Abstract
Purpose
To examine the benefits of a culturally targeted compared with a nontargeted smoking cessation intervention on smoking cessation outcomes among lesbian, gay, bisexual, and transgender (LGBT) smokers.
Methods
A prospective randomized design was used to evaluate the added benefits of an LGBT culturally targeted Courage to Quit (CTQ-CT) smoking cessation treatment (N = 172) compared with the standard intervention (CTQ; N = 173). The smoking cessation program consisted of six treatment sessions combined with 8 weeks of nicotine replacement therapy. The primary smoking cessation outcome was 7-day point prevalence quit rates. Secondary outcomes examined included changes in nicotine dependence, nicotine withdrawal, cigarettes per day, smoking urges, self-efficacy, and readiness to quit.
Results
Overall quit rates were 31.9% at 1 month, 21.1% at 3 months, 25.8% at 6 months, and 22.3% at 12 months. Quit rates did not differ between treatment groups [1 month OR = 0.81 (0.32, 2.09), 3 months OR = 0.65 (0.23, 1.78), 6 months OR = 0.45 (0.17, 1.21), 12 months OR = 0.70 (0.26, 1.91)]. Compared with baseline levels, all secondary smoking cessation outcomes measured were improved at 1 month and were maintained at 12-month follow-up. Compared with the CTQ, the CTQ-CT intervention was more highly rated on program effectiveness (d = 0.2, p = .011), intervention techniques (d = 0.2, p = .014), the treatment manual (d = 0.3, p < .001), and being targeted to the needs of LGBT smokers (d = 0.5, p < .0001).
Conclusions
LGBT smokers receiving the CTQ intervention achieved smoking cessation outcomes in the range reported for other demographic groups. Cultural targeting improved the acceptability of the intervention but did not confer any additional benefit for smoking cessation outcomes.
Implications
Study results have implications for understanding the benefits of culturally targeted compared with nontargeted smoking cessation interventions for improving smoking cessation outcomes among LGBT smokers. Shorter and longer term 7-day point prevalence quit rates associated with the targeted and nontargeted interventions were modest but comparable with other group-based interventions delivered in a community setting. Although cultural targeting improved the overall acceptability of the intervention, no added benefits were observed for the culturally targeted intervention on either the primary or secondary outcomes.
Introduction
Research suggests that lesbian, gay, bisexual, and transgender persons (LGBT) are at elevated risk for tobacco-related health disparities because of disproportionately high rates of tobacco use.1 A recent national study found that gay men were 50% more likely to smoke than heterosexual men, while lesbian and bisexual women were 80%–95% more likely to smoke than heterosexual women.2 Despite these known and persistent disparities, LGBT individuals are not systematically included in tobacco prevention and control efforts or in research evaluating smoking cessation intervention approaches.3 Furthermore, no LGBT-specific cessation interventions were identified in the 2008 Public Health Service Clinical Update4 nor did the Institute of Medicine report on research on LGBT health5 provide guidance on interventions to address this known disparity.6 The paucity of empirical data regarding effective smoking cessation treatments for LGBT smokers has important implications for the persistence of observed smoking disparities, thus making LGBT smokers an important priority group for smoking cessation intervention efforts.
Smoking Cessation Interventions for LGBT Smokers
The extant literature has established that psychosocial variables related to smoking cessation may differ among population subgroups and that considering cultural variation may improve tobacco dependence treatment programs.4,7 The results of these studies have been mixed with some interventions showing positive benefits of targeting for improving behavioral outcomes,8 some for treatment acceptability and satisfaction,9 while others reporting no added benefit of targeted approaches.10 Among LGBT smokers, preliminary research suggests the potential need for targeted interventions because of unique stressors and risk factors experienced by LGBT smokers including elevated rates of discrimination,11 exposure to more tobacco-friendly community norms that may reduce motivation to quit smoking,12 the use of smoking to rebel against or promote particular gender identities,13 low readiness to quit smoking,14 reduced access to LGBT-specific cessation services,12,13 targeted tobacco marketing,15 and a stated preference for LGBT-specific smoking cessation treatments.3
To date, a limited number of targeted intervention approaches have been conducted. The majority of the available studies are minimally targeted and group-based interventions delivered in community-based settings.16–21 The quit rates associated with these interventions range from 6% at 3 months to 36% at 6 months. Although limited, the combined available literature suggests the potential benefit of targeted intervention approaches for reducing smoking behaviors among LGBT smokers. Studies that provide direct comparisons of interventions between LGBT and non-LGBT populations on nontailored treatments are also limited.4 Two programs were not tailored for the lesbian, gay, bisexual, and transgender (LGBTQ) community but tested a program for the general population and provided results for the LGBT community. In the first study, an intensive cessation intervention that was designed for the general population and combined bupropion, individual counseling, and nicotine replacement therapy (NRT) found no significant difference in end of treatment quit rates for heterosexual and gay and/or bisexual male participants (57% vs. 58%, respectively).22 In the second study, LGBT and heterosexual smokers showed no difference in smoking cessation outcomes following participant in an extended and nontailored treatments at the 104th week of follow-up (38% vs. 40%, respectively).23The available literature on targeted and nontargeted interventions for LGBT is equally promising but limited by the relatively few number of studies that have been conducted and observed methodological concerns (eg, small sample sizes, the absence of control groups, and the lack of objective verification of quit rates).4
The need for evidence-based interventions to reduce smoking disparities among LGBT population is compelling; yet, little is known about the benefit of culturally targeted interventions for LGBT people or the effectiveness of existing group interventions in promoting tobacco use cessation among LGBT people. This study addressed an important gap in the literature by comparing the effectiveness of an LGBT culturally targeted versus nontargeted smoking cessation intervention for LGBT smokers. The platform treatment was the Respiratory Health Association of Metropolitan Chicago’s (RHA) Courage to Quit (CTQ) program.24,25 The primary outcome was 7-day point prevalent quit rates at 1-, 3-, 6-, and 12-month follow-up. Secondary outcomes included changes in level of nicotine dependence, number of cigarettes smoked per day (among nonquitters), smoking urges, and nicotine withdrawal symptoms over time. Our primary hypothesis was that the CTQ treatment program would have a positive impact on primary and secondary outcomes and that these outcomes would show better improvement for those individuals randomized to the targeted CTQ program.
Methods
Study Design
The full details of study design and trial implementation have been previously reported (US National Institutes of Health Clinical Trials NCT01633567).26 In brief, a prospective two-group randomized experimental design was conducted to test study hypothesizes related to the added benefit of the culturally targeted CTQ program (CTQ-CT) versus the standard CTQ program. Study activities were reviewed and approved by the institutional review boards of The University of Illinois at Chicago and Howard Brown Health. All study activities took place at Howard Brown Health which is an LGBT-serving federally qualified health care center (FQHC) with nine locations throughout the greater Chicago metropolitan area; the study was conducted at the Lakeview site on the North side of Chicago.
Participants
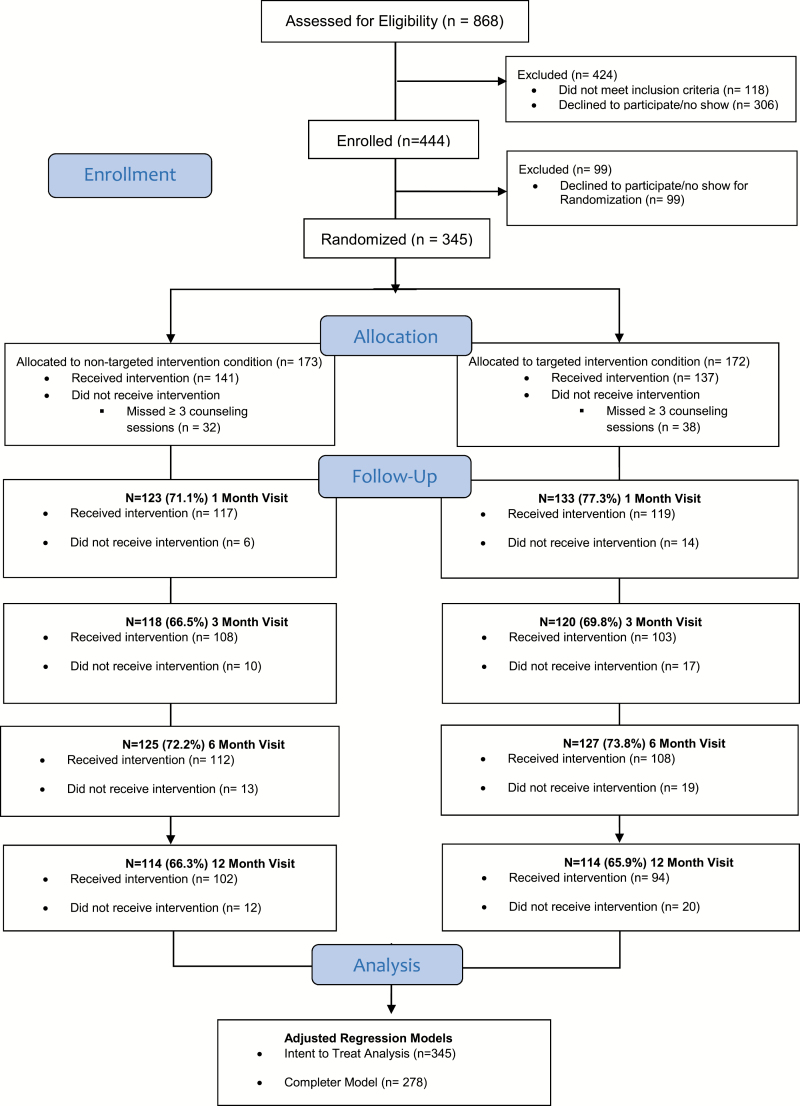
Figure 1 shows a CONSORT diagram of the flow of participants through the study. A total of N = 868 individuals were assessed for eligibility, and N = 345 were randomized into the intervention (N = 173 nontargeted and N = 172 targeted). Forty-two treatment cohorts were completed between July 2012 and July 2015. Inclusion criteria included (1) self-identity as LGBT, (2) age 18–65, (3) current smoker (more than five packs in lifetime AND past year smoking AND 4 or more days per week AND carbon monoxide [CO] expired-air reading of ≥8 ppm), (4) greater than or equal to 5 on a 10-point Likert scale measuring desire to quit smoking, and (6) no prior adverse reactions to nicotine replacement patches.25
Figure 1.
Consort diagram.
Accrual and Enrollment
Recruitment was conducted by a diverse group of LGBT- and non-LGBT–identified research staff. All team members completed a standardized LGBT cultural competency training offered by the education team at Howard Brown Health. This training is required of all individuals who have direct contact with their patient populations (for more information, see https://howardbrown.org/era/education/curriculum/). Participants were recruited using one of four strategies. Active outreach included venue-based recruitment (bars, community events, festivals) and street-intercept approaches (teams of recruiters working in pairs walking in commercial areas serving LGBT patrons). Passive outreach activities included the placement of flyers in locations such as coffee shops, community centers, and organizations serving the LGBT community. Clinic-based strategies including sending mailed letters to smokers identified by the electronic health records at Howard Brown Health, as well as direct provider referral and flyers and other study materials located in the clinic. Finally, word-of-mouth referrals were strongly encouraged by study participants to other LGBT smokers. Participants were enrolled on a continuous basis, and a dedicated research phone line was used to screen interested callers. Interested callers completed a 10-minute semistructured interview to obtain background characteristics and to determine initial study eligibility. Those who met the screening criteria were invited to an in-person screening with the study nurse to review medical exclusionary criteria (ie, untreated hypertension) or contraindications for NRT (ie, Nicoderm CQ patch) use27 and to establish the correct dosing level for NRT. Those screening candidates who were deemed ineligible received a $5.00 cash stipend for their time and those who were eligible and agreed to enroll in the trial completed a computerized battery of baseline self-report questions and received a $20.00 cash stipend.
CTQ Smoking Cessation Treatment Program
All eligible participants were randomized to CTQ-CT or nontargeted CTQ groups at the time of the first treatment session. The CTQ program24,25 is a semistructured and manualized smoking cessation intervention developed in 2007 by a clinical psychologist (A. K. Matthews) in conjunction with RHA. The program has been used as a platform treatment in clinical trials in evaluating the efficacy of experimental medications or in underserved minority communities with 6-month biochemically confirmed point prevalence quit rates ranging from 19% to 35%.24,25,28,29 The CTQ program includes six weekly sessions starting 2 weeks before the designated quit date and proceeding through 4 weeks after the quit date. The treatment modules include a progression of topics incorporating evidence-based behavioral, cognitive, and motivational smoking cessation strategies as outlined in the US Public Health Service Clinical Practice Guidelines for Treating Tobacco Use and Dependence.4
Cultural Targeting of the CTQ Smoking Cessation Treatment Program
Cultural targeting of the CTQ intervention was based on an iterative process of literature review, community engagement, and pilot testing to adapt the CTQ intervention.13,26 First, a literature review was conducted to identify any relevant content. Next, focus groups were conducted with LGBT smokers and health care providers associated with our community partner to identify unique concerns, attitudes, knowledge, and preferences. The adaptation of the CTQ manual was guided by the strategies for cultural targeting outlined by Kreuter et al.30 The five strategies outlined by Kreuter et al.30 were used to target the CTQ program to be culturally relevant to LGBT smokers and included (1) peripheral strategies (eg, culturally appropriate packaging, including images and exemplars with LGBT individuals), (2) evidential strategies (eg, enhancing perceived relevance by presenting evidence of impact of smoking on LGBT), (3) linguistic strategies (eg, using language relevant to the LGBT community), (4) constituent-involving strategies (eg, including facilitators who are LGBT or allies), and (5) sociocultural strategies (eg, discussing smoking-related risks within the context of the broader social and cultural values of LGBT). Once developed, a small pilot test in 10 LGBT smokers was conducted to determine the feasibility and acceptability of the curriculum materials and finalized study procedures. See Table 1 for a comparison of targeted versus nontargeted elements and for program outline and examples of culturally targeted treatment module.
Table 1.
Similarities and Differences Between a Culturally Targeted Versus a Nontargeted Courage to Quit Curriculum Based on Kreuter et al.30.
| Similarities | Targeted smoking | Nontargeted smoking |
|---|---|---|
| Cessation program (CTQ-CT) | Cessation program (CTQ) | |
| Theoretical basis | Stages of change and health beliefs model | Stages of change and health beliefs model |
| Delivery channel | Group and individual peer support | Group and individual peer support |
| Counseling technique | Professionally facilitated | Professionally facilitated |
| Differences | ||
| Purpose | Achieve positive smoking cessation outcomes by addressing general and culturally specific determinants of smoking (eg, beliefs, norms). | Achieve positive smoking cessation outcomes by addressing general population–derived determinants of smoking. |
| Group counseling | Culturally targeted, LGBT specific plus general content | Nontargeted, general content |
| Peer counseling | General support and counseling | General support and counseling |
| Information delivery | Culturally informed and relevant advice and support | General advice and support |
| Packaging of contents | Use of images, color, pictures that convey relevance to the group (Peripheral Targeting) | Generic content presumed to appeal broadly |
| Educational content | Increase perceived relevance by presenting evidence specific to that population group (Evidential Targeting) | Generic content based on aggregated data |
| Educational messages | Delivered in the dominant language or use of language relevant to group (Linguistic Targeting) | Delivered in the language of the majority |
| Context and meaning of messages | Relevant to the cultural values, beliefs, and behaviors of the audience (Sociocultural Targeting) | Generic content based on mainstream culture |
| Involvement of larger community | Involvement of target community (Constituent-Involving Targeting) | Generic model of intervention delivery |
CTQ = Courage to Quit; CTQ-CT = culturally targeted Courage to Quit.
Intervention Procedures
Both the CTQ and CTQ-CT interventions consisted of six weekly group-based smoking cessation therapy sessions each lasting 90 minutes.24,25,29 The CTQ and CTQ-CT group participants all completed the same measures and procedures, including the follow-up schedule. All treatment sessions were cofacilitated by six master’s level clinicians. Group facilitators were a diverse group of LGBT- and non-LGBT–identified individuals selected based on training and experience in group facilitation. All facilitators were trained by the RHA on the CTQ smoking cessation treatment program and cultural competency in working with LGBT populations by staff at Howard Brown Health. A clinical psychologist and LGBT health expert (A. K. Matthews) provided additional training to facilitators on the issues associated with the culturally targeted elements of the curriculum. All staffs were supervised on a weekly basis. All groups were led by a facilitator and cofacilitator who were trained on both the targeted and nontargeted interventions. To ensure fidelity to the intervention protocol, the cofacilitator completed an in-session fidelity rating form that counted whether each specific intervention element required by the study protocol was addressed. Results revealed high levels of treatment fidelity (eg, 90%–100% correct content in each session delivered). Each session was preceded by a short interview with a research assistant (RA) to obtain self-report psychosocial and substance use information and obtain CO measures. At each visit, study staff distributed NRT for each participant per manufacturer’s instructions for dosage. Modifications of dosing level were adjusted, as needed, in conjunction with the study medical provider. Nicotine replacement use began on the quit date and continued for 8 weeks. Post treatment follow-up interviews took place in-person at 1 and 3 months, and telephone or internet-based data collection procedures were used for 6- and 12-month assessment points. CO readings were obtained for all participants reporting abstinence at each weekly session and at 1- and 3-month follow-up. If self-reported abstinence could not be biochemically verified at those time points, participants were conservatively marked as smoking. Participants received $20 cash stipend at each assessment time point and $5 for each of the six weekly sessions to cover transportation costs. Study participants and data collection assistants were blinded to group assignment.
Study Measures
Standard demographic characteristics were collected at baseline. Smoking-related measures were collected at baseline, weekly, and at follow-up. Smoking measures included the Fagerstrom Test for Nicotine Dependence (FTND),31 the brief Questionnaire of Smoking Urges (BQSU-brief),32 Self-Efficacy for Quitting,33and the Minnesota Nicotine Withdrawal Scale (MNWS),27 readiness to quit (Likert scale ranging from 0 to 10, with higher scores indicating more readiness to quit),34 and an adapted Risks and Benefits of Quitting measure.35 Daily smoking frequency and patch use were determined by self-report, as obtained via a self-reported timeline follow-back interview. 36 Weekly and follow-up assessments included readiness to quit, smoking urges, withdrawal symptoms, and 7-day point prevalence quit rates. The primary smoking cessation outcome was 7-day point prevalence smoking quit rates (ie, no smoking during the past 7 days; derived from timeline follow-back interviews).36 Secondary outcomes included level of nicotine dependence, number of cigarettes smoked per day (among nonquitters), smoking urges, nicotine withdrawal symptoms, readiness to quit, abstinence self-efficacy, and perceived risks and benefits of quitting. Biochemical verification of smoking status was performed using expired-air CO at each study visit and at 1- and 3-month follow-up (Smokerlyzer, Bedfont Corp, Medford, NJ).37 If CO was higher than 8 ppm, the participant was conservatively classified as a smoker.38 Program acceptability was measured at the 1-month follow-up visit and included ratings of treatment satisfaction, usefulness of the treatment manual, and whether the program was targeted to their needs (scale 1–10, with higher scores meaning more satisfaction). Treatment elements were evaluated using a 5-point scale (1–5, with higher scores meaning more satisfaction): weekly CO tests, identifying smoking triggers, methods of handling triggers, stress management, cognitive techniques, self-monitoring (‘‘wrap sheets’’), and addressing weight and health concerns. Participants also rated their satisfaction with their smoking cessation counselor using a 5-point Likert scale on the following: competency, communication skills, and overall satisfaction.
Data Analysis
Sample size and power for the study were based on detecting a 20% significant difference in cessation rates between the CTQ-CT and CTQ group in cross-sectional multinomial logistic regression models to show 85% power at p less than .05. We used a comprehensive approach to the analyses of primary and secondary outcomes including running analyses on both the intention to treat and the as-treated samples (ie, including treatment completers only) and running analyses both with and without adjustment for baseline covariates known to be related to the outcome, treatment completion, or found to differ by treatment arm despite randomization. The covariates for adjusted analyses included race/ethnicity, age, gender identity, sexual identity, readiness to quit stage, and menthol cigarette use. All data were analyzed using SAS software (SAS, Cary, NC).39 We examined baseline demographic and smoking variables by treatment condition using descriptive statistics. Similarly, we examined if these variables were associated with treatment completion defined as attending three or more group sessions using independent t tests and Pearson chi-squared statistics. Treatment main effects on the primary and secondary outcomes were examined using bivariate tests at each of the measurement time points (ie, baseline, 1-, 3-, 6-, and 12-month after the program specified quit date). For the primary outcome of 7-day point prevalence smoking, a longitudinal intention to treat analysis with all randomized participants was conducted using a generalized linear mixed model with logit link, with separate intercepts for each participant to account for correlated repeated measurements. Previously, the strategy for a missing smoking cessation measurement was to assume the participant was smoking;40 however, this approach has been criticized on a statistical basis for its potential to increase Type I errors by artificially inflating the sample size and reducing standard errors.41 To bridge the findings of this study with past research, we include the missing = smoking approach in the simple bivariate tests (Table 2) but apply modern missing data methods for our formal analysis. In our formal models of the primary outcome, missing values were handled using an inclusive multiple imputation approach (m = 100 datasets). Results were imputed separately by treatment arm, and all Table 2 variables were included in the multiple imputation procedures to enhance prediction outcomes. Model parameters were pooled across the m = 100 analyses using the multiple imputation analyze procedure utilizing standard errors adjusted for sample fluctuations because of missing data.42,43 Sensitivity analyses were also conducted varying the assumptions about the relationship between missingness and smoking,41 assuming 50%, 70%, and 90% of missing were smoking via different rounding criteria applied to the imputed 7-day point prevalence smoking values estimated assuming a normal variable.42 Secondary outcomes were analyzed using linear covariance pattern regression models essentially applied the full information maximum likelihood approach to missing outcome data.43
Table 2.
Baseline Characteristics by Experimental Group and Treatment Completion
| Characteristic | Experimental group | Treatment completion | ||||
|---|---|---|---|---|---|---|
| Targeted n = 172 | Control n = 173 | p Value | Yes n = 278 | No n = 166 | p Value | |
| Demographic | ||||||
| Age, m (SD) | 38.6 (11.7) | 39.4 (11.3) | .535 | 39.4 (11.6) | 37.5 (11.1) | .091 |
| BMI, m (SD) | 26.9 (6.7) | 27.0 (6.7) | .924 | 26.8 (6.4) | 27.0 (6.5) | .722 |
| Gender identity, n (%) | .850 | .489 | ||||
| Male | 122 (71.3) | 126 (73.3) | 202 (73.2) | 113 (68.1) | ||
| Female | 39 (22.8) | 35 (20.3) | 59 (21.4) | 41 (24.7) | ||
| Transgender | 10 (5.8) | 11 (6.4) | 15 (5.4) | 12 (7.2) | ||
| Sexual identity, n (%) | .231 | .034 | ||||
| Only gay | 104 (60.5) | 120 (69.4) | 185 (66.5) | 88 (53.0) | ||
| Mostly gay | 25 (14.5) | 18 (10.4) | 35 (12.6) | 27 (16.3) | ||
| Bisexual | 32 (18.6) | 22 (12.7) | 43 (15.5) | 35 (21.1) | ||
| Other | 11 (6.4) | 13 (7.5) | 15 (5.4) | 16 (9.6) | ||
| Race, n (%) | .158 | <.0001 | ||||
| White | 92 (53.5) | 113 (65.3) | 174 (62.6) | 62 (37.3) | ||
| Black | 45 (26.2) | 34 (19.6) | 59 (21.2) | 70 (42.2) | ||
| Hispanic | 14 (8.1) | 9 (5.2) | 20 (7.2) | 11 (6.6) | ||
| Other | 21 (12.2) | 17 (9.8) | 25 (9.0) | 23 (13.9) | ||
| Education, n (%) | .982 | <.0001 | ||||
| High school or less | 28 (16.3) | 28 (16.2) | 40 (14.4) | 51 (30.7) | ||
| Trade school/some college | 64 (37.2) | 61 (35.3) | 93 (33.4) | 65 (39.2) | ||
| College degree | 50 (29.1) | 52 (30.1) | 88 (31.6) | 35 (21.1) | ||
| Graduate work | 30 (17.4) | 32 (18.5) | 57 (20.5) | 15 (9.0) | ||
| Income, n (%) | .583 | .017 | ||||
| <20 k | 83 (48.5) | 70 (40.5) | 115 (41.5) | 88 (53.0) | ||
| 20–29 k | 23 (13.4) | 29 (16.7) | 43 (15.5) | 32 (19.3) | ||
| 30–39 k | 22 (12.9) | 21 (12.1) | 38 (13.7) | 13 (7.8) | ||
| 40–49 k | 14 (8.2) | 17 (9.8) | 24 (8.7) | 14 (8.4) | ||
| 50+ | 29 (17.0) | 36 (20.8) | 57 (20.6) | 19 (11.4) | ||
| Insurance, n (%) | .666 | .051 | ||||
| Yes | 124 (72.5) | 129 (74.6) | 209 (75.4) | 111 (66.9) | ||
| No | 47 (27.5) | 44 (25.4) | 68 (24.5) | 55 (33.1) | ||
| HIV status, n (%) | .893 | .065 | ||||
| Positive | 54 (31.4) | 51 (29.5) | 81 (29.1) | 66 (39.8) | ||
| Negative | 103 (59.9) | 105 (60.7) | 171 (61.5) | 85 (51.2) | ||
| Don’t know/refused | 15 (8.7) | 17 (9.8) | 26 (9.3) | 15 (9.0) | ||
| Smoking characteristics | ||||||
| CO level, m (SD) | 23.4 (14.0) | 26.1 (18.7) | .143 | 24.8 (17.4) | 23.5 (13.9) | .367 |
| No. of quit attempts, m (SD) | 4.7 (5.8) | 5.1 (6.3) | .576 | 4.3 (6.0) | 3.4 (4.4) | .064 |
| Had previous quit attempts, n (%) | 150 (87.2) | 148 (85.5) | .623 | 245 (88.1) | 124 (75.1) | .0004 |
| Menthol use, n (%) | 83 (48.5) | 61 (35.3) | .013 | 107 (38.6) | 112 (67.9) | <.0001 |
| Nicotine dependence, m (SD) | 4.3 (2.5) | 4.2 (2.3) | .854 | 4.1 (2.4) | 4.8 (2.5) | .001 |
| Cigarettes per day, m (SD) | 12.1 (7.4) | 13.8 (8.1) | .048 | 12.5 (7.4) | 13.5 (9.2) | .224 |
| Smoking urges, m (SD) | 36.2 (13.5) | 36.2 (13.1) | .990 | 35.2 (13.0) | 39.5 (15.3) | .002 |
| Nicotine withdrawal, m (SD) | 15.8 (9.9) | 16.6 (9.4) | .459 | 16.2 (9.5) | 16.2 (10.1) | .965 |
| Readiness to quit, m (SD) | 7.0 (1.2) | 6.9 (1.1) | .416 | 6.9 (1.1) | 7.0 (1.2) | .633 |
| Abstinence self-efficacy, m (SD) | 17.4 (5.8) | 17.8 (5.5) | .522 | 17.5 (5.6) | 18.5 (6.3) | .075 |
| Perceived risks of quitting, m (SD) | 4.2 (0.8) | 4.2 (0.8) | .973 | 4.2 (0.8) | 4.1 (1.0) | .117 |
| Perceived benefits of quitting, m (SD) | 6.2 (0.6) | 6.2 (0.8) | .994 | 6.2 (0.8) | 6.1 (0.7) | .359 |
BMI = body mass index; CO = carbon monoxide; HIV = human immunodeficiency virus; SD = standard deviation.
Treatment completion was defined as attending three or more treatment sessions. Treatment noncompleters included 99 nonrandomized participants who completed baseline measures and 67 randomized participants who did not complete at least three treatment sessions.
Results
Participants
Baseline demographic characteristics of the study sample are displayed in Table 2. Study participants averaged 12.9 (standard deviation [SD] = 7.7) cigarettes per day with 41.9% reporting smoking a mentholated brand of cigarette. Baseline FTND nicotine dependency scores (M = 4.1) and BQSU smoking urge scores (M = 36.2) indicated a moderate degree of physical dependence. Baseline characteristics differed significantly between the CTQ-CT and CTQ group on number of cigarettes smoked per day (M = 12.1 vs. M = 13.8, p = .048) and menthol cigarette use (M = 48.5 vs. M = 35.3, p = .013). In addition, race also differed when considering the proportion of White participants versus non-White with a larger proportion of White in the control group despite randomization [control: 0.653; target: 0.535; χ2(1) = 5.01. p = 0.025].
Treatment Engagement
Overall rates of treatment engagement including treatment completion, NRT initiation and adherence, and study retention rates did not differ by treatment condition (data not shown). Overall treatment completion rates were high (80%, >3 sessions). Study retention rates were 74.2%, 68.1%, 73.0%, and 66.1% at 1, 3, 6, and 12 months, respectively. The proportion of participants who completed each assessment point was unrelated to treatment condition (ps > .25). Treatment completers differed from noncompleters on sexual identity (p = .034, with higher completion among exclusively LGBT and bisexual), race (p < .0001, higher completion among White and Hispanic), education (<.0001, higher completion with higher education), and income (p = .017, higher completion among >30 k). Noncompleters were more likely to smoke mentholated cigarettes (p = <.0001), to be more nicotine dependent (p < .0001), greater smoking urges (p < .001), and less likely to have a history of quit attempts (p = .0004, see Table 2). More than three-fourths of study participants (77.1%) initiated NRT use during the active treatment period. Of those who initiated NRT, 56% used NRT as recommended by the study nurse.
Primary Smoking Cessation Outcomes
Smoking quit rates did not significantly differ between treatment groups in the unadjusted and adjusted analyses. Biochemically verified 7-day point prevalence quit rates were 31.9% at 1 month and 21.1% at 3 months (see Table 3). Self-reported quit rates were 25.8% at 6 months and 22.3% at 12 months, assuming all missing responses were smoking.39 The agreement between self-reported and CO verified quit rates was 78.9% and 79.8% at 1 and 3 months, respectively, using a CO level greater than 8ppm. When self-report and CO did not agree, self-reported quitting was not verified for 28%, while self-reported smoking was not verified for 15.4% (McNemar’s test S = 3.19, p = .074) at 1 month. This trend was not evident at 3 months (unverified quits 20.2%, unverified smoking 18.9%; McNemar’s test S = 0.78, p = .376). Unverified self-reported smoking was analyzed as smoking in our analyses.
Table 3.
Primary and Secondary Outcomes by Experimental Group and Time Point
| 1 Mo | 3 Mo | 6 Mo | 12 Mo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Targeted n = 133 | Control n = 123 | p Value | Targeted n = 120 | Control n = 118 | p Value | Targeted n = 127 | Control n = 125 | p Value | Targeted n = 114 | Control n = 114 | p Value | |
| Quit rates | 52 (40.3) | 58 (47.1) | .273 | 33 (28.7) | 40 (34.5) | .344 | 37 (29.1) | 52 (41.6) | .038 | 35 (30.7) | 42 (37.2) | .303 |
| Completer quit rates | 52 (44.8) | 56 (47.9) | .642 | 32 (32.6) | 39 (36.4) | .568 | 34 (31.5) | 49 (43.7) | .060 | 30 (31.6) | 38 (37.6) | 0.374 |
| Missing as smoking | ||||||||||||
| Quit ratesa, n (%) | 52 (30.2) | 58 (33.5) | .512 | 33 (19.2) | 40 (23.1) | .371 | 37 (21.5) | 52 (30.1) | .070 | 35 (20.3) | 42 (24.3) | .381 |
| Completer quit rates,an (%) | 52 (38.0) | 56 (39.7) | .763 | 32 (23.4) | 39 (27.7) | .411 | 34 (24.8) | 49 (34.7) | .070 | 30 (21.9) | 38 (26.9) | .327 |
| Secondary outcomes | ||||||||||||
| Nicotine dependence,b m (SD) | 1.4 (1.1) | 1.4 (1.0) | .845 | 2.1 (2.5) | 1.8 (2.2) | .335 | 2.0 (2.2) | 1.7 (2.2) | .207 | 1.9 (2.2) | 2.0 (2.4) | .623 |
| Cigarettes per day,b m (SD) | 2.3 (3.4) | 2.5 (4.1) | .673 | 4.7 (5.4) | 3.9 (5.1) | .229 | 5.1 (5.6) | 5.5 (6.7) | .619 | 6.0 (5.9) | 6.5 (7.8) | .615 |
| Smoking urges, m (SD) | 17.5 (11.5) | 18.2(12.3) | .637 | 19.8 (11.9) | 20.5 (13.4) | .642 | 22.4 (14.4) | 22.2 (14.4) | .895 | 23.8 (15.4) | 21.0 (12.1) | .125 |
| Nicotine withdrawal, m (SD) | 8.5 (9.3) | 8.7 (8.1) | .849 | 8.6 (9.0) | 9.5 (9.2) | .427 | 10.9 (10.1) | 10.6 (9.4) | .819 | 12.0 (10.0) | 12.0 (9.7) | .979 |
| Readiness to quit, m (SD) | 8.5 (1.5) | 8.5 (1.5) | .942 | 8.1 (1.5) | 8.1 (1.6) | .860 | 7.8 (1.5) | 8.1 (1.6) | .129 | 7.7 (1.8) | 7.8 (1.7) | .513 |
| Abstinence self-efficacy, m (SD) | 28.4 (7.0) | 28.6 (7.0) | .822 | 22.5 (7.9) | 23.1 (7.8) | .563 | ||||||
| Risks of quitting,c m (SD) | 3.2 (1.2) | 3.5 (1.3) | .229 | 3.3 (1.2) | 3.5 (1.2) | .240 | ||||||
| Benefits of quitting,c m (SD) | 6.3 (0.6) | 6.0 (1.3) | .143 | 6.1 (1.1) | 5.9 (1.2) | .308 | ||||||
| Weight gain concerns,c m (SD) | 10.0 (4.1) | 10.6 (4.5) | .466 | 10.6 (4.6) | 10.1 (4.0) | .491 | ||||||
SD = standard deviation.
aMissing values for quit rates were coded as “not quit” and sample sizes are equal for all time points (targeted n = 172 and control n = 173; completers: targeted n = 137 and control n = 141).
bNonsmokers included as 0.
cOnly collected for current smokers (1 mo: targeted n = 70 and control n = 60; 3 mo: targeted n = 85 and control n = 73). Results are unadjusted.
Overall quit rates were slightly higher for treatment completers at 1 (38.8%), 3 (25.5%), 6 (29.7%), and 12 months (24.4%). Multiple imputation mixed-effects regression models were used to test treatment differences in smoking quit rates (see Table 4). Odds ratios (95% confidence intervals) for treatment group differences for each assessment period for unadjusted and adjusted models for the intention to treat sample (all randomized) and as-treated sample (all completing three or more sessions) consistently show no treatment group differences with the exception of the 6-month adjusted model for treatment completers, where control participants were more likely to be quit. Sensitivity analyses results, conducted to assess how varying the relationship between missingness and smoking affected our findings, yielded the same statistical conclusions. Bayes factors were calculated for 7-day point prevalence of quitting for the chemically verified measurements at 1 and 3 months. We specified our expected findings assuming a 19% quit rate in the comparison condition, with missing data presumed to be smoking as reported by King et al.25 and a 29% quit rate in the tailored condition. Using a half-normal distribution, the Bayes factors for adjusted models were 0.34 and 0.17 for 1- and 3-month measurements, respectively, with slightly lower estimates for our unadjusted results. These findings show support for the null hypothesis, which the treatment groups were not different based on our assumption that a 10% point advantage for the tailored group was important to demonstrate a superior intervention.
Table 4.
Imputed Mixed-Effects Regression Odds Ratios for Treatment Group Differences in Quit Rates by Time Point
| OR | 95% CI | p Value | |
|---|---|---|---|
| Unadjusted | |||
| 1 mo | 0.74 | (0.29 to 1.87) | .521 |
| 3 mo | 0.61 | (0.22 to 1.68) | .340 |
| 6 mo | 0.40 | (0.15 to 1.07) | .067 |
| 12 mo | 0.62 | (0.23 to 1.69) | .355 |
| Adjusted | |||
| 1 mo | 0.81 | (0.32 to 2.09) | .670 |
| 3 mo | 0.65 | (0.23 to 1.78) | .398 |
| 6 mo | 0.45 | (0.17 to 1.21) | .113 |
| 12 mo | 0.70 | (0.26 to 1.91) | .484 |
CI = confidence interval; OR = odds ratios.
Comparison group is the nontailored intervention. The covariates for adjusted analyses included race/ethnicity, age, gender identity, sexual identity, readiness to quit stage, and menthol cigarette use.
Secondary Smoking Cessation Outcomes
Mixed-effects regression models assessing treatment effects showed no group differences for secondary outcomes or mediators, and groups were combined to examine effectiveness of both interventions (Table 5). Compared with baseline levels, combined results from treatment groups for nicotine dependency, smoking urges, nicotine withdrawal levels, and readiness to quit smoking were significantly improved at end of treatment (1-month outcomes), and these benefits remained improved from baseline across all assessment time points (all ps < .0001). Furthermore, compared with baseline, the daily number of smoked cigarettes per week decreased significantly from baseline to end of treatment (1 month) for both the targeted and nontargeted treatment groups (M = 12.9 vs. M = 2.66, p < .0001) and was maintained at 12 months (M = 12.9 vs. M = 6.54, p < .0001).
Table 5.
Combined Results From Treatment Groups for Secondary Outcomes by Time Point
| Mean | 95% CI | p Value | |
|---|---|---|---|
| Cigarette dependency (n = 345) | |||
| Baseline | 4.24 | (3.99 to 4.50) | reference |
| 1 mo | 1.54 | (1.27 to 1.81) | <.0001 |
| 3 mo | 2.06 | (1.78 to 2.34) | <.0001 |
| 6 mo | 1.89 | (1.63 to 2.16) | <.0001 |
| 12 mo | 2.07 | (1.77 to 2.37) | <.0001 |
| Average cigarettes per day (n = 345) | |||
| Baseline | 12.90 | (12.07 to 13.73) | reference |
| 1 mo | 2.66 | (2.20 to 3.12) | <.0001 |
| 3 mo | 4.56 | (3.91 to 5.20) | <.0001 |
| 6 mo | 5.59 | (4.82 to 6.36) | <.0001 |
| 12 mo | 6.54 | (5.57 to 7.52) | <.0001 |
| Smoking urges (n = 345) | |||
| Baseline | 36.26 | (34.85 to 37.67) | reference |
| 1 mo | 18.55 | (17.11 to 19.99) | <.0001 |
| 3 mo | 20.55 | (19.01 to 22.09) | <.0001 |
| 6 mo | 22.25 | (20.48 to 24.03) | <.0001 |
| 12 mo | 22.27 | (20.42 to 24.12) | <.0001 |
| Nicotine withdrawal (n = 345) | |||
| Baseline | 12.63 | (11.84 to 13.42) | reference |
| 1 mo | 6.85 | (6.03 to 7.67) | <.0001 |
| 3 mo | 7.30 | (6.44 to 8.17) | <.0001 |
| 6 mo | 8.64 | (7.72 to 9.55) | <.0001 |
| 12 mo | 9.17 | (8.19 to 10.15) | <.0001 |
| Readiness to quit (n = 345) | |||
| Baseline | 6.92 | (6.80 to 7.04) | reference |
| 1 mo | 8.37 | (8.18 to 8.55) | <.0001 |
| 3 mo | 8.06 | (7.87 to 8.25) | <.0001 |
| 6 mo | 7.87 | (7.68 to 8.07) | <.0001 |
| 12 mo | 7.69 | (7.47 to 7.92) | <.0001 |
| Abstinence self-efficacy (n = 345) | |||
| Baseline | 18.58 | (17.56 to 19.61) | reference |
| 1 mo | 29.16 | (27.96 to 30.36) | <.0001 |
| 3 mo | 23.88 | (22.60 to 25.16) | <.0001 |
| Perceived risks of quittinga (n = 140) | |||
| Baseline | 3.97 | (3.81 to 4.13) | reference |
| 1 mo | 3.21 | (2.97 to 3.44) | <.0001 |
| 3 mo | 3.21 | (2.99 to 3.43) | <.0001 |
| Perceived Benefits of Quittinga (n = 140) | |||
| Baseline | 6.16 | (6.01 to 6.31) | reference |
| 1 mo | 6.09 | (5.91 to 6.28) | .070 |
| 3 mo | 6.07 | (5.87 to 6.27) | .071 |
| Weight gain concernsa (n = 140) | |||
| Baseline | 11.84 | (11.12 to 12.56) | reference |
| 1 mo | 10.03 | (9.11 to 10.95) | <.0001 |
| 3 mo | 10.17 | (9.27 to 11.08) | <.0001 |
CI = confidence interval.
aQuestionnaire only administered to current smokers. Model only includes participants who were smoking at the 3 mo time point. Results are unadjusted.
Acceptability and Satisfaction
Program evaluations were conducted at the 1-month follow-up assessment. The overall quality of the group facilitation [m(SD) = 4.4(0.8) vs. m(SD) = 4.3(0.8), t(251) = −0.6, p = .102] and overall satisfaction with the program were high [m(SD) = 3.7(0.5) vs. m(SD) = 3.6(0.6), t(224) = −1.8, p = .311] and did not differ by group. However, compared with the standard CTQ invention, individuals in the CTQ-CT intervention rated more highly the effectiveness of the program for assisting with quitting smoking [m(SD) = 3.7(0.5) vs. m(SD) = 3.5(0.7), t(217) = −2.6, p = .011], effectiveness of treatment techniques [m(SD) = 3.9(0.8) vs. m(SD) = 3.6(0.9), t(251) = −2.5, p = .014], and the effectiveness of the treatment manual [m(SD) = 8.3(1.6) vs. m(SD) = 7.2(2.1), t(227) = −5.0, p < .0001]. The culturally targeted intervention was also more highly rated on addressing the unique barriers to smoking cessation faced by the LGBT communities [m(SD) = 3.4(0.8) vs. m(SD) = 2.5(1.2), t(219) = −6.8, p < .0001] and being targeted to the specific needs of LGBT smokers [m(SD) = 8.1(2.2) vs. m(SD) = 5.3(3.5), t(203) = −7.4, p < .0001].
Discussion
The current study represents one of the first studies to report on the outcomes of a randomized clinical trial examining the comparative benefits of a targeted versus nontargeted cognitive-behavioral smoking cessation treatment for LGBT smokers. Results suggest that a group-based cognitive-behavioral intervention combined with NRT has benefit for LGBT smokers. However, contrary to study hypothesis, smoking cessation outcomes associated with the targeted and nontargeted interventions did not differ for either primary or secondary outcomes. In the combined CTQ and CTQ-CT samples, overall quit rates were modest, yet encouraging in both the intent to treat and treatment completers analyses. Intervention benefits were also observed with secondary smoking outcomes. Compared with baseline levels, scores for nicotine dependency, smoking urges, nicotine withdrawal levels, and readiness to quit smoking were significantly improved at the 1-month follow-up (end of treatment) and were maintained across all assessment points. There was also a statistically significant reduction in the number of daily cigarettes smoked. Although there are no safe levels of smoking, reduction in daily numbers of cigarettes smoked has been found to be associated with an increased likelihood of future quit attempts.44
Overall, the study findings suggest that an evidence-based cognitive-behavioral treatment combined with nicotine replacement therapies has benefits for LGBT smokers that are on par with the benefits achieved with other populations of smokers. In this study, overall 12-month quit rates were 22.1%, thus doubling the rates of unassisted quit rates.4 Furthermore, the quit rates achieved in this sample of LGBT smokers were similar to other groups of smokers who received the evidence-based CTQ intervention (19% to 35%).25,28,29 Although the quit rates in this study were modest, they were similar to those reported in other large-scale, community-based smoking cessation programs.4 Notably, these mainstream smoking cessation programs included primarily White smokers with little racial diversity. These results suggest that continued efforts should be made to increase awareness of and access to evidence-based smoking cessation treatments among LGBT smokers.
Study findings also have implications for understanding the feasibility and acceptability of smoking cessation interventions for LGBT smokers. Treatment completion rates were acceptable in this intensive group-based intervention that included smokers with known barriers to treatment completion including racial/ethnic minorities and smokers from lower socioeconomic backgrounds. Eighty percentage of the sample completed at least three sessions or 50% of the overall treatment program. Dose of treatment interventions has been shown to impact treatment outcomes among participants in smoking cessation treatment programs.4 In the current study, overall quit rates were on average 5% points higher for treatment completers compared with noncompleters at all time points. This pattern of better outcomes for more intensive behavioral intervention and use of smoking cessation medication is consistent with the Tobacco Treatment Guidelines.4 Predictors of treatment completion mirrored those of general populations of smokers and included demographic and smoking-related characteristics. Demographic characteristics associated with treatment noncompletion included African American race, bisexually identified participants, low educational attainment, and lower income levels. In addition, smoking-related characteristics included fewer prior quit attempts, higher levels of nicotine dependency, higher smoking urges, and use of mentholated cigarettes. Menthol use is higher among LGBT smokers45 and has been associated with higher levels of nicotine dependency and increased difficulty with smoking cessation.46
There were overall high levels of satisfaction with the intervention and smoking cessation counselors, indicating high levels of treatment acceptability among participants. Although the targeted intervention did not produce better smoking outcomes, consistent with the extant literature, there was a preference for the targeted intervention. Participants in the CTQ-CT were more likely to perceive the intervention to be targeted to the specific needs of LGBT smokers and to address the unique barriers to smoking cessation faced by LGBT communities. Furthermore, compared with the standard CTQ invention, individuals in the CTQ-CT intervention had higher ratings for perceived program effectiveness in terms of assisting with quitting smoking, techniques, and the manual. Overall, both CTQ and CTQ-CT interventions were generally favorably viewed, and this may relate to the fact that groups were held in the same community location and offered by counselors and research staff with high levels of training and cultural competency in working with LGBT individuals. Also, both treatments included only LGBT smokers, and any additional benefit of the targeted curriculum may have been obscured. However, it is unknown whether LGBT smokers are less willing to participate in nontargeted tobacco treatment programs offered by providers without strong linkages to the LGBT community or whether the quit outcomes would be equivalent for community cessation groups that include both LGBT and non-LGBT individuals.
Strengths and Study Limitations
Our study extends the current literature by improving upon the methodological rigor of prior studies including the inclusion of a control group, randomization, recruiting a large and diverse sample of LGBT smokers, conducting assessments of the benefit of the treatment intervention over time, and conducting biochemical verification of self-reported quit rates. Additionally, research staff collecting outcome data were blind to treatment allocations, so outcome ascertainment bias was minimized. The study also helps to establish the feasibility and acceptability of offering intensive group-based smoking cessation treatments for LGBT smokers in a community setting, thus making an important contribution to the scientific literature on smoking cessation interventions for underserved populations of smokers. Study participants were overall a diverse sample of urban LGBT smokers. Despite this diversity, White males were over-represented in this sample. Although study participants, data collection staff, and the data manager were all blinded to treatment condition, it was not possible to blind treatment facilitators. High levels of treatment fidelity were obtained to the core elements of the intervention curriculum in both the targeted and nontargeted groups as monitored by the group cofacilitator using a standardized checklist. However, we did not independently assess intervention fidelity such as rating audiotaped sessions. Furthermore, we did not monitor the degree that LGBT-specific issues were spontaneously raised by participants in the nontargeted groups. Biochemical verification of smoking status was made at the 1- and 3-month follow-up assessments points. To increase longer term retention rates, study participants completed 6- and 12-month assessments either by phone or via an online survey. Individuals who reported being smoke-free were asked to attend an in-person appointment to confirm their smoking status. While the remote assessment approaches for months 6 and 12 increased study retention rates, very few self-reported nonsmokers attended a subsequent smoking verification session. As such, overall quit rates for months 6 and 12 may be somewhat inflated. However, studies suggest that the use of CO also has limitations and that self-report is highly accurate except for some high-risk groups (ie, medical patients).47 These findings were consistent with the self-reported and CO agreement obtain in the present study. The study was conducted at an LGBT-serving institution with highly trained staff. Furthermore, all study participants were LGBT. As such, the level of comfort, cohesiveness, and support may have been enhanced for all participants and not just those in the culturally targeted groups. Additional research is needed to compare our results with treatment outcomes achieved by community cessation groups that include both LGBT and non-LGBT individuals. These limitations notwithstanding, our findings have implications for smoking cessation treatments for LGBT smokers.
Conclusions
In conclusion, this evidence-based cognitive-behavioral smoking cessation intervention was highly feasible, acceptable, and had benefits for LGBT smokers. However, similar to studies involving ethnic minority groups,9,10 there was no clear evidence of a benefit of a targeted intervention in promoting smoking cessation among LGBT smokers. Despite the equivalency in the programs in terms of smoking cessation outcomes, acceptability ratings of the targeted intervention were higher. Additional research is needed to improve upon smoking cessation intervention outcomes for LGBT smokers.
Funding
This research was supported by the National Institute on Drug Abuse (R01 DA023935-01A2, AKM), and The University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS) is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR002003 (Tobacman and Mermelstein, 2016), the NCI P20 CA202908-01 (Winn), and National Cancer Institute grant #P30-CA14599 to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the office views of the National Institutes of Health.
Declaration of Interests
King has consulted with the Respiratory Health Association, a nonprofit organization devoted to lung health in the Chicagoland region and served on a Health Advisory Board for Pfizer Inc. None of the other authors has any conflicts or other financial interests to declare.
Acknowledgments
The authors would like to thank the Howard Brown Health for partnering on this study and the Respiratory Health Association of Chicago for providing staff training in tobacco cessation and the use of the Courage to Quit treatment manual. We would also like to thank Frances Aranda, Kyle Jones, Wendy Choure, and John Cesario for their roles in the successful completion of this study.
References
- 1. Johnson SE, Holder-Hayes E, Tessman GK, King BA, Alexander T, Zhao X. Tobacco product use among sexual minority adults: findings from the 2012-2013 National Adult Tobacco Survey. Am J Prev Med. 2016;50(4):e91–e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jamal A, Phillips E, Gentzke AS, et al. . Current cigarette smoking among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(2):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee JG, Matthews AK, McCullen CA, Melvin CL. Promotion of tobacco use cessation for lesbian, gay, bisexual, and transgender people: a systematic review. Am J Prev Med. 2014;47(6):823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fiore MC, Jaen CR, Baker T, et al. . Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- 5. Graham R, Berkowitz B, Blum R, et al. . The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC: Institute of Medicine; 2011. Accessed February 7, 2018 http://www.nationalacademies.org/hmd/Reports/2011/The-Health-of-Lesbian-Gay-Bisexual-and-Transgender-People.aspx [PubMed] [Google Scholar]
- 6. Lee JG, Blosnich JR, Melvin CL. Up in smoke: vanishing evidence of tobacco disparities in the Institute of Medicine’s report on sexual and gender minority health. Am J Public Health. 2012;102(11):2041–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. U.S. Department of Health and Human Services. Reducing Tobacco Use: A Report of the Surgeon General. Atlanta, GA: USDHHS, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2000. Accessed February 12, 2018 https://www.cdc.gov/tobacco/data_statistics/sgr/2000/complete_report/pdfs/fullreport.pdf [Google Scholar]
- 8. Nierkens V, Hartman MA, Nicolaou M, et al. . Effectiveness of cultural adaptations of interventions aimed at smoking cessation, diet, and/or physical activity in ethnic minorities. A systematic review. PLoS One. 2013;8(10):e73373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu JJ, Wabnitz C, Davidson E, et al. . Smoking cessation interventions for ethnic minority groups—a systematic review of adapted interventions. Prev Med. 2013;57(6):765–775. [DOI] [PubMed] [Google Scholar]
- 10. Webb MS. Treating tobacco dependence among African Americans: a meta-analytic review. Health Psychol. 2008;27(3S):S271–S282. [DOI] [PubMed] [Google Scholar]
- 11. McCabe SE, Hughes TL, Matthews AK, et al. . Sexual orientation discrimination and tobacco use disparities in the United States. Nic Tob Res. 2017. Nicotine & Tobacco Research, ntx283. doi: 10.1093/ntr/ntx283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matthews AK, Vargas M, Kuhns L, Shappiva N, King AC. A qualitative examination of barriers and motivators to smoking cessation among HIV positive African American MSM smokers. J Health Dispar Res Prac. 2014;7(2):4. [Google Scholar]
- 13. Matthews AK, Cesario J, Ruiz R, Ross N, King A. A qualitative study of the barriers to and facilitators of smoking cessation among lesbian, gay, bisexual, and transgender smokers who are interested in quitting. LGBT Health. 2017;4(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matthews AK, Hotton A, Aranda F, Kuhns L, Lee JG, Ross N. Predictors of readiness to quit among a diverse sample of sexual minority male smokers. J Health Dispar Res Pract. 2014;7(5):9. [Google Scholar]
- 15. Stevens P, Carlson LM, Hinman JM. An analysis of tobacco industry marketing to lesbian, gay, bisexual, and transgender (LGBT) populations: strategies for mainstream tobacco control and prevention. Health Promot Pract. 2004;5(3 Suppl):129S–134S. [DOI] [PubMed] [Google Scholar]
- 16. Dickson-Spillmann M, Sullivan R, Zahno B, Schaub MP. Queer quit: a pilot study of a smoking cessation programme tailored to gay men. BMC Public Health. 2014;14(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eliason MJ, Dibble SL, Gordon R, Soliz GB. The last drag: an evaluation of an LGBT-specific smoking intervention. J Homosex. 2012;59(6):864–878. [DOI] [PubMed] [Google Scholar]
- 18. Harding R, Bensley J, Corrigan N. Targeting smoking cessation to high prevalence communities: outcomes from a pilot intervention for gay men. BMC Public Health. 2004;4(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matthews AK, Conrad M, Kuhns L, Vargas M, King AC. Project exhale: preliminary evaluation of a tailored smoking cessation treatment for HIV-positive African American smokers. AIDS Patient Care STDS. 2013;27(1):22–32. [DOI] [PubMed] [Google Scholar]
- 20. Matthews AK, Li CC, Kuhns LM, Tasker TB, Cesario JA. Results from a community-based smoking cessation treatment program for LGBT smokers. J Environ Public Health. 2013;2013:984508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walls NE, Wisneski H. Evaluation of smoking cessation classes for the lesbian, gay, bisexual, and transgender community. J Soc Serv Res. 2011;37(1):99–111. [Google Scholar]
- 22. Covey LS, Weissman J, LoDuca C, Duan N. A comparison of abstinence outcomes among gay/bisexual and heterosexual male smokers in an intensive, non-tailored smoking cessation study. Nicotine Tob Res. 2009;11(11):1374–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grady ES, Humfleet GL, Delucchi KL, Reus VI, Muñoz RF, Hall SM. Smoking cessation outcomes among sexual and gender minority and nonminority smokers in extended smoking treatments. Nicotine Tob Res. 2014;16(9):1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. King A, de Wit H, Riley RC, Cao D, Niaura R, Hatsukami D. Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex differences. Nicotine Tob Res. 2006;8(5):671–682. [DOI] [PubMed] [Google Scholar]
- 25. King AC, Cao D, O’Malley SS, et al. . Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. J Clin Psychopharmacol. 2012;32(5):630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matthews AK, McConnell EA, Li CC, Vargas MC, King A. Design of a comparative effectiveness evaluation of a culturally tailored versus standard community-based smoking cessation treatment program for LGBT smokers. BMC Psychol. 2014;2(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nicoderm CQ. [package insert]. Pittsburgh, PA: GlaxoSmithKline Consumer Healthcare; 2006. [Google Scholar]
- 28. Matthews AK, Sánchez-Johnsen L, King A. Development of a culturally targeted smoking cessation intervention for African American smokers. J Community Health. 2009;34(6):480–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asvat Y, Cao D, Africk JJ, Matthews A, King A. Feasibility and effectiveness of a community-based smoking cessation intervention in a racially diverse, urban smoker cohort. Am J Public Health. 2014;104(Suppl 4):S620–S627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kreuter MW, Lukwago SN, Bucholtz RD, Clark EM, Sanders-Thompson V. Achieving cultural appropriateness in health promotion programs: targeted and tailored approaches. Health Educ Behav. 2003;30(2):133–146. [DOI] [PubMed] [Google Scholar]
- 31. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 32. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief Questionnaire of Smoking Urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 33. Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict Behav. 1990;15(3):271–283. [DOI] [PubMed] [Google Scholar]
- 34. Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365. [DOI] [PubMed] [Google Scholar]
- 35. Menon U, Champion VL, Larkin GN, Zollinger TW, Gerde PM, Vernon SW. Beliefs associated with fecal occult blood test and colonoscopy use at a worksite colon cancer screening program. J Occup Environ Med. 2003;45(8):891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12(2):101. [Google Scholar]
- 37.Smokerlyzer, Bedfond Corporation, Medford, New Jersey. https://www.bedfont.com/shop/smokerlyzer
- 38. Shiffman S, Rolf CN, Hellebusch SJ, et al. . Real-world efficacy of prescription and over-the-counter nicotine replacement therapy. Addiction. 2002;97(5):505–516. [DOI] [PubMed] [Google Scholar]
- 39. SAS Institute. SAS/STAT User’s Guide: Version 6 (Vol. 2). SAS Institute: University of Michigan;1990. [Google Scholar]
- 40. Hughes JR, Novy P, Hatsukami DK, Jensen J, Callas PW. Efficacy of nicotine patch in smokers with a history of alcoholism. Alcohol Clin Exp Res. 2003;27(6):946–954. [DOI] [PubMed] [Google Scholar]
- 41. Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102(10):1564–1573. [DOI] [PubMed] [Google Scholar]
- 42. Enders CK. Applied Missing Data Analysis. New York, NY: Guilford Press; 2010. [Google Scholar]
- 43. Allison PD. Missing data techniques for structural equation modeling. J Abnorm Psychol. 2003;112(4):545–557. [DOI] [PubMed] [Google Scholar]
- 44. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11 Accessed March 2, 2018. [DOI] [PubMed] [Google Scholar]
- 45. Fallin A, Goodin AJ, King BA. Menthol cigarette smoking among lesbian, gay, bisexual, and transgender adults. Am J Prev Med. 2015;48(1):93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Delnevo CD, Gundersen DA, Hrywna M, Echeverria SE, Steinberg MB. Smoking-cessation prevalence among U.S. smokers of menthol versus non-menthol cigarettes. Am J Prev Med. 2011;41(4):357–365. [DOI] [PubMed] [Google Scholar]
- 47. Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull. 1992;111(1):23–41. [DOI] [PubMed] [Google Scholar]