Abstract
The fusion by exocytosis of many vesicles to the plasma membrane induces the discharge to the extracellular space of their abundant luminal cargoes. Other exocytic vesicles, however, do not contain cargoes, and thus, their fusion is not followed by secretion. Therefore, two distinct processes of exocytosis exist, one secretory and the other non-secretory. The present review deals with the knowledge of non-secretory exocytosis developed during recent years. Among such developments are the dual generation of the exocytic vesicles, initially released either from the trans-Golgi network or by endocytosis; their traffic with activation of receptors, channels, pumps, and transporters; the identification of their tethering and soluble N-ethylmaleimide-sensitive factor attachment protein receptor complexes that govern membrane fusions; the growth of axons and the membrane repair. Examples of potential relevance of these processes for pathology and medicine are also reported. The developments presented here offer interesting chances for future progress in the field.
Keywords: exocytosis, Golgi complex, vesicles, endocytosis
Introduction
In cell biology, exocytoses are the processes by which cytoplasmic vesicles and granules fuse with the plasma membrane, either spontaneously (constitutive exocytosis) or in response to cell stimulation (regulated exocytosis). Such fusions of vesicles and granules can be followed by the discharge to the extracellular space of the abundant cargoes segregated within their lumen. Because of the latter property, exocytoses are often defined as secretory processes (Sudhof, 2004; Rindler et al., 2007; Thorn et al., 2016). Such definition, however, does not cover all types of exocytosis. Studies started a few decades ago demonstrated, in fact, that among vesicles competent for fusion with the plasma membrane only some are equipped with abundant secretory cargoes, while many others contain only few luminal molecules or completely lack them. Therefore, the plasma membrane fusions of the latter vesicles are not followed by abundant secretion.
For many years, the plasma membrane fusions without secretion were interpreted as processes distinct from exocytosis. Processes of this type, reported in fibroblasts and other cells, were often induced by Ca2+ influx taking place upon surface cell lesion (Steinardt et al., 1994; Miyake and McNeil, 1995; Chieregatti and Meldolesi, 2005). More recently, although expressed by various types of cells, including neurons, macrophages, adipocytes, duct and parietal cells, and reported in organs such as brain, glands, muscle, kidney, stomach (Pfenninger and Friedman, 1993; Holman, 1994; Yeh et al., 1995; Schenk et al., 2003; Watson et al., 2003; Chieregatti and Meldolesi, 2005), these processes were often considered distinct from each other, not only in structure but also mechanistically and in their functional role. The present and other studies (Pfenninger and Friedman, 1993; Holman, 1994; Steinardt et al., 1994; Miyake and McNeil, 1995; Yeh et al., 1995; Gouraud et al., 2002; Clapham, 2003; Schenk et al., 2003; Watson et al., 2003; Uldry et al., 2004; Chieregatti and Meldolesi, 2005), anticipating the concept of peculiar forms of exocytosis, can be envisaged as precursors. In 2002, our discovery of a new plasma membrane fusion vesicle lacking secretion, the enlargeosome (Cocucci et al., 2004), suggested the possibility that the various non-secretory fusions are not distinct processes, but share some critical properties common also to exocytosis rich of secretion. In a subsequent review, we proposed exocytosis to include two families of processes, named secretory and non-secretory exocytosis, characterized by high and low (or absent) degrees of secretion (Chieregatti and Meldolesi, 2005).
Upon our previous review, the investigation of the two families of exocytosis has been developed further. Distinct new information about generation, transport, properties, and functions of the organelles involved have become available. The reinforced and expanded knowledge has been largely that of non-secretory exocytosis. Although distinct in many, but not all, respects from secretory exocytosis, these processes are also relevant. We have therefore decided to reconsider and reorganize in a new review the available information about non-secretory exocytosis. The present review reports about recent developments of the latter processes that took place in cell biology, physiology, and relevant aspects of pathology and medicine.
General organization of the review
The present work on non-secretory exocytosis, accumulated during the last few years, includes two groups of processes, distinct for their generation and function.
The vesicles of the first group are generated by release from the trans-Golgi network (TGN) (Gu et al., 2016; Pick et al., 2017; Roth et al., 2017). Exocytosis of additional non-secretory vesicles, generated from intracellular organelles other than the TGN, has not been demonstrated, however it cannot be excluded. Upon their first exocytosis, non-secretory vesicles are endocytized, and their traffic is prosecuted by recycling from the plasma membrane. The main task of these vesicles is the transport of specific membrane proteins, synthesized in the endoplasmic reticulum (ER), to critical sites of the cell surface. Transported proteins include subunits and/or whole proteins of receptors, channels, pumps, transporters, and others (Flowerdew and Burgoyne, 2009; Dumoulin et al., 2011; Luscher et al., 2011; Tanaka and Hirano, 2012; Jurado et al., 2013; Martinez-Mamol et al., 2013; Hussain and Davanger, 2015; Gu et al., 2016; Gu and Huganir, 2016; Hussain et al., 2016; Langlhofer and Villmann, 2016; Meneses et al., 2016; Vien et al., 2016; Jaldin-Fincati et al., 2017; Klemens et al., 2017; Pick et al., 2017; Roth et al., 2017; Wu et al., 2017).
Generation of the vesicles in place directly by endocytosis at the plasma membrane. Upon generation, also these vesicles undergo exocytosis followed by recycling. Examples of the two groups of these non-secretory vesicles are shown in Table 1.
Table 1.
Examples of non-secretive vesicles and their exo/endocytic processes.
| Markers/processesa | Sites of generationb | Endocytic recyclingc | Exocytic SNAREs | Locationd | Function |
|---|---|---|---|---|---|
| Aa. GluA2 subunit of resting AMPA | TGN | + | VAMP2, SNAP25, Stx1 | Synapses | Receptor |
| Ab. GluA1 subunit of stimulated AMPA | TGN | + | VAMP3, SNAP47, Stx3 | Synapses | Receptor |
| B. KV1.3 | TGN | −−− | VAMP7, Vtil | Synapses | Voltage channel |
| C. GLUT4 | TGN | +++ | VAMP2, SNAP23, Stx4 | Muscle fibers, adipocytes, other cell types | Glucose transport |
| D. Endocytosis | Plasma membrane | VAMP4 and 3, Stx6 and 13 | All cells | Exocytosis | |
| E. Axon growth | Plasma membrane | VAMP4, SNAP23, Stx6 | Axonal growth cones | Surface expansion | |
| F. Plasma membrane repair | Plasma membrane | −−− | wounds in all cells | Uptake of wounds |
aA–C show markers of vesicles of the first group; Aa and Ab refer about subunits of the same receptor before and after stimulation. D–F show processes where vesicles of the second group are involved.
bThe distinct type of generation is the property that distinguishes the vesicles of the first and second groups.
cThe endocytic recycling is typical of the first group vesicles. The number of + indicates the intensity of such recycling. −−− means that recycling has not been demonstrated for a voltage channel (B), or no SNAREs for membrane repair (F).
dLocation means cells or cell areas where the various vesicles operate.
Distribution of non-secretory processes occurs at critical sites of the plasma membrane area or at the whole cell surface. Exocytosis of both vesicle groups induces surface enlargements followed by surface reductions by endocytosis (Doherty and McMahon, 2009; De Curtis and Meldolesi, 2012; Chamberland et al., 2016; Chiu et al., 2017; Fujii et al., 2017; Lesteberg et al., 2017). The general properties of all processes anticipated here are presented in detail in the corresponding sections and summarized critically in the Conclusions section.
Generation and initial traffic of the first group of non-secretory vesicles
Several properties of this group of non-secretory vesicles are generally similar while others are different (see the examples in Table 1). Appearance of their specific proteins in the Golgi complex, with concentration in the TGN, reveals that these vesicles assemble in parallel to secretory organelles. The co-transport of proteins destined to secretory and non-secretory vesicles, however, does not affect their specificity. Along this pathway, in fact, generation and maintenance of distinct specificities take place not only for secretory and non-secretory vesicles but also for other forms of vesicles, including the constitutive vesicles of exocytosis. Therefore, the initial co-existence of proteins destined to secretory and non-secretory vesicles does not result in the induction of mixtures (Hussain and Davanger, 2015; Gu et al., 2016; Roth et al., 2017).
For some non-secretory vesicles, generation from the TGN was demonstrated many years ago. For other vesicles, the same generation has been recognized, however only recently. Such difference was due, at least in part, to the different balance between generation and subsequent recycling typical of the latter vesicles. When recycling is limited, the recently generated vesicles predominate. When, in contrast, predominance is of recycled vesicles, the recently generated vesicles are outnumbered and hard to distinguish (Hussain and Davanger, 2015; Gu et al., 2016; Pick et al., 2017; Roth et al., 2017). The first part of this presentation starts with non-secretory vesicles predominant upon their release from the TGN. Other vesicles, present in lower amounts, are mentioned later.
Ionic receptor subunits of neurones
A frequent property of non-secretory vesicles released from the TGN of neurons consists in their transport of key receptor channel subunits to critical sites of the postsynaptic plasma membrane. Upon their synthesis in the ER, the subunits of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, a stimulatory receptor of glutamate, traffic along Golgi cisternae concentrating at membrane sites coated by the coatomer protein 2 (COPII) (Pick et al., 2017). Upon their accumulation in the TGN, each subunit is released, concentrated at the membrane of distinct, non-secretory vesicles, which navigate through the cytoplasm. The assembly and release of the subunits to the cytoplasm contribute to relevant properties of the receptor, such as distribution, activation, regulation, and turnover.
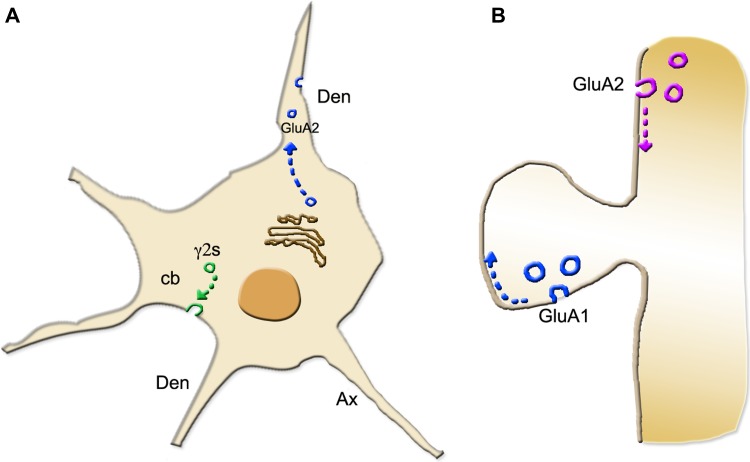
In resting hippocampal pyramidal neurons, the activity of postsynaptic AMPA receptors is small and stable. In these conditions, subunits such as GluA2 of AMPA receptor, and γ2S of gamma-aminobutyric acid inhibitory receptor A (GABAA), emerge from the TGN within distinct constitutive vesicles. These vesicles traffic separately, addressed to different areas of the plasma membrane, located at dendrites and cell body, respectively (Gu et al., 2016; Roth et al., 2017) (Figure 1A). These constitutive vesicles differ not only for their traffic but also for the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes of their exocytosis. The complex of GluA2-specific vesicles is known since long to include the vesicle-associated membrane protein 2 (VAMP2), synaptosome-associated protein 25 (SNAP25), and syntaxin 1 (Stx1), three SNAREs analogous to those of the complex that induces the regulated secretory exocytosis of synaptic vesicles. In contrast, the SNARE complex specific of γ2S subunit is not entirely analogous. Together with VAMP2 and Stx1, this complex includes SNAP23 instead of SNAP25 (Hussain and Davanger, 2015; Gu et al., 2016). In the postsynaptic compartment of resting neurons, therefore, traffic and exocytic fusions specific for GluA2- and γ2S-specific vesicles are clearly different.
Figure 1.
Transport and non-secretory exocytosis of vesicles positive for receptor subunits in neurons. (A) The traffic of two receptor subunits, GluA2 of the stimulatory AMPA receptor and γ2S of the inhibitory GABAA receptor, in resting hippocampal pyramidal neurons. The two subunits, released from the TGN in distinct vesicles (blue and green), move to different sites of the plasma membrane: dendrites (den) for GluA2; the cell body (cb) for γ2S. The subsequent exocytic fusion of the two subunit-positive vesicles occurs with SNARE complexes different for t-SNARE, SNAP25, and SNAP23, respectively. (B) The traffic of two AMPA subunits in the same dendrite, taking place during stimulation. GluA1 (blue), transported by a vesicle addressed to the dendritic spine near the dense protein complex, moves rapidly to the postsynaptic membrane. GluA2 (violet), which has been transported by a vesicle addressed to a dendritic site away from the spine, moves along the plasma membrane towards the spine, reaching it, however, after some time. Interestingly, the SNARE complex used by GluA-positive vesicles in stimulated neurons is different from the complex used by GluA-positive vesicles in the same neurons at rest.
What happens in the postsynaptic compartment of the same neurons upon stimulation? Three AMPA receptor subunits, GluA1, GluA2, and GluA3, traffic via distinct vesicles of the regulated type. Comparatively, these vesicles are brighter, less frequent and slower than the constitutive GluA2-specific vesicles of resting neurons (Hussain and Davanger, 2015; Gu et al., 2016; Roth et al., 2017). Interestingly, the GluA1-specific vesicles are targeted directly to dendritic spines, where exocytosis occur at sites adjacent to synaptic densities (Figure 1B). In contrast, the vesicles specific for GluA2 and GluA3 are targeted to dendritic plasma membrane sites away from the spines. In order to participate in the assembly of the AMPA receptor, therefore, the latter subunits have to slip along the plasma membrane, reaching the spines with a significant delay (Gu et al., 2016; Roth et al., 2017) (Figure 1B). The exocytosis of the non-secretory vesicles specific for the various regulated AMPA receptor subunits is governed by the same SNARE complex, including VAMP2 together with SNAP47 and Stx3, two t-SNAREs different from the SNAP25 and Stx1 of resting vesicles (Gu et al., 2016; Roth et al., 2017). Interestingly, the SNARE complex including SNAP47 and Stx3 operates for plasma membrane fusion of GluA1-specific vesicles also in the course of LTP processes (Tanaka and Hirano, 2012; Jurado et al., 2013; Wu et al., 2017).
Recently, properties analogous to those discovered for AMPA receptor subunits have been reported also for subunits of another glutamate receptor, the N-methyl-D-aspartate (NMDA) receptor. Also in this case, the SNARE complexes that govern the exocytosis of resting and stimulated vesicles are different (Gu and Huganir, 2016; Hussain et al., 2016). The vesicles of subunits of other receptors, the inhibitory GABAA and glycine receptors, are also involved in non-secretory exocytosis, analogous to those of glutamate receptors (Dumoulin et al., 2011; Luscher et al., 2011; Langlhofer and Villmann, 2016; Vien et al., 2016). In neurons, therefore, the specific roles of the receptors and their subunits strongly suggest that the exocytosis of many non-secretory vesicles, distinct in terms of specificity, participates in distinct physiological functions.
Channels
The physiology of many cells depends on ion fluxes induced by the activation of their channels. However, the intracellular pathways of many such channels, including their traffic to the plasma membrane, are still unclear. Here we present an example of voltage-gated channels transported by non-secretory vesicles. Two K+ channels, Kv1.3 and Kv4, traffic within neurons and other cell types by small vesicles moving from the ER to the Golgi complex. Vesicles analogous to those of the ER–Golgi traffic reach the presynaptic plasma membrane and undergo a non-secretory exocytosis regulated by a SNARE complex including the v-SNARE VAMP7 (also known as Tetanus toxin-insensitive VAMP, Ti-VAMP) together with the t-SNARE Vti1 (Martinez-Mamol et al., 2013; Meneses et al., 2016). This SNARE complex is distinct from the complexes already described for other non-secretory exocytosis (Flowerdew and Burgoyne, 2009).
Additional non-secretory vesicles participate in the transport of other types of channel. Upon their generation at the TGN, the vesicles of epithelial sodium channels (ENaCs), the channel of Na+ resorption across tight epithelia, accumulate in the cytoplasm during recycling. For their targeting, the newly generated forms of these vesicles do not require the support of cytoskeletal proteins. In contrast, cytoskeletal proteins are needed for recycling of these vesicles (Klemens et al., 2017). Analogous dependence may exist for vesicles specific for other channels such as the Cl− channel cystic fibrosis transmembrane conductance regulator (CFTR) and aquaporin2. The latter is a water channel present in the kidney and other organs. Similar generation appears to operate for transporters, in particular for glucose transporter-4 (GLUT4) (Jaldin-Fincati et al., 2017), whose dynamic vesicles operate under insulin regulation.
Recycling of non-secretory vesicles generated at the TGN
The non-secretory processes, initiated by vesicle generation at the TGN, are then operated by endocytic recycling. The two subsequent processes, generation and operation, can differ not only in the two starting steps. Often, in fact, they include additional distinct events. Upon endocytosis, many non-secretory vesicles of the first group are heterogeneous. They recycle, in fact, from distinct cups of the plasma membrane: clathrin, caveolae, flotillin and other operative/marker proteins, associated to their specific membrane proteins. In the cytoplasm, the endocytic vesicles undergo repeated events, including homotypic and heterotypic fusions (Doherty and McMahon, 2009; De Curtis and Meldolesi, 2012; Chamberland et al., 2016). Due to the complexity of their intracellular life, the targets of these vesicles are variable. Some vesicles fuse with lysosomes, and thus undergo digestion, while other vesicles undergo rapid fusion with the plasma membrane, with ensuing expansion of the cell surface (Figure 2A). Here we will consider non-secretory vesicles including in their membrane important proteins of receptors, channels, transporters, pumps. Upon their first exocytosis, these vesicles keep on by their recycling. Thus, they operate by multiple cycles of exo/endocytosis. The differential properties observed with these processes depend on both the specificity of their vesicles and the nature of the involved cells.
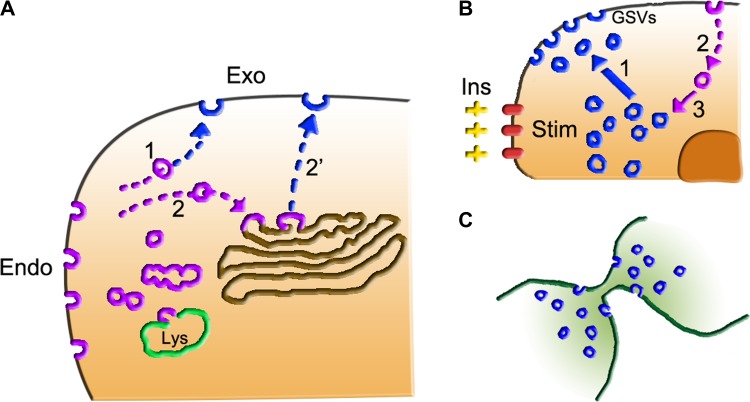
Figure 2.
Non-secretory exocytosis of endocytic origin. (A) After endocytosis (Endo, violet), most endocytic vesicles and tubules are spread in the cytoplasm, where some of them fuse with lysosomes (Ly, green). A fraction of these organelles, however, proceeds to the plasma membrane, either directly (1) or after fusion with the TGN (2), by processes designed as recycling. The exocytic fusion (Exo, blue) of recycled organelles takes place by two alternative SNARE complexes. (B) Events that appear in adipocytes and muscle fibers upon stimulation (stim) by insulin (Ins). A population of GSV vesicles (blue), enriched in the transporter GLUT4, is concentrated near the nucleus of resting cells. After insulin stimulation, the GSVs move (1) to the proximity of the plasma membrane. The ensuing GSV exocytosis (blue) induces great increase of GLUT4 at the cell surface, with ensuing marked increase of glucose uptake by the cell. The exocytized GLUT4-rich vesicles are then taken up by the endocytosis process of the cell (violet, 2) followed by the conversion of endosomes into GSVs (3), the vesicles ready to be re-exocytized upon continued or re-stimulation of the cell by insulin. (C) The role of non-secretory exocytosis in cytokinesis, the final step of mitosis. This process, resulting in the detachment of the two daughter cells, requires the participation of many vesicles (blue) of endocytic origin, which are first concentrated in the cleavage furrow along the central spindle of the cell, and then fuse locally to the plasma membrane.
Ionic receptors and channels
In neuronal cells, non-secretory vesicles, addressed to ionic receptors, navigate after their TGN generation. Some recycling, documented by a number of recent demonstrations, is important for these receptors (Chiu et al., 2017; Fujii et al., 2017; Lesteberg et al., 2017). Many types of channel, in particular voltage-gated channels, do not operate by recycling of non-secretory vesicles. In contrast, vesicle transport takes place for other channels, distinct in structure and function. Three such channels have already been mentioned for the TGN generation of their vesicles. Here we report about the recycling of the vesicles specific for these channels.
ENaCs are abundant in endocytic vesicle pools adjacent to the apical plasma membrane, regulated by a small GTPase, Rab11b. In several epithelial cell types, the specific accumulation of ENaC-rich vesicles in apical cytoplasmic pools depends on their binding to the cytoskeletal protein ankyrin G (Klemens et al., 2017). Stimulated, non-secretory exocytosis of these vesicles, triggered by hormones active via cyclic AMP (cAMP), induce transfer of ENaC to the apical plasma membrane. After reduction of stimulation, ENaC-rich vesicles recycle by endocytosis and accumulate in cytoplasmic pools near the cell surface (Butterworth et al., 2012; Edinger et al., 2012).
CFTR, crucial for ion and water transport in epithelial cells, moves by cytoplasmic vesicle, reaching the apical plasma membrane by exocytosis controlled by cAMP active via protein kinase A (PKA) type II. Backward transport of the vesicles occurs when cAMP declines (Tang et al., 2011; Cihil et al., 2012; Zinn et al., 2015). Exocytosis of CFTR vesicles occurs by SNARE complexes including SNAP23 together with Stx1A (Tang et al., 2011).
Another channel, that resembles in function and regulation both ENaC and CFTR, is aquaporin2, a water channel present in the kidney and other organs. This channel is stimulated by cAMP, which drives vesicle exocytosis at the apical plasma membrane. At the baso-lateral plasma membrane some fusion can occur, however only without the cAMP increase (Rice et al., 2015). The surface deployment of the aquaporin vesicles depends on another v-SNARE, VAMP8 (Wang et al., 2010).
ATPase pumps
In resting cells of various organs, ATPases of endocytic vesicles and tubules are associated to adjacent transporters. Upon stimulation, these vesicles increase their apical non-secretory exocytosis, with ensuing changes of pH in the extracellular tubules. In gastric parietal cells, the H+, K+-ATPase vesicles accumulate the K+-Cl− cotransporter chlorochloro-5 (ClCl-5), and then move to the plasma membrane. By Cl− release, this transporter contributes to the acidity of the gastric lumen (Takahashi et al., 2014). Analogous interaction occurs in the A-type intercalated cells scattered at the renal collecting duct surface. During transport of vesicles, the H+-ATPase pump associates the kidney/brain anion transporter (KBAT) and thus strengthens their non-secretory exocytosis (Xu et al., 2011).
Transporters
In addition to those associated to ion pumps, endocytic transporters exist and operate independently. Among these transporters, the best known is GLUT4 (Figure 2B), initially released within specific non-secretory vesicles generated at the TGN. The main effect of GLUT4, the intracellular accumulation of glucose accompanied by its progressive decrease in the extracellular space, is due almost exclusively to their recycling under the control of insulin (Beaton et al., 2015; Fazakerley et al., 2015; Brewer et al., 2016; Sun et al., 2016; Hatakeyama and Kanzaki, 2017; Jaldin-Fincati et al., 2017; McNally et al., 2017; Pan et al., 2017; Uhm et al., 2017; Zhou et al., 2017; Li et al., 2018; Pearson-Leary et al., 2018). At rest, many cells, especially muscle fibers and adipocytes, contain large populations of recycling vesicles, the GLUT4-positive vesicles (GSVs), distributed in the cytoplasmic area proximal to nuclei. Fewer vesicles of this type are present in a variety of other cell types, including heart and smooth muscle fibers, neurons, and hepatocytes. Accurate analyses have revealed the role of the GTPase Rab11 in early steps of GSV transport. Further steps of this transport are controlled by other types of GTPases, Rab8A, Rab13, and Rab14 (Jaldin-Fincati et al., 2017; Li et al., 2018). Insulin treatment induces in GSVs the activation of two signals identified recently: phosphorylation of the Exo84 protein, a component of the exocyst tethering complex, by the TANK-binding kinase 1 (TBK1); and facilitation of GLUT4 recycling by the tumor suppressor candidate 5 (TUSC5), during prolonged stimulation (Beaton et al., 2015; Fazakerley et al., 2015; Jaldin-Fincati et al., 2017; Uhm et al., 2017). Upon docking and subsequent tethering by the exocyst complex (Sun et al., 2016), GSVs undergo a regulated, non-secretory exocytosis dependent on both Rab13 and Rab28 (Sun et al., 2016; Zhou et al., 2017), operated by the SNARE complex including the v-SNARE VAMP2 associated to the t-SNAREs SNAP23 and Stx4 (Jaldin-Fincati et al., 2017). Such exocytosis is interesting also in the brain. In hippocampal neurons, the GLUT4 stimulation by insulin induces a relevant increase of the spatial working memory (Pearson-Leary et al., 2018).
Following their relatively long stay at the plasma membrane, the exocytized GLUT4s move to endocytic cups, where they accumulate. The retrograde transport of the endocytic vesicles occurs by heterotypic fusions (Hatakeyama and Kanzaki, 2017). The concomitant interaction with sorting proteins, sortilin, retriever, and retromer, favors recycling and rescues the vesicles upon their possible fusion with lysosomes (McNally et al., 2017; Pan et al., 2017). The final target of the vesicle traffic is a population of endosomes, slowly converted to GSVs by an insulin-sensitive process dependent on Rab14 (Brewer et al., 2016) (Figure 2C). A proteomic analysis, made during the conversion of endocytic vesicles to GSVs, revealed an enrichment of over 50 proteins (Fazakerley et al., 2015). Such analysis confirms the molecular specificity of GSVs. In addition, conversions such as those from endosomes to GSVs, may occur also with other non-secretory vesicles during their post-exocytic recycling.
Non-secretory exocytosis of the second group
The vesicles of this group differ in some respects from those of the first group. Their generation occurs not by release from the TGN but by endocytosis at the plasma membrane (see the examples in Table 1). The task of these vesicles does not depend only on the transfer of critical membrane subunits/proteins to the plasma membrane but include changes in the cell surface area. For this task, vesicles of the second group can be larger than those of the first group. The group includes also vesicles of small size involved in specific functions (an example in Figure 2C). Recent progresses about these vesicles, however, have been limited. In this review, therefore, their presentation is very short. Many properties of the second group vesicles, including their recycling by various forms of endocytosis, are analogous to those of the first group vesicles (Doherty and McMahon, 2009; De Curtis and Meldolesi, 2012; Chamberland et al., 2016). Therefore, their presentation will not be repeated here. Other aspects, such as the dual pathways of vesicle traffic and the tethering of their fusions, are best known for the second than for the first group. Therefore, they are mentioned in some detail in this Section.
Exocytosis of recycling vesicle takes place by two forms, corresponding to the two loops of the traffic, one long, including a step at the TGN, the other short, directly addressed to the plasma membrane (Figure 2A). The key relevance in these processes of small G proteins, particularly of Rab11a, was known since a few decades. Additional Rabs involved, Rab4, Rab8, and Rab37, participate in the control of traffic pathways and membrane fusions, including the mechanisms of their pore opening and subsequent closing (Jullié et al., 2014; Lesteberg et al., 2017). The latter result could be due to the binding of Rab11a to Munc13-4, a protein associated to the SNARE complexes (Johnson et al., 2016).
Several multi-subunit complexes make possible the specific passage of vesicles through distinct compartments, corresponding to the steps preceding exocytosis. These complexes differ depending on the compartments involved. For example, the core vacuole-endosome tethering complex (COVET) operates at the C-core of lysosome/endosome fusions. Another tethering complex, homotypic fusion and vacuole protein sorting complex (HOPS), operates at the homotypic endosome fusions (Chou et al., 2016; Spang, 2016). Two other complexes are necessary for the passage of vesicles along the recycling loops. The Golgi-associated retrograde protein complex (GARP) makes possible the TGN fusion of the long recycling loop vesicles (Hirata et al., 2015; Spang, 2016); the endosome associated retrograde protein complex (EARP) makes possible the plasma membrane tethering of the vesicles from both the long and short pathways (Schindler et al., 2015; Spang, 2016). Interestingly, the GARP and EARP complexes include several common proteins. Upon tethering, the vesicles of the two loops proceed rapidly to plasma membrane fusion (Spang, 2016).
The SNARE complex of exocytosis by the second group vesicles includes the v-SNARE VAMP4 together with the t-SNAREs Stx6 (Tran et al., 2007; Riggs et al., 2012). Two additional SNAREs, VAMP3 and Stx13, have been identified for these processes (Riggs et al., 2012; Jovic et al., 2014). All these proteins differ from those previously reported for the TGN-generated vesicles. The multiplicity of the SNAREs involved in the fusion of second group vesicles documents the heterogeneity of these fusions possibly dependent, at least in part, on the multiplicity of the participating cell types.
Non-secretory exocytosis during integrated processes
So far, we have presented several single cell processes dependent on the non-secretory exocytosis of various types of vesicles, generated either at the TGN or at the plasma membrane. Non-secretory processes, however, are not always simple. They also occur during events that include various types of vesicles and cells. Here we present a few examples of these processes.
Small non-secretory vesicles undergo exocytosis at limited areas of many cell surfaces
These exocytoses differ in many respects from those of recycling loops. The vesicles involved are smaller and their exocytosis, delayed upon their generation, occur at limited areas of the cell surface. Some processes, such as the cellularization of Drosophila embryos, by which a large syncytium is converted into about 6000 distinct cells, and the late step of macrophage phagocytoses, have been extensively characterized during the last decades (Chieregatti and Meldolesi, 2005). Therefore, details of these studies are not included in the present review. In contrast, the final step of cytokinesis has been recently clarified, revealing new aspects of exocytosis.
Results of several years ago had demonstrated during mitosis the strict localization and increased number of small endocytic vesicles (Figure 2C), taking place when the separation of daughter cells begins (Chieregatti and Meldolesi, 2005). The exocytosis of these vesicles has been shown to start at the cleavage furrow, along the central spindle of the cells, upon redistribution of two signaling molecules, the small GTPase Rap1 and the guanine nucleotide exchange factor (RhoGEF) epithelial cell transforming factor 2 (ECT2), which govern the structure of the cytoskeleton (Kotynkova et al., 2016; Gibieza and Prekeris, 2018). Concomitantly, the action of the endosomal vesicles equipped with the protein family-interacting protein 3 (FIP3), induces the formation of a secondary ingression and thus mediates the recruitment of endosomal sorting complex required for transport III (ESCRT III). Here the ESCRT III complex ultimately governs the final step of cytokinesis, the cell membrane abscission (Schiel et al., 2012).
Distinct structures and functions of dendrites and axons
The properties acquired by dendrites and axons during differentiation are necessary for the assembly of synapses by mature neurons. At their very beginning, the fibers developed by neurons and neural cells, largely sustained by the exocytosis of non-secretory vesicles, are very similar to each other. In neural cells, such as PC12, all fibers remain of the dendritic type. In each neuron, however, at an early stage of development a single dendrite is converted into an axon, different from the remaining dendrites (Chieregatti and Meldolesi, 2005).
Numerous non-secretory vesicles of the first group, transporting to the plasma membrane subunits of ionic receptors necessary for their postsynaptic function, have already been described (Dumoulin et al., 2011; Luscher et al., 2011; Tanaka and Hirano, 2012; Jurado et al., 2013; Hussain and Davanger, 2015; Gu et al., 2016; Gu and Huganir, 2016; Hussain et al., 2016; Langlhofer and Villmann, 2016; Vien et al., 2016; Chiu et al., 2017; Fujii et al., 2017; Lesteberg et al., 2017; Roth et al., 2017; Wu et al., 2017). The events occurring on axons are more complex (Figure 3A–C). Growth cones start expanding their surface by exocytosis of large, non-secretory vesicles stimulated by various agents, including the chemoattractant protein netrin1 (Rios et al., 2015) (Figure 3A). Interestingly, the exocytic fusion of these non-secretory vesicles takes place by the v-SNARE VAMP4 together with the t-SNAREs SNAP23 and Stx6 (Grassi et al., 2015), completely different from the SNARE proteins predominant in the fusion of synaptic vesicles at mature synapses. Non-secretory vesicles are not the only ones developed at growth cones. Additional vesicles are regulated by Rab33 and Rho GTPase TC10, working via the tethering of the exocyst component Exo7 (Nakazawa et al., 2012; Fujita et al., 2013).
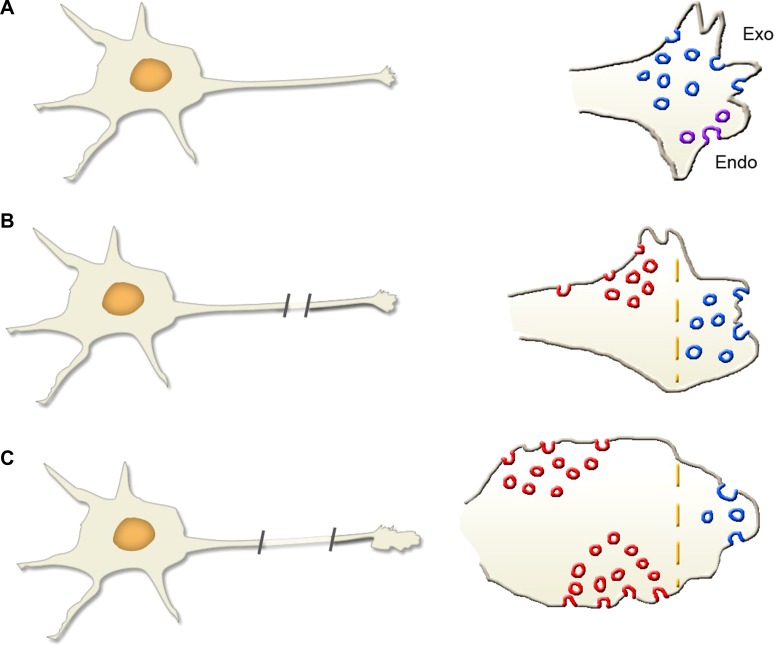
Figure 3.
Axon growth requires non-secretory exocytosis during neuronal development. This figure illustrates three steps of axon growth in the same cultured neuron. (A) The axon just starts to increase its length. At its tip (enlarged on the right), the growth cone is filled with moderately large vesicles (blue) that undergo intense non-secretory exocytosis followed by some endocytosis (violet). A consequence is a progressive elongation of the fiber. (B) A few days later, the length of the axon is significantly increased. At the growth cone, the less numerous vesicles undergoing non-secretory exocytosis coexist with groups of small synaptic vesicles (red) undergoing rapid secretory exocytosis, followed by endocytosis. (C) In the neuron cultured for several days, the growth cone is further grown up. However, non-secretory exocytosis (blue) have almost disappeared, while synaptic cycles (secretory exocytosis of small vesicles, red, followed by endocytosis) have increased, both at the cone (shown on the right) and at earlier sites along the axon (not shown).
Concomitantly to exocytosis, the development of axonal growth cones depends on various forms of macropinocytic endocytosis, with accumulation of intracellular pools of specific vesicles (Bonanomi et al., 2008). The balance in favor of exocytic with respect to endocytic processes appears important for the operation of neuronal growth cones, which depends on Ca2+ influx into the cells (Bonanomi et al., 2008; Tojima et al., 2014). At a later stage, approaching the establishment of synapses, both non-secretory exocytic and macropinocytic systems progressively decrease and then disappear from growth cones, which establish connections via the development of cell adhesion proteins. At this stage, the non-secretory vesicles are progressively replaced by the secretory exocytic systems of synaptic vesicles, which operate by recycling via their two types of endocytosis (Li et al., 2017) (Figure 3C). During further time, variable exocytosis/endocytosis balances do participate in important processes including axon branching by intracellular neuronal morphogenesis and intracellular regulation of synaptic plasticity (Winkle et al., 2016).
Exocytosis in plasma membrane repair
Plasma membrane wounding is a process that occurs with some frequency during physiological processes of different types of cells and tissues, including blood circulation and prolonged muscle contraction. The demonstration that, upon wounding, many cells do not die but undergo long-term survival, documents the existence of repair processes. Identification of the tools and mechanisms involved in these processes attracted therefore strong interest. For decades, repair was believed to start upon rapid Ca2+ influx through the wounds, with ensuing expansion and decreased tension of the plasma membrane due to exocytosis of lysosomes and/or vesicles of the non-secretory type. This type of wound sealing was defined the membrane patch model (Chieregatti and Meldolesi, 2005; Corrotte et al., 2013).
New studies have revealed the existence of additional mechanisms of repair. The increased cytosolic Ca2+ and the ensuing exocytosis of organelles, with expansion and decreased tension of the plasma membrane, stimulate marked generation of non-secretory vesicles. Such responses involve also the uptake and intracellular accumulation of the wounded areas, leaving the plasma membrane intact (Corrotte et al., 2013; Andrews and Corrotte, 2018). This endocytic process depends on the conversion of the surface phospholipid sphingomyelin into ceramide, induced by the released lysosomal enzyme acid sphingomyelinase (Tam et al., 2010).
Recent analyses of plasma membrane repair processes have emphasized the multiplicity of the mechanisms and procedures involved. In addition to the membrane patch and the endocytosis processes, new procedures have been proposed based on the removal of the wounded areas. The mechanisms of these processes could involve the local recruitment of ESCRT III, with accumulation of proteins and protein complexes (Babiychuk et al., 2011; Jimenez et al., 2014). The role of transmembrane proteins, reported to promote plasma membrane repair by interacting intracellularly with exocytic organelles, may also be relevant (Babiychuk et al., 2011; Rezvanpour et al., 2011).
Potential role of non-secretory vesicles in pathology and therapy
Non-secretory vesicles, tools in physiology and specific pathologies, can be considered also for their role in new therapies. Up to now, such role of vesicles had been considered only in a few cases. More often, relevance has been attributed to their transported cargoes. The examples reported here document the extended role of vesicles in biological and physiological processes.
Let us start with gene mutations. In a limited number of diseases, the possibility has been excluded that specific pathology depends, at least partially, on the traffic of non-secretory vesicles. This is the case of the renal polycystic disease, induced by numerous mutations of the channel CFTR. In this disease, the structure and traffic of the specific vesicles are unaffected (Zhang et al., 2017). Alterations of brain ionic receptors, glutamate and GABA receptors, play critical roles in neurodegenerative diseases, including the Alzheimer’s disease. Whether defects of the specific, non-secretory vesicles play roles in the alterations of these receptors is unknown (Bading, 2017; Ramirez-Jarquin and Tapia, 2017; Wang and Reddy, 2017).
In other diseases, roles played by transported proteins appear more likely. The GLUT4 transporter is critical for the pathology of both type 2 diabetes and various forms of cancer. In these cases, the circulation of non-secretory GSV vesicles, induced by insulin, appears to play relevant roles (Adekola et al., 2012; Sayem et al., 2018; Wasik and Lehtonen, 2018). Other important cases deal with traumas and pathological conditions of peripheral nerves. In these neurons, the endocitized vesicles play very important roles due to their continuous exocytosis needed for axon regeneration. The latter processes prevail for weeks, when the turnover of axonal vesicle components remains inappreciable (Hausott and Klimaschewski, 2016). Finally membrane repair, which takes place already under specific physiological conditions, is greatly reinforced in cells affected by various diseases, such as dystrophies of muscle fibers, membrane injuries of guts, and many others. Also in these cases, the reinforced repairs have been attributed to non-secretory vesicles (Carmeille et al., 2016; Demonbreun and McNally, 2016; Basson, 2017).
Conclusions
The present review, based on the important developments identified during the last few years, has illustrated the present knowledge about non-secretory exocytosis. In the past, this approach had been rare. Most studies, in fact, concerned non-secretory vesicles expressed by only single types of cell. At the beginning of this review, non-secretory vesicles have been presented as a family of exocytic organelles, distinct from the family of secretory vesicles. This sort of classification, chosen initially in our original review (Chieregatti and Meldolesi, 2005), has been emphasized here. The two families of vesicles structurally distinct are involved in different but critical functions.
The relevance of non-secretory exocytosis was confirmed by recent results. For example, the transfer to the cell surface of critical membrane proteins, a property known since long for secretory vesicles, has been demonstrated also for the vesicles of the non-secretory type. Even more relevant is another property of the latter vesicles, heterogeneity. Particularly convincing is the distinction of the neuronal vesicles employed for the traffic of different receptor subunits (Hussain and Davanger, 2015; Gu et al., 2016; Roth et al., 2017), and of the vesicles employed for the same subunit, trafficking however either in resting or stimulated cells (Gu and Huganir, 2016; Gu et al., 2016; Hussain et al., 2016; Roth et al., 2017). Another heterogeneity of non-secretory vesicles depends on their origin. In our previous review (Chieregatti and Meldolesi, 2005), we had reported the opinion, at the time widely accepted, that most non-secretory vesicles are generated separately at two sites, the TGN and the plasma membrane. Recent studies, however, have demonstrated that most vesicles follow an integrated pathway, with generation at the TGN followed by recycling from the plasma membrane. Interestingly, the pathways of the various vesicles can exhibit important differences (see comparative properties of vesicles in Table 1). For example, recycling of brain receptor vesicles is short, whereas recycling of the GLUT4 transporter vesicles is long and complex. Also for non-secretory vesicles, really generated at the plasma membrane by endocytosis, the intracellular pathway can be variable. Recycling of these vesicles occurs along two loops, one long, with a step at the TGN, the other short, addressed directly to the plasma membrane (Hirata et al., 2015; Schindler et al., 2015; Chou et al., 2016; Spang, 2016). Heterogeneity exists also at the mechanistic level. For example, the small G protein Rab11 plays a role in the endocytosis of several vesicles. Other proteins of the Rab and Rho type, identified for the recycling of GLUT4-specific vesicles (Sun et al., 2016; Jaldin-Fincati et al., 2017; Li et al., 2017; Zhou et al., 2017) do not appear to participate in the recycling of other vesicles (Bonanomi et al., 2008; Adekola et al., 2012, Tojima et al., 2014; Carmeille et al., 2016; Demonbreun and McNally, 2016; Hausott and Klimaschewski, 2016; Bading, 2017; Basson, 2017; Ramirez-Jarquin and Tapia, 2017; Wang and Reddy, 2017; Sayem et al., 2018; Wasik and Lehtonen, 2018). Even the SNARE complexes that govern the exocytosis of all vesicles are mostly heterogeneous.
Based on the exciting properties reported in the present review, we expect that interest about non-secretory vesicles will increase in the near future. This also because various properties in the field, concerning both physiology and pathology of vesicles, are still unclear. Many of such properties will be clarified in the near future. In pathology, many studies deal to the development of integrated processes such as axon growth/regeneration, membrane repair (Bonanomi et al., 2008; Tam et al., 2010; Babiychuk et al., 2011; Rezvanpour et al., 2011; Nakazawa et al., 2012; Corrotte et al., 2013; Fujita et al., 2013; Jimenez et al., 2014; Tojima et al., 2014; Grassi et al., 2015; Rios et al., 2015; Winkle et al., 2016; Li et al., 2017; Andrews and Corrotte, 2018) and other processes. Interest will grow also about therapy. Non-secretory vesicles could become drug targets, potentially useful in a number of diseases.
In conclusion, various non-secretory vesicle types presented here often exhibit heterogeneous properties. Because of their recognized role in distinct functions, these vesicles are certainly more important than widely believed until now. In our comprehensive presentation, we have emphasized the properties of these vesicles, including their origin and various functions. Growing interest, based on increased knowledge about their relevance in physiology, pathology, and medicine, is widely expected for the future.
Acknowledgements
We are grateful to colleagues who have discussed with us the properties, the exocytosis, and the role of non-secretory vesicles.
Conflict of interest
none declared.
References
- Adekola K., Rosen S.T., and Shammugam M. (2012). Glucose transporters in cancer metabolism. Curr. Opin. Oncol. 24, 650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N.W., and Corrotte M. (2018). Plasma membrane repair. Curr. Biol. 28, R392–R397. [DOI] [PubMed] [Google Scholar]
- Babiychuk E.B., Monastyrskaya K., Potez S., et al. (2011). Blebbing confers resistance against cell lysis. Cell Death Differ. 18, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H. (2017). Therapeutic targeting of the pathological triad of extrasynaptic NMDA receptor signaling in neurodegeneration. J. Exp. Med. 214, 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson M.D. (2017). Hiearchies of healing in gut mucosal injury. J. Physiol. Pharmacol. 68, 789–795. [PubMed] [Google Scholar]
- Beaton N., Rudigier C., Moest H., et al. (2015). TUSC5 regulates insulin-mediated adipose tissue glucose uptake by modulation of GLUT4 recycling. Mol. Metab. 4, 795–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanomi D., Fornsiero E.F., Valdez C., et al. (2008). Identification of a developmentally regulated pathway of membrane retrieval in neuronal growth cones. J. Cell Sci. 121, 3757–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer P.D., Hablemichaele E.N., Romenskaia I., et al. (2016). Rab14 limits the sorting of Glut4 from endosomes into insulin-sensitive regulated compartments in adipocytes. Biochem. J. 473, 1315–1327. [DOI] [PubMed] [Google Scholar]
- Butterworth M.B., Edinger F.S., Silvis M.S., et al. (2012). Rab11b regulates the trafficking and recycling of the epithelial sodium channel (ENaC). Am. J. Physiol. Renal Physiol. 302, F581–F590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeille R., Bouvet F., Tan S., et al. (2016). Membrane repair of human skeletal muscle cells requires annexin-A5. Biochim. Biophys. Acta 1863, 2267–2279. [DOI] [PubMed] [Google Scholar]
- Chamberland J.P., Antonov L.T., Dias Santos M., et al. (2016). NECAP2 controls clathrin coat recruitment to early endosomes for fast endocytic recycling. J. Cell Sci. 129, 2625–2637. [DOI] [PubMed] [Google Scholar]
- Chieregatti E., and Meldolesi J. (2005). Regulated exocytosis: new organelles for non-secretory purposes. Nat. Rev. Mol. Cell Biol. 6, 181–187. [DOI] [PubMed] [Google Scholar]
- Chiu S.L., Diering G.H., Ye B., et al. (2017). GRASP1 regulates synaptic plasticity and learning through endosomal recycling of AMPA receptors. Neuron 93, 1405–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou H.T., Dukovski D., Chambers M.G., et al. (2016). CATCHR, HOPS and CORVET tethering complexes share a similar architecture. Nat. Struct. Mol. Biol. 23, 761–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihil K.M., Ellinger E.P., Fellows A., et al. (2012). Disabled-2 protein facilitates assembly of polypeptide-2-independent recruitment of cystic fibrosis transmembrane conductance regulator to endocytic vesicles in polarized airway epithelial cells. J. Biol. Chem. 287, 15087–15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. (2003). TRP channels as cellular sensor. Nature 426, 517–524. [DOI] [PubMed] [Google Scholar]
- Cocucci E., Racchetti G., Podini P., et al. (2004). Enlargeosome, an exocytic vesicle resistant to non-ionic detergents, undergoes endocytosis via a non-acidic route. Mol. Biol. Cell 15, 5356–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrotte M., Almeida P.E., Tam C., et al. (2013). Caveolae internalization repairs wounded cells and muscle fibers. eLife 2, e00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Curtis I., and Meldolesi J. (2012). Cell surface dynamics—how Rho GTPases orchestrate the interplay between the plasma membrane and the cortical cytoskeleton. J. Cell Sci. 125, 4435–4444. [DOI] [PubMed] [Google Scholar]
- Demonbreun A.R., and McNally E.M. (2016). Plasma membrane repair in health and disease. Curr. Top. Membr. 77, 67–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty G.J., and McMahon H.T. (2009). Mechanisms of endocytosis. Ann. Rev. Biochem. 278, 857–902. [DOI] [PubMed] [Google Scholar]
- Dumoulin A., Triller A., and Kneussel M. (2011). Cellular transport and membrane dynamics of the glycine receptor. Front. Mol. Neurosci. 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger R.S., Bertrand C.A., Rondadino C., et al. (2012). The epithelial sodium channel (ENaC) establishes a trafficking vesicle pool responsible for its regulation. PLoS One 7, e46593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazakerley D.J., Naghiloo S., Chaudhuri R., et al. (2015). Proteomic analysis of Glut4 storage vesicles reveals tumor suppressor candidate 5 (TUSK5) as a novel regulator of insulin action on adipocytes. J. Biol. Chem. 290, 23528–23542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowerdew S.E., and Burgoyne R.D. (2009). A VAMP7/Vti1A SNARE complex distinguishes a non-conventional traffic route to the cell surface used by KChiP1 and Kv4 potassium channels. Biochem. J. 418, 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Tanaka H., and Hirano T. (2017). Detection and characterization of individual endocytosis of AMPA-type glutamate receptor around postsynaptic membrane. Genes Cells 22, 583–590. [DOI] [PubMed] [Google Scholar]
- Fujita A., Koinuma S., Yasuda S., et al. (2013). GTP hydrolysis of TC10 promotes neurite outgrowth through exocyst fusion of Rab11 and L1-containing vesicles by releasing exocyst component Exo7. PLoS One 8, e79689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibieza P., and Prekeris R. (2018). Rab GTPases and cell division. Small GTPases 9, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouraud S., Laera A., Calamita G., et al. (2002). Functional involvement of VAMP/synaptobrevin2 in cAMP-stimulated aquaporin-2 translocation in renal collecting duct cells. Mol. Cell. Biol. 23, 961–974. [DOI] [PubMed] [Google Scholar]
- Grassi D., Plonka F.B., Oksdath M., et al. (2015). Selected SNARE proteins are essential for the polarized membrane insertion of Igf-1 receptor and the regulation of initial axon outgrowth in neurons. Cell Discov. 1, 15023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Chiu S.L., Liu B., et al. (2016). Differential vesicular sorting of AMPA and GABAA receptors. Proc. Natl Acad. Sci. USA 113, E922–E931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., and Huganir R.L. (2016). Identification of the SNARE complex mediating the exocytosis of NMDA receptor. Proc. Natl Acad. Sci. USA 113, 12280–12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama H., and Kanzaki M. (2017). Heterotypic endosomal fusion as an initiator of insulin induces glucose transporter-4 (GLUT4) translocation in skeletal muscle. J. Physiol. 595, 5603–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausott B., and Klimaschewski L. (2016). Membrane turnover and receptor trafficking in regenerating axons. Eur. J. Neurosci. 43, 309–317. [DOI] [PubMed] [Google Scholar]
- Hirata T., Fujita M., Nakamura S., et al. (2015). Post-Golgi anterograde transport requires GARP-dependent endosome-to-TGN retrograde transport. Mol. Biol. Cell 26, 3071–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman G.D. (1994). Insulin-stimulated GLUT4 glucose transported recycling. A problem in membrane protein subcellular trafficking through multiple pools. J. Biol. Chem. 169, 17516–17524. [PubMed] [Google Scholar]
- Hussain S., and Davanger S. (2015). Postsynaptic VAMP/synaptobrevin facilitates differential vesicle trafficking of GluA1 and GluA2 AMPA receptor subunits. PLoS One 10, e140868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S., Ringsevjen H., Eqbenya D.L., et al. (2016). SNARE protein syntaxin-1 colocalizes closely with NMDA receptor subunit NR2B in postsynaptic spines in the hippocampus. Front. Mol. Neurosci. 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaldin-Fincati J.R., Pavarotti M., Frendo-Cumbo S., et al. (2017). Update on GLUT4 vesicle traffic: a cornerstone of insulin action. Trends Endocrinol. Metab. 28, 597–611. [DOI] [PubMed] [Google Scholar]
- Jimenez A.J., Maiuri P., Lafaurie-Janvore J., et al. (2014). ESCRT machinery is required for plasma membrane repair. Science 343, 1247136. [DOI] [PubMed] [Google Scholar]
- Johnson J., He H., Ramadass M., et al. (2016). Munc13-4 is a Rab11-binding protein that regulates Rab11-positive vesicle trafficking and docking at the plasma membrane. J. Biol. Chem. 291, 3423–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovic M., Kean M.J., Dubanskova A., et al. (2014). Endosomal sorting of VAMP3 is regulated by PI4K2A. J. Cell Sci. 127, 3745–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullié D., Choquet D., and Parrais D. (2014). Recycling endosomes undergo rapid closure of a fusion pore on exocytosis in neuronal dendrites. J. Neurosci. 34, 11106–11118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S., Goswami D., Zhang Y., et al. (2013). LTP requires a unique postsynaptic SNARE fusion machinery. Neuron 77, 542–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemens C.H., Edinger R.S., Kightinger L., et al. (2017). Ankyrin G expression regulates apical delivery of the epithelial sodium channel (ENaC). J. Biol. Chem. 292, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotynkova K., Su K.C., West S.C., et al. (2016). Plasma membrane association but not midzone recruitment of RhoGEF ECT2 is essential for cytokinesis. Cell Rep. 17, 2672–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlhofer G., and Villmann C. (2016). The intracellular loop of the glycine receptor: it’s not all about the size. Front. Mol. Neurosci. 9, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesteberg K., Orange J., and Makedonas G. (2017). Recycling endosomes in human cytotoxic T lymphocytes constitute an auxiliary intracellular trafficking pathway for newly synthesized perforin. Immunol. Res. 65, 1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Han W., Pelkev K.A., et al. (2017). Molecular dissection of neuroligin2 and Slitrk3 reveals an essential framework for GABAergic synapse development. Neuron 96, 808–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Yue Y., Hu F., et al. (2018). Electrical pulse stimulation induces GLUT4 glucose transporter translocation in C2C12 myotubes that depends on Rab8A, Rab13 and Rab14. Am. J. Physiol. Endocrinol. Metab. 314, E478–E493. [DOI] [PubMed] [Google Scholar]
- Luscher B., Fuchs T., and Kilpatrick C.L. (2011). GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron 70, 385–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Mamol R., Perez-Verdaguer R., Roig S.R., et al. (2013). A non-canonical di-acidic signal at the C-terminus of Kv1.3 determines anterograde trafficking and surface expression. J. Cell Sci. 126, 5681–5691. [DOI] [PubMed] [Google Scholar]
- McNally K.E., Faulkner R., Steinberg F., et al. (2017). Retriever is a multipotent complex for retromer-independent endosomal cargo recycling. Nat. Cell Biol. 18, 1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses D., Vega A.V., Torres-Cruz F.M., et al. (2016). KV1 and KV3 potassium channels identified at presynaptic terminals of the corticostriatal synapses in the rat. Neural Plast. 2016, 872518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., and McNeil P.L. (1995). Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J. Cell Biol. 131, 1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa H., Sada T., Toriyama M., et al. (2012). Rab33a mediates anterograde vesicular transport for membrane exocytosis and axon outgrowth. J. Neurosci. 32, 12712–12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Zaarur N., Singh M., et al. (2017). Sortilin and retromer mediate retrograde transport of Glut4 in 3T3-L1 adipocytes. Mol. Biol. Cell 28, 1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson-Leary J., Jahagirdar V., Sage J., et al. (2018). Insulin modulates hippocampally-mediated spatial working memory via glucose transporter-4. Behav. Brain Res. 338, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfenninger K.H., and Friedman L.B. (1993). Sites of plasmalemma expansion in growth cones. Brain Res. Dev. Brain Res. 71, 181–192. [DOI] [PubMed] [Google Scholar]
- Pick J.E., Khatri L., Sathler M.F., et al. (2017). mGluR long-term depression regulates GluA2 association with COPII vesicles and exit from the endoplasmic reticulum. EMBO J. 36, 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Jarquin U.N., and Tapia R. (2017). Chronic GABArgic blockade in the spinal cord in vivo induces motor alterations and neurodegeneration. Neuropharmacology 117, 85–92. [DOI] [PubMed] [Google Scholar]
- Rezvanpour A., Santamaria-Kisiel L., Shaw G.S., et al. (2011). The S100A10-annexin A2 complex provides a novel asymmetric platform for membrane repair. J. Biol. Chem. 286, 40174–40183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.L., Li W., Mamuya F., et al. (2015). Polarized trafficking of AQP2 revealed in three dimensional epithelial cultures. PLoS One 10, e0131719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs K.A., Hasan N., Hamphrey D., et al. (2012). Regulation of integrin endocytic recycling and chemotactic cell migration by syntaxin 6 and VAMP3 interaction. J. Cell Sci. 125, 3827–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindler M.J., Xu C.F., Gumper I., et al. (2007). Proteomic analysis of pancreatic zymogen granules: identification of new granule proteins. J. Proteome Res. 6, 2978–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios O., Coitrufo T., Martinez-Marmol R., et al. (2015). Regulation of patterned dynamics of local exocytosis in growth cones by netrin-1. J. Neurosci. 35, 5156–5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R.H., Zhang Y., and Huganir R.L. (2017). Dynamic imaging of AMPA receptors trafficking in vivo and in vitro. Curr. Opin. Neurobiol. 45, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayem A.S.M., Arya A., Karimian K., et al. (2018). Action of photochemicals on insulin signaling pathway accelerating glucose transporter (GLUT4) protein translocation. Molecules 23, E258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk U., Verderio C., Benfenati F., et al. (2003). Regulated delivery of AMPA receptor subunits to the presynaptic membrane. EMBO J. 22, 558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiel J.A., Simon G.C., Zahrris C., et al. (2012). FIP3-endosome dependent formation of the secondary ingression mediates ESCRT recruitment during cytokinesis. Nat. Cell Biol. 14, 1068–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Chen Y., Pu J., et al. (2015). EARP is a multi-subunit tethering complex involved in endocytic recycling. Nat. Cell Biol. 17, 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A. (2016). Membrane tethering complexes in the endosomal system. Front. Cell Dev. Biol. 4, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinardt R.A., Bi G., and Alderton J.M. (1994). Cell Membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science 263, 390–393. [DOI] [PubMed] [Google Scholar]
- Sudhof T.C. (2004). The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547. [DOI] [PubMed] [Google Scholar]
- Sun Y., Jaldin-Fincati J., Liu Z., et al. (2016). A complex of Rab13 with MICAL-L2 and actinin-4 is essential for insulin-dependent GLUT4 exocytosis. Mol. Biol. Cell 27, 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Fujii T., Fugijita K., et al. (2014). Functional coupling of chloride-proton exchanger ClC-5 to gastric H+, K+-ATPase. Biol. Open 3, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam C., Idone V., Devlin C., et al. (2010). Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J. Cell Biol. 189, 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., and Hirano T. (2012). Visualization of subunit-specific delivery of glutamate receptors to postsynaptic membrane during hippocampal long-term potentiation. Cell Rep. 1, 291–298. [DOI] [PubMed] [Google Scholar]
- Tang B.L., Gee H.Y., and Lee M.G. (2011). The cystic fibrosis transmembrane conductance regulator’s expanding SNARE interactome. Traffic 12, 364–371. [DOI] [PubMed] [Google Scholar]
- Thorn P., Zorec R., Retting J., et al. (2016). Exocytosis in non-neuronal cells. J. Neurochem. 137, 849–859. [DOI] [PubMed] [Google Scholar]
- Tojima T., Itofusa R., and Kamiquchi H. (2014). Steering neuronal growth cones by shifting the imbalance between exocytosis and endocytosis. J. Neurosci. 34, 7165–7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T.H., Zeng Q., and Hong W. (2007). VAMP4 cycles from the cell surface to the trans-Golgi network via sorting and recycling endosomes. J. Cell Sci. 120, 1028–1041. [DOI] [PubMed] [Google Scholar]
- Uhm M., Bazuine M., Zhao P., et al. (2017). Phosphorylation of the exocyst protein Exo84 by TBK1 promotes insulin-stimulated GLUT4 trafficking. Sci. Signal. 10, pii: eaah5085. [DOI] [PubMed] [Google Scholar]
- Uldry M., Steiner P., Zurich M.J., et al. (2004). Regulated exocytosis of an H+/myo-inositol symporter at synapses and growth cones. EMBO J. 23, 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vien T.N., Moss S.J., and Davies P.A. (2016). Regulating the efficacy of the inhibition through trafficking of γ-aminobutyric acid type A receptors. Anesth. Analg. 123, 1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.C., Ng C.P., Shi H., et al. (2010). A role of VAMP8/endobrevin in surface deployment of the water channel aquaporin2. Mol. Cell. Biol. 30, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., and Reddy P.H. (2017). Role of glutamate and NMDA receptors in Alzheimer’s disease. J. Alzheimers. Dis. 57, 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasik A.A., and Lehtonen S. (2018). Glucose transporters in diabetic kidney disease-friends or foes? Front. Endocrinol. 9, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R.T., Furukawa M., Chiang S.H., et al. (2003). The exocytic trafficking of TC10 occurs through both classical and non-classical transport pathways in 3T3L1 adipocytes. Mol. Cell. Biol. 23, 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle C.C., Hanlin C.C., and Gupton S.L. (2016). Utilizing combined methodologies to define the role of plasma membrane delivery during axon branching and neuronal morphogenesis. J. Vis. Exp. 2016, 53743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Bacaj T., Morishita W., et al. (2017). Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature 544, 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Barone S., Li H., et al. (2011). Slc26a11, a chloride transporter, localizes with the vacuolar H+-ATPase of A-intercalated cells of the kidney. Kidney Int. 80, 926–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J.I., Verhey K.J., and Bimbaum M.J. (1995). Kinetic analysis of glucose transporter trafficking in fibroblasts and adipocytes. Biochemistry 34, 15523–15531. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Bahash M.M., Finn M.G., et al. (2017). Direct measurement of trafficking of the cystic fibrosis transmitter conductance regulator to the cell surface and binding to a chemical chaperone. Biochemistry 56, 240–249. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Enzel F., Bennighoff T., et al. (2017). Rab28 is a TBC1D1/TBC1D4 substrate involved in GLUT4 trafficking. FEBS Lett. 591, 88–96. [DOI] [PubMed] [Google Scholar]
- Zinn V.Z., Khatri A., Mednieks M.I., et al. (2015). Localization of cystic fibrosis transmembrane conductance regulator signaling complexes in human salivary gland striated duct cells. Eur. J. Oral Sci. 123, 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]