More complete accounting reveals how intact tropical forest loss plays a larger-than-realized role in exacerbating climate change.
Abstract
Intact tropical forests, free from substantial anthropogenic influence, store and sequester large amounts of atmospheric carbon but are currently neglected in international climate policy. We show that between 2000 and 2013, direct clearance of intact tropical forest areas accounted for 3.2% of gross carbon emissions from all deforestation across the pantropics. However, full carbon accounting requires the consideration of forgone carbon sequestration, selective logging, edge effects, and defaunation. When these factors were considered, the net carbon impact resulting from intact tropical forest loss between 2000 and 2013 increased by a factor of 6 (626%), from 0.34 (0.37 to 0.21) to 2.12 (2.85 to 1.00) petagrams of carbon (equivalent to approximately 2 years of global land use change emissions). The climate mitigation value of conserving the 549 million ha of tropical forest that remains intact is therefore significant but will soon dwindle if their rate of loss continues to accelerate.
INTRODUCTION
Large tracts of forest that are free from significant anthropogenic influence, which we term “intact” forests, play a unique and important role in the global carbon cycle (1). Using mapped intact forest landscapes as the best available proxy, only 20% of tropical forests can be considered intact, but these areas store 40% of the aboveground carbon found in all tropical forests (2). The net biomass increase of intact forests also removes large amounts of atmospheric carbon (3)—sequestering at least one petagram of carbon per year, or up to 0.9 Mg of carbon per hectare per year (4)—and thus makes substantial contributions to the residual terrestrial carbon sink phenomenon (3).
When compared to forests that have been degraded by large-scale human activities, intact forests are often more resistant to pressures such as fire and drought events (5) and usually less accessible to logging and agricultural conversion (6). Avoiding the degradation or outright clearance of intact forests (which we collectively term “intact forest loss”) is therefore likely to be an important contributor to the Paris Agreement’s goal of limiting global warming to well below 2°C above preindustrial levels (7), alongside other nature-based climate mitigation actions (8).
Most national governments fail to recognize or prioritize the retention of intact tropical forests as a means of meeting their commitments under the Paris Agreement. For example, reduced emissions from land use and land cover change account for a quarter of all emission reductions planned by countries (9), but intact forest retention [or related approaches such as the prioritization of primary forests (10)] is seldom a specific contributor to these planned reductions (11).
Intact forest retention also rarely attracts funding from schemes designed to avoid land use and land cover change emissions in developing nations. Notably, the Reducing Emissions from Deforestation and Forest Degradation (REDD+) approach enables developing countries to receive financial incentives for enhancing carbon stocks or avoiding the loss of carbon that would otherwise be emitted because of land use and land cover change. Among other activities, “+” indicates support for conservation of tropical forests not under immediate threat of direct clearance or degradation and was formally adopted by parties to the United Nations Framework Convention on Climate Change in 2008 at the 14th Conference of the Parties in Poland (12). Since then, however, financial support and implementation have predominantly focused on areas with high historical rates of deforestation (i.e., “deforestation frontiers”) and hence high predicted rates of emissions in the near future. This is widely believed to deliver more immediate and more clearly demonstrable emission reductions than conserving intact forest areas, which tend to be treated as negligible sources of emissions as a result of the short time scales and conservative assumptions under which REDD+ operates (11).
The relative value of retaining intact tropical forest areas increases if one takes a longer-term view and considers the likely state of the world’s forests by mid-century—a milestone date in the Paris Agreement (13). Far from being stable and free from threat, intact tropical forests have been severely reduced by industrial human activities in recent decades. Agricultural expansion, logging, mining, and anthropogenic fires reduce the global extent of intact forests by 7.2% between 2000 and 2013 (2), yet the carbon emissions associated with intact forest loss have not been comprehensively estimated.
The most obvious and immediate source of emissions from intact forest loss occurs through outright forest clearance (14). The clearance of intact forests also leads to numerous sources of committed emissions. Newly accessible forests are targeted for first-cut selective logging, which can result in substantial emissions (15). Increased accessibility also initiates cryptic sources of emissions that occur more gradually, including the edge effects associated with forest fragmentation (16) and declines of carbon-dense tree species due to overhunting of seed-dispersing animals (i.e., “defaunation”) (17).
The loss of intact forests also forgoes the opportunity for persistent carbon removals, as degradation processes or conversion to nonforest land uses reduces carbon uptake from the atmosphere (5). Intact tropical forests account for nearly half of all the carbon sequestered in global intact forests (4), which absorbed around 28% of anthropogenic carbon emissions from all sources during the period 2007–2016, and hence markedly reduced the net rate of carbon dioxide accumulation in the atmosphere (18). Failing to account for forgone carbon removals potentially distorts priority setting for mitigation action within and beyond the forest sector. Despite these shortcomings, no previous study has accounted for less-readily observed impacts when quantifying the climate mitigation potential of retaining intact forests.
Here, we used a stepwise approach to estimate carbon emissions and forgone carbon removals that result from the loss of intact forests across the tropics. We used reductions in intact forest landscapes (2), which are predominantly forested ecosystems with no satellite-based observations of large-scale human activity and a minimum area of 500 km2, to delineate parcels of intact forest that were lost between 2000 and 2013. Within these lost parcels (49 million ha), we estimated pulse emissions from forest clearance and fire that occurred between 2000 and 2013 (Fig. 1). We then estimated likely committed emissions up to 2050 from selective logging near roads that entered lost parcels between 2000 and 2013. We then estimated committed emissions up to 2050 from edge effects associated with forest fragmentation that occurred in lost parcels between 2000 and 2013 and from reduced faunal abundance and diversity that is expected to occur as intact forests become more accessible to hunters. Last, we compared these pulse and committed emissions to carbon sequestration that would likely have occurred had forested areas affected by clearance, logging, or edge effects (28 million ha) remained intact from year 2000 onward. We demonstrate and quantify the climate mitigation potential of retaining intact tropical forests for individual countries and present approaches for rewarding intact forest retention under the Paris Agreement.
Fig. 1. Areas of tropical forest that remained intact in 2013 or were lost between 2000 and 2013 (2).
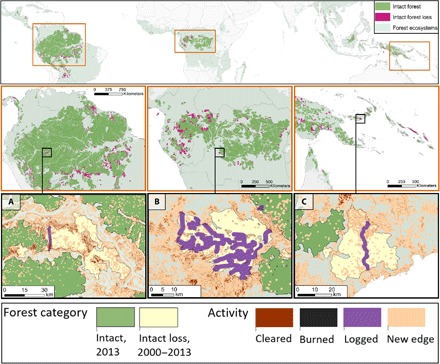
(A to C) Inset maps with orange borders show remnant and lost parcels of intact forest across Latin America, Central Africa, and Asia-Pacific. The second row of inset maps (black borders) shows the spatial distribution of activities that cause carbon emissions and forgo carbon removal in intact forest areas. Forest clearance and burned areas were sourced from (14) and (21), respectively. Selectively logged areas were simulated inside lost parcels by applying a 1-km buffer around roads mapped in the OpenStreetMap dataset (www.openstreetmap.org). New forest edges were simulated by applying 500-m buffers around footprints of burned area and forest clearance.
RESULTS
Our findings show that intact tropical forest loss plays a larger-than-realized role in exacerbating climate change, particularly when forgone carbon removals are considered (Table 1). Annual emissions from forest clearance observed in lost parcels between 2000 and 2013 [26 Tg of carbon per year (Tg C year−1); 1 Tg = 1012 kg] were 3.2% of annual emissions from all deforestation across the pantropics in the 2000s (19). Selective logging within 1 km from roads that entered intact tropical forests in the 2000s was predicted to emit at least 118 (126 to 66) Tg C by 2050. This is likely an underestimate, given that all tropical forests outside protected areas are expected to be logged (20). Emissions could sum to 523 (571 to 304) Tg C if selective logging extends beyond 1 km from roads that entered intact tropical forests in the 2000s to all areas in lost parcels not affected by forest clearance between 2000 and 2013. Further committed emissions from defaunation and newly created forest edges could add an additional 733 (797 to 444) Tg C to the atmosphere by 2050. Had the 28 million ha of forest damaged by clearance, logging, or edge effects remained intact from year 2000 onward, they could have sequestered 972 (1604 to 331) Tg C by 2050. Hence, after accounting for committed emissions and forgone carbon removals, the estimated net carbon impact from intact tropical forest loss in the 2000s increased sixfold over the estimate based on forest clearance alone, from 338 (372 to 208) to 2116 (2854 to 1004) Tg C (see Materials and Methods; Fig. 2). This revised estimate equates to approximately 2 years of global land use change emissions (18) and implies that accounting for clearance alone will underestimate the carbon impact of intact forest loss by 84%.
Table 1. Pantropical and regional estimates of the full carbon impact of intact forest loss.
Pulse emissions include those from forest clearance and fire observed between 2000 and 2013. Disturbance inside intact forests will lead to numerous sources of emissions between 2013 and 2050, including selective logging, edge effects, and defaunation, herein collectively referred to as committed emissions. Committed emissions shown here also account for some carbon sequestration from forest regrowth between 2013 and 2050 (see Materials and Methods). Emission estimates were based on a synthesis map of pantropical aboveground biomass of woody vegetation (22). Upper and lower uncertainty bounds were based on rerunning emission calculations with two original biomass maps (23, 24). Forgone removals are an estimate of the amount of carbon that cleared or degraded forests could have sequestered had they remained intact beyond 2000. Forgone removal estimates and 95% confidence intervals were based on carbon sequestration rates in intact tropical forests estimated for the 2000s (4).
| Carbon emissions (Tg C) | Forgone (Tg C) | ||||
| Region | Carbon impact (Tg C) | Net (2000–2050) | Pulse (2000–2013) | Committed (2013–2050) | Total (2000–2050) |
| Pantropics | 2116 (2854–1004) | 1114 (1250–673) | 338 (372–208) | 806 (878–465) | 972 (1604–331) |
| Latin America | 1132 (1633–455) | 677 (766–420) | 263 (294–168) | 414 (472–252) | 455 (867–35) |
| Africa | 517 (681–239) | 236 (239–117) | 31 (32–15) | 205 (207–103) | 281 (442–122) |
| Asia-Pacific | 467 (540–310) | 231 (245–135) | 44 (46–25) | 188 (199–110) | 236 (295–175) |
Fig. 2. Full accounting of the carbon impact of intact forest loss.
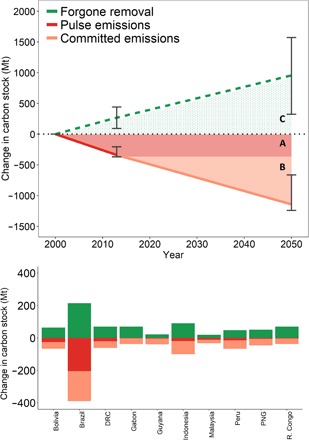
(A, red segments) Conventionally, only emissions from readily observed forest clearance are considered. Forest clearance in intact forest between 2000 and 2013 led to the emission of 338 (372 to 208) Tg C. (B, orange segments) Less readily observed degradation processes that follow forest clearance, including selective logging, edge effects, and defaunation, are rarely accounted for in emission estimates. We expect that these events occurring in intact forest between 2013 and 2050 will lead to the emission of 806 (878 to 465) Tg C. (C, green stippled segments) Forgone carbon removal—carbon sequestration that could have occurred had cleared or degraded forest areas remained intact beyond 2000—is not considered in conventional emission accounting frameworks. If the forested area affected by clearance, logging, or edge effects remained intact beyond 2000, then it could have sequestered 972 (1604 to 331) by 2050. Full accounting of these additional factors (i.e., selective logging, cryptic emissions, and forgone enhancement) led to a 626% increase in cumulative net carbon impact from intact forest loss. Histogram plot shows carbon impacts for the 10 countries with the highest estimated impacts.
Emissions from forest clearance and selective logging
Forest clearance and fire observed in intact tropical forests between 2000 and 2013 led to the emission of 26 Tg C year−1, or 338 (372 to 208) Tg C in total (see Materials and Methods; Table 1). These pulse emissions were largest in Latin America, with Brazil accounting for 61% of all forest clearance emissions. Bolivia, Democratic Republic of Congo, and Indonesia were also large contributors. The ratio of forest loss to gain across the pantropics indicates that reforestation will occur at around 28% of sites that were cleared between 2000 and 2013 (14). After accounting for carbon removed by this amount of forest regrowth (45 Tg C; see Materials and Methods), emissions from forest clearance in intact forests in the 2000s would still result in the net emission of 293 (327 to 163) Tg C by 2050.
We partitioned selective logging footprints inside intact tropical forests into conventional or responsible logging areas based on the extent of responsible forestry practices in tropical countries (table S1; see Materials and Methods). Assuming no change in the relative extent of responsible logging inside national borders, we estimate that conventional logging activities will account for 93% of emissions from selective logging up to 2050. The universal adoption of responsible logging techniques in lost parcels of intact tropical forest would lead to better biodiversity outcomes (25) and lower expected emissions from selective logging by approximately 25%. Yet, even when constrained to only being within 1 km of roads, we estimate that selective logging will cause emissions equivalent to 35% of those resulting from direct forest clearance. This estimate is similar to a previous study that shows that emissions from selective logging in tropical countries can be equivalent to 10 to 50% of those from deforestation (15).
Emissions from cryptic forest degradation
Forest fragmentation reduces the net amount of carbon stored at forest edges (26). Forest clearance and fire observed in intact forests in the 2000s created new forest edges, which, between 2013 and 2050, could lead to the emission of 631 (686 to 352) Tg C, or approximately 18 Tg C year−1. This estimate is based on a 25% loss in aboveground carbon penetrating 500 m from the edge of a deforested or burnt site (16). Should the first 100 m of this 500-m edge incur a 50% loss in aboveground biomass—an effect observed in long-term fragmentation studies in tropical forests (27)—cumulative emissions from edge effects would increase by approximately 25% to 761 (828 to 426) Tg C by 2050. Hence, we expect that cumulative net emissions from edge effects will approximately double those from direct forest clearance events observed in intact forest in the 2000s.
The relative magnitude of emissions from edge effects reported here are higher than previous studies, showing emissions from forest fragmentation across all tropical forests (including nonintact areas) to be 47 to 75% of those from deforestation (28–30). This result is driven by large edge-to-forest clearance ratios found in lost parcels of intact forest (table S2). On average, every 1 ha of intact forest clearance resulted in 7 ha of new forest edge, with much larger ratios found in Gabon (60:1) and Guyana (44:1).
Declines of large-seeded animal-dispersed trees in intact forests could lead to the emission of 102 (111 to 92) Tg C by 2050. This estimate assumes that modeled effects of defaunation on aboveground carbon storage across the pantropics (17) take effect linearly over 100 years and occur in all moist broadleaf intact forests that became degraded (i.e., selectively logged or fragmented) but not cleared between 2000 and 2013 (40 million ha) (table S3). We predict that carbon losses from defaunation will be similar in magnitude between Latin America and Africa despite the extent of intact forest loss being much lower in Africa. This result is due to the stronger relationship between defaunation and carbon storage in tropical moist broadleaf forests across Africa (17).
Forgone carbon removals
We expect that, by 2050, carbon sequestration in 28 million ha of intact forest area will be permanently altered because of forest clearance, edge creation, or selective logging. Had this forested area remained intact, it could have potentially sequestered 19 Tg C year−1, totaling 972 (1604 to 331) Tg C by 2050 (Fig. 2). This estimate is based on carbon sequestration rates in intact forests across Asia-Pacific, Africa, and Latin America continuing at rates estimated for the 2000s (table S4) (4). Brazil was expected to have the largest forgone removals, but high per-hectare rates of carbon sequestration in Asian and African intact forests will also result in large forgone removals for Indonesia and across the Congo Basin.
Tropical tree growth could be unaffected by climate change (31), but under some scenarios, carbon sequestration rates could increase because of decreased cloudiness (32) or carbon dioxide fertilization effects (33) or could slow down due to higher drought frequency and drying trends (34), increased frequency or intensity of pest outbreaks (35), or mortality rates catching up with stagnating growth rates (36). Should the intact forest sink in Latin America become saturated by 2030 (37) (see Materials and Methods; fig. S1), forgone removals would drop by 31% to 674 (1025 to 311) (fig. S2). Should all tropical intact forests reach saturation by 2030, forgone removals would reduce by 66% to 329 (531 to 117) (fig. S3). We note that the onset of saturation by 2030 is quite a conservative assumption; many other studies indicate that the intact forest sink could be maintained or accelerate before reaching saturation late in the 21st century [e.g., (38)].
DISCUSSION
Climate change mitigation funding in the land sector is overwhelmingly concentrated in deforestation frontiers and restoration zones (39). There has been insufficient focus on many intact forest areas despite this analysis, suggesting that they have very significant climate mitigation potential (13). Recognizing how intact forest retention can deliver large-scale emission reductions has broad relevance, because intact tropical forests are found in at least 65 nations and represent ≥25% of the forest cover in at least 18 nations. One group of countries for whom this analysis is especially relevant is those with high amounts of forest and low deforestation rates (e.g., Suriname, Gabon, and Papua New Guinea). These countries have thus far received little attention under REDD+ when compared to high forest cover–high deforestation countries (e.g., Brazil and Indonesia) despite the fact that REDD+ includes, in principle, support for the retention of tropical forests that are not under immediate threat of direct clearance or degradation (40).
To rectify the current neglect of intact forests in global climate finance mechanisms, we encourage national governments to better account for the full carbon impact of intact forest retention. For example, emission baselines that account for selective logging and other more cryptic degradation processes would reduce the disproportionate emphasis on recent forest clearance within countries, which is more readily observed but is concentrated at forest margins. Recent advances in modeling capacity and understanding of forest degradation trends [e.g., (41)] are likely to improve emission baseline estimates in this regard, as are innovative approaches to estimating baselines in the context of areas with low historical rates of forest cover change. Recent examples of such development include the way baselines were calculated for the bilateral emission reduction agreement between Guyana and Norway (42) and the introduction of a “stock-flow” structure to the Amazon Fund in Brazil, which has partially decoupled domestic incentive structures from the more restrictive international framework (43). Furthermore, improved recognition in the scientific community that most forest degradation processes that follow from the opening up of intact forests will play out over a period of decades, rather than occurring instantly, could potentially allow them to be acknowledged and better accounted for in emission baselines.
Looking beyond current carbon accounting frameworks, national governments and the carbon finance sector should also consider complementary funding mechanisms that use innovative accounting frameworks (13). For example, previous studies have proposed a global carbon preservation target that would reward nations who retain standing forests [e.g., (44)]. Among other advantages, this approach would place a value on maintaining carbon sequestration by intact forests. Carbon dioxide removal will be necessary to limit global warming to 1.5°C above preindustrial levels, yet large-scale engineered solutions to carbon removal on land are unproven and risky (45). In contrast, intact tropical forests represent a safe and effective way to achieve meaningful carbon dioxide removals and to neutralize emissions from other sources.
Our study is subject to several caveats, most of which suggest that we have understated the climate mitigation benefits of intact forest retention. We assume that no additional fire and deforestation will occur after 2013 in parcels of intact forest loss, whereas in reality deforestation frontiers and wildfires are likely to expand into forested areas where access has improved (46) or selective logging has occurred (47). Consideration of forest clearance after 2013 would likely increase the carbon impact of intact forest loss, given that these events in many cases imply a near-complete and permanent loss of biomass (48). Should additional forest clearance after 2013 replace areas of degraded forest (e.g., selectively logged areas and new forest edges), it could reduce our estimates of committed emissions between 2013 and 2050. However, additional forest clearance after 2013 would likely displace forest degradation processes and subsequent emissions into previously undisturbed forest areas and also increase the magnitude of foregone removals. It is also likely that we have underestimated the extent of current road networks (and hence the extent of selective logging) and burned area in lost parcels because the road dataset we used omits many known roads in tropical areas (49) and many tropical forest fires are undetected or underestimated (50). We also do not capture emissions from peat fires (51)—a major source of emissions from southeast Asia (52). Emissions from conventional and responsible selective logging practices may also be conservative, given that timber harvest rates often exceed regeneration capacity (53), driving progressive degradation over time (6), and we do not model any additional edge effects along roads also used for selective logging.
CONCLUSION
As of 2013, there were 549 million ha of intact tropical forest remaining globally. Yet, the rate at which intact forests are being lost is increasing, closing off opportunities for countries to use them in mitigation efforts. We show that accounting for clearance alone will underestimate the carbon impact of intact forest loss by 84%. The climate mitigation potential from retaining intact forests increases by at least 626% after accounting for forgone carbon removals, selective logging, and cryptic emissions from edge effects and defaunation. A comparable analysis for extratropical regions is urgently required, given that approximately a half (4) to two-thirds (54) of carbon removals on Earth’s intact ecosystems occur outside the tropics. The climate mitigation potential of retaining intact forests is significant, but without proactive conservation action by national governments, supported by the global community, this potential will continue to dwindle.
MATERIALS AND METHODS
Mapping intact forest loss and aboveground biomass
We used reductions in intact forest landscapes (2), which are defined as predominantly forest ecosystems with no evidence of large-scale human activity observed from satellites and a minimum area of 500 km2, to delineate parcels of intact forest that were lost between 2000 and 2013. Ninety-three million hectares of previously intact forest were lost in this time. “Loss” in this sense entails either outright deforestation or fragmentation into areas smaller than the 500-km2 intact forest landscape threshold.
We focused on intact forests that are found in the pantropics because the majority of current planned actions to reduce emissions from land use and land cover change will occur in this region (9). There are also substantial differences in how biomass in tropical and extratropical forests responds to natural and anthropogenic disturbances (54), which would make a global analysis somewhat intractable. We used a terrestrial ecoregions map to delineate lost parcels located in tropical forest ecoregions (50 million ha) (55). Lost parcels overlapped with three tropical ecoregions, namely, tropical and subtropical moist broadleaf forests, tropical and subtropical dry broadleaf forests, and tropical and subtropical coniferous forests. In cases where more than one ecoregion was found inside a fragmented parcel, we split the parcel into new polygons along ecoregion boundaries. We included, in our analysis, lost parcels of ≥100 ha to match the spatial resolution of aboveground biomass data for the pantropics. This reduced the total area of lost parcels of intact tropical forest analyzed by 1.3%.
We used a synthesis map of pantropical aboveground biomass of woody vegetation (22), which was based on two earlier datasets (23, 24), to calculate mean estimates for biomass inside before their loss. The biomass data, originally mapped at 1-km resolution in forested areas for the 2000s, were resampled to a spatial resolution of 100 m and then overlaid on lost parcels to derive mean biomass estimates. This resampling procedure helped to extract biomass estimates for smaller parcels of forest that did not completely overlap with the 1-km pixels.
To explore the sensitivity of our results to our chosen aboveground biomass dataset, we also derived mean biomass estimates inside lost parcels from the two original pantropical datasets of aboveground biomass—the Saatchi et al. dataset, which maps total carbon stock in live biomass at a spatial resolution of approximately 1 km, and the Baccini et al. dataset, which maps aboveground live woody vegetation carbon density at a spatial resolution of 463 m. Upper and lower uncertainty bounds around emission estimates were based on rerunning emission calculations with these two alternative biomass datasets. Within each fragmented parcel, we applied a stepwise procedure to estimate the respective areas suffering from a number of fates that result in carbon emissions or forgone carbon removals (i.e., data on emission-causing activities were extracted at the scale of individual lost parcels of intact forest).
Mapping forest clearance
We used satellite-derived data to map the spatial footprint of two forest clearance processes inside lost intact forest parcels—burned area and forest clearance. To map forest clearance, we used version 1.1 of the Hansen et al. Global Forest Change dataset (14), which provides forest loss information between 2000 and 2013 at a spatial resolution of 30 m. This dataset also provides a continuous canopy cover band representing tree cover in year 2000. Following previous studies [e.g., (46)], we used a canopy cover threshold of 25% to delineate forested and nonforested areas within lost parcels. We used the MCD45 burned area product to map burned area (21). This MODIS-derived product maps the approximate date of burning at 500-m resolution using multitemporal land surface reflectance data. Monthly burned area data were aggregated from 1 April 2000 (the earliest available date) to 31 December 2013, using only burned areas with the highest pixel detection confidence. Pixels that burned multiple times during aggregation period were treated as only having burned once in our analysis. We removed burned areas that overlapped with forest clearance inside lost parcels to avoid double counting forest clearance processes. Data were extracted at a spatial resolution of 30 m in Google Earth Engine.
Mapping forest degradation from selective logging
The effect of selective logging on terrestrial carbon stocks likely plays a larger-than-expected role in the climate system (15). Information about the spatial extent and intensity of selective logging is not yet available at the pantropical scale, but selective logging operations in the tropics rely on networks of roads to provide access for machinery and the transport of merchantable timber products (56). We simulated the spatial footprint of selective logging inside lost parcels by applying a buffer around roads mapped in the OpenStreetMap dataset (data copyrighted OpenStreetMap contributors and available from www.openstreetmap.org). We applied a 1-km buffer around roads to approximate where selective logging is likely to occur in lost parcels between 2013 and 2050. The 1-km buffer size was based on previous assessments of the relationship between accessibility and the intensity of selective logging (57). We removed selective logging footprints that overlapped with nonforest areas, burned area, or forest clearance inside lost parcels.
Selective logging footprints inside lost parcels were split into conventional logging or responsible logging areas (table S1). We based proportional estimates of responsible logging on nationally reported statistics on how much of the selective logging that occurs in their forests is responsible (58). In cases where responsible logging estimates were not available for a particular country, responsible logging proportional extents were set to mean of estimates from countries in the same continent.
Mapping cryptic forest degradation
Forest fragmentation reduces the net amount of carbon stored at forest edges (26). Many studies indicate that such edge effects penetrate at least 500 m, and potentially well beyond this threshold, from a disturbed site (5, 16, 59). We therefore simulated fragmentation by applying 500-m buffers around the spatial footprints of forest clearance inside lost parcels. In pixels where fragmentation buffers overlapped inside a lost parcel, only one buffer was retained to prevent double counting edge effects. We also removed fragmentation buffers that overlapped with nonforest areas, burned area, forest clearance, or selective logging footprints inside lost parcels. Spatial data on edge effects were extracted at a spatial resolution of 30 m in Google Earth Engine.
Unsustainable harvesting of wild animals (i.e., defaunation) is causing declines of large-seeded animal-dispersed trees across the pantropics and could subsequently reduce the carbon stored in some tropical forest types by between 2 and 12% (17). The roads and forest clearance we observed in lost parcels will make them more susceptible to defaunation (60). We simulated emissions caused by defaunation in lost parcels using published estimates of the extent and intensity of carbon losses from defaunation across the pantropics (17). Osuri et al. use country-scale data on the relative abundance of animal-dispersed tree species and differences in median change in carbon stocks between defaunation and species-based control scenarios. Where these data were not available for all countries that lost intact tropical forest between 2000 and 2013, they were set to the median of values from countries within the same geographic region (i.e., Africa, Asia-Pacific, or Latin America) (table S3). We estimated carbon losses from defaunation in areas of lost parcels that were forested in 2013, and situated in the tropical moist broadleaf ecoregion (40 million ha).
In summary, we considered sources of committed emissions additively, ensuring that there was no overlap or double counting of emissions from selective logging or fragmentation. It was possible, however, for a pixel within a lost parcel to be subject to both defaunation and selective logging concurrently, or defaunation and fragmentation concurrently.
Estimating net emissions from forest clearance and degradation
We estimated emissions from forest clearance that occurred in intact forests between 2000 and 2013 using a “combine and assign” approach (61). Pulse emissions from a disturbance process d in a fragmented parcel i were measured using
| (1) |
where A is the area of disturbance d (in ha), P is the proportion of biomass lost due to disturbance d (i.e., the “carbon emission factor”), and B is the mean aboveground biomass estimated inside parcel i (in T ha−1). The factor of 0.5 is the proportion by weight of carbon in aboveground dry biomass.
In a second time period between 2013 and 2050, we estimated committed emissions from forest degradation processes that require years or decades to take effect and accounted for carbon sequestration that may occur from reforestation beyond 2013. We note, however, that a small fraction of the committed emissions we estimate for this second time period would actually occur in the first time period (2000–2013), given that some forest clearance occurred early in the 2000s.
We used Eq. 1 to estimate committed emissions between 2013 and 2050 from selective logging, fragmentation, and ecological changes triggered by defaunation. Carbon emission factors for selective logging in countries that lost intact tropical forest between 2000 and 2013 were drawn from the peer-reviewed literature (table S1) (20). Where carbon emission factors for selective logging were not available for a particular country, they were set to the median of estimated values from countries within the same geographic region.
Emissions from defaunation in lost parcels were also estimated using Eq. 1. We estimated the area within tropical moist broadleaf forests affected by defaunation, ADFAUN, in parcel i using
| (2) |
where Ai is the forested area of parcel i (in ha), AFC is the area of forest clearance in parcel i (in ha), and Ti is the relative abundance of tree species vulnerable to defaunation in parcel i. We derived Ti values and carbon penalties for defaunation from Osuri et al. (table S3).
The committed period also accounted for carbon sequestration by reforestation that is likely to occur between 2013 and 2050 at sites that were cleared between 2000 and 2013. In tropical countries, the majority of land for agricultural expansion comes from forests, not from previously cleared lands (48), and pressure to continue agricultural expansion in tropical countries is likely to increase in the coming decades (62). We therefore assumed that reforestation will occur in 28% of areas that were deforested in lost parcels located in tropical and subtropical ecoregions (14). We based this estimate on the observed ratio of forest loss to gain in the tropical forest domain for the 2000s (3.6 for >50% tree cover) (14). We assumed that regrowing forests will sequester carbon at rates estimated for the 2000s (table S4) (4).
Most selective logging in the tropics operates on 20- to 40-year harvest cycles (20). We assumed that selective logging footprints within lost parcels will be logged at least once between 2013 and 2050 (37 years). Emissions from selective logging at a specific site are cyclical—an immediate release followed by gradual recovery—but we applied carbon penalties for conventional logging and responsible logging linearly across 37 years. This simplification was based on the typical long-term carbon impact of selective logging activities (20) and is not likely to affect our estimated regional or pantropical emissions from selective logging.
Time scales and trajectories for emissions from fragmentation remain poorly characterized. Previous studies show that emissions of the magnitude we considered (i.e., 25% loss in aboveground carbon penetrating 500 m from a newly created edge, and the first 100 m of this 500-m edge incurring a 50% loss in aboveground biomass) can occur within 10 to 17 years (27). We conservatively assumed a loss residence time for fragmentation of 37 years and, for simplicity, assumed that this loss occurs linearly (table S5). The time frame over which emissions from defaunation may occur is also unknown at this time. These emissions are likely to be much longer term than more immediate drivers of carbon losses such as deforestation or logging (17), yet ecological changes within tropical forests with the potential to result in biomass changes of the magnitude we considered for defaunation (i.e., 0.5 to 13.9% loss in aboveground carbon) can occur within 20 to 30 years (63). We chose to set a conservative time scale for defaunation emission to 100 years and also assumed that this loss occurs linearly. We did not account for any defaunation that potentially occurred in intact forests before year 2000 (64).
Estimating forgone carbon removals
Last, we compared emissions caused by forest clearance, selective logging, and edge effects between 2000 and 2050 to the amount of carbon that could have been removed from the atmosphere had these processes not occurred. We estimated annual foregone removals, Fn, for three geographic regions, j (Asia-Pacific, Africa, and Latin America), using
| (3) |
where E is the total area in lost parcels sited in geographic region, j, that is subject to clearance, selective logging, or edge effects between 2000 and 2050 (in ha) and R is the intact forest sink for each geographic region (in T ha−1 year−1) in year n. We derived Rj values from Pan et al. (4) (table S4), who estimated annual sequestration rates inside tropical intact forests across Asia-Pacific, Africa, and the Americas in the 2000s (table S4). We also derived 95% confidence intervals for Rj values from Pan et al. (4) using
| (4) |
where Uj is the annual carbon stock change uncertainty (in T ha−1 year−1) and Fj is the average annual forested area between (in ha) reported by Pan et al. (4) for different geographic regions between 2000 and 2007. Where possible, Uj values reported by Pan et al. (4) were quantitative estimates of uncertainty, either calculated from sample plot data or reported in the source of data using an acceptable calculation method. If quantitative estimates of uncertainties were not available from the source data or could not be calculated, then Uj values were derived from expert opinion (4).
Spatial variation in the intact forest sink is poorly understood, and some individual areas of intact forest may have markedly different sequestration rates than those estimated here. However, we estimated that, by 2050, aboveground carbon currently held in intact tropical forests would increase by 13% if the intact forest sink continues at rates estimated for the 2000s, which is comparable to observed accumulation rates from long-term studies of intact forest areas in Africa (38), Asia-Pacific (5), and Latin America (3).
We developed two alternative sequestration scenarios to explore how a gradual saturation of the intact forest sink in the 21st century would influence our results. There is some early evidence that intact forests are becoming saturated in Latin America (36), whereas the intact forest sink in Asia (5) and Africa (38) appears to be more robust. Most studies suggest that any sink saturation within tropical intact forests would occur late in the 21st century, with 2030 being a very conservative estimate [e.g., (38)]. Hence, our two alternative scenarios assumed that intact forests located in Latin America will reach saturation by 2030 and that all tropical intact forests will reach saturation by 2030.
Saturation of Rj values between 2013 and 2030 was modeled using an exponential decay function
| (5) |
where Rj(t) is the annual sequestration rate at time t and Rj0 is the annual sequestration rate in year 2013 for geographic region j. To calculate the decay constant, k, for each geographic region, we solved the exponential decay function for k after setting Rj(t) to approximately zero (0.001) and t to 16 (because the 16th time iteration represented year 2029 when we assumed that the sequestration rate would approach zero). Annual sequestration rates between 2030 and 2050 were then set to zero (fig. S1).
Supplementary Material
Acknowledgments
We thank S. Atkinson for technical guidance during the initial stages of this study. We also thank two reviewers and the editor for comments on the manuscript. Funding: This project was supported by the John D. and Catherine T. MacArthur Foundation. Author contributions: S.L.M., T.E., J.E.M.W., A.M., and Y.M. conceived the study. S.L.M., T.E., J.E.M.W., A.M., H.G., N.H., P.P., R.K.R., O.V., S.W., and Y.M. advised on study methodology. S.L.M. and A.D. performed data extraction. S.L.M. compiled and analyzed data. S.M. wrote the manuscript. All authors contributed comments on the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data supporting the findings of this study either are publicly available online via the referenced source or can be obtained directly from the corresponding author upon request.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/10/eaax2546/DC1
Fig. S1. Estimated annual rate of carbon sequestration inside intact forests under alternative scenarios.
Fig. S2. Full accounting of the carbon impact of intact forest loss assuming the onset of carbon saturation in intact forests in Latin America by 2030.
Fig. S3. Full accounting of the carbon impact of intact forest loss assuming the onset of carbon saturation in all tropical intact forests by 2030.
Table S1. Country-level estimates on the proportion of selective logging that uses responsible forest management techniques and emission factors for responsible and conventional logging practices.
Table S2. Intact forest landscape reduction between 2000 and 2013 in countries across the global tropics.
Table S3. Country-level statistics required to estimate carbon emissions from defaunation.
Table S4. Regional carbon sequestration rates for intact forest areas and reforestation.
Table S5. Assumed time scales and trajectories of carbon dynamics from different forest processes.
REFERENCES AND NOTES
- 1.Malhi Y., The carbon balance of tropical forest regions, 1990–2005. Curr. Opin. Environ. Sustain. 2, 237–244 (2010). [Google Scholar]
- 2.Potapov P., Hansen M. C., Laestadius L., Turubanova S., Yaroshenko A., Thies C., Smith W., Zhuravleva I., Komarova A., Minnemeyer S., Esipova E., The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 3, e1600821 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips O. L., Malhi Y., Higuchi N., Laurance W. F., Núñez P. V., Vásquez R. M., Laurance S. G., Ferreira L. V., Stern M., Brown S., Grace J., Changes in the carbon balance of tropical forests: Evidence from long-term plots. Science 282, 439–442 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Pan Y., Birdsey R. A., Fang J., Houghton R., Kauppi P. E., Kurz W. A., Phillips O. L., Shvidenko A., Lewis S. L., Canadell J. G., Ciais P., Jackson R. B., Pacala S. W., McGuire A. D., Piao S., Rautiainen A., Sitch S., Hayes D., A large and persistent carbon sink in the world’s forests. Science 333, 988–993 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Qie L., Lewis S. L., Sullivan M. J. P., Lopez-Gonzalez G., Pickavance G. C., Sunderland T., Ashton P., Hubau W., Salim K. A., Aiba S.-I., Banin L. F., Berry N., Brearley F. Q., Burslem D. F. R. P., Dančák M., Davies S. J., Fredriksson G., Hamer K. C., Hédl R., Kho L. K., Kitayama K., Krisnawati H., Lhota S., Malhi Y., Maycock C., Metali F., Mirmanto E., Nagy L., Nilus R., Ong R., Pendry C. A., Poulsen A. D., Primack R. B., Rutishauser E., Samsoedin I., Saragih B., Sist P., Slik J. W. F., Sukri R. S., Svátek M., Tan S., Tjoa A., van Nieuwstadt M., Vernimmen R. R. E., Yassir I., Kidd P. S., Fitriadi M., Ideris N. K. H., Serudin R. M., Lim L. S. A., Saparudin M. S., Phillips O. L., Long-term carbon sink in Borneo’s forests halted by drought and vulnerable to edge effects. Nat. Commun. 8, 1966 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindenmayer D. B., Hobbs R. J., Likens G. E., Krebs C. J., Banks S. C., Newly discovered landscape traps produce regime shifts in wet forests. Proc. Natl. Acad. Sci. U.S.A. 108, 15887–15891 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adoption of the Paris Agreement Report No. FCCC/CP/2015/L.9/Rev.1 (UNFCCC, 2015); http://unfccc.int/resource/docs/2015/cop21/eng/l09r01.pdf.
- 8.Griscom B. W., Adams J., Ellis P. W., Houghton R. A., Lomax G., Miteva D. A., Schlesinger W. H., Shoch D., Siikamäki J. V., Smith P., Woodbury P., Zganjar C., Blackman A., Campari J., Conant R. T., Delgado C., Elias P., Gopalakrishna T., Hamsik M. R., Herrero M., Kiesecker J., Landis E., Laestadius L., Leavitt S. M., Minnemeyer S., Polasky S., Potapov P., Putz F. E., Sanderman J., Silvius M., Wollenberg E., Fargione J., Natural climate solutions. Proc. Natl. Acad. Sci. U.S.A. 114, 11645–11650 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grassi G., House J., Dentener F., Federici S., den Elzen M., Penman J., The key role of forests in meeting climate targets requires science for credible mitigation. Nat. Clim. Chang. 7, 220–226 (2017). [Google Scholar]
- 10.Mackey B., DellaSala D. A., Kormos C., Lindenmayer D., Kumpel N., Zimmerman B., Hugh S., Young V., Foley S., Arsenis K., Watson J. E. M., Policy options for the world’s primary forests in multilateral environmental agreements. Conserv. Lett. 8, 139–147 (2015). [Google Scholar]
- 11.Watson J. E. M., Evans T., Venter O., Williams B., Tulloch A., Stewart C., Thompson I., Ray J. C., Murray K., Salazar A., McAlpine C., Potapov P., Walston J., Robinson J. G., Painter M., Wilkie D., Filardi C., Laurance W. F., Houghton R. A., Maxwell S., Grantham H., Samper C., Wang S., Laestadius L., Runting R. K., Silva-Chávez G. A., Ervin J., Lindenmayer D., The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2, 599–610 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Goetz S. J., Hansen M., Houghton R. A., Walker W., Laporte N., Busch J., Measurement and monitoring needs, capabilities and potential for addressing reduced emissions from deforestation and forest degradation under REDD+. Environ. Res. Lett. 10, 123001 (2015). [Google Scholar]
- 13.Funk J. M., Aguilar-Amuchastegui N., Baldwin-Cantello W., Busch J., Chuvasov E., Evans T., Griffin B., Harris N., Ferreira M. N., Petersen K., Phillips O., Soares M. G., van der Hoff R. J. A., Securing the climate benefits of stable forests. Clim. Policy 19, 845–860 (2019). [Google Scholar]
- 14.Hansen M. C., Potapov P. V., Moore R., Hancher M., Turubanova S. A., Tyukavina A., Thau D., Stehman S. V., Goetz S. J., Loveland T. R., Kommareddy A., Egorov A., Chini L., Justice C. O., Townshend J. R. G., High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Pearson T. R. H., Brown S., Casarim F. M., Carbon emissions from tropical forest degradation caused by logging. Environ. Res. Lett. 9, 034017 (2014). [Google Scholar]
- 16.Chaplin-Kramer R., Ramler I., Sharp R., Haddad N. M., Gerber J. S., West P. C., Mandle L., Engstrom P., Baccini A., Sim S., Mueller C., King H., Degradation in carbon stocks near tropical forest edges. Nat. Commun. 6, 10158 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osuri A. M., Ratnam J., Varma V., Alvarez-Loayza P., Astaiza J. H., Bradford M., Fletcher C., Ndoundou-Hockemba M., Jansen P. A., Kenfack D., Marshall A. R., Ramesh B. R., Rovero F., Sankaran M., Contrasting effects of defaunation on aboveground carbon storage across the global tropics. Nat. Commun. 7, 11351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Quéré C., Andrew R. M., Friedlingstein P., Sitch S., Pongratz J., Manning A. C., Korsbakken J. I., Peters G. P., Canadell J. G., Jackson R. B., Boden T. A., Tans P. P., Andrews O. D., Arora V. K., Bakker D. C. E., Barbero L., Becker M., Betts R. A., Bopp L., Chevallier F., Chini L. P., Ciais P., Cosca C. E., Cross J., Currie K., Gasser T., Harris I., Hauck J., Haverd V., Houghton R. A., Hunt C. W., Hurtt G., Ilyina T., Jain A. K., Kato E., Kautz M., Keeling R. F., Goldewijk K. K., Körtzinger A., Landschützer P., Lefèvre N., Lenton A., Lienert S., Lima I., Lombardozzi D., Metzl N., Millero F., Monteiro P. M. S., Munro D. R., Nabel J. E. M. S., Nakaoka S.-i., Nojiri Y., Padin X. A., Peregon A., Pfeil B., Pierrot D., Poulter B., Rehder G., Reimer J., Rödenbeck C., Schwinger J., Séférian R., Skjelvan I., Stocker B. D., Tian H., Tilbrook B., Tubiello F. N., van der Laan-Luijkx I. T., van der Werf G. R., van Heuven S., Viovy N., Vuichard N., Walker A. P., Watson A. J., Wiltshire A. J., Zaehle S., Zhu D., Global carbon budget 2017. Earth Syst. Sci. Data 10, 405–448 (2018). [Google Scholar]
- 19.Harris N. L., Brown S., Hagen S. C., Saatchi S. S., Petrova S., Salas W., Hansen M. C., Potapov P. V., Lotsch A., Baseline map of carbon emissions from deforestation in tropical regions. Science 336, 1573–1576 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Putz F. E., Zuidema P. A., Synnott T., Peña-Claros M., Pinard M. A., Sheil D., Vanclay J. K., Sist P., Gourlet-Fleury S., Griscom B., Palmer J., Zagt R., Sustaining conservation values in selectively logged tropical forests: The attained and the attainable. Conserv. Lett. 5, 296–303 (2012). [Google Scholar]
- 21.Roy D. P., Jin Y., Lewis P. E., Justice C. O., Prototyping a global algorithm for systematic fire-affected area mapping using MODIS time series data. Remote Sens. Environ. 97, 137–162 (2005). [Google Scholar]
- 22.Avitabile V., Herold M., Heuvelink G. B. M., Lewis S. L., Phillips O. L., Asner G. P., Armston J., Ashton P. S., Banin L., Bayol N., Berry N. J., Boeckx P., de Jong B. H. J., DeVries B., Girardin C. A. J., Kearsley E., Lindsell J. A., Lopez-Gonzalez G., Lucas R., Malhi Y., Morel A., Mitchard E. T. A., Nagy L., Qie L., Quinones M. J., Ryan C. M., Ferry S. J. W., Sunderland T., Laurin G. V., Gatti R. C., Valentini R., Verbeeck H., Wijaya A., Willcock S., An integrated pan-tropical biomass map using multiple reference datasets. Glob. Chang. Biol. 22, 1406–1420 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Baccini A., Goetz S. J., Walker W. S., Laporte N. T., Sun M., Sulla-Menashe D., Hackler J., Beck P. S. A., Dubayah R., Friedl M. A., Samanta S., Houghton R. A., Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Chang. 2, 182–185 (2012). [Google Scholar]
- 24.Saatchi S. S., Harris N. L., Brown S., Lefsky M., Mitchard E. T. A., Salas W., Zutta B. R., Buermann W., Lewis S. L., Hagen S., Petrova S., White L., Silman M., Morel A., Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. U.S.A. 108, 9899–9904 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vidal E., West T. A. P., Putz F. E., Recovery of biomass and merchantable timber volumes twenty years after conventional and reduced-impact logging in Amazonian Brazil. For. Ecol. Manage. 376, 1–8 (2016). [Google Scholar]
- 26.Pütz S., Groeneveld J., Henle K., Knogge C., Martensen A. C., Metz M., Metzger J. P., Ribeiro M. C., de Paula M. D., Huth A., Long-term carbon loss in fragmented Neotropical forests. Nat. Commun. 5, 5037 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Laurance W. F., Laurance S. G., Ferreira L. V., Rankin-de Merona J. M., Gascon C., Lovejoy T. E., Biomass collapse in Amazonian forest fragments. Science 278, 1117–1118 (1997). [Google Scholar]
- 28.Baccini A., Walker W., Carvalho L., Farina M., Sulla-Menashe D., Houghton R. A., Tropical forests are a net carbon source based on aboveground measurements of gain and loss. Science 358, 230–234 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Asner G. P., Powell G. V. N., Mascaro J., Knapp D. E., Clark J. K., Jacobson J., Kennedy-Bowdoin T., Balaji A., Paez-Acosta G., Victoria E., Secada L., Valqui M., Hughes R. F., High-resolution forest carbon stocks and emissions in the Amazon. Proc. Natl. Acad. Sci. U.S.A. 107, 16738–16742 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berenguer E., Ferreira J., Gardner T. A., Cruz Aragão L. E. O., Barbosa De Camargo P., Cerri C. E., Durigan M., Cosme De Oliveira Junior R., Vieira I. C. G., Barlow J., A large-scale field assessment of carbon stocks in human-modified tropical forests. Glob. Chang. Biol. 20, 3713–3726 (2014). [DOI] [PubMed] [Google Scholar]
- 31.van der Sleen P., Groenendijk P., Vlam M., Anten N. P. R., Boom A., Bongers F., Pons T. L., Terburg G., Zuidema P. A., No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat. Geosci. 8, 24–28 (2015). [Google Scholar]
- 32.Saleska S. R., Didan K., Huete A. R., da Rocha H. R., Amazon forests green-up during 2005 drought. Science 318, 612–612 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Schimel D., Stephens B. B., Fisher J. B., Effect of increasing CO2 on the terrestrial carbon cycle. Proc. Natl. Acad. Sci. U.S.A. 112, 436–441 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips O. L., Aragão L. E. O. C., Lewis S. L., Fisher J. B., Lloyd J., López-González G., Malhi Y., Monteagudo A., Peacock J., Quesada C. A., van der Heijden G., Almeida S., Amaral I., Arroyo L., Aymard G., Baker T. R., Bánki O., Blanc L., Bonal D., Brando P., Chave J., Alves de Oliveira Á. C., Cardozo N. D., Czimczik C. I., Feldpausch T. R., Freitas M. A., Gloor E., Higuchi N., Jiménez E., Lloyd G., Meir P., Mendoza C., Morel A., Neill D. A., Nepstad D., Patiño S., Peñuela M. C., Prieto A., Ramírez F., Schwarz M., Silva J., Silveira M., Thomas A. S., ter Steege H., Stropp J., Vásquez R., Zelazowski P., Dávila E. A., Andelman S., Andrade A., Chao K.-J., Erwin T., Fiore A. D., C. E. H., Keeling H., Killeen T. J., Laurance W. F., Cruz A. P., Pitman N. C. A., Vargas P. N., Ramírez-Angulo H., Rudas A., Salamão R., Silva N., Terborgh J., Torres-Lezama A., Drought sensitivity of the Amazon rainforest. Science 323, 1344–1347 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Kurz W. A., Dymond C. C., Stinson G., Rampley G. J., Neilson E. T., Carroll A. L., Ebata T., Safranyik L., Mountain pine beetle and forest carbon feedback to climate change. Nature 452, 987–990 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Brienen R. J. W., Phillips O. L., Feldpausch T. R., Gloor E., Baker T. R., Lloyd J., Lopez-Gonzalez G., Monteagudo-Mendoza A., Malhi Y., Lewis S. L., Vásquez Martinez R., Alexiades M., Álvarez Dávila E., Alvarez-Loayza P., Andrade A., Aragão L. E. O. C., Araujo-Murakami A., Arets E. J. M. M., Arroyo L., Aymard C. G. A., Bánki O. S., Baraloto C., Barroso J., Bonal D., Boot R. G. A., Camargo J. L. C., Castilho C. V., Chama V., Chao K. J., Chave J., Comiskey J. A., Cornejo Valverde F., da Costa L., de Oliveira E. A., Di Fiore A., Erwin T. L., Fauset S., Forsthofer M., Galbraith D. R., Grahame E. S., Groot N., Hérault B., Higuchi N., Coronado E. N. H., Keeling H., Killeen T. J., Laurance W. F., Laurance S., Licona J., Magnussen W. E., Marimon B. S., Marimon-Junior B. H., Mendoza C., Neill D. A., Nogueira E. M., Núñez P., Pallqui Camacho N. C., Parada A., Pardo-Molina G., Peacock J., Peña-Claros M., Pickavance G. C., Pitman N. C. A., Poorter L., Prieto A., Quesada C. A., Ramírez F., Ramírez-Angulo H., Restrepo Z., Roopsind A., Rudas A., Salomão R. P., Schwarz M., Silva N., Silva-Espejo J. E., Silveira M., Stropp J., Talbot J., ter Steege H., Teran-Aguilar J., Terborgh J., Thomas-Caesar R., Toledo M., Torello-Raventos M., Umetsu R. K., van der Heijden G. M. F., van der Hout P., Guimarães Vieira I. C., Vieira S. A., Vilanova E., Vos V. A., Zagt R. J., Long-term decline of the Amazon carbon sink. Nature 519, 344–348 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Phillips O. L., Baker T. R., Arroyo L., Higuchi N., Killeen T. J., Laurance W. F., Lewis S. L., Lloyd J., Malhi Y., Monteagudo A., Neill D. A., Núñez Vargas P., Silva J. N. M., Terborgh J., Vásquez Martínez R., Alexiades M., Almeida S., Brown S., Chave J., Comiskey J. A., Czimczik C. I., Di Fiore A., Erwin T., Kuebler C., Laurance S. G., Nascimento H. E. M., Olivier J., Palacios W., Patiño S., Pitman N. C. A., Quesada C. A., Saldias M., Torres Lezama A., Vinceti B., Pattern and process in Amazon tree turnover, 1976–2001. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359, 381–407 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis S. L., Lopez-Gonzalez G., Sonké B., Affum-Baffoe K., Baker T. R., Ojo L. O., Phillips O. L., Reitsma J. M., White L., Comiskey J. A., Djuikouo K M.-N., Ewango C. E. N., Feldpausch T. R., Hamilton A. C., Gloor M., Hart T., Hladik A., Lloyd J., Lovett J. C., Makana J.-R., Malhi Y., Mbago F. M., Ndangalasi H. J., Peacock J., Peh K. S.-H., Sheil D., Sunderland T., Swaine M. D., Taplin J., Taylor D., Thomas S. C., Votere R., Wöll H., Increasing carbon storage in intact African tropical forests. Nature 457, 1003–1006 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Venter O., Koh L. P., Reducing emissions from deforestation and forest degradation (REDD+): Game changer or just another quick fix? Ann. N. Y. Acad. Sci. 1249, 137–150 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Griscom B., Shoch D., Stanley B., Cortez R., Virgilio N., Sensitivity of amounts and distribution of tropical forest carbon credits depending on baseline rules. Environ. Sci. Policy 12, 897–911 (2009). [Google Scholar]
- 41.Taubert F., Fischer R., Groeneveld J., Lehmann S., Müller M. S., Rödig E., Wiegand T., Huth A., Global patterns of tropical forest fragmentation. Nature 554, 519–522 (2018). [DOI] [PubMed] [Google Scholar]
- 42.N. Birdsall, J. Busch, Assessing Performance-Based Payments for Forest Conservation: Six Successes, Four Worries, and Six Possibilities to Explore of the Guyana-Norway Agreement (Climate and Forest Paper Series, Center for Global Development, Washington, DC, 2014).
- 43.D. Lee, P. Llopis, R. Waterworth, G. Roberts, T. Pearson, Approaches to REDD+ Nesting: Lessons Learned from Country Experiences (World Bank, 2018), pp. 1–38.
- 44.Mollicone D., Achard F., Federici S., Eva H. D., Grassi G., Belward A., Raes F., Seufert G., Stibig H.-J., Matteucci G., Schulze E.-D., An incentive mechanism for reducing emissions from conversion of intact and non-intact forests. Clim. Change 83, 477–493 (2007). [Google Scholar]
- 45.IPCC, Summary for Policymakers, in Global Warming of 1.5°C. An IPCC Special Report on the Impacts of Global Warming of 1.5°C above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty, V. Masson-Delmotte, P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy, Maycock, M. Tignor, T. Waterfield Eds., (World Meteorological Organization, 2018), 32 pp.
- 46.Busch J., Engelmann J., Cost-effectiveness of reducing emissions from tropical deforestation, 2016–2050. Environ. Res. Lett. 13, 015001 (2017). [Google Scholar]
- 47.Zimmerman B. L., Kormos C. F., Prospects for sustainable logging in tropical forests. Bioscience 62, 479–487 (2012). [Google Scholar]
- 48.Gibbs H. K., Ruesch A. S., Achard F., Clayton M. K., Holmgren P., Ramankutty N., Foley J. A., Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc. Natl. Acad. Sci. U.S.A. 107, 16732–16737 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hughes A. C., Have Indo-Malaysian forests reached the end of the road? Biol. Conserv. 223, 129–137 (2018). [Google Scholar]
- 50.Withey K., Berenguer E., Palmeira A. F., Espírito-Santo F. D. B., Lennox G. D., Silva C. V. J., Aragão L. E. O. C., Ferreira J., França F., Malhi Y., Rossi L. C., Barlow J., Quantifying immediate carbon emissions from El Niño-mediated wildfires in humid tropical forests. Philos. Trans. R. Soc. B Biol. Sci. 373, 20170312 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaveau D. L. A., Salim M. A., Hergoualc'h K., Locatelli B., Sloan S., Wooster M., Marlier M. E., Molidena E., Yaen H., DeFries R., Verchot L., Murdiyarso D., Nasi R., Holmgren P., Sheil D., Major atmospheric emissions from peat fires in Southeast Asia during non-drought years: Evidence from the 2013 Sumatran fires. Sci. Rep. 4, 6112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Page S. E., Siegert F., Rieley J. O., Boehm H.-D. V., Jaya A., Limin S., The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature 420, 61–65 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Ruslandi, Cropper W. P. Jr., Putz F. E., Effects of silvicultural intensification on timber yields, carbon dynamics, and tree species composition in a dipterocarp forest in Kalimantan, Indonesia: An individual-tree-based model simulation. For. Ecol. Manage. 390, 104–118 (2017). [Google Scholar]
- 54.Gauthier S., Bernier P., Kuuluvainen T., Shvidenko A., Schepaschenko D., Boreal forest health and global change. Science 349, 819–822 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Olson D. M., Dinerstein E., Wikramanayake E. D., Burgess N. D., Powell G. V. N., Underwood E. C., D'amico J. A., Itoua I., Strand H. E., Morrison J. C., Loucks C. J., Allnutt T. F., Ricketts T. H., Kura Y., Lamoreux J. F., Wettengel W. W., Hedao P., Kassem K. R., Terrestrial ecoregions of the world: A new map of life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. Bioscience 51, 933–938 (2001). [Google Scholar]
- 56.Laurance W. F., Goosem M., Laurance S. G. W., Impacts of roads and linear clearings on tropical forests. Trends Ecol. Evol. 24, 659–669 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Gaveau D. L. A., Sloan S., Molidena E., Yaen H., Sheil D., Abram N. K., Ancrenaz M., Nasi R., Quinones M., Wielaard N., Meijaard E., Four decades of forest persistence, clearance and logging on Borneo. PLOS ONE 9, e101654 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.International Tropical Timber Organization, Biennial Review and Assessment of the World Timber Situation 2015-2016 (International Tropical Timber Organization, 2016).
- 59.Brinck K., Fischer R., Groeneveld J., Lehmann S., Dantas De Paula M., Pütz S., Sexton J. O., Song D., Huth A., High resolution analysis of tropical forest fragmentation and its impact on the global carbon cycle. Nat. Commun. 8, 14855 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laurance W. F., Croes B. M., Tchignoumba L., Lahm S. A., Alonso A., Lee M. E., Campbell P., Ondzeano C., Impacts of roads and hunting on central African rainforest mammals. Conserv. Biol. 20, 1251–1261 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Goetz S. J., Baccini A., Laporte N. T., Johns T., Walker W., Kellndorfer J., Houghton R. A., Sun M., Mapping and monitoring carbon stocks with satellite observations: A comparison of methods. Carbon Balance Manag. 4, 2 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laurance W. F., Sayer J., Cassman K. G., Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 29, 107–116 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Esquivel-Muelbert A., Baker T. R., Dexter K. G., Lewis S. L., Brienen R. J. W., Feldpausch T. R., Lloyd J., Monteagudo-Mendoza A., Arroyo L., Álvarez-Dávila E., Higuchi N., Marimon B. S., Marimon-Junior B. H., Silveira M., Vilanova E., Gloor E., Malhi Y., Chave J., Barlow J., Bonal D., Cardozo N. D., Erwin T., Fauset S., Hérault B., Laurance S., Poorter L., Qie L., Stahl C., Sullivan M. J. P., ter Steege H., Vos V. A., Zuidema P. A., Almeida E., de Oliveira E. A., Andrade A., Vieira S. A., Aragão L., Araujo-Murakami A., Arets E., Aymard C G. A., Baraloto C., Camargo P. B., Barroso J. G., Bongers F., Boot R., Camargo J. L., Castro W., Moscoso V. C., Comiskey J., Valverde F. C., da Costa A. C. L., del Aguila Pasquel J., Fiore A. D., Duque L. F., Elias F., Engel J., Llampazo G. F., Galbraith D., Fernández R. H., Coronado E. H., Hubau W., Jimenez-Rojas E., Lima A. J. N., Umetsu R. K., Laurance W., Lopez-Gonzalez G., Lovejoy T., Cruz O. A. M., Morandi P. S., Neill D., Vargas P. N., Pallqui Camacho N. C., Gutierrez A. P., Pardo G., Peacock J., Peña-Claros M., Peñuela-Mora M. C., Petronelli P., Pickavance G. C., Pitman N., Prieto A., Quesada C., Ramírez-Angulo H., Réjou-Méchain M., Correa Z. R., Roopsind A., Rudas A., Salomão R., Silva N., Espejo J. S., Singh J., Stropp J., Terborgh J., Thomas R., Toledo M., Torres-Lezama A., Gamarra L. V., van de Meer P. J., van der Heijden G., van der Hout P., Martinez R. V., Vela C., Vieira I. C. G., Phillips O. L., Compositional response of Amazon forests to climate change. Glob. Chang. Biol. 25, 39–56 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benítez-López A., Santini L., Schipper A. M., Busana M., Huijbregts M. A., Intact but empty forests? Patterns of hunting-induced mammal defaunation in the tropics. PLOS Biol. 17, e3000247 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.S. Brown, T. Pearson, M. Delaney, D. Shoch, R. Vaca, J. Quispe, The 2003 Carbon Offsets Analysis and Status Report for the Noel Kempff Climate Action Project (Report for The Nature Conservancy, Winrock International, Ecosystem Services Unit, 2003), pp. 1–15.
- 66.West T. A. P., Vidal E., Putz F. E., Forest biomass recovery after conventional and reduced-impact logging in Amazonian Brazil. For. Ecol. Manage. 314, 59–63 (2014). [Google Scholar]
- 67.M. K. Keller, G. P. Asner, N. Silva, M. Palace, Sustainability of selective logging of upland forests in the Brazilian Amazon: Carbon budgets and remote sensing, in Working Forests in the Neotropics, D. J. Zarin, J. R. R. Alavalapati, F. E. Putz, M. Schmink, Eds. (Columbia Univ. Press, 2004), pp. 41–63. [Google Scholar]
- 68.Miller S. D., Goulden M. L., Hutyra L. R., Keller M., Saleska S. R., Wofsy S. C., Figueira A. M. S., da Rocha H. R., de Camargo P. B., Reduced impact logging minimally alters tropical rainforest carbon and energy exchange. Proc. Natl. Acad. Sci. U.S.A. 108, 19431–19435 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Figueira A. M. e S., Miller S. D., de Sousa C. A. D., Menton M. C., Maia A. R., da Rocha H. R., Goulden M. L., Effects of selective logging on tropical forest tree growth. J. Geophys. Res. Biogeosci. 113, G00B05 (2008). [Google Scholar]
- 70.Mazzei L., Sist P., Ruschel A., Putz F. E., Marco P., Pena W., Ferreira J. E. R., Above-ground biomass dynamics after reduced-impact logging in the Eastern Amazon. For. Ecol. Manage. 259, 367–373 (2010). [Google Scholar]
- 71.Asner G. P., Knapp D. E., Broadbent E. N., Oliveira P. J. C., Keller M., Silva J. N., Selective logging in the Brazilian Amazon. Science 310, 480–482 (2005). [DOI] [PubMed] [Google Scholar]
- 72.T. Pearson, S. Walker, S. Grimland, S. Brown, Impact of Logging on Carbon Stocks of Forests: The Brazilian Amazon as a Case Study (Report for USAID by Winrock International, Ecosystem Services Unit., 2006), pp. 1–33.
- 73.Huang M., Asner G. P., Long-term carbon loss and recovery following selective logging in Amazon forests. Global Biogeochem. Cycles 24, GB3028 (2010). [Google Scholar]
- 74.Blanc L., Echard M., Herault B., Bonal D., Marcon E., Chave J., Baraloto C., Dynamics of aboveground carbon stocks in a selectively logged tropical forest. Ecol. Appl. 19, 1397–1404 (2009). [DOI] [PubMed] [Google Scholar]
- 75.Medjibe V. P., Putz F. E., Starkey M. P., Ndouna A. A., Memiaghe H. R., Impacts of selective logging on above-ground forest biomass in the Monts de Cristal in Gabon. For. Ecol. Manage. 262, 1799–1806 (2011). [Google Scholar]
- 76.B. Griscom, D. Ganz, N. Virgilio, F. Price, J. Hayward, R. Cortez, G. Dodge, J. Hurd, F. L. Lowenstein, B. Stanley, The Hidden Frontier of Forest Degradation: A Review of the Science, Policy and Practice of Reducing Degradation Emissions (Report for The Nature Conservancy, 2010).
- 77.Pinard M. A., Putz F. E., Retaining forest biomass by reducing logging damage. Biotropica 28, 278–295 (1996). [Google Scholar]
- 78.Berry N. J., Phillips O. L., Lewis S. L., Hill J. K., Edwards D. P., Tawatao N. B., Ahmad N., Magintan D., Khen C. V., Maryati M., Ong R. C., Hamer K. C., The high value of logged tropical forests: Lessons from northern Borneo. Biodivers. Conserv. 19, 985–997 (2010). [Google Scholar]
- 79.Bryan J., Shearman P., Ash J., Kirkpatrick J. B., Impact of logging on aboveground biomass stocks in lowland rain forest, Papua New Guinea. Ecol. Appl. 20, 2096–2103 (2010). [DOI] [PubMed] [Google Scholar]
- 80.S. Stanley, Preliminary Biomass Estimate in Pt. Mamberamo Alas Mandiri Concession Papua, Indonesia (Report for Conservation International, 2009).
- 81.Fox J. C., Yosi C. K., Nimiago P., Oavika F., Pokana J. N., Lavong K., Keenan R. J., Assessment of aboveground carbon in primary and selectively harvested tropical forest in Papua New Guinea. Biotropica 42, 410–419 (2010). [Google Scholar]
- 82.Lasco R. D., MacDicken G., Pulhin F., Guillermo I. Q., Sales R. F., Cruz R. V. O., Carbon stocks assessment of a selectively logged dipterocarp forest and wood processing mill in the Philippines. J. Trop. For. Sci. 18, 212–221 (2006). [Google Scholar]
- 83.S. Brown, T. Pearson, N. Moore, A. Parveen, S. Ambagis, D. Shoch, Impact of Logging on Carbon Stocks of Forests: Republic of Congo as a Case Study (Report for USAID by Winrock International, Ecosystem Services Unit, 2005), pp. 1–21.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/10/eaax2546/DC1
Fig. S1. Estimated annual rate of carbon sequestration inside intact forests under alternative scenarios.
Fig. S2. Full accounting of the carbon impact of intact forest loss assuming the onset of carbon saturation in intact forests in Latin America by 2030.
Fig. S3. Full accounting of the carbon impact of intact forest loss assuming the onset of carbon saturation in all tropical intact forests by 2030.
Table S1. Country-level estimates on the proportion of selective logging that uses responsible forest management techniques and emission factors for responsible and conventional logging practices.
Table S2. Intact forest landscape reduction between 2000 and 2013 in countries across the global tropics.
Table S3. Country-level statistics required to estimate carbon emissions from defaunation.
Table S4. Regional carbon sequestration rates for intact forest areas and reforestation.
Table S5. Assumed time scales and trajectories of carbon dynamics from different forest processes.


