Abstract
Background and Aims
The use of woody crops for Quad-level (approx. 1 × 1018 J) energy production will require marginal agricultural lands that experience recurrent periods of water stress. Populus species have the capacity to increase dehydration tolerance by lowering osmotic potential via osmotic adjustment. The aim of this study was to investigate how the inherent genetic potential of a Populus clone to respond to drought interacts with the nature of the drought to determine the degree of biochemical response.
Methods
A greenhouse drought stress study was conducted on Populus deltoides ‘WV94’ and the resulting metabolite profiles of leaves were determined by gas chromatography–mass spectrometry following trimethylsilylation for plants subjected to cyclic mild (–0.5 MPa pre-dawn leaf water potential) drought vs. cyclic severe (–1.26 MPa) drought in contrast to well-watered controls (–0.1 MPa) after two or four drought cycles, and in contrast to plants subjected to acute drought, where plants were desiccated for up to 8 d.
Key Results
The nature of drought (cyclic vs. acute), frequency of drought (number of cycles) and the severity of drought (mild vs. severe) all dictated the degree of osmotic adjustment and the nature of the organic solutes that accumulated. Whereas cyclic drought induced the largest responses in primary metabolism (soluble sugars, organic acids and amino acids), acute onset of prolonged drought induced the greatest osmotic adjustment and largest responses in secondary metabolism, especially populosides (hydroxycinnamic acid conjugates of salicin).
Conclusions
The differential adaptive metabolite responses in cyclic vs. acute drought suggest that stress acclimation occurs via primary metabolism in response to cyclic drought, whereas expanded metabolic plasticity occurs via secondary metabolism following severe, acute drought. The shift in carbon partitioning to aromatic metabolism with the production of a diverse suite of higher order salicylates lowers osmotic potential and increases the probability of post-stress recovery.
Keywords: Populus deltoides ‘WV94’, cyclic drought, acute drought, gas chromatography–mass spectrometry, metabolite profiles, osmotic adjustment, acclimation, metabolic perturbation
INTRODUCTION
Use of woody, perennial crops for Quad-level (1015 BTU; approx. 1 × 1018 J) energy production will require use of marginal agricultural lands (Tuskan 1998), where periods of water stress are frequent. Populus species are widespread in North America, have high productivity, especially their interspecific hybrids, and are ideal candidate biomass crops for bioenergy production. In the Pacific Northwest, they readily exceed long-held productivity targets of the US Department of Energy of 10 Mg ha–1 year–1 oven dry weight of above-ground biomass (Tuskan and Walsh, 2001) when grown on mesic sites or on dry sites supplemented with irrigation. Productivity declines in many areas in the USA where water availability is lacking, and, in such areas, sustainable biomass production will need drought-tolerant species/clones. Populus species have the capacity to their increase dehydration tolerance by lowering the osmotic potential via osmotic adjustment, the active accumulation of solutes under stress, allowing turgor and growth maintenance under mild to moderate stress (Tschaplinski et al., 2006).
There is a need to understand the phenotypic plasticity in the drought tolerance of Populus species, and this plasticity can be assessed across large numbers of individuals as in a structured pedigree of P. trichocarpa × deltoides (TD) (Tschaplinski et al., 2006) or in a large, range-wide collection of native populations, such as P. nigra cultured in a common garden (Viger et al., 2016). In the case of the former study, P. deltoides imparted its drought tolerance capacity on the F1 hybrids that were intermediate of the parents, with the trait further segregating into a wide range of responses in the resulting F2 population. The superior allelic effect arose from the grandparent with the lowest value of osmotic potential at full saturation (πo); P. deltoides ‘ILL-129’ (male) for four of the seven statistically significant quantitative trait loci (QTLs) that were identified and the parent with the highest value of πo; and P. trichocarpa ‘93-968’ (female) for one QTL. The importance of osmotic potential to productivity is evidenced in that it explained 20 % of the phenotypic variation in relative growth rate (RGR) in stem diameter at breast height in an F2 TD × TD pedigree 331 during its second growing season in the field, and 15 % of clones displaying osmotic adjustment (lowering of πo by solute accumulation) to drought. In the case of the latter common garden study, phenotypic variation in drought tolerance was found across the P. nigra population in leaf morphology, stomatal responses and gene expression responses to drought stress. Specifically, southern genotypes from drier regions of France and Spain typically had smaller leaves, reduced biomass production, rapid stomatal closure, higher water use efficiency (WUE) and lower levels of leaf abscission. On the other hand, ‘North Eastern’ genotypes from more mesic sites typically responded to drought stress with reduced biomass growth, slow stomatal closure and lower WUE.
The inherent genetic potential of a given Populus species/clone to respond to drought interacts with the nature of the drought exposure to determine the degree of the biochemical response. With P. deltoides being one of the most drought-tolerant Populus species in North America (if not the most tolerant), and with its drought tolerance being conferred, in part, by its low osmotic potential and capacity for osmotic adjustment, it was selected for an in-depth study of its transcriptomic, proteomic and metabolomic responses to drought stress, with this paper focusing on its metabolomic responses. Given that pre-conditioning plants with multiple stress cycles enhances their capacity to tolerate drought (Silim et al., 2009), a key uncertainty is whether the metabolomic response observed in response to cyclic drought differs from that observed in response to an acute drought, where no additional water is added after drought stress is imposed. Given such considerations, it was hypothesized that the largest osmotic adjustment in P. deltoides would be observed following cyclic stress. Whereas many controlled drought stress studies have been conducted on Populus sp., very few studies have determined whether the nature of drought imposition alters the nature and degree of the metabolomic responses, which, whether adaptive or perturbational, have consequences for leaf senescence and plant post-stress recovery. Overall, such responses determine the dehydration tolerance of specific Populus genotypes and their ability to sustain biomass production in an environment with fluctuating water availability.
MATERIALS AND METHODS
Cyclic and acute drought experiments
The plant materials, growth conditions and drought treatments were as recently reported in Abraham et al. (2018). Briefly, in the cyclic drought experiment, twenty 6-month-old eastern cottonwood poplar (Populus deltoides ‘WV94’) plants were regenerated from 15 cm long cuttings and were approx. 88 cm in height when assigned to one of three watering regimes, including a well-watered control with eight plants kept near field capacity (approx. 0.10 MPa) and 12 plants subjected to cyclic drought treatments with target pre-dawn leaf water potentials of –0.5 MPa for the mild drought treatment, and < –0.8 MPa for the severe drought stress treatment, as determined with a Scholander pressure chamber (PMS Instrument Co., Albany, GA, USA). Whereas field capacity is widely accepted to be > –0.1 MPa and from what we have observed in both greenhouse and field studies (Tschaplinski et al., 2006), the thresholds for the mild and severe treatments were based on our experience and that of others reported in the literature. Stress initiation in well-watered poplars typically ranges from –0.3 MPa (Tschaplinski et al., 2006) to –0.5 MPa (Pezeshki and Hinckley, 1982) and is comparable with other fast-growing hardwood species (Tschaplinski et al., 1995); hence, we selected –0.5 MPa for mild stress, with the severe stress threshold at –0.8 MPa. The severe stress threshold typically acclimates to lower levels with continued drought exposure, and thus we further lowered our target to be –1.0 MPa, as was observed for poplar hybrids in the field (Tschaplinski et al., 1998b). Plants in drought treatments were re-watered after hitting the target pre-dawn water potentials on days 6, 12 and 20, with the experiment ending after 31 d. Leaves were collected at mid-day for metabolomic analyses at the end of drought cycle two and cycle four, and then processed as described below. There were four biological replicates for each treatment at each sampling time point. The pre-dawn water potentials for the cyclic drought treatments are shown in Table 1.
Table 1.
Average leaf pre-dawn water potential (± s.e.m.) for each cyclic drought treatment and the well-watered control
| Drought cycle | Treatment | Pre-dawn water potential (MPa) |
| 2 | Control | –0.09 ± 0.02 |
| 2 | Mild | –0.49 ± 0.14 |
| 2 | Severe | –0.84 ± 0.24 |
| 4 | Control | –0.11 ± 0.02 |
| 4 | Mild | –0.50 ± 0.04 |
| 4 | Severe | –1.26 ± 0.12 |
There were four biological replicates per treatment at each sampling time.
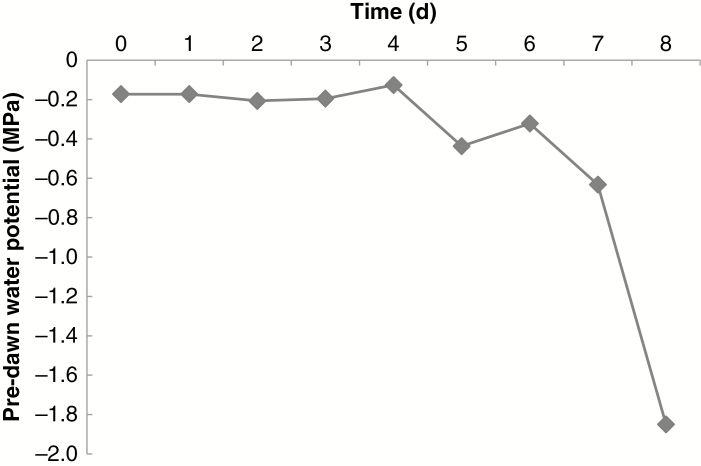
For the acute drought experiment, nine 6-month-old P. deltoides ‘WV94’ plants that were of similar stature (quantitatively) to those used in the cyclic drought study were kept well watered to field capacity (approx. –0.1 MPa) until the start of the acute drought experiment, when all plants were allowed to dehydrate for up to 8 d. Leaves of leaf plastochron index (LPI) 9–12 of three biological replicates per day were sampled for pre-dawn leaf water potential at each time point from day 1 through 8, as described in Abraham et al. (2018). Pre-dawn leaf water potentials over the 8 d of the acute drought experiment are shown in Fig. 1.
Fig. 1.
Leaf pre-dawn water potential over the 8 d of the acute drought experiment. Values on days 5, 7 and 8 were significantly lower (P ≤ 0.05) than that on day 0. There were three biological replicates sampled for each day.
Metabolite profiling by gas chromatography–mass spectrometry (GC-MS)
Leaves of LPI 10 were sampled on each of the 8 d of the acute drought experiment (three replicates per sampling day) and at the end of the second and fourth drought cycles in the cyclic drought treatments (four replicates for the controls; six replicates for the mild and severe treatments). They were fast-frozen on dry ice and stored at −80 °C until they were ground in liquid nitrogen, with about 50 mg f. wt of tissue subsequently extracted with 80 % ethanol and an aliquot of the extracts dried and silylated to generate trimethylsilyl (TMS) derivatives, as described previously (Tschaplinski et al., 2012; Abraham et al., 2016). After 2 d, 1 μL aliquots were injected into an Agilent Technologies Inc. (Santa Clara, CA, USA) 5975C inert XL gas chromatograph–mass spectrometer, fitted with an Rtx-5MS with Integra-guard (5 % diphenyl/95 % dimethyl polysiloxane) 30 m × 250 μm × 0.25 μm film thickness capillary column, using operating conditions described previously (Tschaplinski et al., 2012). Metabolite peaks were extracted using a key selected ion, characteristic mass-to-charge (m/z) ratio, with peaks of known metabolites scaled back up to the total ion current using pre-determined scaling factors. Peaks of TMS-derivatized metabolites thus generated by electron impact ionization (70 eV) were identified and quantified as described previously (Tschaplinski et al., 2012). Unidentified metabolites were denoted by their retention time as well as key m/z ratios and partial naming given the typical identity of a specific m/z.
Statistical analyses
Each Populus plant was considered an experimental unit. Principal component analysis (PCA) was performed on the metabolite data using the JMP®, Version 14 (SAS Institute Inc., Cary, NC, USA), to identify biologically relevant metabolite features in both the cyclic and acute metabolite data sets. Additionally, the average and standard error of the mean of the pre-dawn water potential data and metabolite data were determined by treatment. Treatment differences were analysed by Student’s t-tests, with differences considered significant at P ≤ 0.05. Each drought treatment (acute or cyclic) was contrasted with its well-watered control. Thus, metabolite fold changes were reported relative to the control of each experiment.
RESULTS
Cyclic drought treatments
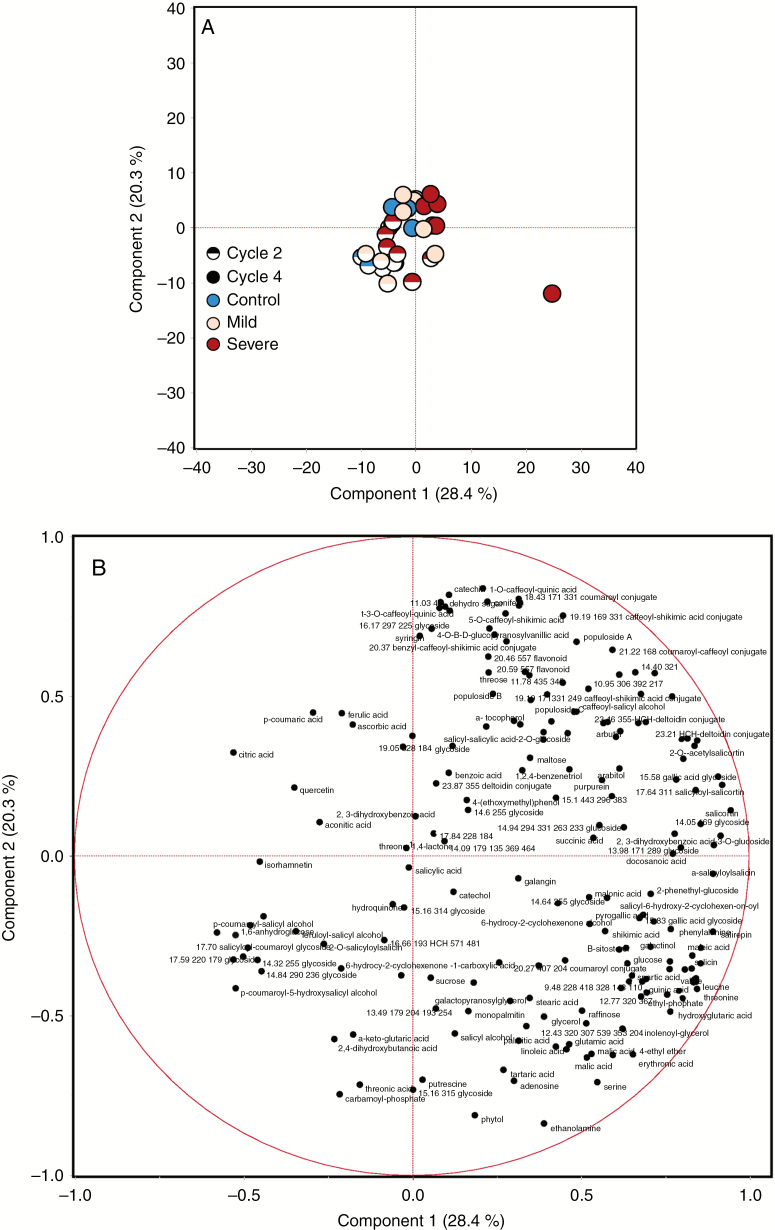
The PCA of the metabolite data of the cyclic drought experiment indicates discrete grouping between the two cycle data sets, as well variation across the water deficit severity (Fig. 2). Both PC1 and PC2 explain a high proportion of the variation in the multivariate analysis; 28.4 and 20.3 %, respectively. In general, the loading plots for the metabolite data of the cyclic drought indicate that PC1 is driven in the positive direction by higher order salicylates [e.g. salicortin, 17.64 min 311 salicyloyl-salicortin, hydroxycyclohexenoyl (HCH)-deltoidin, 21.47 min salicyloyl-HCH-deltoidin, isosalicin, salirepin, α-salicyloylsalicin, salicin, 23.31 min HCH-deltoidin conjugate] and amino acids (e.g. threonine, valine, leucine, isoleucine, tryptophan and glutamine), and in the negative direction by a number of flavonoids (e.g. kaempferol, isorhamnetin and quercetin) and a number of unidentified glycosides (specific loadings are given in Supplementary data Table S1). PC2 is driven in the positive direction by hydroxycinnamate conjugates, flavonoids, lignans and monolignol glucosides, and in the negative direction by simple organic acids, amino acids, other nitrogen-containing metabolites and fatty acids (Fig. 2; Supplementary data Table S1). Overall, soluble carbohydrates were the most abundant organic solutes in P. deltoides leaves, led by sucrose constituting about a third of the pool size in the control, followed by glucose, myo-inositol and fructose (Supplementary data Table S2). Also abundant were (1) organic acids, including citric acid and malic acid; and (2) phenolic glycosides, including salicortin, a HCH-deltoidin conjugate eluting at 23.31 min, populoside, α-salicyloylsalicin and HCH-deltoidin, with this latter class of metabolites having a major effect on sample clustering as described above.
Fig. 2.
Principal component analysis of the metabolite data of the cyclic drought experiment: (A) sample clustering in response to drought severity and number of drought cycles; and (B) loading plots for the specific metabolites.
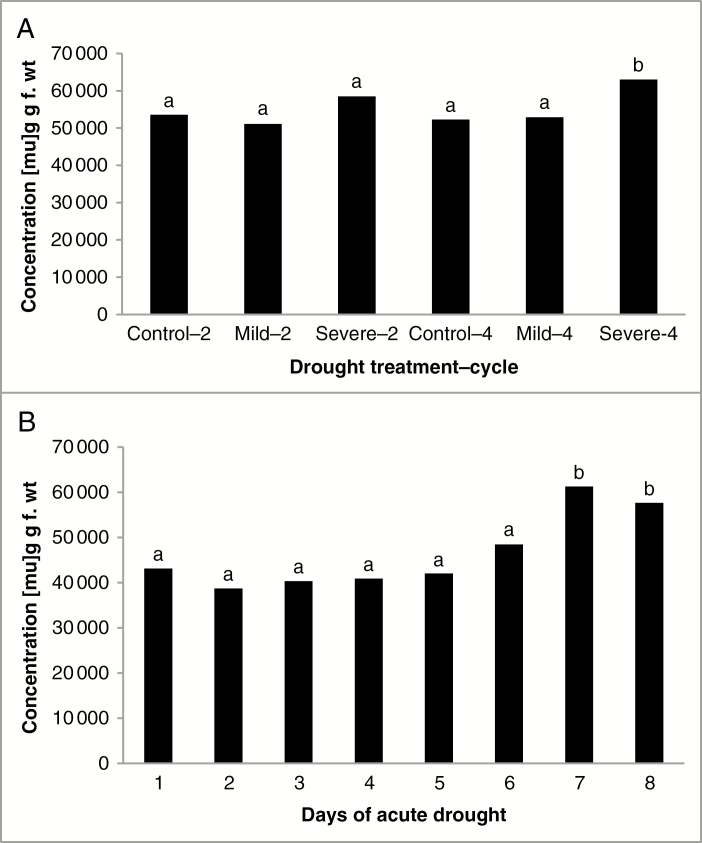
With respect to specific metabolite responses, after two cycles of mild drought, out of the 186 metabolites quantified, there was only one statistically significant accumulation relative to the well-watered control and that was a 1.47× increase in a partially identified coumaroyl glycoside eluting at 20.27 min with key m/z 407 204 (Supplementary data Table S2). There were, however, modest declines in several abundant metabolites, including sucrose (0.86×) and salicylic acid (0.76×). The largest declines were observed for 6-hydroxy-2-cyclohexenone-1-carboxylic acid (0.52×), 6-hydroxy-2-cyclohexenone alcohol (0.63×), both salicortin precursors or breakdown products; a number of hydroxycinnamates, including caffeic acid, p-coumaric acid and ferulic acid, and their salicylate conjugates, p-coumaroyl-5-hydroxysalicyl alcohol, p-coumaroyl-salicyl alcohol and feruloyl-salicyl alcohol that were all reduced to 0.48×–0.63×; and flavonoids, including galangin, quercetin, kaempferol and isorhamnetin that were reduced to 0.60×–0.66×. Despite these numerous declining components, the overall total concentration of metabolites did not differ from the well-watered control, nor did the total metabolites observed in the severe treatment after two cycles (Fig. 3). Conversely, raffinose, the galactose-containing oligosaccharide, was increased 2.50× in this latter treatment, and other accumulating identified metabolites included 5-oxo-proline (1.83×), ascorbic acid glucoside (1.51×), salicortin (1.38×) and gallic acid (1.27×) (Supplementary data Table S2). The metabolites that declined in this treatment were largely the same as in the mild treatment after two cycles, and the declines were of a similar magnitude.
Fig. 3.
Total organic solute concentration (μg g f. wt in sorbitol equivalents) as determined by gas chromatography–mass spectrometry for leaves of Populus deltoides ‘WV94’ subjected to (A) cyclic drought treatment or (B) acute drought treatment. The cyclic drought treatments are designated by the number of drought cycles, and the data are the means (control treatment with four replicates; mild and severe treatments with six replicates). The acute drought treatments are designated by day since the last watering, with day1 being the well-watered control with three replicates for each treatment. Treatments with different letters are significantly different (P ≤ 0.05) from the respective well-watered control treatment.
Although the total organic solutes in the mild drought treatment after four cycles was again not significantly different from the controls (Fig. 3), specific metabolites were elevated, including two lipid-related metabolites, monogalactosylglycerol (2.12×) and digalactosylglycerol (1.66×), as well as the aforementioned coumaroyl glycoside eluting at 20.27 min with key m/z 407 204 (1.80×) (Supplementary data Table S2). Other notable components accumulating were putrescine (1.29×) and salicortin (1.20×). Many of the same metabolites that declined after two drought cycles also declined after four cycles of mild drought, but the magnitude of the declines was not great. It was only in the severe treatment after four drought cycles that the total metabolite concentration was significantly greater (1.20×) than that of the control (Fig. 3). The largest accumulations were observed for soluble carbohydrates, including maltose (8.63×), fructose (3.35×), galactose (3.06×), galactinol (2.67×) and glucose (2.52×) (Supplementary data Table S2). Other metabolites that were also increased included quinic acid (2.60×), digalactosylglycerol (1.84×), succinic acid (1.83×) and ascorbic acid glucoside (1.50×). Again, the same metabolites declined in this treatment as in the others, but the declines were not as great. These included declines in p-coumaroyl-salicyl alcohol (0.57×), p-coumaroyl-5-hydroxysalicyl alcohol (0.68×), quercetin (0.68×) and kaempferol (0.75×) (Supplementary data Table S2).
Acute drought treatments
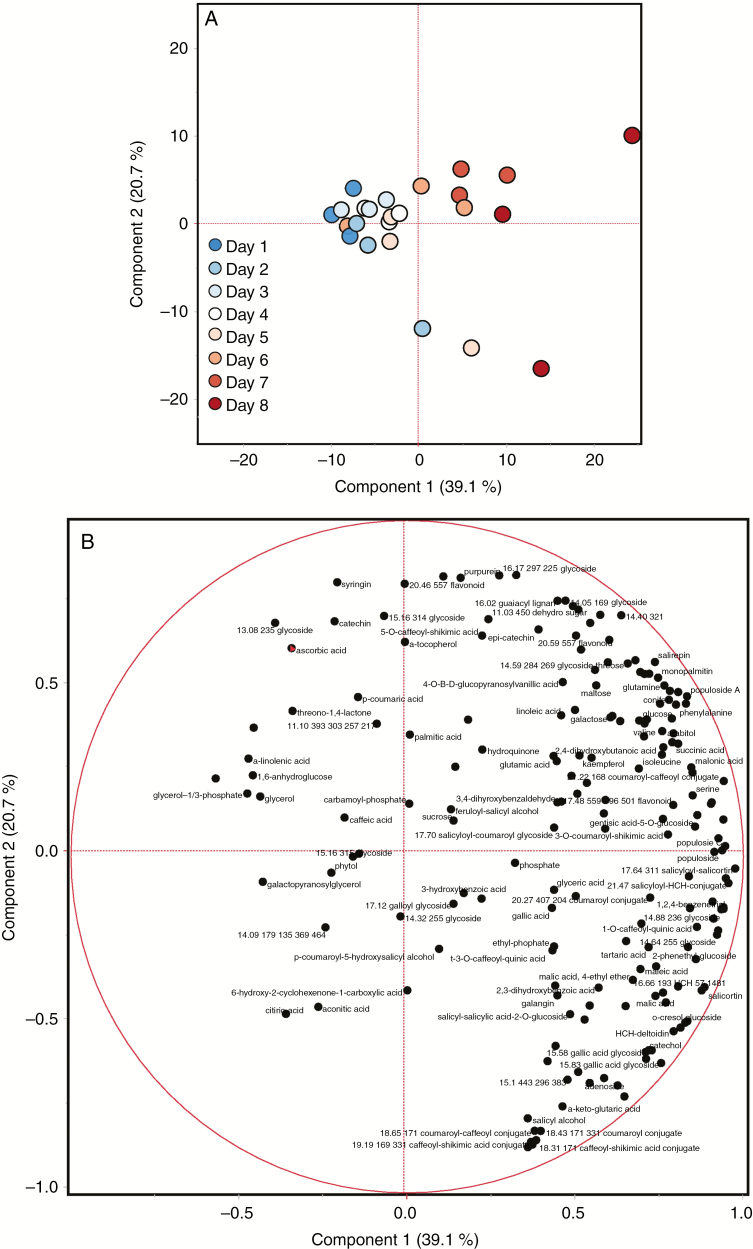
Although the pre-dawn water potential differed from the controls on days 5, 7 and 8, the total metabolite concentration differed from the well-watered control only on days 7 and 8 (Fig. 3), when pre-dawn water potential had dropped to below –0.6 MPa (Fig. 1). As observed in the cyclic drought study, PCA indicates that both PC1 and PC2 explain a high proportion of the variation in the multivariate analysis of the metabolite data in the acute drought study; 39.1 and 20.7 %, respectively, with sample clustering largely dependent on PC1 corresponding to the variation observed across water severity, and with days 7–8 showing the highest variability (Fig. 4). In general, the loading plots for the metabolite data of the acute drought experiment indicate that PC1 is driven in the positive direction by higher order salicylates and their related hydroxycinnamate conjugates, the populosides and their precursors (Fig. 4; Supplementary data Table S1). The former was also evident under cyclic drought, but the latter was not. PC1 is driven in the negative direction by fatty acid-related metabolites (e.g. digalactosylglycerol, glycerol-1/3-phosphate, α-linolenic acid, glycerol and galactopyranosylglycerol) and organic acids (e.g. citric acid, ascorbic acid and aconitic acid). PC2 is driven in the positive direction by glucosides (e.g. ascorbic acid glucoside, grandidentatin, purpurein, syringin and arbutin) and other unidentified glycosides, and in the negative direction by a number of partially identified caffeoyl-shikimate conjugates and closely related hydroxycinnamate conjugates (Fig. 4; Supplementary data Table S1).
Fig. 4.
Principal component analysis of the metabolite data of the acute drought experiment: (A) sample clustering in response to drought severity over time; and (B) loading plots for the specific metabolites.
Overall, the total metabolite concentration peaked at day 7 at 1.42× that of the well-watered control and was not as great a difference on day 8 at 1.34× (Fig. 3), but these osmotic adjustments were much greater than the 1.20× response observed for the severe treatment after four drought cycles. Although total metabolites did not differ until day 7 (Fig. 3), specific metabolite responses were evident early in the acute drought treatment (Supplementary data Table S3). As early as day 2, populoside A was detected and significantly differed from the control, which did not have detectable levels of any of the populosides, other than populoside itself, the most abundant of these hydroxycinnamate conjugates of salicin, which was elevated 6.73×. Other phenolics that were increased included a flavonoid at retention time 17.48 min with key m/z 559 496 501 (3.81×), α-salicyloylsalicin (3.30×), caffeoyl-salicyl alcohol (3.10×), a likely precursor of populoside, 17.59 min m/z 220 179 glycoside (2.99×), and isorhamnetin (1.77×). It is interesting to note also that phosphate was elevated 1.50× on day 2, and later on days 4 and 8 at a similar magnitude. There were not many declining metabolites, but the greatest decline was in the flavonoid catechin (0.37×), followed by ferulic acid (0.42×), aconitic acid (0.52×), ascorbic acid (0.67×) and a related metabolite, threono-1,4-lactone (0.68×).
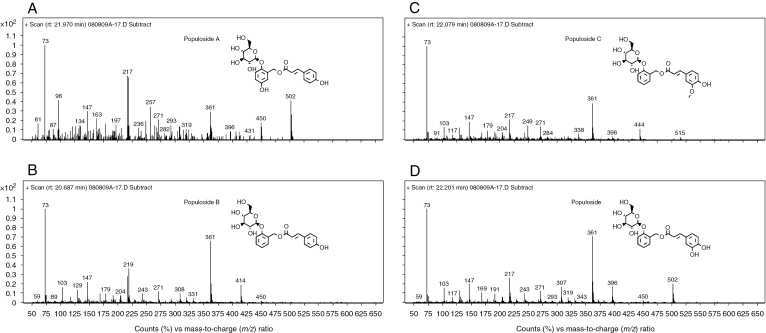
As the acute drought progressed through day 8, a consistent pattern emerged, with the large-scale accumulation of the populosides and numerous higher order salicylates and hydroxycinnamate conjugates (Supplementary data Table S3). Populosides are hydroxycinnamate conjugates of salicin. Specifically, populoside is caffeoylsalicin and was the most abundant of this class of metabolite in P. deltoides. Populosides A, B and C are coumaroyl-5-hydroxysalicin, coumaroylsalicin and feruloylsalicin, respectively. They all exist in P. deltoides leaves as the more abundant trans isomer and the less abundant cis isomer. The concentrations of populosides (Supplementary data Tables S2 and S3) include both the cis and trans isomers pooled together. The structures of each of these populosides and the fragmentation patterns of their trans isomers are depicted in Fig. 5. Populosides were readily detected by the presence of their stable aglycones, m/z 502 in the case of populoside and populoside A, m/z 414 for populoside B and m/z 444 for populoside C, as were their aglycone precursors that eluted earlier in the runs. The fold change of populoside increased over time to peak at 57.93× by day 8 of acute drought, and a similar response was evident for the other populosides, with the next most abundant being populosides B, C and finally A, with all of these not detectable in the well-watered control plants. Also present in high concentration and displaying large fold changes were several late-eluting, partially identified HCH-deltoidin conjugates, including metabolites 23.31 min HCH-deltoidin conjugate, 23.46 min m/z 355 HCH-deltoidin conjugate (23.75×), 23.69 min m/z 283 salicyloyl-HCH-deltoidin conjugate (27.03×) and 23.87 min m/z 355 deltoidin conjugate (26.42×), with the latter three probably conjugated to a dihydroxybenzoic acid generating m/z 283 and m/z 355 (Supplementary data Table S3). These metabolites are all higher order salicylate conjugates. Other closely related metabolites that displayed high fold changes late (day 8) in the acute drought included 2-O-acetylsalicortin (16.78×), 21.47 min salicyloyl-HCH conjugate (14.98×), caffeoyl-salicyl alcohol (10.21×), salicortin (7.08×), α-salicyloylsalicin (5.12×) and HCH-deltoidin (4.19×) (Supplementary data Table S3). In addition to higher order salicylates and their conjugates with hydroxycinnamates, several hydroxycinnamate conjugates also had large fold changes, including 21.22 min m/z 168 coumaroyl-caffeoyl conjugate (14.97×), 19.64 min m/z 171 caffeoyl-shikimic acid conjugate (4.12×) and 20.27 min m/z 407 204 coumaroyl glycoside (2.98×), the same metabolite that accumulated in cyclic drought.
Fig. 5.
Structure and electron impact ionization (70 eV) fragmentation pattern of trimethylsilyl-derivatized (A) populoside A, (B) populoside B, (C) populoside C and (D) populoside. In each case, the most abundant high mass-to-charge ratio corresponds to the aglycone of each metabolite.
For the primary carbon metabolism compounds, several of which responded to cyclic drought, many also responded to acute drought, but their fold changes were overwhelmed by that of the classes of aromatic metabolites just described. Although few nitrogenous metabolites responded to cyclic drought, several amino acids responded to acute drought. Such responsive primary carbon and nitrogen metabolism compounds included isoleucine (7.10×), valine (5.60×), raffinose (4.19×), threonine (4.09×), fructose (3.48×), galactose (3.05×), quinic acid (2.78×), 5-oxo-proline (2.59×), glucose (1.98×), docosanoic acid (1.96×) and serine (1.92×) (Supplementary data Table S3). Overall, there were few metabolites that declined under severe drought, but those that did were similar to those observed under cyclic drought. These included ferulic acid (0.29×), 1,6-anhydroglucose (0.30×), catechin (0.33×), aconitic acid (0.44×), threono-1,4-lactone (0.49×), ascorbic acid (0.55×), caffeic acid (0.61×) and three lipid-related metabolites, namely glycerol-1/3-phosphate (0.61×), glycerol (0.62×) and α-linolenic acid (0.64×) (Supplementary data Table S3).
DISCUSSION
Cyclic drought induced osmotic adjustment once the severe stress threshold of ≤ –1.0 MPa was achieved after several stress cycles, as expected. The cycling of stress is thought to provide the opportunity to allow osmotic adjustment to occur if the capacity for adjustment exists in the genotype. Our previous field study in an F2 pedigree of P. trichocarpa × P. deltoides family 331 containing 59 genotypes demonstrated that osmotic adjustment was relatively rare, with only 15 % of the genotypes displaying adjustment to reduced irrigation at a hot, dry site east of the Cascade Mountain Range near Boardman, OR (Tschaplinski et al., 2006). Nonetheless, the capacity for osmotic adjustment was attributed to the P. deltoides ‘ILL-129’ male grandparent, which maintained low osmotic potentials at full turgor (–1.9 MPa) that were as much as approx. 0.4 MPa lower than those of the P. trichocarpa female grandparent, and hence was much more drought tolerant. Interestingly, many clones showed a shift to higher (less negative) osmotic potentials in the dry treatment, indicating a decline in foliar metabolite concentrations and lessened overall drought tolerance capacity under the severe stress conditions that also included heat stress. In this study, a direct link between organic solute accumulation, as determined by GC-MS-based metabolite profiling, with the lowering of osmotic potential is made because P. deltoides is a glycophytic species, wherein the bulk of its solute potential and its adjustment in leaves are determined by changes in organic solute concentrations (Tschaplinski and Tuskan, 1994, and other citations, below).
The nature of the organic solutes that accumulated in the present study was similar to what has been frequently reported, wherein the bulk of the osmotic adjustment resulted from accumulation of soluble sugars and organic acids (Tschaplinski and Blake, 1989; Tschaplinski and Tuskan, 1994; Gebre et al., 1998; Marron et al., 2002). Maltose displayed the largest fold change, suggesting that starch mobilization, which has been demonstrated previously for P. nigra drought response (Regier et al., 2009), fuelled the maltose accumulation. Subsequent maltose metabolism probably contributed to the observed accumulation in monosaccharides which were the next biggest contributors to osmotic adjustment. Similarly, metabolism of the monosaccharides probably led to the accumulation of the tricarboxylic acid (TCA) cycle organic acids. Overall, osmotic adjustment to cyclic drought was largely constituted from primary carbon metabolism as observed previously (Tschaplinski and Tuskan, 1994). An evident consequence of the increased carbon partitioning to primary metabolites under cyclic drought was a decline in a number of secondary metabolites, including several flavonoids, hydroxycinnamates of the lignin biosynthetic pathway and precursors of salicortin that together had a negative effect on the total osmotic adjustment and was also evident in the earlier drought cycles.
Although a case can be made that the application of acute drought does not provide an opportunity to allow the expression of the innate osmotic adjustment capacity, the greater osmotic adjustment in organic solutes after seven consecutive days of drought vs. that observed after four drought cycles allowed us to reject our working hypothesis that the largest osmotic adjustment would be observed following cyclic stress. Whereas both cyclic and acute drought had similar increases in soluble sugars and organic acids of primary carbon metabolism, acute drought induced a large-scale accumulation of phenolic metabolites of secondary carbon metabolism that was not observed in response to cyclic drought, nor was the accumulation of several amino acids that was also observed under acute drought. Drought-tolerant P. euphratica also primarily accumulated amino acids when growing in the driest and most saline soils of its native environment, but most of the major carbon metabolites were non-responsive, with the exception of glyceric acid, glycerol and myo-inositol (Brosché et al., 2005), indicating that osmotic adjustment was limited and not the key trait underlying its drought tolerance. Accumulation of phenolics in response to drought in the drought-tolerant Populus × euramericana Dorskamp clone has been reported (Marron et al., 2002), but the specific metabolites involved were not characterized.
In the present study, the largest accumulations were observed in the populosides (in response to acute drought), which are conjugates of salicin with hydroxycinnamates of the lignin biosynthetic pathway. As such, these metabolites are generated from two unrelated pathways of secondary carbon metabolism. It could be argued that these conjugates could arise as a result of simple conjugation from accumulating pools of upstream lignin precursors. Such a simple explanation is unlikely, as it would be expected to be widespread across Populus spp., all of which produce salicin and the hydroxycinnamates, but few of which are reported to produce populosides. In addition to being detected in the leaves of P. deltoides in this study, they have been reported to be produced in the bark of some Populus species, including P. grandidentata (Erickson et al., 1970), P. davidiana (Zhang et al., 2006) and P. nigra (Lee et al., 2010). However, a recent report confirms the presence of populosides in leaves of both P. deltoides and P. grandidentata as detected by leaf spray ionization tandem mass spectrometry (Snydera et al., 2015). Populosides were not observed in the >850 P. trichocarpa genotypes that we have recently analysed (unpubl. data). In addition to detecting large fold changes in populosides in response to acute drought in the present study, their aglycone precursors were also detected, including p-coumaroyl-salicyl alcohol, p-coumaroyl-5-hydroxysalicyl alcohol, trace amounts of feruloyl-salicin and caffeoyl-salicyl alcohol. These data suggest that the populosides are synthesized first by the conjugation of the aromatic moieties, followed by glucosyl conjugation. Whereas it could be argued that the accumulation of populosides was an artefact of perturbed metabolism under acute drought, this is unlikely given that the capacity for synthesizing populosides is inherent in P. deltoides, and that their presence was evident under milder, cyclic drought stress and in the early stages of acute drought, but it was only under severe, acute drought that their concentrations greatly increase. The results of this study and others together suggest that Populus species containing populosides must first be able to couple the aromatics, and then have a unique glucosyl transferase that conjugates the aromatic conjugates to glucose, allowing their accumulation.
In addition to glucose and fructose of primary carbon metabolism that heavily contributed to the total osmotic adjustment observed in both cyclic and acute drought in P. deltoides leaves, the large fold change responses of populosides, higher order salicylate conjugates of secondary carbon metabolism, acute drought also elicited large-scale accumulation of other higher order salicylates of secondary carbon metabolism. In addition to salicortin, salicin and α-salicyloylsalicin that are widely observed across Populus spp. (Pearl and Darling, 1971; Abreu et al., 2013; Payyavula et al., 2014; Tschaplinski et al., 2014; Keefover-Ring et al., 2014; Feistel et al., 2015) and were moderately responsive to acute drought, P. deltoides contained a number of relatively unique metabolites, including deltoidin (2-O-salicyloylsalicin) and HCH-deltoidin that were moderately responsive; and four partially identified, late-eluting (23–24 min retention times) conjugates of deltoidin and HCH-deltoidin that were highly responsive to acute drought. All four metabolites are conjugated to an unidentified dihydroxybenzoic acid, but it is most likely to be 2,5-dihydroxybenzoic acid (gentisic acid) or 2,3-dihydroxybenzoic acid, the most abundant dihydroxybenzoic acids in P. deltoides, including their glucosides. These metabolites elute in a region of the GC-MS chromatogram that is similar to, albeit later than, where tremulacin that is often found in Populus spp. is located, but tremulacin was not detected in P. deltoides leaves. The absence of tremulacin in P. deltoides leaves was adeptly reported by Lindroth et al. (1987). Instead, P. deltoides accumulates conjugates of deltoidin and HCH-deltoidin in leaves as complex higher order salicylates. Is this large-scale accumulation of complex aromatic metabolites under acute drought truly adaptive or indicative of perturbed metabolism leading to foliar senescence? Given that these aromatic metabolites steadily accumulated with the progression of acute drought with increased carbon partitioning to secondary metabolism, we infer that this adjustment is adaptive, leading to increased organic solute accumulation and a lowering of osmotic potential that increases drought tolerance. It could be argued that maximum solute accumulation would favour the accumulation of the smaller conjugates (e.g. deltoidin and HCH-deltoidin) vs. their later-eluting, larger higher order deltoidin conjugates, but the larger conjugates may be a detoxification reaction that accommodates the continued enhanced production of aromatic metabolites under continued drought progression. The fact that these higher order deltoidin conjugates are normally produced under well-watered conditions also argues against these reactions being strictly the result of metabolic perturbation.
Solute accumulation leading to osmotic adjustment in response to drought lowers the osmotic potential of leaves and enhances the ability to extract water from drying soils (Kramer, 1980; Tschaplinski et al., 1998a). Significant osmotic adjustments were only observed when drought stress was severe, whether induced by cyclic or acute stress. Such osmotic adjustments are thought to enhance post-stress recovery and are characteristic of dehydration-tolerant species (Kramer, 1980). The increased concentrations and diversity of aromatic metabolites in leaves under acute drought have potential consequences on plant–microbe interactions, particularly with respect to beneficial endophytes that must now tolerate a very different milieu of constituents, which has become potentially more inhibitory or toxic to microbes than what was present under well-watered conditions. Similarly, with populosides being astringent compounds, their large-scale accumulation in severely drought stressed plants is likely to increase the foliar defence against herbivory.
In summary, the nature of drought, frequency of drought and the severity of drought dictated the degree of osmotic adjustment and the nature of the organic solutes that accumulated. Acute onset of prolonged, severe drought induced the greatest accumulation of foliar metabolites observed. Cyclic drought induced the largest responses in primary carbon metabolism (sugars and organic acids). Acute drought induced the largest responses in secondary carbon metabolism, especially conjugates of hydroxycinnamates with salicin, leading to the accumulation of populosides. Also accumulating under acute drought are numerous higher order salicylates, such as salicortin and α-salicyloylsalicin, and deltoidin and its conjugates with HCH, salicylic acid, salicyl alcohol and probably gentisic acid, yielding a unique suite of constituents in the leaves of P. deltoides. In conclusion, the extent of metabolic plasticity was evident in the leaves of P. deltoides under acute drought, which adaptively led to the accumulation of a large number of metabolites from diverse pathways of primary and secondary carbon metabolism, but with aromatic metabolites, including salicin conjugates of hydroxycinnamates (populosides) and higher order salicylates (e.g. deltoidin and its conjugates) particularly responsive, contributing to lowering the osmotic potential and increasing drought tolerance.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: PCA loadings for specific metabolites in response to cyclic drought and acute drought. Table S2: metabolite profiles of Populus deltoides leaves subjected to two or four cycles of mild or severe drought vs. the well-watered control plants. Table S3: metabolite profiles of Populus deltoides leaves subjected to acute drought vs. the well-watered control plants.
ACKNOWLEDGEMENTS
This work was supported by the Plant-Microbe Interfaces Science Focus Area and the Center for Bioenergy Innovation (CBI, FWP ERKP886), which were both funded by the US Department of Energy, Office of Science, Biological and Environmental Research. This manuscript has been authored by UT-Battelle, LLC under Contract no. DE-AC05-00OR22725 with the US Department of Energy.
The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide licence to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
LITERATURE CITED
- Abraham P, Yin H, Borland AM, et al. 2016. Transcript, protein and metabolite temporal dynamics in the CAM plant Agave. Nature Plants 2: doi: 10.1038/nplants.2016.178. [DOI] [PubMed] [Google Scholar]
- Abraham PE, Garcia BJ, Gunter LE, et al. 2018. Quantitative proteome profile of water deficit stress responses in eastern cottonwood (Populus deltoides) leaves. PLoS One 13: e0190019. doi: 10.1371/journal.pone.0190019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu IN, Ahnlund M, Moritz T, Albrectsen BR. 2013. UHPLC-ESI/TOFMS determination of salicylate-like phenolic gycosides in Populus tremula leaves. Journal of Chemical Ecology 37: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosché M, Vinocur B, Alatalo ER, et al. 2005. Gene expression and metabolite profiling of Populus euphratica growing in the Negev desert. Genome Biology 6: R101. doi.org/10.1186/gb-2005-6-12-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RL, Pearl IA, Darling SF. 1970. Populoside and grandidentoside from the bark of Populus grandidentata. Phytochemistry 9: 857–863. [Google Scholar]
- Feistel F, Paetz C, Lorenz S, Schneider B. 2015. The absolute configuration of salicortin, HCH-salicortin and tremulacin from Populus trichocarpa × deltoides Beaupré. Molecules 20: 5566–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebre GM, Tschaplinski TJ, Tuskan GA, Todd DE. 1998. Clonal and seasonal differences in leaf osmotic potentials and organic solutes of five hybrid poplar clones grown under field conditions. Tree Physiology 18: 645–652. [DOI] [PubMed] [Google Scholar]
- Keefover-Ring K, Ahnlund M, Abreu IN, Jansson S, Moritz T, Albrectsen BR. 2014. No evidence of geographical structure of Salicinoid chemotypes within Populus tremula. PLoS One 9: e107189. doi: 10.1371/journal.pone.0107189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PJ. 1980. Drought stress and the origin of adaptations. In: Turner NC, Kramer PJ, eds. Adaptation of plants to water and high temperature stress. New York: Wiley, 7–22. [Google Scholar]
- Lee YS, Cui C-B, Kim JK, et al. 2010. Inhibitory effect of populoside from the bark of Populus nigra on aldose reductase. Journal of the Korean Society for Applied Biological Chemistry 53: 729–733. [Google Scholar]
- Lindroth RL, Hsia MTS, Scriber JM. 1987. Seasonal patterns in the phytochemistry of three Populus species. Biochemical Systematics and Ecology 15: 687–686. [Google Scholar]
- Marron N, Delay D, Petit JM, et al. 2002. Physiological traits of two Populus × euramericana clones, Luisa Avanzo and Dorskamp, during a water stress and re-watering cycle. Tree Physiology 22: 849–858. [DOI] [PubMed] [Google Scholar]
- Payyavula RS, Tschaplinski TJ, Jawdy SS, Sykes RW, Tuskan GA, Kalluri UC. 2014. Metabolic profiling reveals altered sugar and secondary metabolism in response to UGPase overexpression in Populus. BMC Plant Biology 14: 265. doi: 10.1186/s12870-014-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl I, Darling S. 1971. Studies of the hot water extractives of the bark and leaves of Populus deltoides Bartr. Canadian Journal Chemistry 49: 49–55. [Google Scholar]
- Pezeshki SR, Hinckley TM. 1982. The stomatal response of red alder and black cottonwood to changing water status. Canadian Journal Forest Research 12: 761–71. [Google Scholar]
- Regier N, Streb S, Cocozza C, et al. 2009. Drought tolerance of two black poplar (Populus nigra L.) clones: contribution of carbohydrates and oxidative stress defence. Plant, Cell & Environment 32: 1724–1736. [DOI] [PubMed] [Google Scholar]
- Silim S, Nash R, Reynard D, White B, Schroeder W. 2009. Leaf gas exchange and water potential responses to drought in nine poplar (Populus spp.) clones with contrasting drought tolerance. Trees 23: 959–969. [Google Scholar]
- Snydera DT, Schilling MC, Hochwender CG, Kaufman AD. 2015. Profiling phenolic glycosides in Populus deltoides and Populus grandidentata by leaf spray ionization tandem mass spectrometry. Analytical Methods 7: 870–876. [Google Scholar]
- Tschaplinski TJ, Blake TJ. 1989. Water stress tolerance and late-season organic solute accumulation in hybrid poplar. Canadian Journal of Botany 67: 1681–1688. [Google Scholar]
- Tschaplinski TJ, Tuskan GA. 1994. Water-stress tolerance of black cottonwood and eastern cottonwood clones and four of their hybrid progeny. II. Metabolites and inorganic ions that constitute osmotic adjustment. Canadian Journal of Forest Research 24: 681–687. [Google Scholar]
- Tschaplinski TJ, Stewart DB, Norby RJ. 1995. Interactions between drought and elevated CO2 on osmotic adjustment and solute concentrations of tree seedlings. New Phytologist 131: 169–177. [DOI] [PubMed] [Google Scholar]
- Tschaplinski TJ, Gebre GM, Shirshac TL. 1998a. Osmotic potential of several hardwood species as affected by throughfall manipulation of an upland oak forest during a dry year. Tree Physiology 18: 291–298. [DOI] [PubMed] [Google Scholar]
- Tschaplinski TJ, Tuskan GA, Gebre GM, Todd DE. 1998b. Drought resistance of two hybrid Populus clones grown in a large-scale plantation. Tree Physiology 18: 653–8. [DOI] [PubMed] [Google Scholar]
- Tschaplinski T, Tuskan GA, Sewell MM, Gebre GM, Todd DE, Pendley CD. 2006. Phenotypic variation and QTL identification for osmotic potential in an interspecific hybrid inbred F2 poplar pedigree growing under contrasting environments. Tree Physiology 26: 595–604. [DOI] [PubMed] [Google Scholar]
- Tschaplinski TJ, Standaert RF, Engle NL, et al. 2012. Down-regulation of the caffeic acid O-methyltransferase gene in switchgrass reveals a novel monolignol analog. Biotechnology for Biofuels 5: 71. doi: 10.1186/1754-6834-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschaplinski TJ, Plett JM, Engle NL, et al. 2014. Populus trichocarpa and Populus deltoides exhibit different metabolomic responses to colonization by the symbiotic fungus Laccaria bicolor. Molecular Plant-Microbe Interactions 27: 546–556. [DOI] [PubMed] [Google Scholar]
- Tuskan GA. 1998. Short-rotation forestry in hte United States: what we know and what we need to know. Biomass and Bioenergy 14: 307–315. [Google Scholar]
- Tuskan GA, Walsh M. 2001. Short-rotation woody crop systems, atmospheric carbon dioxide and carbon management. Forestry Chronicle 77: 259–264. [Google Scholar]
- Viger M, Smith HK, Cohen D, et al. 2016. Adaptive mechanisms and genomic plasticity for drought tolerance identified in European black poplar (Populus nigra L.). Tree Physiology 36: 909–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Thuong PT, Min BS, et al. 2006. Phenolic glycosides with antioxidant activity from the stem bark of Populus davidiana. Journal of Natural Products 69: 1370–1373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.