Abstract
Background and aim
This cross-sectional study investigated the prevalence and risk factors of high-risk human papilloma virus (HPV) infection, especially types 16 and 18, and cervical neoplasia in female Inflammatory bowel disease (IBD) patients.
Methods
From July 2014 to January 2017, sexually active, female, Chinese IBD patients (21–60 years) and age-matched controls underwent cervical ThinPrep cytology testing (TCT) and high-risk HPV-DNA detection, and completed questionnaires about awareness of cervical cancer and HPV. Cervical dysplasia was categorized as cervical intraepithelial neoplasia (CIN) 1, 2 and 3.
Results
Of 124 IBD patients (30 ulcerative colitis and 94 Crohn’s disease), 17 (13.7%) had high-risk HPV among whom 9 (7.3%) had HPV 16/18 infection and 4 (3.2%) had cervical CIN (3 CIN 3, 1 CIN 1) by pathology. Among 372 controls, 33 (8.9%) had high-risk HPV and only 1 (0.3%) had HPV 16 infection. Cervical TCT detected atypical squamous cells of unknown significance in one control; no control had CIN. The HPV 16/18 infection rate and CIN prevalence were significantly higher in IBD patients than controls (both P < 0.001). The HPV-infection rate was higher in patients administered methotrexate [P = 0.005, odds ratio (95% confidence interval) 4.76 (1.471–15.402)] or more than two immunosuppressants [P = 0.013, odds ratio (95% confidence interval) 3.64 (1.255–10.562)]. Thiopurine, steroid, infliximab and disease behavior/location were not associated with HPV infection. Only 29.3% of patients had undergone cervical-cancer screening. Awareness of HPV infection and HPV-related cervical cancer was poor (28.2%).
Conclusions
Female IBD patients are at increased risk of high-risk HPV infection and cervical neoplasia, which may be associated with immunosuppressants. Education and routine follow-up with HPV-DNA testing and TCT are recommended, especially in female Chinese IBD patients.
Keywords: Inflammatory bowel disease, high-risk human papilloma virus, cervical intraepithelial neoplasia
Introduction
Cervical cancer is the second most common malignant tumor worldwide [1]. The incidence of cervical cancer is much higher in developing countries because of imperfect screening and vaccination [2, 3]. High-risk types of human papillomavirus (HPV), especially types 16 and 18, have been identified as important pathogenic factors for the development of cervical neoplasms, which cause about 70% of cervical cancers worldwide [4, 5]. Additionally, the prevalence of cervical intraepithelial neoplasia (CIN) is also much higher in patients with autoimmune disease such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) [6, 7]. Moreover, the use of immunosuppressants has been suggested to increase the risk of HPV infection in patients with renal transplantation and immune disease [8, 9].
Inflammatory bowel disease (IBD), encompassing ulcerative colitis (UC) and Crohn’s disease (CD), is characterized by chronic relapsing intestinal inflammation that is considered to be caused by interactions of genetic, environmental and immune system disorder [10]. Immunosuppressants are an important and frequently used regimen for inducing and/or maintaining disease, inhibiting the production of antibodies for biologics [11].
The relationship between IBD and cervical cancer was first reported in 1994 when researchers found out that 2 among 366 IBD patients treated with azathioprine developed invasive cervical neoplasms [12]. A population-based study also showed that the risk of cervical cancer was much higher in IBD patients than in healthy individuals and use of 5-aminosalicylic acid [odds ratio (OR) 1.65], steroid (OR 2.79) or immunosuppressant (OR 3.45) were risk factors for cervical cancer [13]. A case–control study demonstrated that use of oral contraceptives (OCP), steroids or immunosuppressants could increase the risk of cervical cancer in CD patients; however, this correlation was not found in UC patients [14]. In a study of 116 IBD female patients and controls, Bhatia et al. [15] found that the proportion of IBD patients with abnormal cervical cells (18%) was significantly higher than that of healthy controls (5%), while immunosuppressive treatments had no impact on the occurrence of cervical cancer. Some other studies also indicated that the incidence of cervical cancer was not different between IBD patients and healthy individuals, and that immunosuppressant use did not increase the risk of cervical cancer [16].
The findings of previous studies were inconsistent, and none of them evaluated the HPV status in IBD patients. Moreover, even fewer studies had evaluated the risk of cervical cancer in Asian IBD patients. Thus, in this study, we aimed to assess the risk of cervical dysplasia and infection of high-risk HPV types, especially types 16 and 18, in a national cohort of Chinese women with IBD matched with a large sample of women from the general population. We also investigated the risk factors for cervical neoplasia, especially medications, and the knowledge and attitudes about HPV infection in women with IBD.
Materials and methods
Subjects and study design
This prospective observational study was performed at the Six Affiliated Hospital of Sun Yat-sen University and the IBD clinic of the Sir Run Run Shaw hospital from July 2014 to January 2017. The study design was approved by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University. We enrolled 21- to 60-year-old female IBD patients with sexual life into the study. All patients consented to participate and signed written informed consent forms prior to enrollment (ClinicalTrials.gov Identifier: NCT03055130). Age-matched controls without gynecological cancer and autoimmune diseases were enrolled from the Medical Examination Center of our hospital at a 1:3 ratio. We excluded controls who had ever been diagnosed with genital tumors and immunosuppressive diseases such as human immunodeficiency virus (HIV), RA or SLE. Demographic data of participants at the point of cervical examination and HPV testing were collected from IBD database, including disease location, disease behavior, disease duration, surgical history, history of drugs and smoking history.
Medical treatment of IBD
Detailed information regarding medical treatment of IBD was collected, including the use of steroids, mesalazine, azathioprine, mercaptopurine, cyclophosphamide, methotrexate (MTX), infliximab and adalimumab. Exposure to azathioprine, mercaptopurine or methotrexate was defined as treatment for more than 3 months. Exposure to anti-tumor necrosis factor (TNF) therapy was defined as treatment for any duration. Exposure to steroids was defined as treatment for >2 weeks. Data on the total durations of drug treatment were also collected.
Patient questionnaire
Patients were asked to complete questionnaires that included items regarding age, education background, economic and marital status, age at first sexual encounter, number of partners, number of pregnancies/abortions, contraception measures and previous history of cervical disease, including history of sexually transmitted diseases. To help keep the information private, we prepared a blank cover for every questionnaire. Then patients were asked to write down answers in a quiet room, alone and with enough time. They were then asked to hand it to the researcher directly. Only if patients did not have enough time to write down the answers due to being in a hurry would we call them by telephone to get the answer. We also came across a few patients who did not understand questions because of a low education level and, for these patients, we would read and explain every question to them and help them with writing down their oral answers. Awareness of cervical cancer and HPV was gauged using following five questions: Have you ever heard about cervical cancer? (yes or no). Are you aware of the method of cervical-cancer screening? (yes, know exactly or know only a little, or no). How many times have you undergone cervical-cancer screening? (0, 1, 2, >2 times). Have you ever heard about HPV? (yes or no). Are you aware of the relationship between HPV infection and cervical cancer (yes, know exactly or know only a little, or no). The ‘know exactly’ option was chosen when the patient clearly described the screening method and understood that high-risk HPV infection could cause cervical cancer and that the risk could be prevented by HPV vaccine. The ‘know only a little’ option was selected when the patient had only heard about cervical-cancer screening by gynecologic examination and knew that HPV infection may cause genital disease.
Cervical-cancer screening and HPV-DNA detection
The cervical examination and sample collection were performed by an experienced gynecologist. Samples were used for the cervical ThinPrep cytology test (TCT) and high-risk HPV-DNA detection including: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 and 82 [17, 18]. Patients who had been detected with atypical squamous cells of unknown significance (ASC-US) on TCT or those who were HPV 16/18(+) were further evaluated with a colposcope. Cervical dysplasia was categorized as CIN 1, 2 and 3—CIN 1: low-grade squamous intraepithelial lesion, including mild CIN; CIN 2: moderate dysplasia confined to the basal two-thirds of the epithelium; CIN 3: severe dysplasia that spans more than two-thirds of the epithelium and may involve the full thickness [19].
Statistical analysis
All analyses were performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as mean ± standard deviation, and tested by using Student’s t-test. Categorical data were described as numbers and percentages, and tested by using the Pearson χ2 test or Fisher exact test. Univariate analysis was performed after including the following variables: smoking status, OCP use, age at diagnosis, disease duration and immunosuppressant exposure. Multivariate analysis was performed using significant variables detected in the univariate analysis. A P < 0.05 was considered statistically significant.
Results
Demographic characteristics of study participants
A total of 496 participants (mean age 35.56 ± 7.07 years) were included in this study, including 124 IBD patients (30 UC and 94 CD) and 372 age-matched controls. Among them, 99 patients were willing to answer the questionnaire but 1 of them refused to answer questions referring to sexual information due to privacy reasons and, therefore, 98 patients had completed data on education, income, production and sexual-related information. Detailed demographic characteristics of patients are summarized in Table 1.
Table 1.
Difference between high-risk human papilloma virus (HPV)-positive and -negative patients in demographic characteristics
| Patients without high-risk HPV infection | Patients with high-risk HPV infection | P-value | |
|---|---|---|---|
| Crohn’s disease | N = 81 | N = 13 | |
| Age, years | 34.56 ± 4.61 | 36.15 ± 7.25 | 0.427 |
| Disease duration, years | 4.26 ± 3.63 | 8.69 ± 9.17 | 0.14 |
| Family history, n (%) | 0 (0.0) | 0 (0.0) | – |
| Behavior, n (%) | 0.839 | ||
| B1 (non-stricturing, non-penetrating) | 45 (55.6) | 6 (46.2) | |
| B2 (structuring) | 13 (16.0) | 2 (15.4) | |
| B3 (penetrating) | 23 (28.4) | 5 (38.4) | |
| Location, n (%) | 0.505 | ||
| L1 (terminal ileum) | 12 (14.8) | 2 (15.4) | |
| L2 (colon) | 7 (8.6) | 0 (0.0) | |
| L3 (ileocolon) | 48 (59.3) | 10 (76.9) | |
| L4 (upper gastrointestinal tract) | 14 (17.3) | 1 (7.7) | |
| Smoking, n (%) | 8 (9.9) | 1 (7.7) | 1.000 |
| Extra-intestinal manifestation, n (%) | 15 (18.5) | 1 (7.7) | 0.571 |
| Perianal disease, n (%) | 36 (44.4) | 5 (38.5) | 0.686 |
| Ulcerative colitis | N = 26 | N = 4 | |
| Age, years | 39.24 ± 8.72 | 35.75 ± 6.02 | 0.450 |
| Disease duration, years | 4.01 ± 3.57 | 6.17 ± 3.09 | 0.266 |
| Family history, n (%) | 0 (0.0) | 0 (0.0) | – |
| Location, n (%) | 0.662 | ||
| E1 (ulcerative proctitis) | 4 (15.4) | 0 (0.0) | |
| E2 (left-sided) | 4 (15.4) | 1 (25.0) | |
| E3 (extensive) | 18 (69.2) | 3 (75.0) | |
| Smoking, n (%) | 0 (0.0) | 0 (0.0) | – |
| Extra-intestinal manifestation, n (%) | 1 (3.8) | 0 (0.0) | 1.000 |
Prevalence rates of high-risk HPV infection and cervical dysplasia
Out of 124 IBD patients, 17 (13.7%; 13 CD and 4 UC) had been detected as high-risk-type HPV-DNA(+) and 9 of them (7.3%) were infected with HPV 16/18. Among these nine patients with HPV 16/18 infection, three had CIN 3 and one had ASC-US. One patient was infected with HPV 58 and her cervical biopsy results indicated CIN 1. Among the 372 controls, 33 (8.9%) were high-risk HPV-DNA(+) and only 1 (0.3%) was infected with HPV 16. TCT of cervical tissue revealed ASC-US in one control, whose cervical biopsy did not reveal the presence of CIN. In total, the HPV 16/18 infection rate and CIN prevalence were significantly higher in IBD patients than those in controls {HPV 16/18 infection rate: 7.3 vs 0.3%, P < 0.001, OR [95% confidence interval (CI)]: 29.035 [3.64–210.988]; CIN prevalence: 3.2 vs 0.0%, P = 0.004} (Table 2).
Table 2.
Prevalence of high-risk human papilloma virus (HPV) infection and cervical dysplasia
| IBD patients (N = 124) | Controls (N = 372) | P-value | Odds ratio (95% CI) | |
|---|---|---|---|---|
| High-risk type HPV-DNA(+), n (%) | 17 (13.7) | 33 (8.9) | 0.121 | 1.632 (0.874–3.047) |
| HPV 16/18(+), n (%) | 9 (7.3) | 1 (0.3) | <0.001 | 29.035 (3.64–210.988) |
| ASC-US, n (%) | 1 (0.8) | 1 (0.3) | 0.413 | 3.060 (0.187–48.585) |
| Cervical intraepithelial neoplasia, n (%) | 4 (3.2) | 0 (0.0) | 0.004 | – |
IBD, inflammatory bowel disease; ASC-US, atypical squamous cells of unknown significance; CI, confidence interval.
Risk factors of high-risk HPV infection in IBD patients
For further analysis, we stratified patients into HPV-positive group and HPV-negative group. No significant difference was found between the two groups in aspects such as family income, education level, history of genital diseases, reproductive health and sexual practices (all P > 0.05; Table 3). Moreover, the disease location, behavior, duration and smoking were not correlated with high-risk HPV infection (all P > 0.05; Table 1).
Table 3.
Risk factors of high-risk human papilloma virus (HPV) infection in patients with inflammatory bowel disease (IBD)
| HPV- negative (N = 86) | HPV- positive (N = 12) | P-value | |
|---|---|---|---|
| Education, n (%) | |||
| Uneducated | 2 (2.3) | 0 (0.0) | 0.59 |
| No high-school degree | 9 (10.5) | 2 (16.7) | |
| Junior high school | 16 (18.6) | 6 (50.0) | |
| Senior high-school graduate | 31 (36.0) | 3 (25.0) | |
| College degree | 25 (29.1) | 1 (8.3) | |
| Postgraduate degree | 3 (3.5) | 0 (0.0) | |
| Annual income per person, n (%) | |||
| ≤60 000 RMB | 56 (65.1) | 6 (50.0) | 0.39 |
| 60 000–150 000 RMB | 28 (32.6) | 5 (41.7) | |
| >150 000 RMB | 2 (2.3) | 1 (8.3) | |
| Age of first coitus, n (%) | |||
| ≤20 years | 12 (14.0) | 3 (25.0) | 0.46 |
| 21–25 years | 49 (57.0) | 6 (50.0) | |
| 26–30 years | 23 (26.7) | 2 (16.7) | |
| >30 years | 2 (2.3) | 1 (8.3) | |
| Age of first delivery, n (%) | |||
| No delivery | 0 (0.0) | 5 (41.7) | |
| ≤20 years | 12 (14.0) | 1 (8.3) | 0.13 |
| 21–25 years | 49 (57.0) | 2 (16.7) | |
| 26–30 years | 23 (26.7) | 4 (33.3) | |
| >30 years | 2 (2.3) | 0 (0.0) | |
| Number of reproductions, n (%) | |||
| 0 | 44 (51.2) | 5 (41.7) | 0.20 |
| 1 | 32 (37.2) | 6 (50.0) | |
| 2 | 7 (8.1) | 1 (8.3) | |
| 3 | 2 (2.3) | 0 (0.0) | |
| ≥4 | 1 (1.2) | 0 (0.0) | |
| Number of abortions, n (%) | 0.56 | ||
| 0 | 44 (51.2) | 8 (66.7) | |
| 1 | 32 (37.2) | 2 (16.7) | |
| 2 | 7 (8.1) | 2 (16.6) | |
| 3 | 2 (2.3) | 0 (0.0) | |
| ≥4 | 1 (1.2) | 0 (0.0) | |
| Contraception measures, n (%) | 0.89 | ||
| No | 20 (23.3) | 2 (16.7) | |
| Short-acting oral contraceptive | 2 (2.3%) | 0 (0.0) | |
| Long-acting oral contraceptive | 1 (1.2%) | 0 (0.0) | |
| Emergency oral contraceptive | 0 (0.0) | 0 (0.0) | |
| Condom | 35 (40.7%) | 7 (58.3%) | |
| Intrauterine device | 19 (22.1%) | 2 (16.7%) | |
| Tubal ligation | 5 (5.8%) | 0 (0.0) | |
| Safe period | 4 (4.7%) | 1 (8.3%) | |
| Lifetime sexual partners, n (%) | 0.643 | ||
| 0 | 1 (1.2%) | 7 (58.3%) | |
| 1 | 61 (70.9%) | 3 (25.0%) | |
| 2 | 18 (20.9%) | 2 (16.7%) | |
| 3 | 6 (7.0%) | 0 (0.0) | |
| ≥4 | 0 (0.0) | 0 (0.0) | |
| STD history, n (%)a | 0.207 | ||
| Yes | 38 (44.2) | 3 (25.0) | |
| No | 48 (55.8) | 9 (75.0) | |
The table includes data from 98 IBD patients who had complete data on education, income, production and sexual-related information.
aSTD history includes cervical erosion, uterine cervicitis, trichomoniasis, fungal infection, urinary-tract infection, gonorrhea and syphilis. Some patients had several kinds of disease.
Further analysis on medical treatment of the two groups revealed that exposure to MTX [35.3% (6/17) vs 10.3% (11/107), P = 0.005, OR (95% CI): 4.76 (1.471–15.402)] and using more than two types of immunosuppressant [47.1% (8/17) vs 19.6% (21/107), P = 0.013, OR (95% CI): 3.64 (1.255–10.562)] significantly increased the risk of high-risk HPV infection, while the use of thiopurine, steroid and infliximab did not increase the rate of HPV infection (all P > 0.05) (Table 4). Moreover, we did not find any correlation between duration of drug treatment and high-risk HPV infection.
Table 4.
Influence of drugs on the prevalence of high-risk HPV infection in IBD patients
| High-risk HPV exposed group | High-risk HPV Non-exposed group | P-value | |
|---|---|---|---|
| OR (95%CI) | |||
| MTX | 6(17) | 11(107) | 0.005 |
| 4.76 (1.471-15.402) | |||
| more than 2 immunosuppressants | 8(17) | 21(107) | 0.013 |
| 3.64 (1.255-10.562) | |||
| thiopurine | 12(17) | 54(107) | 0.106 |
Awareness of HPV infection and cervical cancer in IBD patients
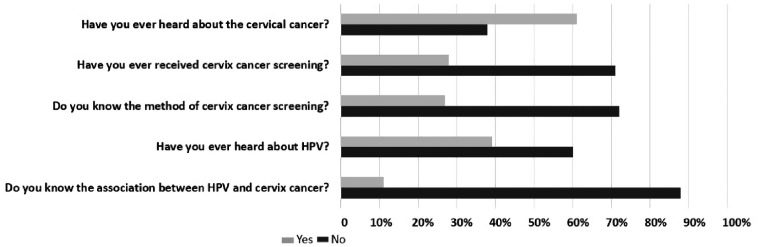
Among 99 patients who were willing to answer the questionnaire, 61 patients (61.6%) had heard about cervical cancer. Only 27 patients (27.3%) knew a little about the screening methods and the remaining (72.7%) did not know anything at all. Moreover, 71 (71.7%) patients have never undergone cervical-cancer screening. Thirty-nine patients (39.3%) had heard about HPV and only a few (11.1%) were aware of the relationship between HPV infection and cervical cancer (Figure 1). Because no HPV vaccine is available in mainland China, none of the patients had received HPV vaccination. Most of the participants had a college degree and no differences in HPV awareness were found among patients with different education levels.
Figure 1.
Awareness of human papilloma virus (HPV) infection and cervical cancer in patients with inflammatory bowel disease (IBD). Among 99 IBD patients who completed the questionnaires, 61.6% had heard about cervical cancer, although only 27.3% knew a little about the screening methods and 71.7% had never undergone cervical-cancer screening. Only 39.3% of patients had heard about HPV and only 11.1% knew that it was an important risk factor for cervical cancer.
Discussion
Because of a lack of regular cervical-cancer screening and HPV vaccination, the incidence of cervical cancer is much higher in most developing countries, including China, compared to that in developed countries. Cervical cancer is the most common genital malignant tumor in Chinese females, with approximately 30 400 female patients dying of the disease in China per year [2, 3]. Results of a few previous studies suggested that immune disorders or drugs used to treat these disorders might increase the incidence of cervical cancer; however, the relationship between IBD and cervical cancer remained unclear. No study has focused on the problem of high-risk HPV infection in female IBD patients. Therefore, in this study, we mainly aimed to investigate the association between IBD and high-risk HPV infection/cervical cancer in order to identify risk factors for HPV infection/cervical-cancer development in IBD patients.
In the present study, the infection rate of HPV 16/18 was significantly higher in IBD patients than that in controls (7.3 vs 0.3%). HPV 16/18 has been reported to be the main risk factor for cervical dysplasia [4, 5]. Consistently with this, we diagnosed CIN in four patients (3 CIN 3 and 1 CIN 1) and ASC-US in one patient with HPV 16/18 infection. The prevalence of cervical dysplasia was also higher in IBD patients than in controls. In a 2001–12 study in American participants, Kim et al. [20] retrospectively analysed the differences in the incidence of CIN among 133 333 females with immune disease and 533 332 controls. They found that the incidence rate of CIN was higher in patients with diagnoses of RA, SLE and IBD, which is consistent with our results. Our results are also supported by the findings of another study by Kane et al. [21], in which Pap smears of 40 female IBD patients (8 UC, 32 CD) and healthy controls were evaluated. The prevalence of abnormal Pap-smear findings in female IBD patients was 42.5 vs 7% in healthy controls (P < 0.001). Moreover, women with IBD were more likely than controls to have higher-grade lesions (P < 0.001). The cytopathology of abnormal lesions also revealed either HPV serotype 16/18 in all specimens. However, in a large case–control study conducted in Scotland, analysis of full cervical-smear histories of 411 female IBD patients and 1644 matched controls revealed no significant difference in the rate of cervical dysplasia between the two groups [16]. Thus, currently, there is no definite conclusion regarding the relation between IBD and cervical dysplasia. Most studies were retrospective and did not investigate the HPV status of patients, which is an important risk factor. Thus, the results of the present case–control study from two large centers in China were of great interest to clinicians, especially gastroenterologists, and suggested that IBD patients may have an increased risk of developing cervical cancer because of the higher infection rate with high-risk HPV types, especially HPV 16/18.
Some previous studies have identified OCP, smoking, number of pregnancies and number of sexual partners as potential risk factors for HPV infection [22–24]. In contrast, in the present study, we found no correlation between high-risk HPV-infection rate in IBD patients and disease behavior, location, smoking, economic status, education level, reproductive status, sexual history or genital disease. This inconsistency might be due to the limited number of high-risk HPV cases included in this study. However, we did find a significant correlation between immunosuppressant use (MTX or more than two types of immunosuppressants) and HPV infection. In contrast, in the aforementioned Scottish study, immunosuppressant therapy was not correlated with the incidence rates of cervical dysplasia or neoplasia [16]. In Kane’s study [21], female IBD patients with a history of exposure to immunosuppressants were significantly more likely to have abnormal Pap-smear findings than controls and cytopathology of abnormal lesions revealed either HPV serotype 16/18 in all specimens. Consistently with this finding, Hutfless et al. [13] reported that 5-ASA (OR 1.65), steroid (OR 2.79) or immunosuppressant use (OR 3.45) might increase the risk of cervical cancer in IBD patients. All of these results suggested that the risk of cervical dysplasia in IBD patients might relate to the use of immunosuppressants; however, they did not investigate the HPV status of these patients. Our study showed exposure to immunosuppression might increase the risk of high-risk HPV infection. Intact and functioning cellular immunity including T cell and natural killer (NK) cell cytotoxicity is important for the host to defense against persistent HPV infection. Mutations in EVER1, EVER2, GATA2, CXCR4 and DOCK8 have been reported to associate with extensive HPV infections [25]. Use of antipsychotics, antidepressants and anxiolytics/sedatives was observed to decrease oral HPV clearance with stronger effects when used concomitantly [26]. Maybe using immunosuppressant especially ever using several kinds also could weaken the ability of HPV clearance. The role of methotrexate (MTX) and infection with HPV is poorly documented. Zumtobel et al. [27] reported that two long-term PUVA-treated patients with severe psoriasis developed widespread cutaneous carcinomas associated with HPV 5, 14 and 20 after the introduction of methotrexate. In our study, we observed using MTX or more than two kinds of immunosuppressant had an effect on HPV infection. Though the exact mechanisms were unclear, our results and previous studies raised the hypothesis that the underlying immunologic changes in IBD or the treatment of IBD with immunosuppressive drugs may lead to increased risk of cervical neoplasia via impairment of the ability to clear high-risk HPV infections or via increased risk of infection with high-risk HPV serotypes.
In the present study, we also evaluated the awareness about HPV and cervical cancer in IBD patients. Most patients (72.7%) were unaware of the screening method used for cervical cancer and 60.7% of patients had never been exposed to any information regarding HPV. Moreover, a majority of IBD patients (88.9%) were unaware of the relationship between HPV and cervical cancer. In contrast, a previous study in Hispanic females with IBD reported that 77% of women were aware of the existence of HPV. Among those who had heard about HPV, 79.6% knew that HPV can cause cervical cancer and 57.5% knew that the virus was sexually transmitted [28]. A letter to the editor of Inflammatory Bowel Diseases reported that a very low proportion of patients in America received information from their physicians about cervical-cancer screening, which thus resulted in a potentially increased risk of HPV infection [29]. The American Cancer Society has clearly documented that reducing the HPV-infection rate could decrease the incidence of cervical cancer [18]. Therefore, physicians and health-care workers should focus on educating IBD patients about the link between HPV, cervical cancer and IBD. As recommend by the European Cron’s and Colitis Organisation (ECCO), patients should be encouraged to undergo regular cervical-cancer screening and to receive HPV vaccination, especially those who use immunomodulators [30].
The presented study had some limitations. First, except for age and important information on previous diseases, we did not collect other personal data such as sexual-related information from patients in the control group, which prevented a multivariate analysis to validate further the significant difference in the HPV-infection rate between healthy controls and IBD female patients. Second, though we tried some methods to keep the information of patients from leaking, some patients still refused to answer the questionnaire because of personal privacy concerns, which may have influenced the identification of factors associated with high-risk HPV infection in female IBD patients. Mailing the questionnaires to patients may be a better design and could improve the response rate and the reality of the answers. Third, the estimated sample size of the study was very large; therefore, we enrolled patients in two large IBD centers to get as many patients as possible. However, the actual sample size was still not large enough, which is partly due to the low incidence of IBD in Chinese females. The relatively small sample size may have had an influence on the study results. A large multi-center study with a larger sample size is warranted to confirm the findings of this study.
In conclusion, our findings indicated that Chinese women with IBD have an increased risk of infection with high-risk HPV types, especially HPV type 16/18, and cervical neoplasia; this increased risk might be associated with the use of immunosuppressants. Knowledge regarding HPV and HPV vaccination was poor in our population of IBD patients. Therefore, IBD patients should be educated about cervical cancer and high-risk HPV infection, and HPV vaccination should be recommended universally to female IBD patients.
Acknowledgements
X.G. contributed to the design of the study and conducting the research comprehensively. M.L. and Q.F.Y. were responsible for following up patients, statistical analysis and drafting the article. J.T., K.C., L.J.H. and Y.Z. helped in collecting part of the patients’ data. X.H.W., X.Y.L. and L.L. helped in performing the cervical examination and sample collection. M.L.S., W.M.H., Y.Y. and S.Y.X. helped in collecting the controls’ data. Y.G. and M.Z. helped with data acquisition. Q.C. contributed to the analysis of the data. P.J.H. supervised the research and revised the article.
Conflicts of interest
None declared.
Funding
This work was supported by National Key Clinical Discipline, the National Nature Science Fund of China (No. 81370498), the Science and Technology Planning Project of Guangdong Province, China (No. 2012B091100455), the Science and Technology Project of Tianhe District, Guangdong Province, China (No.201404KW018) and the Medical Scientific and Technical Foundation of Guangdong Province (A2016322).
References
- 1. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clinc Oncol 2006;24:2137–50. [DOI] [PubMed] [Google Scholar]
- 2. Castellsague X, de Sanjose S, Aguado T. HPV and cervical cancer in the world 2007 report. Vaccine 2007;25:C1–230. [DOI] [PubMed] [Google Scholar]
- 3. Gu XY, Zheng RS, Sun KX. Incidence and mortality of cervical cancer in China, 2014. Zhonghua Zhong Liu Za Zhi 2018;40:241–6. [DOI] [PubMed] [Google Scholar]
- 4. Walboomer JM, Jacobs MV, Manos MM. Human papillomarvirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–9. [DOI] [PubMed] [Google Scholar]
- 5. Bosch FX, Lorincz A, Munoz N. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002;55:244–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu H, Ding Q, Yang K, et al. Meta-analysis of systemic lupus erythematosus and the risk of cervical neoplasia. Rheumatology (Oxford) 2011;50:343–8. [DOI] [PubMed] [Google Scholar]
- 7. Rojo-Contreras W, Olivas-Flores EM, Gamez-Nava JI, et al. Cervical human papillomavirus infection in Mexican women with systemic lupus erythematosus or rheumatoid arthritis. Lupus 2012;21:365–72. [DOI] [PubMed] [Google Scholar]
- 8. Ognenovski VM, Marder W, Somers EC, et al. Increased incidence of cervical intraepithelial neoplasia in women with systemic lupus erythematosus treated with intravenous cyclophosphamide. J Rheumatol 2004;31:1763–7. [PubMed] [Google Scholar]
- 9. Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA 2006;296:2823–31. [DOI] [PubMed] [Google Scholar]
- 10. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34. [DOI] [PubMed] [Google Scholar]
- 11. Lightner AL, Shen B. Perioperative use of immunosuppressive medications in patients with Crohn’s disease in the new ‘biological era’. Gastroenterol Rep (Oxf) 2017;5:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Connell WR, Kamm MA, Dickson M, et al. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet 1994;343:1249–52. [DOI] [PubMed] [Google Scholar]
- 13. Hutfless S, Fireman B, Kane S, et al. Screening differences and risk of cervical cancer in inflammatory bowel disease. Aliment Pharmacol Ther 2008;28:598–605. [DOI] [PubMed] [Google Scholar]
- 14. Singh H, Demers AA, Nugent Z, et al. Risk of cervical abnormalities in women with inflammatory bowel disease: a population-based nested case-control study. Gastroenterology 2009;136:451–8. [DOI] [PubMed] [Google Scholar]
- 15. Bhatia J, Bratcher J, Korelitz B, et al. Abnormalities of uterine cervix in women with inflammatory bowel disease. World J Gastroenterol 2006;12:6167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lees CW, Critchley J, Chee N, et al. Lack of association between cervical dysplasia and IBD: a large case-control study. Inflamm Bowel Dis 2009;15:1621–9. [DOI] [PubMed] [Google Scholar]
- 17. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003;348:518–27. [DOI] [PubMed] [Google Scholar]
- 18. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012;62:147–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crum CP, McLachlin CM. Cervical intraepithelial neoplasia. J Cell Biochem Suppl 1995;23:71–9. [DOI] [PubMed] [Google Scholar]
- 20. Kim SC, Glynn RJ, Giovannucci E, et al. Risk of high-grade cervical dysplasia and cervical cancer in women with systemic inflammatory diseases: a population-based cohort study. Ann Rheum Dis 2015;74:1360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kane S, Khatibi B, Reddy D. Higher incidence of abnormal Pap smears in women with inflammatory bowel disease. Am J Gastroenterol 2008;103:631–6. [DOI] [PubMed] [Google Scholar]
- 22. Hellberg D, Nilsson S, Haley NJ, et al. Smoking and cervical intraepithelial neoplasia: nicotine and cotimne in serum and cervical mucns in smokers and nonsmokers. Am J Obstet Gynecol 1988;158:910–3. [DOI] [PubMed] [Google Scholar]
- 23. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA 2007;297:813–9. [DOI] [PubMed] [Google Scholar]
- 24. Barnhart KT, Sondheimer SJ. Contraception choice and sexually transmitted disease. Curr Opin Obstet Gynecol 1993;5:823–8. [PubMed] [Google Scholar]
- 25. Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol 2012;130:1030–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lam JO, Sugar EA, Cranston RD, et al. The association of medication use with clearance or persistence of oral HPV infection. Cancer Causes Control 2016;27:1491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zumtobel U, Schwarze HP, Favre M, et al. Widespread cutaneous carcinomas associated with human papillomaviruses 5, 14 and 20 after introduction of methotrexate in two long-term PUVA-treated patients. Dermatology (Basel) 2001;202:127–30. [DOI] [PubMed] [Google Scholar]
- 28. Rivera-Acosta JE, Aponte M, Villamil I, et al. Human papilloma virus awareness among Hispanic females with inflammatory bowel disease. J Racial Ethn Health Disparities 2016;3:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greywoode R, LaFond J, Fine S, et al. Women with inflammatory bowel disease do not receive adequate cervical cancer screening or pregnancy counseling. Inflamm Bowel Dis 2013;19:E6–7. [DOI] [PubMed] [Google Scholar]
- 30. Annese V, Beaugerie L, Egan L, et al. European evidence-based consensus: inflammatory bowel disease and malignancies. J Crohns Colitis 2015;9:945–65. [DOI] [PubMed] [Google Scholar]