche or synchronous.
Statistical methods
Variable categorization
Caregivers’ education was categorical, namely, a high school degree or less, associate or bachelor’s degree obtained, and more than a bachelor’s degree obtained. Maternal age of menarche was reported as a yearly age and categorized as <12 years old, 12 years or greater but <14 years old, and at least 14 years old or older. Family history of breast cancer was reported as no or yes that either a mother or maternal second-degree blood relative had breast cancer. As a result of small numbers of Asians and Hispanics included in this cohort, race was reported as black vs all other, including non-Hispanic white, Hispanic, and Asian. Body mass index (BMI), derived from the average of two measurements of height and weight, taken at each study visit, was calculated as weight in kilograms divided by the height in meters squared. BMI z-scores (BMIz) were determined using age and sex-specific 2000 Centers for Disease Control and Prevention growth charts.
Values of hormones, less than the limit of quantification (LOQ), were imputed using LOQ/. This imputation is commonly used for estimating analyte concentrations below the LOQ when the data are not highly skewed, and the percent of values less than the LOQ is <30% (30). The proportion of values below the LOQ in this study was <20%.
Phenotype development
With the use of an agnostic approach, PCA was used to reduce the number of objective predictive variables (hormone levels at time periods). CA was used to assign girls to phenotypes based on the logarithmically transformed sex hormones at the time of breast development and 6 months before and 6 months after thelarche. K-Clustering assigned the girls to disjoint clusters, based on Euclidean distances. We used an iterative approach over a range of clusters (two to six) to determine the optimal number of phenotypes.
Descriptive statistics were used to characterize the study cohort as a whole and each of the hormone phenotypes. The Kruskal-Willis and Wilcoxon signed rank tests were used to evaluate the group- and pair-wise differences among the phenotypes. A χ2 test assessed the difference in distribution for categorical variables.
Relationship between hormone phenotypes and pubertal milestones
Kaplan-Meier estimates of cumulative probability were used to examine the unadjusted survival (to a pubertal milestone) by phenotypes. Factors affecting the age at the pubertal events were assessed by multiple variable Cox proportional hazard models. Included in each model, in addition to the phenotype, were the following variables: BMIz at the visit closest to the pubertal event, race, caregiver’s education, and mother’s age of menarche. The results of the final models were expressed as hazard ratios (HRs) with Wald’s 95% CIs. An HR = 1 indicates no relationship between the predictor and the age of reaching the pubertal milestone. An HR < 1 indicates a reduced risk of reaching the pubertal milestone early, in other words, age of reaching the pubertal milestone is late. An HR > 1 indicates an increased risk of reaching the pubertal milestones or in other words, reaching the pubertal milestone at an earlier age than others. Proportional hazard assumption for each variable was tested using the log of each baseline variable. If needed, interactions with time were added to the models for those variables that failed the proportional hazard assumption. Girls who either dropped out of the study before its end or did not reach the pubertal event during the study period were right censored.
All analyses were conducted using SAS 9.2 (SAS Institute Inc., Cary, NC). A P value 0.05 was considered statistically significant for all analyses.
Results
Identification of hormone phenotypes
Girls (269) were selected for these analyses, because they had blood serum samples assayed for sex hormones at least twice across the five time periods related to thelarche. By the end of follow-up, all of these girls reached thelarche, 93.68% of the girls reached pubarche, and 78.44% of the girls reached menarche. The average age of menarche was 12.3 years (Table 1).
Table 1.
Characteristics of Study Participants (n = 269)
| Characteristic | Mean (SD) | (Maximum, Minimum) |
|---|---|---|
| Age of thelarche, y | 9.02 ± 1.10 | (6.08,12.41) |
| Age of pubarche, y | 9.85 ± 1.35 | (6.17,13.17) |
| Age of menarche, y | 12.30 ± 1.15 | (8.00,15.5) |
| Tempo,a y | 3.25 ± 1.09 | (0, 6.17) |
| BMIz | 0.33 ± 1.02 | (−2.1,2.72) |
| BMI percentile | 58.97 ± 29.56 | (1.78,99.67) |
| Mother’s age of menarche, % | ||
| <12 y old | 20.07 | |
| At least 12 y but <14 y old | 59.85 | |
| At least 14 y or older | 20.07 | |
| Race/ethnicity, % | ||
| Black | 31.60 | |
| Hispanic, White, Asian, all other | 68.40 | |
| First- or second-degree maternal family member breast cancer diagnosis, % | ||
| Diagnosis of breast cancer | 12.64 | |
| No diagnosis of breast cancer | 80.30 | |
| Missing | 7.06 | |
| Caregiver’s education, % | ||
| High school degree or less | 29.00 | |
| Associate’s or bachelor’s degree | 45.35 | |
| More than a bachelor’s degree | 25.62 | |
| Pubertal pathway, % | ||
| Thelarche before pubarche | 69.14 | |
| Pubarche before thelarche | 17.10 | |
| Entered at the same time | 7.43 | |
| Missing as a result of censorship | 6.32 | |
| Mean hormone values at 6 mo before (−6), thelarche (0), and 6 mo after thelarche (+6) | ||
| Testosterone −6 | 4.48 ± 2.69 | |
| Testosterone 0 | 4.96 ± 2.76 | |
| Testosterone 6 | 6.16 ± 3.64 | |
| Estrone −6 | 4.00 ± 2.19 | |
| Estrone 0 | 4.47 ± 2.46 | |
| Estrone +6 | 6.09 ± 4.46 | |
| DHEA-S −6 | 28.23 ± 24.99 | |
| DHEA-S 0 | 30.79 ± 26.08 | |
| DHEA-S +6 | 38.32 ± 29.16 | |
| Estradiol −6 | 2.83 ± 3.32 | |
| Estradiol 0 | 3.37 ± 4.22 | |
| Estradiol +6 | 5.38 ± 7.16 | |
Values for continuous variables are presented as means and SD. Values for categorical variables are presented as a percentage. DHEA-S (µg/dL), estrone and estradiol (pg/mL), and testosterone (ng/dL).
Tempo is the time between age of thelarche and age of menarche.
PCA reduced the variables from five time periods surrounding breast development for four hormones to three time periods for the four hormones. As a result of the extent of the missing data at −18 and −12 months, we focused the PCA on the three time periods with the most data (6 months before thelarche, thelarche, and 6 months after thelarche). Nine girls were excluded from the PCA who did not have hormone measurements for any of the three time periods with the most data. For other missing data during these three time periods, 30 datasets were imputed for the missing hormone measurements. After incorporation of the imputed data, we used 30 PCA and then averaged the eigenvalues of the factor loadings. Results from the PCA analyses indicated that the first three components contributed significantly to explaining the relationships among absolute hormones at three time periods (eigenvalues >1) and explained 74% of the shared variance. All four sex hormones at each of the three time periods loaded on the first three components. Therefore, variable reduction was not possible, and it was necessary to include all hormone values at the three time periods in the CA. A sensitivity PCA analysis, consisting of a subset (n = 67) of girls who had no missing data for all four hormone measurements at three time periods (−6, 0, 6), yielded similar results (data not shown). CA then identified 11 girls as outliers who did not fit into any of the phenotypes. Ultimately, 249 girls were included in the four PCA-CA-identified phenotypes.
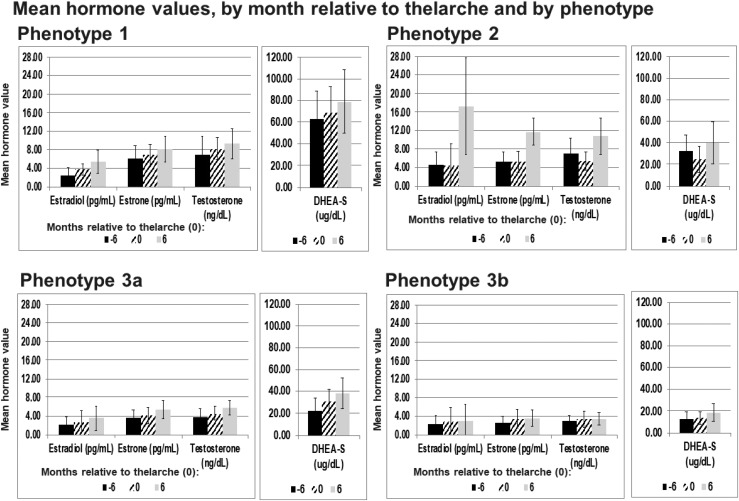
The phenotypes indicated that hormone levels relative to the timing of thelarche are not the same for all girls. Girls were assigned to one of four hormone phenotypes: phenotype 1, characterized by high DHEA-S, E1, and T (n = 42); phenotype 2, characterized by high E2, high E1, and T (n = 37); phenotype 3a, as defined by no high hormone levels (n = 74); and phenotype 3b, characterized as lower hormone levels, except for DHEA-S, than the rest of the cohort (n = 96). Figure 1 details the variables used to define the phenotypes. Girls within each phenotype varied considerably along ages of pubertal milestones, pubertal pathway, and in the hormone levels among the time periods (Table 2). The phenotypes are distinguished by essential features that follow.
Figure 1.
Phenotype 1 had higher hormone values, especially DHEA-S, across the time periods compared with the other phenotypes. Phenotype 2 was distinguished by higher estradiol, estrone, and testosterone at 6 mo after thelarche. Phenotype 3b had very little variation in the hormones across the time periods, whereas although the hormones in phenotype 3a were low, they did increase across the time periods.
Table 2.
Maturation and Clinical Characteristics of Study Participants by Hormone Phenotype
| Phenotype 1 | Phenotype 2 | Phenotype 3a | Phenotype 3b | |||
| Cohort | High DHEA-S, T, and E1 | High E2, T, and E1 | No High Hormones | All Low Hormones | ||
| n = 269 | n = 42 | n = 37 | n = 74 | n = 96 | ||
| Means ± SD | Means ± SD | Means ± SD | Means ± SD | Mean ± SD | P Value | |
| Age of thelarche, y | 9.02 ± 1.10 | 9.44 ± 0.94 | 9.37 ± 1.10 | 8.86 ± 1.08 | 8.67 ± 1.03 | <0.0001 |
| Age of pubarche, y | 9.85 ± 1.35 | 9.74 ± 1.60 | 9.89 ± 0.95 | 9.49 ± 1.43 | 10.13 ± 1.25 | 0.0345 |
| Age of menarche, y | 12.3 ± 1.15 | 12.56 ± 1.28 | 11.87 ± 0.99 | 12.3 ± 1.19 | 12.39 ± 1.02 | 0.0606 |
| Tempo,a y | 3.25 ± 1.09 | 3.03 ± 0.96 | 2.55 ± 0.86 | 3.38 ± 0.91 | 3.76 ± 1.05 | <0.0001 |
| BMIz | 0.33 ± 1.02 | 0.29 ± 0.96 | 0.14 ± 0.97 | 0.42 ± 1.10 | 0.4 ± 1.03 | 0.5245 |
| BMI percentile | 58.97 ± 29.56 | 57.67 ± 27.62 | 52.47 ± 28.56 | 61.46 ± 30.70 | 61.6 ± 30.29 | 0.3667 |
| Mother’s age of menarche, % | 0.4507 | |||||
| <12 y old | 20.07 | 14.29 | 27.03 | 25.68 | 14.58 | |
| At least 12 y but <14 y old | 59.85 | 66.67 | 51.35 | 55.41 | 64.58 | |
| At least 14 y or older | 20.07 | 19.05 | 21.62 | 18.92 | 20.83 | |
| Ethnicity, % | 0.7755 | |||||
| Black | 31.60 | 35.71 | 27.03 | 29.73 | 34.38 | |
| Hispanic, White, Asian, all other | 68.40 | 64.29 | 72.97 | 70.27 | 65.63 | |
| First- or second-degree maternal family member breast cancer diagnosis, % | 0.8573 | |||||
| Diagnosis of breast cancer | 12.64 | 11.90 | 13.51 | 16.21 | 11.46 | |
| No diagnosis of breast cancer | 80.30 | 83.33 | 78.84 | 78.38 | 79.70 | |
| Missing | 7.06 | 4.76 | 8.10 | 5.40 | 9.37 | |
| Caregiver’s education, % | 0.4303 | |||||
| High school degree or less | 29.00 | 40.48 | 16.22 | 27.03 | 29.17 | |
| Associate’s or bachelor’s degree | 45.35 | 38.10 | 54.05 | 44.59 | 43.75 | |
| More than a bachelor’s degree | 25.62 | 21.43 | 29.73 | 28.38 | 27.08 | |
| Pubertal pathway, % | 0.0017 | |||||
| Thelarche before pubarche | 69.14 | 66.67 | 64.86 | 64.86 | 79.17 | |
| Pubarche before thelarche | 17.10 | 26.17 | 16.22 | 28.38 | 5.21 | |
| Entered at the same time | 7.43 | 7.14 | 13.51 | 4.05 | 4.17 | |
| Missing as a result of censorship | 6.32 | 0.00 | 5.41 | 2.70 | 11.46 | |
Values for continuous variables are presented as means and SD. Values for categorical variables are presented as a percentage. P values represent tests for group-wise differences among the phenotypes using Kruskal-Wallis to test the difference between continuous variables and χ2 for categorical variables.
Tempo is the time between age of thelarche and age of menarche.
Phenotype 1 (high DHEA-S, T, and E1)
DHEA-S serum concentrations in this group were at almost twice the cohort mean, and T and E1 values were 50% higher than the cohort mean values. The average age of thelarche in this phenotype (mean = 9.44 years) was later than in the other phenotypes. There were significant increases (∼40%) in DHEA-S, E1, and T from thelarche to +6 months after thelarche when compared with the other phenotypes (Fig. 1).
Phenotype 2 (high E2, T, and E1)
These girls represent the smallest phenotype profile group and are distinguished by their high E2 values compared with the other phenotypes, especially at 6 months after thelarche. They also are distinguished by high T and E1 at 6 months after thelarche (Fig. 1). They were younger at menarche (mean = 11.87) than girls in phenotypes 1, 3a, and 3b (means = 12.64, 12.30, 12.39 years) and consequently, had the shortest tempo (means = 2.54 years). The girls in this phenotype had substantially lower BMIz than girls in the other three phenotypes but not statistically lower (Table 2). These girls also experienced a significant decrease in DHEA-S, E1, and T levels between the time periods, 6 months before thelarche, and thelarche but then, an increase in those hormones from thelarche to 6 months after breast development, a pattern not seen in the other phenotypes. They experienced a substantial increase (over 250%) in E2 from thelarche to 6 months after breast development (Fig. 1).
Phenotype 3a (hormone levels lower than the cohort mean except for DHEA-S)
The girls in this phenotype entered thelarche at a significantly earlier age (means = 8.86 years) than phenotypes 1 and 2 (means = 9.44 and 9.37 years) and also entered pubarche (means = 9.49 years) earlier than any of the other phenotypes (Table 2). The hormone levels on average were 15% lower than the cohort as a whole. DHEA-S levels were the same as the cohort mean at thelarche and 6 months after thelarche (Fig. 1).
Phenotype 3b (all low hormone values)
This is the largest cluster, representing 36.9% of the girls. These girls were significantly the earliest to enter thelarche (means = 8.67 years) but latest to enter pubarche (means = 10.13 years). They also experienced a significantly longer tempo (means = 3.76 years) than the other phenotypes, over 1 year longer than the girls in phenotype 2 (means = 2.55 years; Table 2). The hormone values of these girls were at least 30% lower than those of the cohort as a whole and changed minimally between the time periods when compared with hormone value changes seen in the other phenotypes across time (Fig. 1).
Phenotypes and age of pubertal events
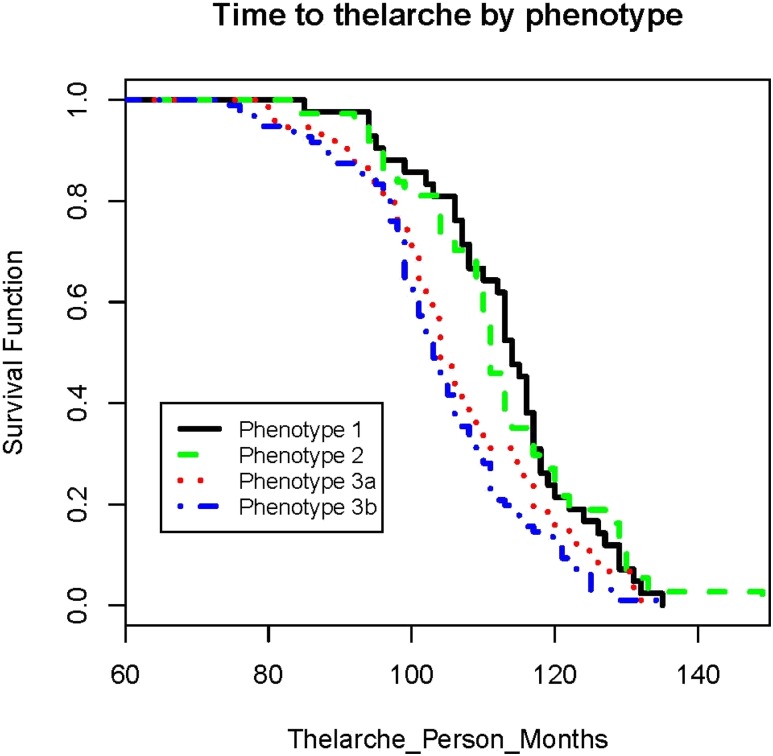
The results of the Kaplan-Meier analyses indicated phenotypes differ in probability of having attained thelarche (Fig. 2) and pubarche (thelarche log-rank P value = 0.0008; pubarche log-rank P value = 0.0333), with phenotypes 3a and 3b reaching thelarche earlier than the others. Although the difference in the age of menarche by phenotype was not significant (menarche log-rank P value = 0.1975), phenotype 2 girls appeared to reach menarche earlier than those of the other phenotypes, which led us to pursue further Cox regression analysis for this pubertal outcome.
Figure 2.
The time to thelarche was longest for the girls in phenotypes 1 and 2, whereas it was the shortest for girls in phenotypes 3a and 3b. The time under observation began at birth for all girls. We have changed the x-axis of the figure to begin at 60 mo so that the individual curves are more visible.
In Cox regression analyses of all girls, with phenotype as a factor in the model, a caregiver’s education had no significant effect on pubertal timing. Race, BMIz, and mother’s age of menarche had significant effects on the ages of thelarche, pubarche, and menarche. A higher BMIz, closest to the pubertal events, was associated with a highly significant earlier age of thelarche (HR = 7.18, 95% CI 2.65 to 19.51), age of pubarche (HR = 1.16, 95% CI 1.05 to 1.30), and age of menarche (HR = 1.57, 95% CI 1.36 to 1.81). Those belonging to the nonblack group (non-Hispanic white, Hispanic, and Asian) were more likely to enter a pubertal event later than black girls for all three pubertal milestones (thelarche HR = 0.67, 95% CI 0.51 to 0.88; pubarche HR = 0.01, 95% CI 0.00 to 0.06; menarche HR = 0.01, 95% CI 0.00 to 0.30). Those who had a mother who reached menarche before the age of 12 years were more likely of an earlier thelarche, pubarche, and menarche over girls whose mothers’ menarche occurred after the age of 14 years (thelarche HR = 1.68, 95% CI 1.10 to 2.57; pubarche HR = 1.58, 95% CI 1.04 to 2.42; menarche HR = 1.61, 95% CI 1.01 to 2.59).
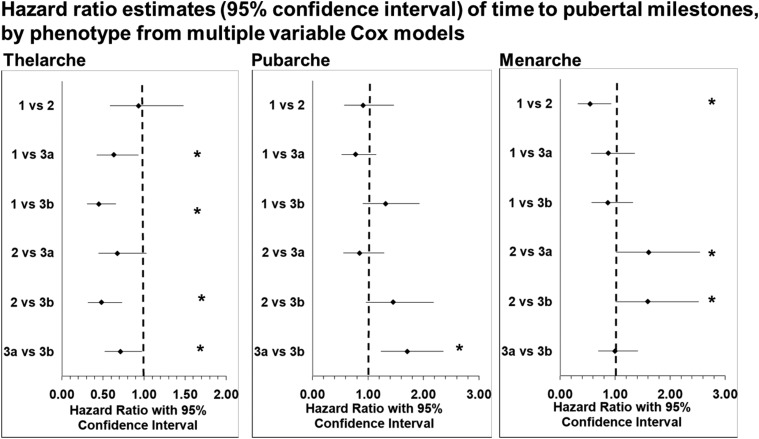
Phenotype 1 had less likelihood of earlier thelarche than the phenotype 3a (HR = 0.63, 95% CI 0.43 to 0.93). Phenotypes 1, 2, and 3a were less likely to have earlier ages of thelarche than phenotype 3b (HR = 0.45, 95% CI 0.31 to 0.66; HR = 0.48, 95% CI 0.32 to 0.73; HR = 0.71, 95% CI 0.52 to 0.97; Fig. 3, left).
Figure 3.
Risk of earlier thelarche was less for phenotypes 1 and 2. Time to pubarche, only differs between phenotypes 3a and 3b, with 3a more likely to enter pubarche earlier than 3b. Phenotype 2 was associated with earlier menarche than any of the other phenotypes. *Significance. Figures created using Clark O, Djulbegovic B. Forest plots in Excel software (data sheet), 2001.
Phenotype 3a was significantly associated a higher likelihood of earlier age of pubarche compared with phenotype 3b (HR = 1.71, 95% CI 1.24 to 2.35). No other phenotypes were associated with the likelihood of earlier pubarche when referenced to other phenotypes (Fig. 3, middle).
Cox proportional hazard, adjusted for other risk factors, did show that girls in phenotype 2 were significantly more likely to have an earlier age of menarche than girls in the other three phenotypes. Membership to phenotype 1 almost halved the likelihood of entering menarche earlier than phenotype 2 (HR = 0.55, 95% CI 0.32 to 0.93). Girls in phenotype 2 were >50% more likely to enter menarche earlier than those in phenotype 3a (HR = 1.61, 95% CI 1.02 to 2.54) and those in phenotype 3b (HR = 1.59, 95% CI 1.01 to 2.52). There was no significant differences in the timing of menarche among the other phenotypes (Fig. 3, right).
Discussion
We applied an objective statistical approach to define sex hormone phenotypes and associate them with the age of pubertal milestones in a longitudinal cohort of young girls. Four clinically relevant heterogeneous hormone phenotypes were identified. Girls across phenotypes varied considerably along measures of hormones, differences in hormone values between time periods, and age of pubertal milestones, as well as other demographic variables.
These findings indicate substantial heterogeneity exists in hormones within young girls around the time of breast development. They also underscore the need to understand better why hormone profiles vary among girls during puberty.
This application of PCA-CA defines hormone phenotypes and follows with the application of survival analysis to determine ages of pubertal milestones by phenotype. Several other studies have used this PCA-CA method to identify successfully relevant phenotypes in patient subgroups, such as chronic obstructive pulmonary disease, asthma, and sleep apnea. In each study, different sets of disease symptoms presented in each of the phenotypes, thus supporting the heterogeneity within a specific disease, much as the different hormones presented differently in our pubertal phenotypes. Others studies have further applied these phenotype analyses, using group membership, to predict age of mortality or risk of developing a comorbidity or hospitalization (31–37). This longitudinal cohort provided a unique opportunity to define phenotypes based on hormones from multiple time points related to age of thelarche, as well as to follow the girls throughout achievement of pubertal events and associate the phenotypes with risk of earlier age of pubertal milestones.
We report substantial variability of hormonal fluctuations among individuals in the course of “normal” puberty. Apart from the analysis that within the spectrum of normal, there are distinct phenotypes of pubertal hormones, this observation allows the opportunity to assess whether these hormonal patterns are harbingers of future disease. These phenotypes are unlikely directly causative of adult-onset diseases, although they may serve as biomarkers of exposure or potential risk.
The concept for the origins of adult disease in early life is well accepted, and a variety of disease states are thought to be related to fetal, newborn, and childhood factors. Several adult disorders are considered to be related to earlier hormonal factors. In women, these include endometrial cancer (with higher lifelong estrogen exposure) (38–40), breast cancer (with higher lifelong estrogen exposure) (41, 42), PCOS (fetal and childhood “hyperandrogenia phenotype”) (43), and optimal bone health (with greater bioavailable estrogen) (44).
Girls in phenotypes 3a and 3b are noted to have greater BMI than the cohort as a whole, but the difference was not substantial, and they have the earliest age of thelarche in the cohort. These two phenotypes have statistically longer pubertal tempos than phenotypes 1 and 2 (mean tempo 3.38 and 3.76 years, contrasted to 3.03 and 2.54 years, respectively; Table 2). This suggests that breast budding may have been driven by local production of estrogens through aromatization of androgens, rather than reactivation of the hypothalamic-pituitary-ovarian axis, presumably with later central activation. Lower circulating estradiol levels in both phenotypes 3a and 3b are also consistent with a lack of hypothalamic-pituitary-ovarian axis activation and therefore, a lack of robust ovarian hormonal production.
The phenotype 1 profile, with high DHEA-S and testosterone levels, as well as a notable rise in DHEA-S at thelarche, may be associated with greater risk of PCOS. This group was noted with latest age of menarche. In addition, higher testosterone and rising levels of DHEA-S may also indicate a future risk of PCOS (45).
Phenotype 2 includes girls with high estradiol levels at all time periods, a large estradiol peak post-thelarche, and significantly earlier menarche than the other phenotypes. This suggests a group with greater lifelong estrogen exposure, lower risk of osteoporosis, and potentially greater risk of breast cancer.
Ultimately, the understanding of varying normal hormonal patterns during puberty may help guide identification of adult disorders and potential of earlier interventions. Each phenotype may serve as a marker to identify pubertal anomalies or populations at risk for adult health issues, such as PCOS, breast cancer, and bone health. In addition, the understanding of these innate hormonal fluctuations may inform a more physiologic approach to hormonal replacement therapy in girls with ovarian insufficiency.
The strengths of this study include the objective statistical approach, the longitudinal ability to assess hormones based on timing around thelarche rather than chronologic age, and the use of HPLC-MS to evaluate hormones that are typically too low to measure in young girls with earlier hormone analytic methods. There are several potential limitations. First, this cohort was from only the greater Cincinnati area and therefore, not nationally representative, but it was racially and socioeconomically diverse. Additionally, the BMI% of the cohort is similar to National Health and Nutrition Examination Survey data for this age group (46). Second, breast tissue can often be confused with fat tissue in prepubertal girls, making some experts question the validity of physical examination to assess breast maturation. The examiners were trained, and agreement on breast maturation was 87%, with the master trainer in a subset of blind assessments (28). Furthermore, as previously documented, in a subset of this cohort, E2 levels differed between girls with high vs low BMI%. Girls with a BMI% higher than the median had significantly lower E2 levels at thelarche than girls with a BMI% lower than the median. This finding necessitated investigation into the validity of the breast maturation staging, as it would imply girls with the higher BMI% but lower E2 might have had breast maturation confused with fat tissue. However, upon further investigation, girls with lower BMI% had pubertal growth spurts and height velocities similar to those with a high BMI% (18), supporting the accuracy of the breast maturation staging. Third, the accuracy of the recall is often a limitation in studies. However, accuracy of the recall of age of menarche is typically quite good among girls, as it is an important event in their lives. In a longitudinal Swedish cohort, 63% of the girls were able to recall the age of menarche within ±3 months after 4 years from the start of menarche (47). Another study found 66.1% of girls were able to recall their age of menarche accurately after a mean time of 323 days had passed (48). Recall is less valid as time passes between menarche and recall. In this study, we asked about age of menarche starting at age 11. We have a reported age of menarche for >78% of the girls; the remainder had not achieved menarche by the end of follow-up. Of the girls who had achieved menarche, menarche occurred before age 11 for 12.86% of the girls. Only 1.9% of the girls had their first menses before age 10, making their recall longer than 1 year. Phenotype 3b had the fewest girls attaining menarche (71.99%).
In conclusion, with the use of PCA-CA on longitudinal sex hormones, values related to the time of thelarche identified four distinct heterogeneous phenotypes. Girls within each phenotype varied along hormone values at the time points, changes in the hormones between time points, age of pubertal milestones, and other demographic characteristics. Distinct differences in the ages of achievement of pubertal milestones were seen across the phenotypes. These findings highlight the need to understand better the impact of these hormone phenotypes on adult-related morbidity.
Acknowledgments
Financial Support: This work was funded by the National Institute of Environmental Health Sciences [Grants U01ES012770 and U01ES019453 (to F.M.B.); U01ES019457, U01ES026119, U0ES029133, and P30ES006096 (to S.M.P.); and T32-ES10957 (to C.M.G.)], National Institutes of Health [Grant T32-GM063483 (to C.M.G.)], and National Center for Advancing Translational Sciences [Grant CSTA-UL1RR026314 (to C.M.G.)]. The funding sources had no input into the study design, collection, analysis, and interpretation of the data; writing of the report; and decision to submit the report for publication.
Additional Information
Disclosure Summary: C.S.F., I.G.-L., C.X., C.M.G., F.M.B., and S.M.P. have nothing to disclose. D.W.C. is an employee of LabCorp and a small stockholder of LabCorp.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Glossary
Abbreviations:
- BMI
body mass index
- BMIz
body mass index z-score
- CA
cluster analysis
- DHEA-S
dehydroepiandrosterone sulfate
- E1
estrone
- E2
estradiol
- HR
hazard ratio
- HPLC-MS
high-performance liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- PCA
principal component analysis
- PCOS
polycystic ovarian syndrome
- T
testosterone
References and Notes
- 1. McDowell MA, Brody DJ, Hughes JP. Has age at menarche changed? Results from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. J Adolesc Health. 2007;40(3):227–231. [DOI] [PubMed] [Google Scholar]
- 2. Cabrera SM, Bright GM, Frane JW, Blethen SL, Lee PA. Age of thelarche and menarche in contemporary US females: a cross-sectional analysis. J Pediatr Endocrinol Metab. 2014;27(1-2):47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biro FM, Greenspan LC, Galvez MP, Pinney SM, Teitelbaum S, Windham GC, Deardorff J, Herrick RL, Succop PA, Hiatt RA, Kushi LH, Wolff MS. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132(6):1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson SE, Dallal GE, Must A. Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics. 2003;111(4):844–850. [DOI] [PubMed] [Google Scholar]
- 5. Bodicoat DH, Schoemaker MJ, Jones ME, McFadden E, Griffin J, Ashworth A, Swerdlow AJ. Timing of pubertal stages and breast cancer risk: the Breakthrough Generations Study. Breast Cancer Res. 2014;16(1):R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clavel-Chapelon F; E3N-EPIC Group. Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer. 2002;86(5):723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garland M, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Spiegelman D, Speizer F, Willett WC. Menstrual cycle characteristics and history of ovulatory infertility in relation to breast cancer risk in a large cohort of US women. Am J Epidemiol. 1998;147(7):636–643. [DOI] [PubMed] [Google Scholar]
- 8. Rockhill B, Moorman PG, Newman B. Age at menarche, time to regular cycling, and breast cancer (North Carolina, United States). Cancer Causes Control. 1998;9(4):447–453. [DOI] [PubMed] [Google Scholar]
- 9. Conley CS, Rudolph KD. The emerging sex difference in adolescent depression: interacting contributions of puberty and peer stress. Dev Psychopathol. 2009;21(2):593–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stice E, Presnell K, Bearman SK. Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Dev Psychol. 2001;37(5):608–619. [DOI] [PubMed] [Google Scholar]
- 11. Rudolph KD, Troop-Gordon W, Lambert SF, Natsuaki MN. Long-term consequences of pubertal timing for youth depression: identifying personal and contextual pathways of risk. Dev Psychopathol. 2014;26(4pt2)1423–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Copeland W, Shanahan L, Miller S, Costello EJ, Angold A, Maughan B. Outcomes of early pubertal timing in young women: a prospective population-based study. Am J Psychiatry. 2010;167(10):1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mendle J, Turkheimer E, Emery RE. Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Dev Rev. 2007;27(2):151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deardorff J, Gonzales NA, Christopher FS, Roosa MW, Millsap RE. Early puberty and adolescent pregnancy: the influence of alcohol use. Pediatrics. 2005;116(6):1451–1456. [DOI] [PubMed] [Google Scholar]
- 15. Downing J, Bellis MA. Early pubertal onset and its relationship with sexual risk taking, substance use and anti-social behaviour: a preliminary cross-sectional study. BMC Public Health. 2009;9(1):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baumrind D. The influence of parenting style on adolescent competence and substance use. J Early Adolesc. 1991;11(1):56–95. [Google Scholar]
- 17. Prentice P, Viner RM. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes. 2013;37(8):1036–1043. [DOI] [PubMed] [Google Scholar]
- 18. Biro FM, Pinney SM, Huang B, Baker ER, Walt Chandler D, Dorn LD. Hormone changes in peripubertal girls. J Clin Endocrinol Metab. 2014;99(10):3829–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goodman E, Dolan LM, Morrison JA, Daniels SR. Factor analysis of clustered cardiovascular risks in adolescence: obesity is the predominant correlate of risk among youth. Circulation. 2005;111(15):1970–1977. [DOI] [PubMed] [Google Scholar]
- 20. Burgel PR, Paillasseur JL, Caillaud D, Tillie-Leblond I, Chanez P, Escamilla R, Court-Fortune I, Perez T, Carré P, Roche N; Initiatives BPCO Scientific Committee. Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. Eur Respir J. 2010;36(3):531–539. [DOI] [PubMed] [Google Scholar]
- 21. Newandee DA, Reisman SS, Bartels AN, De Meersman RE COPD severity classification using principal component and cluster analysis on HRV parameters. In: Proceedings of the IEEE 29th Annual Northeast Bioengineering Conference; 22 March 2003; Newark, NJ. 134–135. [Google Scholar]
- 22. Cho MH, Washko GR, Hoffmann TJ, Criner GJ, Hoffman EA, Martinez FJ, Laird N, Reilly JJ, Silverman EK. Cluster analysis in severe emphysema subjects using phenotype and genotype data: an exploratory investigation. Respir Res. 2010;11(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Just J, Gouvis-Echraghi R, Rouve S, Wanin S, Moreau D, Annesi-Maesano I. Two novel, severe asthma phenotypes identified during childhood using a clustering approach. Eur Respir J. 2012;40(1):55–60. [DOI] [PubMed] [Google Scholar]
- 25. Kurukulaaratchy RJ, Zhang H, Raza A, Patil V, Karmaus W, Ewart S, Arshad SH. The diversity of young adult wheeze: a cluster analysis in a longitudinal birth cohort. Clin Exp Allergy. 2014;44(5):724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ye L, Pien GW, Ratcliffe SJ, Björnsdottir E, Arnardottir ES, Pack AI, Benediktsdottir B, Gislason T. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014;44(6):1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vavougios GD, George D G, Pastaka C, Zarogiannis SG, Gourgoulianis KI. Phenotypes of comorbidity in OSAS patients: combining categorical principal component analysis with cluster analysis. J Sleep Res. 2016;25(1):31–38. [DOI] [PubMed] [Google Scholar]
- 28. Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, Teitelbaum S, Windham GC, Kushi LH, Wolff MS. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. 2010;126(3):e583–e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Biro FM, Pajak A, Wolff MS, Pinney SM, Windham GC, Galvez MP, Greenspan LC, Kushi LH, Teitelbaum SL. Age of menarche in a longitudinal US cohort. J Pediatr Adolesc Gynecol. 2018;31(4):339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- 31. Ahmad T, Pencina MJ, Schulte PJ, O’Brien E, Whellan DJ, Piña IL, Kitzman DW, Lee KL, O’Connor CM, Felker GM. Clinical implications of chronic heart failure phenotypes defined by cluster analysis. J Am Coll Cardiol. 2014;64(17):1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bäckström D, Granåsen G, Domellöf ME, Linder J, Jakobson Mo S, Riklund K, Zetterberg H, Blennow K, Forsgren L. Early predictors of mortality in Parkinsonism and Parkinson disease: a population-based study. Neurology. 2018;91(22):e2045–e2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burgel PR, Paillasseur JL, Peene B, Dusser D, Roche N, Coolen J, Troosters T, Decramer M, Janssens W. Two distinct chronic obstructive pulmonary disease (COPD) phenotypes are associated with high risk of mortality. PLoS One. 2012;7(12):e51048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Howrylak JA, Fuhlbrigge AL, Strunk RC, Zeiger RS, Weiss ST, Raby BA; Childhood Asthma Management Program Research Group. Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications. J Allergy Clin Immunol. 2014;133(5):1289–1300.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang R-C, Mori TA, Burrows S, Le Ha C, Oddy WH, Herbison C, Hands BH, Beilin LJ. Sex dimorphism in the relation between early adiposity and cardiometabolic risk in adolescents. J Clin Endocrinol Metab. 2012;97(6):E1014–E1022. [DOI] [PubMed] [Google Scholar]
- 36. Kendzerska T, Gershon AS, Atzema C, Dorian P, Mangat I, Hawker G, Leung RS. Sleep apnea increases the risk of new hospitalized atrial fibrillation: a historical cohort study. Chest. 2018;154(6):1330–1339. [DOI] [PubMed] [Google Scholar]
- 37. Kim NH, Seo JA, Cho H, Seo JH, Yu JH, Yoo HJ, Kim SG, Choi KM, Baik SH, Choi DS, Shin C, Cho NH. Risk of the development of diabetes and cardiovascular disease in metabolically healthy obese People: The Korean Genome and Epidemiology Study. Medicine (Baltimore). 2016;95(15):e3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Henderson BE, Ross RK, Pike MC, Casagrande JT. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982;42(8):3232–3239. [PubMed] [Google Scholar]
- 39. Brinton LA, Berman ML, Mortel R, Twiggs LB, Barrett RJ, Wilbanks GD, Lannom L, Hoover RN. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstet Gynecol. 1992;167(5):1317–1325. [DOI] [PubMed] [Google Scholar]
- 40. England PC, Skinner LG, Cottrell KM, Sellwood RA. Serum oestradiol-17 beta in women with benign and malignant breast disease. Br J Cancer. 1974;30(6):571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Key TJ, Appleby PN, Reeves GK, Travis RC, Brinton LA, Helzlsouer KJ, Dorgan JF, Gapstur SM, Gaudet MM, Kaaks R, Riboli E, Rinaldi S, Manjer J, Hallmans G, Giles GG, Le Marchand L, Kolonel LN, Henderson BE, Tworoger SS, Hankinson SE, Zeleniuch-Jacquotte A, Koenig K, Krogh V, Sieri S, Muti P, Ziegler RG, Schairer C, Fuhrman BJ, Barrett-Connor E, Laughlin GA, Grant EJ, Cologne J, Ohishi W, Hida A, Cauley JA, Fourkala EO, Menon U, Rohan TE, Strickler HD, Gunter MJ; Endogenous Hormones and Breast Cancer Collaborative Group. Steroid hormone measurements from different types of assays in relation to body mass index and breast cancer risk in postmenopausal women: reanlaysis of eighteen prospective cohorts. Steroids. 2015;99(Pt A):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Endogenous Hormones and Breast Cancer Collaborative Group; Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton LA, Dorgan JF, Dossus L, Dowsett M, Eliassen AH, Fortner RT, Hankinson SE, Helzlsouer KJ, Hoff man-Bolton J, Comstock GW, Kaaks R, Kahle LL, Muti P, Overvad K, Peeters PH, Riboli E, Rinaldi S, Rollison DE, Stanczyk FZ, Trichopoulos D, Tworoger SS, Vineis P. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanlaysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14(10):1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Legro RS, Driscoll D, Strauss JF III, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA. 1998;95(25):14956–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khosla S, Melton LJ III, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83(7):2266–2274. [DOI] [PubMed] [Google Scholar]
- 45. Torchen LC, Legro RS, Dunaif A. Distinctive reproductive phenotypes in perpubertal girls at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2019:104(8):3355–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299(20):2401–2405. [DOI] [PubMed] [Google Scholar]
- 47. Bergsten-Brucefors A. A note on the accuracy of recalled age at menarche. Ann Hum Biol. 1976;3(1):71–73. [DOI] [PubMed] [Google Scholar]
- 48. Koo MM, Rohan TE. Accuracy of short-term recall of age at menarche. Ann Hum Biol. 1997;24(1):61–64. [DOI] [PubMed] [Google Scholar]