Abstract
Human blastocyst nidation in the uterus and successful pregnancy require coordinated endometrial expression of estrogen receptor (ER)-α, progesterone receptors (PR)-A and -B and the gap junction protein, connexin (Cx)43. Our prior work established that inflammation associated with conditions of reduced fecundity, particularly endometriosis, can perturb eutopic decidual function. In the current studies, we have modeled endometrial decidualization in primary human endometrial stromal cell cultures derived from normal controls (NESC) and from the eutopic endometria of women with endometriosis (EESC) to test the hypothesis that a proinflammatory cytokine, interleukin (IL)-1β, can disrupt stromal cell differentiation. The cells were grown under a standard protocol with hormones (10 nM 17β-estradiol, 100 nM progesterone and 0.5 mM dibutyryl cAMP) for up to 7 days in the absence or presence of IL-1β. Time-course experiments showed that IL-1β compromised decidual function in both NESC and EESC, which was accompanied by rapid phosphorylation of ER-α, PR and Cx43 and their cellular depletion. Inhibition of the extracellular signal-regulated kinase (ERK)1/2 pathway by a selective pharmacological blocker (PD98059) or siRNA interference, or the addition of hormones themselves, blocked the phosphorylation of ERK mediators; increased the production of steroid receptors, Cx43, prolactin, insulin-like growth factor binding protein-1 (IGFBP)-1 and vascular endothelial growth factor (VEGF) and accelerated the differentiation. The results indicate that inhibition of IL-1β can enhance decidualization in NESC and EESC in vitro. Strategies to interfere with this pathway might be implemented as an in vivo approach to enhance fertility in women with endometriosis and, potentially, other inflammatory pathologies.
Keywords: estrogen receptor-α, progesterone receptors, connexin 43, interleukin-1β, decidualization, cytokines, signal transduction, uterus, endometriosis, inflammation
Introduction
In viviparous, hemochorial placental mammals, such as women, non-human primates and mice, blastocyst nidation is critically controlled within a narrow window of uterine receptivity that is precisely synchronized to embryo development by the ovarian secretion of 17β-estradiol (E2) and progesterone (P4). Functioning predominantly via their classical nuclear receptors (ER-α and PR-A and B), these steroid hormones orchestrate a genetic program of endometrial proliferation and differentiation. In women, the ‘open’ endometrial interval only lasts ~96 h each month, spanning idealized menstrual cycle days 20–24 (LH +7 to +11), with implantation on days 22–24 rendering the highest likelihood of pregnancy success (Wilcox et al., 1999). This open window is characterized by biochemical and morphological changes in endometrial glands and mesenchyme, including expression of ER-α and PR (Lessey et al., 1988; Perrot-Applanat et al., 1994), apical epithelial protrusions called pinopodes (Nikas and Psychoyos, 1997) and stromal gap junctions composed predominantly of connexin (Cx)43 hemichannels (Ramathal et al., 2010). Other protein markers, many of which are cell type-specific, have been described, for example prolactin (PRL) (Maslar et al., 1980), insulin-like growth factor binding protein-1 (IGFBP)-1 (Rutanen et al., 1985), αvβ3 integrin (Lessey et al., 1994), glycodelin (GdA) (Seppala et al., 2002), homeobox A10 (Taylor et al., 1998), interleukin (IL)-11 (Dimitriadis et al., 2000), signal transducer and activator of transcription (STAT)-3 and CCAAT/enhancer-binding protein β (Wang et al., 2012). Endometrial gene expression profiles also have been interrogated to define optimal uterine receptivity for the timing of embryo transfer (Burney et al., 2007) (Ruiz-Alonso et al., 2013), but available diagnostic tests do not yet enjoy wide clinical acceptance (Bassil et al., 2018). Refining clinically meaningful implantation biomarkers and elucidating their physiological activities are important goals for contemporary reproductive medicine practitioners.
Among the E2- and P4-induced implantation biomarkers that our group has investigated, Cx43 stands out as a functionally interesting candidate. Cx43 is the dominant gap junction protein expressed in the human endometrium, where its synthesis and localization are almost exclusively stromal (Yu et al., 2014b). Cx43 protein concentrations in normal human endometrial stromal cells (NESC) from parous controls are reversibly responsive to hormone treatment and withdrawal, with peak levels following exposure to E2 (10 nM), P4 (100 nM) and dibutyryl cAMP (0.5 mM) for 7 days (Yu et al., 2016). These hormonal conditions, selected to mimic early pregnancy endocrinology, also correspond to NESC acquisition of an epithelioid morphology and maximal expression of the mesenchymal-to-epithelial transition (MET) proteins E-cadherin, GdA and zona occludens-1 (Yu et al., 2016). Unlike many of the extant protein biomarkers of decidualized endometrium mentioned above, whose cellular functions remain unknown, Cx43 is well established as a subunit of functional, transmembrane hemichannels and connexons, capable of intra- or intercellular transport of small molecules < 1 kD in mass or <1.2 nm in Stokes’ radius (Ramathal et al., 2010).
The necessity of Cx43 for embryonic implantation was established in a genetically engineered, loss-of-function murine model. Global Cx43 gene deletion in mice is embryonically lethal due to cardiovascular malformations (Reaume et al., 1995), so our collaborators developed a tissue-specific, conditional knockout of Cx43 in adult mouse uterus, using the progesterone receptor Cre-LoxP system (Laws et al., 2008). Cx43d/d dams had normal ovulation and P4 production, but showed severe fertility defects during breeding; > 60% of dams had reduced litter sizes and >50% never bore live offspring. Analyses of Cx43d/d uteri showed complete absence of Cx43 protein and failure of the endometrium to decidualize or undergo angiogenesis. Blastocyst attachment was supported up to day 7 of gestation, but implantation and embryonic growth were arrested thereafter, ultimately accompanied by resorption of concepti (Laws et al., 2008).
Other revealing findings about Cx43 mediation of decidual function came from studies of endometriosis, an inflammatory gynecologic syndrome known to be associated with impaired endometrial stromal cells (ESC) differentiation (Klemmt et al., 2006) and reduced fecundity (Zondervan et al., 2018). In a cohort of 12 women with endometriosis, homogenates of cultured eutopic endometrial stromal cells (EESC) had less than half the Cx43 concentrations of NESC derived from 12 unaffected controls (Yu et al., 2014b). Several inflammatory factors are reported to be elevated in EESC relative to NESC (Tseng et al., 1996; Hornung et al., 1997; Fang et al., 2009), but among them the IL-1 pathway has been implicated most frequently (Fakih et al., 1987; Mori et al., 1992; Lebovic et al., 2001b; Gajbhiye et al., 2018).
It has been known for some time that IL-1β, derived primarily from infiltrating macrophages, can perturb fertility by disrupting endometrial decidualization (Frank et al., 1995). Overexpression of IL-1β and its actions have been reported extensively in endometriosis and are thoroughly reviewed (Lebovic et al., 2001a). This cytokine has also been invoked in idiopathic primary infertility (Fitzgerald et al., 2016), recurrent miscarriage (Tang and Quenby, 2010) and other complications of pregnancy (Romero et al., 2006). Previous findings (Yu et al., 2017) demonstrated that IL-1β dose-dependently compromises decidualization via the extracellular signal-regulated kinase (ERK)1/2 signal transduction pathway. The current studies were undertaken to evaluate whether this cytokine pathway could induce the downregulation or degradation of ER-α, PR-A and -B and Cx43 directly and to evaluate its mechanisms. The goal of these experiments was to predict interventions that might correct decidual dysfunction in endometriosis and other common syndromes of female subfertility and pregnancy pathology.
Materials and Methods
Source of human tissues
NESC were prepared from six parous (fertile) women with regular menstrual cycles recruited before undergoing elective laparoscopic surgery in the mid-proliferative phase of the cycle for tubal sterilization (n = 4) or for evaluation of subserosal leiomyomata (n = 2). EESC were collected from proliferative phase eutopic endometrial biopsies from six subjects with a history of infertility or chronic pelvic pain who were found to have active endometriosis on laparoscopy. Four of these subjects were nulligravid. Pipelle aspiration was performed under sterile conditions as we have described previously (Ryan et al., 1994) (Yu et al., 2018) from anesthetized subjects who provided written informed consent under an Institutional Review Board-approved study protocol (Wake Forest School of Medicine no. 00019887) and in accordance with the World Medical Association Declaration of Helsinki. All the subjects had regular menstrual cycles and had not received hormonal therapy for at least 3 months before surgery. Biopsies were obtained prior to ovulation to avoid effects of endogenous P4 and were transported to the laboratory in sterile Dulbecco’s modified Eagle’s/Ham’s F-12 medium (catalog no. 10-092cv; CellGro, Manassas, VA).
Immunohistochemistry
Paraffin-embedded endometrial tissue blocks were sectioned 4- to 5-μm thick and subjected to immunohistochemistry (IHC) as described previously (Yu et al., 2016) with some modifications. After mounting, the slides were deparaffinized in xylene and rehydrated in graded concentrations of ethanol. For heat-induced epitope retrieval, slides were incubated in 10 mM citrate buffer (pH 6.0) for 4 min in a pressure cooker and endogenous hydrogen peroxide was quenched in 3% hydrogen peroxide in methanol. Primary antibodies to PR (catalog no. 8757, Cell Signaling Technology, Danvers, MA) and ERK1/2 (catalog no. 4696, Cell Signaling) were diluted to 1:1000 and 1:500, respectively, in Tris buffer, pH 7.4, containing 0.5% casein as a blocking reagent. Sections were incubated overnight at 4°C with the primary antibodies. Mild counterstaining with Mayer’s hematoxylin was performed. The negative staining control was performed by substituting the primary antibody with nonimmune serum (Biogenex, San Ramon, CA).
NESC and EESC cultures, hormone and cytokine treatment and in vitro decidualization
As noted above, NESC were prepared from proliferative-phase endometria of six parous controls and EESC were prepared from proliferative-phase endometria of six cases with surgically documented endometriosis. The cells were subcultured 2–5 passages to eliminate contamination by macrophages or other leukocytes, as described previously (Ryan et al., 1994; Yu et al., 2011). ESC prepared by this protocol are > 93% pure and retain phenotypic markers in vitro (Tseng et al., 1996; Chowdhury et al., 2018), including characteristic microfilament proteins and functional ER and PR (Ryan et al., 1994). Cells were cultured directly on 10-cm polystyrene dishes or Lab-Tek® glass chamber slides (catalog no. 177402; Thermo-Fisher Scientific, Waltham, MA) to 80% confluence in phenol red-free Dulbecco’s modified Eagle medium/Ham’s F-12, supplemented with 5% charcoal-stripped fetal calf serum. To induce decidualization, an established hormone protocol with 10 nM E2, 100 nM P4 and 0.5 mM dibutyryl cAMP for 3 or 7 days (H + 3 or H + 7) (Yu et al., 2011) was used. Previous dose–response studies in NESC indicated that IL-1β (over a wide concentration range of 0–160 ng/ml [0–9.6 nM]) had progressive inhibitory or stimulatory effects on vascular endothelial growth factor (VEGF) or IL-6, respectively, with IC50 or EC50 ~0.2 ng/ml (~12 pM). This concentration is in keeping with the reported Kd of the human IL-1 type I receptor (Yu et al., 2017). Auto-upregulation of pro-IL-1β, a 31-kDa precursor of the mature (17 kDa) cytokine, provided a confirmation of IL-1β action, as reported (Yu et al., 2017).
Western blot analyses
Western blots were performed on whole-cell extracts obtained by vortexing in extraction buffer (catalog no. FNN0011; Life Technologies, Grand Island, NY). Total proteins (20–60 μg per lane), determined using the Thermo Scientific-Pierce BCA Protein Assay Kit (catalog no. PI-23227; Thermo-Fisher Scientific, Waltham, MA), were run on NuPAGE® Novex® 4–12% Bis-Tris protein gels, transferred to polyvinylidene difluoride membranes and blocked with 5% skim milk in phosphate buffered saline with Tween 20. Specific proteins were detected using curated and validated polyclonal or monoclonal antibodies at optimized concentrations, as indicated. Human ER-α (66 kDa) expression was determined using rabbit monoclonal (catalog no. 8644, Cell Signaling Technology, Danvers, MA). Rabbit monoclonal anti-human PR antibodies detected both A (116 kDa) and B (90 kDa) isoforms of the receptor in ESC (catalog no. 8757, Cell Signaling). Total Cx43 was detected using a rabbit polyclonal anti-Cx43 antibody (1:1000 dilution, catalog no. 3512, Cell Signaling). Western blots of ESC were also evaluated using antibodies specific for phospho-serine (Ser)367 (1:1000, catalog no. SAB4504371, Sigma-Aldrich, St. Louis, MO), phospho-Ser368 Cx43 (1:1000, catalog no. 3511, Cell Signaling) and non-phospho-Ser368 Cx43 (1:1000, cat no. 13–8300, Life Technologies). IL-1β antibodies detected both mature (17 kDa) and precursor (31 kDa) forms as reported (Yu et al., 2017). The latter was used to verify the auto-upregulation by IL-1β action. After overnight labeling with primary antisera, blots were then incubated with a secondary goat anti-rabbit or anti-mouse antibody (1:300 000) (catalog no. 31460 and no. 31430; Pierce Biotechnology Inc., Rockford, IL) linked to horseradish peroxidase, and immunoreactive bands were visualized by the Enhanced Chemiluminescence System (Fisher Scientific, Hampton, NH). Blots were washed, reprobed with mouse monoclonal anti-β-actin antibodies (1:1000 dilution, catalog no. A2228, Sigma-Aldrich) and developed in an identical manner to ensure even loading. Molecular weight standards were used to calibrate the migration of immunopositive bands. The mass of each band is indicated in the figures. For quantification, western blot bands were captured by laser densitometry and normalized to the intensity of corresponding, constitutive β-actin band densities for statistical analyses using ImageJ software (version 1.61) from the National Institutes of Health. Full length images of the western blots were provided for the reviewers and editors and are available on request.
Pharmacological and genetic ERK1/2 inhibition
PD98059 (PD) is a 267 Da compound (catalog no. P215, Sigma-Aldrich) that binds to the inactive form of mitogen-activated protein kinase kinase (MEK1/2) and prevents its phosphorylation. Inhibition of MEK1/2 prevents the subsequent phosphorylation and activation of ERK1/2, which is the dominant IL-1β-regulated pathway that represses ESC differentiation (Yu et al., 2017). In experiments using the kinase inhibitor, PD (15 μM) was added to the cultures 1 h prior to the addition of hormones and IL-1β. The cells were allowed to incubate from 20 min to 7 days prior to termination of the experiments. To independently confirm the PD findings, we employed specific double-stranded siRNA constructs to selectively silence translation of ERK1/2 (Cell Signaling, catalog no. 6560) or a scrambled duplex siRNA (Cell Signaling, catalog no. 6568) from the same manufacturer as a control. siRNAs were introduced into ESC grown on six-well dishes by transfection with Lipofectamine RNAiMAX reagent (Thermo-Fisher Scientific) with some modifications to the manufacturer’s specifications and an increase in targeted and control siRNA to 100 nmol per well as described (Yu et al., 2017).
Quantification of cell shape index
Cell morphology was assessed every other day by phase-contrast microscopy. Captured images were photographed with a green filter to enhance clarity and measured using a modified calculation of ‘roundness’ that we have described previously (Yu et al., 2016), where an index of 1.0 = a maximally circular, decidualized cell. Results are presented as mean cell shape index ± SEM.
ELISA to quantify secreted PRL, IGFBP-1 and VEGF
Sensitive and specific sandwich ELISA kits, previously validated for NESC supernatants, were used to measure PRL (Alpha Diagnostic International, San Antonio, TX) and IGFBP-1 (R&D Biosystems, Minneapolis, MN), considered classical biomarkers (Gellersen et al., 2007), as well as VEGF (R&D Biosystems), an emerging biomarker of ESC decidualization (Yu et al., 2011).
Immunofluorescence cytochemistry
To corroborate and localize the expression of phospho-ERK1/2, NESC were cultured on Lab-Tek® chamber slides before and after IL-1β exposure for 20 min and in the absence or presence of the inhibitor PD98059. The cells were fixed in 4% paraformaldehyde and immunofluorescence cytochemistry was performed on an EVOS cellular imaging microscope (Thermo-Fisher Scientific).
Statistical analyses
All of the data represent assays performed in three or more replicates, with each result repeated with at least three independent ESC isolates. The data were normally distributed (Kolmogorov–Smirnov test) and are expressed as means ± SEM. Significant differences were accepted when two-tailed, Student’s t tests with Bonferroni’s correction or ANOVA with Scheffé’s post hoc tests yielded P < 0.05.
Results
Endometrial steroid hormone responsiveness and expression of nuclear receptors
To establish that endometrial specimens from our subjects are sensitive P4 targets, we confirmed the presence of nuclear PR by IHC (Fig. 1, left panel). The tissues also have the capacity to participate actively in cytokine signaling, demonstrated by cytoplasmic and nuclear localization of ERK1/2 (Fig. 1, middle panel), as reported previously by Velarde et al. (Velarde et al., 2009). Fields were selected to demonstrate glandular and stromal elements. A negative IHC control, using nonimmune serum (Fig. 1, right panel), is included. Similar results were noted in biopsies from two independent subjects.
Figure 1.
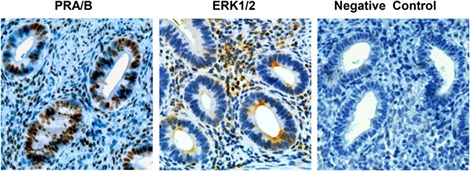
Immunohistochemistry of PR and ERK1/2 in endometrium. Immunoperoxidase histochemistry (brown precipitate) shows nuclear localization of PR in endometrial glands and stroma (left panel). ERK1/2 is identified in the cytoplasm of endometrial glands with both cytoplasmic and nuclear staining in the stroma (middle panel). A negative control, where the primary antibody was substituted with nonimmune serum, is included (right panel). All sections were counterstained with Mayer’s hematoxylin. Magnification ×200. ERK1/2, extracellular signal-regulated kinase (ERK)1/2; PR, progesterone receptors.
Effects of IL-1β on ER-α, PR-A and -B and Cx43 in NESC as detected by western blots
Primary NESC were isolated from the tissue and placed into culture. Figure 2A shows representative results of three independent experiments wherein incubation of NESC with decidualizing hormones for 7 days (H 7d) increased ER-α and PR by 3.8 ± 0.4-fold and 5.8 ± 1.1-fold, respectively, relative to control cells (lane 1) (P < 0.01, ANOVA with Scheffé’s post hoc tests). The gap junction protein Cx43 was increased 3.6 ± 1.0-fold over 7 days, as reported previously (Yu et al., 2016), with a shorter (3 days) incubation with hormones (H 3d) yielding correspondingly lesser increases in both steroid hormone receptors and Cx43 (P < 0.05, ANOVA with Scheffé’s post hoc tests). By contrast, exposure of ESC for 3 or 7 days to 120 pM IL-1β dramatically reduced ER-α, PR and Cx43 expression (Fig. 2A, panels 2 and 3), consistent with the hypothesis that hormone resistance can be attributed to proinflammatory cytokines (Patel et al., 2015).
Figure 2.
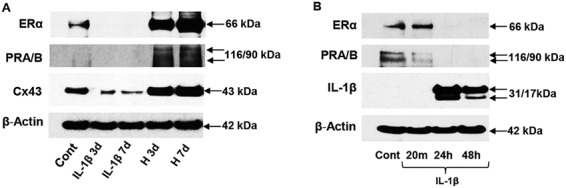
Western blots of endometrial proteins in NESC in response to IL-1β and hormones. (A) NESC treated with E2, P4 and cAMP for 3 (H 3d) or 7 (H 7d) days show upregulation of ER-α (66 kDa, panel 1), PR-A and -B (116 and 90 kDa, panel 2) and Cx43 (43 kDa, panel 3). Exposure to IL-1β for 3 or 7 days suppressed ER-α (panel 1), PR-A and -B (panel 2) and Cx43 (panel 3) expression, relative to control (Cont) cells. β-Actin levels (42 kDa) are shown. (B) A short time-course revealed the effects of IL-1β on ER-α (panel 1), PR-A/B (panel 2) and pro- (31 kDa) and processed IL-1β (17 kDa, panel 3). β-Actin levels are shown (panel 4). Full length images of the western blots are available on request. Cx43, connexin 43; ER-α, estrogen receptor-α; NESC, normal human endometrial stromal cells.
To define the inhibitory effects of IL-1β on ER-α, PR and Cx43, we performed more precise time-course experiments (Fig. 2B), which revealed that ER-α (Fig. 2B, panel 1) and PR (Fig. 2B, panel 2) proteins were nearly fully degraded within 24 h of cytokine exposure. Auto-upregulation of pro- (31 kDa) and mature (17 kDa) IL-1β after 24 h served as a positive control for IL-1β action (Yu et al., 2017; Fig. 2B, panel 3). β-Actin levels were unchanged (Fig. 2A and B, panels 4). Similar results were noted in three independent cell preparations.
By performing a comprehensive analysis of carefully selected inhibitors of mTOR (mechanistic target of rapamycin), p38 MAPK, JNK (Jun N-terminal kinase), NF-κB (nuclear factor-kappa B) and ERK1/2 pathways, our previous study documented that the inhibitory effect of IL-1β on hormone-induced NESC differentiation was mediated predominantly via the ERK1/2 pathway (Yu et al., 2017). Thus, the experiments described in the current paper were focused on the latter signaling cascade, schematized in Figure 3.
Figure 3.
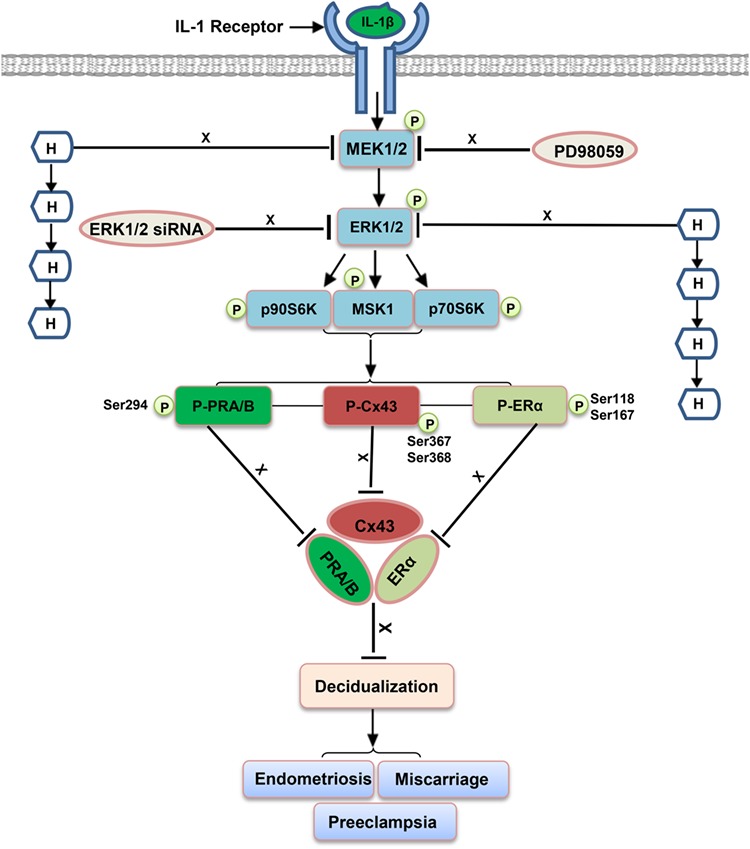
Model describing IL-1β signaling via ERK1/2, endometrial biomarkers and clinical consequences. The ERK1/2 MAP kinase enzyme cascade following IL-1β ligation is depicted, showing intermediates and signaling mechanisms. Arrows (←) indicate stimulatory effects, whereas ‘block’ symbols ( ⊥) represent inhibitory effects. H refers to the combination of E2, P4 and cAMP and postulated, related reproductive pathologies are noted. See text for other abbreviations. MEK1/2, mitogen-activated protein kinase kinase.
Incubation of NESC with the MEK1/2 inhibitor PD98059 (PD) or hormones (H) each time-dependently increased Cx43, but maximal expression relative to control values was noted in response to the combination of both stimuli for 7 days (PD + H 7d). The quantified results yielded a 1.8 ± 0.1-fold increase over the control (P < 0.01, n = 3 independent experiments, see examples in Figure 4A, panel 1 and Fig. 4B, panel 3). The combination of PD and hormones also had a profound, time-dependent, inhibitory effect on phospho-ERK1/2 (Fig. 4A, panel 2) and its downstream effector phospho-p90RSK (Fig. 4A, panel 3). Expression of ER-α (Fig. 4B, panel 1) and PR-A and -B (Fig. 4B, panel 2) was also highest in NESC after exposure to the PD + H 7d combination (Fig. 4B, lane 2). In three independent experiments, ER-α was increased 2.5 ± 0.4-fold and PR was increased 11.9 ± 1.0-fold by the PD + H 7d combination relative to controls (P = 0.03 and P < 0.01, respectively). β-Actin did not change appreciably under any of the conditions (Fig. 4A and B, panels 4).
Figure 4.
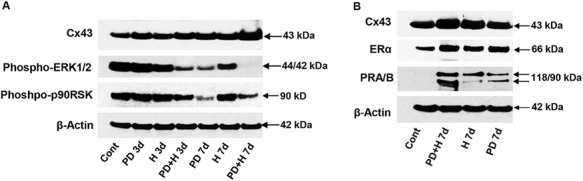
Effects of decidualizing hormones and PD98059 (PD) on NESC proteins: Cx43, ERK1/2, p90RSK, ER-α and PR-A/B (western blots). (A) The ERK1/2 inhibitor (PD), hormones (H) or the combination of the two were incubated for 3 (PD + H 3d) or 7 days (PD + H 7d) and their effects on Cx43 (panel 1), phospho-ERK1/2 (panel 2), phospho-p90RSK (panel 3) and β-actin levels (panel 4) were measured. (B) Cx43 (panel 1), ER-α (panel 2), PR-A/B (panel 3) and β-actin levels (panel 4) were also evaluated, relative to control ESC. Molecular weights (kDa) of the specific protein bands are indicated at the right. Full length images of the western blots are available on request.
Decidual morphology changes and effects of IL-1β and its inhibitor (PD) on NESC
Decidualizing hormones and PD treatment also induced characteristic epithelioid morphological changes consistent with NESC differentiation. The mean ± SEM cell shape index, calculated in three independent cell preparations, progressively increased from 0.29 ± 0.06 (control) to 0.71 ± 0.02 (PD), 0.74 ± 0.02 (H) and 0.76 ± 0.13 (PD + H 7d) over 7 days (Fig. 5, ANOVA, P < 0.01).
Figure 5.
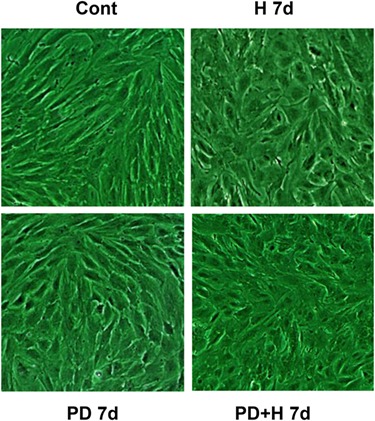
Decidual hormones and PD alter NESC morphology. NESC cultured for 7 days in the presence of E2, P4 and cAMP (H 7d), PD (PD 7d) or the combination (PD + H 7d) were examined for decidual morphology. Cell shape indices were calculated and are presented in the text as mean ± SEM. Magnification ×400.
As reported (Gellersen et al., 2007; Yu et al., 2011), ‘rounded’ morphology correlates with the secretion of classic decidual biomarkers, PRL and IGFBP-1, and the emerging biomarker, VEGF, and also reflects MET. Production of the three secreted proteins was dramatically upregulated by H 7d and further increased by PD + H 7d. The combination treatment resulted in 1.9-fold, 1.4-fold and 1.3-fold stimulations, respectively, over hormone-alone treated cells (Fig. 6A–C, *P < 0.05, ANOVA with Scheffé’s post hoc tests). These results represent the mean ± SEM of triplicate wells and similar findings were noted in three independent NESC preparations.
Figure 6.
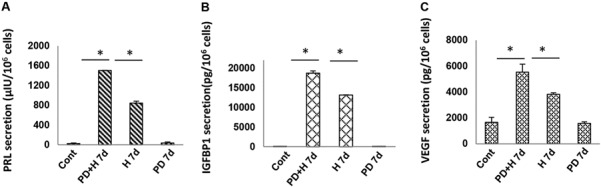
Hormones and PD increase secretion of decidual biomarkers in NESC (ELISA data). (A) Triplicate NESC cultures were incubated for 7 days with E2, P4 and cAMP (H 7d), PD (PD 7d) or the combination (PD + H 7d) and cell supernatants were assayed for (A) PRL, (B) IGFBP-1 and (C) VEGF. Means ± SEM of triplicate wells from a single representative subject are shown. Asterisk depicts differences among comparisons by ANOVA with Scheffé’s post hoc tests (*P < 0.05). Similar findings were noted in three independent NESC preparations. PRL, prolactin; IGFBP-1, insulin-like growth factor binding protein-1; VEGF, vascular endothelial growth factor.
To extend these findings, we performed immunofluorescence cytochemistry in NESC cultures treated without or with IL-1β, either in the absence or presence of PD. Under control conditions, faint cytoplasmic and nuclear localization of phospho-ERK1/2 was observed (Fig. 7, upper left panel), but IL-1β induced a rapid (20 min) and significant increase in nuclear phospho-ERK1/2 translocation and accumulation (Fig. 7, upper right panel). Co-incubation with PD blocked the IL-1β-induced increase and PD alone suppressed phospho-ERK1/2 staining below basal (control) levels (Fig. 7, lower left panel). Quantification of nuclear pixels shown in Table I reveals that IL-1β or PD treatments exerted statistically significant effects on phospho-ERK1/2 concentrations (P < 0.01, ANOVA with Scheffé’s post hoc tests). Similar results were reproduced in three independent NESC preparations.
Figure 7.
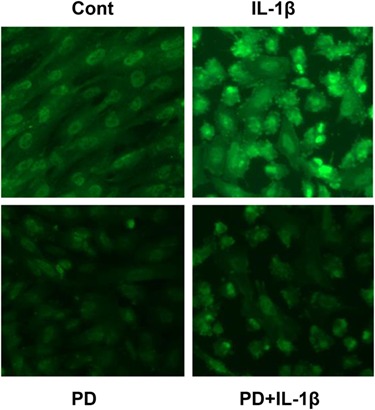
IL-1β and PD98059 differentially affect nuclear translocation and accumulation of phospho-ERK1/2 in NESC. Immunofluorescence cytochemistry in NESC monolayers was performed with anti-phospho-ERK1/2 antibodies (green signal, upper right panel) 20 min following IL-1β exposure. Control (upper left panel), PD (lower left panel) and PD + IL-1β (lower right panel). Magnification ×400. Pixel quantification and statistical analyses are provided in Table I.
Table I.
IL-1β and PD98059 differentially affect nuclear translocation and accumulation of phospho-ERK1/2 in NESC (pixel quantification from immunofluorescence cytochemistry).
| ESC treatment | Phospho-ERK1/2 (nuclear pixels % relative to control) |
|---|---|
| Control | 100 ± 5% |
| IL-1β | 211 ± 7%* |
| PD98059 | 84 ± 4%* |
| IL-1β + PD98059 | 111 ± 4%** |
* P < 0.01 vs control.
** P < 0.05 vs PD98059
Phosphoprotein cascades affected by IL-1β and its pharmacological inhibitor PD in NESC
Western blots confirmed that treatment of NESC with 120 pM IL-1β resulted in a prompt and sustained increase in phospho-ERK1/2 (Fig. 8A, panel 1) along with its downstream effector phospho-p90RSK (Fig. 8A, panel 2). This response was accompanied by transient, subtle increases in phospho-Cx43 isoforms (at Ser367 and Ser368 residues) peaking at 40 and 20 min, respectively, followed by extensive reduction of both phosphoproteins beyond 12 h of IL-1β exposure (Fig. 8A, panels 3 and 4). IL-1β also caused transient (80 min to 2 h) increases in phospho-ER-α (Ser118 and Ser167, Fig. 8A, panels 5 and 6) and phospho-PR (Ser294) (20–40 min, Fig. 8A, panel 7); β-actin levels remained relatively constant (Fig. 8A, panel 8).
Figure 8.
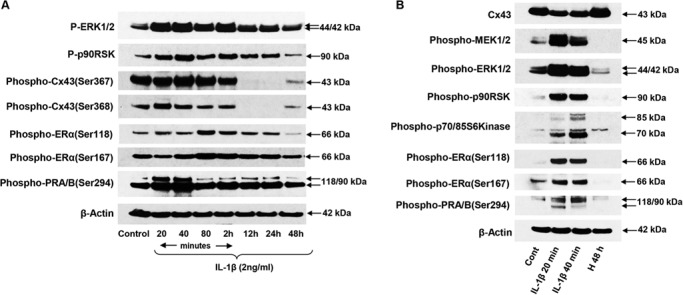
Effects of IL-1β and hormones (H) on NESC phosphoprotein expression. (A) Kinetics of IL-1β effects on phospho-ERK1/2 (P-ERK1/2, panel 1), phospho-p90RSK (P-p90RSK, panel 2), phospho-Cx43 isoforms (at Ser367 and Ser368 residues; panels 3 and 4), phospho-ER-α (Ser118 and Ser167; panels 5 and 6), phospho-PR-A and -B (Ser294, panel 7) and β-actin (panel 8). (B) Brief exposure (20 or 40 min) to IL-1β reduced total Cx43 (panel 1) but increased phospho-MEK1/2, phospho-ERK1/2, phospho-p90 RSK and phosho-p70/85 S6Kinase (panels 2–5). Phosphorylation of ER-α and PR-A and -B is also shown (panels 6–8). β-Actin effects of decidual hormone treatment for 48 h (H 48 h) are presented in the far right lane. Full length images of the western blots are available on request.
In Figure 8B, we show the effects of short-term IL-1β exposure (20 and 40 min) or decidualizing hormones (H 48 h) on NESC using many of the same antibodies. Compared to untreated controls, total Cx43 was inhibited by IL-1β at both time points, whereas hormones for 48 h modestly increased Cx43 (Fig. 8B, panel 1), as we have reported before (Yu et al., 2014a; Yu et al., 2016). IL-1β promptly upregulated the phospho-MEK–ERK pathway (Fig. 8B, panels 2–5) as well as phospho-ER-α and -PR (Fig. 8B, panels 6–8). By contrast, hormone exposure for 48 h reduced expression of the phospho-proteins in the MEK-ERK cascade and dramatically reduced phospho-ER-α and -PR isoforms. β-Actin was not altered (Fig. 8B, panel 9).
In parallel experiments, we evaluated the kinetics of PD by western blotting of critical enzymes in the MAPK cascade (Fig. 9A). Our findings corroborated that only a brief (20 min) period of exposure to the inhibitor was sufficient to reduce phospho-MEK1/2, phospho-ERK1/2 (Fig. 9A, panels 1 and 2) and its downstream effector phospho-p90RSK (Fig. 9A, panel 3) in NESC. Levels of all three enzymes remained suppressed for up to 72 h. Of interest was that within 20 min of PD treatment, reductions in phosphorylated Cx43 at Ser367 and Ser368 (Fig. 9A, panels 4 and 5) and an increase in the active, non-phospho-Cx43 (Ser368) species (Fig. 9A, panel 6) were noted. β-Actin was not altered (Fig. 9A, panel 7).
Figure 9.
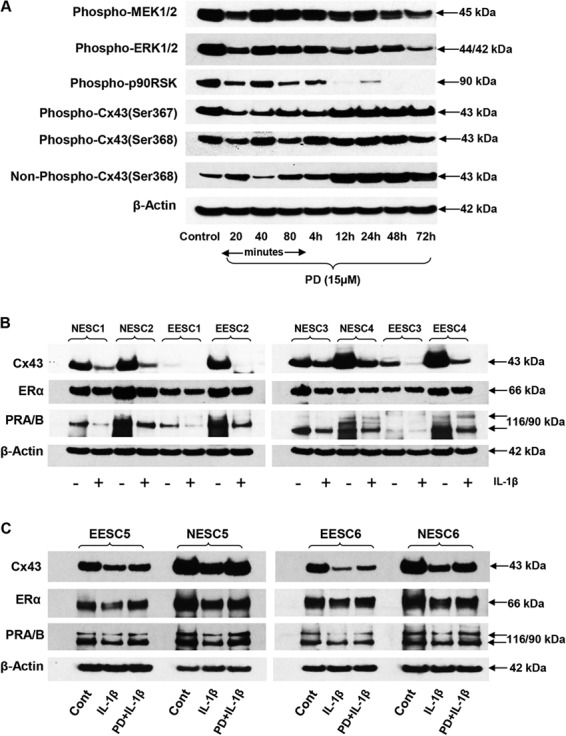
Suppression of MAP kinase signal cascade by PD and effects of IL-1β in NESC and EESC. (A) NESC cultured for 20 min to 72 h with PD were evaluated for phospho-MEK1/2 (panel 1), phospho-ERK1/2 (panel 2), phospho-p90RSK (panel 3), phospho-Cx43 (Ser367 and Ser368 isoforms, panels 4 and 5, respectively), non-phosphorylated Cx43 (Ser368, panel 6) and β-actin (panel 7). Similar findings were noted in two independent ESC preparations. (B) The effects of IL-1β (+) on Cx43 (panel 1), ER-α (panel 2), PR-A and -B (panel 3) and β-actin (panel 4) are shown in four independent preparations each of NESC (1–4) and EESC (1–4). (C) In two additional preparations of EESC (5–6) and NESC (5–6), the effects of IL-1β and PD + IL-1β are compared to the control (Cont) for Cx43 (panel 1), ER-α (panel 2), PR (panel 3) and β-actin (panel 4). Full length images of the western blots are available on request. EESC, eutopic endometrial stromal cells.
Comparison of NESC and EESC responses to IL-1β and its inhibitor PD
The effects of IL-1β on Cx43, ER-α and PR were evaluated in four cases each of NESC and EESC. Gel densitometry indicated that baseline Cx43 concentrations were 1.5 ± 0.2-fold higher in NESC compared to EESC (P < 0.04, Student’s t test), and in all cases incubation with IL-1β resulted in a depletion of Cx43, ER-α and PR proteins (Fig. 9B and C). The ability of PD to reverse the IL-1β action was tested in Fig. 9C. Although co-incubation of PD + IL-1β did not fully restore the control concentrations of Cx43, ER-α and PR, it stimulated recovery of their protein levels in two cases each of NESC and EESC, resulting in increases over IL-1β-treated levels of 1.7 ± 0.2-fold, 2.0 ± 0.2-fold and 2.0 ± 0.1-fold, respectively (ANOVA with Scheffé’s post hoc tests, P < 0.05). β-Actin levels were not affected by IL-1β or PD (Fig. 9B and C, panels 4).
Alternate approach to ERK1/2 inhibition: siRNA interference
To independently validate the salutary effects observed in decidual markers following treatment with the pharmacological inhibitor PD, we employed RNA interference as an alternate strategy to suppress ERK1/2 action. NESC morphology was assessed under control (scrambled siRNA), ERK1/2 siRNA and ERK1/2 siRNA + hormones (H) treatments (Fig. 10A). ERK1/2 siRNA induced morphological changes consistent with decidualization, with even more pronounced changes occurring after the combination of ERK1/2 siRNA + H (Fig. 10A, panel 3). Cell shape indices (mean ± SEM) progressively increased from 0.33 ± 0.06 (control) to 0.66 ± 0.02 (ERK1/2 siRNA) and to 0.88 ± 0.10 (ERK1/2 siRNA + H7) over 7 days. All three conditions differed significantly from each other (ANOVA with Scheffé’s post hoc tests, P < 0.05). Western blotting revealed that, as expected, ERK1/2 siRNA treatment led to a brisk reduction in phospho-ERK1/2 and also suppressed phospho-Cx43 (Ser367) and phospho-Cx43 (Ser368) relative to control NESC (Fig. 10B). By contrast, ERK1/2 siRNA treatment afforded modest increases in ER-α and PR.
Figure 10.
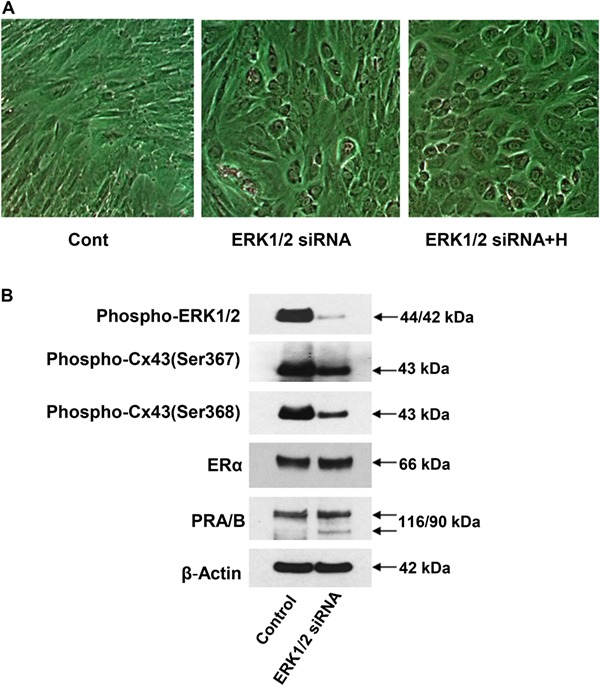
Effects of ERK1/2 siRNA and hormones (H) on NESC morphology and proteins. (A) siRNA used to inhibit ERK1/2 in the absence and presence (+H) of hormones showed prominent morphological changes (panels 2 and 3, respectively) relative to controls treated with scrambled siRNA (panel 1). (B) ERK1/2 siRNA effects on phospho-ERK1/2 (panel 1), phospho-Cx43 isoforms (panels 2 and 3), ER-α (panel 4), PR-A and -B (panel 5) and β-actin (panel 6) are shown.
Discussion
The experiments described in this report extend observations by us (Yu et al., 2017) and others (Frank et al., 1995; Guzeloglu-Kayisli et al., 2015) that the proinflammatory cytokine, IL-1β, can potently inhibit decidualization in vitro. Based on our extensive analyses of endometriosis and the prominent role that IL-1β plays in its etiology, we focused our evaluation on NESC and EESC models. In these experiments, we have shown that inhibition of ERK1/2 using PD98059, or alternatively by antagonizing ERK1/2 with siRNA, can enhance biomarkers of decidual function in vitro. Overall, the results suggest that inflammatory mediators that perturb endometrial differentiation can be partly mitigated using strategies that target the IL-1β-MEK-ERK axis. We postulate that the development of ERK inhibitors might someday provide novel therapies to improve endometrial receptivity and pregnancy success. Obviously, such a goal awaits further validation in animal models and, ultimately, in clinical trials.
In our NESC and EESC models, 120 pM (2 ng/ml) IL-1β phosphorylated ERK1/2 and induced its rapid translocation into the nucleus (Fig. 7). This action was accompanied by phosphorylation of Cx43, ER-α and PR, peaking within 20 min to 2 h of cytokine exposure. These changes were followed by a progressive attenuation of Cx43, ER-α, PR, PRL, IGFBP-1 and VEGF protein levels as well as a reversal of decidual morphology. All of these indicators are well-established correlative biomarkers of endometrial stromal differentiation. As reported by others, differences between NESC and EESC can be subtle (McKinnon et al., 2018). In our small sample, we observed that Cx43 concentrations were significantly higher in NESC (1.5 ± 0.2-fold, P < 0.04, Student’s t test) similar to our prior findings (Yu et al., 2014b). But in agreement with a recent report (Petousis et al., 2018), we failed to observe differences in endometrial stromal cell PR concentrations in women with endometriosis compared to those without endometriosis. We interpret these data to indicate not only that Cx43 in EESC may be particularly sensitive to IL-1β but also that cytokine activation is potentially detrimental to endometrial cell differentiation in women with or without endometriosis.
Phosphorylation is a key post-translational regulator of inflammation and other physiological processes. Relevant to our interests, Cx43 phosphorylation leads to gap junction inactivation, whereas hypophosphorylated species of Cx43 are the most active with respect to gap junction intercellular communication (GJIC) (Laird, 2005). The precise biological function of Cx43 gap junctions in the decidua remains unknown, but these are required for normal murine implantation (Laws et al., 2008) and their expression is regulated by ovarian steroid hormones necessary for endometrial secretory differentiation (Yu et al., 2016). Cx43 is reported to have as many as 21 different phosphorylation sites (Pogoda et al., 2016) with Ser368 being a particularly important amino acid. In the current study, the Ser368 residue was rapidly phosphorylated (~20 min) following IL-1β treatment (Fig. 8A). We previously documented that Cx43 gene silencing (Laws et al., 2008), pharmacological blockade of gap junctions (Yu et al., 2011) or treatment with IL-1β (Yu et al., 2017) all dramatically attenuated GJIC and NESC differentiation. Similar inhibitory effects of IL-1β on Cx43 were reported in murine astrocyte cultures (Watanabe et al., 2016).
ER-α and PR, also key regulators of NESC decidualization (Kaya Okur et al., 2016), can be modified by post-translational phosphorylation (Le Romancer et al., 2011). In our results, IL-1β induced a rapid increase in phospho-forms of the nuclear receptors (Fig. 8A) followed by degradation of total receptor proteins (Figs 2A and B and 9B and C). Similar effects were noted in both NESC and EESC (Fig. 9B). While ERK1/2-mediated phosphorylation temporally precedes ER-α, PR and Cx43 degradation in our studies, we have no direct evidence that this represents a cause–effect relationship and it remains an hypothesis. However, in stably transfected HEK293 cells expressing recombinant ER-α (Valley et al., 2005), phosphorylation at Ser118 targets ligand-activated ER-α complexes to the ubiquitin–proteasome pathway. Similarly, in human breast cancer cells, ERK1/2-induced Ser294 phosphorylation of PR-A and -B led to receptor ubiquitination and degradation via the 26S proteasome (Lange et al., 2000).
Clinical implications of the findings
Our findings support the clinical corollary that gynecological and obstetrical complications associated with elevated IL-1α or -β activity are accompanied by impaired decidualization and poor reproductive performance. These include endometriosis (Lebovic et al., 2001a), polycystic ovary syndrome (Gonzalez, 2012), idiopathic primary infertility (Fitzgerald et al., 2016), recurrent miscarriage (Tang and Quenby, 2010), preeclampsia (Lockwood et al., 2013) and preterm birth (Romero et al., 2006). Taking endometriosis as a prototypical example that we have studied extensively, in vivo levels of phospho-ERK and its downstream signal phospho-p70S6K were documented to be increased in deep infiltrating lesions (Matsuzaki and Darcha, 2015). Moreover, ERK1/2 conferred antiapoptotic (Murk et al., 2008) and proangiogenic (Arlier et al., 2017) properties in endometriosis-derived cell cultures. Uimari et al. (Uimari et al., 2017) identified significant over-representation of candidate genes involved in IL-1 signaling in subjects with endometriosis, including single nucleotide polymorphism variants in the MEK–ERK pathway. In a nested case–control study within the Nurses’ Health Study II cohort, elevated circulating levels of IL-1β were identified to be the most predictive plasma biomarker for this condition (Mu et al., 2018). Recently, it has been suggested that microRNAs can epigenetically regulate ERK pathways, with miR-196a proposed as a candidate that simultaneously activates MEK-ERK and represses PR in endometriosis (Zhou et al., 2016). The experiments reported in our paper provide further evidence that disturbed decidualization, an established pathophysiological correlate of endometriosis (Klemmt et al., 2006; Burney et al., 2007; Yu et al., 2014b; Zondervan et al., 2018), is in part attributable to activation of ERK1/2 by IL-1β and that it involves attenuated expression of Cx43, ER-α and PR, possibly mediating these defects. Conversely, our PD and siRNA experiments indicate that ERK1/2 inhibition can induce decidual morphology and effect changes in ER-α, PR and phospho-Cx43 that are associated with differentiated biochemical function.
This information should be useful for the design and development of future therapeutics targeted to improve decidual response in women with endometriosis or other relevant reproductive conditions. Computational biology approaches to identify natural, small molecule inhibitors of ERK1/2 have been described recently by Yan et al. (Yan et al., 2018). We encourage the evaluation of herbal analogues and establishment of simplified registration procedures for botanical derivatives with a long tradition of safe use (Wieser et al., 2007). Clinical trials will hopefully demonstrate that such compounds can be safely and efficaciously applied to improve uterine factor infertility. By identifying and interfering with its dominant signal transduction pathway (ERK1/2), we hope to mitigate the detrimental effects of IL-1β on human reproduction attributed to endometriosis and other inflammatory conditions that result in poor pregnancy outcomes.
Acknowledgements
The authors thank the operating room and nursing staffs of Wake Forest Baptist Hospital who contributed to the protocol and the generous participation of the study participants.
Authors’ roles
All co-authors contributed substantively to the work. J.Y., S.L.B. and R.N.T. were responsible for the experimental design. S.L.B. and R.N.T. were responsible for the endometrial sample collection. J.Y. and W.Z. performed the experiments. All four co-authors were involved in the data analysis and preparation of the manuscript, table and figures and approved the final version of the paper. R.N.T. secured funding and wrote the manuscript.
Funding
Eunice Kennedy Shriver National Institute of Child Health and Human Development (R21 HD78818 and U01 HD66439).
Conflict of interest
The authors have none to declare.
References
- Arlier S, Murk W, Guzeloglu-Kayisli O, Semerci N, Larsen K, Tabak MS, Arici A, Schatz F, Lockwood CJ, Kayisli UA. The extracellular signal-regulated kinase 1/2 triggers angiogenesis in human ectopic endometrial implants by inducing angioblast differentiation and proliferation. Am J Reprod Immunol 2017;78. [DOI] [PubMed] [Google Scholar]
- Bassil R, Casper R, Samara N, Hsieh TB, Barzilay E, Orvieto R, Haas J. Does the endometrial receptivity array really provide personalized embryo transfer? J Assist Reprod Genet 2018;35:1301–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007;148:3814–3826. [DOI] [PubMed] [Google Scholar]
- Chowdhury I, Banerjee S, Driss A, Xu W, Mehrabi S, Nezhat C, Sidell N, Taylor RN, Thompson WE. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-kappaB signaling pathway. J Cell Physiol 2018;234:6298–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis E, Salamonsen LA, Robb L. Expression of interleukin-11 during the human menstrual cycle: coincidence with stromal cell decidualization and relationship to leukaemia inhibitory factor and prolactin. Mol Hum Reprod 2000;6:907–914. [DOI] [PubMed] [Google Scholar]
- Fakih H, Baggett B, Holtz G, Tsang KY, Lee JC, Williamson HO. Interleukin-1: a possible role in the infertility associated with endometriosis. Fertil Steril 1987;47:213–217. [PubMed] [Google Scholar]
- Fang CL, Han SP, Fu SL, Wang W, Kong N, Wang XL. Ectopic, autologous eutopic and normal endometrial stromal cells have altered expression and chemotactic activity of RANTES. Eur J Obstet Gynecol Reprod Biol 2009;143:55–60. [DOI] [PubMed] [Google Scholar]
- Fitzgerald HC, Salamonsen LA, Rombauts LJ, Vollenhoven BJ, Edgell TA. The proliferative phase underpins endometrial development: altered cytokine profiles in uterine lavage fluid of women with idiopathic infertility. Cytokine 2016;88:12–19. [DOI] [PubMed] [Google Scholar]
- Frank GR, Brar AK, Jikihara H, Cedars MI, Handwerger S. Interleukin-1 beta and the endometrium: an inhibitor of stromal cell differentiation and possible autoregulator of decidualization in humans. Biol Reprod 1995;52:184–191. [DOI] [PubMed] [Google Scholar]
- Gajbhiye R, McKinnon B, Mortlock S, Mueller M, Montgomery G. Genetic variation at chromosome 2q13 and its potential influence on endometriosis susceptibility through effects on the IL-1 family. Reprod Sci 2018;25:1307–1317. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 2007;25:445–453. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids 2012;77:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Kayisli UA, Semerci N, Basar M, Buchwalder LF, Buhimschi CS, Buhimschi IA, Arcuri F, Larsen K, Huang JS et al. . Mechanisms of chorioamnionitis-associated preterm birth: interleukin-1beta inhibits progesterone receptor expression in decidual cells. J Pathol 2015;237:423–434. [DOI] [PubMed] [Google Scholar]
- Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab 1997;82:1621–1628. [DOI] [PubMed] [Google Scholar]
- Kaya Okur HS, Das A, Taylor RN, Bagchi IC, Bagchi MK. Roles of estrogen receptor-alpha and the coactivator MED1 during human endometrial decidualization. Mol Endocrinol 2016;30:302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril 2006;85:564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta 2005;1711:172–182. [DOI] [PubMed] [Google Scholar]
- Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A 2000;97:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws MJ, Taylor RN, Sidell N, DeMayo FJ, Lydon JP, Gutstein DE, Bagchi MK, Bagchi IC. Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development 2008;135:2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Romancer M, Poulard C, Cohen P, Sentis S, Renoir JM, Corbo L. Cracking the estrogen receptor’s posttranslational code in breast tumors. Endocr Rev 2011;32:597–622. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Chao VA, Martini JF, Taylor RN. IL-1beta induction of RANTES (regulated upon activation, normal T cell expressed and secreted) chemokine gene expression in endometriotic stromal cells depends on a nuclear factor-kappaB site in the proximal promoter. J Clin Endocrinol Metab 2001a;86:4759–4764. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril 2001b;75:1–10. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab 1994;79:643–649. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab 1988;67:334–340. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Huang SJ, Chen CP, Huang Y, Xu J, Faramarzi S, Kayisli O, Kayisli U, Koopman L, Smedts D et al. . Decidual cell regulation of natural killer cell-recruiting chemokines: implications for the pathogenesis and prediction of preeclampsia. Am J Pathol 2013;183:841–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslar IA, Kaplan BM, Luciano AA, Riddick DH. Prolactin production by the endometrium of early human pregnancy. J Clin Endocrinol Metab 1980;51:78–83. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Darcha C. Co-operation between the AKT and ERK signaling pathways may support growth of deep endometriosis in a fibrotic microenvironment in vitro. Hum Reprod 2015;30:1606–1616. [DOI] [PubMed] [Google Scholar]
- McKinnon B, Mueller M, Montgomery G. Progesterone resistance in endometriosis: an acquired property? Trends Endocrinol Metab 2018;29:535–548. [DOI] [PubMed] [Google Scholar]
- Mori H, Sawairi M, Nakagawa M, Itoh N, Wada K, Tamaya T. Expression of interleukin-1 (IL-1) beta messenger ribonucleic acid (mRNA) and IL-1 receptor antagonist mRNA in peritoneal macrophages from patients with endometriosis. Fertil Steril 1992;57:535–542. [DOI] [PubMed] [Google Scholar]
- Mu F, Harris HR, Rich-Edwards JW, Hankinson SE, Rimm EB, Spiegelman D, Missmer SA. A prospective study of inflammatory markers and risk of endometriosis. Am J Epidemiol 2018;187:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murk W, Atabekoglu CS, Cakmak H, Heper A, Ensari A, Kayisli UA, Arici A. Extracellularly signal-regulated kinase activity in the human endometrium: possible roles in the pathogenesis of endometriosis. J Clin Endocrinol Metab 2008;93:3532–3540. [DOI] [PubMed] [Google Scholar]
- Nikas G, Psychoyos A. Uterine pinopodes in peri-implantation human endometrium. Clinical relevance. Ann N Y Acad Sci 1997;816:129–142. [DOI] [PubMed] [Google Scholar]
- Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update 2015;21:155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Applanat M, Deng M, Fernandez H, Lelaidier C, Meduri G, Bouchard P. Immunohistochemical localization of estradiol and progesterone receptors in human uterus throughout pregnancy: expression in endometrial blood vessels. J Clin Endocrinol Metab 1994;78:216–224. [DOI] [PubMed] [Google Scholar]
- Petousis S, Prapas Y, Margioula-Siarkou C, Ravanos K, Milias S, Mavromatidis G, Kalogiannidis I, Haitoglou C, Athanasiadis A, Prapas N et al. . Unexplained infertility patients present the mostly impaired levels of progesterone receptors: prospective observational study. Am J Reprod Immunol 2018;79:e12828. [DOI] [PubMed] [Google Scholar]
- Pogoda K, Kameritsch P, Retamal MA, Vega JL. Regulation of gap junction channels and hemichannels by phosphorylation and redox changes: a revision. BMC Cell Biol 2016;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med 2010;28:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume AG, Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science 1995;267:1831–1834. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 2006;11:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Gomez E, Fernandez-Sanchez M, Carranza F, Carrera J, Vilella F, Pellicer A, Simon C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril 2013;100:818–824. [DOI] [PubMed] [Google Scholar]
- Rutanen EM, Koistinen R, Wahlstrom T, Bohn H, Ranta T, Seppala M. Synthesis of placental protein 12 by human decidua. Endocrinology 1985;116:1304–1309. [DOI] [PubMed] [Google Scholar]
- Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab 1994;78:642–649. [DOI] [PubMed] [Google Scholar]
- Seppala M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev 2002;23:401–430. [DOI] [PubMed] [Google Scholar]
- Tang AW, Quenby S. Recent thoughts on management and prevention of recurrent early pregnancy loss. Curr Opin Obstet Gynecol 2010;22:446–451. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest 1998;101:1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, Taylor RN. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab 1996;81:1118–1122. [DOI] [PubMed] [Google Scholar]
- Uimari O, Rahmioglu N, Nyholt DR, Vincent K, Missmer SA, Becker C, Morris AP, Montgomery GW, Zondervan KT. Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum Reprod 2017;32:780–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valley CC, Metivier R, Solodin NM, Fowler AM, Mashek MT, Hill L, Alarid ET. Differential regulation of estrogen-inducible proteolysis and transcription by the estrogen receptor alpha N terminus. Mol Cell Biol 2005;25:5417–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velarde MC, Aghajanova L, Nezhat CR, Giudice LC. Increased mitogen-activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women with endometriosis reduces 3′,5′-cyclic adenosine 5′-monophosphate inhibition of cyclin D1. Endocrinology 2009;150:4701–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Taylor RN, Bagchi IC, Bagchi MK. Regulation of human endometrial stromal proliferation and differentiation by C/EBPbeta involves cyclin E-cdk2 and STAT3. Mol Endocrinol 2012;26:2016–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Masaki K, Yamasaki R, Kawanokuchi J, Takeuchi H, Matsushita T, Suzumura A, Kira JI. Th1 cells downregulate connexin 43 gap junctions in astrocytes via microglial activation. Sci Rep 2016;6:38387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser F, Cohen M, Gaeddert A, Yu J, Burks-Wicks C, Berga SL, Taylor RN. Evolution of medical treatment for endometriosis: back to the roots? Hum Reprod Update 2007;13:487–499. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 1999;340:1796–1799. [DOI] [PubMed] [Google Scholar]
- Yan S, Zhang L, Wang S, Wu T, Gong Z. Inhibition of the Ras/Raf/extracellular signal-regulated kinase 1/2 signaling pathway by compounds of natural origin for possible treatment of spinal cord injury: an in silico approach. Exp Ther Med 2018;15:2860–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Berga SL, Johnston-MacAnanny EB, Sidell N, Bagchi IC, Bagchi MK, Taylor RN. Endometrial stromal decidualization responds reversibly to hormone stimulation and withdrawal. Endocrinology 2016;157:2432–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Berga SL, Zou W, Sun HY, Johnston-MacAnanny E, Yalcinkaya T, Sidell N, Bagchi IC, Bagchi MK, Taylor RN. Gap junction blockade induces apoptosis in human endometrial stromal cells. Mol Reprod Dev 2014a;81:666–675. [DOI] [PubMed] [Google Scholar]
- Yu J, Berga SL, Zou W, Yook DG, Pan JC, Andrade AA, Zhao L, Sidell N, Bagchi IC, Bagchi MK et al. . IL-1beta inhibits connexin 43 and disrupts decidualization of human endometrial stromal cells through ERK1/2 and p38 MAP kinase. Endocrinology 2017;158:4270–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Boicea A, Barrett KL, James CO, Bagchi IC, Bagchi MK, Nezhat C, Sidell N, Taylor RN. Reduced connexin 43 in eutopic endometrium and cultured endometrial stromal cells from subjects with endometriosis. Mol Hum Reprod 2014b;20:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Francisco AMC, Patel BG, Cline JM, Zou E, Berga SL, Taylor RN. Interleukin-1beta stimulates BDNF production in eutopic endometriosis stromal cell cultures a model for cytokine regulation of neuroangiogenesis. Am J Pathol 2018;188:2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Wu J, Bagchi IC, Bagchi MK, Sidell N, Taylor RN. Disruption of gap junctions reduces biomarkers of decidualization and angiogenesis and increases inflammatory mediators in human endometrial stromal cell cultures. Mol Cell Endocrinol 2011;344:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Fu J, Xiao L, Yang S, Song Y, Zhang X, Feng X, Sun H, Xu W, Huang W. miR-196a overexpression activates the MEK/ERK signal and represses the progesterone receptor and decidualization in eutopic endometrium from women with endometriosis. Hum Reprod 2016;31:2598–2608. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers 2018;4:9. [DOI] [PubMed] [Google Scholar]


