Abstract
At least 30% of all pheochromocytomas (PCCs)/paragangliomas (PGLs) arise in patients with a germline predisposition syndrome. Variants in succinate dehydrogenase subunits A, B, C, and D (SDHA, SDHB, SDHC, and SDHD) are the most common pathogenic germline alterations. Few pathogenic variants have been reported in succinate dehydrogenase assembly factor 2 (SDHAF2). Here, we describe a 30-year-old female patient who presented with a left-sided neck mass, which was later characterized as a carotid body PGL. Genetic testing revealed a likely pathogenic SDHAF2 variant (c.347G>A;p.W116X). Two sisters carried the same pathologic variant, and screening protocols were recommended. Whole-body MRI revealed thyroid nodules; this testing was followed by fine-needle aspiration, which confirmed papillary thyroid carcinoma in one sister and a follicular adenoma in the other. The two sisters then underwent hemithyroidectomy and total thyroidectomy, respectively. Because evidence for pathogenic variants in SDHAF2 causing predisposition to PCC/PGL is limited, we discuss the challenges in mutational variant interpretation and decision making regarding screening for associated tumors.
Keywords: carotid body; paraganglioma; SDHAF2, thyroid; neoplasia
Paragangliomas (PGLs) are derived from paraganglial cells of the autonomous nervous system. Unlike their adrenal counterparts, pheochromocytomas (PCCs) or abdominal PGLs, head and neck PGLs (HNPGLs) are less likely to secrete catecholamines. To date, there are >20 PCC/PGL potential susceptibility genes, with pathogenic variants in the succinate dehydrogenase (SDHx) subunits (SDHAF2, SDHA, SDHB, SDHC, SDHD) accounting for 30% to 54% of all cases. A germline loss-of-function mutation in a highly conserved mitochondrial protein (SDH5) required for SDH-dependent respiration and SDH1 flavination resulted in hereditary PGLs in a previously reported Dutch family [1, 2]. SDH5, now known as SDH complex assembly factor 2 (SDHAF2), has since been reported in a limited number of additional families [3–5]. SDHAF2-related PCCs/PGLs are characterized by low metastatic potential, a higher likelihood of HNPGLs, and paternal inheritance [6]. Here, we present and describe an additional family with a likely pathogenic SDHAF2 variant resulting in an HNPGL.
Informed consent was obtained from all individual participants included in the study. All procedures involving human participants were in accordance with the ethical standards and approved by the institutional review board of the University of Michigan (HUM00043430).
1. Case Description
A. Patient III.2
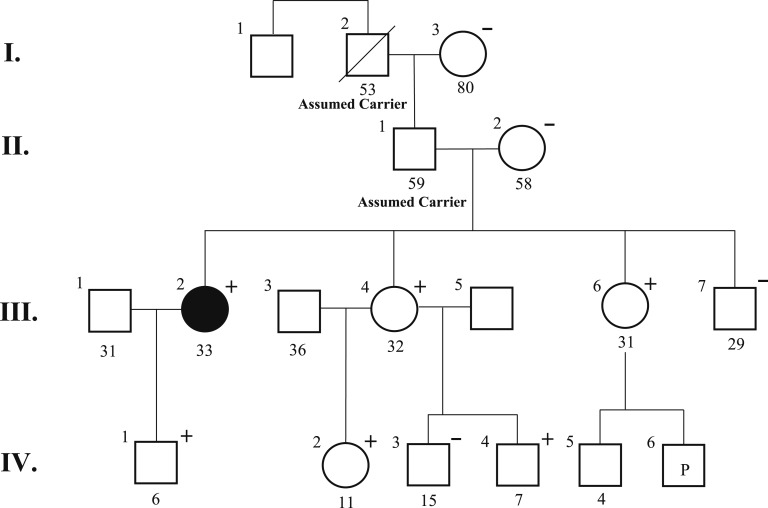
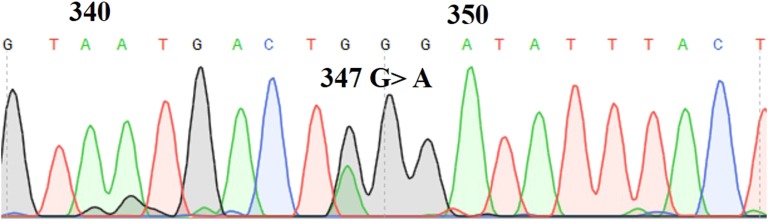
A 30-year-old woman presented with a left-sided neck mass accompanied by headaches, fatigue, and pressure. A CT scan indicated a 3.8 × 2.2-cm mass within the left carotid sheath. MRI showed a 4.5-cm carotid body PGL (Fig. 1A). Plasma metanephrine and normetanephrine levels were within normal limits. A three-generation pedigree (Fig. 2) revealed no family history of PCC/PGLs. Before surgical excision, the patient underwent genetic testing, which included sequencing and deletion/duplication analysis for 10 PCC/PGL susceptibility genes (MAX, NF1, RET, SDHA, SDHAF2, SDHB, SDHC, SDHD, TMEM127, and VHL) via the Ambry Genetics PGLNext panel. Results revealed a likely pathogenic variant in SDHAF2 (c.347G>A;p.W116X). Confirmatory Sanger sequencing of the novel SDHAF2 variant was performed (Fig. 3). She underwent an uncomplicated transcervical excision.
Figure 1.
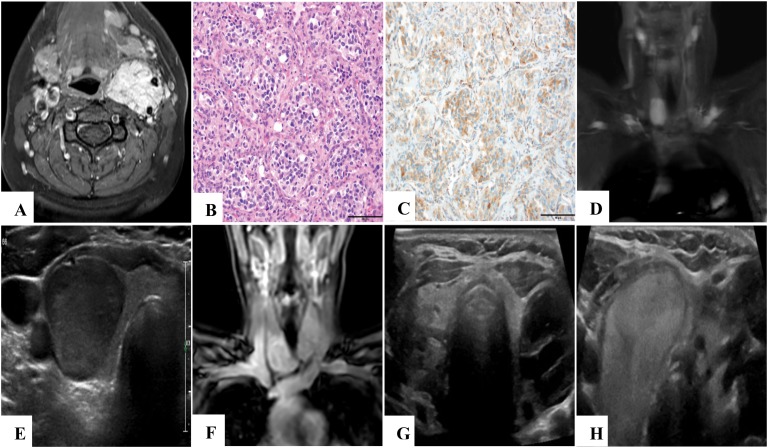
Imaging and SDHB immunohistochemistry findings. T1-weighted fat-saturated axial neck MRI with contrast (A) of patient III.2 revealed a 4.5-cm hypervascular mass in the left carotid sheath splaying the internal and external carotid arteries, consistent with a carotid body paraganglioma. Hematoxylin and eosin–staining (B) and immunohistochemistry (C) show retained SDHB protein expression (original magnification, ×200; scale bars, 100 microns each). In 2015, T2-weighted fat-saturated coronal neck MRI (D) of patient III.4 revealed a 3.0-cm right thyroid mass, which was further characterized by ultrasonography (E) as a hypoechoic solid nodule in the inferior pole of the right thyroid. T1-weighted fat-saturated coronal neck MRI with contrast (F) of patient III.6 in 2015 revealed a potential goiter or bilateral thyroid masses. Definitive characterization with neck ultrasonography showed a large, complex, septated, predominantly cystic nodule in the left lobe (G) and a vascular, solid 3.6 × 2.4 × 3.4-cm nodule (H) in the right lobe.
Figure 2.
Family pedigree.
Figure 3.
Sanger sequencing chromatogram of the novel SDHAF2 variant.
B. Patients III.4 and III.6
A 28-year-old (patient III.4) and 29-year-old (patient III.6) pair of female siblings to patient III.2 were found to carry the familial SDHAF2 variant identified in patient III.2. High-risk screening protocols ensued, which included whole-body MRI and plasma metanephrine and normetanephrine.
In patient III.4, MRI (Fig. 1D) revealed asymmetric enhancement of the thyroid gland and a 5.5-cm uterine fibroid. Ultrasonography confirmed a 1.7 × 1.8 × 2.9-cm hypoechoic solid nodule of the right thyroid gland (Fig. 1E). Ultrasonography-guided fine-needle aspiration cytology indicated a follicular lesion of undetermined significance (Bethesda category 3). She elected to undergo a right hemithyroidectomy for a 2.5-cm papillary thyroid carcinoma. Because pathology indicated low-risk papillary thyroid carcinoma, surveillance without further treatment was recommended.
In Patient III.6, MRI revealed bilateral thyroid region fullness, nodularity, and signal inhomogeneity (Fig. 1F). Ultrasonography showed a 2.1 × 2.2 × 2.7-cm, complex, septated, predominantly cystic nodule of the left lobe and a 3.6 × 2.4 × 3.4-cm vascular, solid nodule of the right lobe (Fig. 1G and 1H, respectively). Fine-needle aspiration revealed nodular hyperplasia on the left and a follicular lesion of undetermined significance on the right. Thyroidectomy revealed a 2.8-cm follicular adenoma on the right.
Through use of a previously validated and published protocol, immunohistochemistry demonstrated retained SDHB protein [7] expression in the PGL from patient III.2 (Fig. 1B and 1C), as well as in the thyroid neoplasms from patients III.4 and III.6 [8] (not shown).
2. Discussion
We describe a family with a CBPGL in the index patient and thyroid neoplasias in two sisters, all of whom are carrying a novel, presumably paternally inherited, SDHAF2 germline variant. The variant is classified as likely pathogenic because it is a nonsense mutation that results in premature protein truncation, affects conserved areas of the gene, is predicted to be deleterious by in silico analysis, and is not present in population databases (ExAC, National Heart, Lung, and Blood Institute Exome Sequencing Project, 1000 Genomes Project). Unfortunately, no data on protein function of the truncated protein variant were available, which would have added substantial evidence in variant classification.
The ClinVar database (accessed 1 September 2018) holds 101 SDHAF2 sequence variants, of which three have been categorized as pathogenic and four as likely pathogenic. Two of the four likely pathogenic variants have conflicting interpretations as a variant of unknown significance. One of the pathogenic variants, c.232G>A, has been associated with SDHAF2-related disease in families of Dutch and Spanish origin, as well as in one Italian individual [1–5, 9]. All patients with this variant presented with HNPGLs without PCCs. A large cohort analysis revealed a single patient from the United States with a CBPGL and vagal PGL carrying the same variant [10]. A literature review revealed c.357_358insT (p.Tyr119LeufsX7), a likely pathogenic mutation (Table 1) [5]. Other variants, such as the c.12C>T in the 3′UTR, likely represent polymorphisms or variants of unknown significance [11].
Table 1.
Reported Likely Pathogenic and Pathogenic SDHAF2 Variants
| Variant | Variant Type | Evidence |
|---|---|---|
| NM_017841.2: c.165G>A (p.Trp55Ter)a | Nonsense | Loss of normal protein function through protein truncation or nonsense-mediated mRNA decay. Not present in population databases and not reported in the literature in individuals associated with SDHAF2-related disease. Cannot rule out possibility of rare benign variant. |
| NM_017841.2: c.177dupT (p.Asp60Terfs) | Frameshift | Premature translational stop signal resulting in an absent or disrupted protein product. Not present in population databases; not reported in the literature in individuals associated with SDHAF2-related disease. |
| NM_017841.2: c.232G>A (p.Gly78Arg) | Missense | Dutch founder mutation with a parent-of-origin inheritance pattern (paternal transmission). Reported to segregate with PGL in multiple families. Functional studies show this variant causes a destabilized SDHAF2 protein and impairs SDHAF2-SDHA interaction. |
| NM_017841.2: c.260+1G>A | Splice site | Sequence change results in alteration of donor splice site in intron 2 that is expected to disrupt RNA splicing and likely results in an absent or disrupted protein product; not reported in individuals with an SDHAF2-related disease. |
| NM_017841.2: c.305_306insA (p.Asn103Glufs) | Frameshift | Premature translational stop signal expected to result in an absent or disrupted protein product; not reported with SDHAF2-related disease. |
| NM_017841.2: c.347G>A (p.Trp116Ter)b | Nonsense | Loss of normal protein function through protein truncation or nonsense-mediated mRNA decay. Not present in population databases and not reported in the literature in individuals associated with SDHAF2-related disease. Variant occurs near 3′ terminus so protein may escape nonsense-mediated mRNA decay. |
| NM_017841.2: c.357_358insT (p.Tyr119Terfs)c | Frameshift | Premature translational stop signal resulting in an absent or disrupted product. Reported in literature in patient with PGL. |
| NM_017841.2: c.371-2A>Ga | Splice site | Affects an acceptor splice site in the last intron (3) of SDHAF2 gene. Not present in population databases and not reported in the literature in individuals associated with SDHAF2-related disease. Likely alters RNA splicing and results in disrupted protein product, but pathogenicity of a splice variant in the last intron is inconclusive because of the uncertain effect on mRNA splicing and protein function. |
Variant also reported as a variant of unknown significance.
Variant discovered in the family of the current report; evidence reported from ClinVar database and testing laboratory.
Variant reported in literature only; not in ClinVar database.
Overall, a cautious review of evidence is required before we can assume association between gene variants and hereditary tumors. The evidence for SDHAF2 as an underlying cause for a hereditary paraganglioma syndrome is based only on a limited number of families and the knowledge that it contributes to the same cellular functions as other well-known pathogenic SDHx genes. In addition, the allele frequency of rare SDHAF2 variants (<0.05%) is 0.4%, and SDHAF2 is loss-of-function tolerant and fairly missense tolerant [12]. However, the high penetrance observed in large pedigrees adds a substantial amount of evidence and makes it unlikely that SDHAF2 is simply coinherited without pathogenicity.
In the present family, the inheritance of the SDHAF2 variant is presumed to be through the paternal lineage as the proband’s mother (II.2) tested negative for the familial variant. Patient III.2 is the only affected individual in a three-generation pedigree. However, there was limited information about paternal relatives, and only the patient’s father (II.1), grandfather (I.2), and great-uncle (I.1) represent potentially at-risk family members. However, none of them were reported to have a phenotype in accordance with hereditary PGL syndrome. Alternatively, the observed variant could represent a low penetrance allele.
Although the family described here had only one HNPGL, both of the proband’s sisters had thyroid neoplasias, which have not been reported in SDHAF2-related PCC/PGL. However, loss of SDHB/C/D gene expression is common in differentiated thyroid cancer, and thyroid cancer has been reported to coexist in patients with SDHB/D-related PCC/PGL [13, 14]. Nevertheless, given the small number of family members, the limited information regarding paternal relatives, and overall rarity of SDHAF2-related PCC/PGLs, we cannot form any conclusions on the association between thyroid neoplasia and an SDHAF2 variant. It remains elusive whether the thyroid neoplasias are truly related to the underlying genetic predisposition or are unrelated incidental findings.
SDHB immunohistochemistry failed to provide further evidence for pathogenic involvement of the germline SDHAF2 variant observed in this family. However, information on SDHB staining in SDHAF2-related paragangliomas is limited; prior studies have been small and yielded inconclusive results [14, 15]. Therefore, the presence of SDHB staining did not influence our decision to regard the germline variant as likely pathogenic.
The Endocrine Society Clinical Practice Guideline on Pheochromocytoma and Paraganglioma notes that all patients with PCC/PGL should consider genetic testing; however, it does not comment on general screening recommendations for unaffected carriers [16]. Because of the limited confirmation of a parent-of-origin effect for SDHAF2, we recommend a limited evaluation by history and physical exam, primarily focusing on the signs and symptoms associated with PCC/PGL for family members with maternal variant inheritance. Additionally, a discussion with the family about increased awareness of potential symptoms should occur. For all carriers who likely inherited the SDHAF2 variant paternally, we continue to recommend annual plasma free metanephrines and biennial whole-body MRI screening. Annual clinic visits are necessary to review variant interpretation and discuss the current evidence for screening protocols.
In summary, we describe a family with anSDHAF2 likely pathogenic variant, few of which have previously been described. Our findings highlight the importance of a thorough variant interpretation of a new germline SDHAF2 variant, while keeping in mind the limited evidence regarding pathogenicity, inheritance, and clinical characteristics of SDHAF2-related hereditary PGL syndrome. Screening carriers with rare variants in SDHAF2 remains individually constructed and should at least focus on symptom monitoring and physical exam.
Glossary
Abbreviations:
- HNPGL
head and neck paraganglioma
- PCC
pheochromocytoma
- PGL
paraganglioma
- SDH
succinate dehydrogenase
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References and Notes
- 1. Hao HX, Khalimonchuk O, Schraders M, Dephoure N, Bayley JP, Kunst H, Devilee P, Cremers CW, Schiffman JD, Bentz BG, Gygi SP, Winge DR, Kremer H, Rutter J. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325(5944):1139–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Baars F, Cremers C, van den Broek P, Geerts S, Veldman J. Genetic aspects of nonchromaffin paraganglioma. Hum Genet. 1982;60(4):305–309. [DOI] [PubMed] [Google Scholar]
- 3. Bayley JP, Kunst HP, Cascon A, Sampietro ML, Gaal J, Korpershoek E, Hinojar-Gutierrez A, Timmers HJ, Hoefsloot LH, Hermsen MA, Suárez C, Hussain AK, Vriends AH, Hes FJ, Jansen JC, Tops CM, Corssmit EP, de Knijff P, Lenders JW, Cremers CW, Devilee P, Dinjens WN, de Krijger RR, Robledo M. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010;11(4):366–372. [DOI] [PubMed] [Google Scholar]
- 4. Kunst HP, Rutten MH, de Mönnink JP, Hoefsloot LH, Timmers HJ, Marres HA, Jansen JC, Kremer H, Bayley JP, Cremers CW. SDHAF2 (PGL2-SDH5) and hereditary head and neck paraganglioma. Clin Cancer Res. 2011;17(2):247–254. [DOI] [PubMed] [Google Scholar]
- 5. Piccini V, Rapizzi E, Bacca A, Di Trapani G, Pulli R, Giachè V, Zampetti B, Lucci-Cordisco E, Canu L, Corsini E, Faggiano A, Deiana L, Carrara D, Tantardini V, Mariotti S, Ambrosio MR, Zatelli MC, Parenti G, Colao A, Pratesi C, Bernini G, Ercolino T, Mannelli M. Head and neck paragangliomas: genetic spectrum and clinical variability in 79 consecutive patients. Endocr Relat Cancer. 2012;19(2):149–155. [DOI] [PubMed] [Google Scholar]
- 6. King KS, Pacak K. Familial pheochromocytomas and paragangliomas. Mol Cell Endocrinol. 2014;386(1-2):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. RRID: AB_2532233, https://antibodyregistry.org/search.php?q=AB_2532233.
- 8. Udager AM, Magers MJ, Goerke DM, Vinco ML, Siddiqui J, Cao X, Lucas DR, Myers JL, Chinnaiyan AM, McHugh JB, Giordano TJ, Else T, Mehra R. The utility of SDHB and FH immunohistochemistry in patients evaluated for hereditary paraganglioma-pheochromocytoma syndromes. Hum Pathol. 2018;71:47–54. [DOI] [PubMed] [Google Scholar]
- 9. Mariman EC, van Beersum SE, Cremers CW, Struycken PM, Ropers HH. Fine mapping of a putatively imprinted gene for familial non-chromaffin paragangliomas to chromosome 11q13.1: evidence for genetic heterogeneity. Hum Genet. 1995;95(1):56–62. [DOI] [PubMed] [Google Scholar]
- 10. Bausch B, Schiavi F, Ni Y, Welander J, Patocs A, Ngeow J, Wellner U, Malinoc A, Taschin E, Barbon G, Lanza V, Söderkvist P, Stenman A, Larsson C, Svahn F, Chen JL, Marquard J, Fraenkel M, Walter MA, Peczkowska M, Prejbisz A, Jarzab B, Hasse-Lazar K, Petersenn S, Moeller LC, Meyer A, Reisch N, Trupka A, Brase C, Galiano M, Preuss SF, Kwok P, Lendvai N, Berisha G, Makay Ö, Boedeker CC, Weryha G, Racz K, Januszewicz A, Walz MK, Gimm O, Opocher G, Eng C, Neumann HPH; European-American-Asian Pheochromocytoma-Paraganglioma Registry Study Group. Clinical characterization of the pheochromocytoma and paraganglioma susceptibility genes SDHA, TMEM127, MAX, and SDHAF2 for gene-informed prevention. JAMA Oncol. 2017;3(9):1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casey R, Garrahy A, Tuthill A, O’Halloran D, Joyce C, Casey MB, O’Shea P, Bell M. Universal genetic screening uncovers a novel presentation of an SDHAF2 mutation. J Clin Endocrinol Metab. 2014;99(7):E1392–E1396. [DOI] [PubMed] [Google Scholar]
- 12. Newey PJ, Berg JN, Zhou K, Palmer CNA, Thakker RV. Utility of population-level DNA sequence data in the diagnosis of hereditary endocrine disease. J Endocr Soc. 2017;1(12):1507–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ni Y, Seballos S, Ganapathi S, Gurin D, Fletcher B, Ngeow J, Nagy R, Kloos RT, Ringel MD, LaFramboise T, Eng C. Germline and somatic SDHx alterations in apparently sporadic differentiated thyroid cancer. Endocr Relat Cancer. 2015;22(2):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papathomas TG, Gaal J, Corssmit EP, Oudijk L, Korpershoek E, Heimdal K, Bayley JP, Morreau H, van Dooren M, Papaspyrou K, Schreiner T, Hansen T, Andresen PA, Restuccia DF, van Kessel I, van Leenders GJ, Kros JM, Looijenga LH, Hofland LJ, Mann W, van Nederveen FH, Mete O, Asa SL, de Krijger RR, Dinjens WN. Non-pheochromocytoma (PCC)/paraganglioma (PGL) tumors in patients with succinate dehydrogenase-related PCC-PGL syndromes: a clinicopathological and molecular analysis. Eur J Endocrinol. 2013;170(1):1–12. [DOI] [PubMed] [Google Scholar]
- 15. Papathomas TG, Oudijk L, Persu A, Gill AJ, van Nederveen F, Tischler AS, Tissier F, Volante M, Matias-Guiu X, Smid M, Favier J, Rapizzi E, Libe R, Currás-Freixes M, Aydin S, Huynh T, Lichtenauer U, van Berkel A, Canu L, Domingues R, Clifton-Bligh RJ, Bialas M, Vikkula M, Baretton G, Papotti M, Nesi G, Badoual C, Pacak K, Eisenhofer G, Timmers HJ, Beuschlein F, Bertherat J, Mannelli M, Robledo M, Gimenez-Roqueplo AP, Dinjens WN, Korpershoek E, de Krijger RR. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: a multicenter interobserver variation analysis using virtual microscopy: a Multinational Study of the European Network for the Study of Adrenal Tumors (ENS@T). Mod Pathol. 2015;28(6):807–821. [DOI] [PubMed] [Google Scholar]
- 16. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K Young WF Jr; Endocrine Society. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942. [DOI] [PubMed] [Google Scholar]