Abstract
Bupropion overdose in the pediatric setting poses significant potential for toxicity. We present the case of a 15-year-old female patient presenting with intentional bupropion overdose resulting in generalized tonic–clonic seizures, severe acidosis, vomiting, and tachycardia after ingestion of between 1,650 to 9,000 mg (24–133 mg/kg) of bupropion. The patient was admitted to pediatric intensive care unit (PICU) where toxicity was resolved promptly following administration of intravenous lipid emulsion (ILE) infusion. ILE is a first-line treatment for other forms of toxicity including unintended local anesthetic administration. ILE use is not a first-line treatment in this setting, but this case presents a positive subsequent patient outcome.
Keywords: Bupropion, Wellbutrin, lipid emulsion therapy, antidepressant, overdose
Introduction
Bupropion (Wellbutrin, GlaxoSmithKline) is a synthetic cathinone norepinephrine/dopamine reuptake inhibitor commonly used as an antidepressant. Its primary toxic effects include seizure, tachycardia, and agitation. Many reports in the literature describe massive ingestions in adolescents presenting challenges in management. 1 We report a case of adolescent bupropion ingestion resulting in multiple seizures with subsequent derangements to blood chemistries managed with intravenous lipid emulsion (ILE) therapy. ILE use as an antidotal agent came into vogue in the past two decades based on pharmacologic principles and case reports, despite sparse high-quality data supporting its use and limited recommendation by the American College of Medical Toxicology (ACMT) and Poison Control Centers (PCC). 2 While recognized off-label as a salvage therapy for bupropion overdose resulting in hemodynamic instability and cardiovascular collapse, ILE use is not standard in the setting of status epilepticus. 3 This case represents a successful application of toxicologist-recommended ILE use with prompt and effective reversal of bupropion induced neurotoxicity and symptomatology.
Case
A 15-year-old, 67.2-kg female patient with no significant past medical or psychiatric history intentionally ingested between an estimated 1,650 to 9,000 mg (24–133 mg/kg) of bupropion in the form of 150 mg bupropion extended-release tablets belonging to her recently deceased grandmother. Approximately 1-hour postingestion, the patient experienced a generalized tonic-clonic seizure. Although ingested pill quantity was unknown, the patient's mother counted approximately eleven pills (1,650 mg) in the patient's emesis. Emergency medical services were called and transported the patient to the emergency department (ED). Upon ED arrival, the patient experienced a self-limited second seizure lasting 30 seconds. Following this seizure, the patient became somnolent with a Glasgow Coma Score of 11; spontaneous eye opening, incomprehensible speech, and purposeless movements. Physical examination revealed the patient to be tachycardic to 138 beats per minute with strong pulses in all extremities. Her airway was intact and she had clear lung sounds. Her abdomen was soft and nontender with normal bowel sounds. Her blood pressure was 121/87 mm Hg; respiratory rate, 24; temperature, 38.6°C; and oxygen saturation, 98% on room air. Electrocardiogram (ECG) revealed sinus tachycardia with borderline prolonged QTc at 461 milliseconds (ms) and normal QRS interval at 84 ms ( Fig. 1 ). Further examination disclosed no other focal abnormalities. Intravenous (IV) access was established and the patient was administered a 20 mL/kg normal saline bolus. Shortly thereafter the patient experienced a third seizure and was administered 2 mg (0.02 mg/kg) IV lorazepam with termination of seizure activity. Initial venous blood gas analysis revealed a pH of 7.02, PCO 2 of 34 mm Hg, HCO 3 of 9 mEq/L, and an anion gap of 32 indicating a mixed respiratory alkalosis with metabolic acidosis. Initial recommendations from the PCC at that time were to provide supportive care with intubation, benzodiazepines for seizure activity, sodium bicarbonate if QRS widening developed on ECG, gastrointestinal decontamination with activated charcoal, and 1.5 mL/kg ILE infusion if refractory to supportive care. Given the unknown time lapse since initial ingestion and severity of ongoing symptomatic presentation, decontamination was considered but not pursued and the patient was not intubated. Abdominal radiograph revealed no pill fragments or concretions. ILE infusion was also deferred as the patient's seizure activity was responsive to 2 mg IV lorazepam. Initial labs detected amphetamines on serum drug assay. This was considered to be likely false-positive due to cross-reactivity with bupropion. 4
Fig. 1.
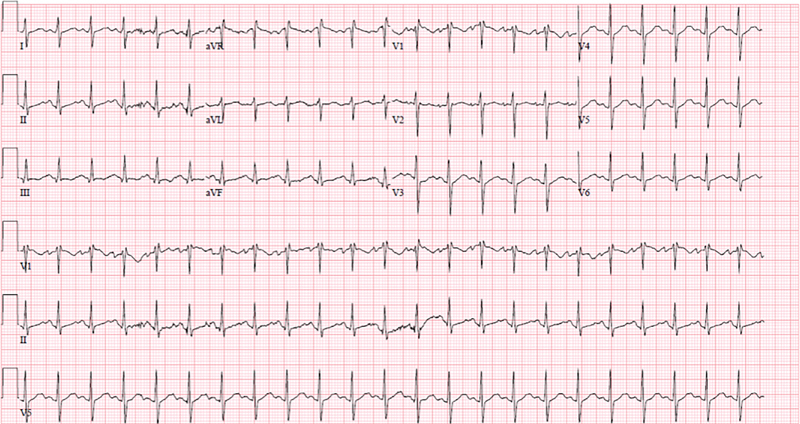
Patient's electrocardiogram on ED presentation showing sinus tachycardia, with bupropion-induced QT prolongation—304 ms (QTc of 461 ms) and QRS of 84 ms. ED, emergency department.
The patient was admitted to PICU. Eight hours following ED presentation, the patient experienced two more generalized seizures in the PICU. She received 2 mg IV lorazepam, becoming postictal afterwards. ECG at this time demonstrated widened QRS at 108 ms and she was administered 50 mEq IV sodium bicarbonate. On day 1, the patient was febrile to 38.8°C with continued somnolence. Her best verbal response was incoherent speech. The decision was then made to administer 1.5 mL/kg (20%) ILE infusion (Intralipid, Baxter). The patient had a marked improvement in her mental status, becoming oriented to person and responding appropriately to questions 30 minutes following ILE administration. Although she demonstrated no more generalized seizure activity, she continued to have myoclonic jerks. On day 3 she was again noted to have alteration in her mental status and received a repeat infusion of 1.5 mL/kg (20%) ILE over 30 minutes. Prompt and lasting return to baseline mental status was observed following administration. Patient denied confusion or suicidal ideation. Myoclonic jerks resolved at this time. The patient had a mild transaminitis (aspartate aminotransferase (AST), 72; alanine aminotransferase (ALT), 134) on day 4, with return to baseline the following day. The patient was discharged from the PICU on day 4 to inpatient psychiatry. Urine amphetamine level was negative one day postdischarge from PICU.
Discussion
Epidemiology
A 2002 analysis of 7,348 bupropion exposures reported to PCCs found clinical effects related to bupropion were noted in 31% of exposures. Among bupropion overdoses resulting in toxicity, symptomatology varied by pediatric age group. 1 Among younger patients, vomiting was most common versus tachycardia among teenagers. Seizures were more frequent sequela for teenagers, occurring in 28% of symptomatic exposures. Of cases with seizure, 70% were single, 27% were multiple, and 3% were status epilepticus. 1 An analysis of single-drug antidepressant exposures reported to United States PCCs from 2000 to 2014 found bupropion overdose to portend the highest morbidity and mortality among second generation antidepressants. 5
Bupropion Pharmacology
Bupropion is a unique synthetic cathinone antidepressant with functions mediated through two theorized mechanisms: stimulation of norepinephrine and dopamine release in the central nervous system (CNS) and inhibition of catecholamine synaptic reuptake. 6 While the drug classes are chemically distinct, these mechanisms may account for the overlap in bupropion psychopharmacology between synthetic cathinones and amphetamines. The CNS features of bupropion play a role in the neurotoxicity manifested in overdose, including the seizures seen in our patient. Bupropion causes generalized seizures in a dose-dependent manner even within therapeutic ranges by lowering seizure threshold in susceptible patients. 7 Serious reactions reported with overdoses of bupropion include hallucinations, seizures, altered mental status, and ECG changes including arrhythmias and QRS and QT prolongation. 8 Fever, clonus, rhabdomyolysis, hypotension, coma, and respiratory failure have been reported. However, these sequelae occurred mainly in cases of multidrug ingestions involving bupropion. Although most patients recover without sequelae, fatal overdoses of bupropion have been reported in cases involving large ingestions. Status epilepticus, hemodynamic collapse, and cardiac arrest preceded death in those cases. 9 Animal testing data suggests a potential for bupropion-related hepatotoxicity. 10 Our patient did have a transient transaminase elevation, but it is unclear whether her brief transaminitis resulted from bupropion, ILE therapy, or a separate unknown cause. 6 During therapeutic use, plasma levels in the extended-release formulation peak approximately five hours postingestion. Extended-release bupropion continues absorption for 12 to 15 hours and the half-life is approximately 21 (±9) hours. 10 Time of seizure onset in our patient is consistent with the expected peak plasma level from the extended-release formulation ingested. The duration of presentation is accounted for by the plasma half-life of bupropion. 11 Bupropion has three major bioactive metabolites, of which hydroxybupropion is the principal and most clinically relevant. Hydroxybuprion bears half the potency of its precursor and similar levels of plasma protein binding. Plasma concentrations of metabolites can be as high as or higher than those of bupropion. 10 Extended-release bupropion presents additional considerations for clinicians in that patients may present clinically stable early in the course of overdose with benign-appearing vital signs, laboratory values, and neurologic exam, while decompensating rapidly later in their course. The true toxicity of a bupropion overdose may not be revealed until days after initial exposure. 7 In the presented case, the patient's serum bupropion level was drawn approximately 16 hours postingestion and was 879 ng/mL, nearly nine times the upper limit of therapeutic serum concentration of 50 to 100 ng/mL. Hydroxybupropion was measured at 5,799.5 ng/mL; nearly three times the upper limit of therapeutic serum concentration of 600 to 2,000 ng/mL. 12 Notably, fatalities have been reported at ingested doses of between 5.4 and 9 g with postmortem serum bupropion concentration of 440 ng/mL. 7
Lipid Emulsion Pharmacology
Intravenous lipid emulsions have been used since the 1970s as a component of parenteral nutrition. The only current Food and Drug Administration (FDA) indication for ILE is as a source of calories and essential fatty acids for such patients. 13 Off-label, ILE has also been used since the late 1990s to reverse local anesthetic toxicity. Recently, off-label ILE use has also expanded to treat other toxidromes. 14 15 16 Multiple ILE formulations exist using different lipid sources and concentrations. The formulation used in our case was 20% soybean oil, 1.2% egg yolk phospholipids, 2.25% glycerin, and water. 13 ILE is well tolerated in many critically ill patients from a nutritional perspective and is used in both the inpatient and outpatient setting in all patient age groups across a wide variety and spectrum of severity of illness. As a reversal agent for acute xenobiotic toxicity, ILE proposed mechanism of action involves sequestering pharmacologically active lipophilic drugs by forming a strong concentration gradient in an expanded plasma lipid compartment, and removal from the serum via biliary excretion into the gut. 17 18 19 Additionally, ILE has a hypothesized cardioprotective role through ion channel modulation, improvement of cardiac fatty acid metabolism, and inhibition of mitochondrial proapoptotic signaling pathways. 20 Toxicity associated with acute ILE use is minimal and the drug has a wide therapeutic range. The ACMT notes that there is no known daily upper limit for safe dosing in humans. 21 Extrapolation from animal studies suggests limiting dosing to approximately 10 to 12 mL/kg over 30 minutes to maintain a reasonable margin of safety. 22 Adverse events are rare but include lung injury such as pulmonary lipid embolism and adult respiratory distress syndrome, 19 lipemia-associated acute pancreatitis, 23 electrolyte disturbances, phlebitis, and allergic reactions. 24 ILE infusion may interfere in laboratory serum analysis, and there is a theoretical risk of nullification of benzodiazepine effects. 2 23 25 Additionally, concerns have been raised that ILE administration may increase lipophilic medication absorption from the gastrointestinal tract, and can agglutinate in extracorporeal membrane oxygenation (ECMO) circuits, infrequently causing obstruction in longer term (≥24 hour) simultaneous use of both modalities. 26 While the use of ILE in xenobiotic-induced toxicity is not one of the FDA-recommended indications, expert panels recommend it on a case-by-case basis based on prior reports, pharmacokinetics, and mechanisms of action. 2 A 2016 international clinical toxicology association consensus statement on the use of ILE in poisonings recommends its use for bupropion toxicity if other therapies fail, or as last resort due to lack of gold standard, as ILE lacks high-powered, randomized controlled evidence supporting its use, and case report literature supporting its use is scant. 2 The consensus statement conditionally recommends that “a trial of ILE seems reasonable … in prolonged and refractory status epilepticus.” As PCC protocol is typically driven by robust data, ILE is most likely to be recommended in more severe cases of xenobiotic toxicity, potentially resulting in a bias towards late recommendation and poor outcomes. 27 Indeed, independent examination of cases from one regional poison center found nine instances of ILE use in severe presentations among 1,274 cases of suspected bupropion ingestion. While only five of the nine patients who received ILE survived, this represents a very small sample size with nearly all of the patients initially presenting in cardiac arrest or hemodynamic instability due to severe poisoning. 3 Given their critical illness, it is difficult to determine whether these poor patient outcomes were ultimately worsened by ILE, or if they were so unstable as to unlikely respond to any form of therapy at all. Overall, it appears that it is not due to the intrinsic pharmacologic or toxicologic profile of ILE that it is not used more frequently but rather the absence of high-quality evidence validating its use in critically ill patients or in specific end-organ toxicity manifestation. This, unfortunately, represents the most likely and biased subset for which ILE may be recommended by medical toxicologists and administered in the clinical setting, limiting future expansion of case report literature upon which current ILE recommendations depend. 27
Management of Bupropion Toxicity
Bupropion does not have a specific antidote or antagonist medication. Pharmacologic management of the toxicity associated with bupropion is typically supportive and tailored to presentation. Benzodiazepines are indicated as first-line therapy for seizures. Barbiturates are superior to phenytoin and other antiepileptic drugs as second-line therapy, described in multiple case reports and case series. 28 29 QRS prolongation can be managed with sodium bicarbonate, 7 but QRS prolongation may be refractory to this therapy as bupropion does not directly block sodium channels 30 but rather, disrupts intrinsic cardiomyocyte conduction via gap junctions. 31 Bupropion and hydroxybupropion are highly lipid-soluble and extensively plasma protein bound (∼84%), excluding dialysis as a viable option for decontamination. 10 32 Plasmapheresis and ECMO are mentioned as salvage therapies in a small number of case reports featuring massive bupropion overdose resulting in cardiac arrest. 33 However, there are no evidence-based guidelines referring to the use of these therapies in bupropion toxicity or other severe poisonings more generally. 34 35 In this instance, use of ILE per medical toxicologist recommendation in a case of benzodiazepine-refractory bupropion neurotoxicity with seizures and prolonged mental status changes resulted in a remarkable improvement in patient condition allowing for prompt discharge from intensive care. Quantitative analysis indicated persistent toxic levels of bupropion and hydroxybupropion would have been present even after several half-lives, likely accounting for the prolonged clinical presentation of seizures and altered mentation on PICU days 2 and 3. Based on pharmacologic principles, it is plausible that administered ILE bound a sufficient quantity of serum bupropion and metabolites to reverse bupropion-induced cathinone neurotoxicity. While this case represents an isolated incident, the potential for use of ILE in management of severe toxicity in the setting of pediatric overdose can be considered on a case-by-case basis as a therapeutic modality in similar cases with the guidance of medical toxicologists and/or a regional PCC. Further high-quality investigation is warranted to expand and strengthen recommendations for antidotal uses of ILE beyond salvage therapy for acute resuscitation in the setting of other specific end-organ toxicities.
Funding Statement
Funding None.
Conflict of Interest None declared.
Note
This report describes a case of intentional severe bupropion overdose in an adolescent patient and successful management of sequelae with intravenous lipid emulsion therapy.
Authors' Contribution
M.A.P. and T.M. conceptualized the case report and provided edits and revision. K.B. drafted the initial manuscript and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- 1.Belson M G, Kelley T R. Bupropion exposures: clinical manifestations and medical outcome. J Emerg Med. 2002;23(03):223–230. doi: 10.1016/s0736-4679(02)00522-x. [DOI] [PubMed] [Google Scholar]
- 2.Gosselin S, Hoegberg L C, Hoffman R S et al. Evidence-based recommendations on the use of intravenous lipid emulsion therapy in poisoning. Clin Toxicol (Phila) 2016;54(10):899–923. doi: 10.1080/15563650.2016.1214275. [DOI] [PubMed] [Google Scholar]
- 3.Chhabra N, DesLauriers C, Wahl M, Bryant S M. Management of severe bupropion poisoning with intravenous lipid emulsion. Clin Toxicol (Phila) 2018;56(01):51–54. doi: 10.1080/15563650.2017.1337909. [DOI] [PubMed] [Google Scholar]
- 4.Casey E R, Scott M G, Tang S, Mullins M E. Frequency of false positive amphetamine screens due to bupropion using the Syva EMIT II immunoassay. J Med Toxicol. 2011;7(02):105–108. doi: 10.1007/s13181-010-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson J C, Spyker D A. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000–2014. Am J Psychiatry. 2017;174(05):438–450. doi: 10.1176/appi.ajp.2016.16050523. [DOI] [PubMed] [Google Scholar]
- 6.Anez-Bustillos L, Dao D T, Baker M A, Fell G L, Puder M, Gura K M. Intravenous fat emulsion formulations for the adult and pediatric patient: understanding the differences. Nutr Clin Pract. 2016;31(05):596–609. doi: 10.1177/0884533616662996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bupropion. Micromedex Solutions. Greenwood Village, CO: Truven Health Analytics. Available at:https://www.micromedexsolutions.com/micromedex2/librarian/CS/958B44/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/5E4665/N. Updated 1/4/2019. Accessed May 8, 2019
- 8.Boora K, Cummings M R, Marshall D R. Generalized seizure in three adolescents with bupropion overdose. J Child Adolesc Psychopharmacol. 2010;20(02):159–160. doi: 10.1089/cap.2009.0016. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Kolawole T, Jimenez X F. Atypical findings in massive bupropion overdose: a case report and discussion of psychopharmacologic issues. J Psychiatr Pract. 2016;22(05):405–409. doi: 10.1097/PRA.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 10.Wellbutrin XL [package insert]. Research Triangle Park N. In. GlaxoSmithKline 2006. Available at:https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018644s043lbl.pdf. Accessed May 8, 2019
- 11.Spiller H A, Schaeffer S E. Multiple seizures after bupropion overdose in a small child. Pediatr Emerg Care. 2008;24(07):474–475. doi: 10.1097/PEC.0b013e31817de2e6. [DOI] [PubMed] [Google Scholar]
- 12.Heise C W, Skolnik A B, Raschke R A, Owen-Reece H, Graeme K A. Two cases of refractory cardiogenic shock secondary to bupropion successfully treated with veno-arterial extracorporeal membrane oxygenation. J Med Toxicol. 2016;12(03):301–304. doi: 10.1007/s13181-016-0539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Intralipid. [package insert]. Deerfield IL. Baxter. Available at:https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/017643s072,018449s039lbl.pdf. Accessed May 9, 2019
- 14.Picard J, Meek T. Lipid emulsion to treat overdose of local anaesthetic: the gift of the glob. Anaesthesia. 2006;61(02):107–109. doi: 10.1111/j.1365-2044.2005.04494.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosenblatt M A, Abel M, Fischer G W, Itzkovich C J, Eisenkraft J B. Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology. 2006;105(01):217–218. doi: 10.1097/00000542-200607000-00033. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg G L, Di Gregorio G, Ripper R et al. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008;108(05):907–913. doi: 10.1097/ALN.0b013e31816d91d2. [DOI] [PubMed] [Google Scholar]
- 17.Rothschild L, Bern S, Oswald S, Weinberg G. Intravenous lipid emulsion in clinical toxicology. Scand J Trauma Resusc Emerg Med. 2010;18:51. doi: 10.1186/1757-7241-18-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kryshtal D O, Dawling S, Seger D, Knollmann B C. In vitro studies indicate intravenous lipid emulsion acts as lipid sink in verapamil poisoning. J Med Toxicol. 2016;12(02):165–171. doi: 10.1007/s13181-015-0511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sirianni A J, Osterhoudt K C, Calello D Pet al. Use of lipid emulsion in the resuscitation of a patient with prolonged cardiovascular collapse after overdose of bupropion and lamotrigine Ann Emerg Med 20085104412–415., 415.e1 [DOI] [PubMed] [Google Scholar]
- 20.Walter E, McKinlay J, Corbett J, Kirk-Bayley J. Review of management in cardiotoxic overdose and efficacy of delayed intralipid use. J Intensive Care Soc. 2018;19(01):50–55. doi: 10.1177/1751143717705802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American College of Medical Toxicology.ACMT position statement: guidance for the use of intravenous lipid emulsion J Med Toxicol 20171301124–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinberg Guy L. Lipid emulsion infusion: resuscitation for local anesthetic and other drug overdose. Anesthesiology. 2012;117(01):180–187. doi: 10.1097/ALN.0b013e31825ad8de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucklin M H, Gorodetsky R M, Wiegand T J. Prolonged lipemia and pancreatitis due to extended infusion of lipid emulsion in bupropion overdose. Clin Toxicol (Phila) 2013;51(09):896–898. doi: 10.3109/15563650.2013.831436. [DOI] [PubMed] [Google Scholar]
- 24.Karcioglu O. Use of lipid emulsion therapy in local anesthetic overdose. Saudi Med J. 2017;38(10):985–993. doi: 10.15537/smj.2017.10.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smolinske S, Hoffman R S, Villeneuve E, Hoegberg L CG, Gosselin S. Utilization of lipid emulsion therapy in fatal overdose cases: an observational study. Clin Toxicol (Phila) 2019;57(03):197–202. doi: 10.1080/15563650.2018.1504954. [DOI] [PubMed] [Google Scholar]
- 26.Lee H MD, Archer J RH, Dargan P I, Wood D M. What are the adverse effects associated with the combined use of intravenous lipid emulsion and extracorporeal membrane oxygenation in the poisoned patient? Clin Toxicol. 2015;53(03):145–150. doi: 10.3109/15563650.2015.1004582. [DOI] [PubMed] [Google Scholar]
- 27.Christian M R, Pallasch E M, Wahl M, Mycyk M B. Lipid rescue 911: are poison centers recommending intravenous fat emulsion therapy for severe poisoning? J Med Toxicol. 2013;9(03):231–234. doi: 10.1007/s13181-013-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu P, Juurlink D. Switzerland: Springer International Publishing; 2016. Bupropion; pp. 1–10. [Google Scholar]
- 29.Shah A SV, Eddleston M. Should phenytoin or barbiturates be used as second-line anticonvulsant therapy for toxicological seizures? Clin Toxicol. 2010;48(08):800–805. doi: 10.3109/15563650.2010.521506. [DOI] [PubMed] [Google Scholar]
- 30.Wills B K, Zell-Kanter M, Aks S E. Bupropion-associated QRS prolongation unresponsive to sodium bicarbonate therapy. Am J Ther. 2009;16(02):193–196. doi: 10.1097/MJT.0b013e3180a5bd83. [DOI] [PubMed] [Google Scholar]
- 31.Caillier B, Pilote S, Castonguay A et al. QRS widening and QT prolongation under bupropion: a unique cardiac electrophysiological profile. Fundam Clin Pharmacol. 2012;26(05):599–608. doi: 10.1111/j.1472-8206.2011.00953.x. [DOI] [PubMed] [Google Scholar]
- 32.Worrall S P, Almond M K, Dhillon S. Pharmacokinetics of bupropion and its metabolites in haemodialysis patients who smoke. A single dose study. Nephron Clin Pract. 2004;97(03):c83–c89. doi: 10.1159/000078635. [DOI] [PubMed] [Google Scholar]
- 33.Tolentino S, Gharpure V, Tsifansky M. 1173: Successful use of lipid infusion and plasmapheresis after massive bupropion overdose. Crit Care Med. 2013;41(12):A298. [Google Scholar]
- 34.Levine M, Hoffman R S, Lavergne V et al. Systematic review of the effect of intravenous lipid emulsion therapy for non-local anesthetics toxicity. Clin Toxicol (Phila) 2016;54(03):194–221. doi: 10.3109/15563650.2015.1126286. [DOI] [PubMed] [Google Scholar]
- 35.Disel N R, Akpinar A A, Sebe A et al. Therapeutic plasma exchange in poisoning: 8 years' experience of a university hospital. Am J Emerg Med. 2015;33(10):1391–1395. doi: 10.1016/j.ajem.2015.07.016. [DOI] [PubMed] [Google Scholar]


