Abstract
Background:
Differentiating etiologies of acute kidney injury is critical in determining the course of care in clinical practice. For example, acute interstitial nephritis (AIN) requires withdrawal of the offending drug and immunosuppressive therapy while acute tubular injury (ATI) does not have any disease-specific therapies. Failure to distinguish AIN from ATI in a timely manner can lead to kidney fibrosis and chronic kidney disease. In this review, we discuss current tests and novel biomarkers to distinguish ATI from AIN.
Summary:
In a prospective cohort study of 32 participants with AIN and 41 with ATI, clinical features and current, laboratory tests did not provide sufficient distinction between the two subpopulations of AKI. The findings in our cohort are consistent with our review of the literature. Given the limitations of clinical features and laboratory assessments, clinical practice relies on kidney biopsy for histological diagnosis, which is not always feasible, and is associated with bleeding complications in high risk populations. In addition, histological diagnosis is prone to sampling errors and inter-rater variability. In the interest of identifying a novel biomarker, we compared urine and plasma levels of cytokines in the Th1, Th2, and Th9 pathways, which have been implicated in the pathogenesis of AIN. Urine TNF-α and interleukin-9 were higher in AIN participants than in ATI controls and help discriminate AIN from ATI (AUC, 0.83 (0.73–0.92)).
Key Messages:
Differentiation between AIN and ATI in patients with AKI using currently available tests is challenging. Urine TNF-α and interleukin-9 may help clinicians separate AIN from ATI.
Keywords: acute renal injury, fibrosis, kidney, nephrology
Introduction
Acute kidney injury (AKI) consists of a group of diseases characterized by loss of kidney function. A major challenge in the clinical care of patients with AKI is differentiating between its underlying etiologies such as acute tubular injury (ATI) and acute interstitial nephritis (AIN). Timely differentiation between these etiologies of AKI is critical due to differences in their management. ATI does not have any disease specific therapies. However, AIN is treated through withdrawal of the offending drug and immunosuppressive therapy. Failure to recognize and treat AIN promptly could lead to fibrosis, permanent kidney damage, and progression to chronic kidney disease (CKD).1
In the present report, we review data from published studies evaluating clinical features and diagnostic tests that could help a clinician differentiate between AIN and ATI. In addition, we present data from a prospective, observational study of participants who underwent a kidney biopsy for evaluation of AKI between 2015–2018 at two Yale University-affiliated hospitals.2 We discuss clinical features, traditional tests (blood eosinophils, urinalysis, and urine microscopy), histological features, and novel biomarkers for differentiating AIN from ATI.
Clinical Features
Due to varying clinical features, latent period of disease presentation, and variable degree of kidney dysfunction, AIN is often confused with other kidney diseases such as ATI or progressive CKD. A few decades ago, AIN occurrence was predominantly by medications such as beta-lactam antibiotics and sulfur-containing drugs and the patient presented with acute or sub-acute onset of allergic features such as fever, rash, and eosinophilia within a few days of starting the drug. Recently, however, other medication classes such as proton pump inhibitors (PPI), non-steroidal anti-inflammatory drugs (NSAID), and cancer immunotherapy agents have become common causes of AIN. AIN from these drugs do not present with the same allergic features and the clinical presentation is protracted. In fact, the classic triad of fever, rash and eosinophilia was reported in only 10% of antibiotic-induced AIN cases and these features were not present in any of the PPI-induced AIN cases.3 Furthermore, AIN cases induced by these new medication classes have significantly longer latent periods than cases caused by beta-lactam antibiotics or sulfur-containing drugs. For example, studies demonstrated that AIN occurs 8–15 days after antibiotic initiation, 76 days after NSAID initiation, and 234 days after PPI initiation.3, 4 This subacute to chronic clinical onset of renal dysfunction in AIN can be hidden under the natural history of progressive CKD, delaying AIN diagnosis and treatment. In a study of biopsy-proven AIN, only half of the participants exhibited a sharp increase in serum creatinine in 48 hours to 7 days (Acute Kidney Injury) whereas over 90% exhibited a serum creatinine increase over a longer time frame of <3 months (Acute Kidney Disease).5
Non-Invasive Diagnostic Tests
There is no reliable, non-invasive diagnostic test for clinical diagnosis of AIN. Urine eosinophil testing was once considered a diagnostic test for AIN. However, a recent study showed that the sensitivity and specificity of urine eosinophil testing were 31% and 68%, respectively, indicating that this test could not reliably differentiate AIN from other causes of AKI.6 Urine eosinophils were found not only in AIN but also in cases with glomerulonephritis, atheroembolic disease, multiple myeloma and sometimes in cases with ATI. Another clinical test of interest for AIN diagnosis is urine sediment examination for sterile pyuria and white blood cell (WBC) casts. However, one case series in AIN showed that only 15% had WBC casts.6 Furthermore, the study did not evaluate the presence of these casts in other causes of AKI and this method has not been systematically evaluated. While markers of tubular injury and dysfunction such as low-grade proteinuria and elevated urine levels of biomarker neutrophil gelatinase-associated lipocalin are present in AIN, these are not specific for AIN and are also elevated in other causes of AKI most notably in ATI.7 Gallium-67 scanning has been proposed as a test of AIN to detect inflammation in the kidney tissue; one study showed that this test had an area under receiver operating characteristic curve (AUC) of 0.75 for AIN diagnosis although less than a third of the patients in this study underwent a kidney biopsy to confirm the diagnosis.8
Results from Yale AIN Study
We compared various clinical, laboratory, urine dipstick, and microscopy features between biopsy-proven, adjudicated AIN and ATI (Table 1). Most clinical features were comparable between AIN and ATI, except for cirrhosis which was only present in patients with ATI. Laboratory features were also comparable between AIN and ATI, including the degree of renal dysfunction, blood eosinophil levels, and urine albumin. However, patients with AIN tended to have lower hemoglobin levels. Urine dipstick features were also comparable between the two groups including dipstick leukocyte esterase levels. AIN participants tended to have more alkaline urine despite having slightly lower serum bicarbonate levels, which may indicate tubular dysfunction that is described with AIN. Urine microscopy is often thought to be crucial in differentiating AIN from ATI. However, review of urine sediment in each case by a trained nephrologist did not reveal any significant differences between the two diseases. Given these findings, it is not surprising that a review of medical charts revealed that the clinician’s pre-biopsy diagnosis had a low AUC for post-biopsy AIN diagnosis [0.58 (0.47, 0.68)].
Table 1.
Comparison of clinical and laboratory features between acute interstitial nephritis and acute tubular injury
| Variable | AIN (N=32) | ATI (N=41) | P-value |
|---|---|---|---|
| Clinical features | |||
| Age (years) | 58 (40, 68) | 56 (42, 65) | 0.70 |
| Female | 18 (56%) | 16 (39%) | 0.14 |
| African-American race | 10 (31%) | 9 (22%) | 0.37 |
| Diabetes | 7 (22%) | 9 (22%) | 0.99 |
| Cirrhosis | 0 (0%) | 6 (15%) | 0.02 |
| Acute kidney injury (AKI) | 18 (56%) | 26 (63%) | 0.53 |
| Severe AKI (Stage 2 or higher) | 2 (7%) | 8 (20%) | 0.12 |
| Dialysis at biopsy | 1 (3%) | 4 (10%) | 0.26 |
| Laboratory features at biopsy | |||
| Serum Creatinine, mg/dl | 4.4 (2.8, 6.3) | 4.1 (2.4, 6.7) | 0.71 |
| Blood Urea Nitrogen, mg/dl | 40 (27, 57) | 37 (28, 61) | 0.85 |
| Hemoglobin level, g/dl | 9.8 (8.1, 10.7) | 10.7 (9.3, 12.4) | 0.02 |
| Platelet count (*1000 per mm3) | 247 (202, 290) | 211 (169, 253) | 0.07 |
| Blood eosinophil count | 234 (149, 466) | 198 (95, 400) | 0.24 |
| Urine albumin to creatinine, mg/g | 157 (44, 1452) | 109 (20, 682) | 0.12 |
| Serum bicarbonate level | 19.7 (17, 22.5) | 21 (18.4, 23.9) | 0.29 |
| Urine dipstick | |||
| Specific gravity | 1.015 (1.015, 1.020) | 1.015 (1.015, 1.025) | 0.58 |
| pH | 6 (5.5, 7) | 6 (5.5, 6.5) | 0.02 |
| Protein, ≥2+ | 19 (59%) | 20 (49%) | 0.65 |
| Leukocytes, ≥2+ | 10 (32%) | 11 (28%) | 0.71 |
| Urine Microscopy | |||
| White blood cell, ≥1/HPF | 8 (27%) | 8 (22%) | 0.63 |
| White blood cell cast, ≥1/HPF | 1 (3%) | 1 (3%) | 0.88 |
| RTE cell, ≥1/HPF | 14 (47%) | 12 (32%) | 0.23 |
| RTE cast, ≥1/HPF | 2 (7%) | 4 (11%) | 0.58 |
| Granular cast, ≥1/HPF | 10 (33%) | 17 (46%) | 0.29 |
| Red blood cells, >5/HPF | 5 (29%) | 10 (39%) | 0.54 |
| Red blood cell cast, ≥1/HPF | 0 (0%) | 3 (12%) | 0.14 |
Fisher’s exact test or Wilcoxon Ranksum test. Median (interquartile range) or n (%) shown. HPF, high power field
Histology
In the absence of a non-invasive biomarker, the diagnosis of AIN relies on performing a biopsy to obtain kidney tissue for histological diagnosis. The typical findings of AIN are predominantly in the tubulointerstitium. AIN is characterized by infiltration of lymphocytes, macrophages, and eosinophils in the renal interstitium. This is also accompanied by presence of inflammatory cells in the renal tubules (“tubulitis”). Tubular injury and interstitial fibrosis often accompany this inflammatory infiltrate. In our study, we noted that the pathologists were more likely to diagnose AIN if the biopsies that had higher severity of interstitial lymphocytic infiltrate, tubulitis, and eosinophils (Table 2). Of the 79 cases with AIN on official biopsy interpretation, all three pathologists agreed on the diagnosis in 32 (41%) cases and two out of three agreed in 23 (29%) cases. In 24 (30%) cases of AIN on official biopsy report, a majority of pathologists reclassified the diagnosis as not AIN. The pathologists were more likely to classify a biopsy as AIN when it was listed as the first numerical diagnosis on the official biopsy report (55%) than when it was listed as second or third (27%) (P=0.01). We noted a modest inter-rater agreement and kappa statistic among the pathologists for AIN diagnosis (agreement 63–70%, Fleiss kappa=0.35). Such modest degree of agreement is not unique to AIN; poor agreement between raters has also been noted in other kidney pathologies and represents a challenge for clinician’s interpreting a biopsy report.9
Table 2.
Histological features associated with acute interstitial nephritis
| Interstitial feature | Severity | Adj. OR (95% CI) |
|---|---|---|
| Interstitial infiltrate | <10% | 1 (ref.) |
| 11–25% | 4.1 (1.0, 17.2) | |
| >25% | 17.6 (3.4, 90.4) | |
| Eosinophils | 0/HPF | 1 (ref.) |
| 1–5/HPF | 8.1 (2.1, 30.8) | |
| >5/HPF | 71.3 (4.3, 1172.2) | |
| Tubulitis | None | 1 (ref.) |
| 1–5/tubule | 9.7 (1.8, 53.8) | |
| Tubular injury | <10% | 1 (ref.) |
| 11–25% | 0.4 (0.0, 3.5) | |
| >25% | 0.1 (0.0, 1.3) |
HPF, high power field; adj. OR, adjusted odds ratio
Logistic regression model for outcome of AIN and predictors as various interstitial histological features reported by the adjudicating pathologists controlling for the pathologist and clustered at participant level.
Novel Biomarkers
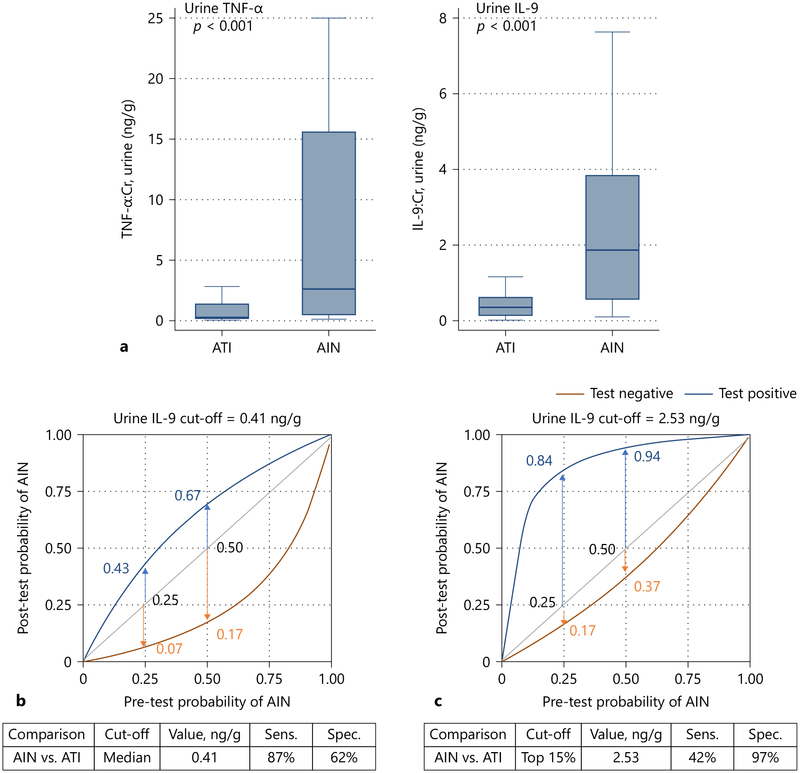
Kidney biopsies for histological diagnoses pose risks for patients with AKI and may not be feasible in some individuals with increased bleeding risk.10 The resulting delay in diagnosis leads to increasing fibrosis and 40–60% of cases of AIN progress to CKD.1, 11 As a result, novel, non-invasive biomarkers are needed to diagnosis AIN. Past studies showed that CD4+T-cells play an important role in the pathogenesis of AIN.12–14 Based on the preliminary data, we selected 12 cytokines in the Th1 (interferon-Y, IL-2, IL-12), Th2 (IL-4, IL-5, IL-13), and Th9 (IL-9) pathways, as well as other generally inflammatory cytokines (TNF-α, IL-iβ, IL-6, IL-8, IL-10) and compared the urine and plasma levels of these cytokines between AIN and ATI. We found that urine TNF-α and interleukin-9 were higher in AIN patients than in ATI controls (Figure 1, Panel A), whereas the other urine or plasma cytokines were not consistently associated with AIN. These two urine biomarkers had an AUC of 0.83 (0.73, 0.92) for AIN diagnosis. We also tested two cut-offs of urine IL-9: the first corresponding to the median value in the cohort (0.41 ng/g) and the second corresponding to the top 15% values (2.53 ng/g). In Figure 1, panels B and C we show how urine IL-9 testing can help avoid a kidney biopsy at various pre-biopsy probabilities of AIN. For example, if the pre-biopsy probability of AIN is 0.25, a value below 0.41 can rule out the diagnosis of AIN (post-test probability 0.07), whereas a value above 2.53 can rule in the diagnosis (post-test probability to 0.84). A value between these two cut-offs would likely require a kidney biopsy for diagnosis.
Figure 1. Tumor necrosis factor-a and interleukin-9 are biomarkers to differentiate acute interstitial nephritis from acute tubular injury.
Panel A shows median (horizontal line), 25th and 75th percentile (box), and 5th and 95th percentile (whiskers) compared between AIN and ATI. Wilcoxon Ranksum test P-values are shown in red.
Panel B and C show post-test probability of AIN at various pre-test probabilities at two cut-offs of interleukin (IL)-9.
Conclusions
Differentiating between ATI and AIN, two common etiologies of AKI, is challenging for clinicians due to the lack of a reliable, non-invasive, diagnostic test. Establishing the diagnosis of AIN requires performing a kidney biopsy to obtain tissue for histological diagnosis, which carries risks and may not always be feasible. Novel biomarkers such as urine TNF-α and interleukin-9 may be able to differentiate AIN from ATI. However, before wider clinical application, further studies are required to validate our findings in external cohorts, in AKI patients who aren’t being considered for a biopsy, and in patients who do not have AKI but are nevertheless at high risk for AIN (eg. users of proton pump inhibitors and immunotherapy agents). Moreover, a kidney biopsy may still be needed when biomarker results are equivocal or to obtain prognostic information such as degree of fibrosis which may guide therapy. Finally, these biomarkers need to be linked to patient outcomes and response to therapy.
Acknowledgement
The authors would like to thank the participants of the Yale biopsy study, without whom this study would not have been possible.
Funding Sources
This work was supported by the National Institutes of Health (K23DK117065 to DGM, P30DK079310 to DGM and CRP; UG3-DK114866 to CRP). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Disclosure Statement
CRP and DGM are named inventors in a provisional patent number 62/716,465 titled “System and methods for diagnosing acute interstitial nephritis.”
References
- 1.Raghavan R, Eknoyan G. Acute interstitial nephritis-a reappraisal and update. Clin Nephrol 2014; 82: 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moledina DG, Wilson FP, Pober JS, et al. Urine TNF-α and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight 2019; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muriithi AK, Leung N, Valeri AM, et al. Clinical characteristics, causes and outcomes of acute interstitial nephritis in the elderly. Kidney Int 2015; 87: 458–464. [DOI] [PubMed] [Google Scholar]
- 4.Bhaumik SK, Kher V, Arora P, et al. Evaluation of clinical and histological prognostic markers in drug-induced acute interstitial nephritis. Ren Fail 1996; 18: 97–104. [DOI] [PubMed] [Google Scholar]
- 5.Chu R, Li C, Wang S, et al. Assessment of KDIGO definitions in patients with histopathologic evidence of acute renal disease. Clin J Am Soc Nephrol 2014; 9: 1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fogazzi GB, Ferrari B, Garigali G, et al. Urinary sediment findings in acute interstitial nephritis. Am J Kidney Dis 2012; 60: 330–332. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Yang L, Su T, et al. Pathological significance of a panel of urinary biomarkers in patients with drug-induced tubulointerstitial nephritis. Clin J Am Soc Nephrol 2010; 5: 1954–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham F, Lord M, Froment D, et al. The use of gallium-67 scintigraphy in the diagnosis of acute interstitial nephritis. Clin Kidney J 2016; 9: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liapis H, Gaut JP, Klein C, et al. Banff Histopathological Consensus Criteria for Preimplantation Kidney Biopsies. Am J Transplant 2017; 17: 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moledina DG, Luciano RL, Kukova L, et al. Kidney Biopsy-Related Complications in Hospitalized Patients with Acute Kidney Disease. Clin J Am Soc Nephrol 2018: CJN.04910418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993–2011: a case series. Am J Kidney Dis 2014; 64: 558–566. [DOI] [PubMed] [Google Scholar]
- 12.Spanou Z, Keller M, Britschgi M, et al. Involvement of drug-specific T cells in acute drug-induced interstitial nephritis. J Am Soc Nephrol 2006; 17: 2919–2927. [DOI] [PubMed] [Google Scholar]
- 13.Zand L, Monaghan M, Griffin BR, et al. The role of type I hypersensitivity reaction and IgE-mediated mast cell activation in acute interstitial nephritis. Clin Nephrol 2015; 84: 138–144. [DOI] [PubMed] [Google Scholar]
- 14.D’Agati VD, Theise ND, Pirani CL, et al. Interstitial nephritis related to nonsteroidal antiinflammatory agents and beta-lactam antibiotics: a comparative study of the interstitial infiltrates using monoclonal antibodies. Mod Pathol 1989; 2: 390–396. [PubMed] [Google Scholar]