Abstract
Background.
Human immunodeficiency virus (HIV) viral suppression (VS) decreases morbidity, mortality, and transmission risk.
Methods.
The Patient-centered HIV Care Model integrated community-based pharmacists with HIV medical providers and required them to share patient clinical information, identify therapy-related problems, and develop therapy-related action plans.
Proportions adherent to antiretroviral therapy (proportion of days covered [PDC] ≥90%) and virally suppressed (HIV RNA <200 copies/mL), before and after model implementation, were compared. Factors associated with postimplementation VS were determined using multivariable logistic regression; participant demographics, baseline viral load, and PDC were explanatory variables. PDC was modified to account for time to last viral load in the year postimplementation, and stratified as <50%, 50% to <80%, 80% to <90%, and ≥90%.
Results.
The 765 enrolled participants were 43% non-Hispanic black, 73% male, with a median age of 48 years; 421 and 649 were included in the adherence and VS analyses, respectively. Overall, proportions adherent to therapy remained unchanged. However, VS improved a relative 15% (75% to 86%, P < .001). Higher PDC (adjusted odds ratio [AOR], 1.74 per 1-level increase in PDC category [95% confidence interval {CI}, 1.30–2.34]) and baseline VS (AOR, 7.69 [95% CI, 3.96–15.7]) were associated with postimplementation VS. Although non-Hispanic black persons (AOR, 0.29 [95% CI, .12–.62]) had lower odds of suppression, VS improved a relative 23% (63% to 78%, P < .001).
Conclusions.
Integrated care models between community-based pharmacists and primary medical providers may identify and address HIV therapy–related problems and improve VS among persons with HIV.
Keywords: HIV, antiretroviral therapy, medication adherence, sustained virologic response, patient-centered HIV care model
Achieving human immunodeficiency virus (HIV) viral suppression is a central means for improving the well-being of those living with HIV, including reducing morbidity and mortality, increasing quality of life, and decreasing the likelihood of transmission to uninfected partners [1–8]. Despite the benefits of viral suppression, it is estimated that only 58% of persons with diagnosed HIV are suppressed [9]. Even fewer are likely to be durably suppressed [10, 11]. Compounding low viral suppression rates are age and racial disparities, with younger and black persons having lower rates of viral suppression, which in turn propagates disparities in morbidity and HIV incidence [12, 13].
Most persons with HIV, who are in care, are prescribed antiretroviral therapy (ART). However, to become and remain suppressed, a person must be on an effective antiretroviral (ARV) regimen and adherent to therapy. Given the complexities in selecting appropriate regimens and the continued monitoring necessary to identify drug resistance, adverse events, drug interactions, contraindicated therapy, and poor adherence, the US Department of Health and Human Services (DHHS) recommends providers work collaboratively with a multidisciplinary team to support patients’ complex needs, including their medication adherence–related needs [14].
Pharmacists are trained to help patients manage and adhere to complex medication regimens and can assist clinical providers with selection or modification of appropriate ARV regimens. With HIV specialty training, community pharmacists also have in-depth knowledge of: interactions between ARV medications and medications used to treat comorbid conditions, medication adverse events that can affect adherence to and effectiveness of treatment, and patient education and adherence counseling. Pharmacists can, therefore, be a key part of the multidisciplinary HIV care team.
In August of 2014, the Centers for Disease Control and Prevention, Walgreen Company, and the University of North Texas Health Science Center System College of Pharmacy implemented the Patient-centered HIV Care Model (PCHCM). The goal of the model was to integrate community-based HIV-trained pharmacists with primary medical providers to provide patient-centered care for people with HIV. There were 3 primary objectives of the model: (1) improve retention in HIV care; (2) improve adherence to ART; and (3) improve HIV viral suppression. These analyses evaluate adherence to ART and viral suppression among the PCHCM participants.
METHODS
The Patient-centered HIV Care Model
The PCHCM was a demonstration project conducted from August 2014 to September 2016. The model is described in detail elsewhere [15]. In summary, the model is built upon the existing medication therapy management (MTM) model. MTM encompasses a range of pharmacist-provided patient-care services such as review of medication regimens for interactions and response to therapy; patient education to improve understanding and appropriate use of medications; monitoring of prescription filling patterns to determine adherence to therapy; and adherence counseling [16]. To build upon MTM, the model required project clinics to share patients’ clinical information (eg, medical histories, laboratory test results) with their partnered pharmacists to enable the pharmacists to more precisely conduct MTM. After a review of patient clinical information, pharmacists conducted an initial comprehensive medication review (CMR) and subsequent quarterly medication reviews, to assess patients’ medication regimens for indication, effectiveness, safety, and adherence [16]. Fundamental to the model was the requirement for collaborative medication-related action planning between pharmacists, medical providers, and patients to address problems identified during pharmacist review of patients’ information or prescription filling patterns, or during pharmacist–participant interactions. No formalized practice agreements were established between pharmacists and prescribers.
The project provided model services to 765 persons with HIV at 10 project sites in: Albany, Georgia; Chicago, Illinois; Fort Lauderdale, Florida; Kansas City, Missouri; Miami, Florida; New York, New York; Palm Springs, California; Philadelphia, Pennsylvania; St Louis, Missouri; and Washington, District of Columbia. Each project site consisted of 1–2 community-based HIV-specialized retail pharmacies partnered with a medical clinic, and each site enrolled a convenience sample of 26 to 107 participants. Each participant received at least 12 months of services. All project pharmacists and pharmacy technicians had previous training on HIV treatment and prevention, stigma, and cultural competency. The HIV treatment and prevention training was developed by Walgreens, the National Alliance of HIV Education and Workforce Development, and the American Academy of HIV Medicine. The HIV treatment training included ARV pharmacology, identification and management of drug resistance, medication contraindications, drug–drug interactions, and adverse effects. The HIV stigma and cultural competency trainings were developed by Walgreens and accredited for continuing education by the Accreditation Council for Pharmacy Education (ACPE). The Office of Research Compliance, on behalf of the Institutional Review Board of the University of North Texas Health Science Center, determined the project met criteria for exempt status.
Measurement of Outcomes and Case Definitions
Adherence to ART
The proportion of persons adherent to ART was calculated using the proportion of days covered (PDC) measure. The PDC is a claims-based metric that determines the proportion of days for which a person has medication available. The ART PDC is calculated by dividing the number of days a person has adequate ART coverage during the measurement period by the length of the measurement period; adjustments are made for fill days’ supply and for days with overlapping medication supply [17]. Adequate ART coverage was defined as 3 ARV medications (excluding boosters), as outlined by treatment guidelines, or one of the following nonstandard regimens: lamivudine used in combination with 2 other ARV drugs or the combined use of darunavir/dolutegravir/ritonavir [14, 18]. Persons who were on ART but not on an adequate regimen were included in the denominator. Data were abstracted from project pharmacy fulfillment records.
Adherence to ART was calculated before and after model implementation and defined as a PDC ≥90%. The preimplementation measurement period began 12 months prior to the first CMR; postimplementation began the day after the first CMR, and extended forward 12 months. Participants were grouped into 1 of 4 PDC categories (<50%, 50% to <80%, 80% to <90%, and ≥90%) and the mean PDC was calculated for each group, before and after model implementation.
HIV Viral Suppression
The proportion of persons virally suppressed was calculated, before and after model implementation. Viral suppression was defined as HIV RNA <200 copies/mL at the last test in the 12-month measurement period [19]. The cutoff value of <200 copies/mL was based on the DHHS recommended definition of virologic failure [14]. Sustained viral suppression was defined as HIV RNA <200 copies/mL at the last 2 test results in the 12-month measurement period. In the pre- and postimplementation periods, the last viral load test was the viral load result closest to and furthest from the first CMR date, respectively. Measurement periods were the same as those used for the adherence analysis. Data were abstracted from clinic records.
In addition, the proportion of persons with viral loads ≥1500 copies/mL (the level above which the risk of transmission significantly increases) was determined, pre- to postimplementation.
Inclusion Criteria and Censoring
Persons were included in the adherence analysis if they had an initial CMR between August 2014 and August 2015, an ARV prescription at one of the project pharmacies, and at least 180 days of ARV fill records in both pre- and postimplementation measurement periods. Persons censored prior to 180 days postimplementation were excluded from the adherence analysis. Persons were included in the viral suppression analysis if they had ≥1 viral load result in each measurement period; the sustained viral suppression analysis required ≥2 viral load results. A description of how persons were censored from the analysis is described in the Supplementary Materials.
Model Interventions
For the purpose of the analysis, model interventions were categorized as (1) adherence support: individualized patient adherence counseling only, without development of a pharmacist-patient or pharmacist-clinic action plan; (2) pharmacist-patient action plan: development of a medication-related or other action plan in collaboration with the patient for the patient’s use (eg, instructions to take medication with food to prevent nausea) and no development of a pharmacist-clinic action plan; (3) pharmacist-clinic action plan: development of a medication-related or other action plan in collaboration with the clinic (eg, change medication regimen). Interventions were not mutually exclusive; a person could have >1 category of intervention during the measurement period. Model interventions were counted from the date of the initial CMR and were abstracted from project pharmacy records.
Statistical Analysis
The relative percentage change of the proportion of persons adherent and virally suppressed was calculated, before and after model implementation. Each pre- and postimplementation PDC was dichotomized (eg, PDC of ≥90% vs <90%) and McNemar test was used to compare the proportions of persons in each PDC category with those not within that category. In addition, the following statistical tests were used to compare the remaining groups, pre- to postimplementation: the difference in the mean PDC of persons within each baseline PDC category was tested using a paired t test; and the proportion of persons virally suppressed and the proportion with sustained viral suppression was tested using McNemar test.
Bivariate and multivariable logistic regression analyses were conducted to determine factors associated with viral suppression in the postimplementation period. Both the bivariate and multivariable models were adjusted for baseline viral suppression. For the bivariate analysis, odds ratios with 95% confidence intervals (CIs) were calculated for each demographic factor (age, sex, race/ethnicity, and insurance status), model intervention category, and number of pharmacist encounters (dichotomized as ≥3 and <3 encounters). Because the model interventions were not mutually exclusive, 5 separate multivariable analyses were conducted. The first model used each demographic factor and the PDC category as explanatory variables. The PDC was modified to account for the time to the last viral load result in the measurement period; the modified PDC was measured from the initial CMR date to the date of the last viral load result in the 12 months following the CMR. A separate model was conducted for number of pharmacist encounters and for each of the 3 categories of model interventions: adherence support, pharmacist–patient action plan, and pharmacist–clinic action plan. Each separate model contained demographic factors and modified PDC category. Backwards selection was used for the multivariable models. All data were analyzed using SAS Enterprise Guide 7.1 or SAS version 9.4 software.
RESULTS
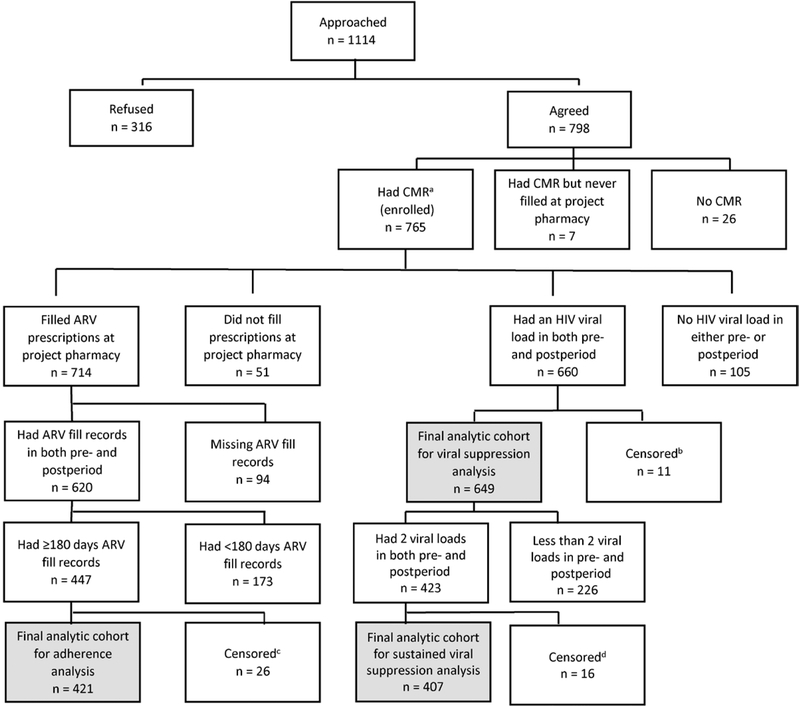
Of the 765 persons enrolled in the project, the largest proportions were non-Hispanic black (43%), male (73%), and Medicaid-insured (34%). The median age was 48 years (interquartile range, 38–55 years). A total of 421, 649, and 407 met inclusion criteria for the adherence, viral suppression, and sustained viral suppression analyses, respectively (Figure 1). Demographics for enrolled participants and the analytic cohorts are shown in Table 1.
Figure 1.
Flow diagram of inclusion in adherence and viral suppression analyses, Patient-centered Human Immunodeficiency Virus (HIV) Care Model, 2014–2016, United States. Abbreviations: ARV, antiretroviral; CMR, comprehensive medication review; HIV, human immunodeficiency virus. aAll persons who completed an initial comprehensive medication review and who filled prescriptions at the project pharmacy were considered enrolled in the project. bPersons were censored in the postimplementation period before a viral load test was drawn and were excluded from the viral suppression analysis. Persons were censored for the following reasons: 1 voluntarily withdrew, 1 was incarcerated, 1 moved out of area, and 8 transferred prescriptions to a nonproject (or nonproject network) pharmacy. cPersons were censored in the postimplementation period and excluded from the adherence analysis. Persons were censored for the following reasons: 5 died, 2 moved out of area, 2 transferred care, 1 was incarcerated, 2 voluntarily withdrew, and 9 transferred prescriptions to a nonproject (or nonproject network) pharmacy. Two project sites did not collect censoring data. For individuals from these 2 sites, persons were censored 1 day after the date of the last clinic visit if a person had no clinic visit or HIV laboratory test drawn for >6 months but continued to fill prescriptions in the last 6 months of the project implementation period; 5 persons were censored for this reason. dPersons were censored in the postimplementation period before 2 viral load tests were drawn and were excluded from the sustained viral suppression analysis. Persons were censored for the following reasons: 1 died, 1 transferred care, 1 moved out of area, 1 was incarcerated, 2 transferred prescriptions to a nonproject (or nonproject network) pharmacy, and 9 were no longer able to fill prescriptions at project pharmacy due to insurance reasons. Two project sites did not collect censoring data. For individuals from these 2 sites, persons were censored 1 day after the date of the last clinic visit if a person had no clinic visit or HIV laboratory test drawn for >6 months but continued to fill prescriptions in the last 6 months of the project implementation period; 1 person was censored for this reason.
Table 1.
Characteristics of Persons Enrolled Within the Patient-centered Human Immunodeficiency Virus Care Model and Characteristics of the Analytic Cohorts—United States, 2014–2016
| Characteristic | Total Enrolled (N = 765) | Included in Adherence Analysis (n = 421) | Included in VL Analysis (n = 649) | Included in Sustained VL Analysis (n = 407) |
|---|---|---|---|---|
| Age, y, median (IQR) | 48 (38–55) | 51 (43–57) | 49 (38–56) | 50 (40–57) |
| Age, y | ||||
| 18–24 | 27 (4) | 11 (3) | 25 (4) | 12 (3) |
| 25–34 | 123 (16) | 47 (11) | 97 (15) | 54 (13) |
| 35–49 | 251 (33) | 123 (29) | 209 (32) | 120 (29) |
| ≥50 | 364 (48) | 240 (57) | 318 (49) | 221 (54) |
| Race/ethnicity | ||||
| Black, non-Hispanic | 331 (43) | 157 (37) | 285 (44) | 172 (43) |
| Hispanic | 101 (13) | 45 (11) | 88 (14) | 56 (14) |
| Other/unknown | 79 (10) | 45 (11) | 68 (10) | 50 (12) |
| White, ethnicity unknown | 69 (9) | 54 (13) | 62 (10) | 45 (11) |
| White, non-Hispanic | 185 (24) | 120 (29) | 146 (22) | 84 (21) |
| Sex | ||||
| Male | 555 (73) | 319 (76) | 471 (73) | 293 (72) |
| Female | 193 (25) | 89 (21) | 164 (25) | 105 (26) |
| Transgender | 17 (2) | 13 (3) | 14 (2) | 9 (2) |
| Medical insurance | ||||
| Medicaid | 257 (34) | 133 (32) | 219 (34) | 135 (33) |
| Medicare | 155 (20) | 103 (24) | 134 (21) | 84 (21) |
| Multiple | 55 (7) | 44 (10) | 48 (7) | 39 (10) |
| Ryan White/ADAP | 113 (15) | 50 (12) | 92 (14) | 55 (14) |
| Uninsured/unknown | 70 (9) | 41 (10) | 61 (9) | 41 (10) |
| Private insurance | 115 (15) | 50 (12) | 95 (15) | 53 (13) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ADAP, AIDS Drug Assistance Program; IQR, interquartile range; VL, viral load.
Adherence to ART
Overall, there was no significant difference in the proportions of persons adherent to ART, pre- to postimplementation (Table 2). However, some persons moved between PDC categories (Table 2 and Supplementary Figure 1). In addition, the mean PDC for persons whose baseline PDC was ≥90% decreased 5% from 98% to 93% (P < .001), whereas the mean PDC of persons with a baseline PDC of <50% increased 121% from 28% to 62% (P < .001), postimplementation. There was no significant change in the mean PDC for persons whose baseline PDC was 50% to <80% or 80% to <90%.
Table 2.
Proportions of Persons in Each Proportion of Days Covered Category, Before and After Model Implementation, Patient-centered Human Immunodeficiency Virus Care Model—United States, 2014–2016
| PDC Categorya | Baseline, No. (%) | Follow-up, No. (%) | % Changeb | PValuec |
|---|---|---|---|---|
| <50% | 30 (7) | 34 (8) | 13 | .555 |
| 50% to <80% | 63 (15) | 66 (16) | 5 | .742 |
| 80% to <90% | 73 (17) | 48 (11) | −34 | .010 |
| ≥90% | 255 (61) | 273 (65) | 7 | .086 |
Abbreviation: PDC, proportion of days covered.
Among people who had at least 180 days of fill data in both the pre- and postimplementation periods before the first comprehensive medication review date.
Relative percentage change.
Each pre- and postimplementation PDC category was dichotomized (eg, PDC of ≥90% and <90%) and McNemar test was used to compare the proportions of persons in each PDC category with those not within that category.
Viral Suppression
Overall, viral suppression improved 15%, from 75% preimplementation to 86% (P < .001) postimplementation. Viral suppression improved within most demographic groups with notable improvements among persons aged 25–34years (26% increase; from 60% to 75%; P = .009), non-Hispanic black persons (23% increase; from 63% to 78%; P < .001), privately insured persons (31% increase;72% to 94%; P < .001) and persons whose care was covered by the Ryan White program (23% increase; 65% to 80%; P < .001) (Table 3). Overall, sustained viral suppression improved 22%, from 65% to 80% (P < .001; Table 3). Notably, the proportion of persons with viral loads >1500 copies/mL decreased 46% from 19% to 10% (data not shown).
Table 3.
Proportion of Persons Virally Suppressed Before and After Model Implementation by Characteristic, Patient-centered Human Immunodeficiency Virus Care Model—United States, 2014–2016
| VL <200 copies/mLa (n = 649) | Sustained VL <200 copies/mLb (n = 407) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Baseline | Follow-up | % Changec | P Valued | Baseline | Follow-up | % Changec | P Valued |
| Total | 486 (75) | 558 (86) | 15 | < .001 | 266 (65) | 324 (80) | 22 | < .001 |
| Age, y | ||||||||
| 18–24 | 12 (48) | 22 (88) | 83 | .002 | 4 (33) | 8 (67) | 100 | .102 |
| 25–34 | 58 (60) | 73 (75) | 26 | .009 | 21 (39) | 37 (69) | 76 | < .001 |
| 35–49 | 144 (69) | 170 (81) | 18 | < .001 | 70 (58) | 89 (74) | 27 | < .001 |
| ≥50 | 272 (86) | 293 (92) | 8 | .001 | 171 (77) | 190 (86) | 11 | .002 |
| Race/ethnicity | ||||||||
| Black, non-Hispanic | 180 (63) | 222 (78) | 23 | < .001 | 92 (53) | 121 (70) | 32 | < .001 |
| Hispanic | 72 (82) | 83 (94) | 15 | < .001 | 36 (64) | 49 (88) | 36 | < .001 |
| Other/unknown | 56 (82) | 58 (85) | 4 | .414 | 36 (72) | 40 (80) | 11 | .248 |
| White, ethnicity unknown | 60 (97) | 57 (92) | −5 | .178 | 39 (87) | 40 (89) | 3 | .739 |
| White, non-Hispanic | 118 (81) | 138 (95) | 17 | < .001 | 63 (75) | 74 (88) | 18 | .012 |
| Sex | ||||||||
| Male | 368 (78) | 419 (89) | 14 | < .001 | 198 (68) | 241 (82) | 22 | < .001 |
| Female | 111 (68) | 127 (77) | 14 | .006 | 63 (60) | 76 (72) | 21 | .005 |
| Transgender | 7 (50) | 12 (86) | 71 | .025 | 5 (56) | 7 (78) | 40 | .157 |
| Medical insurance | ||||||||
| Medicaid | 156 (71) | 178 (81) | 14 | .002 | 75 (56) | 96 (71) | 28 | < .001 |
| Medicare | 111 (83) | 120 (90) | 8 | .029 | 62 (74) | 68 (81) | 10 | .134 |
| Multiple | 47 (98) | 46 (96) | −2 | .317 | 32 (82) | 36 (92) | 13 | .157 |
| Ryan White/ADAP | 60 (65) | 74 (80) | 23 | < .001 | 31 (56) | 42 (76) | 36 | .005 |
| Uninsured/unknown | 44 (72) | 51 (84) | 16 | .020 | 26 (63) | 34 (83) | 31 | .011 |
| Private insurance | 68 (72) | 89 (94) | 31 | < .001 | 40 (75) | 48 (91) | 20 | .033 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ADAP, AIDS Drug Assistance Program; VL, viral load.
Viral suppression was defined as a human immunodeficiency virus (HIV) RNA of <200 copies/mL at the last test in the 12-month measurement period.
Sustained viral suppression was defined as consecutive HIV VLs <200 copies/mL at the last 2 tests in the 12-month measurement period.
Relative percentage change.
McNemar test use to compare groups before and after model implementation.
After adjusting for baseline viral suppression, bivariate analysis showed that persons aged ≥50 years (adjusted odds ratio [AOR], 1.91 [95% CI, 1.12–3.32]), men (AOR, 2.02 [95% CI, 1.20–3.40]), and privately insured persons (AOR, 3.73 [95% CI, 1.62–10.2]) had greater odds of being virally suppressed postimplementation than persons aged <50 years, women, and persons without private insurance, respectively. Persons with a higher modified PDC had greater odds (AOR, 1.89 per 1-level increase in PDC category [95% CI, 1.46–2.45]), and non-Hispanic black persons had lesser odds of being virally suppressed postimplementation (AOR, 0.26 [95% CI, .13–.49]). There was no significant difference in postimplementation viral suppression by any category of model intervention or number of pharmacist encounters (Table 4).
Table 4.
Factors Associated With Viral Suppression, After Model Implementation, Patient-centered Human Immunodeficiency Virus Care Model—United States, 2014–2016
| Bivariatea | Multivariableb (n = 461) | |||
|---|---|---|---|---|
| Characteristic | AOR (95% CI) | P Value | AOR (95% CI) | P Value |
| Age ≥50 y (n = 635)c,d | 1.91 (1.12–3.32) | .020 | … | |
| Black, non-Hispanic race/ethnicity (n = 509)d,e | 0.26 (.13-.49) | < .001 | 0.29 (.12-.62) | .002 |
| Male sex (n = 635)d | 2.02 (1.20–3.40) | .008 | … | |
| Private insurance (n = 578)d,f | 3.73 (1.62–10.2) | .004 | … | |
| Modified PDC (n = 582)d,g | 1.89 (1.46–2.45) | < .001 | 1.74 (1.30–2.34) | < .001 |
| Baseline HIVVL <200 copies/mL (n = 635)d | 11.0 (6.74–18.4) | < .001 | 7.69 (3.96–15.7) | < .001 |
| ≥1 adherence support (n = 564)d,h | 0.65 (.34–1.27) | .199 | … | |
| ≥1 pharmacist-patient action plan (n = 564)d,h | 0.85 (.50–1.46) | .561 | … | |
| ≥1 pharmacist-clinic action plan (n = 564)d,h | 0.25 (.04-.90) | .069 | … | |
| ≥3 pharmacist encounters (n = 559)d,h | 0.88 (.67–1.15) | .343 | … | |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; PDC, proportion of days covered; VL, viral load.
Each bivariate logistic regression model controlled for baseline viral suppression (HIV RNA <200 copies/mL).
Multivariate logistic regression.
Persons aged ≥50 years were compared with persons aged <50 years.
Transgender persons were excluded from the analyses due to small numbers.
Non-Hispanic black persons were compared with all other races/ethnicities combined.
Persons with private insurance were compared to persons without private insurance.
PDC was modified to account for the time to the last viral load in the postimplementation measurement period.
Separate multivariable models were conducted for each model intervention category and for number of pharmacist encounters. Each of the separate models contained demographic factors, modified PDC, and baseline viral suppression.
In the multivariable model that included only demographic factors, baseline viral suppression, and modified PDC, persons with a higher modified PDC (AOR, 1.74 per 1-level increase in PDC category [95% CI, 1.30–2.34]) and persons who were virally suppressed at baseline (AOR, 7.69 [95% CI, 3.96–15.7]) had greater odds of being suppressed, and non-Hispanic black persons (AOR, 0.29 [95% CI, .12–.62]) had lower odds of being virally suppressed, postimplementation. In none of the separate models that contained category of model intervention or number of pharmacist encounters were these variables significantly associated with postimplementation viral suppression (Table 4).
DISCUSSION
The Patient-centered HIV Care Model, implemented at 10 sites, improved viral suppression among participants by a relative 15%. Notably, the improvement was found across most demographic groups and among both persons with private and publicly funded insurance. In addition, the proportion of persons with a viral load >1500 copies/mL decreased 46%, from 19% to 10%. This model, which operated by sharing patient clinical information between partnered community-based HIV-trained pharmacists and medical providers, addressed medication and other therapy-related problems for persons with HIV. While there was no overall improvement in adherence to ART, there was a strong association between adherence to ART and viral suppression, postimplementation.
A potential explanation for the seemingly disparate results–improvement in viral suppression without finding an improvement in adherence–is that viral suppression is affected not only by adherence to therapy, but by the appropriateness and effectiveness of patients’ ARV regimens. An integral part of the PCHCM was pharmacists’ review of patients’ clinical information, such as HIV viral loads, to evaluate effectiveness of therapy. Also pivotal to the conduct of the model was pharmacists’ review of patients’ medication regimens to identify obstacles to effective treatment, such as ARV drug resistance, non-ARV and ARV drug interactions, drug toxicity, and contraindicated therapy. Pharmacists then worked with clinic providers to modify or change regimens to optimize treatment effectiveness, which might have contributed to improved viral suppression. In addition, a previous analysis of model outcomes showed that retention in care improved 13% among project participants; improved retention may have also contributed to improved viral suppression [15].
While there was no significant change in the overall proportion of persons adherent to therapy, the mean PDC for persons whose baseline PDC was ≥90% decreased from 98% to 93%. The mean postimplementation PDC of 93%, however, is still considered adherent. Conversely, the mean PDC of persons with a baseline PDC of <50% increased 121% from 28% to 62%, postimplementation. Some of this improvement may be explained by regression to the mean, but given the magnitude of the change, it is unlikely to account for all of the difference. While a postimplementation average PDC of 62% is not considered adherent, the improvement could imply that the model can help improve adherence in very poorly adherent persons.
Viral suppression improved in most race and ethnicity groups. While non-Hispanic black persons were less likely to be suppressed than the other race and ethnicity groups, the proportion suppressed improved 23%. In addition, 70% of non-Hispanic black participants had sustained postimplementation viral suppression, which was higher than the 53% seen among this population in a surveillance-based study [20]. These improvements are notable because black persons make up the largest proportion of persons with HIV (42% of all infections), and studies have shown persistent racial disparities in viral suppression; from 2011 to 2015, 40%–54% of black persons compared with 53%–67% of white persons were virally suppressed [12, 13, 21–24]. In addition, black persons are more likely to spend longer times with HIV viral loads >1500 copies/mL [25]. Part of this disparity has previously been explained by poor provider cultural competency; studies have shown that providers with low cultural competence have patients with lower self-efficacy to manage medications and lower rates of viral suppression [26]. Each PCHCM pharmacist had received previous ACPE-accredited training on cultural competency and HIV stigma. This training may have been particularly helpful in reaching black participants.
Viral suppression improved among all age groups with large improvements among younger adults. This improvement is noteworthy because younger adults have low viral suppression rates; in 2015, an estimated 51%–54% of persons aged 13–34 years were virally suppressed compared with 63% of persons aged ≥55 years [12]. Low suppression rates, when combined with high-risk behaviors (which are prevalent among younger adults), contribute to age disparities in HIV incidence [13, 27, 28]. In 2015, approximately 60% of all new diagnoses were among persons aged 13–34 years [13]. Because the greatest HIV burden falls upon black and younger persons, achieving and maintaining viral suppression in these populations is critical to controlling HIV in the United States. The improvement in viral suppression among black and younger adults, seen in this study, is therefore encouraging.
Persons who were suppressed at baseline had 7 times the odds of being suppressed postimplementation. This finding is not surprising as studies have shown that people who are suppressed often will remain suppressed [10, 29]. No association was seen between category of model intervention and viral suppression. This might be reflective of how the model interventions were categorized, or indicative that the model as a whole is necessary to improve viral suppression.
The project analyses have limitations. First, the PDC measure is a proxy for adherence; it measures the amount of time a person has medication in hand, not whether a person is actually taking their medication; adherence was, therefore, likely overestimated. Second, the PDC was calculated for persons with between 180 and 365 days of fulfillment data such that comparisons were made for different measurement period lengths. However, there were no significant differences in PDC calculations when comparing persons with at least 180 days to those with 180–365 days of fulfillment data. Persons with no viral loads in either the pre- or postimplementation periods were excluded from the viral suppression analysis. It is possible that persons with no viral load test results were not suppressed and by excluding these persons the proportion of persons virally suppressed was overestimated. Project pharmacists were not reimbursed for model services, which may be required to scale this model. The measurement period for the analysis was 12 months; because time has an impact on both medication adherence and viral suppression, a longitudinal evaluation is needed to determine the durability of the model’s effect. While the analysis of model outcomes showed those willing and able to participate in the model benefited, further studies on participation barriers and of excluded populations may be warranted. Last, the PCHCM was a demonstration project, not a research study; therefore, there were no control groups.
The Patient-centered HIV Care Model integrated community-based HIV specialized pharmacists with primary medical providers to provide patient-centered care for persons with HIV. This model, which seeks to identify and address HIV therapy–related problems, can lead to improved viral suppression among persons with HIV. Despite not finding an overall improvement in adherence to ART, viral suppression improved 15%, which is likely reflective of pharmacist and medical provider efforts to optimize ARV treatment.
Supplementary Material
Financial support.
This work was supported by the Department of Health and Human Services Secretary’s Minority AIDS Initiative fund and the Centers for Disease Control and Prevention through a cooperative agreement (grant number PS13–1315) with the University of North Texas Health Science Center System College of Pharmacy. The Walgreen Company provided all services in-kind.
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Publisher's Disclaimer: Disclaimer. The findings and conclusions of this analysis are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Potential conflicts of interest. J.G. H., R.H., H.K., and A.D.were employees of the Walgreen Company during the conduct of this study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes JP, Baeten JM, Lingappa JR, et al. ; Partners in Prevention HSV/HIV Transmission Study Team. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis 2012; 205:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000; 342:921–9. [DOI] [PubMed] [Google Scholar]
- 4.Rodger AJ, Cambiano V, Bruun T, et al. ; PARTNER Study Group. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016; 316:171–81. [DOI] [PubMed] [Google Scholar]
- 5.Farnham PG, Gopalappa C, Sansom SL, et al. Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr 2013; 64:183–9. [DOI] [PubMed] [Google Scholar]
- 6.Farnham PG, Holtgrave DR, Gopalappa C, Hutchinson AB, Sansom SL. Lifetime costs and quality-adjusted life years saved from HIV prevention in the test and treat era. J Acquir Immune Defic Syndr 2013; 64:e15–8. [DOI] [PubMed] [Google Scholar]
- 7.May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 2014; 28:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine (Baltimore) 2016; 95:e3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marks G, Patel U, Stirratt MJ, et al. Single viral load measurements overestimate stable viral suppression among HIV patients in care: clinical and public health implications. J Acquir Immune Defic Syndr 2016; 73:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crepaz N, Tang T, Marks G, Hall HI. Viral suppression patterns among persons in the United States with diagnosed HIV infection in 2014. Ann Intern Med 2017; 167:446–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2016. HIV surveillance supplemental report. 2018:23 Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 2 July 2018. [Google Scholar]
- 13.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2015. HIV surveillance supplemental report. 2018:23 Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 7 February 2019. [Google Scholar]
- 14.Tanner Z, Lachowsky N, Ding E, et al. ; Canadian Observation Cohort (CANOC) Collaboration. Predictors of viral suppression and rebound among HIV-positive men who have sex with men in a large multi-site Canadian cohort. BMC Infect Dis 2016; 16:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrd KK, Hardnett F, Clay PG, et al. ; Patient-Centered HIV Care Model Team. Retention in HIV care among participants in the patient-centered HIV care model: a collaboration between community-based pharmacists and primary medical providers. AIDS Patient Care STDS 2019; 33:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Pharmacists Association, National Association of Chain Drug Stores Foundation. Medication therapy management in pharmacy practice: core elements of an MTM service model (version 2.0). J Am Pharm Assoc (2003) 2008; 48:341–53. [DOI] [PubMed] [Google Scholar]
- 17.Nau D. Proportion of days covered (PDC) as a preferred method of measuring medication adherence. Available at: http://www.pqaalliance.org/images/uploads/files/PQA%20pdc%20vs%20%20mpr.pdf. Accessed 5 June 2018.
- 18.Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society–USA panel. JAMA 2016; 316:191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Resources Health and Administration Services. HIV/AIDS Bureau performance measures. Available at: https://hab.hrsa.gov/sites/default/files/hab/clinical-quality-management/coremeasures.pdf. Accessed 5 June 2018.
- 20.Crepaz N, Tang T, Marks G, et al. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012–2013. Clin Infect Dis 2016; 63:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2012. HIV surveillance supplemental report. 2014:19 Available at: http://www.cdc.gov/hiv/library/reports/surveillance/. Accessed 8 February 2019. [Google Scholar]
- 22.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2013. HIV surveillance supplemental report. 2015:20 Available at: http://www.cdc.gov/hiv/library/reports/surveillance/. Accessed 8 February 2019. [Google Scholar]
- 23.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2015. HIV surveillance supplemental report. 2017:22 Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 8 February 2019. [Google Scholar]
- 24.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2014. HIV surveillance supplemental report. 2016:21 Available at: http://www.cdc.gov/hiv/library/reports/surveillance/html. Accessed 8 February 2019. [Google Scholar]
- 25.Crepaz N, Dong X, Wang X, Hernandez AL, Hall HI. Racial and ethnic disparities in sustained viral suppression and transmission risk potential among persons receiving HIV care—United States, 2014. MMWR Morb Mortal Wkly Rep 2018; 67:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saha S, Korthuis PT, Cohn JA, Sharp VL, Moore RD, Beach MC. Primary care provider cultural competence and racial disparities in HIV care and outcomes. J Gen Intern Med 2013; 28:622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beer L, Mattson CL, Shouse RL, Prejean J. Receipt of clinical and prevention services, clinical outcomes, and sexual risk behaviors among HIV-infected young adults in care in the United States. AIDS Care 2016; 28:1166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2015. MMWR Surveill Summ 2016; 65:1–174. [DOI] [PubMed] [Google Scholar]
- 29.Crepaz N, Tang T, Marks G, Hall HI. Changes in viral suppression status among US HIV-infected patients receiving care. AIDS 2017; 31:2421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.