ABSTRACT
Background
Environmental enteropathy (EE) refers to villus blunting, reduced absorption, and microbial translocation in children and adults in tropical or deprived residential areas. In previous work we observed an effect of micronutrients on villus height (VH).
Objective
We aimed to determine, in a randomized controlled trial, if amino acid (AA) or multiple micronutrient (MM) supplementation can improve intestinal structure or barrier dysfunction in Zambian adults with EE.
Methods
AA (tryptophan, leucine, and glutamine) and/or MM supplements were given for 16 wk in a 2 × 2 factorial comparison against placebo. Primary outcomes were changes in VH, in vivo small intestinal barrier dysfunction assessed by confocal laser endomicroscopy (CLE), and mechanistic (or mammalian) target of rapamycin complex 1 (MTORC1) nutrient responsiveness in lamina propria CD4+ lymphocytes.
Results
Over 16 wk AA, but not MM, supplementation increased VH by 16% (34.5 μm) compared with placebo (P = 0.04). Fluorescein leak, measured by CLE, improved only in those allocated to both AA and MM supplementation. No effect was seen on MTORC1 activation, but posttreatment MTORC1 and VH were correlated (ρ = 0.51; P = 0.001), and change in MTORC1 was correlated with change in VH in the placebo group (ρ = 0.63; P = 0.03). In secondary analyses no effect was observed on biomarkers of microbial translocation. Metabolomic analyses suggest alterations in a number of microbial- and host-derived metabolites including the leucine metabolite β-hydroxy-β-methylbutyrate, which was increased by AA supplementation and correlated with VH.
Conclusions
In this phase 2 trial, AA supplementation protected against a decline in VH over the supplementation period, and improved barrier function when combined with micronutrients. Leucine and MTORC1 metabolism may be involved in the mechanism of effect. This trial was registered at www.pactr.org as PACTR201505001104412.
Keywords: environmental enteropathy, amino acids, micronutrients, MTOR, confocal endomicroscopy, morphometry, clinical trial, metabolomics, Zambia
Introduction
Environmental enteropathy (EE) was first recognized as “tropical enteropathy,” a phenomenon of uncertain significance (1–3). There is now evidence that it underlies the growth impairment of millions of young people globally (4, 5), impairs the neurocognitive development of children (6), contributes to micronutrient deficiencies through impaired absorption of nutrients (7), and may blunt responses to oral vaccines (5, 8).
The definitive assessment of EE requires small intestinal biopsy (1, 9). Recent work has established that assessment of fecal inflammatory and permeability markers can be used to evaluate the severity of EE (10–12), and these markers correlate with ponderal growth (12, 13). Recent metabolomic studies have found that EE is associated with perturbations in choline and tryptophan metabolism (14, 15). Transcriptomic analysis of stool RNA has shown that transcripts related to cell adhesion and to responses to viral and other pathogens characterize EE (16). We have recently reported that confocal endomicroscopy can be used to quantify leakiness of the epithelium in EE (17). T cell–mediated inflammation is a feature of EE, but the drivers of the mucosal immune response are as yet unknown.
There are currently no established therapies for EE, and even preventive strategies have been unsuccessful to date. Water and sanitation trials have so far proved disappointing (18, 19). Antibiosis using rifaximin did not alter lactulose permeation in Malawian children (20). There is divergent evidence on the effects of antihelminthics (21, 22). Probiotics were ineffective (23), as was anti-inflammatory therapy using mesalazine (24). New treatments are needed (25). We previously reported preliminary evidence that micronutrient supplementation could improve villus morphology (26). There are data which suggest that glutamine can reduce intestinal permeability (27, 28), but there are also negative trial data (29). In view of this preliminary evidence, and evidence that tryptophan metabolism is perturbed in EE (14, 30, 31), we hypothesized that micronutrient supplementation and/or supplementation with glutamine, tryptophan, and leucine can ameliorate the mucosal pathology of EE through modification of immune cell nutrient-sensing systems such as mechanistic (or mammalian) target of rapamycin complex 1 (MTORC1). Here we report the results of that randomized controlled trial.
Methods
Participants and recruitment
Participants were recruited from Misisi, Lusaka, where we have previously conducted studies of EE (9, 17). Recruitment followed a 3-stage process, beginning with door-to-door invitations, followed by a series of focus group discussions, and then individual interviews before written informed consent. At the initial assessment, inclusion and exclusion criteria were assessed, a medical and social history questionnaire was administered, and a physical examination was carried out. A stool sample was provided to exclude helminths and protozoal infection. An HIV test (Determine, Abbott Diagnostics) was administered, with a confirmatory test (Unigold, Trinity Biotech) administered in positive cases. On the day of endoscopy, a blood sample was collected, and body composition assessment was performed using impedance and plethysmography (see below).
Inclusion and exclusion criteria
Participants were eligible for recruitment if between 18 and 60 y of age; male or female; resident in B section, Misisi compound, Lusaka; and able to give informed consent. Exclusion criteria were pregnancy (by self-report), breastfeeding (by self-report), BMI <18 kg/m2, any antibiotic use within the previous 4 wk, regular non-steroidal anti-inflammatory drug use within the previous 4 wk, diarrhea within the previous 4 wk, significant comorbidity precluding endoscopy with sedation, therapeutic anticoagulation or bleeding diathesis precluding endoscopic biopsies, or lack of consent for HIV testing. Untreated helminth or protozoal infection, or helminth or protozoal infection treated within 6 mo, were also exclusion criteria; however, participants with nonpathogenic parasites were eligible (detailed in Supplemental Table 1).
Trial design
The trial was designed as a parallel-group phase 2 trial in which multiple micronutrient (MM) or amino acid (AA) supplements were compared with respective matching placebos in a 2 × 2 factorial design. The study was powered for primary outcomes in HIV-negative individuals, although HIV-positive participants were also included so as to avoid discrimination in this close-knit community. We did not detect any effect of HIV status on any of the outcomes measured, and results are reported here combined, without reference to HIV status unless otherwise stated.
Randomization
Each AA or matched placebo sachet and each MM or matched placebo pillbox was labeled with 1 of 4 letters (A, B, C, D for AA/placebo) or numbers (1, 2, 3, 4 for MM/placebo), respectively. Two numbers and 2 letters were randomly assigned by the manufacturer to the active supplements, with the other 2 representing the placebos. The supplements were then labeled accordingly, and the allocation code was supplied to the study statistician. The code was known to the manufacturer and to the study statistician and was only revealed to the investigators once the databases had been locked at the end of the trial.
After completing enrollment and baseline assessments, participants were randomly allocated in blocks of 16 by the study statistician to 1 of the 16 possible groups (4 possible AA allocations × 4 possible MM allocations) using the randomization algorithms available at www.randomization.com (accessed 9 October, 2015 by DM). Participants were therefore allocated to each of the 4 study arms in a 1:1:1:1 ratio.
Interventions
The MM supplement (Immunace Original) and matched placebo were both manufactured by Vitabiotics Ltd. The AA supplement (29.9 g l-Gln, 2.79 g l-Leu, and 0.28 g l-Trp) and isocaloric taste-matched placebo were both manufactured by Glanbia Nutritionals Deutschland GmbH. Both were given daily for 16 wk (112 d). Participants were advised to take both supplements with their main meal every day, at any time of day, starting the day after the baseline endoscopy. The composition and presentation of the active supplements and matching placebos are given in Tables 1 and 2.
TABLE 1.
Composition of the micronutrient supplement1
| Average dose per tablet | Total daily dose | EC RDA | Multiples of EC RDA per daily dose | |
|---|---|---|---|---|
| Vitamin A RE, IU/µg | 2664/800 | 5328/1600 | 800 | 2 |
| Vitamin D3, IU/µg | 400/10 | 800/20 | 5 | 4 |
| Vitamin E α-TE, mg | 40 | 80 | 12 | 6.66 |
| Vitamin K, μg | 70 | 140 | 75 | 1.86 |
| Vitamin C, mg | 150 | 300 | 80 | 3.76 |
| Thiamin, mg | 18 | 36 | 1.1 | 32.72 |
| Riboflavin, mg | 6 | 12 | 1.4 | 8.58 |
| Niacin NE, mg | 27 | 54 | 16 | 3.38 |
| Vitamin B-6, mg | 10 | 20 | 1.4 | 14.28 |
| Folic acid, μg | 400 | 800 | 200 | 4 |
| Vitamin B-12, μg | 14 | 28 | 2.5 | 11.2 |
| Pantothenic acid, mg | 20 | 40 | 6 | 6.66 |
| Iron, mg | 8 | 16 | 14 | 1.14 |
| Magnesium, mg | 60 | 120 | 375 | 0.32 |
| Zinc, mg | 15 | 30 | 10 | 3 |
| Iodine, μg | 200 | 400 | 150 | 2.66 |
| Copper, μg | 500 | 1000 | 1000 | 1 |
| Manganese, mg | 4 | 8 | 2 | 4 |
| Selenium, μg | 180 | 360 | 55 | 6.54 |
| Chromium, μg | 100 | 200 | 40 | 5 |
| l-Cystine, mg | 40 | 80 | N/A | N/A |
| l-Carnitine, mg | 30 | 60 | N/A | N/A |
| Citrus bioflavonoids, mg | 30 | 60 | N/A | N/A |
| β-Carotene (natural carotenoids), mg | 3 | 6 | N/A | N/A |
1The total daily dose consisted of 2 tablets. The multiple micronutrient supplement included the following excipients: microcrystalline cellulose, hydroxypropylmethylcellulose, ethyl cellulose, propylene glycol, purified talc, titanium dioxide, iron oxides, glucose syrup, magnesium stearate, silicon dioxide, polyvinylpolypyrrolidone, acacia, sucrose, starch, tricalcium phosphate, dicalcium phosphate, medium-chain triglycerides, colloidal silica, maltodextrin, and butylated hydroxyanisole. The micronutrient placebo contained only these excipients. EC RDA, European Community RDA; IU, international units; N/A, not available; NE, niacin equivalents; RE, retinol equivalents; α-TE, α-tocopherol equivalents.
TABLE 2.
Composition of the amino acid supplement1
| Active | Placebo | |
|---|---|---|
| l-Gln | 29.9 g | — |
| l-Trp | 0.28 g | — |
| l-Leu | 2.79 g | — |
| Maltodextrin | — | 32.9 g |
| Sodium hydrogen sulfite (E222) | — | 0.07 g |
| Total daily dose | 33 g | 33 g |
1Sodium hydrogen sulfite was included as a bitterant in the placebo to approximate the slight bitterness of tryptophan.
Primary endpoints
Three primary endpoints were evaluated in this phase 2 trial: 1) change in villus height (VH) measured morphometrically in distal duodenal biopsies collected during endoscopy under sedation; 2) change in in vivo small intestinal barrier leak by measuring fluorescein leak via confocal laser endomicroscopy (CLE); and 3) change in distal duodenal lamina propria mucosal CD4+ T cell MTORC1 activity, measured by flow cytometry.
Morphometry
Endoscopic biopsies from distal duodenum were orientated under a dissecting microscope at the time of endoscopy before being fixed in formalin. Hematoxylin and eosin stained sections of paraffin-embedded tissue were then digitally imaged using a Hamamatsu digital slide scanner (Hamamatsu Photonics UK Ltd.). After identification of the villus–crypt boundary and mucosal length by the user, several parameters were measured: VH, villus width, crypt depth, villus perimeter per unit length of mucosa (as a measure of 3-dimensional villus surface area), and villus cross-sectional area per unit length of mucosa (as a measure of 3-dimensional villus compartment volume) (17).
CLE
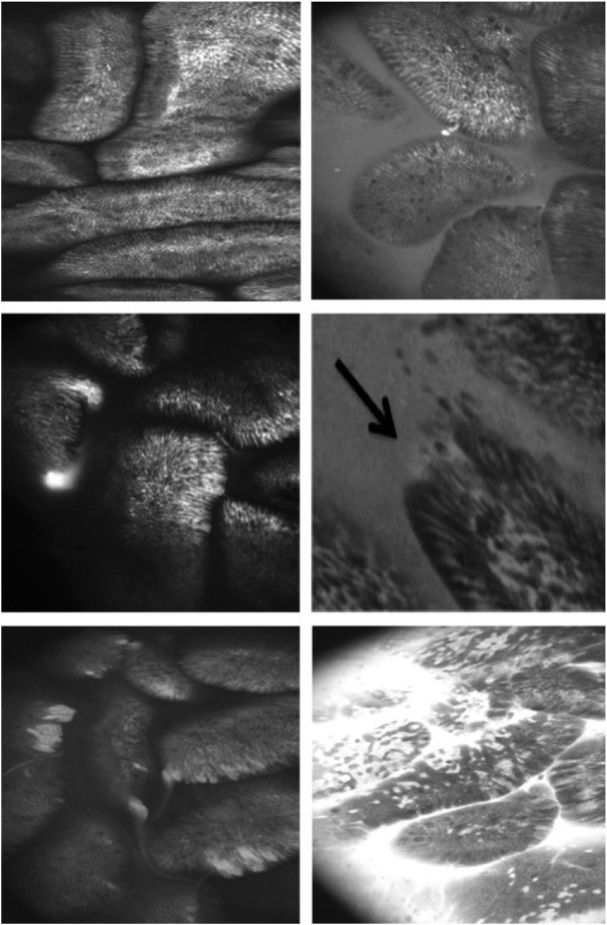
The small intestinal barrier was imaged in vivo using fluorescein-based CLE (Pentax) as previously described (17). The proportion of fields demonstrating fluorescein leak into the lumen (Figure 1) was scored by 2 independent blinded observers, whose reports demonstrated high interobserver agreement (ρ = 0.94; P < 0.001; Supplemental Figure 1).
FIGURE 1.
Examples of reproducible endomicroscopic signs of barrier dysfunction. Top left: intact mucosa; no leak; intervillous space is black (normal; Watson grade I). Top right: background luminal fluorescein (functional defect; Watson grade II) with a single apoptotic cell shedding event (nonpathological). Centre left: fluorescein plume with no mucosal defects (functional defect; Watson grade II). Center right: apical microerosion (multiple cell defect) with background fluorescein leakage (structural defect; Watson grade III). Bottom left: multiple microerosions with multiple fluorescein plumes (structural defect; Watson grade III). Bottom right: florid fluorescein leak within and out of the mucosa with multiple microerosions (structural defect; Watson grade III).
Assessment of lamina propria CD4+ T lymphocyte MTORC1 activity
The ability of lamina propria CD4+ lymphocytes to respond to environmental nutrients through MTORC1 activity was assessed by measuring levels of its phosphorylated downstream transcription factor target, phosphor-4E-binding protein 1 (p4E-BP1) (31), via flow cytometry (see the Supplemental Methods). The percentage of CD4+ T cells responding to nutrient stimulation was calculated in FlowJo (TreeStar), using the “starved” cells as the control population.
Secondary endpoints
Secondary endpoints measured included change in 1) plasma markers of microbial translocation and inflammation; 2) metabolomic profile; and 3) anthropometry and body composition.
Plasma markers of microbial translocation and inflammation
Serum was stored at −80°C for analysis of LPS concentrations using the Limulus amoebocyte lysate assay (Associates of Cape Cod International Inc.) according to the manufacturer's instructions. C-reactive protein (CRP) and soluble CD14 (sCD14) (both from R&D Systems) concentrations were measured by ELISA according to the manufacturer's instructions. Serum glucagon-like peptide 2 (GLP-2; EMD Millipore Corporation) concentrations were measured by ELISA according to the manufacturer's instructions.
1H NMR spectroscopy–based metabolic phenotyping
Urine samples were collected before endoscopy into a container with 1 mL 0.5% chlorhexidine, then stored at −80°C until transportation to London, United Kingdom on dry ice. Analytical details are given in the Supplemental Methods. Multivariate statistical approaches were used to interrogate the complex metabolic data set. This included principal component analysis to identify the main sources of variance in the data and to identify outliers. Orthogonal projection to latent structures (OPLS) discriminant analysis was performed to assess the metabolic variation between the different treatment groups (e.g., placebo compared with AA). Separate OPLS models were also constructed to illuminate metabolic variation associated with VH and MTORC1 activation. The predictive ability (Q2Y) of the models was calculated using a 7-fold cross-validation approach and their validity was assessed by permutation testing (1000 permutations; with a P value threshold of P < 0.05).
Nutritional assessments
Study participants had BMI, midupper arm circumference (MUAC), and grip strength measurements taken before and after the supplementation period. Body composition pre- and postsupplementation was assessed using whole-body air displacement plethysmography (BodPod, COSMED) and bioelectrical impedance (Tanita Corporation).
Data analysis and statistical considerations
The study was powered to demonstrate the superiority of MM or AA supplementation over placebo in HIV-seronegative participants. We expected a 15% difference in morphometry to be associated with a similar degree of change in intestinal permeability, bacterial translocation, and metabolomic profile. Power and sample size calculations were based on morphometric data from 2 studies of adults with enteropathy in this population (17, 26). Sample size calculations were powered for main effects and assumed no negative interaction between AA and MM supplementation. To detect a difference of 15% between an intervention and the corresponding placebo would require 56 HIV-seronegative participants—28 participants given the supplement and 28 given the corresponding placebo (expected difference in VH = 30 µm; SD = 40 µm; power = 0.8; 2-sided α = 0.05). Therefore, 14 participants per combination of interventions was the minimum sample size. Allowing for a 20% dropout rate and 30% HIV-seropositive rate (17), we anticipated that 100 participants were required. Using the Shapiro–Wilk test, measures of morphometry and biomarkers were nonnormally distributed and are therefore reported as median [IQR]. However, measurements of change in morphometry, fluorescein leakage, and MTORC1 activation did not depart significantly from the normal distribution and are reported as mean ± SD.
Clinical trial approvals
The study protocol and trial were approved by the University of Zambia Biomedical Research Ethics Committee (reference 007-11-14, dated 22 January, 2015), the Zambia Medicines Regulatory Authority (reference CT054/15), and the National Health Research Authority of Zambia (29 October, 2015), and registered with the Pan African Clinical Trials Registry (PACTR201505001104412). A favorable nonbinding opinion was also obtained from Queen Mary University of London Ethics of Research Committee (ref. QMERC2014/77).
Results
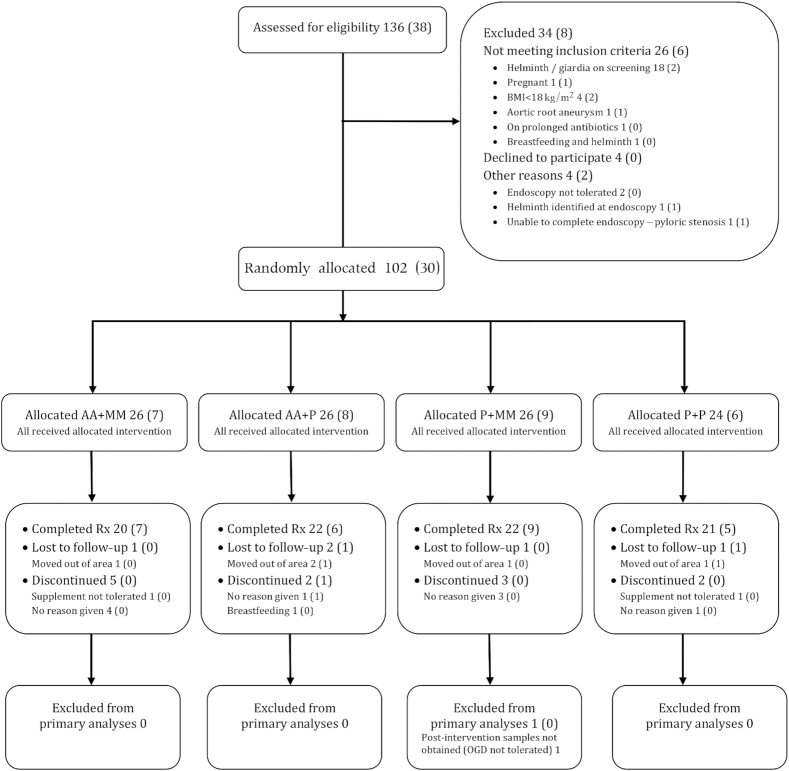
The trial was carried out between October 2015 and May 2016. The timing of recruitment was such that treatment was initiated in November–December 2015 (before the rainy season) and the final measurements after treatment were made in March–April 2016 (after the rainy season). One hundred and two participants were recruited and randomly allocated, and 85 were included in the final analysis (Figure 2). Compliance was high (Supplemental Figure 2).
FIGURE 2.
Consolidated Standards of Reporting Trials flow diagram of participants through the trial. Total numbers of participants are given, with the number of HIV-positive participants in parentheses. AA, amino acid; MM, multiple micronutrient; OGD, oesophagogastroduodenoscopy; P, placebo.
Participant characteristics
Age, sex, nutritional status (BMI, MUAC, fat distribution, grip strength), and other baseline measurements for primary endpoints were comparable between the treatment groups (Table 3).
TABLE 3.
Baseline characteristics1
| AA arm | MM arm | |||
|---|---|---|---|---|
| AA (n = 42) | Placebo (n = 43) | MM (n = 42) | Placebo (n = 43) | |
| Sex, M:F | 16:26 | 11:32 | 12:30 | 15:28 |
| Age, y | 35 [28, 46] | 42 [24, 51] | 40 [21, 47] | 40 [30, 46] |
| HIV seropositive | 13 (31%) | 14 (33%) | 16 (38%) | 11 (26%) |
| BMI, kg/m2 | 23.1 [19.9, 25.1] | 22.3 [20.5, 27.7] | 23.1 [20.2, 26.7] | 22.0 [19.9, 25.9] |
| MUAC, cm | 27.3 [25.4, 30.1] | 28.5 [25.8, 32.0] | 28.3 [25.4, 31.1] | 27.9 [25.4, 31.3] |
| Grip strength, kg | 31.8 [25.0, 39.2] | 29.2 [27.1, 36.9] | 30.7 [27.7, 36.1] | 31.2 [25.7, 37.7] |
| Fat percentage, by impedance | 24.1 [14.7, 30.0] | 25.3 [17.6, 36.2] | 25.2 [16.5, 31.6] | 24.1 [15.7, 34.4] |
| Fat percentage, by plethysmography | 24.5 [17.3, 34.3] | 30.9 [19.3, 40.6] | 30.3 [17.6, 37.6] | 28.5 [17.3, 36.1] |
| VH, μm | 185 [154, 217] | 218 [201, 250] | 202 [181, 252] | 206 [170, 235] |
| CD, μm | 163 [141, 196] | 163 [150, 198] | 157 [135, 194] | 174 [152, 199] |
| VW, μm | 230 [210, 263] | 222 [197, 268] | 221 [202, 253] | 230 [205, 267] |
| VP, μm, per unit mucosa | 865 [702, 1030] | 987 [868, 1134] | 931 [788, 1072] | 913 [772, 1090] |
| VA, μm2, per unit mucosa | 30,061 [23,036, 37,178] | 32,328 [27,992, 40,093] | 31,998 [23,619, 37,642] | 31,288 [24,892, 37,200] |
| Fluorescein leak, % fields | 85 [66, 91] | 79 [57, 89] | 80 [56, 91] | 86 [75, 91] |
| p4E-BP1 positive LP CD4+, % | 48 [41, 57] | 60 [45, 66] | 58 [46, 65] | 49 [36, 62] |
| LPS, EU/mL | 158 [106, 257] | 155 [94, 232] | 173 [108, 251] | 150 [89, 244] |
| CRP, μmol/mL | 1.9 [0.6, 3.6] | 3.9 [1.1, 9.0] | 2.2 [0.6, 7.2] | 2.7 [0.9, 8.5] |
| sCD14, μmol/mL | 1.8 [1.4, 2.4] | 1.9 [1.4, 2.3] | 1.7 [1.4, 2.4] | 1.9 [1.5, 2.3] |
| GLP-2, ng/mL | 0.76 [0, 1.4] | 0.92 [0.5, 1.4] | 0.75 [0.5, 1.5] | 0.88 [0.5, 1.4] |
1Values are given as median [IQR] unless otherwise stated. Fluorescein leak was defined as Watson grade II or III defects (see Methods and Figure 1 for further details), and scored as the percentage of unique endomicroscopic fields displaying leaks. The percentage of p4E-BP1 positive LP CD4+ represents the proportion of LP CD4+ that responds to nutrient stimulation in vitro (see the Methods for details). AA, amino acid; CD, crypt depth; CRP, C-reactive protein; F, female; GLP-2, glucagon-like peptide 2; LP CD4+, lamina propria CD4+ T cell; M, male; MM, multiple micronutrient; MUAC, midupper arm circumference; p4E-BP1, phospho-4E-binding protein 1 (marker of MTORC1 activation); sCD14, soluble CD14; VA, villus cross-sectional area per 100 μm muscularis mucosae; VH, villus height; VP, villus perimeter per 100 μm muscularis mucosae; VW, villus width.
Primary endpoint: mucosal morphometry
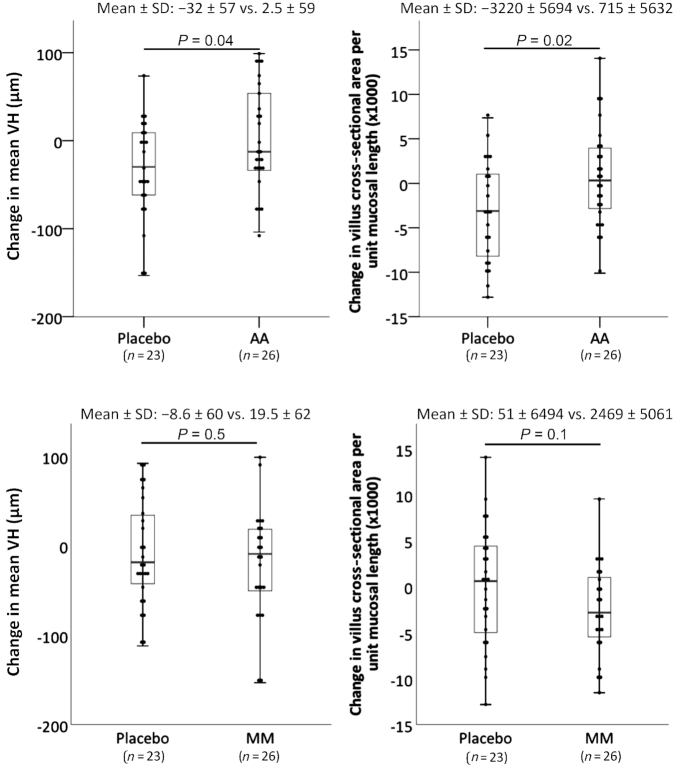
Unfortunately, in several cases it was not possible for an experienced technician to obtain well-orientated sections suitable for morphometry, resulting in a reduction in the number of paired samples available for analysis. Nevertheless, there was a general reduction in villus and crypt measurements in all groups between the first and second biopsies, which may relate to seasonality (9). The reduction in VH and villus cross-sectional area was significantly less in participants given AA supplementation compared with placebo (Table 4; Figure 3). This effect of AA supplementation on VH was confirmed in linear regression models in which MUAC, fat mass, grip strength, and HIV status were included; only AA supplementation remained a significant contributor to the model (P = 0.03; Supplemental Table 2). MM supplementation had no effect on any morphometric measurement (Table 4). HIV status had no effect on morphometric parameters at baseline (data not shown) and had no effect on the change in VH over the supplementation period (mean ± SD: −28 ± 57 in HIV seropositives compared with −8.6 ± 61 in HIV seronegatives; P = 0.30).
TABLE 4.
Changes in primary histological endpoints1
| AAs (Trp, Leu, Gln) vs. placebo | MMs vs. placebo | |||||
|---|---|---|---|---|---|---|
| Change in: | AA (n = 26) | Placebo (n = 23) | P | MM (n = 26) | Placebo (n = 23) | P |
| Villus height, μm | 2.5 ± 59 | −32.0 ± 57 | 0.04 | −19.5 ± 62 | −8.6 ± 60 | 0.53 |
| Crypt depth, μm | −13.3 ± 39 | −31.1 ± 45 | 0.15 | −25.5 ± 43 | −18.3 ± 43 | 0.56 |
| Villus width, μm | −11.5 ± 60 | −13.7 ± 138 | 0.94 | −16.6 ± 72 | −8.9 ± 126 | 0.80 |
| Villus perimeter, μm, per 100 μm muscularis mucosae | −1.9 ± 180 | −89.5 ± 159 | 0.08 | −64.1 ± 137 | −24.4 ± 203 | 0.43 |
| Villus area, μm2, per 100 μm muscularis mucosae | 715 ± 5632 | −3220 ± 5694 | 0.02 | −2469 ± 5061 | 50.8 ± 6494 | 0.14 |
| Villus surface area:volume ratio | −0.0009 ± 0.0007 | 0.0003 ± 0.006 | 0.50 | −0.00004 ± 0.006 | −0.0006 ± 0.006 | 0.76 |
1Values are mean ± SD. Two-tailed independent-samples t test, with normality confirmed by the Shapiro–Wilk test. AA, amino acid; MM, multiple micronutrient.
FIGURE 3.
Change in VH and villus cross-sectional surface area per unit length of mucosa after AA (top panels) or MM (bottom panels) supplementation compared with placebo. Mean ± SD changes are given together with Tukey's hinges. Two-tailed independent-samples t test, with normality confirmed by the Shapiro–Wilk test. AA, amino acid; MM, multiple micronutrient; VH, villus height.
Primary endpoint: assessment of barrier dysfunction by CLE
Barrier leak was assessed using CLE (Figure 1). The median number of fields available for analysis was 72 at baseline and 98 after supplementation (IQR: 49–98 and 78–117.5, respectively). The mean ± SD change in the percentage of fields in which fluorescein leakage was detected was −10.1% ± 31% in the AA group compared with −5.1% ± 36% in the placebo group (P = 0.54). The mean ± SD change in the percentage of fields evaluated in which fluorescein leakage was detected was −11.3% ± 35% in the MM group compared with −4.3% ± 32% in the placebo group (P = 0.39). However, leak was reduced in the group allocated to AA + MM (P = 0.02 across all groups, P = 0.004 for interaction). The percentage of fields in which fluorescein leakage was observed was not correlated with morphometric or nutritional measurements (data not shown).
Primary endpoint: assessment of nutrient sensing by lamina propria CD4+ T cells using p4E-BP1 as an index of MTORC1 activation
The ability of small intestinal lamina propria CD4+ T lymphocytes to respond to an environmental nutrient stimulus was assessed by flow cytometry. Both basal (starved cells) and peak (fed cells) MTORC1 activity [mean fluorescence intensity (MFI) of p4E-BP1+ CD4+ T lymphocytes after 90 min of nutrient deprivation or stimulation, respectively] declined over the course of the study. Basal mean ± SD MFI declined from 286.4 ± 84.7 presupplementation to 145.5 ± 55.7 postsupplementation (P < 0.001) but this did not differ by treatment allocation.
The mean ± SD proportion of CD4+ T lymphocytes that were nutrient responsive (i.e., p4E-BP1+ after nutrient stimulation) was 51.3% ± 16.3% presupplementation. The proportion of mucosal CD4 cells responding to the nutrient stimulus fell after the supplementation period in all groups. The mean ± SD change was −6.1% ± 16% in 9 participants allocated to AA supplementation, which did not significantly differ from −7.4% ± 21% in 16 participants allocated to placebo (P = 0.86). The mean ± SD change was −9.8% ± 19% in 16 participants allocated to MM supplementation, which did not significantly differ from −3.7% ± 20% in 14 participants allocated to placebo (P = 0.40). The small number of paired samples available for analysis reflects the harsh permeabilization required for p4E-BP1 staining, which as an undesired side effect resulted in the destruction of a variable proportion of lymphocytes.
Although MTORC1 activation was not affected by treatment allocation, it was strongly correlated with posttreatment VH (ρ = 0.51; P = 0.001; Supplemental Figure 3). The change in MTORC1 MFI over the period of supplementation was correlated with the change in VH in the placebo group (ρ = 0.63; P = 0.03), but not in the AA group (ρ = 0.26; P = 0.62). MTORC1 activation was not associated with sex, HIV status, fat-free mass, grip strength, or T cell activation as assessed by human leucocyte antigen-DR expression.
Secondary endpoint: serum biomarkers
Serum concentrations of LPS, CRP, sCD14, and plasma GLP-2 did not differ between groups at baseline (Table 3). Changes in serum concentrations during the trial period did not differ in participants allocated to AA or placebo, or MM or placebo (Supplemental Table 3).
Secondary endpoint: metabolomic analysis
Having observed the effect of AA supplementation on VH, the metabolic phenotypes were investigated for features that might explain this effect, or correlate with the changes observed. Significant OPLS models were obtained for the 3 active groups (MM, AA, and both) compared with placebo (Tables 5 and 6, Figure 4).
TABLE 5.
Metabolomic changes in 3 active treatment groups compared with placebo1
| Increased with intervention | Reduced with intervention | P for model | |
|---|---|---|---|
| MM (n = 15) vs. placebo (n = 18) | Pantothenate, HMB, PAG, 4-CS | NMNA, 3-HPHPA | 0.01 |
| AA (n = 16) vs. placebo (n = 18) | BAIBA, PAG, 4-CS, citrate | No significant changes | 0.01 |
| MM + AA (n = 17) vs. placebo (n = 18) | Pantothenate, succinate, HMB, PAG, fumarate, 2-PY | No significant changes | 0.01 |
For details of statistical analysis see Methods. AA, amino acid; BAIBA, β-amino-isobutyric acid; HMB, β-hydroxy-β-methylbutyrate; MM, multiple micronutrient; NMNA, N-methylnicotinc acid; PAG, phenylacetylglutamine; 2-PY, N-methyl-2-pyridone-5-carboxamide; 3-HPHPA, 3-(3-hydroxyphenyl)-3-hydroxypropionic acid; 4-CS, 4-cresyl sulfate.
TABLE 6.
Metabolomic changes over the supplementation period by treatment group1
| Increased over time | Reduced over time | P for model | |
|---|---|---|---|
| MM pre vs. post (n = 18) | Pantothenate, HMB, succinate, 2-PY, NMND | BAIBA, 4-HH, 3-IS | 0.01 |
| AA pre vs. post (n = 16) | Citrate, myo-inositol, formate | Taurine, glycolate, trans-aconitate | 0.01 |
| MM + AA pre vs. post (n = 16) | Pantothenate, HMB, succinate, 2-PY, PAG, citrate, NMND, unknown | BAIBA, 2-HIB, choline, acetylcholine, taurine, hippurate | 0.01 |
| Placebo pre vs. post (n = 18) | No significant changes | No significant changes | N/A |
1Significant metabolomic changes within treatment groups over time. For details of statistical analysis see Methods. AA, amino acid; BAIBA, β-amino-isobutyric acid; HMB, β-hydroxy-β-methylbutyrate; MM, multiple micronutrient; NMND, N-methylnicotinamide; PAG, phenylacetylglutamine; 2-HIB, 2-hydroxyisobutyrate; 2-PY, N-methyl-2-pyridone-5-carboxamide; 3-IS, 3-indoxyl sulfate; 4-HH, 4-hydroxyhippurate.
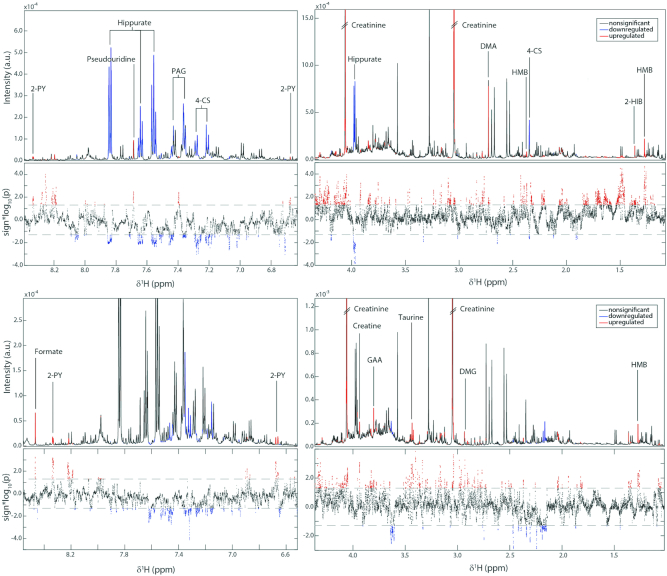
FIGURE 4.
Associations between the urinary metabolic profile and MTORC1 (upper panels) or VH (lower panels) based on the OPLS models. The NMR spectra are divided into 2 (right and left) to show the full range of δ1H. For each comparison the upper section of the panel shows the average 1H NMR spectrum (intensity in arbitrary units) and the lower section shows a Manhattan plot which displays the −log10(P value) for each of the spectral variables. Statistically significant peaks from the OPLS models are colored red if positively associated with MTORC1 or VH, and blue if inversely associated. n = 46 for both VH and MTORC1 models. For details of statistical analysis see the Methods. 2-HIB, 2-hydroxyisobutyrate; 2-PY, N-methyl-2-pyridone-5-carboxamide; 4-CS, 4-cresyl sulfate; DMA, dimethylamine, DMG, dimethylglycine; GAA, guanidinoacetic acid; HMB, β-hydroxy-β-methylbutyrate; MTORC1, mechanistic (or mammalian) target of rapamycin complex 1; PAG, phenylacetylglutamine; OPLS, orthogonal projection to latent structures; VH, villus height.
To explore drivers of our primary endpoints, the profiles were correlated with posttreatment VH and posttreatment MTORC1 activation. β-Hydroxy-β-methylbutyrate (HMB), dimethyglycine, creatine, creatinine, guanidinoacetic acid, taurine, N-methyl-2-pyridone-5-carboxamide (2-PY), and formate excretion were all positively correlated with VH. With respect to MTORC1 activation, HMB, 2-hydroxyisobutyrate, dimethylamine, pseudouridine, and 2-PY correlated positively, whereas 4-cresyl sulfate, hippurate, and phenylacetylglutamine (PAG) correlated negatively (Table 7). A summary of the origins and putative biological roles of these metabolites is given in Supplemental Table 4.
TABLE 7.
Metabolomic changes correlated with postsupplementation VH and/or MTORC1 activity1
| Outcome | Positively correlated | Negatively correlated | P for model |
|---|---|---|---|
| VH (n = 46) | HMB, DMG, creatine, creatinine, GAA, taurine, 2-PY, formate | No significant changes | 0.01 |
| MTORC1 (n = 46) | HMB, 2-HIB, 2-PY, pseudouridine, dimethylamine | 4-CS, hippurate, PAG | 0.01 |
Differentially excreted urinary metabolites correlated with posttreatment VH and posttreatment MTORC1 responsiveness. For details of statistical analysis see Methods. DMG, dimethylglycine; GAA, guanidinoacetic acid; HMB, β-hydroxy-β-methylbutyrate; MTORC1, mechanistic (or mammalian) target of rapamycin complex 1; PAG, phenylacetylglutamine; VH, villus height; 2-HIB, 2-hydroxyisobutyrate; 2-PY, N-methyl-2-pyridone-5-carboxamide; 4-CS, 4-cresyl sulfate.
Secondary endpoint: nutritional assessment
Random allocation to MM supplementation had no impact on change in BMI, MUAC, fat-free mass (by impedance or plethysmography), or grip strength over this time period. There was also no difference in nutritional parameters by random allocation to AA supplementation (data not shown).
Safety and adverse events
All supplements were well tolerated. The most frequent adverse events reported were feeling hungry, cough, diarrhea, nausea, vomiting, and dizziness. There were no serious adverse events and no deaths occurred. Adverse experiences were not more frequent in the AA or MM groups (Supplemental Tables 5 and 6).
Discussion
EE is a subclinical alteration in small intestinal mucosal architecture and function, widely prevalent in the tropics and in disadvantaged communities. Previous trials have identified few interventions which consistently and successfully target any of the domains of pathophysiology, but nutritional or other interventions are needed. In this double-blinded randomized controlled trial, on a background of a seasonal reduction in VH and villus surface area, supplementation with AA but not MM increased VH and surface area compared with placebo. However, the effects on intestinal barrier function (assessed in vivo using CLE) were very modest and only seen in the AA + MM group. There was no effect on plasma biomarkers of microbial translocation. The pronounced correlation between posttreatment p4E-BP1 expression in lamina propria CD4+ cells and VH suggests that nutrient sensing by the MTORC1 pathway in the mucosal immune compartment plays a part in determining villus morphology, but it is also possible that improved absorptive capacity increases MTORC1 activation. The pathway of the effect of AA supplementation on mucosal architecture, if not through MTORC1, remains to be determined.
Our morphometric findings are in contrast to a secondary analysis of 1 of our previous studies which suggested that MM supplementation exerted a protective effect on small bowel histology (26). We cannot offer a robust explanation for this finding, but we speculate that seasonality may contribute to this discrepancy because the first study was conducted during the dry season and this study was conducted over the course of hot and rainy seasons. We and others have consistently demonstrated a seasonal variation in mucosal structure and/or intestinal permeability and microbial translocation (9, 26). A reduction in small bowel absorptive area over the course of the rainy season (which coincided with the supplementation period) was again observed. It is assumed that loss of absorptive capacity is related to the increased pathogen load observed during the rainy season, due to inadequate water and sanitation, but a reduction in dietary quality and quantity and increased food prices during this time may also be relevant, particularly with regards to nutrient-sensing pathways. With hindsight, it would have been better to have spread the trial over a full calendar year to include all seasons.
Confocal endomicroscopy is a novel tool for evaluating epithelial integrity in the intestinal mucosa (17, 32). We found it to be highly reproducible when used to assess leak severity measured by fluorescein efflux. There is evidence that fluorescein leak is a reflection of disturbed tight junction proteins and microerosions (17, 33). However, evaluation of details of epithelial integrity at a cellular level is time-consuming, and less reproducible than assessment of fluorescein leak. Although we found a modest response of epithelial leak to AA + MM, there was no response of a range of biomarkers of microbial translocation to the interventions. It is possible that mucosal barrier function and epithelial integrity are regulated by different factors to VH and villus surface area.
The lack of effect of HIV on mucosal morphometry and on barrier function may seem surprising. However, the data presented here are entirely consistent with our earlier work (9) which showed that only in advanced AIDS did VH fall below the range consistent with EE, whereas crypt depth was increased earlier in HIV infection. We believe that in industrialized countries HIV may affect villus morphology, but that in low- and middle-income countries any such subtle effect is undetectable alongside the EE, which is virtually ubiquitous.
Metabolomic analysis revealed a number of insights which may merit further investigation. A number of host- and gut microbial–derived metabolites were significantly correlated with VH and/or CD4+ T lymphocyte MTORC1 responses. For example, HMB was positively correlated with both MTORC1 and VH. HMB is a physiological leucine metabolite which has been demonstrated to enhance lean muscle mass and strength and decrease muscle protein breakdown via MTORC1 signaling (34). Interestingly, HMB increased with the MM supplement but not the leucine-containing AA supplement, suggesting that such changes were not driven by increased leucine intake. HMB has been previously observed to increase after zinc supplementation in children from Brazil (unpublished data). The gut microbial–host cometabolites 4-cresyl sulfate, PAG, and hippurate were all negatively associated with MTORC1. These metabolites have been found to be excreted in greater amounts by stunted children and are thought to arise from an overgrowth of bacteria and malabsorption in the small intestine preventing their precursors from being absorbed, thus increasing their bioavailability for microbial processing (35). Further work is required to assess their direct potential to activate MTORC1.
The nicotinamide/NAD+ pathway has been previously observed to be perturbed with chronic undernutrition and EE (14, 15). NAD+ is key for many metabolic processes including energy generation but is also important for DNA repair processes. NAD+ can be synthesized from the dietary precursors nicotinic acid (Preiss-Handler pathway), nicotinamide and nicotinamide riboside (salvage pathway), or via tryptophan (de novo biosynthesis pathway). Of note, either tryptophan or nicotinic acid was supplemented in 3 of the 4 trial arms. N-methylnicotinamide (NMND), a urinary marker of NAD+ abundance, has been positively associated with catch-up growth, whereas N-methylnicotinic acid (NMNA; trigonelline) excretion has been found to be reduced in wasted and underweight children. NMNA is a dietary constituent found in legumes and coffee. Although nicotinic acid is not methylated to NMNA in vivo, 1 study observed that when niacin-deficient rats received bound nicotinic acid (niacytin) from cereals, a prolonged excretion of NMNA followed. A defect in small intestinal permeability was implicated in the mechanism. In the present study, MM supplementation [containing nicotinic acid (niacin)] increased the excretion of NMND and its breakdown product 2-PY and decreased the excretion of NMNA. In addition, 2-PY was positively associated with VH and MTORC1 activation.
The tryptophan–indoleamine 2,3-dioxygenase (IDO)–kyneurine immunometabolic axis is currently a subject of great interest. Tryptophan is partly metabolized through host IDO 1 and 2 to produce kynurenines, and this pathway may be subverted by microbial tryptophan uptake and metabolism. High levels of IDO activity promote regulatory immune responses and Treg differentiation and inhibit Teff differentiation (36, 37). In stunted children with EE, plasma tryptophan concentrations are correlated with growth (38), and the kynurenine to tryptophan ratio (KTR, with high ratios indicative of high IDO activity) is correlated with systemic markers of inflammation and inversely correlated to growth (i.e., high KTR and presumed greater IDO activity are associated with greater stunting) (30), suggesting that microbial manipulation of tryptophan signaling and inhibition of kynurenine production may be relevant in the pathophysiology of EE. The metabolic differences observed in this study also suggest a possible role for tryptophan signaling in modulating the villus compartment and immune cell nutrient sensing.
A number of other metabolites correlated with VH, MTORC1 activity, or the interventions were identified. These can be broadly classed as AA–derived (e.g., taurine, 4-cresyl sulfate, PAG), tricarboxylic cycle intermediaries (e.g., succinate, formate), or microbial coproducts (e.g., pseudouridine, hippurate). Although some of these have been identified in undernourished/stunted populations previously, the significance of these findings in this study is unclear.
In recent reviews, it has been proposed that measurement of EE should be considered in several domains—villus morphology, permeability, microbial translocation, mucosal inflammation, systemic inflammation, malabsorption, and changes in the intestinal microbiota (35). Our data suggest that therapies for EE may have a preferential impact on 1 of these domains (villus morphology) without necessarily having a significant impact on the others. Indeed, our previous work has suggested that the observed villous atrophy may in fact have a protective role in EE (17). This suggests that VH, itself determined by enterocyte replication, migration, and apoptosis as well as stromal cells, might be rate-limited by the availability of nutrients such as glutamine, leucine, and tryptophan.
In summary, a safe and well-tolerated AA supplement in adults with EE resulted in increased VH (i.e., changes in gut mucosal structure), but these changes were not matched by improvements in intestinal barrier function, and intestinal barrier function only improved in the AA + MM group. There was consistent evidence of a correlation between VH and MTORC1 responsiveness in lymphocytes. Further work is required to determine whether modest improvements in small intestinal structure have relevant physiological benefits, such as improved absorption of nutrients. Given the promising effects of leucine in improving muscle mass in other contexts (39), work on AA/MM supplementation could provide benefits in >1 physiological system.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to our team in Misisi and in the University Teaching Hospital: John Mbewe, Rose Soko, and Joyce Sibwani. The ImageJ morphometry script was written with assistance from Dr. B Martín-Martín (Blizard Institute, Queen Mary University of London).
The authors’ responsibilities were as follows—JL-A and PK: designed the trial and wrote the first draft of the manuscript; JL-A, EB, KZ, DM, RB, TB, and PK: conducted the trial; AW: designed and trained the team on CLE scoring; JL-A, EB, KZ, JM-P, JS, AW, and PK: analyzed the data; and all authors: take responsibility for, read, and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by CORE, UK (to JL-A) and Bill & Melinda Gates Foundation grant OPP1066118 (to PK). The micronutrient supplement and matching placebo were generously manufactured and donated by Vitabiotics Ltd, UK.
Supplemental Tables 1–6, Supplemental Figures 1–3, and Supplemental Methods are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article (an anonymized database) will be made available upon request to the corresponding author pending application and approval.
Abbreviations used: AA, amino acid; CLE, confocal laser endomicroscopy; CRP, C-reactive protein; EE, environmental enteropathy; GLP-2, glucagon-like peptide 2; HMB, β-hydroxy-β-methylbutyrate; IDO, indoleamine 2,3-dioxygenase; KTR, kynurenine to tryptophan ratio; MFI, mean fluorescence intensity; MM, multiple micronutrient; MTORC1, mechanistic (or mammalian) target of rapamycin complex 1; MUAC, midupper arm circumference; NMNA, N-methylnicotinic acid; NMND, N-methylnicotinamide; OPLS, orthogonal projection to latent structures; p4E-BP1, phospho-4E-binding protein 1; PAG, phenylacetylglutamine; sCD14, soluble CD14; VH, villus height; 2-PY, N-methyl-2-pyridone-5-carboxamide.
References
- 1. Keusch GT, Denno DM, Black RE, Duggan C, Guerrant RL, Lavery JV, Nataro JP, Rosenberg IH, Ryan ET, Tarr PI et al.. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis. 2014;59:S207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prendergast A, Kelly P. Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg. 2012;86:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis-Auguste J, Kelly P. Tropical enteropathies. Curr Gastroenterol Rep. 2017;19:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prendergast AJ, Rukobo S, Chasekwa B, Mutasa K, Ntozini R, Mbuya MNN, Jones A, Moulton LH, Stoltzfus RJ, Humphrey JH. Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS One. 2014;9:e86928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naylor C, Lu M, Haque R, Mondal D, Buonomo E, Nayak U, Mychaleckyj JC, Kirkpatrick B, Colgate R, Carmolli M et al.. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine. 2015;2:1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang NM, Tofail F, Ma JZ, Haque R, Kirkpatrick B, Nelson CA, Petri WA. Early life inflammation and neurodevelopmental outcome in Bangladeshi infants growing up in adversity. Am J Trop Med Hyg. 2017;97:974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manary MJ, Abrams SA, Griffin IJ, Quimper MM, Shulman RJ, Hamzo MG, Chen Z, Maleta K, Manary MJ. Perturbed zinc homeostasis in rural 3–5-y-old Malawian children is associated with abnormalities in intestinal permeability attributed to tropical enteropathy. Pediatr Res. 2010;67:671–5. [DOI] [PubMed] [Google Scholar]
- 8. Church JA, Parker EP, Kosek MN, Kang G, Grassly NC, Kelly P, Prendergast AJ. Exploring the relationship between environmental enteric dysfunction and oral vaccine responses. Future Microbiol. 2018;13:1055–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelly P, Menzies I, Crane R, Zulu I, Nickols C, Feakins R, Mwansa J, Mudenda V, Katubulushi M, Greenwald S et al.. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg. 2004;70:412–19. [PubMed] [Google Scholar]
- 10. Menzies I, Zuckerman MJ, Nukajam WS, Somasundaram SG, Murphy B, Jenkins AP, Crane RS, Gregory GG. Geography of intestinal permeability and absorption. Gut. 1999;44:483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Denno DM, VanBuskirk K, Nelson ZC, Musser CA, Hay Burgess DC, Tarr PI. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin Infect Dis. 2014;59(Suppl 4):S213–19. [DOI] [PubMed] [Google Scholar]
- 12. Kosek M, Haque R, Lima AA, Babji S, Shrestha S, Qureshi S, Amidou S, Mduma E, Lee G, Yori PP et al.. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013;88:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kosek MN; The MAL-ED Network Investigators. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED birth cohort study. EBioMedicine. 2017;18:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mayneris-Perxachs J, Lima AAM, Guerrant RL, Leite ÁM, Moura AF, Lima NL, Soares AM, Havt A, Moore SR, Pinkerton R et al.. Urinary N-methylnicotinamide and β-aminoisobutyric acid predict catch-up growth in undernourished Brazilian children. Sci Rep. 2016;6:19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayneris-Perxachs J, Bolick DT, Leng J, Medlock GL, Kolling GL, Papin JA, Swann JR, Guerrant RL. Protein- and zinc-deficient diets modulate the murine microbiome and metabolic phenotype. Am J Clin Nutr. 2016;104(5):1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu J, Ordiz MI, Stauber J, Shaikh N, Trehan I, Barnell E, Head RD, Maleta K, Tarr PI, Manary MJ. Environmental enteric dysfunction includes a broad spectrum of inflammatory responses and epithelial repair processes. Cell Mol Gastroenterol Hepatol. 2016;2:158–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelly P, Besa E, Zyambo K, Louis-Auguste J, Lees J, Banda T, Soko R, Banda R, Amadi B, Watson A. Endomicroscopic and transcriptomic analysis of impaired barrier function and malabsorption in environmental enteropathy. PLoS Negl Trop Dis. 2016;10:e0004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luby S, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, Stewart CP, Begum F, Hussain F, Benjamin-Chung J et al.. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhea and child growth in rural Bangladesh: a cluster randomized controlled trial. Lancet Glob Health. 2018;6:e302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, Benjamin-Chung J, Clasen T, Dewey KG, Fernald LCH et al.. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhea and child growth in rural Kenya: a cluster-randomized controlled trial. Lancet Glob Health. 2018;6:e316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trehan I, Shulman RJ, Ou CN, Maleta K, Manary MJ. A randomized, double-blind, placebo-controlled trial of rifaximin, a nonabsorbable antibiotic, in the treatment of tropical enteropathy. Am J Gastroenterol. 2009;104:2326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang AZ, Shulman RJ, Crocker AH, Thakwalakwa C, Maleta KM, Devaraj S, Manary MJ, Trehan I. A combined intervention of zinc, multiple micronutrients, and albendazole does not ameliorate environmental enteric dysfunction or stunting in rural Malawian children in a double-blind randomized controlled trial. J Nutr. 2017;147:97–103. [DOI] [PubMed] [Google Scholar]
- 22. Ryan KN, Stephenson KB, Trehan I, Shulman RJ, Thakwalakwa C, Murray E, Maleta K, Manary MJ. Zinc or albendazole attenuates the progression of environmental enteropathy: a randomized controlled trial. Clin Gastroenterol Hepatol. 2014;12(9):1507–13..e1. [DOI] [PubMed] [Google Scholar]
- 23. Galpin L, Manary MJ, Fleming K, Ou CN, Ashorn P, Shulman RJ. Effect of Lactobacillus GG on intestinal integrity in Malawian children at risk of tropical enteropathy. Am J Clin Nutr. 2005;82:1040–5. [DOI] [PubMed] [Google Scholar]
- 24. Jones KD, Hünten-Kirsch B, Laving AM, Munyi CW, Ngari M, Mikusa J, Mulongo MM, Odera D, Nassir HS, Timbwa M et al.. Mesalazine in the initial management of severely acutely malnourished children with environmental enteric dysfunction: a pilot randomized controlled trial. BMC Med. 2014;12:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petri WA, Naylor C, Haque R. Environmental enteropathy and malnutrition: do we know enough to intervene?. BMC Med. 2014;12:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Louis-Auguste J, Greenwald S, Simuyandi M, Soko R, Banda R, Kelly P. High dose multiple micronutrient supplementation improves villous morphology in environmental enteropathy without HIV enteropathy: results from a double-blind randomised placebo controlled trial in Zambian adults. BMC Gastroenterol. 2014;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lima AAM, Brito LFB, Ribeiro HB, Martins MC, Lustosa AP, Rocha EM, Lima NL, Monte CM, Guerrant RL. Intestinal barrier function and weight gain in malnourished children taking glutamine supplemented enteral formula. J Pediatr Gastroenterol Nutr. 2005;40:28–35. [DOI] [PubMed] [Google Scholar]
- 28. Rhoads JM, Wu G. Glutamine, arginine, and leucine signalling in the intestine. Amino Acids. 2009;37:111–22. [DOI] [PubMed] [Google Scholar]
- 29. Williams EA, Elia M, Lunn PG. A double-blind, placebo-controlled, glutamine-supplementation trial in growth-faltering Gambian infants. Am J Clin Nutr. 2007;86:421–7. [DOI] [PubMed] [Google Scholar]
- 30. Kosek MN, Mduma E, Kosek PS, Lee GO, Svensen E, Pan WKY, Olortegui MP, Bream JH, Patil C, Asayag CR et al.. Plasma tryptophan and the kynurenine–tryptophan ratio are associated with the acquisition of statural growth deficits and oral vaccine underperformance in populations with environmental enteropathy. Am J Trop Med Hyg. 2016;95(4):928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guerrant RL, Leite AM, Pinkerton R, Medeiros PH, Cavalcante PA, DeBoer M, Kosek M, Duggan C, Gewirtz A, Kagan JC et al.. Biomarkers of environmental enteropathy, inflammation, stunting, and impaired growth in children in northeast Brazil. PLoS One. 2016;11:e0158772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, Pritchard DM, Galle PR, Neurath MF, Watson AJ. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61(8):1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lim LG, Neumann J, Hansen T, Goetz M, Hoffman A, Neurath MF, Galle PR, Chan YH, Kiesslich R, Watson AJ. Confocal endomicroscopy identifies loss of local barrier function in the duodenum of patients with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2014;20:892–900. [DOI] [PubMed] [Google Scholar]
- 34. Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM et al.. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J Physiol. 2013;591(11):2911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vonaesch P, Morien E, Andrianonimiadana L. Stunted childhood growth is associated with decompartmentalization of the GI tract and overgrowth of oropharyngeal taxa. Proc Natl Acad Sci U S A. 2018;115(36):E8489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yan Y, Zhang G-X, Gran B, Fallarino F, Yu S, Li H, Cullimore ML, Rostami A, Xu H. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol. 2010;185(10):5953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Metz R, Rust S, DuHadaway JB, Mautino MR, Munn DH, Vahanian NN, Link CJ, Prendergast GC. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR. Oncoimmunology. 2012;1(9):1460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Semba RD, Shardell M, Ashour FAS, Moaddel R, Trehan I, Maleta KM, Ordiz MI, Kraemer K, Khadeer MA, Ferrucci L et al.. Child stunting is associated with low circulating essential amino acids. EBioMedicine. 2016;6:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Borack MS, Volpi E. Efficacy and safety of leucine supplementation in the elderly. J Nutr. 2016;146(12):2625S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.