Preface
Transmembrane sodium ion gradients provide energy that can be harnessed by so-called ‘secondary transporters’ to drive the translocation of solute molecules into a cell. Decades of intense study has proven the central role that sodium-coupled transporters play in many physiological processes, making them vital targets to treat many serious diseases. Within the last year, several sodium-coupled transporter crystal structures have been reported from different families, showing a remarkable structural conservation between functionally unrelated transporters. In this review we discuss these atomic resolution structures and how they illuminate the mechanistic principles of sodium-coupled transport of solutes across cellular membranes.
Nature has evolved a multitude of greasy, transmembrane transporter proteins to catalyze the movement of polar or charged small molecules across the ~30 Å thick hydrophobic barrier of the membrane bilayer1. A large class of these proteins, deemed secondary transporters, can couple the selective discharge of an ionic gradient to power the “uphill” translocation of solute molecules across membranes. By coupling solute movement to ion co-transport, secondary transporters can concentrate solutes across a membrane by as much as 106-fold2, and can accelerate solute flux by as much as 105-fold3, 4 over simple passive diffusion.
Secondary transporters are present in all species throughout the kingdoms of life5. In humans, secondary transporters participate in diverse physiological processes, from the uptake of nutrients in the intestine6, to the transport of Na+ and Cl− in the kidney7, and the removal of neurotransmitters from the synaptic cleft8. Consequently, secondary transporters are the target of multiple therapeutic agents that include thiazide diuretics that inhibit a Na+/Cl− symporter in the distal convoluted tubule of the kidney9 and selective serotonin reuptake inhibitors (antidepressants) that block activity of the serotonin transporter10.
At the level of primary structure, amino acid sequence analysis suggests that there are over 100 distinct families of secondary transporters [Transport Commission (TC) system]11, with over 40 families identified in humans alone [Solute Carrier (SLC) system]. With respect to biological function, these amino acid sequences encode uniporters, symporters, and antiporters that act on a myriad of substrates ranging from elemental cations and anions to aromatic neurotransmitters, nutrients, or even di- and tri-peptides12, 13. Transport is most commonly driven by proton or sodium transmembrane gradients, but other ionic gradients such as potassium, calcium, or chloride can also be utilized12, 14.
In this review, we focus on transporters that are coupled to the sodium ion for their function. Our discussion employs the recent crystallographic advances in sodium coupled transporters to address questions pertaining to substrate-ion coupling, conformational states of the transport cycle, mechanisms of inhibition, and how the permeation pathway is alternately gated to maintain a tightly coupled transport mechanism.
Alternating access mechanism and internal symmetry
The mechanism by which secondary transporters couple the chemical potential of an ionic gradient to the translocation of solute has been considered for decades. Peter Mitchell provided early insights into the mechanism of secondary transporters by suggesting that they occupy two alternating structural states: one where the substrate binding pocket is accessible to extracellular solution (open-to-out) and another where the binding pocket is accessible to the cytoplasm (open-to-in)15. In this simple model, the concept of coupled transport (e.g. symport) could be understood by the positive coupling in the binding of substrate and ion to the open-to-out state, followed by isomerization of the transporter to the open-to-in state, allowing release of substrate and ion to the cytoplasm16. Through the late 1950s and the 1960s the basic idea of a two state alternating access mechanism was recast in a number of forms, from the ‘gate type non-carrier’ mechanism of Patlak17, to the two state ‘allosteric model’ of Vidaver18,and the ‘alternating access’ model of Jardetzky19.
Molecular mechanisms of secondary transporters based on atomic structures did not emerge until almost 40 years later, largely due to the fact that these transporters are hydrophobic and dynamic proteins that are difficult to crystallize. In 2002, the first crystal structure of a secondary transporter, the proton-driven multi-drug efflux pump AcrB of the resistance nodulation cell division (RND) family from Escherichia coli was reported20. Shortly thereafter, in 2003, the crystal structures of two major facilitator superfamily (MFS) transporters were solved: the glycerol-3-phosphate/phosphate antiporter GlpT21 and the proton-coupled lactose symporter LacY22. Despite the markedly different fold of AcrB compared to the MFS transporters, each of these structures revealed internal 2-fold structural pseudo-symmetry that relates the N-terminal half of the transporter to the C-terminal half by an axis running through the center of the transporter, approximately perpendicular to the membrane. Furthermore, the inward-facing conformations adopted by GlpT and LacY suggested a mechanism of transport that involves a ‘rocker-switch type’ motion of the two symmetry-related halves, alternately opening and closing ‘gates’ to the extracellular and intracellular solutions.
The first atomic resolution structural insights into the mechanisms of sodium-coupled secondary transporters were reported in 2004 and 2005 with the structures of the aspartate transporter GltPh23 (Dicarboxylate/Amino Acid:Cation Symporter; DAACS family), followed by the Na+/H+ antiporter NhaA from E. coli.24 and the bacterial leucine transporter LeuT25 (Neurotransmitter:Sodium Symporter; NSS family). The structures of GltPh, NhaA, and LeuT not only revealed unique membrane protein folds, but they also underscored the theme of internal 2-fold structural symmetry and discontinuous transmembrane helices26.
GltPh, which assembles as a homotrimer, displays a pseudo 2-fold symmetrical relationship between crucial elements of the protomer architecture that includes 2 re-entrant hairpin loops (HP1, HP2), together with TM7a and the first half of TM823. The relevance of the 2-fold axis to transporter mechanism is particularly striking and it immediately suggests that HP1 and HP2 may undergo alternating, symmetry-related motions that open and close access to the substrate and ion binding sites (Fig. 1a, b, c)23 .
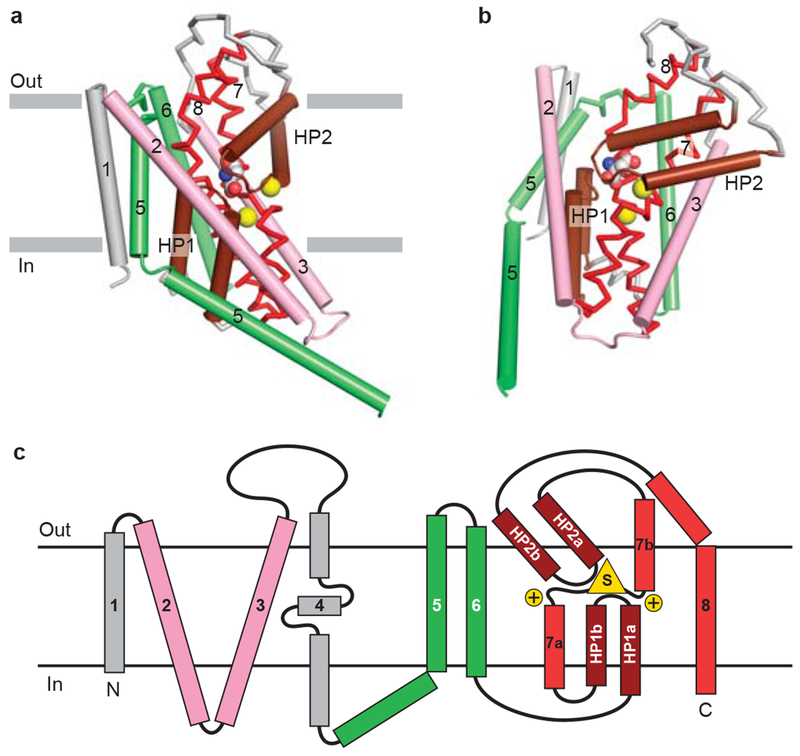
Figure 1 |. Architecture of the GltPh fold.
a, View of core transmembrane helices for GltPh illustrating how the first 6 transmembrane segments form a cradle harboring the elements of the transporter machinery. The functionally essential reentrant hairpin loops (HP1/HP2) are in brown, the partially unwound TM7 and the amphipathic TM8 are in red. View is parallel to the membrane and only one subunit of the GltPh trimer is shown. b, Same elements as panel (a) viewed approximately perpendicular to the membrane. The bound substrate (carbon, gray; oxygen, red; nitrogen, blue) and sodium ions (yellow) are shown in CPK representation. c, Topology diagram for GltPh with substrate and ions depicted as yellow triangle and circles, respectively.
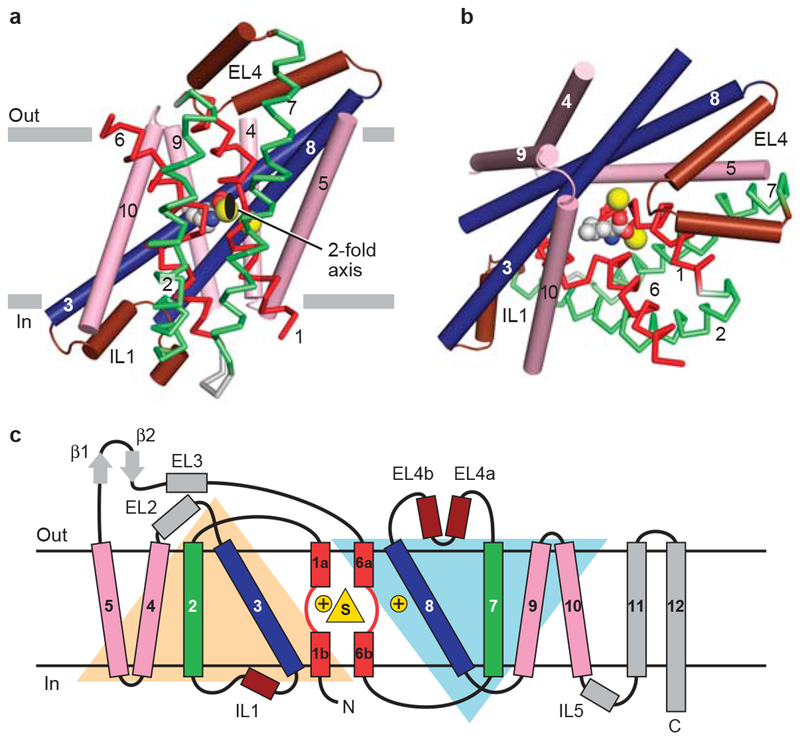
In LeuT, which has a different fold from GltPh, an internal 2-fold pseudo-symmetry axis, running parallel to the membrane plane through the center of the transporter relates the first 5 transmembrane helices (TMs 1-5) to the second 5 helices (TMs 6-10) by ~180° rotation25 (Fig. 2a, b, c). Surprisingly, the same fold as LeuT was observed in the subsequently reported structure of the galactose transporter, vSGLT (Solute:Sodium Symporter; SSS family)27, and in the benzyl-hydantoin transporter Mhp1 (Nucleobase:Cation Symporter; NCS1 family)28, transporters that are unrelated in amino acid sequence to LeuT. Both vSGLT27 and Mhp128 contain the 5+5 inverted structural symmetry motif defined by TMs 1-10 of LeuT even though these three transporters share neither significant amino acid sequence identity nor the same number of TM segments. In vSGLT, with 14 TM helices compared to the 12 for LeuT and Mph1, there is an amino terminal TM helix preceding the 5+5 helix repeat and 3 additional helices following the repeat. That different transporters have the same common helix core yet have additional TM segments on the periphery supports the idea that the 2-fold-related 5+5 TM repeat defines the fundamental machinery of these transporters.
Figure 2 |. Architecture of the LeuT fold.
a, View of the core 5+5 repeat structure for LeuT showing the inverted scaffold of TMs 4/5 and 9/10 (pink) holding the long bracing helices (TMs3/8; blue) and the jointed, finger-like and partially unwound TM1/6 helices (red). Bracing TMs 1/6 are TMs 2/7 (green). Reentrant, pseudo 2-fold related loops that either partially (EL4) or fully (IL1) occluded central binding site are shown in brown. View is parallel to the membrane. b, Same elements as in panel (a) viewed approximately perpendicular to the membrane. The bound substrate (carbon, gray; oxygen, red; nitrogen, blue) and sodium ions (yellow) are shown in CPK representation. c, Topology diagram for LeuT with substrate and ions depicted as yellow triangle and circles, respectively..
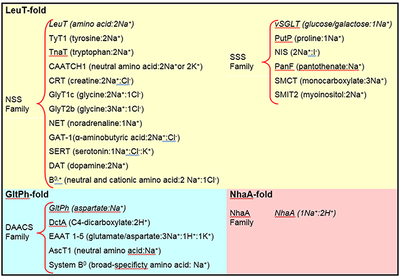
Not only have the structure determinations of vSGLT, LeuT, and Mhp1 effectively ‘collapsed’ the SSS29, NSS30 and NCS1 transporter families into one structural group (Box 1), they also foreshadow the likelihood that other secondary transporters, previously believed to belong to distinct families, may also have LeuT-like folds. The similarity in architecture between LeuT, vSGLT, and Mhp1 further implies commonalities in mechanism, ranging from principles of substrate and ion binding and specificity to conformational changes associated with transport. Atomic models of these functionally disparate yet structurally related transporter families has provided insight into the principles of sodium-coupled transport, and is beginning to clarify an alternating access mechanism that is distinctly different from the MFS family. Box 1 outlines the three known structural folds of sodium-coupled transporters and the members from each family whose crystal structures have been solved. Each fold is named based upon the first transporter structure solved for that fold. Representative homologs in each family are also listed for reference. As highlighted in this review, despite being structurally unrelated, the concept of ‘gates’ and how they might function in the alternate access mechanism has many similarities for transporters with LeuT- and GltPh-folds.
Box 1 |. Families of sodium-coupled transporters (TC classification13, www.tcdb.org) grouped according to structural fold.
NSS, SSS, and NCS1 families adhere to the LeuT-fold, while DAACS family adopts the GltPh-fold, and NhaA family adopts the NhaA-fold. Representative transporters from each family are listed, and the ion stoichiometry is indicated when supported by biochemical data. Transporters whose crystal structures have been determined are italicized. Note that some of the transporters are thought to be H+ rather than Na+ dependent.
Central pathway inside a scaffold
Inspection of LeuT, vSGLT, and Mhp1 structures show that the 5+5 TM motif consists of two interior pairs of symmetry-related helices, TMs 1, 6, and 3, 8, which are nested within an outer ring of helices, TMs 2, 4, 5, 7, 9, and 10 (LeuT numbering). Consistent with mutagenesis and functional studies31–34, these interior pairs largely define the central translocation pathway that contains the binding sites for substrate and ions. The three transporter structures show that the substrate binding site lies in the center of these interior pairs, and is coincident with the internal 2-fold symmetry axis (Fig 1, 2, and 3).
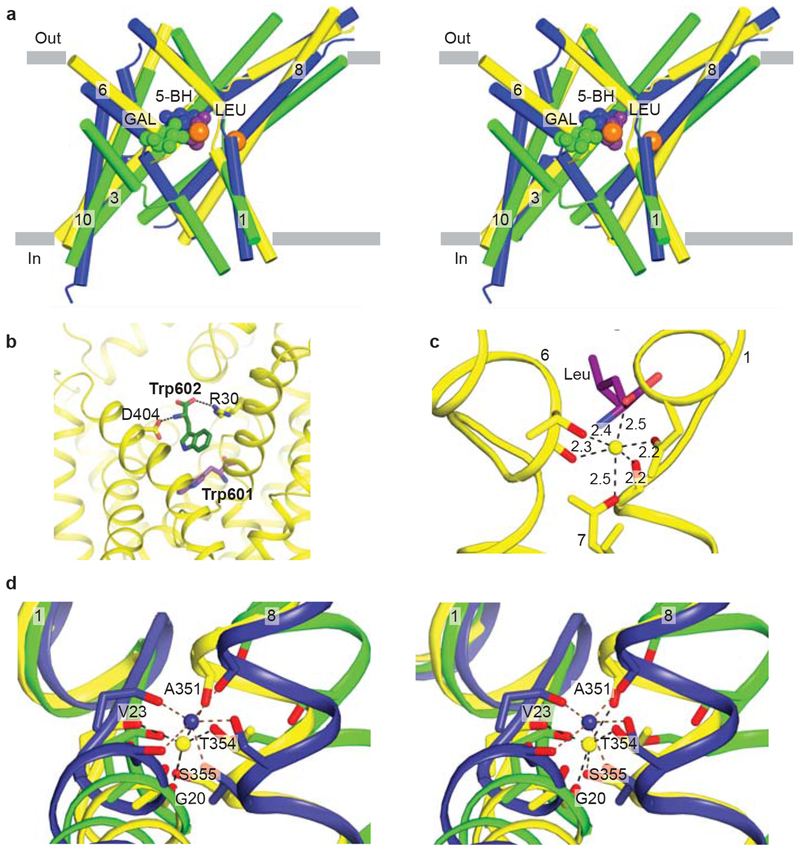
Figure 3 |. Conserved substrate and ion binding sites in LeuT, vSGLT and Mph1.
a, Stereo diagram of the superpositioned occluded structures of LeuT (yellow), Mhp1 (blue), and vSGLT (green) showing the location of their primary substrate binding sites roughly in the middle of the membrane bilayer and close to the discontinuous regions of TMs 1 and 6. Substrates and LeuT Na ions (orange) are shown in CPK representation. For clarity, only TMs 1, 3, 6, 8, and 10 are shown. b, View of the secondary binding site in LeuT. A second Trp molecule, Trp 602, is bound between R30 and D404 in the open-to-out conformation stabilized by Trp 601 in the primary binding site. c, Na1 ion in LeuT is octahedrally coordinationed by residues from TMs 1, 6, and 7 as well as bound leucine (purple). d, Stereo representation of superpositioned LeuT (yellow), vSGLT (green), and Mhp1 (blue) structures shows the location of their Na2 sites. Na2 of LeuT and Mhp1 are shown as yellow and blue spheres, respectively. Residues contributing side chain and main chain oxygens that coordinate Na2 are shown as sticks with LeuT residues labeled.
Among the outer ring of helices, symmetry-related TMs 4, 5 and 9, 10 form inverted V-shaped ‘pincers’ that cradle the interior TM 3, 8 pair, whereas TMs 2 and 7, also related by the 2-fold axis of symmetry, link TMs 1 and 6 with the intracellular and extracellular helix-loop-helix structures, IL1 and EL4. We suggest that the outer ring of helices, which nestle the interior pairs, provides a framework to stabilize the transporter within the lipid membrane and couples conformational changes occurring on one side of the membrane to movements on the other side.
The notion of a central translocation pathway that is surrounded by a protein scaffold is also observed in the GltPh-fold (Fig 1a, b, c). In GltPh, the transport machinery of HP1, HP2, TM7 and TM8, forming a C-terminal domain, is enveloped by a ring of 6 TM helices from the N-terminal domain of the transporter. In this case, the crucial role of the C-terminal domain in defining the transport pathway was suggested by studies showing that functionally important residues were localized to the C-terminus and that the C-terminal domain was more highly conserved than the N-terminal domain35–37. For GltPh and its orthologs, the scaffold of TMs 1-6 not only supports elements of the transport pathway, but it also mediates essential intersubunit contacts in the trimer.
Substrate and ion binding sites
A single substrate binding site was identified in LeuT, at the center of the transporter, surrounded by the interior helices, TMs 1, 3, 6, and 825. The binding sites for galactose in vSGLT27 and benzyl-hydantoin in Mhp128 are also similarly located (Fig. 3a). Directly adjacent to the primary binding site, TMs 1 and 6 (LeuT and Mhp1) or TMs 2 and 7 (vSGLT) have interruptions in their helical conformations, a structural feature encountered in other membrane proteins of ion-transport function24, 38, 39. The interruption in the α-helical structures in the proximity of the binding site exposes main-chain hydrogen-bonding partners and orients the helical dipoles to create a polar environment for coordinating substrate and ions within the lipid bilayer25. These electrostatic elements combined with specific side chains that alter the volume and shape of the binding pocket provide selectivity of these transporters for a substrate based on its size, polarity, and charge40.
Recent experimental ligand binding experiments and steered molecular dynamics simulations (SMD) of LeuT have suggested that there is an additional ‘secondary’ binding site between the primary site and bulk extracellular solution located near R30 and D40441, 42. Shi and colleagues41 proposed that the simultaneous occupancy of this secondary site triggers the intracellular release of substrate and sodium from the primary site. X-ray diffraction studies of LeuT, by contrast, do not show binding of either the substrate leucine or the substrate analog selenomethionine anywhere other than the primary binding site40. However, LeuT complexed with tryptophan, which locks the transporter in an open-to-out conformation, does bind a second tryptophan molecule (Trp 602) between R30 and D40440 (Fig. 3b). Because binding at this site is observed when the transporter is trapped in an open-to-out state, we suggest that this site serves as a transiently occupied site as substrates move from the extracellular vestibule to the primary binding site.
The high resolution structure of LeuT also identified the presence of two Na+ ions, Na1 and Na225. The Na1 ion is octahedrally coordinated by five protein ligands and the carboxylate of the substrate leucine (Fig 3c), demonstrating that ion and substrate binding are directly coupled. By contrast, the Na2 ion is located ~6 Å away from the substrate in LeuT and is bound via a trigonal bipyramidal coordination geometry (Fig. 3d). Intriguingly, by structural comparison, a sodium-ion binding site similar to the site occupied by the Na2 ion in LeuT was identified in both vSGLT and Mhp1, positioned about 10 Å away from the substrates27, 28. Although the resolutions of the vSGLT and Mhp1 structures are not sufficient to unambiguously assign a sodium ion to the site occupied by the Na2 ion, a sodium ion at this position in vSGLT is supported by biochemical and mutagenesis studies on vSGLT and other SSS family members, including the sodium/iodide symporter27, 43, 44. These observations indicate a role for a Na2-like ion not only in substrate binding but also in conformational changes associated with substrate transport. Studies on GAT145 as well as molecular dynamic and free-energy simulations of LeuT46 implicates the site occupied by the Na2 ion as a low affinity site that can readily give up its ion to the bulk phase, thus promoting release of the substrate47.
Sodium-to-substrate stoichiometry not only varies between sodium-coupled transporters but also amongst members of the same family, depending on the thermodynamic driving force required for substrate uptake4. The requirement for transport varies from 1 to 3 sodium ions per substrate in the NSS2, 48–50 and SSS families44, 51–53 (Box 1). Because vSGLT and Mhp1 probably have a sodium ion binding site similar to that occupied by the LeuT Na2 ion, we suggest that it is a common ion site for divergent transporters and is essential for coupled substrate binding and symport. Though the ion site for Na1 is less conserved among these transporters from different families, for members of the NSS family the Na1 ion not only enhances substrate binding, but it also provides favorable interactions with the co-transported chloride ion54, 55. Even though some LeuT orthologs couple substrate transport to 3 sodium ions, there is no direct experimental determination of a third sodium ion binding site4.
Conformational states
Crystal structures of LeuT25, 40, 56, Mhp128, vSGLT27, and GltPh23, 57 provide evidence for the conformations sampled by sodium coupled secondary transporters as they proceed through the transport cycle. These structures support a mechanism of transport (Fig. 4a) where an outward-facing conformation of the transporter (Tout) binds substrate and ions and subsequently isomerizes to an inward-facing conformation (Tin) via substrate-and-ion bound Michaelis-like intermediates (TMSin and TMSout). After the release of substrate and ion(s), the Tin state recycles ‘back’ to the Tout state either in the apo form, or through a potassium bound state (as is the case for glutamate58 and serotonin transporters59), or back through a TMS state, i.e. substrate exchange.
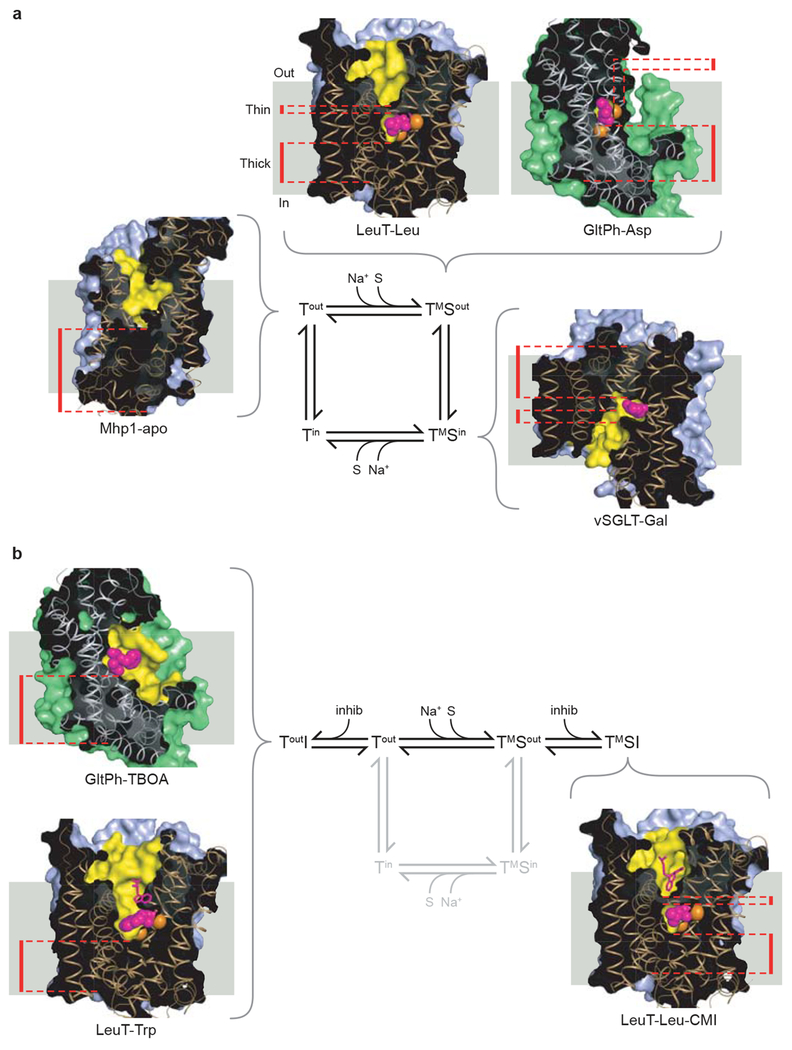
Figure 4 |. Crystal structures of transport intermediates.
a, Transport cycle based upon an alternating access type mechanism together with insights from crystallographic studies. Known transporter structures that represent intermediate states are shown. Clockwise from the Tout state: Mhp1-apo (PDB 2JLN), LeuT-Leu (PDB 2A65), GltPh-Asp (PDB 2NWX), vSGLT-Gal (PDB 3DH4). b, The inhibitory branches of the transport cycle from panel a. On the left, structures of GltPh-TBOA (PDB 2NWW) and LeuT-Trp (PDB 3F3A) represent an open-to-out competitive-inhibitor bound state. On the right, the structure of LeuT-Leu-CMI (PDB 2Q6H) represents a non-competitive inhibitor-bound occluded state. Cross-sectional illustrations of the crystal structures of each transporter are shown associated with the states of the cycle that they represent. The positions of the ‘thin’ gates and ‘thick’ gates are highlighted by red dashed lines. The solvent-accessible surface area, calculated with a probe radius of 1.4 Å, is shown in light blue for the LeuT-fold structures or green for GltPh-fold structures. The yellow regions highlight the surfaces of the binding site and cavities that penetrate the structures. Bound ligands, shown as van der Waals spheres, are colored magenta, with sodium ions colored orange. The view of each transporter is approximately parallel to the membrane plane, with the extracellular side at the top of each figure.
A common and striking observation from the crystallographic studies reviewed here is the presence of a stable occluded state for the substrate-ion bound ternary complex of each of the four transporters (Fig 4a). This state is characterized by the bound substrate residing in a closed or partially occluded binding pocket, where dissociation from the pocket would require a conformational change. Despite the common steric occlusion of substrate, the degree to which the four transporters block solvent accessibility to the binding pocket from the extracellular and cytoplasmic sides varies (Fig. 4a).
In the substrate-bound state of Mhp1 and LeuT (Fig. 4a), the occluded state occupies an outward-facing conformation (TMSout), where the extracellular pathway is kept open to solvent. In LeuT, the extracellular solvent-exposed region is formed by a large hydrophobic vestibule. At the base of this vestibule are two highly conserved residues, Y108 and F253, that close the top of the binding pocket, forming the occluded substrate binding pocket. In Mhp1, the structural elements that occlude the substrate benzyl-hydantoin are different from LeuT and involve the N-terminal half of TM10. GltPh also displays an outward-facing occluded conformation (Fig. 4a). Aspartate is bound between the tips of the HP1 and HP2 loops, which are closed over the binding site like lids, preventing the dissociation of substrate to either side of the transporter.
In contrast to LeuT and Mhp1, the occluded state of vSGLT adopts an inward-facing conformation (TMSin), exposing a region to intracellular solution (Fig. 4a) that is consistent with accessibility studies carried out on PutP43 and SERT60–63, vSGLT and LeuT orthologs, respectively. Akin to leucine binding in LeuT, galactose is bound to vSGLT in a central binding pocket located above the intracellular vestibule, and is occluded from the vestibule by a conserved aromatic residue, Y263. Even though the inward-facing, occluded conformation of galactose-bound vSGLT is fundamentally different from that observed for substrate-bound LeuT or Mhp1, there is a simple relationship between the two distinct states: the outward- and inward-facing states are related by the common 2-fold axis of internal symmetry that relates the 5+5 TM repeats, thus suggesting a symmetrical relationship between the TMSin and TMSout states.
The crystal structure of Mhp1 in the unliganded form shows an open-to-out conformation representing the Tout state of the transport cycle (Fig 4a). Comparison of the ligand-bound occluded form of Mhp1 with the apo open-to-out state shows that the N-terminal half of TM10 bends inward in response to ligand binding to form the occluded state. Further insight into the conformation of the Tout state is provided by crystal structures of LeuT and GltPh bound to competitive inhibitors that trap open-to-out conformations of the transporters40, 57 (Fig 4b).
Mechanisms of inhibition
Crystal structures of LeuT bound to both competitive and non-competitive inhibitors have afforded us a glimpse into the mechanisms of inhibition for NSS family transporters. In 2007, crystal structures of LeuT bound to the tricyclic antidepressants (TCAs) clomipramine, desipramine, and imipramine were reported56, 64. Therapeutically, these molecules are competitive inhibitors of the human serotonin transporter65 and block re-uptake of serotonin from synapses, thereby prolonging activation of the serotonin receptor. For LeuT, however, the mechanism of inhibition by TCAs is purely non-competitive56. The structures of the LeuT-TCA complexes reveal that the TCA molecule binds in the outward-facing vestibule, a partially hydrophobic cavity that binds other non polar molecules, including n-octyl-β-D-glucopyranoside40. The TCA is situated directly above the R30-D404 salt bridge, where the guanidinium head group of the arginine has flipped to form a direct salt bridge with the aspartate, stabilizing the occluded state of LeuT (TMSI) (Fig. 4b), and preventing further conformational changes necessary to progress around the transport cycle. The identification of this inhibitory allosteric site is consistent with a general mechanism of non-competitive inhibition, where the substrate binding site and inhibitor site do not overlap, and thereby trap the transporter in an inactive state. Though the non-competitive mechanism for TCA inhibition of LeuT is different from the competitive mechanism for TCA inhibition of SERT65, the structural principles revealed by the LeuT-TCA complexes define a paradigm for allosteric inhibition of NSS family transporters and, by extension, for other transporters with the LeuT-fold.
The structural basis for competitive inhibition was recently revealed by the crystal structure of LeuT bound to tryptophan40. Tryptophan acts like a strut between TMs 1/6 and TMs 3/8/10 where the bulky indole ring is wedged into the binding pocket and, in so doing, displaces the α-amino and α-carboxylate moieties outward by ~2 Å compared to their positions in the leucine-bound occluded state. With insufficient space to fully accommodate the indole ring in the substrate binding pocket, the transporter is effectively propped open through interactions of the inhibitor’s α-substituents with TMs 1b and 6a and the indole ring with TMs 3, 8, and 10. The transporter is thus locked open (ToutI, Fig. 4b) and thereby blocked from progressing to the occluded TMSout state of the transport cycle.
The crystal structure of GltPh bound to the competitive inhibitor TBOA, a bulky aspartate analog, underscores a similar principle of competitive inhibition for transporters with the GltPh-fold (Fig. 4b)57. In this structure the aspartate group of TBOA binds similarly to the substrate L-aspartate, lodged between TM7, TM8 and HP1. The large benzyl moiety of TBOA, however, sticks out toward HP2, propping HP2 in an open conformation (Fig. 4b), disrupting sodium site 2 and precluding the formation of the occluded state.
The observation of bulky substrate analogs as competitive inhibitors of transport that stabilize an opening of the extracellular side of the transporter is also supported by SCAM assays for the eukaryotic SLC6 homolog GAT1, as well as the human glucose transporter hSGLT of the SLC5 family66. The consistency of these results with the LeuT-Trp crystal structure suggests that the mechanism of inhibition is likely to be similar for other sodium-coupled transporters that share the LeuT-fold, and that a comparable principle seems to be found in other families of structurally disparate transporters, such as those adopting the GltPh-fold.
Permeation pathways and gating mechanisms
In transporters with GltPh-fold (GltPh) and LeuT-fold (LeuT, vSGLT, and Mhp1), the primary substrate and ion binding sites are flanked by two gates, one controlling access to the outside of the cell and the other controlling access to the inside. To allow substrates and ions to reach the primary binding sites yet not open up a continuous transmembrane pore, only one gate can open at a time. Thus, understanding how secondary transporters ‘work’ is fundamentally a question of how the gates work, i.e. what are the principles governing the coordinated alternate opening and closing of extracellular and intracellular gates upon substrate binding from the outside and unbinding from the inside. To answer this question, we consider the conformational changes that occur during transport and the likely pathways that substrates and ions take upon binding and unbinding from their central primary sites.
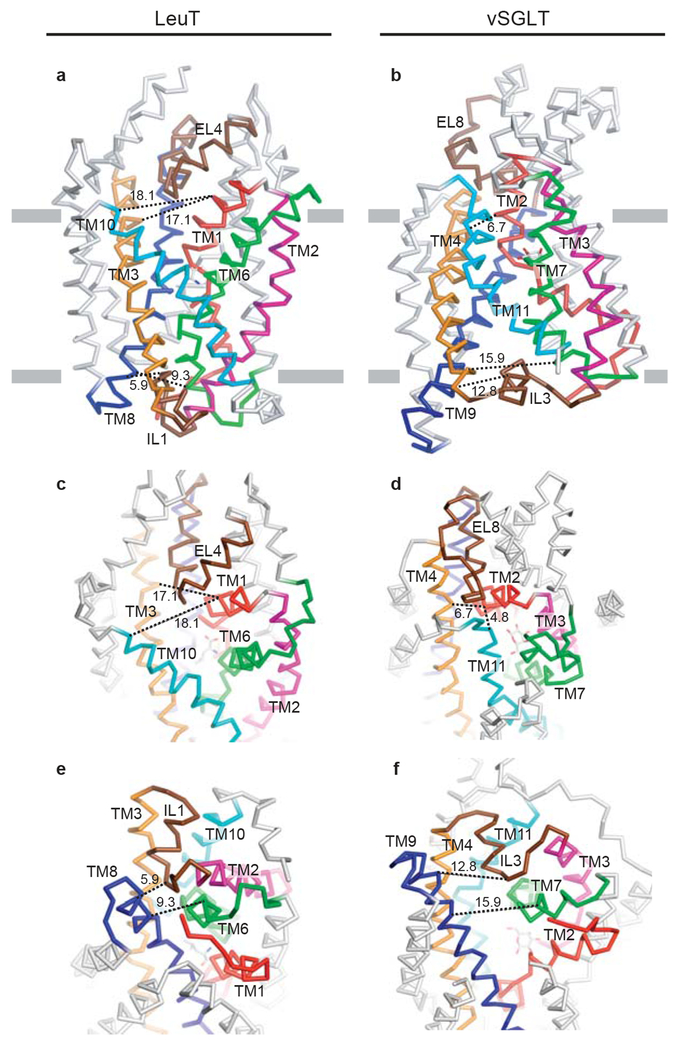
Inspection of the small group of sodium-coupled transporter structures suggests that for a given transporter trapped in a specific state, the gates that control access to and from the primary binding site are often asymmetric, i.e. the extracellular gate is less substantial or ‘thinner’ and the cytoplasmic gate is more substantial or ‘thicker’, and vice versa (Fig. 4a). This is observed for transporters with the GltPh- as well as the LeuT-fold. For example, in the outward-facing occluded leucine-bound LeuT complex, only a few residues directly block access from the primary binding site (Y108, F253), forming a ‘thin’ gate at the base of a solvent-filled cavity to the outside, whereas the cytoplasmic ‘thick’ gate is made up of ~20 Å of packed protein that includes TMs 1a, 3, 6b, 8 and 10, in combination with the amino terminus and IL1 (Fig 5a, c, e). Similarly, in the substrate-bound state of GltPh we find that the extracellular gate is made up of a few residues at the tip of HP2 whereas the cytoplasmic gate is composed of a ~15 Å slab of helices and side chains (HP1, TM7a and TM8).
Figure 5 |. Comparative views of substrate-bound LeuT (PDB 2A65, panels a, c, e) and vSGLT (PDB 3DH4, panels b, d, f).
a, and b, show membrane-parallel views of LeuT and vSGLT. c, and d, show a top-down view of the extracellular pathway. e, and f, show a bottom-up view of the intracellular pathway. Equivalent structural elements are colored the same in both LeuT and vSGLT. To help gauge the re-organization of the extra- and intracellular elements, black dashed lines indicate distance measurements between structural elements, measured from structurally similar residues in the two transporters. Considering the internal two-fold symmetry, note the similar organization of the open LeuT extracellular pathway to the open vSGLT intracellular pathway, and the similarity of the closed vSGLT extracellular pathway to the closed LeuT intracellular pathway.
The substrate-and-ion bound inward-facing occluded state of vSGLT presents the converse situation, with a ‘thick’ extracellular gate formed by TMs 1b, 3, 6a, 10 and EL4 (LeuT numbering) and a ‘thin’ cytoplasmic gate defined by Y262, Y263 and W264 (Fig 5b, d, f). Thus, the ‘thin’ gates are typically defined by the side chain atoms of a few residues whereas the ‘thick’ gates are formed by entire TM helices packing close together, in combination with extracellular and intracellular loops such as amino termini, IL1 or EL4 (LeuT numbering). Importantly, the extracellular and intracellular pathways defined by the open-to-out and outward-facing LeuT/Mhp1 and inward-facing vSGLT structures, are defined precisely by the symmetry-related components to the “thick” gate for each transporter. That is, in any one state, the location of the “thick” gate (either extracellular or intracellular) is reciprocal to the solvent-filled pathway, related fundamentally by the 2-fold axis of internal symmetry.
Instances where the same transporter was captured in different states of the transport cycle provide insight into how the thin gate opens and closes. The structures of LeuT, Mhp1, and GltPh in open-to-out and in substrate-and-ion bound occluded states demonstrate that substrate and ion binding results in relatively small conformational changes. In GltPh, for example, aspartate binding allows HP2 to close over the binding site, whereas TBOA binding holds it open, suggesting that the simple ‘flipping’ movement of HP2 primarily describes the thin gate motion that occludes the binding site during transport. With LeuT, in comparing the open-to-out conformation of the Trp complex with the occluded Leu-bound state, the most substantial change is the rotation of a subdomain of the transporter composed of TMs 1b, 2a, 6b, and EL4, which, together with rotations of several side chains, collectively move inward to close off the substrate binding site from extracellular solution. For Mhp1, the binding of substrate involves the inward bending of the N-terminal half of TM10. Thus, the thin gate opens and closes around the substrate binding pocket by movements localized to the side of the transporter from which substrate is binding or unbinding (i.e., transitions from Tout to TMSout or from TMSin to Tin) .
In contrast to the local changes associated with substrate binding, the isomerization between the outward-facing (TMSout) and inward-facing (TMSin) states involves larger-scale conformational changes spread throughout the transporter. As first illustrated by Yamashita and colleagues25, and further elaborated by Forrest et al.60, one can conceptualize this conformational change by applying the 2-fold axis of internal pseudo-symmetry to the key TM segments 1 and 6. In so doing, one sees that because the conformation of TM1 and 6 deviate from the internal 2-fold axis, a rotation of ~180° about the internal symmetry axis alternatively ‘open’ and ‘closes’ the extracellular and cytoplasmic gates. Forrest and colleagues suggest that the bundle of TMs 1, 2, 6 and 7 moves as a rigid body, in a rocker switch-like mechanism. This simplification, however, is not consistent with the structural comparison of the LeuT-Leu and LeuT-Trp complexes which indicate that there is some degree of independent movement within this bundle and thus further experimental and computational studies are required to validate this hypothesis.
Nevertheless, comparison of the LeuT-leucine (outward-facing, TMSout) and vSGLT-galactose (inward-facing, TMSin) structures suggests that the differences in these states can be described by a reorientation of TMs 1 and 6 (TMs 2 and 7 in vSGLT), together with movement and bending of TMs 2 and 7 (TMs 3 and 8 in vSGLT). In the outward-facing state, near the extracellular opening in LeuT, TM 1, for example, is about 17 Å and 18 Å away from TM3 and 10 respectively (Fig 5a, c) compared to ~ 7 Å and 5 Å for equivalent elements in vSGLT (Fig 5b, d). Similarly, the intracellular cavity is open in vSGLT by ~16 Å, measured between TMs 9 and 7 (Fig 5b, f), whereas the same elements in LeuT (TMs 6 and 8), with the ‘thick’ intracellular gate closed, are ~ 9 Å apart (Fig 5a, e). Thus, the similar magnitude to which the extracellular cavity of LeuT collapses to form the ‘thick’ gate seen in vSGLT and to which the ‘thick’ intracellular gate of LeuT opens to form the cavity in vSGLT supports the idea that the relationship between the cavities and ‘thick’ gates is reciprocally related by the 2-fold internal symmetry of the transporter, and that structures of LeuT and vSGLT largely represent distinct occluded-state ternary intermediates that interconvert during transport. The re-orientation of TMs 1, 2, 6, and 7 (LeuT numbering) between an occluded LeuT-like conformation (TMSout) and an occluded vSGLT-like conformation (TMSin) is therefore likely to approximate the conformational transition that re-orients the ‘thin’ gates of a transporter to the opposite side of the membrane. Additionally, flexing of TMs 3 and 8 may also contribute to opening and closing the gates, with these TMs bending at conserved glycine residues near their midsections and with IL1, EL4, and the amino terminus functioning as flexible ‘flaps’, helping to seal the gates in the closed states.
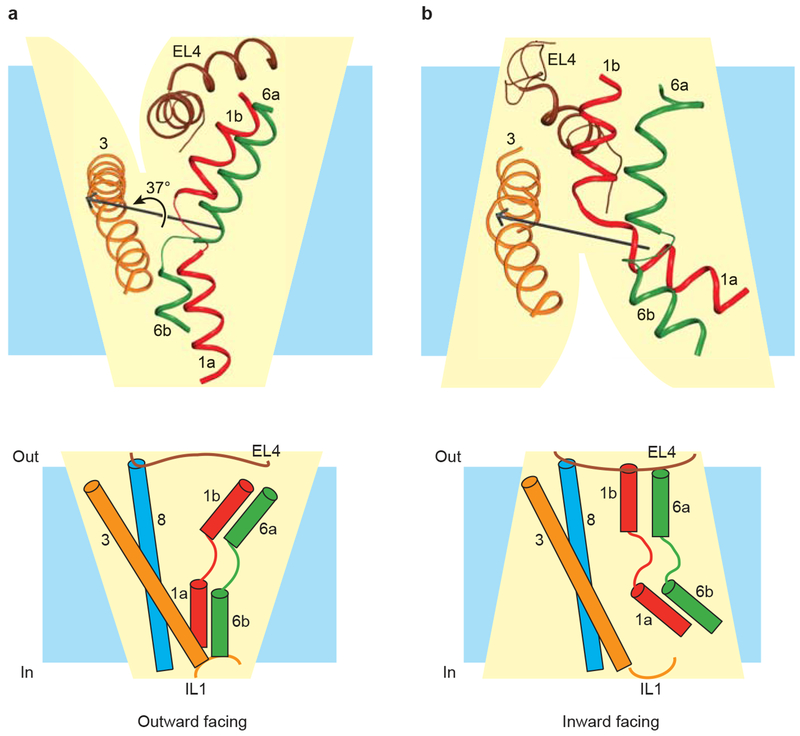
Taken together, the analyses described above from the available structures of the LeuT-fold, namely Mhp1, LeuT, and vSGLT, identify two major classes of transitions that occur during transport. First, substrate binding and unbinding closes and opens, respectively, the ‘thin’ gates to occlude or expose substrate in the primary binding site. Second, opening and closing of the ‘thick’ gates ‘switch’ the transporter from outward-facing to inward-facing states and vice versa. The opening and closing of the ‘thin’ gates stem from relatively local conformational changes, some of which involve helix rotations centered on axes passing through the regions of helical discontinuity. By contrast, the ‘thick’ gate transition reorients the occluded substrate-transporter complex by rotation of entire transmembrane-spanning bundle of helices about a central axis approximately perpendicular to the axis of internal 2-fold symmetry (Fig 6a, b). How do we know that comparing different transporters in different conformations reliably predicts common mechanistic principles? While there is, at present, no unambiguous answer to this question, the fact that transporters with the LeuT-fold share multiple common elements of structure and symmetry suggests that basic mechanistic principles are also likely common. Specific details, related to substrate and transporter interactions, as well as regulation, as examples, will likely differ, however.
Figure 6 |. Transition between outward-facing and inward-facing states in LeuT-fold transporters.
TMs 1 (red), 3 (orange), 6 (green), and 8 line the central translocation pathway with EL4 and IL1 acting as lids that seal the extracellular and intracellular gates, respectively, in their closed states. a, The outward-facing arrangement of central helices in substrate-bound LeuT. b, The inward-facing arrangement of central helices in substrate-bound vSGLT, with LeuT numbering for comparison with panel a. TM 8 and IL1 are omitted from the figure for clarity. TMs 1 and 6 rotate approximately 37 degrees relative to TMs 3 and 8 in transitioning from the outward-facing state adopted by LeuT in (a) to the inward-facing state adopted by vSGLT in (b). The rotation axis, shown in black, and the angle of rotation were calculated using DynDom67. Cartoon representations of outward-facing and inward-facing states, adapted from Yamashita et al, 25 are shown below the corresponding ribbon diagrams.
What prevents both gates from opening simultaneously? We suggest that the discontinuous helical regions of TM1 and 6 provide a hinge around which a small degree of conformational change can occur. This is visualized in the movements that accompany binding of the competitive inhibitor, tryptophan to LeuT in which the ‘thin’ extracellular gate opens by outward movements of TMs1b and 6a. However, TMs 1 and 6 are also adjacent to TMs 2 and 7, which all together form a 4-helix bundle. Thus larger scale movement of TMs 1b and 6a are constrained by TMs 2 and 7, perhaps because the latter are continuous α-helices lacking the non helical, hinge-like regions present in TMs 1 and 6. Consequently, substantial outward (opening) movements of TMs 1b and 6a, or TMs 1a and 6b, are limited by TMs 2 and 7. Thus, while both gates may be closed at the same time, both gates are prevented from simultaneously opening by the conformational rigidity enforced by TMs 2 and 7.
Future Prospects
The recent crystallographic advances in sodium-coupled secondary transporters have greatly advanced our understanding of the structural principles that underlie transporter function. The consistency of these models with the decades of elegant functional studies has allowed us to associate specific conformations with different mechanistic states of the transport cycle. However, this mechanistic description is derived from a patchwork of different transporters fortuitously crystallized in different states. Therefore, an accurate description of the precise conformational changes that a given transporter undergoes during transport must await further structural, biophysical and computational studies of individual secondary transporters. Additionally, multiple questions regarding the fundamental nature of the transport cycle remain outstanding. Although it is straightforward to understand how substrate binding leads to closure of a ‘thin’ gate, what are the chemical and structural principles that drive isomerization of the transporter from outward-facing to inward-facing states, i.e. opening of the ‘thick’ gate? Unlike mechanical models of gating in primary transporters and in ion channels, in secondary transporters there is no apparent source of mechanical force to open the thick gate. What structural changes occur upon only the binding of ions? What is the sequence of events that leads to release of substrate on the cytoplasmic side? How do ions, such as potassium, catalyze the isomerization of glutamate and serotonin transporters from inward facing to outward facing states? Finally, in order to fully understand and appreciate the biological and pharmacological properties unique to the human transporters, crystal structures of eukaryotic homologs will have to be solved.
Acknowledgements
This work was supported by the NIH. E. G. is an investigator with the Howard Hughes Medical Institute. We thank, R. Hibbs, K. Hollenstein, S. Singh and A. Sobolevsky for helpful comments and L. Vaskalis for assistance with the figures.
References
- 1.Quick MW (ed.) Transmembrane Transporters (Wiley-Liss, Inc., Hoboken, NJ, 2002). [Google Scholar]
- 2.Roux MJ & Supplisson S Neuronal and glial glycine transporters have different stoichiometries. Neuron 25, 373–383 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Chakrabarti AC & Deamer DW Permeability of lipid bilayers to amino acids and phosphate. Biochim. Biophys. Acta 1111, 171–177 (1992). [DOI] [PubMed] [Google Scholar]
- 4.Supplisson S & Roux MJ Why glycine transporters have different stoichiometries. FEBS Lett. 529, 93–101 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Sobczak I & Lolkema JS Structural and mechanistic diversity of secondary transporters. Cur. Opin. Microbiol 8, 161–167 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Wright EM & Turk E The sodium/glucose cotransport family SLC5. Pflügers Arch. 447, 510–518 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Hebert SC, Mount DB & Gamba G Molecular physiology of cation-coupled Cl- cotransport: the SLC12 family. Pflugers Arch - Eur. J. Physiol 447, 580–593 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Sonders MS, Quick M & Javitch JA How did the neurotransmitter cross the bilayer? A closer view. Curr. Opin. Neurobiol 15, 296–304 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Gamba G Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol. Rev 85, 423–493 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Murphy DL, Lerner A, Rudnick G & Lesch KP Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol. Interv 4, 109–123 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Busch W & Saier MH Jr. The transporter classification (TC) system, 2002. Crit. Rev. Biochem. Mol. Biol 37, 287–337 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Saier MH Jr. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev 64, 354–411 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saier MH Jr., Tran CV & Barabote RD TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 34, D181–6 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]; Togther with refs 11 and 12, Saier et al provides a comprehensive classification system for membrane transport proteins, that includes ion channels as well as primary and secondary transporters.
- 14.Hediger MA et al. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins. Pflugers Arch. 447, 465–468 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Mitchell P A general theory of membrane transport from studies of bacteria. Nature 180, 134–136 (1957). [DOI] [PubMed] [Google Scholar]
- 16.Mitchell P in Advances in Enzymology and Related Areas of Molecular Biology (ed. Nord FF) 33–87 (Interscience Publishers, Bronx, New York, 1967). [Google Scholar]
- 17.Patlak CS Contributions to the theory of active transport: II. The gate type non-carrier mechanism and generalizations concerning tracer flow, efficiency, and measurement of energy expenditure. Bull. Math. Biophysics 19, 209–235 (1957). [Google Scholar]
- 18.Vidaver GA Inhibition of parallel flux and augmentation of counter flux shown by transport models not involving a mobile carrier. J. Theoret. Biol 10, 301–306 (1966). [DOI] [PubMed] [Google Scholar]
- 19.Jardetzky O Simple allosteric model for membrane pumps. Nature 211, 969–70 (1966). [DOI] [PubMed] [Google Scholar]
- 20.Murakami S, Nakashima R, Yamashita E & Yamaguchi A Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 419, 587–593 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Lemieux MJ, Song J, Auer M & Wang DN Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science 301, 616–20 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Abramson J et al. Structure and mechanism of the lactose permease of Escherichia coli. Science 301, 610–5 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Yernool D, Boudker O, Jin Y & Gouaux E Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature 431, 811–818 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Hunte C et al. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 435, 1197–1202 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Yamashita A, Singh SK, Kawate T, Jin Y & Gouaux E Crystal structure of a bacterial homologue of Na+/Cl- - dependent neurotransmitter transporters. Nature 437, 215–223 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Screpanti E & Hunte C Discontinuous membrane helices in transport proteins and their correlation with function. J. Struct. Biol 159, 261–7 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Faham S et al. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science 321, 810–4 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weyand S et al. Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science 322, 709–13 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright EM, Loo DDF, Hirayama BA & Turk E Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology 19, 370–376 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Chen NH, Reith ME & Quick MW Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflügers Arch. 447, 519–531 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Barker EL, Moore KR, Rakhshan F & Blakely RD Transmembrane domain I contributes to the permeation pathway for serotonin and ions in the serotonin transporter. J. Neurosci 19, 4705–17 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudnick G Serotonin transporters--structure and function. J. Membr. Biol 213, 101–110 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Kanner BI & Zomot E Sodium-coupled neurotransmitter transporters. Chem. Rev (2008). [DOI] [PubMed] [Google Scholar]
- 34.Ben-Yona A & Kanner BI Transmembrane domain 8 of the gamma -aminobutyric acid transporter gat-1 lines a cytoplasmic accessibility pathway into its binding pocket. J Biol. Chem (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slotboom DJ, Lolkema JS & Konings WN Membrane topology of the C-terminal half of the neuronal, glial, and bacterial glutamate transporter family. J. Biol. Chem 271, 31317–21. (1996). [DOI] [PubMed] [Google Scholar]
- 36.Grunewald M, Bendahan A & Kanner BI Biotinylation of single cysteine mutants of the glutamate transporter GLT-1 from rat brain reveals its unusual topology. Neuron 21, 623–632 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Seal RP & Amara SG A reentrant loop domain in the glutamate carrier EAAT1 participates in substrate binding and translocation. Neuron 21, 1487–1498 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Toyoshima C, Nakasako M, Nomura H & Ogawa H Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature 405, 647–55. (2000). [DOI] [PubMed] [Google Scholar]
- 39.Dutzler R, Campbell EB, Cadene M, Chait BT & MacKinnon R X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415, 287–294 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Singh SK, Piscitelli CL, Yamashita A & Gouaux E A competitive inhibitor traps LeuT in an open-to-out conformation. Science 322, 1655–61 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; Crystallographic and functional analysis of LeuT with several different ligands that correlates the occluded state to transportable ligands and identifies a tryptophan as a competitive inhibitor which traps an open-to-out conformation.
- 41.Shi L, Quick M, Zhao Y, Weinstein H & Javitch JA The mechanism of a neurotransmitter:sodium symporter-inward release of Na+ and substrate is triggered by a substrate in a second binding site. Mol. Cell 30, 667–677 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Celik L, Schiott B & Tajkhorshid E Substrate binding and formation of an occluded state in the leucine transporter. Biophys. J 94, 1600–12 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilger D, Bohm M, Hackmann A & Jung H Role of Ser-340 and Thr-341 in transmembrane domain IX of the Na+/proline transporter PutP of Escherichia coli in ligand binding and transport. J. Biol. Chem 283, 4921–9 (2008). [DOI] [PubMed] [Google Scholar]
- 44.De la Vieja A, Reed MD, Ginter CS & Carrasco N Amino acid residues in transmembrane segment IX of the Na+/I- symporter play a role in its Na+ dependence and are critical for transport activity. J. Biol. Chem 282, 25290–8 (2007). [DOI] [PubMed] [Google Scholar]; Mutation of conserved serine and threonin residues in TM 9 of the NIS transporter (SSS family) shows that they are involved in Na+ binding/translocation.
- 45.Zhou Y, Zomot E & Kanner BI Identification of a lithium interaction site in the GABA transporter GAT-1. J. Biol. Chem 281, 22092–9 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Noskov SY & Roux B Control of ion selectivity in LeuT: two Na+ binding sites with two different mechanisms. J. Mol. Biol 377, 804–18 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caplan DA, Subbotina JO & Noskov SY Molecular mechanism of ion-ion and ion-substrate coupling in the Na+-dependent leucine transporter LeuT. Biophys. J 95, 4613–21 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talvenheimo J, Fishkes H, Nelson PJ & Rudnick G The serotonin transporter-imipramine “receptor”. J. Biol. Chem 258, 6115–9 (1983). [PubMed] [Google Scholar]
- 49.Gu HH, Wall S & Rudnick G Ion coupling stoichiometry for the norepinephrine transporter in membrane vesicles from stably transfected cells. J. Biol. Chem 271, 6911–6 (1996). [DOI] [PubMed] [Google Scholar]
- 50.Keynan S & Kanner BI gamma-Aminobutyric acid transport in reconstituted preparations from rat brain: coupled sodium and chloride fluxes. Biochemistry 27, 12–7 (1988). [DOI] [PubMed] [Google Scholar]
- 51.Diez-Sampedro A, Eskandari S, Wright EM & Hirayama BA Na+-to-sugar stoichiometry of SGLT3. Am. J. Physiol. Renal. Physiol 280, F278–82 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Mackenzie B, Loo DDF & Wright EM Relationships Between Na+/Glucose Cotransporter (SGLT1) Currents and Fluxes. J. Membr. Biol 162, 101–106 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Eskandari S et al. Thyroid Na+/I- symporter. Mechanism, stoichiometry, and specificity. J. Biol. Chem 272, 27230–8 (1997). [DOI] [PubMed] [Google Scholar]
- 54.Forrest LR, Tavoulari S, Zhang YW, Rudnick G & Honig G Identification of a chloride ion binding site in Na+/Cl- -dependent transporters. Proc. Natl. Acad. Sci. USA 104, 12761–12766 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zomot E et al. Mechanism of chloride interaction with neurotransmitter:sodium symporters. Nature 449, 726–30 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Singh S, Yamashita A & Gouaux E Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature 448, 952–956 (2007). [DOI] [PubMed] [Google Scholar]; Singh, et al. present a structural paradigm for non-competitive inhibition of NSS transporters by tri-cyclic antidepressants.
- 57.Boudker O, Ryan R, Yernool D, Shimamoto K & Gouaux E Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature 445, 387–393 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Kavanaugh MP, Bendahan A, Zerangue N, Zhang Y & Kanner BI Mutation of an amino acid residue influencing potassium coupling in the glutamate transporter GLT-1 induces obligate exchange. J. Biol. Chem 272, 1703–1708 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Keyes SR & Rudnick G Coupling of transmembrane proton gradients to platelet serotonin transport. J. Biol. Chem 257, 1172–6 (1982). [PubMed] [Google Scholar]
- 60.Forrest LR et al. Mechanism for alternating access in neurotransmitter transporters. Proc. Natl. Acad. Sci. USA 105, 10338–43 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using scanning cysteine accesibility mutagenesis on SERT, this paper proposes a model for an inward-facing conformation of LeuT based upon the structure of outward-facing LeuT-leu and the inverted structural pseudo-symmetry.
- 61.Jacobs MT, Zhang YW, Campbell SD & Rudnick G Ibogaine, a noncompetitive inhibitor of serotonin transport, acts by stabilizing the cytoplasm-facing state of the transporter. J. Biol. Chem 282, 29441–29447 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y-W & Rudnick G The cytoplasmic substrate permeation pathway of serotonin transporter. J. Biol. Chem 281, 36213–36220 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Zhang YW & Rudnick G Cysteine-scanning mutagenesis of serotonin transporter intracellular loop 2 suggests an alpha-helical conformation. J. Biol. Chem 280, 30807–13 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Zhou Z et al. LeuT-desipramine structure reveals how antidepressants block neurotransmitter uptake. Science 317, 1390–1393 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Apparsundaram S, Stockdale DJ, Henningsen RA, Milla ME & Martin RS Antidepressants targeting the serotonin reuptake transporter act via a competitive mechanism. J. Pharmacol. Exp. Ther 327, 982–90 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Hirayama BA, Diez-Sampedro A & Wright EM Common mechanisms of inhibition for the Na+/glucose (hSGLT1) and Na+/Cl-/GABA (hGAT1) cotransporters. Br. J. Pharmacol 134, 484–95 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]; A series of studies is presented that demonstrates a common principle for inhibition between two functionally unrelated families that hints at the underlying structural conservation.
- 67.Hayward S & Berendsen HJ Systematic analysis of domain motions in proteins from conformational change: new results on citrate synthase and T4 lysozyme. Proteins 30, 144–54 (1998). [PubMed] [Google Scholar]