Abstract
Objectives:
We hypothesized that an increased duration of donor brain death may worsen survival following orthotropic heart transplantation.
Methods:
The United Network for Organ Sharing (UNOS) registry was queried for first-time, adult recipients of heart transplant from 2006–2018. Cox Proportional Hazards with penalized smooth splines was used to stratify patients based on donor brain death interval: shorter (<22 hours), reference (22–42 hours), and longer (>42 hours). Overall survival was estimated using Kaplan-Meier and Cox Proportional Hazards models.
Results:
A total of 22,960 patients met study criteria (9.2% shorter, 55.0% reference, 35.8% longer). Longer brain death duration recipients were more likely to have a later year of transplant and have a mechanical bridge to transplant, while longer duration donors were more likely to be Black and die of anoxia compared to shorter duration and reference donors. Compared to reference, neither shorter (Hazard Ratio [HR] 1.02; 95% Confidence Interval [CI] 0.94–1.12) nor longer donor brain death interval (HR 1.01; 95%CI 0.94–1.08) was associated with post-transplant survival in either unadjusted or multivariable analyses (both p>0.6).
Conclusions:
Longer duration of brain death was not associated with worse survival following heart transplantation. Donors with prolonged interval of brain death should not necessarily be excluded based on brain death period alone.
Graphical Abstract
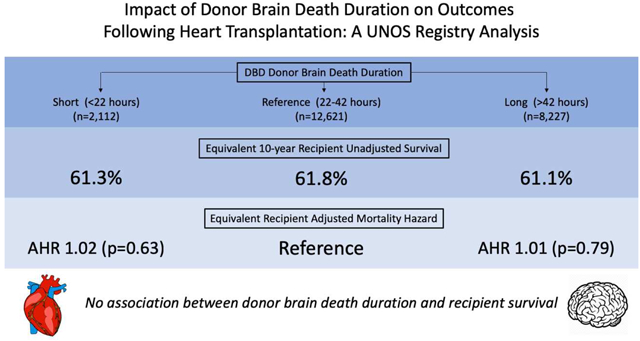
Legend: No association between donor brain death duration and recipient survival. DBD, donation after brain death; AHR, adjusted hazard ratio.
Central Message
Donor brain death duration is not associated with a difference in survival following heart transplantation. Potential donors should not be refused evaluation solely because of prolonged brain death.
Background
In the past decade, there has been a 57% increase in patients listed for heart transplantation, with a 28% decrease in the rate of transplantation1. There are currently 3800 patients on the waiting list, with an estimated 51% of patients receiving a transplant within one year of listing1,2. In an effort to expand the donor pool, increasingly liberal criteria have been applied to evaluation of potential donor organs, though only about a third of donor organ offers are accepted3. In the United States, only donors after brain death (DBD) are accepted for heart transplantation. However, brain death has been associated with several deleterious changes, including autonomic ‘storm’ resulting in hemodynamic instability, inflammatory cascade activation, leukocyte infiltration of the donor organ, endocrine dysregulation, and increased apoptosis and necrosis4–12. These changes have been associated with ischemia-reperfusion injury, rejection, allograft dysfunction, and worse survival in transplant recipients in animal models and observational studies6,10,12–15. In rat models of DBD heart transplantation, cardiac allografts demonstrate subendocardial ischemia, myocytolysis, leukocyte infiltration, and cytokine activation5,7.
While brain death is associated with substantial physiologic changes through multiple mechanisms, it is unclear whether the duration of donor brain death is a significant factor in recipient allograft function. There are no guidelines on the safe duration of donor brain death prior to heart transplantation, and there is a paucity of literature largely limited to single-institution retrospective cohort studies examining the relationship between donor brain death duration and outcomes after heart transplantation, with conflicting results16–18. The majority of these prior studies suggest an association between longer donor brain death duration and poorer recipient outcomes. We used the United Network for Organ Sharing (UNOS) registry to examine the association between interval of brain death and outcomes. We hypothesized that a longer brain death interval would result in worse overall survival following heart transplantation.
Methods
UNOS Registry
The UNOS database provides prospectively collected donor and recipient information for all organ transplants performed in the US. UNOS also separately provided a file containing data about donor brain death and cross clamp time for 98.8% of heart transplant donors since 2006. The study was deemed exempt by our Institutional Review Board.
Patient Selection
The UNOS database was interrogated for all first-time, adult heart transplant recipients between 2006 and 2018 along with their respective donors. Exclusion criteria included transplant donors with missing time of brain death or cross clamp and recipients undergoing multi-organ transplant. In an effort mitigate potential data entry error, donors with brain death duration less than one hour or greater than the 99th percentile were excluded (n=239).
Data Analysis
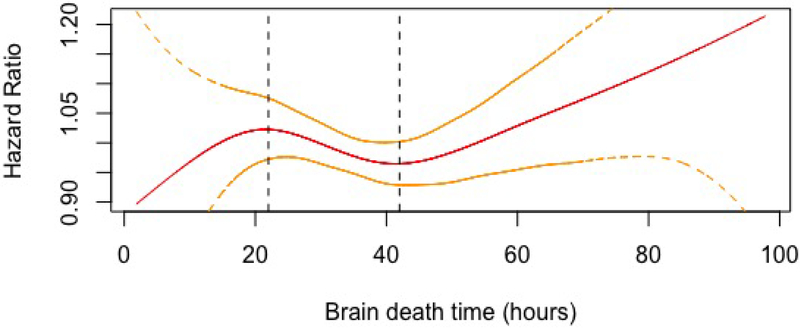
The duration of donor brain death was defined as the difference, in hours, between declaration of donor brain death and subsequent cross clamp prior to organ procurement. The effect of donor brain death duration on recipient survival post-transplant was modeled with Cox Proportional Hazards with penalized smooth splines. The use of splines is a validated technique that uses smoothly joined polynomial functions in regression models while adjusting for covariates without assuming linearity19,20. Covariates were selected a priori based on clinical experience and included donor age, sex, race, ischemic time, brain death duration, preprocurement steroid and T4 administration, and recipient age, sex, race, BMI, history of diabetes, IV antibiotic requirement within two weeks, disposition at transplant, year of transplant, mechanical circulatory support (both durable and temporary extracorporeal devices), and inotrope requirement. The model was used to then define brain death duration intervals for subsequent analyses based on prior similar studies21,22.
Based on the qualitatively defined inflection points from the Cox model, donor brain death duration was divided into three intervals: SH (<22 hours), reference (22–42 hours), and LG (>42 hours). The primary outcome was recipient overall survival with a secondary outcome of acute rejection. Comparisons between cohorts were performed using the Wilcoxon rank sum test for continuous variables and the Pearson chi-squared test or Fisher’s exact test for categorical variables. Unadjusted survival was estimated with the Kaplan-Meier method, and compared with the log-rank test. Cox Proportional Hazards models along with logistic regression models were used to identify independent predictors of survival. Donor brain death was modeled as a categorical variable divided into three intervals as above. Complete case analyses were performed, with 259 cases (1.1%) excluded due to missing covariates. To confirm consistency of results, four independent sensitivity analyses were performed using both unadjusted and adjusted methods. These included (1) restricting the analysis to donors with a brain death duration <5th percentile or greater than the 95th percentile, (2) stratifying the donor brain death interval into categories based upon distribution quartiles, (3) performing Cox Proportional Hazards regression with brain death duration modeled as a continuous variable using restricted cubic splines (RCS), as well as (4) repeating the analysis following propensity matching based upon recipient baseline characteristics. Propensity matching was performed in a 1:1 fashion using the nearest neighbor algorithm. A p value ≤ 0.05 was considered statistically significant. All analyses were performed with R version 3.5.1 (Vienna, Austria).
Results
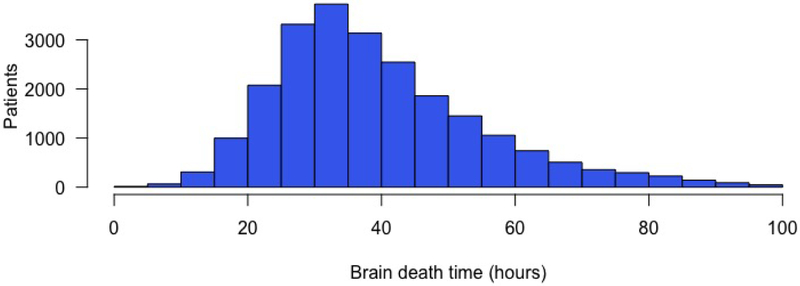
A total of 22,960 donor-recipient pairs met study criteria, of whom 2,112 (9.2%) were classified as SH, 12,621 (55.0%) as reference, and 8,227 (35.8%) as LG (Figures 1–2). Baseline characteristics of recipients are presented in Table 1. Recipients stratified by donor brain death duration differed with regard to majority of studied factors including gender, age, ethnicity, heart failure etiology, pre-transplant status and medical condition, as well as waiting list duration, waiting list status, and transplant year (p<0.05). Compared with SH and reference recipients, LG recipients tended to have a later year of transplant (median year 2015 vs 2011 – reference & 2009 - SH), were more likely to be bridged to transplant with a durable ventricular assist device (46.4% vs 40.6% - reference & 34.7% - SH, p<0.001), were more likely to be status 1A prior to transplant (62.5% vs 57.3% - reference & 55.4% - SH, p<0.001), and were less likely to have a diagnosis of ischemic cardiomyopathy (31.1% vs 34.5% - reference & 37.4% - SH, p < 0.001).
Figure 1.
Distribution of heart transplant donor brain death duration
Figure 2.
Cox Proportional Hazard analysis of donor brain death duration with penalized smooth splines. 95% confidence interval depicted by gold lines. Approximate inflection points identified with vertical dotted lines used to separate population into three cohorts for subsequent analyses.
Table 1.
Recipient baseline characteristics stratified by donor brain death duration
| Time from brain death to cross clamp | ||||
|---|---|---|---|---|
| Variable | < 22 hours | 22–42 hours | > 42 hours | p-value |
| (n=2,112) | (n=12,621) | (n=8,227) | ||
| Male gender | 78.6% (1,660) | 74.5% (9,400) | 73.9% (6,079) | < 0.001 |
| Age (median, IQR) | 55 (16) | 56 (16) | 57 (16) | < 0.001 |
| BMI | 27.1 (7.1) | 27.1 (6.9) | 26.9 (6.9) | 0.224 |
| Ethnicity | < 0.001 | |||
| White | 70.4% (1,487) | 68.1% (8,594) | 64.5% (5,308) | |
| Black | 19.4% (410) | 21.1% (2,660) | 20.3% (1,668) | |
| Hispanic | 6.9% (145) | 7.2% (904) | 9.6% (789) | |
| Other | 3.3% (70) | 3.7% (463) | 5.6% (462) | |
| Recipient history | ||||
| Diabetes | 30.3% (639) | 27.6% (3,488) | 26.6% (2,186) | 0.003 |
| Malignancy | 7.1% (151) | 7.6% (955) | 8.2% (672) | 0.159 |
| Cerebrovascular disease | 5.4% (114) | 5.6% (701) | 5.3% (439) | 0.788 |
| Recipient heart failure etiology | < 0.001 | |||
| Ischemic | 37.4% (790) | 34.5% (4,351) | 31.1% (2,562) | |
| Non-ischemic dilated | 47.1% (995) | 50.2% (6,330) | 52.3% (4306) | |
| Other | 15.5% (327) | 15.4% (1,940) | 16.5% (1,359) | |
| Recipient creatinine (median, IQR) | 1.2 (0.5) | 1.2 (0.5) | 1.2 (0.5) | < 0.001 |
| Recipient bilirubin (median, IQR) | 0.8 (0.7) | 0.8 (0.7) | 0.7 (0.6) | < 0.001 |
| Pre-transplant status | 0.039 | |||
| Intensive care unit | 30.3% (639) | 28.5% (3,595) | 28.0% (2,305) | |
| Hospitalized (non-ICU) | 16.8% (354) | 15.2% (1,922) | 15.9% (1,311) | |
| Not hospitalized | 53.0% (1,119) | 56.3% (7,104) | 56.0% (4,611) | |
| Medical therapy | ||||
| IV antibiotics in two weeks before transplant | 9.8% (208) | 10.6% (1,343) | 9.7% (796) | 0.067 |
| IV inotropes prior to transplant | 45.9% (970) | 39.1% (4,939) | 35.6% (2,925) | < 0.001 |
| Ventilator support prior to transplant | 2.0% (42) | 1.6% (201) | 0.9% (74) | < 0.001 |
| IABP prior to transplant | 6.5% (137) | 6.2% (784) | 5.7% (467) | 0.191 |
| ECMO support prior to transplant | 0.8% (16) | 0.6% (72) | 0.5% (45) | 0.514 |
| VAD prior to transplant | 34.7% (732) | 40.6% (5,127) | 46.4% (3,818) | < 0.001 |
| ABO blood type | 0.008 | |||
| A | 43.8% (926) | 40.9% (5,168) | 39.6% (3,257) | |
| B | 14.0% (295) | 14.3% (1,809) | 15.2% (1,247) | |
| AB | 4.9% (103) | 5.9% (749) | 5.6% (459) | |
| O | 37.3% (788) | 38.8% (4,895) | 39.7% (3,264) | |
| Days on waitlist (median, IQR) | 76 (189) | 92 (226) | 100 (264) | < 0.001 |
| Waitlist status at transplant | < 0.001 | |||
| 1A | 55.4% (1,170) | 57.3% (7,238) | 62.5% (5,141) | |
| 1B | 37.6% (794) | 36.5% (4,602) | 31.4% (2,584) | |
| 2 | 7.0% (148) | 6.2% (780) | 6.1% (502) | |
| Year of transplant (median, IQR) | 2009 (5) | 2011 (5) | 2015 (4) | < 0.001 |
| Acute rejection prior to discharge | 18.3% (386) | 18.7% (2,357) | 18.3% (1,503) | 0.734 |
| Treated for rejection within 1 year* | 24.3% (432) | 21.2% (2,170) | 18.3% (1,043) | < 0.001 |
of transplants performed prior to 2017; IQR, interquartile range; BMI, body mass index; ICU, intensive care unit; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; VAD, ventricular assist device
Donor characteristics are summarized in Table 2. Donor cohorts differed with respect to gender, median age, ethnicity, medical history, use of prerecovery steroids, T4, and cause of death (p<0.05). Compared with SH brain death duration grafts, reference and LG brain death duration was associated with a slightly longer median graft ischemic time (3.2 vs 3.0 hours, p<0.001). Donor ejection fraction (EF) was similar between the three donor cohorts (median 60%).
Table 2.
Donor baseline characteristics stratified by brain death duration
| Time from brain death to cross clamp | ||||
|---|---|---|---|---|
| Variable | < 22 hours | 22–42 hours | > 42 hours | p-value |
| (n=2,112) | (n=12,621) | (n=8,227) | ||
| Male gender | 73.9% (1,560) | 71.0% (8,958) | 70.2% (5,777) | 0.004 |
| Donor age (median, IQR) | 29 (19) | 30 (19) | 30 (19) | < 0.001 |
| Donor BMI (median, IQR) | 26.3 (6.7) | 26.3 (7.1) | 26.2 (7.0) | 0.943 |
| Donor ethnicity | < 0.001 | |||
| White | 76.9% (1,625) | 68.9% (8,691) | 56.2% (4,627) | |
| Black | 11.2% (236) | 15.7% (1,983) | 17.8% (1,462) | |
| Hispanic | 9.9% (21p) | 12.9% (1,633) | 22.0% (1,814) | |
| Other | 1.9% (41) | 2.5% (314) | 3.9% (324) | |
| Donor history | ||||
| Cigarette use | 15.9% (336) | 14.6% (1,845) | 11.2% (923) | < 0.001 |
| Cocaine use | 16.1% (339) | 16.4% (2,071) | 18.5% (1,518) | < 0.001 |
| Alcohol abuse | 14.0% (295) | 15.6% (1,970) | 16.6% (1,367) | 0.008 |
| Diabetes | 2.6% (55) | 3.4% (435) | 3.4% (277) | 0.135 |
| Hypertension | 12.0% (254) | 14.8% (1,862) | 15.7% (1,291) | < 0.001 |
| Cancer | 1.5% (32) | 1.5% (192) | 1.4% (118) | 0.875 |
| Donor creatinine (median, IQR) | 1.0 (0.6) | 1.0 (0.6) | 1.0 (0.7) | < 0.001 |
| Donor bilirubin (median, IQR) | 0.8 (0.9) | 0.7 (0.7) | 0.7 (0.7) | < 0.001 |
| Donor sodium (median, IQR) | 147 (11) | 148 (12) | 148 (11) | < 0.001 |
| Donor ejection fraction (%, median, IQR) | 60 (10) | 60 (10) | 60 (8) | < 0.001 |
| Prerecovery steroids | 80.2% (1,693) | 76.4% (9,646) | 70.2% (5,774) | < 0.001 |
| Prerecovery T4 | 71.7% (1,515) | 74.0% (9,342) | 67.3% (5,537) | < 0.001 |
| Donor cause of death | < 0.001 | |||
| Anoxia | 20.7% (437) | 22.1% (2,787) | 26.9% (2,216) | |
| Cerebrovascular/stroke | 19.6% (415) | 20.5% (2,588) | 19.7% (1,623) | |
| Head trauma | 57.0% (1,203) | 54.6% (6,891) | 50.3% (4,141) | |
| CNS tumor | 0.6% (13) | 0.6% (80) | 0.6% (50) | |
| Other | 2.1% (44) | 2.2% (275) | 2.4% (197) | |
| ABO blood type | 0.059 | |||
| A | 38.7% (818) | 36.0% (4,549) | 35.5% (2,922) | |
| B | 10.4% (220) | 11.1% (1,407) | 10.9% (899) | |
| AB | 2.2% (46) | 2.4% (308) | 2.1% (169) | |
| O | 48.7% (1,028) | 50.4% (6,357) | 51.5% (4,237) | |
| Graft ischemic time (median hours, IQR) | 3.0 (1.4) | 3.2 (1.4) | 3.2 (1.4) | < 0.001 |
IQR, interquartile range; CNS, central nervous system
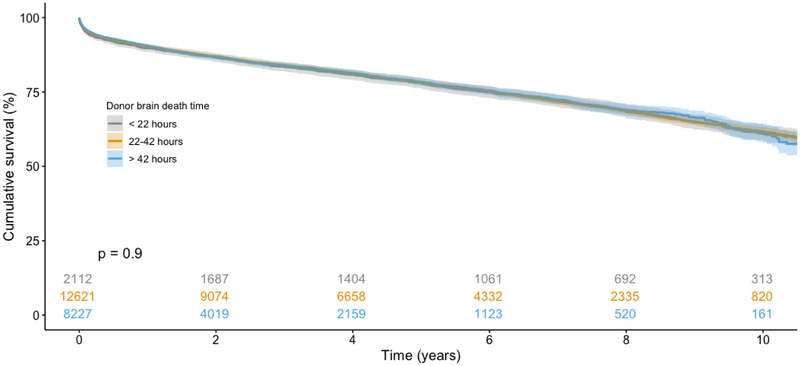
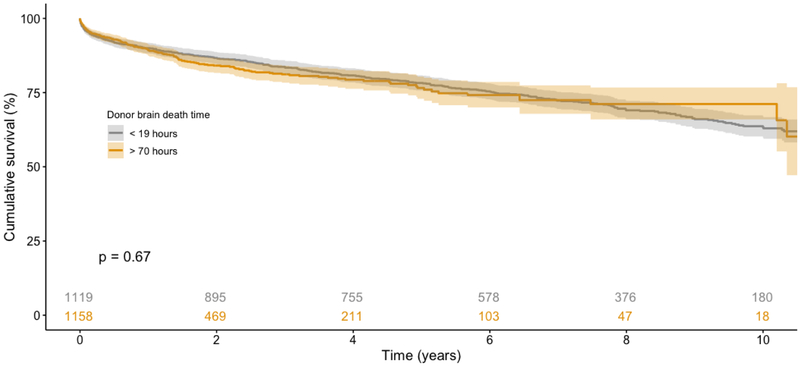
On unadjusted analysis of survival using the Kaplan-Meier method (Figure 3), recipients from all three cohorts had similar post-transplant survival (p=0.9). The adjusted Cox Proportional Hazards model for recipient survival is summarized in Table 3. Compared with the brain death duration reference interval of 22–42 hours, neither SH (HR 1.02, 95% CI 0.94–1.12) nor LG (HR 1.01, 95% CI 0.94–1.08) brain death duration intervals were associated with increased or decreased recipient survival. A sensitivity analysis was subsequently performed with donor brain death duration divided into quartiles based upon distribution. Compared with the shortest interval of less than 29 hours, brain death for 29–36 hours (HR 0.98, 95% CI 0.91–1.05), 36–48 hours (HR 0.93, 95% CI 0.86–1.01), and greater than 48 hours (HR 1.02, 95% CI 0.94–1.12) all did not predict improved or worsened recipient survival. An additional sensitivity analysis was performed restricted to donors with a brain duration less than the 5th percentile (<19 hours) or greater than the 95th percentile (> 70 hours). On Kaplan-Meier analysis (Figure 4), recipients again had similar survival (p=0.67). When modeled as a continuous variable using restricted cubic splines (RCS) in an adjusted Cox Proportional Hazards model, donor brain death duration once again was not independently associated with survival. After performing 1:1 propensity matching of SH and LG cohorts based upon recipient characteristics, donor brain death duration again did not independently predict recipient survival (Supplementary Tables 1–2).
Figure 3.
Kaplan-Meier analysis of recipient survival stratified by donor brain death duration. No survival difference observed between cohorts (p=0.9).
Table 3.
Cox Proportional Hazards regression analysis for post-transplant mortality
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Hazard Ratio | Lower | Upper | p-value |
| Donor/graft characteristics | ||||
| Age (per year) | 1.01 | 1.01 | 1.01 | < 0.001 |
| Male gender (vs female) | 0.96 | 0.90 | 1.03 | 0.232 |
| Ethnicity | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 1.09 | 1.00 | 1.17 | 0.043 |
| Hispanic | 1.05 | 0.97 | 1.14 | 0.237 |
| Other | 1.26 | 1.08 | 1.47 | 0.004 |
| Preprocurement steroids | 1.01 | 0.95 | 1.08 | 0.733 |
| Preprocurement T4 | 1.00 | 0.94 | 1.06 | 0.954 |
| Ischemic time (per hour) | 1.11 | 1.08 | 1.14 | < 0.001 |
| Brain death time | ||||
| 22–42 hours | Ref | Ref | Ref | Ref |
| < 22 hours | 1.02 | 0.94 | 1.12 | 0.627 |
| > 42 hours | 1.01 | 0.94 | 1.08 | 0.792 |
| Recipient characteristics | ||||
| Age (per year) | 1.00 | 1.00 | 1.01 | < 0.001 |
| Male gender (vs female) | 1.00 | 0.94 | 1.08 | 0.896 |
| Ethnicity | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 1.27 | 1.18 | 1.36 | < 0.001 |
| Hispanic | 0.99 | 0.89 | 1.10 | 0.817 |
| Other | 0.96 | 0.83 | 1.12 | 0.634 |
| BMI | 1.01 | 1.01 | 1.02 | < 0.001 |
| Diabetes | 1.23 | 1.15 | 1.31 | < 0.001 |
| VAD at transplant | 1.16 | 1.08 | 1.24 | < 0.001 |
| IV inotropes at transplant | 1.00 | 0.93 | 1.07 | 0.974 |
| IV antibiotics in two weeks before transplant | 1.21 | 1.11 | 1.32 | < 0.001 |
| Pre-transplant recipient status | ||||
| Intensive care unit | Ref | Ref | Ref | Ref |
| Hospitalized (non-ICU) | 0.94 | 0.86 | 1.02 | 0.135 |
| Not hospitalized | 0.84 | 0.79 | 0.91 | < 0.001 |
| Year of transplant (per year) | 0.98 | 0.97 | 0.99 | < 0.001 |
BMI, body mass index; VAD, ventricular assist device; ICU, intensive care unit
Figure 4.
Kaplan-Meier analysis of recipient survival, restricted to donors with brain death duration <5th percentile or >95th percentile, stratified by donor brain death duration. No survival difference observed between cohorts (p=0.67).
Both unadjusted and adjusted logistic regression was performed to evaluate the association between donor brain death interval and post-transplant allograft rejection (Table 4). While there was no significant association observed on unadjusted analysis between brain death interval and the incidence of acute rejection prior to discharge (p>0.05), compared with the reference interval, LG interval recipients were less likely to experience acute rejection prior to discharge (AOR 0.84, 95% CI 0.78–0.91) when adjusting for clinical and demographic covariates. Similarly, on both unadjusted and adjusted logistic regression, LG brain death interval was associated with decreased rates of treatment for rejection within 1 year (AOR 0.85, 95% CI 0.77–0.92) while SH brain death interval was associated with increased 1-year rejection (AOR 1.21, 95% CI 1.07–1.37).
Table 4.
Unadjusted and adjusted logistic regression analysis of recipient acute rejection
| Unadjusted | Adjusted* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% Confidence Interval | 95% Confidence Interval | ||||||||
| Outcome | Brain death time | Odds Ratio | Lower | Upper | p-value | Odds Ratio | Lower | Upper | p-value |
| Acute rejection episode prior to discharge | 24–60 hours | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| < 24 hours | 0.97 | 0.86 | 1.1 | 0.663 | 1.1 | 0.97 | 1.24 | 0.126 | |
| > 60 hours | 0.97 | 0.91 | 1.05 | 0.461 | 0.84 | 0.78 | 0.91 | < 0.001 | |
| Treated for rejection within 1 year | 24–60 hours | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| < 24 hours | 1.2 | 1.06 | 1.35 | 0.003 | 1.21 | 1.07 | 1.37 | 0.002 | |
| > 60 hours | 0.83 | 0.77 | 0.90 | < 0.001 | 0.85 | 0.77 | 0.92 | < 0.001 | |
covariates from multivariable model in Table 3
Discussion
In this UNOS registry analysis, we found no association between increased donor brain death duration and recipient survival following heart transplantation. This finding persisted after utilizing multivariable models to account for differences in baseline donor and recipient characteristics between groups. The existing literature reveals conflicting data about the effect of donor brain death interval on outcomes in heart transplantation. There are few single-institution retrospective cohort studies addressing this question. Cantin and colleagues reviewed 475 heart transplant patients at Stanford and found that brain death duration over 72 hours was associated with decreased one- and five-year survival compared to less than 72 hours18. Ramjug and colleagues analyzed 157 patients undergoing heart transplant and found that longer brain death interval was associated with poor survival (HR 1.15)17. In a study of 215 consecutive heart transplants, Marasco and colleagues found that brain death duration was not associated with rejection or mortality, but that primary graft dysfunction (PGD) occurred less with a shorter donor brain death interval23. Similar observational studies in kidney and lung transplant patients have demonstrated equivocal findings, with increased brain death duration associated with reduced PGD in kidney transplant24 and no change in survival in lung transplant16,25. These studies, however, are limited by their smaller number of patients and less systematic definitions of ‘long’ and ‘short’ brain death intervals. In contrast, our study reports the results of close to 23,000 patients undergoing heart transplant using statistically rigorous methods to categorize brain death interval.
Small animal models comparing brain dead and living donors have revealed that brain death is associated with cytokine and chemokine activation, decreased hormone synthesis, autonomic storm and consequent hemodynamic instability, inflammatory and immune activation along with leukocyte infiltration of donor organ, increased apoptosis, and necrosis4–10,12,14,15,26. These changes have, in turn, been associated with ischemia-reperfusion injury, acute and chronic rejection, PGD, and worse survival in DBD compared to living donor transplants. While brain death is clearly associated with multiple deleterious effects in the donor, we found that the duration of brain death does not worsen survival in recipients of heart transplantation.
There are several possible explanations for this finding. Increased brain death duration may enable hemodynamic recovery from the autonomic and endocrine changes that occur immediately after brain death, thereby mitigating the damage to the donor heart. For instance, Wilhelm and colleagues found myocardial necrosis following donor brain death in a rat model of heart transplant, which they hypothesized was induced by catecholamine-related coronary vasospasm7. Due to autoregulatory mechanisms and critical care, donors likely regain hemodynamic stability shortly after brain death, ensuring donor cardiac myocyte necrosis is restricted to the time of the initial insult. Similarly, the inflammatory and immune activation that occurs immediately after brain death likely also results in rapid injury to the donor organ, but then reaches a threshold or even decays. This may be reflected by the increased usage of prerecovery steroids among short interval brain death donors in the present study. A peak in immune activation shortly after brain death may result in receptor saturation, preventing further injury, and donor immune cells likely undergo apoptosis and necrosis as well, attenuating the immune response with time. Avlonatis and colleagues, in a rat model of lung transplant, found that inflammatory markers were expressed similarly in lungs retrieved early compared to late after brain death, but that lung function, expressed by pulmonary vascular resistance, actually improved with late retrieval26. These time-dependent changes to immune and inflammatory activation likely also account for the reduced incidence of acute and subacute rejection we found patients with longer donor death intervals.
Our study has several limitations. It is a retrospective cohort analysis and therefore subject to confounding factors that we cannot altogether adjust for. For example, we used a proxy calculation to represent donor brain death interval, subtracting the time of cross clamp from the time of declaration of brain death. However, donors may have been brain dead for a variable period of time prior to declaration, which might confound our analysis. An additional potential confounder is the reported practice of organ procurement organizations (OPO) sometimes delaying the organ procurement process after poor cardiac function is observed, providing additional time to allow for organ recovery but also extending the brain death period. Additionally, only brain-dead donors whose organs were successfully transplanted were used in this analysis, introducing significant potential for selection bias. We cannot make any conclusions about brain death interval-related changes that might have rendered donor hearts unsuitable for transplantation or led to functional deterioration prompting allograft discard. As such, there may exist a cohort of donor allografts with prolonged brain death duration that would have resulted in differential recipient outcomes if transplanted. We also excluded from analysis a subset of patients with missing cross clamp or donor brain death declaration time, and the missing data might affect our findings in ways that we cannot estimate. Similarly, our study is limited by variables available in the UNOS registry. PGD, for instance, is not reliably coded in the registry and consequently could not be used as an endpoint in our analysis, although this is of clinical interest. In addition, donor serum sodium as well as hormonal replacement are not coded, which would have helped strengthen the study. Nevertheless, the UNOS registry contains information about 100% of organ transplants performed in the US and therefore serves as a robust source of data.
Conclusion
In this study of the UNOS registry, increased duration of donor brain death was not associated with a difference in survival following orthotropic heart transplantation. Brain dead donors for heart transplant should not be refused evaluation expressly because of prolonged brain death interval.
Supplementary Material
Perspective Statement.
Donor brain death has been associated with multiple deleterious physiologic changes including autonomic storm and inflammatory cascade activation, among others. Multiple animal model studies have demonstrated an impact of donor brain death duration on recipient allograft function. There is a paucity of published literature examining this association in adult heart transplantation, however.
Acknowledgements
The authors would like to thank Dr. Hanghang Wang for contributing her statistical expertise to this project. Dr. Jawitz was supported by a NIH T-32 grant 5T32HL069749 in clinical cardiovascular research. The authors have no disclosures relevant to the presented work. This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Funding for this study was provided by NIH T-32 grant 5T32HL069749
Glossary of abbreviation
- UNOS
United Network for Organ Sharing
- DBD
Donation after brain death
- BMI
Body mass index
- PGD
Primary graft dysfunction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no relevant conflicts of interest
References
- 1.OPTN/SRTR 2016. Annual Data Report: Heart - Colvin - 2018 - American Journal of Transplantation - Wiley Online Library. https://onlinelibrary.wiley.com/doi/full/10.1111/ajt.14561. Accessed December 20, 2018.
- 2.Waiting list candidates by organ type. UNOS. https://unos.org/data/transplant-trends/waiting-list-candidates-by-organ-type/. Published May 11, 2015. Accessed December 20, 2018.
- 3.Mancini D, Goldstein D, Taylor S, et al. Maximizing donor allocation: A review of UNOS region 9 donor heart turn-downs. American Journal of Transplantation. 2017;17(12):3193–3198. doi: 10.1111/ajt.14499 [DOI] [PubMed] [Google Scholar]
- 4.van der Hoeven JAB, Molema G, Ter Horst GJ, et al. Relationship between duration of brain death and hemodynamic (in)stability on progressive dysfunction and increased immunologic activation of donor kidneys. Kidney Int. 2003;64(5):1874–1882. doi: 10.1046/j.1523-1755.2003.00272.x [DOI] [PubMed] [Google Scholar]
- 5.Pratschke J, Wilhelm MJ, Kusaka M, et al. BRAIN DEATH AND ITS INFLUENCE ON DONOR ORGAN QUALITY AND OUTCOME AFTER TRANSPLANTATION1. Transplantation. 1999;67(3):343. [DOI] [PubMed] [Google Scholar]
- 6.Pratschke J, Wilhelm MJ, Laskowski I, et al. Influence of donor brain death on chronic rejection of renal transplants in rats. J Am Soc Nephrol. 2001;12(11):2474–2481. [DOI] [PubMed] [Google Scholar]
- 7.Wilhelm MJ, Pratschke J, Beato F, et al. Activation of the heart by donor brain death accelerates acute rejection after transplantation. Circulation. 2000;102(19):2426–2433. [DOI] [PubMed] [Google Scholar]
- 8.Novitzky D, Wicomb WN, Cooper DKC, Rose AG, Reichart B. Prevention of Myocardial Injury During Brain Death by Total Cardiac Sympathectomy in the Chacma Baboon. The Annals of Thoracic Surgery. 1986;41(5):520–524. doi: 10.1016/S0003-4975(10)63032-9 [DOI] [PubMed] [Google Scholar]
- 9.Dziodzio T, Biebl M, Pratschke J. Impact of brain death on ischemia/reperfusion injury in liver transplantation. Current Opinion in Organ Transplantation. 2014;19(2):108. doi: 10.1097/MOT.0000000000000061 [DOI] [PubMed] [Google Scholar]
- 10.Marasco SF, Sheeran FL, Chaudhuri K, Vale M, Bailey M, Pepe S. Molecular markers of programmed cell death in donor hearts before transplantation. The Journal of Heart and Lung Transplantation. 2014;33(2):185–193. doi: 10.1016/j.healun.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 11.Cooper DK, Novitzky D, Wicomb WN. The pathophysiological effects of brain death on potential donor organs, with particular reference to the heart. Ann R Coll Surg Engl. 1989;71(4):261–266. [PMC free article] [PubMed] [Google Scholar]
- 12.Smith M Physiologic changes during brain stem death--lessons for management of the organ donor. J Heart Lung Transplant. 2004;23(9 Suppl):S217–222. doi: 10.1016/j.healun.2004.06.017 [DOI] [PubMed] [Google Scholar]
- 13.Ritschl PV, Ashraf MI, Oberhuber R, et al. Donor brain death leads to differential immune activation in solid organs but does not accelerate ischaemia–reperfusion injury. The Journal of Pathology. 2016;239(1):84–96. doi: 10.1002/path.4704 [DOI] [PubMed] [Google Scholar]
- 14.Saat TC, Susa D, Roest HP, et al. A Comparison of Inflammatory, Cytoprotective and Injury Gene Expression Profiles in Kidneys From Brain Death and Cardiac Death Donors. Transplantation. 2014;98(1):15. doi: 10.1097/TP.0000000000000136 [DOI] [PubMed] [Google Scholar]
- 15.Zweers N, Petersen AH, van der Hoeven JAB, et al. Donor brain death aggravates chronic rejection after lung transplantation in rats. Transplantation. 2004;78(9):1251–1258. [DOI] [PubMed] [Google Scholar]
- 16.Pecoraro Y, Tsushima Y, Opitz I, et al. Impact of time interval between donor brain death and cold preservation on long-term outcome in lung transplantation. Eur J Cardiothorac Surg. 2016;50(2):264–268. doi: 10.1093/ejcts/ezw028 [DOI] [PubMed] [Google Scholar]
- 17.Ramjug S, Hussain N, Yonan N. Prolonged time between donor brain death and organ retrieval results in an increased risk of mortality in cardiac transplant recipients. Interact Cardiovasc Thorac Surg. 2011;12(6):938–942. doi: 10.1510/icvts.2010.252809 [DOI] [PubMed] [Google Scholar]
- 18.Cantin B, Kwok BWK, Chan MCY, et al. The impact of brain death on survival after heart transplantation: time is of the essence. Transplantation. 2003;76(9):1275. doi: 10.1097/01.TP.0000093445.50624.5A [DOI] [PubMed] [Google Scholar]
- 19.Molinari N, Daurès J-P, Durand J-F. Regression splines for threshold selection in survival data analysis. Statistics in Medicine. 2001;20(2):237–247. doi: [DOI] [PubMed] [Google Scholar]
- 20.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54(3):201–208. [DOI] [PubMed] [Google Scholar]
- 21.Sun Z, Adam MA, Kim J, et al. Determining the Optimal Timing for Initiation of Adjuvant Chemotherapy After Resection for Stage II and III Colon Cancer. Dis Colon Rectum. 2016;59(2):87–93. doi: 10.1097/DCR.0000000000000518 [DOI] [PubMed] [Google Scholar]
- 22.Salazar MC, Rosen JE, Wang Z, et al. Association of Delayed Adjuvant Chemotherapy With Survival After Lung Cancer Surgery. JAMA Oncol. 2017;3(5):610–619. doi: 10.1001/jamaoncol.2016.5829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marasco S, Kras A, Schulberg E, et al. Donor brain death time and impact on outcomes in heart transplantation. Transplant Proc. 2013;45(1):33–37. doi: 10.1016/j.transproceed.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 24.Nijboer WN, Moers C, Leuvenink HGD, Ploeg RJ. How important is the duration of the brain death period for the outcome in kidney transplantation? Transpl Int. 2011;24(1):14–20. doi: 10.1111/j.1432-2277.2010.01150.x [DOI] [PubMed] [Google Scholar]
- 25.Wauters S, Verleden GM, Belmans A, et al. Donor cause of brain death and related time intervals: does it affect outcome after lung transplantation? Eur J Cardiothorac Surg. 2011;39(4):e68–76. doi: 10.1016/j.ejcts.2010.11.049 [DOI] [PubMed] [Google Scholar]
- 26.Avlonitis VS, Wigfield CH, Golledge HDR, Kirby JA, Dark JH. Early hemodynamic injury during donor brain death determines the severity of primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7(1):83–90. doi: 10.1111/j.1600-6143.2006.01593.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.