Abstract
Our objective is to evaluate intravenous (IV) fluid prescription practice patterns in critically ill children in the first 72 hours of pediatric intensive care unit (PICU) admission and to evaluate the incidence and predictors of hyperchloremic metabolic acidemia (HCMA) and the association between HCMA and adverse outcomes. This retrospective cohort study was conducted in two tertiary-care Canadian PICUs. Children aged 0 to 18 years admitted to the PICU between January 2015 and January 2016 who received at least 50% of their calculated maintenance fluid requirements parenterally during the first 24 hours of admission were included. Children with known preexisting conditions associated with HCMA, such as renal tubular acidosis and gastrointestinal bicarbonate losses, were excluded. Of the 771 children screened, 543 met eligibility criteria and were included. The commonest prescribed maintenance fluid was 0.9% NaCl (72.9%) followed by lactated Ringer's solution (19.6%) and hypotonic solutions (4.6%). Balanced salt solutions (i.e., lactated Ringer's and Plasma-Lyte) were as commonly administered as unbalanced solutions (0.9% NaCl) for volume expansion (49.6 vs. 48.5%, respectively). Medications contributed to a significant proportion of total daily intake, in excess of bolus fluids. The incidence of hyperchloremia and HCMA was 94.9% (95% confidence interval [CI]: 93.2–96.9; 470/495) and 38.9% (95% CI: 34.6–43.2; 196/504), respectively. Predictors of HCMA were increasing combined bolus and maintenance 0.9% NaCl intake (odds ratio: 1.13; 95% CI: 1.04–1.23) and increasing severity of illness. HCMA was not associated with an increased risk of acute kidney injury, feeding intolerance, or PICU-acquired weakness. Isotonic fluids, specifically 0.9% NaCl, were the most commonly administered maintenance IV fluid in critically ill children. Sources of chloride load are not isolated to resuscitation fluids as previously suggested. Maintenance fluids and fluids administered with medications and IV flushes (fluid creep) are under-recognized significant sources of fluid and electrolyte intake in critically ill children. HCMA is common, and further prospective research is required to determine whether HCMA is indeed harmful in children. However, all significant sources of fluid should be accounted for in the design of future trials comparing balanced and unbalanced salt solutions.
Keywords: pediatrics, IV solutions, maintenance fluids, hyperchloremia, acidosis
Introduction
Hypotonic solutions were widely accepted as the intravenous (IV) maintenance fluid of choice in children older than 50 years, 1 2 until more recent evidence demonstrated a clear association between the use of hypotonic fluids and iatrogenic hyponatremia related morbidity and mortality. 2 3 4 Consequently, isotonic solutions are now recommended by a number of practice guidelines as a safer and more appropriate empiric IV maintenance fluid choice in children. 5 Whether this has translated into a change in practice and a subsequent reduction in the incidence of iatrogenic hyponatremia remains unclear. 6 7
Although hospital-acquired hyponatremia may be reduced by isotonic fluids, there are growing concerns of iatrogenic hyperchloremia. There is emerging evidence in both adult and pediatric populations that hyperchloremia is increasingly common and may be associated with adverse outcomes in critically ill patients. 8 9 Hyperchloremia has been reportedly associated with acute kidney injury, coagulopathy, gastrointestinal dysfunction, and even increased mortality in critically ill adults and children. 3 10 11 More recent fluid debates have therefore been focused on whether chloride-rich solutions, such as 0.9% NaCl, worsen patient outcomes through the increased risk of hyperchloremic acidosis and whether physiologically balanced salt solutions such as lactated Ringer's or Plasma-Lyte may ameliorate this harm. 9 Previous studies have concluded that the primary source of chloride load is attributed to volume resuscitation. 10 However, we hypothesized that in children, maintenance IV fluids and fluid from medication infusions (i.e., fluid creep) may contribute a significant chloride load.
Our primary objective in this retrospective cohort study of critically ill children was to assess current IV fluid prescription practice patterns in the first 72 hours of pediatric intensive care unit (PICU) admission. Our secondary objectives were to determine the incidence and predictors of hyperchloremia and hyperchloremic metabolic acidemia (HCMA) and to evaluate the association between HCMA and specific adverse events such as acute kidney injury, feeding intolerance, PICU-acquired weakness, coagulopathy, and mortality.
Materials and Methods
Study Design and Participants
We conducted this retrospective cohort study at the PICUs of McMaster Children's Hospital in Hamilton, Ontario, Canada (site 1), and Stollery Children's Hospital in Edmonton, Alberta, Canada (site 2). It was approved by the institutional research ethics boards at both institutions, and the need for informed consent was waived. We included consecutive children younger than 18 years who were admitted to the PICUs at these centers between January 1, 2015, and January 31, 2016, and who received 50% or more of their calculated total maintenance fluid requirements parenterally during the first 24 hours of admission. We excluded patients with a known preexisting condition associated with hyperchloremia and/or HCMA (i.e., renal tubular acidosis and gastrointestinal bicarbonate losses). Data were abstracted from electronic patient medical records from both study sites for the first 72 hours of PICU admission or until 2 days after the resolution of HCMA. The data collected included baseline demographic information (age, gender, weight, reason for admission); severity of illness as indicated by the Pediatric Risk of Mortality III (PRISM III) and Pediatric Logistic Organ Dysfunction-2 (PELOD-2) scores 12 13 ; fluid sources, type, and volume during the first 72 hours of PICU admission; daily biochemical (electrolytes, acid-base variables) and hematological values; and clinical outcomes (occurrence of potential HCMA-associated morbidities, as well as mortality and length of hospital/PICU stay).
Outcomes Measures
The primary outcome was IV fluid prescription patterns during the first 72 hours of PICU admission. Solutions were categorized as isotonic, near isotonic, and hypotonic according to their concentration of sodium relative to that of human serum ( Table 1 ). Balanced crystalloids contain chloride concentrations close to that of plasma (i.e., lactated Rings and Plasma-Lyte), whereas unbalanced crystalloids contain supraphysiological chloride content (i.e., 0.9% NaCl). Secondary outcomes of interest were the incidence and predictors of iatrogenic hyperchloremia and HCMA, and the association between HCMA and the following reported adverse events: acute kidney injury, gastrointestinal dysfunction, new-onset coagulopathy, PICU-acquired muscle weakness occurring at any time in a patient with concurrent HCMA up until 2 days following the resolution of HCMA, and/or PICU mortality. These adverse events were chosen as they are reported in the literature to be associated with HCMA. 14 15 16 17 18 Hyperchloremia was defined as a serum chloride of >107 mmol/L. 19 20 HCMA was defined as hyperchloremia associated with a metabolic acidemia (serum pH of <7.35 and bicarbonate of <23 mmol/L and/or a base deficit of >5 mEq/L). 14 Acute kidney injury was defined by the Kidney Disease: Improving Global Outcomes (KDIGO) criteria for acute kidney injury. 21 Gastrointestinal dysfunction was defined as feeding intolerance requiring withholding of feeds at any time during the study period. Coagulopathy was identified using PELOD-2 cutoffs for this system and/or evidence of any major bleeding events. 13 PICU-acquired weakness was defined according to previously established clinical criteria. 22
Table 1. Intravenous fluid contents.
| IV fluid | Sodium (mEq/L) | Chloride (mEq/L) | Tonicity |
|---|---|---|---|
| 0.9% NaCl | 154 | 154 | Isotonic |
| Plasma-Lyte | 140 | 98 | Isotonic |
| Lactated Ringer's | 130 | 109 | Near isotonic |
| 0.45% NaCl | 77 | 77 | Hypotonic |
Abbreviation: IV, intravenous.
Sample Size
We powered this study to enable the evaluation of the predictors of HCMA, our secondary outcome. Based on existing evidence, we hypothesized the following six potential predictors for HCMA: 0.9% NaCl as the first maintenance fluid, increasing total volume intake of 0.9% NaCl, increasing severity of illness (as measured by PRISM III scores), age, surgical patients, and study site. 8 14 17 23 Using the basic rule of thumb for logistic regression models, we required at least 10 to 12 events (i.e., 60–70 patients with HCMA) per degree of freedom for stable models. 24 Based on the available evidence, we conservatively estimated an HCMA incidence in the range of 7 to 10% 25 and calculated that a sample size of 700 patients would provide us with an ability to evaluate the aforementioned six predictors in the planned multivariable analysis. Following a preliminary analysis that revealed that the actual event rate of HCMA was more than 30%, which was greater than originally anticipated, we revised our sample size to 500 patients.
Statistical Analysis
Descriptive statistics are used to present patient demographics and IV fluid prescription practice patterns, using counts and percentages for categorical variables, and medians and interquartile ranges (IQRs) for non-normal continuous variables. The incidences and corresponding 95% confidence intervals (CIs) are presented for hyperchloremia and HCMA. Unadjusted and adjusted logistic regression analyses were conducted to identify predictors of HCMA on the first day of PICU admission and presented as odds ratios (ORs) along with the 95% CI. Collinearity between PRISM III and total volume of isotonic fluids was assessed using the variance inflation factor (VIF). 26 Lastly, unadjusted logistic regression analyses were performed to explore the relationship between HCMA and the adverse events listed above. Statistical significance was determined by p < 0.05 for all tests.
Results
Patient Characteristics
We screened a total of 771 patients, of whom 543 patients were included in the analyses. Of the 228 excluded patients, 124 were receiving less than 50% of their maintenance fluid requirements parenterally, 13 had pre-existing conditions associated with hyperchloremia or HCMA, and 91 had missing medical record data required for data abstraction. Demographic and baseline characteristics of these patients are presented in Table 2 . The median (IQR) age of the population was 68 (17,160) months, and 307 (56.5%) were males.
Table 2. Demographics and clinical variables.
| Characteristics | Total, n = 543 | Site 1, n = 326 | Site 2, n = 217 |
|---|---|---|---|
| Age (mo); median (IQR) | 68 (17,160) | 83 (23,169) | 54 (14,144) |
| Male; n (%) | 307 (56.5) | 178 (54.6) | 129 (59.4) |
| Primary reason for admission, n (%) | |||
| Medical: 370 (68.6%) | |||
| Respiratory failure | 147 (27.2) | 94 (29.0) | 53 (24.4) |
| Neurologic | 60 (11.1) | 36 (11.1) | 24 (11.1) |
| Endocrine | 35 (6.5) | 25 (7.7) | 10 (4.6) |
| Sepsis | 32 (5.9) | 12 (3.7) | 20 (9.2) |
| Poisoning/overdose | 25 (4.6) | 12 (3.7) | 13 (6.0) |
| Other a | 71 (13.3) | 57 (17.6) | 14 (6.5) |
| Surgical | 141 (26.06) | 71 (21.9) | 70 (32.3) |
| Trauma | 32 (5.9) | 19 (5.9) | 13 (6.0) |
| Preexisting comorbidity, n (%) | 252 (46.6) | 126 (38.9) | 126 (58.1) |
| PRISM III score on admission, median (IQR) | 4 (1,8) | 4 (0,8) | 4 (2,8) |
| PELOD 2 score on the day of admission, median (IQR) | 8 (4,11) | 6 (4,9) | 9 (5,12) |
Abbreviations: PRISM III, Pediatric Risk of Mortality III, PELOD 2, Pediatric Logistic Organ Dysfunction 2, IQR, interquartile range.
Other includes cardiac, malignancy, gastrointestinal, metabolic, nephrological, and other.
Intravenous Fluid Prescription Practices
Maintenance IV fluid prescriptions over the first 72 hours of admission are displayed in Fig. 1 . The most commonly prescribed solution was 0.9% NaCl, comprising 72.9% (1,476/2,024) of all maintenance fluid prescriptions. Of the remaining prescriptions, 19.6% (396/2,024) was lactated Ringer's solution, 4.6% (92/2,024) was hypotonic solutions, 2.3% (46/2,024) was total parenteral nutrition, and 0.7% (14/2,024) was dextrose. Plasma-Lyte was not used as a maintenance fluid.
Fig. 1.
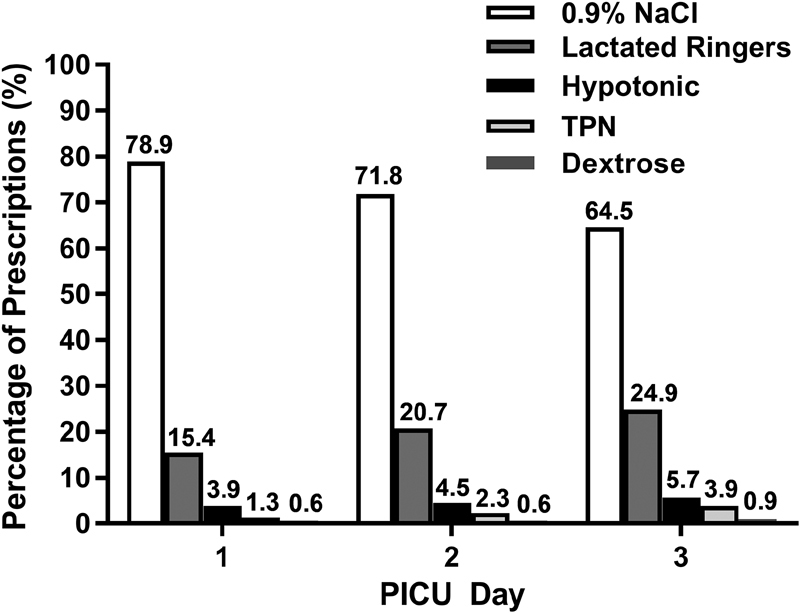
Maintenance intravenous fluid prescriptions over the first 72 hours of admission. PICU, pediatric intensive care unit.
There were a total of 363 bolus IV fluid prescriptions in 382 patients during the first 72 hours of PICU admission. Of these, 48.5% (176/363) were 0.9% NaCl, 14.1% (51/363) were lactated Ringer's, 35.5% (129/363) were Plasma-Lyte, 1.4% (5/363) were albumin, and 0.6% (2/363) were categorized as “other.”
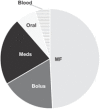
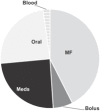
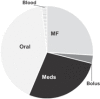
Sources of fluid intake are presented in Table 3 . Of the total fluid intake by volume on PICU day 1, 49.2% was from maintenance IV fluids. As expected, the proportion of total fluid intake from maintenance IV fluids falls with each PICU day as oral intake increases. The contribution of fluid from medications was greater than bolus fluid intake on each day and increased daily from 21.9% of the total intake on PICU day 1 to 27.4% by PICU day 3. Medication fluid volumes (5,965 mL) approximated that of maintenance IV fluids (5,668 mL) by PICU day 3.
Table 3. Total fluid intake by day and type of fluid.
| Fluid type | PICU day 1 | PICU day 2 | PICU day 3 | |||
|---|---|---|---|---|---|---|
| Total volume (mL) | Percentage of daily total (%) | Total volume (mL) | Percentage of daily total (%) | Total volume (mL) | Percentage of daily total (%) | |
| Maintenance fluids | 12,114 | 49.2 | 13804 | 42.6 | 5,668 | 26.0 |
| Bolus fluids | 4,299 | 17.5 | 2,060 | 6.4 | 648 | 2.9 |
| Medications | 5,403 | 21.9 | 7,963 | 24.6 | 5,965 | 27.4 |
| Oral fluids | 1,634 | 6.6 | 7,824.7 | 24.2 | 9,097 | 41.8 |
| Blood products | 1,180 | 4.8 | 744 | 2.3 | 400 | 1.8 |
| Total | 24,634 | 100 | 32,397 | 100 | 21,780 | 100 |
| Distribution of the total volume of IV and oral fluids given per day |
|
|
|
|||
Abbreviations: Blood, blood products; Bolus, bolus fluid; IV, intravenous; MF, maintenance fluid; Meds, medications; Oral, oral fluid; PICU, pediatric intensive care unit.
Hyperchloremia and Hyperchloremic Metabolic Acidemia
Chloride levels were available for 495 of the 543 included patients. The overall incidence of hyperchloremia and HCMA in the first 72 hours of PICU admission was 94.9% (95% CI: 93.2–96.9) and 38.9% (95% CI: 34.6–43.2), respectively. Of the 470 patients with hyperchloremia (defined as serum chloride > 107 mmol/L), 373 (79.4%) had chloride levels > 110 mmol/L. The median (IQR) chloride levels in the study cohort were 112 (109, 116) mmol/L on PICU day 1, 112 (108, 116) mmol/L on PICU day 2, and 109 (105, 113) mmol/L on PICU day 3. The prevalence of hyperchloremia and HCMA decreased daily during this period ( Fig. 2 ), as did the rate of new cases of hyperchloremia (81.8% on PICU day 1, 52% on PICU day 2, and 23.3% on PICU day 3) and HCMA (33.7% on PICU day 1, 7.6% on PICU day 2, 3.1% on PICU day 3).
Fig. 2.
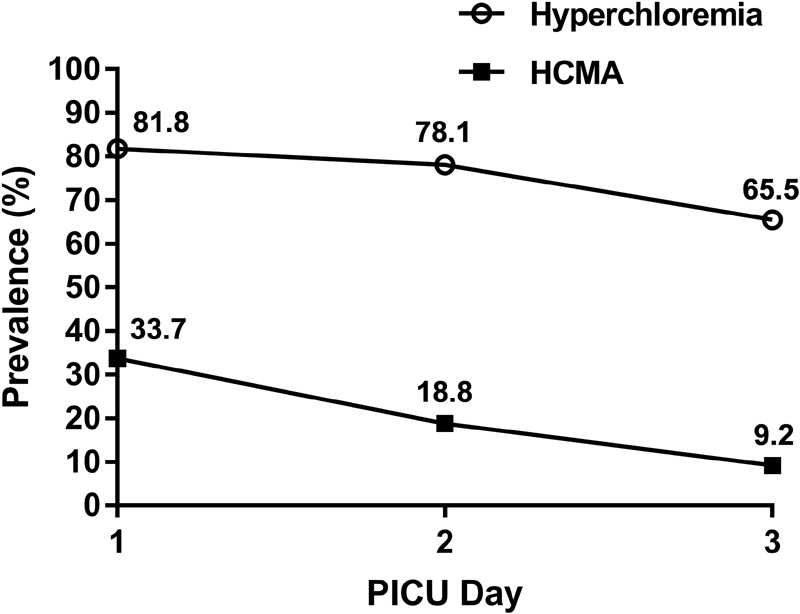
Prevalence of hyperchloremia and hyperchloremic metabolic acidemia over the first 72 hours of admission. HCMA, hyperchloremic metabolic acidemia; PICU, pediatric intensive care unit.
Predictors of Hyperchloremic Metabolic Acidemia
Multivariable logistic regression analysis demonstrated that independent predictors of developing HCMA were increasing total IV fluid intake (combined bolus and maintenance fluids) of 0.9% NaCl (OR: 1.13; 95% CI: 1.04–1.23) and increasing severity of illness ( Table 4 ). Sensitivity analysis assessing increasing volumes of 0.9% NaCl intake as a maintenance fluid only revealed that this was not an independent risk factor for developing HCMA (OR 1.10; 95% CI 0.99–1.23, p = 0.07). We assessed collinearity between PRISM III scores and the total volume of 0.9% NaCl intake and found a variance inflation factor of 1, indicating very low collinearity between the two variables.
Table 4. Predictors of the development of hyperchloremic metabolic acidemia.
| Variable | Multivariable analysis | |
|---|---|---|
| OR (95% CI) | p -Value | |
| Increasing total volume of 0.9% NaCl (bolus and maintenance) a | 1.13 (1.04,1.23) | 0.004 |
| Maintenance fluid type b | ||
| Chloride rich (0.9% NaCl) | 0.87 (0.36, 2.08) | 0.76 |
| PRISM III score categories | ||
| 0 (0–5 points) | Reference | |
| 1 (6–10 points) | 1.48 (0.83, 2.66) | 0.19 |
| 2 (11–15 points) | 6.43 (2.83, 14.65) | <0.001 |
| 3 (16–20 points) | 3.36 (1.02, 11.02) | 0.05 |
| 4 (>20 points) | 13.19 (4.01, 43.35) | <0.001 |
| Study site c | ||
| Site 1 | 0.60 (0.35, 1.01) | 0.05 |
| Surgical patients d | 1.22 (0.71, 2.09) | 0.47 |
| Age e | ||
| Neonate (0 to <1 mo) | 1.58 (0.18, 14.28) | 0.68 |
| Infant (1 mo to 1 y) | 1.15 (0.54, 2.44) | 0.72 |
| Child (1–12 y) | 0.65 (0.36, 1.18) | 0.16 |
Abbreviations: CI, confidence interval, PRISM III, Pediatric Risk of Mortality III; OR, odds ratio.
Per increments of 10 mL/kg.
Maintenance fluid type received on pediatric intensive care unit day 1; reference is combined lower chloride containing solutions (lactated Ringer's and hypotonic solutions).
Reference is site 2.
Reference is medical patients.
Reference age group is adolescent (>12 years).
Association between HCMA and Clinical Outcomes
Using unadjusted logistic regression analyses, we found that HCMA was not associated with an increased risk of acute kidney injury (OR: 1.35; 95% CI: 0.92–1.97), gastrointestinal dysfunction (OR: 1.00; 95% CI: 0.52–1.94), or PICU-acquired muscle weakness (OR: 0.57; 95% CI 0.15–2.14). Bleeding events ( n = 2) and 30-day mortality ( n = 4) could not be assessed because the event rates were too low.
Discussion
While the debate of the previous decades focused on hypotonic versus isotonic maintenance fluids, 3 4 the current debate centers around balanced versus unbalanced salt solutions fueled by emerging evidence suggesting an association between hyperchloremia and adverse patient outcomes. 14 15 16 17 18 It is currently unclear whether the incidence of hyperchloremia and HCMA in critically ill children is rising over time, as previous studies focused primarily on sodium concentration and fluid tonicity. 2 3 4 5 The primary source of hyperchloremia in critically ill children therefore has not been well established. Previous studies have implicated the use of large volumes of unbalanced, chloride-rich solutions (i.e., 0.9% NaCl) administered for the purposes of volume expansion. 9 11 We hypothesized that there are additional sources beyond bolus fluids that can contribute to chloride loading in children, and therefore we sought to evaluate the current fluid prescription practice patterns within two Canadian PICUs. We also sought to evaluate the incidence and predictors of HCMA, as well as any potential adverse outcomes associated with HCMA. The results of this retrospective cohort study demonstrate the following key findings: (1) the preferred maintenance fluid is an isotonic and unbalanced crystalloid (0.9% NaCl), and hypotonic solution use is now rare; (2) fluid from medications contribute to a previously unrecognized significant volume load; (3) HCMA is common and occurs early in critically ill children; (4) the key risk factors for the development of HCMA are increasing 0.9% NaCl intake from combined volumes of bolus and maintenance intake, and increasing severity of illness; and (5) we did not find any association between HCMA and adverse clinical outcomes.
Despite more than 15 years of accumulating clinical trial evidence, systematic reviews, and patient safety alerts warning of the harms of hypotonic solutions, 2 3 4 practice has been slow to change among pediatricians and clinicians who perhaps do not routinely care for children. 7 The results of this study are consistent with more recent observational studies, indicating that isotonic solutions are the preferred maintenance solution, specifically in critically ill children. 4 While an unbalanced crystalloid (0.9% NaCl) was the most commonly used maintenance fluid in our cohort, balanced crystalloids (i.e., lactated Ringer's) were increasingly used over time, likely in response to developing hyperchloremia and HCMA. Although 0.9% NaCl was the preferred maintenance IV fluid, balanced crystalloids (i.e., lactated Ringer's and Plasma-Lyte) were as commonly used for volume expansion as 0.9% NaCl (49.6% vs. 48.5%, respectively).
We hypothesized that the volume of maintenance fluid is the primary contributor to total fluid volume intake and hence a potentially greater source of chloride compared with bolus fluids. We found that an increasing intake of 0.9% NaCl from combined volumes of bolus and maintenance fluids was an independent risk factor for the development of HCMA. 0.9% NaCl from maintenance fluids alone did not increase the risk of HCMA, as this accounted for only half of the total fluid intake on PICU day 1 in this cohort and was reduced daily to 26% by PICU day 3. As there was low collinearity between the severity of illness scores and the total intake of 0.9% NaCl, we hypothesize that the risk of HCMA predicted by increasing PRISM III score categories is explained by renal handling of the chloride load. Our study also revealed that a significant source of fluid (and hence exogenous electrolytes) comes from medications (i.e., IV medication infusions, flushes, and concentrated electrolyte administration). Medication volumes contributed approximately 25% of total daily volume intake, exceeding bolus volumes during the study period and exceeding maintenance volumes by PICU day 3. As the standard solution for medication diluent is 0.9% NaCl, this represents an underrecognized source of chloride. Fluids as drug vehicles and flushes have been coined “fluid creep.” 27 Fluid creep has previously been observed in critically ill infants and children 4 ; however, little emphasis has been placed on the significance of its contribution given the current research focus on restrictive resuscitation and maintenance fluid strategies. 28 29
The majority of patients in this study developed hyperchloremia within the first 72 hours of PICU admission. Hyperchloremia and HCMA have not been well studied in the general PICU population. Previous studies focused on evaluating hyperchloremia in specific subsets of critically ill children, 10 30 31 reporting a 58% incidence in children with septic shock and 59% of children requiring continuous renal replacement therapy. 10 30 Hyperchloremia has been reported to affect between 17 and 58% of critically ill adults. 8 32 Our estimates of hyperchloremia are higher compared with that of previous studies, which we attribute to our a priori defined cutoff of 107 mmol/L. Given the lack of a standardized definition, we based our definition of hyperchloremia on established pediatric reference ranges, 19 20 whereas the previous pediatric studies defined hyperchloremia as a serum chloride of ≥110 mmol/L. Nevertheless, we did find a high incidence (75.4%) of hyperchloremia using the 110 mmol/L cutoff value in our study cohort. Combining the presence of acidemia and hyperchloremia, the incidence of HCMA of 38.9% observed in our study population is similar to that in the study by Abbas et al, who reported an incidence of 31.1% in their cohort. 31
The mechanism of end-organ dysfunction from hyperchloremia is proposed to be mediated through organ-specific vasoconstriction, in particular the renal and splanchnic vasculature 23 33 34 As metabolic acidosis is associated with depressed function in multiple organs and increased pulmonary vascular resistance, it is unclear whether the increased morbidity observed in previous studies is a direct result of HCMA or due to metabolic acidosis per se. 23 34 While previous pediatric studies evaluated the effects of hyperchloremia, we specifically sought to determine if HCMA was associated with increased morbidity and mortality. The adverse event rates observed in our study were low, and hence we were unable to draw any conclusions on the effect of HCMA on these outcomes. While there is evidence in adults from a large, pragmatic, randomized trial suggesting that balanced crystalloids reduce the risk of major adverse kidney events through the postulated mechanism of a reduced chloride load, 35 the evidence from pediatrics to date is currently derived from observational studies and are therefore not conclusive. Studies by Stenson et al 10 and Barhight et al 30 reported worse patient outcomes in hyperchloremic patients with sepsis and acute kidney injury, respectively; however, Abbas et al 31 did not find an increase in morbidity in a general medical-surgical PICU population and in fact found a decrease in mortality in the hyperchloremia group. Indirect evidence from a Pediatric Health Information System based study by Emrath et al 9 suggests improved survival and reduced acute kidney injury in children with sepsis resuscitated with balanced fluids.
This is the first to study to our knowledge to evaluate the incidence, risk factors, and outcomes associated with HCMA specifically in the general medical-surgical PICU population. Limitations of this study include the risk of unaccounted confounding associated with the retrospective observational design. Our definition of hyperchloremia (serum Cl > 107 mmol/L), while the same as that reported in many similar studies, 36 is lower than the 110 mmol/L cutoff of more recent ICU-based studies and accounts, at least in part, for the high incidence of hyperchloremia we observed. While we powered our study to evaluate predictors of HCMA, we acknowledge the potential for imprecision given the relatively small number of patients, particularly with respect to adverse outcomes. Finally, our findings are limited to two Canadian centers and hence may not be generalizable. We are aware that there are regional differences to the availability of balanced salt solutions, and this may be the primary influence on prescription practices. 37
Conclusions
In this Canadian study of critically ill children, an unbalanced salt solution (i.e., 0.9% NaCl) was the most commonly used maintenance IV fluid, whereas balanced salt solutions were as commonly administered as unbalanced solutions for volume expansion. Sources of chloride load are not isolated to resuscitation fluids in critically ill children as previously suggested; maintenance fluids and fluid creep are underrecognized and substantial sources of fluid intake. HCMA is common, and further prospective research is required to determine whether HCMA is indeed harmful in children. However, all significant sources of fluid should be accounted for in the design of future trials comparing balanced and unbalanced salt solutions.
Acknowledgments
We would like to thank Ji Cheng, Puru Panchal, Sam Laskey, and Katina Zheng for their help with data collection and statistical services.
Funding Statement
Funding This study was conducted without any funding.
Conflict of Interest None declared.
Note
The study was conducted at McMaster Children's Hospital and Stollery Children's Hospital.
References
- 1.Holliday M A, Segar W E. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(05):823–832. [PubMed] [Google Scholar]
- 2.Choong K, Arora S, Cheng J et al. Hypotonic versus isotonic maintenance fluids after surgery for children: a randomized controlled trial. Pediatrics. 2011;128(05):857–866. doi: 10.1542/peds.2011-0415. [DOI] [PubMed] [Google Scholar]
- 3.Choong K, Kho M E, Menon K, Bohn D. Hypotonic versus isotonic saline in hospitalised children: a systematic review. Arch Dis Child. 2006;91(10):828–835. doi: 10.1136/adc.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster B A, Tom D, Hill V. Hypotonic versus isotonic fluids in hospitalized children: a systematic review and meta-analysis. J Pediatr. 2014;165(01):163–16900. doi: 10.1016/j.jpeds.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 5.McNab S, Ware R S, Neville K A et al. Isotonic versus hypotonic solutions for maintenance intravenous fluid administration in children. Cochrane Database Syst Rev. 2014;12(12):CD009457. doi: 10.1002/14651858.CD009457.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bihari S, Gelbart B, Seppelt I et al. Maintenance fluid practices in paediatric intensive care units in Australia and New Zealand. Crit Care Resusc. 2017;19(04):310–317. [PubMed] [Google Scholar]
- 7.Lee J M, Jung Y, Lee S E et al. Intravenous fluid prescription practices among pediatric residents in Korea. Korean J Pediatr. 2013;56(07):282–285. doi: 10.3345/kjp.2013.56.7.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neyra J A, Canepa-Escaro F, Li X et al. Association of hyperchloremia with hospital mortality in critically ill septic patients. Crit Care Med. 2015;43(09):1938–1944. doi: 10.1097/CCM.0000000000001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emrath E T, Fortenberry J D, Travers C, McCracken C E, Hebbar K B. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(07):1177–1183. doi: 10.1097/CCM.0000000000002365. [DOI] [PubMed] [Google Scholar]
- 10.Stenson E K, Cvijanovich N Z, Anas N et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(02):155–160. doi: 10.1097/PCC.0000000000001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sen A, Keener C M, Sileanu F E et al. Chloride content of fluids used for large-volume resuscitation is associated with reduced survival. Crit Care Med. 2017;45(02):e146–e153. doi: 10.1097/CCM.0000000000002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollack M M, Patel K M, Ruttimann U E. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(05):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F; Groupe Francophone de Réanimation et d'Urgences Pédiatriques (GFRUP).PELOD-2: an update of the PEdiatric logistic organ dysfunction score Crit Care Med 201341071761–1773. [DOI] [PubMed] [Google Scholar]
- 14.Toyonaga Y, Kikura M. Hyperchloremic acidosis is associated with acute kidney injury after abdominal surgery. Nephrology (Carlton) 2017;22(09):720–727. doi: 10.1111/nep.12840. [DOI] [PubMed] [Google Scholar]
- 15.Suetrong B, Pisitsak C, Boyd J H, Russell J A, Walley K R. Hyperchloremia and moderate increase in serum chloride are associated with acute kidney injury in severe sepsis and septic shock patients. Crit Care. 2016;20(01):315. doi: 10.1186/s13054-016-1499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhury A H, Cox E F, Francis S T, Lobo D N. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and Plasma-Lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256(01):18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 17.Waters J H, Gottlieb A, Schoenwald P, Popovich M J, Sprung J, Nelson D R. Normal saline versus lactated Ringer's solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;93(04):817–822. doi: 10.1097/00000539-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Wilkes N J, Woolf R, Mutch M et al. The effects of balanced versus saline-based hetastarch and crystalloid solutions on acid-base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg. 2001;93(04):811–816. doi: 10.1097/00000539-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Gregory G A, Andropoulus D B. San Francisco, CA: Blackwell Publishing Ltd.; 2012. Gregory's Pediatric Anesthesia. 5th ed. [Google Scholar]
- 20.DuBose T D., Jr . Philadelphia, PA: Saunders Elsevier; 2008. Disorders of acid-base balance; pp. 505–546. [Google Scholar]
- 21.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(04):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 22.Choong K, Fraser D, Al-Harbi S et al. Functional recovery in critically ill children, the “WeeCover” multicenter study. Pediatr Crit Care Med. 2018;19(02):145–154. doi: 10.1097/PCC.0000000000001421. [DOI] [PubMed] [Google Scholar]
- 23.Burdett E, Roche A, Mythen M. Hyperchloremic acidosis: pathophysiology and clinical impact. Transfus Altern Transfus Med. 2003;5(04):424–430. [Google Scholar]
- 24.Peduzzi P, Concato J, Kemper E, Holford T R, Feinstein A R. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 25.Durward A, Tibby S M, Skellett S, Austin C, Anderson D, Murdoch I A. The strong ion gap predicts mortality in children following cardiopulmonary bypass surgery. Pediatr Crit Care Med. 2005;6(03):281–285. doi: 10.1097/01.PCC.0000163979.33774.89. [DOI] [PubMed] [Google Scholar]
- 26.Midi H, Sarkar S K, Rana S. Collinearity diagnostics of binary logistic regression model. J Interdiscip Math. 2010;13(03):253–267. [Google Scholar]
- 27.Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens P G. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018;44(04):409–417. doi: 10.1007/s00134-018-5147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maitland K, Kiguli S, Opoka R O et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 29.Díaz F, Nuñez M J, Pino P, Erranz B, Cruces P. Implementation of preemptive fluid strategy as a bundle to prevent fluid overload in children with acute respiratory distress syndrome and sepsis. BMC Pediatr. 2018;18(01):207. doi: 10.1186/s12887-018-1188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barhight M F, Lusk J, Brinton J et al. Hyperchloremia is independently associated with mortality in critically ill children who ultimately require continuous renal replacement therapy. Pediatr Nephrol. 2018;33(06):1079–1085. doi: 10.1007/s00467-018-3898-2. [DOI] [PubMed] [Google Scholar]
- 31.Abbas Q, Ul Ain N, Ehsan L et al. Hyperchloremia and its association with outcomes in critically ill children. SM Emerg Med Crit Care. 2017;1(04):1018. [Google Scholar]
- 32.Boniatti M M, Cardoso P RC, Castilho R K, Vieira S R. Is hyperchloremia associated with mortality in critically ill patients? A prospective cohort study. J Crit Care. 2011;26(02):175–179. doi: 10.1016/j.jcrc.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Wilcox C S. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71(03):726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stengl M, Ledvinova L, Chvojka J et al. Effects of clinically relevant acute hypercapnic and metabolic acidosis on the cardiovascular system: an experimental porcine study. Crit Care. 2013;17(06):R303. doi: 10.1186/cc13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semler M W, Self W H, Wanderer J P et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(09):829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tani M, Morimatsu H, Takatsu F, Morita K. The incidence and prognostic value of hypochloremia in critically ill patients. Scientific World Journal. 2012;2012:474185. doi: 10.1100/2012/474185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNab S, Duke T, South Met al. 140 mmol/L of sodium versus 77 mmol/L of sodium in maintenance intravenous fluid therapy for children in hospital (PIMS): a randomised controlled double-blind trial Lancet 2015385(9974):1190–1197. [DOI] [PubMed] [Google Scholar]


