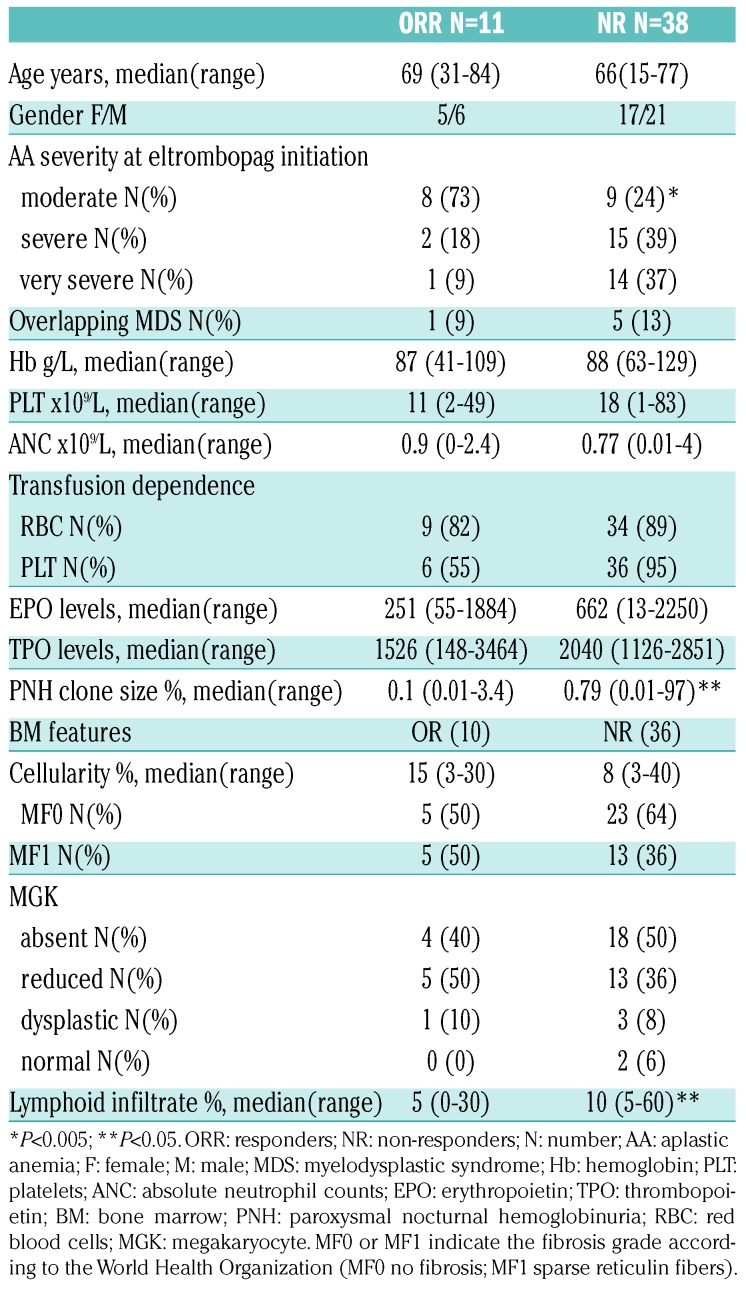
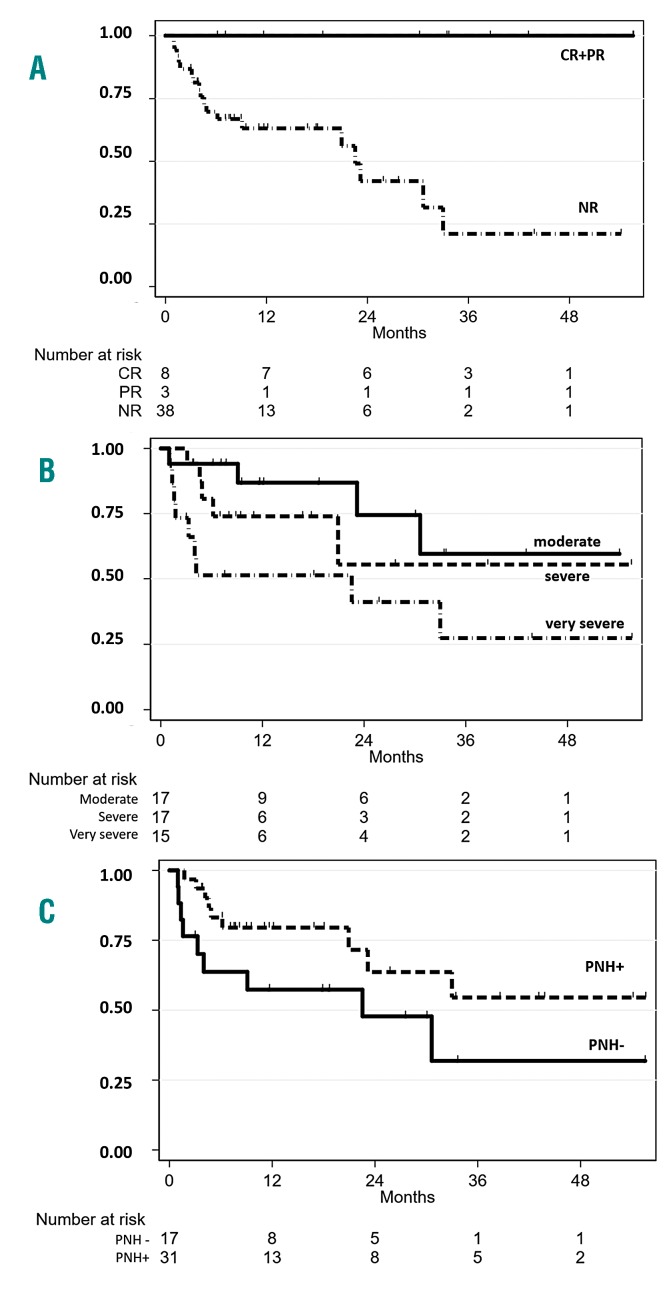
Treatment of aplastic anemia (AA) in the elderly is particularly challenging due to higher toxicity and lower efficacy of the available therapies. In fact, 30-40% of patients eligible for intensive immunosuppressive treatment (IST) with horse antithymocyte globulin (ATG) plus cyclosporine (CyA) will relapse and only a few cases would be candidates for hematopoietic stem cell transplant (HSCT).1,2 Moreover, ATG might be contraindicated in frail patients or in those with infectious comorbidities, and CyA alone is of limited benefit.3,4 Recently, prospective trials show the clinical efficacy of eltrombopag in refractory/relapsed AA, with up to 40% hematologic improvement,5 and, intriguingly, persistent remission upon discontinuation in robust responders.6 Moreover, eltrombopag was added to front-line IST, with an overall response rate exceeding 80%.7 Clinical and laboratory characteristics predicting eltrombopag response in AA are still largely unknown, and data about real-life use of this drug are scarce. Here we analyzed the baseline hematologic and morphological (bone marrow aspirate and trephine) variables and clinical course of 49 AA cases treated with eltrombopag at two tertiary hematologic institutions in the UK, from January 2012 to January 2018. We focused on efficacy, safety, impact on disease outcome, and on potential clinical and laboratory predictors of treatment response (Online Supplementary Methods). Median age was 67 years [interquartile range (IQR): 50.5-71.5, 82% >60 years], 56% of cases had severe or very severe AA, and the majority were either red cell or platelet transfusion dependent (Table 1). Endogenous erythropoietin (EPO) and thrombopoietin (TPO) levels were markedly elevated in all cases, and telomere length (TL) reduced (<10th percentile) in 11. Regarding bone marrow (BM) features, all cases displayed reduced cellularity; the relative percentage of lymphoid cells was >10% in 20 cases, with no clonal lymphoid infiltrate or cases of T-cell large granular lymphocytic (TLGL) leukemia by immunohistochemistry or immunophenotyping. Cytogenetics were abnormal in four patients (2 with del13q, one each with trisomy 8 and loss of Y), and failed in eight cases (16%) of whom one showed presence of the +8 by fluorescence in situ hybridization (FISH). Paroxysmal nocturnal hemoglobinuria (PNH) clone was detected in 32 patients (65%), and clone size was <1% in 11 cases, 1-10% in 12, 10-50% in 5, >50% in 3 cases (one requiring eculizumab). Seven patients were treatment naïve and 42 had received prior therapy (median: 1; range: 1-3), of whom eight patients had relapsed and 34 were refractory AA (Online Supplementary Figure S1). The median time from diagnosis to eltrombopag was 330 days (IQR: 108-695), shorter for the treatment naïve group. Patients received eltrombopag for a median of four months (range: 3.5-30 months) at median dose of 150 mg/day, and CyA was continued in three patients. Overall 11 patients (22%) responded to eltrombopag, nine partial response (PR) and two complete response (CR) according to the criteria of the European Group for Blood and Marrow Transplantation (EBMT) (first response) at 12 weeks. Six patients converted their PR into CR (best response) on continuing treatment. Mean time to first response was 4.4+2 months and to best response was 12.3+10.5 months. Interestingly, all responders attained trilineage hematologic improvement and robust response, as by National Institutes of Health criteria. Mean Hb level increase from baseline was 34+12.4 g/L, platelet count increase was 80+53×109/L, and ANC increase 1.25+0.95x109/L (P<0.001, P<0.001, and P=0.02, respectively). Moreover, 21% and 14% cases became red cell and platelet transfusion independent, respectively. BM re-evaluation after eltrombopag (n=25) showed significantly increased cellularity (30+26% vs. 16+13%; P=0.004), more prominent in responders, and one responding patient displayed increased fibrosis (from MF0 to MF2). Considering PNH clone size dynamic after eltrombopag treatment, median granulocyte size increased in ten cases (mean increase 12%) and decreased in eight (mean decrease 11%) while all other patients showed stable clones before and after eltrombopag; no relationships were observed with eltrombopag response. Regarding predictors of response (Table 2), overall response (OR) was significantly related with a moderate disease, lower BM lymphoid percentage, and a smaller PNH clone. Considering previous therapies, treatment naïve cases showed significantly better OR compared to relapse/refractory patients (42% vs. 15%; P=0.03). Refractory cases showed a lower OR compared to relapsed ones [17.6% vs. 37.5%; not significant (ns)], particularly among CyA-treated patients (7% vs. 18%; P=0.05). Finally, among those tested for TL, patients with TL >1st percentile showed a higher OR (22.2%) compared to the others (0%), although this difference was not significant. As regards safety, we observed mostly grade I/II adverse events in up to 29% of cases; 11 patients are still on treatment, whereas 38 patients discontinued eltrombopag after a median of four months (range: 1-32 months) (Online Supplementary Results). Three patients relapsed after 7, 15 and 34 months of treatment, and received various therapies (Online Supplementary Figure S1). The first case was then treated with danazol and reached PR. The second case had concomitant autoimmune thrombocytopenia and was treated with steroids and intravenous immunoglobulin with platelet count recovery, and then re-challenged with eltrombopag until CR. The third patient received CyA and then tacrolimus with intolerance/no response, and continues to be transfusion dependent. Further therapies for NR cases are detailed in Online Supplementary Figure S1. Nineteen patients (39%) died, and median OS from the beginning of eltrombopag was nine months (range: 4-54 months). Patients died because of progressive disease complications including sepsis (n=10), severe cytopenias with multi-organ failure (n=7), chest fungal infection (n=1), and bacterial pneumonia (n=1). OS (censored for HSCT) was significantly longer in patients responding to eltrombopag [33.3 (95%CI: 21.4-45.2) vs. 7.5 (4.07-10.9) months; P=0.005], in those with moderate AA (at diagnosis and before eltrombopag treatment; P=0.001 and P=0.01), and in the presence of a PNH clone (P=0.05) (Figure 1). Finally, patients with normal or reduced BM megakaryocytes showed a better outcome compared to cases with absent megakaryocytes (84% vs. 41%; P=0.001); likewise, patients with TL >1% and normal karyotype also showed better OS, although the difference was not significant.
Table 1.
Baseline clinical and laboratory characteristics of aplastic anemia patients enrolled.
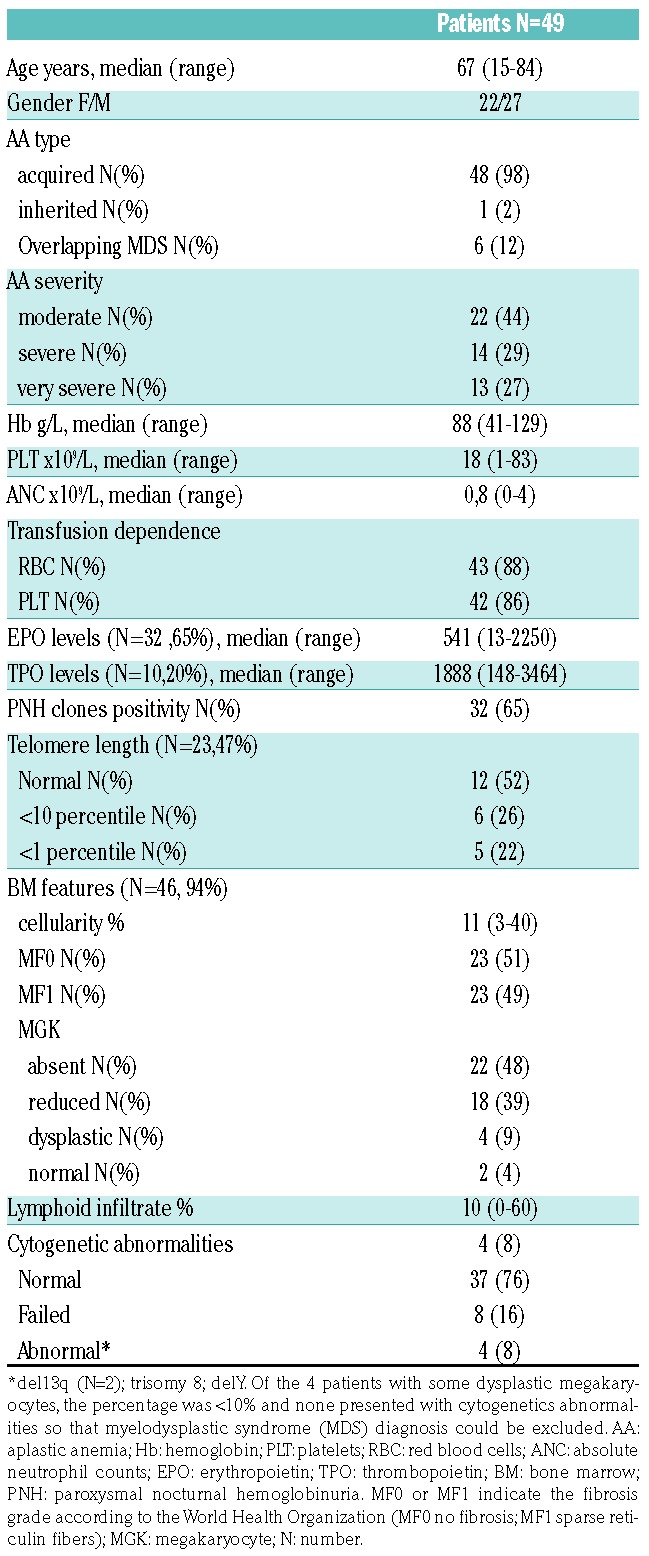
Table 2.
Response evaluation: relationship with baseline clinical and laboratory characteristics.
Figure 1.
Overall survival in aplastic anemia patients. Overall survival by Kaplan-Meyer method in eltrombopag-treated patients divided according to (A) response to eltrombopag (P=0.005), (B) disease severity (P=0.001), and (C) paroxysmal nocturnal hemoglobinuria (PNH) positivity (P=0.05) at the time of eltrombopag initiation. CR: complete responders; PR: partial responders; NR: non-responders.
In this real-world study, we report eltrombopag efficacy in approximately 25% of AA cases, mostly relapsed after or refractory to IST. Response was lower than that reported in early phase studies6,7 likely mirroring the heterogeneity and complexity of real-world series, and the older age in our study. Our data are more similar to the recent French study evaluating 46 cases treated outside clinical trials, that showed trilineage hematologic improvement in 27% IST naïve and 34% refractory cases.8 Survival of older patients with AA is only 38% at five years using IST with ATG and CyA. Moreover, a large study from Sweden5 using CyA alone in AA reported no survival difference compared to supportive care alone. Hence the need for new approaches to treatment in this older cohort of patients. We demonstrate for the first time a favorable impact of non-severe disease, lower polyclonal BM lymphoid infiltrate, and of a smaller PNH clone on the response to eltrombopag. Previous studies showed inconsistent predictors of response: in prospective trials, better OR has been associated with higher reticulocyte counts in refractory cases,6 and with longer TL and younger age in naïve patients.7 Our results, although preliminary and in a small series, suggest that eltrombopag is more effective in the setting of less severe BM exhaustion, when stem cell renewal capability and residual cellularity are greater. This is in line with the reported ability of eltrombopag to stimulate hematopoiesis at the level of primitive hematopoietic cells.9,10 We found that responders displayed higher cellularity and better megakaryocytic morphology (i.e. reduced compared to absent megakaryocytes), suggesting that eltrombopag may act better in the presence of a greater bone marrow reserve. Consistently, a possible impact of BM cellularity on OR has been recently reported in primary immune thrombocytopenia patients.11 Moreover, we observed a better response in patients with lower BM lymphoid percentage, and in those previously responding to CyA. These findings may reflect the influence of the immune system on OR and might suggest a role for combining eltrombopag with IST in these cases; data from the RACE trial (IST vs. IST+eltrombopag front line) will possibly clarify this point.
More than one-third of patients died, with survival being significantly related to response to eltrombopag and presence of less severe disease, suggesting that the BM stem cell pool is critical for survival. Interestingly, OS significantly correlated with PNH positivity, in line with recent reports in a large cohort of patients with AA and myelodysplastic syndrome.12 Although these observations need to be confirmed in prospective studies, they might be consistent with the presence of a stronger immune surveillance against disease progression in PNH-positive cases.12
In our study, we confirmed that eltrombopag can be discontinued with persistent response and re-administered after interruption obtaining a new response, in line with previous reports in AA and ITP.6 In spite of the concerns about clonal evolution during TPO-mimetics stimulation, we did not observe disease transformation, not even in patients with aberrant karyotype, except for one who evolved to refractory cytopenia with multilineage dysplasia. The limited follow up does not allow definitive conclusions to be drawn, as the appearance of new cytogenetic abnormalities, including chromosome 7 loss/partial deletion, has been described after longer follow up.6
In conclusion, here we describe a clinically challenging elderly population, with high mortality and limited treatment options beyond best supportive care. In this setting, eltrombopag is an effective and safe option, with better results in treatment naïve patients, non-severe disease, and a lower BM lymphoid infiltrate. Finally, we showed that eltrombopag impacts on disease natural history, prolonging survival in responding patients.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Killick SB, Bown N, Cavenagh J, et al. British Society for Standards in Haematology Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172(2):187–207. [DOI] [PubMed] [Google Scholar]
- 2.Marsh JC, Bacigalupo A, Schrezenmeier H, et al. European Blood and Marrow Transplant Group Severe Aplastic Anaemia Working Party Prospective study of rabbit antithymocyte globulin and cyclosporine for aplastic anemia from the EBMT Severe Aplastic Anaemia Working Party. Blood. 2012;119(23):5391–5396. [DOI] [PubMed] [Google Scholar]
- 3.Marsh J, Schrezenmeier H, Marin P, et al. Prospective randomized multicenter study comparing cyclosporin alone versus the combination of antithymocyte globulin and cyclosporin for treatment of patients with nonsevere aplastic anemia: a report from the European Blood and Marrow Transplant (EBMT) Severe Aplastic Anaemia Working Party. Blood. 1999;93(7):2191–2195. [PubMed] [Google Scholar]
- 4.Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000-2011. Haematologica. 2017;102(10):1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367(3):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desmond R, Townsley DM, Dumitriu B, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014;123(12):1818–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N Engl J Med. 2017;376(16):1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lengline E, Drenou B, Peterlin P, et al. Nationwide survey on the use of eltrombopag in patients with severe aplastic anemia: a report on behalf of the French Reference Center for Aplastic Anemia. Haematologica. 2018;103(2):212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87(6):2162–2170. [PubMed] [Google Scholar]
- 10.Qian H, Buza-Vidas N, Hyland CD, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1(6):671–684. [DOI] [PubMed] [Google Scholar]
- 11.Uto Y, Fujiwara S, Arai N, et al. Age and bone marrow cellularity are associated with response to eltrombopag in japanese adult immune thrombocytopenia patients: a retrospective single-center study. Rinsho Byori. 2015;63(5):548–556. [PubMed] [Google Scholar]
- 12.Fattizzo B, Dunlop A, Ireland R, et al. Clinical significance of PNH clones in 3085 patients with cytopenia: a large single-center experience. [Abstract PF304] 23rd Meeting of the European Hematology Association, Stockholm June 2018. [Google Scholar]