ABSTRACT
Background
Determining the human vitamin E [α-tocopherol (α-T)] requirement is difficult, and novel approaches to assess α-T absorption and trafficking are needed.
Objective
We hypothesized that the dual-isotope technique, using 2 deuterium-labeled [intravenous (IV) d6- and oral d3-] α-T, would be effective in determining α-T fractional absorption. Further, defined liquid meal (DLM) fat or fasting would modulate α-T fractional absorption and lipoprotein transport.
Methods
A 3-phase cr ossover design was used. At 0 h, participants received IV d6-α-T and consumed d3-α-T with a 600-kcal DLM (40% or 0% fat) followed by controlled meals or by the 0% fat DLM, a 12-h fast, and then controlled meals. Blood samples and fecal samples were collected at intervals and analyzed by LC-MS. Pharmacokinetic parameters were calculated from plasma tracer concentrations and enrichments. Fractional absorption was calculated from d3- to d6-α-T areas under the curve, from a novel mathematical model, and from the balance method (oral d3-α-T minus fecal d3-α-T excreted).
Results
Estimated α-T fractional absorption during the 40% fat intervention was 55% ± 3% (mean ± SEM; n = 10), which was 9% less than during the 0% fat intervention (64% ± 3%, n = 10; P < 0.02). Fasting had no apparent effect (56% ± 3%, n = 7), except it slowed plasma oral d3-α-T appearance. Both balance data and model outcomes confirmed that the DLM fat did not potentiate d3-α-T absorption. During the IV emulsion clearance, HDL rapidly acquired d6-α-T (21 ± 2 nmol/L plasma per minute). During the first 8 h postdosing, triglyceride-rich lipoproteins (TRLs) were preferentially d3-α-T enriched relative to LDL or HDL, showing the TRL precursor role.
Conclusions
Quantitatively, α-T absorption is not limited by fat absence or by fasting. However, α-T leaves the intestine by a process that is prolonged during fasting and potentiated by eating, suggesting that α-T absorption is highly dependent on chylomicron assembly processes. This trial was registered at clinicaltrials.gov as NCT00862433.
Keywords: chylomicrons, fecal α-T analysis, dual-isotope ratio method, HDL and LDL, triglyceride-rich lipoproteins
Introduction
Determining human vitamin E [α-tocopherol (α-T)] dietary requirements has proven difficult (1). Healthy, normal-weight women were chosen for the present study because current vitamin E requirements (1) have been based on a study that only investigated men (2). The current vitamin E RDA may be higher than necessary. Novotny et al. (3) have suggested that absorption of dietary α-T is greater than estimated previously. Thus, accurate estimates of α-T fractional absorption are needed (1).
Orally administered stable isotope [deuterium (d)]–labeled α-T has been used to measure kinetics of vitamin E, their esters, or different α-T stereoisomers and to report relative bioavailabilities. Previously, we found that as the dietary fat given with the labeled vitamin E (α-T-acetate) decreased, the maximum plasma labeled d-α-T concentration (Cmax) also decreased (4, 5). Dietary fat is required to induce mechanisms to hydrolyze α-T-acetate (6), and Cheeseman et al. (7) demonstrated that when vitamin E was consumed with fat, there were no effects on the relative bioavailabilities of α-T compared with α-T-acetate.
Various approaches can be used to quantitate intestinal absorption. The balance method estimates the differences between intake and excretion. Limitations with regards to α-T include 1) the fecal collection may not be complete and α-T losses are miscounted as absorbed; 2) losses could occur due to bacterial degradation (8); 3) α-T undergoes enterohepatic circulation, and excreted recirculating α-T is considered unabsorbed (9, 10); and 4) delays in α-T absorption could occur due to the presence of fat droplets in the enterocyte (11). Balance studies using radioactive α-T have estimated α-T fractional absorption (3, 12, 13) with estimates as high as 80%. These values are higher than the ∼30% based on plasma Cmax (4, 14), suggesting there were unexplained losses or incomplete fecal collections that inflated the α-T absorption values.
α-T fractional absorption measured using the dual-isotope method is based on the ratio of the plasma concentrations following administration of differently labeled oral and intravenous (IV) doses. The ratios of the oral to the IV (equals 100%) are expected to equal fractional absorption. A challenge is that a lipid emulsion containing the α-T dose that has characteristics of chylomicron-like particles (15, 16) and delivers α-T to the liver in a physiologically relevant manner (17) must be developed.
We describe a new stable isotope-labeled [hexadeuterium (d6)]-α-T for IV administration to humans and show its efficacy with simultaneous administration of an oral stable isotope-labeled α-T [trideuterium (d3)-α-T]. We validate this new dual-isotope method for our primary outcome of α-T fractional absorption with pharmacokinetic parameters estimated from α-T administered by IV (d6-α-T) compared with oral (d3-α-T) routes. Further, we assess the fractional α-T absorption measured using 3 techniques: 1) the dual-isotope method, 2) the balance method, and 3) modeling of the plasma enrichment percentage of the 2 isotopes (d6- and d3-α-T). Primary outcomes also included the effects of fat ingestion and meal ingestion on α-T fractional absorption. Secondary outcomes included the effects of these factors on standard pharmacokinetic parameters and on α-T lipoprotein kinetics.
Methods
Clinical research trial
This clinical trial, registered at clinicaltrials.gov as NCT00862433, was conducted at the NIH Clinical Research Center (CRC). The NIH National Institute of Diabetes and Digestive Kidney Diseases/National Institute of Arthritis and Musculoskeletal and Skin Diseases Institutional Review Board (IRB) for Protection of Human Subjects approved the protocol (09-DK-0097). The study was performed under an Investigational New Drug protocol (110033) issued by the US Food and Drug Administration to ML. Oregon State University IRB deferred to the NIH-IRB. Sample collection for the trial described herein took place between January 2015 and August 2016. Sample analyses took place at the NIH and at the Linus Pauling Institute.
Research population description
Participants were adult normotensive (<160/90 mm Hg), nonobese (BMI <29.9 kg/m2) nondiabetic women (aged 18–40 y) able to give informed consent (Table 1). The participants were not users of regular medications (other than hormonal contraceptives) and were willing to use effective contraceptive methods. They had urinary isoprostane levels within normal ranges (18).
TABLE 1.
Subject baseline characteristics, shown by intervention1
| Characteristic | 40% fat (n = 10) | 0% fat (n = 10) | 0% fat-fast (n = 7) |
|---|---|---|---|
| Age, y | 23 (19–32) | 24 (19–32) | 23 (19–33) |
| Body weight, kg | 60 (48–75) | 65 (57–75) | 66 (58–77) |
| BMI, kg/m2 | 22 (17–25) | 24 (21–26) | 24 (22–27) |
| Body fat,2 % | 31 (14–45) | 34 (23–45) | 36 (23–45) |
| Blood pressure (systolic), mm Hg | 106 (96–121) | 112 (96–120) | 112 (103–120) |
| Blood pressure (diastolic), mm Hg | 67 (45–76) | 66 (53–75) | 67 (55–80) |
| Total cholesterol, mg/dL | 145 (96–195) | 151 (101–190) | 170 (115–195) |
| Triglycerides, mg/dL | 76 (38–120) | 58 (32–204) | 62 (45–86) |
| HDL cholesterol, mg/dL | 60 (35–82) | 59 (38–74) | 61 (38–82) |
| LDL cholesterol, mg/dL | 72 (40–102) | 72 (56–110) | 89 (66–120) |
| Glucose, mg/dL | 88 (78–99) | 91 (80–97) | 90 (73–91) |
| Hematocrit, % | 36 (31–40) | 36 (32–39) | 36 (33–38) |
| 8-IsoPGF2a,3 ng/g creatinine | 0.38 (0.22–0.63) | 0.41 (0.16–0.77) | 0.35 (0.25–0.75) |
| Prostaglandin F2a,3 ng/g creatinine | 1.12 (0.83–2.73) | 1.29 (0.47–2.51) | 1.24 (0.57–2.05) |
Values are medians (ranges). Intervention groups included the following participants: 40% fat (n = 10), subject numbers 1 to 9 and 12; 0% fat (n = 10), subject numbers 1 to 5, 8 to 11, and 13; and 0% fat-fast (n = 7), subject numbers 1, 2, 4, 5, 9, 10, and 11. Five women participated in all 3 interventions, subject numbers 1, 2, 4, 5, and 9. The medians were calculated from the baseline data of the individuals participating in that group.
Body fat was determined by dual-energy X-ray absorptiometry whole-body composition analyses (Hologic Discovery QDR; Hologic).
Urinary isoprostanes measured as described (18). Values shown in the table are within the ranges for nonsmoking, healthy women.
We excluded women with digestive abnormalities, such as malabsorption or chronic diarrhea; organ malfunction, including (but not limited to) liver disease, pulmonary disease, ischemic heart disease, heart failure, stroke, and peripheral vascular disease; hypertension (blood pressure >160/90 mm Hg); anemia (hematocrit <30%); current or history of serious or chronic illness, including hyperlipidemia or hypercholesterolemia; coronary artery disease; or peripheral vascular disease. Additionally, participants could not smoke tobacco, use medications (including insulin, but not contraceptives or medications taken only on an as-needed basis), use alcohol, or abuse drugs. Pregnancy or lactation was not allowed (a urine pregnancy test was performed on all women before each intervention). Also excluded were women with positive HIV or hepatitis (B or C) screening tests; allergies to soy, egg, milk protein (casein), or wheat/gluten; or a known coagulopathy.
Study recruitment and assignment
We used a crossover design but did not randomize the order, and there was no blinding. We initially recruited subjects to the 40% fat intervention (see "Contolled metabolic diet" section) to validate the dual-isotope method prior to testing other fat interventions (Figure 1). Although we hypothesized that absence of fat would decrease fractional absorption, we did not observe significant differences between the 0% and the 40% fat intervention outcomes (see Results). For this reason, we added an additional objective, which was to study the effect of fasting to unmask effects of food ingestion.
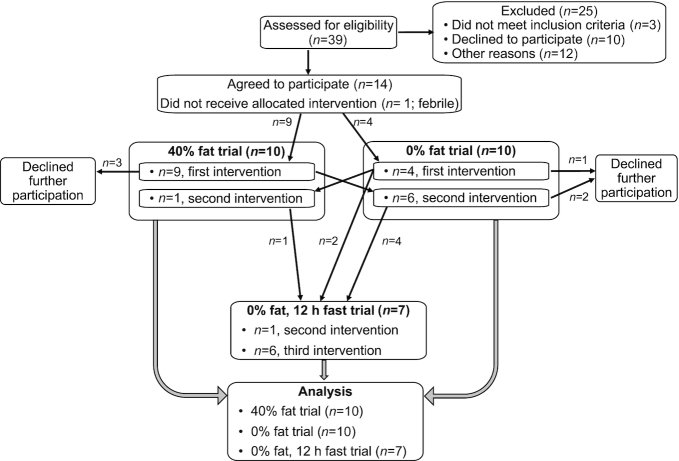
FIGURE 1.
Recruitment scheme. The recruitment scheme for this clinical intervention (NCT00862433), carried out at the NIH Clinical Research Center, is shown. Data from all participants are included in the analyses. Statistical methods were used to correct for missing subjects (SAS, PROC MIXED; see Methods). Where indicated, comparisons (paired t-test) were made between the subjects who repeated an intervention [see footnotes to Tables 6 and 7, 40% fat and 0% fat (n = 7 or 6, respectively) or 0% fat and 0% fat-fast (n = 7)] . Five women participated in all 3 interventions.
Participants in the 40% fat intervention were invited to enroll in the 0% fat intervention, but some chose not to participate further, necessitating that additional subjects be recruited to the 0% fat intervention. One new participant in the 0% fat intervention agreed to also participate in the 40% fat intervention. Last, participants who completed the 0% fat intervention were invited to enroll in the 0% fat intervention with a 12-h fast following oral dosing. Hereafter, these interventions are called 40% fat, 0% fat, or 0% fat-fast interventions, respectively. Interventions included the following participants: 40% fat (n = 10), subject numbers 1 to 9 and 12; 0% fat (n = 10), subject numbers 1 to 5, 8 to 11, and 13; and 0% fat-fast (n = 7), subject numbers 1, 2, 4, 5, 9, 10, and 11. Five women participated in all 3 interventions, subject numbers 1, 2, 4, 5, and 9.
To avoid confounding results with variable vitamin C status (19), vitamin C stores were saturated in all subjects (20–22). Two weeks prior to admission, participants consumed 1 g vitamin C daily for 7 d, and then the dose was decreased to 250 mg daily and continued for the duration of the subject's participation.
Controlled metabolic diet
During the stays, all meals were controlled and were provided and monitored by the NIH CRC Metabolic Kitchen. Participants were admitted the evening prior to d3- and d6-α-T administration and consumed a low vitamin E dinner and snack. On the dosing day (first full CRC day), participants consumed a 600-kcal defined liquid meal (DLM) at 0945. The DLM contained 1) 40% fat, 2) 0% fat, or 3) 0% fat followed by a 12-h fast with dinner at 2200. The fat percentage in the DLMs was modified by using skim compared with whole milk (lactose-free), as well as sorbet compared with ice cream (lactose-free). Thereafter, unless participating in the fasting condition, participants received meals at 1400 and 1800 and followed the meal schedule described below. When blood, urine, or fecal sampling occurred at mealtimes, sampling was completed prior to food consumption. Minimal amounts of vitamin E (on average, <6 mg α-T per day and ∼12 mg γ-T per day total in all meals, snacks, and beverages) were consumed daily (see Supplemental Table 1 and Supplemental Figure 1). Participants in the 40% and 0% interventions received ∼1800 kcal in 3 meals (breakfast 1000, lunch 1400, dinner 1800) and a snack (2200). Participants in the 0% fat-fast intervention only received a meal at 2200, 12 h after the DLM; on subsequent study days, they received 3 meals daily. Macronutrient composition of the diets was 55% carbohydrate, 15% protein, and 30% fat. On the dosing day, the 600-kcal DLM limited the remaining meals to ∼1200 kcal (1800 kcal total for the day), except during the 0% fat-fast intervention, where the 2200 dinner contained ∼600 kcal (1200 kcal total for the day). Foods rich in vitamin E (wheat germ oil, almonds, sunflower seeds, snack chips, and spinach) were not included in the diets. Five diet options meeting these criteria were given as options to the participants, who selected preferred menu options. The diets were analyzed prior to the study to confirm vitamin E content (Supplemental Table 1).
Oral and IV deuterium-labeled α-T doses
Because α-T is naturally found in food, α-T is the vitamin E form chosen for investigation in this study. The oral dose contained d3-α-T, which was synthesized from γ-T (to ensure that the resulting d3-α-T was in the RRR-stereoisomeric form) using SnCl2-catalyzed deuteromethylation with perdeutero-paraformaldehyde (23) under good manufacturing practices for use in humans (PCI Pharma). The isotopic distribution was 0.8% d0-α-T, 98.4% d3-α-T, and 0.8% d6-α-T, with >99% deuterium-labeled α-T. The d3-α-T was diluted with soybean oil (final concentration 50 mg/mL oil) and the precise dose (30 mg d3-α-T) weighed and placed in a dosing syringe for oral administration. The oral dose preparation was carried out by the NIH Pharmacy Pharmaceutical Development Service. The IV dose contained d6-α-T synthesized from δ-T (to ensure that the resulting d6-α-T was in the RRR-stereoisomeric form) under good manufacturing practices for IV use in humans (ChemCon), as described (24). The isotopic distribution was 0% d0-α-T, 0% d3-α-T, 97.6% d6-α-T, and 0.2% d7-α-T, with >99% deuterium-labeled α-T. The equivalence of the 2 tracers (d3-RRR-α-T and d6-RRR-α-T) has been reported previously (25).
The d6-α-T (5.4 g/L, final concentration) was suspended in oil from which an oil/water emulsion containing soybean oil and phospholipids (egg yolk lecithin) was prepared under nitrogen (15). The IV emulsion (particles <0.5 nm) was aliquoted into 10-mL quantities in single-use vials, then sterilized by autoclaving (Fresenius-Kabi).
The emulsion in individual vials was stored protected from light in a closed box and in a dark cabinet at 22–23°C. Emulsion stability and α-T purity were monitored throughout the study by the Traber laboratory and the NIH Pharmacy. Particle size distribution at manufacture was on average 225 nm, with 100% ≤5 µm and 98% ≤1.5 µm, as measured by Fresenius-Kabi. From July 2013 to August 2016, as monitored by the NIH Pharmacy, 80% of the particles were ≤0.5 µm, 20% were between >0.5 and <1.2 µm, and 100% were ≤5 µm. The pH remained within limits (pH 7.5–8.5). Malondialdehyde was routinely measured, as described (26), and remained between 2.0 and 4.2 µmol/L. The d3- and d6-α-T purities and concentrations remained unchanged, no increases in oxidation products were detected, and the emulsion remained stable.
The dose sizes were 30 mg each of the oral d3-α-T (69.3 μmol) and IV d6-α-T (68.8 μmol). Each of these doses is double the 15-mg current vitamin E RDA (1) but substantially smaller than commonly used vitamin E supplements (e.g., hundreds of milligrams). The dose was chosen to allow the labels to be followed for up to 30 d. No adverse effects were observed.
Study procedures
Participants were housed in the NIH CRC for up to 5 d (Figure 2) for each phase of the study. Outpatient studies with sampling 2–3 times per week continued in some subjects up to 30 d (data not shown). Participants were allowed to watch TV, read, take naps, or rest, but they were not allowed to perform any strenuous physical activity.
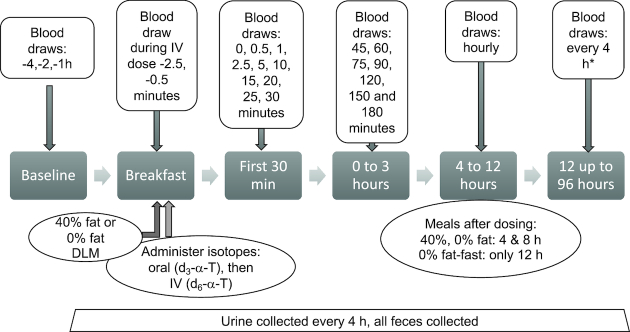
FIGURE 2.
Study design. Participants stayed in the Clinical Research Center (CRC) for 5–6 d. Upon admission to the CRC, participants consumed the same dinner and fasted for 12 h prior to dosing the following morning. We studied participants under 3 dietary interventions: a defined liquid meal (DLM) with 40% fat, 0% fat, or 0% fat followed by a 12-h fast (0% fat-fast). After an overnight fast on the morning of dosing, subjects were fitted with an intravenous (IV) cannula in each arm. Three fasting baseline blood samples 1 h apart were obtained to allow calculation of the background stable isotopes and estimates of enrichment percentages. The participant consumed approximately two-thirds of the DLM, and then the oral dose was administered as a measured amount of oil placed on the participant's tongue followed by consumption of the remaining DLM. Immediately following the oral dosing, the IV dose was administered via 1 IV cannula by slow injection over ∼6 min. Blood sample collection from the contralateral IV cannula commenced with the beginning of IV dosing. Upon completion of the IV dose administration as follows, blood sampling occurred at 0, 0.5, 1, 3, 5.0, 10, 15, 20, 25, 30, 45, and 60 min; 1.25, 1.5, 2, and 2.5 h; and then hourly from 3 to 12 h, then every 4 to 72 h. *For the 0% fat-fast intervention, hourly sampling was continued until 18 h. All feces were collected up to 96 h.
Following an overnight fast, an IV cannula was placed into each arm. Three fasting baseline blood samples were obtained approximately 1 h apart beginning at –3 to –4 h to allow calculation of the background stable isotopes and estimates of enrichment percentages.
Deuterated-α-T was administered via 2 routes, as follows. At 0945 for every intervention, each participant consumed approximately two-thirds of the DLM. The oral dose was then administered by placing it on the participant's tongue, who then consumed the remaining DLM. The IV d6-α-T dose (5.6 mL) was drawn from a single-use vial into a syringe. Immediately upon finishing the DLM, the IV dose was administered via an IV cannula by slow injection over ∼6 min. Blood sample collection from the contralateral IV cannula commenced with the beginning of IV dosing. The schedule included multiple blood, urine, and fecal samples (Figure 2).
Blood volumes were ∼10 mL per sample for the first 24 h, then approximately 6 mL per sample for the remainder of each intervention. Blood collection tubes (containing sodium heparin) were kept on ice for under 30 min prior to plasma and erythrocyte (RBC) isolation by centrifugation. Plasma was further processed to obtain lipoprotein fractions, as described below. The remaining plasma was aliquoted into cryovials, frozen in liquid nitrogen, and kept frozen at –80°C.
RBCs were washed twice with PBS–EDTA (0.01 M PBS, pH 7.4 with 1% EDTA) by centrifugation at 500 × g at 4°C, then resuspended in PBS. The final time, RBCs were centrifuged, resuspended with an equal volume of PBS, mixed by inversion, aliquoted, and frozen at –80°C. RBC α-T are reported as the ratio of α-T to cholesterol (data not shown), as well as by d3- and d6-α-T enrichment percentages (see analytical section below).
Urine was collected every 4 h during the first 24 h, then every 8 h up to 96 h (no urine data are reported); feces were collected during the CRC stay (up to 96 h). Some participants chose not to collect feces; some subjects collected feces at 1, 2, or 3 times up to 96 h. Fecal samples collected from subjects in the 0% fat-fast intervention were insufficient for analysis. Both urine and fecal samples were stored frozen at –80°C.
The plasma, RBC, lipoprotein (see below), urine, and fecal samples were shipped on dry ice by overnight freight to the Traber laboratory.
Analytical procedures
Lipoprotein isolation by ultracentrifugation and by precipitation
Lipoprotein density fractions [triglyceride-rich fraction (TRL; protein-free density <1.006 g/mL), LDL (1.006 < density < 1.063 g/mL), HDL (1.063 < density < 1.21 g/mL), and infranate (density >1.21 g/mL)] were isolated using discontinuous salt density-gradient ultracentrifugation as described (27). Isolated lipoprotein fractions were frozen and stored at –80°C until analyzed for unlabeled and labeled α-T (see below), as well as total cholesterol concentrations, as described (28). Attempts to isolate the emulsion from the TRL were unsuccessful (data not shown).
For lipoprotein isolation for ex vivo incubation studies, four 10-mL blood samples from each subject were drawn into sodium heparin tubes 30 min prior to isotope dosing. To produce a final concentration of ∼7 µM, 5.5 µL d6-α-T emulsion (12.3 µmol/µL) was added ex vivo to each 10-mL blood sample, followed by incubation at 37°C for 0, 2, 10, and 60 min. Plasma was then isolated by centrifugation and used for HDL isolation. HDLs were isolated from ex vivo and in vivo plasma samples (collected up to 20 min postinfusion) by using magnesium/dextran sulfate to precipitate apolipoprotein (apo) E- and apoB-containing lipoproteins (29, 30) but not apoE-HDL (31). Briefly, 500 µL plasma was mixed with 50 µL HDL cholesterol precipitation reagent (Stanbio, catalog #0599–020) and the mixture kept on ice for 5 min to allow lipoprotein precipitation. The mixture was then centrifuged at 1000 × gat 4°C for 5 min and the resulting supernatant (HDL) transferred to a new tube. The apoB/apoE pellet was washed once using 500 μL water and reprecipitated as apoB/apoE particles. Both parts of each sample were stored frozen at –80°C until analyzed. The percent HDL cholesterol in the samples varied minimally (38%; range: 36–44%, n = 9).
Vitamin E analyses
Labeled [(d3-, d6-, and d9-α-T, an internal standard (IS)] and unlabeled (d0) α-T were measured by LC-MS in plasma, lipoprotein fractions, and RBC, as described (32–34). RBC cholesterol was measured, as described (28). Standard operating procedures were followed, including preparation of standards and samples, use of IS, and quality control samples.
Sample preparation included defrosting the samples at room temperature immediately prior to use and saponifying the aliquots at 70°C with alcoholic KOH (8.2% final concentration) in 1% ascorbic acid. The samples were then cooled, antioxidants (1% ascorbic acid in water and 25 μg butylated hydroxytoluene in ethanol) and IS [d9-α-T, Isotec Labs; the isotopic distribution was 0% d0-α-T, 0% d3-α-T, 0% d6-α-T, 0.6% d7-α-T, 11.0% d8-α-T, and 88.4% d9-α-T; >99% deuterium-labeled α-T (5)] added, and samples extracted with hexane. The extracts were dried under nitrogen, resuspended in 1:1 ethanol/methanol, and then injected either into a single quadrupole instrument, LC-MS (Waters 2695 Separations Module and a Micromass ZQ2000) or into 1 of 2 triple quadrupole instruments, LC-MS/MS (either an Applied Biosystems/MDS Sciex API 3000 or a Waters XEVO TQD); all MS was used with an atmospheric pressure chemical ionization source operated in negative mode. The limit of detection/limit of quantitation for all 3 of the instruments used was in the range of 20–50 fmol on column and were found to have similar accuracies for plasma α-T analyses (32). For single-ion recording detection, mass-to-charge ratio (m/z) data were obtained for α-Ts (d0-α-T, m/z 429; d3-α-T,m/z 432; d6-α-T, m/z 435; and d9-α-T,m/z 438) and d0-γ-T (m/z 415). For MS/MS detection, analytes were detected using multiple reaction monitoring of transitions: d0-α-T,m/z 429/163; d3-α-T,m/z 432/166; d6-α-T,m/z 435/169; d9-α-T,m/z 438/172; and d0-γ-T,m/z 415/149. Fecal samples (described below) were also analyzed for d0-δ-T (m/z 401/135) and d0-γ-T (m/z 415/149) by comparison to authentic compounds used as standards.
Fecal samples were collected by participants, frozen by nursing staff, and kept frozen until analysis. Frozen fecal samples were transferred directly from –80°C storage to tared, quart-sized plastic buckets and weighed. Then, a 2:1 ratio to sample weight homogenizing solution [1% ascorbic acid in ultrapure water (w/v), 10 μmol diethylenetriaminepenta-acetic acid, and 12.42 μmol δ-T (recovery standard)] was added to the bucket. The buckets were weighed again for total solution weight and sealed shut. Samples were allowed to thaw for a maximum 18 h in a cold room (4°C). Each bucket containing the entire collected fecal sample in homogenizing solution was mixed for 15 min in a VR-1 Digital Paint Mixer (The Cary Company). Aliquots were taken from the homogenate and frozen in a –80°C freezer for future analyses.
Fecal homogenate aliquots were prepared for extraction and analysis by LC-MS/MS. Duplicate samples for each collection of ∼0.25 g of fecal homogenate were quickly thawed, weighed, and then saponified before extraction. For the saponification step, samples were incubated with 10 mL ethanol, 5 mL 1% ascorbic acid in ultrapure water (w/v), and 1.5 mL saturated KOH in a water bath at 70°C for 30 min. Upon cooling, 5 mL water (1% ascorbic acid, w/v), 100 μL butylated hydroxy toluene (1 mg/mL in ethanol), and an additional IS 20 μL d9-α-T (800 μmol) were added. After hexane extraction, an aliquot was evaporated under nitrogen and the dried residue resuspended in 1 mL 1:1 EtOH/MeOH. An appropriate aliquot was analyzed by LC-MS/MS, as described for plasma samples. Samples were corrected for δ-tocopherol recovery, which was 60–90%. The total amount excreted daily was calculated from the averages for each collection, then summed over the study period. The fraction d3-α-T absorbed was calculated from the difference between the amount administered and that excreted.
Each subject's samples for a given type (e.g., plasma) were analyzed in singlicate in a single run, and sets of external standards (the d3- and d6-α-T used for incorporation into the oral dose and IV emulsion, respectively) of varying concentrations were analyzed at the beginning and end of each run; both sets were used for calculating standard curves. Because labeled α-T can vary ∼100-fold, a high and low standard curve, based on area responses that bracketed the sample area responses, was used to estimate the pmol injected, and the aliquots were used to calculate the sample concentrations. The IS was used to correct for variations in MS operation. Thus, sample tocopherol concentrations were calculated from the peak area of the corresponding ion to those of the authentic compound peaks, with correction for the IS (d9-α-T) by comparison to the average IS area counts for the samples. Outcomes for quantitation were validated by comparison to the expected concentration of the quality control sample. Data are expressed as μmol/L.
Data were also calculated as enrichment percentages by identification of the peaks according to the external standards but without any additional corrections, with the assumption that all forms of α-T are equally detected by the MS, as follows:
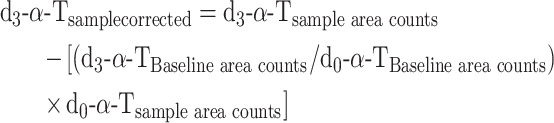 |
(1) |
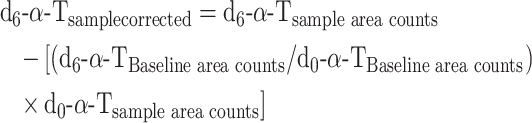 |
(2) |
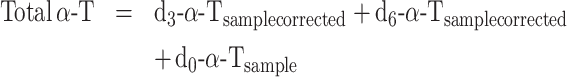 |
(3) |
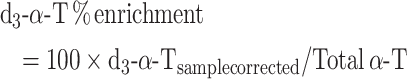 |
(4) |
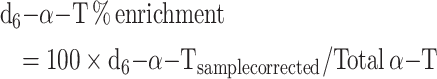 |
(5) |
Approximately 1-g samples of DLM or food were extracted and analyzed for the α-T and γ-T contents using standard LC/electrochemical methodologies for vitamin E analysis (32).
Pharmacokinetic analyses, mathematical modeling, and statistical analyses
Dual-isotope ratio method
Pharmacokinetic (PK) parameters were calculated for each participant from plasma concentration–time data after simultaneous IV (d6-α-T) and oral (d3-α-T) administration. This method assumes that the injected dose is 100% absorbed and that the ratio of the oral to the injected dose represents fractional absorption. The fractional absorption of the oral dose was calculated using the d3-α-T to d6-α-T ratios of area under the plasma vitamin E concentration–time (0–72 h) curves (AUC) times 100.
To maximize the accuracy of the data, analysis of PK data was performed separately on both 1) d6- and d3-α-T concentrations and 2) enrichment percentages of the 2 different deuterated tracers after IV and oral doses, respectively. A program (PK Solutions) was used to estimate the PK parameters (Tables 2–4), as we described previously (14).
TABLE 2.
Pharmacokinetic parameters derived from the plasma d3- and d6-α-T concentrations following oral d3- and IV d6-α-T administration during 3 interventions1
| Parameter | DLM interventions | IV d6-α-T | Compared with 0% fat,2P value | Oral d3-α-T | Compared with 0% fat,3P value | Oral d3- compared with IV d6-α-T per intervention,4P value | Overall interaction,5P value |
|---|---|---|---|---|---|---|---|
| Elimination rate, Ke | 40% fat | 0.022 ± 0.001 | 0.1801 | 0.024 ± 0.002 | 0.6961 | 0.1242 | 0.0500* |
| 0% fat | 0.020 ± 0.001 | 0.023 ± 0.002 | 0.0867 | ||||
| 0% fat-fast | 0.024 ± 0.001 | 0.0034* | 0.022 ± 0.002 | 0.6701 | 0.1197 | ||
| Half-life, h | 40% fat | 32.7 ± 1.4 | 0.0973 | 30.0 ± 2.1 | 0.6784 | 0.0994 | 0.0075* |
| 0% fat | 34.6 ± 1.4 | 31.2 ± 2.0 | 0.0512 | ||||
| 0% fat-fast | 30.3 ± 1.5 | 0.0019* | 32.5 ± 2.6 | 0.6939 | 0.0217* | ||
| Cmax, μmol/L | 40% fat | 7.1 ± 0.6 | 0.8734 | 4.1 ± 0.4 | 0.3149 | <0.0001* | 0.0483* |
| 0% fat | 6.9 ± 0.6 | 4.7 ± 0.4 | <0.0001* | ||||
| 0% fat-fast | 7.4 ± 0.7 | 0.6520 | 3.6 ± 0.5 | 0.0927 | <0.0001* | ||
| Tmax,6 h | 40% fat | 8.0 ± 0.6 | 0.2513 | 12.4 ± 1.5 | 0.9374 | 0.0177* | 0.0565 |
| 0% fat | 7.1 ± 0.5 | 12.3 ± 1.5 | 0.0069* | ||||
| 0% fat-fast | 8.7 ± 0.6 | 0.0677 | 19.5 ± 1.8 | 0.0041* | <0.0001* | ||
| AUC0–72,7 μmol · h/L | 40% fat | 272 ± 25 | 0.9873 | 147 ± 17 | 0.2130 | <0.0001* | 0.3414 |
| 0% fat | 273 ± 25 | 176 ± 17 | <0.0001* | ||||
| 0% fat-fast | 253 ± 30 | 0.6217 | 139 ± 20 | 0.1551 | <0.0001* | ||
| Fractional absorption (0–72 h)8 | 40% fat | 55% ± 3% | 0.0212* | ||||
| 0% fat | 64% ± 3% | ||||||
| 0% fat-fast | 56% ± 3% | 0.0670 |
Pharmacokinetic (PK) parameters (mean ± SEM) were calculated from the d6-α-T and d3-α-T concentrations as described in Methods from data shown in Figure 3A–C from women in the following interventions: 40% fat, n = 10; 0% fat, n = 10; and 0% fat, fasting 12 h, n = 7. *Statistically significant values. Cmax, maximum concentration; DLM, defined liquid meal; K e, post-Cmax elimination rate; IV, intravenous; Tmax, time of Cmax. The statistical analysis was carried out to answer the questions in the footnotes shown below.
What is the effect of the intervention on the IV d6-α-T dose PK parameter? Pharmacokinetic parameters derived from d6-α-T concentrations were analyzed as an incomplete crossover design in SAS PROC MIXED with treatment (40% fat, 0% fat, and 0% fat-fast) as fixed effect and subject as random effect. Using the ESTIMATE statements, the effect of DLM fat was evaluated using the contrast between 40% fat and 0% fat interventions, while the effect of fasting was evaluated using the contrast between 0% fat and 0% fat-fast interventions.
What is the effect of the intervention on the oral d3-α-T dose PK parameter? Pharmacokinetic parameters derived from d3-α-T concentrations were analyzed as an incomplete crossover design in SAS PROC MIXED with treatment (40% fat, 0% fat, and 0% fat-fast) as fixed effect and subject as random effect. Using the ESTIMATE statements, the effect of DLM fat was evaluated using the contrast between 40% fat and 0% fat interventions, while the effect of fasting was evaluated using the contrast between 0% fat and 0% fat-fast interventions.
Are there differences in the IV compared with oral α-T responses for each PK parameter? For comparisons between d6-α-T and d3-α-T pharmacokinetic parameters, the differences between d6-α-T and d3-α-T for each parameter were calculated, and then the data were analyzed as a crossover design in SAS PROC MIXED with treatment (40% fat, 0% fat, 0% fat-fast) as fixed effect and subject as random effect.
Did the response differ between the intervention and the route (IV d6-α-T and oral d3-α-T) for the PK parameter? To determine whether the effect of the route of α-T administration differed across treatments, the overall interaction was analyzed as a Latin square design with double repeated measures (Kronecker product) in SAS PROC MIXED. Fixed effects were interventions (40% fat, 0% fat, 0% fat-fast), the route of α-T administration (oral, IV) and their interaction. The variance–covariance structure within subjects was modeled by using a Kronecker product, in which the intervention was modeled using a compound symmetry matrix and the type of treatment within treatment was modeled using an unstructured variance–covariance matrix.
Tmax is postnadir for the IV dose.
AUC0–72 is calculated from the plasma concentrations from 0 to 72 h.
From the dual-isotope method (% absorption = oral AUC0–72/IV AUC0–72 × 100).
TABLE 4.
Pharmacokinetic parameters derived from the RBC d3- and d6-α-tocopherol enrichment percentages1
| Parameter | DLM fat | IV d6-α-T | Compared with 0% fat,2P value | Oral d3-α-T | Compared with 0% fat,3P value | Oral d3- compared with IV d6-α-T,4P value | Interaction,5P value |
|---|---|---|---|---|---|---|---|
| %Max (enrichment) | 40% fat | 20.4% ± 0.8% | 0.4201 | 12.9% ± 0.7% | 0.0023* | <0.0001* | 0.0014* |
| 0% fat | 20.0% ± 0.8% | 15.4% ± 0.8% | 0.0004* | ||||
| 0% fat-fast | 21.2% ± 0.8% | 0.0236* | 13.2% ± 0.8% | 0.0062* | <0.0001* | ||
| Tmax,6 h | 40% fat | 14.7 ± 1.0 | 0.3959 | 22.4 ± 1.4 | 0.6019 | 0.0055* | 0.0032* |
| 0% fat | 13.4 ± 1.0 | 21.4 ± 1.4 | 0.0002* | ||||
| 0% fat-fast | 14.1 ± 1.2 | 0.6580 | 30.8 ± 1.7 | 0.0050* | <0.0001* | ||
| AUC0–72 h,7 % · h | 40% fat | 1012 ± 40 | 0.5831 | 621 ± 38 | <0.0001* | <0.0001* | <0.0001* |
| 0% fat | 999 ± 40 | 737 ± 39 | <0.0001* | ||||
| 0% fat-fast | 1108 ± 42 | 0.0003* | 620 ± 42 | <0.0001* | <0.0001* | ||
| Fractional absorption (0–72 h)8 | 40% fat | 62% ± 3% | 0.0020* | ||||
| 0% fat | 73% ± 3% | ||||||
| 0% fat-fast | 56% ± 3% | <0.0001* |
Pharmacokinetic (PK) parameters (mean ± SEM) were calculated as described in Methods from data shown in Figure 4B, D, F from women consuming a DLM containing 40% fat, n = 10; 0% fat, n = 10; and 0% fat, fasting 12 h, n = 7. Statistical analyses of the pharmacokinetic parameters derived from RBC d 6-α-T and d3-α-T enrichments were performed as described for Table 2. *Statistically significant values. DLM, defined liquid meal; IV, intravenous; Tmax, time of %Max. The statistical analysis was carried out to answer the questions in the footnotes shown below.
What is the effect of the intervention on the IV d6-α-T dose PK parameter?
What is the effect of the intervention on the oral d3-α-T dose PK parameter?
Are there differences in the IV compared with oral α-T responses for each PK parameter?
Did the response differ between the intervention and the route (IV d6-α-T and oral d3-α-T) for the PK parameter?
Tmax is postnadir for the IV dose.
AUC0–72 is calculated from the RBC d6-α-T and d3-α-T enrichments from 0 to 72 h.
From the dual-isotope method (% absorption = oral AUC0–72/IV AUC0–72 × 100).
Mathematical modeling
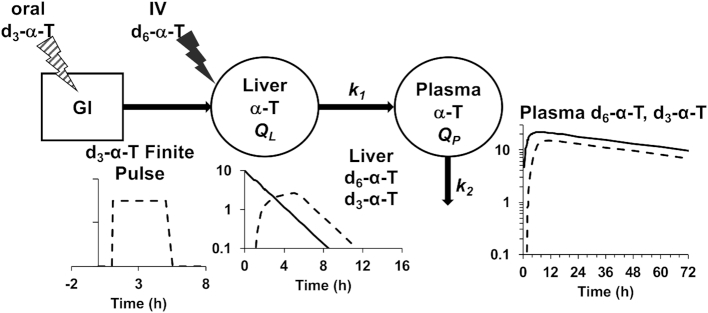
A mathematical model was developed using the plasma d6- and d3-α-T enrichment percentages in all participants from all interventions. Remarkably, a very simple combined model for the 2 tracers with just 7 parameters [turnover rate constants of the pools QL (liver) and QP (plasma), the magnitudes of the 2 doses and pulse durations, and, for the oral dose, an initial delay] fitted the plasma d6- and d3-α-T enrichment data very well.
For each tracer, denoting the intestinal delay (only for d3-α-T) by Δ, the pulse duration by τ, the effective dose divided by the product of k2 times the traceable α-T by D, and u = t – Δ – τ, then y, the tracer enrichment at time t is given by the following equations (the first before the delay is over with no enrichment to be seen; the second during the pulse where the formula is for the plasma response to a constant pulse infusion into the liver; and the third for the period after the pulse ends where the formula is for the plasma response to a finite-duration pulse infusion into the liver, obtained algebraically as the difference between the plasma responses to 2 constant pulse infusions into the liver, one beginning at Δ and the other at Δ + τ):
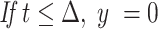 |
(6) |
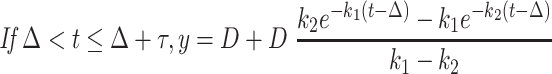 |
(7) |
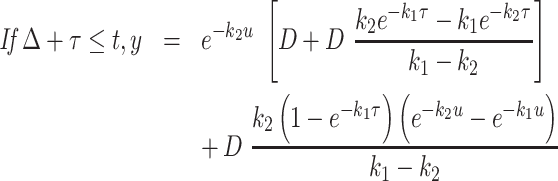 |
(8) |
The equations for the 2 tracers have the same k1 and k2, but each tracer has its own Δ, τ, and effective dose. The delay Δ for d6-α-T is zero, and thus, there are 7 unknown parameters in the model to be estimated from the data. The model was fitted to the data and parameters estimated by the method of least squares, using the program Poolfit, developed by one of the authors and used in past collaborations with the first author (35). The program fits tracer data by compartmental models of the type used here by solving the model in closed form, if possible, with algebraic equations for tracer enrichments as done here (35).
The statistical analysis of the parameters was limited to those subjects who participated in at least 2 interventions [e.g., 40% and 0% fat (n = 7), or 0% and 0% fat-fast (n = 7)] and was carried out as paired t-tests (Microsoft Excel).
Study statistical design
The sample size (n) for the study was designed using the Cmax values (means = 3.47 μmol/L, standard deviation = 1.0) based on data obtained from our previous study (4). Although our primary outcome is fractional absorption, no data were available as this measure has not been evaluated previously. To have 90% power to find a 50% increase in Cmax as a result of fat inclusion in the DLM with a P ≤ 0.05, the power calculation estimated that 7 participants were required. Because we did not observe significant differences between 0% and 40% fat in the interim analysis with 7 participants per group, we did not test intermediate fat levels and instead focused on a new objective, which was to study the effect of a postdosing 12-h fast on α-T absorption. To observe a 50% difference in Cmax, such a study would require 7 participants, as well.
Data were analyzed using Statistical Analysis System (SAS, version 9.4; SAS Institute) by 4 different analyses. 1) To determine the effect of the intervention on PK parameters within the route of α-T administration (oral and IV, independently), pharmacokinetic data on d6-α-T and d3-α-T concentrations and enrichment percentages were each analyzed, as an incomplete crossover design in SAS PROC MIXED with intervention (40% fat, 0% fat, and 0% fat-fast) as a fixed effect and subject as a random effect. Numbers of participants analyzed in these interventions were 40% fat (n = 10), 0% fat (n = 10), or 0% fat-fast (n = 7), as identified in Table 1. Using the ESTIMATE statements, the effect of DLM fat was evaluated using the contrast between 40% fat and 0% fat interventions, while the effect of fasting was evaluated using the contrast between 0% fat and 0% fat-fast interventions. 2) To determine the effect of the route of α-T (oral compared with IV) or for the comparisons between plasma and RBC parameters [for each d6- and d3-α-T AUC, %max (the maximum enrichment percentage observed) and Tmax (the maximum concentration observed), respectively], the differences between d6-α-T and d3-α-T for each parameter were calculated, and then the differences were analyzed as a crossover design in SAS PROC MIXED, with the intervention (40% fat, 0% fat, 0% fat-fast) as a fixed effect and subject as a random effect. 3) To determine whether the effect of the route of α-T administration differed across treatments, the overall interaction was analyzed as a Latin square design with double repeated measures (Kronecker product) in SAS PROC MIXED. Fixed effects were interventions (40% fat, 0% fat, 0% fat-fast), the route of α-T administration (oral, IV), and their interaction. The variance–covariance structure within subjects was modeled by using a Kronecker product, in which the intervention was modeled using a compound symmetry matrix and the type of treatment within treatment was modeled using an unstructured variance–covariance matrix. 4) To determine the α-T disposition into the lipoprotein fractions, a Latin square design with double repeated measures (Kronecker product) in SAS PROC MIXED was used independently for each route of α-T administration. Fixed effects were the intervention (40% fat, 0% fat, 0% fat-fast), the lipoprotein fractions (TRL, LDL, HDL), and their interaction. The variance–covariance structure within subjects was modeled by using a Kronecker product in which the treatment was modeled using a compound symmetry matrix and the lipoprotein fractions within treatment was modeled using an unstructured variance–covariance matrix. The Kenward–Roger approximation was used to obtain the correct degrees of freedom, and a priori comparisons were used to assess our hypothesis.
Data are reported in Table 1 as medians and ranges and in Tables 2–5 as least squares means ± SEMs. Statistical tests were 2-sided, and statistical significance was set as P ≤ 0.05. In Tables 6 and 7, model parameters and fecal data, respectively, were compared within subjects using a paired t test (mean ± SEM; P values are shown for 40% compared with 0% fat, n = 7 and 6, respectively; for 0% compared with 0% fat-fast, n = 7); data for each individual's model fits are given in Supplemental Table 2 and a representative subject in Supplemental Figure 2.
TABLE 5.
AUC0–8 h derived from plasma lipoprotein d3- and d6-α-tocopherol enrichment percentages1
| Lipoprotein | 40% fat | 0% fat | 0% fat-fast | Intervention, P value | Lipoprotein, P value | Interaction, P value |
|---|---|---|---|---|---|---|
| d6-α-T AUC0–8 h (% · h) | ||||||
| IV dose | ||||||
| TRL2 | 160 ± 13 | 171 ± 8 | 174 ± 13 | 0.7145 | 0.0183* | 0.4964 |
| LDL3 | 158 ± 13 | 167 ± 8 | 171 ± 13 | |||
| HDL | 154 ± 13 | 162 ± 8 | 169 ± 13 | |||
| d3-α-T AUC0–8 h (% · h) | ||||||
| Oral dose | ||||||
| TRL2 | 82 ± 13 | 62 ± 5 | 34 ± 5 | 0.0022* | <0.0001* | 0.2926 |
| LDL3 | 28 ± 13 | 34 ± 5 | 12 ± 5 | |||
| HDL | 30 ± 13 | 34 ± 5 | 12 ± 5 | |||
AUC0–8 h (% · h, mean ± SEM) calculated from the lipoprotein d3- and d6-α-tocopherol enrichment percentages from 0 to 8 h following oral d3- and IV d6-α-tocopherol administration to women consuming different fat levels during the defined liquid meal [40% fat, n = 7 (lipoproteins were not obtained for subjects 1–3); 0% fat, n = 10; 0% fat, fasting 12 h, n = 7]. To determine the α-T disposition into the lipoprotein fractions, a Latin square design with double repeated measures (Kronecker product) in SAS PROC MIXED was used independently for each route (oral compared with IV) of α-T administration. Fixed effects were the intervention (40% fat, 0% fat, 0% fat-fast), the lipoprotein fractions (TRL, LDL, HDL), and their interaction. The variance–covariance structure within subjects was modeled by using a Kronecker product in which the treatment was modeled using a compound symmetry matrix, and the lipoprotein fractions within treatment were modeled using an unstructured variance–covariance matrix. *Statistically significant values. IV, intravenous.
TRL, density <1.006 g/L.
LDL, 1.006 < density > 1.063 g/mL.
TABLE 6.
Kinetic parameters derived from modeling the plasma d3- and d6-α-T enrichment percentages following oral d3- and the IV d6-α-T administration1
| Parameter | 40% fat | 0% fat | 40% compared with 0% fat, P value | 0% fat | 0% fat-fast | 0% fat compared with 0% fat-fast, P value |
|---|---|---|---|---|---|---|
| k1 (shared)2 | 0.797 ± 0.085 | 0.775 ± 0.094 | 0.8616 | 0.732 ± 0.089 | 0.797 ± 0.069 | 0.4009 |
| k2 (shared) | 0.016 ± 0.001 | 0.017 ± 0.001 | 0.4481 | 0.017 ± 0.001 | 0.016 ± 0.001 | 0.5347 |
| Half-life (shared), h | 42.8 ± 1.7 | 41.8 ± 3.3 | 0.7421 | 41.5 ± 2.3 | 43.0 ± 2.0 | 0.5486 |
| Pulse duration (d3-α-T), h | 4.9 ± 1.0 | 4.2 ± 0.8 | 0.4622 | 4.2 ± 0.7 | 15.4 ± 0.8 | <0.0001* |
| Pulse duration (d6-α-T), h | 0.04 ± 0.01 | 0.03 ± 0.00 | 0.6891 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.5098 |
| Delay (d3-α-T), h | 1.8 ± 0.4 | 2.8 ± 0.4 | 0.0786 | 2.7 ± 0.4 | 2.5 ± 0.6 | 0.7411 |
| d3-α-T fractional absorption3 | 63 ± 6 | 73 ± 3 | 0.1351 | 74 ± 3 | 68 ± 3 | 0.0392* |
Kinetic parameters (mean ± SEM) are from the model shown in Figure 7. Individual values and for a representative subject fitted graphs are provided in the Supplemental Table 2 and Supplemental Figure 2. Model parameters were compared within subjects using a paired t-test (mean ± SEM; P values are shown; for 40% compared with 0% fat, n = 7; for 0% compared with 0% fat-fast, n = 7). *Statistically significant values.
k1 (from liver to plasma) and k2 (leaving plasma) rates are set to be shared between d3- and d6-α-T, respectively; plasma volume is estimated for subjects in 40% fat intervention (2.71 ± 0.09 L, mean ± SEM) compared with 0% fat intervention (2.71 ± 0.10 L); 0% fat intervention (2.71 ± 0.20 L) compared with 0% fat-fast intervention (2.79 ± 0.10 L); plasma volume = 0.04167 times body weight (kg); weights were measured at the start of each intervention.
d3-α-T fractional absorption is corrected for the recovery of d6-α-T.
TABLE 7.
Fecal d0-γ-T, d0-, d3-, and d6-α-T excreted1
| Paired subjects (n = 6) | |||
|---|---|---|---|
| Characteristic | 40% fat | 0% fat | P value |
| d0-γ-T | 33 ± 7 | 42 ± 14 | 0.4125 |
| d0-α-T | 22 ± 4 | 17 ± 3 | 0.3844 |
| d3-α-T | 9.9 ± 1.9 | 8.8 ± 1.2 | 0.6589 |
| d6-α-T | 0.59 ± 0.20 | 0.66 ± 0.09 | 0.7504 |
| Percent dose d3-α-T excreted2 | 33% ± 6% | 29% ± 4% | 0.6568 |
| d3-α-T fractional absorption3 | 67% ± 6% | 71% ± 4% | 0.6568 |
| Percent dose d6-α-T excreted4 | 2.0% ± 0.6% | 2.2% ± 0.3% | 0.7516 |
The excreted quantity (mg per 96 h; mean ± SEM) of d0-γ-T, d0-, d3-, and d6-α-T. Fecal data were compared within subjects using a paired t-test (mean ± SEM; P values are shown for 40% compared with 0% fat, n = 6, subjects 1–5, 8). Molecular weights for d0-γT = 417, d0-α-T = 430, d3-α-T = 433, and d6-α-T = 436 g/mol.
Percent dose d3-α-T excreted = mg d3-α-T excreted/30 mg × 100.
d3-α-T fractional absorption = 100% – percent dose d3-α-T excreted.
Percent dose d6-α-T excreted = mg d6-α-T excreted/30 mg × 100.
Results
α-T absorption estimated using dual-isotope method
Method validation by comparing d3- and d6-α-T concentration-time data following a 40% fat DLM: dual-isotope method
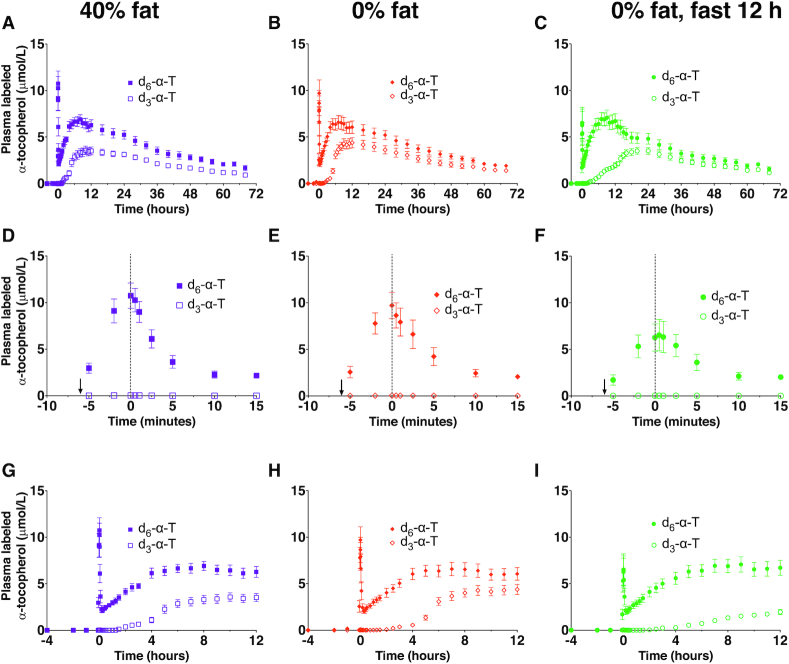
Plasma α-T concentrations were measured in healthy adult women following oral d3-α-T consumed with 40% fat DLM and simultaneous IV d6-α-T administration. Plasma d6-α-T concentrations declined from the time = 0 peak by ∼80–90%, reaching a nadir within ∼10 min of the completed injection (Figure 3A, D). Subsequently, plasma d6-α-T concentrations increased over the next several hours (Figure 3A). Once the plasma d6-α-T concentrations reached Cmax, they declined exponentially. By contrast, the plasma appearance of oral d3-α-T was delayed (Figure 3G). Post-Cmax, the plasma d3- and d6-α-T concentrations decreased exponentially.
FIGURE 3.
Plasma d3- and d6-α-T concentrations following simultaneous administration of oral d3-α-T and intravenous (IV) d6-α-T. Plasma d3- and d6-α-T concentrations were measured in healthy adult women following α-T administration via 2 routes: slow IV injection of 30 mg (68.8 μmol) d6-α-T given in an oil–water emulsion and 30 mg (69.3 μmol) d3-α-T given orally with a defined liquid meal containing 40% fat (A, D, G), with 0% fat (B, E, H) or with 0% fat followed by a 12-h fast (C, F, I). Panels A–C, G–I show time in hours, while panels D–F show time in minutes; an arrow at –6 min shows the start of the IV infusion, and a dotted vertical line at 0 h indicates the end of the emulsion injection. Concentrations are shown as mean ± SEM (40% fat, n = 10; 0% fat, n = 10; 0% fat-fast, n = 7). Some subjects participated in multiple interventions (see Table 1 for participants’ allocation to interventions, and see Table 2 for statistical interpretation of differences).
α-T fractional absorption estimated using the dual-isotope method is dependent on the equilibration of the oral and IV α-Ts and that the 2 delivery routes are similar with regard to α-T distribution, elimination, and other undefined factors. We calculated d3- and d6-α-T Cmax, times of Cmax (Tmax), elimination rates (Ke, post-Cmax), half-lives, and the AUC (Table 2). The d6-α-T Tmax preceded that of the d3-α-T, and both the Cmax and AUC were greater for IV d6- compared with oral d3-α-T (Table 2).
The elimination rates (Ke) of the oral d3- compared with IV d6-α-Ts were not significantly different, nor were the half-lives calculated from these rates for the 40% fat intervention (Table 2). Thus, the equilibration criteria for the dual-isotope technique were met. Fractional α-T absorption was estimated to be 55% ± 3% (mean ± SEM, n = 10) from the ratios of the AUC0–72 oral to IV (d3- to d6-α-T).
Role of DLM fat in modulating deuterated α-T concentrations and absorption: dual-isotope method
To evaluate the effect of fat on d3-α-T absorption, the DLM was decreased from 40% to 0% fat. Controlled α-T meals containing ∼30% fat were consumed at 4 and 8 h postdosing and on subsequent days at standardized times.
Comparing the 0% fat with the 40% intervention, neither the appearances of the plasma concentration graphs (Figure 3B, E, H compared with Figure 3A, D, G, respectively) nor the PK parameters (Table 2) were different. However, the ratios of the d3- to d6-α-T AUC showed that the intestinal absorption during the 0% fat intervention was significantly greater by ∼9% compared with that observed during the 40% fat intervention (Table 2). This outcome contradicts the hypothesis that increased fat would improve α-T absorption and suggests that the meal fat at 4 h postdosing affects the observations.
Role of fasting in modulating deuterated α-T concentrations and absorption: dual-isotope method
To evaluate the role of the meals subsequent to the oral dose on α-T kinetics, participants fasted for 12 h following the 0% fat DLM (Figure 3C, I compared with Figure 3B, H, respectively). Comparing the 0% fat and 0% fat-fast interventions, fasting resulted in a significantly more rapid decline in d6-α-T concentrations from the IV dose, as measured by Kes and the ∼4 h shorter half-life; there were no other statistically significant effects of fasting on d6-α-T Cmax, Tmax, or the AUCs (Table 2).
Fasting caused a major delay in the plasma d3-α-T appearance from the oral dose. Specifically, plasma d3-α-T concentrations during the 0% fat-fast intervention increased more slowly than during the 0% fat intervention (Figure 3C, I compared with Figure 3B, H, respectively). These differences were documented by the d3-α-T Tmax, which occurred ∼7 h later (Table 2). Thus, fasting during the intervention apparently delayed intestinal d3-α-T absorption.
Surprisingly, fasting had no significant effects on the d3-α-T Cmax, despite the delay in d3-α-T Tmax (Table 2). After the meal at 2200 was consumed, the d3-α-T concentrations rose to those observed during the fed state (Figure 3C compared with Figure 3B). Based on the d3-/d6-α-T AUC0–72 ratios, fasting for 12 h had no apparent effect on the d3-α-T fractional absorption (Table 2). Thus, fasting delayed and eating restored d3-α-T absorption (see lipoprotein studies below).
Plasma and erythrocyte deuterated α-T enrichment: dual-isotope method
Plasma d3- and d6-α-T enrichments were calculated independently for each sample for each of the interventions from the original LC/MS data without inclusion of IS or calibration by standards (Figure 4A, C, E). The purpose of both modes of calculating the data is to show the consistency of the mass spectrometry analysis. There were some inconsistencies in the statistical outcomes, but largely trends were similar between outcomes.
FIGURE 4.
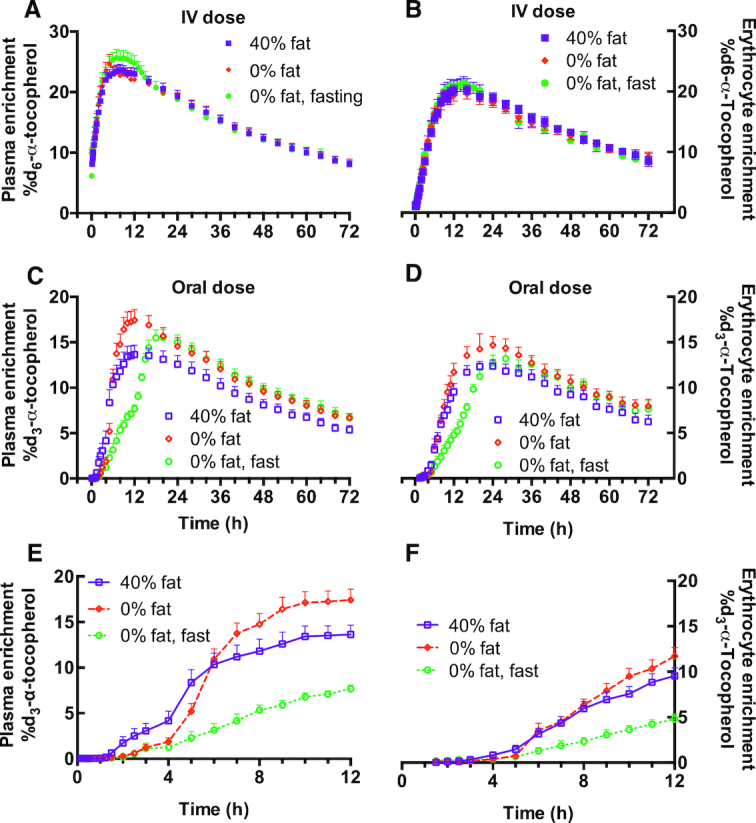
Plasma and erythrocyte d3- and d6-α-T enrichment percentages following simultaneous administration of oral d3-α-T and intravenous (IV) d6-α-T. The enrichments were calculated (described in Subjects and Methods) from the area responses of d3- and d6-α-T measured by LC-MS/MS in plasma and RBC obtained from healthy adult women, as described in Figure 3. Plasma d6-α-T (A) or d3-α-T (C, E) enrichments or RBC d6-α-T (B) or d3-α-T (D, F) enrichments during the 40% fat, 0% fat, or 0% fat-fast interventions. Enrichments are shown as mean ± SEM (40% fat, n = 10; 0% fat, n = 10; 0% fat-fast, n = 7). Some subjects participated in multiple interventions (see Table 1 for participants’ allocation to interventions, and see Tables 3 and 4, respectively, for statistical interpretation of differences).
The outcomes for the oral d3-α-T %Max enrichment, the AUC0–72, and the intestinal absorption were significantly greater in the 0% fat compared with either the 40% fat or the 0% fat-fast interventions (Table 3). Relative to the 40% fat intervention, the d3-α-T enrichment was delayed but increased rapidly following the meal in the 0% fat intervention (Figure 4E) or at the meal that ended the fast in the 0% fat-fast intervention (Figure 4C), but only the latter caused the Tmax to increase (Table 3). Based on d3-/d6-α-T enrichment AUC0–72 ratios, fractional absorption was highest during the 0% fat intervention compared with the 40% fat or the 0% fat-fast interventions (Table 3).
TABLE 3.
Pharmacokinetic parameters derived from the plasma d3- and d6-α-T enrichment percentages following oral d3- and IV d6-α-T administration during 3 interventions1
| Parameter | DLM fat interventions | IV d6-α-T | Compared with 0% fat,2P value | Oral d3-α-T | Compared with 0% fat,3P value | Oral d3- compared with IV d6-α-T,4P value | Interaction,5P value |
|---|---|---|---|---|---|---|---|
| Elimination rate, Ke | 40% fat | 0.023 ± 0.002 | 0.3062 | 0.020 ± 0.002 | 0.7828 | 0.0601 | 0.5609 |
| 0% fat | 0.021 ± 0.002 | 0.020 ± 0.002 | 0.4862 | ||||
| 0% fat-fast | 0.026 ± 0.002 | 0.0551 | 0.021 ± 0.002 | 0.7353 | 0.0336* | ||
| Half-life, h | 40% fat | 30.9 ± 2.2 | 0.2264 | 34.2 ± 2.7 | 0.6058 | 0.1564 | 0.2066 |
| 0% fat | 34.5 ± 2.2 | 36.2 ± 2.7 | 0.4862 | ||||
| 0% fat-fast | 29.0 ± 2.6 | 0.0982 | 40.4 ± 3.3 | 0.3273 | 0.0336 | ||
| %Max (enrichment) | 40% fat | 23.5% ± 1.1% | 0.1816 | 14.7% ± 0.9% | 0.0008* | <0.0001* | 0.0166* |
| 0% fat | 24.9% ± 1.1% | 18.1% ± 0.9% | <0.0001* | ||||
| 0% fat-fast | 25.4% ± 1.3% | 0.6238 | 15.5% ± 1.0% | 0.0082* | 0.0004* | ||
| Tmax,6 h | 40% fat | 7.8 ± 0.6 | 0.1057 | 12.9 ± 1.3 | 0.9809 | 0.0306* | 0.0003* |
| 0% fat | 6.5 ± 0.7 | 12.9 ± 1.3 | 0.0005* | ||||
| 0% fat-fast | 9.0 ± 0.8 | 0.0055* | 20.0 ± 1.4 | <0.0001* | <0.0001* | ||
| AUC0–72 h,7 % · h | 40% fat | 1101 ± 46 | 0.9274 | 657 ± 39 | 0.0037* | <0.0001* | 0.1032 |
| 0% fat | 1105 ± 47 | 781 ± 39 | 0.0001* | ||||
| 0% fat-fast | 1121 ± 50 | 0.6622 | 693 ± 43 | 0.0323* | <0.0001* | ||
| Fractional absorption (0–72 h)8 | 40% fat | 61% ± 3% | 0.0120* | ||||
| 0% fat | 70% ± 3% | ||||||
| 0% fat-fast | 62% ± 4% | 0.0370* |
Pharmacokinetic (PK) parameters (mean ± SEM) were calculated as described in Methods from data shown in Figure 4A, C from women in the following interventions: 40% fat, n = 10; 0% fat, n = 10; and 0% fat, fasting 12 h, n = 7. Statistical analyses of the PK parameters derived from either d6-α-T or d3-α-T enrichment percentages were performed as described for Table 2. *Statistically significant values. Cmax, maximum concentration; DLM, defined liquid meal; Ke, post-Cmax elimination rate; IV, intravenous; Tmax, time of Cmax. The statistical analysis was carried out to answer the questions in the footnotes shown below.
What is the effect of the intervention on the IV d6-α-T dose PK parameter?
What is the effect of the intervention on the oral d3-α-T dose PK parameter?
Are there differences in the IV compared with oral α-T responses for each PK parameter?
Did the response differ between the intervention and the route (IV d6-α-T and oral d3-α-T) for the PK parameter?
Tmax is postnadir for the IV dose.
AUC0–72 is calculated from the plasma d6-α-T and d3-α-T enrichments from 0 to 72 h.
From the dual-isotope method (% absorption = oral AUC0–72/IV AUC0–72 × 100).
Erythrocyte α-T enrichments (Figure 4B, D, F; Table 4), as well as the ratios of d3- and d6-α-T relative to erythrocyte cholesterol (data not shown), were also measured. The general patterns of the d3- and d6-α-T enrichments observed between plasma and erythrocytes were similar (Figure 4A, C, E compared with Figure 4B, D, F, respectively). However, Tmax for both the d3- and d6-α-T enrichments in erythrocytes occurred later than the respective enrichments observed in the plasma (Table 3 compared with Table 4). These data show that the plasma is labeled first with the α-T and subsequently the erythrocytes become enriched, but the similarities in the AUC0–72 suggest that there are no differences between plasma and erythrocytes with regard to the extent of enrichment over the 3-d study. Based on the d3-/d6-α-T RBC enrichment AUC0–72 ratios, the fractional absorption was highest during the 0% fat intervention compared with the 40% fat or the 0% fat-fast interventions (Table 3).
Key role of the liver in α-T trafficking identified by analyzing α-T transport in lipoproteins
The data shown above suggested that there were marked differences in the liver-derived lipoprotein α-T transport that are dependent on meal fat and timing. Therefore, mechanistic studies of lipoprotein α-T enrichment were undertaken.
Emulsion d6-α-T clearance assessed by HDL isolation by precipitation of particles containing apoB/apoE
We hypothesized that the rapid plasma d6-α-T decreases during the first 20 min of emulsion clearance (Figure 3D, E, F) were likely dependent on apoE acquisition from plasma by the emulsion (36), resulting in liver clearance of the d6-α-T emulsion particles. To investigate this hypothesis, we carried out both in vitro and in vivo analyses of HDL isolated by apoB/apoE precipitation with magnesium/dextran sulfate.
In vitro experiments
In vitro incubation of blood with the d6-α-T emulsion at room temperature was stopped at 0, 2.5, 10, and 60 min by plasma isolation and apoB/E precipitation. At 0 time, the in vitro d6-α-T enrichment in the apoB/E precipitate was 43% ± 2% and remained unchanged over time, while the d6-α-T enrichment at time 0 in the HDL (supernatant fraction) was 1.2% ± 0.2% (mean ± SEM, n = 9) and increased linearly at ∼0.08% per minute, reaching 6.3% ± 0.5% by 60 min. Thus, there is some spontaneous d6-α-T transfer from the emulsion to HDL in vitro. Based on these studies, the emulsion, which has no precipitable components, rapidly acquired apoE in vitro from plasma (36), causing the emulsion to be precipitated.
In vivo outcomes
Plasma samples were obtained from 0 to 20 min following IV d6-α-T emulsion administration to participants (during the 40% and 0% fat interventions), then were precipitated (Figure5). At time 0, the d6-α-T enrichment in the apoB/E precipitate was 40–50% and decreased exponentially with similar half-lives in the 40% (4.4 ± 0.8 minutes, least-squares means ± SEM) compared with the 0% fat (3.8 ± 0.6) interventions (Figure 5C); similar half-lives were obtained when calculated using the concentration data (Figure 5A). Taken together, the in vitro and in vivo data show that the rapid removal of the emulsion from the plasma is likely dependent on the emulsion acquiring apoE and being taken up by receptor mediated-processes, as has been reported previously (36).
FIGURE 5.
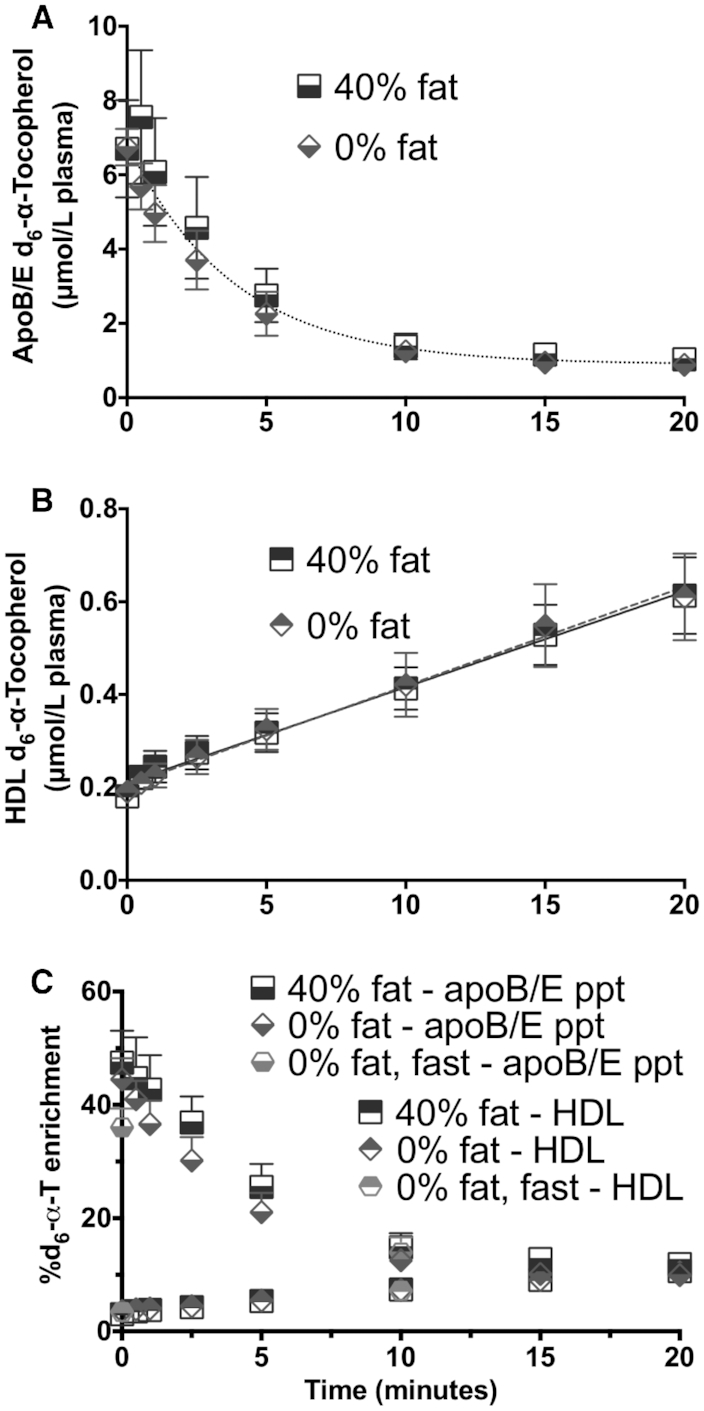
Apolipoprotein B/E (apoB/E) precipitation and HDL isolation. HDLs were isolated from in vivo plasma samples (collected up to 20 min postinfusion) by precipitating apoE- and apoB-containing lipoproteins using magnesium/dextran sulfate (n = 10 per group, except subject 5’s sample was lost for 40% intervention due to technical difficulties). (A) ApoB/apoE precipitation, concentrations (mean ± SEM), d6-α-T half-life averaged 3.0 min; none of the curve parameters were significantly different between the 2 interventions, F-test P = 0.3826. (B) HDL, concentrations [40% fat, d6-α-T (nmol/L plasma) = 20.6X + 21.0; 0% fat, d6-α-T = 21.4X + 20.5; where X = minutes]; neither the slopes nor the y-intercepts were significantly different between the 2 interventions, F-test P = 0.9805. (C) d6-α-T enrichments in apoB/E and HDL particles. Only 2 samples were obtained for the 0% fat-fast intervention and are shown in C as a circle at 0 and 10 min that overlaps HDL data.
We also observed that the HDL fraction in vivo acquired d6-α-T from 0 to 20 min at a linear rate of 21 ± 2 nmol/L plasma per minute during the 40% fat intervention (n = 7) and at a similar rate (21 ± 3 nmol/L plasma per minute, n = 10) during the 0% fat intervention (Figure 5B). The HDL d6-α-T enrichment also increased linearly (Figure 5C). The rate of increase in the HDL d6-α-T enrichment in vivo (0.31% ± 0.05%) was ∼4 times greater than in vitro (0.08% ± 0.01%, P = 0.0005, paired t-test, n = 9), suggesting that in vivo the d6-α-T transfer to HDL occurred during the emulsion lipolysis and was not just a result of the passive process observed in vitro.
Estimation of α-T disposition in lipoproteins: isolation by ultracentrifugation
Plasma lipoproteins were also isolated using standard ultracentrifugation techniques to follow the α-T labels into the lipoprotein fractions over time (Figure 6). The purpose of these studies was to evaluate how dietary fat or fasting affected the secretion of the IV dose by the liver into the lipoproteins and to determine the impact of meal fat on the intestinal absorption of the oral dose.
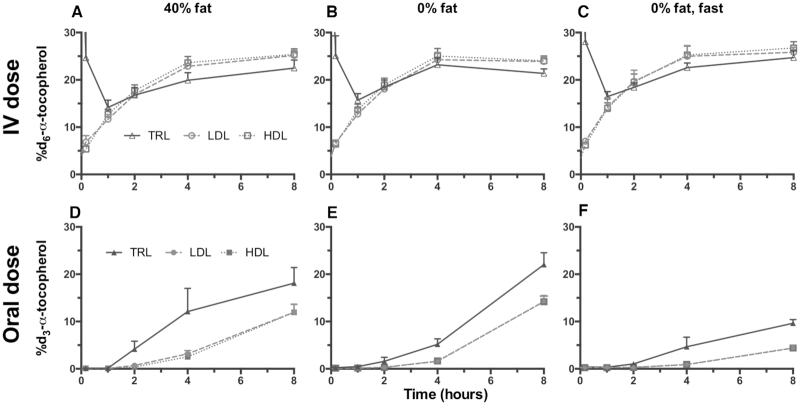
FIGURE 6.
Lipoprotein fractions following the intravenous (IV) and oral d-α-T dosing. The enrichments for the d6-α-T from the IV (A, B, C) and the d3-α-T from the oral (D, E, F) dosing of the lipoprotein density fractions: triglyceride-rich fraction [TRL (triangle), density (d < 1.006 g/mL)], LDL (circle, 1.006 < d > 1.063 g/mL), and HDL (square, 1.063 < d > 1.21 g/mL), isolated using discontinuous salt density-gradient ultracentrifugation, are shown (mean ± SEM). Statistical evaluations are given in Table 5.
To compare the d6-α-T enrichments of the lipoprotein fractions, the AUC from 0 to 8 h (AUC0–8) of each (TRL, LDL, and HDL) was calculated. The AUC0–8 of the TRL d6-α-T enrichments were greater than in LDL or HDL (Table 5, Figure 6A–C).
Dietary fat consumption and fasting had marked effects on the distribution of the d3-α-T enrichments in the lipoproteins from the orally administered dose (Table 5, Figure 6D–F). For all interventions, the AUC0–8 of the TRL d3-α-T enrichments were significantly greater than in LDL or HDL, which were similar to each other (Table 5, Figure 6D–F).
Mathematical modeling of the plasma d3- and d6-α-T enrichments
A mathematical model was developed using the plasma d3- and d6-α-T enrichment percentages and the observations from lipoprotein and fecal studies (described below). Key observations included the rapid uptake of the d6-α-T emulsion by the liver, similarity of the plasma disappearance curves of the 2 labeled α-tocopherols and the limited d6-α-T excretion.
Based on the shape of the enrichment curves (Figure 4A, C), we evaluated a model where the gastrointestinal tract converts the oral d3-α-T into a finite pulse going into the liver—that is, at a constant rate for a period of time (following an initial delay, Figure 7). We assigned the kinetics of the 2 tracers to be identical in the plasma α-T pool (QP) and in the liver α-T pool (QL), which is the source for the plasma α-T (37). Remarkably, a very simple combined model, described in detail under Subjects and Methods, for the 2 tracers with just 7 parameters (turnover rate constants of the pools QL and QP, the magnitudes of the 2 doses and pulse durations, and, for the oral dose, an initial delay) fitted the plasma d6- and d3-α-T enrichment data very well.
FIGURE 7.
Model to fit the plasma d3- and d6-α-T enrichments. Based on the shape of the enrichment curves from the oral and intravenous doses, we evaluated a model where the gastrointestinal (GI) tract converts the oral d3-α-T into a finite pulse going into the liver—that is, at a constant rate for a period of time (following an initial delay). We assigned the kinetics of the 2 tracers to be identical in the plasma α-T pool (QP) and in the liver α-T pool (QL), which is the source for the plasma α-T (36). Remarkably, a very simple combined model for the 2 tracers with just 7 parameters (turnover rate constants of the pools QL and QP, the magnitudes of the 2 doses and pulse durations, and, for the oral dose, an initial delay) fitted the plasma d6- and d3-α-T enrichment data. GI box converts the bolus capsule into a finite pulse (with an initial delay) entering the liver pool. 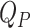 (μmoles) = plasma volume (L) multiplied by the plasma concentration of α-tocopherol (μM);
(μmoles) = plasma volume (L) multiplied by the plasma concentration of α-tocopherol (μM); 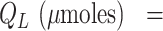
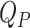
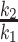 . Model fits are shown in Table 6.
. Model fits are shown in Table 6.
Model parameters estimated for 40% compared with 0% fat interventions were similar and were similar for the 0% fat compared with 0% fat-fast intervention, with the exception that the 12-h fast increased the d3-α-T pulse duration by 11 h (Table 6). This latter finding is consistent with prolonged retention of the oral d3-α-T by the intestine and the slow appearance of the d3-α-T in plasma lipoproteins.
The percentage d6-α-T recovered for each person, as estimated by the model, was 25%–30% lower than the 100% expected, presumably due to the volume of distribution being larger than the plasma volume. A correction for d6-α-T recovery was applied to the d3-α-T dose fraction to account for the larger than expected volume of distribution. This correction seems appropriate given the low (∼2% of d6-α-T dose) excretion estimated from the fecal data (see below, Table 7).
Outcomes from the model (after correction for d6-α-T recovery) for the 40% compared with 0% fat intervention showed that d3-α-T fractional absorption did not vary with the DLM fat. However, fasting for 12 h decreased d3-α-T fractional absorption by ∼6% compared with outcomes for the 0% fat intervention (Table 6). Individual values and fitted graphs are provided (Supplemental Table 2 and Supplemental Figure 1).
The model outcomes support the findings that the fat consumed in the DLM with the administered dose does not alter α-T absorption, but the fat in the subsequent meals is the driver of α-T absorption.
Fecal excretion of labeled and unlabeled tocopherols: balance method for estimation of α-T absorption
Participants were asked to collect all fecal material excreted from the time of dosing until they were discharged. In the 0% compared with 40% fat interventions (n = 6, paired subjects), similar amounts of each d0-γT, d0-α-T, d3-α-T, and d6-α-T, respectively, were excreted by the participants (Table 7). The time course of excretion from all subjects demonstrated that 72 h was sufficient during the 40% intervention to collect >90% of the total excreted, while during the 0% fat intervention, collection up to 96 h was necessary (data not shown).
d3-α-T fractional absorption was calculated from the differences in the dose administered and the d3-α-T excreted over the 96 h. Fractional absorption in the 40% and 0% fat interventions averaged ∼69%; outcomes were not significantly different between these 2 interventions (Table 7).
About 2% of the d6-α-T IV administered dose was excreted over the 96-h study period with daily excretion ranging between 0.3 and 1.2 μmol. Thus, the d6-α-T was not excreted in large amounts via the bile and feces over the 96 h.
Discussion
Fractional α-T absorption in healthy women ranged between 55% and 74% (Tables 2–4, 6, 7), with the various methodologies yielding a similar range of values. These α-T absorption values are higher than those previously estimated from plasma concentrations following oral deuterated-α-T dosing alone (4) but not as high as those estimated using radioactive α-T (3).
Dietary fat (40% compared with 0% fat in the DLM) had no positive quantitative effect on α-T absorption. The 0% fat DLM intervention increased α-T fractional absorption to 64% absorption from 55% during the 40% fat intervention (Table 2). When participants consumed d3-α-T with the 0% fat DLM and then undertook a 12-h fast, the effects of fasting seemed to be largely overcome by mechanisms to potentiate fat absorption when the next meal was consumed. We had hypothesized the “no-fat” condition would limit α-T absorption based on previous studies using α-tocopheryl acetate (4, 38), as well as the observation that fat malabsorption causes α-T malabsorption and vitamin E deficiency in humans (39, 40). By contrast, this “lack of fat effect” was observed also when α-T was used to assess bioavailability when varying levels of milk fat consumed with the vitamin E dose (14). Taken together, these findings suggest that hydrolysis of the α-tocopheryl acetate (as in vitamin supplements and fortified food) may be limited when consumed without fat, as has been observed in vitro (41).
To our knowledge, this is the first time that vitamin E kinetics have been studied using an intravenously administered, deuterium-labeled-α-T in an oil–water emulsion. Studies in mice show rapid plasma clearance of soy–oil emulsions with uptake primarily by the liver (42). The rapid plasma clearance of the d6-α-T emulsion during the first 15–20 min post-IV administration (Figure 3) is likely dependent on apoE acquisition by the emulsion (36). Emulsion clearance, along with lipolysis (43), appears similar to receptor-mediated TRL particle clearance (44). The IV d6-α-T emulsion allows measurement of α-T transfer to HDL during TRL lipolysis. HDL isolation by precipitation from plasma obtained during the first 20 min after d6-α-T administration shows for the first time, to our knowledge, that there is substantial d6-α-T transfer (approximately 1 μmol/h) to HDL (Figure 5B).
The clearance of the d6-α-T emulsion is estimated at a half-life of ∼3–4 min (Figure 5); thus, by 30 min, more than 90% of the d6-α-T emulsion is cleared from the circulation. The flattening of the d6-α-T enrichment curve in the apoB/E fraction at 15–20 min (Figure 5A, C) is similar to that in the plasma (Figure 3D–F). Rapid d6-α-T equilibration in circulating lipoproteins is supported by their nearly uniform d6-α-T enrichment by 1 h (Figure 6A–C). Notably, α-T can transfer spontaneously from HDL to TRL and between LDL and HDL (45). This process is more rapid than LDL and/or HDL turnover (46), so is likely due to equilibration of the labeled-α-T between particles (45, 47), potentially facilitated by the phospholipid transfer protein (48).
Very different patterns for IV d6- and oral d3-α-T lipoprotein trafficking are observed during the first 8 h after dosing. Following hepatic uptake of the d6-α-T emulsion, there was continuous VLDL secretion (49) containing d6-α-T, a process not modulated by either DLM fat consumption or fasting (Figure 6A–C, Table 5). All of the plasma lipoproteins became equally d6-α-T enriched after IV dosing and showed no effect of DLM fat or fasting (Figure 6A–C, Table 5). Liver α-T secretion depends on the function of the hepatic α-tocopherol transfer protein (α-TTP) to mediate α-T transfer to VLDL for transport in plasma, as reviewed (17). Notably, Smagris et al. (50) report that VLDL is secreted despite a 3-fold decrease in VLDL–triglyceride (TG) secretion rate without any associated reduction in hepatic apoB secretion. Thus, it is plausible that d6-α-T is continuously secreted from the liver in VLDL. An alternative scenario is that HDLs are the d6-α-T acceptor in the liver and HDLs rapidly transfer d6-α-T to VLDL. This proposal is based on the interaction of α-TTP with phosphatidylinositol phosphates in the membrane (51, 52), the membrane exchanging phosphatidylinositol phosphates for d6-α-T, with the ATP-binding cassette transporter A1 (53–55) transferring d6-α-T to an acceptor lipoprotein, possibly either VLDL or HDL, outside the hepatocyte.
The distribution of the oral d3-α-T in these same lipoproteins is also reported (Figure 6D–F, Table 5). In all three dietary treatments, the TRL AUCs of the d3-α-T enrichment were double to more than triple those of either LDL or HDL during the same intervention (Table 5). The TRL d3-α-T are not in rapid equilibrium with the other lipoproteins and thus are likely chylomicrons.
We speculate that the oral d3-α-T was taken up by small intestinal cells by mechanisms that are potentiated by various transporters (56) and retained until sufficient fat was consumed to promote chylomicron secretion (57, 58). Studies on fat absorption have documented that lipid droplets are rapidly formed inside enterocytes during fat consumption (59), potentially controlling TG fluctuations (60), rather than immediately secreting TG in chylomicrons (61). Because α-T is not appreciably secreted into plasma in the absence of apoB48 and lipid droplet accumulations are found in enterocytes (58), it seems likely that the d3-α-T could have been retained in the enterocytes. Potentially, the meal after dosing (e.g., lunch, 4 h postdosing) stimulates chylomicron secretion from enterocytes that have retained d3-α-T. In the fasting intervention, a similar increase in plasma d3-α-T enrichment is observed once the meal is eaten at 2200 to end the fast (Figure 4D).
Our investigations into the role of fat on α-T absorption have provided an explanation for the observation that the liver but not the intestine requires an α-TTP to facilitate α-T secretion (37, 62, 63). Our findings suggest that the various vitamin E forms can accumulate in lipid droplets and that these droplets can then coalesce with nascent chylomicrons to enrich their TG contents with vitamin E, and in the process, vitamin E absorption is facilitated. A similar process has been shown for cholesterol (64) and for carotenoids (56). Once the chylomicron remnants reach the liver, then α-TTP is needed to facilitate preferential α-T secretion into plasma. The other vitamin E forms are metabolized and/or excreted in bile, as we showed for γ-tocopherol (65).
This study has limitations, including small sample size, lack of randomization or blinding, and compliance issues, leading to imbalance with attendant potential for baseline and residual confounding.
In summary, α-T fractional absorption is about 55% when it is consumed with a 40% fat meal. α-T leaves the intestine by a process that takes several hours or longer to complete, suggesting slow chylomicron α-T secretion. Once α-T reaches the liver, where α-T secretion in VLDL occurs, it is swiftly distributed to all of the lipoprotein fractions. Research into mechanisms for α-T secretion from enterocytes, as well as the role that the lipid droplets play in hindering or promoting α-T secretion in chylomicrons, is warranted.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—MGT, MN, SP, RS, SS, and ML: designed the research (project conception, development of overall research plan, and study oversight); SP, MN, YW, HT, AC, SB, IE, RS, SS, and ML: conducted the research (hands-on conduct of the experiments and data collection); AC and SB: designed the low vitamin E meals and defined liquid diets; MGT, SWL, P-CV, YW, JC, IE, and ML: analyzed the data; RR: designed the mathematical model; RR and GB: performed the statistical analyses. All authors wrote the paper. MGT had primary responsibility for final content. All authors gave approval of final version of the article. The authors state that they have no conflicts of interest.
Notes
This study was supported by National Institutes of Health (NIH) DK081761 and Office of Dietary Supplements grant to MGT; Intramural Research Programs, National Institute of Diabetes and Digestive Kidney Diseases and National Heart, Blood, and Lung Institute, NIH; Metabolic Unit Staff, NIDDK Clinical Core Staff, and Clinical Center Nutrition and Nursing Department Staff, NIH; and grant DK053213-11 to ML. The deuterated-α-tocopherol for intravenous administration and the intravenous emulsion were provided as a gift by Dr Manfred Eggersdorfer, DSM Nutritional Products AG, Kaiseraugst, Switzerland. DSM also provided a gift in support of the purchase of a mass spectrometer for the Traber laboratory. The funders had no input into study outcomes.
Supplemental Tables 1 and 2 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
MGT and ML contributed equally to this work.
Individual data described in the manuscript will be made available upon request.
Abbreviations used: apo, apolipoprotein; Cmax, maximum concentration; CRC, Clinical Research Center; d, deuterium; DLM, defined liquid meal; IRB, institutional review board; IS, internal standard; IV, intravenous; Ke, post-Cmax elimination rate; PK, pharmacokinetic; Tmax, time of Cmax; TG, triglyceride; TRL, triglyceride-rich lipoprotein; α-T, α-tocopherol; α-TTP, α-tocopherol transfer protein.
References
- 1. Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 2. Horwitt MK, Harvey CC, Duncan GD, Wilson WC. Effects of limited tocopherol intake in man with relationships to erythrocyte hemolysis and lipid oxidations. Am J Clin Nutr. 1956;4:408–19. [DOI] [PubMed] [Google Scholar]
- 3. Novotny JA, Fadel JG, Holstege DM, Furr HC, Clifford AJ. This kinetic, bioavailability, and metabolism study of RRR-alpha-tocopherol in healthy adults suggests lower intake requirements than previous estimates. J Nutr. 2012;142(12):2105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bruno RS, Leonard SW, Park S, Zhao YY, Traber MG. Human vitamin E requirements assessed with the use of apples fortified with deuterium-labeled alpha-tocopheryl acetate. Am J Clin Nutr. 2006;83(2):299–304. [DOI] [PubMed] [Google Scholar]
- 5. Leonard SW, Good CK, Gugger ET, Traber MG. Vitamin E bioavailability from fortified breakfast cereal is greater than that from encapsulated supplements. Am J Clin Nutr. 2004;79(1):86–92. [DOI] [PubMed] [Google Scholar]
- 6. Traber MG. Mechanisms for the prevention of vitamin E excess. J Lipid Res. 2013;54(9):2295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheeseman KH, Holley AE, Kelly FJ, Wasil M, Hughes L, Burton G. Biokinetics in humans of RRR-alpha-tocopherol: the free phenol, acetate ester, and succinate ester forms of vitamin E. Free Radic Biol Med. 1995;19(5):591–8. [DOI] [PubMed] [Google Scholar]
- 8. Rontani J-F, Nassiry M, Michotey V, Guasco S, Bonin P. Formation of pristane from α-tocopherol under simulated anoxic sedimentary conditions: a combination of biotic and abiotic degradative processes. Geochim Cosmochim Acta. 2010;74(1):252–63. [Google Scholar]
- 9. Bjorneboe A, Bjorneboe GE, Drevon CA. Serum half-life, distribution, hepatic uptake and biliary excretion of alpha-tocopherol in rats. Biochim Biophys Acta. 1987;921(2):175–81. [DOI] [PubMed] [Google Scholar]
- 10. Mustacich DJ, Shields J, Horton RA, Brown MK, Reed DJ. Biliary secretion of alpha-tocopherol and the role of the mdr2 P-glycoprotein in rats and mice. Arch Biochem Biophys. 1998;350(2):183–92. [DOI] [PubMed] [Google Scholar]
- 11. Larsen LF, Marckmann P, Kornerup Hansen A, Bukhave K. Fish oil feeding is associated with an increased accumulation of dietary lipids in enterocytes: results from an in vivo study in rats. Scand J Gastroenterol. 2003;38(7):712–8. [DOI] [PubMed] [Google Scholar]
- 12. Clifford AJ, de Moura FF, Ho CC, Chuang JC, Follett J, Fadel JG, Novotny JA. A feasibility study quantifying in vivo human alpha-tocopherol metabolism. Am J Clin Nutr. 2006;84(6):1430–41. [DOI] [PubMed] [Google Scholar]
- 13. Chuang JC, Matel HD, Nambiar KP, Kim SH, Fadel JG, Holstege DM, Clifford AJ. Quantitation of [5-14CH3]-(2R, 4′R, 8′R)-alpha-tocopherol in humans. J Nutr. 2011;141(8):1482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mah E, Sapper TN, Chitchumroonchokchai C, Failla ML, Schill KE, Clinton SK, Bobe G, Traber MG, Bruno RS. alpha-Tocopherol bioavailability is lower in adults with metabolic syndrome regardless of dairy fat co-ingestion: a randomized, double-blind, crossover trial. Am J Clin Nutr. 2015;102(5):1070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lassnigg A, Punz A, Barker R, Keznickl P, Manhart N, Roth E, Hiesmayr M. Influence of intravenous vitamin E supplementation in cardiac surgery on oxidative stress: a double-blinded, randomized, controlled study. Br J Anaesth. 2003;90(2):148–54. [DOI] [PubMed] [Google Scholar]
- 16. Bartels M, Biesalski HK, Engelhart K, Sendlhofer G, Rehak P, Nagel E. Pilot study on the effect of parenteral vitamin E on ischemia and reperfusion induced liver injury: a double blind, randomized, placebo-controlled trial. Clin Nutr. 2004;23(6):1360–70. [DOI] [PubMed] [Google Scholar]
- 17. Kono N, Arai H. Intracellular transport of fat-soluble vitamins A and E. Traffic. 2015;16(1):19–34. [DOI] [PubMed] [Google Scholar]
- 18. Taylor AW, Bruno RS, Traber MG. Women and smokers have elevated urinary F(2)-isoprostane metabolites: a novel extraction and LC-MS methodology. Lipids. 2008;43(10):925–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruno RS, Ramakrishnan R, Montine TJ, Bray TM, Traber MG α-Tocopherol disappearance is faster in cigarette smokers and is inversely related to their ascorbic acid status. Am J Clin Nutr. 2005;81(1):95–103. [DOI] [PubMed] [Google Scholar]
- 20. Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J et al.. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93:3704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levine M, Wang Y, Padayatty S, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA. 2001;98:9842–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bruno RS, Leonard SW, Atkinson J, Montine TJ, Ramakrishnan R, Bray TM, Traber MG. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic Biol Med. 2006;40(4):689–97. [DOI] [PubMed] [Google Scholar]
- 23. Ingold KU, Hughes L, Slaby M, Burton GW. Synthesis of 2R,4′R,8′R-a-tocopherols selectively labelled with deuterium. J Labelled Comp Radiopharm. 1987;24:817–31. [Google Scholar]
- 24. Termath AO, Sebode H, Schlundt W, Stemmler RT, Netscher T, Bonrath W, Schmalz HG. Total synthesis of (R,R,R)-alpha-tocopherol through asymmetric Cu-catalyzed 1,4-addition. Chemistry. 2014;20(38):12051–5. [DOI] [PubMed] [Google Scholar]
- 25. Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, Ingold KU. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998;67(4):669–84. [DOI] [PubMed] [Google Scholar]
- 26. Hong YL, Yeh SL, Chang CY, Hu ML. Total plasma malondialdehyde levels in 16 Taiwanese college students determined by various thiobarbituric acid tests and an improved high-performance liquid chromatography-based method. Clin Biochem. 2000;33(8):619–25. [DOI] [PubMed] [Google Scholar]
- 27. Redgrave TG, Roberts DC, West CE. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975;65(1-2):42–9. [DOI] [PubMed] [Google Scholar]
- 28. Mazalli MR, Sawaya AC, Eberlin MN, Bragagnolo N. HPLC method for quantification and characterization of cholesterol and its oxidation products in eggs. Lipids. 2006;41(6):615–22. [DOI] [PubMed] [Google Scholar]
- 29. Hafiane A, Genest J. High density lipoproteins: measurement techniques and potential biomarkers of cardiovascular risk. BBA Clin. 2015;3:175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Warnick GR, Nguyen T, Albers AA. Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterol. Clin Chem. 1985;31(2):217–22. [PubMed] [Google Scholar]
- 31. Gibson JC, Rubinstein A, Brown WV. Precipitation of apo E-containing lipoproteins by precipitation reagents for apolipoprotein B. Clin Chem. 1984;30(11):1784–8. [PubMed] [Google Scholar]
- 32. Leonard SW, Bobe G, Traber MG. Stability of antioxidant vitamins in whole human blood during overnight storage at 4 degrees C and frozen storage up to 6 months. Int J Vitam Nutr Res. 2018;88(3-4):151–7. [DOI] [PubMed] [Google Scholar]
- 33. Vaule H, Leonard SW, Traber MG. Vitamin E delivery to human skin: studies using deuterated alpha-tocopherol measured by APCI LC-MS. Free Radic Biol Med. 2004;36(4):456–63. [DOI] [PubMed] [Google Scholar]
- 34. Traber MG, Leonard SW, Bobe G, Fu X, Saltzman E, Grusak MA, Booth SL. alpha-Tocopherol disappearance rates from plasma depend on lipid concentrations: studies using deuterium-labeled collard greens in younger and older adults. Am J Clin Nutr. 2015;101(4):752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramakrishnan R, Ramakrishnan JD.. A state space transformation can yield identifiable models for tracer kinetic studies with enrichment data. Bull Math Biol. 2010;72(8):2019–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bradley WA, Gianturco SH. ApoE is necessary and sufficient for the binding of large triglyceride-rich lipoproteins to the LDL receptor; apoB is unnecessary. J Lipid Res. 1986;27(1):40–8. [PubMed] [Google Scholar]
- 37. Traber MG, Ramakrishnan R, Kayden HJ. Human plasma vitamin E kinetics demonstrate rapid recycling of plasma RRR-alpha-tocopherol. Proc Natl Acad Sci U S A. 1994;91(21):10005–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeanes YM, Hall WL, Ellard S, Lee E, Lodge JK. The absorption of vitamin E is influenced by the amount of fat in a meal and the food matrix. Br J Nutr. 2004;92(4):575–9. [DOI] [PubMed] [Google Scholar]
- 39. Sokol RJ, Heubi JE, Iannaccone S, Bove KE, Balistreri WF. Mechanism causing vitamin E deficiency during chronic childhood cholestasis. Gastroenterology. 1983;85(5):1172–82. [PubMed] [Google Scholar]
- 40. Sokol RJ, Balistreri WF, Hoofnagle JH, Jones EA. Vitamin E deficiency in adults with chronic liver disease. Am J Clin Nutr. 1985;41(1):66–72. [DOI] [PubMed] [Google Scholar]
- 41. Desmarchelier C, Tourniaire F, Preveraud DP, Samson-Kremser C, Crenon I, Rosilio V, Borel P. The distribution and relative hydrolysis of tocopheryl acetate in the different matrices coexisting in the lumen of the small intestine during digestion could explain its low bioavailability. Mol Nutr Food Res. 2013;57(7):1237–45. [DOI] [PubMed] [Google Scholar]
- 42. Ton MN, Chang C, Carpentier YA, Deckelbaum RJ. In vivo and in vitro properties of an intravenous lipid emulsion containing only medium chain and fish oil triglycerides. Clin Nutr. 2005;24(4):492–501. [DOI] [PubMed] [Google Scholar]
- 43. Traber MG, Olivecrona T, Kayden HJ. Bovine milk lipoprotein lipase transfers tocopherol to human fibroblasts during triglyceride hydrolysis in vitro. J Clin Invest. 1985;75(5):1729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40(1):1–16. [PubMed] [Google Scholar]
- 45. Traber MG, Lane JC, Lagmay NR, Kayden HJ. Studies on the transfer of tocopherol between lipoproteins. Lipids. 1992;27(9):657–63. [DOI] [PubMed] [Google Scholar]
- 46. Gotto AM., Jr Interrelationship of triglycerides with lipoproteins and high-density lipoproteins. Am J Cardiol. 1990;66(6):20A–3A. [DOI] [PubMed] [Google Scholar]
- 47. Hacquebard M, Vandenbranden M, Malaisse WJ, Ruysschaert JM, Deckelbaum RJ, Carpentier YA. Vitamin E transfer from lipid emulsions to plasma lipoproteins: mediation by multiple mechanisms. Lipids. 2008;43(7):663–71. [DOI] [PubMed] [Google Scholar]
- 48. Kostner GM, Oettl K, Jauhiainen M, Ehnholm C, Esterbauer H, Dieplinger H. Human plasma phospholipid transfer protein accelerates exchange transfer of alpha-tocopherol between lipoproteins and cells. Biochem J. 1995;305:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dixon JL, Ginsberg HN. Regulation of hepatic secretion of apolipoprotein B-containing lipoproteins: information obtained from cultured liver cells. J Lipid Res. 1993;34(2):167–79. [PubMed] [Google Scholar]
- 50. Smagris E, Gilyard S, BasuRay S, Cohen JC, Hobbs HH. Inactivation of Tm6sf2, a gene defective in fatty liver disease, impairs lipidation but not secretion of very low density lipoproteins. J Biol Chem. 2016;291(20):10659–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kono N, Ohto U, Hiramatsu T, Urabe M, Uchida Y, Satow Y, Arai H. Impaired alpha-TTP-PIPs interaction underlies familial vitamin E deficiency. Science. 2013;340(6136):1106–10. [DOI] [PubMed] [Google Scholar]
- 52. Chung S, Ghelfi M, Atkinson J, Parker R, Qian J, Carlin C, Manor D. Vitamin E and phosphoinositides regulate the intracellular localization of the hepatic alpha-tocopherol transfer protein. J Biol Chem. 2016;291(33):17028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oram JF, Vaughan AM, Stocker R. ATP-binding cassette transporter A1 mediates cellular secretion of alpha-tocopherol. J Biol Chem. 2001;276(43):39898–902. [DOI] [PubMed] [Google Scholar]
- 54. Nicod N, Parker RS.. Vitamin E secretion by Caco-2 monolayers to APOA1, but not to HDL, is vitamer selective. J Nutr. 2013;143(10):1565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shichiri M, Takanezawa Y, Rotzoll DE, Yoshida Y, Kokubu T, Ueda K, Tamai H, Arai H. ATP-binding cassette transporter A1 is involved in hepatic alpha-tocopherol secretion. J Nutr Biochem. 2010;21(5):451–6. [DOI] [PubMed] [Google Scholar]
- 56. Reboul E. Vitamin E bioavailability: mechanisms of intestinal absorption in the spotlight. Antioxidants (Basel). 2017;6(4):E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Di Filippo M, Collardeau Frachon S, Janin A, Rajan S, Marmontel O, Decourt C, Rubio A, Nony S, Dumont S, Cuerq C et al.. Normal serum ApoB48 and red cells vitamin E concentrations after supplementation in a novel compound heterozygous case of abetalipoproteinemia. Atherosclerosis. 2019;284:75–82. [DOI] [PubMed] [Google Scholar]
- 58. Young SG, Cham CM, Pitas RE, Burri BJ, Connolly A, Flynn L, Pappu AS, Wong JS, Hamilton RL, Farese RV Jr. A genetic model for absent chylomicron formation: mice producing apolipoprotein B in the liver, but not in the intestine. J Clin Invest. 1995;96(6):2932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Soayfane Z, Terce F, Cantiello M, Robenek H, Nauze M, Bezirard V, Allart S, Payre B, Capilla F, Cartier C et al.. Exposure to dietary lipid leads to rapid production of cytosolic lipid droplets near the brush border membrane. Nutr Metab (Lond). 2016;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Demignot S, Beilstein F, Morel E. Triglyceride-rich lipoproteins and cytosolic lipid droplets in enterocytes: key players in intestinal physiology and metabolic disorders. Biochimie. 2014;96:48–55. [DOI] [PubMed] [Google Scholar]
- 61. Khalifeh-Soltani A, Gupta D, Ha A, Iqbal J, Hussain M, Podolsky MJ, Atabai K. Mfge8 regulates enterocyte lipid storage by promoting enterocyte triglyceride hydrolase activity. JCI Insight. 2016;1(18):e87418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Traber MG, Sokol RJ, Burton GW, Ingold KU, Papas AM, Huffaker JE, Kayden HJ. Impaired ability of patients with familial isolated vitamin E deficiency to incorporate alpha-tocopherol into lipoproteins secreted by the liver. J Clin Invest. 1990;85(2):397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Traber MG, Sokol RJ, Kohlschutter A, Yokota T, Muller DPR, Dufour R, Kayden HJ. Impaired discrimination between stereoisomers of alpha-tocopherol in patients with familial isolated vitamin-E-deficiency. J Lipid Res. 1993;34(2):201–10. [PubMed] [Google Scholar]
- 64. Beaumier-Gallon G, Dubois C, Senft M, Vergnes MF, Pauli AM, Portugal H, Lairon D. Dietary cholesterol is secreted in intestinally derived chylomicrons during several subsequent postprandial phases in healthy humans. Am J Clin Nutr. 2001;73(5):870–7. [DOI] [PubMed] [Google Scholar]
- 65. Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled alpha- and gamma-tocopherols demonstrate faster plasma gamma-tocopherol disappearance and greater gamma-metabolite production. Free Radic Biol Med. 2005;38(7):857–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.