Abstract
Seeking smaller and indistinct incisions, physicians have attempted endoscopic breast surgery in breast cancer patients. Unfortunately, there are some limitations in the range of movement and visualization of the operation field. Potentially addressing these limitations, we investigated the outcomes of gas and gasless robot-assisted nipple-sparing mastectomy (RANSM) with immediate breast reconstruction (IBR). Ten patients underwent 12 RANSM with IBR between November 2016 and April 2018. Patients with tumors measuring >5 cm in diameter, tumor invasion of the skin or nipple-areolar complex, proven metastatic lymph nodes, or planned radiotherapy were excluded. Age, breast weight, diagnosis, tumor size, hormone receptor status, and operation time were retrospectively collected. Postoperative outcomes including postoperative complications and final margin status of resected were analyzed. The median total operation time and console time were 351 min (267–480 min) and 51 min (18–143 min), respectively. The learning curve presented as a cumulative sum graph showed that the console time decreased and then stabilized at the eighth case. There was no open conversion or major postoperative complication. One patient had self-resolved partial nipple ischemia, and two patients experienced partial skin ischemia. We deemed that RANSM with IBR is safe and feasible for early breast cancer, benign disease of the breast, and BRCA 1/2 mutation carriers. RANSM is an advanced surgical method with a short learning curve.
Subject terms: Breast cancer, Breast cancer, Surgical oncology
Introduction
Nipple-sparing mastectomy (NSM) is popular secondary to the excellent cosmetic effects achieved in appropriately selected patients1. However, a conspicuous scar on the breast dome and a change in the shape of the breast are unavoidable. An inframammary fold or lateral or periareolar incision ensures an inconspicuous scar; however, removal of adequate quantities of breast tissue is technically challenging2. The risk of nipple necrosis associated with a periareolar incision is higher than that with a non-periareolar incision3. Aesthetically, endoscopic breast surgery is expected to achieve complete cancer clearance with preservation of the patient’s body image4. However, restricted maneuverability (because of inflexible endoscopic equipment) and inadequate operative field visualization (because of 2-dimensional cameras) are limitations of this technique4,5.
Robotic surgery is widely used across various fields since its introduction in 19856 because of its several incomparable advantages. High-resolution, 10-fold image magnification, and 3-dimensional optics enable accurate visualization and differentiation of fine structures including intercostal perforators and lymphatics7. The sophisticated and intuitive motion of robotic arms allows microscale manipulation, and surgeons can perform delicate tasks accurately even in a limited operative field. Thus, robotic surgery scores over endoscopic surgery and is widely used for several intracorporeal procedures. Toesca et al. first introduced robotic breast surgery in 2015 after which this technique was attempted globally5,8. Only a few studies have evaluated the feasibility and safety of robot-assisted nipple-sparing mastectomy (RANSM) with immediate breast reconstruction (IBR) to treat breast cancer. Not many studies have analyzed the learning curve of RANSM. We investigated the outcome of RANSM with IBR and analyzed the learning curve for this procedure.
Patients and Methods
Cadaveric study
Prior to evaluating the feasibility and safety of RANSM with IBR in humans, RANSM with a latissimus dorsi flap and/or implant insertion was performed on cadavers between December 2013 and November 2014 (Fig. 1). The sources of cadavers were body donation programs or unclaimed bodies provided by the Severance robot and MIS center9,10. Six breasts in 3 cadavers were used for the initial RANSM with IBR, and feasibility and safety of the procedure were evaluated. The cadaveric study was performed by 2 breast surgeons and 2 plastic surgeons. A Chung’s or modified Chung’s retractor for endoscopic and robotic surgeries was utilized during the gasless technique. The detailed procedure of a gasless RANSM has been described in a previous case report11. During the first attempted cadaveric RANSM, the breast surgeons could not identify the anatomical borders of the breast. Thus, indigo carmine was injected into the borders of the breast parenchyma to identify the anatomical borders of the breast. After injection of the dye, the anatomical borders of the breast could be identified within the working space without help from assistants during the second and third cadaveric studies. Barring this aforementioned technical issue, no significant failure or safety issue occurred during cadaveric RANSM with IBR. In addition to the cadaveric study, the surgeons also participated in the essential robot-training programs conducted by the Severance Robot & Minimally Invasive Surgery center to gain experience in basic and advanced robotic surgical skills.
Figure 1.
The cadaveric study of robot-assisted nipple-sparing mastectomy with immediate breast reconstruction.
Patient selection
Between November 2016 and April 2018, 10 patients underwent RANSM with IBR at a single center. This study was approved by the Institutional Review Board of Severance Hospital including waiver of consent for the current study because the study involved only a retrospective chart review of anonymous patients.
A single breast surgeon and 2 plastic surgeons performed 12 RANSM with IBR (2 patients underwent bilateral procedures). Selected patients underwent physical examination, mammogram, breast ultrasonography and magnetic resonance imaging. Chest or abdominopelvic computed tomography, abdominal ultrasonography, or whole body bone scan were performed to assess metastasis. Patients with tumors measuring >5 cm in diameter, those with skin or nipple-areolar complex (NAC) tumor invasion, those showing metastatic lymph nodes, or those scheduled for radiotherapy were excluded. We recorded age, body mass index, breast weight, diagnosis, tumor size and grade, number of metastatic lymph nodes, hormone receptor status, and type of adjuvant therapy. Postoperative complications and final margin status of resected specimens were evaluated to investigate postoperative outcomes. The operation time was measured as the interval between the creation of the skin incision and the end of the reconstructive surgery. The console time was defined as the time spent by a surgeon to operate the console for mastectomy.
Surgical technique
The procedure was performed in the first 10 patients using a gasless technique with self-retractors used for robotic and endoscopic surgery. Briefly, a patient was placed in the supine position using a shoulder pad. The ipsilateral arm was straightened with internal rotation and abduction and fixed to the head. Indigo carmine was injected around the NAC to detect sentinel lymph nodes preoperatively. A 3.5–6 cm sized longitudinal skin incision was made in the anterior axillary line below the axillary fossa. Sentinel lymph node biopsy was performed manually without robotic assistance, and the subcutaneous skin flap was dissected toward the NAC using monopolar electrocautery. After an adequate working space was obtained, the retroareolar ductal tissue was resected for frozen section examination to evaluate tumor involvement. Before docking the da Vinci Xi® or Si® Surgical System (Intuitive Surgical Inc., California, US), indigo carmine was injected into the borders of the breast to delineate the anatomical boundaries of the breast.
In the gasless method, the retractor was hung to lift the skin flap, and the robot was docked on the contralateral side of the patient. Dissection was performed using fenestrated bipolar forceps on the left side and a permanent cautery spatula on the right under visual guidance of a dual-channel 30° down endoscope of the robotic system11.
After dissecting the subcutaneous flap, a Chung’s or modified Chung’s retractor was repositioned over the pectoralis major fascia and drawn up to expose the posterior aspect of the breast. The breast tissue was detached from the fascia and removed through the 3.5–6 cm axillary skin incision.
RANSM with IBR using gas insufflation was performed in the 2 most recent cases as described in a previous study7. In brief, carbon dioxide (CO2) gas was insufflated through a single port (Lapsingle®, Sejong Medical Inc., Korea). The retroareolar ductal tissue was resected for frozen section examination under robotic vision. Dissection of the skin flap was performed using the ProGraspTM forceps (Intuitive Surgical, Sunnyvale, USA) on the left side and the Hot ShearsTM monopolar curved scissors (Intuitive Surgical, Sunnyvale, USA) on the right.
Assistants guided the surgeon by providing verbal information/guidance regarding the location of the instrument’s tip and the flap thickness. After the entire breast tissue was removed, the subcutaneous skin flap was inspected for bleeding and thickness, and the IBR was performed by the plastic surgeons.
Statistical analysis
This study used 3 case moving average curves (MAC) and cumulative sum (CUSUM) analysis to identify the learning curve for RANSM except 2 gas methods of RANSM because different technique can affect the result of the learning curve. Previous studies have reported the utility of MAC and CUSUM in analyzing the learning curves12–14. The slope of the CUSUM graph represents the trend of learning performance, and the plateau represents the proficiency acquired.
The SPSS software, version 23 (SPSS Inc., Chicago, IL) was used for statistical analysis. The polynomial curves of the MAC and CUSUM diagram were obtained using the Trendline function of Microsoft Excel 2010, and we subsequently determined the curve gradients.
Results
Clinical and pathological characteristics
The median age of patients was 46 years (29–51 years), and the median weight of the resected breast specimens was 225.5 g (150–436 g). Ten mastectomies were performed to treat breast cancer—9 cases (75%) presented with invasive ductal carcinoma and 1 case (8.3%) with ductal carcinoma in situ (DCIS). The patient with DCIS underwent a bilateral procedure, and a bilateral procedure was also performed in a patient with interstitial mastitis secondary to a foreign body injection. The median tumor size was 1.35 cm (0.5–4.2 cm). Sentinel lymph node biopsies were performed during all operations except in 1 patient with interstitial mastitis. Axillary dissection without robotic assistance was performed in a patient showing a positive frozen section biopsy result. One patient with a metastatic lymph node that was not identified by the frozen section underwent postoperative radiation therapy. All patients with breast cancer showed estrogen receptor positivity and received hormonal therapy. Human epidermal growth factor receptor 2 was overexpressed in 3 patients, and 1 of them received targeted therapy. Left breast surgeries were performed in 5 and right breast surgeries in 7 cases. Direct-to-implant breast reconstruction was performed in 3 and reconstruction using tissue expanders in 9 cases.
Adjuvant chemotherapy was administered in 4 and radiotherapy in 2 patients.
All frozen sections were examined, and histopathological changes were detected in 2 patients. One patient with a superficial focal abutting of the skin margin underwent radiotherapy. The other patient showed ductal carcinoma of the NAC and underwent excision of the NAC (Tables 1 and 2).
Table 1.
General characteristics of the study population.
| RANSM with IBR (n = 12) | |
|---|---|
| Age (years) | 46 (29–51) |
| BMI (kg/m2) | 20.8 (18.59–23.93) |
| Breast weight (g) | 225.5 (150–436) |
| Diagnosis | |
| Benign | 2 (16.7) |
| DCIS | 1 (8.3) |
| IDC | 9 (75) |
| Tumor size (cm) (n = 10)* | 2.3 (0.5–4.2) |
| No. of metastatic lymph nodes (n = 10)* | |
| 0 | 8 (80) |
| 1 | 2 (20) |
| Histopathological grade (n = 10)* | |
| 1 | 0 (0) |
| 2 | 6 (60) |
| 3 | 4 (40) |
| Estrogen receptor status (n = 10)* | |
| Negative | 0 (0) |
| Positive | 10 (100) |
| Progesterone receptor status (n = 10)* | |
| Negative | 1 (10) |
| Positive | 9 (90) |
| HER2 status (n = 10)* | |
| Negative | 7 (70) |
| Positive | 3 (30) |
| Ki 67 (n = 10)* | |
| Low (<14%) | 5 (50) |
| High (≥14%) | 5 (50) |
| Adjuvant chemotherapy (n = 10)* | |
| No | 6 (60) |
| Yes | 4 (40) |
| Radiotherapy (n = 10)* | |
| No | 8 (80) |
| Yes | 2 (20) |
| Hormonal therapy (n = 10)* | |
| No | 0 (0) |
| Yes | 10 (100) |
| Targeted therapy (n = 10)* | |
| No | 9 (90) |
| Yes | 1 (10) |
Values are represented as median (minimum–maximum) or N (percentage).
*2 cases showed a benign presentation.
BMI: body mass index, DCIS: ductal carcinoma in situ, HER: human epidermal growth factor receptor, IBR: immediate breast reconstruction, IDC: invasive ductal carcinoma, RANSM: robot-assisted nipple-sparing mastectomy.
Table 2.
Surgical methods and postoperative outcomes.
| RANSM with IBR (n = 12) | |
|---|---|
| Operated site | |
| Left | 5 (41.7) |
| Right | 7 (58.3) |
| Reconstruction | |
| Tissue expander insertion | 9 (75) |
| Direct-to-implant | 3 (25) |
| Gas insufflation | |
| No | 10 (83.3) |
| Yes | 2 (16.7) |
| SLNB (n = 10)* | |
| No | 0 (0) |
| Yes | 10 (100) |
| ALND (n = 10)* | |
| No | 9 (90) |
| Yes | 1 (10) |
| Length of hospitalization (days) | 11 (9–13) |
| Total operation time (min) | 351 (267–480) |
| Console time (min) | 51 (18–143) |
| Margin status | |
| Negative | 10 (83.3) |
| Positive | 2 (16.7) |
| Complication | |
| None | 9 (75) |
| Skin ischemia | 2 (16.7) |
| Nipple ischemia | 1 (8.3) |
Values are represented as median (minimum–maximum) or N (percentage).
*2 cases showed a benign presentation.
ALND: axillary lymph node dissection, IBR: immediate breast reconstruction, RANSM: robot-assisted nipple-sparing mastectomy, SLNB: sentinel lymph node biopsy.
Operation time
The median length of hospitalization was 11 days (9–13 days). The median operation time was 351 min (267–480 min). The median console time was 51 min (18–143 min) (Table 2). The first 5 patients who underwent the operation showed a shorter total operation and console time. However, from the sixth case onwards, we observed fluctuations in the operation times. The MAC graph of the 3 cases representing the total operation time and the console time also decreased until the fifth case and increased from the sixth case onwards. CUSUM analysis showed that the total operation time reduced until the ninth case and increased thereafter (Fig. 2A). The CUSUM graph of the console time decreased until the eighth case and stabilized thereafter (Fig. 2B).
Figure 2.
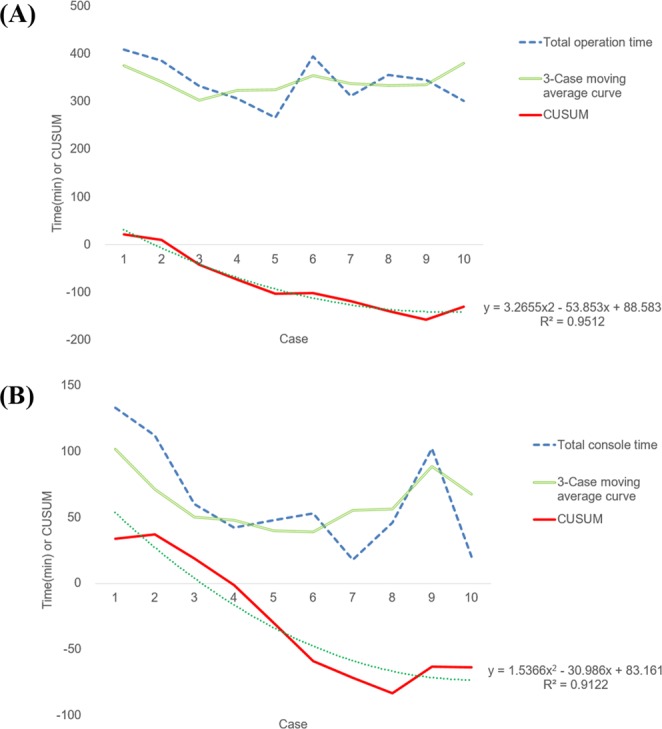
The learning curve of the robot-assisted nipple-sparing mastectomy with immediate breast reconstruction. (A) The learning curve of the total operation time. (B) The learning curve of the console time. CUSUM: cumulative sum.
Postoperative complications
Conversion to open surgery and mortality were not reported. Major postoperative complications including hematoma, infection, and total nipple or skin necrosis did not occur. Self-limited partial nipple ischemia occurred in 1 and partial skin ischemia in 2 patients (Table 2).
Discussion
This study showed that RANSM with IBR is safe and feasible for patients with early breast cancer. With improved disease-free and overall survival rates, patients with early breast cancer are increasingly concerned regarding postoperative cosmetic effects and overall quality of life5. Therefore, newer surgical methods that ensure oncologic safety without compromising body image are required. We introduced robotic breast surgery to achieve these goals. The new surgical method facilitates RANSM with IBR for patients with early breast cancer via a small axillary incision. No major postoperative complications including total nipple necrosis, wound infection, and implant removal were observed. No negative effects on cosmetic outcomes and quality of life were identified. Previous studies have reported similar results of RANSM with IBR5,8,15.
Gasless RANSM with IBR was performed for the first time in our study. The gasless and the CO2-insufflation techniques are both associated with specific advantages and disadvantages. The gasless technique utilizes a self-retractor (Chung’s retractor) to maintain the working space as an alternative to CO2 gas insufflation16. A disadvantage of the CO2-insufflation method is that CO2 gas leakage impairs optimal availability and visualization of the working space, and robotic vision can be affected by the smoke released during electrocauterization. The gasless technique utilizes retractors to maintain the working space; thus, bleeding or air suction associated with this procedure does not significantly impair the availability and visualization of the working space. The smoke from electrocauterization causes lesser technical difficulties (lesser visual disturbances) with the gasless technique thereby facilitating a clearer view of the operative field. Postoperative subcutaneous emphysema and hypercapnia can complicate the gas insufflation technique5. Although these complications can be managed with supportive care15, the gasless robotic procedure eliminates the risks of CO2 insufflation-related complications. Another benefit of the gasless technique is that the retroareolar ductal tissue can be resected to confirm tumor invasion by performing frozen section examination prior to docking of the robotic system.
A few disadvantages of the gasless technique must be mentioned. Prolonged retraction can cause skin flap ischemia. The length of a single incision required for the gasless technique is longer than that used for the gas-insufflated procedure. In this study, we used an axillary incision measuring 2.5–4 cm for the CO2-insufflation technique compared with an incision measuring approximately 3.5–6 cm for the gasless technique.
Robotic surgery is more ergonomically comfortable than conventional surgery owing to the 3-dimensional optics and intuitive robotic arm motion6, and robotic surgery is easier to learn than conventional surgery. As reported by previous studies, learning curves for RANSM are rapidly declining5,15,17. In this study, the console time was investigated to evaluate proficiency of surgical skills in robotic surgery. CUSUM analyses of the console time revealed that it was rapidly stabilized at the eighth case. It can be concluded that surgeons can acquire appropriate surgical skills within a relatively short time.
Owing to limited data regarding long-term outcomes, RANSM is performed only in highly selected patients, primarily patients with early breast cancer, healthy carriers showing BRCA1/2 mutation, and those with benign disease. In this study, nearly all patients demonstrated early breast cancer. Toesca et al. enrolled patients with early breast cancer and carriers with BRCA1/2 mutations5,7. Sarfati et al. suggested that unaffected carriers with BRCA1/2 mutation could be good candidates for RANSM with IBR8,17. Indications outlined in the previous study were similar to those in our study. In the current study, 1 patient underwent RANSM with IBR because of bilateral interstitial mastitis. A previous study has suggested that NSM with IBR can be used to treat this condition18. We reported the first attempt to treat bilateral interstitial mastitis using robotic surgery (Table 3).
Table 3.
Comparison between previous studies and the current study describing robot-assisted nipple-sparing mastectomy with immediate breast reconstruction.
| Study | Type of study | Number of procedures performed (N) | Age (years) | Indications (N, %) | Method of RANSM | Reconstruction method | Breast volume (g) | Open conversion (N, %) | Blistering of skin (N, %) | NAC necrosis (N, %) | Infection or delayed wound healing (N, %) | f/u (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Toesca A. et al. (2017) |
Prospective | 29 | 42 (30–55) |
BRCA mutation carrier (11, 38%) DICS (9, 31%) Invasive carcinoma (9, 31%) |
with gas | expander or DTI | range 200–300 | 2, 6.9% | 2, 6.9% | 0 | 0 | 8 (1–14) |
|
Sarfati B. et al. (2018) |
Prospective | 63 | 37 (24–52) |
DCIS (1, 1.6%) BRCA mutation carrier (62, 98.4%) |
with gas | expander or DTI |
range 78–330 |
1, 1.6% | 2, 3.2% | 0 | 3, 4.8% | 9 (NA) |
|
Lai HW. et al. (2018) |
Retrospective | 23 | 48.9* | NA | with gas | DTI | 284.3* | 0 | 2, 8.6% |
partial (3, 13%) |
1, 4.3% | 6.9* |
|
Park HS et al. (Present) |
Retrospective | 12 |
46 (29–51) |
Benign (2, 16.7%) DCIS (1, 8.3%) IDC (9, 75%) |
with or without gas | expander or DTI | range 150–436 | 0 | 2, 16.7% |
partial (1, 8.3%) |
0 | 8 (5–22) |
Values are expressed as median (minimum–maximum) or (N, percentage).
*These are mean values.
DCIS: ductal carcinoma in situ, DTI: direct-to-implant, f/u: follow-up, IBR: immediate breast reconstruction, IDC: invasive ductal carcinoma, NA: not available, NAC: nipple-areolar complex, RANSM: robot-assisted nipple-sparing mastectomy.
No major complications were reported among patients who underwent RANSM with IBR. A previous study reported a postoperative complication rate of 10.3%5. Skin blistering, partial necrosis of the skin and nipple, and temporary neurapraxia are common postoperative complications of RANSM5,15,17. These complications can be managed conservatively as reported by previous studies, which concurs with our results. Toesca et al. reported that the conversion rate to open surgery was 6.9% in those who underwent RANSM; however, no patient required conversion to open surgery in our study5 (Table 3).
The limitations of this study are as follows: Owing to the small size and retrospective design, the role of a selection bias and uncontrolled confounders cannot be completely excluded. Furthermore, long-term survival outcomes remain unknown because this is our initial experience of RANSM with IBR. RANSM with IBR was performed only in a highly selected group of patients such as those with early breast cancer and benign disease. To overcome these limitations, prospective trials are warranted to evaluate the stability and the cosmetic effects of the surgery. RANSM with IBR has not been standardized because of the insufficient evidence regarding the safety and efficacy of the various methods. Large prospective randomized studies are needed to definitively establish a more accurate and stable surgical procedure.
Conclusion
RANSM with IBR is a feasible and safe surgical treatment for early breast cancer and benign conditions. Although robotic technology is a relatively recent introduction, RANSM with IBR showed a rapid learning curve.
Acknowledgements
This study was presented as a poster at the 16th St. Gallen International Breast Cancer Conference. The abstract of the poster presentation for this study was published in the Breast in 2019.
Author contributions
H.S.P. designed and supervised the study. J.L. collected and assembled data. H.S.P. and J.L. contributed equally to write the manuscript. H.S.P., D.W.L. and S.Y.S. performed robot-assisted nipple sparing mastectomy with immediate breast reconstruction. All authors reviewed and approved the manuscript.
Data availability
The data of the current study are available from the corresponding author on reasonable request.
Competing interests
Hyung Seok Park received consulting fee from Intuitive Surgical Korea, which was not related with the data and analysis of this study. Jeea Lee, Dong Won Lee, Seung Yong Song, Dae Hyun Lew, Seung Il Kim, and Young Up Cho declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harris, J. R. Disease of the breast 5 edn (Wolters Kluwer Health, 2014).
- 2.Tokin C, Weiss A, Wang-Rodriguez J, Blair SL. Oncologic safety of skin-sparing and nipple-sparing mastectomy: a discussion and review of the literature. International journal of surgical oncology. 2012;2012:921821. doi: 10.1155/2012/921821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawlani V, et al. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. The Canadian journal of plastic surgery = Journal canadien de chirurgie plastique. 2011;19:129–133. doi: 10.1177/229255031101900410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leff DR, et al. Endoscopic breast surgery: where are we now and what might the future hold for video-assisted breast surgery? Breast cancer research and treatment. 2011;125:607–625. doi: 10.1007/s10549-010-1258-4. [DOI] [PubMed] [Google Scholar]
- 5.Toesca A, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: Feasibility and safety study. Breast (Edinburgh, Scotland) 2017;31:51–56. doi: 10.1016/j.breast.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu HY, Friedlander DF, Patel S, Hu JC. The current status of robotic oncologic surgery. CA: a cancer journal for clinicians. 2013;63:45–56. doi: 10.3322/caac.21160. [DOI] [PubMed] [Google Scholar]
- 7.Toesca A, et al. Robotic Nipple-sparing Mastectomy and Immediate Breast Reconstruction With Implant: First Report of Surgical Technique. Annals of surgery. 2017;266:e28–e30. doi: 10.1097/sla.0000000000001397. [DOI] [PubMed] [Google Scholar]
- 8.Sarfati B, et al. Robotic da Vinci Xi-assisted nipple-sparing mastectomy: First clinical report. The breast journal. 2018;24:373–376. doi: 10.1111/tbj.12937. [DOI] [PubMed] [Google Scholar]
- 9.Habicht JL, Kiessling C, Winkelmann A. Bodies for Anatomy Education in Medical Schools: An Overview of the Sources of Cadavers Worldwide. Academic medicine: journal of the Association of American Medical Colleges. 2018;93:1293–1300. doi: 10.1097/acm.0000000000002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JT, et al. The trend of body donation for education based on Korean social and religious culture. Anatomical sciences education. 2011;4:33–38. doi: 10.1002/ase.198. [DOI] [PubMed] [Google Scholar]
- 11.Park HS, et al. Gasless Robot-Assisted Nipple-Sparing Mastectomy: A Case Report. J Breast Cancer. 2018;21:334–338. doi: 10.4048/jbc.2018.21.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park HS, Jeon CW. Learning curve for breast mass excision using a vacuum-assisted biopsy system. Minimally invasive therapy & allied technologies: MITAT: official journal of the Society for Minimally Invasive Therapy. 2014;23:235–240. doi: 10.3109/13645706.2014.894918. [DOI] [PubMed] [Google Scholar]
- 13.Park EJ, et al. Long-term oncologic outcomes of laparoscopic right hemicolectomy during the learning curve period: comparative study with cases after the learning curve period. Surgical laparoscopy, endoscopy & percutaneous techniques. 2015;25:52–58. doi: 10.1097/sle.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 14.Lim SM, Park HS, Jeon CW. Cumulative sum analysis for learning curve for breast mass excision using an ultrasound-guided vacuum-assisted biopsy system. Journal of Breast Disease. 2015;3:43–47. doi: 10.14449/jbd.2015.3.2.43. [DOI] [Google Scholar]
- 15.Lai Hung-Wen, Chen Shou-Tung, Lin Shih-Lung, Chen Chih-Jung, Lin Ya-Ling, Pai Shu-Hsin, Chen Dar-Ren, Kuo Shou-Jen. Robotic Nipple-Sparing Mastectomy and Immediate Breast Reconstruction with Gel Implant: Technique, Preliminary Results and Patient-Reported Cosmetic Outcome. Annals of Surgical Oncology. 2018;26(1):42–52. doi: 10.1245/s10434-018-6704-2. [DOI] [PubMed] [Google Scholar]
- 16.Kim MJ, et al. Yonsei Experience of 5000 Gasless Transaxillary Robotic Thyroidectomies. World journal of surgery. 2018;42:393–401. doi: 10.1007/s00268-017-4209-y. [DOI] [PubMed] [Google Scholar]
- 17.Sarfati B, et al. Robotic Prophylactic Nipple-Sparing Mastectomy with Immediate Prosthetic Breast Reconstruction: A Prospective Study. Annals of surgical oncology. 2018;25:2579–2586. doi: 10.1245/s10434-018-6555-x. [DOI] [PubMed] [Google Scholar]
- 18.Chen TH. Silicone injection granulomas of the breast: treatment by subcutaneous mastectomy and immediate subpectoral breast implant. British journal of plastic surgery. 1995;48:71–76. doi: 10.1016/0007-1226(95)90099-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of the current study are available from the corresponding author on reasonable request.


