Abstract
Background
Cross-sectional studies have repeatedly shown impaired white matter integrity in patients with major depressive disorder. Longitudinal analyses are missing from the current research and are crucial to elucidating the impact of disease trajectories on white matter impairment in major depressive disorder.
Methods
Fifty-nine patients with major depressive disorder receiving inpatient treatment, as well as 49 healthy controls, took part in a prospective study. Participants were scanned twice (baseline and follow-up), approximately 2.25 years apart, using diffusion tensor imaging. We analyzed diffusion metrics using tract-based spatial statistics.
Results
At baseline, patients had higher mean diffusivity in a large bilateral frontal cluster comprising the body and genu of the corpus callosum, the anterior and superior corona radiata, and the superior longitudinal fasciculus. A significant group × time interaction revealed a decrease of mean diffusivity in patients with major depressive disorder over time, abolishing group differences at follow-up. This effect was observed irrespective of disease course in the follow-up period.
Limitations
Analyzing the course of illness is challenging because of recollection biases in patients with major depressive disorder.
Conclusion
This study reports follow-up diffusion tensor imaging data in patients with major depressive disorder after an acute depressive episode. We demonstrated impaired prefrontal white matter microstructure (higher mean diffusivity) at baseline in patients with major depressive disorder, which normalized at follow-up after 2 years, irrespective of disease course. This might have been due to a general treatment effect and might have reflected recovery of white matter integrity.
Introduction
Major depressive disorder (MDD) is expected to become the second-highest contributor to global disease burden by the year 2020,1 and yet our understanding of its pathophysiological basis remains incomplete. Multimodal neuroimaging evidence has shown an integrated pattern of structural and functional brain abnormalities in affective disorders in regions such as the prefrontal cortex, the hippocampus and the amygdala.2–4
A key step forward in the field has been the understanding that abnormal structural and functional connectivity between these regions is likely as important to the pathogenesis of MDD as isolated changes in volume and functional activity.5,6 The lower integrity of white matter tracts connecting these cortical and subcortical regions supports the concept of MDD as a “disconnection syndrome.”7–9 In MDD, recent studies have implicated alterations in certain structural and functional subnetworks with specific depression symptom dimensions. 10,11 Diffusion tensor imaging (DTI) techniques such as tract-based spatial statistics (TBSS) allow for the investigation of diffusion in the brain, which is employed to derive information about white matter integrity.12 Studies using DTI have repeatedly shown impaired white matter,7,13,14 especially in frontal tracts such as the genu of the corpus callosum, as well as longitudinal tracts; some studies have shown that increasing age, longer illness duration and early age of onset might be associated with lower fractional anisotropy (FA).15–17 This notion is further corroborated by findings from a meta-analysis, which has shown an association between illness severity and reduced FA in MDD.14 Studies demonstrating white matter disturbances in adolescents before the onset of depression18,19 and in healthy participants at familial risk for MDD20 also suggest that white matter alterations are risk factors and predate the onset of disease.
However, even though these alterations have been extensively studied in cross-sectional designs, little is known from longitudinal designs about the causality between onset and course of depression on one hand, and white matter changes over time on the other. Whereas longitudinal studies focusing on grey matter trajectories in MDD have emerged in recent years,3,21,22 longitudinal studies on white matter in MDD are lacking. We investigated white matter in MDD in a longitudinal design (2 years) to disentangle trait and state influences on white matter alterations. To that end, we also conducted an exploratory analysis to evaluate whether changes in white matter were associated with relapse in the follow-up period or with changes in depressive symptom severity between the 2 time points. We hypothesized that (1) white matter integrity would be impaired at baseline in MDD compared with healthy controls, especially in the corpus callosum; and (2) patients would display differential changes of white matter integrity over time compared with controls, and that patients with a relapse would show more pronounced white matter deficits than patients without a relapse at follow-up.
Methods
Participants and procedure
The present study comprised 59 patients diagnosed with MDD and 49 healthy controls. Patients were recruited from the inpatient service of the Department of Psychiatry, University of Münster. All participants were evaluated using the Structured Clinical Interview for DSM-IV-TR23 to confirm psychiatric diagnosis or lack thereof at both time points. For study inclusion and exclusion criteria see Appendix 1, Supplementary Methods 1, available at jpn.ca/180243-a1. All participants underwent structural diffusion-weighted MRI at baseline and approximately 2 years later at follow-up (time between scans, mean ± standard deviation [SD]: 2.25 ± 0.26 years). We applied the Hamilton Depression Rating Scale (HDRS),24 a measure of depressive symptom severity, at both time points. We also performed structured interviews with all patients to assess the course of illness during the follow-up interval (e.g., relapse). At baseline, all patients were experiencing a moderate or severe depressive episode and received inpatient treatment. We assessed the type and dose of psychopharmacological treatment at baseline and follow-up to calculate a medication index for both time points. We used these variables to compute an established medication load index,8,25 a composite measure of total medication load reflecting the dose and number of prescriptions, irrespective of active components. For more information on the calculation of the medication index, see Appendix 1, Supplementary Methods 1.
Two patients and 2 healthy controls were excluded during MRI preprocessing (see Image Processing). The final sample consisted of 57 patients with MDD and 47 healthy controls.
For an exploratory analysis based on interview information at follow-up, we subdivided the MDD sample into patients with MDD who had another depressive episode during the follow-up period (relapse; n = 32) and patients who did not have another episode (no relapse; n = 25). For further information, see Appendix 1, Table S1.
This study was approved by the ethics committee of the Medical Faculty of Münster University, and all participants gave written informed consent before the examination. They received financial compensation for participation after the testing session.
DTI data acquisition
We acquired data using a 3 T whole body MRI scanner (Gyroscan Intera, Philips Medical Systems). The DTI data were acquired in 36 axial slices, 3.6 mm thick with no gap (acquired matrix 128 × 128), resulting in a voxel size of 1.8 × 1.8 × 3.6 mm3. The echo time was 95 ms, and the repetition time was 9473 ms. We used a b-value of 1000 s/mm2 for 20 diffusion-weighted images, with isotropic gradient directions plus 1 non–diffusion-weighted (b0 = 0) image. In sum, we used 21 images per slice for diffusion tensor estimation. The total data acquisition time was approximately 8 minutes per participant. During the experiment, participants lay supine in the MRI scanner with their head position stabilized. We used the same scanner and sequences at both time points.
Image processing
We performed preprocessing and analysis using the FSL FMRIB Software Library v5.0.10 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/, FMRIB, Oxford Centre for Functional MRI of the Brain26). Preprocessing followed the established FSL TBSS pipeline and has been described in detail earlier.27 Briefly, images were corrected for motion and eddy-current distortions and underwent automated skull stripping before diffusion tensor estimation using the FSL FDT.28 This yielded an estimation of FA, mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD) for each voxel.
To ensure data quality, we visually inspected all raw DTI and FA images. We excluded 2 participants whose estimated mean displacement provided by the eddy correct log file was more than 3 times the standard deviation of all participants’ mean displacement. We also excluded 2 outliers based on FA maps using the homogeneity of covariance test (> 2 SD) provided by the VBM8-toolbox (http://dbm.neuro.unijena.de/vbm).
Tract-based spatial statistics
We performed standard TBSS preprocessing.29 The FA images were registered to the FMRIB58 FA template and averaged to create a mean FA image. A white matter skeleton was created with an FA threshold of 0.2 and overlaid onto each participant’s registered FA image. We warped individual FA values onto this mean skeleton mask by searching perpendicular from the skeleton for maximum FA values. We performed the same registration on MD, RD and AD values.
To test for statistical significance, we used the nonparametric permutation testing implemented in FSL’s “randomize,” with 5000 permutations. We used threshold-free cluster enhancement30 to correct for multiple comparisons using the default values provided by the –T2 option optimized for TBSS. This allowed us to estimate cluster sizes corrected for family-wise error (FWE; p < 0.05, 5000 permutations). We derived Montreal Neurological Institute coordinates and cluster size at peak voxel using FSL Cluster and retrieved the corresponding white matter tract from the ICBM-DTI-81 white matter atlas.31
Analysis
We performed statistical analyses on the demographic data using IBM SPSS Statistics 25 (SPSS Inc.).
We established cross-sectional differences (baseline, follow-up) between MDD and healthy controls in diffusion metrics (FA, MD, RD, AD) using independent t tests implemented in “FSL randomize,” correcting for age, sex, time between scans and total intracranial volume. We established longitudinal changes in diffusion metrics in both samples using paired t tests in FSL randomize.
To analyze differential changes over time between healthy controls and MDD, we performed a repeated-measures analysis of covariance (ANCOVA). Permutation of ANCOVAs via FSL randomize has not been implemented yet (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/GLM); therefore, to analyze a group × time interaction, we extracted mean diffusion values from significant clusters at baseline and analyzed them in SPSS. There, we performed repeated-measures ANCOVAs with group (patients with MDD, healthy controls) as the between-subjects factor and time (baseline, follow-up) as a within-subjects factor. We included age, sex and total intracranial volume as covariates, as estimated by the Computational Anatomy Toolbox (www.neuro.uni-jena.de/cat, v933) based on the corresponding T1 images.3 For an alternative approach to investigating whole-brain changes over time between groups, we calculated difference images, subtracting baseline from follow-up voxel values employing fslmaths. To compare changes in diffusion metrics (ΔFA, ΔMD, ΔAD, ΔRD) over time between groups, we analyzed difference images using independent t tests within FSL comparing patients with MDD to healthy controls, correcting for age, sex, time between scans and total intracranial volume. Finally, we repeated the same analyses in a region-of-interest analysis restricted to the mask from significant clusters at baseline.
In an exploratory analysis based on the interview information at follow-up, we subdivided the MDD sample into “relapse” and “no relapse” groups. We performed independent t tests in FSL to test for differences at baseline and follow-up. Again, we analyzed difference images using independent t tests in FSL, comparing the 2 groups using age, sex and total intracranial volume as covariates.
To test whether baseline values were associated with clinical characteristics and medication, we performed a correlation analysis in SPSS with mean MD (from the significant baseline cluster), number of depressive episodes before baseline, number of hospitalizations before baseline and medication index at baseline. To further investigate the observed changes over time in the MDD sample, we correlated changes in MD scores (Δ MD [follow-up – baseline]; extracted from the significant baseline cluster) with changes in medication (Δ medication index [follow-up – baseline]) and changes in depression severity (Δ HDRS [follow-up – baseline]).
Results
Demographic data
Groups did not differ with respect to sex, age, time between scans or years of education (Table 1). Patients had lower HDRS and medication index scores at follow-up (HDRS [baseline] 22.43, HDRS [follow-up] 8.73, t56 = 12.60, p < 0.001; medication index [baseline] 1.95, medication index [follow-up] 1.32, t56 = 2.7, p = 0.008). For more detailed information on patients’ medication and comorbidities, see Appendix 1, Table S1 and Table S2.
Table 1.
Sample characteristics
| Characteristic | MDD (n = 57) | Healthy controls (n = 47) | p value |
|---|---|---|---|
| Sex, M/F | 30/27 | 22/25 | 0.69* |
| Age, yr | 34.68 ± 12.08 | 37.38 ± 7.95 | 0.19 |
| Education, yr | 14.75 ± 2.42 | 15.00 ± 2.35 | 0.75 |
| Time between scans, yr | 2.24 ± 0.26 | 2.26 ± 0.26 | 0.37 |
F = female; M = male; MDD = major depressive disorder.
Values are mean ± standard deviation unless otherwise indicated.
χ2 test.
Cross-sectional findings
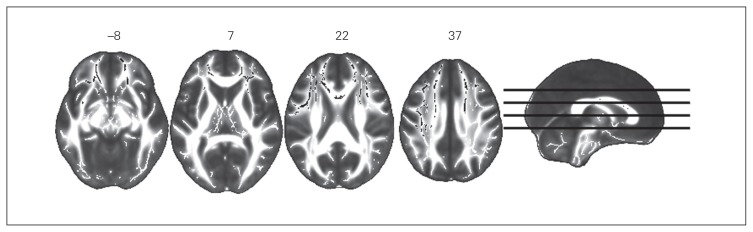
At baseline, compared to healthy controls, patients with MDD had significantly higher MD and RD in a large bilateral frontal cluster comprising the body and genus of the corpus callosum, the anterior and superior corona radiata, and the left superior longitudinal fasciculus. (Fig. 1, Fig. 2, Table 2, Table 3). We observed a trend of lower FA in the patient group (p = 0.088).
Fig. 1.
Increased mean diffusivity (MD) in patients with major depressive disorder at baseline. Axial slices with corresponding y-axis values (Montreal Neurological Institute) are presented. Black areas in white matter tracts represent voxels where significantly higher MD was detected in patients with MDD compared to healthy controls (pFWE < 0.05). FWE = family-wise error.
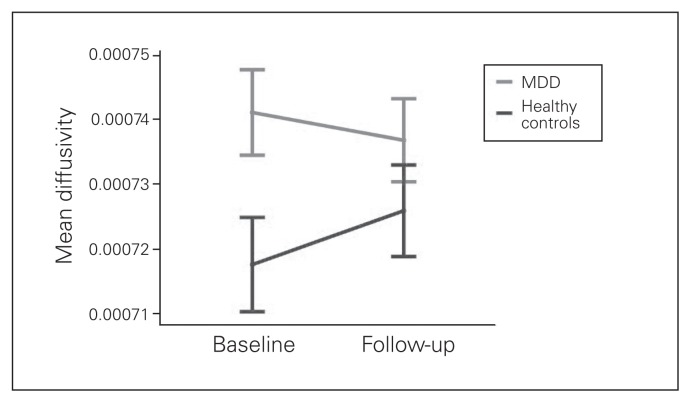
Fig. 2.
Time × group interaction of mean diffusivity (MD) in a significant baseline cluster. Extracted mean MD values from all significant voxels of the baseline contrast major depressive disorder (MDD) > healthy controls. Repeated-measures analysis of covariance revealed a significant time × group interaction (F1,99 = 16.80; p < 0.001). Error bars indicate ± 2 standard errors.
Table 2.
Results from cross-sectional and longitudinal analyses, mean diffusivity*
| Voxels, no. | Max 1 – p | MNI x, y, z (mm) |
|---|---|---|
| Cross-sectional results | ||
| Baseline | ||
| MDD > healthy controls | ||
| 6526 | 0.964 | −35, 8, 20 |
| 3266 | 0.955 | 19, 22, 38 |
| Healthy controls > MDD, no significant results | ||
| Follow-up | ||
| MDD > healthy controls, no significant results | ||
| Healthy controls > MDD, no significant results | ||
| Longitudinal results | ||
| Healthy controls | ||
| Follow up > baseline | ||
| 4048 | 0.003 | −14, 35, 6 |
| 1342 | 0.97 | 32, 34, 6 |
| 2 | 0.95 | 4, 28, 11 |
| Baseline > follow-up, no significant results | ||
| MDD | ||
| Follow-up > baseline, no significant results | ||
| Baseline > follow-up, no significant results | ||
MDD = major depressive disorder; MNI = Montreal Neurological Institute.
Dimensions of clusters (number of voxels) and localization of signal peaks (MNI coordinates) are given for regions showing maximal differences in tract-based spatial statistics values (signal peak).
Table 3.
Results from cross-sectional and longitudinal analyses, lateriality and probability of affected tracts*
| Region | Laterality | Probability, % |
|---|---|---|
| Cross-sectional results | ||
| Baseline | ||
| MDD > healthy controls | ||
| Genu of corpus callosum | — | 3.27 |
| Body of corpus callosum | — | 5.80 |
| Anterior limb of internal capsule | R | 0.01 |
| Anterior limb of internal capsule | L | 0.02 |
| Anterior corona radiata | R | 6.62 |
| Anterior corona radiata | L | 7.60 |
| Superior corona radiata | R | 1.86 |
| Superior corona radiata | L | 4.20 |
| Posterior thalamic radiation (includes optic radiation) | L | 0.05 |
| External capsule | R | 0.20 |
| External capsule | L | 0.61 |
| Superior longitudinal fasciculus | R | 0.03 |
| Superior longitudinal fasciculus | L | 3.36 |
| Superior fronto-occipital fasciculus | R | 0.11 |
| Superior fronto-occipital fasciculus | L | 0.02 |
| Unclassified | — | 66.22 |
| Healthy controls > MDD, no significant results | ||
| Follow-up | ||
| MDD > healthy controls, no significant results | ||
| Healthy controls > MDD, no significant results | ||
| Longitudinal results | ||
| Healthy controls | ||
| Follow up > baseline | ||
| Genu of corpus callosum | — | 11.40 |
| Body of corpus callosum | — | 0.33 |
| Anterior limb of internal capsule | R | 0.69 |
| Anterior limb of internal capsule | L | 0.61 |
| Anterior corona radiata | R | 8.46 |
| Anterior corona radiata | L | 15.69 |
| External capsule | R | 0.13 |
| External capsule | L | 1.54 |
| Unclassified | — | 61.15 |
| Baseline > follow-up, no significant results | ||
| MDD | ||
| Follow-up > baseline, no significant results | ||
| Baseline > follow-up, no significant results | ||
L = left; MDD = major depressive disorder; R = right.
White matter tracts in the cluster based on Johns Hopkins University ICBM-DTI-81 white matter labels (as implemented in FSL).31 Probability of affected tracts: (average) probability of all significant voxels being a member of the labelled regions in the atlas, calculated with the FSL tool atlasquery.
At follow-up, we detected no significant differences between groups. For additional information on this trend-level finding, see Appendix 1, Supplementary Results 3.
Longitudinal findings
Repeated-measures t tests within FSL revealed a significant increase in MD, AD and RD in a widespread cluster containing the corpus callosum and the corona radiata (Table 2, Table 3), as well as a trend of decreased FA (p = 0.068) at follow-up in the healthy control sample. In the MDD sample, we did not detect any significant time effects. Repeated-measures ANCOVA using baseline and follow-up mean MD values (extracted from the significant baseline cluster) revealed a significant group × time interaction (F1,99 = 16.80, p < 0.001; Fig. 2); post-hoc t tests showed an increase of MD over time in the healthy control sample (p = 0.02) and a decrease of MD over time in the MDD sample (p < 0.001).
Whole-brain analysis using difference images (ΔFA, ΔMD, ΔAD and ΔRD) did not yield significant group differences. In a region-of-interest analysis using the significant clusters at baseline as a mask (Fig. 1), the same analyses revealed significantly higher ΔMD, ΔAD and ΔRD in the healthy control group compared to the MDD group (Appendix 1, Table S4), reflecting a higher increase in those metrics over time in the healthy control group.
Exploratory analysis: effects of relapse, depression severity and medication index
At baseline, mean MD from the significant cluster was not associated with number of depressive episodes before baseline (r = 0.16, p = 0.24), number of hospitalizations before baseline (r = 0.11, p = 0.42) or medication index at baseline (r = −0.09, p = 0.51) in patients with MDD. Furthermore, ΔMD was not correlated with ΔHDRS (r = 0.05, p = 0.77) or Δ medication index (r = −0.13, p = 0.34).
We detected no significant cross-sectional or longitudinal differences between patients with MDD who had a relapse in the follow-up period and patients with MDD who did not have a relapse.
Discussion
At baseline we found higher MD in patients with MDD compared with healthy controls in several frontal white matter regions. At follow-up, MD values increased in this cluster in healthy controls, but it declined in patients with MDD, resulting in diminished MD differences between the 2 groups at follow-up.
Cross-sectional studies have repeatedly demonstrated impaired white matter fibre integrity in patients with MDD compared with healthy controls,8,13 with most studies focusing on FA as the primary DTI measure. We could show only a trend of reduced FA in many frontal white matter tracts, but analysis of MD revealed widespread increases in frontal white matter in patients with MDD, including the body and genu of the corpus callosum, the anterior and superior corona radiata, and the left superior longitudinal fasciculus. These affected white matter tracts have been consistently reported in white matter studies in patients with MDD13 and are thought to connect crucial prefrontal brain regions central to current theories of mood dysregulation in affective disorders.32,33
Although MD was less frequently investigated in the past, our findings were in line with previous evidence of increased MD in patients with MDD.34–36 Also conceptualized as apparent diffusion coefficient,12 MD reflects the total amount of diffusion in a voxel and is considered to represent demyelination or edema.37 In contrast, FA represents the degree of anisotropy in a voxel and is thought to be high in organized, coherent white matter tracts. However, given the complexity of neuronal architecture and the numerous factors influencing diffusion metrics (e.g., myelination, packing density, axonal diameter, membrane permeability, crossing of fibres) it is challenging to draw definite conclusions about specific biological brain alterations using DTI metrics.38 Nonetheless, there is evidence that axonal membranes and myelin behave as major barriers to water diffusion, and that increases in MD could reflect less restricted diffusion caused by demyelination. The investigation of MD in other diseases revealed that MD was increased in acute multiple sclerosis white matter lesions and might therefore be more closely related to inflammatory brain processes (for a review, see Alexander and colleagues37). Furthermore, our data showed that the MD differences were partly based on higher RD in patients with MDD at baseline. A measure of diffusion perpendicular to the main diffusion direction, RD has been shown to offer specific assessment of demyelination of the corpus callosum in a mouse model.39 Moreover, higher RD and MD have been associated with increased proinflammatory cytokines in major depressive episodes in bipolar disorder. 40 Taken together, these findings could further support a concept of disturbed white matter integrity (possibly through demyelination) in MDD during an acute depressive episode. Future research is needed to investigate whether these changes might be related to inflammatory processes associated with affective disorders.41–43
As expected, MD increased over time in the healthy control group. This finding is supported by previous studies showing an increase of AD, RD and MD, and a decrease of FA, with age,44,45 and it likely represents normal aging processes. Interestingly, MD decreases in patients with MDD after 2 years (Fig. 2) leading to a significant group × time interaction. This could be seen as a possible neurobiological sign of recovery after an acute depressive episode. Regardless of clinical development over the follow-up period, all patients with MDD at baseline were experiencing an acute moderate to severe depressive episode that required hospitalization. On average, patients with MDD showed clinical improvement reflected in lower HDRS score and a lower medication index at follow-up. However, we could not demonstrate differences between patients with relapse and those without, and we could not show a correlation between MD decrease and changes in HDRS scores or changes in medication scores. This may point toward a global, nonspecific effect of treatment during and after the acute depressive episode, because all patients received psychotherapeutic treatment during the initial hospitalization and all but 4 patients received psychotropic medication. Future studies with bigger sample sizes (ideally with more treatment-naïve patients) need to be conducted to further elucidate possible longitudinal effects of psycho- and pharmacological treatment on white matter integrity.
Because we have observed only baseline differences and a group × time interaction in MD (and not FA), it could be argued that — compared with FA — MD is a more dynamic DTI metric, more likely to represent flexible white matter changes over time in a disease such as MDD, which has a relapsing-remitting pattern. Future studies with longer follow-up periods could shed light on the long-term development of MD based on the clinical course of the disease.
Limitations
We did not detect any differences in FA between patients with MDD and healthy controls, even though we found a trend of decreased FA in the MDD sample at baseline. This might be due to the small sample size, and our study might have been underpowered to detect reliable cross-sectional differences. Regarding the longitudinal analysis, it was a challenge to assess a precise course of illness, because self-reported clinical measures often have low accuracy because of recollection biases in patients with MDD.46 When we compared longitudinal group differences using difference images (e.g., ΔMD), analyses did not reach significance. This was likely because of the reduced variance in analysis using differences instead of a proper repeated-measures general linear model, reducing the sensitivity of detecting subtle changes between groups over time. However, when restricting analyses to a region-of-interest analysis of all regions that showed baseline differences, we could demonstrate significantly higher ΔMD, ΔAD and ΔRD in the healthy controls group. Diffusion tensor imaging sequences with higher numbers of diffusion directions should be used in the future for higher signal-to-noise ratio and improved estimation of the diffusion tensor.
Conclusion
This study reports follow-up data on patients with MDD after an acute depressive episode. We demonstrated impaired prefrontal white matter (higher MD) at baseline in patients with MDD, which seemed to normalize at follow-up after 2 years, irrespective of disease course. This might have been due to a general treatment effect. Future research with longer follow-up periods and larger sample sizes is needed to disentangle treatment factors that might have exerted possible neuroprotective effects (e.g., medication or psychotherapy) and to explore a possible link between inflammation and white matter integrity in MDD.
Acknowledgements
We acknowledge support from the Open Access Publication Fund of the University of Münster.
Footnotes
Funding: This work was funded by the German Research Foundation (grants FOR2107 DA1151/5-1 and DA1151/5-2 to U. Dannlowski; SFB- TRR58, Projects C09 and Z02 to U. Dannlowski), the Interdisciplinary Centre for Clinical Research of the medical faculty of Münster (grant Dan3/012/17 to U. Dannlowski), IMF Münster RE111604 and RE111722 to R. Redlich, IMF Münster RE 221707 to J. Repple and the Deanery of the Medical Faculty of the University of Münster.
Competing interests: H. Kugel has received consultation fees from MR:comp GmbH, Testing Services for MR Safety, outside the submitted work. V. Arolt is a member of the advisory boards of Allergan, Janssen-Cilag, Lundbeck, Otsuka, Servier and Trommsdorff and has received speaker fees from Janssen-Cilag, Lundbeck, Otsuka, Servier, outside the submitted work. No other competing interests declared.
Contributors: J. Repple, R. Redlich, K. Dohm, H. Kugel and U. Dannlowski designed the study. J. Repple, S. Meinert, D. Grotegerd, R. Redlich, K. Förster, K. Dohm, N. Opel. T. Hahn, V. Enneking, E. Leehr, J. Böhnlein, F. Dzvonyar, L. Sindermann, N. Winter, J. Goltermann, H. Kugel, J. Bauer, W. Heindel and V. Arolt acquired the data, which J. Repple, D. Zaremba, S. Meinert, D. Grotegerd, R. Redlich, K. Förster, K. Dohm, V. Arolt and U. Dannlowski analyzed. J. Repple and U. Dannlowski wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–86. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 2.Redlich R, Dohm K, Grotegerd D, et al. Reward Processing in unipolar and bipolar depression: a functional MRI study. Neuropsychopharmacology. 2015;40:2623–31. doi: 10.1038/npp.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaremba D, Dohm K, Redlich R, et al. Association of brain cortical changes with relapse in patients with major depressive disorder. JAMA Psychiatry. 2018;75:484–92. doi: 10.1001/jamapsychiatry.2018.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delvecchio G, Fossati P, Boyer P, et al. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol. 2012;22:100–13. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korgaonkar MS, Fornito A, Williams LM, et al. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol Psychiatry. 2014;76:567–74. doi: 10.1016/j.biopsych.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Liao Y, Huang X, Wu Q, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38:49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Repple J, Meinert S, Grotegerd D, et al. A voxel-based diffusion tensor imaging study in unipolar and bipolar depression. Bipolar Disord. 2017;19:23–31. doi: 10.1111/bdi.12465. [DOI] [PubMed] [Google Scholar]
- 9.Post RJ, Warden MR. Melancholy, anhedonia, apathy: the search for separable behaviors and neural circuits in depression. Curr Opin Neurobiol. 2018;49:192–200. doi: 10.1016/j.conb.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li BJ, Friston K, Mody M, et al. A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci Ther. 2018;24:1004–19. doi: 10.1111/cns.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helm K, Viol K, Weiger TM, et al. Neuronal connectivity in major depressive disorder: a systematic review. Neuropsychiatr Dis Treat. 2018;14:2715–37. doi: 10.2147/NDT.S170989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soares JM, Marques P, Alves V, et al. A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci. 2013;7:1–14. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wise T, Radua J, Nortje G, et al. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol Psychiatry. 2016;79:293–302. doi: 10.1016/j.biopsych.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Murphy ML, Frodl T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol Mood Anxiety Disord. 2011;1:3. doi: 10.1186/2045-5380-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Y, Xu J, Yu H, et al. Delineation of early and later adult onset depression by diffusion tensor imaging. PLoS One. 2014;9:e112307. doi: 10.1371/journal.pone.0112307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor WD, MacFall JR, Gerig G, et al. Structural integrity of the uncinate fasciculus in geriatric depression: relationship with age of onset. Neuropsychiatr Dis Treat. 2007;3:669–74. [PMC free article] [PubMed] [Google Scholar]
- 17.De Diego-Adeliño J, Pires P, Gómez-Ansón B, et al. Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol Med. 2014;44:1171–82. doi: 10.1017/S003329171300158X. [DOI] [PubMed] [Google Scholar]
- 18.Ganzola R, McIntosh AM, Nickson T, et al. Diffusion tensor imaging correlates of early markers of depression in youth at high-familial risk for bipolar disorder. J Child Psychol Psychiatry Allied Discip. 2018;59:917–27. doi: 10.1111/jcpp.12879. [DOI] [PubMed] [Google Scholar]
- 19.Vulser H, Paillère Martinot M-L, Artiges E, et al. Early variations in white matter microstructure and depression outcome in adolescents with subthreshold depression. Am J Psychiatry. 2018;175:1255–64. doi: 10.1176/appi.ajp.2018.17070825. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Fan X, Williamson DE, et al. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology. 2011;36:684–91. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohm K, Redlich R, Zwitserlood P, et al. Trajectories of major depression disorders: a systematic review of longitudinal neuroimaging findings. Aust N Z J Psychiatry. 2017;51:441–54. doi: 10.1177/0004867416661426. [DOI] [PubMed] [Google Scholar]
- 22.Frodl T, Jäger M, Smajistrlova I, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:423–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Wittchen H, Zaudig M, Fydrich T. Strukturiertes Klinisches Interview für DSM-IV. Hogrefe, Göttingen, Germany: 1997. [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redlich R, Almeida JJR, Grotegerd D, et al. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. JAMA Psychiatry. 2014;71:1222. doi: 10.1001/jamapsychiatry.2014.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkinson M, Beckmann C, Behrens TE, et al. FSL. Neuroimage. 2012;62:782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Repple J, Opel N, Meinert S, et al. Elevated body-mass index is associated with reduced white matter integrity in two large independent cohorts. Psychoneuroendocrinology. 2018;91:179–85. doi: 10.1016/j.psyneuen.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Le Bihan D. Looking at the functional architechture of the brain with diffusion. Nat Rev Neurosci. 2003;4:469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 31.Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–47. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wise T, Cleare AJ, Herane A, et al. Diagnostic and therapeutic utility of neuroimaging in depression: an overview. Neuropsychiatr Dis Treat. 2014;10:1509–22. doi: 10.2147/NDT.S50156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korgaonkar MS, Grieve SM, Koslow SH, et al. Loss of white matter integrity in major depressive disorder: evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum Brain Mapp. 2011;32:2161–71. doi: 10.1002/hbm.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu F, Tang Y, Xu K, et al. White matter abnormalities in medication-naive subjects with a single short-duration episode of major depressive disorder. Psychiatry Res Neuroimaging. 2011;191:80–3. doi: 10.1016/j.pscychresns.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimony JS, Sheline YI, D’Angelo G, et al. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66:245–52. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–54. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 39.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–40. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 40.Benedetti F, Poletti S, Hoogenboezem TA, et al. Inflammatory cytokines influence measures of white matter integrity in bipolar disorder. J Affect Disord. 2016;202:1–9. doi: 10.1016/j.jad.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 41.Eyre H, Baune BT. Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology. 2012;37:1397–416. doi: 10.1016/j.psyneuen.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Leboyer M, Soreca I, Scott J, et al. Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord. 2012;141:1–10. doi: 10.1016/j.jad.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yap QJ, Teh I, Fusar-Poli P, et al. Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. J Neural Transm. 2013;120:1369–95. doi: 10.1007/s00702-013-0971-7. [DOI] [PubMed] [Google Scholar]
- 45.Sexton CE, Walhovd KB, Storsve AB, et al. Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. J Neurosci. 2014;34:15425–36. doi: 10.1523/JNEUROSCI.0203-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barsky AJ. Forgetting, fabricating, and telescoping. Arch Intern Med. 2002;162:981–4. doi: 10.1001/archinte.162.9.981. [DOI] [PubMed] [Google Scholar]