Abstract
Background and Aims:
The transcription factors Foxf1 and Foxf2 have been implicated in the development of the gastrointestinal tract but their role in adults or in gastrointestinal diseases is poorly understood. We have recently shown that expression of serum response factor (SRF), a transcription factor whose activity is modulated by Foxf proteins, is decreased in the stomach muscularis of patients with gastroparesis. The aim of the current study was to determine if FOXF expression is decreased in gastroparesis patients and if loss of Foxf1 and/or Foxf2 from adult smooth muscle is sufficient to impair gastric emptying in mice.
Methods:
Full thickness stomach biopsy samples were collected from control subjects and from patients with gastroparesis. mRNA was isolated from the muscularis externa and FOXF mRNA expression levels were determined by quantitative reverse transcriptase (RT)-PCR. Foxf1 and Foxf2 were knocked out together and separately from smooth muscle cells in adult mice and the subsequent effect on liquid gastric emptying and contractile protein expression were determined.
Key Results:
Expression of FOXF1 and FOXF2 is decreased in smooth muscle tissue from gastroparesis patients. Knockout of Foxf1 and Foxf2 together, but not alone, from mouse smooth muscle resulted in delayed liquid gastric emptying. Foxf1/2 double knockout mice had decreased expression of smooth muscle contractile proteins, SRF and myocardin in stomach muscularis.
Conclusions and Inferences:
Our findings suggest that decreased expression of Foxf1 and Foxf2 may be contributing to the impaired gastric emptying seen in gastroparesis patients.
Gastroparesis defined as delayed gastric emptying in the absence of a physical obstruction, is estimated to occur in about 1.8% of the adult population, with the majority of cases being in women.1,2 Symptoms of gastroparesis can be debilitating, dramatically lowering quality of life.3 Overall, gastrointestinal motility and functional bowel disorders cause a huge burden on the US healthcare system.4,5 Etiologies of gastroparesis are approximately 36% idiopathic (unknown etiology), 29% diabetic and 13% postsurgical.6,7 Gastric emptying is dependent on an intricate interaction of neural cells, interstitial cells of Cajal (ICC), platelet derived growth factor α positive (PDGFRα+) fibroblasts and smooth muscle cells (SMCs).8–12 ICC are the pacemaker cells in the stomach that transmit slow wave depolarization to the gastric smooth muscle cells.10,13 The PDGFRα+ fibroblasts are a more recently recognized component of the electrical syncytium that controls gastric contractility. These cells transmit inhibitory neural signals to gastric smooth muscle cells.8 It is likely that defects in any of these cell types could impair gastric emptying resulting in gastroparesis.
Histopathological analyses have suggested decreases in numbers of ICCs and increases in numbers of CD45 and CD68 positive cells in gastric full-thickness biopsies from diabetic and idiopathic gastroparesis patients.14 In contrast, it has been reported that there are no changes in the PDGFRα+ cell number in either group of gastroparesis patients.9 It has also been proposed that an increased ratio of M1 relative to M2 macrophages may contribute to delayed gastric emptying in diabetic gastroparesis.15 We have recently challenged some of these findings using qRT-PCR analysis of RNA isolated from the stomach muscularis of control and gastroparesis patients. We found that idiopathic gastroparesis is associated with decreased mRNA encoding smooth muscle cell contractile proteins and PDGFRα+ without a change in mRNA encoding ICC markers.16 The altered expression of mRNA encoding contractile proteins was correlated with decreased expression of serum response factor (SRF) a transcription factor that is crucial for regulating the differentiation state of smooth muscle cells.17 Knockout of SRF from adult smooth muscle cells has been show to impair intestinal motility resulting in pseudo-obstruction.18,19 SRF can promote the proliferation, migration or differentiation of smooth muscle cells depending on which other transcription factors and co-regulators that it interacts with.20 One critical co-regulator that promotes SRF’s myogenic activity is myocardin.21 During the biphasic remodeling that occurs in the intestine following partial obstruction, the proliferative phase is associated with decreased expression of myocardin and SRF and decreased binding of SRF to the promoters of smooth muscle contractile protein genes.22 In contrast, in the hypertrophic phase myocardin expression is increased and more SRF is found at the promoters of contractile protein genes.22
We have previously shown that the transcription factors Foxf1 and Foxf2 also interact with SRF and myocardin to regulate transcriptional activity within gastrointestinal smooth muscle cells.23,24 Loss of Foxf1 from smooth muscle cells during embryonic development resulted in early postnatal lethality that was associated with severe abnormalities in the gastrointestinal tract, including markedly dilated esophagus and stomach.24 In contrast, loss of Foxf2 from smooth muscle cells during embryonic development resulted in an expansion of the myenteric nerve plexus that may have been associated with increased expression of PDGFb.23
Given the known roles of Foxf, myocardin and SRF complexes in regulating the phenotype of smooth muscle cells and our previous findings of altered expression of mRNAs encoding some smooth muscle contractile proteins in gastric muscle of patients with gastroparesis, we set out to determine the potential roles of the Foxf proteins in regulating gastric emptying and contributing to the pathology of gastroparesis.
Materials and Methods
Clinical assessment and human full-thickness stomach biopsies.
This study received IRB approval from the Indiana University Institutional Review Board (protocol #1309147354R004). Biopsies from the control group of human subjects were obtained from either non-diabetic or diabetic individuals without gastroparesis symptoms that were undergoing bariatric weight loss surgery as described previously (normal HbA1c level < 1 month prior to surgery in non-diabetic group)16. Control and gastroparesis subjects were age and sex matched. The average ages of the non-diabetic control and gastroparesis patients were 42.8±7.8 years and 34.6±10.1 years, respectively, with 11 of 14 (79%) control subjects being female compared to 14 out of 16 (87%) patients with gastroparesis. The average ages of the diabetic control and gastroparesis patients were 52.1±12.1 years and 40.9±10.5 years, respectively with 78% of the diabetic control patients and 82% of the diabetic gastroparesis patients being female. Gastroparesis was defined as patients with symptoms of gastroparesis ≥ 3 months with delayed gastric emptying by 4-hour gastric scintigraphy or gastric bezoar with undigested foods by upper endoscopy after an overnight fast. Gastric scintigraphy was performed following consumption of a low fat egg-white meal with imaging at 0, 2 and 4hrs as described previously.25,26 Abnormal gastric emptying was defined as 2-hour retention ≥60% or 4-hour retention ≥10%.
Surgical full-thickness biopsies from the gastroparesis patients were obtained at the time of routine pathological analysis in patients with severe idiopathic gastroparesis or at the time of gastric stimulator placement. In each case (control and gastroparesis patients), the biopsy was taken about 10cm from the pylorus on the anterior aspect midway between the greater and lesser curvatures of the stomach. All laproscopic biopsies were obtained using linear cutting staplers. For sleeve gastrectomy subjects the sample was obtained after the stomach was removed from the abdomen. Tissues were collected into ice cold DMEM (Dullbeco’s modified eagle media) or RNAlater (Ambion) and immediately transferred to the investigator’s laboratory. Muscle, submucosal and mucosal layers were separated by dissection, flash frozen in liquid nitrogen and ground to a powder under liquid nitrogen prior to storage at −80°. A small amount of frozen muscle tissue powder (~150mg) was homogenized in Trizol (Invitrogen) and a Purelink Micro RNA isolation kit was used to isolate total RNA. Contaminating DNA was removed by on-column DNase treatment. The integrity and purity of the isolated RNA was determined using an Agilent Bioanalyzer. Samples used had an RNA integrity number of >7.
qRT-PCR.
500ng of RNA was used in reverse transcription reactions (High Capacity RT-cDNA Kit, Life Technologies). Real time PCR was performed using Syber Green (Roche). For samples from human subjects levels of mRNA expression were normalized to expression of TATA binding protein (TBP) as an internal control and are expressed relative to the mean values seen in samples from control patients. For samples from mice levels of mRNA expression were normalized to expression of Hprt as an internal control and are expressed relative to the mean values seen in samples from control mice (either corn oil treated Foxf1ff Myh11creER(T2)+/−, Foxf2ff Myh11creER(T2)+/− or Foxf1/2ff Myh11creER(T2)+/− mice or tamoxifen treated Foxf1ff, Foxf2ff or Foxf1/2ff mice, as indicated). Primers used for PCR are indicated in Supplemental Table 1.
Smooth muscle-specific knockout of Foxf1 and Foxf2 in adult mice.
All mouse studies were performed in accordance with procedures approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Foxf1ff and Foxf2ff mice, described previously23,24, were crossed with Myh11-creER(T2) mice27 to generate Foxf1ff Myh11creER(T2)+/−, Foxf2ff Myh11creER(T2)+/− or Foxf1/2ff Myh11creER(T2)+/− mice. In order to induce knockout of Foxf genes at 5–6 weeks of age these mice were treated with tamoxifen once a day for 5 days (100μl of 10mg mL−1 tamoxifen in corn oil given IP). Control mice were either mice of the same genotype given corn oil injections instead of tamoxifen or were Foxf1ff, Foxf2ff of Foxf1ff/f2ff mice treated with tamoxifen. Only male mice were used for all experiments as the Myh11creER(T2) transgene is on the Y chromosome. For some experiments we crossed the floxed mice onto myocdmyc-HA/myc-HA mice in which a myc-HA epitope tag was introduced into the carboxy-terminus of the endogenous myocardin using Crispr.28 Ultimately this resulted in generation of Foxf1fff2ff Myocdmyc-HA/myc-HA Myh11cre ER(T2)−/+ that were used in experiments. The Myocdmyc-HA/myc-HA mice were generated by co-injection of a guide RNA (acagcagugguaaacacccg), targeting oligonucleotide (caacatcgattttctggatgttacagatcttaatctgaattcccctatggatctccacttacagcagtgggaacaaaaactcatctcagaagaggatctgaattacccatacgatgttccagattacgcttgaacacccgaggtacaagagctacgagagctcagtgggaattcaatggaggaaagcacgataccggaaa) and Cas9 protein (obtained from SAGE Labs – now Horizon Discovery) into fertilized oocytes obtained from C57BL/6J mice. Insertion of this sequence into the 3’ end of myocardin results in expression of myocardin with carboxyl-terminal myc and HA epitope tags (carboxyl-terminal amino acid sequence: LQQW-EQKLISEEDLNYPYDVPDYA*, myc and HA epitopes are underlined). Correctly targeted mice were identify by PCR and confirmed by direct DNA sequencing. Mice were outbred to C57BL/6J mice for 3 generations before homozygous Myocdmyc-HA/myc-HA and Foxf1fff2ffMyocdmyc-HA/myc-HA were generated. Myocdmyc-HA mice are available from JAX (Stock # 030703, C57BL/6J-Myocd<em1Bph>/J).
Immunofluorescence imaging.
For confocal imaging, mouse tissues (stomach body or aorta) were fixed in 4% paraformaldehyde for 2 hours on ice. Following fixation, tissues were washed in phosphate buffered saline for 3× 5 minutes and then cryoprotected by incubation in PBS containing 20% sucrose overnight at 4° before being frozen in OCT tissue freezing media (Tissue-Tech) on a bed of dry ice/2-methylbutane. Cryosections (8μm) were cut and stored at −80°. For immuno-fluorescent staining cryosections were permeabilized in 0.2% triton in tris buffered saline (TBS) for 5 minutes, washed in TBS for 5 minutes them blocked in 5% donkey serum diluted in TBS at room temperature for 1 hour. Blocked sections were incubated for 48 hours at 4° with primary antibodies to Foxf1 (1:400, Goat polyclonal antibody AF4798, 1:800, R&D Systems, Minneapolis, MN), Foxf2 (1:200, Sheep polyclonal antibody AF6988, R&D Systems, Minneapolis, MN), HA (1:100, Rabbit monoclonal antibody C29F4, Cell Signaling, Danvers, MA), kit (Rabbit monoclonal antibody D13A2, Cell Signaling, Danvers, MA), PDGFRα (1:200, rabbit polyclonal antibody, 3164, Cell Signaling, Danvers, MA), or PGP9.5 (1:200, Rabbit polyclonal antibody A01398–40, Genscript, Piscataway, NJ) diluted in 5% donkey serum/TBS. Some sections were incubated without primary antibody as a negative control. Following washing in TBS, primary antibodies were detected by incubation with anti-goat, rabbit or sheep Alexa Fluor 647, Alexa Fluor 488 or Rhodamine-X (1:1,000, Jackson ImmunoResearch) as appropriate. For slides in which smooth muscle α-actin was visualized, Cy3 conjugated anti-smooth muscle α-actin antibody (1:250, Mouse monoclonal clone 1A4, #C6198 MilliporeSigma, Burlington, MA) was added along with the other secondary antibodies. After washing slides were mounted in Prolong Gold containing DAPI (Invitrogen) and visualized by confocal microscopy. Images shown are single confocal slices.
Western blotting.
For Western blotting protein lysates were generated by homogenizing frozen stomach antrum (in which the mucosa had been removed by careful dissection prior to freezing) in RIPA lysis buffer (1%NP-40, 1% sodium deoxycholate, 0.1% SDS, 0.15M NaCl, 0.01M sodium phosphate pH7.2, 2mM EDTA, 50mM NaFl) in a glass homogenizer on ice. Insoluble material was removed by a brief centrifugation and protein concentrations of the lysates determined using a BCA assay (Biorad). Either 5 or 25μg aliquots of lysates were separated on 7.5% acrylamide gels. Proteins were transferred to nitrocellulose membranes overnight at 70mA in a buffer containing 0.192M glycine, 0.25M Tris, 0.05%SDS, 20% methanol, following transfer, blots were stained with Ponceau S to visualized the transferred proteins then blocked in 5% BSA dissolved in 10mM Tris ph7.5, 150mM NaCl, 0.05% tween 20 (TBST). Blots were incubated with primary antibodies in the same solution overnight at 4°C. Primary antibodies used for western blotting were against; myh11 (SM1 and SM2 isoforms),29 HA (NB600–363, 1μg/ml, Novus Biologicals, Littleton, CO), calponin (13938–1-AP, 0.2μg mL−1, Proteintech), SM22α (1:10,000, rabbit polyclonal antibody raised against the peptide CGPDVGRPDRGRLGFQVW (residues 38–55 of mouse, rat and human SM22α, Proteintech), ACTA2 (Clone 1A4, 1:10000, Sigma), vinculin (Clone VIN11-A5, 1:10000, Sigma), gamma1 tubulin (Clone GTU-88, 1:1000, Millipore-Sigma). Following washing with TBST (3× 5minutes) blots were incubated with the appropriate horse radish peroxidase conjugated secondary antibodies diluted in TBST containing 2% gelatin at room temperature for 45 minutes. Protein bands were visualized on a G-box imager (Syngene) following incubation with ECL Western blotting substrate (Pierce).
Liquid gastric emptying assays.
Prior to liquid gastric emptying assays mice were deprived of food and bedding for about 18 hours. Two hours following removal of water from the mice they were given an oral gavage of 100μl of Rhodamine dextran in methylcellulose (2.5mg mL−1 Rhodamine B dextran 70K dissolved in 1% methylcellulose). 15 minutes following the gavage the mice were euthanized by cervical dislocation and the esophagus, duodenum and ileum quickly clamped. The stomach and small intestine were then removed from the mouse. The stomach was placed in 4mL of saline and the small intestine was divided into 8 equal length portions and each portion added to a tube containing 4mL of saline. Stomach and intestinal segments were homogenized using a polytron (Kineticore), samples were clarified by centrifugation (13,000g for 5 minutes). Rhodamine B was detected in 100μL aliquots of the lysates using a fluorescence plate reader (579nm excitation, 590nm emission.) % gastric emptying = [sum of all fluorescence – stomach fluorescence] / all fluorescence ×100
Statistical analysis
Significant differences between the means values of the gastroparesis and control patients or wild type and knockout mice were determined using a two-tailed, Welch corrected student T-test in order to account for potential differences in the variance in each group. P values <0.05 were considered significant. Experiments which compared expression between more than two groups used a one-way Anova analysis to determine statistical significance (Kruskal Wallis test with a Dunn’s Multiple Comparison post test).
Results
FOXF expression is decreased in human patients with gastroparesis.
Expression of FOXF1 and FOXF2 mRNA was measured by qRT-PCR in the isolated stomach muscularis obtained from full thickness biopsies of control patients with no symptoms of gastroparesis and from patients with gastroparesis. Two groups of control patients were used. The first group included 14 patients undergoing bariatric weight loss surgery, 5 patients from sleeve gastrectomy and 9 patients from Roux-en-Y bypass surgery. None of these 14 controls subjects had diabetes (normal HbA1c < 1 month prior to surgery) (Figure 1A). The clinical description of these patients was described previously.16 The second group included 11 patients undergoing bariatric weight loss surgery that had diabetes. (Figure 1B). We also analyzed samples from two groups of patients with gastroparesis one group with no diabetes (16 patients with idiopathic gastroparesis described previously,16 Figure 1A) and one group in which the patients also had diabetes (9 patients with diabetic gastroparesis, Figure 1B). This analysis revealed that FOXF1 and FOXF2 mRNA levels were significantly decreased in the patients with idiopathic gastroparesis (Figure 1A) and FOXF1 mRNA was also significantly decreased in the patients with diabetic gastroparesis (Figure 1B).
Figure 1. Expression of FOXF1 and FOXF2 in stomach muscle from human subjects with gastroparesis.
Total RNA was isolated from the muscle layer of biopsies obtained from 14 control subjects and 16 subjects with idiopathic gastroparesis, with no diabetes (A) or 11 control subjects with diabetes and 9 subjects with diabetic gastroparesis (B). qRT-PCR was used to measure the expression of each of the mRNAs indicated. Data presented were normalized to an internal control encoding TBP and are expressed relative to levels in the respective control subjects. Relative expression =2−ΔΔCt, where ΔΔCt = (CtGastroparesis-CtTBP) – (Ctcontrol-CtTBP). Each circle represents an individual subject with the control subjects represented as open circles and the gastroparesis subjects as closed circles. The mean±SEM are also indicated. Student T-tests were used to identify statistical significance. P-values are indicated.
Knockout of Foxf1 and Foxf2 from adult gastric smooth muscle cells.
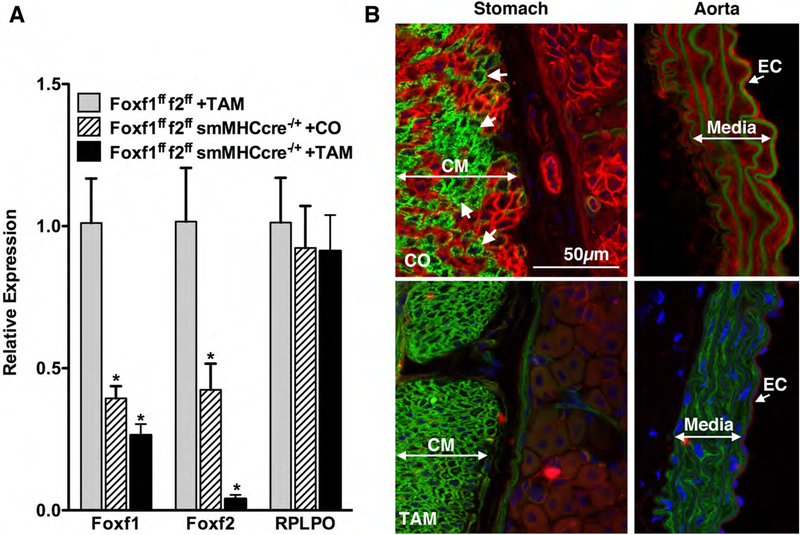
In order to determine if loss of FOXF1 and/or FOXF2 may be contributing to the impaired gastric motility seen in human patients with gastroparesis we generated a mouse model in which Foxf1 and/or Foxf2 were specifically ablated in adult smooth muscle cells. To generate these mice the previously described Foxf1 and Foxf2 floxed mice23,24 were crossed with mice expressing a tamoxifen regulated cre recombinase driven by the smooth muscle myosin heavy chain (Myh11) promoter (Myh11creER(T2)27, as described in ‘Methods’. The efficacy of the knockout of Foxf1 and Foxf2 proteins was determined by qRT-PCR and immunohistological analysis of cryosections cut from the stomach body of tamoxifen treated male Foxf1fff2ff Myh11creER(T2)−/+ mice compared to control mice either of the same genotype treated with corn oil or from Foxf1fff2ff mice treated with tamoxifen (Figures 2 and 3). Surprisingly we observed a significant decrease in Foxf1 and Foxf2 expression in Foxf1fff2ff Myh11creER(T2)−/+ mice treated with corn oil, suggesting that the creER(T2) transgene is at least partially active in the absence of tamoxifen (Figure 2A). To further examine this possibility we visualized the activity of the cre recombinase by crossing the Myh11creER(T2) mice with a cre-dependent mTmG reporter mouse as described previously.30 Fluorescence imaging of corn oil (CO) or tamoxifen (TAM) treated Myh11creER(T2)−/+ mTmG+/− mice revealed cre-dependent expression of the mEGFP reporter uniformly in the circular muscle layer (CM) of stomach and in the media of the aorta upon tamoxifen treatment. However, patchy cre-dependent expression of the mEGFP was also seen in stomach circular muscle of corn oil treated mice, although the aorta remained mEGFP negative and mTomato positive. The partial loss of Foxf1 and Foxf2 in the corn oil treated Foxf1fff2ff Myh11creER(T2)−/+ mice was further confirmed by immunofluorescence localization of the Foxf proteins (Figure 3). This analysis further confirmed the effective deletion of Foxf1 and Foxf2 from gastric SMCs in tamoxifen treated Foxf1fff2ff Myh11creER(T2)−/+ mice (Figure 3). Both Foxf1 and Foxf2 co-localized in gastric SMCs of control mice (Figure 3 and Supplemental data figure 1). The residual Foxf1 and to a lesser extent Foxf2 mRNA expression detected in the gastric antrum muscularis of knockout mice (Figure 2A), appears to result from Foxf expression in other cell types (Figure 3). In support of this, Foxf1 and Foxf2 expression was also detectable in kit positive interstitial cells of Cajal (ICC) within the gastric muscularis, and this expression was not altered following tamoxifen treatment of Foxf1fff2ff Myh11creER(T2)−/+ mice (Figure 4). Lower levels of Foxf1 and Foxf2 expression were also detectable in PDGFRα positive fibroblasts (Supplemental data figure 2) and in submucosal and mucosal fibroblasts, although neural ganglia did not express detectable levels of Foxf1 or Foxf2 (Supplemental data figure 2).
Figure 2. Myh11creER(T2) exhibits tamoxifen independent activity in the stomach.
A. qRT-PCR analysis of Foxf1 and Foxf2 expression in stomach antrum isolated from the indicated mouse strains four weeks after treatment with tamoxifen or corn oil (1mg (100μl) daily for 5 days). Data presented were normalized to an internal control encoding Hprt and are expressed relative to levels in the Foxf1fff2ff mice treated with tamoxifen(control). * indicates significant differences (P<0.05) from Foxf1fff2ff + TAM mice determined by one-way Anova analysis. Relative expression =2−ΔΔCt, where ΔΔCt = (Cttest-CtHprt) – (Ct control-CtHprt). An additional housekeeping gene (RPLPO, Ribosomal protein lateral stalk subunit P0) was analyzed as a further control. B. To visualize the activity of the cre recombinase encoded by the smHCcreER(T2) transgene we crossed these mice to the mTmG reporter mouse as described previously.30 Fluorescence imaging of corn oil (CO) or tamoxifen (TAM) treated Myh11creER(T2)−/+ mTmG mice revealed cre-dependent expression of the mEGFP reporter uniformly in the circular muscle layer (CM) of stomach body and in the media of the aorta upon tamoxifen treatment. However, patchy cre-dependent expression of the mEGFP was also seen in stomach body circular muscle of corn oil treated mice, although the aorta remained mTomato positive. White arrows indicate patches of mEGFP positive smooth muscle cells in the corn oil treated mice.
Figure 3. Foxf proteins are efficiently knocked out of smooth muscle cells in Foxf1fff2ff Myh11creER(T2)−/+ mice treated with tamoxifen.
Immunofluorescence staining of Foxf1 (white nuclei), Foxf2 (green nuclei) and ACTA2 (red) in stomach body of tamoxifen (TAM) treated Foxf1/2ff mice and Foxf1ff Myh11creER(T2)+/− mice treated with corn oil (CO) or tamoxifen (TAM). Mice were treated with tamoxifen or corn oil at 5 weeks of age, once a day for 5 days. Four weeks later tissues were harvested and processed for immunofluorescence microscopy as described in ‘Methods’. White arrows indicate Foxf1 and Foxf2 positive smooth muscle cells. Yellow arrows indicate other cells that are Foxf1 and Foxf2 positive (likely ICCs).
Figure 4. Foxf proteins are efficiently knocked out of smooth muscle cells but remain expressed in ICC and mucosal fibroblasts.
Immunofluorescence staining of Foxf1 (red), Foxf2 (green), Kit (cyan) and nuclei (blue) in stomach body of tamoxifen treated, Foxf1ff/2ff control mice and Foxf1ff/2ff Myh11creER(T2)+/− mice (Foxf1f2 smKO). Mice were treated with tamoxifen at 5 weeks of age, once a day for 5 days. Four weeks later tissues were harvested and processed for immunofluorescence microscopy as described in ‘Methods’. Yellow arrows indicate Foxf1 and Foxf2 positive ICCs (Kit positive). CM- circular muscle, MG- myenteric ganglia.
Ablation of Foxf1 and Foxf2 from adult mouse gastric smooth muscle cells results in impaired liquid gastric emptying.
To determine if loss of FOXF1 or FOXF2 in humans could be contributing to impaired gastric emptying in gastroparesis patients we examined the rate of liquid gastric emptying in mice lacking Foxf1, Foxf2 or both proteins. This analysis revealed that mice lacking Foxf1 and Foxf2 had significantly impaired liquid gastric emptying compared to control mice (Figure 5). Neither single knockout or double heterozygous mice exhibited this phenotype and all control mice examined had similar liquid gastric emptying rates (Figure 5).
Figure 5. Knockout of Foxf1 and Foxf2 results in delayed liquid gastric emptying.
Foxf1ff Myh11creER(T2)+/− (smFoxf1 KO), Foxf2ff Myh11creER(T2)+/− (smFoxf2 KO), Foxf1f+/2f+ Myh11creER(T2)+/− (smFoxf1/2 Het) or Foxf1ff/2ff Myh11creER(T2)+/− (smFoxf1/2 KO) mice were treated with corn oil (CO) or tamoxifen (TAM) at 5 weeks of age, once a day for 5 days. The smFoxF1/2 KO MyocdHA/HA graph shows data from Foxf1ff/2ff Myocd HA/HA mice (grey bar) and Foxf1ff/2ff Myh11creER(T2)+/− MyocdHA/HA mice (black bar) both treated with tamoxifen. Four weeks later liquid gastric emptying was assayed following oral gavage of rhodamine dextran. The mean±SD percent gastric emptying is shown. The numbers in brackets represent the number of mice in each group. * indicates significant difference between CO and Tam treated mice as determined by T test. No significant differences were observed between the gastric emptying rates of control mice (One-way Anova).
Foxf1 and Foxf2 double knockout mice exhibit marked decreases in mRNAs encoding contractile proteins.
qRT-PCR analysis of stomach antrum obtained from Foxf1/2 double knockout mice revealed a significant decrease in mRNAs encoding contractile proteins such as Myh11, Acta2, calponin and SM22α (Figure 6). This was accompanied by decrease in mRNAs encoding myocardin and SRF, two transcriptional activators that are known to be required for the expression of these smooth muscle proteins. We also observed a small but significant decrease of Kitlg mRNA and a marked decrease in BMP4 mRNA (Figure 6). A significant decrease in myocardin mRNAs was also observed in the single Foxf2 knockout mice whereas in the single Foxf1 knockout mice we observed significant decreases in expression of calponin and myocardin (Figure 6).
Figure 6. Knockout of Foxf1 and Foxf2 from adult mouse smooth muscle results in decreased expression of mRNAs encoding smooth muscle contractile proteins.
qRT-PCR analysis of mRNA expression in antrum smooth muscle from knockout mice. Analysis was performed as described in figure 2, mRNA levels in each of the knockout mice were expressed relative to a common group of tamoxifen treated Foxf1ff/f2ff, control mice (n=15). Foxf1ff Myh11creER(T2)+/− (F1KO) (n=8), Foxf2ff Myh11creER(T2)+/− (F2KO) (n=6) or Foxf1ff/2ff Myh11creER(T2)+/− (F12KO) (n=12) were treated with tamoxifen (TAM) as described in figure 2. Data shown are mean±SD * P<0.05 determined by one-way Anova analysis.
SRF and myocardin proteins are decreased in Foxf1/2 double knockout mice.
To determine if the transcriptional changes that we observed in figure 6 also reflect changes in protein levels we performed a Western blot analysis. As there are no commercially available antibodies that can reliably detect endogenous myocardin protein we first generated a knockin mouse in which we knocked in myc-HA epitope tags into the 3’ end of the endogenous myocd gene (Methods). This allowed us to use antibodies to HA to detect the endogenous myocardin protein. The MyocardinHA/HA mice were crossed into the Foxf1ff2ff mice so that the knockout mice had one or both alleles of myocd tagged with HA. Western blot analysis revealed a significant decrease in myocardin, SRF, myh11 (SM1 and SM2 isoforms), calponin and SM22α protein levels in the stomach antrum of Foxf1/2 double knockout mice (Figure 7). We also observed a decrease in the SMC selective meta-vinculin. Confocal microscopy also revealed decreased myocardin-HA expression in gastric SMCs of the Foxf1/2 double knockout mice (Supplemental data figure 3).
Figure 7. Knockout of Foxf1 and Foxf2 from adult mouse smooth muscle results in decreased expression of myocardin and SRF proteins.
A. Western blot analysis of protein expression in antrum smooth muscle from control Foxf1ff2ff MyocdHA/HA mice, smF12KO MyocdHA/HA mice and Foxf1ff/2ff control mice. All mice were treated with tamoxifen as described in methods. 1μg (SM1, SM2, vinculin, calponin, SM22α, GAPDH) or 25μg (HA, SRF, GAPDH, γ-tubulin) of protein were separated on 7.5% (SM1, SM2, HA, SRF, vinculin, ACTA2) and 15% (calponin, SM22α, GAPDH) polyacrylamide gels and analyzed by western blotting. Molecular mass markers are indicated to the left of the blots (kDa). B. Quantitation of the levels of protein expression observed in the blots in panel A. Expression levels were normalized relative to γ-tubulin. P-values from student T-tests between F1ffF2ff MyocdHA/HA and smF12KO MyocdHA/HA samples are indicated.
Discussion
Results from our studies show that FOXF1 and FOXF2 mRNA is decreased in the stomach of human patients with gastroparesis and that loss of Foxf1 and Foxf2 in adult mice is sufficient to result in delayed liquid gastric emptying. Although it is unlikely that the observed decreased expression of FOXF1 and FOXF2 in humans is in itself sufficient to cause gastroparesis our findings from knockout mice suggest that the decreased FOXF expression may be contributing to the pathogenesis of gastroparesis in humans. This proposal must, however, be interpreted within the limitations of our study. Firstly, we have not been able to confirm that the decreased FOXF1 and FOXF2 mRNA expression in humans is mirrored by decreased FOXF protein expression, as we have not been able to detect these proteins by Western blotting using our antibodies. Secondly, although the Foxf1/f2 double knockout mice had impaired liquid gastric emptying and decreased expression of smooth muscle contractile proteins, we have not directly measured the contractility of the stomach muscle in these mice. Although, as we have previously shown that smooth muscle-specific knockout of Foxf1 during embryonic development results in decreased contractile protein expression and impaired intestinal contractility, it seems reasonable to suggest that stomach contractility may be impaired in the adult smooth muscle-specific Foxf1/f2 double knockout mice described in the current study.24 Finally, although gastroparesis is more prevalent in females than males in humans we have not been able to access the effects of Foxf1/f2 knockout in female mice as the Myh11 creER(T2) transgene used in our study is located on the Y chromosome.27
In the samples obtained from humans with gastroparesis, there was reduced but not abolished expression of FOXF1 and FOXF2 mRNA (Figure 1). However, in mice we only observed impaired gastric emptying in the homozygous knockout mice, neither heterozygous mice or corn oil treated Foxf1fff2ff Myh11creER(T2)−/+ mice that had~50% reduction in Foxf1 and Foxf2 mRNA levels exhibited any detectable defects in liquid gastric emptying (Figures 2,5). Moreover both these latter two groups of mice had similar rates of liquid gastric emptying compared to control tamoxifen treated Foxf1fff2ff mice (Figure 5). However, it is important to note that in mice we measured gastric emptying of a liquid meal whereas in humans a solid meal is used for gastric emptying studies. It is possible that this difference in methodology is affecting the sensitivity of our detection of delayed emptying in mice. Alternatively, our findings may suggest that either humans are more sensitive to decreased FOXF expression, or more likely that there are other changes that occur in the human subjects that act together with decreased smooth muscle FOXF1/2 levels leading to impaired gastric emptying. Consistent with this latter possibility, it has recently been shown that FOXF1 plays a critical role in ICC, which would not be predicted to be affected in our smooth muscle-specific Foxf1/2 knockout mice.31 Furthermore, both Foxf1 and Foxf2 are readily detectable in ICC of both control and smFoxf1/2 knockout mice (Figure 4). In the smFoxf1/2 knockout mice we did observe statistically significant decrease in Kitlg mRNA, although there was no change in Kit or Ano1(Anoctamin 1) mRNA, two established markers of ICC.32 The decreased Kitlg expression would be consistent with previous studies that showed decreased Kitlg expression in a rodent diabetic gastroparesis model.33 However, the lack of change in the mRNAs encoding ICC markers is not consistent with the proposal that the decreased Kitlg results in decreased Kit signaling in ICC, leading to their death.33 The role of ICC loss or dysfunction in human gastroparesis is also not fully resolved, although several immunohistological studies have suggested that gastroparesis is associated with a loss of ICC,14,33–35 in a recent study we did not observe any detectable changes in mRNA levels of ICC markers KIT or ANO1 in patients with idiopathic gastroparesis.16 Whether these results reflect differences between protein and mRNA expression or other differences in the methodologies employed remains to be determined.
Previously we showed that Foxf2 knockout from SMCs during development results in elevated PDGFb expression and increased proliferation of SMCs in the longitudinal layer of the GI tract and an expansion of the myenteric plexus.23 Although we did not observe any changes in PDGFb expression when Foxf2 was knocked out from adult smooth muscle, we did see a small but significant increase in the pan neural marker, nestin in the Foxf2 KO mice, suggesting that there may be some neural expansion in these mice (Figure 6). Surprisingly, increased nestin expression was also seen in the Foxf1 KO mice yet not in the double knockout mice (Figure 6). The meaning of these findings are unclear. Although the lack of change in the double knockout mice suggest that the increased nestin expression in the single knockout mice is not simply a compensatory effect due to potentially impaired muscle activity, as this would be expected to be worse in the double knockout mice.
One surprising technical finding from our study was the tamoxifen independent activity of the Myh11creER(T2) transgene in gastric SMCs (Figure 2). This transgenic mouse line has been used extensively to achieve regulated smooth muscle-specific ablation of genes and most of these previous studies have shown tight tamoxifen dependent regulation of the cre recombinase activity.27,30,36 Our current findings suggest that although cre activity appears to be completely tamoxifen dependent in vascular smooth muscle there is a significant tamoxifen independent cre activity in GI smooth muscle (Figure 2). This highlights the importance of utilizing several different mouse control lines to both monitor potential effects of tamoxifen itself and to evaluate possible hypomorphic effects of partial gene deletion from GI smooth muscle in the absence of tamoxifen. Future studies utilizing this cre transgene should thus be interpreted with caution if they do not address these issues.
Early studies on Foxf proteins showed that they are part of a signaling pathway through which sonic hedgehog (shh) regulates patterning of the GI tract during embryonic development.37,38 In this pathway, shh released from epithelial cells stimulates the transcription of Foxf1 in adjacent mesodermal cells. In turn Foxf1 drives transcription of BMP4 which is released from the developing mesodermal cells and then feeds back to the GI epithelial cells to regulate their proliferation. Consistent, with these observations we have previously shown that deletion of Foxf1 from SMCs during development leads to impaired BMP4 expression. In the current study, we show that Foxf1 and Foxf2 appear to play partly redundant roles in regulating BMP4 expression in that we only observed a significant decrease in BMP4 mRNA expression in the double knockout mice (Figure 6). Examining the effects of the decreased BMP4 on the mucosa of adult mouse stomach will be an interesting avenue for future investigation.
In summary, we have shown that expression of mRNA encoding FOXF1 and FOXF2 is decreased in human subjects with gastroparesis and that loss of both these proteins from adult smooth muscle in mice is sufficient to impair liquid gastric emptying. These findings together with our previous studies showing Foxf regulation of SRF/myocardin transcriptional complexes23,24 and altered transcriptional activity of contractile protein genes in the stomach of patients with gastroparesis16 suggest that SRF containing transcriptional complexes may play a central role in pathological changes that lead to gastroparesis.
Supplementary Material
Key Points.
Gastroparesis is a debilitating disease with complex etiology including altered transcription regulation in gastric smooth muscle. We aimed to determine the role of forkhead transcription factors in gastric smooth muscle in the pathology of gastroparesis.
Forkhead transcription factors FOXF1 and FOXF2 are decreased in smooth muscle tissue from gastroparesis patients and knockout of Foxf1 and Foxf2 together, but not alone, from mouse smooth muscle resulted in delayed liquid gastric emptying associated with decreased expression of smooth muscle contractile proteins.
Our findings suggest that FOXF1 and FOXF2 are required for the normal function of the stomach and that loss of these proteins may contribute to the development of gastroparesis.
Acknowledgments, Funding and Disclosures.
We would like to thank Sarah Griffith for expert technical assistance and for mouse husbandry.
Grant support and other assistance: The study was supported by a Project Development Team within the ICTSI NIH/NCRR Grant Number UL1TR001108.
Abbreviations:
- ICC
Interstitial Cells of Cajal
- GI
gastrointestinal
- PDGFRα+ fibroblasts
Platelet derived growth factor receptor alpha positive fibroblasts
- SMCs
smooth muscle cells
- SRF
serum response factor
Footnotes
Disclosures: all of the authors attest that they have no competing interests
References
- 1.Rey E, Choung RS, Schleck CD, Zinsmeister AR, Talley NJ, Locke GR 3rd. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. Journal of neurogastroenterology and motility. 2012;18(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung HK, Choung RS, Locke GR 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkman HP, Hasler WL, Fisher RS, American Gastroenterological A. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127(5):1592–1622. [DOI] [PubMed] [Google Scholar]
- 4.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136(2):376–386. [DOI] [PubMed] [Google Scholar]
- 5.Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol. 2008;103(2):313–322. [DOI] [PubMed] [Google Scholar]
- 6.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140(1):101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43(11):2398–2404. [DOI] [PubMed] [Google Scholar]
- 8.Baker SA, Hennig GW, Salter AK, Kurahashi M, Ward SM, Sanders KM. Distribution and Ca(2+) signalling of fibroblast-like (PDGFR(+)) cells in the murine gastric fundus. J Physiol. 2013;591(24):6193–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grover M, Bernard CE, Pasricha PJ, et al. Platelet-derived growth factor receptor alpha (PDGFRalpha)-expressing “fibroblast-like cells” in diabetic and idiopathic gastroparesis of humans. Neurogastroenterol Motil. 2012;24(9):844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111(2):492–515. [DOI] [PubMed] [Google Scholar]
- 11.Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281(3):G602–611. [DOI] [PubMed] [Google Scholar]
- 12.Bhetwal BP, An C, Baker SA, Lyon KL, Perrino BA. Impaired contractile responses and altered expression and phosphorylation of Ca(2+) sensitization proteins in gastric antrum smooth muscles from ob/ob mice. J Muscle Res Cell Motil. 2013;34(2):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ordog T, Ward SM, Sanders KM. Interstitial cells of cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518(Pt 1):257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140(5):1575–1585 e1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neshatian L, Gibbons SJ, Farrugia G. Macrophages in diabetic gastroparesis--the missing link? Neurogastroenterol Motil. 2015;27(1):7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herring BP, Hoggatt AM, Gupta A, et al. Idiopathic gastroparesis is associated with specific transcriptional changes in the gastric muscularis externa. Neurogastroenterol Motil. 2018;30(4):e13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ro S. Multi-Phenotypic Role of Serum Response Factor in the Gastrointestinal System. Journal of neurogastroenterology and motility. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mericskay M, Blanc J, Tritsch E, et al. Inducible Mouse Model of Chronic Intestinal Pseudo-Obstruction by Smooth Muscle-Specific Inactivation of the SRF Gene. Gastroenterology. 2007;133(6):1960–1970. [DOI] [PubMed] [Google Scholar]
- 19.Angstenberger M, Wegener J, Pichler B, et al. Severe Intestinal Obstruction on Induced Smooth Muscle–Specific Ablation of the Transcription Factor SRF in Adult Mice. Gastroenterology. 2007;133(6):1948–1959. [DOI] [PubMed] [Google Scholar]
- 20.Miano J. Serum response factor: toggling between disparate programs of gene expression. Journal of Molecular and Cellular Cardiology. 2003;35(6):577–593. [DOI] [PubMed] [Google Scholar]
- 21.Miano JM. Myocardin in biology and disease. Journal of biomedical research. 2015;29(1):3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Chen H, Sanders KM, Perrino BA. Regulation of SRF/CArG-dependent gene transcription during chronic partial obstruction of murine small intestine. Neurogastroenterol Motil. 2008;20(7):829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolte C, Ren X, Tomley T, et al. Forkhead box F2 regulation of platelet-derived growth factor and myocardin/serum response factor signaling is essential for intestinal development. J Biol Chem. 2015;290(12):7563–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoggatt AM, Kim JR, Ustiyan V, et al. The transcription factor Foxf1 binds to serum response factor and myocardin to regulate gene transcription in visceral smooth muscle cells. J Biol Chem. 2013;288(40):28477–28487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95(6):1456–1462. [DOI] [PubMed] [Google Scholar]
- 26.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Journal of nuclear medicine technology. 2008;36(1):44–54. [DOI] [PubMed] [Google Scholar]
- 27.Wirth A, Benyo Z, Lukasova M, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14(1):64–68. [DOI] [PubMed] [Google Scholar]
- 28.Lyu Q, Dhagia V, Han Y, et al. CRISPR-Cas9 Mediated Epitope Tagging Provides Accurate and Versatile Assessment of Myocardin. Arterioscler Thromb Vasc Biol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herring BP, Hoggatt AM, Smith AF, Gallagher PJ. Targeted expression of SV40 large T-antigen to visceral smooth muscle induces proliferation of contractile smooth muscle cells and results in megacolon. J Biol Chem. 1999;274(25):17725–17732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herring BP, Hoggatt AM, Burlak C, Offermanns S. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vascular cell. 2014;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ran L, Chen Y, Sher J, et al. FOXF1 Defines the Core-Regulatory Circuitry in Gastrointestinal Stromal Tumor. Cancer Discov. 2018;8(2):234–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296(6):G1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horvath VJ, Vittal H, Lorincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130(3):759–770. [DOI] [PubMed] [Google Scholar]
- 34.Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9(1):102–108. [DOI] [PubMed] [Google Scholar]
- 35.Battaglia E, Bassotti G, Bellone G, et al. Loss of interstitial cells of Cajal network in severe idiopathic gastroparesis. World J Gastroenterol. 2006;12(38):6172–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albinsson S, Skoura A, Yu J, et al. Smooth Muscle miRNAs Are Critical for Post-Natal Regulation of Blood Pressure and Vascular Function. PLoS One. 2011;6(4):e18869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahlapuu M, Enerback S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128(12):2397–2406. [DOI] [PubMed] [Google Scholar]
- 38.Madison BB, McKenna LB, Dolson D, Epstein DJ, Kaestner KH. FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. The Journal of biological chemistry. 2009;284(9):5936–5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.