Abstract
Recent, rapid changes in the treatment of type 1 diabetes have allowed for commercialization of an “artificial pancreas” that is better described as a closed-loop controller of insulin delivery. This review presents the current state of closed-loop control systems and expected future developments with a discussion of the human factor issues in allowing automation of glucose control. The goal of these systems is to minimize or prevent both short-term and long-term complications from diabetes and to decrease the daily burden of managing diabetes. The closed-loop systems are generally very effective and safe at night, have allowed for improved sleep, and have decreased the burden of diabetes management overnight. However, there are still significant barriers to achieving excellent daytime glucose control while simultaneously decreasing the burden of daytime diabetes management. These systems use a subcutaneous continuous glucose sensor, an algorithm that accounts for the current glucose and rate of change of the glucose, and the amount of insulin that has already been delivered to safely deliver insulin to control hyperglycemia, while minimizing the risk of hypoglycemia. The future challenge will be to allow for full closed-loop control with minimal burden on the patient during the day, alleviating meal announcements, carbohydrate counting, alerts, and maintenance. The human factors involved with interfacing with a closed-loop system and allowing the system to take control of diabetes management are significant. It is important to find a balance between enthusiasm and realistic expectations and experiences with the closed-loop system.
Essential Points
The goal of automated insulin delivery is to minimize or prevent short-term and long-term complications from diabetes and to decrease the daily burden of managing diabetes
Current systems provide good glucose control overnight; however, the patient is still required to enter meal boluses
Significant improvements in continuous glucose monitoring have made commercialization of these systems possible
Automated full closed-loop systems that can automatically manage meals may significantly benefit from faster acting insulins with a shorter duration of action
Bihormonal systems with the addition of glucagon and/or amylin replacement may allow for full closed-loop control
It will be key for systems to offer automatic adaptability to the individual’s changes in not only diurnal patterns of insulin sensitivity but also to automatically adapt to changes resulting from illness, exercise routines, menstrual cycles, and eating habits
We need multiple systems so people can choose what best fits their needs: tubeless pumps, implanted sensors, systems allowing for user adjustment of glycemic goals, communication to significant others, phone-based or pump-based controller systems, more complicated systems and simple systems based on the personality of the user, systems that allow for significant user input and systems that take over control of most diabetes tasks, and choices to cover a range of costs and the need to wear multiple devices and infusion sets
The Need for Closed-Loop Control
Type 1 diabetes and insulin replacement
Type 1 diabetes is generally thought to be precipitated by an immune-associated, if not directly immune-mediated, destruction of insulin-producing pancreatic β cells (1). The loss of β-cells leads to loss of insulin and amylin secretion and dysfunctional glucagon secretion. Insulin is the only hormone in the body that lowers glucose levels. Before the discovery of insulin, children diagnosed with diabetes had a very short lifespan. Insulin replacement therapy has been lifesaving. In the 90 years since the discovery of insulin, there have been progressive improvements in insulin replacement therapy and the ability to measure blood glucose levels to guide more physiologic insulin delivery. Physiologic replacement means providing rapid increases in insulin when carbohydrate-containing meals are consumed and basal insulin at other times of the day. Today, insulin is replaced by either insulin injections or by continuous insulin infusion via an insulin pump. With multiple daily injection (MDI) therapy a long-acting insulin is given to meet basal needs, and injections of a rapid-acting insulin are given to meet meal insulin requirements and correct hyperglycemia. Insulin infusion pump therapy was introduced in the late 1970s (2, 3) and provided more flexibility around meal doses. Because only short-acting insulin is infused, when the infusion catheter becomes clogged or displaced or there is local inflammation at the infusion site, insulin delivery can be disrupted, which can lead to diabetic ketoacidosis. The rate of diabetic ketoacidosis is not higher in people using pump therapy (T1D Exchange data) (4), but it does occur and is also a risk with automated insulin delivery.
The long-term risk of diabetes related complications
Although insulin replacement therapy has significantly improved the lifespan of people with diabetes, it is not a cure. As people with diabetes began living longer, they began to develop long-term complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disease. It was debated whether it was the insulin therapy that was leading to these complications or higher than normal glucose levels. People with diabetes have higher blood insulin levels because insulin given in the subcutaneous space must first circulate through the systemic circulation before it reaches the liver, whereas insulin secreted by the pancreas goes directly to the liver, one of the major organs affected by insulin action. To determine whether it was higher insulin requirements or higher glucose levels leading to long-term complications, the Diabetes Control and Complications Trial (DCCT) was initiated in 1975 (5). It clearly demonstrated that improved glycemic control, measured by 8 point glucose profiles and by hemoglobin A1c (HbA1c) levels, decreased this risk of diabetes complications, including microvascular and coronary artery disease (5, 6).
Burden of diabetes management
Insulin doses need to be carefully adjusted. Insulin is a replacement hormone with a narrow therapeutic margin. If insulin replacement is inadequate, or increasing insulin needs with stress and illness are not met, then metabolic decomposition can lead to ketoacidosis, which can be fatal (7). Overtreatment with insulin can lead acutely to impaired cognition, hypoglycemic seizures, and can also lead to death (dead-in-bed syndrome) (8). In a meal, the glycemic index of the carbohydrates and the amount of protein and fat can significantly change postprandial glucose control (9). Exercise can have both immediate and delayed effects on glucose levels. Short bursts of aerobic activity can raise glucose levels, whereas sustained exercise can cause both acute and delayed hypoglycemia, as well as having an impact on insulin sensitivity lasting days (10). To achieve current goals for glycemic control, the person with diabetes is making multiple, complex decisions each day based on food composition, exercise (planned or past), as well as factors such as their ability to recognize hypoglycemia and menstrual cycles.
It is the current recommendation for diabetes treatment to maintain an HbA1c level of <7% (53 mmol/mol) to decrease the risk of long-term complications. In a study from the T1D Exchange clinical registry in the United States in 2015, only 14% to 30% (depending on age group) of the 16,061 participants were meeting this goal (11). In a recent report on HbA1c levels on 8186 pediatric participants <18 years old in 2016, only 19% had an HbA1c <7.5% (58 mmol/mol). In 2011 the percent reaching target was higher at 22%, and increasing pump use (from 56% to 64%) and continuous glucose monitoring (CGM) use (from 3% to 22%) between 2011 and 2016 did not lower mean HbA1c levels [8.5% ± 1.5% (70 ± 16 mmol/mol) in 2011 and 8.8% ± 1.6% (72 ± 18 mmol/mol) in 2016]. The use of CGM, regardless of MDI or pump therapy, however, did result in lower HbA1c levels [8.1% (65 mmol/mol) for CGM users compared with 9.0% (75 mmol/mol) for nonusers; P < 0.001] (12). Automated insulin delivery could offer significant improvements in outcomes, but this has yet to be documented across a broad population.
Assessing outcomes: beyond HbA1c
In the DCCT, glucose control was measured by periodically obtaining seven glucose readings throughout a day, and by measuring glycosylated Hb. At the time this study was conducted the technology for CGM had not been developed. As a result of the DCCT study, HbA1c became the standard measurement to determine the risk of long-term diabetes complications; however, it is an indirect measure of the glucose levels. HbA1c measurements are affected by ethnicity, red blood cell lifespan, and turnover rates (13), and people can have significant differences in their HbA1c levels with similar mean glucose levels (13, 14). CGM provides a more direct measure of the glucose levels bathing the eyes and kidneys where nonenzymatic glycosylation leads to long-term complications. Nonenzymatic glycosylation is directly correlated with the degree and duration of hyperglycemia, that is, mean glucose levels. When advanced glycosylation end products were measured in skin collagen biopsies from the DCCT, these levels were robust predictors of retinopathy, nephropathy, and neuropathy, and A1c lost significance in predicting these long-term complications (15). It has been recommended that we now consider CGM metrics when defining glucose control and go beyond using an HbA1c level, which has significant issues based on red blood cell turnover rates and does not measure the risk for hypoglycemia (16). We have therefore chosen to report the commonly reported outcomes of automated insulin delivery studies in terms of CGM levels: mean glucose, time in range [70 to 180 mg/dL (3.9 to 10.0 mmol/L)], and time <70 mg/dL (3.9 mmol/L). CGM metrics have also been accepted by the US Food and Drug Administration (FDA) as outcome measures when assessing automated insulin delivery. When automated insulin delivery studies were of at least 3 months in duration, we have also provided HbA1c outcomes. There have been several consensus statements on the reporting of CGM data (16, 17). These metrics are provided below in Table 1. In studies reported before 2018, these metrics were not consistently used.
Table 1.
Outcome Metrics
| Outcome | Value/Measure |
|---|---|
| Hypoglycemia | <70 mg/dL (3.9 mmol/L) |
| <54 mg/dL (3.0 mmol/L) | |
| Seizure or loss of consciousness | |
| Time in range | 70–180 mg/dL (3.9–10.0 mmol/L) |
| 70–140 mg/dL (3.9–7.8 mmol/L) (as a second measure) | |
| Hyperglycemia | >180 mg/dL (10.0 mmol/L) |
| >250 mg/dL (13.9 mmol/L) | |
| Diabetic ketoacidosis | |
| Overall | Mean glucose |
| Glycemic variability | Coefficient of variation |
| Sleep/wake blocks | Midnight to 6:00 am/6:00 am to midnight |
| CGM data sufficiency | 2 wk of collection with at least 70% of possible readings |
| Hypo and hyper events | Duration of at least 15 min, separated by at least 15 min of intervening normal values |
Glycemic targets may vary with specific patients or providers. In assessing how an automated insulin delivery system is performing, or how a patient is doing in meeting glycemic goals, we have set these goals:
Mean glucose of ≤154 mg/dL (8.5 mmol/L)
≥70% of overall readings between 70 and 180 mg/dL (3.9 to 10.0 mmol/L)
<4% of all readings <70 mg/dL (3.9 mmol/L)
<25% of readings >180 mg/dL (10.0 mmol/L)
Coefficient of variation of <36%
No diabetic ketoacidosis, seizures, or loss of consciousness
Closed-Loop Component Technology
A closed loop refers to a feedback control system that attempts to keep a measurable quantity within a desired target range. There is some instrument that enables modification of the measured quantity. Finally, there are rules that control the operation of the instrument to try to move the measurable quantity toward the desired target. In the case of artificial pancreas technology, glucose is the quantity that can be measured continuously with CGM, an insulin pump is the instrument that can alter glucose, and the rules are computer algorithms that command the insulin pump. These three components provide the minimum components for automated glucose-mediated insulin delivery. Additional factors that affect glucose may also be recorded (e.g., carbohydrate intake, exercise), and other instruments (e.g., glucagon) may also be included. Full closed-loop systems require no additional data entry from the user, whereas current hybrid closed-loop configurations require users to enter data such as the amount of carbohydrates consumed.
Continuous glucose monitoring
Commercial CGM has been available since 1999 (18). Since then, there has been marked improvements in sensor accuracy, reliability, wearability. and features. Sensor accuracy is commonly measured by the mean (or median) absolute relative difference (MARD) of sensor readings to a reference glucose. Initial sensors had an MARD of 26%, and current sensors generally have a MARD of <10%. Using in silico modeling, Kovatchev et al. (19) determined that a sensor with a MARD of ≤10% would be accurate enough for insulin dose decisions. Currently, sensors from two companies have been used in most reported automated insulin delivery studies, and each sensor has a reported MARD of <10%. Both the Dexcom G5, with two calibrations daily, and the Dexcom G6, with no calibrations, have a MARD of 9% (20). The Medtronic Guardian sensor with three to four calibrations a day has a MARD of 9.6% (21).
The Dexcom and Medtronic sensors are based on an electrochemical glucose oxidase reaction to measure glucose. The Eversense implanted subcutaneous sensor is coated with a fluorescent chemical that produces light proportional to the glucose concentration. The fluorescence is measured optically with a miniaturized spectrofluorometer, and the data are then transmitted via near-field communication to a receiver worn over the sensor. In a recent study testing 90 days of sensor performance after implantation, the overall MARD was 8.8% (22), and there are now ongoing trials testing the use of this sensor with automated insulin delivery (23). The FreeStyle® Libre™ provides a factory-calibrated glucose oxidase–based sensor that allows for intermittent reading of the glucose values by swiping a handheld near-field communication device over the sensor. The current glucose value is displayed along with historic glucose readings obtained every 15 minutes for up to 8 hours and has a MARD of 10.7% during days 2 to 14 of wear (24). Because it only provides intermittent readings, the sensor in the present configuration would not work for automated insulin delivery; however, this well-established sensor is being configured to work in an automated insulin delivery system developed by Bigfoot Biomedical (25).
The Dexcom G6 has been approved as an integrated continuous glucose monitoring (iCGM) system (26). This means it can be used in multiple closed-loop systems without the need to provide full manufacturing and performance data on the sensor when building the system (i.e., a plug-and-play sensor).
The above sensors are placed subcutaneously and measure glucose concentration in the interstitial fluid rather than blood glucose, which introduces an ∼5- to 10-minute delay in sensor readings compared with a blood glucose (27). Intravascular glucose kinetics are instantaneous, and IP glucose-sensing kinetics are significantly faster than subcutaneous kinetics (28). The sensor kinetics in these spaces may provide added benefit to implanted closed-loop systems.
Insulin infusion pumps
All insulin infusion pump therapy makes it easier to have multiple courses (such as dessert) and to cover snacks, because additional food does not require separate injections. Some pumps allow greater flexibility with meal insulin coverage, so that meals with a high fat content and delayed food absorption can be covered with a “dual wave bolus,” with a portion of the insulin given as a usual bolus and the remainder of the bolus given during several hours. This is also effective in dosing young children who do not always consume all of their food. If this occurs, the extended bolus can be cancelled. Basal insulin needs can also be adjusted throughout the day and night to adjust for changes in insulin sensitivity due to exercise, early morning insulin resistance (the dawn phenomenon), illnesses, and periods of fasting.
Currently, there are three manufacturers selling pumps in the United States, with closed-loop insulin delivery in various phases of development:
Medtronic: commercial automated insulin delivery with a hybrid closed-loop system (670G)
Tandem: FDA-approved predictive low-glucose suspend, and initiating trials with a hybrid closed-loop system; also seeking FDA approval to function as an interoperable alternate controller–enabled (ACE) infusion pump so that it could be configured to work in multiple closed-loop systems (29)
Insulet: OmniPod tubeless “patch pump” with a hybrid closed-loop system under development
Important worldwide pump manufacturers selling pumps outside the United States are:
Roche: Having recently left the United States market, Roche Accu-Chek remains available in Europe. The Accu-Chek Spirit Combo pump can receive commands via Bluetooth and is compatible with an open-source control system called AndroidAPS.
SOOIL Development: The Diabecare RS insulin pump can receive Bluetooth commands from a smartphone, and the company supports the use of do-it-yourself (DIY) artificial pancreas software, such as AndroidAPS.
Control algorithms for glucose control
Control systems have been used since antiquity and only recently applied to the field of diabetes care. The described techniques have been used in industrial processes and adapted for maintaining a target blood sugar. In industry, they provide control for practically any variable that can be measured. These systems are discussed individually and a summary is provided in Table 2.
Table 2.
Comparison of Control Strategies
| Proportional, Integral, Derivative Controller | Model Predictive Control | Fuzzy Logic | |
|---|---|---|---|
| Method | Evaluate deviation from target glucose and use operations on these data to change insulin delivery | Model future glucose and deliver insulin to bring the predicted glucose into target range | Establish specific rules for what to do to insulin delivery based on available data |
| Systems | Medtronic 670G | Tandem | MD-Logic |
| OmniPod | |||
| Beta Bionics | |||
| Loop | |||
| OpenAPS | |||
| AndroidAPS | |||
| Benefits | Easy to implement | Good performance in subcutaneous hybrid closed loop | Can add additional data sources or perform operations on existing data sources to add finer levels of control |
| Does not require information about carbohydrate intake | Can add information from various sources to better predict future glucose | Possible optimizations with machine learning | |
| In theory, models can be tailored to an individual | |||
| Drawbacks | In unaltered state usually has worse performance vs other strategies | More difficult to implement | Often starts with generalized “expert” opinion to establish baseline rules. |
| Current commercial systems require additional modeling of insulin-on-board | Usually based on a “standard” model of absorption, which may not be applicable to all users | Difficult to perfect |
Although systems are designated by their primary control strategy, many use combinations of techniques.
Proportional, integral, derivative controllers
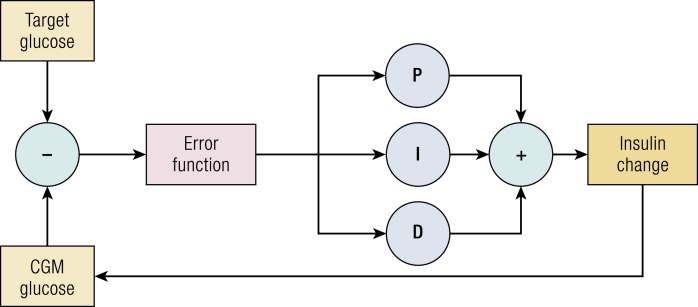
Proportional, integral, derivative (PID) control is among the most basic control systems. At each point in time, the controller assesses how far the current glucose is from the desired glucose. Insulin delivery is based on the difference at the current point of time (proportional), the rate of change over time (derivative), and the cumulative deviation above or below target (integral). Each of these three terms is weighted with a multiplier, which may be determined beforehand or tuned over time. These individual terms are summed to modify insulin delivery and thereby alter the measured glucose value (Fig. 1). In closed-loop insulin delivery, the difference term is the current CGM value subtracted from a defined goal blood sugar; the integral is the area under the curve of the difference term providing memory of prior controller action; and the derivative is the rate of change in the error term anticipating the trajectory of current changes.
Figure 1.
Diagram of a simple PID controller applied to closed-loop glucose control. P indicates proportional term (scalar factor multiplied by the difference between current CGM and target glucose); I indicates integral term (scalar factor multiplied by the area under the error function); and D indicates derivative term (scalar factor multiplied by the current rate of change in the error function).
In commercial systems, such as the Medtronic 670G, the standard PID algorithm has been combined with an insulin-on-board estimate. This method is often used to constrain the maximum insulin delivery and limit hypoglycemia (30).
Model predictive control controllers
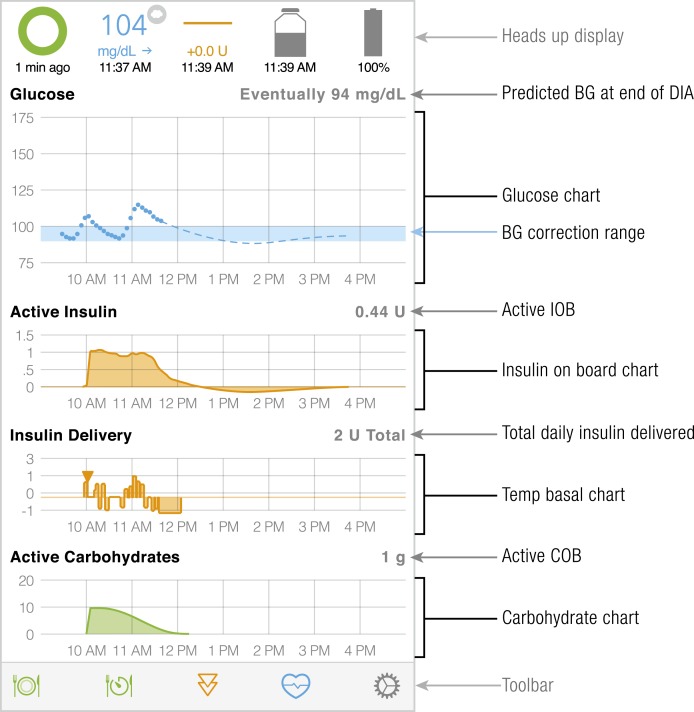
Model predictive control (MPC) relies on dynamic multicompartmental modeling of a system to predict an outcome after a fixed period of time. With respect to artificial pancreas systems, glucose is the dependent outcome being modeled, which is affected by carbohydrate intake, endogenous glucose production, and insulin-on-board. In closed-loop systems predictions are made for some period in the future, and the prediction is updated every 5 to 15 minutes with new sensor glucose measurement and new information on insulin delivery, carbohydrate intake, or any other available data. Some controllers will model diurnal variations (31), exercise, and accept additional inputs from an accelerometer or heart rate monitor. One of the main constraints on insulin delivery is the risk of hypoglycemia. A control strategy that minimizes hyperglycemia and hypoglycemia is applied and the process repeats itself when a new measurement becomes available. This process is illustrated in Fig. 2 for the main user interface of Loop, an open-source DIY artificial pancreas system.
Figure 2.
Status screen for Loop, an open-source DIY MPC that runs on Apple iOS. Glucose values received from the CGM are denoted with dots, and the dashed lines reflect the predicted glucose dependent on active insulin and active carbohydrates modeled in the graphs below. The system alters insulin delivery in an effort to keep the eventual glucose within target (reflected by the shaded blue area in the glucose graph). BG, blood glucose; COB, carbohydrates on board; DIA, duration of insulin action; IOB, insulin on board.
A 2016 study comparing MPC to PID controllers in 30 adults with type 1 diabetes using subcutaneous systems revealed greater time in the range of 74.4% with MPC controller vs 63.7% with the PID controller used in this study (P = 0.02) (32).
Beta Bionics is developing a bihormonal insulin and glucagon system that utilizes MPC for insulin delivery to a glucose goal of 100 mg/dL (5.6 mmol/L) and a proportional-derivative control algorithm for glucagon delivery to prevent or treat glucose levels <100 mg/dL (5.6 mmol/L) (33). Since the original description of the algorithm by Damiano and colleagues (33) in 2010, the insulin dosing algorithm has received feedback from the glucagon administration subroutine (34).
Multivariable adaptive artificial pancreas systems describe the addition of other measures to better model future glucose and improve MPC. Turksoy et al. (35) expanded the MPC system to include energy expenditure and galvanic skin response measured by the SenseWear Pro3 armband. In comparison with an open loop, the system significantly reduced severe hypoglycemia and increased time in the range from 54% to 58% (36). A few research groups reported the use of heart rate monitor or accelerometer to detect exercise. Although results indicate that exercise can be detected, evidence for changes in postexercise hypoglycemia is more limited (37–41). There is ongoing research on the measurement of physiologic stress as a variable in closed-loop systems (42). The use of automatic meal detection using various devices have also been proposed (43).
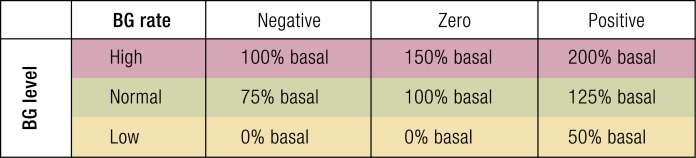
Fuzzy logic controllers
Fuzzy control systems take a set of inputs and apply conditional logic to produce an output control that is often based on “expert” opinion. Several such systems have been implemented for closed-loop glucose control. The inputs to these systems are CGM data and some of their derivatives, and the output is a dose of insulin to be delivered. MD-Logic, a proprietary implementation by Phillip and colleagues, has been shown to mitigate nocturnal hypoglycemia and increase time in the range of 90 to 140 mg/dL (5.0 to 7.8 mmol/L) (44). MD-Logic provides correction boluses in real time, which is now being tested on the Medtronic 670G pump. Another system, reported by Mauseth et al. (45), used current glucose, glucose velocity, and glucose acceleration to deliver insulin based on clinician expert opinion (45). A very basic example of a fuzzy-logic control system is illustrated in Fig. 3.
Figure 3.
A simple fuzzy logic controller using current blood glucose (BG) level and rate of change in blood glucose.
Overview of Current Controllers and Controller Configurations
Closing the loop: specific system configurations
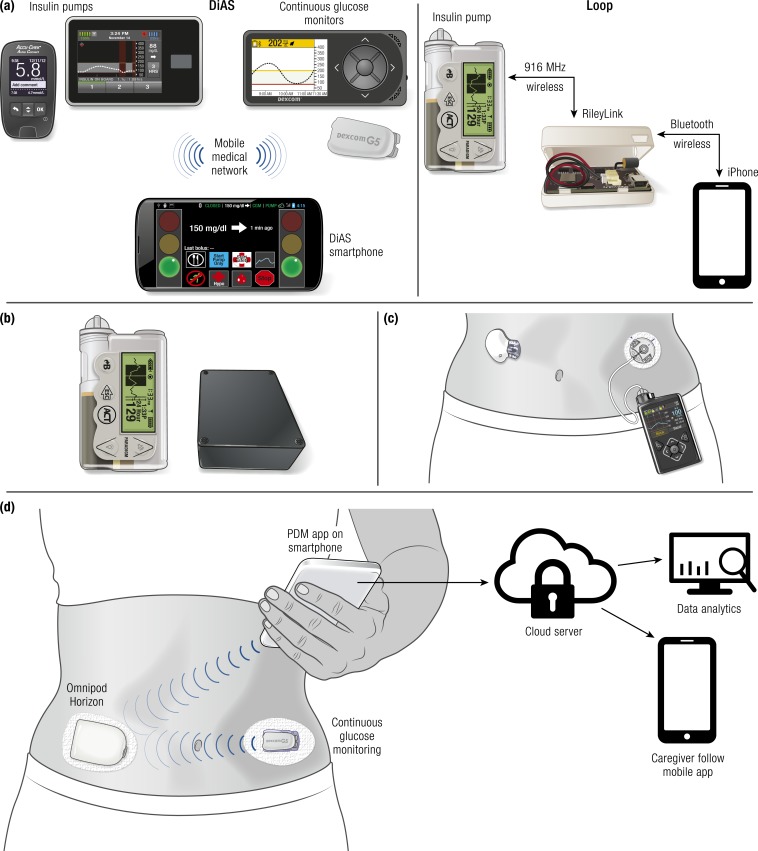
In an automated insulin delivery system there are multiple configurations for the functions of an insulin pump (see Fig. 4):
The pump can be a “servant” that reliably executes commands of a remote controller that has the algorithm to determine insulin doses (such as a smart phone or a dedicated controller). In hybrid automated insulin delivery, the meal boluses would be entered on the remote controller [Fig. 4(a)].
All interaction remains on the pump, but two-way communication with a remote controller (such as a smart phone or dedicated controller) adjusts insulin delivery on the pump based on CGM data. In hybrid automated insulin delivery, the meal boluses are entered on the pump and these data are received on the controller [Fig. 4(b)].
The algorithm for controlling insulin doses resides on the pump. In this configuration the pump receives the CGM data directly and does not communicate with another device [Fig. 4(c)].
The algorithm for controlling insulin doses resides on the pump and the pump receives the CGM data directly, but the pump also communicates with a remote device (such as a smartphone). In this configuration the sensor and pump data could be displayed remotely and could be sent to the cloud for real-time analysis, which could allow long-term adaptability of the algorithm [Fig. 4(d)].
Figure 4.
Automated insulin delivery configurations, with representative systems. (a) University of Virginia Diabetes Assistant (DiAS) (left): The user interacts only with the controller (Android phone). In this system all communication occurs through native Bluetooth without the need for any intermediary devices. Loop (right): The user interacts exclusively with the controller (iPhone) where he or she enters meal information. In this case, the iPhone commands the insulin pump through a Bluetooth-to-radio bridge known as the RileyLink. (b) Open Artificial Pancreas System (OpenAPS): The user interacts with the pump where he or she enters meal information. The “black box” modulates delivery based on data received from CGM and pump. (c) Medtronic 670G: The user interacts exclusively with the pump where he or she enters meal information. The pump is in direct communication with the proprietary sensor and holds the control algorithms. (d) OmniPod Horizon (planned future configuration): The user interacts with the smartphone where he or she enters meal information. The smartphone, insulet “patch pump,” and CGM communicate with each other directly via Bluetooth. The pump holds the control algorithms and data are sent from the smartphone to the cloud for additional services.
Independent microcontroller with independent insulin pump and sensor
Early and DIY systems use a CGM that can send data remotely to a separate microcontroller (computer, tablet, smartphone, or system on a chip) and a separate insulin pump that can be commanded by the same microcontroller. One of the first platforms developed by Dassau for multiple academic groups to use in testing algorithms was the artificial pancreas system. It was run on a personal computer under MATLAB and could be configure to work with Dexcom or Navigator sensors driving an OmniPod pump and could be used to test PID, MPC, and fuzzy logic algorithms (46). The University of Virginia Diabetes Assistant (DiAS) system utilizes a Dexcom sensor, Tandem or Roche Spirit Combo insulin pump and an Android smartphone controller with the ability to communicate with both CGM and pump (47). Systems using a smartphone allow for remote monitoring of sensor values and insulin boluses. Insulet recently tested their model predictive control algorithm using a tablet that received data from a Dexcom sensor transmitting wirelessly to a Dexcom receiver wired to a tablet and transmitting wireless to the OmniPod personal diabetes manager, and then to the OmniPod pump (48). The ultimate goal is for sensors to communicate directly with the pump and the pump to have Bluetooth connection to a smartphone for remote monitoring.
The #WeAreNotWaiting DIY movement is focused on empowering the diabetes community by providing tools to use existing devices and data to improve health outcomes. Several open-source platforms have been developed to provide artificial pancreas technology that is not FDA approved. One such system, OpenAPS, utilizes a system on a chip running a Linux variant with wireless capabilities. CGM data are transmitted via Nightscout and commands are issued to a pump (49). The Nightscout project puts CGM data on a personal Web site, through a variety of interfaces depending on the specific CGM system (50). Loop is another DIY artificial pancreas system that utilizes an iOS device to acquire Dexcom data and issues commands to an insulin pump through a Bluetooth bridge. The Dana Diabecare R is an insulin pump that can be issued commands directly over Bluetooth. The DIY AndroidAPS system makes use of an Android phone, acquiring CGM data through a variety of software methods and can issue commands directly to the Dana pump.
These hardware implementations allow for rapid prototyping and testing of new control systems in the research setting. Often the microcontroller running on this type of setup is far more powerful than what could be placed on an integrated circuit within the existing devices. DIY systems that combine existing proprietary hardware must often rely on this configuration to allow interoperability of several devices. The major problem with these systems is their reliance on multiple hardware components and resultant communication errors. When any single piece is inoperative, or there is any lack of communication between devices, the whole system fails.
Integrated systems with algorithm on the insulin pump and a separate sensor
After initial algorithm design has been performed on a general purpose microcontroller, the code is often ported to a specialized integrated circuit that is part of the insulin pump system. If the pump manufacturer does not produce the glucose sensor, data must be communicated from the CGM to the pump/controller. Insulet and Tandem are currently investigating such systems that pair with the Dexcom CGM via Bluetooth, and Bigfoot Biomedical is working with the Abbott FreeStyle® Libre™. These systems encourage collaboration between sensor and pump manufacturers. Indeed, the Dexcom G6 was labeled as an iCGM system used to describe CGM that reliably and securely transmits measurements to digitally connected devices (51). Likewise, Tandem is seeking approval for an integrated insulin delivery (iPump) system (ACE pump) that would allow for interoperability with a variety of closed-loop systems (29).
Fully integrated with sensor, pump, and algorithm on one device
A fully integrated system includes sensor and pump that communicate seamlessly and are built to work together. Medtronic is currently the only pump manufacturer producing their own sensors. The Medtronic 670G system has controller hardware inside the pump and utilizes proprietary communication with their CGM transmitter. The benefits of such a design are assured interoperability, optimization of power usage, and interface and communication between devices. Although reliable, the operation of this system is less transparent to the end user. Theoretical benefits of this type of system are software updates or customizations that can carry over to all hardware. Although not currently implemented, the same hardware may be optimized for level of aggressiveness or age of the patient, offering a unique experience depending on the user.
In automated hybrid insulin delivery systems the basal rates are adjusted in real time based on CGM data and meal boluses are provided by the patient. Correction doses for high glucose may be given by the automated insulin delivery system or may require user input. Some of the features of a standard insulin pump may be lost with automated insulin delivery systems. In the first commercial automated insulin delivery system, the Medtronic 670G, it is not possible to give extended boluses or to adjust basal insulin delivery, however the glucose set point can be raised for activity. To be effective this change in set point for exercise should be done 30 to 90 minutes before onset of activity.
IP systems
Beginning in the 1970s, several groups added insulin to dialysate in patients with diabetes undergoing peritoneal dialysis (52–54). IP insulin delivery results in faster drug pharmacokinetics and pharmacodynamics. By the early 1980s, the peritoneal route of insulin delivery was used in those with diabetes not receiving concurrent peritoneal dialysis. In these patients, a catheter was placed percutaneously 5 to 7 mm into the peritoneum and connected to an externally worn pump (55–58). Implanted pumps manufactured by Siemens, Infusaid, and MiniMed soon followed (59). The last implanted IP pump manufactured was the Medtronic MIP 2007. This pump was combined with an IV glucose sensor for full closed-loop control but had limited performance due to the newly developed sensor (60). Subsequent studies combined the implanted pump with a subcutaneous sensor and demonstrated greater time in range during closed loop with a PID controller vs open loop (61). The most recent studies have used the Accu-Check DiaPort peritoneal catheter that connects to an external insulin pump and a subcutaneous CGM. Using a zonal MPC controller, Dassau et al. (62) demonstrated superior full closed-loop control using the IP system with regular insulin vs a subcutaneous system with fast-acting insulin analog. Additional research in control theory, insulin, and sensor kinetics is required to develop fully closed-loop implanted insulin delivery.
First-Generation Hypoglycemia Prevention and Overnight Controllers
In the DCCT, 55% of severe hypoglycemic events occurred at night (63), and in children 75% of seizures occurred overnight (64). As of 2015, 6% of T1D Exchange Clinic Registry participants reported having experienced a seizure or loss of consciousness due to hypoglycemia in the prior 3 months (11). Fear of hypoglycemia limits therapy intensification efforts and can adversely affect the lives of patients with type 1 diabetes and their families (65). Using real-time continuous glucose monitoring, people with diabetes can monitor their glucose values frequently during the day and respond to alarms for hypoglycemia and hyperglycemia. When they are sleeping, however, they fail to awaken to 71% of nocturnal alarms (66). In a randomized trial in children and adults with 36,467 nights of CGM data, the glucose level was <60 mg/dL (3.3 mmol/L) for at least 10 consecutive minutes on 8.5% of nights, and on 23% of those nights, the duration was >2 hours (67). There is a greater frequency of severe hypoglycemia during sleeping than during waking hours (63, 64, 68). Prolonged nocturnal hypoglycemia may result in the “dead-in-bed” syndrome, which is possible due to cardiac arrhythmias triggered by hypoglycemia and/or associated hypokalemia (69). One of the obvious first uses of an automated insulin delivery system was to decrease the incidence of nocturnal hypoglycemia by stopping insulin delivery when there is low blood glucose (threshold suspend). These systems were successful in reducing nocturnal hypoglycemia by 38% without an increase in HbA1c levels (70). The next step in protection against hypoglycemia was to use a predictive low-glucose suspend system. In 5332 randomized nights of testing a predictive low-glucose suspend system in patients with type 1 diabetes from 4 to 45 years of age, the number of nights with glucose <60 mg/dL (3.3 mmol/L) for >120 minutes was reduced by 60% (11- to 45-year-olds) to 80% (4- to 10-year-olds) when compared with nights when the system was not active (71, 72). Medtronic Diabetes has a commercial predictive low-glucose suspend system (640G) (73–76). It has been tested in two randomized clinical trials in children and adolescents (77, 78), and in both trials there was an ∼40% decrease in the time <65 mg/dL (3.6 mmol/L) and <54 mg/dL (3.0 mmol/L). In both studies, however, there were mild increases in hyperglycemia, although HbA1c levels were not increased during 6 months (78). Tandem Diabetes has also evaluated a predictive low-glucose suspend algorithm, which showed a 31% reduction in percent CGM time <70 mg/dL (3.9 mmol/L) without a significant increase in hyperglycemia (79). They received FDA approval for this algorithm (Basal-IQ) on their pump in June 2018, and it was released to patients via a downloadable upgrade to their existing pumps that can be performed at home. Tandem’s Basal-IQ pump works with the factory-calibrated Dexcom G6 sensor.
The next step in overnight glucose control was to minimize both hypoglycemia and hyperglycemia with automated insulin delivery. In one of the first major publications for automated insulin delivery, Phillip et al. (80) demonstrated in a diabetes camp that automated insulin delivery decreased overnight hypoglycemia and hyperglycemia when compared with sensor-augmented pump therapy without automated insulin delivery. The Cambridge group, led by Roman Hovorka, subsequently demonstrated in a randomized controlled outpatient trial without remote monitoring that automated insulin delivery significantly decreased both hypoglycemia and hyperglycemia and improved mean glucose values when compared with sensor-augmented pump therapy without automated insulin delivery (81). The same group subsequently conducted a 3-month randomized in-home closed-loop study for children and adolescents and again demonstrated significant improvements in overnight hypoglycemia and hyperglycemia, as well as increased time in range [70 to 145 mg/dL (3.9 to 8.0 mmol/L)], compared with sensor-augmented pump therapy (82). However, without automated insulin delivery during the day, there was no improvement in HbA1c.
These studies have demonstrated that automated insulin delivery at night, whether by threshold suspend, predicted low-glucose suspend, or hybrid closed loop, can significantly decrease the risk for nocturnal hypoglycemia. Additionally, the hybrid closed-loop systems significantly improve time in range and fasting glucose values. Subsequent studies described below have used hybrid closed-loop control 24/7 to not only improve nocturnal glycemic control, but also to improve daytime glycemic control. As an example, Hovorka and colleagues (83) have subsequently conducted a 3-week cross-over study of day-and-night closed loop in suboptimal controlled adolescents and showed a significant improvement in time in range, as well as decreased hyperglycemia and an improved mean glucose. Using the same algorithm but a new hardware configuration with an Android phone and a 640G pump, they conducted a 3-month study showing improved HbA1c levels of 7.4% compared with 7.7% in the closed-loop group when compared with the sensor-augmented pump group, improved time in range [70 to 180 mg/dL (3.9 to 10 mmol/L)] of 65% compared with 54%, and a lower time <70 mg/dL (3.9 mmol/L) of 2.6% compared with 3.9% (84). In the subsequent section we have provided a table of 24/7 hybrid closed-loop control studies showing mean glucose values, time in range, and percent time <70 mg/dL (3.9 mmol/L) when these measurements were reported in the study outcomes.
24/7 Closed-Loop Control
Trials testing the efficacy and safety of the closed-loop systems have progressed from initial studies in adults to studies in adolescents and young children, and from inpatient settings to transitional, supervised studies in camps, rental homes, and hotels to free-range in-home and work environments with and without remote monitoring by research staff. Hybrid closed-loop systems automate insulin delivery based on CGM glucose values that adapt daily but they require user-initiated boluses for carbohydrates and/or optional correction doses. Overall, closed-loop systems typically reduce mean glucose, increase time in range, and reduce the risk of hypoglycemia. However, despite improved overnight and preprandial glycemic control, postprandial glucose excursions remain challenging.
Insulin-only hybrid closed loop
Tables 3–6 (47, 48, 80–83, 85–108) include the clinical trials testing insulin-only closed-loop systems. In addition to considering methodological differences, these studies were written prior to the development of standardized glycemic outcomes. We have therefore denoted when median instead of mean values were used and when the currently accepted standards for time in range and hypoglycemia thresholds were not used. Some of the study designs did not include a control arm and compared the results to the participants’ baseline information using their usual care system. With enrollment into a study there can often be a significant improvement in glucose control during the “run-in” phase (84), and many studies did not incorporate a “run-in” period. Remote CGM is an important factor that may affect outcomes depending on when the remote monitor intervenes. We have therefore separated the results of the studies to those with and without remote monitoring.
Table 3.
Insulin-Only Closed-Loop Inpatient Studies
| System | n | Study Outcomes: Closed Loop vs Open Loop | ||
|---|---|---|---|---|
| Average Glucose ± SD [mg/dL (mmol/L)] or Median (IQR1, IQR3) | % CGM Time 70–180 mg/dL (3.9–10.0 mmol/L) | % CGM Time <70 mg/dL (3.9 mmol/L) | ||
| Inpatient studies | ||||
| MD-Logic 2012 (85)a | 7 | CGM 122 ± 16 (6.8 ± 0.9) | 63–140 mg/dL (3.5–7.8 mmol/L) | <63 mg/dL (3.5 mmol/L) |
| Glucose meter | 83% vs 34% | 0% vs 7% | ||
| 129 ± 12 (7.2 ± 0.7) vs 160 ± 57 (8.9 ± 3.2) | ||||
| Medtronic 2012 (86) | 4 | No control | No control | No control |
| PID + IFB: 153 ± 54 (8.5 ± 3.0) | PID + IFB 70% | PID + IFB 2% | ||
| PID: 133 ± 56 (7.4 ± 3.1) | PID 73% | PID 9% | ||
| Medtronic 2015 (87) | 8 | No control | No control | No control |
| 152 ± 54 (8.4 ± 3) | 67.6% during the day | 2% during the day | ||
| Medtronic 2016 (88)a | 16 | Not reported | No control | No control |
| 70–150 mg/dL (3.9–8.3 mmol/L) | 0% | |||
| 63% | ||||
| DiAs-MMPC algorithm 2017 (47) | 10 | Inpatient: 142 (7.9) | Inpatient: 78% | <50 mg/dL (2.8 mmol/L) |
| Hotel: 152 (8.4) | Hotel: 73% | Inpatient: 0.05% | ||
| Open Loop: 160 (8.9) | Open Loop: 62% | Hotel: 0.2% | ||
| Open loop: 0.4% | ||||
| Insulet 2018 (48) | 58 | No control (vs prior open loop) | No control (vs prior open loop) | No control (vs prior open loop) |
| Adults: 161.5 ± 20.1 (8.9 ± 1.1) vs 155 ± 22.6 (8.6 ± 1.3) | Adults: 69.5% vs 63.8% | Adults: 0.7% vs 5.2% | ||
| Adolescents: 153.4 ± 21.6 (8.5 ± 1.2) vs 165.3 ± 28.3 (9.2 ± 1.6) | Adolescents: 72.6% vs 60% | Adolescents: 2% vs 3.5% | ||
| Pediatrics: 156.9 ± 20.4 (8.7 ± 1.1) vs 160.7 ± 21.1 (8.9 ± 1.2) | Pediatrics: 70.1% vs 63.5% | Pediatrics: 2% vs 3.2% | ||
We use italics when our standards for time in range and hypoglycemia thresholds were not used.
Abbreviations: IFB, insulin feedback algorithm; IQR, interquartile range; MMPC, modular model predictive control.
Overnight glycemic outcomes.
Table 6.
Insulin-Only Closed-Loop Outpatient Studies Without Monitoring (Free-Living Without Direct Supervision or Real-Time Remote Monitoring)
| System | n | Study Outcomes: Closed Loop vs Open Loop | ||
|---|---|---|---|---|
| Average Glucose ± SD (mg/dL (mmol/L)) or Median (Interquartile Range) | % CGM Time 70–180 mg/dL (3.9–10.0 mmol/L) | % CGM Time <70 mg/dL (3.9 mmol/L) | ||
| Outpatient studies <2 wk without monitoring | ||||
| Florence 2014 (104) | 17 | 146 ± 18 (8.1 ± 1.0) vs 158 ± 18 (8.8 ± 1.0) | 75% vs 62% | 3.7% vs 5.0% |
| Florence 2016 (105) | 12 | 156.6 ± 19.8 (8.7 ± 1.1) vs 181.8 ± 23.4 (10.1 ± 1.3) | 72% vs 53% | 2.9% vs 1.7% |
| Outpatient studies 2 wk–3 mo without monitoring | ||||
| Florence 2014 (106) | 24 | 148 ± 16 (8.2 ± 0.9) vs 162 ± 23 (9.0 ± 1.3) | 73.2% vs 61.2% | 1.8% vs 2.1% |
| Florence 2014 (81) | 16 | 137 ± 32 (7.6 ± 1.8) vs 151 ± 52 (8.4 ± 2.9) | 85% vs 69% | 0.9% vs 1.4% |
| Florence 2015 (82) | 58 | 157 ± 19 (8.7 ± 1.1) vs 168 ± 28 (9.3 ± 1.6) | 68% vs 57% | 2.9% vs 3% |
| Florence 2016 (83) | 12 | 157 ± 16 (8.7 ± 0.9) vs 189 ± 32 (10.5 ± 1.8) | 66.6% vs 47.7% | 4.3% vs 2.4% |
| Outpatient studies >3 mo without monitoring | ||||
| Medtronic 2016 (107) | 124 | 150.8 ± 13.7 (8.4 ± 0.8) vs 150.2 ± 22.7 (8.3 ± 1.3) (baseline) | 72.2% vs 66.7% | 3.3% vs 5.9% |
| Medtronic 2018 (108) | 31 | Not reported | 67.4%–69% vs 55.3% (Time in range was reported for four 7-d time points: days 1–7, 22–28, 50–56, and 78–84) | Not reported |
We use italics when median instead of mean values were reported and when the currently accepted standards for time in range and hypoglycemia thresholds were not used.
Table 4.
Insulin-Only Closed-Loop Transitional Studies Conducted in Camp, Hotel, or Airbnb Settings
| System | n | Study Outcomes: Closed Loop vs Open Loop | ||
|---|---|---|---|---|
| Average Glucose ±SD [mg/dL (mmol/L)] or Median (IQR1, IQR3) | % CGM Time 70–180 mg/dL (3.9–10.0 mmol/L) | % CGM Time <70 mg/dL (3.9 mmol/L) | ||
| Transitional studies <48 h | ||||
| MD-Logic 2013 (80)a | 54 | 126.4 (115.7–139.1) [7 (6.4–9.3)] vs 140.4 (105.7–167.4) [7.9 (5.9–9.3)] | 70–140 mg/dL (3.9–7.8 mmol/L) | Time reported, rather than percent 7.6 min vs 16.4 min |
| Time reported, rather than percent 4.4 h vs 2.8 h | ||||
| DiAs 2014 (89) | 6 | Not reported | 94.8% vs 68.2% | 1.25% vs 11.9% |
| DiAs 2014 (90)a | 20 | 147 ± 34 (8.2 ± 1.9) vs 146 ± 42 (8.1 ± 2.3) | 70–150 mg/dL (3.9–8.3 mmol/L) | Not reported |
| 62% vs 55% | ||||
| DiAs 2014 (91) | 18 | 161.3 ± 2.49 (9.0 ± 0.1) vs 152.1 ± 2.44 (8.4 ± 0.1) | 66.1% vs 70.7% | 0.7% vs 1.25% |
| Transitional studies >48 h | ||||
| MD-Logic 2014 (92)a | 15 | 133.5 (123.9–145.8) [7.4 (6.9–8.1)] vs 130 (113.1–152.4) [7.2 (6.3–8.5)] | Time reported, rather than percent 4.4 h vs 3.1 h | Time reported, rather than percent 3.8 min vs 48.7 min |
| DiAs 2015 (93)a | 10 | 139.0 (123–158) [7.7 (6.8–8.8)] vs 170.3 (133–200) [9.5 (7.4–11.1)] | 85.4% vs 59.1% | 0.55% vs 1.56% |
| DiAs 2016 (94) | 33 | 143 ± 3 (7.9 ± 0.2) vs 156 ± 5 (8.7 ± 0.3) | 78.6% vs 65.4% | 1.8% vs 4.2% |
| DiAs 2016 (95) | 30 | 169 ± 23 (9.4 ± 1.3) vs 147 ± 23 (8.2 ± 1.3) | 56.8% vs 63.1% | 2% vs 6.7% |
| Medtronic 2015 (87) | 21 | Reported on daily basis | 69.9% vs 73.1% | Not reported |
| Medtronic 2016 (88)a | 21 | 132 (119–144) [7.3 (6.6–8.0)] vs 128 (115–141) [7.1 (6.4–7.8)] | 79.9% vs 60% | 5.4% vs 19.5% |
| Medtronic 2016 Android-based hybrid closed loop (96) | 9 | No control | 80% | 0.79% |
| 145 ± 43 (8.0 ± 2.4) | ||||
We use italics when our standards for time in range and hypoglycemia thresholds were not used.
Abbreviation: IQR, interquartile range.
Overnight glycemic outcomes.
Table 5.
Insulin-Only Closed-Loop Outpatient Studies With Monitoring (Participants’ Glucose Data Monitored in Real Time Remotely by Study Providers)
| System | n | Study Outcomes: Closed Loop vs Open Loop | ||
|---|---|---|---|---|
| Average Glucose ± SD [mg/dL (mmol/L)] or Median (Interquartile Range) | % CGM Time 70–180 mg/dL (3.9–10.0 mmol/L) | % CGM Time <70 mg/dL (3.9 mmol/L) | ||
| Outpatient studies <2 wk with monitoring | ||||
| Medtronic 2015 (97) | 8 | 158.4 ± 55.8 (8.8 ± 3.1) vs 165.6 ± 61.2 (9.2 ± 3.4) | 67.4% vs 61.0% | <60 mg/dL (3.3 mmol/L) |
| 0.54% vs 1.13% | ||||
| Medtronic 2017 (98) | 9 | 152 ± 14 (8.4 ± 0.8) vs 144 ± 15 (8.0 ± 0.8) | 72% vs 68% | 2% vs 7.6% |
| Medtronic 2017 (98) | 15 | 153 ± 17 (8.5 ± 0.9) vs 171 ± 30 (9.5 ± 1.7) | 70% vs 55% | 2.5% vs 2.7% |
| Android hybrid closed loop 2016 (99) | 28 | 144.1 ± 18.9 (8.0 ± 1.0) vs 142.1 ± 24.3 (7.9 ± 1.3) | 70–140 mg/dL (3.9–7.8 mmol/L) | <72 mg/dL (4.0 mmol/L) |
| 59.4% vs 53.2% | 0% vs 0% | |||
| Outpatient studies 2 wk–3 mo with monitoring | ||||
| MD-Logic 2014 (100)a | 24 | 147.72 ± 15.84 (8.2 ± 0.9) vs 161.28 ± 25.1 (9.0 ± 1.4) | 72.87% vs 52.72% | 2.53% vs 5.16% |
| DiAs 2016 (101) | 30 | 153 ± 12 (8.5 ± 0.7) vs 157 ± 18 (8.7 ± 1.0) | 73% vs 65% | 1.7% vs 4.1% |
| DiAs 2015 (102) | 32 | 160.2 ± 9 (8.9 ± 0.5) vs 163.8 ± 9 (9.1 ± 0.5) | 63.7% vs 59.4% | 2.6% vs 3.6% |
| Outpatient studies >3 mo with monitoring | ||||
| DiAs 2017 (103) | 14 | 149 ± 10.8 (8.3 ± 0.6) vs 155 ± 19.8 (8.6 ± 1.1) | 77% vs 66% | 1.30% vs 4.1% |
We use italics when our standards for time in range and hypoglycemia thresholds were not used.
Indicates overnight glycemic outcomes.
Bihormonal hybrid closed loop
The current sensor-augmented pump technology and insulin-only closed-loop systems allow for temporary insulin suspension when a patient’s blood glucose is trending toward, or is already in, the hypoglycemic range. Such measures can reduce the rate of hypoglycemic episodes compared with standard pump therapy. However, because even the most rapid-acting insulin has relatively slow absorption, an insulin suspension system cannot halt the actions of preadministered insulin, which may continue to lower blood glucose even when the patient is already trending toward hypoglycemia. Thus, although insulin suspensions may help reduce the severity and duration of hypoglycemia, they will not always be successful in preventing hypoglycemia (109). Within a year of type 1 diabetes diagnosis there is a loss of the glucagon response to hypoglycemia as well as a dysregulation of glucagon secretion with high levels immediately after a meal and decreased secretion of glucagon when the glucose is falling in the latter stages of a meal (110).
Dual-hormone closed-loop delivery systems have been proposed to provide more physiologic islet cell replacement (111–114). These systems combine subcutaneous insulin and glucagon delivery by pump to further reduce the risk of hypoglycemia. These bihormonal (also known as dual-hormone) closed-loop systems deliver subcutaneous glucagon when hypoglycemia is detected or predicted. Bihormonal systems can be adjusted to administer insulin in the same way as insulin-only closed-loop systems or more “aggressively,” anticipating that glucagon may mitigate insulin over delivery (115). A dual-hormone system will allow a lower average glucose concentration with a lower risk of hypoglycemia because aggressive insulin delivery can be countered with glucagon. The requirement for a second infusion pump makes the bihormonal systems more complicated. The infusion set and the glucagon cartridge need to be replaced daily because the current glucagon preparations are unstable in the infusion sets after 24 hours (116). Potential cytotoxic amyloid fibril formation of glucagon in aqueous solution, pump, and infusion set occlusions limits the usage of glucagon in bihormonal systems (117). Stable and novel formulation of glucagon for a dual-hormone pump is key to the development of bihormonal closed-loop systems (116, 118). Long-term safety studies are required to evaluate the effect of glucagon on the liver and cardiovascular system and to assess long-term tolerability. Increased glucagon delivery might also be associated with increased gastrointestinal symptoms such as nausea or vomiting. Longer-term studies are required to compare the usage of glucagon in terms of dosage and timing in addition to the potential side effects. A summary of outcomes of bihormonal systems (119) is provided in Table 7 (33, 34, 120–130).
Table 7.
Bihormonal Closed-Loop Studies in Various Settings
| System | n | Study Outcomes: Closed Loop vs Open Loop | ||
|---|---|---|---|---|
| Average Glucose ± SD [mg/dL (mmol/L)] | % CGM Time 70–180 mg/dL (3.9–10.0 mmol/L) | % CGM Time <70 mg/dL (3.9 mmol/L) | ||
| Median (IQR1, IQR3) or Median (IQR) | ||||
| Bionic Pancreas 2010 (33)a | 6 | Fast PK controller 140 ± 9 (7.8 ± 0.5) | Fast PK controller 74% | Fast PK controller <1% |
| Slow PK controller 173 ± 18 (9.6 ± 1.0) | Slow PK controller 56% | Slow PK controller <1% | ||
| Bionic Pancreas 2014 (120)b | 24 | Meal priming 129 ± 8 (7.2 ± 0.4) | Meal priming 80% | 5.1% vs 3.6% |
| No meal priming 140 ± 8 (7.8 ± 0.4) | No meal priming 70% | |||
| Bionic Pancreas 2014 (121) | 20 | Adults (no control group) 138 ± 14 (7.7 ± 0.8) | Day 2–5 86.5% | 4.8% |
| 32 | Adolescents 138 ± 18 (7.7 ± 1.0) vs 157 ± 27 (8.7 ± 1.5) | Day 2–5 86.9% vs 66.7% | 2.6% vs 3.3% | |
| Bionic Pancreas 2016 (122) | 19 | Day 2–5 136.8 ± 10.8 (7.6 ± 0.6) vs 167.4 ± 30.6 (9.3 ± 1.7) | 80.6% vs 57.6% | <2.9% vs 6.1% |
| Bionic Pancreas 2017 (34) | 43 | 140.4 ± 10.8 (7.8 ± 0.6) vs 162 ± 28.8 (9.0 ± 1.6) | 78.4% vs 61.9% | <60 mg/dL (3.3 mmol/L) |
| 0.65% vs 1.9% | ||||
| Inreda bihormonal closed loop 2010 (123) | 5 | Not reported | 60% vs 31% | 11% vs 19% |
| Inreda bihormonal closed loop 2012 (124) | 10 | 156.6 vs 162 (8.7 vs 9.0) (SD not reported) | 61.2% vs 62.3% | 4.1% vs 5.3% |
| Bihormonal: Oregon University 2014 (125) | 7 | 153 (8.5) | 73.10% | 1.30% |
| Inreda bihormonal closed loop 2014 (126) | 11 | Day 1: 132 (40) [7.38 (2.23)] vs 149 (15) [8.27 (0.83)] | Day 1: 79.2% vs 67.2% | Day 1: 2.1% vs 0.7% |
| Day 2: 139 (41) [7.70 (2.29)] vs 159 (16) [8.84 (0.87)] | Day 2: 76.5% vs 66.0% | Day 2: 0% vs 2.8% | ||
| Inreda Diabetic 2016 (127) | 10 | 133.2 (131.4–145.8) [7.4 (7.3–8.1)] vs 145.8 (133.2–167.4) [8.1 (7.4–9.3)] | 84.7% vs 68.5% | 1.3% vs 2.4% |
| Haidar 2013 (128) | 15 | 140 ± 20 (7.8 ± 1.1) vs 142 ± 34 (7.9 ± 1.9) | 70.7% (46.4%–88.4%) vs 57.3 (25.2–71.8) | 0.0% (0.0%–3.0%) vs 10.2% (0.0%–13.0%) |
| Haidar 2015 (129) | 25 | 167.4 ± 25.2 (9.3 ± 1.4) | 72–180 mg/dL (4.0–10 mmol/L) | <72 mg/dL (4.0 mmol/L) |
| Single hormone: 145.8 ± 30.6 (8.1 ± 1.7) | Conventional 29% | 3.4% (0–11.0) conventional therapy | ||
| Dual hormone: 138.6 ± 30.6 (7.7 ± 1.7) | Single hormone 55% | 3.1% (0.0–6.9) insulin only | ||
| Dual hormone 63% | 0% dual hormone | |||
| Haidar 2016 (130) | 28 | Open Loop 121 (104–140) [6.7 (5.8–7.8)] | 72–180 mg/dL (4.0–10 mmol/L) | <72 mg/dL (4.0 mmol/L) |
| Single hormone 112 (104–122) [6.2 (5.8–6.8)] | Open loop 70% (58%–81%) | Open loop 14% (4%–28%) | ||
| Dual hormone 112 (104–126) [6.2 (5.8–7.0)] | Single hormone 91% (76%–97%) | Single hormone 5% (0%–13%) | ||
| Dual hormone 93% (81%–99%) | Dual hormone 1% (0%–8%) | |||
We use italics when our accepted standards for time in range and hypoglycemia thresholds were not used.
Abbreviation: IQR, interquartile range.
“Fast PK Group”: Model parameters include a 33-min time-to-peak (tmax) and 3.25-h time to 95% clearance (t95%). “Slow PK Group”: tmax of 65 min, t95% of 6.5 h.
Participants were randomized either to receive or not receive automatically adaptive meal-priming boluses.
Full closed-loop systems
A fully automated closed-loop system should not require meal or physical activity announcements to the algorithm, and it would significantly reduce the burden of diabetes. Short-term studies [see Table 8 (131–137)] have shown that a fully automated system can improve glycemic control and decrease hypoglycemia. However, ideal glycemic control following unannounced meals is not feasible with the current delays associated with subcutaneous insulin delivery.
Table 8.
Full Closed-Loop Studies
| System | n | Study Outcomes: Closed Loop vs Open Loop | ||
|---|---|---|---|---|
| Average Glucose ± SD [mg/dL (mmol/L)] | % CGM Time 70–180 mg/dL (3.9–10.0 mmol/L) | % CGM Time <70 mg/dL (3.9 mmol/L) | ||
| Steil 2006 (131) | 10 | 133 ± 52 (7.4 ± 2.9) | 75% vs 63% | Not reported |
| 133 ± 63 (7.4 ± 3.5) | ||||
| Medtronic 2008 (132) | 17 | HCL: 135 ± 45 (7.5 ± 2.5) | 85% | 3% |
| FCL: 141 ± 55 (7.8 ± 3.1) | ||||
| MD-Logic 2010 (133) | Not reported | 73% vs 58% | 0% vs 9% | |
| Fuzzy logic controller 2013 (134) | 3 | Not reported | 56.10% | 1.40% |
| DiAs 2012 (135) | 38 | sCTR: 150.1 ± 5.1 (8.3 ± 0.3) | sCTR: 74.4% | Not reported |
| eCTR: 120.2 ± 5.1 (6.7 ± 0.3) | eCTR: 90.1% | |||
| MMPC 2013 (136) | 39 | 132 ± 47 (7.3 ± 2.6) | 80–180 mg/dL (4.4–10 mmol/L) | 2% |
| 68% | ||||
| Maseuth 2013 (45) | 7 | 165 (9.2) (SD not reported) | 65.00% | 0.10% |
| MMPC 2014 (137) | 4 | 167 (9.3) cohort 1 (SD not reported) | 62% cohort 1 (initial cohort) | 0.1% cohort 1 |
| 6 | 142 (7.9) cohort 2 (SD not reported) | 78% cohort 2 (revised algorithm) | 2.1% cohort 2 | |
We use italics when our accepted standards for time in range were not used.
Abbreviations: eCTR, enhanced control to range; FCL, full closed loop; HCL, hybrid closed loop; MMPC, modular model predictive control; sCTR, standard control to range.
“…automated insulin delivery has advanced from closely monitored research studies to become the standard of care for subcutaneous insulin delivery.”
There have been several systematic reviews and meta-analysis of randomized trials comparing artificial pancreas systems with conventional pump therapy in outpatient settings in adults and children with type 1 diabetes. In a review in 2017 by Weisman et al. (138), they made 27 comparisons from 24 studies, which included 585 participants. Use of artificial pancreas systems resulted in a robust 12% greater time in blood glucose target range [70 to 180 mg/dL (3.9 to 10 mmol/L)], which is equivalent to a reduction in HbA1c by a minimum of 0.3% (3.3 mmol/mol) and reduced the percent of time in hypoglycemia [<70 mg/dL (3.9 mmol/L)] to 2.45%, a 50% reduction when compared with the control group (138). In a review and meta-analysis by Bekiari et al. (139) in 2018, they made 44 comparisons from 40 studies that included 1027 participants. Use of artificial pancreas systems resulted in a 9.6% increased time in the target blood glucose range [70 to 180 mg/dL (3.9 to 10 mmol/L)], which is equivalent to a reduction in HbA1c by a minimum of 0.3% (3.3 mmol/mol) and reduced the percent of time in hypoglycemia [<70 mg/dL (3.9 mmol/L)] by 1.34% or ∼20 minutes each day when compared with the control group. In both meta-analyses improvement in overnight glucose control was very significant, and the greatest improvements in control were seen overnight.
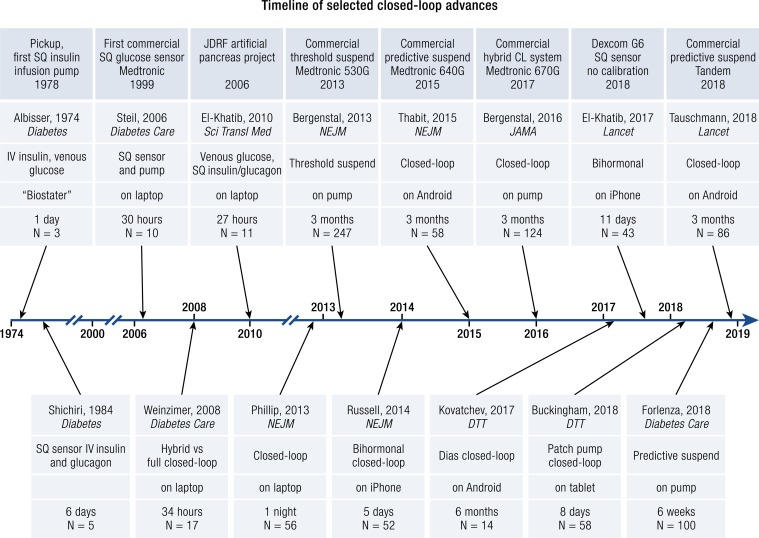
Timeline of selected achievements in the field of automated closed-loop systems
To provide a perspective on these studies, we present a timeline of some selected publications and milestones in the development of automated insulin delivery in Fig. 5. The first artificial pancreas used for research and inpatient patient care was developed in 1972. It used continuous withdrawal of blood for glucose measurements and IV insulin and glucose infusions to reach a glucose set point (140, 141). This system became a commercial product, the “Biostater,” which has been used in >200 publications and is still used today for glucose clamp studies, although there is no longer commercial support for the system. With the development of subcutaneous insulin delivery by infusion pumps, and the ability to measure glucose subcutaneously, ambulatory outpatient automated insulin delivery became feasible. These systems were first tested in inpatient settings using laptop computers (131) and then moved to closely monitored outpatient studies and then longer outpatient studies without remote monitoring. Initially, commercial systems were designed to prevent hypoglycemia by suspending insulin delivery based on the sensor glucose, and then based on predicted hypoglycemia. As the subcutaneous sensor accuracy improved, these systems were able to mitigate both hyperglycemia and hypoglycemia. Now all future insulin pumps will incorporate automated insulin delivery, and this has become the new standard of care for subcutaneous ambulatory insulin pump therapy.
Figure 5.
Timeline. Selected references of automated insulin delivery are noted across the timeline, with selected commercial milestones highlighted across the top. CL, closed loop; JDRF, Juvenile Diabetes Research Foundation; SQ, subcutaneous.
Adjunctive Therapy Integrated Into Closed-Loop Control
As discussed below, the addition of other medications may improve time in range while using a closed-loop system. These gains must be balanced against the additional burden placed on patients in administering additional agents.
Pramlintide
Pramlintide, a synthetic analog of amylin, is another protein therapeutic agent used to control blood glucose levels. Amylin is a protein that is cosecreted with insulin from pancreatic β-cells and is also absent in type 1 patients with diabetes (142). Amylin slows gastric emptying, suppresses the mobilization of glycogen stores by inhibiting glucagon excretion, and prolongs fullness. Pramlintide reaches peak serum concentrations in 20 minutes and has been shown to reduce postprandial spikes in blood glucose more effectively than insulin alone (143). In 2012, Weinzimer et al. (144) assessed the effect of 30 μg of pramlintide subcutaneously before meals with a PID closed-loop system vs closed loop alone. Eight adolescent and young adults with type 1 diabetes used the closed-loop device alone for 24 hours followed by 24 hours with the pramlintide intervention. There was a statistically significant reduction in time-to-peak blood glucose from 2.5 ± 0.9 hours to 1.5 ± 0.5 hours and decreased glycemic excursion from 113 ± 32 mg/dL (6.3 ± 1.8 mmol/L) to 88 ± 42 mg/dL (4.9 ± 2.3 mmol/L) with pramlintide. During the 2018 American Diabetes Association meeting, Haidar presented clinical trial data in which adults underwent three 24-hour inpatient experiments with automated insulin delivery and the time in range [70 to 180 mg/dL (3.9 to 10 mmol/L)] with regular insulin and pramlintide was 72%, with rapid-acting insulin and pramlintide was 85%, and with rapid insulin alone was 71% (145). In these studies, the pramlintide and insulin were infused through separate insulin infusion pumps because of pH incompatibility. Pramlintide, which is formulated at pH 4, cannot currently be coformulated with insulin owing to poor stability at pH 7.13. A coformulation of insulin and pramlintide has the potential to reduce patient burden and allows for the adoption of replacement therapy that more closely mimics endogenous hormone secretion from the β-cell for improved glycemic control. There are several companies working on coformulation of a rapid-acting insulin with pramlintide. Adocia presented data at the 2018 European Association for the Study of Diabetes meeting in Berlin using a coformulation of 10 U of lispro with 60 µg of pramlintide that resulted in a 97% reduction in postprandial glucose excursions for the first 2 hours compared with lispro alone.
Glucagon-like peptide-1 agonists
Glucagon-like peptide-1 (GLP-1) agonists slow gastric emptying, enhance satiety, reduce postprandial glucagon, and increase glucose-dependent insulin secretion (in those with intact endogenous insulin production). A 2016 study compared prandial glycemic excursions with pramlintide and the GLP-1 agonist liraglutide in a PID closed-loop controller. With 30 µg of pramlintide given subcutaneously before meals there was a statistically significant reduction in time-to-peak blood glucose from 2.9 ± 0.9 hours to 1.6 ± 0.5 hours, a 39% decrease in peak postprandial glucose, and a 40% decrease in area under curve for pramlintide. With 1.8 mg of daily subcutaneous liraglutide there was no decrease in time-to-peak blood glucose but a statistically significant 22% reduction in peak postprandial glucose and a 39% decrease in area under curve. Liraglutide therapy also led to a weight loss of 3.2 ± 1.8 kg after 4 weeks (146).
Another study by Ilkowitz et al. (147) performed a closed-loop study among 15 adult patients with type 1 diabetes. They found that 1.2 mg of liraglutide vs closed-loop control alone decreased the average blood glucose from 160 ± 51 mg/dL (8.9 ± 2.8 mmol/L) to 145 ± 36 mg/dL (8.0 ± 2.0 mmol/L) and decreased areas under the curve after breakfast and lunch without increasing the incidence of hypoglycemia (147).
Barriers to Full Closed Loop
Delayed insulin absorption and prolonged insulin action
Insulin analogs
The greatest challenge for full closed-loop control is the pharmacokinetics and pharmacodynamics of rapid-acting insulin. Without announcing meals, current systems require a glucose change to start delivering insulin. The longer the onset of insulin action, the more difficult it is to act on a rapidly changing glucose (148). Insulin analogs have been available in the United States since the approval of lispro in 1996. Approved rapid-acting insulin analogs (lispro, aspart, and glulisine) and have been shown to reduce rates of hypoglycemia and decrease HbA1c in those with diabetes (149, 150). The ideal fast-acting insulin would instantly correct a rising blood sugar.
Novo Nordisk received FDA approval for a fast-acting insulin aspart (Fiasp) in September 2017. It contains the excipients niacinamide and l-arginine hydrochloride. Recent publications have demonstrated small [0.1% (1.1 mmol/mol)] but significant (P = 0.0424) decreases in HbA1c and 1-hour postprandial plasma glucose levels among 381 subjects with type 1 diabetes (151) using MDIs. When used in an insulin pump there is an additional improvement in the pharmacokinetics and pharmacodynamics of insulin aspart (152). Multiple trials are underway to investigate the effect of Fiasp on closed-loop systems.
IP insulin delivery
IP insulin delivery results in faster drug pharmacokinetics and pharmacodynamics, with peak insulin efficacy 15 minutes after administration (153, 154) vs 82 minutes with subcutaneous insulin aspart and 57 minutes with subcutaneous Fiasp (152). The insulin is absorbed through blood vessels of the visceral peritoneum and is detectable within the portal system 1 minute after administration (155). IP delivery partially restores glucagon response to hypoglycemia and exercise (156–159). Additionally, there is long-term evidence of increased IGF-1 concentrations closer to reference populations without diabetes (160). Significant research on larger populations is needed to elucidate the purported benefits of IP insulin with closed-loop delivery.
Lived Experience of Automation: Role of Human Factors
Excitement and enthusiasm for closed loop is driven by the hope and promise of removing significant daily burden of diabetes management (e.g., vigilance to glucose levels, dietary intake, and physical activity) and decreasing risk of complications via improved time spent in the target glucose range. Stakeholders in closed loop, including people with type 1 diabetes, system developers, device manufacturers, and diabetes care clinicians, presently face a challenge of balancing this enthusiasm with the practical limitations of early generation systems, as noted earlier in this review. Our experiences with multiple closed-loop systems and assessment of the available data on the lived experience of closed loop led us to conclude that two topics are worth considering as the field evolves and matures: human factors associated with diabetes device use and the lived experience of automated insulin delivery (so far).
Human side of diabetes devices and technologies
“The best predictor of future behavior is past behavior” (161) can be adapted for diabetes devices to note that the best predictors of future use are predictors of past use. Considering this framework, the future forecast is less enthusiastic than most stakeholders presently endorse. For example, there has been rapid development of devices and digital health applications, as well as unparalleled access, yet uptake of the main components of closed loop is low. CGM rates of use are increasing, but most show values below one-fourth of the patient population (wide variation across countries and clinics). Insulin pump use approaches three-fourths of the patient population in many places, again with wide variability. Smartphone uptake nears 80% of the general population, and a 2015 survey by the Pew Research Center revealed that 62% used their phone to search for health information. However, there has been little sustained use for health and diabetes apps. This is particularly relevant when closed-loop system algorithms operate on a smartphone. So, what are the reasons for low uptake of closed-loop components and how can that knowledge be used to optimize uptake and sustain use of closed loop?
Through collaboration with the T1D Exchange and Jaeb Center for Health Research, we explored barriers to CGM and pump use with 1503 adults with type 1 diabetes (162). In addition to reported cost barriers, the most common modifiable barriers of users and potential users were physical discomfort, having to wear devices all the time, the way devices look on the body, and worries about devices not working correctly. For those users who stopped using CGM or an insulin pump, they noted lack of accuracy, nuisance from alarms, and mental burden as reasons for discontinuing use. In addition to the inspection of barriers, common “hidden” factors were correlated with device use; those include psychological distress related to having type 1 diabetes (such as diabetes distress), concerns about hypoglycemia, and attitudes about diabetes devices and technologies (162, 163).
Related to this work is the concept of device readiness, which we termed the degree to which a person is ready to use diabetes devices. Our research has revealed four personas of use based on statistical clustering of human and behavioral factors (164). For example, a “free ranger” who is being asked by the diabetes care provider to consider CGM would likely benefit from education on the device paired with some type of simulation, whether that is wearing CGM on a trial basis or leveraging other technology like virtual reality. Experiencing CGM alarms and alerts and the demands from continuous data will likely ease the reluctant device user with negative technology attitudes, whether it is real or virtual. Furthermore, teaching problem-solving techniques (165) will help this type of potential user to be ready and able to deal with common device issues and failures. Stepping this person up to closed loop may require another round of simulation, expectation setting, and new problem-solving techniques. The investment of upfront and staggered support will show a return on investment of sustained use and should contribute to optimized glycemic outcomes.
As an example, we have recently completed a series of studies with adolescents and adults using the Bionic Pancreas, which does not require carbohydrate counting, and the patient cannot give correction doses. Our adolescent population was immediately grateful that they no longer had to perform these tasks and readily gave control over to the Bionic Pancreas control algorithm. A number of adults with long-standing diabetes who were very active in their diabetes management initially had difficulty relinquishing control to the Bionic Pancreas, that is, they wanted to give correction doses, and they wanted to have control of how much insulin was given at meals based on their carbohydrate counts. After 4 to 5 days, however, the adults who were initially hesitant to relinquish control of their diabetes to the closed-loop system were no longer apprehensive and were happy to let the system take over a significant portion of their diabetes tasks. All subjects in our closed-loop studies have been very happy and grateful for the overnight glucose control these systems have provided.
Although most of our work has focused on people with type 1 diabetes, Tanenbaum et al. (166) surveyed 209 diabetes care clinicians about their perspectives on patient barriers to diabetes device use. It was noteworthy that clinicians perceived similar barriers for their patients, as did the patients themselves in some cases. For example, both reported common barriers of not liking diabetes devices on the body as well as how they look. What was more interesting was that there was a significant mismatch between clinicians and people with type 1 diabetes on whether they knew what to do with information from devices; clinicians saw it as a major barrier whereas few patients did. The implication of this finding is that clinicians perceive an information gap and likely recommend or offer more education; however, that is unlikely to be what the patient needs or wants. Although clinicians are often the gatekeepers to diabetes devices and education is necessary, it is unlikely that education is sufficient for successful onboarding and sustained use.
There are notable systematic reviews of studies in type 1 diabetes about diabetes device and app use (167). In general, they support findings of satisfaction with use vs nonuse of CGM and pumps and note similar barriers of body image and wearing devices all the time. However, this review noted additional concerns on the social side with devices negatively drawing attention from others. This review along with one in Lancet (168) noted ongoing struggles to both understand sustained use of digital technologies (e.g., smartphone apps) and explore how to optimize engagement with them. There are notable benefits, however, on social connectedness and quality of life. Thus, leveraging apps and support programs for diabetes devices may optimize uptake and sustained use.
Lived experience of closed loop
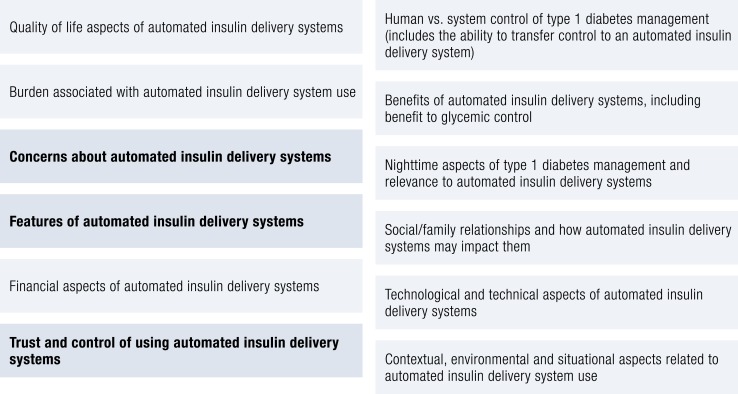
The Stanford group under the direction of Dr. Hood has also been integrally involved in efforts to better understand the lived experience of closed loop. This has cut across large-scale qualitative work to understand preferences and expectations of potential closed-loop users and integration of human factor assessments into closed-loop trials. For example, they report on focus groups and interviews with 289 potential end users and stakeholders of closed-loop systems (169). Results show that people with type 1 diabetes (8 to 80 years of age) and stakeholders (such as parents and partners) revealed 12 main themes about closed-loop systems (Fig. 6).
Figure 6.
Closed-loop themes.
What is noteworthy is the breadth of the themes as well as the three bolded themes, which were the most cited of the respondents. Concerns about the closed-loop systems, what features they will have, and how to trust the systems were primary concerns for people considering whether they would use closed loop themselves or in their families. Additionally, it was noteworthy that patient age and stakeholder type (parent vs partner) reported different primary concerns. For example, teenagers noted wearability and comfort as main factors, whereas adults were primarily focused on reliability and safety. This further supports using a developmental lens to an approach to starting and maintaining use of closed loop. Data on parents and partners are lacking in this area (170, 171); however, multiple studies with parents of youth with type 1 diabetes suggest benefits on quality of life from pumps (172) and mixed, yet mostly positive, results from using CGM (173, 174). Results in these areas suggest that for closed loop, taking a broader family approach by including parents and caregivers and teaching all involved in closed loop use the same problem-solving skills taught to the person with type 1 diabetes will prove beneficial to closed-loop uptake and sustained use.
Human factor assessments have also been integrated into a number of closed-loop trials and have produced important results about the lived experience of these systems to go along with the glycemic results. For example, focus groups and surveys have been embedded into several studies (65, 103, 175–178). Results confirm that topics such as mental burden, diabetes distress, trust, and hypoglycemia worries are at the forefront of the minds of closed-loop users in these trials. In most studies, there are improvements in these areas with closed-loop system use when compared with baseline (e.g., run-in levels of distress), yet controlled studies show similar improvement in closed-loop and comparison groups (177, 178). More controlled studies are needed, but preliminary findings suggest satisfaction with closed-loop use and that it is important to understand expectations of potential closed-loop users along with the development of trust in automation.
Summary and Future Directions
The field of automated insulin delivery systems has shown rapid progress in the last several years with significant improvements in CGM and the FDA fast-tracking testing and approval of these systems. Multiple systems will be on the market within the next several years that will offer unique configurations, giving consumers many choices, assuming their health care plans will provide coverage. All systems will begin to offer adaptability to the individual and to changes in insulin sensitivity during the day and overnight, and with cloud computing there can be adaptability to weekly or monthly patterns of insulin sensitivity. There will be a shift from having systems with multiple alarms (used as safety measures) to systems that have fewer alarms, more personalization, and individualization of tuning parameters, and the focus will be on decreasing the overall burden of diabetes. Glucose set points or algorithm aggressiveness will be adjustable to the individual and incorporate diurnal targets.
The FDA has developed standards for the individual components of an integrated insulin delivery system so that a sensor and/or pump could be configured to work in multiple systems, the ACE infusion pump and iCGM. This could mean the approval by the FDA of systems developed by the do-it-yourself community, which has a growing user base.
A full closed-loop system without the need for meal announcement may occur using multiple configurations, including the use of more rapid-acting insulins, perhaps incorporating inhaled insulins, as well as the use of additional hormones such as glucagon, pramlintide (especially if it could be coformulated with insulin), GLP-1 agonists, and perhaps sodium-glucose cotransporter inhibitors. Delivery of insulin at the onset of eating could be triggered through recognition of the hand motions associated with eating to deliver an early, small bolus before CGM values begin to rise (using a watch worn on the dominant hand to detect eating). Full closed loop may not be able to achieve mean glucose levels as low as 138 mg/dL (7.7 mmol/L), which can be achieved with premeal announcement and use of glucagon, but it may be able to achieve mean glucose levels of 154 mg/dL (8.6 mmol/L), which would be equivalent to an estimated A1c of 7% (8.6 mmol/L), which would be an improvement for 70% of the type 1 community without the burden of meal announcement and carbohydrate counting.
Sensors
CGM will continue to improve, factory calibration will be the standard, and sensors will become smaller with longer duration of wear. Implanted sensors lasting at least a year, and perhaps multiple years, will be a reality. Smaller, less expensive sensors will be developed for the type 2 diabetes community. It may even be conceivable that a commercial electronics company could develop a noninvasive glucose sensor incorporated into a watch-like device. The connectivity between sensors and other components of a closed-loop system should be robust, with minimal dropout of sensor values.
Pumps
Insulin infusion devices will continue to improve with Bluetooth communication becoming standard, automated insulin delivery algorithms will be incorporated into the pumps, and remote upload of software updates (which Tandem is now doing) will become standard. Pumps with dual hormone capability are being developed (iLet by Bionic Pancreas) that allow for lower glucose targets while maintaining safety. Patch pumps (such as OmniPod) will be entering the closed-loop space and could offer multiple configurations in the future (algorithm on the pump or on a phone or handheld device). IP insulin delivery offers many pharmacokinetic advantages, and with the improved insulin kinetics could be used in a full closed-loop configuration. An implanted pump could be combined with an implanted sensor to allow for a full closed-loop system without the need for inserting and wearing multiple devices on the body. This configuration could be appealing to the 40% of people with type 1 diabetes who use MDIs to avoid wearing a pump.
Infusion sets
Failure of an insulin infusion set results in loss of glucose control and a closed-loop system is no longer functional. Infusion sets need to be developed that do not kink on insertion, have a longer duration of wear, and have fewer occlusions. Ideally, a sensor and infusion set could be combined into a single insertion site with a 10- to 14-day duration of wear. Algorithms need to be developed that are robust in detecting an infusion set failure before metabolic decompensation (179–182).
Consumer electronics
Devices that incorporate an accelerometer and heart rate can adjust for changes in insulin sensitivity associated with activity. It is even possible that a closed-loop system could be developed by companies such as Apple or Google where the user interface would be well integrated into smartphones and watches.
Cost
Once they are approved, reimbursement for integrated insulin delivery systems by the FDA could impose limitations on who will have access to this care. The Medtronic 670G hybrid closed-loop system was introduced to the marketplace at the same price as the standard Medtronic pump, and therefore there was minimal resistance to insurance acceptance of the closed-loop system. If additional hormones (such as glucagon or pramlintide) are included in closed-loop systems, the additional cost for these hormones could limit who is able to receive insurance coverage. The cost of real-time sensors has remained at ≈$10 per day. Because these systems all require real-time continuous monitoring, this could impose additional consumable costs to the health care system beyond the initial purchase of the pump (≈$14,000 over 4 years for sensors, and infusion sets and reservoirs add an additional ≈$7000 over 4 years). These costs may eventually lead to insurance companies applying restrictions on who will have access to these systems. Fortunately, there is the potential for good data on the reduction of health care costs by avoiding emergency room visits for hypoglycemia and acute admissions for diabetic ketoacidosis, as well as decreasing the long-term costs of diabetes. These long-term data still need to be published with postmarketing follow-up of approved commercial devices. The combination of lowering both the HbA1c and the risk of hypoglycemia should make closed-loop systems cost-effective, depending on the horizon used for projecting cost savings.
Human factors
To achieve a balance of enthusiasm and realistic expectations and experiences with closed loop, we suggest that human and psychosocial variables should be considered when starting and supporting closed loop use. Furthermore, it appears important that attention be paid equally to supporting the components of closed loop as the overarching system is supported. Brief surveys and qualitative interviews or focus groups are effective tools for understanding the contribution of these variables in the uptake and use of closed loop.
Conclusion
In a span of 5 years, automated insulin delivery has advanced from closely monitored research studies to become the standard of care for subcutaneous insulin delivery. Currently the only pump sold in the United States that does not have automated insulin delivery is a “patch” pump, and the next generation of this pump will have automated hybrid closed-loop control. In the future, any new pump coming onto the market for type 1 diabetes will have automated insulin delivery (such as systems being developed by Insulet, Beta Bionics, Lilly, Bigfoot Biomedical, Tidepool Loop, Roche, and Diabeloop). As glucose sensors continue to improve in accuracy and reliability, the glucose targets for these systems will be lowered. This will allow for use in pregnancy and more adjustable glucose targets by the user, allowing greater individualization of glycemic control. As systems become more robust, there will be a significant decrease in alerts and alarms, allowing them to work quietly in the background. Systems will be developed for inpatient care not only for people with diabetes, but for patients who have hyperglycemia associated with surgeries, chemotherapies, transplantations, and steroid treatment. For the person with type 1 diabetes, there will be quicker adaptability to changes in insulin sensitivity with stress, activity, illness, and menstrual cycles. The goal for the next generation of automated insulin delivery systems will be to provide full closed loop, without the need for meal boluses using more rapid-acting insulins, and bihormonal systems incorporating glucagon and/or hormone analogs such as pramlintide. Insulins that are more concentrated would allow both patch and tethered pumps to become smaller. Integration and communication with consumer electronics such as smart watches and smartphones will allow for better user interaction, incorporation of activity monitors into the algorithms, as well as remote notification to significant others. One of the challenges in the future will be to make these devices available worldwide in the face of high costs. The DIY community promotes the ethical principle of justice by offering controller software free of charge. We are finally at a point where closed-loop control can reduce the burden of diabetes.
Acknowledgments
Financial Support: R.A.L. is a Stephen Bechtel Endowed Adult and Pediatric Endocrinology Fellow through the Stanford Child Health Research Institute and is supported by Diabetes, Endocrinology and Metabolism Training Grant T32 DK007217 from the National Institute of Diabetes and Digestive and Kidney Diseases. B.B. has research support from the National Institutes of Health (Grants DP3DK104059, DP3DK101055, 3UC4DK108483, and 1UK4DK108520), the Helmsley Foundation, Juvenile Diabetes Research Foundation, Medtronic Diabetes, Insulet, Dexcom, Convatec, and Tandem.
Additional Information
Disclosure Summary: R.A.L. has consulted for GlySens and Abbott Diabetes Care. B.B. has served on medical advisory boards for Novo Nordisk, Convatec, and Profusa; has received consultation fees from Tandem Diabetes, Insulet, Medtronic Diabetes, and Dexcom; and has received research funding from Medtronic, Tandem, Insulet, Dexcom, and Convatec. K.H. receives research funding from Dexcom for investigator-initiated studies, as well as consulting fees from Bigfoot Biomedical, Lilly Innovation Center, Insulet, and J&J Diabetes Institute. The remaining author has nothing to disclose.
Glossary
Abbreviations:
- ACE
alternate controller–enabled
- CGM
continuous glucose monitoring
- DCTT
Diabetes Control and Complications Trial
- DiAs
Diabetes Assistant
- DIY
do-it-yourself
- FDA
US Food and Drug Administration
- GLP-1
glucagon-like peptide-1
- iCGM
integrated CGM
- MARD
mean (or median) absolute relative difference
- MDI
multiple daily injection
- MPC
model predictive control
- PID
proportional
- integral
derivative
References and Notes
- 1. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pickup JC, Keen H, Parsons JA, Alberti KG. Continuous subcutaneous insulin infusion: an approach to achieving normoglycaemia. BMJ. 1978;1(6107):204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tamborlane WV, Sherwin RS, Genel M, Felig P. Reduction to normal of plasma glucose in juvenile diabetes by subcutaneous administration of insulin with a portable infusion pump. N Engl J Med. 1979;300(11):573–578. [DOI] [PubMed] [Google Scholar]
- 4. Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case-control study. Diabetologia. 2013;56(11):2392–2400. [DOI] [PubMed] [Google Scholar]
- 5. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C; Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 6. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delaney MF, Zisman A, Kettyle WM. Diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic syndrome. Endocrinol Metab Clin North Am. 2000;29(4):683–705, V. [DOI] [PubMed] [Google Scholar]
- 8. Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Characterizing sudden death and dead-in-bed syndrome in type 1 diabetes: analysis from two childhood-onset type 1 diabetes registries. Diabet Med. 2011;28(3):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bell KJ, Smart CE, Steil GM, Brand-Miller JC, King B, Wolpert HA. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care. 2015;38(6):1008–1015. [DOI] [PubMed] [Google Scholar]
- 10. Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, Kowalski A, Rabasa-Lhoret R, McCrimmon RJ, Hume C, Annan F, Fournier PA, Graham C, Bode B, Galassetti P, Jones TW, Millán IS, Heise T, Peters AL, Petz A, Laffel LM. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5(5):377–390. [DOI] [PubMed] [Google Scholar]
- 11. Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, Maahs DM, Tamborlane WV; T1D Exchange Clinic Network. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–978. [DOI] [PubMed] [Google Scholar]
- 12. DeSalvo DJ, Miller KM, Hermann JM, Maahs DM, Hofer SE, Clements MA, Lilienthal E, Sherr JL, Tauschmann M, Holl RW; T1D Exchange and DPV Registries. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: international comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes. 2018;19(7):1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson DM, Xing D, Beck RW, Block J, Bode B, Fox LA, Hirsch I, Kollman C, Laffel L, Ruedy KJ, Steffes M, Tamborlane WV; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Hemoglobin A1c and mean glucose in patients with type 1 diabetes: analysis of data from the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Care. 2011;34(3):540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Genuth S, Sun W, Cleary P, Gao X, Sell DR, Lachin J, Monnier VM; DCCT/EDIC Research Group. Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes. Diabetes. 2015;64(1):266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beyond A1C Writing Group. Need for regulatory change to incorporate beyond A1C glycemic metrics. Diabetes Care. 2018;41(6):e92–e94. [DOI] [PubMed] [Google Scholar]
- 17. Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, Garg S, Heinemann L, Hirsch I, Amiel SA, Beck R, Bosi E, Buckingham B, Cobelli C, Dassau E, Doyle FJ III, Heller S, Hovorka R, Jia W, Jones T, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Maahs D, Murphy HR, Nørgaard K, Parkin CG, Renard E, Saboo B, Scharf M, Tamborlane WV, Weinzimer SA, Phillip M. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bode BW, Gross TM, Thornton KR, Mastrototaro JJ. Continuous glucose monitoring used to adjust diabetes therapy improves glycosylated hemoglobin: a pilot study. Diabetes Res Clin Pract. 1999;46(3):183–190. [DOI] [PubMed] [Google Scholar]
- 19. Kovatchev BP, Patek SD, Ortiz EA, Breton MD. Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol Ther. 2015;17(3):177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6):428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christiansen MP, Garg SK, Brazg R, Bode BW, Bailey TS, Slover RH, Sullivan A, Huang S, Shin J, Lee SW, Kaufman FR. Accuracy of a fourth-generation subcutaneous continuous glucose sensor. Diabetes Technol Ther. 2017;19(8):446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christiansen MP, Klaff LJ, Brazg R, Chang AR, Levy CJ, Lam D, Denham DS, Atiee G, Bode BW, Walters SJ, Kelley L, Bailey TS. A prospective multicenter evaluation of the accuracy of a novel implanted continuous glucose sensor: PRECISE II. Diabetes Technol Ther. 2018;20(3):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Russell S. The insulin-only bionic pancreas bridging study. Available at: https://clinicaltrials.gov/show/NCT03565666. Accessed 1 February 2019.
- 24. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17(11):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abbott. Abbott and Bigfoot Biomedical announce collaboration to develop breakthrough diabetes technologies. Available at: https://abbott.mediaroom.com/2017-07-13-Abbott-and-Bigfoot-Biomedical-Announce-Collaboration-to-Develop-Breakthrough-Diabetes-Technologies. Accessed 1 February 2019.
- 26. U.S. Food and Drug Administration. FDA authorizes first fully interoperable continuous glucose monitoring system, streamlines review pathway for similar devices. Available at: https://www.fda.gov/news-events/pressannouncements/fda-authorizes-first-fully-interoperablecontinuous-glucose-monitoring-system-streamlinesreview. Accessed 1 February 2019.
- 27. Sinha M, McKeon KM, Parker S, Goergen LG, Zheng H, El-Khatib FH, Russell SJ. A comparison of time delay in three continuous glucose monitors for adolescents and adults. J Diabetes Sci Technol. 2017;11(6):1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burnett DR, Huyett LM, Zisser HC, Doyle FJ III, Mensh BD. Glucose sensing in the peritoneal space offers faster kinetics than sensing in the subcutaneous space. Diabetes. 2014;63(7):2498–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. FDA News Release, February 14, 2019. Available at: www.fda.gov/news-events/press-announcements/fda-authorizes-first-interoperable-insulin-pump-intended-allow-patients-customize-treatment. Accessed 14 Februrary 2019.
- 30. Hu R, Li C. An improved PID algorithm based on insulin-on-board estimate for blood glucose control with type 1 diabetes. Comput Math Methods Med. 2015;2015:281589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toffanin C, Zisser H, Doyle FJ III, Dassau E. Dynamic insulin on board: incorporation of circadian insulin sensitivity variation. J Diabetes Sci Technol. 2013;7(4):928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pinsker JE, Lee JB, Dassau E, Seborg DE, Bradley PK, Gondhalekar R, Bevier WC, Huyett L, Zisser HC, Doyle FJ III. Randomized crossover comparison of personalized MPC and PID control algorithms for the artificial pancreas. Diabetes Care. 2016;39(7):1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2(27):27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, Mondesir D, Esmaeili A, Hartigan C, Thompson MJ, Malkani S, Lock JP, Harlan DM, Clinton P, Frank E, Wilson DM, DeSalvo D, Norlander L, Ly T, Buckingham BA, Diner J, Dezube M, Young LA, Goley A, Kirkman MS, Buse JB, Zheng H, Selagamsetty RR, Damiano ER, Russell SJ. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet. 2017;389(10067):369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turksoy K, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive identification and control for artificial pancreas systems. IEEE Trans Biomed Eng. 2014;61(3):883–891. [DOI] [PubMed] [Google Scholar]
- 36. Turksoy K, Quinn LT, Littlejohn E, Cinar A. An integrated multivariable artificial pancreas control system. J Diabetes Sci Technol. 2014;8(3):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Breton MD, Brown SA, Karvetski CH, Kollar L, Topchyan KA, Anderson SM, Kovatchev BP. Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther. 2014;16(8):506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cichosz SL, Frystyk J, Hejlesen OK, Tarnow L, Fleischer J. A novel algorithm for prediction and detection of hypoglycemia based on continuous glucose monitoring and heart rate variability in patients with type 1 diabetes. J Diabetes Sci Technol. 2014;8(4):731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jacobs PG, Resalat N, El Youssef J, Reddy R, Branigan D, Preiser N, Condon J, Castle J. Incorporating an exercise detection, grading, and hormone dosing algorithm into the artificial pancreas using accelerometry and heart rate. J Diabetes Sci Technol. 2015;9(6):1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dasanayake IS, Bevier WC, Castorino K, Pinsker JE, Seborg DE, Doyle FJ III, Dassau E. Early detection of physical activity for people with type 1 diabetes mellitus. J Diabetes Sci Technol. 2015;9(6):1236–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stenerson M, Cameron F, Payne SR, Payne SL, Ly TT, Wilson DM, Buckingham BA. The impact of accelerometer use in exercise-associated hypoglycemia prevention in type 1 diabetes. J Diabetes Sci Technol. 2015;9(1):80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gonder-Frederick LA, Grabman JH, Kovatchev B, Brown SA, Patek S, Basu A, Pinsker JE, Kudva YC, Wakeman CA, Dassau E, Cobelli C, Zisser HC, Doyle FJ III. Is Psychological Stress a Factor for Incorporation Into Future Closed-Loop Systems? J Diabetes Sci Technol. 2016;10(3):640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Samadi S, Rashid M, Turksoy K, Feng J, Hajizadeh I, Hobbs N, Lazaro C, Sevil M, Littlejohn E, Cinar A. Automatic detection and estimation of unannounced meals for multivariable artificial pancreas system. Diabetes Technol Ther. 2018;20(3):235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nimri R, Bratina N, Kordonouri O, Avbelj Stefanija M, Fath M, Biester T, Muller I, Atlas E, Miller S, Fogel A, Phillip M, Danne T, Battelino T. MD-Logic overnight type 1 diabetes control in home settings: a multicentre, multinational, single blind randomized trial. Diabetes Obes Metab. 2017;19(4):553–561. [DOI] [PubMed] [Google Scholar]
- 45. Mauseth R, Hirsch IB, Bollyky J, Kircher R, Matheson D, Sanda S, Greenbaum C. Use of a “fuzzy logic” controller in a closed-loop artificial pancreas. Diabetes Technol Ther. 2013;15(8):628–633. [DOI] [PubMed] [Google Scholar]
- 46. Dassau E, Zisser H, C Palerm C, A Buckingham B, Jovanovic L, J Doyle F III. Modular artificial β-cell system: a prototype for clinical research. J Diabetes Sci Technol. 2008;2(5):863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cameron FM, Ly TT, Buckingham BA, Maahs DM, Forlenza GP, Levy CJ, Lam D, Clinton P, Messer LH, Westfall E, Levister C, Xie YY, Baysal N, Howsmon D, Patek SD, Bequette BW. Closed-loop control without meal announcement in type 1 diabetes. Diabetes Technol Ther. 2017;19(9):527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buckingham BA, Forlenza GP, Pinsker JE, Christiansen MP, Wadwa RP, Schneider J, Peyser TA, Dassau E, Lee JB, O’Connor J, Layne JE, Ly TT. Safety and feasibility of the OmniPod hybrid closed-loop system in adult, adolescent, and pediatric patients with type 1 diabetes using a personalized model predictive control algorithm. Diabetes Technol Ther. 2018;20(4):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lewis D, Leibrand S; #OpenAPS Community. Real-world use of open source artificial pancreas systems. J Diabetes Sci Technol. 2016;10(6):1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee JM, Hirschfeld E, Wedding J. A patient-designed do-it-yourself mobile technology system for diabetes: promise and challenges for a new era in medicine. JAMA. 2016;315(14):1447–1448. [DOI] [PubMed] [Google Scholar]
- 51. U.S. Food and Drug Administration. FDA authorizes first fully interoperable continuous glucose monitoring system, streamlines review pathway for similar devices. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm602870.htm. Accessed 1 February 2019.
- 52. Crossley K, Kjellstrand CM. Intraperitoneal insulin for control of blood sugar in diabetic patients during peritoneal dialysis. BMJ. 1971;1(5743):269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abe T, Kusumi H, Kanazawa M. Peritoneal dialysis for diabetic nephropathy: a case of intraperitoneal insulin administration [in Japanese]. Hinyokika Kiyo. 1972;18(3):133–136. [PubMed] [Google Scholar]
- 54. Flynn CT, Nanson JA. Intraperitoneal insulin with CAPD—an artificial pancreas. Trans Am Soc Artif Intern Organs. 1979;25(1):114–117. [DOI] [PubMed] [Google Scholar]
- 55. Eaton RP, Friedman NM, Spencerr WJ. Intraperitoneal delivery of insulin by a portable microinfusion pump. Metabolism. 1980;29(8):699–702. [DOI] [PubMed] [Google Scholar]
- 56. Irsigler K, Kritz H, Hagmüller G, Franetzki M, Prestele K, Thurow H, Geisen K. Long-term continuous intraperitoneal insulin infusion with an implanted remote-controlled insulin infusion device. Diabetes. 1981;30(12):1072–1075. [DOI] [PubMed] [Google Scholar]
- 57. Schade DS, Eaton RP, Friedman NM, Spencer WJ, Standefer JC. Five-day programmed intraperitoneal insulin delivery in insulin-dependent diabetic man. J Clin Endocrinol Metab. 1981;52(6):1165–1170. [DOI] [PubMed] [Google Scholar]
- 58. Pozza G, Spotti D, Micossi P, Cristallo M, Melandri M, Piatti PM, Monti LD, Pontiroli AE. Long-term continuous intraperitoneal insulin treatment in brittle diabetes. Br Med J (Clin Res Ed). 1983;286(6361):255–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Diagnostic and therapeutic technology assessment. Continuous peritoneal insulin infusion and implantable insulin infusion pumps for diabetic control. JAMA. 1989;262(22):3195–3198. [PubMed] [Google Scholar]
- 60. Renard E, Costalat G, Chevassus H, Bringer J. Artificial β-cell: clinical experience toward an implantable closed-loop insulin delivery system. Diabetes Metab. 2006;32(5 Pt 2):497–502. [DOI] [PubMed] [Google Scholar]
- 61. Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas. Diabetes Care. 2010;33(1):121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dassau E, Renard E, Place J, Farret A, Pelletier MJ, Lee J, Huyett LM, Chakrabarty A, Doyle FJ III, Zisser HC. Intraperitoneal insulin delivery provides superior glycaemic regulation to subcutaneous insulin delivery in model predictive control-based fully-automated artificial pancreas in patients with type 1 diabetes: a pilot study. Diabetes Obes Metab. 2017;19(12):1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. The DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med. 1991;90(4):450–459. [PubMed] [Google Scholar]
- 64. Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care. 1997;20(1):22–25. [DOI] [PubMed] [Google Scholar]
- 65. Barnard KD, Wysocki T, Allen JM, Elleri D, Thabit H, Leelarathna L, Gulati A, Nodale M, Dunger DB, Tinati T, Hovorka R. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diabetes Res Care. 2014;2(1):e000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Buckingham B, Block J, Burdick J, Kalajian A, Kollman C, Choy M, Wilson DM, Chase P; Diabetes Research in Children Network. Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther. 2005;7(3):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Prolonged nocturnal hypoglycemia is common during 12 months of continuous glucose monitoring in children and adults with type 1 diabetes. Diabetes Care. 2010;33(5):1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sovik O, Thordarson H. Dead-in-bed syndrome in young diabetic patients. Diabetes Care. 1999;22(Suppl 2):B40–B42. [PubMed] [Google Scholar]
- 69. Novodvorsky P, Bernjak A, Chow E, Iqbal A, Sellors L, Williams S, Fawdry RA, Parekh B, Jacques RM, Marques JLB, Sheridan PJ, Heller SR. Diurnal differences in risk of cardiac arrhythmias during spontaneous hypoglycemia in young people with type 1 diabetes. Diabetes Care. 2017;40(5):655–662. [DOI] [PubMed] [Google Scholar]
- 70. Bergenstal RM, Welsh JB, Shin JJ. Threshold insulin-pump interruption to reduce hypoglycemia. N Engl J Med. 2013;369(15):1474. [DOI] [PubMed] [Google Scholar]
- 71. Buckingham BA, Raghinaru D, Cameron F, Bequette BW, Chase HP, Maahs DM, Slover R, Wadwa RP, Wilson DM, Ly T, Aye T, Hramiak I, Clarson C, Stein R, Gallego PH, Lum J, Sibayan J, Kollman C, Beck RW; In Home Closed Loop Study Group. Predictive low-glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care. 2015;38(7):1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maahs DM, Calhoun P, Buckingham BA, Chase HP, Hramiak I, Lum J, Cameron F, Bequette BW, Aye T, Paul T, Slover R, Wadwa RP, Wilson DM, Kollman C, Beck RW; In Home Closed Loop Study Group. A randomized trial of a home system to reduce nocturnal hypoglycemia in type 1 diabetes. Diabetes Care. 2014;37(7):1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Abraham MB, Nicholas JA, Ly TT, Roby HC, Paramalingam N, Fairchild J, King BR, Ambler GR, Cameron F, Davis EA, Jones TW. Safety and efficacy of the predictive low glucose management system in the prevention of hypoglycaemia: protocol for randomised controlled home trial to evaluate the Suspend before low function. BMJ Open. 2016;6(4):e011589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Abraham MB, de Bock M, Paramalingam N, O’Grady MJ, Ly TT, George C, Roy A, Spital G, Karula S, Heels K, Gebert R, Fairchild JM, King BR, Ambler GR, Cameron F, Davis EA, Jones TW. Prevention of insulin-induced hypoglycemia in type 1 diabetes with predictive low glucose management system. Diabetes Technol Ther. 2016;18(7):436–443. [DOI] [PubMed] [Google Scholar]
- 75. Abraham MB, Davey R, O’Grady MJ, Ly TT, Paramalingam N, Fournier PA, Roy A, Grosman B, Kurtz N, Fairchild JM, King BR, Ambler GR, Cameron F, Jones TW, Davis EA. Effectiveness of a predictive algorithm in the prevention of exercise-induced hypoglycemia in type 1 diabetes. Diabetes Technol Ther. 2016;18(9):543–550. [DOI] [PubMed] [Google Scholar]
- 76. Choudhary P, Olsen BS, Conget I, Welsh JB, Vorrink L, Shin JJ. Hypoglycemia prevention and user acceptance of an insulin pump system with predictive low glucose management. Diabetes Technol Ther. 2016;18(5):288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Battelino T, Nimri R, Dovc K, Phillip M, Bratina N. Prevention of hypoglycemia with predictive low glucose insulin suspension in children with type 1 diabetes: a randomized controlled trial. Diabetes Care. 2017;40(6):764–770. [DOI] [PubMed] [Google Scholar]
- 78. Abraham MB, Nicholas JA, Smith GJ, Fairchild JM, King BR, Ambler GR, Cameron FJ, Davis EA, Jones TW; PLGM Study Group. Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care. 2018;41(2):303–310. [DOI] [PubMed] [Google Scholar]
- 79.Forlenza GP, Li Z, Buckingham BA, Pinsker JE, Cengiz E, Wadwa RP, Ekhlaspour L, Church MM, Weinzimer SA, Jost E, Marcal T, Andre C, Carria L, Swanson V, Lum JW, Kollman C, Woodall W, Beck RW. Predictive low glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care. 2018;41(10):2155–2161. [DOI] [PubMed] [Google Scholar]
- 80. Phillip M, Battelino T, Atlas E, Kordonouri O, Bratina N, Miller S, Biester T, Stefanija MA, Muller I, Nimri R, Danne T. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368(9):824–833. [DOI] [PubMed] [Google Scholar]
- 81. Hovorka R, Elleri D, Thabit H, Allen JM, Leelarathna L, El-Khairi R, Kumareswaran K, Caldwell K, Calhoun P, Kollman C, Murphy HR, Acerini CL, Wilinska ME, Nodale M, Dunger DB. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37(5):1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thabit H, Tauschmann M, Allen JM, Leelarathna L, Hartnell S, Wilinska ME, Acerini CL, Dellweg S, Benesch C, Heinemann L, Mader JK, Holzer M, Kojzar H, Exall J, Yong J, Pichierri J, Barnard KD, Kollman C, Cheng P, Hindmarsh PC, Campbell FM, Arnolds S, Pieber TR, Evans ML, Dunger DB, Hovorka R. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373(22):2129–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tauschmann M, Allen JM, Wilinska ME, Thabit H, Acerini CL, Dunger DB, Hovorka R. Home use of day-and-night hybrid closed-loop insulin delivery in suboptimally controlled adolescents with type 1 diabetes: a 3-week, free-living, randomized crossover trial. Diabetes Care. 2016;39(11):2019–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, Ruan Y, Sibayan J, Kollman C, Cheng P, Beck RW, Acerini CL, Evans ML, Dunger DB, Elleri D, Campbell F, Bergenstal RM, Criego A, Shah VN, Leelarathna L, Hovorka R; APCam11 Consortium. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392(10155):1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nimri R, Atlas E, Ajzensztejn M, Miller S, Oron T, Phillip M. Feasibility study of automated overnight closed-loop glucose control under MD-Logic artificial pancreas in patients with type 1 diabetes: the DREAM Project. Diabetes Technol Ther. 2012;14(8):728–735. [DOI] [PubMed] [Google Scholar]
- 86. Ruiz JL, Sherr JL, Cengiz E, Carria L, Roy A, Voskanyan G, Tamborlane WV, Weinzimer SA. Effect of insulin feedback on closed-loop glucose control: a crossover study. J Diabetes Sci Technol. 2012;6(5):1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ly TT, Roy A, Grosman B, Shin J, Campbell A, Monirabbasi S, Liang B, von Eyben R, Shanmugham S, Clinton P, Buckingham BA. Day and night closed-loop control using the integrated Medtronic hybrid closed-loop system in type 1 diabetes at diabetes camp. Diabetes Care. 2015;38(7):1205–1211. [DOI] [PubMed] [Google Scholar]
- 88. Ly TT, Keenan DB, Roy A, Han J, Grosman B, Cantwell M, Kurtz N, von Eyben R, Clinton P, Wilson DM, Buckingham BA. Automated overnight closed-loop control using a proportional-integral-derivative algorithm with insulin feedback in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Technol Ther. 2016;18(6):377–384. [DOI] [PubMed] [Google Scholar]
- 89. Del Favero S, Bruttomesso D, Di Palma F, Lanzola G, Visentin R, Filippi A, Scotton R, Toffanin C, Messori M, Scarpellini S, Keith-Hynes P, Kovatchev BP, Devries JH, Renard E, Magni L, Avogaro A, Cobelli C; AP@home Consortium. First use of model predictive control in outpatient wearable artificial pancreas. Diabetes Care. 2014;37(5):1212–1215. [DOI] [PubMed] [Google Scholar]
- 90. Ly TT, Breton MD, Keith-Hynes P, De Salvo D, Clinton P, Benassi K, Mize B, Chernavvsky D, Place J, Wilson DM, Kovatchev BP, Buckingham BA. Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care. 2014;37(8):2310–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kovatchev BP, Renard E, Cobelli C, Zisser HC, Keith-Hynes P, Anderson SM, Brown SA, Chernavvsky DR, Breton MD, Mize LB, Farret A, Place J, Bruttomesso D, Del Favero S, Boscari F, Galasso S, Avogaro A, Magni L, Di Palma F, Toffanin C, Messori M, Dassau E, Doyle FJ III. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014;37(7):1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nimri R, Muller I, Atlas E, Miller S, Kordonouri O, Bratina N, Tsioli C, Stefanija MA, Danne T, Battelino T, Phillip M. Night glucose control with MD-Logic artificial pancreas in home setting: a single blind, randomized crossover trial-interim analysis. Pediatr Diabetes. 2014;15(2):91–99. [DOI] [PubMed] [Google Scholar]
- 93. Brown SA, Kovatchev BP, Breton MD, Anderson SM, Keith-Hynes P, Patek SD, Jiang B, Ben Brahim N, Vereshchetin P, Bruttomesso D, Avogaro A, Del Favero S, Boscari F, Galasso S, Visentin R, Monaro M, Cobelli C. Multinight “bedside” closed-loop control for patients with type 1 diabetes. Diabetes Technol Ther. 2015;17(3):203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ly TT, Buckingham BA, DeSalvo DJ, Shanmugham S, Satin-Smith M, DeBoer MD, Oliveri MC, Schertz E, Breton MD, Cherñavvsky DR. Day-and-night closed-loop control using the unified safety system in adolescents with type 1 diabetes at camp. Diabetes Care. 2016;39(8):e106–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Del Favero S, Boscari F, Messori M, Rabbone I, Bonfanti R, Sabbion A, Iafusco D, Schiaffini R, Visentin R, Calore R, Moncada YL, Galasso S, Galderisi A, Vallone V, Di Palma F, Losiouk E, Lanzola G, Tinti D, Rigamonti A, Marigliano M, Zanfardino A, Rapini N, Avogaro A, Chernavvsky D, Magni L, Cobelli C, Bruttomesso D. Randomized summer camp crossover trial in 5- to 9-year-old children: outpatient wearable artificial pancreas is feasible and safe. Diabetes Care. 2016;39(7):1180–1185. [DOI] [PubMed] [Google Scholar]
- 96. Grosman B, Ilany J, Roy A, Kurtz N, Wu D, Parikh N, Voskanyan G, Konvalina N, Mylonas C, Gottlieb R, Kaufman F, Cohen O. Hybrid closed-loop insulin delivery in type 1 diabetes during supervised outpatient conditions. J Diabetes Sci Technol. 2016;10(3):708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. de Bock MI, Roy A, Cooper MN, Dart JA, Berthold CL, Retterath AJ, Freeman KE, Grosman B, Kurtz N, Kaufman F, Jones TW, Davis EA. Feasibility of outpatient 24-hour closed-loop insulin delivery. Diabetes Care. 2015;38(11):e186–e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ly TT, Weinzimer SA, Maahs DM, Sherr JL, Roy A, Grosman B, Cantwell M, Kurtz N, Carria L, Messer L, von Eyben R, Buckingham BA. Automated hybrid closed-loop control with a proportional-integral-derivative based system in adolescents and adults with type 1 diabetes: individualizing settings for optimal performance. Pediatr Diabetes. 2017;18(5):348–355. [DOI] [PubMed] [Google Scholar]
- 99. Sharifi A, De Bock MI, Jayawardene D, Loh MM, Horsburgh JC, Berthold CL, Paramalingam N, Bach LA, Colman PG, Davis EA, Grosman B, Hendrieckx C, Jenkins AJ, Kumareswaran K, Kurtz N, Kyoong A, MacIsaac RJ, Speight J, Trawley S, Ward GM, Roy A, Jones TW, O’Neal DN. Glycemia, treatment satisfaction, cognition, and sleep quality in adults and adolescents with type 1 diabetes when using a closed-loop system overnight versus sensor-augmented pump with low-glucose suspend function: a randomized crossover study. Diabetes Technol Ther. 2016;18(12):772–783. [DOI] [PubMed] [Google Scholar]
- 100. Nimri R, Muller I, Atlas E, Miller S, Fogel A, Bratina N, Kordonouri O, Battelino T, Danne T, Phillip M. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care. 2014;37(11):3025–3032. [DOI] [PubMed] [Google Scholar]
- 101. Anderson SM, Raghinaru D, Pinsker JE, Boscari F, Renard E, Buckingham BA, Nimri R, Doyle FJ III, Brown SA, Keith-Hynes P, Breton MD, Chernavvsky D, Bevier WC, Bradley PK, Bruttomesso D, Del Favero S, Calore R, Cobelli C, Avogaro A, Farret A, Place J, Ly TT, Shanmugham S, Phillip M, Dassau E, Dasanayake IS, Kollman C, Lum JW, Beck RW, Kovatchev B; Control to Range Study Group. Multinational home use of closed-loop control is safe and effective. Diabetes Care. 2016;39(7):1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kropff J, Del Favero S, Place J, Toffanin C, Visentin R, Monaro M, Messori M, Di Palma F, Lanzola G, Farret A, Boscari F, Galasso S, Magni P, Avogaro A, Keith-Hynes P, Kovatchev BP, Bruttomesso D, Cobelli C, DeVries JH, Renard E, Magni L; AP@home consortium. 2 Month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol. 2015;3(12):939–947. [DOI] [PubMed] [Google Scholar]
- 103. Kovatchev B, Cheng P, Anderson SM, Pinsker JE, Boscari F, Buckingham BA, Doyle FJ III, Hood KK, Brown SA, Breton MD, Chernavvsky D, Bevier WC, Bradley PK, Bruttomesso D, Del Favero S, Calore R, Cobelli C, Avogaro A, Ly TT, Shanmugham S, Dassau E, Kollman C, Lum JW, Beck RW. Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther. 2017;19(1):18–24. [DOI] [PubMed] [Google Scholar]
- 104. Leelarathna L, Dellweg S, Mader JK, Allen JM, Benesch C, Doll W, Ellmerer M, Hartnell S, Heinemann L, Kojzar H, Michalewski L, Nodale M, Thabit H, Wilinska ME, Pieber TR, Arnolds S, Evans ML, Hovorka R; AP@home Consortium. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care. 2014;37(7):1931–1937. [DOI] [PubMed] [Google Scholar]
- 105. Tauschmann M, Allen JM, Wilinska ME, Thabit H, Stewart Z, Cheng P, Kollman C, Acerini CL, Dunger DB, Hovorka R. Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2016;39(7):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Thabit H, Lubina-Solomon A, Stadler M, Leelarathna L, Walkinshaw E, Pernet A, Allen JM, Iqbal A, Choudhary P, Kumareswaran K, Nodale M, Nisbet C, Wilinska ME, Barnard KD, Dunger DB, Heller SR, Amiel SA, Evans ML, Hovorka R. Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol. 2014;2(9):701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bergenstal RM, Garg S, Weinzimer SA, Buckingham BA, Bode BW, Tamborlane WV, Kaufman FR. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407–1408. [DOI] [PubMed] [Google Scholar]
- 108. Messer LH, Forlenza GP, Sherr JL, Wadwa RP, Buckingham BA, Weinzimer SA, Maahs DM, Slover RH. Optimizing hybrid closed-loop therapy in adolescents and emerging adults using the MiniMed 670G system. Diabetes Care. 2018;41(4):789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, Ahmann AJ, Welsh JB, Lee SW, Kaufman FR; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224–232. [DOI] [PubMed] [Google Scholar]
- 110. Cryer PE. Minireview: glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. 2012;153(3):1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Reiband HK, Schmidt S, Ranjan A, Holst JJ, Madsbad S, Nørgaard K. Dual-hormone treatment with insulin and glucagon in patients with type 1 diabetes. Diabetes Metab Res Rev. 2015;31(7):672–679. [DOI] [PubMed] [Google Scholar]
- 112. McCall AL, Farhy LS. Treating type 1 diabetes: from strategies for insulin delivery to dual hormonal control. Minerva Endocrinol. 2013;38(2):145–163. [PMC free article] [PubMed] [Google Scholar]
- 113. Castle JR, Engle JM, El Youssef J, Massoud RG, Ward WK. Factors influencing the effectiveness of glucagon for preventing hypoglycemia. J Diabetes Sci Technol. 2010;4(6):1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Russell SJ, El-Khatib FH, Nathan DM, Damiano ER. Efficacy determinants of subcutaneous microdose glucagon during closed-loop control. J Diabetes Sci Technol. 2010;4(6):1288–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Haidar A, Smaoui MR, Legault L, Rabasa-Lhoret R. The role of glucagon in the artificial pancreas. Lancet Diabetes Endocrinol. 2016;4(6):476–479. [DOI] [PubMed] [Google Scholar]
- 116. Steiner SS, Li M, Hauser R, Pohl R. Stabilized glucagon formulation for bihormonal pump use. J Diabetes Sci Technol. 2010;4(6):1332–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bakhtiani PA, Zhao LM, El Youssef J, Castle JR, Ward WK. A review of artificial pancreas technologies with an emphasis on bi-hormonal therapy. Diabetes Obes Metab. 2013;15(12):1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jackson MA, Caputo N, Castle JR, David LL, Roberts CT Jr, Ward WK. Stable liquid glucagon formulations for rescue treatment and bi-hormonal closed-loop pancreas. Curr Diab Rep. 2012;12(6):705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Trevitt S, Simpson S, Wood A. Artificial pancreas device systems for the closed-loop control of type 1 diabetes: what systems are in development? J Diabetes Sci Technol. 2016;10(3):714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. El-Khatib FH, Russell SJ, Magyar KL, Sinha M, McKeon K, Nathan DM, Damiano ER. Autonomous and continuous adaptation of a bihormonal bionic pancreas in adults and adolescents with type 1 diabetes. J Clin Endocrinol Metab. 2014;99(5):1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Russell SJ, El-Khatib FH, Sinha M, Magyar KL, McKeon K, Goergen LG, Balliro C, Hillard MA, Nathan DM, Damiano ER. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371(4):313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Russell SJ, Hillard MA, Balliro C, Magyar KL, Selagamsetty R, Sinha M, Grennan K, Mondesir D, Ekhlaspour L, Zheng H, Damiano ER, El-Khatib FH. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016;4(3):233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. van Bon AC, Hermanides J, Koops R, Hoekstra JB, DeVries JH. Postprandial glycemic excursions with the use of a closed-loop platform in subjects with type 1 diabetes: a pilot study. J Diabetes Sci Technol. 2010;4(4):923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Van Bon AC, Jonker LD, Koebrugge R, Koops R, Hoekstra JB, DeVries JH. Feasibility of a bihormonal closed-loop system to control postexercise and postprandial glucose excursions. J Diabetes Sci Technol. 2012;6(5):1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jacobs PG, El Youssef J, Castle J, Bakhtiani P, Branigan D, Breen M, Bauer D, Preiser N, Leonard G, Stonex T, Ward WK. Automated control of an adaptive bihormonal, dual-sensor artificial pancreas and evaluation during inpatient studies. IEEE Trans Biomed Eng. 2014;61(10):2569–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. van Bon AC, Luijf YM, Koebrugge R, Koops R, Hoekstra JB, DeVries JH. Feasibility of a portable bihormonal closed-loop system to control glucose excursions at home under free-living conditions for 48 hours. Diabetes Technol Ther. 2014;16(3):131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Blauw H, van Bon AC, Koops R, DeVries JH; on behalf of the PCDIAB consortium. Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab. 2016;18(7):671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Haidar A, Legault L, Dallaire M, Alkhateeb A, Coriati A, Messier V, Cheng P, Millette M, Boulet B, Rabasa-Lhoret R. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ. 2013;185(4):297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Haidar A, Legault L, Matteau-Pelletier L, Messier V, Dallaire M, Ladouceur M, Rabasa-Lhoret R. Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(8):595–604. [DOI] [PubMed] [Google Scholar]
- 130. Haidar A, Rabasa-Lhoret R, Legault L, Lovblom LE, Rakheja R, Messier V, D’Aoust É, Falappa CM, Justice T, Orszag A, Tschirhart H, Dallaire M, Ladouceur M, Perkins BA. Single- and dual-hormone artificial pancreas for overnight glucose control in type 1 diabetes. J Clin Endocrinol Metab. 2016;101(1):214–223. [DOI] [PubMed] [Google Scholar]
- 131. Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):3344–3350. [DOI] [PubMed] [Google Scholar]
- 132. Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–939. [DOI] [PubMed] [Google Scholar]
- 133. Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-Logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care. 2010;33(5):1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther. 2013;15(5):386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Breton M, Farret A, Bruttomesso D, Anderson S, Magni L, Patek S, Dalla Man C, Place J, Demartini S, Del Favero S, Toffanin C, Hughes-Karvetski C, Dassau E, Zisser H, Doyle FJ III, De Nicolao G, Avogaro A, Cobelli C, Renard E, Kovatchev B; International Artificial Pancreas Study Group. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61(9):2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Dassau E, Zisser H, Harvey RA, Percival MW, Grosman B, Bevier W, Atlas E, Miller S, Nimri R, Jovanovic L, Doyle FJ III. Clinical evaluation of a personalized artificial pancreas. Diabetes Care. 2013;36(4):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Cameron F, Niemeyer G, Wilson DM, Bequette BW, Benassi KS, Clinton P, Buckingham BA. Inpatient trial of an artificial pancreas based on multiple model probabilistic predictive control with repeated large unannounced meals. Diabetes Technol Ther. 2014;16(11):728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501–512. [DOI] [PubMed] [Google Scholar]
- 139. Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, Haidich AB, Hovorka R, Tsapas A. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Albisser AM, Leibel BS, Ewart TG, Davidovac Z, Botz CK, Zingg W, Schipper H, Gander R. Clinical control of diabetes by the artificial pancreas. Diabetes. 1974;23(5):397–404. [DOI] [PubMed] [Google Scholar]
- 141. Albisser AM, Leibel BS, Ewart TG, Davidovac Z, Botz CK, Zingg W. An artificial endocrine pancreas. Diabetes. 1974;23(5):389–396. [DOI] [PubMed] [Google Scholar]
- 142. Moore CX, Cooper GJ. Co-secretion of amylin and insulin from cultured islet beta-cells: modulation by nutrient secretagogues, islet hormones and hypoglycemic agents. Biochem Biophys Res Commun. 1991;179(1):1–9. [DOI] [PubMed] [Google Scholar]
- 143. Kruger DF, Gloster MA. Pramlintide for the treatment of insulin-requiring diabetes mellitus: rationale and review of clinical data. Drugs. 2004;64(13):1419–1432. [DOI] [PubMed] [Google Scholar]
- 144. Weinzimer SA, Sherr JL, Cengiz E, Kim G, Ruiz JL, Carria L, Voskanyan G, Roy A, Tamborlane WV. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012;35(10):1994–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Haidar A, Tsoukas M, Sarah T, Strauss N, Yale Jf, Rutkowski J, Bossy A, Pytka E, Nguyen HT, Legault L. Insulin-plus-pramlintide artificial pancreas in type 1 diabetes—randomized controlled trial. Diabetes. 2018;67(Suppl 1). Abstract 210-OR. [Google Scholar]
- 146. Sherr JL, Patel NS, Michaud CI, Palau-Collazo MM, Van Name MA, Tamborlane WV, Cengiz E, Carria LR, Tichy EM, Weinzimer SA. Mitigating meal-related glycemic excursions in an insulin-sparing manner during closed-loop insulin delivery: the beneficial effects of adjunctive pramlintide and liraglutide. Diabetes Care. 2016;39(7):1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ilkowitz JT, Katikaneni R, Cantwell M, Ramchandani N, Heptulla RA. Adjuvant liraglutide and insulin versus insulin monotherapy in the closed-loop system in type 1 diabetes: a randomized open-labeled crossover design trial. J Diabetes Sci Technol. 2016;10(5):1108–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gingras V, Taleb N, Roy-Fleming A, Legault L, Rabasa-Lhoret R. The challenges of achieving postprandial glucose control using closed-loop systems in patients with type 1 diabetes. Diabetes Obes Metab. 2018;20(2):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Davidson MB. Insulin analogs—is there a compelling case to use them? No! Diabetes Care. 2014;37(6):1771–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Grunberger G. Insulin analogs—are they worth it? Yes! Diabetes Care. 2014;37(6):1767–1770. [DOI] [PubMed] [Google Scholar]
- 151. Mathieu C, Bode BW, Franek E, Philis-Tsimikas A, Rose L, Graungaard T, Birk Østerskov A, Russell-Jones D. Efficacy and safety of fast-acting insulin aspart in comparison with insulin aspart in type 1 diabetes (onset 1): a 52-week, randomized, treat-to-target, phase III trial. Diabetes Obes Metab. 2018;20(5):1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Heise T, Zijlstra E, Nosek L, Rikte T, Haahr H. Pharmacological properties of faster-acting insulin aspart vs insulin aspart in patients with type 1 diabetes receiving continuous subcutaneous insulin infusion: a randomized, double-blind, crossover trial. Diabetes Obes Metab. 2017;19(2):208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Selam JL, Bergman RN, Raccah D, Jean-Didier N, Lozano J, Charles MA. Determination of portal insulin absorption from peritoneum via novel nonisotopic method. Diabetes. 1990;39(11):1361–1365. [DOI] [PubMed] [Google Scholar]
- 154. Nathan DM, Dunn FL, Bruch J, McKitrick C, Larkin M, Haggan C, Lavin-Tompkins J, Norman D, Rogers D, Simon D. Postprandial insulin profiles with implantable pump therapy may explain decreased frequency of severe hypoglycemia, compared with intensive subcutaneous regimens, in insulin-dependent diabetes mellitus patients. Am J Med. 1996;100(4):412–417. [DOI] [PubMed] [Google Scholar]
- 155. Radziuk J, Pye S, Seigler DE, Skyler JS, Offord R, Davies G. Splanchnic and systemic absorption of intraperitoneal insulin using a new double-tracer method. Am J Physiol. 1994;266(5 Pt 1):E750–E759. [DOI] [PubMed] [Google Scholar]
- 156. Selam JL, Medlej R, M’bemba J, Chevalier A, Guyon F, Ashworth L, Slama G. Symptoms, hormones, and glucose fluxes during a gradual hypoglycaemia induced by intraperitoneal vs venous insulin infusion in type I diabetes. Diabet Med. 1995;12(12):1102–1109. [DOI] [PubMed] [Google Scholar]
- 157. Oskarsson PR, Lins PE, Wallberg Henriksson H, Adamson UC. Metabolic and hormonal responses to exercise in type 1 diabetic patients during continuous subcutaneous, as compared to continuous intraperitoneal, insulin infusion. Diabetes Metab. 1999;25(6):491–497. [PubMed] [Google Scholar]
- 158. Oskarsson PR, Lins PE, Backman L, Adamson UC. Continuous intraperitoneal insulin infusion partly restores the glucagon response to hypoglycaemia in type 1 diabetic patients. Diabetes Metab. 2000;26(2):118–124. [PubMed] [Google Scholar]
- 159. Wan CK, Giacca A, Matsuhisa M, El-Bahrani B, Lam L, Rodgers C, Shi ZQ. Increased responses of glucagon and glucose production to hypoglycemia with intraperitoneal versus subcutaneous insulin treatment. Metabolism. 2000;49(8):984–989. [DOI] [PubMed] [Google Scholar]
- 160. van Dijk PR, Logtenberg SJ, Chisalita SI, Hedman CA, Groenier KH, Gans RO, Kleefstra N, Arnqvist HJ, Bilo HJ. After 6 years of intraperitoneal insulin administration IGF-I concentrations in T1DM patients are at low-normal level. Growth Horm IGF Res. 2015;25(6):316–319. [DOI] [PubMed] [Google Scholar]
- 161. Mischel W. Personality and Assessment. New York, NY: Wiley; 1968. [Google Scholar]
- 162. Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care. 2017;40(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Naranjo D, Tanenbaum ML, Iturralde E, Hood KK. Diabetes technology: uptake, outcomes, barriers, and the intersection with distress. J Diabetes Sci Technol. 2016;10(4):852–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Tanenbaum ML, Adams RN, Lanning MS, Hanes SJ, Agustin BI, Naranjo D, Hood KK. Using cluster analysis to understand clinician readiness to promote continuous glucose monitoring adoption. J Diabetes Sci Technol. 2018;12(6):1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kumah-Crystal YA, Hood KK, Ho YX, Lybarger CK, O’Connor BH, Rothman RL, Mulvaney SA. Technology use for diabetes problem solving in adolescents with type 1 diabetes: relationship to glycemic control. Diabetes Technol Ther. 2015;17(7):449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Tanenbaum ML, Adams RN, Hanes SJ, Barley RC, Miller KM, Mulvaney SA, Hood KK. Optimal use of diabetes devices: clinician perspectives on barriers and adherence to device use. J Diabetes Sci Technol. 2017;11(3):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Waite M, Martin C, Franklin R, Duce D, Harrison R. Human factors and data logging processes with the use of advanced technology for adults with type 1 diabetes: systematic integrative review. JMIR Human Factors. 2018;5(1):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Duke DC, Barry S, Wagner DV, Speight J, Choudhary P, Harris MA. Distal technologies and type 1 diabetes management. Lancet Diabetes Endocrinol. 2018;6(2):143–156. [DOI] [PubMed] [Google Scholar]
- 169. Naranjo D, Suttiratana SC, Iturralde E, Barnard KD, Weissberg-Benchell J, Laffel L, Hood KK. What end users and stakeholders want from automated insulin delivery systems. Diabetes Care. 2017;40(11):1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Barnard KD, Weissberg-Benchell J. Diabetes and partners. Diabetes Technol Ther. 2016;18(5):278–279. [DOI] [PubMed] [Google Scholar]
- 171. Garza KP, Jedraszko A, Weil LEG, Naranjo D, Barnard KD, Laffel LMB, Hood KK, Weissberg-Benchell J. Automated insulin delivery systems: hopes and expectations of family members. Diabetes Technol Ther. 2018;20(3):222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Barnard KD, Lloyd CE, Skinner TC. Systematic literature review: quality of life associated with insulin pump use in type 1 diabetes. Diabet Med. 2007;24(6):607–617. [DOI] [PubMed] [Google Scholar]
- 173. Harrington KR, Boyle CT, Miller KM, Hilliard ME, Anderson BJ, Van Name M, DiMeglio LA, Laffel LM; T1D Exchange Clinic Network. Management and family burdens endorsed by parents of youth <7 years old with type 1 diabetes. J Diabetes Sci Technol. 2017;11(5):980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Petrie JR, Peters AL, Bergenstal RM, Holl RW, Fleming GA, Heinemann L. Improving the clinical value and utility of CGM systems: issues and recommendations: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care. 2017;40(12):1614–1621. [DOI] [PubMed] [Google Scholar]
- 175. Iturralde E, Tanenbaum ML, Hanes SJ, Suttiratana SC, Ambrosino JM, Ly TT, Maahs DM, Naranjo D, Walders-Abramson N, Weinzimer SA, Buckingham BA, Hood KK. Expectations and attitudes of individuals with type 1 diabetes after using a hybrid closed loop system. Diabetes Educ. 2017;43(2):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Tanenbaum ML, Iturralde E, Hanes SJ, Suttiratana SC, Ambrosino JM, Ly TT, Maahs DM, Naranjo D, Walders-Abramson N, Weinzimer SA, Buckingham BA, Hood KK. Trust in hybrid closed loop among people with diabetes: perspectives of experienced system users. J Health Psychol. 2017 (Jul 1):1359105317718615. doi: 10.1177/1359105317718615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Barnard KD, Hood KK, Weissberg-Benchell J, Aldred C, Oliver N, Laffel L. Psychosocial assessment of artificial pancreas (AP): commentary and review of existing measures and their applicability in AP research. Diabetes Technol Ther. 2015;17(4):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Barnard KD, Venkat MV, Close K, Heinemann L, Weissberg-Benchell J, Hood KK, Kubiak T, Kowalski AJ, Laffel L. PsychDT working group: report psychosocial aspects of artificial pancreas systems. J Diabetes Sci Technol. 2015;9(4):925–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Cameron F, Buckingham BA, Wilson DM, Bequette BW. Extending threshold-based detection of infusion set failures. J Diabetes Sci Technol. 2012;7(1):A17. [Google Scholar]
- 180. Cescon M, DeSalvo DJ, Ly TT, Maahs DM, Messer LH, Buckingham BA, Doyle FJ III, Dassau E. Early detection of infusion set failure during insulin pump therapy in type 1 diabetes. J Diabetes Sci Technol. 2016;10(6):1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Howsmon DP, Cameron F, Baysal N, Ly TT, Forlenza GP, Maahs DM, Buckingham BA, Hahn J, Bequette BW. Continuous glucose monitoring enables the detection of losses in infusion set actuation (LISAs). Sensors (Basel). 2017;17(1):E161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Howsmon DP, Baysal N, Buckingham BA, Forlenza GP, Ly TT, Maahs DM, Marcal T, Towers L, Mauritzen E, Deshpande S, Huyett LM, Pinsker JE, Gondhalekar R, Doyle FJ III, Dassau E, Hahn J, Bequette BW. Real-Time detection of infusion site failures in a closed-loop artificial pancreas. J Diabetes Sci Technol. 2018;12(3):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]