Abstract
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease characterized by joint involvement, extra-articular manifestations, comorbidities, and increased mortality. In the last few decades, the management of RA has been dramatically improved by the introduction of a treat-to-target approach aiming to prevent joint damage progression. Moreover, the increasing knowledge about disease pathogenesis allowed the development of a new drug class of biologic agents targeted on immune cells and proinflammatory cytokines involved in RA network. Despite the introduction of several targeted drugs, a significant proportion of RA patients still fail to achieve the clinical target; so, more recently the focus of research has been shifted toward the inhibition of kinases involved in the transduction of the inflammatory signal into immune cells. In particular, two Janus kinase (JAK) inhibitors, baricitinib and tofacitinib, have been licensed for the treatment of RA as a consequence of a very favorable profile observed in randomized controlled trials (RCTs) conducted across different RA subpopulations. Both these new compounds are active on the majority of four JAK family members (JAK1, JAK2, JAK3, and TYK2), whereas the most recent emerging approach is directed toward the development of JAK1 selective inhibitors (upadacitinib and filgotinib) with the aim to improve the safety profile by minimizing the effects on JAK3 and, especially, JAK2. In this narrative review, we discuss the rationale for JAK inhibition in RA, with a special focus on the role of JAK1 selective blockade and a detailed description of available data from the results of clinical trials on upadacitinib and filgotinib.
Keywords: arthritis, filgotinib, Janus kinase 1, rheumatoid, upadacitinib
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease characterized by joint involvement, high morbidity, and increased mortality, with a worldwide prevalence of 0.5–1% of the population.1 Although RA etiology is unknown, several genetic polymorphisms and environmental factors have been associated with increased susceptibility and disease severity.2 RA primarily affects peripheral joints, with aberrant inflammatory proliferation of the synovial tissue leading to cartilage damage and bone erosion.3,4 Moreover, RA chronic systemic inflammation can also lead to the development of extra-articular manifestations such as chronic anemia, fatigue, and interstitial lung disease, and of comorbidities such as osteoporosis, infections, cancer, increased cardiovascular disease, type-2 diabetes mellitus, and psychological impairment.5,6 As a consequence, RA is characterized by progressive disability over time and is associated with an increased risk of mortality compared with the general population.7 The natural course of RA is characterized by a close association between persistent high disease activity and progression of joint damage, making the introduction of a treat-to-target approach to achieve a state of clinical remission/low disease activity (LDA) in all diagnosed patients fundamental to prevent the adverse long-term consequences of RA.8,9 Since the late 1990s, methotrexate has been identified as the most effective conventional synthetic disease-modifying antirheumatic drug (csDMARD) and is still considered as the ‘anchor’ drug for the treatment of all newly diagnosed RA patients.10 In the last few decades, the increasing knowledge of RA pathogenesis has dramatically improved the management of the disease with a better definition of the role of several immune cells (mainly T- and B-lymphocytes) and key proinflammatory cytokines, such as tumor necrosis factor (TNF) and interleukin 6 (IL-6).11,12 These advances opened the way to the development of biologic disease-modifying antirheumatic drugs (bDMARDs), a new drug class consisting of agents targeted on the main cellular and extracellular mediators of RA pathogenetic network.13 The progressive introduction of five TNF inhibitors, two IL-6 blockers, one T-cell costimulation modulator, one IL-1 soluble receptor, and one B-cell depleting monoclonal antibody has made low disease activity and remission achievable targets even in methotrexate (MTX)-insufficient responder (IR) patients.14 Despite this abundance of therapeutic options, real-world data demonstrated that about 50–70% of treated patients still fail to achieve clinical remission or to maintain an initially good response over time.15–19 Moreover, observational registries are still populated by a non-negligible proportion of patients presenting a ‘difficult-to-treat’ RA pattern refractory to the majority of available mechanisms of action,20 as a result of the complexity and the variety of the pathogenetic mechanisms accounting for RA clinical manifestation. Considering the lack of head-to-head comparative clinical trials,21 in this scenario, the right choice of the first-line targeted agent in MTX-IR patients22 and the strategy for managing bDMARD failures still remain as critical unmet needs in the treatment of RA.23–27 More recently, the focus of the research has been shifted from outside to inside of the cell and in particular on kinases-mediated effect on the transduction of the signal into the cell produced by the interaction between some proinflammatory mediators and their specific transmembrane receptors on immune cells.28 Although several different kinases have been evaluated as potential treatment target for RA, to date only Janus kinase inhibitors (JAKis) have become part of the armamentarium for the management of the disease and are classified as targeted synthetic disease-modifying antirheumatic drug (tsDMARD).29 The JAK family comprises four members (JAK1, JAK2, JAK3, and tyrosine kinase 2 [Tyk2]), whose specific activities are related by their association with intracellular domains of different cytokine receptors. Therefore, the available JAKis can be classified according to different selectivity for each JAK subtype.30 To date, two pan-JAK inhibitors demonstrated a solid efficacy/safety profile in clinical trials conducted in different RA subsets and are now licensed for clinical use in RA with the same positioning of bDMARDs.14,31 Tofacitinib is a selective JAK1,3 inhibitor with minor activity on JAK2 and TYK232; whereas baricitinib (generated by modifying the structure of tofacitinib) is a selective JAK1,2 inhibitor with moderate activity versus TYK2 and minimal activity against JAK3.32,33 Given the favorable results encountered with tofacitinib and baricitinib, JAKis are expected to become the next-generation compounds for treating RA, and a number of new JAKis are currently under evaluation in clinical trials (Table 1). In particular, it has been hypothesized that more specific selectivity of JAKis toward the inhibition of JAK1 might only reduce dose-related toxicity, without a significant detriment to efficacy.34 The goal could be to selectively inhibit only JAK1 so as to obtain the same clinical efficacy as a non-selective pan-JAK inhibitor, but with a better safety profile potentially guaranteed by the non-inhibition of JAK3.34 This is the reason why two JAK1 selective drugs (upadacitinib and filgotinib) are now considered as the two most promising new small molecules in development for the management of RA.
Table 1.
The development program of main JAK inhibitors.
| JAK inhibitor | JAK selectivity | Disease | Clinical status |
|---|---|---|---|
| Baricitinib | 1,2 | RA Atopic Dermatitis Alopecia SLE JIA Psoriasis Giant cell arteritis |
Approved (EMA, FDA) Phase III Phase III Phase III Phase III Phase II Phase II |
| Tofacitinib | 3,1,2 | RA SpA Psoriasis JIA SLE CD UC Alopecia areata Uveitis, scleritis SLE, DLE Dermatomyositis Systemic sclerosis |
Approved (EMA, FDA) Phase IV Phase III Phase III Phase II Phase II Phase III Phase IV Phase II Phase II Phase I Phase I |
| Upadacitinib | 1 | RA PsA AS UC AD CD Giant cell arteritis Pediatric AD JIA |
Approved FDA, submitted EMA Phase III Phase II Phase III Phase III Phase III Phase III Phase I Phase I |
| Filgotinib | 1 | RA AS PsA UC CD Small bowel CD Fistulizing CD Sjögren syndrome Cutaneous lupus Lupus nephropathy Uveitis |
Phase III Phase II Phase II Phase III Phase III Phase II Phase II Phase II Phase II Phase II Phase II |
AD, atopic dermatitis; CD, Crohn’s disease; DLE, discoid lupus erythematosus; EMA, European Medicine Agency; FDA, Food and Drug Administration; JAK, Janus kinase; JIA, juvenile idiopathic arthritis; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SpA, spondyloarthritis; UC, ulcerative colitis.
Methods
In this narrative review, we discuss the rationale for JAK inhibition in RA with a special focus on the role of JAK1 selective blockade. Moreover, we describe the available data on upadacitinib and filgotinib from clinical trials and the potential positioning of these two new compounds in the treatment algorithm of RA.
Mechanism of action
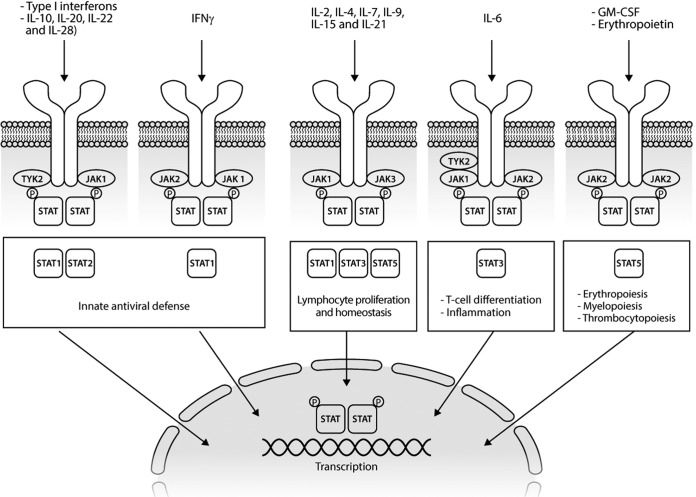
The JAK–STAT pathway is implicated in the pathogenesis of a broad spectrum of autoimmune and inflammatory diseases because of its role in the downstream transduction of the signal of multiple cytokines crucial for the development of these immune-mediated disorders35 (Figure 1). The JAK–STAT pathway is composed of four nonreceptor protein tyrosine kinases, namely, JAK1, JAK2, JAK3, and TYK2, and seven inactive cytoplasmic proteins STAT (STAT1–4, STAT5A, STAT5B, and STAT6).36 The signal cascade is promoted by the binding of JAKs to type-1 and -2 cytokine receptors. Different receptors signal through different JAKs with variable selectivity. The receptor–JAK interaction leads to oligomerization and separation of the receptor from JAK.29 Accordingly, upon its activation, the JAK phosphorylates itself and the intracellular subunits of the receptor and STAT, allowing for the formation of active STAT homodimers, heterodimers, or tetramers.37 Finally, the phosphorylated STAT dimers move within the nucleus where, acting as transcriptional factors, they regulate the transcription of specific target genes.38
Figure 1.
The JAK-STAT signaling pathway.
GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; JAK, Janus kinase; STAT, signal transducer of activation; TYK, tyrosine kinase.
The JAK–STAT signaling pathway is characterized by a very complex organization that could lead to redundancies in some cases but, to date, with no identified compensatory pathways. JAKs exhibit one true kinase domain (JH1) and one inactive pseudokinase domain (JH2), conduiting thus their name from the two-faced Roman god of doors and new beginnings, Janus. Several cytokine receptors bind more than one JAK and the inhibition of a specific JAK could target different cytokine pathways. The pairing of JAKs with a given cytokine receptor is determined by their association with specific receptor chains.39 For example, JAK3 and JAK1 are always paired and associated with adaptive immunity interleukins (IL-2, IL-4, IL7, IL-9, IL-15, and IL-21). Nevertheless, JAK1 regulates innate immunity when it pairs with JAK2 and TYK2, regulating several proinflammatory cytokines such as IL-6 and the type-I interferons. Differently from all other JAKs, JAK2 can pair with itself, modulating different cytokines and growth factors (IL-3, IL-5, granulocyte macrophage colony-stimulating factor [GM-CSF], erythropoietin, and thrombopoietin). The primary negative regulator of JAK–STAT signaling is a class of proteins, named suppressor of cytokine signaling (SOCS). In RA, but also in cancer and certain immunodeficiency syndromes, the activity of SOCS is importantly dysfunctional.40 The crucial roles of the JAK–STAT pathway in proinflammatory cytokine signaling were a major driver for the development of targeted therapies for the management of RA. The first JAKis approved for treatment of RA were two pan-JAK blockers, tofacitinib and baricitinib.41 Given the promising performance of the first-generation JAKis, a second more selective generation of JAKis is now under development for inflammatory diseases, with the aim to increase the effectiveness and reduced adverse events related to simultaneous multi-JAK inhibition and regulation of multiple cytokines action.42 For example, a potential plus for anti-JAK selectivity could be the avoidance of inhibition of JAK2, with preclusion of the typical hematological adverse events (AEs). Otherwise, this benefit could be in collision with a minor drug efficacy due to a major selectivity on cytokines blocking.34 The inhibition of JAK1 could be more effective in RA because it modulates the signal transmission of several proinflammatory cytokines involved in the pathogenesis of the disease, especially IL-6, the pleiotropic effect of which seems to be crucial for the development of articular and main extra-articular manifestations of the disease.43,44
Efficacy of JAK-1 inhibition
To date, two JAKis with greater affinity for JAK1 (upadacitinib and filgotinib) have been evaluated in the treatment of RA patients. Upadacitinib is a next-generation JAKi selective for JAK1 74-fold over JAK2.45 This characteristic is due to its ability to bind JAK1 outside and on the adenosine triphosphate-binding site of JH1. Upadacitinib has been evaluated for the treatment of RA in the huge phase III development program SELECT, which included six RCTs covering different RA subpopulations from early MTX-naïve to bDMARD-IR patients (Table 2). Filgotinib is a selective JAKi with a selectivity for JAK1 versus JAK2 of near 30-fold.45 Furthermore, filgotinib exerts a dose-dependent inhibition of Th1–Th2 and to a lesser extent Th17 cell differentiation. After the completion of phase II studies (DARWIN 1 and 2 trials, along with the open-label extension DARWIN 3 trial), filgotinib is now under evaluation in the FINCH program, encompassing five clinical trials conducted in different RA patient types (Table 3).
Table 2.
Overview of upadacitinib rheumatoid arthritis phase III program.
| Study | SELECT-Early | SELECT-Monotherapy | SELECT-Compare | SELECT- Next | SELECT- Beyond | SELECT-Choice |
|---|---|---|---|---|---|---|
| Population | MTX-naïve | MTX-IR | MTX-IR | csDMARD-IR | bDMARD-IR | bDMARD-IR |
| Type of therapy | Mono | Mono | Combo | Combo | Combo | Combo |
| Concomitant background | – | – | MTX | csDMARDs | csDMARDs | csDMARDs |
| Active comparator | MTX | MTX | ADA | – | – | ABT |
| Arms |
|
|
|
|
|
|
| Duration Period 1 | 12 weeks | 14 weeks | 26 weeks | 12 weeks | 12 weeks | 24 weeks |
| Actual enrollment | 1002 | 648 | 1629 | 661 | 499 | 614 |
ABT, abatacept; ADA, adalimumab; bDMARD, biologic disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; EOW, every other week; IR, insufficient responder; MTX, methotrexate; PBO, placebo; QD, once daily; UPA, upadacitinib. Study details from https://clinicaltrials.gov
Table 3.
Overview of filgotinib rheumatoid arthritis phase III program.
| Study | FINCH1 | FINCH2 | FINCH3 | FINCH4 |
|---|---|---|---|---|
| Population | MTX-IR | bDMARD-IR | MTX-naïve | LTE |
| Type of therapy | Combo | Combo | Mono versus Combo | Combo |
| Concomitant background | MTX | csDMARDs | MTX | csDMARDs |
| Active comparator | ADA | csDMARDs | MTX | – |
| Arms |
|
|
|
|
| Duration Period 1 | 12 weeks | 24 weeks | 26 weeks | 78 weeks |
| Enrollment | 1759 | 449 | 1252 | 2800 |
ADA, adalimumab; bDMARD, biologic disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; EOW, every other week; FIL, filgotinib; IR, insufficient responder; LTE, long-term extension; MTX, methotrexate; PBO, placebo; QD, once daily. Study details from https://clinicaltrials.gov
Upadacitinib
Combination therapy in MTX- and bDMARDs-IR patients: overall efficacy
The clinical performance of upadacitinib as a combination therapy with csDMARDs was analyzed in the SELECT-NEXT and SELECT-BEYOND trials.46,47 The SELECT-NEXT study randomly assigned 661 RA csDMARD-IR patients to upadacitinib 15 or 30 mg/day or to placebo.46 At week 12, patients in the two treatment groups achieved a significantly higher ACR20 response compared with placebo (64%, 66%, and 36%, respectively; p<0.0001 for each dose versus placebo). The other primary endpoint (disease activity score on 28 joints using C-reactive protein [DAS28-CRP] ≤3.2) was met by 48% of patients in both upadacitinib treatment groups versus 17% in the placebo one (p<0.0001 for each dose versus placebo). Moreover, a significantly higher proportion of patients in the two upadacitinib groups achieved low disease activity or clinical remission versus placebo at week 12 when considering the more stringent efficacy measures aligned with the treat-to-target strategy: DAS28-CRP<2.6, clinical disease activity index (CDAI), and simplified disease activity index (SDAI). The onset of activity was significantly faster for both doses of upadacitinib versus that for placebo, with an ACR20 response rate at week 1 of 22%, 28%, and 9%, respectively (p<0.0001 for each dose versus placebo). This trend was confirmed from week 2 onward for ACR50/70. These results were consistent with the data observed in the SELECT-BEYOND study, conducted in bDMARD-IR RA patients on stable csDMARD therapy.47 In this study, 498 RA patients were randomized to upadacitinib 15 or 30 mg, or placebo followed by upadacitinib 15 or 30 mg from week 12 onward. Overall, 47% of patients had been treated with one earlier bDMARD, 28% with two earlier bDMARDs, and 25% with at least three earlier bDMARDs; 90% had inadequate response or intolerance to at least one tumor necrosis factor inhibitor (TNFi) and 18% had lack of efficacy with an anti-IL6. At week 12, upadacitinib reported a significantly better clinical response at both regimens (15 and 30 mg) compared with placebo according to ACR20 (65 and 56 versus 28%, respectively; p<0.0001 for both dosage versus placebo), DAS28-CRP≤3.2 (43 and 42 versus 14%, respectively; p<0.0001 for both dosages versus placebo), and ACR50 (34 and 36 versus 12%, respectively; p<0.0001 for both dosages versus placebo). After stratification of population for earlier treatments, these results were similar regardless of the number and the different mechanisms of action of earlier bDMARDs. At week 24, clinical response was maintained over time in upadacitinib groups and patients who switched from placebo to upadacitinib at week 12 achieved a similar ACR response to those receiving upadacitinib from the baseline. Even in this specific RA population with a complex treatment history, upadacitinib showed a rapid response with a significantly higher ACR20 rate at week 1 in both dose groups than that in the placebo group (27 and 25 versus 11%; p<0.0001 and p<0.0006, respectively).
Upadacitinib monotherapy
The efficacy of upadacitinib as a monotherapy was assessed in two RCTs conducted in MTX-naïve (SELECT-EARLY)48 and MTX-IR (SELECT-MONOTHERAPY)48,49 active RA patients. In the SELECT-EARLY 945 MTX-naïve RA patients with poor prognostic factors (double seropositivity for rheumatoid factor and anti-citrullinated protein antibody and one or more joint erosions) were randomized in the ratio 1:1:1 to receive upadacitinib (15 or 30 mg) or MTX.48 Upadacitinib showed a significantly greater clinical response versus that in MTX in ACR50 rate (52.1, 56.4, and 28.3%, respectively; p<0.001) and DAS28-CRP<2.6 rate (35.6, 40.8, and 13.7%, respectively) at week 12 and the same positive trend was confirmed at week 24 (ACR20: 60.3, 65.6, and 33.4%, respectively; p<0.001. DAS28-CRP<2.6: 48.3, 50, and 18.5%, respectively; p<0.001). Moreover, the proportion of patients showing no 24-week radiographic progression was significantly higher in both upadacitinib groups versus that in MTX (87.5, 89.3, and 77.7%, respectively). The second RCT, the SELECT-MONOTHERAPY, enrolled 648 patients randomized in the ratio 1:1:1 to receive upadacitinib 15 or 30 mg monotherapy versus continuing MTX at prior stable dose.49 At week 14, both primary endpoints were achieved: a significantly higher rate of patients on upadacitinib (15 and 30 mg) achieved ACR20 versus that in MTX (67.7 and 71.2 versus 41.2%, respectively; p<0.001) and DAS28-CRP≤3.2 (44.7 and 53 versus 19.4%, respectively; p<0.001). Similar tendency was confirmed by other more stringent efficacy and remission criteria, as ACR50/70, DAS28-CRP<2.6, and CDAI≤10.49
Head-to-head comparison with active comparator
The SELECT-COMPARE study is a phase III superiority RCT head-to-head comparing upadacitinib to adalimumab and placebo in MTX-IR RA patients while continuing stable background MTX.50 The study population (n=1629) was randomized in the ratio 2:2:1 to once daily upadacitinib 15 mg, placebo, or ADA 40 mg every other week. All primary and key secondary endpoints were met. In particular, at week 12, superiority was met for upadacitinib versus placebo (ACR20 70.5 versus 36.4%, respectively; p<0.001. DAS28CRP<2.6 28.7 versus 6.1%, respectively; p<0.001) and versus adalimumab (ACR20 70.5 versus 63%, respectively; p<0.05. ACR50 45.2 versus 29.1%, respectively; p<0.001. DAS28CRP≤3.2 45.0 versus 28.7%, respectively). All these differences were maintained through to the end of the double-blind phase (26 weeks). Moreover, the radiographic progression was significantly reduced in patients receiving an active treatment compared with placebo, with no significant difference between upadacitinib and adalimumab in the rate of non-progressor patients (83.5 versus 86.8%, respectively).50
Patient-reported outcomes
In the last few years, increasing interest in the role of patient-reported outcomes (PROs) for defining clinical response and disease effect in treatment decision emerged.51 Upadacitinib demonstrated, in RCTs, an important effect on PROs producing a significant improvement in the quality of life (QoL) indices, such as pain, fatigue, and disability. In particular, the effect of upadacitinib on pain and morning stiffness was extrapolated from the SELECT-NEXT, SELECT-BEYOND, and SELECT-MONOTHERAPY RCTs.46,47,52 Across all studies, a significant least squares mean (LSM) percent change from the baseline to week 12/14 in pain and morning stiffness was reported by upadacitinib-treated patients compared with placebo or MTX at as early as week 2. In particular, both upadacitinib doses reported a significantly higher improvement of ³50% in pain (41–51% for 15 mg and 42–56% for 30 mg) with no or mild pain in 30–36% of patients for 15 mg and in 36–44% for 30 mg versus 14–15% for MTX or placebo (p<0.05). A similar trend was observed for morning stiffness duration. Strand and colleagues analyzed the association between PROs and composite outcomes in the same populations evidencing an overall improvement from the baseline in pain, physical function, and fatigue correlated to individual physician-derived measures and composite disease outcomes, thus underlying the additional value of PROs in RCTs.52 Furthermore, in the SELECT-MONOTHERAPY STUDY, a treatment with upadacitinib 15 or 30 mg demonstrated a significant improvement in Patient Global Assessment of Disease Activity (PtGA), visual analog scale (VAS), pain VAS, Health Assessment Questionnaire Disability Index (HAQ-DI), and health-related quality of life (HRQoL) by 36-Item Short Form Health Survey (SF-36) compared with MTX monotherapy in MTX-IR.52 In conclusion, the clinical benefits of upadacitinib gain additional value when combined with patient insights as control of pain and morning stiffness, which confirm the potential of upadacitinib to improve patients’ QoL as well as the signs and symptoms of the disease.
Filgotinib
To date, two phase IIb trials have evaluated the performance of filgotinib as an add-on therapy (DARWIN 1 study) or as a monotherapy (DARWIN 2 study) in MTX-IR RA patients.53,54 The DARWIN 1 study evaluated filgotinib efficacy at week 24 in 594 MTX-IR patients at different doses and regimens (50, 100, or 200 mg once or twice daily) versus placebo.53 At week 12, the ACR20 rates were significantly higher for 100 mg once daily (64%), 200 mg once daily (69%), and 100 mg twice daily (79%) versus placebo (44%; p=0.0435, p=0.0068, p<0.0001, respectively). Furthermore, significant dose-dependent improvements in ACR50, ACR-N index of improvement, DAS28-CRP, CDAI, and SDAI for filgotinib versus placebo were reported at week 12 and maintained through week 24. The filgotinib efficacy, as evaluated by DAS28-CRP, was observed from week 1 onward in the 100 and 200 mg once daily groups. A similar efficacy was observed between once or twice daily regimens.
The efficacy of filgotinib as a monotherapy was investigated in the DARWIN 2 study.54 Moreover, 283 MTX-IR patients were randomized to receive filgotinib 50, 100, or 200 mg monotherapy once daily versus placebo and after a washout period from MTX of more than 4 weeks. The primary endpoint (ACR20 response rate) was achieved in all the active treatment groups versus placebo (67, 66, and 73 versus 31%, respectively; p<0.001) at week 12. Similar performances were reported for all filgotinib dose groups when considering ACR-N, DAS28-CRP, SDAI, and European League Against Rheumatism (EULAR) good response at week 12. The mean change from the baseline of disease activity (measured by both DAS28-CRP and SDAI) and the remission rates were greater for the highest filgotinib dosages. All responses were maintained or improved through week 24. An early onset of response was observed at week 1 for ACR20 in the filgotinib 200 mg group and in DAS28-CRP and CDAI in all dose groups, at week 2 for ACR50 in the filgotinib 200 mg and at week 4 for ACR70 in the filgotinib 200 mg dose group. Genovese and colleagues analyzed the effect of filgotinib on PROs in RA patients selected from DARWIN 1 and 2 studies.55 At week 12, all PROs, except for the SF-36 mental component in the DARWIN 1 study, were significantly improved in patients treated with filgotinib compared with placebo, with a very early onset of clinical response since the first week of therapy. Filgotinib reduced HAQ-DI by 0.58–0.84 points, FACIT-Fatigue by 6.9–11.4, pain by 24.2–37.9 mm, and PtGA by 25.2–35.6. These outcomes were sustained up to week 24. In placebo patients reassigned to filgotinib 100 mg at week 12, similar improvements in PROs were observed between weeks 12 and 24.
The FINCH 2 trial is the only filgotinib phase III study with available presented data.56 The enrolled 448 active RA patients were randomized to receive filgotinib (200 or 100 mg one daily) or placebo for 24 weeks. At week 12, a significantly higher ACR20 response rate was observed in both filgotinib groups versus placebo (66 and 57.5 versus 31.1%, respectively; p<0.001). These positive trends were confirmed at week 24. The reduction from baseline of HAQ-DI, SF36 physical component score and the fatigue component of the functional assessment of chronic illness (FACIT-Fatigue) were significantly greater in filgotinib 200 and 100 mg versus placebo at weeks 12 and 24.
Preliminary data on the long-term efficacy of filgotinib in RA patients are available from the DARWIN 3 trial, a phase IIb open-label extension study including 739 eligible patients from the DARWIN 1 and 2 studies enrolled to receive filgotinib 200 mg once daily, 100 mg twice daily or, in the U.S. males only, 100 mg once daily.57 Week 132 efficacy data showed a maintenance of clinical response in the long term, with an ACR20/50/70 response in 89, 70, and 49% of patients, respectively, while DAS28-CRP£3.2 was achieved in 69% of patients.57
Safety profile of JAK-1 inhibition
The majority of available safety data on JAKis come from RCTs and open-label, long-term extensions of clinical trials, with the only exception of tofacitinib, the real-life data of which from postmarketing experience outside Europe are already available.58,59 Overall, the safety profile of JAKis seem to be quite similar to the one observed for bDMARDs.60 JAKis are involved in the simultaneous control of signal transduction of several cytokines active in immune cell homeostasis and in physiological functions as erythrocyte, lymphocyte, and platelet proliferation.61 Consequently, safety concerns related to the use of JAKis could be a direct consequence of their mechanism of action, with perturbations of hematopoiesis, innate and adaptive immunity, and growth.62 The inhibition of different components of the JAK family conveys in several different potential AEs. JAK3 is selectively expressed on epithelial and hematopoietic cells, and its genetic lack results in severe combined immunodeficiency disease. JAK2 inhibition could block erythropoietin signal and affect the functions of GM-CSF. Therefore, the selectivity for JAK1 inhibition could minimize the potential toxicities of pan-JAK blockade.30 Despite these theoretical assumptions, RCTs seemed to demonstrate a general overlap in the safety profile among the available JAKis, irrespective of JAK selectivity.62
The most frequently reported AEs with JAK1 inhibition were nausea, headache, upper respiratory and urinary tract infection, and changes in laboratory parameters such as dose-related neutropenia (more frequently observed with upadacitinib 30 mg/day) and increase in serum creatinine and liver enzymes levels.46,53,54,57 Differently from what described in tofacitinib clinical experiences, an increase in hemoglobin level was reported especially with filgotinib, most likely as a result of the anti-inflammatory efficacy of selective JAK1 inhibitors combined with the lack of erythropoietin blockade mediated by JAK2 inhibition.63 Moreover, no reduction in lymphocytes or natural killer cells’ absolute values was described, probably due to a minor effect of JAK1 selective products on the IL-15 signal.64 A perturbation of lipid profile with elevation of both high- and low-density lipoprotein cholesterol was reported, consistently with the safety profiles of other targeted therapies working on the IL-6 pathway.19,43,65 The observation of serious AEs leading to drug discontinuation was only numerically greater in patients receiving the highest upadacitinib and filgotinib doses. In particular, rare cardiovascular events, venous thromboembolism, and pulmonary embolism were reported only in treated patients carrying specific risk factors. Malignancies (lymphoma or cancer) were not observed in the filgotinib clinical trials, whereas 7 cases (versus 3 in the placebo group) were described in patients treated with upadacitinib.46–50,53,54 The overall risk of serious infections observed with JAK1 inhibitors is basically the same reported with tofacitinib and baricitinib. Although most infections observed in patients treated with JAKis are bacterial, the major concern specifically associated with the use of this drug class is the potential reactivation of Herpes Zoster virus (HZV) in already infected subjects. This kind of complication is more frequent in RA patients compared with the general population and is significantly influenced by age and chronic use of corticosteroids.66 In the tofacitinib-pooled population enrolled in RCTs, the incidence rate of HZV was 1.5–2-fold higher than that expected for RA and generally higher compared with data observed in patients treated with bDMARDs.67 However, the incidence rate is strongly influenced by the endemicity of HZV in the geographic area where the study population was enrolled, being increased in Asia (9.2 per 100 patient-years) and India (8.9 per 100 patient-years), and significantly lower in the Western countries (from 2.7 in Western Europe to 3.3 per 100 patient-years in North America).67 Notably, multidermatomal or disseminated herpes zoster on tofacitinib therapy were uncommon, and no cases exited in visceral disease or death.67 In the preliminary short-term experience with JAK1 selective drugs, the incidence of HZV reactivation is higher in active arms compared with placebo for both upadacitinib (16 and 15 patients in upadacitinib 15 and 30 mg groups versus 7 in the placebo group in the pooled population of the SELECT program) and filgotinib (5 versus only 1 patient in the overall population of DARWIN 1 and 2 studies).46–50,53,54 These findings seem to confirm for JAK1 selective inhibitors the well described effect on HZV infections, even if the lack of long-term and real-world data is still a limitation in the comparative safety profile of selective versus nonselective compounds.
Conclusion
The development and introduction of JAKis in the therapeutic armamentarium for RA represented the edge of a new era in the management of the disease, with the potential to address some of the persistent unmet needs in this area. The opportunity to target simultaneously several proinflammatory mediators by using the same small molecule in a very complex and multifactorial disease, as RA is the revolutionary aspect related to the use of this new drug class. After the marketing of the first two pan-JAK inhibitors (tofacitinib and baricitinib), the more recent research has been focused toward the development of more selective drugs with the ability to modulate the activity of only one JAK family member (JAK1), with the aim to improve the safety profile by minimizing the effects on JAK3 and especially JAK2. Available phase II and III data on upadacitinib and filgotinib are very promising and seemed to confirm the efficacy data observed with the first-generation JAKis. In particular, upadacitinib demonstrated a statistical superiority over adalimumab in a head-to-head RCT conducted on top of MTX, thus replicating the same results observed with baricitinib in the RA-BEAM trial. The preliminary data about safety profile of JAK1 selective inhibitors seem to be consistent with the previous experience observed with tofacitinib and baricitinib. In the absence of clinical trials directly comparing the available JAKis, indirect comparison of real-life data from observational registries is crucial for better understanding the real potential benefit of JAK1 selective inhibition over pan-JAK blockade.
Acknowledgments
None.
Footnotes
Contributions: EGF designed the review methods and drafted and revised the paper. MB, CC, EA, and AB drafted and revised the paper. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: EGF served as a consultant and/or speaker for BMS, Eli-Lilly, Celgene, UCB, Pfizer, Novartis, and Sanofi-Genzyme. MB, CC, EA, and AB have declared no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at http://www.drugsincontext.com/wp-content/uploads/2019/10/dic.212595-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2019 Biggioggero M, Becciolini A, Crotti C, Agape E, Favalli EG. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 1 July 2019
References
- 1.Myasoedova E, Crowson CS, Kremers HM, et al. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002;4(Suppl 3):S265–S272. doi: 10.1186/ar578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 4.Lefevre S, Knedla A, Tennie C, et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med. 2009;15(12):1414–1420. doi: 10.1038/nm.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 6.Firestein GS. The disease formerly known as rheumatoid arthritis. Arthritis Res Ther. 2014;16(3):114. doi: 10.1186/ar4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmqvist M, Ljung L, Askling J. Mortality following new-onset rheumatoid arthritis: has modern rheumatology had an impact? Ann Rheum Dis. 2018;77(1):85–91. doi: 10.1136/annrheumdis-2017-212131. [DOI] [PubMed] [Google Scholar]
- 8.Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75(1):3–15. doi: 10.1136/annrheumdis-2015-207524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11(5):276–289. doi: 10.1038/nrrheum.2015.8. [DOI] [PubMed] [Google Scholar]
- 10.Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: still an “anchor” drug? Autoimmun Rev. 2014;13(11):1102–1108. doi: 10.1016/j.autrev.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. doi: 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 12.McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis - shaping the immunological landscape. Nat Rev Rheumatol. 2016;12(1):63–68. doi: 10.1038/nrrheum.2015.171. [DOI] [PubMed] [Google Scholar]
- 13.Chighizola CB, Favalli EG, Meroni PL. Novel mechanisms of action of the biologicals in rheumatic diseases. Clin Rev Allergy Immunol. 2014;47(1):6–16. doi: 10.1007/s12016-013-8359-x. [DOI] [PubMed] [Google Scholar]
- 14.Smolen JS, Landewé R, Bijlsma J, et al. Eular recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 15.Biggioggero M, Favalli EG. Ten-year drug survival of anti-TNF agents in the treatment of inflammatory arthritides. Drug Dev Res. 2014;75(Suppl 1):S38–S41. doi: 10.1002/ddr.21192. [DOI] [PubMed] [Google Scholar]
- 16.Favalli EG, Pregnolato F, Biggioggero M, et al. Twelve-year retention rate of first-line tumor necrosis factor inhibitors in rheumatoid arthritis: real-life data from a local registry. Arthritis Care Res (Hoboken) 2016;68(4):432–439. doi: 10.1002/acr.22788. [DOI] [PubMed] [Google Scholar]
- 17.Sarzi-Puttini P, Antivalle M, Marchesoni A, et al. Efficacy and safety of anti-TNF agents in the Lombardy rheumatoid arthritis network (LORHEN) Reumatismo. 2008;60(4):290–295. doi: 10.4081/reumatismo.2008.290. [DOI] [PubMed] [Google Scholar]
- 18.Iannone F, Sinigaglia L, Favalli EG, et al. Drug survival of adalimumab in patients with rheumatoid arthritis over 10 years in the real-world settings: high rate remission together with normal function ability. Clin Rheumatol. 2016;35(11):2649–2656. doi: 10.1007/s10067-016-3349-z. [DOI] [PubMed] [Google Scholar]
- 19.Iannone F, Ferraccioli G, Sinigaglia L, et al. Real-world experience of tocilizumab in rheumatoid arthritis: sub-analysis of data from the Italian biologics’ register GISEA. Clin Rheumatol. 2018;37(2):315–321. doi: 10.1007/s10067-017-3846-8. [DOI] [PubMed] [Google Scholar]
- 20.de Hair MJH, Jacobs JWG, Schoneveld JLM, et al. Difficult-to-treat rheumatoid arthritis: an area of unmet clinical need. Rheumatology (Oxford) 2017 doi: 10.1093/rheumatology/kex349. [DOI] [PubMed] [Google Scholar]
- 21.Favalli EG, Bugatti S, Biggioggero M, et al. Treatment comparison in rheumatoid arthritis: head-to-head trials and innovative study designs. Biomed Res Int. 2014;2014 doi: 10.1155/2014/831603. 831603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cantini F, Niccoli L, Nannini C, et al. Tailored first-line biologic therapy in patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis. Semin Arthritis Rheum. 2016;45(5):519–532. doi: 10.1016/j.semarthrit.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Sebastiani M, Anelli MG, Atzeni F, et al. Efficacy and safety of rituximab with and without methotrexate in the treatment of rheumatoid arthritis patients: results from the GISEA register. Joint Bone Spine. 2014;81(6):508–512. doi: 10.1016/j.jbspin.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Cantini F, Niccoli L, Nannini C, et al. Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Semin Arthritis Rheum. 2017;47(2):183–192. doi: 10.1016/j.semarthrit.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Favalli EG, Biggioggero M, Marchesoni A, et al. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology (Oxford) 2014;53(9):1664–1668. doi: 10.1093/rheumatology/keu158. [DOI] [PubMed] [Google Scholar]
- 26.Todoerti M, Favalli EG, Iannone F, et al. Switch or swap strategy in rheumatoid arthritis patients failing TNF inhibitors? Results of a modified Italian Expert Consensus. Rheumatology (Oxford) 2018;57(Suppl 7):vii42–vii53. doi: 10.1093/rheumatology/key195. [DOI] [PubMed] [Google Scholar]
- 27.Favalli EG, Raimondo MG, Becciolini A, et al. The management of first-line biologic therapy failures in rheumatoid arthritis: current practice and future perspectives. Autoimmun Rev. 2017;16(12):1185–1195. doi: 10.1016/j.autrev.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson FM, Gray NS. Kinase inhibitors: the road ahead. Nat Rev Drug Discov. 2018;17(5):353–377. doi: 10.1038/nrd.2018.21. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz DM, Bonelli M, Gadina M, et al. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016;12(1):25–36. doi: 10.1038/nrrheum.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirahara K, Schwartz D, Gadina M, et al. Targeting cytokine signaling in autoimmunity: back to the future and beyond. Curr Opin Immunol. 2016;43:89–97. doi: 10.1016/j.coi.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Taylor PC. Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford) 2019;58(Suppl 1):i17–i26. doi: 10.1093/rheumatology/key225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014;57(12):5023–5038. doi: 10.1021/jm401490p. [DOI] [PubMed] [Google Scholar]
- 33.Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374(13):1243–1252. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- 34.Yamaoka K. Janus kinase inhibitors for rheumatoid arthritis. Curr Opin Chem Biol. 2016;32:29–33. doi: 10.1016/j.cbpa.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz DM, Kanno Y, Villarino A, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;17(1):78. doi: 10.1038/nrd.2017.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liongue C, Ward AC. Evolution of the JAK-STAT pathway. JAKSTAT. 2013;2(1):e22756. doi: 10.4161/jkst.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368(2):161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Shea JJ, Kontzias A, Yamaoka K, et al. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii111–ii115. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofmann SR, Ettinger R, Zhou YJ, et al. Cytokines and their role in lymphoid development, differentiation and homeostasis. Curr Opin Allergy Clin Immunol. 2002;2(6):495–506. doi: 10.1097/01.all.0000044534.45448.bf. [DOI] [PubMed] [Google Scholar]
- 40.Malemud CJ. Negative regulators of JAK/STAT signaling in rheumatoid arthritis and osteoarthritis. Int J Mol Sci. 2017;18(3) doi: 10.3390/ijms18030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghoreschi K, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib (cp-690,550) J Immunol. 2011;186(7):4234–4243. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raimondo MG, Biggioggero M, Crotti C, et al. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des Devel Ther. 2017;11:1593–1603. doi: 10.2147/DDDT.S100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biggioggero M, Crotti C, Becciolini A, et al. Tocilizumab in the treatment of rheumatoid arthritis: an evidence-based review and patient selection. Drug Des Devel Ther. 2019;13:57–70. doi: 10.2147/DDDT.S150580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs. 2014;23(8):1067–1077. doi: 10.1517/13543784.2014.918604. [DOI] [PubMed] [Google Scholar]
- 46.Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (select-next): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503–2512. doi: 10.1016/S0140-6736(18)31115-2. [DOI] [PubMed] [Google Scholar]
- 47.Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (select-beyond): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513–2524. doi: 10.1016/S0140-6736(18)31116-4. [DOI] [PubMed] [Google Scholar]
- 48.van Vollenhoven R, Takeuchi T, Pangan AL, et al. A phase 3, randomized, controlled trial comparing upadacitinib monotherapy to MTX monotherapy in MTX-naïve patients with active rheumatoid arthritis. Arthritis Rheumatol. 2018;70(Suppl 10) doi: 10.1002/art.41384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smolen JS, Cohen S, Emery P, et al. Upadacitinib as monotherapy: a phase 3 randomized controlled double-blind study in patients with active rheumatoid arthritis and inadequate response to methotrexate. Arthritis Rheumatol. 2018;70(Suppl 10) [Google Scholar]
- 50.Fleischmann R, Pangan AL, Mysler E, et al. A phase 3, randomized, double-blind study comparing upadacitinib to placebo and to adalimumab, in patients with active rheumatoid arthritis with inadequate response to methotrexate. Arthritis Rheumatol. 2018;70(Suppl 10) doi: 10.1002/art.41032. [DOI] [PubMed] [Google Scholar]
- 51.Crotti C, Biggioggero M, Becciolini A, et al. Sarilumab: patient-reported outcomes in rheumatoid arthritis. Patient Related Outcome Measures. 2018;9:275–284. doi: 10.2147/PROM.S147286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strand V, Buch M, Tundia N, et al. Upadacitinib monotherapy improves patient-reported outcomes in patients with rheumatoid arthritis and inadequate response to methotrexate. Arthritis Rheumatol. 2018;70(Suppl 10) [Google Scholar]
- 53.Westhovens R, Taylor PC, Alten R, et al. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1) Ann Rheum Dis. 2017;76(6):998–1008. doi: 10.1136/annrheumdis-2016-210104. [DOI] [PubMed] [Google Scholar]
- 54.Kavanaugh A, Kremer J, Ponce L, et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2) Ann Rheum Dis. 2017;76(6):1009–1019. doi: 10.1136/annrheumdis-2016-210105. [DOI] [PubMed] [Google Scholar]
- 55.Genovese M, Westhovens R, Meuleners L, et al. Effect of filgotinib, a selective JAK1 inhibitor, with and without methotrexate in patients with rheumatoid arthritis: patient-reported outcomes. Arthritis Res Ther. 2018;20(1):57. doi: 10.1186/s13075-018-1541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Genovese MC, Kalunian KC, Walker D, et al. Safety and efficacy of filgotinib in a phase 3 trial of patients with active rheumatoid arthritis and inadequate response or intolerance to biologic dmards. Arthritis Rheumatol. 2018;70(Suppl 10) [Google Scholar]
- 57.Kavanaugh A, Genovese MC, Winthrop K, et al. Rheumatoid arthritis treatment with filgotinib: week 132 safety data from a phase 2b open-label extension study. Arthritis Rheumatol. 2018;70(Suppl 10) [Google Scholar]
- 58.Cohen S, Curtis JR, DeMasi R, et al. Worldwide, 3-year, post-marketing surveillance experience with tofacitinib in rheumatoid arthritis. Rheumatol Ther. 2018;5(1):283–291. doi: 10.1007/s40744-018-0097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caporali R, Zavaglia D. Real-world experience with tofacitinib for treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2019;37(3):485–495. [PubMed] [Google Scholar]
- 60.Cohen S, Radominski SC, Gomez-Reino JJ, et al. Analysis of infections and all-cause mortality in phase ii, phase iii, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(11):2924–2937. doi: 10.1002/art.38779. [DOI] [PubMed] [Google Scholar]
- 61.O’Shea JJ, Laurence A, McInnes IB. Back to the future: oral targeted therapy for ra and other autoimmune diseases. Nat Rev Rheumatol. 2013;9(3):173–182. doi: 10.1038/nrrheum.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13(4):234–243. doi: 10.1038/nrrheum.2017.23. [DOI] [PubMed] [Google Scholar]
- 63.Park SO, Wamsley HL, Bae K, et al. Conditional deletion of jak2 reveals an essential role in hematopoiesis throughout mouse ontogeny: implications for jak2 inhibition in humans. PLoS One. 2013;8(3):e59675. doi: 10.1371/journal.pone.0059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/s0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 65.Md Yusof MY, Emery P. Targeting interleukin-6 in rheumatoid arthritis. Drugs. 2013;73(4):341–356. doi: 10.1007/s40265-013-0018-2. [DOI] [PubMed] [Google Scholar]
- 66.Smitten AL, Choi HK, Hochberg MC, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the united states and the united kingdom. Arthritis Rheum. 2007;57(8):1431–1438. doi: 10.1002/art.23112. [DOI] [PubMed] [Google Scholar]
- 67.Winthrop KL, Yamanaka H, Valdez H, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(10):2675–2684. doi: 10.1002/art.38745. [DOI] [PMC free article] [PubMed] [Google Scholar]