Improved oncology treatment has dramatically increased the number of long-term cancer survivors, but the potential cardiovascular side effects from the cancer treatment are increasing their morbidity and premature mortality.1 This is particularly true for paediatric cancers patients who are commonly treated with anthracycline chemotherapy (AC) and/or radiotherapy to the chest (RT), because the developing children’s heart appears more sensitive to the cardiotoxic effects.2,3 Acute (early-onset) AC-induced cardiotoxicity (AIC) interferes with optimal cancer treatment if interruptions or changes to cancer treatment are required.
However, for paediatric cancer survivors the major clinical problem is cardiovascular disease years after treatment [late-onset cardiotoxicity (LOC)]. Second malignancies are also more common in paediatric cancer survivors, further increasing the risk for additional cardiotoxic cancer therapies in patients with prior cardiac injury.4 Late heart failure following anthracyclines is particularly treatment-resistant and is associated with a poor prognosis. Therefore, recent guidelines recommend lifelong follow-up of paediatric cancer survivors and especially of those at risk for cardiovascular toxicity.5
Currently, the clinical approach is to treat heart failure after it has presented. However, if we can develop an improved understanding of the molecular pathophysiology then the opportunity to treat and prevent heart failure in cancer survivors becomes a new option. In this regard, toxic mitochondria iron accumulation is induced by AC in cardiomyocytes and dexrazoxane has been shown to protect cardiomyocytes from doxorubicin induced toxicity (Figure 1).6 Dexrazoxane has recently been approved for use in paediatric patients7 and a recent systematic review in paediatric cancer survivors treated with anthracyclines suggests that administration of dexrazoxane may also reduce LOC.8
Figure 1.
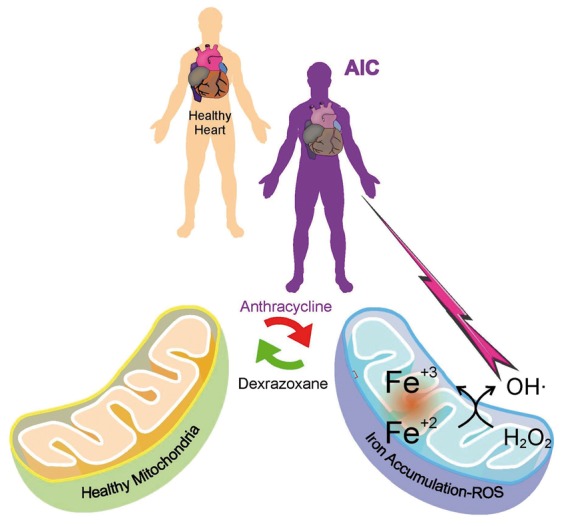
Mitochondria with and without Iron.
The dose of AC and/or RT, the patient’s age and cancer type, treatment duration, and disease relapse are risk factors for LOC.8 There is no proven diagnostic test to identify patients with higher risk LOC prior its clinical manifestation, although emerging data suggests reduction in global longitudinal strain on speckle echocardiography in paediatric cancer survivors may precede heart failure presentation. Furthermore, also knowledge on additional ‘second hit’ risk factors that may trigger clinical manifestation of LOC.
In addition to the risk associated with the growing heart in children, genetic risk factors may play an important role. These include cardiomyopathy-related genes or therapy-induced changes in metabolic pathways leading to prolonged and/or higher serum concentrations of toxic chemotherapeutic substances. Genome-wide association studies have shown variants in the retinoic acid receptor gamma (rarg), cugbp elav-like family member 4 (celf4) as possible genetic factors predisposing to cardiac damage following anthracycline therapy.9,10 Several other studies have focused on the identification of genetic predictors using a candidate gene-based approach11 showing for example that polymorphism in the carbonyl reductase genes (cbr3)12 and (cbr4),3electron transfer flavoprotein beta subunit (etfb),13G protein-coupled receptor 35 (gpr35),14 and hyaluronan synthase 3 (has3)3 may influence genetic predisposition for LOC in paediatric cancer survivors.
Recent work has demonstrated that human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) accurately recapitulate a patient’s predisposition to AIC.15 This exciting finding confirms that AIC has a genomic basis and that hiPSC-CM can be used for elucidating the genetic variants responsible. In addition, genetic predisposition to tumour disease may also impact on the risk for LOC. For example, the tumour suppressor breast cancer1 (brca1), which is mutated in early onset familial breast and ovarian cancer and plays an important role in DNA repair mechanisms of the cardiomyocyte.16 Mutations in such genes may on the one hand lead to cancer but may also affect the response of the cardiomyocyte to cardiotoxic treatment, as it may play a role in anti-tumour therapy induced damage and damage repair, thereby modulate the risk for LOC. It has also been demonstrated that erythropoietin may promote repair mechanisms in the heart, especially in cardiac progenitor cells,17 thereby reduce the risk for LOC. Recent findings indicate that specific pathways including PI3Kγ, can be up-regulated in the heart after anthracycline therapy and may contribute to heart failure development which not only involve cardiomyocytes but also inflammatory cells (leucocytes), thus potentially providing a new strategy to develop biomarkers for AIC and LOC risks.18
Lifestyle measures are also important factors determining the risk for late onset cardiotoxicity. For example, exercise is beneficial in cancer patients during and after chemotherapy and may reduce the risk for LOC.19 This is particularly important as childhood cancer survivors are less physically active than the general population and adult survivors are more likely to report a sedentary lifestyle than their siblings.20 In addition, future cardiovascular stress insults may trigger cardiovascular disease at a lower threshold in patients previously treated with cardiotoxic chemotherapy or RT.
One common and important issue specifically for female paediatric cancer survivors, especially with the advances in oocyte preservation and reproductive science, is pregnancy. Pregnancy induces high mechanical and neurohormonal stress on the maternal cardiovascular system and needs complex signalling networks to prevent damage of the heart.21 Impairment of these cardioprotective networks, which includes pathways typically targeted by anti-cancer treatment, such as Vascular Endothelial Growth Factor A (VEGFA), Signal Transducer And Activator Of Transcription 3 (STAT3), or Neuregulin-1 (Nrg1) signalling, lead to peripartum cardiomyopathy.22 Indeed, there is accumulating evidence that anti-cancer treatment could predispose women for peripartum heart failure later in life.23,24
The appearance of AIC and LOC in cancer survivors together with the complexity of acute cardiovascular toxicity from many novel cancer therapies has led to the development of cardio-oncology as a new medical subspecialty, with specialist cardio-oncology centres created to address this growing medical problem.25 New strategies to detect toxicity earlier with sensitive cardiac biomarkers and imaging are being implemented.26 However, to date the molecular mechanisms of late onset cardiotoxicity are incompletely understood and no biomarkers are available for monitoring and prediction for LOC disease risk and no approved specific co-treatments to prevent LOC are available. Exploring both the mechanisms of acute and long-term cardiovascular side effects of anti-tumour therapies, to develop early markers and preventive and curative treatment is needed not only to reduce cancer disease but to improve morbidity and mortality of cancer survivors.
We believe this can be achieved by combined interdisciplinary efforts of clinicians and scientist from oncology and cardiology working together in cardio-oncology science.

Conflict of interest: A.R.L. has received speaker, advisory board or consultancy fees and/or research grants from Pfizer, Novartis, Servier, Amgen, Clinigen Group, Takeda, Roche, Eli Lily, Eisai, Bristol Myers Squibb, Ferring Pharmaceuticals and Boehringer Ingelheim. E.H. is a co-founder of Kither Biotech an academic spin-off focusing on PI3K inhibitors. The other authors have nothing to disclose.
References
References are available as supplementary material at European Heart Journal online.
Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


