Abstract
While efficient at treating B-cell malignancies, Bruton tyrosine kinase (BTK) inhibitors are consistently reported to increase the risk of bleeding. Analyzing platelet aggregation response to collagen in platelet-rich plasma allowed us to identify two groups in the healthy population characterized by low or high sensitivity to ibrutinib in vitro. Inhibition of drug efflux pumps induced a shift from ibrutinib low-sensitive platelets to high-sensitive ones. At a clinically relevant dose, acalabrutinib, a second-generation BTK inhibitor, did not affect maximal collagen-induced platelet aggregation in the ibrutinib low-sensitive group but did inhibit aggregation in a small fraction of the ibrutinib high-sensitive group. Consistently, acalabrutinib delayed aggregation, particularly in the ibrutinib high-sensitive group. In chronic lymphocytic leukemia patients, acalabrutinib inhibited maximal platelet aggregation only in the ibrutinib high-sensitive group. Acalabrutinib inhibited collagen-induced tyrosine-753 phosphorylation of phospholipase Cγ2 in both groups, but, in contrast to ibrutinib, did not affect Src-family kinases. Acalabrutinib affected thrombus growth under flow only in the ibrutinib high-sensitive group and potentiated the effect of cyclooxygenase and P2Y12 receptor blockers in both groups. Since the better profile of acalabrutinib was observed mainly in the ibrutinib low-sensitive group, replacement therapy in patients may not systematically reduce the risk of bleeding.
Introduction
Bruton tyrosine kinase (Btk) inhibitors are efficient therapeutic agents for the treatment of chronic lymphocytic leukemia (CLL), mantle-cell lymphoma and Waldenström macroglobulinemia.1–3 However, these drugs are recognized to increase the rate of bleeding in up to 50% of treated patients.4,5 Most bleeding events are of grade 1-2, and include spontaneous bruising, petechiae and hematomas, but, in 5% of patients, they are of grade 3 or higher.6–9 Such an incidence of bleeding warrants concerns, particularly during invasive procedures or surgery or when Btk inhibitors are associated with antithrombotic therapy.4,5
Several studies have now clearly shown that the first-in-class Btk inhibitor, ibrutinib, causes platelet dysfunction in a significant proportion of treated patients. In vitro, in normal platelets, the drug has been shown to affect activation mechanisms downstream of the collagen receptor GPVI, GPIb and αIIbβ3 integrin.10–13 Btk and Tec are two members of the same family of tyrosine kinases involved in platelet activation, at least via their contribution to phospholipase Cγ2 (PLCγ2) phosphorylation.14,15 Experimental mouse models of Btk invalidation have shown that Btk is involved in collagen/GPVI and von Willebrand factor/GPIb-IX-V-induced platelet activation.14,16 However, patients with X-linked agammaglobulinemia do not have a bleeding phenotype and their Btk-deficient platelets exhibit only a weak defect, suggesting compensation of Btk by Tec.14,17,18 Accordingly, invalidation of both Btk and Tec in mice was required to impair platelet responses evoked by GPVI agonists.15
Like ibrutinib, the second-generation Btk inhibitor, acalabrutinib, inhibits Btk by covalent modification of the cysteine residue C481 in the ATP binding domain. While ibrutinib is known to irreversibly inhibit both Btk and Tec, acalabrutinib exhibits a higher specificity towards Btk and less activity on Tec,9,19,20 although a recent study suggests that its selectivity for Btk over Tec is not substantial.21 At the highest clinically achievable doses, ibrutinib has also been shown to inhibit Src-kinases in washed human platelets.10–13 Src-kinases are essential for platelet activation and act upstream of Btk and Tec in the GPVI signaling pathway.22 Their inhibition has been shown to induce bleeding in vivo.23 Of note, acalabrutinib seems to have less inhibitory potential than ibrutinib on Src-kinases.19,24 A clinical trial that enrolled 61 relapsed CLL patients demonstrated the efficacy of acalabrutinib and did not document any major bleeding although a significant incidence of low-grade bleeding including petechiae (16%) and contusions (18%) was observed.9 Using an elegant experimental mouse model of arterial thrombosis and platelets from acalabrutinib-treated patients, it was also shown in this study that this second-generation Btk inhibitor has fewer anti-platelet effects than ibrutinib. Another recent study in a small cohort of patients also documented significant low-grade bleeding in acalabrutinib-treated patients and less platelet dysfunction in these patients than in ibrutinib-treated patients.24 Of note, a very recent report suggests that ibrutinib and the second generation Btk inhibitors, acalabrutinib and tirabrutinib (ONO/GS-4059), have the capacity to prevent platelet thrombus formation on human atherosclerotic plaque homogenate.25
There are only limited available data on the effects of second-generation Btk inhibitors to guide physicians who switch from ibrutinib to another kinase inhibitor (e.g. in the case of bleeding, or co-medication with antithrombotic drugs). Therefore, we evaluated in this study whether acalabrutinib could represent a safer option. We first identified two groups of healthy donors based on their sensitivity to collagen-induced platelet aggregation inhibition by ibrutinib in vitro. We then characterized the differences and similarities in the effects of acalabrutinib and ibrutinib in the two groups and analyzed the impact of an association of acalabrutinib with antiplatelet drugs. Our data suggest that switching Btk therapy may not be a systematically good option for patients who bleed under ibrutinib treatment, and that the association of any of these Btk inhibitors with antiplatelet drugs significantly potentiates the inhibition of collagen-induced platelet aggregation.
Methods
Reagents
The sources of the reagents used in this study are provided in the Online Supplement.
Preparation of human platelets
Human platelets from adult healthy volunteers who had not taken aspirin or any anti-platelet or anti-inflammatory drugs in the preceding 10 days or CLL patients were isolated from blood collected under citrate. All experiments were performed within 1 h after blood sample collection for healthy donors and within 2 h after blood sample collection for CLL patients. Platelet-rich plasma (PRP) and washed platelets were prepared as indicated in the Online Supplement and elsewhere.23
Light transmission aggregometry
Platelet aggregation was monitored in siliconized glass cuvettes under continuous stirring (1000 rpm) at 37°C using a turbidimetric method in a multi-channel aggregometer (SD Medical, France). Platelet aggregation was monitored for 10 min and the extent of platelet aggregation and area under curve (AUC) were analyzed using Thrombosoft 1.6 software (SD Medical, France).
Ex vivo model of thrombosis under flow conditions
Glass microcapillaries (Cellix System, New York, NY, USA) were coated with 50 μg/mL type I collagen from equine tendon overnight at 4°C and saturated with a solution of 1% bovine serum albumin (fatty acid-free) in phosphate-buffered saline for 30 min. Heparin-anticoagulated whole blood from healthy human donors was pre-treated with ibrutinib, acalabrutinib or vehicle (dimethylsulfoxide) for 60 min at 37°C and platelets were labeled with DiOC6 (2 μM, 10 min at 37°C). Blood was then perfused through the microfluidic system for the indicated times at an arterial shear rate of 1500 s−1 as previously described.26 Platelet adhesion and thrombus formation were measured in real time with an epi-fluorescence microscope (Axiovert 200; Zeiss) with a 40X oil immersion objective (Plan-Apo 40x/1.3 Oil DIC UVVIS-IR) and a Colibri LED System light source (Zeiss, Jena, Germany). The results were recorded in real time (acquisition rate: 1 frame every 30 s) using a high resolution CCD cooled camera (Orca-R2, Hamamatsu, Hamamatsu City, Japan). Image sequences of the time-lapse recording and surface coverage were analyzed using Image J software.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA) with the Bonferroni post-test (GraphPad PRISM software, San Diego, CA, USA). P-values <0.05 were considered statistically significant. Two-way ANOVA with the Bonferroni post-test was used for statistical analysis of the surface coverage in the thrombus formation assay and a one sample t-test was applied to analyze thrombus volume at 180 s.
Ethical approval
Ethical approval for collecting blood from patients and healthy volunteers was granted by the Hémopathies Inserm Midi-Pyrénées (HIMIP) collection declared to the French Ministry of Higher Education and Research (DC 2008-307 collection 1) and a transfer agreement (AC 2008-129) was obtained after approval from the ethical committee Comité de Protection des Personnes Sud-Ouest et Outremer II and the Toulouse Hospital Bio-Resources biobank, declared to the Ministry of Higher Education and Research (DC 2016-2804). In accordance with French law, clinical and biological annotations of patients’ samples were declared to the Comité National Informatique et Libertés (CNIL), the French data protection authority.
Results
Effect of ibrutinib and acalabrutinib on collagen-induced platelet aggregation in healthy donors
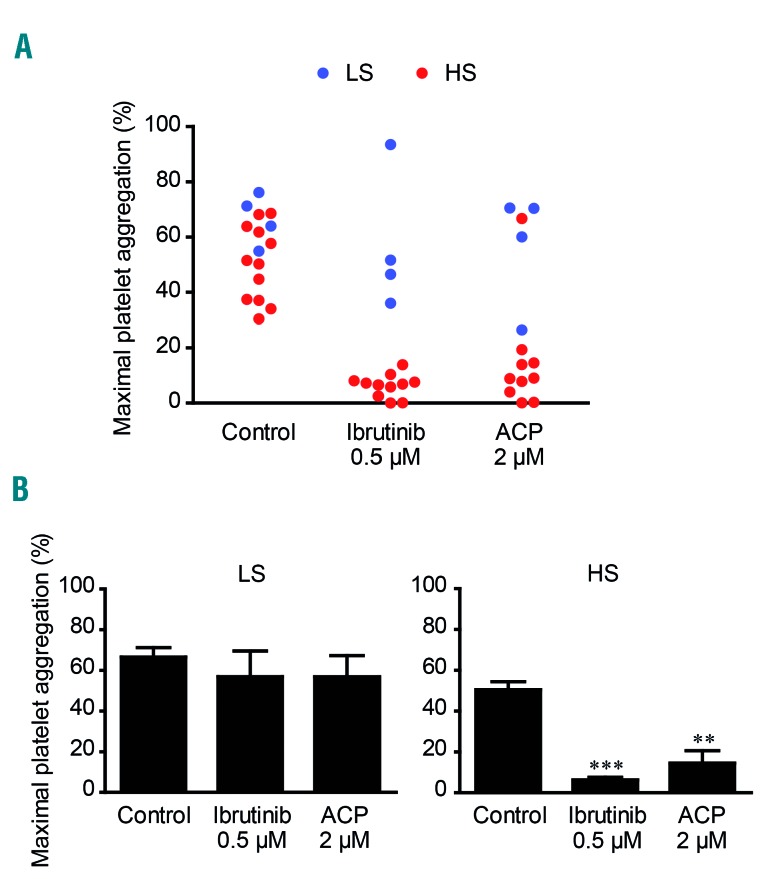
It is important to note that only a subset of ibrutinib-treated patients develop spontaneous bleeding and have a defect in collagen-induced platelet aggregation.3,10,11 Moreover, we consistently observed great heterogeneity in the intensity of the in vitro effect of ibrutinib on collagen-induced platelet aggregation in PRP from healthy donors (unpublished observation). We therefore first tested the effect of ibrutinib at the clinically achievable dose of 0.5 μM on collagen-induced platelet aggregation in PRP from 70 healthy volunteers. Ibrutinib inhibited collagen-induced platelet aggregation (maximal platelet aggregation <50%) in 56% of healthy donors while it had no or little effect (maximal platelet aggregation >50%) in the remaining 44% (Figure 1A). Interestingly, when we performed this aggregometry assay again 6 months later in 29 out of the 70 donors, the response profile was comparable. Indeed, the same donors were either sensitive or resistant to ibrutinib (Figure 1A). Figure 1B highlights the important difference in the dose-dependent effect of ibrutinib in the two groups. This effect was probably not related to an apparent difference of collagen sensitivity among the healthy donor population since the maximal platelet aggregation in response to a low dose of collagen (3.3 μg/mL) was not significantly different in the two groups. Moreover, increasing the collagen concentration from 3.3 μg/mL to 6 μg/mL reduced but did not overcome the inhibitory effect of ibrutinib in the high sensitivity group (Online Supplementary Figure S1). These two groups will hereafter be referred to as ibrutinib “high sensitive” (HS) and “low sensitive” (LS) donors. To further characterize this marked difference, similar experiments were performed in the presence of drug efflux pump inhibitors, reserpine and verapamil, in LS donors (Online Supplementary Figure S2). While these two drugs alone or in combination had no effect on collagen-induced platelet aggregation, each drug significantly increased ibrutinib sensitivity. In combination, they induced high ibrutinib sensitivity in LS donors. Since it was recently shown that the effects of ibrutinib are incubation time-dependent,27 we also performed a time-course analysis of the effects of ibrutinib treatment (Online Supplementary Figure S2C). We found that incubation with ibrutinib for 1 h caused the maximal inhibition and that the effect of the efflux pump inhibitors was visible at 30 min and maximal at 1 h in the LS group. These data are in agreement with those from Nicolson et al.27 and suggest that the intra-platelet concentration of the drug correlates with the aggregation defect.
Figure 1.
Effect of ibrutinib and acalabrutinib on collagen-induced platelet aggregation in vitro, in healthy donors. Platelet-rich plasma (PRP) from healthy volunteers was treated or not with ibrutinib (A-D) or acalabrutinib (ACP) (C-E) at the indicated concentrations for 1 h at 37°C and stimulated with collagen 3.3 μg/mL. Platelet aggregation was assessed by turbidimetry during 10 min and results, expressed as percentage of maximal platelet aggregation and area under the curve, are mean ± standard error of mean. A maximal platelet aggregation response below 50% indicated ibrutinib high-sensitive donors (HS, in red, n=39) while a maximal aggregation response above 50% indicated ibrutinib low-sensitive donors (LS, in blue, n=31). The same analysis was performed 6 months later (After 6 months) in 29 out of the 70 healthy donors (A). Platelet aggregation curves showing representative platelet responses to ACP and ibrutinib on PRP from ibrutinib HS healthy donors are shown (D). The numbers of donors analyzed in each experiment were: (A) n=70, after 6 months: n=29; (B) LS: n=15, HS: n=7; (C) n=52; (E) LS: n=10, HS: n= 12. *P<0.05, **P<0.01, ***P<0.001, #P<0.05, ##P<0.01, ###P<0.001 according to one-way analysis of variance.
Thus, with regard to collagen-induced platelet aggregation in the normal population, two groups of individuals were distinguished based on their in vitro sensitivity to ibrutinib, as previously found in CLL patients treated with this drug.10,11
Since acalabrutinib could be an option for patients requiring a switch from ibrutinib therapy, we analyzed its effect at the clinically relevant dose of 2 μM in the two groups. Acalabrutinib was less efficient than ibrutinib on maximal platelet aggregation induced by collagen (Figure 1C, D). The ibrutinib LS donors were not affected by acalabrutinib and a large proportion of ibrutinib HS donors were not or only weakly affected. In a small percentage of donors (10%) both drugs strongly inhibited collagen-induced platelet aggregation. Dose-dependent curves illustrate the lack of effect of acalabrutinib in the LS group and its relatively weak effect in the HS group (Figure 1E). However, while acalabrutinib was less efficient than ibrutinib on maximal platelet aggregation, it consistently delayed the aggregation response (Figure 1D). This is illustrated by a decrease in the area under the aggregation curve (Figure 1C, D). This effect was dose-dependent and more pronounced in the ibrutinib HS group (Figure 1E). It is noteworthy that acalabrutinib had no impact on platelet aggregation induced by thrombin receptor activating peptide (TRAP), the thromboxane A2 analog U46619 or ADP but did affect to some extent platelet aggregation induced by the GPVI agonist, collagen-related peptide, particularly in the HS group (Online Supplementary Figure S3). Of note, the drug efflux pump inhibitors alone or in combination did not significantly amplify the effect of acalabrutinib on maximal platelet aggregation but tended to increase its impact on the delay of aggregation in response to collagen (3.3 μg/mL) in the LS group (data not shown).
The effect of acalabrutinib on collagen-induced platelet aggregation was also tested in vitro in PRP from 16 Btk inhibitor-naïve CLL patients. The maximal platelet aggregation evoked by collagen was reduced compared to that of healthy donors but two groups, ibrutinib LS (n=4) and ibrutinib HS (n=12), were again identified (Figure 2A). While acalabrutinib had no significant effect in ibrutinib LS CLL patients, it significantly decreased the maximal platelet aggregation in ibrutinib HS CLL patients (Figure 2B). In the ibrutinib HS group, only one patient was not sensitive to acalabrutinib.
Figure 2.
Effect of ibrutinib and acalabrutinib on collagen-induced platelet aggregation in vitro in patients with chronic lymphocytic leukemia. Platelet-rich plasma from 16 Bruton kinase inhibitor-naïve patients with chronic lymphocytic leukemia was treated or not with ibrutinib or acalabrutinib (ACP) for 1 h at 37°C and stimulated with collagen 3.3 μg/mL. Platelet aggregation was assessed by turbidimetry during 10 min. (A) Four patients were identified as having low sensitivity (LS) to ibrutinib (reduction of maximal platelet aggregation <50%, blue) and 12 as having high sensitivity (HS) to ibrutinib (reduction of maximal platelet aggregation >50%, red). (B) Results, expressed as percentage of maximal aggregation in the two groups, are mean ± standard error of mean. **P<0.01, ***P<0.001 according to one-way analysis of variance.
Acalabrutinib is less efficient than ibrutinib at inhibiting signaling events downstream of GPVI
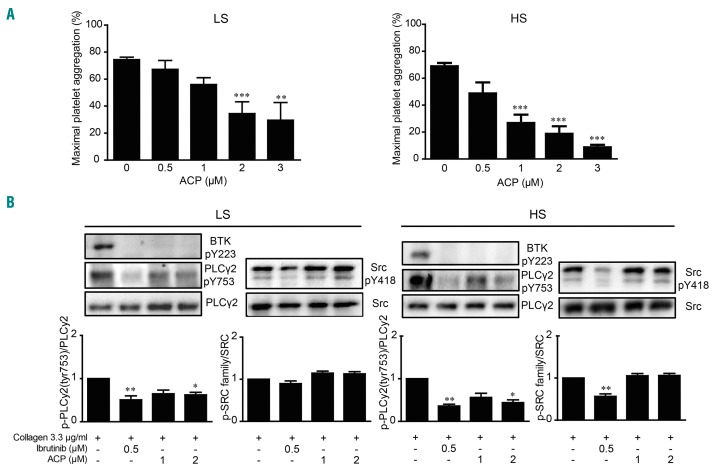
We then compared the impact of acalabrutinib and ibrutinib on tyrosine phosphorylation events downstream of GPVI using washed platelets. Compared with PRP, in which plasma proteins are known to bind and sequester the drug, acalabrutinib was more efficient at inhibiting collagen-induced aggregation of washed platelets. In ibrutinib LS donors the half maximal inhibitory concentration (IC50) was 1.07 ± 0.35 μM, while it was 0.69 ± 0.44 μM in HS donors (Figure 3A). Both ibrutinib and acalabrutinib strongly inhibited Btk autophosphorylation in ibrutinib LS and HS groups (Figure 3B). Ibrutinib was very efficient at blocking PLCγ2 phosphorylation on the Btk-dependent phosphorylation site Tyr753 in both groups. Acalabrutinib at 1 or 2 μM significantly inhibited PLCγ2 Tyr753 phosphorylation in both groups (Figure 3B). The observed stronger effect of ibrutinib on PLCγ2 phosphorylation would be consistent with an off-target effect of this drug on Tec and possibly Src kinases. Indeed, consistent with previous reports,10,24 ibrutinib significantly affected Src activation as assessed by the intensity of its tyrosine 418 phosphorylation. The effect of ibrutinib was more pronounced in the ibrutinib HS group (44 ± 6% inhibition in the HS group vs. 15 ± 5% in the LS group, P<0.01, n=7 for HS and n=10 for LS). Interestingly, while ibrutinib inhibited Src particularly in the HS group, acalabrutinib had no or very little effect on Src activation in both groups. The improved profile of acalabrutinib over ibrutinib on global tyrosine phosphorylation events was confirmed by western blot analysis of the pan-tyrosine phosphorylation pattern in response to collagen stimulation (Online Supplementary Figure S4).
Figure 3.
Effect of ibrutinib and acalabrutinib on tyrosine phosphorylation events. (A) Washed platelets from healthy donors were treated or not with increasing doses of acalabrutinib (ACP) for 1 h at 37°C and stimulated with collagen 3.3 μg/mL. Platelet aggregation was assessed by turbidimetry during 10 min and results, expressed as percentage of maximal aggregation, are mean ± standard error of mean (SEM). Ten donors with low sensitivity (LS) and eight with high sensitivity (HS) to ibrutinib were analyzed. **P<0.01, according to one-way analysis of variance (ANOVA). Half maximal inhibitory concentrations (IC50) were determined using GraphPad Prism software. (B) In parallel to aggregation, the effect of ibrutinib and acalabrutinib on platelet tyrosine phosphorylation events (PLCγ2 phosphorylation on Tyr-753 and Src phosphorylation on Tyr-418) in response to 1 min stimulation with collagen 3.3 μg/mL was assessed by western blotting. The results of the western blot quantification by densitometric analysis are shown as means ± SEM from ten independent experiments for LS and seven independent experiments for HS. *P<0.05, **P<0.01, according to one-way ANOVA. Representative western blots are shown for each group.
Weak impact of acalabrutinib on thrombus formation on collagen under flow
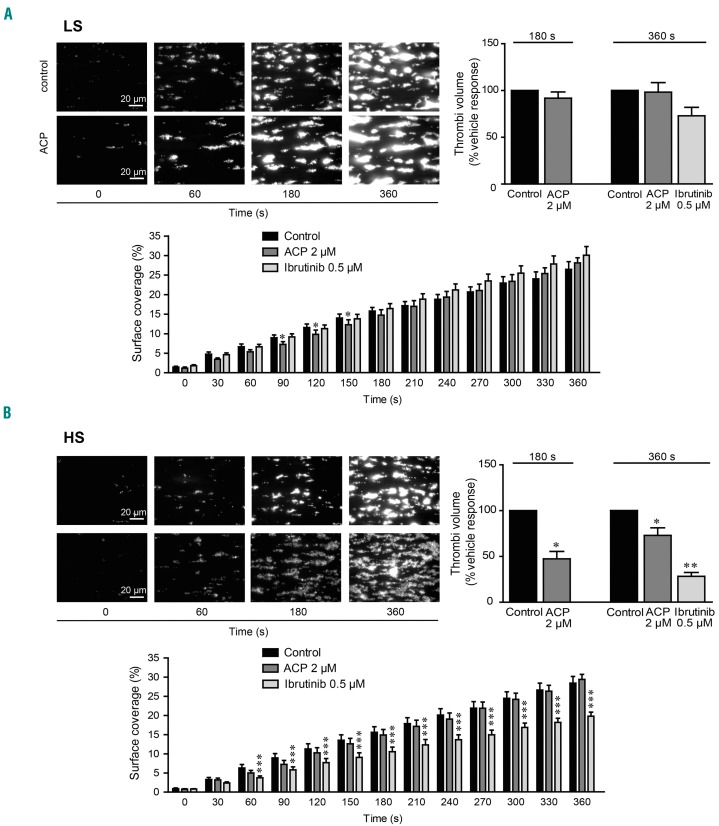
Ibrutinib has been shown to affect thrombus formation and stability on a collagen matrix under flow12,24 and firm platelet adhesion on von Willebrand factor.10 Given the difference of effects of acalabrutinib observed in the two groups of healthy donors, we performed platelet adhesion and thrombus formation assays under an arterial shear rate to mimic the in vivo situation. Whole blood, treated or not with 0.5 μM ibrutinib or 2 μM acalabrutinib for 1 h, was perfused over a collagen matrix and platelet adhesion and thrombus formation were monitored by real-time imaging. Acalabrutinib had no effect on platelet surface coverage in both groups, indicating that platelet adhesion was spared (Figure 4A, B). However, while this drug had no impact on the thrombus volume in the ibrutinib LS group of healthy donors, it significantly decreased thrombus volume in the HS group (Figure 4A, B). As expected, in similar conditions, ibrutinib significantly decreased surface coverage and thrombus volume in the ibrutinib HS group and tended to decrease thrombus volume in the LS group.
Figure 4.
Effect of acalabrutinib on platelet thrombus formation ex vivo on collagen under arterial flow. (A, B) DIOC6-labeled platelets from healthy donors with (A) low sensitivity (LS) or (B) high sensitivity (HS) to ibrutinib (determined on the basis of their aggregation response as in Figure 1) pre-incubated with acalabrutinib (ACP) 2 μM, ibrutinib 0.5 μM or dimethylsulfoxide (control) for 1 h at 37°C were perfused through a collagen-coated microcapillary at a physiological arterial shear rate of 1500 s−1 for 180 or 360 s. Surface coverage (%) and thrombi volume (% of vehicle response) were analyzed using ImageJ software. Results are presented as mean ± standard error of mean of three or four independent experiments. *P<0.05, **P<0.01, ***P<0.001 according to two-way analysis of variance (ANOVA) for surface coverage, one-way ANOVA for thrombi volume at 360 s and a one sample t-test for thrombi volume at 180 s.
Effect of associations of antiplatelet drugs and ibrutinib or acalabrutinib
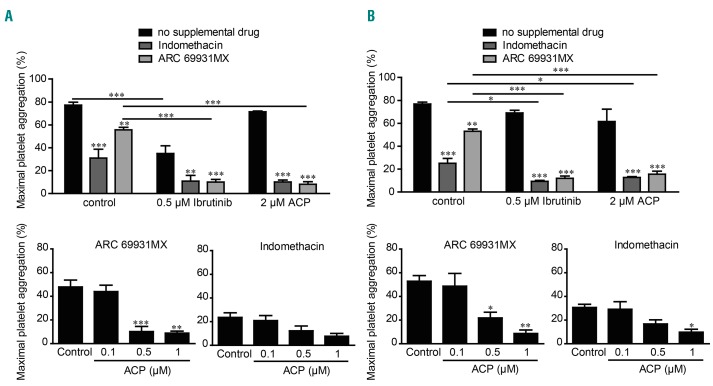
The management of bleeding risk in patients with cardiovascular disease under dual antiplatelet therapy for primary or secondary prevention treated with Btk inhibitors is of concern in clinical practice.4,5,28 There is currently little information to guide clinicians in making decisions about antiplatelet therapy concurrently with Btk inhibitors. We therefore tested the effect of combinations of ibrutinib or acalabrutinib with indomethacin (an aspirin-like drug) or cangrelor (ARC69931MX), an antagonist of the P2Y12 ADP receptor, on platelet aggregation evoked by collagen in PRP from the two groups of healthy donors (Figure 5). In both groups, platelet aggregation was significantly inhibited by indomethacin and to a lesser extent by cangrelor. Importantly, the combination of indomethacin or cangrelor with ibrutinib at a clinically relevant dose amplified the inhibition of platelet aggregation in the ibrutinib HS group. In the ibrutinib LS group, ibrutinib at 0.5 μM did not have a significant effect on the maximal platelet aggregation induced by collagen but increased the effect of indomethacin or cangrelor (in accordance with the 6-8% grade 3-4 bleeding events reported in clinical trials). Interestingly, acalabrutinib, at a clinically relevant dose which had no impact on maximal platelet aggregation induced by collagen, also strongly potentiated, in a dose-dependent manner, the effect of indomethacin and cangrelor in both groups (Figure 5A, B). These data indicate that these two Btk inhibitors potentiated the effect of cyclooxygenase inhibition and P2Y12 antagonism, even in the ibrutinib LS group (Figure 5).
Figure 5.
Effect of ibrutinib and acalabrutinib in association with anti-platelet drugs. (A, B) Platelet-rich plasma from healthy donors with (A) high sensitivity (HS) or (B) low sensitivity (LS) to ibrutinib was pre-treated or not with the indicated doses of acalabrutinib (ACP) or ibrutinib for 1 h at 37°C and anti-platelets drugs (10 μM indomethacin and/or 10 μM ARC69931MX) were added 10 min before stimulation with collagen 3.3 μg/mL. Platelet aggregation was assessed by turbidimetry during 10 min and results, expressed as percentage of maximal aggregation, are the mean ± standard error of mean of three to five independent experiments. *P<0.05, **P<0.01, ***P<0.001 according to one-way and two-way analyses of variance.
Discussion
The first-generation Btk inhibitor ibrutinib has revolutionized the therapy of CLL and mantle cell lymphoma but the drug can cause some side effects such as atrial fibrillation and bleeding.1–3,28 The occurrence of side effects is the major reason for discontinuing ibrutinib during the first year of treatment, with this translating into shorter progression-free and overall survivals.29 Management of bleeding is therefore of paramount importance, especially in the 10-12% of cases who develop atrial fibrillation and require anticoagulation (which increases the risk of grade 3-4 bleeding). The second-generation irreversible Btk inhibitor, acalabrutinib, with less off-target kinase inhibition, is expected to improve the safety profile, including bleeding, of Btk inhibition.9,19,24 In this study we characterized the effects of acalabrutinib on platelet functions in vitro and ex vivo and compared these effects with those of ibrutinib.
We performed a series of assays on two populations of healthy donors characterized by high or low sensitivity to ibrutinib, based on the degree of inhibition of collagen-induced platelet aggregation in PRP achieved by the drug.
We provide evidence that one factor contributing to predispose platelets to ibrutinib sensitivity in vitro is the drug efflux pump system. Indeed, inhibition of drug efflux pumps was sufficient to induce ibrutinib sensitivity in the LS group of healthy donors. This suggests that the actual dose of ibrutinib reaching intracellular targets is critical and will determine the extent of inhibition of Btk and Tec and possibly other undesired targets such as Src-kinases. These data are in line with those of a very recent study suggesting that the ibrutinib-mediated increase of bleeding is due to off-targets effects of the drug occurring because of unfavorable pharmacodynamics.27 The authors propose that the bleeding side effect of ibrutinib may be avoided by reduction of the dose. Our data suggest that an efficient platelet drug efflux pump system may limit the multifactorial antiplatelet effects of ibrutinib. This is important new information to take into consideration when interpreting the results of in vitro experiments with ibrutinib. Moreover, it could be interesting to analyze a potential link between polymorphisms of drug efflux pumps and the risk of bleeding in patients treated with ibrutinib. These data may also stimulate pharmacists to look for intake of P-glycoprotein inhibitors among co-medications in patients with prolonged bleeding under ibrutinib. Since verapamil increases the plasma concentrations of amiodarone and likely the intra-platelet concentration of ibrutinib, cardiologists may favor the use of β-blockers when prescribing drugs to lower the heart rate. Evaluating the sensitivity of a patient’s platelets to ibrutinib before starting therapy could also help clinicians to establish a personalized therapeutic strategy.
Our standard in vitro aggregation tests indicated that acalabrutinib had no effect on the maximal platelet aggregation response in the ibrutinib LS group of healthy donors. In the ibrutinib HS group of healthy donors, acalabrutinib affected maximal platelet aggregation only in a few cases. However, acalabrutinib consistently delayed platelet aggregation in both groups. As expected, acalabrutinib had no effect on platelet aggregation induced by TRAP, U46619 or ADP. These data point to a better profile of acalabrutinib on platelets from healthy donors compared to that of ibrutinib.
Importantly, when acalabrutinib was tested on PRP from CLL patients, it had no effect on the maximal platelet aggregation response in the ibrutinib LS group, but significantly inhibited platelet aggregation in the ibrutinib HS group of patients. These data suggest that a switch from ibrutinib to acalabrutinib therapy may not be systematically appropriate to prevent bleeding in CLL patients.5 The results from clinical trials show that, although no grade 3 bleeding was observed in relapsed CLL patients treated with acalabrutinib, low-grade hemorrhages occurred in a significant proportion of patients, comparable with those observed with ibrutinib.4,9,24
Ibrutinib has been shown to reduce the stability of platelet thrombus on collagen24 and firm platelet adhesion on the von Willebrand factor matrix.10 Consistent with the data reported by Bye et al.,24 we found here that acalabrutinib had no effect on thrombus formation on collagen in the ibrutinib LS group of healthy donors. However, in the ibrutinib HS group, while acalabrutinib did not affect platelet adhesion it was able to significantly reduce thrombus volume. Again these data show a better profile of acalabrutinib, although with some significant impact in the ibrutinib HS group.
The effects of ibrutinib and acalabrutinib on the Btk-mediated tyrosine phosphorylation of PLCγ2 on Tyr-753 and on the Src autophosphorylation site Tyr-418 (activated form of Src-kinases) were investigated in washed platelets from the two groups of healthy donors. Ibrutinib, at the clinically relevant dose of 0.5 μM, inhibited phosphorylation at both sites, with a significantly more intense effect in the ibrutinib HS group. The strong inhibition of phosphorylation observed in the ibrutinib HS group correlated with the inhibition of platelet aggregation. The weaker inhibition of phosphorylation in the ibrutinib LS group was accompanied by a weaker decrease of aggregation. Acalabrutinib efficiently inhibited Btk phosphorylation, significantly decreased PLCγ2 Tyr-753 but had no effect on Src phosphorylation in either group, even at a dose of 2 μM which is above the 1.3 μM mean peak of free plasma drug concentrations measured in patients.9 These data are consistent with the better selectivity of acalabrutinib on Btk, also shown by the whole tyrosine phosphorylation profile of collagen-stimulated platelets. However, it is worth noting that while a dose of 2 μM acalabrutinib had no effect on Src activation it did decrease platelet aggregation significantly in the ibrutinib HS group.
A relevant clinical scenario is the association of Btk inhibitors with dual antiplatelet therapy in patients with cardiovascular diseases, particularly after percutaneous coronary intervention with stent placement. The current dual antiplatelet therapy is based on aspirin and a P2Y12 ADP receptor antagonist such as clopidogrel, prasugrel, ticagrelor or cangrelor. Our data are consistent with those of a previous study showing that ibrutinib amplifies the effect of cangrelor on platelets12 and also demonstrate that acalabrutinib strongly potentiated the effect of indomethacin or cangrelor on platelet aggregation induced by collagen both in the ibrutinib HS and LS groups of healthy donors. This is important information for guiding therapeutic strategies in patients under antiplatelet therapy at high risk of bleeding.
In conclusion, this study provides new insights into the impact of the first- and second-generation Btk inhibitors, ibrutinib and acalabrutinib, on platelets and contributes to the improvement of evidence-based recommendations for a safer use of these targeted therapies.
Acknowledgments
This work was supported by grants from Inserm, Fondation pour la Recherche Médicale (DEQ20170336737) and Janssen. We thank the Genotoul imaging facility of I2MC (Inserm U1048). BP is a scholar of the Institut Universitaire de France.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/11/2292
References
- 1.Thompson PA, Burger JA. Bruton’s tyrosine kinase inhibitors: first and second generation agents for patients with chronic lymphocytic leukemia (CLL). Expert Opin Investig Drugs. 2018;27(1):31–42. [DOI] [PubMed] [Google Scholar]
- 2.Seiler T, Dreyling M. Bruton’s tyrosine kinase inhibitors in B-cell lymphoma: current experience and future perspectives. Expert Opin Investig Drugs. 2017;26(8):909–915. [DOI] [PubMed] [Google Scholar]
- 3.Chakraborty R, Kapoor P, Ansell SM, Gertz MA. Ibrutinib for the treatment of Waldenström macroglobulinemia. Expert Rev Hematol. 2015;8(5):569–579. [DOI] [PubMed] [Google Scholar]
- 4.Shatzel JJ, Olson SR, Tao DL, McCarty OJT, Danilov AV, Deloughery TG. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J Thromb Haemost. 2017;15(5):835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use : a systematic review and meta-analysis. Blood Adv. 2017;1(12):772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rushworth SA, MacEwan DJ, Bowles KM. Ibrutininb in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(13):1277–1278. [DOI] [PubMed] [Google Scholar]
- 7.Byrd JC, Fruman RR, Coutre SE, et al. Targeting BTK with ibrutininb in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang ML, Rule S, Martin P, Goy A, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levade M, David E, Garcia C, et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124(26):3991–3995. [DOI] [PubMed] [Google Scholar]
- 11.Kamel S, Horton L, Ysebaert L, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29(4):783–787. [DOI] [PubMed] [Google Scholar]
- 12.Bye AP, Unsworth AJ, Vaiyapuri S, Stainer AR, Fry MJ, Gibbins JM. Ibrutinib inhibits platelet integrin αIIbβ3 outside-in signaling and thrombus stability but not adhesion to collagen. Arterioscler Thromb Vasc Biol. 2015;35(11):2326–2335. [DOI] [PubMed] [Google Scholar]
- 13.Rigg RA, Aslan JE, Healy LD, et al. Oral administration of Bruton’s tyrosine kinase inhibitors impairs GPVI-mediated platelet function. Am J Physiol Cell Physiol. 2016;310(5):C373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quek LS, Bolen J, Watson SP. A role for Bruton’s tyrosine kinase (Btk) in platelet activation by collagen. Cur Biol. 1998;8(20): 1137–1140. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson BT, Ellmeier W, Watson SP. Tec regulates platelet activation by GPVI in the absence of Btk. Blood. 2003;102(10):3592–3599. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Fitzgerald ME, Berndt MC, Jackson CW, Gartner TK. Bruton tyrosine kinase is essential for botrocetin/VWF-induced signaling and GPIb-dependent thrombus formation in vivo. Blood. 2006;108(8):2596–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Futatani T, Watanabe C, Baba Y, Tsukada S, Ochs HD. Bruton’s tyrosine kinase is present in normal platelets and its absence identifies patients X-linked agammaglobulinaemia and carrier females. Br J Haematol. 2001;114(1):141–149. [DOI] [PubMed] [Google Scholar]
- 18.Winkelstein JA, Marino MC, Lederman HM, et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine. 2006;85(4):193–202. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol. 2016;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barf T, Covey T, Izumi R, et al. Acalabrutinib (ACP-196): a covalent bruton tyrosine kinase inhibitor with a differentiat ed selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363(2):240–252. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Kinoshita T, Gururaja T, et al. The effect of Bruton’s tyrosine kinase (BTK) inhibitors on collagen-induced platelet aggregation, BTK, and tyrosine kinase expressed in hepatocellular carcinoma (TEC). Eur J Haematol. 2018. June 20 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Senis YA, Mazharian A, Mori J. Src family kinases: at the forefront of platelet activation. Blood. 2014;124(13):2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gratacap MP, Martin V, Valéra MC, et al. The new tyrosine-kinase inhibitor and anticancer drug dasatinib reversibly affects platelet activation in vitro and in vivo. Blood. 2009;114(9):1884–1892. [DOI] [PubMed] [Google Scholar]
- 24.Bye AP, Unsworth AJ, Desborough MJ, et al. Severe platelet dysfunction in NHL patients receiving ibrutinib is absent in patients receiving acalabrutinib. Blood Adv. 2017;1(26):2610–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busygina K, Jamasbi J, Seiler T, et al. Oral Bruton tyrosine kinase inhibitors selectively block atherosclerotic plaque-triggered thrombus formation. Blood. 2018;131(24): 2605–2616. [DOI] [PubMed] [Google Scholar]
- 26.Laurent PA, Séverin S, Hechler B, et al. Platelet PI3kbeta and GSK3 regulate thrombus stability at a high shear rate. Blood. 2015;125(5):881–888. [DOI] [PubMed] [Google Scholar]
- 27.Nicolson PLR, Hughes CE, Watson S, et al. Inhibition of Btk-specific concentrations of ibrutinib and acalabrutinib delays but does not block platelet aggregation to GPVI. Haematologica. 2018;103(12):2097–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguilar C. Ibrutinib-related bleeding: pathogenesis, clinical implications and management. Blood Coagul Fibrinolysis. 2018;29(6):481–487. [DOI] [PubMed] [Google Scholar]
- 29.Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the united states: a real-world analysis. Haematologica. 2018;103(5): 874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]