Abstract
The majority of patients with newly diagnosed chronic myeloid leukemia (CML) will enjoy a life expectancy equivalent to that of unaffected individuals, but will remain on life-long treatment with a concomitant requirement for on-going hospital interactions for molecular monitoring and drug dispensing. In order to determine more accurately the frequency of monitoring required, we performed a ‘real-life’ retrospective single-center cohort study of 450 patients with CML in at least major molecular remission (MR3) to analyze the risk of loss of MR3 [defined as at least 2 consecutive real-time quantitative polymerase chain reaction (RT-qPCR) results >0.1% International Scale (IS)]. Patients who achieved sustained MR4 (sMR4, BCR-ABL1 RT-qPCR <0.01% IS for 12 months) had a probability of loss of MR3 at 1 and 5 years of 0 and 2.6% (95%CI: 1.2-5.4) respectively, compared to 4.4% (95%CI: 1.9-9.8) and 25.4% (95%CI: 16.7-36.7) respectively, in those who achieved sustained MR3 (sMR3) but not sMR4 (P<0.001). No patient who improved their response to a deep molecular level (at least MR4) lost MR3 if they were considered compliant, had no history of resistance and remained on standard dose tyrosine kinase inhibitor (TKI). MR4 maintained for at least one year represents a secure response threshold for patients with CML, after which no MR3 loss occurs if certain conditions are satisfied (standard TKI dose, full compliance, and lack of previous TKI resistance). This finding may justify reduction of the frequency of hospital interaction, with an associated positive impact on quality of life, survivorship, and economic burden to both patients and healthcare providers.
Introduction
Since the advent of tyrosine kinase inhibitors (TKI), chronic myeloid leukemia (CML) has indeed become a ‘chronic’ disorder. With a life expectancy comparable to unaffected individuals,1,2 management of CML typically involves daily oral TKI, together with regular hospital attendances for molecular monitoring, control of side effects and dispensing of prescription medicines. Whilst this dramatic change is a welcome testament to the power of molecularly targeted agents, further thought can now be given to decreasing the number of interactions between patient and healthcare professionals in order to restore patient autonomy and reduce the financial burden of long-term care.
In the first year of treatment, molecular monitoring at a minimum of 3-monthly intervals is recommended to assess response against international guidelines [European LeukemiaNet (ELN), National Comprehensive Cancer Network (NCCN].3,4 Several studies have shown the importance of early molecular responses (EMR) at 3, 6 and 12 months for their ability to predict the probability of achievement of deep molecular responses, progression-free survival (PFS) and overall survival (OS).5–10 Indeed, the achievement of a major molecular response (MMR, MR3) is often used as a surrogate end point in clinical trials,11 as failure to reach MR3 by 12 months is associated with lower rates of deeper molecular responses, PFS and OS.1, 11–13
Confirmed major molecular response (defined as MR3 on at least two consecutive occasions) has been described as a ‘safe haven’, in that patients who reach this level of response are highly unlikely to experience disease progression.14–19 However, few studies have investigated the predictive value of a stable MR3 with respect to subsequent loss of MR and the requirement for a change in therapy. Furthermore, there are no studies addressing the requirement for, and frequency of, molecular monitoring in patients who have achieved a stable MR3. We interrogated our single center database to determine if we could identify a level of MR that is highly unlikely to be lost provided the patient remains on treatment, a molecular threshold that would render frequent molecular monitoring unnecessary.
Methods
Patient selection
We included all patients diagnosed with CML in chronic phase, treated with TKI from January 2000 until December 2015, who achieved sustained MR3 (sMR3). We excluded patients treated with chemotherapy and/or stem cell transplantation before TKI. TKI were commenced at standard doses and may have been dose reduced subsequently to manage adverse events. Because some of the patients were treated prior to the availability of second-generation TKI, doses of imatinib > 400 mg daily (HD) were prescribed for less than optimal responses in 15% of patients. Episode of noncompliance with treatment was recorded in our database when patients admitted to taking less than 100% of the prescribed therapy. The data cut-off was 31st October 2017. This retrospective observational study was approved by the research ethics committee, and all patients gave written informed consent for their data to be stored and analyzed in our institutional database.
Response definitions
Standard definitions of response and resistance were used.3 Sustained MR3 (sMR3) and sustained MR4 (sMR4) were defined as BCR-ABL1 real-time quantitative polymerase chain reaction (RT-qPCR) of ≤0.1% and <0.01% (on International Scale, IS), respectively, maintained for at least one year. The BCR-ABL1 RT-qPCR was performed according to our previously established methodology.20 At least three consecutive assessments, at a frequency of 3-4 monthly, were required to define sustained response.
Single RT-qPCR results above MR3 or MR4 were considered fluctuations. The date of loss of MR3 and MR4 was the first of at least two consecutive RT-qPCR levels >0.1% and ≥0.01%, respectively.
A BCR-ABL1 RT-qPCR <1% was considered to be equivalent to complete cytogenetic response (CCyR).21 The date of loss of CCyR was the first of two consecutive RT-qPCR determinations ≥1% (IS).
Patient were monitored every three months throughout the entire follow up, according to current guidelines.3
Statistical analysis
Times to achievement of sMR3 and sMR4 were calculated from the date of starting TKI until the first day of RT-qPCR ≤0.1% and <0.01% IS, respectively, maintained for at least one year. The probabilities of loss of MR3, MR4 and CCyR after the first year of sustained response were calculated using the Kaplan-Meier method, with patients being censored at last follow up on treatment. Potential prognostic factors for loss of MR3 were investigated using the log-rank test, with continuous variables split into groups using either quartiles or median values. Variables significant at the P<0.20 level were then included in a Cox proportional hazard regression analysis to find the best model.
Baseline characteristics were compared using the Mann-Whitney test for continuous variables, and Fisher Exact test for categorical variables.
Overall survival (OS) and the event-free survival (EFS) were estimated using the Kaplan-Meier method. For the analysis of survival from CML-related deaths (CML-OS), patients dying from any cause other than CML progression were censored at the date of death; all the other subjects were censored at the date of last follow up. Both OS and CML-OS were calculated from the date of diagnosis, whereas EFS was calculated from the first year in MR3. For EFS, progression to either accelerated or blast phases and death from any cause were considered as events.
P<0.05 was considered statistically significant. All analyses were performed with SPSS software (version 24; IBM, USA).
Results
Achievement of sustained MR3 and MR4
A total of 450 patients achieved sMR3 (Table 1) at a median time from start of TKI of 15.7 months (range: 2-184 months). The median number of RT-qPCR assays in the first year of MR3 was five (range: 3-12), with a median interval between samples of two months (range: 0.5-4 months). The majority of patients, 336 of 450 (74.7%), were on first-line therapy at the time of achievement of sMR3, and most of the remainder, 96 of 114 (84.2%) had changed treatment because of resistance. Of the total cohort, 324 achieved sMR4 at a median of 11.4 months (range: 0-120 months) from sMR3 and 29.1 months (range: 3.3-172.1 months) from start of TKI therapy. The median number of RT-qPCR assays in the first year of MR4 was four (range: 3-11), with a median interval among samples of 2.8 months (range: 0.5-4). After the achievement of sMR3, the median interval between RT-PCR assays was 2.7 months (range: 0.5-9 months) and was identical in both groups. The 5-year probability of sMR4 after the achievement of sMR3 was 74% (95%CI: 69.3-78.2).
Table 1.
Study cohort characteristics (n=450).
Online Supplementary Table S1 shows a comparison of clinical and CML-related characteristics between patients who achieved sMR3 only and those who reached sMR4. We observed a significant difference in the number of patients treated with high-dose imatinib and in the proportion of those with a history of resistance to first-line TKI (P=0.02 and P=0.0002, respectively), both more common in the sMR3 only group.
Patients who achieved sMR4 reached sMR3 more rapidly (median 12.3 months, range: 1-135.2) than those whose best response was sMR3 only (median 24.5 months, range: 2.8-184.4; P<0.001). The median follow up of patients in sMR3 only was shorter (56.9 months, range: 12.3-155) than those in sMR4 (108.1 months, range: 14.6-199.7).
Patients expressing the BCR-ABL1 e14a2 transcript achieved sMR3 more rapidly at 12.2 months (range: 1-130 months) than those expressing e13a2 at 21.4 months (range: 1-144 months; P=0.019) and were more likely to achieve sMR4, with 5-year probabilities of 81% (range: 74-86.5%) compared to 72.8% (95%CI: 64.8-79.6; P=0.009) for those with the e13a2 transcript (Online Supplementary Figure S1A and B).
Loss of sustained responses
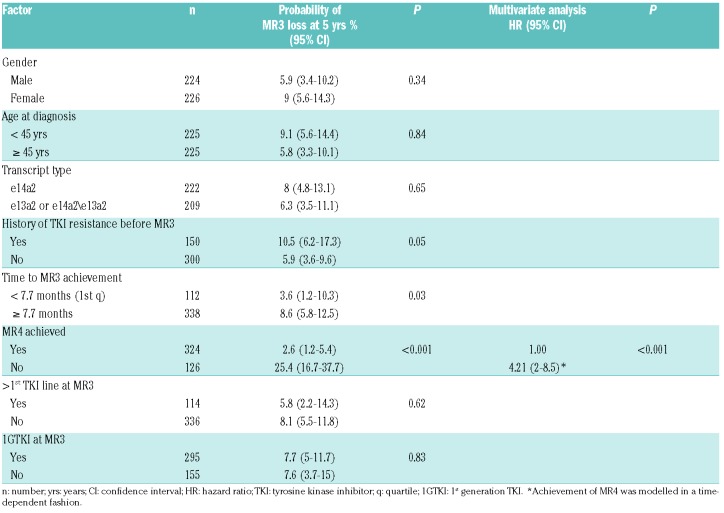
Among the total cohort of 450 patients, the 1-and 5-year probabilities of loss of MR3 were 1.1% (95%CI: 0.5-2.6) and 7.5% (95%CI: 5.2-10.7), respectively (Figure 1B), and of loss of CCyR 0.7% (95%CI: 0.2-2) and 5.9% (95%CI: 4-8.7), respectively. In univariate analysis, a history of resistance to any TKI, time to sMR3 longer than 7.7 months (corresponding to the 1st quartile of the time of achievement of sMR3 in the whole patient cohort), and failure to reach sMR4 were significantly associated with loss of MR3. In multivariate analysis, only failure to reach sMR4 remained statistically significant (Table 2). Patients who achieved sMR3 after an increase in the dose of imatinib were more likely to lose MR3 than patients who achieved sMR3 after a change to a 2GTKI (Online Supplementary Table S2).
Figure 1.
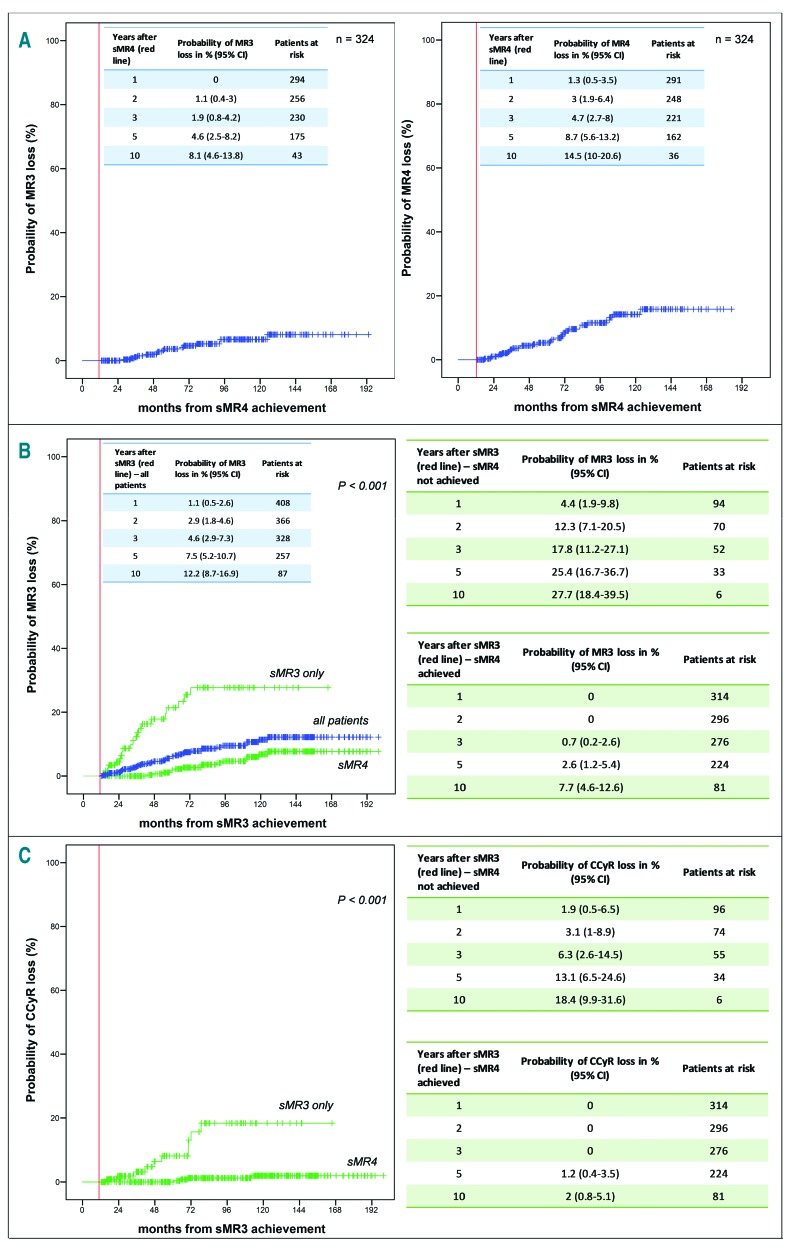
Probabilities of loss of responses after achieving sustained molecular response (MR)-3 (sMR3) and sMR4. (A) Probability of loss of MR3 and MR4 after the first year in MR4 (vertical line indicates time of sMR4, i.e. time at which MR4 was sustained for 12 months). (B) Probability of loss of MR3 for all 450 patients (vertical line indicates time of sMR3, i.e. time at which MR3 was sustained for 12 months) (blue curve); probability of loss of MR3 for patients who achieved sMR3 only (n=126) and those who achieved sMR4 (n=324) (green curves). (C) Probability of loss of complete cytogenetic response (CCyR) for patients who achieved sMR3 only (n=126) and those who achieved sMR4 (n=324). n: number; CI: Confidence Interval.
Table 2.
Univariate and multivariate analysis of factors possibly associated with MR3 loss.
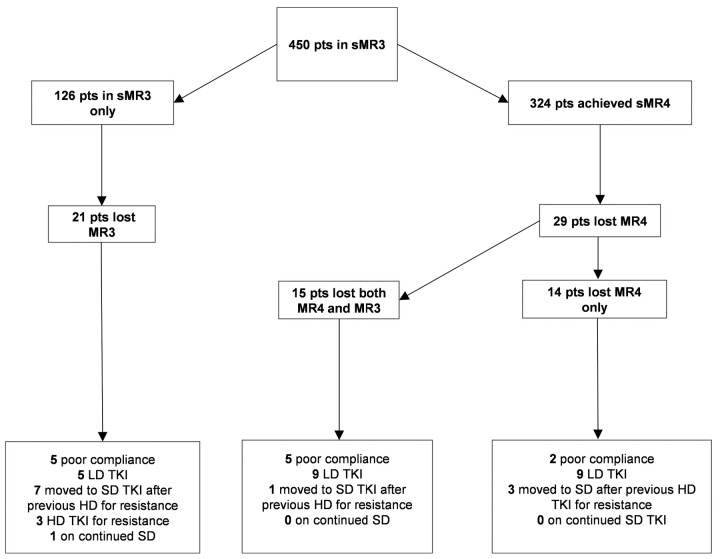
Of the 324 patients in sMR4, 29 (8.9%) lost MR4 at a median of 49.9 months (range: 2.5-111.4 months) after the first year in MR4, of whom 15 also lost MR3 at a median of 41.3 months (range: 15.3-112.7 months) after the first year in MR4 and 2.1 months (range: 0-23.3 months) from loss of MR4. At the time of MR4 loss and MR3 loss, no patient had remained on standard dose of TKI throughout their disease course. Seven were known to be non-compliant with therapy, 18 were on lower doses of TKI, and four had previously received TKI doses greater than standard doses for resistance (Figure 2). The probabilities of loss of MR3 at 1 and 5 years were 0 and 4.6% (95%CI: 2.5-8.2), and of loss of MR4 were 1.3% (95%CI: 0.5-3.5) and 8.7% (95%CI: 5.6-13.2), respectively (Figure 1A).
Figure 2.
Patient outcomes. Flow diagram showing outcomes of 450 patients after the achievement of sustained molecular response (MR)-3 (sMR3). The boxes in the lower part of the image contain information about the tyrosine kinase inhibitor (TKI) dose at the time of response loss. LD: lower TKI dose (compared to the standard recommended doses for first or subsequent lines); SD: standard TKI dose; HD: higher TKI dose; sMR4: sustained MR4; pts: patients.
Of the 126 patients who achieved sMR3 only, 21 (16.7%) lost MR3 at a median of 21.5 months (range: 1-60 months) after the first year in MR3. The probabilities of loss of MR3 at 1 and 5 years were 4.4% (95%CI: 1.9-9.8) and 25.4% (95%CI: 16.7-36.7), respectively, which were higher than in the sMR4 cohort (P<0.001) (Figure 2B). At the time of MR3 loss, only one patient had remained on standard dose of TKI throughout their disease course. Five were known to be non-compliant with therapy, five were on lower doses of TKI, and ten had previously received TKI doses greater than standard doses for resistance (Figure 2).
Durability of response in patients achieving sMR4
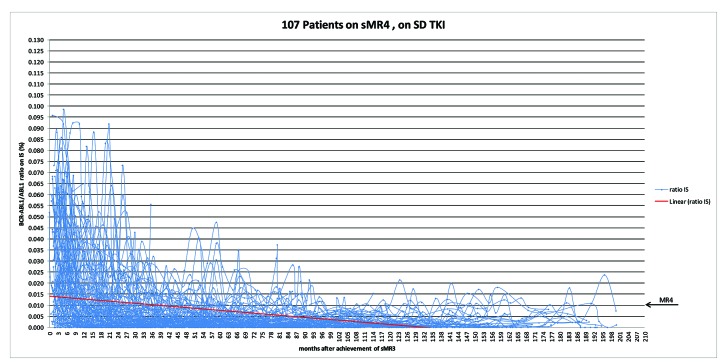
Of the 324 patients in sMR4, 107 patients (23.7%) had remained on standard dose TKI after the achievement of sMR3 and sMR4. None of these patients lost MR3. In order to address the subsequent pattern of BCR-ABL1 RT-qPCR responses after their achievement of sMR3, we plotted a total of 3,305 consecutive results, with a median number of tests per patient of 29 (range: 6-93 tests) and a median interval between tests of three months (range: 1-12 months). Most of the results (2,573, 77.8%) fell below the MR4 threshold, and demonstrated a continuing decline over time (Figure 3).
Figure 3.
A total of 3,305 consecutive real-time quantitative polymerase chain reaction (RT-qPCR) results in patients achieving sustained molecular response (MR)-4 (sMR4) and on standard dose tyrosine kinase inhibitor (TKI) (n=107). The starting point is the date of achievement of sMR3. The red line depicts the linear trend-line of BCR-ABL1/ABL1 RT-qPCR values (expressed in % on International Scale, IS) over time. n: number.
Events subsequent to the loss of MR3 and of MR4
In 36 patients who lost MR3, the median follow up since the loss was 24.5 months (range: 1.3-135.4 months). Of these, 14 also lost CCyR at a median of six months (range: 1.3-55.4 months) from loss of MR3. The 1-and 5-year probabilities of loss of CCyR were 1.9% (95%CI: 0.5-6.5) and 13.1% (95%CI: 6.5-24.2) for those who achieved only sMR3 and 0 and 1.2% (95%CI: 0.4-3.5) for those who reached sMR4 (Figure 1C). Three of these patients experienced progression to accelerated phase, of whom two died subsequently in blast phase and one achieved MR4 on a different TKI. Of the remaining 11 patients, at last follow up four were in complete hematological response, two in CCyR, two in MR3, one in MR4, and two in MR4.5. The median follow up of the 22 patients who lost MR3 but not CCyR was 33.4 months (range: 5.4-135.4 months). At last follow up, ten had achieved MR3, five were in MR4, and seven in MR4.5. Of the 14 patients who lost MR4 but not MR3, none had lost CCyR at a median follow up of 39.2 months (range: 26-89.3 months). At last follow up, five patients were in MR3, five in MR4, and four in MR4.5.
The response status at last follow up on TKI and treatment status at last follow up are summarized in Online Supplementary Figure S2.
Overall survival
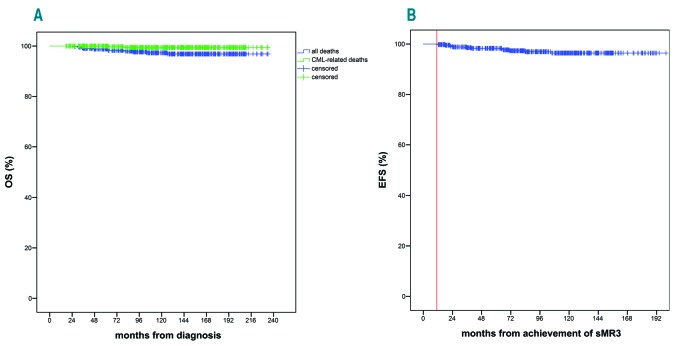
The 5-and 10-year OS were 98.8% (95%CI: 98.7-98.9) and 96.8% (95%CI: 94.2-97.3), while CML-OS were 100% and 99.4% (95%CI: 98-99.8) (Figure 4A). The 5-year EFS after the first year in MR3 was 97.3% (95%CI: 95.2-98.5) (Figure 4B). Overall, 11 deaths were recorded at a median follow up from diagnosis of 122.5 months (range: 17.5-234 months). Of these, only two were directly related to CML. Other causes of death included chronic obstructive pulmonary disease (n=2), second cancer (n=5: non-Hodgkin lymphoma, glioblastoma, mesothelioma, colonic adenocarcinoma, esophageal adenocarcinoma), autoimmune disease (n=1), unknown (n=1).
Figure 4.
Overall survival (OS) (A) and event-free survival (EFS) (B) for the entire patient cohort (n=450). (A) The green line depicts the chronic myeloid leukemia (CML)-OS, whereas the blue line shows the OS. (B) EFS was calculated from the first year in sustained molecular response (MR)-3 (sMR3) (red vertical line indicates time of sMR3, i.e. time at which MR3 was sustained for 12 months).
Discussion
One of the real-life outcomes of the successful use of TKI has been the increase in the number of patients living with CML. Estimates of prevalence of CML suggest that more than 100,000 individuals will be on TKI in the USA alone by 2020,22 requiring in excess of 300,000 out-patient interactions annually. Data from Phase III randomized studies of imatinib versus dasatinib12 and imatinib versus nilotinib11 show that the majority of patients achieve MR3 on at least one occasion by five years (imatinib 64%, dasatinib 76% in DASISION; imatinib 60.4%, nilotinib 77% in ENESTnd) and that many of these also reach MR4 or deeper.11,12 Although the cumulative incidences of molecular responses by pre-defined time points are not identical to the achievement of sustained responses, it seems reasonable to assume that with continued treatment and changes of treatment for less than optimal responses, the number of patients achieving deep and durable responses will continue to increase.
We used our single center cohort of patients who had reached and sustained for 12 months the ‘safe-haven’ of MR3 to investigate the durability of this response,14 in order to provide some evidence for recommendations for the frequency of PCR monitoring once these deep responses have been established. In using a ‘real-life’ patient cohort, we appreciate that there are complexities of decision-making that are confounding compared to the rigorous clinical trial environment. In clinical practice, many patients are managed on lower than standard doses of the various TKI because of intolerance, some admit to non-compliance so that actual dosing is difficult to estimate, and in the early TKI era when only imatinib was available and when the various milestones for response had not been established, some patients were given higher than standard doses to try to deepen their responses. These varying conditions should be taken into account during response monitoring, such that patients may require more frequent RTq-PCR testing if doses are reduced, patients admit to or are thought to be non-compliant or where there is previous evidence of resistance.
We found that patients who had achieved sMR3, who were known to be compliant and who were treated throughout their disease course with standard dose TKI had a low probability of loss of MR3. Furthermore, no patient satisfying these criteria and who achieved sMR4 experienced loss of MR3, and over time they gradually deepened their molecular responses. This leads us to suggest that the achievement of sMR4 in this group identifies patients at negligible risk of loss of molecular responses and that the frequency of molecular monitoring could be at 6-monthly intervals.
In contrast, the much higher rate of MR3 loss in the sMR3 only group (25.4% at 5 years) and the potential to subsequently lose CCyR, would favor continuation of molecular monitoring at a minimum frequency of 3-monthly in this group. Some of the patients who lost MR3 were on a reduced dose of their TKI and our observations are supported by the findings of the DESTINY study,23 in which approximately 30% of patients in sMR3 who reduced their TKI dose by 50% subsequently lost MR3. Although only one patient in the sMR3 group who was on standard dose TKI and thought to be compliant lost MR3, we are aware that we can never be certain of individual patient compliance, and would not recommend reduced vigilance in this group. We have previously reported a close association between compliance and major molecular response.24 We think it unlikely that there would be an unexpected and clinically significant degree of non-compliance in patients in sMR4. In contrast, lack of compliance might be a contributing factor to the failure of patients in sMR3 to gain deeper responses. In fact, this latter group are clearly responsive to TKI and the reasons for their failure to gain deeper responses are unclear.
It is possible that we have underestimated the probability of loss of MR3 in the patients who achieved only sMR3 because this group may contain two distinct sub-populations: those who are destined to achieve sMR4 with longer follow up (and therefore have a better outcome) and those who do not have the biological potential to achieve deeper responses. We consider this unlikely because the median duration of follow up in patients who achieved sMR3 only was long (56.9 months), whereas the median time to sMR4 was 29.1 months (range: 3.3-172.1 months) from start of TKI therapy, such that sMR4 should have been observed had it been destined to occur.
We observed some differences between the patients who achieved sMR3 only and those who reached sMR4 in that the former group were more likely to express the e13a2 BCR-ABL1 transcript, more frequently demonstrated resistance to their first-line TKI and were more likely to have required a higher dose of imatinib before the achievement of sMR3. Moreover, the time to sMR3 was longer in patients in whom this was the best response than in those who achieved sMR4. Although these factors may be useful in identifying a group of patients with a lower probability of achieving sMR4 after reaching sMR3, the fact remains that once sMR4 is reached, it is highly unlikely to be lost if the patient remains on standard doses of TKI. The only factor predictive for loss of MR3 in multivariate analysis was the failure to achieve sMR4, leading us to conclude that sMR4 is a secure position for patients with CML.
The impact of the various levels of molecular response on patient outcome has been studied by others. For some time it was difficult to demonstrate an improvement in survival in patients who had achieved MR3 or better compared to those whose best response was CCyR.25–27 Most recently, data from the German CML Study IV showed that there were no progressions among patients achieving MR4.5, as compared to one, nine and 13 events in patients whose deepest responses were MR4, MR3 and CCyR, respectively.10 Our results confirm the excellent outcome of patients who achieve sMR4 or better, but this was not the focus of our study; instead, we used our database of prolonged molecular monitoring to try to provide an evidence base for the frequency of molecular monitoring and a practical guide for clinical management.
One criticism of the use of our findings is that we are recommending reduced frequency of monitoring and hospital interactions in a group of patients who are candidates for trials of dose-reduction and/or discontinuation for treatment-free remission (TFR). We deliberately defined sMR4 as MR4 sustained for 12 months as this is identical to the criterion used to confirm eligibility for a trial of treatment discontinuation in the EURO-SKI study.28 However, many patients are reluctant to discontinue therapy, and of those who do cease treatment, approximately half will have to recommence TKI because of loss of MR3,29–31 resulting in the majority continuing to require life-long treatment and monitoring. These patients, who have responded well and durably to TKI, may welcome fewer hospital visits.
We acknowledge some limitations in our study. We are aware that it is a retrospective observational study. In addition, we included all patients who achieved sMR3, including those who had at some point received higher or lower than standard doses of TKI and those who were known to be non-compliant. However, we feel that this reflects the ‘real-life’ clinical situation, and it is not surprising that these groups are more likely to lose molecular responses. Our study does confirm the requirement to adhere to more rigorous monitoring in these vulnerable patient groups.
In summary, we have shown that risk of loss of MR3 is negligible in patients who have achieved sMR4, particularly in those who remain on standard dose TKI who have not demonstrated prior resistance and who are known to be compliant. As a consequence, we suggest that sMR4 is regarded as a secure molecular threshold, representing a level of response that may justify less frequent monitoring in patients who are not considering, or who have failed, a trial of treatment discontinuation. For patients and healthcare providers, the identification of a secure level of molecular response has a number of direct and indirect benefits. For patients, the knowledge that they have reached a level of residual disease that is associated with a negligible risk of loss of response will not only be reassuring, improving morale and quality of life, but also facilitate the acceptance of alternative management styles, including remote care. The consequent reduction in hospital visits will be cost saving to both patients and healthcare providers.
There are, of course, reasons other than molecular monitoring, for continuing to provide close interactions between patients and healthcare professionals: continued close supervision may promote compliance, allow recognition and management of adverse events and co-morbidities, optimize the use of expensive medication, facilitate advice regarding parenting, and provide valuable reassurance of on-going response. Our results simply provide support for patients and healthcare professionals who wish to consider relaxing the need for hospital visits.
Acknowledgments
JFA and DM acknowledge the support of the Imperial College NIHR Biomedical Research Centre. JFA is a NIHR Senior Investigator. SC acknowledges the support of Ariad (Incyte) and Pfizer. GN acknowledges the support of ARIAD (Incyte). The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/11/2206
References
- 1.Hochhaus A, Larson RA, Guilhot F, et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017;376(10):917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM. Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J Clin Oncol. 2016;34(24):2851–2857. [DOI] [PubMed] [Google Scholar]
- 3.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(9):1108–1135. [DOI] [PubMed] [Google Scholar]
- 5.Hanfstein B, Muller MC, Hehlmann R, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia. 2012;26(9): 2096–2102. [DOI] [PubMed] [Google Scholar]
- 6.Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for pre dicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012; 30(3):232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branford S, Yeung DT, Ross DM, et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood. 2013; 121(19):3818–3824. [DOI] [PubMed] [Google Scholar]
- 8.Jain P, Kantarjian H, Nazha A, et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: results with four tyrosine kinase inhibitor modalities. Blood. 2013;121(24):4867–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014; 123(9):1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hehlmann R, Muller MC, Lauseker M, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32(5):415–423. [DOI] [PubMed] [Google Scholar]
- 11.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients Trial. J Clin Oncol. 2016; 34(20):2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hehlmann R, Lauseker M, Saussele S, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31(11):2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes TP, Branford S. Monitoring disease response to tyrosine kinase inhibitor therapy in CML. Hematology Am Soc Hematol Educ Program. 2009:477–487. [DOI] [PubMed] [Google Scholar]
- 15.Press RD, Love Z, Tronnes AA, et al. BCR-ABL mRNA levels at and after the time of a complete cytogenetic response (CCR) predict the duration of CCR in imatinib mesylate-treated patients with CML. Blood. 2006;107(11):4250–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Press RD, Galderisi C, Yang R, et al. A halflog increase in BCR-ABL RNA predicts a higher risk of relapse in patients with chronic myeloid leukemia with an imatinib-induced complete cytogenetic response. Clin Cancer Res. 2007; 13(20):6136–6143. [DOI] [PubMed] [Google Scholar]
- 17.Iacobucci I, Saglio G, Rosti G, et al. Achieving a major molecular response at the time of a complete cytogenetic response (CCgR) predicts a better duration of CCgR in imatinib-treated chronic myeloid leukemia patients. Clin Cancer Res. 2006;12(10):3037–3042. [DOI] [PubMed] [Google Scholar]
- 18.Palandri F, Iacobucci I, Soverini S, et al. Treatment of Philadelphia-positive chronic myeloid leukemia with imatinib: importance of a stable molecular response. Clin Cancer Res. 2009;15(3):1059–1063. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian HM, Shan J, Jones D, et al. Significance of increasing levels of minimal residual disease in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in complete cytogenetic response. J Clin Oncol. 2009;27(22): 3659–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foroni L, Wilson G, Gerrard G, et al. Guidelines for the measurement of BCR-ABL1 transcripts in chronic myeloid leukaemia. Br J Haematol. 2011;153(2):179–190. [DOI] [PubMed] [Google Scholar]
- 21.Branford S. Chronic myeloid leukemia: molecular monitoring in clinical practice. Hematology Am Soc Hematol Educ Program. 2007:376–383. [DOI] [PubMed] [Google Scholar]
- 22.Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68(2): 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark RE, Polydoros F, Apperley JF, et al. Deescalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY): an interim analysis of a non-randomised, phase 2 trial. Lancet Haematol. 2017;4(7):e310–e316. [DOI] [PubMed] [Google Scholar]
- 24.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. [DOI] [PubMed] [Google Scholar]
- 26.Kantarjian H, O’Brien S, Shan J, et al. Cytogenetic and molecular responses and outcome in chronic myelogenous leukemia: need for new response definitions¿ Cancer. 2008;112(4):837–845. [DOI] [PubMed] [Google Scholar]
- 27.Marin D, Milojkovic D, Olavarria E, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112(12):4437–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19(6):747–757. [DOI] [PubMed] [Google Scholar]
- 29.Claudiani S, Apperley JF, Gale RP, et al. E14a2 BCR-ABL1 transcript is associated with a higher rate of treatment-free remission in individuals with chronic myeloid leukemia after stopping tyrosine kinase inhibitor therapy. Haematologica. 2017;102(8):e297–e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etienne G, Guilhot J, Rea D, et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. J Clin Oncol. 2017;35(3):298–305. [DOI] [PubMed] [Google Scholar]
- 31.Rea D, Nicolini FE, Tulliez M, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129(7):846–854. [DOI] [PubMed] [Google Scholar]