Abstract
It has been postulated that monitoring measurable residual disease (MRD) could be used as a surrogate marker of progression-free survival (PFS) in chronic lymphocytic leukemia (CLL) patients after treatment with immunochemotherapy regimens. In this study, we analyzed the outcome of 84 patients at 3 years of follow-up after first-line treatment with fludarabine, cyclophosphamide and rituximab (FCR) induction followed by 36 months of rituximab maintenance thearpy. MRD was assessed by a quantitative four-color flow cytometry panel with a sensitivity level of 10−4. Eighty out of 84 evaluable patients (95.2%) achieved at least a partial response or better at the end of induction. After clinical evaluation, 74 patients went into rituximab maintenance and the primary endpoint was assessed in the final analysis at 3 years of follow-up. Bone marrow (BM) MRD analysis was performed after the last planned induction course and every 6 months in cases with detectable residual disease during the 36 months of maintenance therapy. Thirty-seven patients (44%) did not have detectable residual disease in the BM prior to maintenance therapy. Interestingly, 29 patients with detectable residual disease in the BM after induction no longer had detectable disease in the BM following maintenance therapy. After a median followup of 6.30 years, the median overall survival (OS) and PFS had not been reached in patients with either undetectable or detectable residual disease in the BM, who had achieved a complete response at the time of starting maintenance therapy. Interestingly, univariate analysis showed that after rituximab maintenance OS was not affected by IGHV status (mutated vs. unmutated OS: 85.7% alive at 7.2 years vs. 79.6% alive at 7.3 years, respectively). As per protocol, 15 patients (17.8%), who achieved a complete response and undetectable peripheral blood and BM residual disease after four courses of induction, were allowed to stop fludarabine and cyclophosphamide and complete two additional courses of rituximab and continue with maintenance therapy for 18 cycles. Surprisingly, the outcome in this population was similar to that observed in patients who received the full six cycles of the induction regimen. These data show that, compared to historic controls, patients treated with FCR followed by rituximab maintenance have high-quality responses with fewer relapses and improved OS. The tolerability of this regime is favorable. Furthermore, attaining an early undetectable residual disease status could shorten the duration of chemoimmunotherapy, reducing toxicities and preventing long-term side effects. The analysis of BM MRD after fludarabine-based induction could be a powerful predictor of post-maintenance outcomes in patients with CLL undergoing rituximab maintenance and could be a valuable tool to identify patients at high risk of relapse, influencing further treatment strategies. This trial is registered with EudraCT n. 2007-002733-36 and ClinicalTrials.gov Identifier: NCT00545714.
Introduction
Chronic lymphocytic leukemia (CLL) is a mature B-cell neoplasm characterized by a clonal proliferation and compartmentalized accumulation of neoplastic B cells within the blood, bone marrow and secondary lymphatic organs. The neoplastic B cells typically co-express CD5, and CD19, CD20, and CD23; compared with normal B cells, the levels of CD20 and CD79b on CLL cells are usually diminished.1–4 Mutations of immunoglobulin heavy variable chain (IGHV) genes and chromosomal abnormalities are the most important predictors of disease course.
For physically fit patients requiring treatment according to the International Workshop on CLL criteria, the combination of fludarabine, cyclophosphamide and the chimeric anti-CD20 antibody rituximab (FCR) is the standard of care for first-line treatment, based on the improvement of progression-free survival (PFS) and overall survival (OS) of patients treated with this combination compared with those treated with combination chemotherapy alone.5 Following the introduction of purine analogs as a treatment option, higher response rates and a higher proportion of complete remissions were observed, and even better outcomes have been reported in patients carrying mutated IGHV genes (excluding 11q or 17p deletions); patients treated with combination therapies such as FCR may achieve a life expectancy close to that observed in the matched normal general population.6–8
Achieving higher CR rates with chemoimmunotherapy has translated into a documented increase in PFS and seems to lead to an OS benefit, as shown in the CLL8 trial, which reported a 33% reduced risk of death (P=0.01) with the FCR regimen when compared to fludarabine plus cyclophosphamide as first-line therapy. However, while attainment of a CR has historically been considered the gold-standard for treatment response, many of these patients have persistent disease that cannot be easily identified by routine testing approaches.9 This, coupled with the development of extremely sensitive testing technologies, has led to the emergence of measurable residual disease (MRD) as an important endpoint in the treatment of CLL, especially in the era of chemoimmunotherapy. Indeed, achieving undetectable MRD after chemoimmunotherapy is a desirable goal, as MRD below a threshold of 10−4 (0.01%) results in improvement of PFS and OS.8 We hypothesized that using MRD as a surrogate of treatment effectiveness would allow determination of the efficacy of new treatments without the need for prolonged observation.
Several studies have shown that sequential use of induction/maintenance treatment can improve the quality of response achieved with induction. Abrisqueta et al. recently reported an analysis of whether maintenance therapy can improve the response achieved with induction chemotherapy.10 Sixty-seven patients responding to induction therapy with FCR plus mitoxantrone (R-FCM) received rituximab maintenance therapy (375 mg/m2) every 3 months for 2 years. Approximately 40.6% of patients achieved a CR with undetectable MRD at the end of the maintenance treatment. It is important to note that 21% of the patients who had detectable MRD at the end of R-FCM induction had an improved response after rituximab maintenance therapy. Another study showed that after responding to a fludarabine induction, patients who had detectable MRD and were consolidated with four monthly cycles of rituximab followed by a maintenance regimen of 12 monthly rituximab doses had significantly longer responses,compared to those who did not receive consolidation (5-year OS: 87% vs. 32%; P<0.001). The estimated 5-year PFS after induction was 73%.11
However, despite the improvements achieved with rituximab maintenance therapy, there are some biological features which confer a poor response to consolidation plus maintenance therapy. Dal Bo et al. showed that patients harboring the NOTCH1 mutation had a significantly shorter OS compared with those with unmutated NOTCH1. The independent prognostic impact of NOTCH1 mutation on OS was confirmed in multivariate analysis.12
In the light of these observations, we conducted a multicenter, non-randomized phase II clinical trial that aimed to evaluate the efficacy, in terms of CR rate, of FCR as first-line treatment for CLL, and to investigate the impact of rituximab maintenance therapy on the response rate and PFS following FCR. A key secondary objective was to analyze MRD status after chemoimmunotherapy and rituximab maintenance.
Methods
Physically fit patients between 18 and 70 years old with active CD20+ CLL according to the World Health Organization classification, with an Eastern Cooperative Oncology Group Performance Status ≤2, were recruited into the REM (rituximab in maintenance) trial and received treatment with fludarabine (25 mg/m2 iv on days 1-3), cyclophosphamide (250 mg/m2 iv on days 1-3) and rituximab (375 mg/m2 iv cycle 1 and 500 mg/m2 iv cycles 2-6) every 28 days, for up to six cycles. Major exclusion criteria were prior treatment for CLL, severe cardiac, pulmonary, neurological, psychiatric, or metabolic disease, continuous systemic corticosteroids, active autoimmune hemolytic anemia or thrombocytopenia, active severe infection, creatinine clearance <50 mL/min, or transformation to an aggressive B-cell malignancy. All cases were CD20+ as analyzed by flow cytometry, with a mean fluorescence intensity lower than the expression found in normal mature B lymphocytes in peripheral blood and bone marrow (BM).
At the 3-month post-induction clinical response evaluation, patients achieving a CR, partial response (PR) or nodular PR (nPR), based on International Workshop on CLL guidelines, were treated with rituximab 375 mg/m2 iv every 2 months for 3 years (18 cycles). Anti-microbial prophylaxis included trimethoprim-sulfamethoxazole and acyclovir during treatment and until the level of CD4+ lymphocytes reached 0.3x109/L. Patients achieving a CR and undetectable MRD in both peripheral blood and BM after four courses of FCR were allowed to stop fludarabine plus cyclophosphamide and complete two courses of rituximab and continue with rituximab maintenance therapy.
The primary endpoint was the CR rate after FCR treatment. Secondary endpoints included PFS, OS, correlation of response with the level of MRD after FCR and rituximab maintenance therapy, adverse events, and the prognostic impact of the biological markers CD38 and ZAP70, IGHV mutational status, cytogenetic abnormalities and BM-MRD on the course of the disease. Fluorescence in situ hybridization and IGHV analysis were performed locally in accredited laboratories using standardized procedures.
The study protocol was approved by the institutional review board of each participating institution and complied with the Declaration of Helsinki, and existing Good Clinical Practice guidelines, laws and regulations. All participants provided written informed consent before enrollment.
Flow cytometry and measurable residual disease analysis
Samples were stained and lysed using a direct immunofluorescence technique as previously described.13 The following antibody combinations were used: (i) CD22/CD23/CD19/CD5; (ii) FMC7/CD43/CD19/CD5; (iii) CD103/CD25/CD19/CD5; (iv) CD10/CD11c/CD19/CD5; (v) CD20/CD38/CD19/CD5; (vi) CD81/CD22/CD19/CD5; (vii) CD20/CD49d/CD19/CD5; (viii) sIgk/sIgl/CD19/CD5, and (ix) ZAP70/CD3+CD56/CD19/CD5. All monoclonal antibodies except ZAP70 were provided by Becton Dickinson (San José, CA, USA). ZAP70 was purchased from Immunotech (Marseille, France). Samples were acquired in a FACSCalibur flow cytometer (Becton Dickinson) and analyzed using Paint-A-Gate PRO software (Becton Dickinson). At least 20,000 events were acquired. B-lymphocytes were identified according to their SSC/CD19+ distribution and the total percentage of pathological CD38 and CD49d B cells was reported. ZAP70 was quantified using a cut-off of ≥20% to define the ZAP70+ subset of B cells.14
MRD was analyzed in samples from peripheral blood and BM after induction and from BM during rituximab maintenance therapy, with a combination of monoclonal antibodies slightly modified from that in the European Research Initiative on CLL (ERIC) protocol: (i) CD20/CD38/CD19/CD5; (ii) CD81/CD22/CD19/CD5; (iii) sIgL/sIgK/CD19/CD5; and (iv) CD22/CD79b/CD19/CD5. CD43 was not included in the analysis: we included the last combination instead of CD43/CD79b/CD19/CD5 based on our previous experience with that combination in the analysis of MRD in CLL.13 The minimum number of pathological B cells acquired was that in the ERIC recommendations.15 To achieve a limit of detection of 0.01%, at least 200,000 events were acquired if the minimum population size was 20 and 500,000 events if the minimum population size was 50. We prepared the necessary number of tubes for each combination to acquire at least 200,000 events. The complete gating strategy is described in the Online Supplement.
Statistical analysis
This was a two-staged, Simon optimal phase II clinical trial. Based on a CR rate observed in previous trials of first-line therapy ranging around 30%, the inactivity cut-off was chosen to equal 30% and the activity cut-off at least 50%. Hence, the hypotheses of interest were H0: r≤0.3 against Ha: r≥0.5%, where r is the CR rate. Using a type I error rate (α, probability of accepting an insufficiently active treatment, a false positive outcome) set at 0.05, and a type II error rate (β, probability of rejecting an active treatment, a false negative outcome) set at 0.20, we estimated that 90 patients should be enrolled into this trial, assuming a 10% loss.
A descriptive analysis of continuous and qualitative variables was performed. PFS, OS and duration of response were summarized descriptively and graphically using the Kaplan and Meier method in the overall population and separately by biological factors, genetic profiles and MRD status. The log-rank test was used for comparisons of PFS and OS curves. The χ2 test was used to assess the frequencies and differences of biological and cytogenetic abnormalities. The relationship between these abnormalities and MRD level was analyzed using logistic regression models. Safety data were summarized for all treated patients during induction, maintenance and combined. All hypothesis tests were two-sided and a P-value <0.05 was considered statistically significant. All statistical computations were carried out with SPSS version 14.0 or subsequent versions.
Results
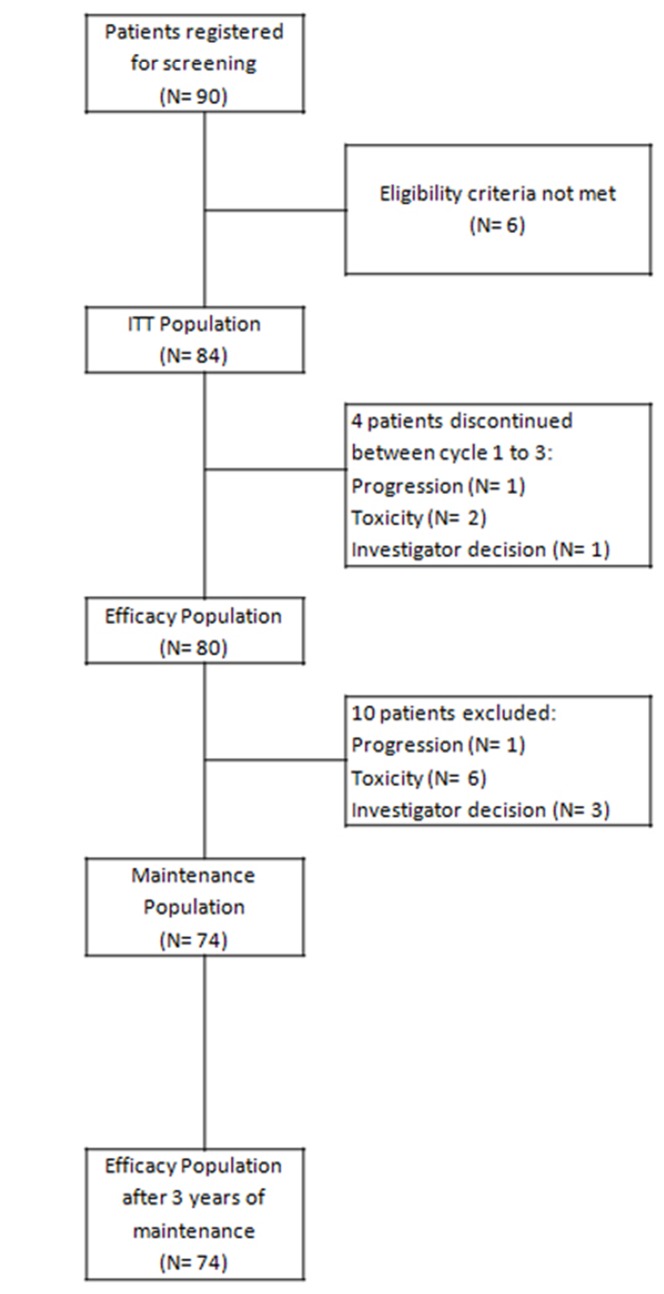
We present the results of an end-of-study analysis at 3 years of follow-up after 36 months of rituximab maintenance therapy following FCR induction. Between October 2007 and December 2012, 90 patients were assessed for eligibility in 29 center across Spain, and 84 were assigned to FCR (6 patients did not meet the eligibility criteria, of whom 2 after 1 treatment infusion) and were evaluable for response in an intent-to-treat analysis (Figure 1). Overall, 79.8% (n=67) of the enrolled patients were aged 64 years or younger, 67.9% (n=57) were male and 83.3% (n=70) had Binet stage B or C disease. The median age of trial participants was 59.5 years (range, 37-70), and 70.5% (n=55) of participants were in a good state of health with an Eastern Cooperative Oncology Group Performance Status of 0-1. Overall, 53.7% (n=45) of the trial population had B symptoms.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram. ITT: intention-to-treat.
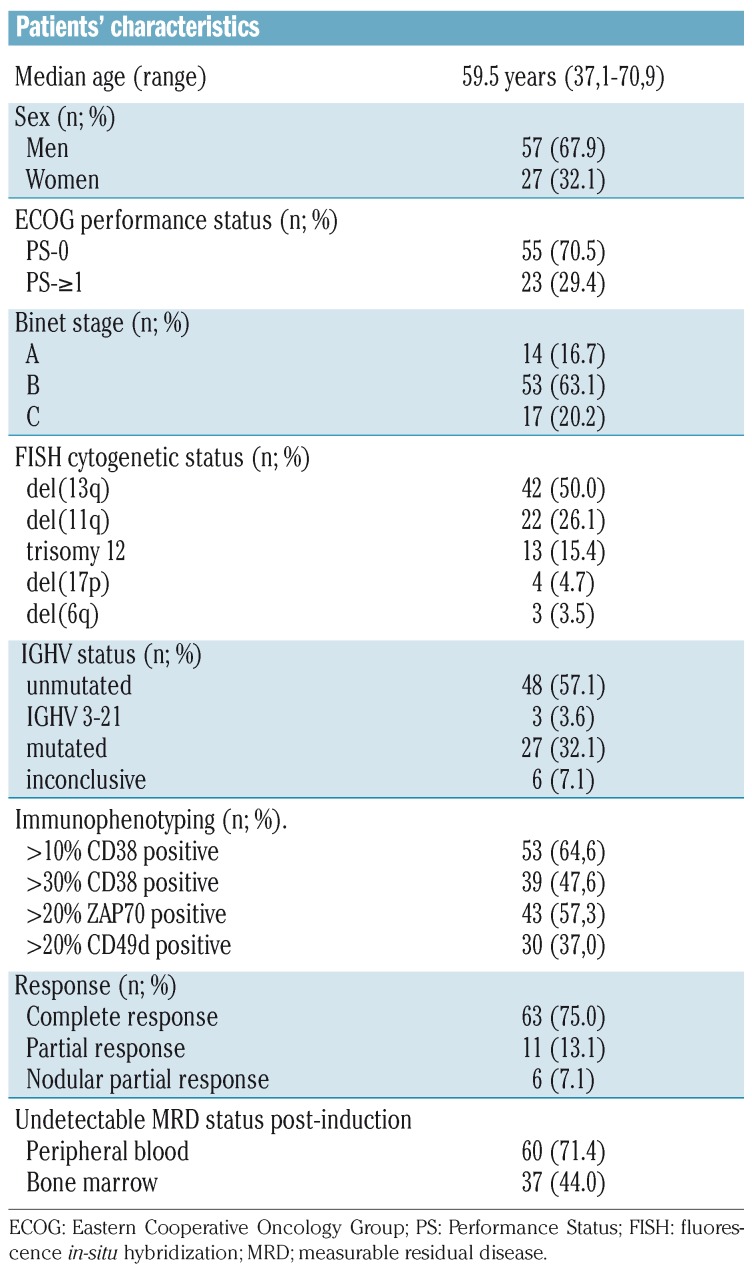
Table 1 summarizes the biological and genetic abnormalities assessed at baseline that were considered to be prognostic for outcome. Overall, 57.14% (n=48) of patients had unmutated IGHV, 47.6% (n=39) were CD38+ and 57.3% (n=43) were ZAP70+. Forty-two (50.0%) patients had a 13q14 deletion, 22 (26.1%) had a 11q22-q23 deletion, 13 (15.4%) harbored trisomy 12, four (4.7%) patients had a 17p deletion, and three (3.5%) had a 6q deletion.
Table 1.
Patients’ baseline characteristics.
Overall, 12 patients (14%) ended treatment induction prematurely. The reasons for discontinuation included toxicity (n=6), progressive disease (n=1), ineligibility (n=2), and investigators’ decision (n=3: 1 patient with ischemic cerebrovascular disease, 1 patient with concomitant idiopathic thrombocytopenic purpura and 1 patient with a karyotype with chromosomal random losses). The median number of FCR cycles was six, and complete treatment was administered to 80% of the patients.
Response and treatment outcomes
Induction
Of 84 evaluable patients in an intent-to-treat analysis of the effects of FCR induction treatment, 80 patients had a CR/CR with incomplete hematologic recovery (CRi), PR or nPR for an overall response rate of 95.2% (75.0% CR/CRi(2), n=63; 7.1% nPR, n=6; 13.1% PR, n=11) while four patients failed to respond to FCR. Of the 80 patients evaluable for BM-MRD status, 44.1% (n=37) had undetectable MRD at 3 months after induction, of whom 35 (41.7%) had a CR and two (2.4%) had a PR, while 43 had detectable MRD, of whom 28 (35.0%) had a CR, eight (10.0%) had a nPR, and seven (8.8%) had a PR.
Rituximab maintenance
Of the 80 patients with CR or PR after FCR induction, 74 entered the maintenance study. At the end of the maintenance phase, 52 patients had a CR and seven had a PR (2 nPR; 5 PR). At cycle 12 of treatment, 29 patients had a CR and four patients had attained a PR. At cycle 9, 42 patients had a CR and five had a PR. Reasons for discon tinuation were myelotoxicity (n=14; 18.9%), clinical progression (n=8; 10.8%), consent withdrawal (n=3; 4.0%) investigator’s decision (n=1; 1.3%), protocol violation (n=1; 1.3%), infection (n=3; 4.0%) and death (n=2; 2.7%). During the follow-up period, all patients who were analyzed maintained CR or PR.
At the end of maintenance therapy, MRD assessed at cycles 9, 12, 15 and 18 was negative in 44 of the 72 patients (61.1%) evaluable for response. Interestingly, 29 patients who had detectable BM-MRD immediately after induction converted to an undetectable BM-MRD status following rituximab maintenance therapy. In detail, after nine cycles, 13 patients with detectable BM-MRD converted to having undetectable BM-MRD and two with undetectable BM-MRD became MRD-positive. After 18 cycles, 16 patients with detectable BM-MRD converted to being MRD-negative and five with undetectable BM-MRD became BM-MRD-positive (2 CR relapsed after 18 months, 1 CR interrupted treatment at 12 months because of toxicity, 1 PR progressed after 13 months and 1 CR relapsed after 4 months) (Table 2).
Table 2.
Measurable residual disease assessment.
Survival
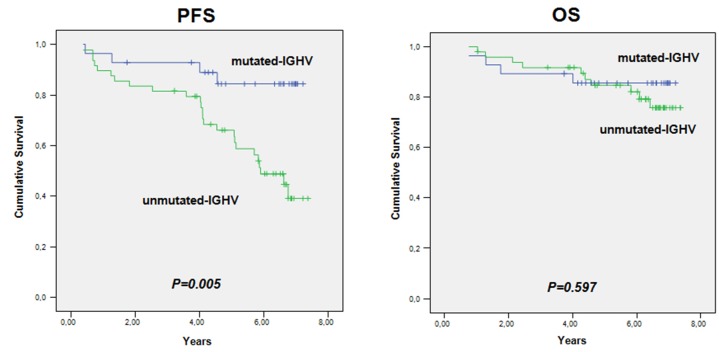
At the end of the study, with a median follow-up of 6.3 years, the estimated proportions of patients who were alive and progression-free were 0.76 and 0.61, respectively. Analyzed according to their MRD status, patients with a CR and either undetectable or detectable MRD did not reach the median PFS and OS, while for patients with detectable MRD and a PR the median PFS was 2.04 years (95% CI: 0-4.3 years) and the median OS was 4.60 years (95% CI: 3.0-6.1 years). Regarding response duration, a total of 56 patients (71.2%) maintained their response throughout the whole study: the median response duration was 6.4 years (95%CI: 6.08-6.68). Univariate Cox regression analysis showed that IGHV status affected PFS: the PFS rate at 7.3 years in patients with mutated IGHV was 0.85, whereas it was 0.39 in those with unmutated IGHV. However, the median OS for patients with either mutated or unmutated IGHV was not reached (Figure 1). No correlations were identified between the other clinical, biological or molecular factors and the achievement of undetectable MRD.
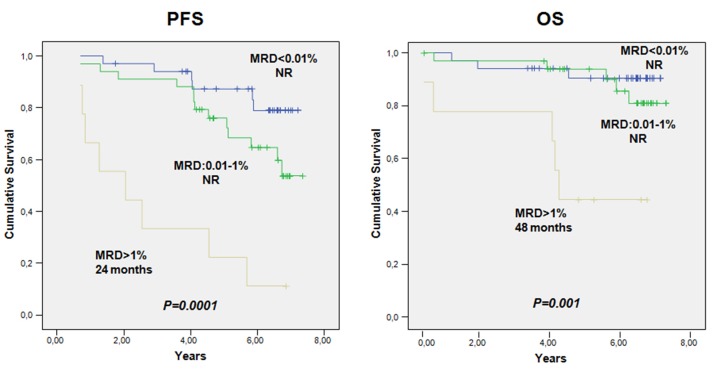
When MRD values were categorized into low (<0.01%, i.e. less than 1 CLL cell per 10,000 leukocytes), intermediate (0.01% to 1%) and high (>1%), the median PFS and OS were not reached in patients with low and intermediate MRD levels and were 2.0 years (95% CI: 0-4.3) and 4.6 years (95% CI: 4.2-4.9), respectively, in patients with high MRD levels (Figure 2, and Table 3A and 3B).
Figure 2.
Progression-free survival and overall survival according to IGHV mutational status. PFS: progression-free survival; OS: overall survival.
Table 3A.
Progression-free and overall survival according to measurable residual disease group assessment at the staging following treatment with fludarabine, cyclophosphamide, and rituximab.
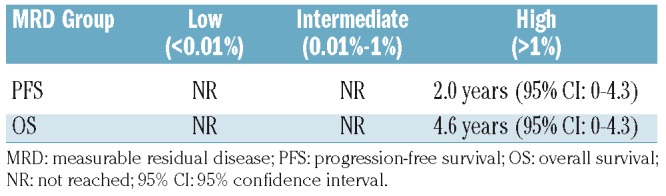
Table 3B.
Seven-year progression-free and overall survival rates after 36 months of maintenance therapy according to measurable residual disease group.
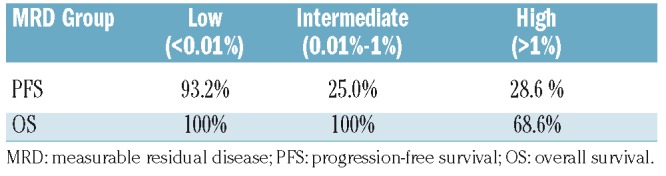
Figure 3.
Progression-free survival and overall survival according to measurable residual disease status. PFS: progression-free survival; OS: overall survival; MRD: measurable residual disease; NR: not reached.
Safety
As per protocol, 86 patients were evaluated for safety after FCR induction. The most common adverse events were grade 1-2 rituximab infusion reactions (65.1%), grade 3-4 myelosuppression (29 patients, 33.7%) and infections (grade 1-2: 30 patients, 34.9%; grade 3-4: 3 patients, 3.5%). In addition, there were 11 (12.8%) grade 3-4 non-hematologic serious adverse events.
The most common adverse event during rituximab maintenance therapy was grade 3-4 myelosuppression, which occurred in 28 patients (37.8%). In more detail, neutropenia between cycles and anemia were observed in 27 patients (36.5%) and one patient (1.4%), respectively. Grade 1-2 infections were detected in 43 patients (58.1%), while grade 3-4 infections were documented in ten patients (13.5%) and were pneumonia (n=5), respiratory tract infections (n=2), meningitis (n=1), viral myocarditis (n=1), and gastroenteritis (n=1). Febrile neutropenia was observed in five patients (6.8%).
Discussion
Despite the improved efficacy of currently approved chemoimmunotherapy in CLL patients, the majority of patients, including those who achieve CR, eventually relapse as a consequence of residual malignant cells still present after therapy. The high CR rate recorded in this study indicates that FCR induction followed by rituximab maintenance therapy produces a high overall response rate in patients considered fit for fludarabine-based therapy. By increasing the quality of clinical responses through obtaining a high undetectable MRD CR rate, the PFS of patients with a clinical response is prolonged. Ultimately, this confirms the role and value of undetectable MRD status in CLL.
In our study, MRD in BM was undetectable at the 10−4 level in 44% of the 80 patients evaluated after the induction treatment and in 68% of 59 patients at the end of maintenance therapy. Furthermore, rituximab maintenance therapy significantly increased the number of patients with undetectable MRD in BM. Indeed, 40 patients with detectable BM-MRD converted to an undetectable BM-MRD status from cycle 9 and subsequent cycles. Additionally, although small numbers limited our subgroup analysis, it is remarkable that 41%, 58% and 60% of patients with undetectable MRD following rituximab maintenance therapy (n=40) harbored factors well-known to be associated with lower response and poor long-term outcomes.16–18 Our data suggest a PFS benefit from rituximab maintenance therapy in IGHV-mutated vs. unmutated patients (PFS at 7.2 year: 84.5% vs. 39.1%, respectively). Overall, clinical outcomes were encouraging in this study as the median OS and PFS were not reached. The estimated 7-year PFS and OS rates were 56.2% and 78.0%, respectively. Of note, the median OS and PFS for patients with a CR and either undetectable BM-MRD or detectable BM-MRD were not reached. However, despite these data suggesting a benefit on time-to-event curves, it is important to note that this was not a randomized study. Nevertheless, although direct comparisons between trials is not recommended, these results (along with published data), suggest that maintenance treatment with a chemotherapy-free approach would improve long-term outcomes with acceptable toxicity. Furthermore, when MRD values were categorized into low (<0.01%), intermediate (0.01% to 1%) and high (>1%), low and intermediate MRD levels were associated with longer PFS and OS following rituximab maintenance therapy (OS: P<0.0001) compared with higher MRD levels which were associated with significantly shorter PFS and OS (2.0 and 4.6 years, respectively), suggesting a favorable prognostic effect of MRD level for patients given rituximab maintenance therapy.
The relationship between undetectable MRD following frontline therapy and long-term outcomes, namely PFS and OS, has been investigated extensively in recent years. Nevertheless, to our knowledge, our study has one of the longest maintenance phases given to CLL patients in first remission. The German CLL Study Group (GCLLSG) updated the CLL8 trial which compared FCR to fludarabine plus cyclophosphamide in untreated CLL patients. With a median follow-up of 5.9 years, the median PFS for the patients treated with FCR was 56.8 months, and the median OS was not reached in the FCR group.5,6 Furthermore, patients achieving undetectable MRD had a significantly longer PFS (64.0 months) while the median OS was not reached. The rituximab-containing arm produced twice the number of patients achieving undetectable MRD.
In our study, median PFS and OS were not reached for patients with undetectable BM-MRD. We hypothesized that this difference was probably due to the source of samples. These data suggest that higher response rates and longer response durations could be expected by intensifying therapy through prolonged maintenance treatment with anti-CD20 immunotherapy.19 In addition, a combined analysis of the CLL8 and CLL10 trials showed that PFS was significantly longer in patients with undetectable MRD than in those with detectable MRD, despite being unaffected by the residual tumor load, thus supporting the prognostic significance of undetectable MRD in CLL patients.20 In another study Greil et al. enrolled patients who had achieved a CR, CRi or PR after first- or second-line rituximab-based chemoimmunotherapy.21 PFS was significantly longer in the rituximab maintenance arm (47.0 vs. 35.5 months, HR 0.50, 95% CI: 0.38-0.66; P<0.0001), suggesting that remission maintenance is an effective and safe option for CLL patients. In that study, MRD progression was documented more frequently in patients on observation than in those on rituximab maintenance therapy (P<0.0001) and, interestingly, conversion to undetectable MRD status occurred more frequently in the rituximab maintenance arm (12 patients vs. 1 patient; P=0.003). Based on these data, it seems that maintenance therapy may improve the quality of remission in CLL subjects and prolong PFS.
Although firm conclusions are limited by the number of subjects in our trial, patients who discontinued chemoimmunotherapy after achieving undetectable BM-MRD CR at cycle 4 and continued with the maintenance phase had similar PFS and OS rates to those of patients who achieved undetectable MRD but continued treatment for all six cycles: 93.3% and 76.5% were alive at 7 years, while 80.0% and 60.6% were free of disease at 7 years, respectively. In the light of these data, it might be useful to evaluate the efficacy and efficiency of a strategy that adapts the duration of treatment to achieve undetectable MRD. Although a high rate of falsely negative MRD in peripheral blood up to 12 months has been reported with rituximab-containing regimens (20-30%), our hypothesis was based on the predictive model published by Dimier et al., testing the effect of treatment on PFS using MRD as a surrogate endpoint.22 Thus, we only stopped FCR after four cycles when MRD was undetectable in both peripheral blood and BM. Although MRD-tailored therapy has not been validated prospectively, Strati et al. showed that patients who discontinued frontline FCR after achieving undetectable MRD after three cycles of treatment had similar PFS and OS to those who achieved undetectable MRD but continued treatment for all six cycles.23 Furthermore, PFS in the subgroup of patients who discontinued frontline FCR after three cycles of treatment because they had achieved undetectable MRD was better than the PFS of patients who remained with detectable MRD at the end of the course of six cycles, despite the shorter duration of treatment in the former group. In addition, Thompson et al. recently reported that undetectable MRD after course 3 of FCR predicted a greater likelihood of undetectable MRD at the end of therapy.24
Although the study was designed before the ERIC recommended diagnostic markers were published, with a median number of BM leukocytes of around 410,000 (range, 150,000-610,000) and a sensitivity of MRD detection of ≥10−4, the immunophenotypic CLL analysis performed in this study was robust. The methodology for assessing MRD was similar to the flow cytometry methodology established by the ERIC consortium, as three of the tubes were similar to the subsequently published ERIC recommendations.15,25,26 The potential limitation of the flow cytometry assay is the need for at least 106 cells per tube and a total of four tubes, which may be an issue to keep in mind when small samples are available. In addition, selection of the sample source remains a challenge, as a significant discrepancy between MRD status determined in peripheral blood and BM has been reported.27 A paired analysis of peripheral blood and BM samples in our study revealed that 60 patients did not have detectable MRD in peripheral blood, while only 37 achieved undetectable MRD in the BM. This discrepancy is partially a result of the compartmental nature of CLL, with disease reservoirs in the BM, blood, lymph nodes, liver, and spleen. As rituximab targets CD20 on mature, malignant and benign B cells, rituximab-based therapy will achieve undetectable MRD much more rapidly in peripheral blood than in BM. Indeed, in the REM trial we decided to use the CD20 marker in two of our MRD tubes for two reasons, (i) CD20 as a single marker provides the most powerful separation of CLL cells from normal B cells, and (ii) in patients treated with rituximab-containing regimens, the correlation between real-time quantitative polymerase chain reaction findings and the results of assays with combinations including the CD20 marker was not weaker than that with combinations not including the CD20 marker.28
Based on the results described above, it appears that 3 years of rituximab maintenance therapy was beneficial for enrolled patients, improving the quality of remissions and prolonging survival. The reason for the high response rates, undetectable MRD and favorable PFS and OS rates compared to those from clinical trials with similar entry criteria is probably multifactorial, but may include the age and Performance Status of the patients: their median age was 59.5 years and up to 70.5% patients had an Eastern Cooperative Oncology Group Performance Status of 0. Additionally, the protocol-defined anti-microbial prophylaxis scheduled for this trial allowed a treatment compliance of around 80%. Furthermore, the secondary endpoint of undetectable MRD is strongly associated with outcome: at the post-maintenance assessment, 68% of assessed patients had undetectable MRD. Finally, the median follow-up of 75.6 months is long enough to allow solid interpretation of both PFS and OS.
A weakness of this trial is that, since its design, new drugs targeting signaling pathways, and newer monoclonal antibodies have become available, and the interest in chemoimmunotherapy, such as FCR, has weakened. Additionally, since OS findings have been inconsistent and one could argue that prolonged maintenance use of certain molecules could expose CLL patients to increased toxicity and ultimately reduce their quality of life, the debate should be whether to use rituximab for maintenance or to watch and wait and give these therapies when the patients relapse. Upon closer examination of our results, patients with unfavorable cytogenetics, unmutated somatic IGHV genes, and CD38 and ZAP70 expression benefited from rituximab maintenance therapy. Further research is now needed to identify subgroups of patients who may benefit while on maintenance therapy.
Of the 957 treatment-emergent adverse events, 54% occurred during induction treatment; most were classed as neutropenia or lymphopenia, and almost half (47.4%) were suspected to be related to rituximab. However, the same adverse events could be related to more than one of the drugs administered. The most frequent of the 440 treatment-emergent adverse events recorded during the maintenance period was neutropenia, which was recorded in 43.3% of the patients. Of the total of 957 treatment-emergent adverse events, 26.6% were assessed as grade ≥3 and the majority were associated with disorders in the blood and lymphatic systems. Sixteen out of 20 deaths reported in the study occurred during the maintenance period. There was only one treatment-related death, which happened during the maintenance period. Overall, the safety profile of rituximab in the maintenance setting was consistent with its expected safety profile and no new unexpected adverse events were reported.
In summary, this study provides the first insights into the potential clinical use of FCR treatment followed by a 3-year period of rituximab maintenance as a treatment strategy. Our study suggests that maintenance therapy with rituximab further prolongs responses in CLL patients with detectable MRD (when judged against historical outcomes after FCR treatment), with significantly improved PFS and OS for patients who achieved at least a PR after FCR induction. Based on these results, undetectable MRD is confirmed as a predictive biomarker associated with treatment response following rituximab maintenance therapy. Prospective studies aimed at evaluating long-term outcomes following early treatment discontinuation and the potential benefit in terms of reducing acute and delayed toxicity are necessary before MRD testing can be used to guide treatment decisions in clinical practice.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/11/2249
Funding
This work was funded by Roche Farma, S.A., Madrid, Spain.
References
- 1.Müller-Hermelink HK, Montserrat E, Catovsky D, et al. Chronic lymphocytic leukaemia/small lymphocytic lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. pp.180–182. [Google Scholar]
- 2.Ginaldi L, De Martinis M, Matutes E, Farahat N, Morilla R, Catovsky D. Levels of expression of CD19 and CD20 in chronic B cell leukaemias. J Clin Pathol. 1998;51(5):364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreau EJ, Matutes E, A’Hern RP, et al. Improvement of the chronic lymphocytic leukemia scoring system with the monoclonal antibody SN8 (CD79b). Am J Clin Pathol. 1997;108(4):378–382. [DOI] [PubMed] [Google Scholar]
- 4.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. [DOI] [PubMed] [Google Scholar]
- 6.Rossi D, Terzi-di-Bergamo L, De Paoli L, et al. Molecular prediction of durable remission after first-line fludarabine-cyclophosphamide-rituximab in chronic lymphocytic leukemia. Blood. 2015;126(16):1921–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–215. [DOI] [PubMed] [Google Scholar]
- 8.Thompson PA, Tam CS, O’Brien SM, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127(3): 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson PA, Wierda WG. Eliminating minimal residual disease as a therapeutic end point: working toward cure for patients with CLL. Blood. 2016;127(3):279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrisqueta P, Villamor N, Terol MJ, et al. Rituximab maintenance after first-line therapy with rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) for chronic lymphocytic leukemia. Blood. 2013;122(24):3951–3959. [DOI] [PubMed] [Google Scholar]
- 11.Del Poeta G, Del Principe MI, Buccisano F, et al. Consolidation and maintenance immunotherapy with rituximab improve clinical outcome in patients with B-cell chronic lymphocytic leukemia. Cancer. 2008;112(1):119–128. [DOI] [PubMed] [Google Scholar]
- 12.Bo MD, Del Principe MI, Pozzo F, et al. NOTCH1 mutations identify a chronic lymphocytic leukemia patient subset with worse prognosis in the setting of a rituximab-based induction and consolidation treatment. Ann Hematol. 2014;93(10): 1765–1774. [DOI] [PubMed] [Google Scholar]
- 13.Garcia Vela JA, Delgado I, Benito L, et al. CD79b expression in B cell chronic lymphocytic leukemia: its implication for minimal residual disease detection. Leukemia. 1999;13(10):1501–1505. [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, Fan L, Xu W, Li JY. Detection methods of ZAP70 in chronic lymphocytic leukemia. Clin Exp Med. 2012;12(2):69–77. [DOI] [PubMed] [Google Scholar]
- 15.Rawstron AC, Fazi C, Agathangelidis A. et al. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European Research Initiative on CLL study. Leukemia. 2016;30(4):929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–1854. [PubMed] [Google Scholar]
- 17.Hamblin TJ, Orchard JA, Ibbotson RE, et al. CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood. 2002;99(3):1023–1029. [DOI] [PubMed] [Google Scholar]
- 18.Dürig J, Nuckel H, Cremer M, et al. ZAP-70 expression is a prognostic factor in chronic lymphocytic leukemia. Leukemia. 2003;17(12):2426–2434. [DOI] [PubMed] [Google Scholar]
- 19.Böttcher S, Ritgen M, Fischer K, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30(9):980–988. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs G, Robrecht S, Fink AM, et al. Minimal residual disease assessment improves prediction of outcome in patients with chronic lymphocytic leukemia (CLL) who achieve partial response: comprehensive analysis of two phase III studies of the German CLL Study Group. J Clin Oncol. 2016;34(31):3758–2765. [DOI] [PubMed] [Google Scholar]
- 21.Greil R, Obrtlíková P, Smolej L, et al. Rituximab maintenance versus observation alone in patients with chronic lymphocytic leukaemia who respond to first-line or second-line rituximab-containing chemoimmunotherapy: final results of the AGMT CLL-8a Mabtenance randomised trial. Lancet Haematol. 2016;3(7):e317–e329. [DOI] [PubMed] [Google Scholar]
- 22.Dimier N, Delmar P, Ward C, et al. A model for predicting effect of treatment on progression-free survival using MRD as a surrogate end point in CLL. Blood. 2018;131(9):955–962. [DOI] [PubMed] [Google Scholar]
- 23.Strati P, Keating MJ, O’Brien SM, et al. Eradication of bone marrow minimal residual disease may prompt early treatment discontinuation in CLL. Blood. 2014;123(24):3727–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson PA, Peterson CB, Strati P, et al. Serial minimal residual disease (MRD) monitoring during first-line FCR treatment for CLL may direct individualized therapeutic strategies. Leukemia. 2018;32(11): 2388–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rawstron AC, Kreuzer KA, Soosapilla A, et al. Reproducible diagnosis of chronic lymphocytic leukemia by flow cytometry: an European Research Initiative on CLL (ERIC) & European Society for Clinical Cell Analysis (ESCCA) harmonisation project. Cytometry B Clin Cytom. 2018;94(1):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawstron AC, Villamor N, Ritgen M, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21(5):956–964. [DOI] [PubMed] [Google Scholar]
- 27.Rawstron A, Cohen D, de Tute R, et al. Bone marrow is more sensitive than peripheral blood for detection of MRD in CLL and provides a more reliable prediction of outcome across different treatments. Haematologica. 2015;100(Suppl 1):Abstract S794. [Google Scholar]
- 28.Rawstron AC, Böttcher S, Letestu R, et al. Improving efficiency and sensitivity: European Research Initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL. Leukemia. 2013;27(1):142–149. [DOI] [PubMed] [Google Scholar]