Abstract
Several molecular modeling programs including Pep-Fold 3, Vienna RNA, RNA Composer, Avogadro, PatchDock, RasMol and VMD were used to define the 3-dimensional and basic binding characteristics of an extant sandwich DNA aptamer assay complex for human brain natriuretic peptide (BNP). In particular, the theoretical question of demonstrating likely binding of 72 base capture and reporter aptamers to at least two separate “epitopes” or binding sites on the small 32 amino acid BNP target was addressed and the data support the existence of separate aptamer binding sites on BNP. The binding model was based on first docking BNP to the capture aptamer based on shape complementarity with PatchDock, followed by docking the capture aptamer-BNP complex with the reporter aptamer in PatchDock. Although, shape complementarity clearly dominated this binding model and aptamers are known to be somewhat flexible, the model demonstrates hydrogen bond stabilization within each of the two different aptamers and between the aptamers and the BNP target, thus suggesting a strong binding and high affinity sandwich assay which matches the author’s former published assay results (Bruno et al., Microchem. J. 2014;115:32–38) with sub-picogram per ml sensitivity and good specificity. Other aspects such as capture and reporter aptamer interactions in the absence of BNP are illustrated and suggest means for potentially improving the existing assay by truncating the capture and reporter aptamers where they overlap to further decrease background signal levels.
Keywords: aptamer, Avogadro, PatchDock, RasMol, RNA Composer, sandwich assay
1. Introduction
Aptamers are short nucleic acid (DNA or RNA) oligonucleotides selected to bind specific target molecules much like antibodies with high affinity and specificity. However, aptamers obviate the need for animals during development or production and can be produced entirely in vitro with great consistency from batch to batch by chemical synthesis. As such, aptamers have tremendous potential in clinical diagnostics and therapeutics which the medical community appears to be increasingly realizing based on the sheer number of aptamers in the diagnostic and therapeutic pipelines including potential cancer treatments.1,2
While many software tools exist for 3D protein modeling and are free via internet-based servers or otherwise readily downloadable and available to the scientific community, creation of new tools or adaptation of existing software tools for use in nucleic acid aptamer modeling has lagged. Herein, the author describes the use of several existing web-based or free downloadable 3D software programs with several adaptations for use in aptamer modelling.3–9 The author has chosen an extant sandwich assay for human brain natriuretic peptide (BNP) which his former laboratory developed to determine low levels of BNP to aid in the diagnosis and prognosis formulation of cardiac hypertrophy.10 This sandwich assay system on the surface of magnetic microbeads has thus been studied extensively and shown to bind sub-picogram per ml levels of BNP even in human serum with good specificity as determined by cross-reactivity analyses.10
It was actually surprising to the author and his former colleagues that the small 32-amino acid BNP target was even able to participate in a sandwich assay given that at least two different “epitopes” or sites were required for the capture and reporter aptamers to bind the small target peptide in a sandwich format. The present docked 3D sandwich assay model confirms that two different aptamer binding sites exist on BNP despite its small size. The 3D model also shows evidence of intra-aptamer hydrogen bonding that may stabilize the capture and reporter aptamer structures for target binding based largely on geometric fit and several hydrogen bonds between the aptamers and the BNP target in addition to a number of obvious weaker van der Waal’s interactions between the aptamers and BNP.
2. Materials and Methods
2.1. DNA aptamer and BNP sequences
Table 1 gives the DNA nucleotide sequences of the capture (designated 25cF) and reporter (designated 2F) aptamers as well as the 32 amino acid sequence of human BNP used for 3D modeling. F stands for “forward” in reference to the directionality of PCR primers during the aptamer amplification process.10
TABLE 1.
DNA aptamer sequences (5’→ 3’) and the BNP amino acid sequence
| DNA Aptamer | Sequence (5’ → 3’) |
| Capture Aptamer (25cF) | 5’-ATACGGGAGCCAACACCA-CCTCTCACATTATATTGTG AATACTTCGTGCTGTTT-AGAGCAGGTGTGACGGAT-3’ |
| Reporter Aptamer (2F) | 5’-ATACGGGAGCCAACACCA-CGTTGCGCAGCTGGGGGC AGTGCTCTTTCGATTTGG-AGAGCAGGTGTGACGGAT-3’ |
| Target Peptide | Amino Acid Sequence |
| BNP (32 amino acids) | Ser-Pro-Lys-Met-Val-Gln-Gly-Ser-Gly-Cys-Phe-Gly-Arg-Lys-Met-Asp-Arg-Ile-Ser-Ser-Ser-Ser-Gly-Leu-Gly-Cys-Lys-Val-Leu-Arg-Arg-His |
Notes: Eighteen base constant sequence PCR primer ends built into the aptamers are in bolded.
2.2. Computer and software programs
All computer software programs were either used for on line web-based computations or downloaded free of charge to a standard 64-bit 4.2 GHz Dell PC with 32 GB RAM running Windows Pro 10 operating system. In particular, the author used Pep-Pro 3 (Univ. of Paris),4 Vienna RNA web server (Univ. of Vienna),5 RNA Composer (Univ. of Technology, Poznan, Poland),6 Avogadro7 ver. 1.2.0, PatchDock web server (Beta ver. 1.3),8,9 VMD ver. 1.9.3 (Beckman Institute for Advanced Science and Technology, National Institutes of Health, National Science Foundation, Physics, Computer Science, and Biophysics at University of Illinois at Urbana-Champaign), and RasMol ver. 2.7.5.2.
2.3. Analysis methods
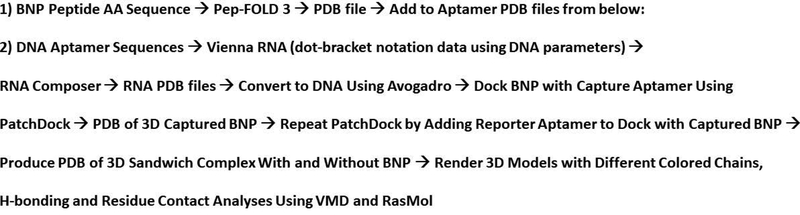
The general flow chart for 3D characterization studies is elucidated in Figure 1. The general philosophy was to create individual PDB files of the BNP peptide and each of the aptamers (25cF capture aptamer and 2F reporter aptamer; Figure 2) from the previously published magnetic bead electrochemiluminescence (ECL) sandwich assay.10 Next, the BNP target would be docked via PatchDock with the capture aptamer (designated 25cF in Bruno’s prior publication)10 to produce a 3D captured analyte complex PDB file (Figure 3A) and this file would be used to dock again with the reporter aptamer (designated 2F) PDB file using PatchDock to produce a 3D model of the sandwich complex (Figure 3B). A 3D PDB file of the sandwich complex without the captured BNP target was also produced (Figure 4) to illustrate potential nonspecific binding of the capture and reporter aptamers. Of course this aptamer complex minus BNP (Figure 4) was based entirely on shape complementarity in PatchDock of two flexible single-stranded (ss) DNA oligonucleotides that were selected to avoid nucleotide hybridization of the component nucleotides between the capture and reporter reagents (i.e., both were F or forward primed complexes with identical 18 base 3’ and 5’ PCR primer end sequences). One complication to this approach is that software does not exist for direct DNA aptamer to 3D PDB file conversion. Thus, the author previously developed a method1 to first model the DNA aptamers as secondary stem-loop structures using the Vienna RNA web server using DNA parameters at 25°C. The resultant dot-bracket notations from Vienna RNA were then imported the dot-bracket data into RNA Composer to produce 3D PDB files which modelled the aptamers as RNA instead of DNA structures. To correct for this, the author used Avogadro software7 to painstakingly delete all 2’ hydroxyl groups from each ribose and to add methyl groups to each uracil ring as appropriate to convert these bases to thymines and the overall aptamers from RNA to their DNA counterparts prior to any additional modeling.
FIGURE 1.
General flow chart for 3D model development and analyses using various free internet software programs.
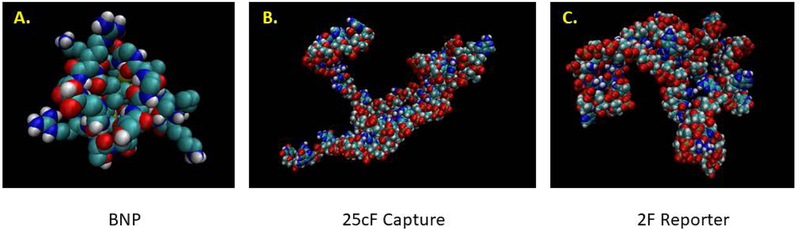
FIGURE 2.
Representative 3D images of the (A) target BNP analyte and the (B) capture (25cF) and (C) reporter (2F) DNA aptamer PDB files imaged in VMD as space filling models.
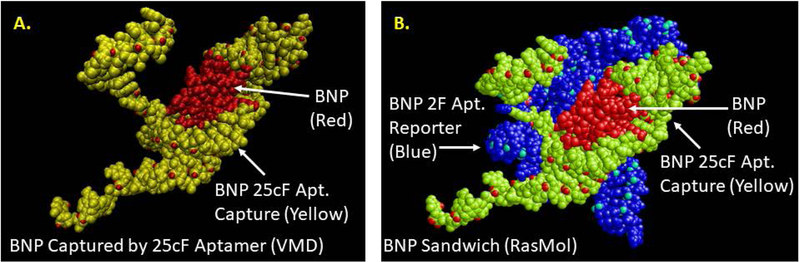
FIGURE 3.
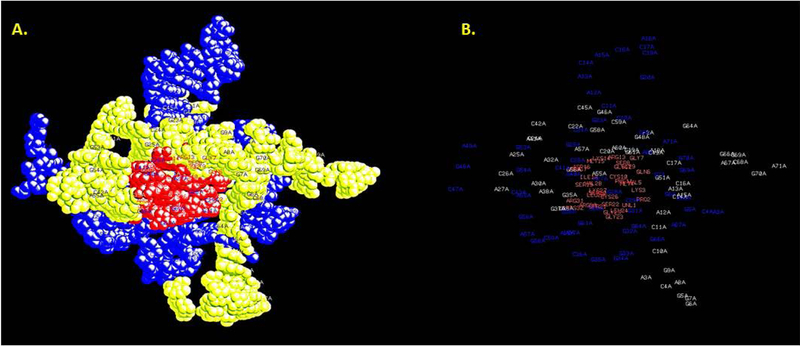
(A) Results of 3D docking for the BNP target with the 25cF capture aptamer following PatchDock simulation and (B) results of docking the 2F reporter aptamer to the captured BNP-25cF aptamer complex from a second PatchDock analysis. In order to visualize three different colored chains for each component of the full sandwich complex, RasMol had to be used for imaging in Figure 3B.
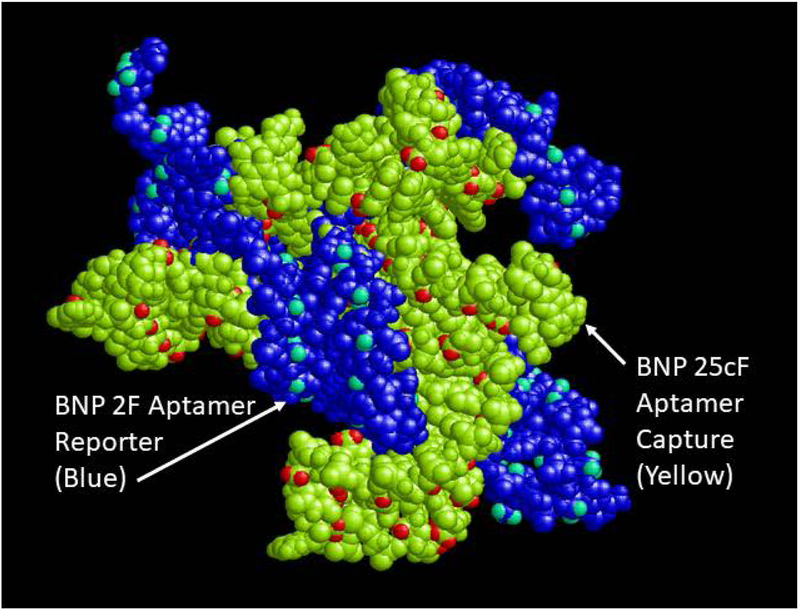
FIGURE 4.
3D docked PDB model of the capture and reporter aptamer fit following PatchDock analysis without the BNP target analyte visualized in RasMol.
Once the docked DNA aptamer sandwich complexes with and without bound BNP were obtained, these complexes were analysed using chain coloration tools in VMD and RasMol to clearly demarcate the BNP, capture, and reporter aptamers which aided in determining if more than one binding site (“epitopes”) for the aptamers existed on BNP and what sorts of bonds (hydrogen or van der Waals) might be responsible for target binding.
3. RESULTS AND DISCUSSION
Figure 2 illustrates the 3D PDB files of the individual components of the sandwich assay, namely the 32-amino acid human BNP target peptide generated by Pep-Fold 3 and the capture and reporter DNA aptamers generated through the longer process of Vienna RNA dot-bracket notation data being input into RNA Composer followed by tedious conversion to DNA aptamers via removal of all 2’ ribose hydroxyl groups and addition of methyl groups to each uracil ring at the 5 position to convert those bases to thymines using Avogadro editing software and visualization of the PDB files in VMD.
The author emulated the published BNP sandwich assay protocol sequence of steps8 by first binding or docking the BNP target analyte PDB file with the 25cF capture aptamer PDB file using PatchDock. Only the first (best or highest scored) docking model is shown in Figure 3A, but it is clearly a tight fit to about half of the target peptide surface. This captured BNP-aptamer complex was then treated as the “receptor” in PatchDock and docked with the 2F reporter aptamer “ligand” in a second PatchDock simulation resulting in the sandwich complex shown in Figure 3B. The author could only image this sandwich complex as three distinctly colored chains (BNP plus two different aptamers) using RasMol. But, Figure 3B shows the 2F reporter aptamer making distinct contact with the other side of the exposed BNP, thus suggesting two distinct binding sites on this small peptide target.
Somewhat unexpectedly though, the capture and reporter DNA aptamers in Figure 3B also appear to interact significantly with each other at least based on shape complementarity in the PatchDock simulation. This observation was further supported by Figure 4 which shows the sandwich complex with omission of the BNP target and yet the capture and reporter aptamers appear to dock together fairly well. This could be significant for the background or baseline ECL level that largely determines the limit of detection (LOD) for the assay, but it is based solely on shape complementarity from PatchDock analysis. In reality, the nonspecific capture and reporter aptamer docking in the absence of the BNP analyte may be insignificant, because the DNA is flexible and the model is not considering attractive forces (only shape or geometric fit). Indeed, the author chose aptamer sequences with little, if any complementarity to avoid nonspecific hybridization of the capture and reporter aptamers (i.e., both a forward or F aptamers with identical 18 base end sequences on the 3’ and 5’ ends as shown in Table 1) to keep background ECL levels low and sensitivity high as was observed empirically and reported.10
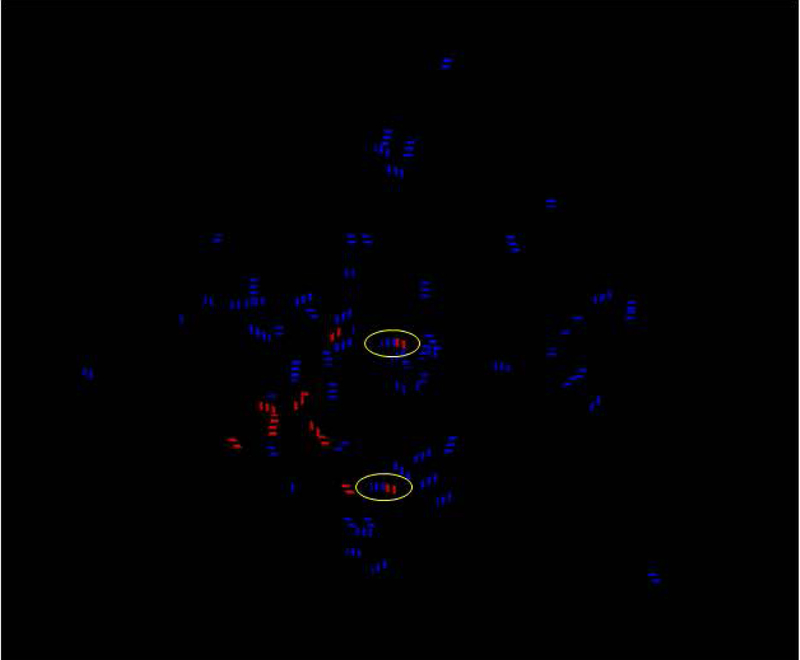
While it is difficult to determine the exact “epitope” aptamer binding sites on BNP with the current modeling, labeling analysis in RasMol suggested that interactions of the purine bases (adenine and guanine) from Figure 5B tended to dominate binding to the peptide target. Further analysis of hydrogen bonds shown in Figure 6 suggest at least two hydrogen bonds (circled blue-red hybrid dashed lines) between the aptamers and the peptide target after VMD analysis using the H-bond tool and chain coloration. Purely red dashed lines in Figure 6 represent hydrogen bonds within BNP, while purely blue dashes lines represent intra-aptamer hydrogen bonds. The binding strength of these two or more aptamer-target hydrogen bonds (circled in Figure 6) would only be enhanced by weaker additional van der Waals interactions (not shown) between the aptamers and target. The blue-labeled hydrogen bonds in Figure 6 may stabilize the capture and reporter aptamer 3D conformations and make target peptide binding more dependent on shape complementarity versus “induced fit.”
FIGURE 5.
Residue contact labeling analysis of the full sandwich complex performed by RasMol suggests that interactions between the labeled amino acids in BNP and the pale yellow or blue nucleotides of the aptamers in panel B are probably dominated by binding to the purines: adenines (As) and guanines (Gs).
FIGURE 6.
Hydrogen bonding analysis performed in VMD using chain coloration demonstrate at least two potential hydrogen bonds between the BNP target and each of the aptamers (circled blue-red hybrid dashed lines) and potential stabilizing intra-aptamer hydrogen bonds shown as blue dashed lines. Purely red dashed lines represent hydrogen bonds within BNP itself. Of course, these analyses were performed in vacuo without consideration for the abundant aqueous solvent, although water could interfere with any of the possible hydrogen bonds or exclusion of water during aptamer-ligand could be a thermodynamic (positive entropy change or + ΔS) driver for BNP-aptamer binding.11
But, one must be careful in evaluating any hydrogen bond contributions to aptamer-BNP binding, because water is not taken into consideration in these simple computer docking models. It is possible as illustrated by Hayashi et al.11 in a similar RNA aptamer-peptide system that water could interfere in binding or that the exclusion of water during aptamer-ligand binding could encourage binding by increasing overall entropy of the system. We simply cannot determine the entropy effects of water upon aptamer-BNP binding with the computational tools utilized here.
4. CONCLUSIONS
While no current 3D model of aptamer-ligand binding is likely to be completely accurate, the present attempt to dock an important analyte such as BNP, which has empirical binding data to support its validity already,10 using PatchDock provides some potentially useful insights. First, the presently reported sandwich model verifies that there could be two or more aptamer binding sites (“epitopes”) on BNP, despite its small 32-amino acid size, and that the selected capture and reporter DNA aptamer pair can probably bind these sites tightly via shape complementarity. In addition, there appear to two or more predicted hydrogen bonds between the aptamers and the target peptide in the absence of water, but this in vacuo model is not realistic, since the system exists in aqueous solvent. The model also shows stabilizing hydrogen bonds within each aptamer to help maintain their 3D conformations, but again the effects of water upon aptamer-ligand binding are not taken into account in PatchDock or YASARA software.
The model suggests possibly significant nonspecific interaction between the capture and reporter aptamers themselves even in the absence of the target peptide (BNP, Figure 4), but this is not born out in reality by Bruno et al.’s published data,10 which demonstrated very low background ECL in the absence of BNP and a very high degree of assay sensitivity (low pg/ml limit of detection). One should remain mindful that the PatchDock analysis is based solely on shape complementarity between supposedly hydrogen bond-stabilized, yet still somewhat flexible aptamers, which were selected to avoid even partial A-T and G-C hybridization (both aptamers are forward primed (labelled with an “F”) and have identical 18-base ends to avoid partial hybridization) and maintain low ECL background. However, even if the capture and reporter aptamer nonspecific binding was significant, the 3D models shown here in Figures 3–6 would be useful for determining where to truncate the aptamers to minimize capture and reporter aptamer binding and decrease background ECL signal in the absence of the BNP target. Thus, this general method demonstrates great practical utility for initially characterizing, evaluating and possibly improving or enhancing aptamer-ligand sandwich or other similar binding assays.
It may seem odd to any reader at first glance to first convert a DNA aptamer to RNA for development of 3D models, but this is necessitated by the lack of software to predict the 3D folding of DNA aptamers directly. Such software is rare or non-existent which forces the modeler to turn to 3D RNA structure prediction software such as RNA Composer and then to alter the resultant 3D RNA structure by removal of 2’ hydroxyl groups on ribose and conversion of uracils to thymines by addition of methyl groups to the 5 position on the ring structure using editing software such as Avogadro or Discovery Studio Visualizer and to then use the resultant polymer as the presumed 3D DNA aptamer structure for subsequent docking analyses. The precedent for this rather convoluted RNA aptamer intermediate structure modelling approach can be found in the published work of Heiat et al.12 Again, this seemingly artificial approach is unfortunately necessitated by a lack of available web-based or free software downloads that will directly accept DNA sequences for 3D structure prediction without an RNA intermediate.
As a footnote following the initial drafting of this manuscript, the author has subsequently discovered an effort at the Institut National des Sciences Appliquées (INSA) in Lyon, France to essentially automate the processes described in this article with free web-based software that will go to various sites and servers on the internet to develop 3D structures of DNA aptamers and aid in their docking or binding analysis. The INSA software is not yet available, but will be free of charge at this site in the future: http://2016.igem.org/Team:INSA-Lyon/Software. The only major differences between the INSA approach and the algorithm shown in Figure 1 of this work appear to be the use of Rosetta software instead of RNA Composer to generate 3D RNA versions of DNA aptamers and a custom script written by INSA to replace Avogadro for the tedious editing of 3D RNA structures back into their DNA forms. The author applauds this effort at INSA, because it firstly validates the current approach described herein and secondly will make the task of generating 3D DNA aptamer structures and their associated ligand docking models much faster and more facile in the future by simply entering a DNA aptamer nucleotide sequence and pressing a button on one’s computer. Hopefully, additional future 3D web-based or free downloadable modeling tools will also include more thermodynamic (entropy) evaluation features and consider the impact of water11 and dissolved salts upon aptamer-ligand binding in addition to shape complementarity.
ACKNOWLEDGMENTS
No funding was provided for the currently reported computer modeling analyses. However, funding for the BNP aptamer and assay development was provided by NIH SBIR grant no. HHSN268201000028C.
Footnotes
Competing interests: The author declares that there are no competing or conflicting interests.
REFERENCES
- 1.Kaur H, Bruno JG, Kumar A, Sharma TK Aptamers in the therapeutics and diagnostics pipelines. Theranostics. 2018;8(15):4016–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur H, Li JJ, Bay BH, Yung LY Investigating the antiproliferative activity of high affinity DNA aptamer on cancer cells. PLoS One. 2013;8(1):e50964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruno JG Do It Yourself 3-Dimensional aptamer-ligand molecular modeling. J Bionanosci. 2017;11:183–186. 10.1166/jbns.2017.1437 [DOI] [Google Scholar]
- 4.Shen Y, Maupetit J, Derreumaux P, Tufféry P Improved PEP-FOLD approach for peptide and miniprotein structure prediction. J Chem Theor Comput. 2014;10:4745–4758. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz R, Bernhart SH, Höner zu Siederdissen C, Tafer H, Flamm C, Stadler PF, Hofacker IL. Vienna RNA package 2.0. Algorithms for Molec Biol. 2011;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popenda M, Szachniuk M, Antczak M, Purzycka KJ, Lukasiak P, Bartol N, Blazewicz J, Adamiak RW Automated 3D structure composition for large RNAs. Nucleic Acids Res. 2012;40(14):e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J Cheminformatics. 2012;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duhovny D, Nussinov R, Wolfson HJ (2002) Efficient Unbound Docking of Rigid Molecules. In Gusfield et al., Ed. Proceedings of the 2’nd Workshop on Algorithms in Bioinformatics(WABI). Rome, Italy, Lecture Notes in Computer Science. Springer Verlag. 2002;2452:185–200. [Google Scholar]
- 9.Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ PatchDock and SymmDock: servers for rigid and symmetric docking. Nucl Acids Res. 2005;3: W363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruno JG, Richarte AM., Phillips T. Preliminary development of a DNA aptamer-magnetic bead capture electrochemiluminescence sandwich assay for Brain Natriuretic Peptide. Microchem J. 2014;115:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi T, Oshima H, Mashima T, Nagata T, Katahira M, Kinoshita M Binding of an RNA aptamer and a partial peptide of a prion protein: crucial importance of water entropy in molecular recognition. Nucleic Acids Res. 2014;42(11):6861–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heiat M, Najafi A, Ranjbar R, Latifi AM, Rasaee MJ J. Biotechnol. Computational approach to analyze isolated ssDNA aptamers against angiotensin II. 2016;230:34–39. [DOI] [PubMed] [Google Scholar]