Abstract
Observational studies demonstrate that women with severe periodontitis have a higher risk of adverse pregnancy outcomes like preterm birth and low birthweight. Standard treatment for periodontitis in the form of scaling and root planing during the second trimester failed to reduce the risk of preterm or low birthweight. It is premature to dismiss the association between periodontitis and adverse pregnancy outcomes because one explanation for the failure of scaling and root planing to reduce the risk of adverse pregnancy outcomes is that periodontal pathogens spread to the placental tissue prior to periodontal treatment. In the placenta, orally derived organisms could cause direct tissue damage or mediate a maternal immune response that impairs the growth of the developing fetus. Sequencing studies demonstrate the presence of organisms derived from the oral microbiome in the placenta, but DNA-based sequencing studies should not be the only technique to evaluate the placental microbiome because they may not detect important shifts in the metabolic capability of the microbiome. In humans, polymerase chain reaction and histology have detected periodontal pathogens in placental tissue in association with multiple adverse pregnancy outcomes. We conclude that both placental and oral microbiomes may play a role in periodontitis-associated adverse pregnancy outcomes. However, the measure to determine the association between periodontal pathogens in the placenta and adverse pregnancy outcomes should be the amount and prevalence, not the mere presence of such microorganisms. Placental colonization with periodontal pathogens thus potentially represents the missing link between periodontitis and adverse pregnancy outcomes.
Keywords: adverse pregnancy outcomes, low birthweight, periodontitis, preterm birth
Adverse pregnancy outcomes (APOs) including preterm birth (PTB), low birthweight (LBW), and comorbid preterm low birthweight (PLBW) occur in a significant number of women without an apparent etiology, suggesting that undiscovered risk factors for APOs exist. Periodontitis has been proposed as a novel risk factor for APOs.1
Disagreement on the association between periodontitis and APOs derives from three major sources. First, there has been heterogeneity in the clinical definition of severity and extent of periodontitis used to distinguish cases vs controls.2–4 Second, studies fail to control for shared risk factors or confounders between periodontitis and APOs.2–4 Lastly, most studies did not consider the spread and survival of periodontal pathogens to the placenta as a mechanism that could induce APOs independently of ongoing disease in the oral cavity.2–4
Defining periodontitis
Periodontitis is evaluated as a permanent loss of clinical attachment level (CAL).5–10 CAL measures the distance between an anatomical reference on tooth crown and the bottom of the periodontal pocket. Probing depth (PD) is a measure related to CAL that estimates the depth of the periodontal pocket. The deeper the pocket, the more inflammation is present around a diseased tooth. These 2 parameters are generally measured at 6 independent sites around each tooth.
The strength of association between periodontitis and APOs varies, depending on the severity (amount of CAL and/or PD) and the extent of the disease (number of sites within the mouth with a given level of CAL or PD) used to separate cases from controls.11 One astute study demonstrated this principle by applying multiple published definitions of periodontitis to the same data set. Six definitions of periodontitis resulted in statistically significant associations with APOs, whereas 8 did not.11 Heterogeneity in the periodontitis definition also means that it is difficult to combine data across multiple studies for a meta-analysis.12,13 This problem may be mitigated if future studies utilize the 2017 consensus classification of periodontal diseases.10
Periodontitis in pregnancy should not be confused with pregnancy gingivitis. Pregnancy gingivitis is a common, reversible condition of gingival inflammation associated with high levels of estrogens and blooms of microbial species such as Prevotella intermedia.14–19 In contrast, periodontitis is associated with a fundamental shift in the relative composition of the oral microbiome induced by anaerobic keystone pathogens.20 In periodontitis the modification of the microbial composition is unrelated to pregnancy status or pregnancy hormones. When good oral hygiene practices are implemented, pregnancy gingivitis resolves within a few months of birth with no permanent changes in CAL. Pregnancy gingivitis is not considered a risk factor for adverse pregnancy outcomes.16,17,19
Risk factors
The drive to discover whether periodontitis represents a risk factor for APOs stems from the need to develop interventions that reduce the impact of APOs on families, children, and society. Because APOs and periodontitis have many risk factors in common, shared risk factors must be considered in any valid attempt to establish periodontitis as causative of APOs12,13 (Table 1). Interestingly, many of these risk factors are associated with inflammation or inflammation-induced pathologies. There has been some suggestion that individuals with a more proinflammatory genotype may be more susceptible to inflammation-mediated disorders like periodontitis or chorioamnionitis. For example, polymorphisms of the interleukin (IL)-1 gene are associated with both periodontitis and APOs.21–29
TABLE 1.
Risk factors for APOs and periodontitis in the United States
| Risk factor | APOs (reference) |
Periodontitise (reference) |
|---|---|---|
| Age greater than 34 years | ✔120 | ✔121, 122 |
| Age less than 17 years | ✔120 | |
| Low socioeconomic status | ✔120 | ✔121, 122 |
| Recreational drug abuse | ✔120 | ✔123 |
| Alcohol use | ✔120 | ✔124 |
| Smoking or other tobacco use | ✔120 | ✔121, 122, 124 |
| Hypertension/cardiovascular disease | ✔120 | ✔121 |
| Diabetes | ✔120 | ✔121, 122, 124 |
| Obesity/metabolic syndrome | ✔120 | ✔121, 122, 124 |
| Osteoporosis | ✔124 | |
| Deficiencies in dietary calcium or vitamin D | ✔124 | |
| Stress | ✔120 | ✔121, 124 |
| Inadequate prenatal care | ✔120 | |
| Multiple pregnancies | ✔120 | |
| Previous incidence of APOs | ✔120 | |
| Racial/ethnic group | ✔120 | ✔121, 122 |
| Anaerobic bacterial infections | ✔32, 48, 49 | ✔55, 56, 92, 93, 121, 125–129 |
| Polymorphisms of IL-1 gene | ✔21–24 | ✔25–29 |
APO, adverse pregnancy outcome; IL, interleukin.
Because complications associated with APOs are a leading cause of infant mortality in the United States, identifying novel risk factors for APOs are important.30,31 Some racial and ethnic groups are especially susceptible to APOs and subsequent infant mortality (Table 2).32 Notably, increased maternal age was associated with both increased prevalence of periodontitis and increased infant mortality rate.31,33
TABLE 2.
Prevalence of APOs, periodontitis, and infant mortality rate by race
| Racial/ethnic group | Prevalence of periodontitis,a 1999–200433 |
Infant mortality rate,b 200531 |
Percent of live births, 201331 |
|
|---|---|---|---|---|
| Non-Hispanic white | 2.0% | 5.06 | PTB | 10.2% |
| LBW <2500 g | 7.0% | |||
| LBW <1500 g | 1.13% | |||
| Non-Hispanic black | 2.2% | 13.63 | PTB | 16.3% |
| LBW <2500 g | 13.2% | |||
| LBW <1500 g | 2.96% | |||
| Mexican American | 9.3% | 5.62 | PTB | 10.8% |
| LBW <2500 g | 6.6% | |||
| LBW <1500 g | 1.15% |
APO, adverse pregnancy outcome; CAL, clinical attachment level; LBW, low birthweight; NHANES, National Health and Nutrition Examination Survey; PD, probing depth; PTB, preterm birth.
A patient having periodontitis was defined by having 2 teeth with each having at least 1 periodontal site with both ≥3 mm of CAL and ≥4 mm PD measuring 2 of 4 possible quadrants.33 A follow-up study suggested NHANES examinations using recordings in 2 of 4 quadrants may have underestimated the prevalence of periodontal disease by 50% or more.130
Number of deaths per 1000 live births.
APOs can also lead to life-long consequences. For example, 52% of infants born at 24—28 weeks have neurodevelopment impairment as compared with 5% of those born at 32—36 weeks.34 Additionally, young adults who were born with very low birthweight (<1500 g) had lower bone mineral density than term-birth, same-age peers.35
In summary, if severe periodontitis represents a potential novel risk factor for APOs, then treating periodontitis has the potential to be a low-cost public intervention to reduce the impact of APOs on society.
Periodontitis as a risk factor for APOs
One meta-analysis of 15 observational studies found an increased risk of PTB (odds ratio [OR], 2.73, 95% confidence interval [CI], 2.06—3.6) and LBW (OR, 1.5, 95% CI 1.26—1.79) in women with periodontitis.36 Other systematic reviews of case-control and cohort studies support an association between periodontitis and APOs.13,37 Again, these studies must be considered with caution because of heterogeneity in periodontitis definitions.11 The observational evidence associating periodontitis with APOs leads to the hypothesis that treating periodontitis through standard periodontal therapy could result in a significant reduction in the devastating impact of PLBW on individuals and families.
Scaling and root-planing trials
Several randomized, controlled treatment trials using scaling and root planing (SRP) tested whether a periodontal therapeutic intervention could reduce the risk of APOs in women with periodontal disease. Studies of high quality, low risk of bias38 in multiple meta-analysis reports agreed that SRP does not reduce the risk of PTB or the risk of LBW.39–42 These findings prompted researchers to question the mechanism behind the association between periodontitis and increased risk of APOs found in observational studies.12,13,36,37
Why did SRP therapy fail?
SRP changes the composition of the periodontal microbiota by mechanically disrupting the biofilm within the periodontal pocket. SRP is strictly a local intervention because it does not prevent hematogenous spread of periodontal pathogens. Because gingival inflammation increases in periodontitis, the likelihood of transient bacteremia also increases.43–45 It is therefore reasonable to speculate that periodontal pathogens seeding the placental tissue prior to second-trimester SRP treatment may impair the developing fetus.4
Infection and inflammation associated with overgrowth of urinary tract and vaginal microorganisms have been well characterized as a risk factor for PLBW. Interestingly, both bacterial vaginosis and periodontitis involve a change in the microbiome that favors overgrowth of anaerobic species normally found in low numbers in their respective human ecological niches. This observation leads to the possibility that anaerobic species originating from the oral cavity and now found in the placenta could be involved in APOs, paralleling the observation that overgrowth of anaerobic species from or in the vagina is involved in APOs.46,47
Anaerobic species of APOs and periodontitis
Bacterial vaginosis is an independent risk factor for PLBW.32,48,49 Bacterial vaginosis results from an overgrowth of organisms such as the anaerobes Gardnerella vaginalis and Mycoplasma hominis in the vaginal canal. These low-frequency organisms grow to replace the normal Lactobacillus-dominated vaginal microbiome.32,46
Anaerobic periodontal pathogens have also been detected in the vaginal microbiome in individuals with bacterial vaginosis.50,51 Interestingly, black women were more likely to have vaginal microbiomes dominated by anaerobes, while white women were more likely to have a vaginal flora dominated by Lactobacillus species.52 This suggests the possibility that the increased rate of PLBW in black women (Table 2) is associated with the increased proportions of vaginal anaerobes. The increased prevalence of vaginal anaerobes may also influence the higher rate of bacterial vaginosis in black vs white women (51% vs 23%, respectively).53
Porphyromonas gingivalis and Fusobacterium nucleatum are two anaerobic species commonly associated with periodontitis that have been detected in the vaginal or placental microbiome in association with APOs.50,51,54–56 The amount of P gingivalis is higher in the oral cavity of pregnant women who subsequently experienced PTB as compared with women with term births.47 Both P gingivalis and F nucleatum are also found in the oral cavity of periodontally healthy individuals, albeit in significantly lower numbers.55,56
Perhaps because anaerobic species from the vagina can lead to inflammation associated with bacterial vaginosis, anaerobic species from the oral cavity can lead to inflammation-mediated placental destruction and APOs. The anaerobic periodontal pathogens F nucleatum and P gingivalis are two prime candidates that could potentially induce placental inflammation and cause subsequent placental damage.
Relevance of Fusobacteria and Porphyromonas species
The periodontal pathogen F nucleatum has been frequently detected in association with APOs.57–61 Experiments using a mouse model have demonstrated biological plausibility for spread of F nucleatum to the murine placenta43 and subsequent murine PTB or stillbirth.62,63 The adhesion complexes FadA64 and Fap265 may be critical to Fusobacterium invasion of placental tissue. Higher vaginal IL-8 concentrations have been found in women with Fusobacterium infections of the amniotic fluid, suggesting Fusobacterium may also elicit an immune response that can be detrimental to fetal development.66
In comparison, P gingivalis, a low-abundance keystone pathogen, is capable of altering nutrients in the local environment in a way that shifts the composition of the microbial community.20 The microbiome is essential to the pathogenesis of P gingivalis—mediated periodontitis because germ-free mice monocolonized with P gingivalis do not develop alveolar bone loss.67
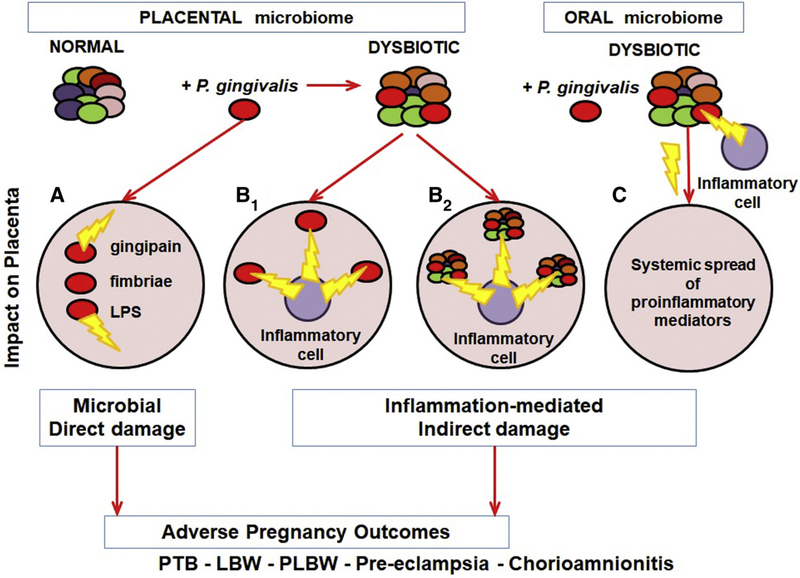
P gingivalis may similarly be capable of shifting the placental microbiome toward a more virulent profile. PLBW could be directly induced through bacterial products or through disruption the normal placental microbiome composition, resulting in damage to placental structures and nutrient transfer (Figure, A).
FIGURE. Model connecting periodontitis to APOs.
Damage to placental structures could result from direct action of P gingivalis virulence factors (A), proinflammatory cells responding to P gingivalis (B1), proinflammatory cells responding to the altered placental microbiome (B2), systemic spread of proinflammatory mediators responding to a dysbiotic oral microbiome (C), or damage to the placental structure may then lead to various APOs.
APO, adverse pregnancy outcome.
Alternatively, the more virulent placental microbiome may attract the attention of the immune system. When the balance of microbiome, trophoblast, and immune cells at implantation site is disturbed, the resulting maternal inflammatory condition has the potential to result in indirect placental collateral damage and APOs (Figure, B and C).1,68 For example, subcutaneous inoculation of BALB/c mice with P gingivalis leads to fetal growth restriction in correlation with an increase in CD4+ T-helper cells expressing interferon gamma.69,70
P gingivalis is also detrimental to fetal development in hamsters and rats71–73 but not to rabbits, despite the ability to translocate to the placenta.74,75 Systemic interferon gamma is hypothesized to be detrimental for pregnancy outcomes in multiple species, although not all studies are in agreement.76–85
As described earlier, many of the shared risk factors between periodontitis and APOs involve an increase in local or systemic inflammation. Indeed, labor it-self involves the upregulation of inflammatory mediators in a positive feedback mechanism. It is possible therefore that preterm labor may be induced by too much inflammation too early in pregnancy. Inflammation induced by periodontal pathogens or the influence of periodontal pathogens on the normal placental microbiome is consequently central to the hypothesized connection between periodontitis and APOs.
Detection of periodontal pathogens in PLBW placentas
Polymerase chain reaction
Placental tissue samples taken from women with PLBW infants yielded a higher prevalence of microorganisms than controls.86 More specifically, F nucleatum was detected in 94% of placentas from mothers with periodontitis and PLBW as compared with 36.4% of full-term placentas from mothers without periodontitis.
The same clonotype of F nucleatum was detected in the placenta of a stillborn infant and in the mouth of its mother, who experienced excessive gingival bleeding during pregnancy.63 Parvimonas micra, a known periodontal pathogen, was detected only in placentas of mothers with PLBW and was not detected in placentas of full-term mothers without periodontitis.86 Interestingly, Actinomyces israelii, a microorganism found in healthy mouths, was detected more frequently in mothers without periodontitis with full-term, normal-weight births.
Preeclampsia is an APO that has been associated with pathological inflammation.87–89 Among the genera of micro-organisms detected in placentas from preeclampsia patient were Bacillus, Variovorax, Prevotella, Porphyromonas, the proposed periodontal pathogen Dialister,56 and Lactobacillus.90
In another study, Aggregatibacter (formerly Actinobacillus) action-mycetemcomitans, F nucleatum, and P intermedia were detected only in placental samples from the preeclampsia patient group.91 P gingivalis, F nucleatum, Tannerella forsythensis, and Treponema denticola were found in significantly higher numbers in the preeclampsia patients than in controls.91 A actinomycetemcomitans and T denticola are also strongly associated with periodontitis,56,92,93 and T denticola in vaginal swabs has also been associated with PTB.47 Increased amounts of P intermedia have been implicated in the pathogenesis of pregnancy gingivitis.14–19 These studies thus demonstrate that a few select periodontal pathogens can be detected in placentas associated with APOs via polymerase chain reaction (PCR)—based methods.
Because the placenta receives most of the 10% of uterine blood flow with each stroke volume, there is the possibility that the placenta filters out microbes present in the blood like the spleen. However, oral pathogens were not detected in the maternal blood in any subjects with pre-eclampsia or controls.90 This suggests that oral microorganisms can enter the blood stream infrequently or at below detectable levels after manipulation of the gingiva with brushing or even after chewing food.43–45 Ultimately, these bacteria maybe able colonize and grow to detectable levels only upon reaching the microbial niche of the placenta.
Immunohistochemistry and immunofluorescence
As compared with PCR, immunohisto-chemistry and immunofluorescence have the advantage of being able to definitively detect whether periodontal pathogens in the placenta are transient visitors within the maternal blood vessels or whether true placental tissue invasion has occurred.
Placental sections from chorioamnionitis-affected placentas associated with PTB showed 30% more intense immunostaining for P gingivalis antigens than normal placental controls.94 A second study reported that P gingivalis antigens in the umbilical cord were associated with preeclampsia. In this study, P gingivalis in the placenta was associated with PTB and delivery by caesarean delivery but not with chorioamnionitis or preeclampsia.95 These findings collectively suggest that well-established periodontal pathogens can be detected within the cells and tissue of placentas associated with inflammation-mediated APOs and not merely within the placental blood vessels.
Ultimately though, we must interpret the PCR, immunohistochemical, and immunofluorescent data with caution because the mere presence of oral P gingivalis does not indicate presence or risk of developing periodontitis.44 Rather, periodontitis is associated with an increase in P gingivalis abundance in the oral microbiome. Perhaps it is a similar situation in the placenta, in which an increase in abundance rather than simple presence of a periodontal pathogen determines the development of APOs.96
The placental microbiome
Given that an oral microbiome is necessary for P. gingivalis to induce inflammation that drives the pathology of periodontitis, a local microbiome would likely be necessary for P. gingivalis to induce APOs. The existence of a placental microbiome is a recent, and still controversial, discovery.57–59,97–100 One side of the controversy ascertains that a unique, low-abundance, placental microbiome exists. In contrast, some believe the detection of bacterial species in placental samples represents environmental contamination and not true placental colonization.57,101–110
On the “pro-placental microbiome” side, placentas obtained from elective caesarian sections harbor distinct microbial populations in the amniotic fluid, placenta, and infant meconium (first feces). These three populations shared enough features to suggest that placental and amniotic fluid species transferred at the feto-maternal interface are the first colonizers of the infant gut.97 Among the low abundance microbes present in amniotic fluid or placenta, Proteobacteria and Entero-bacteriaceae were the most represented phyla. The most predominant genera of the Proteobacteria phyla were Enterobacter, Escheria, and Shigella. Another study verified that Proteobacteria was the most abundant placental phyla, while also reporting that the microbial composition of placentas differed between women with and without gestational diabetes mellitus.98 Additionally, more than half of placentas delivered between the 24th week of gestation and one-third of those delivered between of the 27th week harbored one or more microbial species.99 In this study, the number of microorganisms recovered declined with increasing gestational age among placentas recovered after labor in the absence of preeclampsia, whether the infant was delivered vaginally or by caesarean section.
On the other side, contamination of placental samples from environmental microbial sources has been a contentious issue when it comes to characterizing the placental microbiome.57,101–108 Some publications have even suggested that all or most sequencing reads detected from “placental microbiomes” actually represent environmental and reagent contamination.109,110 However, these studies utilized V1-V2 16s bacterial primers, which are biased towards detecting environmental contaminants.107
To illustrate this issue, one study that detected numerous species associated with the vaginal microflora in the placenta, including the Lactobacillus genera, Mycoplasma hominis, and Gardenerella vaginalis, did not discard fetal membranes.60 As fetal membranes are the placental tissues most likely to be contaminated by exposure to maternal body surfaces, we cannot determine with certainty whether these organisms represented true placental colonization or maternal microbiome contamination. Another study that detected Mycoplasma spp. and Ureaplasma spp. in placentas of spontaneous PTB infants found the same taxonomic reads in reagent and environmental control samples.110 Lastly, detection of the Lactobacillus genera has been strongly associated with vaginal but not caesarean section placentas, suggesting vaginal microbiome contamination of the placenta.
Therefore, studies that included fetal membranes or used maternal sections of the placenta such as the maternal basal plate are not considered here in this review.60,111,112 Instead, this review focuses on studies utilizing only placentas from sterile cesarean births or studies that removed the placental membranes, as these are the most likely to be contaminated with bacteria from maternal, amniotic fluid, or environmental surfaces (Supplementary Table S1). Additionally, these studies sampled areas of the placenta with a low chance of maternal microbial or blood contamination such as the placental parenchyma, or chorionic and intervillous spaces. Finally, given that the placentas were harvested and processed in a random fashion, any environmental or reagent contamination would be the same in both, term placentas and placentas associated with APOs. Thus, if we focus only on those species that vary in abundance we can remove environmental contaminant noise.
Placental Microbiome and APOs
At the phylum level, the normal placental microbiome was found to be more closely related to human non-pregnant oral microbiomes than to the vaginal, skin, or gut microbiomes (Bray-Curtis dissimilarity <0.3).57,59 However, a limitation of this data set is that the oral samples were not collected from the same pregnant mothers but from a cohort of non-pregnant subjects of the Human Microbiome Project. Thus, the periodontal pathogens in the PLBW placentas could not be directly associated with maternal periodontal disease. The placental microbiome detected within this data set was mostly composed of Firmicutes, Tenericutes, Proteobacteria, Bacteroidetes, and Fusobacteria phyla.57,59 A separate study utilizing a different patient population confirmed within the same mother that placental microbial communities are more similar to the ones found in the oral microbiome than the gut microbiome, especially at higher levels of taxonomy.107 This study utilized matched maternal oral, gut, and placental samples. Additionally, this study verified that the placental microbiome has a distinct metabolic profile. Lastly, in humans the taxa of the placental microbiome varied by gestational age between week 24 to 41 (Adonis [PERMANOVA], p = 0.001).57 This suggests that the placental microbiome also changes over time.
Chorioamnionitis-associated placentas of preterm infants universally had less diverse microbiomes.58 Such decreased species richness may be important since similar decreased diversity in the gut has been associated with inflammatory phenotypes and infection.113–116 Sequencing studies also revealed that the placental microbiome changes associated with PTB are not the same as the changes associated with LBW, suggesting a unique microbial community shift for each condition (Supplementary Table S2).
Microbiome metabolic pathways altered in APOs
In APOs, differences in bacterial metabolic pathways have been detected between term and preterm placental samples.57 Changes in the metabolic pathways of the placental microbiome may partially explain inflammation of the placenta in subjects with chorioamnionitis.58 In periodontitis, disease-associated microbial communities have highly conserved metabolic and virulence factor gene expression profiles, even though microbial species within the disease-associated microbiome were not conserved from patient to patient. Therefore, changes in the metabolic capabilities of an altered oral and an altered placental microbiome may be the drivers of both periodontitis and certain APOs. It may be more important to know which metabolic pathways are altered in dysbiotic placental microbiomes than the individual species represented.
For example, in both chronic periodontitis subgingival microbiome and the preterm placental microbiome, methane metabolism was increased.57,117 However, some pathway alterations differ between APOs and periodontitis. Butyrate or butanoate metabolism is increased in aggressive periodontitis but decreased in preterm placentas from women with excess gestational weight gain as well as being lower in term placentas without chorioamnionitis as compared with those with chorioamnionitis.58,59,118
Benzoate degradation was also decreased in term placentas without chorioamnionitis as compared with those with the condition, whereas it was increased in the subgingival plaque of chronic periodontitis patients.58,119 To date, we have very few studies with limited numbers of patients analyzing the metabolic capabilities of placental or oral microbiomes. It is therefore premature to draw definitive conclusions of specific metabolic pathways influencing APOs.
In summary, the microbiome data and preliminary metabolic data indicate that it may not be the presence of a specific periodontal pathogen in the placenta that leads to APOs. Instead, multiple keystone periodontal pathogens may be able to alter the nutrient environment in a way that leads to a disruption of the normal microbial metabolic balance and creates a more proinflammatory environment.
Discussion and conclusion
A causal relationship between periodontitis and APOs was prematurely dismissed by some authors because SRP improved periodontal outcomes but did not reduce the risk of APOs.39–42 This conclusion was predicated on the concept that oral pathogens persisting in the periodontal pocket are the sole contributors to periodontitis-associated APOs.
The evidence we presented here introduces the possibility that the placental microbiome is similar to the oral microbiome and may be amendable to colonization by oral pathogens via hematogenous spread. Clearly standard scaling and root planing treatment would not be effective at eliminating oral pathogens that have seeded the placental tissues prior to periodontal treatment.
Because periodontal pathogens are measured in the placental tissue at delivery, it is unclear exactly when the placenta is seeded. However, the failure of second-trimester therapeutic interventions to prevent APOs suggests that pathogens colonize the placenta within the first trimester. Preventing periodontal pathogens from reaching the placenta by treating periodontitis prior to pregnancy may be safer and more effective than trying to prevent APOs by treating ongoing severe periodontitis during pregnancy. Thus, while standard periodontal therapy at the second trimester is safe during pregnancy and does not increase the risk of PLBW, it should not be recommended to pregnant women with severe periodontitis as a means of preventing PLBW.1,4
As previously discussed, the mere presence of P gingivalis and F nucleatum does not necessarily imply periodontal pathology.55,96 Rather, increased frequency to levels greater than those found in the healthy state is necessary for progression to periodontitis. It is reasonable to assume that these periodontal bacteria would act similarly in a placental environment. Therefore, a key issue raised by this review is that the measure to determine the association between periodontal pathogens in the placenta and APOs should be amount and prevalence, not mere presence of such microorganisms.
There are multiple things that can be done to make future studies of the association between placental-periodontal pathogens and APOs more rigorous. First, studies need more rigorous contamination controls, in the form of collecting maternal blood, vaginal, environmental, and reagent microbiome samples simultaneously or within a short time frame of collecting the placental samples. Studies should also define a null hypothesis, specifying what results would be expected if the isolated micro-organisms represent contamination and not true placental colonization.99 Lastly, while it is difficult to distinguish contamination from true colonization at the genus or family level, it is possible to discern contamination when working at the strain level. Shotgun metagenomics, strain culture, and/or strain-directed sequencing may be utilized to make this distinction between contaminators and colonizers.
If future studies intend to prove that the placental microbiome resembles the maternal oral microbiome, maternal oral microbiome samples should also be taken within a short time frame of collecting the placental samples. Importantly, DNA-based sequencing studies should not be the sole technique used to evaluate the placental microbiome. DNA-based studies may miss important shifts in the metabolic capability of the altered microbiome that can be assessed only by transcriptomics or proteomics. Once potential pathogens of interest are identified, immunohistochemical or immunofluorescent methods should be utilized to verify whether the species detected were transients contained within maternal blood or true placental tissue residents. This is especially important if the same species can be detected in both placental tissue and maternal blood samples.
Lastly, studies should utilize the new 2017 World Workshop consensus definitions of periodontitis to allow for greater clarity within the data set and enable combination of data sets across multiple patient populations for future meta-analysis.10 It may really be that only women with severe forms of periodontitis are at risk of PLBW or would benefit the most from therapeutic intervention.
Supplementary Material
SUPPLEMENTAL TABLE 1
Sample collection method anwd adverse pregnancy outcomes
SUPPLEMENTAL TABLE 2
Changes in placental microbiome in association with APOs as compared with normal for gestational weight term births
Acknowledgments
This study was supported by the Oral Biology program and Schaffer Chair for Periodontal Research at the University of Minnesota, and NIH grants R21 DE022858 (M.C.) and R21 DE026209 (M.C.).
Footnotes
The authors report no conflict of interest.
Contributor Information
Lori A. Fischer, Division of Periodontology, Department of Developmental and Surgical, Sciences, School of Dentistry, University of Minnesota, Minneapolis MN..
Ellen Demerath, Division, of Epidemiology and Community Health, School, of Public Health, University of, Minnesota, Minneapolis MN..
Peter Bittner-Eddy, Division of Periodontology,Department of Developmental and Surgical, Sciences, School of Dentistry, University of Minnesota, Minneapolis MN..
Massimo Costalonga, Division of Periodontology, Department of Developmental and Surgical, Sciences, School of Dentistry, University of Minnesota, Minneapolis MN..
REFERENCES
- 1.Sanz M, Kornman K. Periodontitis and adverse pregnancy outcomes: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol 2013;84:S164–9. [DOI] [PubMed] [Google Scholar]
- 2.Fogacci MF, Barbirato Dda S, Amaral Cda S, et al. No association between periodontitis, preterm birth, or intrauterine growth restriction: experimental study in Wistar rats. Am J Obstet Gynecol 2016;214:749.e1–11. [DOI] [PubMed] [Google Scholar]
- 3.Fogacci MF, Cardoso EOC, Barbirato DDS, de Carvalho DP, Sansone C. No association between periodontitis and preterm low birth weight: a case-control study. Arch Gynecol Obstet 2018;297:71–6. [DOI] [PubMed] [Google Scholar]
- 4.Xiong X, Buekens P, Goldenberg RL, Offenbacher S, Qian X. Optimal timing of periodontal disease treatment for prevention of adverse pregnancy outcomes: before or during pregnancy? Am J Obstet Gynecol 2011;205: 111.e1–6. [DOI] [PubMed] [Google Scholar]
- 5.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999;4:1–6. [DOI] [PubMed] [Google Scholar]
- 6.Armitage GC. Development of a classification system for periodontal diseases and conditions. Northwest Dent 2000;79:31–5. [PubMed] [Google Scholar]
- 7.Armitage GC. Diagnosis of periodontal diseases. J Periodontol 2003;74:1237–47. [DOI] [PubMed] [Google Scholar]
- 8.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000 2004;34:9–21. [DOI] [PubMed] [Google Scholar]
- 9.Diagnosis Highfield J. and classification of periodontal disease. Aust Dent J 2009;54(Suppl 1):S11–26. [DOI] [PubMed] [Google Scholar]
- 10.Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018;89(Suppl 1): S173–82. [DOI] [PubMed] [Google Scholar]
- 11.Manau C, Echeverria A, Agueda A, Guerrero A, Echeverria JJ. Periodontal disease definition may determine the association between periodontitis and pregnancy outcomes. J Clin Periodontol 2008;35:385–97. [DOI] [PubMed] [Google Scholar]
- 12.Vettore MV, Lamarca GDEA, Leao AT, Thomaz FB, Sheiham A, Leal Mdo C. Periodontal infection and adverse pregnancy outcomes: a systematic review of epidemiological studies. Cad Saude Publica 2006;22:2041–53. [DOI] [PubMed] [Google Scholar]
- 13.Xiong X, Buekens P, Fraser WD, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG 2006;113:135–43. [DOI] [PubMed] [Google Scholar]
- 14.Hugoson A Gingival inflammation and female sex hormones. A clinical investigation of pregnant women and experimental studies in dogs. J Periodontal Res Suppl 1970;5:1–18. [PubMed] [Google Scholar]
- 15.Hugoson A Gingivitis in pregnant women. A longitudinal clinical study. Odontol Revy 1971;22:65–84. [PubMed] [Google Scholar]
- 16.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand 1963;21:533–51. [DOI] [PubMed] [Google Scholar]
- 17.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and perodontal condition. Acta Odontol Scand 1964;22:121–35. [DOI] [PubMed] [Google Scholar]
- 18.Kornman KS, Loesche WJ. The subgingival microbial flora during pregnancy. J Periodontal Res 1980;15:111–22. [DOI] [PubMed] [Google Scholar]
- 19.Tilakaratne A, Soory M, Ranasinghe AW, Corea SM, Ekanayake SL, de Silva M. Periodontal disease status during pregnancy and 3 months post-partum, in a rural population of Sri Lankan women. J Clin Periodontol 2000;27: 787–92. [DOI] [PubMed] [Google Scholar]
- 20.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol 2012;10:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal AA, Kapley A, Yeltiwar RK, Purohit HJ. Assessment of single nucleotide polymorphism at IL-1A+4845 and IL-1B+3954 as genetic susceptibility test for chronic periodontitis in Maharashtrian ethnicity. J Periodontol 2006;77:1515–21. [DOI] [PubMed] [Google Scholar]
- 22.Ari G, Cherukuri S, Namasivayam A. Epigenetics and periodontitis: a contemporary review. J Clin Diagn Res 2016;10: Ze07–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grigoriadou ME, Koutayas SO, Madianos PN, Strub JR. Interleukin-1 as a genetic marker for periodontitis: review of the literature. Quintessence Int 2010;41:517–25. [PubMed] [Google Scholar]
- 24.Huynh-Ba G, Lang NP, Tonetti MS, Salvi GE. The association of the composite IL-1 genotype with periodontitis progression and/or treatment outcomes: a systematic review. J Clin Periodontol 2007;34:305–17. [DOI] [PubMed] [Google Scholar]
- 25.Gomez LM, Sammel MD, Appleby DH, et al. Evidence of a gene-environment interaction that predisposes to spontaneous preterm birth: a role for asymptomatic bacterial vaginosis and DNA variants in genes that control the inflammatory response. Am J Obstet Gynecol 2010;202:386.e1–6. [DOI] [PubMed] [Google Scholar]
- 26.Kalish RB, Vardhana S, Gupta M, Chasen ST, Perni SC, Witkin SS. Interleukin-1 receptor antagonist gene polymorphism and multifetal pregnancy outcome. Am J Obstet Gynecol 2003;189:911–4. [DOI] [PubMed] [Google Scholar]
- 27.Kayar NA, Alptekin NO, Erdal ME. Interleukin-1 receptor antagonist gene polymorphism, adverse pregnancy outcome and periodontitis in Turkish women. Arch Oral Biol 2015;60:1777–83. [DOI] [PubMed] [Google Scholar]
- 28.Langmia IM, Apalasamy YD, Omar SZ, Mohamed Z. Interleukin 1 receptor type 2 gene polymorphism is associated with reduced risk of preterm birth. J Matern Fetal Neonatal Med 2016;29:3347–50. [DOI] [PubMed] [Google Scholar]
- 29.Macones GA, Parry S, Elkousy M, Clothier B, Ural SH, Strauss JF 3rd. A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am J Obstet Gynecol 2004;190:1504–8. discussion 3A. [DOI] [PubMed] [Google Scholar]
- 30.Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics 2006;118: 1566–73. [DOI] [PubMed] [Google Scholar]
- 31.Matthews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl Vital Stat Rep 2015;64:1–30. [PubMed] [Google Scholar]
- 32.Paige DM, Augustyn M, Adih WK, Witter F, Chang J. Bacterial vaginosis and preterm birth: a comprehensive review of the literature. J Nurse Midwifery 1998;43:83–9. [DOI] [PubMed] [Google Scholar]
- 33.Azofeifa A, Yeung LF, Alverson CJ, Beltran-Aguilar E. Dental caries and periodontal disease among US pregnant women and nonpregnant women of reproductive age, National Health and Nutrition Examination Survey, 1999–2004. J Public Health Dent 2016;76:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blencowe H, Lee AC, Cousens S, et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res 2013;74(Suppl 1): 17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hovi P, Andersson S, Jarvenpaa AL, et al. Decreased bone mineral density in adults born with very low birth weight: a cohort study. PLoS Med 2009;6:e1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konopka T, Paradowska-Stolarz A. Periodontitis and risk of preterm birth and low birthweight—a meta-analysis. Ginekol Pol 2012;83:446–53. [PubMed] [Google Scholar]
- 37.Shanthi V, Vanka A, Bhambal A, Saxena V, Saxena S, Kumar SS. Association of pregnant women periodontal status to preterm and low-birth weight babies: a systematic and evidence-based review. Dent Res J (Isfahan) 2012;9:368–80. [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim AJ, Lo AJ, Pullin DA, Thornton-Johnson DS, Karimbux NY. Scaling and root planing treatment for periodontitis to reduce preterm birth and low birth weight: a systematic review and meta-analysis of randomized controlled trials. J Periodontol 2012;83: 1508–19. [DOI] [PubMed] [Google Scholar]
- 40.Uppal A, Uppal S, Pinto A, et al. The effectiveness of periodontal disease treatment during pregnancy in reducing the risk of experiencing preterm birth and low birth weight: a meta-analysis. J Am Dent Assoc 2010;141:1423–34. [DOI] [PubMed] [Google Scholar]
- 41.Chambrone L, Pannuti CM, Guglielmetti MR, Chambrone LA. Evidence grade associating periodontitis with preterm birth and/or low birth weight: II: a systematic review of randomized trials evaluating the effects of periodontal treatment. J Clin Periodontol 2011;38:902–14. [DOI] [PubMed] [Google Scholar]
- 42.Polyzos NP, Polyzos IP, Zavos A, et al. Obstetric outcomes after treatment of periodontal disease during pregnancy: systematic review and meta-analysis. BMJ 2010;341: c7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fardini Y, Chung P, Dumm R, Joshi N, Han YW. Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Infect Immun 2010;78:1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen DW, Shapiro J, Friedman L, Kyle GC, Franklin S. A longitudinal investigation of the periodontal changes during pregnancy and fifteen months post-partum. II. J Periodontol 1971;42:653–7. [DOI] [PubMed] [Google Scholar]
- 45.Gursoy M, Pajukanta R, Sorsa T, Kononen E. Clinical changes in periodontium during pregnancy and post-partum. J Clin Periodontol 2008;35:576–83. [DOI] [PubMed] [Google Scholar]
- 46.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis 1993;16(Suppl 4):S273–81. [DOI] [PubMed] [Google Scholar]
- 47.Cassini MA, Pilloni A, Condo SG, Vitali LA, Pasquantonio G, Cerroni L. Periodontal bacteria in the genital tract: are they related to adverse pregnancy outcome? Int J Immunopathol Pharmacol 2013;26:931–9. [DOI] [PubMed] [Google Scholar]
- 48.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol 2003;189: 139–47. [DOI] [PubMed] [Google Scholar]
- 49.Flynn CA, Helwig AL, Meurer LN. Bacterial vaginosis in pregnancy and the risk of prematurity: a meta-analysis. J Fam Pract 1999;48: 885–92. [PubMed] [Google Scholar]
- 50.Holst E, Goffeng AR, Andersch B. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol 1994;32: 176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Africa CW, Nel J, Stemmet M. Anaerobes and bacterial vaginosis in pregnancy: virulence factors contributing to vaginal colonisation. Int J Environ Res Public Health 2014;11:6979–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou X, Brown CJ, Abdo Z, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. Isme j 2007;1:121–33. [DOI] [PubMed] [Google Scholar]
- 53.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001—2004; associations with symptoms, sexual behaviors, and reproductive health. SexTransm Dis 2007;34:864–9. [DOI] [PubMed] [Google Scholar]
- 54.Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett 2014;162:22–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol 1998;25: 134–44. [DOI] [PubMed] [Google Scholar]
- 56.Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol 2005;43:3944–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med 2014;6: 237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prince AL, Ma J, Kannan PS, et al. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol 2016;214:627.e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antony KM, Ma J, Mitchell KB, Racusin DA, Versalovic J, Aagaard K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am J Obstet Gynecol 2015;212:653.e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doyle RM, Alber DG, Jones HE, et al. Term and preterm labour are associated with distinct microbial community structures in placental membranes which are independent of mode of delivery. Placenta 2014;35:1099–101. [DOI] [PubMed] [Google Scholar]
- 61.Zheng J, Xiao X, Zhang Q, Mao L, Yu M, Xu J. The placental microbiome varies in association with low birth weight in full-term neonates. Nutrients 2015;7:6924–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun 2004;72:2272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han YW, Fardini Y, Chen C, et al. Term stillbirth caused by oral Fusobacterium nucleatum. Obstet Gynecol 2010;115:442–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fardini Y, Wang X, Temoin S, et al. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol Microbiol 2011;82:1468–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol 2007;179:2501–8. [DOI] [PubMed] [Google Scholar]
- 66.Hitti J, Hillier SL, Agnew KJ, Krohn MA, Reisner DP, Eschenbach DA. Vaginal indicators of amniotic fluid infection in preterm labor. Obstet Gynecol 2001;97:211–9. [DOI] [PubMed] [Google Scholar]
- 67.Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 2011;10:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mor G, Kwon JY. Trophoblast-microbiome interaction: a new paradigm on immune regulation. Am J Obstet Gynecol 2015;213:S131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin D, Smith MA, Champagne C, Elter J, Beck J, Offenbacher S. Porphyromonas gingivalis infection during pregnancy increases maternal tumor necrosis factor alpha, suppresses maternal interleukin-10, and enhances fetal growth restriction and resorption in mice. Infect Immun 2003;71:5156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin D, Smith MA, Elter J, et al. Porphyromonas gingivalis infection in pregnant mice is associated with placental dissemination, an increase in the placental Th1/Th2 cytokine ratio, and fetal growth restriction. Infect Immun 2003;71:5163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collins JG, Smith MA, Arnold RR, Offenbacher S. Effects of Escherichia coli and Porphyromonas gingivalis lipopolysaccharide on pregnancy outcome in the golden hamster. Infect Immun 1994;62:4652–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michelin MC, Teixeira SR, Ando-Suguimoto ES, Lucas SR, Mayer MP. Porphyromonas gingivalis infection at different gestation periods on fetus development and cytokines profile. Oral Dis 2012;18:648–54. [DOI] [PubMed] [Google Scholar]
- 73.Liang S, Ren H, Guo H, et al. Periodontal infection with Porphyromonas gingivalis induces preterm birth and lower birth weight in rats. Mol Oral Microbiol 2018;33: 312–21. [DOI] [PubMed] [Google Scholar]
- 74.Boggess KA, Madianos PN, Preisser JS, Moise KJ Jr, Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am J Obstet Gynecol 2005;192:554–7. [DOI] [PubMed] [Google Scholar]
- 75.Boggess KA, Price WA, Preisser JS, Moise KJ Jr, Offenbacher S. Insulin-like growth factor and interleukin-1 beta levels and subsequent fetal size in response to chronic Porphyromonas gingivalis exposure in the pregnant rabbit. Am J Obstet Gynecol 2005;193: 1219–23. [DOI] [PubMed] [Google Scholar]
- 76.Almeida A, Correia-da-Silva G, Cepa M, Bell SC, Teixeira NA. Synergistic induction of apoptosis in primary rat decidual cells by INF-gamma and TNF. Mol Reprod Dev 2007;74: 371–7. [DOI] [PubMed] [Google Scholar]
- 77.Almeria S, Araujo R, Tuo W, Lopez-Gatius F, Dubey JP, Gasbarre LC. Fetal death in cows experimentally infected with Neospora caninum at 110 days of gestation. Vet Parasitol 2010;169:304–11. [DOI] [PubMed] [Google Scholar]
- 78.Ashkar AA, Croy BA. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol 2001;13:235–41. [DOI] [PubMed] [Google Scholar]
- 79.Bell MJ, Hallenbeck JM, Gallo V. Determining the fetal inflammatory response in an experimental model of intrauterine inflammation in rats. Pediatr Res 2004;56:541–6. [DOI] [PubMed] [Google Scholar]
- 80.Chaouat G, Ledee-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining theTH1/TH2 paradigm. Int Arch Allergy Immunol 2004;134:93–119. [DOI] [PubMed] [Google Scholar]
- 81.Fujihashi K, Yamamoto M, Hiroi T, Bamberg TV, McGhee JR, Kiyono H. Selected Th1 and Th2 cytokine mRNA expression by CD4(+) T cells isolated from inflamed human gingival tissues. Clin Exp Immunol 1996;103: 422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res 2010;89:1349–63. [DOI] [PubMed] [Google Scholar]
- 83.Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J Immunol 1996;156:644–52. [PubMed] [Google Scholar]
- 84.Krishnan L, Guilbert LJ, Wegmann TG, Belosevic M, Mosmann TR. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions. Correlation with increased IFN-gamma and TNF and reduced IL-10 production by placental cells. J Immunol 1996;156: 653–62. [PubMed] [Google Scholar]
- 85.Lopez-Gatius F, Almeria S, Donofrio G, et al. Protection against abortion linked to gamma interferon production in pregnant dairy cows naturally infected with Neospora caninum. Theriogenology 2007;68:1067–73. [DOI] [PubMed] [Google Scholar]
- 86.Blanc V, O’Valle F, Pozo E, Puertas A, Leon R, Mesa F. Oral bacteria in placental tissues: increased molecular detection in pregnant periodontitis patients. Oral Dis 2015;21:905–12. [DOI] [PubMed] [Google Scholar]
- 87.Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med 2006;11:309–16. [DOI] [PubMed] [Google Scholar]
- 88.Ramma W, Ahmed A. Is inflammation the cause of pre-eclampsia? Biochem Soc Trans 2011;39:1619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sarween N, Drayson MT, Hodson J, et al. Humoral immunity in late-onset Pre-eclampsia and linkage with angiogenic and inflammatory markers. Am J Reprod Immunol 2018;80: e13041. [DOI] [PubMed] [Google Scholar]
- 90.Amarasekara R, Jayasekara RW, Senanayake H, Dissanayake VH. Microbiome of the placenta in pre-eclampsia supports the role of bacteria in the multifactorial cause of preeclampsia. J Obstet Gynaecol Res 2015;41: 662–9. [DOI] [PubMed] [Google Scholar]
- 91.Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J Periodontol 2007;78: 670–6. [DOI] [PubMed] [Google Scholar]
- 92.Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyro-sequencing. Isme J 2012;6:1176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumar PS, Griffen AL, Barton JA, Paster BJ, Moeschberger ML, Leys EJ. New bacterial species associated with chronic periodontitis. J Dent Res 2003;82:338–44. [DOI] [PubMed] [Google Scholar]
- 94.Katz J, Chegini N, Shiverick KT, Lamont RJ. Localization of P. gingivalis in preterm delivery placenta. J Dent Res 2009;88:575–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vanterpool SF, Been JV, Houben ML, et al. Porphyromonas gingivalis within placental villous mesenchyme and umbilical cord stroma is associated with adverse pregnancy outcome. PLoS One 2016;11:e0146157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Steel JH, Malatos S, Kennea N, et al. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res 2005;57:404–11. [DOI] [PubMed] [Google Scholar]
- 97.Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep 2016;6:23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng J, Xiao X, Zhang Q, et al. The placental microbiota is altered among subjects with gestational diabetes mellitus: a pilot study. Front Physiol 2017;8:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Onderdonk AB, Hecht JL, McElrath TF, Delaney ML, Allred EN, Leviton A. Colonization of second-trimester placenta parenchyma. Am J Obstet Gynecol 2008;199:52.e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aagaard KM. Author response to comment on “the placenta harbors a unique microbiome.” Sci Transl Med 2014;6:254lr3. [DOI] [PubMed] [Google Scholar]
- 101.Wassenaar TM, Panigrahi P. Is a foetus developing in a sterile environment? Lett Appl Microbiol 2014;59:572–9. [DOI] [PubMed] [Google Scholar]
- 102.Walker RW, Clemente JC, Peter I, Loos RJF. The prenatal gut microbiome: are we colonized with bacteria in utero? Pediatr Obes 2017;12(Suppl 1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pelzer E, Gomez-Arango LF, Barrett HL, Nitert MD. Review: maternal health and the placental microbiome. Placenta 2017;54:30–7. [DOI] [PubMed] [Google Scholar]
- 104.Willyard C Could baby’s first bacteria take root before birth? Nature 2018;553:264–6. [DOI] [PubMed] [Google Scholar]
- 105.Hornef M, Penders J. Does a prenatal bacterial microbiota exist? Mucosal Immunol 2017;10:598–601. [DOI] [PubMed] [Google Scholar]
- 106.Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 2017;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Nitert MD. Contributions of the maternal oral and gut microbiome to placental microbial colonization in overweight and obese pregnant women. Sci Rep 2017;7:2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kliman HJ. Comment on “the placenta harbors a unique microbiome.” Sci Transl Med 2014;6:254le4. [DOI] [PubMed] [Google Scholar]
- 109.Lauder AP, Roche AM, Sherrill-Mix S, et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 2016;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leon LJ, Doyle R, Diez-Benavente E, et al. Enrichment of clinically relevant organisms in spontaneous preterm delivered placenta and reagent contamination across all clinical groups in a large UK pregnancy cohort. Appl Environ Microbiol 2018;84(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stout MJ, Conlon B, Landeau M, et al. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol 2013;208: 226.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parnell LA, Briggs CM, Cao B, Delannoy-Bruno O, Schrieffer AE, Mysorekar IU. Microbial communities in placentas from term normal pregnancy exhibit spatially variable profiles. Sci Rep 2017;7:11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006;55:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lepage P, Hasler R, Spehlmann ME, et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 2011;141:227–36. [DOI] [PubMed] [Google Scholar]
- 115.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 2007;104:13780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jeffery IB, O’Toole PW, Ohman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012;61:997–1006. [DOI] [PubMed] [Google Scholar]
- 117.Kirst ME, Li EC, Alfant B, et al. Dysbiosis and alterations in predicted functions of the subgingival microbiome in chronic periodontitis. Appl Environ Microbiol 2015;81:783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jorth P, Turner KH, Gumus P, Nizam N, Buduneli N, Whiteley M. Metatranscriptomics of the human oral microbiome during health and disease. MBio 2014;5:e01012–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu B, Faller LL, Klitgord N, et al. Deep sequencing of the oral microbiome reveals signatures of periodontal disease. PLoS One 2012;7:e37919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes. The National Academies Collection: reports funded by National Institutes of Health In: Behrman RE, Butler AS, eds. Preterm birth: causes, consequences, and prevention. Washington (DC): National Academies Press, National Academy of Sciences; 2007. [Google Scholar]
- 121.AlJehani YA. Risk factors of periodontal disease: review of the literature. Int J Dent 2014;2014:182513. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122.Eke PI, Wei L, Thornton-Evans GO, et al. Risk indicators for periodontitis in US adults: NHANES 2009 to 2012. J Periodontol 2016;87: 1174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Saini GK, Gupta ND, Prabhat KC. Drug addiction and periodontal diseases. J Indian Soc Periodontol 2013;17:587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000 2013;62: 59–94. [DOI] [PubMed] [Google Scholar]
- 125.Kumar PS. From focal sepsis to periodontal medicine: a century of exploring the role of the oral microbiome in systemic disease. J Physiol 2017;595:465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000 1994;5:78–111. [DOI] [PubMed] [Google Scholar]
- 127.Haffajee AD, Socransky SS, Patel MR, Song X. Microbial complexes in supragingival plaque. Oral Microbiol Immunol 2008;23: 196–205. [DOI] [PubMed] [Google Scholar]
- 128.Mdala I, Olsen I, Haffajee AD, Socransky SS, Thoresen M, de Blasio BF. Comparing clinical attachment level and pocket depth for predicting periodontal disease progression in healthy sites of patients with chronic periodontitis using multi-state Markov models. J Clin Periodontol 2014;41:837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol 2000;27: 648–57. [DOI] [PubMed] [Google Scholar]
- 130.Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA. Accuracy of NHANES periodontal examination protocols. J Dent Res 2010;89:1208–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL TABLE 1
Sample collection method anwd adverse pregnancy outcomes
SUPPLEMENTAL TABLE 2
Changes in placental microbiome in association with APOs as compared with normal for gestational weight term births