ABSTRACT
Background
Depressive symptoms and impaired physical functioning are prevalent among older adults. Supplementation with vitamin D might improve both conditions, particularly in persons with low vitamin D status.
Objective
The D-Vitaal study primarily aimed to investigate the effect of vitamin D supplementation on depressive symptoms, functional limitations, and physical performance in a high-risk older population with low vitamin D status. Secondary aims included examining the effect of vitamin D supplementation on anxiety symptoms, cognitive functioning, mobility, handgrip strength, and health-related quality of life.
Methods
This study was a randomized placebo-controlled trial with 155 participants aged 60–80 y who had clinically relevant depressive symptoms, ≥1 functional limitations, and serum 25-hydroxyvitamin D [25(OH)D] concentrations of 15–50/70 nmol/L (depending on season). Participants received 1200 IU/d vitamin D3 (n = 77) or placebo tablets (n = 78) for 12 mo. Serum 25(OH)D was measured at baseline and 6 mo; outcomes were assessed at baseline, 6 mo, and 12 mo. Linear mixed-models analyses were conducted to assess the effect of the intervention.
Results
The supplementation increased serum 25(OH)D concentrations in the intervention group to a mean ± SD of 85 ± 16 nmol/L compared with 43 ± 18 nmol/L in the placebo group after 6 mo (P < 0.001). No relevant differences between the treatment groups were observed regarding depressive symptoms, functional limitations, physical performance, or any of the secondary outcomes.
Conclusions
Supplementation with 1200 IU/d vitamin D for 12 mo had no effect on depressive symptoms and physical functioning in older persons with relatively low vitamin D status, clinically relevant depressive symptoms, and poor physical functioning. This trial is registered with the Netherlands Trial Register (www.trialregister.nl) under NTR3845.
Keywords: vitamin D, 25(OH)D, depressive symptoms, physical functioning, functional limitations, physical performance, older adults, randomized clinical trial, prevention, supplementation
Introduction
Depressive symptoms and poor physical functioning are 2 common and burdensome health conditions among older persons (1–3). Simple and safe prevention strategies for these conditions are lacking. In addition, the prevalence of inadequate vitamin D status (serum 25-hydroxyvitamin D [25(OH)D] concentrations <50 nmol/L) (4) is high in this population (5, 6).
Previous research has suggested that low vitamin D status may be associated with depressive symptoms as well as decreased physical health, but the many studies in this field have often yielded conflicting results. Although prospective cohort studies suggest that lower vitamin D status is associated with more depressive symptoms (7), functional limitations (8), and poorer physical performance (9), evidence from randomized controlled trials (RCTs) remains inconclusive regarding causality (10).
Biological evidence for the association of vitamin D with depressive symptoms and poor physical functioning is given by the presence of the activating enzyme 1α-hydroxylase (CYP27B1)—which converts 25(OH)D into 1,25-dihydroxyvitamin D [1,25(OH)2D]—and the vitamin D receptor (VDR) in brain areas, such as the hippocampus, hypothalamus, and cerebellum (11, 12), and the presence of 1,25(OH)2D and the VDR in muscle cells (13). Furthermore, 1,25(OH)2D facilitates the production of serotonin in the brain and has a general protective and stimulating effect on brain and muscle tissue (12–15), whereas severe vitamin D deficiency causes myopathy (16). Additional evidence comes from studies with VDR knockout mice: these rodents showed abnormal muscle development, decreased muscle size and mass, altered grooming behavior, and increased anxiety (16).
A review of 37 previous RCTs examining the effects of vitamin D supplementation on depressive symptoms or physical functioning concluded that the study designs were too heterogeneous to draw conclusions (10). Supplementation dose, study duration, and participant inclusion criteria differed substantially between trials. Moreover, most of the previously conducted RCTs used a general population sample, whereas it can be expected that vitamin D supplementation is more effective and appropriate for persons with insufficient 25(OH)D concentrations and actual emotional and/or physical complaints. Furthermore, depressive symptoms and physical problems are highly interrelated and can reinforce each other (2, 3), which argues for a combined approach. For these reasons, the present RCT was designed.
The D-Vitaal trial investigated whether vitamin D supplementation could improve depressive symptoms, functional limitations, and physical performance in a high-risk older population with relatively low vitamin D status, clinically relevant depressive symptoms, and difficulties with physical functioning.
Methods
Study design
The D-Vitaal study is a randomized, double-blind, placebo-controlled trial designed to investigate the effects of vitamin D supplementation on depressive symptoms and physical functioning in older adults. The design and methods have been described elsewhere (10). The Medical Ethics Committee of the VU University Medical Center approved the study, and all participants provided written informed consent. The study is registered with the Netherlands Trial Register as NTR3845.
Setting and participants
The study was performed in Amsterdam and surroundings in the Netherlands from 2013 to 2016. Participants (n = 155) were community-dwelling persons aged 60–80 y recruited from the general population (81%) or through general practitioners (19%). Inclusion criteria were presence of depressive symptoms [as indicated by a Center of Epidemiological Studies–Depression scale (CES-D) score of ≥16 (17)], ≥1 functional limitation (e.g., difficulties with walking, climbing stairs, or dressing oneself), and a serum 25(OH)D concentration between 15 and 50 nmol/L in winter (October–March) or between 15 and 70 nmol/L in summer (April–September). These 25(OH)D cutoffs were based on the study by van Schoor et al. (18) that showed that the mean seasonal variation (winter–summer difference) of serum 25(OH)D in this age group is ∼20 nmol/L. This implies that persons with serum 25(OH)D concentrations of <70 nmol/L in summer will have inadequate vitamin D status (<50 nmol/L) in winter. Persons with a current major depressive disorder (MDD) diagnosis or life-threatening illness as well as persons currently using antidepressant medication, vitamin D supplements of >400 IU/d, or calcium supplements of >1000 mg/d were excluded.
Randomization and blinding
All participants who fulfilled the inclusion criteria were randomly allocated by an independent pharmacist in a 1:1 ratio in blocks of 4 to receive either vitamin D or placebo.
Participants were stratified by sex, and women were further stratified by age (60–70 y compared with 71–80 y), as we expected to include more women than men in the study (19–21). Participants, researchers, and research nurses were blinded to group allocation during the study. Group assignment was concealed until completion of the statistical analyses.
Intervention
The intervention consisted of a daily dose of 1200 IU vitamin D3 (3 tablets of 400 IU cholecalciferol; Devaron) for 12 mo. The placebo group received identical tablets without vitamin D. All participants were allowed to take a (multi)vitamin D supplement with a maximum of 400 IU/d in addition to the study tablets. Furthermore, all participants were advised to use ≥3 dairy consumptions daily to ensure adequate calcium intake of ∼1000 mg/d. In case of <2 dairy consumptions per day, a calcium tablet of 500 mg/d was prescribed for the duration of the study.
Outcomes and follow-up
Primary outcomes
Primary outcomes of the D-Vitaal study were depressive symptoms, functional limitations, and physical performance.
Depressive symptoms were assessed using the CES-D scale (17). This scale consists of 20 items and ranges from 0 to 60, with a higher score indicating more depressive symptoms. A score of ≥16 is indicative of clinically relevant depressive symptoms. This outcome was defined as the difference in the 12-mo course of the depressive symptoms score between the 2 treatment groups.
Functional limitations were assessed using the Longitudinal Aging Study Amsterdam (LASA) Functional Limitations questionnaire (22). The participants were asked about their ability and degree of difficulty to perform the following functions of daily life: climbing stairs, cutting toenails, walking 5 min outdoors without resting, rising from a chair, dressing/undressing oneself, and using own or public transport. Two scores can be derived from this questionnaire: the number of functional limitations (score 0–6) and the severity of functional limitations (score 0–24). A higher score represents more functional limitations or more severe functional limitations, respectively. These outcomes were defined as the difference in the 12-mo course of the functional limitation scores between the 2 treatment groups.
Physical performance was assessed using a modified version of the Short Physical Performance Battery (SPPB) (9, 23). The SPPB includes a walking test, a repeated chair stand test, and a balance test. Participants could score 0–4 points on each test. Total scores range from 0 to 12, with higher scores representing better performance. This outcome was defined as the difference in the 12-mo course of the physical performance score between the 2 treatment groups.
Secondary outcomes
Secondary outcomes included incidence of MDD, anxiety symptoms, cognitive function, health-related quality of life (HR-QoL), functional mobility, and muscle strength. In addition, we analyzed depressive symptoms and the number of functional limitations dichotomously.
The presence of MDD was assessed using the depression section of the Composite International Diagnostic Interview [version 2.1 (24)], which was administered only for CES-D scores of ≥16. Anxiety was assessed using the Beck Anxiety Inventory (BAI) (25). Cognitive function (i.e., information processing speed and executive functioning) was assessed using the Stroop Color-Word Test (26). HR-QoL was assessed using the EuroQol-5 Dimensions (EQ-5D) (27) and the Short Form–36 Health Survey (SF-36) (28). The timed up-and-go (TUG) test (29) was assessed to test functional mobility. Muscle strength was measured with a handgrip strength test using a strain-gauged dynamometer (Takei TKK 5401; Takei Scientific Instruments). Finally, in addition to the continuous primary analyses, depressive symptoms and number of functional limitations were analyzed dichotomously, with a CES-D cutoff of 16 (presence compared with absence of clinically relevant depressive symptoms) and a functional limitations cutoff of 1 (0 compared with ≥1 functional limitation).
Serum 25(OH)D measurements
Serum 25(OH)D concentrations were measured at screening (baseline sample) and after 6 mo. Blood samples were drawn in the morning by a trained research nurse. Measurements were carried out using a well-standardized liquid chromatography followed by tandem mass spectrometry method (30, 31). Serum 25(OH)D concentrations at baseline were determined immediately after blood draw, whereas the 6-mo samples were measured all at once at the end of the study to ensure blinding. The 6-mo samples were stored at −80°C, with storage times until determination ranging from 6 to 27 mo.
Compliance
Compliance was assessed by tablet count after 6 and 12 mo. A participant was considered compliant if ≥80% of the tablets had been taken during the 12 mo of follow-up. In addition, compliance of the participants in the intervention group was indicated by their serum 25(OH)D concentrations after 6 mo of supplementation. If these concentrations had increased by <10 nmol/L compared with baseline, and their 25(OH)D concentration at 6 mo was <75 nmol/L, participants were also considered noncompliant. During the study, compliance was stimulated by contacting the participants at 2 wk, 3 mo, and 9 mo by telephone and by reminding them during follow-up visits.
Adverse events
Adverse events (AEs) were registered by telephone or face-to-face contact after 2 wk and after 3, 6, 9, and 12 mo. If necessary, the course of the AE was followed up by telephone.
Baseline characteristics and covariables
Baseline characteristics included age, sex, season, marital status, educational level, smoking (yes/no), alcohol use [categories according to the Garretsen index (32)], BMI, waist and calf circumference, blood pressure and pulse rate, physical activity, chronic diseases, medication and supplement use, number of previous depressive episodes, use of psychological counseling, and predictors of vitamin D (sunlight exposure, skin pigmentation, fatty fish consumption).
Statistical analyses
Baseline characteristics were compared between the treatment groups with Pearson χ2 tests (dichotomous or categorical variables) or Mann-Whitney tests (skewed continuous variables). Participants who dropped out were compared with participants who completed the study with respect to age, sex, serum 25(OH)D, and the primary outcome variables. These differences were tested with Mann-Whitney or Pearson χ2 tests.
Differences between treatment groups in the total number of AEs were tested using a Mann-Whitney test, whereas group differences with regard to categories of AEs were tested with Pearson χ2 tests.
Alcohol consumption was dichotomized into no/mild/moderate compared with (very) excessive alcohol use because the treatment groups differed in the latter category.
To assess the effects of the intervention, linear mixed-model analyses were conducted with the continuous primary and secondary outcome scores at 6 and 12 mo as a longitudinal outcome variable, treatment group as a fixed independent variable, and the baseline value of the outcome as a fixed covariate (model 1). In an additional model (model 2), we adjusted for any potential confounding variable that differed (P < 0.10) between treatment groups at baseline. We used an unstructured covariance structure and added a random intercept to the models to adjust for the dependency of the measures at 6 and 12 mo.
If the distribution of an outcome variable was skewed, a natural logarithmic (ln) transformation was performed. If this improved the distribution, analyses were conducted with the transformed variable. To interpret the results of the analyses with logarithmically transformed outcome variables, the resulting B values and CIs were transformed back. This back-transformation changes the B value into a ratio, here representing the difference between the intervention and placebo groups. For example, a ratio of 1.05 should be interpreted as a 5% higher outcome score in the intervention group compared with the placebo group.
Potential effect modification of age (continuous), sex, and baseline serum 25(OH)D concentrations (dichotomous, cutoffs of 50 and 30 nmol/L) was examined in the crude models by adding an interaction term (treatment × potential effect modifier). A time interaction term (treatment group × time point) was added to the crude models to investigate potential differences in effects between 6 and 12 mo of follow-up. If an interaction term had a P value of <0.10, stratified analyses were conducted. As the study was not powered for stratified analyses, we emphasize that these analyses are mainly exploratory.
Secondary dichotomous effect analyses for the CES-D score and number of functional limitations were conducted with general estimating equation analyses with an exchangeable correlation structure. Models and effect modification methods were similar to the continuous linear mixed-model analyses.
In preplanned sensitivity analyses, we examined whether change in serum 25(OH)D was associated with parallel change in the primary outcomes, irrespective of treatment group. For these analyses, change scores were created for 25(OH)D and the primary outcomes by calculating the difference between the values at baseline and 6 mo. Subsequently, multiple linear regression analyses were conducted with the change scores of the primary outcomes as dependent variables, change in serum 25(OH)D as the independent variable, and the baseline values of the independent and outcome variables as covariates. In a second model, we also adjusted for age, sex, season, marital status (CES-D analyses only), education level, alcohol use, smoking status, physical activity, and number of chronic diseases.
The intention-to-treat (ITT) analyses included all participants with ≥1 follow-up measurement. In the per-protocol effect analyses, we excluded participants who were not compliant according to the tablet count or otherwise not compliant with the study protocol. All results are ITT results unless otherwise stated. A double-sided P value of <0.05 was regarded as statistically significant. IBM SPSS Statistics version 22 (SPSS, Inc.) was used to perform all data analyses.
Power calculation
The statistical power analysis has been described in detail elsewhere (10). In short, the power calculation was based on the primary outcomes of depressive symptoms, functional limitations, and physical performance. To calculate the number of participants needed, a power of 80%, a 2-sided α of 0.05, and an intraclass correlation coefficient of 0.70 between baseline and follow-up measures were assumed. Based on a study with similar sample characteristics (33), ≥40 participants per group would be needed to detect a change of 0.5 SD (2.5 points) on the CES-D. For the number of functional limitations, we would need ≥28 participants per group to detect a meaningful change of 1 point, assuming a SD of 1.7. Regarding the severity of functional limitations, ≥48 participants per group would be needed to detect a change of 2 points, assuming a SD of 4.5. For physical performance, 22 persons per group would be needed to detect a meaningful change of 1 point on the SPPB, assuming a SD of 1.5. Taking into account an expected dropout of ∼25% and uncertainty of the 25(OH)D assay, ≥70 persons per group would be needed, adding up to ≥140 participants.
Results
Participant characteristics
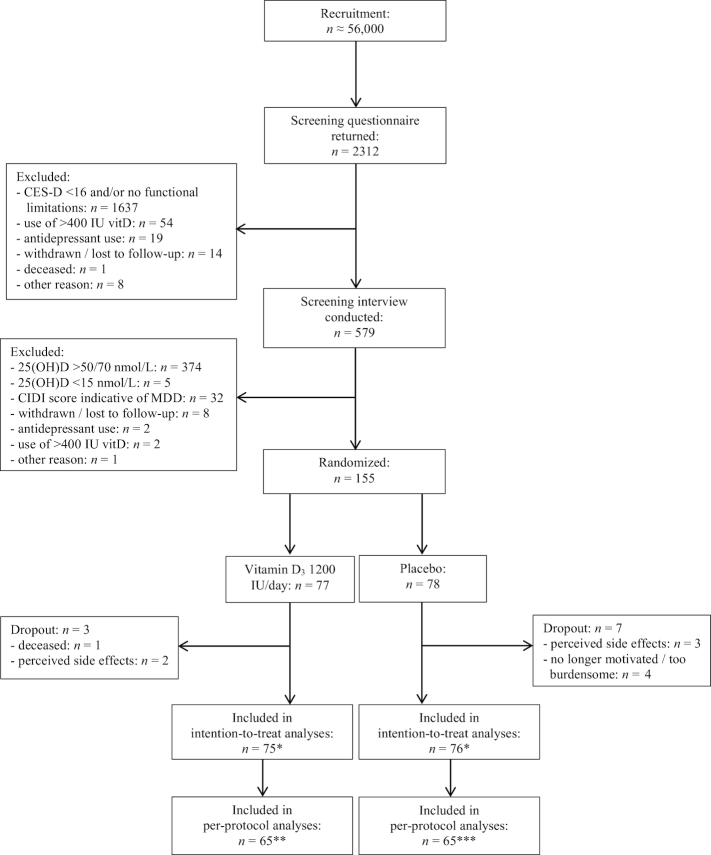
Figure 1 displays the participant flow of the D-Vitaal study. We included 155 participants: 77 in the intervention group and 78 in the placebo group.
FIGURE 1.
Recruitment, selection, randomization, and follow-up of participants in the D-Vitaal study. *n = 2 excluded from the intention-to-treat analyses due to insufficient data. **In the CES-D, BAI, SF-36 MCS, and EQ-5D analyses, an additional n = 2 were excluded completely, and the 12-mo measurements were excluded for another n = 2. ***In the CES-D, BAI, SF-36 MCS, and EQ-5D analyses, an additional n = 1 was excluded completely, and the 12-mo measurements were excluded for another n = 3 in all per-protocol analyses. BAI, Beck Anxiety Inventory; CES-D, Center of Epidemiological Studies–Depression scale; CIDI, Composite International Diagnostic Interview; EQ-5D, EuroQol-5 Dimensions; MDD, major depressive disorder; SF-36 MCS, Short Form–36 Health Survey Mental Component Summary; vitD, vitamin D; 25(OH)D, 25-hydroxyvitamin D.
Baseline characteristics of the participants are presented in Table 1. The placebo group contained more heavy drinkers and current smokers than the intervention group. Participants in the intervention and placebo groups were not different regarding predictors of vitamin D status, use of vitamin D supplements, or other baseline characteristics (data partly shown in Table 1).
TABLE 1.
Baseline characteristics of the D-Vitaal participants1
| Characteristic | Intervention group (n = 77) | Placebo group (n = 78) |
|---|---|---|
| Serum 25(OH)D,2 nmol/L | 46 [32.5–57] | 44 [36–55.25] |
| Baseline 25(OH)D <50 nmol/L3 | 47 (61.0) | 52 (66.7) |
| Baseline 25(OH)D <30 nmol/L3 | 12 (15.6) | 13 (16.7) |
| Age2 | 67.8 [65.4–71.7] | 67.3 [63.4–72.0] |
| Women3 | 45 (58.4) | 44 (56.4) |
| Season of baseline blood collection3 | ||
| Winter | 33 (42.9) | 30 (38.5) |
| Summer | 44 (57.1) | 48 (61.5) |
| Marital status3 | ||
| Never married/divorced | 13 (16.9) | 20 (26.0) |
| Married/living together/registered partner | 49 (63.6) | 46 (59.7) |
| Widowed | 15 (19.5) | 11 (14.3) |
| Education level3,4 | ||
| Low | 22 (29.3) | 21 (26.9) |
| Intermediate | 36 (48.0) | 34 (43.6) |
| High | 17 (22.7) | 23 (29.5) |
| Alcohol consumption3, 5# | ||
| None | 17 (22.1) | 12 (15.4) |
| Light | 36 (46.8) | 33 (42.3) |
| Moderate | 21 (27.3) | 20 (25.6) |
| (Very) excessive | 3 (3.9) | 13 (16.7) |
| Smoking3* | ||
| No | 70 (90.9) | 59 (75.6) |
| Yes | 7 (9.1) | 19 (24.4) |
| BMI,2 kg/m2 | 27.1 [25.1–31.0] | 26.9 [23.9–30.5] |
| Physical activity,2 kcal/d | 509 [332–802] | 494 [271–791] |
| Number of chronic diseases2 | 2 [1–2] | 1 [1–2] |
| Number of medications2 | 4 [2–6] | 4 [2–6] |
| Former depression3 | 34 (44.7) | 34 (43.6) |
| Anxiety (BAI)2 | 10 [5–18] | 11 [6–18.75] |
| Stroop test (interference score)2 | 17.5 [12–23] | 18.5 [14–25.5] |
| Timed up-and-go test2 | 7 [6–8.75] | 7 [6–8] |
| Handgrip strength2 | 27.1 [21.8–38.1] | 26.1 [21.9–37.9] |
| SF-362 | ||
| Physical functioning subscale | 70 [50–85] | 70 [45–85] |
| Physical component summary score | 46.4 [40.8–52.1] | 46.0 [42.2–49.1] |
| Mental component summary score | 39.6 [33.3–43.8] | 37.3 [33.6–41.3] |
| EQ-5D2 | ||
| Index score | 0.72 [0.65–0.84] | 0.78 [0.69–0.84] |
| Visual analog scale | 70 [50–80] | 67 [50–75] |
| CES-D score2 | 22 [18–26.75] | 21 [19–27] |
| Functional limitations2 | ||
| Number | 2 [0.25–4] | 1.5 [0–3.25] |
| Severity | 2 [0.25–4.75] | 2 [0–5] |
| Physical performance (SPPB)2 | 8 [6–10] | 8.5 [6.75–11.0] |
Values are displayed as n (%) or as median [IQR]. #P < 0.10. *P < 0.05. BAI, Beck Anxiety Inventory; CES-D, Center for Epidemiological Studies–Depression scale; EQ-5D, EuroQoL-5 Dimensions; SF-36, Short Form–36 Health Survey; SPPB, Short Physical Performance Battery; 25(OH)D, 25-hydroxyvitamin D.
Differences between treatment groups tested with Mann-Whitney test.
Differences between treatment groups tested with Pearson χ2 test.
Education level categories: low (less than elementary, elementary, or lower vocational education), intermediate (general intermediate, intermediate vocational, or general secondary education), or high (higher vocational, college, or university education).
Dichotomous analysis of alcohol use [no/mild/moderate compared with (very) excessive drinking]: P < 0.01.
At baseline, 9 participants (5.8%) made use of any form of psychological counseling: 5 in the intervention group and 4 in the placebo group. Twenty-six participants (16.8%) started with any form of psychological counseling during the study: 12 in the intervention group and 14 in the placebo group (P for group difference = 0.69). Furthermore, 5 participants (3.2%, 3 in the intervention group and 2 in the placebo group) started with antidepressant medication during the study.
Forty-four participants (18 in the intervention group and 26 in the placebo group, P for group difference = 0.17) received a 500-mg/d calcium supplement in addition to the study tablets because they did not take ≥2 dairy consumptions per day [procedure as described in the study protocol (10)].
Ten participants dropped out during the study: 3 in the intervention group and 7 in the placebo group. Dropouts did not differ significantly from participants who completed the study with respect to age, sex, depressive symptoms, functional limitations, or physical performance (all P > 0.05), but participants who dropped out more often had serum 25(OH)D concentrations <30 nmol/L (P = 0.034).
In total, only 151 of the 155 initially randomly assigned participants could be included in the ITT analyses. Four participants (2 in both groups) had to be excluded because of insufficient data due to dropout shortly after the start of the study. Therefore, we refer to these analyses as modified ITT analyses, as not all initially randomly assigned participants could be included.
Compliance and serum 25(OH)D
The average compliance according to tablet counts was 89.7%: 139 of 155 participants had a tablet intake of ≥80% throughout the study year, similar in both groups (P for group difference = 0.30). Furthermore, 4 participants in the intervention group had a <10-nmol/L increase in serum 25(OH)D in addition to a serum 25(OH)D concentration <75 nmol/L at 6 mo, which brings the total compliance to 87.1%.
In the intervention group, the mean ± SD serum 25(OH)D concentration after 6 mo of intervention was 85 ± 16 nmol/L compared with 43 ± 18 nmol/L in the placebo group (P for group difference < 0.001). The mean ± SD difference in 25(OH)D concentration after 6 mo compared with baseline was 40 ± 23 nmol/L for the intervention group compared with −2 ± 20 nmol/L for the placebo group. After 6 mo, all participants in the intervention group had reached serum 25(OH)D concentrations >50 nmol/L; 74.7% (n = 56) had reached ≥75 nmol/L, and 29.3% (n = 22) had reached ≥90 nmol/L. In the placebo group, 35.1% (n = 26) had a serum 25(OH)D concentration >50 nmol/L after 6 mo, 5.4% (n = 4) had reached ≥75 nmol/L, and 25.7% (n = 19) had a serum 25(OH)D concentration <30 nmol/L after 6 mo.
AEs
One participant in the intervention group died during the study. No statistically significant differences were observed between treatment groups with regard to the total number of AEs (P = 0.24) or categories of AEs (all P > 0.05, Supplemental Table 1).
Primary outcomes
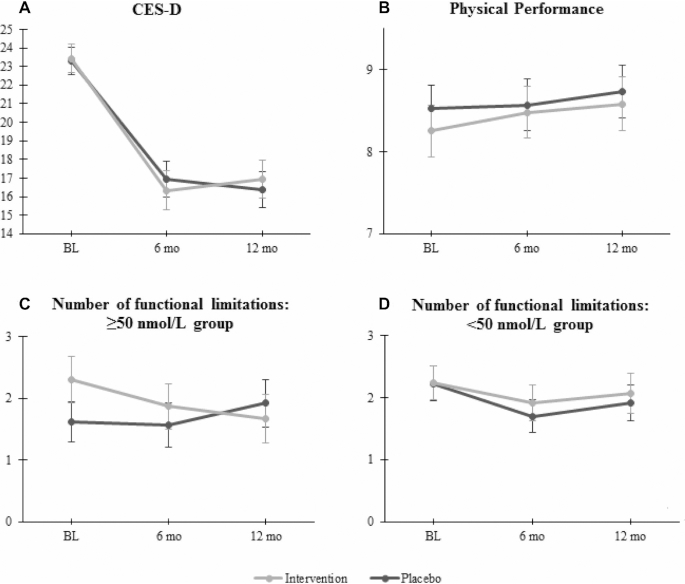
As can be seen in Table 2 and Figure 2, depressive symptoms and physical performance did not significantly differ between the 2 treatment groups over 12 mo. All interaction terms for these 2 outcomes were not significant (P > 0.10).
TABLE 2.
Effect analyses of 12 mo of vitamin D supplementation compared with placebo on the primary and secondary outcomes (modified ITT)1
| Crude model2 | Adjusted model3 | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | n | B/ratio | 95% CI | P | B/ratio | 95% CI | P |
| Primary outcomes | B | B | |||||
| Depressive symptoms (CES-D) | 150 | −0.25 | −2.37, 1.87 | 0.82 | −0.14 | −2.36, 2.08 | 0.90 |
| Functional limitations | |||||||
| Number | 150 | −0.12 | −0.42, 0.18 | 0.42 | −0.11 | −0.42, 0.20 | 0.48 |
| Baseline 25(OH)D <50 nmol/L | 94 | 0.15 | −0.25, 0.54 | 0.46 | 0.22 | −0.20, 0.63 | 0.31 |
| Baseline 25(OH)D ≥50 nmol/L | 56 | −0.62 | −1.08, −0.17 | 0.008 | −0.65 | −1.11, −0.19 | 0.006 |
| Severity | 150 | 0.02 | −0.45, 0.50 | 0.93 | −0.03 | −0.52, 0.46 | 0.91 |
| Baseline 25(OH)D <50 nmol/L | 94 | 0.35 | −0.25, 0.96 | 0.25 | 0.30 | −0.35, 0.94 | 0.36 |
| Baseline 25(OH)D ≥50 nmol/L | 56 | −0.61 | −1.39, 0.18 | 0.13 | −0.64 | −1.44, 0.16 | 0.12 |
| Physical performance (SPPB) | 151 | −0.05 | −0.63, 0.54 | 0.88 | −0.20 | −0.79, 0.38 | 0.49 |
| Secondary outcomes | |||||||
| Health-related quality of life | B | B | |||||
| EQ-5D index score | 150 | −0.02 | −0.07, 0.02 | 0.35 | −0.02 | −0.06, 0.03 | 0.52 |
| Men | 64 | 0.03 | −0.03, 0.10 | 0.31 | 0.03 | −0.04, 0.11 | 0.34 |
| Women | 86 | −0.06 | −0.12, −0.00 | 0.046 | −0.05 | −0.11, 0.01 | 0.11 |
| EQ-5D visual analog scale | 149 | −3.14 | −6.99, 0.72 | 0.11 | −3.31 | −7.32, 0.70 | 0.11 |
| SF-36 Mental Component | 148 | −0.31 | −2.01, 1.39 | 0.72 | −0.22 | −1.97, 1.54 | 0.81 |
| SF-36 Physical Component | 148 | −0.26 | −1.67, 1.14 | 0.71 | −0.22 | −1.67, 1.23 | 0.77 |
| SF-36 Physical functioning subscale | 140 | 0.32 | −3.79, 4.43 | 0.88 | 0.27 | −3.94, 4.48 | 0.90 |
| Handgrip strength | 150 | −0.56 | −1.66, 0.53 | 0.31 | −0.65 | −1.80, 0.50 | 0.27 |
| Ratio | Ratio | ||||||
| Anxiety symptoms (BAI)4 | 147 | 1.05 | 0.86, 1.27 | 0.65 | 1.03 | 0.84, 1.26 | 0.79 |
| Cognitive function (Stroop test)4 | 146 | 0.98 | 0.86, 1.13 | 0.83 | 0.97 | 0.84, 1.12 | 0.67 |
| Timed up-and-go test4 | 148 | 1.02 | 0.95, 1.08 | 0.63 | 1.03 | 0.97, 1.10 | 0.32 |
| Younger-old group (60–70 y) | 100 | 1.00 | 0.93, 1.08 | 0.99 | 1.03 | 0.95, 1.11 | 0.47 |
| Older-old group (71–80 y) | 48 | 1.02 | 0.92, 1.13 | 0.66 | 1.03 | 0.92, 1.14 | 0.62 |
Linear mixed-model analyses. Explorative stratified analyses are italicized. Ps for interaction for the stratified analyses: number of functional limitations: P = 0.020; severity of functional limitations: P = 0.084; EQ-5D index score: P = 0.041; timed up-and-go test: P = 0.087. BAI, Beck Anxiety Inventory; CES-D, Center for Epidemiological Studies–Depression scale; EQ-5D, EuroQoL-5 Dimensions; ITT, intention to treat; SF-36, Short Form–36 Health Survey; SPPB, Short Physical Performance Battery.
Adjusted for the baseline value of the outcome variable.
Additionally adjusted for alcohol use and smoking.
Analyzed with ln-transformed outcome variable, the Bs and 95% CIs were transformed back and should be interpreted as ratios, here representing the difference between the intervention and placebo groups. For example, a ratio of 1.05 should be interpreted as a 5% higher outcome score in the intervention group compared with the placebo group.
FIGURE 2.
Mean scores of the primary outcome variables over time for the intervention and placebo groups. The error bars represent SEMs. n per group: (A) intervention: n = 75, placebo: n = 75; (B) intervention: n = 75, placebo: n = 76; (C) intervention: n = 30, placebo: n = 26; (D) intervention: n = 44, placebo: n = 50. P for interaction for the stratified analyses of number of functional limitations: P = 0.020. BL, baseline; CES-D, Center of Epidemiological Studies–Depression scale.
For both the number and severity of functional limitations, the interaction term of serum 25(OH)D (cutoff of 50 nmol/L) with treatment group was significant (P for interaction = 0.020 and 0.084, respectively), so the analyses for these outcomes were stratified by baseline 25(OH)D concentration, with a cutoff of 50 nmol/L. In the higher 25(OH)D group (≥50 nmol/L at baseline), the intervention group experienced fewer functional limitations compared with the placebo group over 12 mo, but the difference in the severity of functional limitations between the groups was not statistically significant. In the low 25(OH)D group (<50 nmol/L at baseline), both the number and severity of functional limitations did not differ significantly between the treatment groups (Table 2).
Secondary outcomes
Five participants (3.4%) developed MDD according to the Composite International Diagnostic Interview criteria in the course of the study period: 3 in the intervention group and 2 in the placebo group. Because of these low numbers, no further analyses were conducted.
In the dichotomous CES-D analyses, the time interaction term was significant (P for interaction = 0.072), indicating that the effect of the intervention at 6 mo was different from the effect at 12 mo. At 6 mo, more participants in the intervention group scored below the cutoff (no clinically relevant depressive symptoms) compared with the placebo group (55.6% compared with 44.4%, P in adjusted model = 0.09), indicating that the intervention group tended to experience fewer depressive symptoms. However, at 12 mo, this difference was no longer evident (48.8% compared with 51.3%, P in adjusted model = 0.63). The dichotomous analyses for the number of functional limitations yielded similar results as the continuous analyses: the P for interaction with baseline serum 25(OH)D was 0.056, so stratified analyses were performed. In the group with serum 25(OH)D concentrations ≥50 nmol/L, the intervention group more often had no functional limitations compared with the placebo group (OR in adjusted model = 0.21; 95% CI: 0.05, 0.88; P = 0.032) over 12 mo, whereas there was no significant difference between the treatment groups in the <50-nmol/L group (OR in adjusted model = 1.92; 95% CI: 0.46, 8.04; P = 0.37).
The analyses of the BAI anxiety questionnaire, Stroop test, and TUG test were conducted with ln-transformed scores, as their original distributions were skewed to the right. Significant effect modifiers included sex for the EQ-5D index score analysis (P for interaction = 0.041) and age in the TUG test analysis (P for interaction = 0.087). Therefore, the analyses of the EQ-5D index score were stratified for sex, and the TUG test analyses were stratified for age (60–69 compared with 70–80 y).
As Table 2 shows, the treatment groups did not significantly differ with regard to anxiety symptoms, cognitive functioning, the TUG test, handgrip strength, the SF-36 HR-QoL measures, and the EQ-5D Visual Analog Scale score over 12 mo (all P > 0.10). Only the EQ-5D index score showed a significantly lower score in women in the intervention group compared with women in the placebo group, but this effect disappeared in the adjusted analyses (Table 2).
Per-protocol analyses
Two data sets were created for the per-protocol analyses. In the first per-protocol data set, 21 participants were excluded completely for the following reasons: not compliant according to tablet count (n = 16), <10-nmol/L increase of serum 25(OH)D after 6 mo while being in the intervention group and having a serum 25(OH)D <75 nmol/L after 6 mo (n = 4), or otherwise not compliant with the study protocol (n = 1). For another 3 participants, only the 12-mo measurements were excluded due to noncompliance with the study protocol between 6 and 12 mo. This first data set was used to analyze all physical outcomes and the Stroop test. The second data set was created for the per-protocol analyses of the CES-D, BAI, SF-36 Mental Component Summary, and EQ-5D. This second data set was similar to the first data set, but an additional 3 participants were excluded due to antidepressant medication use (n = 1) or baseline CES-D scores below the inclusion cutoff (15 and 14, respectively, n = 2). Furthermore, the 12-mo measurements of another 2 participants were excluded due to antidepressant medication use between 6 and 12 mo in this second data set (the remaining 2 of 5 participants who started using antidepressant medication during the study were already excluded due to noncompliance for other reasons) (Figure 1).
All per-protocol analyses yielded results similar to the modified ITT analyses, although the effect of the supplementation on the number of functional limitations in the 25(OH)D ≥50-nmol/L group was somewhat attenuated (crude model: B = −0.51, 95% CI: −0.98, −0.03, P = 0.036; adjusted model: B = −0.53, 95% CI: −1.02, −0.03, P = 0.037).
Additional analyses
Six-month change in serum 25(OH)D, independent of group assignment, was not significantly associated with parallel change in the CES-D score (B in adjusted model: −0.01, SE: 0.03, P = 0.73), functional limitations (number: B in adjusted model: −0.001, SE: 0.003, P = 0.85; severity: B in adjusted model: −0.004, SE: 0.01, P = 0.51), or physical performance (B in adjusted model: −0.01, SE: 0.01, P = 0.32).
Discussion
The D-Vitaal study investigated whether vitamin D3 supplementation of 1200 IU/d for 12 mo would improve depressive symptoms, functional limitations, and physical performance in older persons with relatively low vitamin D status, clinically relevant depressive symptoms, and problems with physical functioning. The supplementation increased serum 25(OH)D concentrations in the intervention group to a mean of 85 nmol/L after 6 mo, whereas the placebo group remained stable at a mean of 43 nmol/L. However, the intervention had no significant effect on depressive symptoms, physical performance, the severity of functional limitations, or any of the secondary outcomes of the study (anxiety symptoms, cognitive functioning, mobility, grip strength, HR-QoL). Vitamin D supplementation had a small positive effect on the number of functional limitations in participants with serum 25(OH)D concentrations ≥50 nmol/L at baseline.
Similar to our study, several other RCTs did not observe an effect of vitamin D on depressive symptoms either (34–36). In a recent trial, Jorde and Kubiak (37) compared 4 mo of 20,000 IU/wk vitamin D with placebo in 408 participants and found no effect of the supplementation on the Beck Depression Inventory. Baseline vitamin D status was <42 nmol/L, but relatively few participants had depressive symptoms. Moreover, the authors attributed positive findings from their previous RCTs on this topic (38, 39) to chance. In contrast, 3 smaller trials that included persons with both low vitamin D status and MDD did demonstrate a reduction in depression after vitamin D supplementation (40–42). These 3 studies included participants with a depression diagnosis, as opposed to subthreshold depression in our trial, which may explain the discrepancy in results.
Regarding the effect of vitamin D supplementation on physical functioning, recent RCTs reported no effect on gait speed, balance, physical performance, or muscle strength either (43–45), even though these studies included participants with lower vitamin D status and impaired physical functioning. On the contrary, a study with postmenopausal women aged 50–65 y with mean baseline 25(OH)D concentrations of 40 nmol/L and a history of falling showed that 1000 IU/d for 9 mo had a positive effect on lower extremity muscle strength and balance (46, 47). Possibly, these differences between trial results can be attributed to differences in measurement and sample characteristics.
There is a growing consensus that for many outcomes, including depression and physical functioning, supplementation with vitamin D is beneficial only in persons with low serum 25(OH)D concentrations (<50 nmol/L) (48–50). In an extensive review of trial data, Rejnmark et al. (51) evaluated the current evidence regarding the extraskeletal effects of vitamin D and noted that most studies were conducted with persons with replete 25(OH)D concentrations, which may explain the null findings of many trials. Nevertheless, the D-Vitaal study shows the absence of an effect of vitamin D supplementation in a high-risk sample with relatively low vitamin D status.
The small but statistically significant positive effect of the intervention on the number of functional limitations in the group with baseline 25(OH)D concentrations ≥50 nmol/L was surprising. We expected an effect of the supplementation on the outcomes to be more pronounced in persons with the lowest baseline 25(OH)D concentrations. Furthermore, the ≥50-nmol/L group did not reach higher 25(OH)D concentrations after 6 mo compared with the <50-nmol/L group. We therefore believe that, besides not being clinically relevant (52), this effect is most likely a chance finding. Furthermore, our study was not specifically powered for stratified analyses, so these analyses were mainly exploratory.
Strengths of the D-Vitaal study include the double-blind, randomized, placebo-controlled design, with inclusion of persons hypothesized to optimally benefit from the supplementation: an older population with depressive symptoms, poor physical functioning, and relatively low vitamin D status. Dropout was relatively low (6.5%), and compliance was high (87%). To examine the effects of the intervention, we used longitudinal statistical techniques to make optimal use of the available data.
A potential limitation of the present study is the relatively small n of 155 persons, although this number was sufficient according to the power calculation (10). Nevertheless, considering the small effect sizes, it is doubtful that a larger sample size would have yielded different results. Another potential limitation is that we included participants with a maximum serum 25(OH)D concentration of 70 nmol/L in summer. In winter, the limit was set to 50 nmol/L. We checked for interaction effects with baseline 25(OH)D (cutoffs of 50 and 30 nmol/L), but the group with <30 nmol/L at baseline may have been too small to detect an interaction effect. It is possible that our inclusion criteria regarding serum 25(OH)D were too liberal. Potentially, a limit of <50 nmol/L in summer and <30 nmol/L in winter would yield different results, but this would also complicate recruitment considerably. As an additional explorative analysis, we tested whether analyses stratified at a baseline serum 25(OH)D of 30 nmol/L would show a different picture for the primary outcomes, even though the interaction effect was not statistically significant. However, all results were nonsignificant (data not shown), but due to the small n in the <30-nmol/L group, the validity of these results is uncertain.
Presence of MDD and use of antidepressant medication were exclusion criteria in our study; we included only persons with subthreshold depression (CES-D ≥16). Therefore, we may have included persons who had only short-lived symptoms at the time of inclusion and improved naturally over time. This could explain the drop in CES-D scores in both groups between baseline and 6 mo (Figure 2A). However, the mean CES-D score remained around 16 in both groups at 6 and 12 mo, demonstrating that a substantial proportion of participants had clinically relevant depressive symptoms throughout the study period.
Compared with other supplementation trials, our vitamin D dose of 1200 IU/d is relatively low. Nevertheless, participants in the intervention group were replete at 6 mo, with a mean 25(OH)D concentration of 85 nmol/L. As serum 25(OH)D concentrations tend to reach a plateau after a few months of supplementation (53), similar concentrations are assumed after 12 mo. Recent research indicates that higher doses are not more effective and can even be harmful, for instance, by increasing the number of falls or reducing muscle strength (50, 54, 55).
It is remarkable that observational studies have demonstrated rather consistent associations between vitamin D status and numerous health outcomes, whereas this is often not confirmed by trial evidence (56, 57). Therefore, it is more likely that vitamin D status is a marker for poor health or inflammation instead of a cause of disease (58).
At the moment, several large-scale, long-term RCTs are being conducted to examine the effects of vitamin D supplementation on multiple outcomes in older persons: the Vitamin D Assessment Study (ViDa) trial (n = 5110, 100,000 IU/mo for a median period of 3.3 y) (59), the VITamin D and OmegA-3 (VITAL) trial and its ancillary the VITamin D and OmegA-3 Trial–Depression Endpoint Prevention (VITAL–DEP) study (n = 25,874, 2000 IU/d for a mean period of 5 y) (60, 61), and the Vitamin D3–Omega3–Home Exercise –HeALTHy Ageing and Longevity Trial (DO-HEALTH) (n = 2152, 2000 IU/d for 3 y) (62). Although these trials use general population samples, the n is large enough to allow for subgroup analyses. The outcomes of these trials will shed more light on the complex relationship of vitamin D with depression and physical functioning. In addition, there may be some potential in the combination of antidepressants with vitamin D in the treatment of depression (63), although a recent report suggests that it can be challenging to conduct such an RCT (64).
The D-Vitaal recruitment phase was also challenging. We had to send out ∼56,000 information brochures to include 155 participants, which underlines the difficulty of recruiting for this type of RCT in the general population. Most of the potential participants did not respond to our invitation, which might have been due to ineligibility or lack of interest to participate in an RCT. Including participants with even lower baseline serum 25(OH)D concentrations (<30 nmol/L) would be of great scientific interest but will be complicated to accomplish, both ethically and practically.
Based on the results of this study, supplementation with vitamin D for the prevention of depression and poor physical functioning cannot be recommended. However, it is important to continue research for effective and acceptable prevention strategies for these health problems in older persons.
In conclusion, this randomized placebo-controlled trial found no effect of 1200 IU/d vitamin D supplementation for 1 y on depressive symptoms or physical functioning in a high-risk population with relatively low vitamin D status.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all participants for their contributions to the D-Vitaal study. We are grateful to all general practitioners and municipalities that helped us with the recruitment of participants. We also thank our research assistants, Ans Nicolaas and Nicolette Pliester, for their dedicated work and valuable assistance. Jan Smit assisted with the power analysis and Jos Twisk and Maurits van Tulder provided statistical advice, for which we are grateful. Finally, we are grateful to our colleagues and students of the Longitudinal Aging Study Amsterdam who helped with the D-Vitaal study.
The authors’ responsibilities were as follows—NMvS and PL: primarily designed the D-Vitaal study, and all other authors contributed to the design; PL: was the principal investigator; NMvS: was the trial coordinator; EJdK, NMvS, HWJvM, BWJHP, PJME, and PL: conducted the research; ACH: coordinated the serum 25(OH)D determinations; PMB: coordinated the issuing of the study tablets; MdH: assisted with the power analysis; EJdK: collected the data (together with the research assistants), performed the statistical analyses, and wrote the paper; and all authors read and approved the final manuscript. BWJHP has received research funding from Jansen Research and Boehringer Ingelheim outside the submitted work. All other authors have nothing to disclose.
Notes
The D-Vitaal study was funded by The Netherlands Organization for Health Research and Development (ZonMw), the Hague, the Netherlands (grant number 200210022). ZonMw had no role in the design of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Changed affiliations: HWJ van Marwijk no longer works for the Amsterdam UMC Department of General Practice and Elderly Care Medicine.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AE, adverse event; BAI, Beck Anxiety Inventory; CES-D, Center of Epidemiological Studies–Depression scale; EQ-5D, EuroQol-5 Dimensions; HR-QoL, health-related quality of life; ITT, intention to treat; MDD, major depressive disorder; RCT, randomized controlled trial; SF-36, Short Form–36 Health Survey; SPPB, Short Physical Performance Battery; TUG, timed up-and-go; VDR, vitamin D receptor; 1,25(OH)2D, 1,25 dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
References
- 1. Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58(3):249–65. [DOI] [PubMed] [Google Scholar]
- 2. Braam AW, Prince MJ, Beekman ATF, Delespaul P, Dewey ME, Geerlings SW, Kivela SL, Lawlor BA, Magnusson H, Meller I et al.. Physical health and depressive symptoms in older Europeans: results from EURODEP. Br J Psychiatry. 2005;187:35–42. [DOI] [PubMed] [Google Scholar]
- 3. Penninx BW, Deeg DJ, van Eijk JT, Beekman AT, Guralnik JM. Changes in depression and physical decline in older adults: a longitudinal perspective. J Affect Disord. 2000;61(1–2):1–12. [DOI] [PubMed] [Google Scholar]
- 4. Institute of Medicine Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academies Press, 2011. [PubMed] [Google Scholar]
- 5. Kuchuk NO, Pluijm SMF, van Schoor NM, Looman CWN, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94(4):1244–50. [DOI] [PubMed] [Google Scholar]
- 6. Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501. [DOI] [PubMed] [Google Scholar]
- 7. Anglin RES, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100–7. [DOI] [PubMed] [Google Scholar]
- 8. Sohl E, van Schoor NM, de Jongh RT, Visser M, Deeg DJ, Lips P. Vitamin D status is associated with functional limitations and functional decline in older individuals. J Clin Endocrinol Metab. 2013;98(9):E1483–90. [DOI] [PubMed] [Google Scholar]
- 9. Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJH, Smit J, Knol DL, Lips P. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92(6):2058–65. [DOI] [PubMed] [Google Scholar]
- 10. de Koning EJ, van Schoor NM, Penninx BW, Elders PJ, Heijboer AC, Smit JH, Bet PM, van Tulder MW, den Heijer M, van Marwijk HW et al.. Vitamin D supplementation to prevent depression and poor physical function in older adults: study protocol of the D-Vitaal study, a randomized placebo-controlled clinical trial. BMC Geriatr. 2015;15:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. [DOI] [PubMed] [Google Scholar]
- 12. Fernandes de Abreu DA, Eyles D, Feron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009;34(Suppl 1):S265–S77. [DOI] [PubMed] [Google Scholar]
- 13. Annweiler C, Montero-Odasso M, Schott AM, Berrut G, Fantino B, Beauchet O. Fall prevention and vitamin D in the elderly: an overview of the key role of the non-bone effects. J Neuroeng Rehabil. 2010;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eyles DW, Burne THJ, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47–64. [DOI] [PubMed] [Google Scholar]
- 15. Kesby JP, Eyles DW, Burne THJ, McGrath JJ. The effects of vitamin D on brain development and adult brain function. Mol Cell Endocrinol. 2011;347(1–2):121–7. [DOI] [PubMed] [Google Scholar]
- 16. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 18. van Schoor NM, Knol DL, Deeg DJ, Peters FP, Heijboer AC, Lips P. Longitudinal changes and seasonal variations in serum 25-hydroxyvitamin D levels in different age groups: results of the Longitudinal Aging Study Amsterdam. Osteoporos Int. 2014;25(5):1483–91. [DOI] [PubMed] [Google Scholar]
- 19. Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2–3):85–96. [DOI] [PubMed] [Google Scholar]
- 20. Merrill SS, Seeman TE, Kasl SV, Berkman LF. Gender differences in the comparison of self-reported disability and performance measures. J Gerontol A Biol Sci Med Sci. 1997;52(1):M19–26. [DOI] [PubMed] [Google Scholar]
- 21. Sohl E, Heymans MW, de Jongh RT, den Heijer M, Visser M, Merlijn T, Lips P, van Schoor NM. Prediction of vitamin D deficiency by simple patient characteristics. Am J Clin Nutr. 2014;99(5):1089–95. [DOI] [PubMed] [Google Scholar]
- 22. Bisschop MI, Kriegsman DMW, van Tilburg TG, Penninx BWJH, van Eijk JT, Deeg DJH. The influence of differing social ties on decline in physical functioning among older people with and without chronic diseases: the Longitudinal Aging Study Amsterdam. Aging Clin Exp Res. 2003;15(2):164–73. [DOI] [PubMed] [Google Scholar]
- 23. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 24. Andrews G, Peters L. The psychometric properties of the Composite International Diagnostic Interview. Soc Psychiatry Psychiatr Epidemiol. 1998;33(2):80–8. [DOI] [PubMed] [Google Scholar]
- 25. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–7. [DOI] [PubMed] [Google Scholar]
- 26. Klein M, Ponds RW, Houx PJ, Jolles J. Effect of test duration on age-related differences in Stroop interference. J Clin Exp Neuropsychol. 1997;19(1):77–82. [DOI] [PubMed] [Google Scholar]
- 27. Brooks R, Rabin R, de Charro Feds. The measurement and valuation of health status using EQ-5D: a European perspective. Dordrecht (Netherlands): Kluwer Academic, 2003. [Google Scholar]
- 28. Ware JEJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 29. Lin MR, Hwang HF, Hu MH, Wu HDI, Wang YW, Huang FC. Psychometric comparisons of the timed up and go, one-leg stand, functional reach, and Tinetti balance measures in community-dwelling older people. J Am Geriatr Soc. 2004;52(8):1343–8. [DOI] [PubMed] [Google Scholar]
- 30. Heijboer AC, Blankenstein MA, Kema IP, Buijs MM. Accuracy of 6 routine 25-hydroxyvitamin D assays: influence of vitamin D binding protein concentration. Clin Chem. 2012;58(3):543–8. [DOI] [PubMed] [Google Scholar]
- 31. Dirks NF, Vesper HW, van Herwaarden AE, van den Ouweland JM, Kema IP, Krabbe JG, Heijboer AC. Various calibration procedures result in optimal standardization of routinely used 25(OH)D ID-LC-MS/MS methods. Clin Chim Acta. 2016;462:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garretsen H, Knibbe R. Probleemdrinken: prevalentiebepaling, beinvloedende factoren en preventiemogelijkheden: theoretische overwegingen en onderzoek in Rotterdam. [Problem drinking: determinations of prevalence, influencing factors and prevention possibilities: theoretical considerations and research in Rotterdam] Lisse: Swets en Zeitlinger, 1983. Dutch. [Google Scholar]
- 33. van ’t Veer-Tazelaar PJ, van Marwijk HWJ, van Oppen P, van Hout HPJ, van der Horst HE, Cuijpers P, Smit F, Beekman ATF. Stepped-care prevention of anxiety and depression in late life: a randomized controlled trial. Arch Gen Psychiatry. 2009;66(3):297–304. [DOI] [PubMed] [Google Scholar]
- 34. Bertone-Johnson ER, Powers SI, Spangler L, Larson J, Michael YL, Millen AE, Bueche MN, Salmoirago-Blotcher E, Wassertheil-Smoller S, Brunner RL et al.. Vitamin D supplementation and depression in the women's health initiative calcium and vitamin D trial. Am J Epidemiol. 2012;176(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanders KM, Stuart AL, Williamson EJ, Jacka FN, Dodd S, Nicholson G, Berk M. Annual high-dose vitamin D3 and mental well-being: randomised controlled trial. Br J Psychiatry. 2011;198(5):357–64. [DOI] [PubMed] [Google Scholar]
- 36. Yalamanchili V, Gallagher JC. Treatment with hormone therapy and calcitriol did not affect depression in older postmenopausal women: no interaction with estrogen and vitamin D receptor genotype polymorphisms. Menopause. 2012;19(6):697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jorde R, Kubiak J. No improvement in depressive symptoms by vitamin D supplementation: results from a randomised controlled trial. J Nutr Sci. 2018;7:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599–609. [DOI] [PubMed] [Google Scholar]
- 39. Kjaergaard M, Waterloo K, Wang CEA, Almas B, Figenschau Y, Hutchinson MS, Svartberg J, Jorde R. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201(5):360–8. [DOI] [PubMed] [Google Scholar]
- 40. Alavi NM, Khademalhoseini S, Vakili Z, Assarian F. Effect of vitamin D supplementation on depression in elderly patients: a randomized clinical trial. Clin Nutr. 2018, Sept 19 (Epub ahead of print; doi:10.1016/j.clnu.2018.09.011). [DOI] [PubMed] [Google Scholar]
- 41. Mozaffari-Khosravi H, Nabizade L, Yassini-Ardakani SM, Hadinedoushan H, Barzegar K. The effect of 2 different single injections of high dose of vitamin D on improving the depression in depressed patients with vitamin D deficiency: a randomized clinical trial. J Clin Psychopharmacol. 2013;33(3):378–85. [DOI] [PubMed] [Google Scholar]
- 42. Sepehrmanesh Z, Kolahdooz F, Abedi F, Mazroii N, Assarian A, Asemi Z, Esmaillzadeh A. Vitamin D supplementation affects the beck depression inventory, insulin resistance, and biomarkers of oxidative stress in patients with major depressive disorder: a randomized, controlled clinical trial. J Nutr. 2016;146(2):243–8. [DOI] [PubMed] [Google Scholar]
- 43. El Hajj C, Fares S, Chardigny JM, Boirie Y, Walrand S. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: a randomized controlled trial. Arch Osteoporos. 2018;14(1):4. [DOI] [PubMed] [Google Scholar]
- 44. Levis S, Gomez-Marin O. Vitamin D and physical function in sedentary older men. J Am Geriatr Soc. 2017;65(2):323–31. [DOI] [PubMed] [Google Scholar]
- 45. Vaes AMM, Tieland M, Toussaint N, Nilwik R, Verdijk LB, van Loon LJC, de Groot L. Cholecalciferol or 25-hydroxycholecalciferol supplementation does not affect muscle strength and physical performance in prefrail and frail older adults. J Nutr. 2018;148(5):712–20. [DOI] [PubMed] [Google Scholar]
- 46. Cangussu LM, Nahas-Neto J, Orsatti CL, Bueloni-Dias FN, Nahas EA. Effect of vitamin D supplementation alone on muscle function in postmenopausal women: a randomized, double-blind, placebo-controlled clinical trial. Osteoporos Int. 2015;26(10):2413–21. [DOI] [PubMed] [Google Scholar]
- 47. Cangussu LM, Nahas-Neto J, Orsatti CL, Poloni PF, Schmitt EB, Almeida-Filho B, Nahas EA. Effect of isolated vitamin D supplementation on the rate of falls and postural balance in postmenopausal women fallers: a randomized, double-blind, placebo-controlled trial. Menopause. 2016;23(3):267–74. [DOI] [PubMed] [Google Scholar]
- 48. Dawson-Hughes B. Vitamin D and muscle function. J Steroid Biochem Mol Biol. 2017;173:313–6. [DOI] [PubMed] [Google Scholar]
- 49. Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56–61. [DOI] [PubMed] [Google Scholar]
- 50. Reid IR. High-dose vitamin D: without benefit but not without risk. J Intern Med. 2018;284(6):694–6. [DOI] [PubMed] [Google Scholar]
- 51. Rejnmark L, Bislev LS, Cashman KD, Eiriksdottir G, Gaksch M, Grubler M, Grimnes G, Gudnason V, Lips P, Pilz S et al.. Non-skeletal health effects of vitamin D supplementation: a systematic review on findings from meta-analyses summarizing trial data. PLoS One. 2017;12(7):e0180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoogendijk E, van Groenou MB, van Tilburg T, Deeg D. Educational differences in functional limitations: comparisons of 55-65-year-olds in the Netherlands in 1992 and 2002. Int J Public Health. 2008;53(6):281–9. [DOI] [PubMed] [Google Scholar]
- 53. Lips P, Wiersinga A, van Ginkel FC, Jongen MJ, Netelenbos JC, Hackeng WH, Delmas PD, van der Vijgh WJ. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67(4):644–50. [DOI] [PubMed] [Google Scholar]
- 54. Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175–83. [DOI] [PubMed] [Google Scholar]
- 55. Bislev LS, Langagergaard Rodbro L, Rolighed L, Sikjaer T, Rejnmark L. Effects of vitamin D3 supplementation on muscle strength, mass, and physical performance in women with vitamin D insufficiency: a randomized placebo-controlled trial. Calcif Tissue Int. 2018;103(5):483–93. [DOI] [PubMed] [Google Scholar]
- 56. Okereke OI, Singh A. The role of vitamin D in the prevention of late-life depression. J Affect Disord. 2016;198:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wimalawansa SJ. Non-musculoskeletal benefits of vitamin D. J Steroid Biochem Mol Biol. 2018;175:60–81. [DOI] [PubMed] [Google Scholar]
- 58. Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76–89. [DOI] [PubMed] [Google Scholar]
- 59. Scragg R, Waayer D, Stewart AW, Lawes CMM, Toop L, Murphy J, Khaw KT, Camargo CA Jr. The Vitamin D Assessment (ViDA) study: design of a randomized controlled trial of vitamin D supplementation for the prevention of cardiovascular disease, acute respiratory infection, falls and non-vertebral fractures. J Steroid Biochem Mol Biol. 2016;164:318–25. [DOI] [PubMed] [Google Scholar]
- 60. Okereke OI, Reynolds CF III, Mischoulon D, Chang G, Cook NR, Copeland T, Friedenberg G, Buring JE, Manson JE. The VITamin D and OmegA-3 TriaL-Depression Endpoint Prevention (VITAL-DEP): rationale and design of a large-scale ancillary study evaluating vitamin D and marine omega-3 fatty acid supplements for prevention of late-life depression. Contemp Clin Trials. 2018;68:133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pradhan AD, Manson JE. Update on the Vitamin D and OmegA-3 trial (VITAL). J Steroid Biochem Mol Biol. 2016;155(Pt B):252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. DO-HEALTH clinical trial [Internet] 2019. Available from: http://do-health.eu/wordpress. [Google Scholar]
- 63. Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, Ng CH. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry. 2016;173(6):575–87. [DOI] [PubMed] [Google Scholar]
- 64. Aucoin M, Cooley K, Anand L, Furtado M, Canzonieri A, Fine A, Fotinos K, Chandrasena R, Klassen LJ, Epstein I et al.. Adjunctive vitamin D in the treatment of non-remitted depression: lessons from a failed clinical trial. Complement Ther Med. 2018;36:38–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.