Abstract
Catecholamine-secreting tumors are rare among the pediatric population but are increasingly being reported in children with sustained hypoxia secondary to cyanotic congenital heart disease (CCHD). With this review, we report the clinical characteristics of these tumors in children with CCHD. The articles included in the present review were identified using PubMed through February 2019. A manual search of the references retrieved from relevant articles was also performed. Pheochromocytomas and paragangliomas (PPGL) in children are commonly associated with high-risk germline or somatic mutations. There is evidently a higher risk of tumorigenesis in children with CCHD as compared with the general pediatric population, even in the absence of susceptible gene mutations. This is due to molecular mechanisms involving the aberrant activation of hypoxia-response elements, likely secondary to sustained hypoxemia, resulting in tumorigenesis. Due to overlapping symptoms with CCHD, the diagnosis of PPGL may be delayed or missed in these patients. We studied all previously reported PPGL cases in children with CCHD and reviewed phenotypic and biochemical features to assess for contributing factors in tumorigenesis. Larger studies are needed to help determine other potential predisposing factors and to establish screening guidelines in this high-risk population. A delay in diagnosis of the PPGL tumors can lead to exacerbation of cardiac failure, and therefore early diagnosis and intervention may provide better outcomes in these patients, necessitating the need for regular surveillance. We recommend routine biochemical screening in patients with sustained hypoxia secondary to CCHD.
Keywords: pheochromocytoma, paraganglioma, catecholamine secreting tumors, hypoxia induced tumorigenesis, cyanotic congenital heart disease
Pheochromocytomas and paragangliomas (PPGL) are catecholamine-producing tumors of the autonomic nervous system that arise from the chromaffin cells in the adrenal medulla or extra-adrenal paraganglionic tissue, respectively. About 27% to 40% of these tumors are associated with germline mutations in the Von Hippel–Lindau (VHL), Rearranged during Transfection (RET), Myc-Associated Factor X (MAX), Neurofibromin 1 (NF1), Succinate Dehydrogenase complex Assembly Factor 2 (SDHAF2), Succinate Dehydrogenase A (SDHA), Succinate Dehydrogenase B (SDHB), Succinate Dehydrogenase C (SDHC), Succinate Dehydrogenase D (SDHD), and Transmembrane Protein 127 (TMEM127) genes [1, 2]. There are multiple reports of chronic hypoxemia triggering the development of such tumors, especially in patients with cyanotic congenital heart disease (CCHD). PPGL are rare entities in the pediatric population; however, their incidence is significantly higher among patients with CCHD. Although most patients with CCHD present with PPGL during adulthood after prolonged periods of hypoxemia, some may present earlier, and all clinicians caring for these patients must be mindful of this complication.
We report a 12-year-old female patient with a history of hypoplastic left heart status after a lateral tunnel nonfenestrated Fontan procedure. The patient presented with classic symptoms of a catecholamine-producing tumor, which was confirmed to be a paraganglioma on subsequent pathology. We review the characteristics of pediatric patients with CCHD with PPGL reported previously in the literature and the proposed molecular mechanisms of hypoxia-induced tumorigenesis. This review targets pediatric cardiology, endocrinology, and nephrology providers, with the aim to increase awareness of this complication.
1. Case Description
A 12-year-old female patient with a history of hypoplastic left heart had a lateral tunnel nonfenestrated Fontan procedure with mitral and tricuspid valvuloplasty performed at 4 years of age. At presentation, her resting oxygen saturation was 86% to 92%, and she had an elevated hematocrit of 48% to 50% (reference: 35% to 45%). She complained of frequent episodes of sweating, anxiety, and chest pain over several months. On admission, she was noted to have repeated episodes of tachycardia between 90 to 120 beats per minute (normal for patient’s age: 60 to 100 beats per minute) and hypertension up to 220/130 mm Hg (95th percentile for patient’s age, sex, and height: 131/88 mm Hg). Hypertension was refractory to antihypertensive medications including amlodipine, enalapril, nicardipine drip, and hydralazine.
The endocrine team was consulted for the evaluation of hypertension. Based on the blood and urine test results as shown in Table 1, diagnosis of a catecholamine-producing tumor was made.
Table 1.
Preoperative Urinary Catecholamine Levels in Our Patient
| Laboratory Result | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| Plasma normetanephrine, nmol/L (reference: 0–0.89 nmol/L) | 32.7 | 37.8 | 37.4 |
| Plasma metanephrine, nmol/L (reference: 0–0.49 nmol/L) | 0.46 | 0.51 | 0.43 |
| 24-h urine normetanephrine, μg (reference: 67–503 μg/24 h) | 4433 | 4449 | 5125 |
| 24-h urine metanephrine, μg (reference: 51–275 μg/24 h) | 106 | 101 | 130 |
Computed tomography (CT) scan with contrast showed an enhancing abdominal mass in proximity to the left adrenal gland (Fig. 1). Subsequently, a positron emission tomography (PET)-CT scan, using the 68Ga-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-TATE–conjugated somatostatin receptor–targeting peptide tracer, confirmed the presence of PPGL (Fig. 2). Preoperatively, the patient was medically managed with phenoxybenzamine, which was initiated at a dose of 0.2 mg/kg every 12 hours and was slowly increased to 0.4 mg/kg every 6 hours. Metyrosine was added 1 week prior to the scheduled procedure at 125 mg every 8 hours and was slowly increased to 500 mg every 6 hours. While on α-blockade therapy, the patient had orthostatic hypotension and was placed on a high-sodium diet (minimum of 3 g/d of salt). Upon surgery, a left para-aortic mass was found separate from the left adrenal gland, and cytology confirmed a paraganglioma tumor. To prevent a hypotensive crisis, which is common after PPGL resection due to catecholamine withdrawal, the patient was maintained on plasmalyte, epinephrine, and vasopressin drip. These were slowly tapered and discontinued 2 to 3 days after surgery.
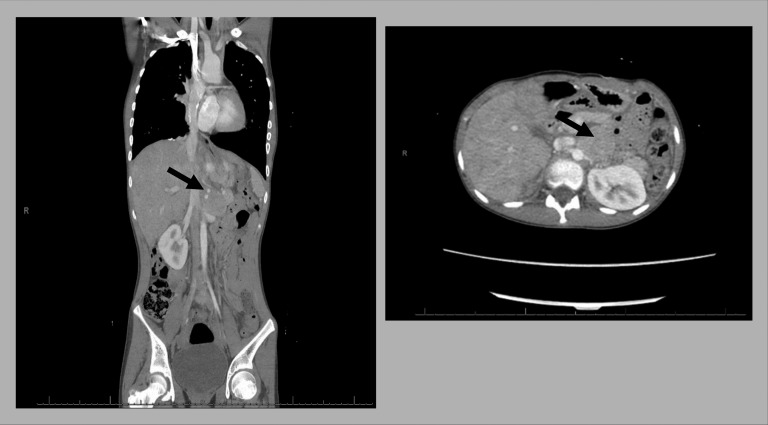
Figure 1.
CT scan with contrast showed an enhancing mass arising from or in proximity to the left adrenal gland measuring 3.6 × 3.7 × 6.6 cm (arrows).
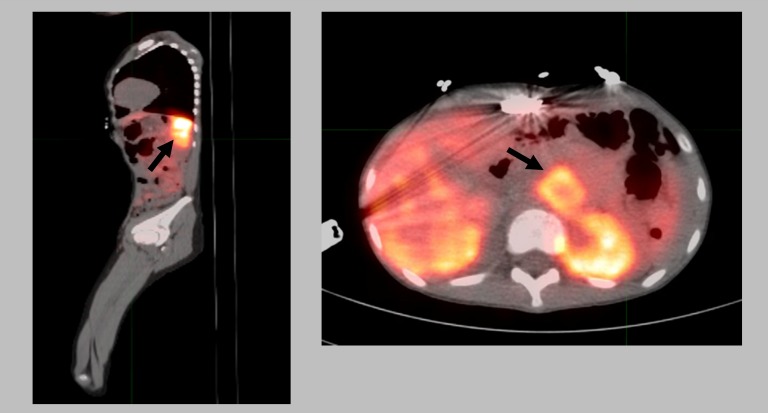
Figure 2.
PET-CT imaging from the skull vertex to the base of feet the performed after the injection of 1.82 mCi of Ga-68-DOTA-TATE. Injection-to-scan interval: 1.5 h. Focal tracer uptake within the left upper quadrant mass possibly arising from the left adrenal gland (arrows).
Blood and urine tests were repeated 1 month postoperatively. Plasma normetanephrine and metanephrine levels decreased to normal (0.34 and 0.20 nmol/L, respectively). The 24-hour urine normetanephrine and metanephrine levels normalized to 142 and 62 μg/24 h, respectively. Genetic testing revealed no germline mutations in the genes tested by a commercial hereditary PPGL panel. The panel tests for gene mutations commonly associated with hereditary PPGL, such as MAX, NF1, RET, SDHAF2, SDHA, SDHB, SDHC, SDHD, TMEM127, and VHL. Targeted next-generation sequencing of the tumor specimen (genes included in the Pediatric Solid Tumor Cancer Mutation Panel) did not reveal any clinically important alterations in the targeted genes but showed mutations in the Lysine N-methyltransferase 2C (KMT2C), Lysine N-methyltransferase 2D (KMT2D), n-myc proto-oncogene (MYCN) and Smoothened, Frizzled Class Receptor (SMO) genes, which were reported as variants of unknown significance. These genetic panels did not include testing for the Endothelial PAS Domain Protein 1/Hypoxia-inducible Factor 2A (EPAS1/HIF2A), Prolyl Hydroxylase Domain-Containing Protein 1 (PHD1), Prolyl Hydroxylase Domain-Containing Protein 2 (PHD2), or Iron-Responsive Element-Binding Protein 1 (IRP1) genes.
2. Materials and Methods
A comprehensive literature search was conducted, and data were retrieved using PubMed. This search was last performed in February 2019. The following search terms were used: “Pheochromocytoma” or “Paraganglioma” and “Hypoxia induced tumorigenesis” or “Pseudohypoxia Signature” or “Cyanotic congenital heart disease” or “Pseudo-hypoxia.” We included 13 articles (20 unique pediatric cases with symptom onset <18 years of age) that reported PPGL in patients with CCHD, which have been referenced in our report. A manual search of the references retrieved from relevant articles was also performed.
3. Prevalence of PPGL in Patients With CCHD
PPGL are rare catecholamine-producing tumors with a reported prevalence of about 1.7% in children [3] and about 0.1% to 0.6% in adults presenting with hypertension [4–7]. A study, including data from the Dutch pathology registry, reported a general annual incidence of 0.46 (95% CI, 0.39 to 0.53) per every 100,000 persons among The Netherlands population between years 2011 and 2015 [8]. Despite being a rarity among the general population, it is relatively more common in the CCHD cohort.
PPGL in association with CCHD were first reported in 1964 [9]. Of the 31,227 autopsies performed at Johns Hopkins hospital over a 61-year period, only 21 cases of PPGL were histologically confirmed. This further emphasizes that these tumors are relatively rare in the general population. However, 3 of those 21 (14%) patients had a history of a cyanotic heart disease, which was unlikely to be an incidental occurrence [9]. A recent study from Korea showed that the incidence of PPGL among Fontan patients older than 10 years of age was 2.5%, which is almost fourfold higher than the general population [10]. The age of onset in these patients was also significantly earlier compared with those with no history of congenital heart disease (CHD).
Another multicenter study, analyzing more than 40 million hospitalizations, reported a higher association of PPGL among patients hospitalized with CCHD when compared with noncyanotic CHD hospitalizations (0.3 ± 0.1% vs 0.05 ± 0.01%). The OR of hospitalization with PPGL among patients with CCHD was 6.0 (95% CI, 2.6 to 13.7; P < 0.0001), whereas the odds of PPGL in noncyanotic CHD was similar to patients without CHD (OR, 0.9; P = 0.48) [11].
4. Genetic Susceptibility for PPGL
PPGL are commonly associated with germline and/or somatic mutations in the susceptible genes resulting in these tumors. More than a third of these tumors are associated with at least one of the common germline mutations, including VHL, RET, NF1, MAX, SDHA, SDHB, SDHC, SDHD, SDHAF2, and TMEM127 genes, and about 25% to 30% are associated with somatic mutations, such as RET, VHL, NF1, MAX, EPAS1/HIF2A, and Harvey rat sarcoma viral oncogene homolog (HRAS) genes [1, 12–16]. Some of these somatic mutations are linked with the processes involved in hypoxia adaptation.
PPGL of various genetic backgrounds are categorized into two clusters based on their downstream transcription profiles [17]. Cluster 1 includes genes (VHL, SDH, and EPAS1/HIF2A) that are associated with the hypoxic response and have a common hypoxia-inducible factor (HIF) activation pathway. Cluster 2 includes genes (RET, NF1, TMEM127, and MAX) that involve tumorigenesis through activation of kinase signaling pathways and protein translation.
Under chronic hypoxic conditions, a series of adaptive or protective responses are inactivated inside the cell, mainly involving the Cluster 1 genes and the degradation pathways of the HIF. Somatic gain-of-function mutations in the EPAS1/HIF2A gene (encoding for HIF2α) have been reported to result in the upregulation of several hypoxia-related genes encoding for vascular endothelial growth factors, erythropoietin, etc. [18–20]. Additionally, this upregulation in the EPAS1/HIF2A gene has been reported to occur in association with chronic hypoxia, causing tumorigenesis and development of PPGL [21–23]. Vaidya et al. [21] reported somatic EPAS1/HIF2A gene mutations in four out of the five cases with CCHD in their report, which points toward the hypoxia-induced somatic mutations playing a role in tumor causation.
5. Hypoxia-Induced Tumorigenesis: “Pseudohypoxia Signature”
HIFs are heterodimers consisting of an O2-labile α-subunit and a stable β-subunit. Three isoforms of HIFα exist in humans: HIF1α, HIF2α, and HIF3α. HIF1α is ubiquitously expressed, whereas HIF2α and HIF3α are selectively expressed in certain types of cells. High intracellular levels or impaired degradation of HIFα can activate transcription of genes potentiating tumorigenesis.
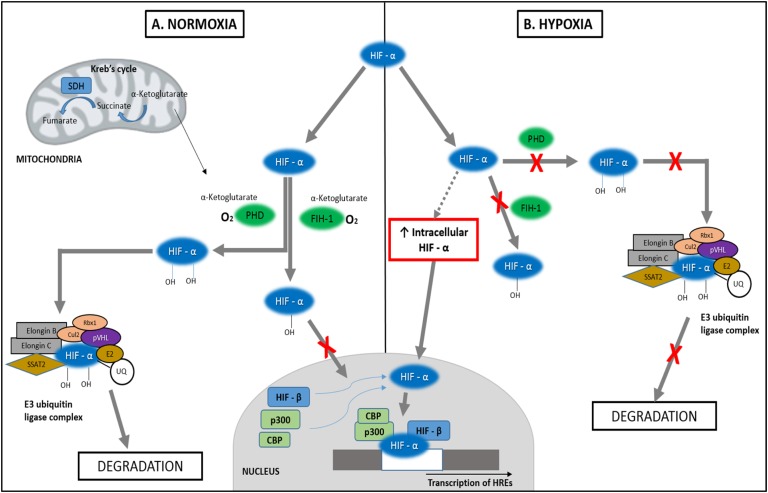
Two types of dioxygen-dependent modification pathways for HIFα have been identified, both of which eventually inhibit transcription of hypoxia related genes under normoxic conditions (Fig. 3). The first one involves the hydroxylation of HIFα subunits at their two proline residues (Pro-402 and Pro-564 in HIF-1α), specifically at their 4′-location. This hydroxylation is mediated by α-ketoglutarate-dioxygen–dependent prolyl hydroxylase domain proteins. Once hydroxylated, it is recognized and binds to the von Hippel-Lindau tumor suppressor protein and ubiquitin, a reaction that is catalyzed by the E2-conjugating ubiquitin enzyme (E2). Additionally, it binds with elongin B, elongin C, and various other proteins to form the E3 ubiquitin ligase complex. The complex is then degraded by proteasomes and thus fails to activate the HIF target genes and hypoxia-response elements (HREs) that play a role in tumorigenesis [24–29].
Figure 3.
Hypoxia-induced tumorigenesis. (A) Under normoxic conditions, HIFα is hydroxylated by PHDs at the two prolyl residues, bound to the E3 ubiquitin complex, and then degraded by proteosomes. It can also be hydroxylated at the asparagine residue by FIH-1, which prevents its interaction with CBP and p300 in the nucleus, ultimately inhibiting transcription. (B) PHDs and FIH-1 are oxygen-dependent enzymes and therefore are unable to hydroxylate HIFα under hypoxic conditions. This leads to decreased in degradation of HIFα and increased transcription of hypoxia related genes. CBP, cAMP-response element binding protein; Cul2, Cullin 2; E2, E2 ubiquitin-conjugating enzyme; FIH-1, factor inhibiting HIF-1; p300, histone acetyltransferase p300; PHD, prolyl hydroxylase domain protein; pVHL, von Hippel-Lindau protein; Rbx1, Ring box protein 1; SDH, succinate dehydrogenase; SSAT2, spermidine/spermine N1-acetyltransferase 2; UQ, ubiquitin.
The second pathway involves the hydroxylation of asparagine 803 residue at the C-terminal transactivation domain of HIFα, mediated by another α-ketoglutarate-dioxygen–dependent hydroxylase factor–inhibiting HIF-1. This prevents the interaction of HIFα with its coactivators, histone acetyltransferase p300 (p300) and cAMP response element–binding protein (CBP), which results in an inhibition of transcription of the HREs [24–28].
In patients with cyanotic heart disease and chronic hypoxia, HIFα is stabilized by several molecular aberrations (evades degradation) and heterodimerizes with the HIFβ subunit. The heterodimer interacts with the coactivators p300 and cAMP response element–binding protein, binds to HREs in the target genes, activates their transcription, and potentiates tumor development [15, 25]. This has been described as the “pseudohypoxia signature.” Recent studies have established a role of gain-of-function mutations in the EPAS1/HIF2A gene, in association with chronic hypoxia, which stabilizes HIFα, causing an upregulation of the genes involved in tumorigenesis and in the development of PPGL [21–23]. Somatic gene mutations in other hypoxia-regulated genes, including PHD1, PHD2, and IRP1, involved in the stabilization of HIFα have been reported to be associated with erythrocytosis and PPGL tumors [30–33].
6. Phenotypic and Biochemical Features of PPGL in Patients With CCHD
Studies of patients with CCHD who developed PPGL have shown that, at diagnosis, the median age was 24 to 31 years, mean resting oxygen saturation was 87.1% to 87.4%, and cyanosis duration was 6 to 25 years [10, 11]. However, these studies included adult presentations as well. We reviewed 13 case reports and have characterized the clinical features of 21 pediatric patients (including our case) with CCHD who were diagnosed with PPGL ante- or postmortem (Table 2).
Table 2.
Clinical Characteristics of Reported PPGL Cases in Children With CCHD
| Case No. | Study | Age at Diagnosis/Sex | CHD | Mode and Age of Repair | SpO2 | Age of Onset; Symptoms; Highest BP | Diagnosis | Elevated Biochemistry | Treatment/Complications |
|---|---|---|---|---|---|---|---|---|---|
| Patients Who Presented With Any Symptoms | |||||||||
| 1 | Folger et al., (1964) [9] | 14/F | TGA | Not repaired | 77% | 12 y; headache, sweating, tachycardia, insomnia, vomiting; 150/110 | Left adrenal pheochromocytoma with metastasis to the regional LN and liver | N/A | Deceased (diagnosed on autopsy) |
| 2 | Folger et al., (1964) [9] | 20/M | TOF | MBTS; 7 y | 80% | 15 y; epistaxis, headache, sweating, insomnia, nervousness; 200/150 | Left adrenal pheochromocytoma | Urine NE: ↑; urine E: = | Surgery |
| 3 | Reynolds and Gilchrist (1966) [34] | 12/F | TGA and VSD | Not repaired | 48% | 11 y; severe headaches, sweating, vomiting, nervousness; 142/112 | Extra-adrenal paraganglioma with metastasis to bone marrow | N/A | Deceased (diagnosed on autopsy) |
| 4 | Cherqaoui et al., (2006) [35] | 13/M | SRV, TA | BTS: 1 y; modified Glenn: 8 y | 83% | 13 y; paroxysmal symptoms; high (not reported) | Two extra-adrenal retroperitoneal paraganglioma | Blood NE: ↑; blood E: =; urine NE: ↑; urine NM: ↑; urine E: = | Surgery |
| 5 | Cheung and Spevack (2008) [36] | 14/F | PA, SLV | MBTS: 2 mo, 18 mo; Glenn: 6 mo; Fontan: 8 y | 75% | 14 y; chest pain, sweating, headache, dyspnea, fatigue; 180/120 | Right adrenal pheochromocytoma | Blood NE: ↑; blood E: =; blood DA: =; urine NM: ↑ | Surgery; metastasis to bone after several months |
| 6 | Chung et al., (2008) [37] | 13/M | Single ventricle, PS, PDA | F-Fontan: 3 y, 13 y; aortic root reconstruction and AV valve repair: 9 y | 78% | 13 y; abdominal pain, nausea, vomiting (diagnosed as cholecystitis); 200 (systolic) (HTN crisis during cholecystectomy procedure) | Right adrenal pheochromocytoma; incidentally diagnosed during a cholecystectomy procedure | Blood NE: ↑; blood E: ↑; urine MN: ↑; urine VMA: ↑ | Surgery; metastasis to bone after 1 y |
| 7 | Hwang et al., (2012) [38] | 18/M | Complex CCHD | TAPVR repair: 1 y; M-Fontan: 3 y | Cyanotica | 18 y; dyspnea, tachycardia; 141/81 | Single extra-adrenal left para-aortic paraganglioma | Blood NE: ↑; blood E: =; blood DA: =; urine VMA: ↑ | Surgery |
| 8 | Kasaliwal et al., (2014) [39] | 14/F | TOF | MBTS: 4 mo | Cyanotica | 14 y; headaches, sweating, abdominal pain, nausea, palpitations; 200 (systolic) | Single right adrenal pheochromocytoma | Blood NM: ↑; blood MN: ↑; RET mut.: neg | Refused surgery; medical treatment (prazosin and amlodipine) |
| 9 | Opotowsky et al., (2015) [11] | 16/M | DILV, PA, VSD, D-TGA | BTS: 3 d; Glenn: 4 y; UF-Fontan: 7 y | 89% | 16 y; paroxysmal and resistant hypertension; high (not reported) | Single adrenal pheochromocytoma (side unknown) | Not available | Surgery |
| 10 | Opotowsky et al., (2015) [11] | 18/F | PA/IVS, EA of TV, absent IVC and common iliac veins | Blalock-Hanlon septostomy: 1 mo; BDG and thromboexclusion of RV: 11 mo; F-Fontan: 2 y | 88% | 18 y; atrial arrhythmia, sweating, flushing; not reported | Single extra-adrenal left retroperitoneal paragangliomna | Urine NE: ↑; urine NM: ↑; urine E: =; urine MN: =; urine DA: =; germ-line mutationb: neg | Surgery |
| 11 | Opotowsky et al., (2015) [11] | 15/M | TOF/ PS, ASD, bicuspid aortic valve | TOF repair: 9 y | 95% (9 y of cyanosis prior) | 15 y; paroxysmal and resistant hypertension, ventricular arrhythmia, worsening HF; high (not reported) | Multiple lesions; single left adrenal pheochromocytoma and two right adrenal pheochromocytoma | Urine NE: ↑; urine E: =; urine DA: = | Surgery |
| 12 | Yamamoto et al., (2016) [40] | 15/F | Type Ic TA | Hemi-Fontan with pulmonary artery banding: 9 mo; M-Fontan: 2 y; pacemaker implant: 3 y; coil embolization of veno-venous shunts: 10 y | 90% | 15 y; paroxysmal sweating, dizziness, transient hypertension; 180/106 | Single right adrenal pheochromocytoma | Plasma NE: ↑; urine NE: ↑; urine NM: ↑; germ-line mutationc: neg | Surgery |
| 13 | Song et al., (2018) [10] | 13/M | Left isomerism, uAVSD, SRV | F-Fontan: 3 y | 71% | 13 y; abdominal pain; normal | Single right extra-adrenal paraganglioma (very close proximity to right adrenal gland); incidental finding on CT scan | Plasma NE: ↑; plasma E: ↑; plasma DA: ↑; urine NE: ↑; urine NM: ↑; urine E: =; urine MN: =; urine DA: ↑; urine VMA: ↑ | Surgery; multiple metastasis to bone and liver after 2 y → death at 18 y |
| 14 | Song et al., (2018) [10] | 16/F | TA | Pulmonary artery banding: 13 d; BCPC: 1 y; ECC: 8 y | 90% (8 y of cyanosis) | 16 y; palpitations, syncope, headaches; normal | Single left adrenal pheochromocytoma | Plasma NE: ↑; plasma NM: ↑; plasma E: =; plasma MN: =; plasma DA: =; urine NE: =; urine NM: ↑; urine E: =; urine DA: =; urine VMA: ↑ | Surgery |
| 15 | Song et al., (2018) [10] | 18/M | Right isomerism, uAVSD, SRV, TAPVR | BCPC: 10 mo; F-Fontan: 3 y | 90% | 18 y; palpitations, junctional tachycardia, sweating, paroxysmal hypertension; high (not reported) | Bilateral extra-adrenal paraganglioma | Plasma NE: ↑; plasma E: =; plasma DA: =; urine NE: ↑; urine NM: ↑; urine E: =; urine MN: =; urine DA: =; urine VMA: ↑ | Surgery |
| 16 | Deshpande et al., (2018) [41] | 12/F | HLH | ECC: 4 y | 92% | 12 y; Fontan dysfunction and RV systolic dysfunction (was diagnosed as a pancreatic mass on MRI); normal | Single right extra-adrenal paraganglioma (retroperitoneal and adherent to IVC); incidentally found during Whipple’s surgery | None | Surgery |
| 17 | Vaidya et al., (2018) [21] | 13/F | PA, DORV, ASD, VSD | BTS: 3 d; central shunt: 7 y; pulmonary arterioplasty with AV valvuloplasty and central shunt closure: 17 y | 85% | 13 y; hypertension, diaphoresis, palpitations, dyspnea; N/A | Single left adrenal pheochromocytoma | Plasma NM: ↑; plasma MN: =; germ-line mutation: neg; somatic mutation: EPAS1 | Surgery |
| 18 | Our case | 12/F | HLH | UF-Fontan with mitral and tricuspid valvuloplasty: 4 y | 89% | 12 y; episodes of sweating, anxiety, chest pain, palpitations; 200/90 | Single extra-adrenal left para-aortic paraganglioma | Plasma NM: ↑; plasma MN: =; urine NM: ↑; urine MN: =; germ-line mutationd: neg | Surgery |
| Patients Who Were Diagnosed Only on Autopsy (No Reported Symptoms) | |||||||||
| 19 | Folger et al., (1964) [9] | 16/M (autopsy report) | Origin of great vessels from RV, dextrocardia, PS | MBTS – 3 y | 43% | None; 110/70 | Bilateral adrenal pheochromocytoma | N/A | Deceased (diagnosed on autopsy) |
| 20 | de la Monte et al., (1985) [42] | 14/M (autopsy report) | TOF | None | N/A | None; N/A | Adrenal pheochromocytoma | N/A | Deceased (diagnosed on autopsy) |
| 21 | de la Monte et al., (1985) [42] | 16/M (autopsy report) | AV canal | None | N/A | None; N/A | Adrenal pheochromocytoma | N/A | Deceased (diagnosed on autopsy) |
Abbreviations: ASD, atrial septal defect; AV, atrio-ventricular; BCPC, bidirectional cavopulmonary connection; BDG, bidirectional Glenn; BP, blood pressure; BTS, Blalock-Taussig shunt; DA, dopamine; DILV, double inlet left ventricle; DORV, double-outlet right ventricle; E, epinephrine; EA, Ebstein anomaly; ECC, extracardiac conduit Fontan; F, female; F-Fontan, fenestrated Fontan; HF, heart failure; HLH, hypoplastic left heart; HTN, hypertension; IVC, inferior vena cava; IVS, intact ventricular septum; LN, lymph node; M, male; MBTS, modified Blalock-Taussig shunt; M-Fontan, modified Fontan; MN, metanephrine; NE, norepinephrine; NM, normetanephrine; PA, pulmonary atresia; PDA, patent ductus arteriosus; PS, pulmonary stenosis; RV, right ventricle; S/S, signs and symptoms; SLV, single left ventricle; SRV, single right ventricle; TA, tricuspid atresia; TAPVR, total anomalous pulmonary venous return; TGA, transposition of great vessels; TOF, Tetralogy of Fallot; TV, tricuspid valve; uAVSD, unbalanced atrioventricular septal defect; UF-Fontan, unfenestrated Fontan; VMA, vanillylmandellic acid; VSD, ventricular septal defect.
Reported to be cyanotic; SpO2 was not reported.
PPGL genetic panel for germ-line mutations: RET, SDHA, SDHB, SDHC, SDHD, SDHAF2, TMEM127, MAX, VHL.
PPGL genetic panel for germ-line mutations: RET, SDHB, SDHD, TMEM127, MAX, VHL.
PPGL genetic panel for germ-line mutations: RET, SDHA, SDHB, SDHC, SDHD, SDHAF2, TMEM127, MAX, VHL, NF1.
A review of the reported pediatric cases of PPGL with CCHD reveals that there have been no reported cases that presented before 10 years of age. The mean and median age of onset of symptoms was 14 years among the cases that presented antemortem. However, the mean age of diagnosis was 14.8 years, and there was no sex predominance. The rarity of these tumors in pediatric patients and the overlapping symptoms with the underlying cardiac pathology may lead to a delay in diagnosis.
Six of the 21 cases (including three postmortem) were diagnosed incidentally. The cause of death, or any prior symptoms, was not reported in the three deceased cases. Three of the 18 cases (cases 6, 13, and 16; Table 2), diagnosed antemortem, were incidentally found during the evaluation of abdominal pain or during surgery for conditions other than PPGL. These three cases did not have the classic paroxysmal symptoms of catecholamine-secreting tumors or hypertension. Interestingly, two of these three cases (cases 6 and 13) had no biochemical predominance for either of the catecholamines, and case 16 did not report any biochemical testing. Case 6, despite being normotensive, was reported to have a hypertensive crisis during a cholecystectomy procedure. Therefore, blood pressure monitoring has poor sensitivity as a sole screening tool due to the episodic nature of hormone production from these tumors and desensitization of adrenoreceptors [43, 44].
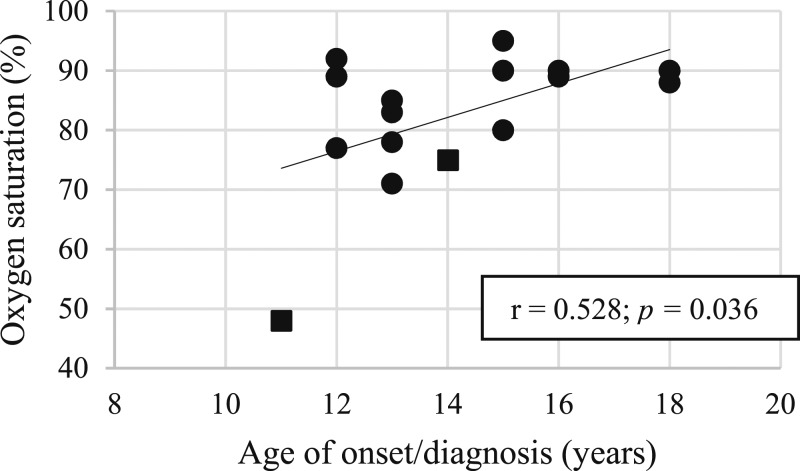
Reynolds and Gilchrist [34] reported on the youngest patient with an unrepaired heart defect, who presented at the age of 11 years. The youngest patient reported also had the lowest oxygen saturation (SpO2 < 50%). We noted a modest positive correlation (r = 0.528, P = 0.036) between the age of onset of symptoms and the oxygen saturation (Fig. 4). This suggests that a lower chronic tissue oxygen level leads to tumor development and symptomatic presentation at a younger age.
Figure 4.
Correlation of age at onset and SpO2. Box: deceased, diagnosed on autopsy (n = 2). Circle: presented with symptoms (n = 14). Excluded were three patients diagnosed incidentally on autopsy (reason for death unknown) and two patients with no reported SpO2.
Biochemical testing was performed in 14 of the 21 cases described in this report (Table 3). Of these 14 patients, 11 had a predominantly norepinephrine-secreting tumor; high levels of plasma norepinephrine, urine normetanephrine, and urine vanillylmandelic acid were noted in these patients. The other three patients did not show predominance of either of the catecholamines in their biochemical profiles and were biochemically more mixed type tumors. Plasma and urine dopamine levels were normal in all patients with one exception, suggesting its low diagnostic utility in the evaluation of PPGL.
Table 3.
Biochemical Characteristics of Catecholamine-Secreting Tumors in Patients With CCHD
| Primarily NE-Secreting Tumors (n = 11) | Mixed NE/E-Secreting Tumors (n = 3) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 2 | Case 4 | Case 5 | Case 7 | Case 10 | Case 11 | Case 12 | Case 14 | Case 15 | Case 17 | Case 18 | Case 6 | Case 8 | Case 13 | |
| Plasma E | =a | = | = | = | = | = | = | ↑b | ||||||
| Plasma MN | = | = | = | ↑ | ||||||||||
| Plasma NE | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||||
| Plasma NM | ↑ | ↑ | ↑ | ↑ | ||||||||||
| Plasma DA | = | = | = | = | ↑ | |||||||||
| Urine E | = | = | = | = | = | = | ||||||||
| Urine MN | = | = | = | ↑ | = | |||||||||
| Urine NE | ↑ | ↑ | ↑ | ↑ | = | ↑ | ↑ | |||||||
| Urine NM | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||
| Urine VMA | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||||
| Urine DA | = | = | = | = | ↑ | |||||||||
Blank boxes denote levels not obtained.
Abbreviations: DA, dopamine; E, epinephrine; MN, metanephrines; NE, norepinephrine; NM, normetanephrine; VMA, vanillylmandellic acid.
Normal.
Increased.
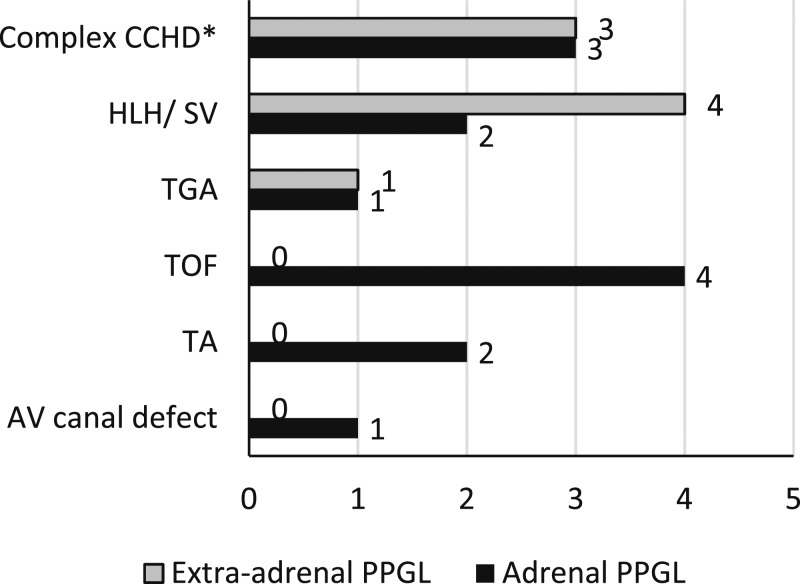
Thirteen of these 21 cases were adrenal pheochromocytomas, and eight were extra-adrenal paragangliomas. Figure 5 shows a detailed description of the type of CCHD in relation to the location of the tumor. Five of the 21 cases had distant metastases to the bone or liver, of which two cases presented with metastasis at initial presentation. Four of the 21 cases had genetic testing; results were reported to be negative for the common germline mutations associated with PPGL. One of the cases (case 17) was positive for a somatic mutation in the EPAS1/HIF2A gene but was negative for germline mutations.
Figure 5.
Type of CCHD vs location of PPGL. AV, atrio-ventricular; HLH/SV, hypoplastic left heart; TA, tricuspid atresia; TGA, transposition of great vessels; TOF, Tetralogy of Fallot. *Complex CCHD (patients with more than one etiology for cyanosis).
7. Surveillance in Patients with CCHD
Considering the apparent increased risk of PPGL among patients with chronic hypoxemia and cyanotic heart disease and the potential for serious morbidity and mortality, routine surveillance is warranted. Although these patients are closely followed by cardiologists and have regular monitoring of their blood pressures and cardiac function, some of these patients may not present with the classic paroxysmal symptoms associated with PPGL. Because many symptoms of catecholamine excess (hypertension, palpitations, sweating, etc.) may also be seen in patients with cyanotic heart diseases, physicians may attribute these symptoms to the underlying cardiac condition and may not consider evaluation for PPGL, which is a relatively rare diagnosis. This may lead to a delay in diagnosis of PPGL, which not only exacerbates cardiac failure and hypoxemic symptoms but may also lead to death. Therefore, regular biochemical and radiologic surveillance testing in these high-risk patients is needed to facilitate early diagnosis.
We reviewed several surveillance recommendations by various physician groups that are targeted toward patients with risk factors for developing PPGL. In normoxic patients with a history of PPGL, the Endocrine Society Practice Guidelines recommend life-long annual biochemical surveillance with plasma or urine metanephrine levels because these patients are at increased risk of recurrent or metastatic disease [4].
The American Thyroid Association guidelines for pheochromocytoma surveillance in patients with Multiple Endocrine Neoplasia type 2 syndromes recommend screening high-risk and moderate-risk patients starting at the age of 11 and 16 years, respectively. They recommend screening with either plasma metanephrine and normetanephrine or 24-hour urinary metanephrine and normetanephrine, with additional CT or MRI of the abdomen for patients with positive biochemical testing [45].
In 2018, Wong et al. [46] published practice guidelines on the surveillance of PPGL among patients carrying an SDHx (SDHA, SDHB, SDHC, or SDHD) variant mutation who are at an increased risk of developing these tumors. The age at presentation varied depending upon the specific mutation, with the SDHB mutation carriers presenting at younger ages. However, the mean age of presentation in patients carrying any of the SDHx mutations was 13.5 years. Wong et al. [46] recommended screening with an annual physical examination, including blood pressure, as well as biochemical testing for all high-risk patients. However, guidelines for the age at initiation of screening were different for SDHB mutation carriers as compared with SDHA, SDHC, or SDHD. Screening was recommended starting at 5 years of age for SDHB mutation carriers and at 10 years of age for patients carrying a mutation in SDHA, SDHC, or SDHD. Radiologic surveillance using MRI every 2 to 3 years was also recommended, starting at age 10 years for SDHB and 15 years for patients in whom biochemical screening was unremarkable [46].
There are not enough data on PPGL in pediatric patients with CCHD, and larger-cohort studies are needed to establish guidelines for surveillance testing. When considering screening tools or diagnostic testing for a disease, the following factors need to be addressed: (i) practicality, invasiveness, sensitivity, and cost of the test; (ii) age of commencement of screening; and (iii) frequency of screening. Specifically for patients with CCHD, the degree of hypoxia, the cumulative duration, and the sustained vs episodic nature of hypoxia likely affect tumorigenesis. These factors that define the “hypoxia pattern” would potentially influence surveillance guidelines.
Based on our review of cases and the surveillance recommendations available in the literature for other high-risk groups, we suggest routine surveillance for patients with a known history of CCHD. We propose performing regular physical examination, including blood pressure monitoring along with measuring plasma free metanephrine or 24-hour urinary fractionated metanephrine levels annually, after 10 years of persistent hypoxia with an average ambulatory oxygen saturation of ≤92%. Blood should be drawn in the supine position, and results should always be correlated clinically [47]. We also recommend obtaining these tests in patients who develop an unexplained deterioration of cardiac function. Radiologic surveillance, including CT scan or MRI, should be reserved for patients who have clinical and/or biochemical suspicion of the tumor.
In a study by Dzimir et al. [48], patients with CHD (cyanotic or acyanotic) had relatively elevated plasma catecholamine levels when compared with a control group of patients with patent ductus arteriosus, although the difference was not statistically significant. There were no differences in the catecholamine levels between the acyanotic and cyanotic patients with CHD. This may lead to a high rate of false-positive results with biochemical testing in the CCHD population. However, a three- to fourfold increase in either plasma metanephrine or normetanephrine levels above the upper limit of normal should raise suspicion of a tumor and warrant closer clinical and biochemical monitoring [49].
8. Diagnostic Imaging and Management of PPGL
In addition to anatomical imaging with CT/MRI, there is an increased use of functional imaging in confirming the diagnosis as well as in staging and defining the extent of PPGL. The 2014 Clinical Endocrine Society Practice Guidelines recommend 123I-metaiodobenzylguanidine single-photon emission computed tomography (SPECT) as one of the modes of imaging, which is widely available [4]. However, in general, SPECT imaging has lower resolution compared with PET imaging in detecting very small lesions. In the last decade, there has been a rapid increase in the use of PET imaging using various types of tracers specific to PPGL. 18F-Fluorodeoxyglucose PET and 18F-fluorohydroxyphenylalanine PET are among the newer imaging modalities recommended for the diagnosis of PPGL and are superior to 123I-metaiodobenzylguanidine SPECT imaging. However, these techniques are not widely available and have variable detection rates depending on tumor location and associated genetic mutations [50, 51].
68Ga-DOTA somatostatin receptor–targeting peptide PET targets the somatostatin receptor, which is abundantly expressed in PPGL tumors. It has shown improved accuracy in detecting such tumors irrespective of the genetic mutation, etiology (familial or sporadic), tumor size, extent, or metastasis. A recent meta-analysis demonstrated that 68Ga-DOTA PET has superior detection rates when compared with other modes of imaging [52]. The 2012 European Academy of Nuclear Medicine guidelines recommend the use of 18F-fluorohydroxyphenylalanine PET, 18F-fluorodeoxyglucose PET, and 68Ga-DOTA PET as the preferable modes of imaging for the accurate detection of these tumors [51].
Surgical resection is the mainstay for management of these tumors, similar to other catecholamine-producing tumors. A minimally invasive surgery (laparoscopic) is preferred; however, an open surgery may be required for large tumors (>6 cm in size) to ensure complete resection and to prevent tumor rupture [4]. Preoperatively, just like other PPGL, patients should be managed medically with α-adrenergic blockade for at least 7 to 10 days before proceeding with the surgery. It is imperative to normalize blood pressure and heart rate before surgery to avoid a hypertensive crisis during the procedure. Also, given the history of CCHD, a pediatric cardiologist must also be consulted and involved in the patient’s care.
9. Conclusion
Despite occasional reports of the association between hypoxia and PPGL, a systematic approach for screening is currently not integrated into the management of patients with CCHD with chronic hypoxemia. A screening strategy based on the available literature should provide for early detection and intervention of PPGL, resulting in improved outcomes for these patients.
Genetic alterations are shown to be involved in the intracellular signaling pathways leading to the aberrant activation of HREs and tumorigenesis. However, it is unclear why all patients with chronic hypoxia do not develop this complication. Although no germline mutations were detected in our patient or in other cases reported in the literature, directed studies to investigate specific “hypoxia patterns” and other potential environmental and/or tumor specific molecular mechanisms of tumorigenesis may reveal predisposing factors.
In summary, with this review, we aim to heighten awareness of this association among pediatric cardiologists, nephrologists, endocrinologists, and other providers who take care of this patient population.
Glossary
Abbreviations:
- CCHD
cyanotic congenital heart disease
- CHD
congenital heart disease
- DOTA
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- HIF
hypoxia-inducible factor
- HRE
hypoxia-response element
- PET
positron emission tomography
- PPGL
pheochromocytomas and paragangliomas
- SPECT
single-photon emission computed tomography
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References and Notes
- 1. Fishbein L, Leshchiner I, Walter V, Danilova L, Robertson AG, Johnson AR, Lichtenberg TM, Murray BA, Ghayee HK, Else T, Ling S, Jefferys SR, de Cubas AA, Wenz B, Korpershoek E, Amelio AL, Makowski L, Rathmell WK, Gimenez-Roqueplo AP, Giordano TJ, Asa SL, Tischler AS, Pacak K, Nathanson KL, Wilkerson MD; Cancer Genome Atlas Research Network. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017;31(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44(5):328–333. [DOI] [PubMed] [Google Scholar]
- 3. Wyszyńska T, Cichocka E, Wieteska-Klimczak A, Jobs K, Januszewicz P. A single pediatric center experience with 1025 children with hypertension. Acta Paediatr. 1992;81(3):244–246. [DOI] [PubMed] [Google Scholar]
- 4. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr; Endocrine Society. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942. [DOI] [PubMed] [Google Scholar]
- 5. Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27(3):193–202. [DOI] [PubMed] [Google Scholar]
- 6. Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12(5):609–615. [DOI] [PubMed] [Google Scholar]
- 7. Sinclair AM, Isles CG, Brown I, Cameron H, Murray GD, Robertson JW. Secondary hypertension in a blood pressure clinic. Arch Intern Med. 1987;147(7):1289–1293. [PubMed] [Google Scholar]
- 8. Berends AMA, Buitenwerf E, de Krijger RR, Veeger NJGM, van der Horst-Schrivers ANA, Links TP, Kerstens MN. Incidence of pheochromocytoma and sympathetic paraganglioma in the Netherlands: a nationwide study and systematic review. Eur J Intern Med. 2018;51:68–73. [DOI] [PubMed] [Google Scholar]
- 9. Folger GM Jr, Roberts WC, Mehrizi A, Shah KD, Glancy DL, Carpenter CC, Esterly JR. Cyanotic malformations of the heart with pheochromocytoma: a report of five cases. Circulation. 1964;29(5):750–757. [DOI] [PubMed] [Google Scholar]
- 10. Song MK, Kim GB, Bae EJ, Lee YA, Kim HY, Min SK, Kim JH, Won JK. Pheochromocytoma and paraganglioma in Fontan patients: common more than expected. Congenit Heart Dis. 2018;13(4):608–616. [DOI] [PubMed] [Google Scholar]
- 11. Opotowsky AR, Moko LE, Ginns J, Rosenbaum M, Greutmann M, Aboulhosn J, Hageman A, Kim Y, Deng LX, Grewal J, Zaidi AN, Almansoori G, Oechslin E, Earing M, Landzberg MJ, Singh MN, Wu F, Vaidya A. Pheochromocytoma and paraganglioma in cyanotic congenital heart disease. J Clin Endocrinol Metab. 2015;100(4):1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burnichon N, Buffet A, Parfait B, Letouzé E, Laurendeau I, Loriot C, Pasmant E, Abermil N, Valeyrie-Allanore L, Bertherat J, Amar L, Vidaud D, Favier J, Gimenez-Roqueplo A-P. Somatic NF1 inactivation is a frequent event in sporadic pheochromocytoma. Hum Mol Genet. 2012;21(26):5397–5405. [DOI] [PubMed] [Google Scholar]
- 13. Welander J, Larsson C, Bäckdahl M, Hareni N, Sivlér T, Brauckhoff M, Söderkvist P, Gimm O. Integrative genomics reveals frequent somatic NF1 mutations in sporadic pheochromocytomas. Hum Mol Genet. 2012;21(26):5406–5416. [DOI] [PubMed] [Google Scholar]
- 14. Toledo RA, Qin Y, Srikantan S, Morales NP, Li Q, Deng Y, Kim SW, Pereira MA, Toledo SP, Su X, Aguiar RC, Dahia PL. In vivo and in vitro oncogenic effects of HIF2A mutations in pheochromocytomas and paragangliomas. Endocr Relat Cancer. 2013;20(3):349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14(2):108–119. [DOI] [PubMed] [Google Scholar]
- 16. Stenman A, Welander J, Gustavsson I, Brunaud L, Bäckdahl M, Söderkvist P, Gimm O, Juhlin CC, Larsson C. HRAS mutation prevalence and associated expression patterns in pheochromocytoma. Genes Chromosomes Cancer. 2016;55(5):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS, Hodin R, Heitritter S, Moore F, Dluhy R, Sosa JA, Ocal IT, Benn DE, Marsh DJ, Robinson BG, Schneider K, Garber J, Arum SM, Korbonits M, Grossman A, Pigny P, Toledo SP, Nosé V, Li C, Stiles CDA. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1(1):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pacak K, Jochmanova I, Prodanov T, Yang C, Merino MJ, Fojo T, Prchal JT, Tischler AS, Lechan RM, Zhuang Z. New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J Clin Oncol. 2013;31(13):1690–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33(4):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaidya A, Flores SK, Cheng Z-M, Nicolas M, Deng Y, Opotowsky AR, Lourenço DM Jr, Barletta JA, Rana HQ, Pereira MA, Toledo RA, Dahia PLM. EPAS1 mutations and paragangliomas in cyanotic congenital heart disease. N Engl J Med. 2018;378(13):1259–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lorenzo FR, Yang C, Ng Tang Fui M, Vankayalapati H, Zhuang Z, Huynh T, Grossmann M, Pacak K, Prchal JT. A novel EPAS1/HIF2A germline mutation in a congenital polycythemia with paraganglioma. J Mol Med (Berl). 2013;91(4):507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT, Pacak K. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367(10):922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. [DOI] [PubMed] [Google Scholar]
- 25. Jochmanová I, Yang C, Zhuang Z, Pacak K. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst. 2013;105(17):1270–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472. [DOI] [PubMed] [Google Scholar]
- 27. Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–468. [DOI] [PubMed] [Google Scholar]
- 28. Hewitson KS, McNeill LA, Elkins JM, Schofield CJ. The role of iron and 2-oxoglutarate oxygenases in signalling. Biochem Soc Trans. 2003;31(3):510–515. [DOI] [PubMed] [Google Scholar]
- 29. Favier J, Gimenez-Roqueplo AP. Pheochromocytomas: the (pseudo)-hypoxia hypothesis. Best Pract Res Clin Endocrinol Metab. 2010;24(6):957–968. [DOI] [PubMed] [Google Scholar]
- 30. Pang Y, Gupta G, Yang C, Wang H, Huynh TT, Abdullaev Z, Pack SD, Percy MJ, Lappin TRJ, Zhuang Z, Pacak K. A novel splicing site IRP1 somatic mutation in a patient with pheochromocytoma and JAK2V617F positive polycythemia vera: a case report. BMC Cancer. 2018;18(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang C, Zhuang Z, Fliedner SM, Shankavaram U, Sun MG, Bullova P, Zhu R, Elkahloun AG, Kourlas PJ, Merino M, Kebebew E, Pacak K. Germ-line PHD1 and PHD2 mutations detected in patients with pheochromocytoma/paraganglioma-polycythemia. J Mol Med (Berl). 2015;93(1):93–104. [DOI] [PubMed] [Google Scholar]
- 32. Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, Feunteun J, Pouysségur J, Richard S, Gardie B. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359(25):2685–2692. [DOI] [PubMed] [Google Scholar]
- 33. Ladroue C, Hoogewijs D, Gad S, Carcenac R, Storti F, Barrois M, Gimenez-Roqueplo AP, Leporrier M, Casadevall N, Hermine O, Kiladjian JJ, Baruchel A, Fakhoury F, Bressac-de Paillerets B, Feunteun J, Mazure N, Pouysségur J, Wenger RH, Richard S, Gardie B. Distinct deregulation of the hypoxia inducible factor by PHD2 mutants identified in germline DNA of patients with polycythemia. Haematologica. 2012;97(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reynolds JL, Gilchrist TF. Congenital heart disease and pheochromocytoma. Am J Dis Child. 1966;112(3):251–255. [DOI] [PubMed] [Google Scholar]
- 35. Cherqaoui I, Raux O, Dehour L, Rochette A, Dadure C, Capdevila X. Transpulmonary thermodilution hemodynamic monitoring for pheochromocytoma surgery in a child with complex congenital heart disease. Paediatr Anaesth. 2006;16(12):1277–1280. [DOI] [PubMed] [Google Scholar]
- 36. Cheung YW, Spevack DM. Single left ventricle and pheochromocytoma. Congenit Heart Dis. 2008;3(5):355–358. [DOI] [PubMed] [Google Scholar]
- 37. Chung SJ, Lee YA, Shin CH, Yang SW, Bae EJ, Noh JI. Pheochromocytoma associated with cyanotic congenital heart disease. Korean J Pediatr. 2008;51(1):93–97. [Google Scholar]
- 38. Hwang BH, Kim HY, Jung SE, Park KW. Extra-adrenal pheochromocytoma after operation of congenital heart disease: a case report of 18-year-old boy. J Korean Surg Soc. 2012;83(1):65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kasaliwal R, Sarathi V, Pandit R, Budyal SR, Bukan A, Kakade H, Jagtap VS, Lila AR, Bandgar T, Menon PS, Shah NS. Pheochromocytoma and tetralogy of Fallot: a rare but potentially dangerous combination. Endocr Pract. 2014;20(5):e80–e85. [DOI] [PubMed] [Google Scholar]
- 40. Yamamoto K, Namba N, Kubota T, Usui T, Takahashi K, Kitaoka T, Fujiwara M, Hori Y, Kogaki S, Oue T, Morii E, Ozono K. Pheochromocytoma complicated by cyanotic congenital heart disease: a case report. Clin Pediatr Endocrinol. 2016;25(2):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deshpande SR, Patel P, Videlefsky N, Soler Rodriguez DM, Romero R, Clifton MS. Retroperitoneal paraganglioma in a patient with Fontan: the hypoxia connection. Ann Pediatr Cardiol. 2018;11(2):197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de la Monte SM, Hutchins GM, Moore GW. Peripheral neuroblastic tumors and congenital heart disease. Possible role of hypoxic states in tumor induction. Am J Pediatr Hematol Oncol. 1985;7(2):109–116. [PubMed] [Google Scholar]
- 43. Streeten DH, Anderson GH Jr. Mechanisms of orthostatic hypotension and tachycardia in patients with pheochromocytoma. Am J Hypertens. 1996;9(8):760–769. [DOI] [PubMed] [Google Scholar]
- 44. Tsujimoto G, Manger WM, Hoffman BB. Desensitization of beta-adrenergic receptors by pheochromocytoma. Endocrinology. 1984;114(4):1272–1278. [DOI] [PubMed] [Google Scholar]
- 45. Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong MY, Andrews KA, Challis BG, Park SM, Acerini CL, Maher ER, Casey RT. Clinical Practice Guidance: surveillance for phaeochromocytoma and paraganglioma in paediatric succinate dehydrogenase gene mutation carriers. Clin Endocrinol (Oxf). 2019;90(4):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lenders JW, Willemsen JJ, Eisenhofer G, Ross HA, Pacak K, Timmers HJ, Sweep CG. Is supine rest necessary before blood sampling for plasma metanephrines? Clin Chem. 2007;53(2):352–354. [DOI] [PubMed] [Google Scholar]
- 48. Dzimiri N, Galal O, Moorji A, Bakr S, Abbag F, Fadley F, Almotrefi AA. Regulation of sympathetic activity in children with various congenital heart diseases. Pediatr Res. 1995;38(1):55–60. [DOI] [PubMed] [Google Scholar]
- 49. Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, Pacak K. Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. J Clin Endocrinol Metab. 2003;88(6):2656–2666. [DOI] [PubMed] [Google Scholar]
- 50. Kong G, Schenberg T, Yates CJ, Trainer A, Sachithanandan N, Iravani A, Ravi Kumar A, Hofman MS, Akhurst T, Michael M, Hicks RJ. The role of 68Ga-DOTA-Octreotate (GaTate) PET/CT in follow-up of SDH-associated pheochromocytoma and paraganglioma (PPGL). J Clin Endocrinol Metab. 2019;jc.2019-00018. [DOI] [PubMed] [Google Scholar]
- 51. Taïeb D, Timmers HJ, Hindié E, Guillet BA, Neumann HP, Walz MK, Opocher G, de Herder WW, Boedeker CC, de Krijger RR, Chiti A, Al-Nahhas A, Pacak K, Rubello D; European Association of Nuclear Medicine. EANM 2012 guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39(12):1977–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Han S, Suh CH, Woo S, Kim YJ, Lee JJ. Performance of 68Ga-DOTA-conjugated somatostatin receptor-targeting peptide PET in detection of pheochromocytoma and paraganglioma: a systematic review and metaanalysis. J Nucl Med. 2019;60(3):369–376. [DOI] [PubMed] [Google Scholar]