Abstract
During pregnancy, the supply of thyroid hormone (TH) to the fetus is critically important for fetal growth, neural development, metabolism, and maintenance of pregnancy. Additionally, in cases where maternal and placental TH regulation is significantly altered, there is an increased risk of several adverse pregnancy outcomes. It is unclear what may be disrupting placental TH regulation; however, studies suggest that environmental contaminants, such as polybrominated diphenyl ethers (PBDEs), could be playing a role. In this study, Wistar rats were gestationally exposed to a mixture of PBDEs for 10 days. THs and PBDEs were quantified in paired maternal serum, dissected placenta, and fetuses, and mRNA expression of transporters in the placenta was assessed. Significantly higher concentrations of PBDEs were observed in the fetal portion of the placenta compared with the maternal side, suggesting that PBDEs are actively transported across the interface. PBDEs were also quantified in 10 recently collected human maternal and fetal placental tissues; trends paralleled observations in the rat model. We also observed an effect of PBDEs on T3 levels in dam serum, as well as suggestive changes in the T3 levels of the placenta and fetus that varied by fetal sex. mRNA expression in the placenta also significantly varied by fetal sex and dose. These observations suggest the placenta is a significant modifier of fetal exposures, and that PBDEs are impacting TH regulation in a sex-specific manner during this critical window of development.
The placenta is an ephemeral organ composed of both maternally and fetally derived tissue that facilitates the exchange of nutrients, gases, hormones, and waste between the mother and developing fetus. In order for these molecules to reach the fetus, they must cross the syncytiotrophoblast, a semipermeable membrane that separates maternal from fetal blood and expresses numerous uptake and efflux transporters. Additionally, it protects the fetus from pathogens, xenobiotics, and excess hormones. However, numerous studies have detected polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls, and DDT in cord serum, suggesting that this barrier is permeable to xenobiotics (1–7). It is also widely accepted that the placenta is a sink for persistent organic pollutants (POPs), with concentrations in the placenta sometimes exceeding maternal serum concentrations (1–3, 7–9). It is unclear how these compounds are transported from maternal to fetal circulation; however, it is generally assumed that passive transport is the predominant mechanism (10–13). A limitation of each of these studies is that POP accumulation was measured in full-thickness placenta tissues rather than specific tissue layers; therefore, it is unclear whether these contaminants are homogeneously distributed within the placenta. By understanding the disposition of these contaminants in specific tissues, we can better understand the toxicokinetics of POPs in the fetal placental unit and their potential effects.
PBDEs have been shown to cause thyroid hormone (TH) dysfunction through various mechanisms, such as displacement of T4 from serum transporters, disruption of the hypothalamic–pituitary–thyroid axis, inhibition of deiodinases, and binding to hepatic influx transporter OATP1A4, an organic anion-transporting protein (OATP) that is responsible for clearing T4 metabolites from serum (14). When circulating levels of TH in maternal serum are disrupted, there is an increased risk for adverse pregnancy outcomes, such as intrauterine growth restriction (IUGR) (15–17). Kilby et al. (15) observed reduced fetal serum levels of free T3 and T4 in fetuses affected by IUGR compared with normal pregnancies without any differences in TSH levels. They also measured increased expression of TRα and TRβ in placentas from IUGR pregnancies compared with normal pregnancies. Loubière et al. (16) observed increased expression of placental TH transporter MCT8 expression in IUGR pregnancies compared with normal pregnancies. Chan et al. (17) also measured an upregulation of MCT8 in IUGR placenta compared with normal pregnancy, which they hypothesized was a compensatory mechanism to increase T3 uptake. Although these studies have investigated compensatory mechanisms for low T3 and T4, it was not clear what was initially driving the low TH levels. One hypothesis could be that environmental contaminants, such as PBDEs, are responsible for the TH disruption observed during pregnancy. To our knowledge, only two other studies have measured THs in placental tissue and assessed associations with PBDEs (18, 19). Previously, we first reported on the concentrations of THs in 102 human placental tissues and found that several of the brominated flame retardants were significantly higher in placentas associated with male infants, compared with placentas associated with female infants. We also observed an inverse relationship between placental PBDEs and T3 among males but a positive relationship between PBDEs and T3 among females. Furthermore, deiodinase type III activity was significantly higher in placentas associated with male infants relative to females, and TH sulfotransferase activity was significantly higher in females relative to males, however the mechanisms responsible for these differences are unclear (18).
These observations raise several questions about the impacts of PBDEs on placental thyroid function as well as fetal sex differences. To date, no study has measured PBDEs and THs in paired maternal serum, placental tissue, and fetal tissue. To investigate this knowledge gap mechanistically, we conducted an experiment using the Wistar rat as a model because (i) the development of the thyroid gland occurs in the same phases and order as humans, (ii) timing of fetal reliance on maternal supply of THs is similar to that of humans, (iii) pregnancy alters maternal thyroid status similarly in both rats and humans, (iv) Wistar rats have the same TH synthesis pathways and enzymes, and (v) they express the same TH transporters in the placenta (20–23). Furthermore, Wistar rats are an advantageous model because of their short gestational period, and they have a hemochorial placental barrier allowing the fetal and maternal placental tissues to be easily isolated. Therefore, the goal of this study was to evaluate (i) tissue-specific differences in PBDE accumulation in the placenta, (ii) sex-specific differences in PBDE accumulation, (iii) sex and tissue-specific changes in TH transporter gene expression, and (iv) effects of PBDE exposure on TH levels in maternal serum, placental tissues, and the fetus. Based on our observations in the rodent study, and for further confirmation, we also collected and analyzed 10 anonymous human placentas to determine whether the PBDE partitioning between maternal and fetal layers of the placenta was similar in both humans and rats.
Methods
Animal husbandry
Animal care, maintenance, and experimental protocols met the standards of the Animal Welfare Act and the US Department of Health and Human Services Guide for the Care and use of Laboratory Animals and were approved by the North Carolina State University Institutional Animal Care and Use Committee. A supervising veterinarian approved and monitored all procedures throughout the duration of the project.
Adult Wistar rats (n = 20 females and 10 males) were paired and monitored for the presence of a sperm plug for 48 hours. Dams were paired with males in late diestrus (as determined by vaginal cytology) to ensure that they reached the proestrus/estrus (peak sexual receptivity) stage while with a male. The males were then removed and the dams housed individually. All paired females except one were successfully impregnated. Pups were generated via in-house breeding and dosed from gestational day (GD) 6 to 15 (gestational age estimated from when dams were sperm plug positive). This exposure window was selected because it is similar to our previous research on other flame retardants, and because, at GD 15, the fetus and placenta are roughly equivalent in weight, with robust architecture on both the fetal and maternal sides (24, 25). Evaluation of the fetuses at necropsy revealed that the distribution of gestational ages ranged from GD 12 to 15. Pregnant Wistar rats were orally dosed with either a mixture of PBDEs or a vehicle control for 10 days during gestation (n = 10 dams per exposure). The mixture of PBDEs included BDE-28, -47, -99, -100, -153, and -209 dissolved in ethanol with 10% pure corn oil ranging in concentration from 5.3 to 30.4 μg/kg/d, resulting in a total dose of 105.3 μg/kg/d (26). The actual doses used in the animal study are higher than those to which humans are typically exposed; however, the intended doses selected here were designed to result in serum and placental concentrations that are ∼10- to 100-fold higher than what is measured in human serum and placenta (1, 9), to account for the 100-fold safety factor included in most risk assessments. Dams were orally exposed by adding 20 μL of the dosing solution onto a ¼ of a soy-free food treat pellet (chocolate-flavored AIN-76A rodent diet test tabs; TestDiet, Richmond, IN) once daily (within an hour beginning at 9:00 am). Dams were euthanized 4 hours after the final dose, and the dam sera, placentas, and fetuses were extracted and weighed. Each placenta was dissected to separate fetal from maternal tissue and immediately frozen. The maternal side consists of the decidua and metrial gland, which play a role in anchoring the developing embryo to the uterine wall and housing the spiral arteries that provide nutrients to the placenta and fetus. The fetal side consists of the junctional zone (containing the trophospongium, and sometimes called the basal zone) and labyrinth zone, which contains the syncytiotrophoblast layer that separates maternal and fetal circulation and provides an interface for exchange (22, 24, 27). One whole placenta and one dissected placenta and their paired fetus per dam from each sex were used for this study. Fetal mass was measured immediately following necropsy. Fetal age was assigned using Witschi, Theiler, and Carnegie staging. The target fetal age of GD 15 in the rat is equivalent to GD 13.5 in the mouse. Placentas, two from the control group (both males) and two from the treated group (one of each sex), were sent to the histopathology core at the North Carolina State College of Veterinary Medicine to look for any evidence of gross pathology (none was found) and provide an independent assessment of gestational age. Placentas were paraffin embedded, cut into 5-µm sections, slide mounted, and labeled with hematoxylin and eosin. Endpoints examined included presence and concentration of glycogen cells, levels of necrosis in the decidual and metrial gland layers, development of the basal zone, and degree of vascular invasion by trophoblasts in the myometrium.
Sample extraction
To extract brominated flame retardants from serum, we used a method previously developed by Butt et al. (28). Briefly, 500 μL of serum was thawed, transferred to a glass test tube, and spiked with internal standards FBDE-69 and 13C-BDE-209. Serum proteins were denatured using formic acid and water, followed by 20 minutes of sonication. Analytes were purified using a Waters Oasis HLB followed by a 1-g Waters Sep-Pak silica column (Waters Corporation, Milford, MA), which was conditioned and eluted with hexane. The analytes were then blown down to ∼100 μL under a gentle stream of N2 and spiked with the recovery standard, 13C-CDE-141, and analyzed using gas chromatography/electron-capture negative-ionization mass spectrometry.
To extract brominated flame retardants from the placenta and fetus, the tissues were homogenized using a mortar and pestle in 5 g of sodium sulfate and spiked with an internal standard, FBDE-69. Tissues were then sonicated with CH2Cl2 and centrifuged to separate the solvent from the solid tissue. After concentrating the extract to ∼1 mL using nitrogen evaporation, 50 μL was aliquoted for gravimetric lipid analysis and another 50 μL was aliquoted for a bicinchoninic acid assay to quantify protein content. The remaining extract was purified through a column packed with Florisil (Sigma-Aldrich, St. Louis, MO) and eluted with 30:70 hexane/CH2Cl2. The elution solvent was then concentrated to 0.5 mL using nitrogen evaporation and spiked with a recovery standard 13C-CDE-141. Extracts were analyzed using gas chromatography/electron-capture negative-ionization mass spectrometry (9). Owing to solubility issues in the dosing vehicle, BDE-209 was not detected in any samples and was not included in any analyses.
TH analyses
To extract THs from dam serum, we used a method previously developed by Wang and Stapleton (29). Briefly, 0.5 mL of serum was added to a glass centrifuge tube containing 120 μL of an antioxidant solution consisting of ascorbic acid, citric acid, and dithiothreitol. Serum was deprotonated by adding 1 mL of acetone and vortexing for 30 seconds, and then left on the bench for 30 minutes. Internal standards 13C12-T4 and 13C6-T3 were spiked into each sample and centrifuged for 5 minutes at 3500 rpm. The supernatant was transferred to an amber vial and serum was extracted twice more using 1 mL of 1:1 acetone/H2O, combining the supernatants each time. Extracts were purified using SampliQ Optimized Polymer Technology cartridges (Thomas Scientific, Swedesboro, NJ), reconstituted in 400 μL of 1:1 H2O/MeOH and filtered using a 0.2-μm GE Healthcare polytetrafluoroethylene UniPrep vial (GE Healthcare Life Sciences, Marlborough, MA). A recovery standard of 13C6-T4 was added to the samples and analyzed using liquid chromatography–tandem mass spectrometry electron spray ionization. TH levels in placental and fetal tissue were quantified using methods previously developed by Leonetti et al. (18). Briefly, ∼0.2 g of tissue was transferred to a Safe-Lock centrifuge tube (Thermo Fisher Scientific, Waltham, MA) containing 0.5-mm glass beads and homogenized using a BBX24 Bullet Blender (Next Advance, Troy, NY). The tissue was then digested using pronase digestion solution consisting of protease, l-glutathione, n-phenylthiourea, and Tris(hydroxymethyl)aminomethane in a hot water bath for 16 hours at 37°C. The mixture was spiked with internal standards 13C12-T4 and 13C6-T3. The reaction was stopped with cold acetone, followed by the addition of an antioxidant solution consisting of citric acid, ascorbic acid, and dithiothreitol. Liquid–liquid extraction using acetone, cyclopentane, and ethyl acetate followed by centrifugation was used to isolate TH from lipids and interfering matrices. The purified extract was reconstituted in 3 mL of 0.01 M HCl with 10% MeOH. Extracts were purified using SampliQ Optimized Polymer Technology cartridges, reconstituted in 400 μL of 1:1 H2O/MeOH, and filtered using a 0.2-μm GE Healthcare polytetrafluoroethylene UniPrep vial. A recovery standard of 13C6-T4 was added to the samples and analyzed using liquid chromatography–tandem mass spectrometry electron spray ionization.
Transporter expression was measured on dissected placentas from both treatment groups [vehicle and PBDEs; n = 7 per sex per group for controls and 8 per sex per group for treated (one per sex per litter)]. Transporters included Abcc1, Abcc4, Slc16a2, and Slco1b2 because they are the predominant transporters expressed in the placenta, they are unidirectional (efflux or uptake), they are localized in either apical or basal membranes, they transport substrates similar in structure to PBDEs, and they have also been shown to be expressed in human placenta. RNA extraction was performed with the Qiagen RNeasy miniprep kit according to the manufacturer’s protocol (catalog no. 74134; Qiagen, Valencia, CA), RNA quality and quantity were estimated using a Thermo Fisher Scientific NanoDrop 1000, and all samples were normalized to a concentration of 200 ng/μL prior to cDNA synthesis. Incubation for reverse transcription reactions was 60 minutes at 37°C, 5 minutes at 95°C, and the cDNA was stored at −20°C until use. An ABI StepOnePlus real-time PCR system (Thermo Fisher Scientific) and TaqMan probes were used for quantitative real-time PCR. Triplicate reactions were run as well as negative controls for each TaqMan assay. A housekeeping gene (18S rRNA) was used to normalize CT values for differences in starting concentrations of cDNA. Relative changes in expression were determined using the Livak ΔΔCT method.
Human study
Ten anonymous human placenta samples from pregnant women who underwent cesarean sections for noncomplicated reasons (e.g., previous cesarean section) were obtained from the Duke University Medical Center. Immediately following the cesarean section, the tissue was subsampled in a systematic fashion to minimize heterogeneity among samples. Using a scalpel, three full-thickness placenta tissues ∼7.6 cm in length and width were incised 5 cm from the insertion of the umbilical cord and 5 cm from the perimeter of the placenta. Two of the placental tissue subsamples were then transferred to a glass dish where the top 1 cm of the fetal portion and the bottom 1 cm of the maternal portion were incised using a clean scalpel. The maternal and fetal portions were then transferred to their own clean amber vial and freeze-dried for 72 hours. The tissue was extracted, purified, and quantified using the same methods as described above for the rodent study. All protocols were approved by the Duke University Institutional Review Board prior to study initiation.
Quality control/quality assurance
For both PBDE and TH analyses, laboratory processing blanks were included in all tissue and serum analyses. The method detection limits for each analyte were calculated from three times the SD of the laboratory blank values and ranged from 0.016 to 0.39 ng. Recovery of the PBDE internal standard (F-BDE-69) was >80% for all congeners. Extraction efficiency and accuracy of our PBDE measurements were evaluated using a Standard Reference Material (SRM 1947, Lake Michigan fish tissue) purchased from the National Institute of Standards and Technology (Gaithersburg, MD). The accuracy of certified values ranged from 101% to 120% for PBDE congeners evaluated. TH recovery was calculated using recovery standard 13C6-T4, and recovery was >70% for all THs.
Data analysis
Statistical analyses were performed using SAS 9.4 and GraphPad Prism version 8 (GraphPad Software, La Jolla, CA). Concentrations of PBDEs in rat serum and tissue were normally distributed, and therefore we used a one-way ANOVA for rat serum and a repeated measures ANOVA for placental tissue and fetus, followed by post hoc comparisons using a Fisher protected least significant differences test. PBDE concentrations in human placenta were not normally distributed, and therefore we used nonparametric statistics (Wilcoxon signed-rank test) to compare PBDE concentrations in maternal and fetal placental tissue. TH analyses were performed using a mixed model analysis with a random effect for dam, dose, tissue type, sex, and GD as multiplicative interactions. The litter was the statistical unit for all analyses, and data were stratified by sex, tissue type, and GD. Differences in expression of transporters in the placenta were evaluated using a Mann–Whitney U analysis. The variable used in the Mann–Whitney U analysis was dose (compared controls to PBDE-treated dams), and CIs for these differences were based off of the medians. A P value of <0.05 was considered statistically significant.
Results
PBDE exposure reduced fetal mass early on in gestation
Results from our multiple linear regression analysis (26) suggest that exposure to PBDEs resulted in a significantly increased fetal mass at GD 14 (P < 0.001) and significantly reduced fetal mass at GD 12 (P < 0.001). Dosed fetuses from GD 13 and 15 did not have significantly different masses relative to controls (P = 0.52 and P = 0.19, respectively).
PBDE concentrations in tissues
PBDEs were detected in all tissues from exposed animals. Concentrations of PBDEs measured in maternal serum, placenta, and fetal tissues are presented in Fig. 1. Results from a repeated measures ANOVA revealed that the PBDE concentrations measured in the fetal portion of the placenta were significantly greater (about twofold) than concentrations measured in the maternal portion, despite no differences in the lipid content of the placental layers (P < 0.001). The relative contribution of each PBDE accumulating in the tissues was similar to the relative contribution in the dosing mixture. PBDE concentrations in the fetus were ∼10-fold lower than that in the fetal placenta and ∼3-fold lower than in the maternal portion of the placenta. This trend was similar for each PBDE congener. There was a statistically significant difference in the relative composition of the PBDEs in maternal and fetal placental tissues for BDE-28, -47, and -99. Specifically, there was a greater proportion of BDE-28 and -47 in maternal compared with fetal placental tissues; however, the proportion of BDE-99 was higher in fetal than in maternal placental tissues. The percentage composition of PBDEs in the fetus differed significantly from the fetal placenta for BDE-28, -47, and -100 (P < 0.001) and differed from the maternal portion for BDE-47, -100, and -99 (P < 0.001) (26).
Figure 1.
Concentration of PBDEs in dam serum, maternal placenta, fetal placenta, and fetus. Numbers above brackets represent fold difference in concentration between maternal and fetal placenta. n = number of litters (not pups); error bars represent SEM of litters. *P < 0.05 between maternal and fetal placental concentrations.
PBDE partitioning in human placenta
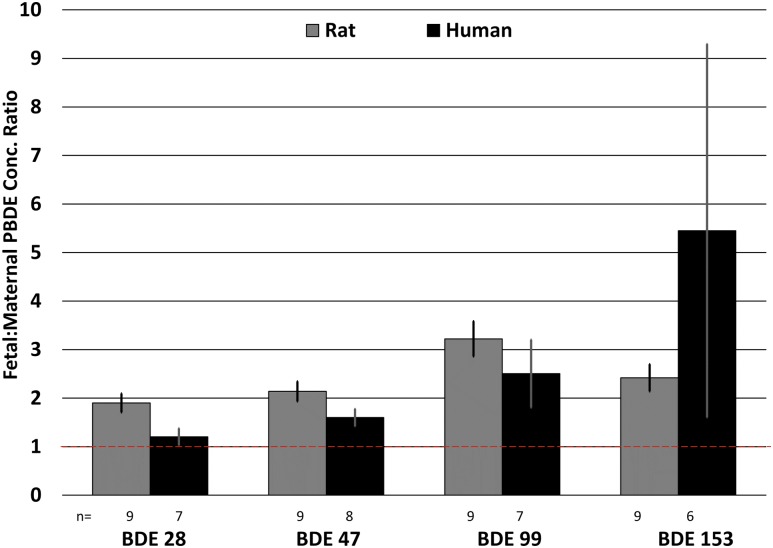
Anonymous human placental tissues (n = 10) were analyzed to determine whether placental PBDE partitioning behavior (between maternal and fetal layers) would be consistent with the observations from the rat study. Tissues were dissected to roughly isolate maternal and fetal layers and separately analyzed for PBDEs. Several PBDE congeners were below the method detection limit in three of the placentas. For the remaining PBDE congeners, the measured concentrations in the fetal and maternal layers were compared and the ratio of the two concentrations was calculated (Fig. 2). PBDE concentrations were higher in the fetal layers relative to the maternal layers, about twofold to fivefold higher, despite no differences in lipid content between the two tissue layers. All measured PBDEs appeared to preferentially accumulate on the fetal side of the placenta. The concentration ratios ranged from 1.2 to 5.5 for humans and 1.9 to 3.2 for rats, and were significantly different than 1 (P ≤ 0.03).
Figure 2.
Ratio of PBDEs in fetal vs maternal placentas measured in both rat (control experiment) and human placentas. The dashed line indicates the 1:1 line. n = number of placentas analyzed; error bars represent SEM of individual placentas.
TH measurements
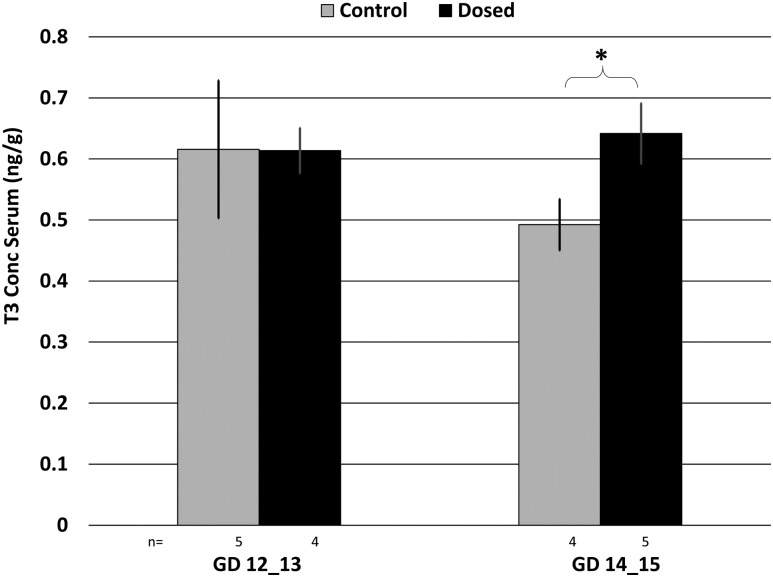
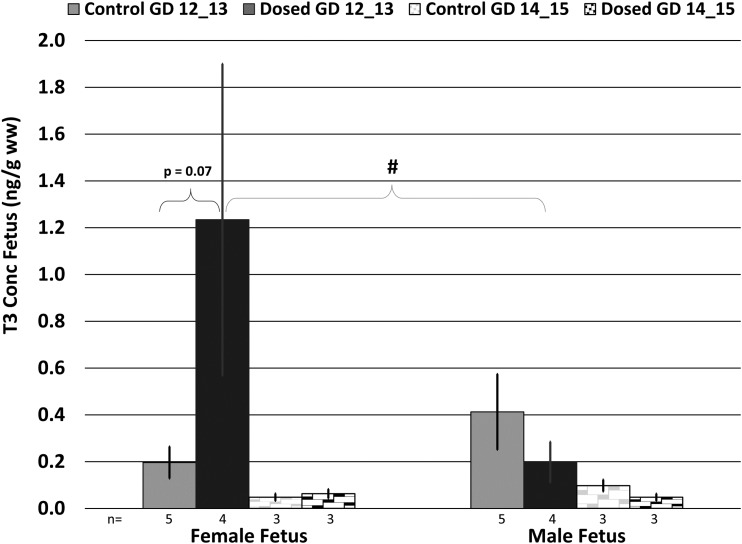
Evaluation at necropsy revealed a distribution of gestational ages in the collected fetuses ranging from GD 12 to 15. Therefore, the effects of dose, tissue type, sex, and gestational age on TH levels in dam serum, the dissected placenta, and fetus were evaluated using a mixed model analysis. Circulating T3 levels in the dosed dams were significantly increased relative to controls at GD 14/15 (P = 0.012) but not at GD 12/13 (P = 0.986) (Fig. 3). Analysis of THs in the placental tissues revealed significant differences in T3 and T4 based on tissue location (e.g., fetal vs maternal). We observed a significant decrease in T3 levels in the fetal placental tissue relative to the maternal placental tissue for all control and exposed groups (P < 0.001); however, we did not have the statistical power to evaluate TH concentrations in the placenta from control female fetuses on GD 14/15 where we only had n = 1 dam. We also observed a significant decrease in T4 levels in the fetal placental tissue relative to the maternal placental tissue in control females on GD12/13. T3 levels in the maternal and fetal placenta also significantly differed from one another based on the GD of the fetus. Specifically, we observed a significant increase in T3 levels in control as well as dosed maternal placentas from males from GD14/15 relative to GD12/13 (control: P = 0.021, ∼1.6-fold greater at GD14/15; dosed: P = 0.029, ∼2.2-fold greater at GD14/15). We also observed a significant GD effect in dosed maternal placenta from females between GD12/13 and GD14/15 (P < 0.001), with T3 levels ∼3.1-fold greater at GD14/15 (Fig. 4). We did not observe any significant dose, tissue type, or GD effect on rT3 levels in the placenta. Overall, there was a suggestive decrease in the T3 levels in exposed placental tissues. In the fetal tissues, we observed a suggestive increase in T3 in female fetuses on GD 12/13 (P = 0.07). Overall, there was an increase in T3 in female exposed fetuses and a decrease in male exposed fetuses, but these differences were not statistically significant (Fig. 5). All TH concentrations in each compartment are summarized in an online repository (26).
Figure 3.
T3 concentration in dam serum, stratified by GD and dose group. n = number of dams; error bars represent SEM of dam. *P < 0.05 in T3 concentrations in dam serum between control and dosed animals at GD 14_15.
Figure 4.
T3 concentration in maternal and fetal placenta, stratified by GD, sex, and dose group. n = number of litters (not pups); error bars represent SEM of litter. *P < 0.05 between maternal and fetal placental tissue type; #P < 0.05 between GD.
Figure 5.
T3 concentration in the fetus, stratified by GD, sex, and dose group. n = number of litters (not pups); error bars represent SEM of litter. #P < 0.05 indicates a significant sex difference.
Transporter expression in placenta
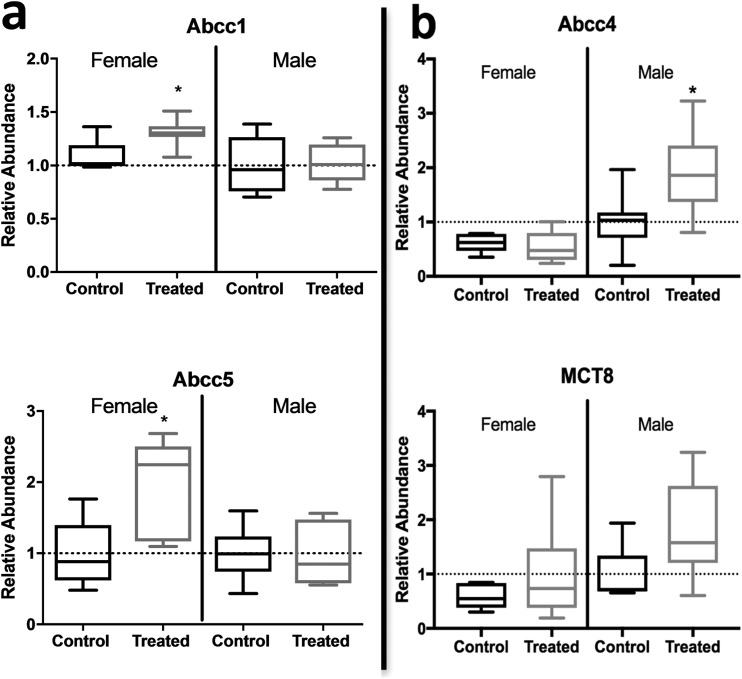
mRNA expression was evaluated for several transporters separately in maternal and fetal placental tissues. We observed a significant upregulation of efflux transporter ABCC4 (P ≤ 0.02) and nearly significant upregulation of uptake transporter MCT8 (P = 0.13) in the fetal portion of the placenta from exposed males only. Efflux transporters ABCC1 and ABCC5 were significantly upregulated in the maternal portion from exposed females only (P ≤ 0.01) (Fig. 6). Expression of placenta transporters OATP1B2, OATP4A1, and OATP1A2 were also evaluated; however, no significant effects of dose or sex were observed. Transporter expression was also assessed for baseline sex differences (control female vs control male). Although we did not observe any significant differences, there was a suggestive difference in expression of MCT8 (P = 0.13).
Figure 6.
Transporter gene expression in the (a) maternal and (b) fetal portion of the placenta (n = 7/8). n = number of litters (not pups); error bars represent SEM of litter. *P < 0.05 between dose groups.
Discussion
This study sought to understand the localization of PBDEs in the fetal placenta and their effects on circulating THs in paired dam serum, dissected placenta, and fetuses in the rat model. Our data demonstrated that PBDEs are (i) concentrating in the fetal portion of the placenta at significantly higher levels than in the maternal portion, (ii) disrupting placenta transporters in a sex-specific manner, and (iii) potentially disrupting TH homeostasis in dam serum and placental and fetal tissues, although the variation in gestational age obfuscates the trends. Our data also demonstrated the importance of considering tissue, sex, and gestational age when evaluating effects of exposure.
PBDE exposure generally resulted in decreased fetal mass
Our data suggest that PBDE exposure resulted in decreased fetal mass in male and female fetuses on GD 12; however, we lacked the statistical power to determine whether the reduction in fetal mass was significant. Zhao et al. (30) exposed 10 Sprague-Dawley rats to 1000 mg/kg/d of decabromodiphenyl ether prior to conception and through gestation. They also observed a significant reduction in body weight of newborn pups and hypothesized that the reduction in fetal mass may be attributed to TH disruption.
Environmental contaminants are concentrating in the fetal portion of the placenta
Our data showed that PBDE congeners concentrate in the fetal portion of the placenta, despite no differences in lipid content. As lipophilic compounds, we would expect the PBDE concentrations to be in equilibrium between the fetal and maternal layers of the placenta if passive diffusion was responsible for the movement of these chemicals. Because they are not in equilibrium, this suggests that these compounds are actively transported across the placental barrier between the mother and fetus. Although some studies suggest that PBDEs are passively transported across the placental barrier, others provide data that suggest otherwise (1, 2, 11–13). To our knowledge, no one has evaluated active transport at the maternal/fetal interface of the placental barrier. Unfortunately, all prior studies used whole or homogenate placental samples for analysis of contaminants, rather than analyzing specific tissue layers. We hypothesize that transporters expressed in the syncytiotrophoblast are actively transporting PBDEs across the placental membrane and against a concentration gradient. There is already evidence that PBDEs are substrates for OATPs, which belong to the SLC superfamily, in hepatocytes (31). BDE-47, -99, and -153 were able to inhibit the cellular uptake of estradiol 17β-glucuronide by OATP1B3 and OATP1B1 as well as estrone 3-sulfate by OATP2B1 at environmentally relevant concentrations [BDE-47 Michaelis constant (Km) of 0.31 μM]. This suggests that PBDEs are capable of binding OATP transporters strongly enough to displace endogenous substrates. We hypothesize that PBDEs may also be capable of competing with TH for MCT8 transport across the placenta and may be a potential mechanism of TH disruption in the placenta, although this needs to be evaluated mechanistically in future studies.
Another novel finding was the difference in the relative composition of congeners in maternal serum, placental tissues, and fetal tissues relative to the composition of each congener in the dosing mixture (26). Accounting for the relative amounts in the dosing mixtures explains some of the variability in the accumulation of the tissues; however, the data clearly show that there is a positive association between the octanol-water partition coefficient (Kow) of the congener and the percentage of the dose in each compartment. Additionally, we observed higher concentrations of BDE-99 in the fetal portion of the placenta relative to the maternal portion and higher concentrations of BDE-28 and -47 in the maternal portion of the placenta relative to the fetal portion (26). Meanwhile, the percentage composition of BDE-153 was relatively equal in each of the compartments quantified. Our data indicate that the placenta, particularly the fetal portion, is a sink for all BDE congeners tested in our mixture.
Importantly, we found that PBDEs concentrate on the fetal side of the placenta in a similar manner in both humans and rats. Therefore, this effect is not specific to rats, and suggests that the rat may be a good model for exploring the mechanism and effects of this phenomena. Although it is possible that metabolism could be influencing the relative accumulation in the maternal and fetal side of the placenta, we think this is unlikely, as this trend was observed for all PBDE congeners measured, regardless of whether that congener is known to be metabolized (e.g., BDE-99) or is relatively recalcitrant to metabolism (e.g., BDE-153) (32). In contrast, we hypothesize that transporters are moving PBDEs actively from one side of the placenta to the other. If true, this would imply that endogenous substrates, such as nutrients and hormones, may have to compete for transport across the placenta barrier, which could have profound implications for the developing fetus. PBDE exposure during prenatal periods have been associated with numerous adverse health outcomes, such as neurodevelopmental delays, behavioral complications, and endocrine disruption. Studies by Herbstman et al. (33) and Eskenazi et al. (34) presented strong evidence that prenatal and early-life exposure to PBDEs can impair neurodevelopment. Several studies have observed associations between cord blood as well as lactational PBDE concentrations with behavioral problems, such as aggression, impulsivity, hyperactivity, attention deficit, and increased anxiety (35–37). PBDEs have also been associated with adverse pregnancy outcomes, such as reduced birthweight, yet the mechanisms driving adverse health outcomes remain poorly understood (38).
PBDE exposure upregulates placental transporters in a sex-specific manner
We quantified mRNA expression of several uptake and efflux transporters in the apical and basal membranes of the fetal and maternal portion of the placenta to provide more insight into the effect of PBDE exposure on placenta function. We observed significant upregulation of efflux transporter ABCC4 and nearly significant upregulation of uptake transporter MCT8 in the fetal portion of the placenta from males only. MCT8 is a specific TH transporter that is responsible for transporting THs to the developing fetus. Our results showed decreased, although not significant, T3 levels in male fetuses, but increased T3 levels in female fetuses, relative to controls. We hypothesize that this upregulation of MCT8 may be a compensatory response to lowered T3 in the male fetus. In a study that evaluated protein and mRNA expression of TH-specific transporter MCT8, Chan et al. (17) observed an upregulation of MCT8 in human placenta from fetuses that were diagnosed with IUGR compared with normal placenta. They hypothesized that the upregulation of MCT8 was a compensatory mechanism to increase T3 delivery to the fetus. Previous literature has also shown that PBDEs upregulate efflux transporters in hepatocytes by activating CAR and RXR, leading to rapid elimination of T4 and T3 from hepatocytes (39). We also observed a significant upregulation of ABCC1 and ABCC5 in the maternal portion from females only. These transporters are responsible for the efflux of folates, biliary substances, and cell signaling molecules such as cAMP and cGMP (23). To our knowledge, this is the first study to identify sex-specific differences in placental transporter activity and, given their important roles in fetal development, highlights a need to understand these basal sex differences and how they are affected by environmental exposures.
PBDE exposure, sex, tissue type, and GD influence TH levels
Numerous studies have shown that PBDEs disrupt TH regulation; however, to our knowledge, no study has quantified THs in paired serum, placenta, and fetus. We observed significantly increased levels of serum T3 in dosed animals on GD14/15 but not on GD 12/13. Although this positive association is discordant with several other studies evaluating the effect of PBDEs on TH in pregnant Wistar rats, we hypothesize that the difference in trends could be attributed to differences in the doses used, with our study using a significantly lower dose (37, 40). Furthermore, these studies collected serum at the end of gestation, whereas we collected dam serum at mid-gestation. Results from Andrade et al. (41) showed that the time point in which serum was collected influenced whether there was a positive or negative association between PBDE exposure and T4 levels. To our knowledge, our data are the first to demonstrate dose, tissue, sex, and GD effects on T3 in the placenta. This finding is critically important for future studies to take into consideration. Our data demonstrate that it is necessary that the exact GD is identified using a fetal index when quantifying TH levels, given that a significant effect can be obfuscated or hidden not because of a dose effect, but because of the sex of the fetus, its GD, or the specific tissue layer sampled from the placenta. Although this study alone cannot give an explanation as to why we see differences in TH levels across gestational ages, we hypothesize that the differences observed could be related to differences in TH transport to the fetus, differences in TH metabolism, and the maturation of the TH hormone system in the fetus as it develops; however, future studies are necessary to understand these trends in greater detail. We also found that T3 concentrations in the fetus increased with PBDE exposure in the female fetuses but decreased in the male fetuses. These differences were not statistically significant, as we were underpowered due to the differences in GD among the replicate dams. However, it is interesting to note that Leonetti et al. (18) observed a similar sex difference in PBDEs and THs quantified in whole human placenta. They found a statistically significant positive association between BDE-99 and T3 in females and a negative association between BDE-99 and T3 in males. These observations warrant more research to elucidate the complicated dynamics between PBDE exposure, TH regulation, and development during gestation.
Study limitations
Although this study has many novel findings and significant implications for future studies, it is important to consider its limitations. Our sample size is limited for some of the dose groups due to the range in GD of the animals used in this study. Therefore, some of our analyses are underpowered, making it difficult to determine whether these trends are statistically significant. Additionally, we could not explore sex differences in PBDE accumulation in the human placenta samples due to confidentiality of the samples. Lastly, there are currently no studies that have identified the localization of placental transporters in the maternal portion of the placenta. Therefore, it is not possible to conclude in which direction substrates are moving and how a change in expression affects their transport.
Implications
This research is significant because it demonstrates that PBDEs are concentrating in the fetal portion of the placenta, which is the source of multiple hormones needed to maintain pregnancy, enzymes for nutrient metabolism and detoxification, and transporters for nutrient and hormone exchange. Therefore, further research is needed to determine whether the accumulation of contaminants on the fetal side is disrupting important biological functions related to fetal development and health. Furthermore, more research should be conducted to determine whether other contaminants (e.g., polychlorinated biphenyls, DDT) behave similar to PBDEs. This study is significant because it explores potential sex-specific accumulation and transport of PBDEs in the placenta and provides strong evidence that the rat is a relevant model to study the transfer of POPs in placenta. Our data challenge currently accepted mechanisms of POP transport in the placenta and demonstrate that it is critical that the sex, exact GD, and tissue type are characterized when quantifying TH levels.
Acknowledgments
The authors acknowledge the contributions made by Christine Crute for assistance with analyzing PBDEs in fetal tissue, and the staff of the North Carolina State University Biological Resource Facility for overseeing animal care and husbandry.
Financial Support: This work was funded by National Institute of Environmental Health Sciences Grants R01 ES028110 (to H.B.P.) and T32-ES021432 (to H.M.S.).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- GD
gestational day
- IUGR
intrauterine growth restriction
- OATP
organic anion-transporting polypeptide
- PBDE
polybrominated diphenyl ether
- POP
persistent organic pollutant
- TH
thyroid hormone
References and Notes
- 1. Vizcaino E, Grimalt JO, Fernández-Somoano A, Tardon A. Transport of persistent organic pollutants across the human placenta. Environ Int. 2014;65:107–115. [DOI] [PubMed] [Google Scholar]
- 2. Kim JT, Son MH, Lee DH, Seong WJ, Han S, Chang YS. Partitioning behavior of heavy metals and persistent organic pollutants among feto-maternal bloods and tissues. Environ Sci Technol. 2015;49(12):7411–7422. [DOI] [PubMed] [Google Scholar]
- 3. Zheng MY, Li XH, Zhang Y, Yang YL, Wang WY, Tian Y. Partitioning of polybrominated biphenyl ethers from mother to fetus and potential health-related implications. Chemosphere. 2017;170:207–215. [DOI] [PubMed] [Google Scholar]
- 4. Meijer L, Weiss J, Van Velzen M, Brouwer A, Bergman A, Sauer PJ. Serum concentrations of neutral and phenolic organohalogens in pregnant women and some of their infants in the Netherlands. Environ Sci Technol. 2008;42(9):3428–3433. [DOI] [PubMed] [Google Scholar]
- 5. Zhao F, Chen M, Gao F, Shen H, Hu J. Organophosphorus flame retardants in pregnant women and their transfer to chorionic villi. Environ Sci Technol. 2017;51(11):6489–6497. [DOI] [PubMed] [Google Scholar]
- 6. Jakobsson K, Fång J, Athanasiadou M, Rignell-Hydbom A, Bergman A. Polybrominated diphenyl ethers in maternal serum, umbilical cord serum, colostrum and mature breast milk. Insights from a pilot study and the literature. Environ Int. 2012;47:121–130. [DOI] [PubMed] [Google Scholar]
- 7. Jeong Y, Lee S, Kim S, Park J, Kim HJ, Choi G, Choi S, Kim S, Kim SY, Kim S, Choi K, Moon HB. Placental transfer of persistent organic pollutants and feasibility using the placenta as a non-invasive biomonitoring matrix. Sci Total Environ. 2018;612:1498–1505. [DOI] [PubMed] [Google Scholar]
- 8. Nanes JA, Xia Y, Dassanayake RM, Jones RM, Li A, Stodgell CJ, Walker C, Szabo S, Leuthner S, Durkin MS, Moye J, Miller RK; National Children’s Study Placenta Consortium. Selected persistent organic pollutants in human placental tissue from the United States. Chemosphere. 2014;106:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leonetti C, Butt CM, Hoffman K, Miranda ML, Stapleton HM. Concentrations of polybrominated diphenyl ethers (PBDEs) and 2,4,6-tribromophenol in human placental tissues. Environ Int. 2016;88:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim YR, Harden FA, Toms LM, Norman RE. Health consequences of exposure to brominated flame retardants: a systematic review. Chemosphere. 2014;106:1–19. [DOI] [PubMed] [Google Scholar]
- 11. Kang CS, Lee JH, Kim SK, Lee KT, Lee JS, Park PS, Yun SH, Kannan K, Yoo YW, Ha JY, Lee SW. Polybrominated diphenyl ethers and synthetic musks in umbilical cord serum, maternal serum, and breast milk from Seoul, South Korea. Chemosphere. 2010;80(2):116–122. [DOI] [PubMed] [Google Scholar]
- 12. Mathiesen L, Mørck TA, Zuri G, Andersen MH, Pehrson C, Frederiksen M, Mose T, Rytting E, Poulsen MS, Nielsen JK, Knudsen LE. Modelling of human transplacental transport as performed in Copenhagen, Denmark. Basic Clin Pharmacol Toxicol. 2014;115(1):93–100. [DOI] [PubMed] [Google Scholar]
- 13. Kawashiro Y, Fukata H, Omori-Inoue M, Kubonoya K, Jotaki T, Takigami H, Sakai S, Mori C. Perinatal exposure to brominated flame retardants and polychlorinated biphenyls in Japan. Endocr J. 2008;55(6):1071–1084. [DOI] [PubMed] [Google Scholar]
- 14. Kodavanti PRS, Curras-Collazo MC. Neuroendocrine actions of organohalogens: thyroid hormones, arginine vasopressin, and neuroplasticity. Front Neuroendocrinol. 2010;31(4):479–496. [DOI] [PubMed] [Google Scholar]
- 15. Kilby MD, Verhaeg J, Gittoes N, Somerset DA, Clark PMS, Franklyn JA. Circulating thyroid hormone concentrations and placental thyroid hormone receptor expression in normal human pregnancy and pregnancy complicated by intrauterine growth restriction (IUGR). J Clin Endocrinol Metab. 1998;83(8):2964–2971. [DOI] [PubMed] [Google Scholar]
- 16. Loubière LS, Vasilopoulou E, Bulmer JN, Taylor PM, Stieger B, Verrey F, McCabe CJ, Franklyn JA, Kilby MD, Chan SY. Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta. 2010;31(4):295–304. [DOI] [PubMed] [Google Scholar]
- 17. Chan SY, Franklyn JA, Pemberton HN, Bulmer JN, Visser TJ, McCabe CJ, Kilby MD. Monocarboxylate transporter 8 expression in the human placenta: the effects of severe intrauterine growth restriction. J Endocrinol. 2006;189(3):465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leonetti C, Butt CM, Hoffman K, Hammel SC, Miranda ML, Stapleton HM. Brominated flame retardants in placental tissues: associations with infant sex and thyroid hormone endpoints. Environ Health. 2016;15(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li ZM, Hernandez-Moreno D, Main KM, Skakkebæk NE, Kiviranta H, Toppari J, Feldt-Rasmussen U, Shen H, Schramm KW, De Angelis M. Association of in utero persistent organic pollutant exposure with placental thyroid hormones. Endocrinology. 2018;159(10):3473–3481. [DOI] [PubMed] [Google Scholar]
- 20. Choksi NY, Jahnke GD, St Hilaire C, Shelby M. Role of thyroid hormones in human and laboratory animal reproductive health. Birth Defects Res B Dev Reprod Toxicol. 2003;68(6):479–491. [DOI] [PubMed] [Google Scholar]
- 21. Fisher DA, Klein AH. Thyroid development and disorders of thyroid function in the newborn. N Engl J Med. 1981;304(12):702–712. [DOI] [PubMed] [Google Scholar]
- 22. Soares MJ, Chakraborty D, Karim Rumi MA, Konno T, Renaud SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta. 2012;33(4):233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walker N, Filis P, Soffientini U, Bellingham M, O’Shaughnessy PJ, Fowler PA. Placental transporter localization and expression in the human: the importance of species, sex, and gestational age differences. Biol Reprod. 2017;96(4):733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furukawa S, Tsuji N, Sugiyama A. Morphology and physiology of rat placenta for toxicological evaluation. J Toxicol Pathol. 2019;32(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baldwin KR, Phillips AL, Horman B, Arambula SE, Rebuli ME, Stapleton HM, Patisaul HB. Sex specific placental accumulation and behavioral effects of developmental Firemaster 550 exposure in Wistar rats. Sci Rep. 2017;7(1):7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruis MT, Rock KD, Hall SM, Horman B, Patisaul HB, Stapleton HM. Data from: PBDEs concentrate in the fetal portion of the placenta: implications for thyroid hormone dysregulation. OSF 2019. Deposited 3 September 2019. https://osf.io/vhz3u. [DOI] [PMC free article] [PubMed]
- 27. Furukawa S, Hayashi S, Usuda K, Abe M, Hagio S, Ogawa I. Toxicological pathology in the rat placenta. J Toxicol Pathol. 2011;24(2):95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Butt CM, Miranda ML, Stapleton HM. Development of an analytical method to quantify PBDEs, OH-BDEs, HBCDs, 2,4,6-TBP, EH-TBB, and BEH-TEBP in human serum. Anal Bioanal Chem. 2016;408(10):2449–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang D, Stapleton HM. Analysis of thyroid hormones in serum by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2010;397(5):1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao X, Peng S, Xiang Y, Yang Y, Li J, Shan Z, Teng W. Correlation between prenatal exposure to polybrominated diphenyl ethers (PBDEs) and infant birth outcomes: a meta-analysis and an experimental study. Int J Environ Res Public Health. 2017;14(3):E268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pacyniak E, Roth M, Hagenbuch B, Guo GL. Mechanism of polybrominated diphenyl ether uptake into the liver: PBDE congeners are substrates of human hepatic OATP transporters. Toxicol Sci. 2010;115(2):344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lupton SJ, McGarrigle BP, Olson JR, Wood TD, Aga DS. Human liver microsome-mediated metabolism of brominated diphenyl ethers 47, 99, and 153 and identification of their major metabolites. Chem Res Toxicol. 2009;22(11):1802–1809. [DOI] [PubMed] [Google Scholar]
- 33. Herbstman JB, Sjödin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118(5):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjödin A, Bradman A. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121(2):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shy CG, Huang HL, Chang-Chien GP, Chao HR, Tsou TC. Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl ethers. Bull Environ Contam Toxicol. 2011;87(6):643–648. [DOI] [PubMed] [Google Scholar]
- 36. Hoffman K, Adgent M, Goldman BD, Sjödin A, Daniels JL. Lactational exposure to polybrominated diphenyl ethers and its relation to social and emotional development among toddlers. Environ Health Perspect. 2012;120(10):1438–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuriyama SN, Wanner A, Fidalgo-Neto AA, Talsness CE, Koerner W, Chahoud I. Developmental exposure to low-dose PBDE-99: tissue distribution and thyroid hormone levels. Toxicology. 2007;242(1–3):80–90. [DOI] [PubMed] [Google Scholar]
- 38. Harley KG, Chevrier J, Aguilar Schall R, Sjödin A, Bradman A, Eskenazi B. Association of prenatal exposure to polybrominated diphenyl ethers and infant birth weight. Am J Epidemiol. 2011;174(8):885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PR, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Czerska M, Zieliński M, Kamińska J, Ligocka D. Effects of polybrominated diphenyl ethers on thyroid hormone, neurodevelopment and fertility in rodents and humans. Int J Occup Med Environ Health. 2013;26(4):498–510. [DOI] [PubMed] [Google Scholar]
- 41. Martino-Andrade A, Kuriyama SN, Akkoc Z, Talsness CE, Chahoud I. Effects of developmental low dose PBDE 47 exposure on thyroid hormone status and serum concentrations of FSH and inhibin B in male rats. Organohalogen Compd. 2004;66:3858–3863. [Google Scholar]