ABSTRACT
Background
Although available data suggest that some dietary flavan-3-ol sources reduce cardiometabolic risk, to our knowledge no review has systematically synthesized their specific contribution.
Objective
We aimed to examine, for the first time, if there is consistent evidence that higher flavan-3-ol intake, irrespective of dietary source, reduces cardiometabolic risk.
Methods
MEDLINE, Cochrane Central, and Commonwealth Agricultural Bureau abstracts were searched for prospective cohorts and randomized controlled trials (RCTs) published from 1946 to March 2019 on flavan-3-ol intake and cardiovascular disease (CVD) risk. Random-effects models meta-analysis was used. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach assessed the strength of evidence.
Results
Of 15 prospective cohorts (23 publications), 4 found highest compared with lowest habitual intakes of flavan-3-ols were associated with a 13% reduction in risk of CVD mortality and 2 found a 19% reduction in risk of chronic heart disease (CHD) incidence. Highest compared with lowest habitual intakes of monomers were associated with a reduction in risk of type 2 diabetes mellitus (T2DM) (n = 5) and stroke (n = 4) (10% and 18%, respectively). No association was found for hypertension. Of 156 RCTs, flavan-3-ol intervention resulted in significant improvements in acute/chronic flow-mediated dilation (FMD), systolic (SBP) and diastolic blood pressure (DBP), total cholesterol (TC), LDL and HDL cholesterol, triglycerides (TGs), hemoglobin A1c (HbA1c), and homeostasis model assessment of insulin resistance (HOMA-IR). All analyses, except HbA1c, were associated with moderate/high heterogeneity. When analyses were limited to good methodological quality studies, improvements in TC, HDL cholesterol, SBP, DBP, HOMA-IR, and acute/chronic FMD remained significant. In GRADE evaluations, there was moderate evidence in cohort studies that flavan-3-ol and monomer intakes were associated with reduced risk of CVD mortality, CHD, stroke, and T2DM, whereas RCTs reported improved TC, HDL cholesterol, SBP, and HOMA-IR.
Conclusions
Available evidence supports a beneficial effect of flavan-3-ol intake on cardiometabolic outcomes, but there was considerable heterogeneity in the meta-analysis. Future research should focus on an integrated intake/biomarker approach in cohorts and high-quality dose–response RCTs. This review was registered at www.crd.york.ac.uk/PROSPERO/ as CRD42018035782.
Keywords: flavan-3-ols, flavonoids, cardiovascular, diabetes, blood pressure
Introduction
Interest in the role of dietary flavonoids (naturally occurring plant bioactives) in reducing the risk of cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) stems from findings from a range of interdisciplinary sources (prospective studies, clinical trials, and mechanistic studies in animal and in vitro) (1–4). However, to date, the majority of the data has focused on specific flavonoid-rich food sources like tea, cocoa, fruits, and soy, all of which contain a range of bioactive constituents beyond flavonoids (5–7). Although a number of systematic reviews have collated the available data for these individual flavonoid-rich food sources (5–13), to our knowledge no review has systematically examined the specific contribution of the flavonoid content of the foods in relation to cardiometabolic health.
One subclass of dietary flavonoids that is of significant interest is the flavan-3-ols, although to date both population-based studies and clinical trials have mainly focused on their 2 main food sources, tea and cocoa, with a number of systematic reviews and meta-analyses published for these individual foods (5–13). Because these foods contain a range of other potentially bioactive constituents, we recently undertook a comprehensive and systematic evidence-mapping exercise which provided the evidence base to suggest that sufficient evidence is now available to conduct a systematic review and meta-analysis on the potential cardiometabolic health effects of flavan-3-ols per se (irrespective of source) (14).
Our aim, using a combination of evidence from both randomized controlled trials (RCTs) and prospective cohort data, was to examine if there was consistent evidence that a higher intake of flavan-3-ols reduced the risk of CVD. We also examined if there was a dose–response relation, a minimum intake for a vascular benefit, if food source or form (food compared with supplement/pure compound) affected the magnitude of the health effect, and if any individual flavan-3-ol components were driving the cardiometabolic health effects.
Methods
This study is a systematic review of published literature evaluating the effects of flavan-3-ol intake on CVD outcomes and risk factors. The review was conducted in 2 phases: a first phase of evidence mapping (14) followed by a full systematic review. A technical expert panel was convened that served as key informants to identify issues relevant to flavan-3-ols, refine key questions, identify interventions of interest, define eligibility criteria, and develop the analytic framework (14). A standard protocol was developed and registered in the PROSPERO database (https://www.crd.york.ac.uk/PROSPERO) as CRD42018035782. This review was conducted using standard methodology and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (15).
Data sources and study eligibility
A comprehensive search of the scientific literature was conducted in MEDLINE, the Cochrane Central databases, and the Commonwealth Agricultural Bureau from inception through 26 April, 2016 for evidence mapping (14) and this search was further updated until 9 March, 2019. The reference lists of any previously published systematic reviews on flavan-3-ols and health outcomes were screened to identify additional studies. Search key terms for monomer, proanthocyanidin, and foods and beverages (i.e., tea, red wine) rich in flavan-3-ols were combined with the outcomes of interest and limited by terms for study designs of interest (i.e., randomized trials and prospective cohort studies) and by studies conducted in humans. No language restriction was applied. The detailed search strategy is reported elsewhere (14). Citations were screened in duplicate using predefined eligibility criteria and any disagreements regarding study inclusion or exclusion were resolved in group meetings.
Study inclusion criteria
We included RCTs and prospective cohort studies conducted in adults (≥18 y) that quantified the amount of flavan-3-ols consumed per day or per week. Studies that provided sufficient serving size data were also included, provided estimation of total flavan-3-ol consumption was possible using the USDA (16, 17) or Phenol Explorer databases (www.phenol-explorer.eu) (18). Interventions included monomers, proanthocyanidins, and foods and beverages rich in flavan-3-ol content such as fruits (i.e., apples, berries, and grapes), dark chocolate, cocoa, teas, and red wine. Comparators included low flavan-3-ol content or no flavan-3-ols or placebos. The primary outcomes of interest included CVD clinical outcomes [e.g., CVD mortality, chronic heart disease (CHD), stroke, diabetes, and hypertension]; secondary outcomes included risk factors [serum lipids, blood pressures, glucose metabolism, and flow-mediated dilation (FMD)] and biomarkers [high-sensitivity CRP (hsCRP), IL-6, and TNF-α]. The full study eligibility criteria are described elsewhere (14).
Study exclusion criteria
We excluded retrospective studies, cross-sectional studies, case series, case reports, and narrative reviews. Studies evaluating interventions that were not clearly specified, including in relation to type of wine (i.e., red or white), type of tea (i.e., black or green), type of chocolate (i.e., dark or white), and cinnamon, were excluded.
In addition to the aforementioned common eligibility criteria, we specified the following additional criteria according to study design. We included prospective cohort studies that reported multivariable results adjusting for any potential confounders and had ≥6 mo of follow-up time.
We included RCTs that recruited participants who were healthy, at an increased risk of CVD, or had existing CVD. Minimum intervention duration was applied according to outcomes of interest. For studies with eligible interventions such as extracts that reported insufficient data on flavan-3-ol content, study authors or product manufacturers were contacted to obtain additional information on flavan-3-ols expressed as mg/d.
Data extraction
A standardized, prepiloted data extraction form was created for extracting data in the Systematic Review Data Repository (https://srdr.ahrq.gov). The forms were designed to capture all relevant elements from the descriptions on study population characteristics, enrolled and analyzed sample sizes, study design features, interventions and comparators, relevant outcomes, results, and risk of bias assessment. Data from each study were extracted independently by 1 of 6 investigators and confirmed by ≥1 other. Data discrepancies were identified and resolved through group discussion.
Risk of bias/quality assessment
Two reviewers independently assessed the risk of bias for each included study. For RCTs, we used the Cochrane risk-of-bias tool for clinical trials along with nutrition-specific items from a critical appraisal of micronutrient systematic reviews (19, 20). For prospective cohort studies, we assessed the following domains: risk of selection, performance, attrition, detection, and selective outcome reporting biases (21). Each methodological quality item was rated as Yes, No, or Unclear.
Data synthesis
All included studies were summarized in narrative form and in summary tables that tabulated important features of the study populations, design, intervention, outcomes, and results.
Summary tables were organized by key questions and by outcome of interest. We performed meta-analyses where there were ≥3 unique studies deemed to be sufficiently similar in population and outcomes (22). The results from RCTs and prospective cohort studies were meta-analyzed separately.
For categorical outcomes, we conducted meta-analysis using a random-effects model and reported the results as summary RRs, comparing the extreme categories of flavan-3-ols (highest compared with lowest, as defined within each study). When ≥3 studies reported their results stratified by age or sex, we performed subgroup meta-analyses for age and sex categories.
For continuous outcomes, we extracted the mean baseline levels in both intervention and control arms and measures of variance (SD or SE). We estimated the within-cohort changes (i.e., intervention final − intervention initial, or control final − control initial). We calculated the SE for within-cohort change. We then calculated net differences for continuous outcomes using the following equation: net change = (intervention final − intervention initial) − (control final − control initial).
When studies reported sufficient information, we conducted stratified analysis by monomers, proanthocyanidins, types of interventions, and quartiles of median flavan-3-ol intake. The types of interventions were categorized into cocoa, extract, fruit, pure form, tea, and wine. To evaluate possible dose effects and heterogeneity in results, we also performed several meta-regression analyses with continuous flavan-3-ol dosage (in milligrams per day) and outcomes of interest.
We tested between-study heterogeneity with the Q statistic (significant when P < 0.10) and quantified its extent with the I2 statistic (23). I2 statistics were further classified in terms of heterogeneity as follows: >25% as low; >50% as moderate; and >75% as high. Analyses were performed in Stata version 13 (StataCorp) with the metan, metareg, and metabias functions.
Grading overall strength of evidence
A structured approach modeled after the GRADE (Grading of Recommendations Assessment, Development and Evaluation) was employed to grade the quality of overall evidence for each outcome as 1 of the following 4 grades: High, Moderate, Low, or Very Low (24).
Results
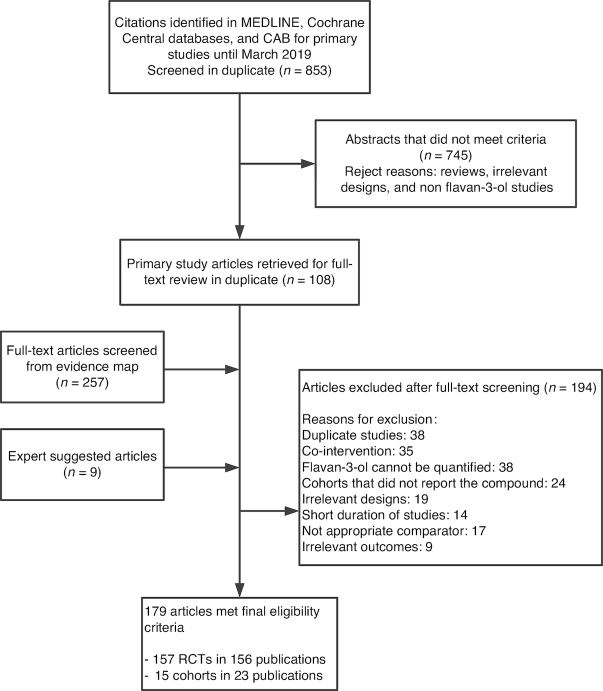
The database searches identified 4047 abstracts. After title and abstract screening, 365 articles (108 articles from the updated search and 257 articles identified from the evidence map) were retrieved for full-text review. An additional 9 articles were identified by the expert. A total of 23 prospective cohort articles and 157 RCTs (156 publications) met the eligibility criteria. The study flow diagram is depicted in Figure 1.
FIGURE 1.
Study flow diagram depicting the review process. CAB, Commonwealth Agricultural Bureau; RCT, randomized controlled trial.
Cohort studies
Fifteen different cohorts [in 23 publications (25–47)] reported the following outcomes: CVD mortality (25, 26, 29, 32, 35–37, 39, 47), incident CHD (31, 38, 39, 41), stroke (25, 31, 37–39, 41), hypertension (27, 34, 39, 43), and T2DM (33, 40, 42, 44–46). One RCT was analyzed as an observational cohort study (38, 44). Details about baseline characteristics are available in Table 1. Four studies included only postmenopausal women (26, 32, 35, 36). All studies assessed flavan-3-ol intake by semiquantitative FFQ administered at baseline, but only a few also assessed flavan-3-ol intake at follow-up time points (25, 27, 29, 40). All adjusted for variables including participant characteristics such as age or CVD risk factors such as T2DM or hypertension. All cohorts except 4 did not adjust for diet factors (26, 32, 34, 36). The methodological quality of the eligible cohort studies was rated good, reflecting low risk of bias (Supplemental Table 1). A summary of the overall effects across the meta-analysis of all eligible outcomes from the cohort studies can be found in Figure 2.
TABLE 1.
Study characteristics of included cohorts1
| Authors | Country | Cohort name | Follow-up years | n enrolled (n analyzed) | Male, % | Mean age,2 y | BMI,3kg/m2 | Funding source | Comorbidities |
|---|---|---|---|---|---|---|---|---|---|
| Adriouch et al. (41) | France | NutriNet-Santé | 18 | 84,158 (84,158) | 21.3 | 44.1 ± 14.5 | NR | Government, academia | Diabetes, hypertension, hypercholesterolemia |
| Arts et al. (25) | Netherlands | The Zutphen Elderly Study | 10 | 939 (806) | 100 | 71 (65–84) | 25.5 (NR) | Government | Hypertension, hypercholesterolemia |
| Arts et al. (26) | United States | Iowa Women's Health Study | 12 | 34,492 (32,857) | 0 | 61.5 (55–69) | 26.9 (NR) | Government | Diabetes, hypertension, CVD |
| Cassidy et al. (27) | United States | NHS, NHS II, HPFS | 14 | 156,957 (156,957) | 14.7 | 44.6 (25–75) | 24.6 (NR) | Government | Hypertension |
| Cassidy et al. (28) | United States | NHS | 18 | 93,600 (93,600) | 0 | 36.6 (25–42) | 24.6 (NR) | Government | Diabetes, hypertension, hypercholesterolemia |
| Dower et al. (29) | Netherlands | The Zutphen Elderly Study | 25 | 774 (774) | 100 | 71.9 (65–84) | 25.6 (NR) | Government, industry | Diabetes, CVD, hypertension, hypercholesterolemia |
| Goetz et al. (30) | United States | REGARDS | 6.5 | 30,239 (20,024) | 44 | 64.6 (NR) | 29.1 (NR) | Government | Diabetes, hypertension |
| Goetz et al. (31) | United States | REGARDS | 6 | 16,678 (16,678) | 41.2 | 64.3 (NR) | 29 (NR) | Government | NR |
| Grosso et al. (42) | Poland | HAPIEE subcohort | 4 | 10,728 (5806) | 47 | 57.4 (45–69) | 27.7 (NR) | Government, foundation | NR |
| Grosso et al. (43) | Poland | HAPIEE subcohort | 4 | 2725 (2725) | 42.2 | 55.6 (45–69) | 26.4 (NR) | Government, foundation | Hypertension |
| Ivey et al. (32) | Australia | Calcium Intake Fracture Outcome Study | 5 | 1063 (1063) | 0 | 80.1 (NR) | 27.1 (NR) | Government, foundation, hospital | Diabetes, hypertension |
| Jacques et al. (33) | United States | Framingham Heart Study Offspring cohort | 11.9 | 2915 (2915) | 45.5 | 54.2 (28–62) | 26.7 (NR) | Government, foundation | Diabetes, CVD |
| Lajous et al. (34) | France | EPIC | 13.8 | 40,574 (40,574) | 0 | 51.6 (NR) | 22.2 (NR) | Government, foundation | Diabetes, CVD, hypertension, hypercholesterolemia |
| McCullough et al. (35) | United States | American Cancer Society's CPS-II Nutrition Cohort | 7 | 98,469 (98,469) | 38.77 | 69.1 (NR) | NR | Government | Diabetes, CVD, hypertension, hypercholesterolemia |
| Mink et al. (36) | United States | Iowa Women's Health Study | 12 | 34,492 (34,492) | 0 | 61 (55–69) | 26.9 (NR) | Foundation | Diabetes, CVD, hypertension |
| Mursu et al. (37) | Finland | The Kuopio Ischaemic Heart Disease Risk Factor Study | 15.2 | 2682 (1950) | 100 | 52.4 (42–60) | 26.7 (NR) | Foundation | Diabetes, CVD |
| Ponzo et al. (47) | Italy | NR | 12 | 1658 (1658) | 47 | 54.6 (45–64) | 26.6 (NR) | Government | CVD, diabetes, hypercholesterolemia, hypertension |
| Tresserra-Rimbau et al. (38) | Spain | PREDIMED | 4.3 | 7447 (7172) | 42.65 | 67 (NR) | 30 (NR) | Government | Diabetes, CVD, hypertension |
| Tresserra-Rimbau et al. (44) | Spain | PREDIMED | 5.51 | 3430 (3430) | 38.3 | 66.6 (NR) | 29.9 (NR) | Government, industry | Hypercholesterolemia, hypertension |
| Vogiatzoglou et al. (39) | United Kingdom | EPIC-Norfolk | 11.1 | 25,639 (24,885) | 34 | 60 (40–75) | 27 (NR) | Government, industry | Diabetes, CVD, hypertension, hypercholesterolemia |
| Wedick et al. (40) | United States | NHS, NHS II, HPFS | 12–18 | 289,900 (199,980) | 20.6 (0, 0, 100) | 44.3 (25–75) | 24.9 (NR) | Government | Diabetes, CVD, hypercholesterolemia, hypertension |
| Zamora-Ros et al. (45, 46) | Eight European countries | EPIC-Interact subcohort | NR | 16,154 (15,268) | 37.8 | 52.4 (NR) | 26.0 (NR) | Government | CVD, diabetes, hypercholesterolemia, hypertension |
1CPS, Cancer Prevention Study; CVD, cardiovascular disease; EPIC, European Prospective Investigation into Cancer and Nutrition; HAPIEE, Health, Alcohol and Psychosocial factors in Eastern Europe; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NR, not reported; PREDIMED, Prevención con Dieta Mediterránea study; REGARDS, REasons for Geographic and Racial Differences in Stroke.
Mean ± SD or mean (range).
Mean ± SD.
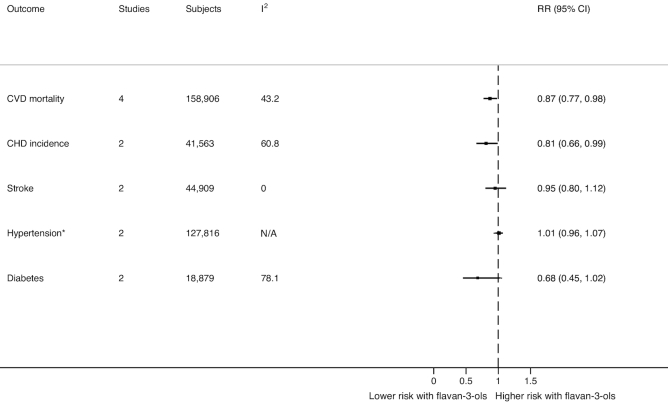
FIGURE 2.
The effect of flavan-3-ols on cardiometabolic health outcomes from prospective cohort studies. Data reported are RRs and 95% CIs for a fully adjusted random-effects meta-analysis model for each outcome. *Hypertension outcome included men and women subpopulations from the same cohort from 1 article that reported total flavan-3-ol intake and blood pressure. CHD, chronic heart disease; CVD, cardiovascular disease.
CVD mortality
Seven cohorts in 9 publications (25, 26, 29, 32, 35–37, 39, 47) reported an association between flavan-3-ol intake and risk of CVD mortality (196,148 participants who were followed for 5–25 y) (Table 1). Four cohorts reported data on total flavan-3-ol intake (32, 35, 36, 39) and 5 cohorts [in 6 studies (25, 26, 29, 35, 37, 47)] reported data on monomers; of these, 1 reported data on components of monomers [gallated compared with nongallated forms (29)]. Ascertainment of CVD mortality was by examination of hospital records (25, 29), national death index or registry (26, 32, 35–37), or death certificates (39, 47).
Higher flavan-3-ol intake was significantly associated with a reduction in risk of CVD mortality in 4 cohorts (summary RR: 0.87; 95% CI: 0.77, 0.98; I2 = 43.2%). In subgroup analysis, a higher flavan-3-ol intake was significantly associated with a reduction in risk of CVD mortality among women, but no similar association was found in men (Supplemental Table 2). Higher monomer intake was significantly associated with lower risk of CVD mortality among women, but no similar association was found in men (Supplemental Table 2).
CHD incidence
Three cohorts (31, 38, 39) reported an association between flavan-3-ol intake and risk of incident CHD (Table 1). The studies included 48,735 participants who were followed for 4–11 y. All studies included both sexes. Two studies reported total flavan-3-ol intake (38, 39) and 1 study reported data for monomers and proanthocyanidins (31). Ascertainment of incident CHD in all studies was either by examination of hospital records or through adjudication.
A higher habitual flavan-3-ol intake was also significantly associated with a reduction in risk of CHD incidence in 2 cohorts that reported data stratified by sexes (summary RR: 0.81; 95% CI: 0.66, 0.99; I2 = 60.8%). In subgroup analysis, higher flavan-3-ol intake was significantly associated with lower CHD incidence among men, but no similar association was found in women. There was no association between monomer intake and CHD incidence in the whole population or by sex (Supplemental Table 2).
Stroke
Six cohorts (25, 31, 37–39, 41) reported the association between flavan-3-ol intake and risk of stroke (Table 1). The studies included 173,487 participants who were followed for 4–18 y. Two studies included all men (25, 37), whereas the others included both sexes. Four studies reported data on the association between monomer intake and risk of stroke (25, 30, 37, 41). Ascertainment of stroke in all studies was either by examination of hospital records or through adjudication, but only 1 cohort reported stroke outcome adjudication by a neurologist (37).
There was no association between higher flavan-3-ol intake and incidence of stroke in 2 cohorts that reported data stratified by sexes (summary RR: 0.95; 95% CI: 0.80, 1.12; I2 = 0.0%). Insufficient data precluded additional sex-specific subgroup analyses. Higher monomer intake was associated with a significant reduction in risk of stroke in 4 cohorts (summary RR: 0.82; 95% CI: 0.68, 0.99; I2 = 0.0%).
Hypertension
Six cohorts [Nurses’ Health Study (NHS), NHS II, Health Professionals Follow-Up Study (HPFS), French-European Prospective Investigation into Cancer and Nutrition (EPIC); EPIC-Norfolk; Health, Alcohol and Psychosocial factors in Eastern Europe (HAPIEE)] in 4 studies (27, 34, 39, 43) reported on the association between monomer or polymer intake and risk of hypertension (Table 1). Only 1 cohort reported data on total flavan-3-ol intake and blood pressure [systolic (SBP) and diastolic blood pressure (DBP)] as a continuous outcome (39). The remaining 5 cohorts reported data on the association between monomer or proanthocyanidin intake and incident hypertension. The studies included 225,141 participants who were followed for 11–14 y. Three cohorts included only women (French-EPIC; NHS; NHS II), 1 cohort included only men (HPFS), and 2 cohorts included both sexes (EPIC-Norfolk; HAPIEE). Ascertainment of hypertension was by self-reported data (27, 34) or done at the time of the baseline visit (43); but this was not reported in 1 cohort (39).
There was no association between higher monomer intake (summary RR: 1.01; 95% CI: 0.96, 1.07; I2 = 65.8%) or between proanthocyanidin intake (summary RR: 0.98; 95% CI: 0.93, 1.04; I2 = 70.4%) and incident hypertension. Insufficient data precluded additional sex-specific subgroup analyses.
T2DM
Two cohorts (HAPIEE and EPIC-Interact) reported an association between total flavan-3-ol intake and risk of T2DM. Six cohorts (NHS, NHS II, HPFS, Framingham Offspring, EPIC-Interact, Prevención con Dieta Mediterránea) in 5 studies reported on the association between monomer intake and risk of T2DM (Table 1). Of these, 1 study reported on the association between subclasses of monomers or proanthocyanidins and risk of T2DM (46). The studies included 233,207 participants who were followed for 5–18 y. Ascertainment of T2DM was by using multiple data sources including questionnaire evaluation of symptoms, diagnostic tests, hospital admissions, and hypoglycemic therapy in most of the studies (40, 42, 44–46), or via confirmation by a physician at each study examination (33).
There was no association between total flavan-3-ol intake and T2DM in 2 studies (summary RR: 0.68; 95% CI: 0.45, 1.02; I2 = 78.1%). Higher monomer intake was significantly associated with a reduction in risk of T2DM in 5 studies (summary RR: 0.90; 95% CI: 0.83, 0.97; I2 = 52.8%). Insufficient data precluded additional sex-specific subgroup analyses. Higher proanthocyanidin intake was significantly associated with a reduction in risk of T2DM in 3 studies (summary RR: 0.89; 95% CI: 0.81, 0.98; I2 = 0.0%).
Overall strength of evidence among cohort outcomes
We then graded the overall strength of evidence for the outcomes reported in cohort studies. There was moderate strength of evidence that higher habitual intake of flavan-3-ols is associated with a reduction in risk of CVD mortality (total flavan-3-ol and monomer intake), CHD incidence (total flavan-3-ol intake), and stroke (monomer intake), and high strength of evidence for an association of higher monomer and proanthocyanidin intake with a reduction in risk of T2DM incidence (Supplemental Table 3). No difference in outcomes was observed for hypertension (data not shown; graded low strength of evidence).
RCTs
Only 1 long-term RCT (48) among 157 eligible RCTs (from 156 publications) reported clinical outcomes that evaluated flavan-3-ol intervention and CVD risk factors (references of the included RCTs are in Supplemental Table 4). The duration of the RCTs included in the meta-analysis ranged from 3 to 26 wk for the majority of the outcomes. The intervention dosages ranged from 40 to 1540 mg/d. Descriptions of study characteristics and outcomes of interest are presented in Table 2. Only 48 RCTs were rated good quality (Supplemental Table 5) and their references are given in Supplemental Table 6. A summary of the overall effects across the meta-analysis of all eligible outcomes can be found in Figure 3.
TABLE 2.
Description of study-level characteristics of eligible RCTs1
| Total | RCT parallel | RCT crossover | |
|---|---|---|---|
| Publications | 157 (100.00) | 92 (58.6) | 65 (41.4) |
| Blinding | |||
| Double-blind | 95 (60.5) | 59 (64.1) | 36 (55.4) |
| Single-blind | 26 (16.6) | 10 (10.8) | 16 (24.6) |
| Not blinded | 17 (10.8) | 11 (12.0) | 6 (9.2) |
| Unclear | 19 (12.1) | 12 (13.0) | 7 (10.7) |
| Mean age | |||
| <50 y | 54 (34.4) | 28 (30.4) | 26 (40.0) |
| ≥50 y | 79 (50.3) | 43 (46.7) | 36 (55.4) |
| Baseline health | |||
| Healthy | 53 (33.8) | 23 (25.0) | 30 (46.2) |
| At risk of CVD | 86 (54.8) | 62 (67.4) | 24 (36.9) |
| Existing CVD | 8 (5.1) | 2 (2.2) | 6 (9.2) |
| Mixed | 10 (6.4) | 5 (5.4) | 5 (7.7) |
| Study region | |||
| Africa | 3 (1.9) | 2 (2.2) | 1 (1.5) |
| Asia | 20 (12.7) | 17 (18.5) | 3 (4.6) |
| Australia | 7 (4.5) | 4 (4.3) | 3 (4.6) |
| Eastern Europe | 6 (3.8) | 4 (4.3) | 2 (3.1) |
| Western Europe | 60 (38.2) | 24 (26.1) | 36 (55.4) |
| Middle East | 12 (7.6) | 12 (13.0) | 0 (0.0) |
| North America | 47 (29.9) | 27 (29.3) | 20 (30.8) |
| South America | 2 (1.3) | 2 (2.2) | 0 (0.0) |
| Diabetes | |||
| 100% | 20 (12.7) | 17 (18.5) | 3 (4.6) |
| None | 87 (55.4) | 38 (41.3) | 49 (75.4) |
| Mixed | 16 (10.2) | 8 (8.7) | 8 (12.3) |
| Not reported | 34 (21.7) | 29 (31.5) | 5 (7.7) |
| Hypertension | |||
| 100% | 14 (8.9) | 6 (6.5) | 8 (12.3) |
| None | 50 (31.8) | 20 (21.7) | 30 (46.2) |
| Mixed | 22 (14.0) | 13 (14.1) | 9 (13.8) |
| Not reported | 71 (45.2) | 53 (57.6) | 18 (27.7) |
| Hypercholesterolemia | |||
| 100% | 9 (5.7) | 7 (7.6) | 2 (3.1) |
| None | 37 (23.5) | 12 (13.0) | 25 (38.5) |
| Mixed | 13 (8.3) | 8 (8.7) | 5 (7.7) |
| Not reported | 98 (62.4) | 65 (70.6) | 33 (50.7) |
| CVD | |||
| 100% | 9 (6.0) | 2 (2.2) | 7 (10.8) |
| None | 91 (60.3) | 52 (56.5) | 45 (69.2) |
| Mixed | 2 (1.3) | 1 (1.1) | 1 (1.5) |
| Not reported | 49 (32.4) | 37 (41.6) | 12 (18.5) |
| Intervention | |||
| Cocoa | 50 (31.9) | 24 (26.1) | 26 (40.0) |
| Extract | 26 (16.5) | 20 (21.7) | 6 (9.2) |
| Fruit | 40 (25.5) | 21 (22.8) | 19 (29.2) |
| Pure form | 5 (3.2) | 2 (2.2) | 3 (4.6) |
| Tea | 27 (17.2) | 20 (21.7) | 7 (10.7) |
| Wine | 9 (5.7) | 5 (5.4) | 4 (6.2) |
Values are numbers/frequencies (percentages) of study-level variables that represent the reporting characteristics of RCTs. CVD, cardiovascular disease; RCT, randomized controlled trial.
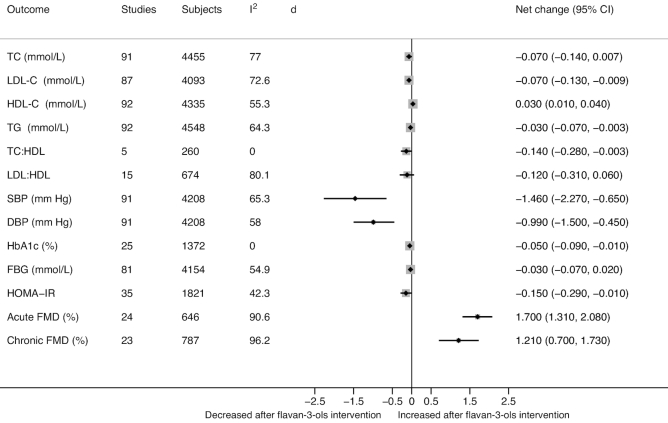
FIGURE 3.
The effect of flavan-3-ols on cardiometabolic risk biomarkers from RCTs. Data estimates are from meta-analysis of the net change and 95% CI for each outcome. DBP, diastolic blood pressure; FBG, fasting blood glucose; FMD, flow-mediated dilation; HbA1c, hemoglobin A1c; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; RCT, randomized controlled trial; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Lipids
Meta-analysis of RCTs comparing the effect of flavan-3-ols with controls on serum lipids showed significant decreases in LDL cholesterol (net change: −0.07 mmol/L; 95% CI: −0.13, −0.009 mmol/L; I2 = 72.6%), triglycerides (TGs) (net change: −0.03 mmol/L; 95% CI: −0.07, −0.003 mmol/L; I2 = 64.3%), and total cholesterol (TC):HDL cholesterol (net change: −0.14 mmol/L; 95% CI: −0.28, −0.003 mmol/L; I2 = 0.0%), and a significant increase in HDL cholesterol (net change: 0.03 mmol/L; 95% CI: 0.01, 0.04 mmol/L; I2 = 55.3%). There was no difference in serum TC and LDL cholesterol:HDL cholesterol. All lipid outcomes, except TC:HDL cholesterol, were associated with moderate to high heterogeneity.
When stratified by studies that reported components of flavan-3-ols, the change in serum LDL cholesterol, TC:HDL cholesterol, and HDL cholesterol remained significant comparing monomer intake with control, but all with moderate to high heterogeneity (I2 = 72.6%, P < 0.001; I2 = 0.0%, P = 0.614; I2 = 66%, P < 0.003, respectively). When stratified by studies that reported proanthocyanidins compared with control, only changes in serum TC:HDL cholesterol and HDL cholesterol remained significant—both without heterogeneity (I2 = 0.0% and I2 = 16.0%, respectively). Supplemental Tables 7–10 list data on TC, LDL cholesterol, HDL cholesterol, and TGs.
Blood pressure
Meta-analysis of 91 RCTs comparing the effect of flavan-3-ols with controls on blood pressure showed a significant decrease in SBP (net change: −1.46 mm Hg; 95% CI: −2.27, −0.65 mm Hg; I2 = 65.3%) and DBP (net change: −0.99 mm Hg; 95% CI: −1.50, −0.45 mm Hg; I2 = 58.0%); both results were associated with moderate heterogeneity.
When stratified by studies that reported components of flavan-3-ols, the change in SBP and DBP remained significantly decreased for the comparison of monomers with controls, both with moderate heterogeneity (I2 = 65.9% and I2 = 57.9%, respectively). When stratified by studies that reported proanthocyanidins compared with controls, the changes in SBP and DBP remained significantly decreased, but with moderate heterogeneity (I2 = 52.3% and I2 = 61.2%, respectively) (Supplemental Tables 11 and 12).
Glucose metabolism
Meta-analysis of RCTs comparing the effect of flavan-3-ols and controls on glucose metabolism parameters showed significant decreases in hemoglobin A1c (HbA1c) (27 RCTs, net change: −0.05%; 95% CI: −0.09%, −0.01%; I2 = 0.0%) without heterogeneity and in HOMA-IR (35 RCTs, net change: −0.15; 95% CI: −0.29, −0.01; I2 = 42.3%) with moderate heterogeneity. However, there was no difference in fasting blood glucose between groups.
When stratified by studies that reported components of flavan-3-ols, for the comparisons of monomers with controls, there was no difference in HbA1c, HOMA-IR, and fasting blood glucose outcomes. When stratified by studies that reported proanthocyanidins compared with controls, only HbA1c remained significantly decreased, without heterogeneity (I2 = 0%), and for both other outcomes there was no difference between proanthocyanidins and controls (Supplemental Tables 13–15).
Endothelial function
Acute FMD
Meta-analysis of 24 RCTs comparing the effects of flavan-3-ols with controlsshowed a significant increase in the acute FMD response measured from 1 to 6 h (net change: 1.70%; 95% CI: 1.31%, 2.08%; I2 = 90.6%), with high statistical heterogeneity. When stratified by studies that reported components of flavan-3-ols, there was a significant increase in acute FMD with monomer or proanthocyanidin interventions, as compared with the control group—both with high heterogeneity (I2 = 90.6% and I2 = 91.5%, respectively) (Supplemental Table 16).
Chronic FMD
Meta-analysis of 23 RCTs comparing the effect of flavan-3-ols with controls showed a significant improvement in chronic FMD measured after a few weeks of intervention (1–26 wk) (net change: 1.21%; 95% CI: 0.70%, 1.73%; I2 = 96.2%), with high heterogeneity. When stratified by studies that reported components of flavan-3-ols, there was a significant increase in chronic FMD with monomer or proanthocyanidin interventions, as compared with the control group—both with high heterogeneity (I2 = 96.4% and I2 = 92.1%, respectively) (Supplemental Table 17).
Inflammatory biomarkers
Meta-analysis of RCTs on inflammatory biomarkers found no difference between flavan-3-ols and controls for these outcomes: hsCRP (net change: 0.008 mg/L; 95% CI: −0.015, 0.030 mg/L; I2 = 0.0%), IL-6 (net change: −0.05 pg/mL; 95% CI: −0.13, 0.22 pg/mL; I2 = 11.8%), and TNF-α (net change: −0.20 pg/mL; 95% CI: −0.48, 0.08 pg/mL; I2 = 65.4%).
Sensitivity analyses
In sensitivity analyses, limiting analyses to RCT studies with a low risk of bias (good methodological quality), although there was no difference between flavan-3-ols and controls for serum lipids including LDL cholesterol and TGs, or glucose metabolism outcomes including HbA1C or fasting blood glucose (and there was no heterogeneity in these results) (Supplemental Tables 7–17), the following outcomes remained significant after flavan-3-ol interventions: TC (net change: −0.06 mmol/L; 95% CI: −0.11, −0.001 mmol/L; I2 = 0.0%); HDL cholesterol (net change: 0.02 mmol/L; 95% CI: 0.001, 0.05 mmol/L; I2 = 0.0%); SBP (net change: −1.29 mm Hg; 95% CI: −2.45, −0.13 mm Hg; I2 = 44.4%) and DBP (net change: −1.24 mm Hg; 95% CI: −2.13, −0.34 mm Hg; I2 = 58.7%); HOMA-IR (net change: −0.29; 95% CI: −0.48, −0.10; I2 = 0.0%), and acute (net change: 1.15%; 95% CI: 0.71%, 1.59%; I2 = 89.0%) and chronic FMD (net change: 1.30%; 95% CI: 0.59%, 2.00%; I2 = 81.7%).
In additional sensitivity analyses, excluding studies that recruited participants with existing CVD did not alter the main results for all outcomes (Supplemental Tables 7–17).
Dose–response
When studies were stratified by quartiles of median flavan-3-ol intake, intervention dosages in the first quartile (<220 mg/d) significantly decreased SBP and DBP and significantly increased FMD and HDL cholesterol. Intervention dosages in the second quartile (401–661 mg/d) significantly decreased HbA1c; in the third quartile between ∼550 and 850 mg/d they significantly decreased LDL cholesterol, SBP, and HOMA-IR and significantly increased HDL cholesterol and FMD. Intervention dosages >800 mg/d significantly decreased TC, SBP, and DBP and significantly increased FMD (Supplemental Tables 7–17). There was no difference in TGs or fasting blood glucose by quartiles of median flavan-3-ol intake (Supplemental Tables 7–17). Meta-regression found no linear relations between net change in all outcomes and flavan-3-ol dosage.
Subgroup analyses
Analyses by duration of intervention found that interventions >3 mo long significantly increased HDL cholesterol and decreased TGs, SBP, DBP, HbA1c, and HOMA-IR, but results were nonsignificant for TC, LDL cholesterol, and FMD.
In terms of the dietary form by which the flavan-3-ols were consumed, cocoa and tea significantly increased both the acute and chronic FMD responses, but fruit interventions only significantly increased the acute FMD response. Cocoa and fruit significantly decreased SBP and cocoa, fruit, and pure form significantly decreased DBP. Extracts decreased TC, LDL cholesterol, and TGs. In addition to extracts, cocoa and fruit also decreased TGs. For HDL cholesterol, there was no difference across intervention types. Cocoa also significantly decreased HOMA-IR and the pure form of flavan-3-ol significantly decreased HbA1c (Supplemental Tables 7–17).
Overall strength of evidence among RCTs
There was moderate strength of evidence that flavan-3-ol intake increases HDL cholesterol and decreases TC, SBP, and HOMA-IR and a low strength of evidence that flavan-3-ol intake decreases DBP and improves FMD (both acute and chronic) (Supplemental Table 18).
Discussion
To our knowledge, this is the first review that has systematically examined the potential cardiometabolic health effects of flavan-3-ols per se (irrespective of source), integrating all available evidence from both prospective cohort studies and RCTs. We showed that higher habitual flavan-3-ol intakes were associated with a reduction in risk of CVD mortality (13%) and incident CHD (19%); higher habitual intakes of monomers were associated with a reduction in risk of T2DM (10%) and stroke (18%); and these data were calculated to be of moderate strength after GRADE analyses. In RCTs, when we restricted analyses to good methodological quality interventions (low risk of bias), flavan-3-ols improved FMD, the gold-standard measure of endothelial function, acute (1.7%) and chronic FMD (1.14%), TC (−0.06 mmol/L), HDL cholesterol (0.02 mmol/L), SBP and DBP (−1.29 and −1.24 mm Hg, respectively), and HOMA-IR (−0.18). Although many of the biomarkers assessed were associated with significant statistical heterogeneity, after GRADE analyses, the strength of evidence was moderate that flavan-3-ol intervention decreases TC, SBP, and HOMA-IR and increases HDL cholesterol, whereas the strength of evidence for FMD and DBP was graded low.
Even though the evaluated populations in the prospective cohorts were high in number, the evidence primarily came from a few cohort studies and the number of cohorts was sparse. However, the available population-based data together with the supportive mechanistic data from RCTs provide growing evidence that flavan-3-ols may be important for cardiometabolic health. The magnitude of improvement in blood pressure and conduit artery endothelial function in the RCTs merits discussion because this translates into a significant reduction in future cardiovascular events based on previous meta-analysis (49). The authors showed that each 1% increase in FMD was associated with a relative risk of cardiovascular events of 0.87 (95% CI: 0.83, 0.91); the 1.14% chronic improvement in FMD would have important consequences for CVD risk and in combination with the small, but robust, improvements in HDL cholesterol, blood pressure, and HOMA-IR may be substantial. Our RCT results concur with 2 previous meta-analyses that only evaluated a limited number of foods and reported beneficial effects on outcomes including HDL cholesterol, blood pressure, HOMA-IR, and FMD (7, 50). Similar to their review, we did not find a linear relation between flavan-3-ol intake and outcomes of interest. Although our results concurred for LDL cholesterol, the beneficial effects of flavan-3-ol effects disappeared in sensitivity analyses of good methodological quality (low risk of bias) studies.
The molecular mechanisms by which dietary flavan-3-ols mediate these effects on vascular health include nitric oxide–dependent arterial function (51, 52), anti-inflammatory effects (53), and effects on glucose metabolism, β-cell function, and inhibition of glucose transporters (54–57). However, early mechanistic studies did not consider the absorption, distribution, metabolism, and excretion of the flavan-3-ols. After ingestion flavan-3-ols are extensively metabolized and transformed, with recent data suggesting that the gut microbiome–derived metabolites mediate the vascular-protective effects; recent data provide evidence for endothelium-protective effects of gut microbiome–derived circulating flavan-3-ol metabolites specifically through dynamic regulation of endothelial cell monocyte adhesion and permeability (52).
The GRADE analyses highlight an issue which affects many nutrition research trials: the fact that many conducted studies are of poor quality (high risk of bias). These data, like for many other areas of nutrition research, highlight the need to design and conduct higher-quality RCTs. For example, 70–72% of the trials that assessed lipid profiles were calculated to be of low quality (Supplemental Tables 7–10); for blood pressure 65% were of low quality although 30 trials had good-quality evidence (Supplemental Tables 11–12). Although systematic review and meta-analysis approaches give a valuable insight into the state of the art for the flavan-3-ol evidence base, an inevitable limitation of the approach is the quality and scientific rigor of the included data, which needs to be considered in interpreting the results. However, the focus on only good methodological studies and the use of GRADE have allowed us to highlight the findings that remained significant when sensitivity analyses were restricted to the good-quality data.
We reviewed a large number of RCTs that either reported or had estimable flavan-3-ol content; our results are generalizable across a variety of sources rich in flavan-3-ols. Included cohorts examined a wide range of dosages of flavan-3-ols; the mean daily intake of flavan-3-ols in the United States is <200 mg/d (58), whereas in some cohorts dosages ≤1400 mg/d (36) were observed and total flavan-3-ol dosages in included RCTs ranged from 40 to 1540 mg/d. Only a handful of cohorts conducted both baseline and subsequent flavan-3-ol intake assessment. Most cohorts relied on a single baseline flavan-3-ol intake measure to evaluate an association between flavan-3-ol intake and CVD outcomes during follow-up, thereby making an assumption that flavan-3-ol intake did not change over time. Only 1 long-term RCT evaluated clinical outcomes: risk of CHD and stroke (48). From the currently available data, flavan-3-ol content from cocoa and fruits found the greatest number of improved outcomes, followed by the pure form, tea, and extracts.
Current epidemiological data solely rely on self-reported intake, with estimates of flavan-3-ol exposure calculated from food composition databases. Although these data can clearly differentiate between extremes of intake, current data do not account for the extensive metabolism these compounds undergo after ingestion. Future cohort studies should therefore integrate biomarker and dietary assessment methods to more accurately determine the relative importance of specific flavan-3-ols and their metabolites for cardiovascular health. This would also allow a more accurate determination of the relative importance of the different components of flavan-3-ol intake. Another limitation of the available data relates to the number of RCTs that had not independently calculated the flavan-3-ol content (estimated in 75 RCTs). By estimating intakes using a standardized approach, this allowed us to include these 75 RCTs in our analyses. There was considerable heterogeneity in terms of the dosages examined in both cohorts and RCTs, which precludes firm conclusions on the dosage required for health effects. Furthermore, the significance of many of the results from RCTs disappeared in sensitivity analyses of good methodological quality studies; only the data for TC, HDL cholesterol, BP, HOMA-IR, and FMD remained significant. A major strength of our approach includes the integration of flavan-3-ol intake from a range of sources and by focusing on good methodological studies and conducting GRADE we highlight the key research gaps in the field.
In conclusion, the beneficial effect of flavan-3-ols on cardiometabolic outcomes is evident from both cohort studies and RCTs. However, it is imperative that future cohort studies integrate biomarker and dietary assessment methods to more accurately determine the relative importance of specific flavan-3-ols and their metabolites for cardiovascular health. Moreover, high-quality dose–response RCTs are needed to further understand the relative importance of flavan-3-ol source and metabolism for cardiometabolic health.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—GR, JAN, and AC: conceived and designed the study; GR, EEA, SC, JW, JM, and BG: performed the data extraction and quality assessment; GR and EEA: synthesized and analyzed the data; GR, EEA, and AC: interpreted and further analyzed the data; GR, EEA, BG, and AC: wrote the paper; GR: had primary responsibility for final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by International Life Sciences Institute North America.
The funder played no role in the study selection, quality assessment, data synthesis, or manuscript preparation.
Supplemental Tables 1–18 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CHD, chronic heart disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; EPIC, European Prospective Investigation into Cancer and Nutrition; FMD, flow-mediated dilation; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HAPIEE, Health, Alcohol and Psychosocial factors in Eastern Europe; HbA1c, hemoglobin A1c; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; RCT, randomized controlled trial; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; T2DM, type 2 diabetes mellitus.
References
- 1. Cassidy A, Minihane AM. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr. 2017;105:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Del RD, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013;18:1818–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perez-Vizcaino F, Fraga CG. Research trends in flavonoids and health. Arch Biochem Biophys. 2018;646:107–12. [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D, Crozier A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol. 2014;88:1803–53. [DOI] [PubMed] [Google Scholar]
- 5. Greyling A, Ras RT, Zock PL, Lorenz M, Hopman MT, Thijssen DH, Draijer R. The effect of black tea on blood pressure: a systematic review with meta-analysis of randomized controlled trials. PLoS One. 2014;9:e103247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. [DOI] [PubMed] [Google Scholar]
- 7. Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–51. [DOI] [PubMed] [Google Scholar]
- 8. Buitrago-Lopez A, Sanderson J, Johnson L, Warnakula S, Wood A, Di AE, Franco OH. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;343:d4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology. 2012;79:1223–9. [DOI] [PubMed] [Google Scholar]
- 10. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;4:CD008893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shrime MG, Bauer SR, McDonald AC, Chowdhury NH, Coltart CE, Ding EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr. 2011;141:1982–8. [DOI] [PubMed] [Google Scholar]
- 12. Zhang C, Qin YY, Wei X, Yu FF, Zhou YH, He J. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103–13. [DOI] [PubMed] [Google Scholar]
- 13. Zhao Y, Asimi S, Wu K, Zheng J, Li D. Black tea consumption and serum cholesterol concentration: systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2015;34:612–19. [DOI] [PubMed] [Google Scholar]
- 14. Raman G, Shams-White M, Avendano EE, Chen F, Novotny JA, Cassidy A. Dietary intakes of flavan-3-ols and cardiovascular health: a field synopsis using evidence mapping of randomized trials and prospective cohort studies. Syst Rev. 2018;7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 16. Bhagwat S, Haytowitz DB. USDA's Database for the Proanthocyanidin Content of Selected Foods. Release 2. Beltsville, MD: USDA, Agricultural Research Service Nutrient Data Laboratory; 2015. Available from:http://www.ars.usda.gov/nutrientdata/flav. [Google Scholar]
- 17. Bhagwat S, Haytowitz DB. USDA Database for the Flavonoid Content of Selected Foods, Release 3.2. [Internet] Beltsville, MD: USDA, Agricultural Research Service Nutrient Data Laboratory; 2015. Available from: http://www.ars.usda.gov/nutrientdata/flav. [Google Scholar]
- 18. Neveu V, Perez-Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D et al.. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database. 2010:bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition. J Nutr. 2008;138:2297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Viswanathan M, Patnode CD, Berkman ND, Bass EB, Chang S, Hartling L, Murad MH, Treadwell JR, Kane RL. Recommendations for assessing the risk of bias in systematic reviews of health-care interventions. J Clin Epidemiol. 2018;97:26–34. [DOI] [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D et al.. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arts IC, Hollman PC, Feskens EJ, Bueno de Mesquita HB, Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74:227–32. [DOI] [PubMed] [Google Scholar]
- 26. Arts IC, Jacobs DR Jr, Harnack LJ, Gross M, Folsom AR. Dietary catechins in relation to coronary heart disease death among postmenopausal women. Epidemiology. 2001;12:668–75. [DOI] [PubMed] [Google Scholar]
- 27. Cassidy A, O'Reilly EJ, Kay C, Sampson L, Franz M, Forman JP, Curhan G, Rimm EB. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr. 2011;93:338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation. 2013;127:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dower JI, Geleijnse JM, Hollman PC, Soedamah-Muthu SS, Kromhout D. Dietary epicatechin intake and 25-y risk of cardiovascular mortality: the Zutphen Elderly Study. Am J Clin Nutr. 2016;104:58–64. [DOI] [PubMed] [Google Scholar]
- 30. Goetz ME, Judd SE, Hartman TJ, McClellan W, Anderson A, Vaccarino V. Flavanone intake is inversely associated with risk of incident ischemic stroke in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Nutr. 2016;146:2233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goetz ME, Judd SE, Safford MM, Hartman TJ, McClellan WM, Vaccarino V. Dietary flavonoid intake and incident coronary heart disease: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Clin Nutr. 2016;104:1236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ivey KL, Lewis JR, Prince RL, Hodgson JM. Tea and non-tea flavonol intakes in relation to atherosclerotic vascular disease mortality in older women. Br J Nutr. 2013;110:1648–55. [DOI] [PubMed] [Google Scholar]
- 33. Jacques PF, Cassidy A, Rogers G, Peterson JJ, Meigs JB, Dwyer JT. Higher dietary flavonol intake is associated with lower incidence of type 2 diabetes. J Nutr. 2013;143:1474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lajous M, Rossignol E, Fagherazzi G, Perquier F, Scalbert A, Clavel-Chapelon F, Boutron-Ruault MC. Flavonoid intake and incident hypertension in women. Am J Clin Nutr. 2016;103:1091–8. [DOI] [PubMed] [Google Scholar]
- 35. McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012;95:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR Jr. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. [DOI] [PubMed] [Google Scholar]
- 37. Mursu J, Voutilainen S, Nurmi T, Tuomainen TP, Kurl S, Salonen JT. Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr. 2008;100:890–5. [DOI] [PubMed] [Google Scholar]
- 38. Tresserra-Rimbau A, Rimm EB, Medina-Remón A, Martínez-González MA, de la Torre R, Corella D, Salas-Salvadó J, Gómez-Gracia E, Lapetra J, Arós F et al.. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr Metab Cardiovasc Dis. 2014;24:639–47. [DOI] [PubMed] [Google Scholar]
- 39. Vogiatzoglou A, Mulligan AA, Bhaniani A, Lentjes MAH, McTaggart A, Luben RN, Heiss C, Kelm M, Merx MW,Spencer JPE et al.. Associations between flavan-3-ol intake and CVD risk in the Norfolk cohort of the European Prospective Investigation into Cancer (EPIC-Norfolk). Free Radic Biol Med. 2015;84:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, Willett W, Hu FB, Sun Q, van Dam RM. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr. 2012;95:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adriouch S, Lampure A, Nechba A, Baudry J, Assmann K, Kesse-Guyot E, Hercberg S, Scalbert A, Touvier M, Fezeu LK. Prospective association between total and specific dietary polyphenol intakes and cardiovascular disease risk in the Nutrinet-Santé French Cohort. Nutrients. 2018;10:E1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grosso G, Stepaniak U, Micek A, Kozela M, Stefler D, Bobak M, Pajak A. Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the Health, Alcohol and Psychosocial factors in Eastern Europe (HAPIEE) study. Br J Nutr. 2017;118:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grosso G, Stepaniak U, Micek A, Kozela M, Stefler D, Bobak M, Pajak A. Dietary polyphenol intake and risk of hypertension in the Polish arm of the HAPIEE study. Eur J Nutr. 2018;57:1535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tresserra-Rimbau A, Guasch-Ferré M, Salas-Salvadó J, Toledo E, Corella D, Castañer O, Guo X, Gómez-Gracia E, Lapetra J, Arós F et al.. Intake of total polyphenols and some classes of polyphenols is inversely associated with diabetes in elderly people at high cardiovascular disease risk. J Nutr. 2016;146:767–77. [DOI] [PubMed] [Google Scholar]
- 45. Zamora-Ros R, Forouhi NG, Sharp SJ, Gonzalez CA, Buijsse B, Guevara M, van der Schouw YT, Amiano P, Boeing H, Bredsdorff L et al.. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European populations: the EPIC-InterAct study. Diabetes Care. 2013;36:3961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zamora-Ros R, Forouhi NG, Sharp SJ, Gonzalez CA, Buijsse B, Guevara M, van der Schouw YT, Amiano P, Boeing H, Bredsdorff L et al.. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J Nutr. 2014;144:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ponzo V, Goitre I, Fadda M, Gambino R, De Francesco A, Soldati L, Gentile L, Magistroni P, Cassader M, Bo S. Dietary flavonoid intake and cardiovascular risk: a population-based cohort study. J Transl Med. 2015;13:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care. 2012;35:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26:631–40. [DOI] [PubMed] [Google Scholar]
- 50. Kay CD, Hooper L, Kroon PA, Rimm EB, Cassidy A. Relative impact of flavonoid composition, dose and structure on vascular function: a systematic review of randomised controlled trials of flavonoid-rich food products. Mol Nutr Food Res. 2012;56:1605–16. [DOI] [PubMed] [Google Scholar]
- 51. Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Milenkovic D, Berghe WV, Morand C, Claude S, van de Sandt A, Gorressen S, Monfoulet LE, Chirumamilla CS, Declerck K, Szic KSV et al.. A systems biology network analysis of nutri(epi)genomic changes in endothelial cells exposed to epicatechin metabolites. Sci Rep. 2018;8:15487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Claude S, Boby C, Rodriguez-Mateos A, Spencer JP, Gerard N, Morand C, Milenkovic D. Flavanol metabolites reduce monocyte adhesion to endothelial cells through modulation of expression of genes via p38-MAPK and p65-Nf-kB pathways. Mol Nutr Food Res. 2014;58:1016–27. [DOI] [PubMed] [Google Scholar]
- 54. Jalil AM, Ismail A, Pei CP, Hamid M, Kamaruddin SH. Effects of cocoa extract on glucometabolism, oxidative stress, and antioxidant enzymes in obese-diabetic (Ob-db) rats. J Agric Food Chem. 2008;56:7877–84. [DOI] [PubMed] [Google Scholar]
- 55. Strat KM, Rowley TJ, Smithson AT, Tessem JS, Hulver MW, Liu D, Davy BM, Davy KP, Neilson AP. Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders. J Nutr Biochem. 2016;35:1–21. [DOI] [PubMed] [Google Scholar]
- 56. Fernández-Millán E, Cordero-Herrera I, Ramos S, Escrivá F, Alvarez C, Goya L, Martín MA. Cocoa-rich diet attenuates beta cell mass loss and function in young Zucker diabetic fatty rats by preventing oxidative stress and beta cell apoptosis. Mol Nutr Food Res. 2015;59:820–4. [DOI] [PubMed] [Google Scholar]
- 57. Sun Q, Wedick NM, Tworoger SS, Pan A, Townsend MK, Cassidy A, Franke AA, Rimm EB, Hu FB, van Dam RM. Urinary excretion of select dietary polyphenol metabolites is associated with a lower risk of type 2 diabetes in proximate but not remote follow-up in a prospective investigation in 2 cohorts of US women. J Nutr. 2015;145:1280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim K, Vance TM, Chun OK. Estimated intake and major food sources of flavonoids among US adults: changes between 1999–2002 and 2007–2010 in NHANES. Eur J Nutr. 2016;55:833–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.