Abstract
Background and Aims
Hydraulic and chemical signals operate in tandem to regulate systemic plant responses to drought. Transport of abscisic acid (ABA) through the xylem and phloem from the root to shoot has been suggested to serve as the main signal of water deficit. There is evidence that ABA and its ABA-glycosyl-ester (ABA-GE) are also formed in leaves and stems through the chloroplastic 2-C-methylerythritol-5-phosphate (MEP) pathway. This study aimed to evaluate how hormonal and hydraulic signals contribute to optimize stomatal (gs), mesophyll (gm) and leaf hydraulic (Kleaf) conductance under well-watered and water-stressed conditions in Populus nigra (black poplar) plants. In addition, we assessed possible relationships between ABA and soluble carbohydrates within the leaf and stem.
Methods
Plants were subjected to three water treatments: well-watered (WW), moderate stress (WS1) and severe stress (WS2). This experimental set-up enabled a time-course analysis of the response to water deficit at the physiological [leaf gas exchange, plant water relations, (Kleaf)], biochemical (ABA and its metabolite/catabolite quantification in xylem sap, leaves, wood, bark and roots) and molecular (gene expression of ABA biosynthesis) levels.
Key Results
Our results showed strong coordination between gs, gm and Kleaf under water stress, which reduced transpiration and increased intrinsic water use efficiency (WUEint). Analysis of gene expression of 9-cis-epoxycarotenoid dioxygenase (NCED) and ABA content in different tissues showed a general up-regulation of the biosynthesis of this hormone and its finely-tuned catabolism in response to water stress. Significant linear relationships were found between soluble carbohydrates and ABA contents in both leaves and stems, suggesting a putative function for this hormone in carbohydrate mobilization under severe water stress.
Conclusions
This study demonstrates the tight regulation of the photosynthetic machinery by levels of ABA in different plants organs on a daily basis in both well-watered and water stress conditions to optimize WUEint and coordinate whole plant acclimation responses to drought.
Keywords: Abscisic acid (ABA), ABA-GE, bioenergy crop, gene expression, intrinsic water-use efficiency (WUEint), water deficit, leaf gas exchange, leaf hydraulic conductance, 9-cis-epoxycarotenoid dioxygenase (NCED), Populus nigra, soluble carbohydrates
INTRODUCTION
The ideal biomass crop is not only fast growing but also drought tolerant (McKendry, 2002). These attributes may be incompatible, as the rapid growth of the most productive biomass crop species/varieties is sustained by high rates of water use (Blum, 2005). The plant hormone abscisic acid (ABA) plays a central role in signalling reduced water availability and coordinating plant responses to drought stress (Wilkinson and Davies, 2002). The synthesis of ABA was previously considered to occur largely in the roots prior to transport in the xylem sap to the leaves as a signal of soil drying (Davies and Zhang, 1991). However, recent studies have suggested that the ‘whole plant’ dynamics of ABA are more complex, with the leaves and stems playing a major role in ABA synthesis and catabolism (Manzi et al., 2015; Mitchell et al., 2016; Marino et al., 2017; Zhang et al., 2018). Analysis of the temporal and spatial dynamics of ABA content in different plant tissues under drought is critical to understanding the function of this hormone in coordinating physiological responses to reduced water availability. Furthermore, elucidation of the genetic responses that underpin ABA dynamics and the biochemical/physiological responses elicited by ABA are critical to enhance drought tolerance in fast growing biomass species.
As the availability of water within soil declines, plants decrease the aperture of the stomatal pores to reduce stomatal conductance (gs), thus minimizing water loss via transpiration. As gs declines, diffusion of CO2 into the internal leaf air-space is impaired, resulting in reduced net photosynthesis (An) (Flexas et al., 2002; Chaves et al., 2009; Centritto et al., 2011; Lauteri et al., 2014). The stress associated with drought is often the result of an excess of energy that triggers formation of oxidative species as photochemistry declines (Pinheiro and Chaves, 2011). This effect is particularly clear in fast growing biomass species that have high radiation use efficiencies under optimal growth conditions (Silim et al., 2009). Plant responses to drought are regulated by a complex interaction of hydraulic and chemical signals (Comstock, 2002; Rodrigues et al., 2008; Tombesi et al., 2015). Early studies used split-root systems to demonstrate that ABA acts as a chemical signal of soil drying transported from the root to the shoot and acting independently of hydraulic signals in inducing stomatal closure (Zhang et al., 1987; Davies and Zhang, 1991). However, it has been recently shown that gs values and foliar ABA content in black poplar (Populus nigra) grown in a split-root system did not change when half of the root-zone dried (Marino et al., 2017). Similar patterns of gs and ABA content were observed in leaves of olive trees (Olea europaea) subjected to partial root-zone drying (Dbara et al., 2016) in an agronomic extrapolation of the split-root studies. In addition, analysis of Vitis vinifera drought responses suggest that initial stomatal closure is induced by hydraulic signals, followed by an increase in foliar levels of ABA that also maintained stomatal closure after leaf water potentials (ΨL) had returned to pre-stress levels (Correia et al., 1995; Tombesi et al., 2015). This is consistent with observations in other woody plants (Acer pseudoplatanus and Fagus sylvatica) that ABA does not act as an immediate root-to-shoot signal of soil drying to induce stomatal closure, but it is preceded by a hydraulic signal (Christmann et al., 2007). Furthermore, changes in the pH of xylem sap have also been proposed to signal soil drying by altering the partitioning of ABA between the leaf apoplast and symplast (Wilkinson and Davies, 1997). The content of free-ABA within a leaf is not only inversely related to gs, but also modifies stomatal behaviour to environmental stimuli (Haworth et al., 2018).
Mesophyll conductance to CO2 (gm) is also related to the content of free-ABA in the leaf. The ABA-induced alterations in gm are more rapid than those observed in gs (Sorrentino et al., 2016) and are possibly associated with a reduced activity of aquaporins involved in the transport of CO2 across the mesophyll (Jang et al., 2004; Flexas et al., 2006; Perez-Martin et al., 2014). ABA has also been shown to decrease leaf hydraulic conductance (Kleaf) through reduced biochemical activity of aquaporins involved in the regulation of the permeability of transport tissues to the movement of water (Shatil-Cohen et al., 2011; Pantin et al., 2013).
In addition to the regulation of gs, gm and Kleaf, ABA could play a role in sugar metabolism, affecting carbohydrate partitioning during drought (Rook et al., 2001). For example, in drought-stressed plants, ABA induces an increase in the activities of β-amylase and vacuolar invertase, leading to higher starch degradation and the release of hexoses in the cytosol (Pelleschi et al., 1999; Kempa et al., 2008).
The levels of ABA involved in all of these processes are regulated by multiple mechanisms: in situ ABA biosynthesis by NCED (9-cis-epoxycarotenoid dioxygenase) (Pierce and Raschke, 1981; Iuchi et al., 2001; Mitchell et al., 2016; Zhang et al., 2018), root-to-shoot transport (e.g. Zhang et al., 1987), conversion of biologically inactive glucose-conjugated ABA (ABA-GE) (Dietz et al., 2000; Lee et al., 2006; Seiler et al., 2011), and catabolism of free-ABA to phaseic acid (PA) and dihydrophaseic acid (DPA) (Saito et al., 2004; Nambara and Marion-Poll, 2005). Interestingly, de novo biosynthesis of ABA may serve as the main mechanism involved in the regulation of the content of this hormone following a reduction in turgor during an air vapour pressure deficit (VPD) transition (Bauerle et al., 2004; Xie et al., 2006; McAdam et al., 2016).
Analysis of the daily dynamic patterns of ABA content in different plant tissues as soil dries may illustrate the complex function of this plant hormone in eliciting and coordinating a wide variety of physiological responses to alleviate the deleterious impact of drought. We subjected black poplar (Populus nigra) plants to moderate and severe water stress to: (1) investigate the role of hormonal ABA and hydraulic signals of reduced water availability on plant physiology, in particular on the regulation of gs and gm; (2) determine the concomitant diurnal variations in ABA, its breakdown products and regulation of the de novo ABA-biosynthetic gene within different plant organs, to understand how these compounds are regulated during water stress progression; and (3) test the hypothesis of a possible relationship between ABA and soluble carbohydrates within the stem.
MATERIAL AND METHODS
Plant material and drought-stress treatment
One-year-old cuttings of Populus nigra L. were grown outside in Sesto Fiorentino (Italy, 43°49′N, 11°37′E) in 20-litre pots filled with sandy soil (sand/peat, 60: 40, v/v). The plants were maintained at pot water capacity and fertilized with Hoagland solution once a week to supply mineral nutrients at free access rates, until the onset of the water stress treatment (end of August). The experiment was conducted under minimum/maximum air temperatures of 16.4 ± 2.4/30.8 ± 3.2 °C (mean ± s.d.), midday irradiance of 803 ± 20 W m−2 and relative humidity of 27 ± 3.6 %. During the experimental period sunrise occurred between 0625 and 0636 h, and sunset between 2011 and 1954 h. Air temperature, solar irradiance and relative humidity were recorded by the weather station located at the experimental site of the Laboratory of Monitoring and Environmental Modelling for Sustainable Development (http://www.lamma.rete.toscana.it). Forty plants were equally spaced and randomly assigned to three experimental categories: well-watered (WW, 20 plants), moderately water stressed (WS1, ten plants) and severely water stressed (WS2, ten plants). The different water treatments were applied to the plants on the basis of preliminary leaf gas exchange measurements which allowed us to exclude significant differences in gas exchange parameters among plants (P > 0.05, data not shown). Water deficit was then imposed by withholding water (WS1 and WS2 plants), whereas well-watered plants (WW, control) were irrigated to pot capacity each day during the experimental period. Moderate (WS1) and severe (WS2) water-stress conditions were reached when gs values at 0900 h were respectively ~32 and ~ 6 % of the values observed in WW plants. These declines in gs were achieved after withholding water for 3–4 and 7–8 d, respectively. To assess their daily trend, physiological measurements and the sampling of plant material for biochemical analyses were conducted on four replicates at three times of the day: 0900, 1300 and 1800 h (solar time).
Measurements of gas exchange and plant water status
Leaf gas exchange was measured on fully developed and intact leaves using a LI-6400 portable photosynthesis system fitted with a 6400-40 2-cm2 leaf cuvette (Li-Cor, Lincoln, NE, USA). Measurements were performed at a photosynthetic photon flux density (PPFD) of 1000 µmol m−2 s−1at 0900 h, 1800 µmol m−2 s−1 at 1300 h and 1000 µmol m−2 s−1 at 1800 h, thus simulating the recorded environmental PPFD. Photosynthesis (An), stomatal conductance (gs) and the intercellular CO2 concentration (Ci) were calculated using the LI-6400 software; instantaneous water use efficiency (WUEins) was then calculated as the ratio of net CO2 assimilation to stomatal conductance (An/gs). Mesophyll conductance to CO2 (gm) was calculated using the variable J method (Harley et al., 1992; Loreto et al., 1992) as follows:
where Γ*, representing the CO2 compensation point to photorespiration, was measured on leaves of intact plants by determining An/Ci curves at four levels of photosynthetically active radiation and constant temperature (Brooks and Farquhar, 1985), while daytime respiration (Rd) was calculated using the Kok method at PPFD steps of 300, 200, 150, 100, 80, 60, 30 and 0 μmol m−2 s−1 (Kok, 1948). Electron transport rate (JF) was calculated from chlorophyll fluorescence:
where ΦPSII is the actual photochemical efficiency of photosystem II (Genty et al., 1989), the partitioning factor (β) between photosystems I and II was considered to be 0.5 and leaf absorbance (α) was 0.85.
Leaf hydraulic conductance (Kleaf, mmol s−1 MPa−1 kg−1) was measured in the laboratory (at constant temperature of 25 °C) on detached leaves according to the method described by Brodribb and Holbrook (2003):
where C is the capacitance, Ψo is the leaf water potential prior to partial rehydration, Ψf is the leaf water potential after partial rehydration and t is the duration of rehydration. Values of C were calculated from pressure–volume curves (Scholander et al., 1965; Tyree and Hammel, 1972) using the procedure outlined by Johnson et al. (2009) and normalized by leaf dry weight (Nardini et al., 2012). Leaf water potential (ΨL) was measured on fully developed leaves using a pressure chamber (PMS Instrument, Albany, OR, USA). To quantify the effect of the daily ΨL sensed by plants, a cumulative integrated ΨL was calculated for each water treatment as reported by Basile et al. (2003).
Sample collection for biochemical analyses and pH measurements
Stem xylem sap was collected according to the method described by Secchi and Zwieniecki (2012). Briefly, the stems were initially cut in the air and all leaves were removed. Then, after removing the bark from the first 5-cm basal end of the stem, this was connected to a small vacuum chamber. A vacuum pressure of 0.03 MPa allowed the water to be sucked from the stem during progressive cutting from the top of the base. The exuded xylem sap was collected in an Eppendorf tube sitting in ice and then stored at −80 °C prior to measurement of pH and ABA metabolites. After sap collection, bark (including cambium) and wood tissues were carefully separated using a razor blade, immediately frozen in liquid nitrogen to stop enzymatic activity and then lyophilized. Xylem sap pH was measured with a pH-meter (Mettler-Toledo, Switzerland) equipped with a micro electrode (InLab Micro electrode, Mettler Toledo).
Measurements of free-ABA and its metabolites/catabolites
Abscisic acid (free-ABA), its conjugated form (ABA glucoside ester, ABA-GE), phaseic acid (PA) and dihydrophaseic acid (DPA) were analysed in bark, wood and xylem sap. Sixty milligrams of lyophilized tissue was ground in liquid nitrogen, supplemented with 40 ng of d6-ABA, 40 ng of d5-ABA-GE, 40 ng of d3-PA and 40 ng of d3-DPA (National Research Council of Canada, Ottawa, Canada) and extracted three times with 1 mL of CH3OH/H2O (50: 50; v/v, pH 2.5) at 4 °C for 30 min. The extracts were then defatted with 3 × 3 mL of n-hexane and purified through Sep Pak C18-cartridges (Waters, Milford, MA, USA) utilizing ethylacetate as solvent. The eluates were evaporated to dryness, rinsed with 500 µL CH3OH/H2O (50: 50 v/v, pH 2.5) and injected (3 μL) into an LC–DAD-MS/MS system, composed of a Shimadzu Nexera HPLC and a Shimadzu LCMS-8030 quadrupole mass spectrometer, functioning in the negative electrospray ionization (ESI) mode (Kyoto, Japan). Mobile phases consisted of H2O (added with 0.1 % HCOOH, solvent A) and CH3CN/CH3OH (1: 1, v/v, added with 0.1 % HCOOH, solvent B). The analysis was performed using a 18-min gradient run (passing from 95 % solvent A to 100 % solvent B) on a Poroshell 120 SB C18 column (2.7 µm, 100 × 3 mm, Agilent Technologies) at a flow rate of 0.3 mL min−1. Quantification was conducted in multiple reaction mode (López-Carbonell et al., 2009). For analysis of xylem sap ABA, samples of 50 µL were used omitting the Sep-Pak C18 purification step. The recovery rates for deuterated standards are 23 % for d6-ABA and 15 % for d5-ABA-GE, d3-PA and d3-DPA. The ABA delivery flux to the leaves was calculated by multiplying the transpiration rate determined by the Licor device with the concentration of ABA found in xylem sap, as reported by Jackson et al. (1995).
Measurements of non-structural carbohydrates
Samples for sugar analysis in leaves were prepared as previously described (Silvente et al., 2012). Briefly, leaves (25 mg of powdered lyophilized tissues) were mixed with 1 mL of CH3CN/H2O mixture (1: 1 v/v). After centrifugation at 6700 g for 4 min and filtration, the supernatant was dried under N2 flux. The dry residue was dissolved in 0.7 mL of 400 mm D2O phosphate buffer (pD = 6.5) containing 1.0 mm of 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid sodium salt (TSPA). A Bruker AVANCE 600 spectrometer operating at a proton frequency of 600.13 MHz was used to measure the nuclear magnetic resonance (NMR) spectra of the extracts at 300k. The 1H spectra were obtained by co-adding 256 transients with a recycle delay of 7 s, a 45° flip angle pulse of 8.0 μs and 32k data points. A presaturation during the last 2 s of relaxation delay with a long single soft pulse was used to suppress the residual HDO signal. A small line broadening factor (0.4 Hz) was applied before Fourier transformation followed by manual phase and baseline corrections. The spectra were referenced to the signals of TSPA methyl group at δ = 0.00 p.p.m. Bruker TOPSPIN software version 1.3 was utilized for processing of spectra. The integrals of selected signals belonging to sucrose (4.23 p.p.m.), fructose (4.12 p.p.m.), α- and β-glucose (3.42 p.p.m.), and myo-inositol (3.29 p.p.m.) were measured and normalized with respect to the TSPA integral (at 0.00 p.p.m.) set to 100. The sugar content (in mg g–1 dry weight) was calculated using TSPA as an internal standard taking into account the proportionality between the integrals and molar concentrations.
Sugars were extracted from 30 mg of lyophilized bark and 30 mg of lyophilized wood. Samples were ground with liquid nitrogen and extracted twice with ethanol/water (80: 20). The determination of sucrose, fructose and glucose was performed by means of enzymatic test analysis (Boehringer Mannheim / R-Biopharm; Roche, Darmstadt, Germany) as reported by Steegmans et al. (2004). The analysis was full automatized on a ChemWell 2910 Chemistry Analyzer (Awareness Technologies, Palm City, FL, USA).
Gene expression analysis
A total of 144 leaf, root, xylem and bark tissues (50–100 mg each) were homogenized using a bead beater for two 30-s runs, flash-frozen in liquid nitrogen and vortexed in 0.8 mL cetyltrimethylammonium bromide (CTAB) buffer (Vincelli and Amsden, 2013). Total RNA was then extracted using the Maxwell 16 LEV simply RNA Tissue Kit (Promega) following the manufacturer’s instructions and treated with DNAase to eliminate contaminating genomic DNA. RNA quantity and quality were determined with the Agilent Bioanalyzer. A total of 2 µg of RNA was used for cDNA synthesis (Invitrogen) in a 30-μL system (65 °C, 5 min; 4 °C, 1 min; 40 °C, 2 min; 40 °C, 90 min; 70 °C, 15 min) (Johnson et al., 2012). The resulting cDNA was diluted to 20 ng μL–1 and used for quantitative real-time PCR (qPCR) using a Bio-Rad iQ5 real-time PCR detection system and iQ SYBR Green Supermix under the following conditions: 95 °C, 3 min; then 40 cycles of 10 s at 95 °C and 1 min at 60 °C. qPCR was performed in 12.5 µL of reaction mixture, composed of 2 µL of a given cDNA (20 ng μL–1), 6.25 µL 2× iQ SYBR Green Supermix, 0.15 µL each primer (10 μm) and 5.95 µL RNase-free water. BLAST (Altschul et al., 1990) searches for sequence similarities to identify poplar homologues using A. thaliana NCED3 and 18 sRNA as queries against the Populus trichocarpa v3.0 curated in the Phytozome (v9.1) database were performed. Gene-specific primers were then designed using Primer3 (http://bioinfo.ut.ee/primer3-0.4.0/) to work under the same PCR conditions. The following primer sequences were used (forward, reverse): NCED3: 5′-ATCCGGGGAGAATCTGAAGT-3′, 5′-TGTTCGTGTACTGCCCTCTG-3′; 18S rRNA: 5′-TCAACTTTCGATGGTAGGATAGTG-3′, 5′-CCGTGTCAGGATTGGGTAATTT-3′. A melting curve was produced at the end of every reaction cycle through a temperature ramp from 66 to 95 °C with increments of 0.5 °C s–1 and with continuous fluorescence collection to ensure that only single products were formed. PCR products were also separated on a 3 % (w/v) agarose gel to ensure that only a single band was present. Negative controls included the substitution of cDNA with nuclease-free water. All qPCRs were prepared in three biological replicates and technical triplicates/duplicates of each cDNA. The cycle threshold (CT) values indicating in real-time PCR the cycle number at which fluorescence emission reached a threshold above the baseline emission were determined and the mean CT values were considered. For data normalization, the data were normalized to the most stable reference gene 18 sRNA (Xu et al., 2011). Data were analysed using iQ5 Optical System Software v2.1 (Bio-Rad). Fold changes in gene expression were calculated using the 2−∆∆CT method (Pfaffl, 2001).
Statistical analysis
Data were analysed using a repeated-measures ANOVA, with water treatment considered to be the between-subjects effect and time to represent the within-subjects effect (SPSS v.20; IBM, Chicago, IL, USA) for the non-destructive parameters studied (gas exchange). A one-way ANOVA was used to evaluate the effects of water stress on all other parameters. Significant differences among means were estimated at the 5 % (P < 0.05) level, using Tukey’s test. Linear regression analysis using Sigmaplot (SPSS, Inc., Chicago, IL, USA) was used to assess possible relationships between ABA and gs/gm and between ABA and soluble carbohydrates.
RESULTS
Daily trends in gas exchange and water relationships
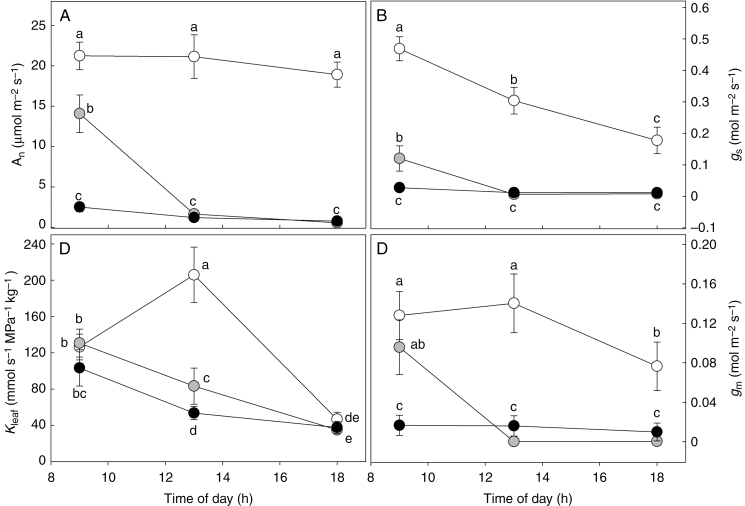
Water stress induced significant reductions in An, gs, gm and Kleaf, with a strong decrease of all parameters by midday (Fig. 1). In WW plants, An consistently remained above 20 μmol m−2 s−1 during the day, while gs declined from the morning until 1800 h, gm remained stable from 00900 to 1300 h before declining by 46.4 % by 1800 h. In WS1 plants, the highest values of An, gs and gm were recorded at 0900 h, but all parameters dropped by midday, approaching zero in the late afternoon. In WS2 plants, despite gs and gm values being close to zero through the day, some photosynthetic activity (An = 2.5 μmol m−2 s−1) was still recorded at 0900 h. In WW plants, Kleaf increased from early morning to midday (+63 %) and declined late in the afternoon. The daily trend of Kleaf in WS1 and WS2 plants showed the highest values at 0900 h (157.6 and 83.3 mmol s−1 MPa−1 kg−1, respectively) prior to significant reductions during the day (Fig. 1D). The leaf to air VPD experienced by the plants was lowest in the morning at 00900h (1.6 ± 0.24 kPa) before rising at midday (3.06 ± 0.62 kPa) until early evening (3.08 ± 0.45 kPa). No significant differences were observed in VPD values among WW, WS1 and WS2 plants. During measurements, the air temperature was 18 ± 3.1 °C at 0900 h, 27 ± 3.3 °C at midday and 21 ± 2.4 °C at 1800 h.
Fig. 1.
Daily trend of net photosynthesis (An, A), leaf hydraulic conductance (Kleaf, B), stomatal conductance (gs, C) and mesophyll conductance (gm, D) in Populus nigra leaves under well-watered (WW, open circles), moderate water stress (WS1, grey circles) and severe water stress (WS2, black circles) conditions. Data (means ± s.d., n = 4) were subjected to a repeated-measures ANOVA; different letters indicate significant differences between groups at a significance level of P < 0.05 using Tukey’s test.
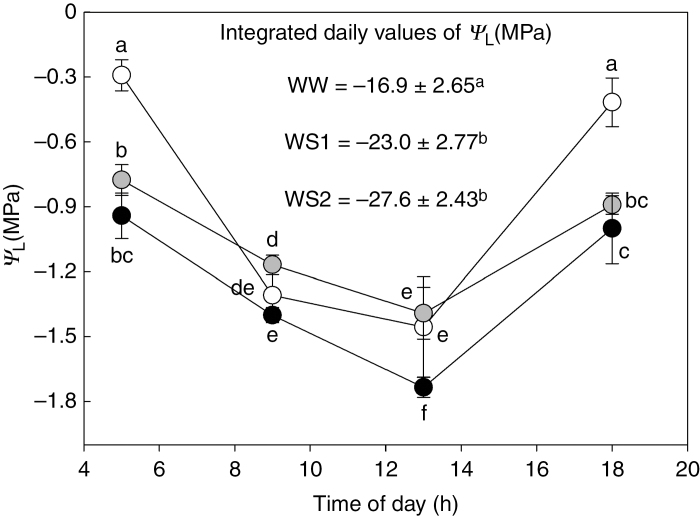
Leaf water potential (ΨL) showed a typical trend in all treatments, with the highest values at pre-dawn and towards sunset, and significantly lower values during the day as VPD increased (Fig. 2). As expected, pre-dawn ΨL was less negative in WW than in WS1 and WS2 plants. During the middle of the day, ΨL also decreased in WW leaves, at levels similar to those of WS1 leaves. There were no significant differences in ΨL values of WS1 and WS2 plants measured at pre-dawn and sunset. By contrast, ΨL was significantly lower in WS2 than in WS1 plants in the morning and at midday. Significantly lower values of integrated daily ΨL were detected in WW plants compared to WS1 and WS2 plants (Fig. 2).
Fig. 2.
Daily trend of leaf water potential (ΨL) in Populus nigra plants subject to well-watered (WW, open circles), moderate water stress (WS1, grey circles) and severe water stress (WS2, black circles) conditions. In the box the integrated daily values of ΨL represent the cumulative difference in water potential from 0 MPa integrated over a 24-h period. Data (means ± s.d., n = 4) were subjected to one-way ANOVA; different letters indicate significant differences between groups at a significance level of P < 0.05 using Tukey’s test.
Daily trend of ABA content
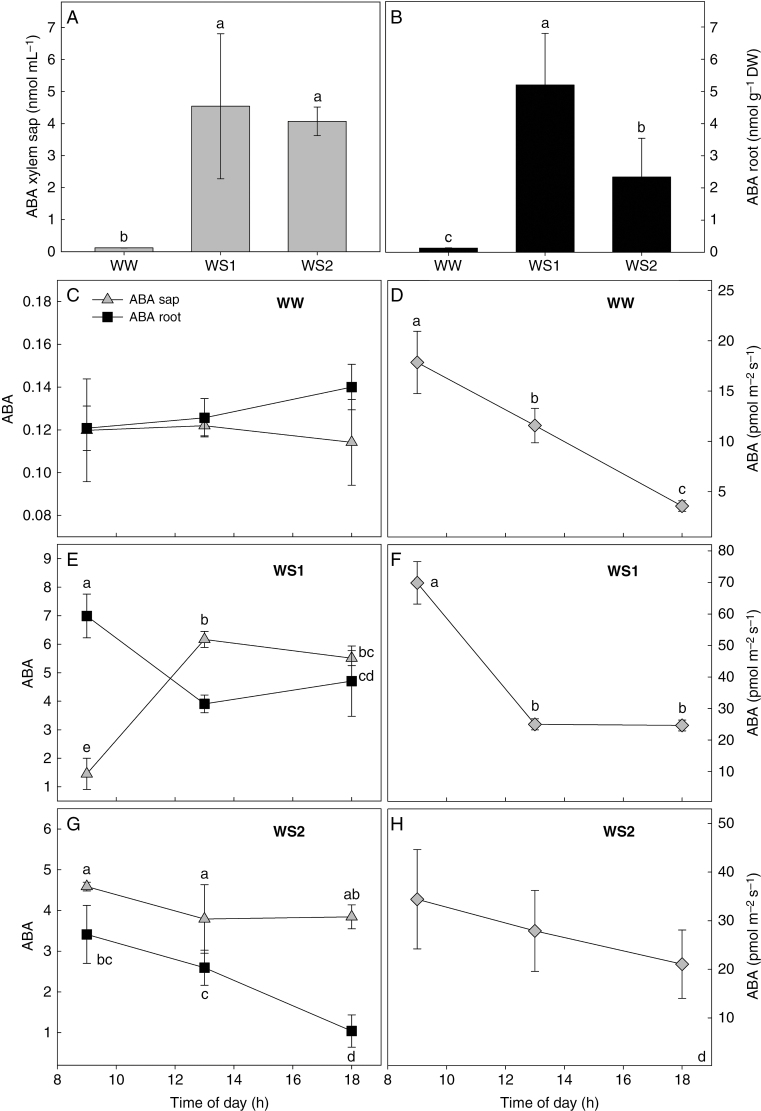
Water stress induced an order of magnitude increase in the concentration of ABA in xylem sap (ABAsap) (+97 % in both WS1 and WS2) and roots (ABAroots) (+97 % in WS1 and +94 % in WS2) (Fig. 3A, B). The daily patterns of ABAsap, ABAroots and ABA fluxes varied among irrigation treatments (Fig. 3C–H). In WW plants, ABAsap and ABAroots were an order of magnitude lower than those observed in the WS1 and WS2 treatments (Fig. 3C). ABA increased to a greater extent under moderate (WS1) than severe (WS2) water stress (Fig. 3E, G). In addition, in WW plants, ABAsap was constant throughout the day, while ABAroots rose slightly from 1300 to 1800 h (Fig. 3C). In contrast, in WS1 plants, ABAsap was low in the morning and increased significantly during the day, while ABAroots showed the opposite trend, declining during the day from a very high morning concentration (Fig. 3E). In WS2 plants, ABAroots again declined significantly during the day, while ABAsap did not change, showing values similar to those in WS1 plants (Fig. 3A). Plants grown under all water treatments showed similar patterns in the flow of ABA delivered to the leaf by xylem sap, with higher values in the morning and subsequent reductions during the afternoon. The overall flux of ABA into the leaves of WS1 plants was ~380 and 200 % greater than in WW and WS2 plants, respectively (Fig. 3D–F).
Fig. 3.
Daily mean of ABA in xylem sap (nmol mL−1) (A) and roots (nmol g−1DW) (B) and daily trend of ABA concentration in xylem sap (ABA sap, grey triangles) and roots (ABA root, nmol g−1DW, black squares) (C, E, G) and flux of ABA into leaf (D, F, H) in Populus nigra plants subject to well-watered (WW), moderate water stress (WS1) and severe water stress (WS2) conditions. Data (means ± s.d., n = 4) were subjected to one-way ANOVA; different letters indicate significant differences between groups at a significance level of P < 0.05 using Tukey’s test.
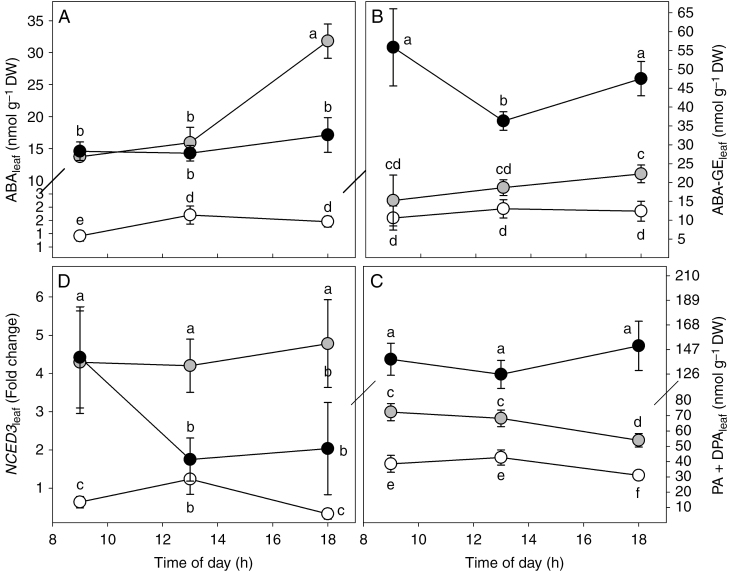
To investigate the dynamics of ABA content in the plants, ABA, ABA-GE, ABA catabolites (PA and DPA) and expression of the NCED3 gene were determined in different tissues (leaves, wood and bark). Water stress induced a marked boost in leaf ABA content (ABAleaf) (Fig. 4A and Supplementary Data Table S1), reaching values of ~14 nmol g−1 DW in both WS1 and WS2 plants between 0900 h and midday. Moreover, a further increase of ABAleaf was observed in the afternoon in WS1 plants (+50 %), while ABAleaf did not change through the day in WS2 plants (Fig. 4A). Water stress also promoted higher metabolism and catabolism of leaf ABA, particularly in WS2 plants where the content of ABA-GE (ABA-GEleaf) and ABA catabolites (PA+DPAleaf) were almost four-fold greater than those observed in WW plants (Supplementary Data Table S1). It is noteworthy that ABA-GE in WS2 plants showed a different daily trend in comparison to other plants, with the minimum value observed at midday (Fig. 4B). Finally, in plants exposed to moderate water stress (WS1), the expression of NCED3 was four times higher than in WW plants throughout the day, and higher than WS2 plants at midday and in the afternoon (Fig. 4D).
Fig. 4.
Daily trend of ABA (ABAleaf, A), ABA-GE (ABA-GEleaf, B), ABA catabolites (PA+DPAleaf, C) and NCED3 expression (NCED3leaf, D) in leaves of Populus nigra plants subjected to well-watered (WW, open circles), moderate water stress (WS1, grey circles) and severe water stress (WS2, black circles) conditions. Data (means ± s.d., n = 4) were subjected to one-way ANOVA; different letters indicate significant differences between groups at a significance level of P < 0.05 using Tukey’s test.
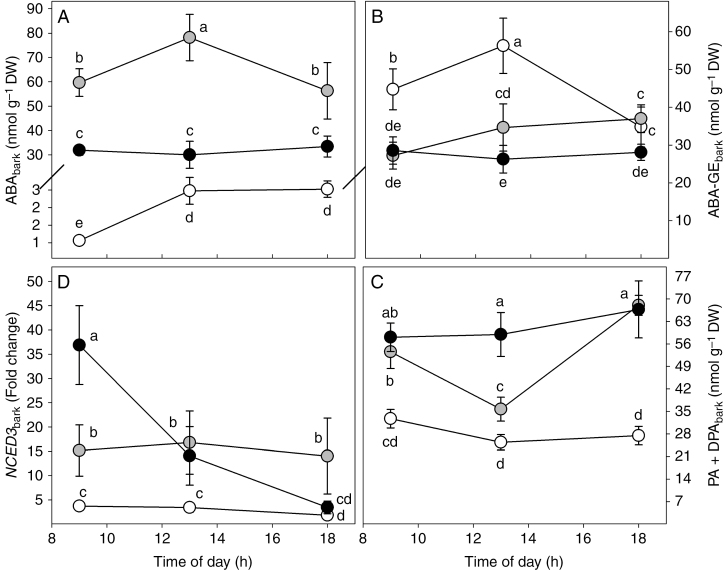
The ABA content in bark (ABAbark) increased significantly under water stress, resulting in respectively 26-fold and 13-fold higher levels in WS1 and WS2 plants in comparison to WW plants (Supplementary Data Table S1). Furthermore, the daily trend of ABAbark differed between plants subjected to the three water treatments (Fig. 5A). In contrast to the leaf, ABA-GE content in the bark (ABA-GEbark) was higher in WW plants compared to WS1 (+27 %) and WS2 (+38 %) plants (Table S1). Additionally, the maximum daily values of ABA-GEbark were observed at midday in WW plants (Fig. 5B). The sum of ABA catabolites in the bark (PA+DPAbark) of water-stressed plants was more than double those found with WW treatments (Table S1). No statistically significant daily changes were found in PA+DPAbark in WW and WS2 plants; however, PA+DPAbark decreased significantly at midday and then increased again at 1800 h in WS1 plants (Fig. 5C). An increase in NCED3 expression similar to that recorded in the leaf was also observed in the bark in response to water stress (Fig. 5D). Expression of NCED3 at 0900 h in WS2 plants was ten-fold higher than in WW plants. The expression of NCED3 then declined during the day in WS2 plants. In contrast, the daily expression of NCED3 was five-fold higher in WS1 plants and remained relatively stable during the day (Fig. 5D).
Fig. 5.
Daily trend of ABA (ABAbark, A), ABA-GE (ABA-GEbark, B), ABA catabolites (PA+DPAbark, C) and NCED3 expression (NCED3bark, D) in the bark of Populus nigra plants subject to well-watered (WW, open circles), moderate water stress (WS1, grey circles) and severe water stress (WS2, black circles) conditions. Data (means ± s.d., n = 4) were subjected to one-way ANOVA; different letters indicate significant differences between groups at a significance level of P < 0.05 using Tukey’s test.
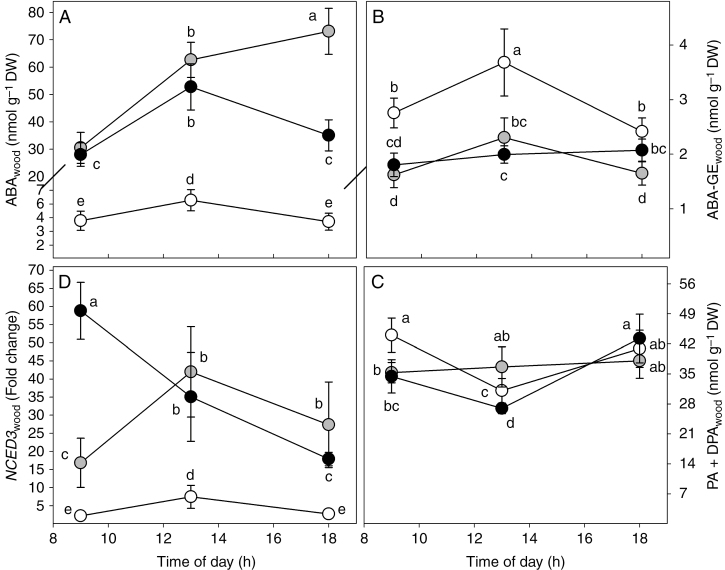
The content of ABA in wood (ABAwood) increased strongly under water stress (Supplementary Data Table S1), reaching its maximum at 1800 h in WS1 plants (73 nmol g−1 DW) and at 1300 h in WS2 plants (53 nmol g−1 DW) (Fig. 6A). The trend of ABA-GE content in wood (ABA-GEwood) was similar to that observed in bark. In fact, ABA-GEwood was more than 50 % higher in WW plants than in WS1 and WS2 plants (Table S1). However, ABA-GEwood was more than 30-fold lower than ABA-GEbark (Table S1). The mean content of ABA catabolites in wood (PA+DPAwood) did not differ among plants from the three water treatments (Table S1). However, in WW and in WS2 plants, levels of PA+DPAwood were at a minimum at midday, while no significant changes were observed during the day in WS1 plants (Fig. 6C). Similar to the other tissues, water stress increased expression of NCED3 in wood. The expression of NCED3 was respectively two- and three-fold higher in WS1 and WS2 plants in comparison to WW plants (Fig. 6D). The expression of NCED3 underwent different diurnal variations in all water treatments. In particular, in WS1 plants, NCED3 expression reached its maximum at 1300 h, whereas in WS2 the highest value was observed at 0900 h.
Fig. 6.
Daily trend of ABA (ABAwood, A), ABA-GE (ABA-GEwood, B), ABA catabolites (PA+DPAwood, C) and NCED3 expression (NCED3wood, D) in the wood of Populus nigra plants subjected to well-watered (WW, open circles), moderate water stress (WS1, grey circles) and severe water stress (WS2, black circles) conditions. Data (means ± s.d., n = 4) were subjected to one-way ANOVA; different letters indicate significant differences between groups at a significance level of P < 0.05 using Tukey’s test.
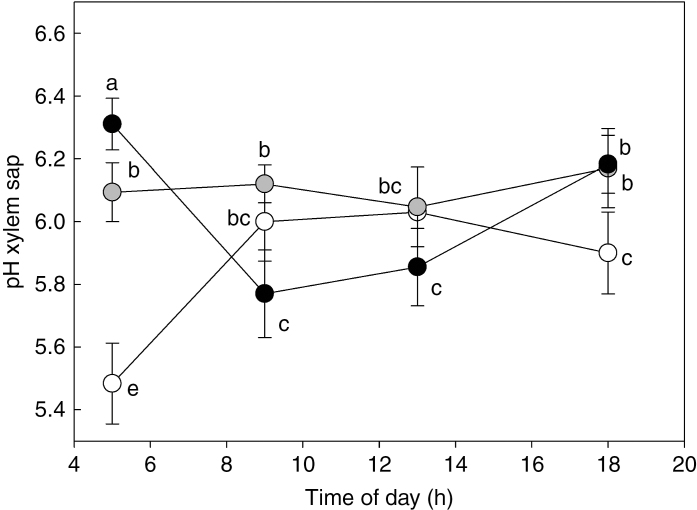
The lowest value of xylem sap pH was observed at pre-dawn in WW plants (Fig. 7). During the day, values of pH in the xylem sap remained relatively unchanged in all treatments, ranging between 6.0 and 6.3, except at 0900 h when WS2 plants showed significantly lower values (pH 5.7).
Fig. 7.
Daily trend of xylem sap pH in Populus nigra plants subject to well-watered (WW, open circles), moderate water stress (WS1, grey circles) and severe water stress (WS2, black circles) conditions. Data (means ± s.d., n = 4) were subjected to one-way ANOVA; different letters indicate significant differences between groups at a significance level of P < 0.05 using Tukey’s test.
Relationship between ABA and diffusional limitations to CO2 transport
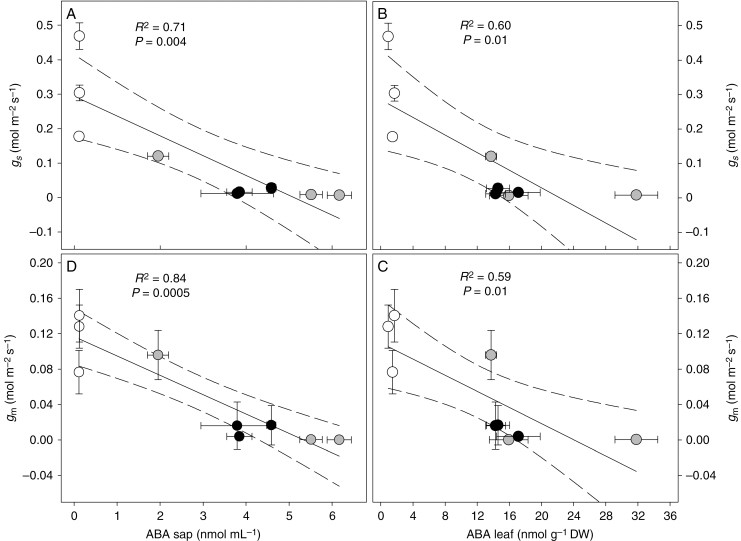
Increased ABA concentration in the sap and in the leaf of water-stressed plants was significantly and positively correlated to both gs (Fig. 8A, B) and gm (Fig. 8C, D). The highest correlation was found between ABA in xylem sap and gm (R2 = 0.84).
Fig. 8.
Relationships between ABA content in the xylem sap (A, B) and leaf (C, D) with stomatal conductance (gs) and mesophyll conductance (gm) in Populus nigra plants under well-watered (WW, open circles), moderate water stress (WS1, grey circles) and severe water stress (WS2, black circles) conditions measured in the morning (0900 h), midday (1300 h) and afternoon (1800 h) sampling points. P and R2 values indicate the results of linear regression. The central black line indicates the line of best fit. The dotted lines either side of the best-fit line indicate 95 % confidence intervals of the mean. Data are the means ± s.d. (n = 4).
Relationship between ABA and soluble carbohydrates in leaves and stem
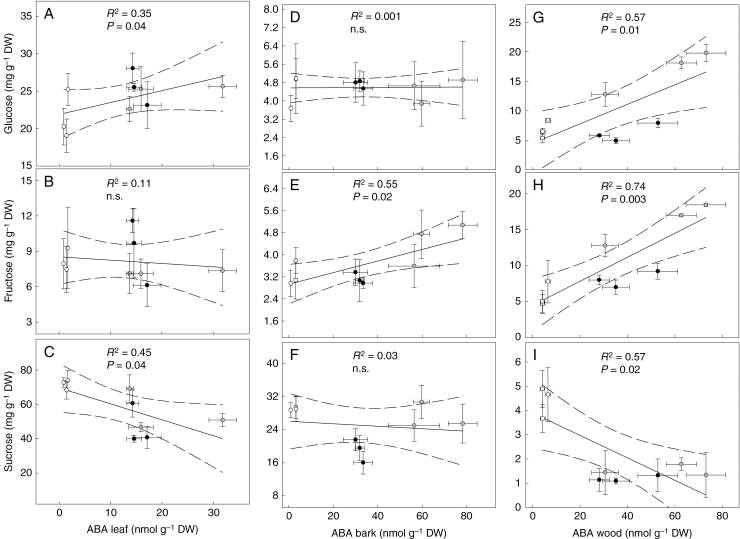
The content of soluble carbohydrates in the different organs was affected by both water treatment and hour of the day (Supplementary Data Fig. S1). The ABA content in leaves showed a significant positive relationship with glucose (P ≤ 0.05) and a significant negative relationship with sucrose (P ≤ 0.05) (Fig. 9A–C). In bark, a significant positive relationship was only found between ABA and fructose (P ≤ 0.05) (Fig. 9D–F). Stronger linear relationships were found in woody tissues between ABAwood with fructose (P = 0.003), sucrose (P = 0.02) and glucose (P = 0.01) (Fig. 9G–I).
Fig. 9.
Relationship between ABA content and soluble carbohydrates in the leaf (A–C), bark (D–F) and wood (G–I) of Populus nigra plants subjected to well-watered (WW, open circles), moderate water stress (WS1, grey circles) and severe water stress (WS2, black circles) conditions measured in the morning (0900 h), midday (1300 h) and afternoon (1800 h) sampling points. P and R2 values indicate the results of linear regression. The central black line indicates the line of best fit. The dotted lines either side of the best-fit line indicate 95 % confidence intervals of the mean. Data are the means ± s.d. (n = 4).
DISCUSSION
In vascular plants, tight coordination between hydraulic and photosynthetic systems allows optimization of water transport and CO2 assimilation in the leaf (Brodribb, 2009; McDowell, 2011). In particular, gs, gm and Kleaf all contribute to regulate maximum rates of photosynthesis and water balance under drought (Flexas et al., 2008, 2013a; Gago et al., 2016). Indeed, Kleaf represents the efficiency of water flow through the leaf mesophyll towards the sites of evaporation, and thus influences stomatal aperture, as it accounts for as much as 80 % of the whole plant hydraulic resistance (Nardini, 2001). Concurrently, gm represents the CO2 transfer conductance from the intercellular airspaces of the leaf into the chloroplast, thus strongly influencing biochemical assimilation rates (Centritto et al., 2003; Flexas et al., 2013a).
ABA and hydraulic coordination of the physiological response to water deficit
In the present study, photosynthesis was constant during the day in WW plants, despite a decline in gs values through the day (Fig. 1). The observed reduction in gs was probably associated with the pronounced increase in VPD (above 1.4 kPa) (Bauerle et al., 2004). Moreover, ABAleaf increased at midday (Fig. 4A), suggesting participation of a biochemical factor in reducing gs (Fig. 8). Even if the precise temporal pattern of this signalling cascade is not clear, the strict regulation of gs in this poplar genotype allowed maintenance of high intrinsic water-use efficiency (WUEint) without an excessive ΨL drop during the day to minimize the risk of hydraulic failure (Fig. 2 and Supplementary Data Table S2). We suggest that the high photosynthetic rates of WW plants throughout the day derived from the synchronous optimization of gm and Kleaf at midday (Fig. 1C, D). Indeed, previous studies have shown that gm and Kleaf respond positively to both light (Lo Gullo et al., 2005; Scoffoni et al., 2008; Sellin et al., 2008; Loreto et al., 2009; Ben Baaziz et al., 2012; Xiong et al., 2018) and temperature (Sellin and Kupper, 2007; Flexas et al., 2013b; Scafaro et al., 2011). Specifically, the increase in temperature (+8 °C) from 0900h to 1300h may have increased the permeability of membranes in the outside-xylem pathway (Sack et al., 2004), also enhancing gm by reducing the resistance to diffusion of HCO3− through a solution. Plasma membrane intrinsic proteins (PIPs) are trans-membrane water channels that facilitate water transport across membranes and determine the permeability of membranes and cell tissues to water, and thus strongly influence Kleaf (Uehlein et al., 2003; Cochard et al., 2007; Voicu et al., 2008; Heckwolf et al., 2011; Maurel and Prado, 2017). Several PIP isoforms have been detected in poplar, which are modulated differently on a daily basis (Lopez et al., 2013). The diurnal expression or activation patterns of PIPs could have been coordinated to maximize Kleaf and gm under WW and drought conditions (Perez Martin et al., 2014; Sade et al., 2015). The strict interconnection of hydraulic and chemical signals may also be due to the effects of ABA on gm and Kleaf through the inactivation of bundle sheath acquaporins during the day (Shatil-Cohen et al., 2011; Grondin et al., 2015; Mizokami et al., 2015; Sorrentino et al., 2016; Qiu et al., 2017). We surmise that the observed daily Kleaf and gm adjustments in the leaves of moderately water stressed plants (WS1) allowed the optimization of carbon gain in the morning before the subsequent rise in temperature and VPD (Flexas et al., 2013b; Meitern et al., 2017).
Analysis of the integral of diurnal ΨL values indicates that under water deficit poplar showed typical isohydric behaviour, with plants subject to the WS1 and WS2 treatments maintaining a near constant minimum daily ΨL by reducing gs in both WS1 and WS2 conditions (Fig. 2) (Attia et al., 2015). Nonetheless, the watering regimes induced different degrees of water stress, as evidenced by significant differences in predawn ΨL between water-stressed and well-watered plants and also in midday ΨL between WS1 and WS2 plants. Furthermore, irrespective of the severity of water stress, gm and Kleaf decreased strongly in poplar as soil water availability declined, consistent with previous results observed in a wide range of species (Brodribb and Holbrook, 2003; Diaz-Espejo et al., 2007; Misson et al., 2010; Galle et al., 2011; Scoffoni et al., 2012; Wang et al., 2018). It is noteworthy that at 0900 h WS1 plants showed An values of ~15 μmol m−2 s−1 (Fig. 1) and lower Ci values than WW plants and WS2 plants, which resulted in a higher WUEint (Supplementary Data Table S2). Conversely, in WS2 plants gs and gm were strongly depressed (Figs 1C, D and 8), imposing a higher limitation to photosynthesis during the whole day (Centritto et al., 2009).
ABA dynamics in different plant tissues under water stress
Alterations in delivery, synthesis and catabolism mechanisms regulated the content of foliar ABA among different water treatments. In WW leaves, ABAsap contributed to total ABAleaf content mostly in the morning, before a subsequent reduction in transpiration flow that decreased the amount of ABAsap delivered to leaves. Therefore, the increase in ABAleaf observed at midday was probably associated with higher in situ ABA biosynthesis (Bauerle et al., 2004; Xie et al., 2006; Marino et al., 2017), probably directly made from the dedicated chloroplastic isoprenoid (MEP) pathway, as first argued by Barta and Loreto (2006). Reduced ΨL at 1300 h and higher VPD may have triggered ABA biosynthesis in WW leaves, thus decreasing gs to prevent excessive dehydration. This could indicate that, under WW conditions, even a ‘low’ ABAleaf pool (~0.80 nmol g−1 DW) leads to a strong gs decrease (−36 %) (Figs 1B and 4A). This reduction, which was paralleled by an increase in Kleaf and a reduction in Ci, could optimize WUEint (Yang et al., 2016) and reduce the risk of xylem embolism formation on a daily basis in WW plants (Bond and Kavanagh, 1999; Nardini and Salleo, 2000; Davies et al., 2002) (Fig. 1 and Supplementary Data Table S2).
The up-regulation of NCED3 is likely to be the major cause of higher levels of ABAleaf found under water stress conditions (Fig. 4). It is notable that at 0900 h, the highest daily values of Kleaf, gs and gm were also recorded (Fig. 1) in WS1 plants, when conditions were most suited to photosynthetic activity as ΨL was relatively high. However, ABAleaf at 0900 h was identical to values at midday, while gs declined, and this is consistent with previous suggestions that stomata are less sensitive to ABA in the morning (Correia et al., 1995). We also observed the maximum level of ABA in the roots of water-stressed plants at 0900 h. Previous studies have shown that ABA stimulates root hydraulic conductivity (Lp) (Aroca et al., 2006), thus enabling water influx from the soil and promoting stomatal opening early in the morning (Hose et al., 2000; Thompson et al., 2007; Parent et al., 2009; Tardieu et al., 2015). At midday, the decrease in gs, gm and Kleaf might depend mostly on the maintenance of high ABA levels, as reported in isohydric grapevine (Coupel-Ledru et al., 2017), but the significant reduction in ΨL in WS2 could have also contributed to this regulation. In addition, WS2 leaves utilized ABA-GE to maintain constantly high values of ABAleaf throughout the day (Fig. 4B) (Hansen and Dörffling, 1999; Lee et al., 2006; Xu et al., 2011), whereas WS1 plants showed a peak of ABAleaf at 1800 h, probably due to lower catabolism (Fig. 4C) and/or reduced ABA export to the bark.
Levels of ABA found in the bark could indicate the potential flow of ABA and ABA-GE loaded into the phloem from leaves (Mwange et al., 2003). In fact, we observed a midday increase of ABA and higher levels of ABA-GE within the bark without changes in NCED3 expression, making the leaves the most likely source of ABA and ABA-GE under WW conditions. Furthermore, ABA-GE decreased in both bark and wood under water stress, while the increase in the content of this metabolite in leaf under severe water stress could be due to reduced rates of phloem loading (Dinat and Lemoine, 2010; Sevanto, 2018) or to an increased rate of ABA glycosylation (Xu et al., 2011). Conversely, ABA appears to be synthesized locally in the wood of WW plants, in which the midday drop of water potential may be related to the peak of ABAwood at 1300 h (Pantin et al., 2013; Fig. 6A). Water stress boosted ABA biosynthesis in both the bark and the wood (Endo et al., 2008; Galvez-Valdivieso et al., 2009; Shatil-Cohen et al., 2011). In these tissues, the catabolism of ABA probably played a major role in the regulation of ABA levels as observed in both WS1 and WS2 plants (Figs 5D and 6D). In particular, it is possible that water stress-induced ABA production in vascular parenchyma (Endo et al., 2008) resulted in the secretion of ABA to the xylem vessels, thus contributing to the strong increase of ABAsap. This ABA secretion could be the result of the release of the uncharged form of the hormone from vascular parechyma cells to xylem vessels (Lacombe and Achard, 2016; Kuromori et al., 2018) induced by the reduction in sap pH by 0.3 units (Fig. 7). The decline in sap pH recorded in our study is similar to results observed in poplar by Secchi and Zwieniecki (2016) and in other deciduous species by Thomas and Eamus (2002). The reduced pH in the xylem sap of poplar contrasts with observations of the opposite trend in other species (Wilkinson and Davies, 1997), suggesting that pH signals of water stress are species-specific, with the charged form of the ABA molecule being more prevalent in herbaceous and evergreen species (Bahrun et al., 2002; Thomas and Eamus, 2002), whilst in woody deciduous species the uncharged form is more commonly utilized as a signal of reduced water availability (Boursiac et al., 2013). Nonetheless, xylem sap pH is related to numerous factors other than ABA concentration, such as the degree of respiration (Salomón et al., 2016), flow rate and the content of minerals/compounds (Peuke, 2016), possibly accounting for the wide variation observed in pre-dawn xylem sap pH values in the present study.
Our results are consistent with the model proposed by Pantin et al. (2013), as the observed increase in ABAwood and ABAsap from 0900 to 1300 h in WS1 plants coincided with a decrease in Kleaf. According to this model, under drought conditions, ABA chemical signals could be converted into a hydraulic signal in the leaf by down-regulation of the activity of bundle-sheath aquaporins, resulting in the decreased permeability of vascular bundle-sheath cells (Shatil-Cohen et al., 2011; Negin and Moshelion, 2016).
Exploring the role of ABA in carbohydrate metabolism
It is noteworthy that similar ABA levels in both leaf and wood correspond to different water status in plants characterized by different gs values. To investigate different roles of ABA, possible correlations between ABA and soluble carbohydrates were analysed. In addition to the activation of a multitude of genes involved in primary carbohydrate metabolism (Zeller et al., 2009; Choudhury and Lahiri, 2011; Yoshida et al., 2015), the accumulation of ABA in leaves under drought increases the activity of β-amylase and vacuolar invertase (Pelleschi et al., 1999; Kempa et al., 2008; Thalmann et al., 2016), thus leading to an increase in hexoses. Our results support the hypothesis that ABA may be responsible for the alteration of soluble carbohydrate metabolism (Fig. 9) (Finkelstein and Gibson, 2002). It is possible that during the night ABA induced remobilization of starch reserves from the chloroplast (Robertson et al., 2009) and maintained the size of leaf sugar pools during water stress (Smith and Stitt, 2007). The maintenance of high levels of sugars in the leaf may result in the down-regulation of photosynthetic genes during water deficit (Van Oosten et al., 1994). This relationship between ABA and sugars emerged not only in the leaf, as previously found in transgenic tobacco leaves exposed to water stress (Tattini et al., 2014), but also in the stem, particularly in the wood (Fig. 9C). We suggest a possible role for this hormone in mobilizing non-structural carbohydrates in the wood to preserve xylem hydraulic integrity under water stress (Secchi et al., 2013). It is important to mention that a putative connection between ABA, PIPs and hexoses has been recently put forward (Kelly et al., 2017; Wang et al., 2017), suggesting that a cross-talk between the glucose and ABA signalling pathways may regulate the level of sucrose (Wang et al., 2017), and in turn the level of hexoses may affect the expression of PIP genes and reduce leaf hydraulic conductance (Kelly et al., 2017). As a consequence, a fine tuning of carbohydrate metabolism by ABA to modulate Kleaf under drought is likely to occur.
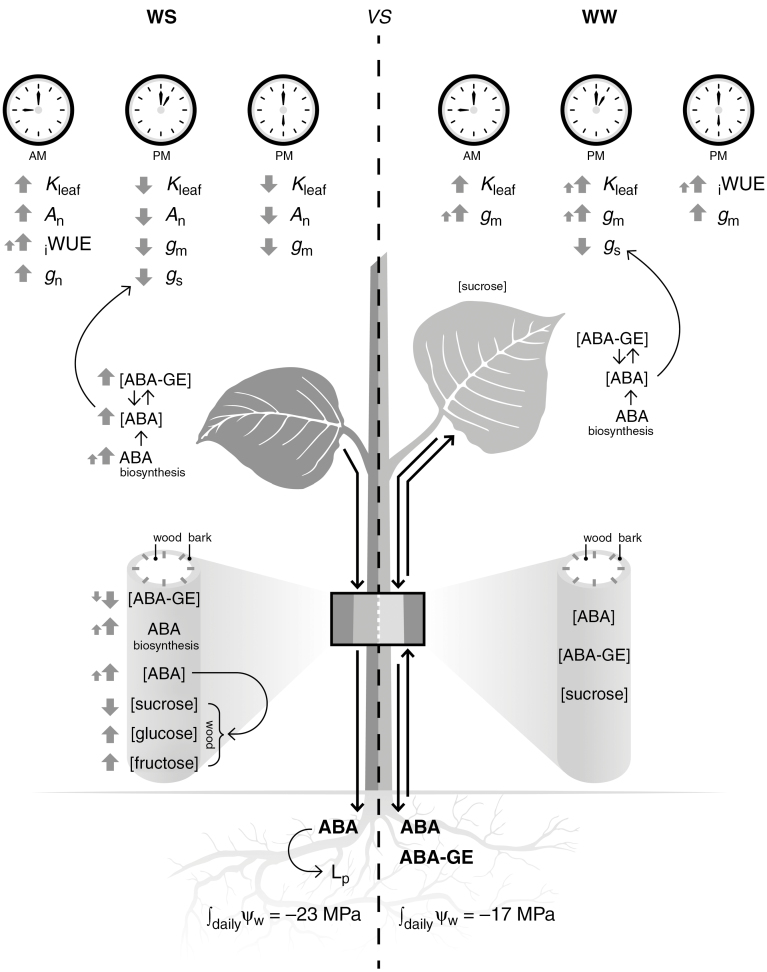
In conclusion, the results of this study expand our understanding of the impact of water stress on the daily trends of gs, gm and Kleaf and the close coordination of these parameters in the optimization of water transport and CO2 assimilation in water-stressed plants. In addition, the finding that WW plants restrict gs during the day while keeping photosynthesis constant provides evidence that unstressed plants can operate in a more water-efficient mode by increasing WUEint without curbing CO2 fixation (Fig. 10). This study supports previous results showing a role of both hydraulic and hormonal signals in the regulation of gs under water stress (Wilkinson and Davies, 2002; Christmann et al., 2007). Moreover, these two signals are strictly coupled and have a different influence on gs and gm depending on the time of the day and the intensity of the stress. In particular, we suggest that in well-watered plants a slight increase in leaf ABA content is enough to decrease gs, but reducing gm in water-stressed plants may require higher leaf levels of ABA, probably associated with reductions in water potential. The hypothesis of a key role for ABA in directly or indirectly regulating stomatal closure in isohydric plants is also supported, as both ABA biosynthesis/catabolism and ABA-GE metabolism are fine-tuned to adjust the content of free-ABA in leaves.
Fig. 10.
Schematic overview of the main experimental results on Populus nigra plants under moderate water stress (WS) and well-watered (WW) conditions: (1) in the leaf there is a clear daily trend where ABA regulates physiological parameters [stomatal conductance (gs), mesophyll conductance (gm) and leaf hydraulic conductance (Kleaf)] to optimize water transport and CO2 assimilation; (2) in the stem ABA content may have a role in the regulation of water transport and carbohydrate metabolism; (3) ABA accumulates in the roots due to transport from the leaves where this hormone is synthesized. The increase in ABA content in the roots under water-stress conditions may enhance root hydraulic conductivity (Lp).
Finally, ABA content and diurnal pattern in different plant organs were also modified in response to water stress. Our study corroborates the current hypothesis that ABA synthesis takes place in several plant organs such as leaves, roots and vascular tissues, and ABA can move to target cells through both the xylem and the phloem, allowing a two-way transportation between roots and shoots (Fig. 10). To the best of knowledge, our data provide the first report of a possible relationship between ABA and soluble carbohydrates in the leaves and stem, suggesting further potential roles of this hormone in carbohydrate metabolism.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: Daily trend of soluble carbohydrates in the leaf (A–C), wood (D, E) and bark (F, G) in Populus nigra plants subject to well-watered (WW, open circles), moderate water stress (WS1, grey circles) and severe water stress (WS2, black circles) conditions. Data (means ± s.d., n = 4) were subjected to one-way ANOVA; different letters indicate significant differences between groups at a significance level of P < 0.05 using Tukey’s test.
FUNDING
This research received funding from the European Union’s Seventh Programme for research, technological development and demonstration under grant agreement No. FP7-311929 for the WATBIO project (Development of improved perennial non-food biomass and bioproduct crops for water stressed environments) and the Brain Gain (Rientro dei Cervelli) MIUR professorship for A.H.
LITERATURE CITED
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Aroca R, Ferrante A, Vernieri P, Chrispeels MJ. 2006. Drought, abscisic acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Annals of Botany 98: 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia Z, Domec JC, Oren R, Way DA, Moshelion M. 2015. Growth and physiological responses of isohydric and anisohydric poplars to drought. Journal of Experimental Botany 66: 4373–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrun A, Jensen CR, Asch F, Mogensen VO. 2002. Drought-induced changes in xylem pH, ionic composition, and ABA concentration act as early signals in field-grown maize (Zea mays L.). Journal of Experimental Botany 53: 251–263. [DOI] [PubMed] [Google Scholar]
- Barta C, Loreto F. 2006. The relationship between the methyl-erythritol phosphate pathway leading to emission of volatile isoprenoids and abscisic acid content in leaves. Plant Physiology 141: 1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile B, Marsal J, DeJong TM. 2003. Daily shoot extension growth of peach trees growing on rootstocks that reduce scion growth is related to daily dynamics of stem water potential. Tree Physiology 23: 695–704. [DOI] [PubMed] [Google Scholar]
- Bauerle W, Whitlow T, Setter T, Vermeylen F. 2004. Abscisic acid synthesis in Acer rubrum L. leaves–a vapour-pressure-deficit-mediated response. Journal of the American Society for Horticultural Science 129: 182–187. [Google Scholar]
- Ben Baaziz K, Lopez D, Rabot A, et al. . 2012. Light-mediated K leaf induction and contribution of both the PIP1s and PIP2s aquaporins in five tree species: walnut (Juglans regia) case study. Tree Physiology 32: 423–434. [DOI] [PubMed] [Google Scholar]
- Blum A. 2005. Drought resistance, water-use efficiency, and yield potential–are they compatible, dissonant, or mutually exclusive? Australian Journal of Agricultural Research 56: 1159–1168. [Google Scholar]
- Bond BJ, Kavanagh KL. 1999. Stomatal behavior of four woody species in relation to leaf-specific hydraulic conductance and threshold water potential. Tree Physiology 19: 503–510. [DOI] [PubMed] [Google Scholar]
- Boursiac Y, Léran S, Corratgé-Faillie C, Gojon A, Krouk G, Lacombe B. 2013. ABA transport and transporters. Trends in Plant Science 18: 325–333. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ. 2009. Xylem hydraulic physiology: the functional backbone of terrestrial plant productivity. Plant Science 177: 245–251. [Google Scholar]
- Brodribb TJ, Holbrook NM. 2003. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology 132: 2166–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A, Farquhar GD. 1985. Effects of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165: 397–406. [DOI] [PubMed] [Google Scholar]
- Centritto M, Loreto F, Chartzoulakis K. 2003. The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant, Cell & Environment 26: 585–594. [Google Scholar]
- Centritto M, Lauteri M, Monteverdi MC, Serraj R. 2009. Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. Journal of Experimental Botany 60: 2325–2339. [DOI] [PubMed] [Google Scholar]
- Centritto M, Tognetti R, Leitgeb E, Střelcová K, Cohen S. 2011. Above ground processes: Anticipating climate change influences. In: Bredemeier M, Cohen S, Godbold DL, Lode E, Pichler V, Schleppi P, eds. Forest management and the water cycle: an ecosystem-based approach. Ecological Studies 212. Dordrecht: Springer, 31–64. [Google Scholar]
- Chaves M, Flexas J, Pinheiro C. 2009. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany 103: 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A, Lahiri A. 2011. Comparative analysis of abscisic acid‐regulated transcriptomes in Arabidopsis. Plant Biology 13: 28–35. [DOI] [PubMed] [Google Scholar]
- Christmann A, Weiler EW, Steudle E, Grill E. 2007. A hydraulic signal in root‐to‐shoot signalling of water shortage. The Plant Journal 52: 167–174. [DOI] [PubMed] [Google Scholar]
- Cochard H, Venisse JS, Barigah TS, et al. . 2007. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiology 143: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock JP. 2002. Hydraulic and chemical signalling in the control of stomatal conductance and transpiration. Journal of Experimental Botany 53: 195–200. [DOI] [PubMed] [Google Scholar]
- Correia MJ, Pereira JS, Chaves MM, Rodrigues ML, Pacheco CA. 1995. ABA xylem concentrations determine maximum daily leaf conductance of field‐grown Vitis vinifera L. plants. Plant Cell & Environment 18: 511–521. [Google Scholar]
- Coupel-Ledru A, Tyerman S, Masclef D, et al. . 2017. Abscisic acid down-regulates hydraulic conductance of grapevine leaves in isohydric genotypes only. Plant Physiology 75: 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WJ, Zhang JH. 1991. Root signals and the regulation of growth and development of plants in drying soil. Annual Review of Plant Physiology and Plant Molecular Biology 42: 55–76. [Google Scholar]
- Davies WJ, Wilkinson S, Loveys B. 2002. Stomatal control by chemical signalling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytologist 153: 449–460. [DOI] [PubMed] [Google Scholar]
- Dbara S, Haworth M, Emiliani G, Mimoun MB, Gómez-Cadenas A, Centritto M. 2016. Partial root-zone drying of olive (Olea europaea var. ‘Chetoui’) induces reduced yield under field conditions. PloS One 11: e0157089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Espejo A, Nicolás E, Fernández JE. 2007. Seasonal evolution of diffusional limitations and photosynthetic capacity in olive under drought. Plant Cell & Environment 30: 922–933. [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Sauter A, Wichert K, Messdaghi D, Hartung W. 2000. Extracellular β‐glucosidase activity in barley involved in the hydrolysis of ABA glucose conjugate in leaves. Journal of Experimental Botany 51: 937–944. [PubMed] [Google Scholar]
- Dinant S, Lemoine R. 2010. The phloem pathway: new issues and old debates. Comptes Rendus Biologies 333: 307–319. [DOI] [PubMed] [Google Scholar]
- Endo A, Sawada Y, Takahashi H, et al. . 2008. Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiology 147: 1984–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Gibson SI. 2002. ABA and sugar interactions regulating development: cross-talk or voices in a crowd? Current Opinion in Plant Biology 5: 26–32. [DOI] [PubMed] [Google Scholar]
- Flexas J, Bota J, Escalona JM, Sampol B, Medrano H. 2002. Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Functional Plant Biology 29: 461–471. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Hanson DT, et al. . 2006. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2in vivo. The Plant Journal 48: 427–439. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbo M, Diaz-Espejo A, Galmes J, Medrano H. 2008. Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell & Environment 31: 602–621. [DOI] [PubMed] [Google Scholar]
- Flexas J, Niinemets Ü, Gallé A, et al. . 2013a. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynthesis Research 117: 45–59. [DOI] [PubMed] [Google Scholar]
- Flexas J, Scoffoni C, Gago J, Sack L. 2013b. Leaf mesophyll conductance and leaf hydraulic conductance: an introduction to their measurement and coordination. Journal of Experimental Botany 64: 3965–3981. [DOI] [PubMed] [Google Scholar]
- Gago J, de Menezes Daloso D, Figueroa CM, Flexas J, Fernie AR, Nikoloski Z. 2016. Relationships of leaf net photosynthesis, stomatal conductance, and mesophyll conductance to primary metabolism: a multispecies meta-analysis approach. Plant Physiology 171: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galle A, Flórez-Sarasa I, El Aououad H, Flexas J. 2011. The Mediterranean evergreen Quercus ilex and the semi-deciduous Cistus albidus differ in their leaf gas exchange regulation and acclimation to repeated drought and re-watering cycles. Journal of Experimental Botany 62: 5207–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez-Valdivieso G, Fryer MJ, Lawson T, et al. . 2009. The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. The Plant Cell 21: 2143–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- Grondin A, Rodrigues O, Verdoucq L, Merlot S, Leonhardt N, Maurel C. 2015. Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. The Plant Cell 27: 1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H, Dörffling K. 1999. Changes of free and conjugated abscisic acid and phaseic acid in xylem sap of drought-stressed sunflower plants. Journal of Experimental Botany 50: 1599–1605. [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD. 1992. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiology 98: 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth M, Cosentino SL, Marino G, et al. . 2018. Increased free abscisic acid during drought enhances stomatal sensitivity and modifies stomatal behaviour in fast growing giant reed (Arundo donax L.). Environmental and Experimental Botany 147: 116–124. [Google Scholar]
- Heckwolf M, Pater D, Hanson DT, Kaldenhoff R. 2011. The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. The Plant Journal 67: 795–804. [DOI] [PubMed] [Google Scholar]
- Hose E, Steudle E, Hartung W. 2000. Abscisic acid and hydraulic conductivity of maize roots: a study using cell-and root-pressure probes. Planta 211: 874–882. [DOI] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T. 2001. Regulation of drought tolerance by gene manipulation of 9‐cis‐epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. The Plant Journal 27: 325–333. [DOI] [PubMed] [Google Scholar]
- Jackson GE, Irvine J, Grace J, Khalil AAM. 1995. Abscisic acid concentrations and fluxes in droughted conifer saplings. Plant Cell & Environment 18: 13–22. [Google Scholar]
- Jang JY, Kim DG, Kim YO, Kim JS, Kang H. 2004. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Molecular Biology 54: 713–725. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Woodruff DR, McCulloh KA, Meinzer FC. 2009. Leaf hydraulic conductance, measured in situ, declines and recovers daily: leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiology 29: 879–887. [DOI] [PubMed] [Google Scholar]
- Johnson MT, Carpenter EJ, Tian Z, et al. , 2012. Evaluating methods for isolating total RNA and predicting the success of sequencing phylogenetically diverse plant transcriptomes. PloS One 7: 50226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G, Sade N, Doron‐Faigenboim A, et al. . 2017. Sugar and hexokinase suppress expression of PIP aquaporins and reduce leaf hydraulics that preserves leaf water potential. The Plant Journal 91: 325–339. [DOI] [PubMed] [Google Scholar]
- Kempa S, Krasensky J, Dal Santo S, Kopka J, Jonak C. 2008. A central role of abscisic acid in stress- regulated carbohydrate metabolism. PLoS One 3: 3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B. 1948. A critical consideration of the quantum yield of Chlorella photosynthesis. Enzymologia 13: 1–56. [Google Scholar]
- Kuromori T, Seo M, Shinozaki K. 2018. ABA transport and plant water stress responses. Trends in Plant Science 23: 513–522. [DOI] [PubMed] [Google Scholar]
- Lacombe B, Achard P. 2016. Long-distance transport of phytohormones through the plant vascular system. Current Opinion in Plant Biology 34: 1–8. [DOI] [PubMed] [Google Scholar]
- Lauteri M, Haworth M, Serraj R, Monteverdi MC, Centritto M. 2014. Photosynthetic diffusional constraints affect yield in drought stressed rice cultivars during flowering. PLoS One 9: e109054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Piao HL, Kim HY, et al. . 2006. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126: 1109–1120. [DOI] [PubMed] [Google Scholar]
- Lo Gullo MA, Nardini A, Trifilò P, Salleo S. 2005. Diurnal and seasonal variations in leaf hydraulic conductance in evergreen and deciduous trees. Tree Physiology 25: 505–512. [DOI] [PubMed] [Google Scholar]
- Lopez D, Venisse JS, Fumanal B, et al. . 2013. Aquaporins and leaf hydraulics: poplar sheds new light. Plant and Cell Physiology 54: 1963–1975. [DOI] [PubMed] [Google Scholar]
- López-Carbonell M, Gabasa M, Jáuregui O. 2009. Enhanced determination of abscisic acid (ABA) and abscisic acid glucose ester (ABA-GE) in Cistus albidus plants by liquid chromatography-mass spectrometry in tandem mode. Plant Physiology and Biochemistry 47: 256–261. [DOI] [PubMed] [Google Scholar]
- Loreto F, Harley PC, Di Marco G, Sharkey TD. 1992. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiology 98: 1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Tsonev T, Centritto M. 2009. The impact of blue light on leaf mesophyll conductance. Journal of Experimental Botany 60: 2283–2290. [DOI] [PubMed] [Google Scholar]
- Manzi M, Lado J, Rodrigo MJ, Zacarías L, Arbona V, Gómez-Cadenas A. 2015. Root ABA accumulation in long-term water-stressed plants is sustained by hormone transport from aerial organs. Plant and Cell Physiology 56: 2457–2466. [DOI] [PubMed] [Google Scholar]
- Marino G, Brunetti C, Tattini M, et al. . 2017. Dissecting the role of isoprene and stress-related hormones (ABA and ethylene) in Populus nigra exposed to unequal root zone water stress. Tree Physiology 12: 1637–1647. [DOI] [PubMed] [Google Scholar]
- Maurel C, Prado K. 2017. Aquaporins and leaf water relations. In: Chaumont F, Tyerman SD, eds. Plant aquaporins from transport to signalling. Berlin: Springer, 155–165. [Google Scholar]
- McAdam SA, Sussmilch FC, Brodribb TJ. 2016. Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell & Environment 39: 485–491. [DOI] [PubMed] [Google Scholar]
- McDowell NG. 2011. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiology 155: 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendry P. 2002. Energy production from biomass (part 1): overview of biomass. Bioresource Technology 83: 37–46. [DOI] [PubMed] [Google Scholar]
- Meitern A, Õunapuu-Pikas E, Sellin A. 2017. Circadian patterns of xylem sap properties and their covariation with plant hydraulic traits in hybrid aspen. Journal of Plant Physiology 213: 148–156. [DOI] [PubMed] [Google Scholar]
- Misson L, Limousin J, Rodriguez R, Letts MG. 2010. Leaf physiological responses to extreme droughts in Mediterranean Quercus ilex forest. Plant Cell & Environment 33: 1898–1910. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, McAdam SA, Pinkard EA, Brodribb TJ. 2016. Significant contribution from foliage-derived ABA in regulating gas exchange in Pinus radiata. Tree Physiology 37: 236–245. [DOI] [PubMed] [Google Scholar]
- Mizokami Y, Noguchi KO, Kojima M, Sakakibara H, Terashima I. 2015. Mesophyll conductance decreases in the wild type but not in an ABA‐deficient mutant (aba1) of Nicotiana plumbaginifolia under drought conditions. Plant Cell & Environment 38: 388–398. [DOI] [PubMed] [Google Scholar]
- Mwange KNK, Hou HW, Cui KM. 2003. Relationship between endogenous indole‐3‐acetic acid and abscisic acid changes and bark recovery in Eucommia ulmoides Oliv. after girdling. Journal of Experimental Botany 54: 1899–1907. [DOI] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. 2005. Abscisic acid biosynthesis and catabolism. Annual Review of Plant Biology 56: 165–185. [DOI] [PubMed] [Google Scholar]
- Nardini A. 2001. Are sclerophylls and malacophylls hydraulically different? Biologia Plantarum 44: 239–245. [Google Scholar]
- Nardini A, Salleo S. 2000. Limitation of stomatal conductance by hydraulic traits: sensing or preventing xylem cavitation? Trees 15: 14–24. [Google Scholar]
- Nardini A, Pedá G, Salleo S. 2012. Alternative methods for scaling leaf hydraulic conductance offer new insights into the structure–function relationships of sun and shade leaves. Functional Plant Biology 39: 394–401. [DOI] [PubMed] [Google Scholar]
- Negin B, Moshelion M. 2016. The evolution of the role of ABA in the regulation of water-use efficiency: from biochemical mechanisms to stomatal conductance. Plant Science 251: 82–89. [DOI] [PubMed] [Google Scholar]
- Pantin F, Monnet F, Jannaud D, et al. . 2013. The dual effect of abscisic acid on stomata. New Phytologist 197: 65–72. [DOI] [PubMed] [Google Scholar]
- Parent B, Hachez C, Redondo E, Simonneau T, Chaumont F, Tardieu F. 2009. Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiology 149: 2000–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleschi S, Guy S, Kim JY, et al. . 1999. Ivr2, a candidate gene for a QTL of vacuolar invertase activity in maize leaves. Gene-specific expression under water stress. Plant Molecular Biology 39: 373–380. [DOI] [PubMed] [Google Scholar]
- Perez-Martin A, Michelazzo C, Torres-Ruiz JM, et al. . 2014. Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: correlation with gene expression of carbonic anhydrase and aquaporins. Journal of Experimental Botany 65: 3143–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuke AD. 2016. ABA flow modelling in Ricinus communis exposed to salt stress and variable nutrition. Journal of Experimental Botany 67: 5301–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Research 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M, Raschke K. 1981. Synthesis and metabolism of abscisic acid in detached leaves of Phaseolus vulgaris L. after loss and recovery of turgor. Planta 153: 156–165. [DOI] [PubMed] [Google Scholar]
- Pinheiro C, Chaves MM. 2011. Photosynthesis and drought: can we make metabolic connections from available data? Journal of Experimental Botany 62: 869–882. [DOI] [PubMed] [Google Scholar]
- Qiu C, Ethier G, Pepin S, Dubé P, Desjardins Y, Gosselin A. 2017. Persistent negative temperature response of mesophyll conductance in red raspberry (Rubus idaeus L.) leaves under both high and low vapour pressure deficits: a role for abscisic acid? Plant Cell & Environment 40: 1940–1959. [DOI] [PubMed] [Google Scholar]
- Robertson FC, Skeffington AW, Gardner MJ, Webb AA. 2009. Interactions between circadian and hormonal signalling in plants. Plant Molecular Biology 69: 419–427. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, Santos TP, Rodrigues AP, et al. . 2008. Hydraulic and chemical signalling in the regulation of stomatal conductance and plant water use in field grapevines growing under deficit irrigation. Functional Plant Biology 35: 565–579. [DOI] [PubMed] [Google Scholar]
- Rook F, Corke F, Card R, Munz G, Smith C, Bevan MW. 2001. Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. The Plant Journal 26: 421–433. [DOI] [PubMed] [Google Scholar]
- Sack L, Streeter CM, Holbrook NM. 2004. Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiology 134: 1824–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade N, Shatil-Cohen A, Moshelion M. 2015. Bundle-sheath aquaporins play a role in controlling Arabidopsis leaf hydraulic conductivity. Plant Signaling & Behavior 10: e1017177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, Mizutani M. 2004. Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiology 134: 1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomón R, Valbuena-Carabaña M, Teskey R, et al. . 2016. Seasonal and diel variation in xylem CO2 concentration and sap pH in sub-Mediterranean oak stems. Journal of Experimental Botany 67: 2817–2827. [DOI] [PubMed] [Google Scholar]
- Scafaro AP, Von Caemmerer S, Evans JR, Atwell BJ. 2011. Temperature response of mesophyll conductance in cultivated and wild Oryza species with contrasting mesophyll cell wall thickness. Plant Cell & Environment 34: 1999–2008. [DOI] [PubMed] [Google Scholar]
- Scholander PF, Hammel HT, Bradstreet ED, Hemmington EA. 1965. Sap pressure in vascular plants. Science 148: 339–346. [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Pou A, Aasamaa K, Sack L. 2008. The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods. Plant Cell & Environment 31: 1803–1812. [DOI] [PubMed] [Google Scholar]
- Scoffoni C, McKown AD, Rawls M, Sack L. 2012. Dynamics of leaf hydraulic conductance with water status: quantification and analysis of species differences under steady-state. Journal of Experimental Botany 63: 643–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchi F, Zwieniecki MA. 2012. Analysis of xylem sap from functional (nonembolized) and nonfunctional (embolized) vessels of Populus nigra: chemistry of refilling. Plant Physiology 160: 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchi F, Zwieniecki MA. 2016. Accumulation of sugars in the xylem apoplast observed under water stress conditions is controlled by xylem pH. Plant Cell & Environment 39: 2350–2360. [DOI] [PubMed] [Google Scholar]
- Secchi F, Perrone I, Chitarra W, Zwieniecka AK, Lovisolo C, Zwieniecki MA. 2013. The dynamics of embolism refilling in abscisic acid (ABA)-deficient tomato plants. International Journal of Molecular Sciences 14: 359–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler C, Harshavardhan VT, Rajesh K, et al. . 2011. ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions. Journal of Experimental Botany 62: 2615–2632. [DOI] [PubMed] [Google Scholar]
- Sellin A, Kupper P. 2007. Temperature, light and leaf hydraulic conductance of little-leaf linden (Tilia cordata) in a mixed forest canopy. Tree Physiology 27: 679–688. [DOI] [PubMed] [Google Scholar]
- Sellin A, Õunapuu E, Kupper P. 2008. Effects of light intensity and duration on leaf hydraulic conductance and distribution of resistance in shoots of silver birch (Betula pendula). Physiologia Plantarum 134: 412–420. [DOI] [PubMed] [Google Scholar]
- Sevanto S. 2018. Drought impacts on phloem transport. Current Opinion in Plant Biology 43: 76–81. [DOI] [PubMed] [Google Scholar]
- Shatil-Cohen A, Attia Z, Moshelion M. 2011. Bundle‐sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: a target of xylem‐borne ABA? The Plant Journal 67: 72–80. [DOI] [PubMed] [Google Scholar]
- Silim S, Nash R, Reynard D, White B, Schroeder W. 2009. Leaf gas exchange and water potential responses to drought in nine poplar (Populus spp.) clones with contrasting drought tolerance. Trees 23: 959–969. [Google Scholar]
- Silvente S, Sobolev AP, Lara M. 2012. Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLoS One 7: e38554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Stitt M. 2007. Coordination of carbon supply and plant growth. Plant Cell & Environment 30: 1126–1149. [DOI] [PubMed] [Google Scholar]
- Sorrentino G, Haworth M, Wahbi S, Mahmood T, Zuomin S, Centritto M. 2016. Abscisic acid induces rapid reductions in mesophyll conductance to carbon dioxide. PLoS One 11: e0148554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmans M, Iliaens S, Hoebregs H. 2004. Enzymatic, spectrophotometric determination of glucose, fructose, sucrose, and inulin/oligofructose in foods. Journal of AOAC International 87: 1200–1207. [PubMed] [Google Scholar]
- Tardieu F, Simonneau T, Parent B. 2015. Modelling the coordination of the controls of stomatal aperture, transpiration, leaf growth, and abscisic acid: update and extension of the Tardieu–Davies model. Journal of Experimental Botany 66: 2227–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattini M, Velikova V, Vickers C, et al. . 2014. Isoprene production in transgenic tobacco alters isoprenoid, non‐structural carbohydrate and phenylpropanoid metabolism, and protects photosynthesis from drought stress. Plant Cell & Environment 37: 1950–1964. [DOI] [PubMed] [Google Scholar]
- Thalmann MR, Pazmino D, Seung D, et al. . 2016. Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. The Plant Cell 28: 1860–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DS, Eamus D. 2002. Seasonal patterns of xylem sap pH, xylem abscisic acid concentration, leaf water potential and stomatal conductance of six evergreen and deciduous Australian savanna tree species. Australian Journal of Botany 50: 229–236. [Google Scholar]
- Thompson AJ, Andrew J, Mulholland BJ, et al. . 2007. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiology 143: 1905–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombesi S, Nardini A, Frioni T, et al. . 2015. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Scientific Reports 5: 12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Hammel HT. 1972. The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. Journal of Experimental Botany 23: 267–282. [Google Scholar]
- Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R. 2003. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425: 734–737. [DOI] [PubMed] [Google Scholar]
- Van Oosten JJ, Wilkins D, Besford RT. 1994. Regulation of the expression of photosynthetic nuclear genes by CO2 is mimicked by regulation by carbohydrates – a mechanism for the acclimation of photosynthesis to high CO2. Plant Cell and Environment 17: 913–923. [Google Scholar]
- Vincelli P, Amsden B. 2013. Comparison of tissue-disruption methods for PCR-based detection of plant pathogens. Plant Disease 97: 363–368. [DOI] [PubMed] [Google Scholar]
- Voicu MC, Zwiazek JJ, Tyree MT. 2008. Light response of hydraulic conductance in bur oak (Quercus macrocarpa) leaves. Tree Physiology 28: 1007–1015. [DOI] [PubMed] [Google Scholar]
- Wang X, Du T, Huang J, Peng S, Xiong D. 2018. Leaf hydraulic vulnerability triggers the decline in stomatal and mesophyll conductance during drought in rice. Journal of Experimental Botany 69: 4033–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Zheng LL, Lin H, Yu F, Sun LH, Li LM. 2017. Grape hexokinases are involved in the expression regulation of sucrose synthase- and cell wall invertase-encoding genes by glucose and ABA. Plant Molecular Biology 94: 61–78. [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. 1997. Xylem sap pH increase: a drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiology 113: 559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. 2002. ABA‐based chemical signalling: the co‐ordination of responses to stress in plants. Plant Cell & Environment 25: 195–210. [DOI] [PubMed] [Google Scholar]
- Xie XD, Wang YB, Williamson L, et al. . 2006. The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Current Biology 16: 882–887. [DOI] [PubMed] [Google Scholar]
- Xiong D, Douthe C, Flexas J. 2018. Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species. Plant Cell & Environment 41: 436–450. [DOI] [PubMed] [Google Scholar]
- Xu M, Zhang B, Su X, Zhang S, Huang M. 2011. Reference gene selection for quantitative real-time polymerase chain reaction in Populus. Analytical Biochemistry 408: 337–339. [DOI] [PubMed] [Google Scholar]
- Yang Z, Liu J, Tischer SV, et al. . 2016. Leveraging abscisic acid receptors for efficient water use in Arabidopsis. Proceedings of the National Academy of Sciences USA 113: 6791–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Mogami J, Yamaguchi-Shinozaki K. 2015. Omics approaches toward defining the comprehensive abscisic acid signaling network in plants. Plant and Cell Physiology 56: 1043–1052. [DOI] [PubMed] [Google Scholar]
- Zeller G, Henz SR, Widmer CK, Sachsenberg T, Rätsch G, Weigel D, Laubinger S. 2009. Stress-induced changes in the Arabidopsis thaliana transcriptome analyzed using whole-genome tiling arrays. The Plant Journal 58: 1068–1082. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Sussmilch F, Nichols DS, Cardoso AA, Brodribb TJ, McAdam SA. 2018. Leaves, not roots or floral tissue, are the main site of rapid, external pressure-induced ABA biosynthesis in angiosperms. Journal of Experimental Botany 69: 1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Schurr U, Davies W. 1987. Control of stomatal behaviour by abscisic acid which apparently originates in the roots. Journal of Experimental Botany 38: 1174–1181. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.