Abstract
Acute kidney injury (AKI) is highly prevalent among hospitalized patients and is associated with serious consequences with limited pharmacological treatment options. Pannexin 1 (Panx1) channel is a ubiquitously expressed non-selective membrane transport channel that efficiently effluxes ATP and plays a central role in the progression of inflammatory diseases. Animal models that target Panx1 through pharmacological inhibition or genetic deficiency have better outcomes in minimizing inflammation and associated pathology. Given the involvement of Panx1 at multiple steps of inflammatory pathology, Panx1 could be a potential therapeutic target in the treatment of AKI. Further research is needed in elaborating the mechanisms and identifying Panx1 specific inhibitor molecules to better understand the role of Panx1 in AKI pathology arising due to diverse insults.
Introduction
Acute kidney injury (AKI) results as a secondary complication to various renal and non-renal pathologies including diabetes, hypertension, surgeries, viral and bacterial infections, cardiovascular diseases, and nephrotoxic drugs. In 2014, the number of hospitalizations due to AKI were nearly 5 million[1]. AKI cases that do not resolve may progress to chronic kidney diseases, renal failure requiring dialysis, or death in some case. AKI could also leads to other organ dysfunctions including lungs, heart, or liver causing further pathology and increased financial burden to the patients[2]. Despite the high incidence rate, huge economic burden, and significant deterioration in quality of life, there are no specific pharmacological or therapeutic regimen for prevention and/or treatment of AKI other than supportive therapy or dialysis in severe cases. The complexity of initiation and progression of AKI pathology due to renal microenvironment, type of insult, and physiological/immunological status of the individual further hinder the efforts to identify a common therapy for AKI. Pannexin (Panx) channels have emerged as a “pan-” player in progression of inflammation by regulating multiple steps in inflammatory pathway. Targeting Panx channel could be one potential pharmaceutical approach in management of AKI.
Pannexins:
Pannexins were first identified in invertebrates as a family of proteins referred to as innexins. The first mammalian isoforms of pannexins were cloned from brain tissues of mice[3]. Due to widespread expression across various phyla, these molecules were named pannexins incorporating “Pan” “innexins”[3]. Pannexins share structure and sequence homology to gap junction connexin family proteins, but their role in forming gap junction intercellular channels is still controversial. Three proteins of mammalian Panx family have been described so far; Panxl- 3. The expression of Panx proteins in various tissues and cell types have been already reviewed in details in [4]. Panx1 is ubiquitously expressed in epithelial cells, stromal cells, neurons as well as immune cells, while Panx2 is exclusive to neurons and Panx3 to cartilages, bones, skin, brain, and kidneys. Only Panx1 and Panx3 are expressed in the kidneys[5]. Since their first identification in 2000, Pannexin proteins were extensively studied in regard to their function in nervous system development and neuron differentiation. The contribution of Panx1 channels in inflammation was first reported by Pelegrin and Surprenant by showing that macrophages express Panx1 and that inhibition of Panx1 channels in macrophages blocks caspase-1 cleavage and IL1β-inflammasome formation[6]. Since then multiple groups have investigated the pro-inflammatory role of Panx1 channels. Genetic knockdown of Panx1 and or pharmacological inhibition of Panx1 have established distinct roles of Panx1-mediated ATP release in calcium signaling, tumorigenesis, immune cell activation and migration, ischemic injury, and apoptosis[4].
Panxl channels in AKI pathophysiology
Structurally, Panx1 forms homo-oligomers and/or heteromers with P2X7 purinergic receptors to form non-selective transport channel in the cell membranes through which nucleotides, ions, and biomolecules smaller than 1.5KDa pass[7]. Panx1 is also present in the endoplasmic reticulum membrane where it is believed to act as a leaky Ca2+ channel[8]. Panx1 channel is activated in apoptotic cells during inflammation/injury via mechanical stimulation and/or posttranslational modifications; for example, phosphorylation of C-terminal cytoplasmic domain or irreversible activation by various caspase-mediated cleavage (previously reviewed in [9]). Activation of Panxl channel causes ATP efflux and increases extracellular ATP, a DAMP molecule, and initiates inflammation by recruiting inflammatory cells to the site of injury [7]. Moreover, the released ATP molecules act in an auto- and/or paracrine fashion to rapidly activate purinergic signaling, inflammasome formation, and hence, further enhance inflammation. In addition, activation of Panx1 channel in other cell types during inflammatory settings further aggravates the pathology. In the vascular endothelium, activation of endothelial Panx1 channels facilitate leukocyte tethering and emigration into the injury site[10]. Activation of Panx1 channels in immune cells facilitate their migration capacity[11]. In AKI the cell types that release ATP via Panx1 channels are not known, however the observation that we observe protection in proximal tubule cell and endothelial cell specific knockout of Panx 1 suggests that at least these two cell are important [14].
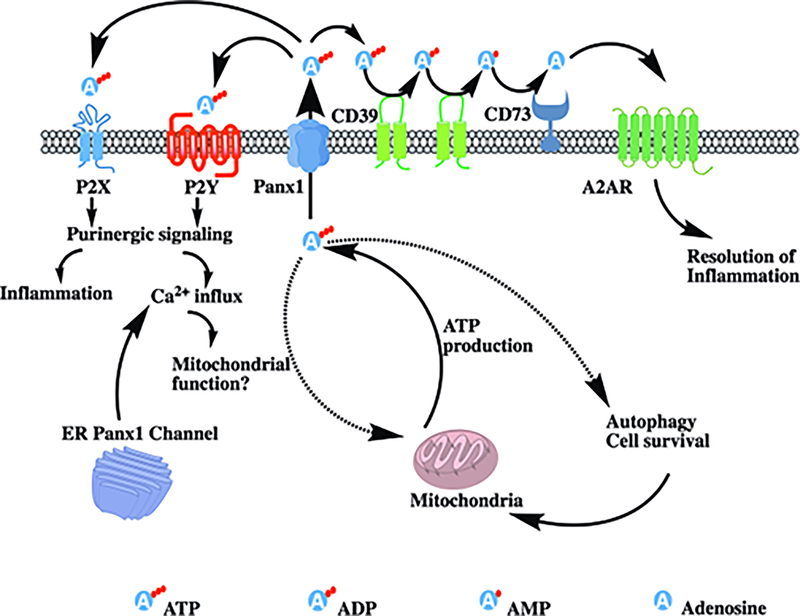
While the initial release of extracellular ATP initiates rapid inflammation via purinergic signaling, cell surface ectonucleotidase CD39 and CD73 expressed by infiltrating immune cells convert extracellular ATP into ADP, AMP, and subsequently into adenosine, which binds with A2A and A2B adenosine receptors to execute immunosuppressive function (Fig 1). Adenosine has been known to inhibit functions of inflammatory immune cells including CD4+ T cells, dendritic cells, NK cells, and macrophages [12] as well as enhance function of regulatory T cells[13] to collectively attenuate inflammation and permit initiation of reparative processes. Thus, during AKI, Panx1 channel-mediated ATP release initiates pro-inflammatory effects during early stages, but also inhibits inflammation that allows for repair processes at later stage.
Figure 1: Panx1 and ATP contribute to inflammation:
Panx1 and ATP play central roles in the inflammatory pathway. During injury, activation of Panx1 channel causes efflux of extracellular ATP, which activates the purinergic P2X and P2Y signaling pathway and initiates inflammation. Extracellular ATP can also be cleaved by CD39 to from ADP, AMP, which is then converted into adenosine by CD73. Adenosine binds with to A2A adenosine receptors that leads to an anti-inflammatory effect. Cytoplasmic calcium influx due to purinergic signaling can alter mitochondrial function. Intracellular ATP is necessary for maintenance of mitochondrial function, autophagy and cell survival. Activation of Panx1 channel causes reduced intracellular ATP pool which negatively affects cell survival. Panx1 channel in endoplasmic reticulum acts as leaky calcium channel and aids in intracellular calcium influx.
In addition to ATP efflux, intracellular ATP pool is reduced due to irreversible activation of Panx1 channels [14]. During AKI, the reduced intracellular ATP pool in conjunction with reduced substrate availability and increased energy demands for maintaining basic cellular functions, mitigating inflammation and initiating reparative process, all in combination, exert metabolic stress in the kidneys, especially proximal tubule epithelium that are highly metabolically active. Metabolic stress results in mitochondrial dysfunction, increased ROS production, apoptosis, and necrosis, all of which are key determinants of severity of AKI. While depletion of ATP can initiate the survival pathways; severe ATP depletion is detrimental to the cells and results in apoptosis and/or necrosis. We have shown that genetic deletion of Panx1 in murine proximal tubule derived TKPTS cells, a proximal tubule cell line, results in consistently higher intracellular ATP during normal physiology as well as hypoxia-reperfusion injury[14]. Panx1 channels could therefore be a central player in the development of AKI not only by exacerbating inflammation via the release of ATP to the extracellular space but also by depleting intracellular ATP and altering cellular energetics leading to irreversible cellular damage (Fig 1). Inhibition of Panx1 channels could therefore serve the dual purpose of reducing inflammation as well as maintaining tubular cell survival during AKI.
Pharmacological targeting of Panxl channels during AKI:
Pharmacological inhibition or genetic knockout of Panx1 has been shown to improve disease outcomes in various murine models. We have recently shown that pharmacological inhibition using carbenoxolone as well as genetic deletion of Panx1 globally, in proximal tubule or in vascular endothelium in mouse are protective against ischemic AKI[14]. We have also shown that transplantation of bone marrow derived myeloid cells from Panx1−/− mice did not protect wildtype mice from AKI. These findings suggest that the protection is primarily rendered by absence of Panxl in the endothelium and proximal tubule epithelial cells[14]. The mechanismsbehind the protection are still under investigation.
A list of pharmacological compounds that can inhibit or activate Panx1 channel have been reviewed [15]. The mechanism of action of most of these molecules are not well understood and the specificity has not been studied rigorously. There are FDA approved drugs for treatment of various diseases that do have activity against Panx 1 channels but their nonselective activity precludes their use as Panx1 inhibitors. Carbenoxolone is FDA approved for inflammation and gastrointestinal ulcers, probenecid for gout, spironolactone for hypertension, trovafloxacin for bacterial infections. However due to the high sequence homology, Panx1 inhibitors also have affinity towards connexin channels, albeit low. Formulation of more selective Panx1 compounds is needed for safe therapeutic use.
The time point of treatment during AKI is another key factor that determines outcomes. We have established that pretreatment of mice with Panx1 inhibitor is protective against IRI, but could Panx1 inhibition be beneficial in patients after onset of AKI is not known yet. Also whether Panx1 inhibition can protect from other models of AKI, for example: nephrotoxins, hypertension, sepsis needs further investigation.
Conclusions.
Panx1 channels regulate multiple steps during AKI and inhibiting Panx1 channels is beneficial in attenuating damage during experimental AKI in mouse. Numerous pharmacological agents that are already FDA approved for other conditions have been shown to inhibit Panx1 channels; some of them have shown promising results in minimizing inflammation and other diseases.
Panx1 inhibition could be a novel therapeutic strategy to attenuate AKI. Further research is needed to investigate physiological role of Panx1, formulate/identify more Panx1 selective inhibitors, and test potential of Panx1 inhibitors as a therapy in different models of AKI as well as for therapy in established AKI.
Acknowledgement:
The authors are grateful for the original research contributions provided by Mr Jakub Jankowski and Dr. Heather Perry for which this review is based. Research conducted to support data described in this review was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) R01DK062324 and T32 DK072922 (MDO). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Pavkov ME, Harding JL, Burrows NR: Trends in Hospitalizations for Acute Kidney Injury - United States, 2000–2014. MMWR Morbidity and mortality weekly report 2018;67:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostermann M, Cerda J: The Burden of Acute Kidney Injury and Related Financial Issues. Contrib Nephrol 2018;193:100–112. [DOI] [PubMed] [Google Scholar]
- 3.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S: A ubiquitous family of putative gap junction molecules. Curr Biol 2000;10:R473–R474. [DOI] [PubMed] [Google Scholar]
- 4.Penuela S, Gehi R, Laird DW: The biochemistry and function of pannexin channels. Biochim Biophys Acta 2013;1828:15–22. [DOI] [PubMed] [Google Scholar]
- 5.Lohman AW, Billaud M, Straub AC, Johnstone SR, Best AK, Lee M, Barr K, Penuela S, Laird DW, Isakson BE: Expression of pannexin isoforms in the systemic murine arterial network. J Vasc Res 2012;49:405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelegrin P, Surprenant A: Pannexin-1 mediates large pore formation and interleukin-ip release by the ATP-gated P2X7 receptor. EMBO J 2006;25:5071–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS: Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010;467:863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Hondt C, Ponsaerts R, De Smedt H, Vinken M, De Vuyst E, De Bock M, Wang N, Rogiers V, Leybaert L, Himpens B, Bultynck G: Pannexin channels in ATP release and beyond: An unexpected rendezvous at the endoplasmic reticulum. Cell Signal 2011;23:305–316. [DOI] [PubMed] [Google Scholar]
- 9.Penuela S, Simek J, Thompson RJ: Regulation of pannexin channels by post-translational modifications. FEBS Lett 2014;588:1411–1415. [DOI] [PubMed] [Google Scholar]
- 10.Lohman AW, Leskov IL, Butcher JT, Johnstone SR, Stokes TA, Begandt D, DeLalio LJ, Best AK, Penuela S, Leitinger N, Ravichandran KS, Stokes KY, Isakson BE: Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat Commun 2015;6:7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledderose C, Liu K, Kondo Y, Slubowski CJ, Dertnig T, Denicolo S, Arbab M, Hubner J, Konrad K, Fakhari M, Lederer JA, Robson SC, Visner GA, Junger WG: Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J Clin Invest 2018;128:3583–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, Moser EK, Jaworska K, Kinsey GR, Day YJ, Linden J, Lobo PI, Rosin DL, Okusa MD: Dendritic cells tolerized with adenosine A(2)AR agonist attenuate acute kidney injury. J Clin Invest 2012;122:3931–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinsey GR, Huang L, Jaworska K, Khutsishvili K, Becker DA, Ye H, Lobo PI, Okusa MD: Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephrol 2012;23:1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jankowski J, Perry HM, Medina CB, Huang L, Yao J, Bajwa A, Lorenz UM, Rosin DL, Ravichandran KS, Isakson BE, Okusa MD: Epithelial and Endothelial Pannexin1 Channels Mediate AKI. J Am Soc Nephrol 2018;29:1887–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu Y-H, Schappe MS, Desai BN, Bayliss DA: Revisiting multimodal activation and channel properties of Pannexin 1. J Gen Physiol 2018;150:19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]