Abstract
Detection of antihypertensive drugs in biological samples is an important tool to assess the adherence of hypertensive patients. Urine and serum/plasma screenings based on qualitative results may lead to misinterpretations regarding drugs with a prolonged detectability. The aim of the present study was to develop a method that can be used for therapeutic drug monitoring (TDM) of antihypertensive drugs with focus on adherence assessment. Therefore, a method for quantification of four diuretics and four β-blockers using high-performance liquid chromatography-mass spectrometric analysis (LC-MS/MS) of combined acidic and basic serum extracts was developed and validated. The method was applied to 40 serum samples from 20 patients in a supervised medication setting (trough and peak serum samples). Literature data on therapeutic concentration ranges, as well as dose-related drug concentrations (calculated from data of pharmacokinetic studies) were used to evaluate adherence assessment criteria. Concentrations were measured for bisoprolol (n = 9 patients), metoprolol (n = 7), nebivolol (n = 1), canrenone (n = 2, metabolite of spironolactone), hydrochlorothiazide (n = 10) and torasemide (n = 8). The measured concentrations were within the therapeutic reference ranges, except for 24% of the samples (mainly β-blockers). In contrast, all measured concentrations were above the lower dose-related concentration (DRC), which appears superior in evaluating adherence. In conclusion, the quantitative analysis of antihypertensive drugs in serum samples and its evaluation on the basis of the individually calculated lower DRC is a promising tool to differentially assess adherence. This method could possibly detect a lack of adherence or other causes of insufficient therapy more reliably than qualitative methods.
Subject terms: Drug therapy, Hypertension, Laboratory techniques and procedures
Introduction
Arterial hypertension is a major risk factor for cardiovascular disease worldwide and remains the leading cause of death in the western world1. In Europe approximately 4 million people die due to cardiovascular disorders every year2.
For therapy, drugs of five different classes are typically used (ACE-inhibitors, AT1 antagonists, β-blockers, calcium-channel blockers and diuretics). Non-adherence to a medication plan leads to poor blood pressure control in antihypertensive therapy3. This results in frequent visits to the doctor and increased hospital stays4. This in return causes greater health care cost3. Adherence rates appear to differ between the drug classes. In a meta-analysis by Kronish et al. of studies based on medication refill data, poorest adherence was found for diuretics (51%) and β-blockers (28.4%)5. This is supported by Gupta et al. revealing that the number of prescribed drugs alongside with the classes of antihypertensives, especially diuretics, are the main risk factors contributing to non-adherence6.
There are different methods of adherence assessment (i.e. self-reporting, prescription records, pill count, electronic monitoring systems and toxicological analyses)7, whereas urine and serum/plasma screenings are the only direct and specific methods8,9. However, the qualitative nature may lead to misclassification of adherence in case of excretion much longer than the dosing interval. A diagnostic advancement was achieved by implementing a quantitative method for 21 antihypertensive drugs in serum that was recently published by Gundersen et al.10. They set up a therapeutic drug monitoring (TDM) system and used pharmacokinetic data to establish calibration ranges to classify measured serum concentrations with respect to adherence.
For the present study a quantitative assay of the main diuretics and β-blockers according to German prescription data was developed and applied to serum of hypertensive patients with confirmed adherence. The concentrations were evaluated with respect to the diagnosis of non-adherence according to two concepts: published reference ranges and the calculated lower dose-related concentration as established for psychiatric TDM11.
Materials and Methods
Chemicals and reference standards
Reference substances furosemide, torasemide, atenolol, bisoprolol and metoprolol were obtained from Sigma-Aldrich GmbH (Steinheim, Germany). Hydrochlorothiazide (HCT), canrenone and nebivolol, as well as the deuterated internal standards (IS) ketamine-d4, haloperidol-d4, diazepam-d5, quetiapine-d8, oxazepam-d5 and methadone-d9 were purchased from LGC Standards GmbH (Wesel, Germany). HCT-d2 was obtained from Toronto Research Chemicals (North York, Canada).
Acetonitrile was obtained from Karl Roth GmbH (Karlsruhe, Germany) and ethyl acetate from AppliChem (Darmstadt, Germany). Further chemicals and solvents used were supplied by Sigma-Aldrich GmbH (Steinheim, Germany). All reagents and solvents were either of analytical or LC grade.
Serum samples
Patients (15 males, 5 females) aged 32 to 81 (median 57) years treated with a constant dosing regimen of antihypertensive drugs at the nephrological ward at the University Hospital Frankfurt/Main (Germany) participated in this study. Two blood samples were collected in serum tubes in the morning. The first one shortly before (trough level) and the second approximately two hours after monitored oral administration of the medication (peak level). After centrifugation (2,000 × g for 10 min) the separated serum was stored at −20 C until analysis. For data evaluation information on hospital admission, medication regimen (dose, dosing interval, date of last dose adjustment, co-medication), times of drug intake and of blood sampling were collected. Hospital admission was documented to ensure that steady-state concentrations were reached by the time of blood sampling. The patients’ drug ingestion was monitored by the nurses. The study protocol was approved by the competent ethics committee of the Goethe University Frankfurt (reference no. 19/18) and the study was in accordance with the 1964 Helsinki declaration and its later amendments. Written informed consent was obtained from all individual participants included in the study.
Sample preparation
An aliquot of 200 µl serum was transferred to a 2 ml polypropylene reaction tube and 1 ml ethyl acetate, 50 µl internal standard working solution (0.25 ng/µl ketamine-d4 and methadone-d9; 0.5 ng/µl haloperidol-d4, diazepam-d5, protriptyline-d3, quetiapine-d8 and oxazepam-d5 and 0.05 ng/µl HCT-d2), 10 µl of acetonitrile (or mixed standard solution or quality control mix, see below) and 50 µl formic acid (10%) were added. After mixing for 2 min and centrifugation at 13,000 × g for 10 min the organic phase was transferred to a silanized glass tube. Another 1 ml ethyl acetate and 50 µl of aqueous ammonia (25%) were added to the aqueous phase followed by mixing for 2 min, centrifugation and transferring to the glass tube. The combined extracts were evaporated at 25 °C with nitrogen using TurboVap LV (Biotage, Uppsala, Sweden). The dry residue was reconstituted with 100 µl of 0.1% formic acid/acetonitrile (80:20, v/v) and transferred to 300 µl glass vials of which 5 µl were analysed.
Calibration standards and quality controls
Human drug-free serum for preparation of quality controls (QC) and calibration standards was provided by healthy volunteers. Stock solutions of furosemide and canrenone were prepared in acetonitrile, whereas torasemide, atenolol, bisoprolol, metoprolol, HCT and nebivolol were dissolved in methanol at concentrations of 1 mg/ml (drug) and kept refrigerated at −20 °C. These were used for preparation of mixed standard solutions and quality controls in acetonitrile. The calibration range was 0.1–20 ng/ml for nebivolol, 2.5–100 ng/ml for bisoprolol, 1–200 ng/ml for metoprolol, 5–1000 ng/ml for atenolol, canrenone, furosemide and HCT and 10–2000 ng/ml for torasemide. The corresponding QC levels (low, medium, high) are listed in Table 1.
Table 1.
Validation data: lower limit of quantification (LLOQ), limit of detection (LOD), intra- and inter-day precision, accuracy, recovery and matrix effects (±SD) measured using the given quality control levels.
| Analyte | LLOQ (LOD) [ng/ml] | quality control [ng/ml] | intra-day precision [%] | inter-day precision [%] | accuracy [%] | recovery [%] | matrix effects ± SD [%] |
|---|---|---|---|---|---|---|---|
| Atenolol | 0.027 | 62.5 | 7.1 | 7.1 | 4.2 | 50.0 | 92.1 ± 3.1 |
| (0.007) | 250.0 | 3.9 | 6.8 | 5.9 | |||
| 625.0 | 5.3 | 5.9 | −4.0 | 60.6 | 85.7 ± 2.7 | ||
| Bisoprolol | 0.006 | 18.75 | 5.2 | 7.4 | 2.9 | 85.8 | 91.8 ± 4.7 |
| (0.003) | 62.5 | 7.9 | 7.9 | −2.7 | |||
| 87.5 | 4.3 | 8.5 | −3.7 | 93.6 | 99.9 ± 5.4 | ||
| Metoprolol | 0.011 | 12.5 | 7.1 | 7.1 | 1.5 | 58.3 | 100.4 ± 2.0 |
| (0.003) | 50.0 | 7.0 | 7.0 | 4.7 | |||
| 125.0 | 6.0 | 6.0 | −4.0 | 80.1 | 96.5 ± 4.3 | ||
| Nebivolol | 0.045 | 1.25 | 7.7 | 8.1 | 0.4 | 54.5 | 81.8 ± 7.9 |
| (0.018) | 5.0 | 5.0 | 6.1 | 5.0 | |||
| 12.5 | 7.7 | 7.7 | 2.1 | 85.3 | 89.7 ± 7.1 | ||
| Canrenone | 0.023 | 62.5 | 5.4 | 6.8 | 2.5 | 59.2 | 92.0 ± 4.1 |
| (0.008) | 250.0 | 4.3 | 4.6 | 6.8 | |||
| 625.0 | 5.0 | 5.0 | −0.9 | 86.0 | 94.1 ± 6.8 | ||
| Furosemide | 0.093 | 62.5 | 4.6 | 8.7 | 6.0 | 60.0 | 109.3 ± 14.8 |
| (0.034) | 250.0 | 6.3 | 7.2 | 5.9 | |||
| 625.0 | 6.2 | 8.0 | 1.0 | 83.6 | 94.1 ± 9.8 | ||
| HCT | 1.592a | 187.5 | 4.0 | 4.0 | −1.3 | 91.3 | 93.3 ± 9.2 |
| (0.525)a | 625.0 | 3.4 | 3.4 | 0.6 | |||
| 875.0 | 2.5 | 2.7 | 0.6 | 92.5 | 101.2 ± 2.3 | ||
| Torasemide | 0.009 | 187.5 | 4.4 | 8.4 | −8.9 | 66.1 | 101.7 ± 5.7 |
| (0.016) | 625.0 | 8.4 | 8.4 | −2.5 | |||
| 875.0 | 5.1 | 5.8 | −3.7 | 94.5 | 93.0 ± 6.5 |
aDetermined according to ICH guidelines33.
Liquid chromatography-mass spectrometry (LC-MS/MS)
The analysis was performed on an Agilent (Waldbronn, Germany) LC-MS/MS system consisting of a 1290 Infinity Liquid Chromatograph coupled via JetStream Electrospray Interface (ESI) to a G6460A Triple Quadrupole Mass Spectrometer. Extracts were kept at 20 °C on the autosampler and analytes were separated on a Kinetex® 2.6 µm XB-C18 100 Å LC column (30 × 2.1 mm) plus corresponding guard column from Phenomenex (Aschaffenburg, Germany) at 55 °C.
Gradient elution was performed at a flow rate of 0.4 ml/min using 0.01% formic acid containing 5 mM ammonium formate (A) and acetonitrile containing 0.1% formic acid (B). Gradient elution started with 5% B kept for 0.5 min, increased to 40% B during 2.7 min, maintained 0.3 min, enhanced to 50% B within 0.5 min, held for 0.5 min and increased during 1.5 min to 95% B, maintained 2 min and followed by re-equilibration for 2 min, resulting in a total run time of 8 min.
Source parameters were selected as follows: gas temperature 300 °C, gas flow 11 l/min, nebulizer 45 psi, sheath gas temperature 400 °C, sheath gas flow 12 l/min and capillary voltage 3500 V. Detection was performed in the dynamic multiple reaction monitoring mode (dMRM) according to the mass spectrometry parameters listed in Table 2. Data acquisition and evaluation was performed using Agilent MassHunter Software (version B.07.00). For identification of analytes a deviation of retention time of less than 0.05 min and a qualifier to quantifier ratio below 20% deviation compared to reference standards were required.
Table 2.
Mass spectrometry parameters for the detection of β-blockers and diuretics using LC-MS/MS operated in dynamic MRM mode with two transitions for analytes and one for the corresponding internal standard. Retention times, MRM transitions and collision energies (CE) were as follows.
| Analyte | Retention Time [min] | Precursor Ion [m/z] | Quantifier [m/z] (CE [eV]) | Qualifier [m/z] (CE [eV]) | Internal standard |
|---|---|---|---|---|---|
| Atenolol | 1.62 | 267.2 | 145.0 (24) | 74.1 (20) | Ketamine-d4 |
| HCT | 2.04 | 295.9 | 268.9 (12) | 78.0 (32) | HCT-d2 |
| Metoprolol | 2.79 | 268.2 | 74.1 (20) | 116.1 (16) | Ketamine-d4 |
| Bisoprolol | 3.27 | 326.2 | 116.1 (16) | 74.1 (24) | Haloperidol-d4 |
| Torasemide | 3.47 | 349.1 | 264.0 (12) | 183.2 (32) | Methadone-d9 |
| Furosemide | 3.82 | 329.0 | 205.0 (16) | 78.0 (4) | Oxazepam-d5 |
| Nebivolol | 4.14 | 406.2 | 151.0 (32) | 103.1 (72) | Quetiapine-d8 |
| Canrenone | 5.44 | 341.2 | 107.1 (36) | 91.1 (70) | Diazepam-d5 |
| Internal Standard | |||||
| HCT-d2 | 2.06 | 298.0 | 270.0 (12) | ||
| Ketamine-d4 | 2.53 | 242.1 | 129.0 (28) | ||
| Quetiapine-d8 | 3.67 | 392.2 | 258.1 (20) | ||
| Haloperidol-d4 | 3.84 | 380.2 | 169.1 (20) | ||
| Oxazepam-d5 | 4.19 | 292.1 | 246.0 (20) | ||
| Methadone-d9 | 4.28 | 319.3 | 268.1 (8) | ||
| Diazepam-d5 | 5.12 | 290.1 | 198.1 (32) | ||
Method validation
The method was validated according to current guidelines12. Statistical evaluation was performed with Valistat 2.0 Software (Arvecon GmbH, Walldorf, Germany).
In order to find appropriate internal standards for the analytes, 41 deuterated medical and illicit drugs were tested. Thus, a broad spectrum of substances with different chemical and chromatographic properties was evaluated. Internal standards were assigned regarding linearity and compensation of matrix effects. Retention time was the decisive factor if deuterated substances yield similar results.
Matrix effects were evaluated by comparing peak areas of spiked extracts with those of standard solutions and recovery by comparing spiked matrix samples to spiked extracts. Both were determined in low and high quality control samples. Each QC sample was measured six times using blank serum samples of different donors.
Selectivity was assessed with human serum samples from eight different drug-free volunteers. Six samples were prepared without (blank samples) and another two by adding internal standard solution (zero samples). To show the absence of interferences serum samples with exogenous substances including typical therapeutic drugs and metabolites, as well as a range of psychoactive substances were analyzed. Sensitivity was assessed by analysing five calibrator concentrations evenly spaced in the range of the expected limit of detection (LOD) and lower limit of quantification (LLOQ) as previously described13.
Evaluation of linearity was done by six-fold determination in one sequence of seven calibration levels evenly distributed across the calibration range. The calibration was checked for outliers (Grubbs test), homogeneity (Cochran test) and linearity (Mandel test).
For verification of accuracy and precision homogenous pools of low, medium and high quality control samples (relative to calibration range) were prepared by spiking blank matrix and dividing into aliquots. Thereafter two quality controls of each concentration level were measured on eight different days. Results were tested for accuracy (bias ≤15%) and intra- and inter-day precision (relative standard deviation ≤15%).
The analytes are sufficiently stable during long-term storage and during freeze-thaw cycles10,14–16. Stability of extracted analytes was tested under autosampler conditions for 72 h. The decrease in concentration of low and high QC samples was checked by repeated injection of an aliquot.
Evaluation of concentrations
The measured concentrations were evaluated by comparison with therapeutic reference ranges as well as with lower limits of calculated dose-related concentrations. Therapeutic reference ranges, indicating therapeutic efficacy and acceptable tolerability, were retrieved from the list of Schulz et al.17 and Repetto et al.18. If the literature data did not match, the larger reference range was selected for evaluation (Table 3).
Table 3.
The data from pharmacokinetic studies refer to healthy volunteers (n = total number of volunteers) with data on bioavailability (f), dosing interval (τ), apparent total clearance (CLt/f) and its standard deviation (SD), average elimination half-life (t½), the mean dose related concentration (DRC) factor with its lower limit for two time intervals between last dose and blood sampling (Δt). The last column cites the therapeutic reference range as retrieved from Schulz et al.17.
| Drug | n | f | τ [h] | CLt/f [ml/min] | SD [ml/min] | t1/2 [h] | Δt [h] | DRC factor [ng/ml/mg] | lower DRC factor [ng/ml/mg] | reference | therapeutic range [ng/ml]17 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atenolol | 30 | 0.55 | 24 12 | 178.3a | 38.4 | 6.1 |
24 12 |
0.404 0.998 |
0.356 0.880 |
28, 34, 35 | 200–450 |
| Bisoprolol | 32 | 0.88 | 24 12 | 337.0a | 76.2 | 14.7 |
24 12 |
1.111 1.533 |
0.860 1.186 |
36, 37 | 10–100 |
| Metoprolol tartrate | 10 | 0.55 | 24 12 | 1454.6a | 181.8 | 4.1 |
24 12 |
0.034 0.147 |
0.030 0.128 |
14, 38 | 20–60018 |
| Metoprolol succinateb | 24 | 0.45 | 24 12 | 2857.2a | 478.0 | 3.0 |
24 12 |
0.005 0.045 |
0.004 0.037 |
39, 40 | |
| Nebivolol | 69 |
0.12 EMd |
24 12 | 7166.7a | 1611.1 | 10.3 |
24 12 |
0.039 0.063 |
0.030 0.049 |
26, 41, 42 | <20 |
|
0.96 PMd |
24 12 |
307.3a | n/a | 33.0 |
24 12 |
1.738 1.987 |
n/a n/a |
||||
| Canrenonec | 25 | 0.25 | 24 12 | 1208.0a | 520.0 | 14.9 |
24 12 |
0.312 0.429 |
0.178 0.244 |
43, 44 | 100–250 |
| Furosemide | 11 | 0.47 | 24 12 | 589.5a | 150.0 | 1.9 |
24 12 |
0.002 0.066 |
0.001 0.049 |
45 | 2000–5000 |
| HCT | 58 | 0.65 | 24 12 | 569.4a | 172.5 | 10.6 |
24 12 |
0.501 0.802 |
0.349 0.559 |
14, 26 | 40–2000 |
| Torasemide | 37 | 0.79 | 24 12 | 43.0 | 9.8 | 3.7 |
24 12 |
0.819 4.287 |
0.632 3.310 |
46– 48 | n/a |
aClearance is calculated by dividing the dose by the AUC.
bSustained-release formulation.
cAdministered as spironolactone.
dGenetic polymorphism: data for extensive metabolizers (EM) was used in the present study which differ markedly from those for poor metabolizers (PM).
The approach to use dose-related concentrations (DRC) consists of comparing measured concentrations with trough serum drug concentrations calculated individually for each patient. To simplify the calculation of expected serum levels, first a factor (DRC factor) was calculated depending on the dosing interval (τ) which is equal to the blood sampling time before the next dose (∆t, 12 or 24 h for both parameters). The necessary pharmacokinetic parameters were retrieved from pharmacokinetic studies on patients without comorbidities, co-medication or genetic abnormalities after oral administration (Table 3): bioavailability f, total body clearance CLt, elimination rate constant ke.
| 1 |
To cover inter-individual variabilities, the standard deviation (SD) of the apparent total clearance (CLt/f) as correlate of elimination was incorporated in the calculation (lower DRC factor, based on the concept of Hiemke et al.11) in a second step.
| 2 |
For each patient the expected trough serum concentration (lower DRC in ng/ml) was calculated by multiplication of the total daily dose in mg with the lower DRC factor. This lower limit of the dose-related concentration was used as a cut-off to evaluate concentrations with regard to adherence assessment.
Results
Method validation
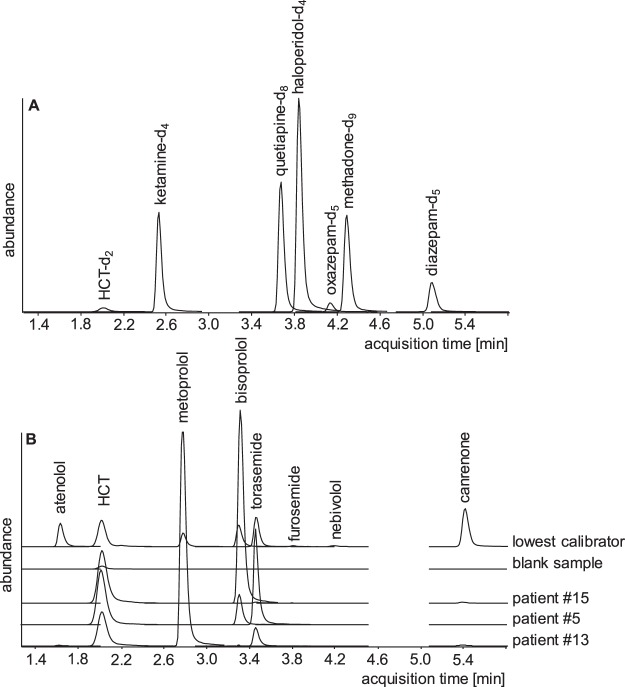
This method based on a two-step liquid liquid-extraction at acidic and basic pH was validated for quantification of four β-blockers and four diuretics. Blank and zero serum samples showed no significant interference in terms of endogenous substances at retention times of analytes or internal standards except that a signal at the retention time of HCT was detected (c.f. chromatogram of a blank sample in Fig. 1) which obviously resulted from non-deuterated HCT present in the HCT-d2. In this case the LOD and LLOQ were determined based on the standard deviation of the response and the slope according to ICH guidelines (Table 1). Apart from this, neither the spiked nor the analysed samples from patients exhibited signals from other drugs.
Figure 1.
Representative extracted ion chromatograms of internal standards are given in (A) and of all analytes in the lowest calibrator, a blank sample, and trough serum samples of patient #15 on HCT (140.5 ng/ml) and bisoprolol (30.2 ng/ml), patient #5 on HCT (139.5 ng/ml), bisoprolol (8.7 ng/ml) and torasemide (439.6 ng/ml) and patient #13 on HCT (108.4 ng/ml), metoprolol (48.5 ng/ml) and torasemide (1752.9 ng/ml) in (B), all signals in equal scale.
Analyte signals were not affected by ion suppression or enhancement. The limit of detection, lower limit of quantification, intra- and inter-day precision, accuracy and recovery as part of the validation procedure are summarized in Table 1. The LLOQs were below expected serum concentrations. The requirements of the Grubbs test (95% significance level), Cochran test (99% significance) and Mandel test (99% significance) were fulfilled and a non-weighted calibration model excluding the origin was used. The calibration curves covered therapeutic ranges and were linear with regression coefficients of at least 0.999. Intra- and inter-day precisions were less than 8.7%, accuracies less than 8.9% (mostly <5.0%) and recoveries higher than 50%. The concentration of the extracted analytes decreased less than 25% during 72 hours of measurement, except of torasemide (48 h), nebivolol (24 h) and canrenone (16 h). Therefore, analysis was always completed within half a day. Representative chromatograms are shown in Fig. 1.
Serum samples
In this study serum of 20 patients on β-blockers and/or diuretics were evaluated. All expected drugs could be quantitated (Table 4) where the trough levels before medication were of special interest. As expected, a marked increase in serum concentrations was observed in the second serum samples representing the time around peak concentrations with a few exceptions (HCT in #16, all metoprolol concentrations).
Table 4.
Concentrations of β-blockers and diuretics in serum samples of patients shortly before and about 2 h after observed ingestion (trough/peak). Concentrations below published therapeutic reference ranges are indicated by “↓” (except for torasemide due to missing reference data), no concentrations below the lower DRC (lower DRC factor * daily dose) were observed.
| Patient # | Drug | Daily dose (single) [mg] | lower DRC [ng/ml] | Bisoprolol [ng/ml] | Metoprolol [ng/ml] | Nebivolol [ng/ml] | HCT [ng/ml] | Torasemide [ng/ml] | Canrenone [ng/ml] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Metoprolol | 50 (25) | 1.9 | 6.6↓/7.3↓ | |||||
| 2 | Bisoprolol | 2.5 | 2.2 | 8.9↓/19.2 | |||||
| 3 | Bisoprolol | 5 (2.5) | 5.9 | 15.8/16.5 | |||||
| HCT | 12.5 | 4.4 | 100.5/200.2 | ||||||
| 4 | HCT | 12.5 | 4.4 | 159.8/264.6 | |||||
| Metoprolol | 200 (100) | 7.4 | 38.7/42.0 | ||||||
| Torasemide | 20 | 12.6 | 50.9/1779.2 | ||||||
| 5 | Bisoprolol | 1.25 | 1.1 | 3.3↓/8.7↓ | |||||
| HCT | 12.5 | 4.4 | 44.2/139.5 | ||||||
| Torasemide | 5 | 3.2 | 91.3/439.6 | ||||||
| 6 | Metoprolol | 100 (50) | 3.7 | 12.6↓/10.7↓ | |||||
| 7 | Metoprolol | 47.5 | 0.2 | 5.8↓/7.3↓ | |||||
| 8 | HCT | 25 | 8.7 | 286.6/542.5 | |||||
| 9 | Bisoprolol | 5 (2.5) | 5.9 | 16.4/23.1 | |||||
| Torasemide | 10 | 6.3 | 320.4/1592.4 | ||||||
| 10 | Spironolactone | 25 | 4.5 | 47.5↓/100.4 | |||||
| Torasemide | 5 | 3.2 | 371.5/1829.2 | ||||||
| 11 | Bisoprolol | 2.5 (1.25) | 3.0 | 15.4/10.9 | |||||
| Torasemide | 5 | 3.2 | 35.1/86.2 | ||||||
| 12 | Nebivolol | 5 | 0.2 | 0.4/1.1 | |||||
| 13 | HCT | 25 | 8.7 | 75.2/108.4 | |||||
| Metoprolol | 190 (95) | 7.0 | 49.4/48.5 | ||||||
| Torasemide | 20 | 12.6 | 24.8/1752.9 | ||||||
| 14 | Metoprolol | 200 (100) | 7.4 | 110.8/92.8 | |||||
| Torasemide | 5 | 3.2 | 17.6/1277.9 | ||||||
| 15 | Bisoprolol | 5 | 4.3 | 12.9/30.2 | |||||
| HCT | 12.5 | 4.4 | 81.0/140.5 | ||||||
| 16 | HCT | 12.5 | 4.4 | 69.5/60.8 | |||||
| 17 | Bisoprolol | 10 (5) | 11.9 | 21.5/53.8 | |||||
| 18 | Spironolactone | 25 | 4.5 | 25.5↓/44.7↓ | |||||
| HCT | 25 | 8.7 | 317.9/606.3 | ||||||
| Metoprolol | 200 (100) | 7.4 | 30.0/24.5 | ||||||
| 19 | Bisoprolol | 10 (5) | 11.9 | 41.1/53.8 | |||||
| HCT | 12.5 | 4.4 | 113.9/167.5 | ||||||
| Torasemide | 20 | 12.6 | 39.6/1570.4 | ||||||
| 20 | Bisoprolol | 10 | 8.6 | 9.8↓/25.1 | |||||
| HCT | 25 | 8.7 | 15.5↓/96.4 |
Reference data on therapeutic plasma levels were used from various sources17,18 (Table 3) with the exception of torasemide for which no data was available. A high proportion of values were within the expected therapeutic reference ranges (75.9% of all determined concentrations). None exceeded the higher limit, but canrenone concentrations were mostly (75.0%) below the reported range, as well as 42.9% of metoprolol serum levels. For bisoprolol trough and peak concentrations of patient #5 were both below the therapeutic range (<10 ng/ml17) as were the trough samples of patients #2 and #20. In one case a HCT concentration (patient #20, trough) was lower than expected (<40 ng/ml17).
In addition to evaluation of concentrations with regard to published reference ranges, the data was also compared with the expected lower limit of the trough serum concentration (lower DRC). This value was individually calculated on the basis of the patient’s drug dose and the drug’s lower DRC factor (Table 3). The lower DRC includes a diminution by one standard deviation of the apparent total clearance to reflect interindividual variations in excretion. All serum concentrations (trough and peak) of bisoprolol, nebivolol, metoprolol, canrenone, HCT, and torasemide were above these calculated limits.
Discussion
Hypertension is the leading factor for cardiovascular morbidity and mortality19,20. Even though there are several pharmacological treatment options, effective high blood pressure management is an ongoing real concern. Since hypertension causes only few symptoms, there is a risk of poor adherence to drugs with unpleasant side effects. Patients not complying with their medication scheme (non-adherence) risk exhibiting a treatment resistant hypertension (TRH). Non-adherence is not easy to diagnose with current methods. Assessment of adherence by direct methods such as toxicological urine or blood analysis is available only occasionally. Based on our detailed previous experience with antihypertensive drug testing in urine8,21 this methodology is well suited to detect non-adherence, but still has some limitations. One problem is, that a few substances are excreted mainly as metabolites (especially dihydropyridine derivatives22,23) and a failure in detection of the drug might lead to misclassification as non-adherent. On the other hand, substances with prolonged excretion may be detectable despite poor adherence (e.g. HCT24). As a potential solution it was hypotheticized whether quantitative assays of the drugs in blood would reflect adherence more precisely. In addition, other causes of TRH like malabsorption or individual differences in excretion could be diagnosed more accurately with such an approach.
As a first step a quantitative chromatographic-mass spectrometric target compound analysis procedure was developed and validated. The assay focused on the mainly prescribed diuretics and β-blockers in Germany according to the annual Drug Prescription Report25. In a pilot study this method was applied to serum samples obtained from patients in the University Hospital Frankfurt/Main (Germany) with confirmed medication adherence. Results were evaluated with regard to two aspects: (1) does the analytical method yield concentrations that are in accordance with results from published studies and (2) can adherence be confirmed? For this latter aspect the measured concentrations were compared with published reference ranges and with individually calculated cut-off concentrations on the basis of the applied doses.
Concentrations
Of the 20 patients 9 were treated with bisoprolol, which was confirmed in concentrations (trough and peak) of 3.3 to 53.8 ng/ml, 7 with metoprolol (5.8 to 110.8 ng/ml), one with nebivolol (0.36 and 1.08 ng/ml), 10 with HCT (15.5 to 606.3 ng/ml), 8 with torasemide (17.6 to 1829.2 ng/ml) and 2 with canrenone (25.5 to 100.4 ng/ml, active metabolite of administered spironolactone). The measured concentration ranges are in accordance with those found in samples of a routine TDM10 for bisoprolol (8.14–44.6 ng/ml), metoprolol (3.74–267 ng/ml), canrenone (14.0–91.2 ng/ml) and HCT (7.44–298 ng/ml). However, no data on daily doses or times of blood sampling was provided for a more detailed comparison. The larger concentration ranges in the present data are in agreement with the sampling scheme targeting the minimal (trough) and maximal (peak) concentrations in the patients. The present results also match reported serum concentrations of nebivolol and torasemide26,27. Rather high peak and trough concentrations of HCT were found for two patients (#8 and #18, Table 4) which is in agreement with a mean of 673.17 ng/ml that has been reported for elderly hypertensive patients on a daily dose of 25 mg28. Therefore, the concentrations measured in the present study are in agreement with published data from patients taking β-blockers and/or diuretics.
Assessment of adherence on the basis of serum concentrations
Adherence assessment is an important part of the diagnosis of treatment resistant hypertension. Several methods have been published to assess patients’ adherence29. However, so far none of those employed quantitative data in combination with cut-off values.
Adherence rates appear to differ between the classes of antihypertensive medications. Especially low adherence was found for diuretics and β-blockers based on medication refill data5 which contrasts data from studies using urine or plasma analysis, where these classes were among those with the highest adherence rates8,30. In the evaluation of this discrepancy it must be taken into account that toxicological analyses are qualitative in nature and may still be positive even if some time passed since the last drug ingestion. Therefore, it appears necessary to extend toxicological analysis by a quantitative feature and evaluate concentrations in terms of pharmacological activity.
Data on the therapeutic concentration ranges have been reported only for five of the six drugs assayed, torasemide concentrations were therefore excluded from evaluation. None of the concentrations measured exceeded the upper therapeutic limits, but 14 of the total of 58 values (24.1%, 31.0% of the 29 trough values) fell below the lower limit of the concentration range considered therapeutic17,18. This affected mainly the β-blockers bisoprolol (especially low doses) and metoprolol, as well as the spironolactone metabolite canrenone (Table 4) and would lead to classification as non-adherent. Since in all cases drug ingestion was monitored this renders the published data as not reliable to differentiate drug ingestion by comparison with the lower limit of the therapeutic reference range. Obviously, reference ranges reflect pharmacologically effective concentrations but cannot be used to evaluate adherence.
For therapeutic drug monitoring (TDM) of antidepressants and neuroleptics this has been improved by Hiemke et al.11. Expected trough concentrations under steady-state conditions were calculated using a function described by Gex-Fabry et al.31 taking into account dose and dosing interval. In the present study this established concept was applied to evaluate concentrations of antihypertensive drugs. Therefore, lower limits of expected therapeutic concentrations were calculated for different dosing regimens. This based on the concept of Hiemke et al.11 for neuropsychopharmacology where complex dosing regimens were simplified by calculating the total daily dose with a hypothetical dosing interval of 24 h. In the present context this was extended by inclusion of the dosing interval as different doses during a day are rather rare in antihypertensive therapy. This leads to a more appropriate estimation of trough concentrations which are used as cut-offs and are thus more reliable for differentiation of adherence state.
All measured values were above the calculated minimum concentrations expected for the respective dosage schemes. The superiority of this evaluation concept has also been shown for TDM in neuropsychopharmacology11. However, limitations of this concept should not be disregarded. For metoprolol, it was striking that serum concentrations of each patient showed hardly any variation from trough to peak. From the patients records it was retrieved that all participants received metoprolol succinate as a sustained-release formulation. From this, a much longer time to maximal concentrations (tmax) and smaller peak-trough fluctuations in serum concentrations are expected. Due to the continuous release over 20 hours, a postabsorptive phase, as with the other drugs, does not occur. Since the DRC concept relies on a forecast of the elimination which is different with sustained-release formulations the lower DRC was calculated using pharmacokinetic parameters from appropriate studies (Table 3). Therefore, this concept still allows comparison with measured serum concentrations but an underestimation cannot be excluded. Another limitation that arises in qualitative as well as quantitative methods are substances with low plasma levels and short half-lives. In the present study for instance, doses of furosemide once daily may result in trough concentrations which are very close to the LOD. It is therefore recommended to critically consider the applicability of the quantitative method for adherence assessment in such cases. A peculiarity which remains problematic is that adherence varies over time32 and the rare ingestion of drugs, especially prior to a doctor’s visit (white coat adherence), results in therapeutic serum concentrations which may lead to the false assumption of continuous adherence.
Conclusion
The present proof-of-concept study was performed to evaluate an improved strategy for the assessment of adherence based on quantitative serum drug concentrations. The results with patients on supervised medication adherence show, that established ranges of therapeutic concentrations as far as they are available are not applicable to multi-drug regimens as used for treatment of hypertension. The calculation of lower limits of dose-related concentrations can be used as cut-off values. Concentrations beneath these thresholds may indicate non-adherence or deviations in pharmacokinetics (e.g. malabsorption or rapid drug elimination) which could be used for adaptation of the dosage scheme.
The superiority of evaluating quantitative serum results for assessment of adherence will be investigated in comparison to qualitative results in urine analysis in a study with outpatients without controlled adherence. In addition, the application of this approach to a wider range of antihypertensive drugs is in progress.
Author contributions
Sabrina Ritscher developed the method, analysed and interpreted the data. Prepared the figures and tables and wrote the main manuscript. Stefan W. Toennes designed the study and contributed to the data analysis and interpretation, the preparation of the figures and tables and the manuscript writing and revision. Milena Hoyer acquired and supervised the patients. Cora Wunder contributed to the method development and validation. Nicholas Obermüller supervised the clinical procedures and revised the manuscript. All authors approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robert Koch-Institute. Health in Germany – the most important trends. Available at, https://www.rki.de/EN/Content/Health_Monitoring/Health_Reporting/HealthInGermany/Health-in-Germany_most_important_developments.pdf?__blob=publicationFile (2016).
- 2.Wilkins, E. et al. European Cardiovascular Disease Statistics 2017. European Heart Network, 1–192 (2017).
- 3.Sabaté, E. Adherence to long-term therapies. Evidence for action. Available at, https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1 (2003).
- 4.Mennini FS, et al. Cost of poor adherence to anti-hypertensive therapy in five European countries. The European journal of health economics. 2015;16:65–72. doi: 10.1007/s10198-013-0554-4. [DOI] [PubMed] [Google Scholar]
- 5.Kronish IM, et al. Meta-Analysis: Impact of Drug Class on Adherence to Antihypertensives. Circulation. 2011;123:1611–1621. doi: 10.1161/CIRCULATIONAHA.110.983874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta P, et al. Risk Factors for Nonadherence to Antihypertensive Treatment. Hypertension. 2017;69:1113–1120. doi: 10.1161/HYPERTENSIONAHA.116.08729. [DOI] [PubMed] [Google Scholar]
- 7.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clinical therapeutics. 1999;21:1074–1090. doi: 10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 8.Jung O, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. Journal of hypertension. 2013;31:766–774. doi: 10.1097/HJH.0b013e32835e2286. [DOI] [PubMed] [Google Scholar]
- 9.Gupta P, et al. How to Screen for Non-Adherence to Antihypertensive Therapy. Current hypertension reports. 2016;18:89. doi: 10.1007/s11906-016-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gundersen POM, Helland A, Spigset O, Hegstad S. Quantification of 21 antihypertensive drugs in serum using UHPLC-MS/MS. Journal of chromatography. B. 2018;1089:84–93. doi: 10.1016/j.jchromb.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 11.Hiemke C, et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry. 2018;51:9–62. doi: 10.1055/s-0043-116492. [DOI] [PubMed] [Google Scholar]
- 12.Peters, F. T. et al. Requirements for the validation of analytical methods. Appendix B: To the GTFCh Guidelines for quality assurance in forensic-toxicological analyses. Available at, https://www.gtfch.org/cms/images/stories/files/Appendix%20B%20GTFCh%2020090601.pdf (2009).
- 13.Kolb M, Bahr A, Hippich S, Schulz W. Calculation of Detection Limit, Identification Limit and Determination Limit according to DIN 32645 with the Aid of a Computer Programs. Acta hydrochim. hydrobiol. 1993;21:308–311. doi: 10.1002/aheh.19930210603. [DOI] [Google Scholar]
- 14.Gao F, et al. Simultaneous quantitation of hydrochlorothiazide and metoprolol in human plasma by liquid chromatography-tandem mass spectrometry. Journal of pharmaceutical and biomedical analysis. 2010;52:149–154. doi: 10.1016/j.jpba.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Sora DI, Udrescu S, Albu F, David V, Medvedovici A. Analytical issues in HPLC/MS/MS simultaneous assay of furosemide, spironolactone and canrenone in human plasma samples. Journal of pharmaceutical and biomedical analysis. 2010;52:734–740. doi: 10.1016/j.jpba.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Liu K-H, et al. Simple and Sensitive Assay of Torasemide in Human Plasma by High-Performance Liquid Chromatography Using a Monolithic Silica Column. Chromatographia. 2004;60:639–643. doi: 10.1365/s10337-004-0427-1. [DOI] [Google Scholar]
- 17.Schulz M, Iwersen-Bergmann S, Andresen H, Schmoldt A. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Critical Care. 2012;16:1–134. doi: 10.1186/cc11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Repetto MR, Repetto M. Therapeutic, Toxic, and Lethal Concentrations in Human Fluids of 90 Drugs Affecting the Cardiovascular and Hematopoietic Systems. Clinical Toxicology. 1997;35:345–351. doi: 10.3109/15563659709043365. [DOI] [PubMed] [Google Scholar]
- 19.Salem H, et al. Worldwide Prevalence of Hypertension: A Pooled Meta-Analysis of 1670 Studies In 71 Countries With 29.5 Million Participants. Journal of the American College of Cardiology. 2018;71:A1819. doi: 10.1016/S0735-1097(18)32360-X. [DOI] [Google Scholar]
- 20.Rahimi K, Emdin CA, MacMahon S. The epidemiology of blood pressure and its worldwide management. Circulation research. 2015;116:925–936. doi: 10.1161/CIRCRESAHA.116.304723. [DOI] [PubMed] [Google Scholar]
- 21.Schmieder, R. E. et al. Adherence to Antihypertensive Medication in Treatment-Resistant Hypertension Undergoing Renal Denervation. Journal of the American Heart Association5; 10.1161/JAHA.115.002343 (2016). [DOI] [PMC free article] [PubMed]
- 22.Barchielli M, et al. Clinical Pharmacokinetics of Lercanidipine. Journal of Cardiovascular Pharmacology. 1997;29:S1–S15. doi: 10.1097/00005344-199729002-00002. [DOI] [Google Scholar]
- 23.Kleinbloesem CH, van Harten J, van Brummelen P, Breimer DD. Liquid chromatographic determination of nifedipine in plasma and of its main metabolite in urine. Journal of Chromatography B: Biomedical Sciences and Applications. 1984;308:209–216. doi: 10.1016/0378-4347(84)80210-8. [DOI] [PubMed] [Google Scholar]
- 24.Barbhaiya RH, Craig WA, Perri Corrick-West H, Welling PG. Pharmacokinetics of Hydrochlorothiazide in Fasted and Nonfasted Subjects: A Comparison of Plasma Level and Urinary Excretion Methods. Journal of Pharmaceutical Sciences. 1982;71:245–248. doi: 10.1002/jps.2600710226. [DOI] [PubMed] [Google Scholar]
- 25.Schwabe, U., Paffrath, D., Ludwig, W.-D. & Klauber, J. Arzneiverordnungs-Report 2018 (Springer Berlin Heidelberg, Berlin, Heidelberg, 2018).
- 26.Vespasiano CFP, et al. Bioequivalence study between a fixed-dose single-pill formulation of nebivolol plus hydrochlorothiazide and separate formulations in healthy subjects using high-performance liquid chromatography coupled to tandem mass spectrometry. Biomedical chromatography. 2017;31:1–9. doi: 10.1002/bmc.3884. [DOI] [PubMed] [Google Scholar]
- 27.Knauf H, Mutschler E. Clinical pharmacokinetics and pharmacodynamics of torasemide. Clinical pharmacokinetics. 1998;34:1–24. doi: 10.2165/00003088-199834010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Sabanathan K, Castleden CM, Adam HK, Ryan J, Fitzsimons TJ. A comparative study of the pharmacokinetics and pharmacodynamics of atenolol, hydrochlorothiazide and amiloride in normal young and elderly subjects and elderly hypertensive patients. European journal of clinical pharmacology. 1987;32:53–60. doi: 10.1007/BF00609957. [DOI] [PubMed] [Google Scholar]
- 29.Eskås PA, et al. Adherence to medication and drug monitoring in apparent treatment-resistant hypertension. Blood pressure. 2016;25:199–205. doi: 10.3109/08037051.2015.1121706. [DOI] [PubMed] [Google Scholar]
- 30.Ewen S, et al. Blood pressure reductions following catheter-based renal denervation are not related to improvements in adherence to antihypertensive drugs measured by urine/plasma toxicological analysis. Clinical Research in Cardiology. 2015;104:1097–1105. doi: 10.1007/s00392-015-0905-5. [DOI] [PubMed] [Google Scholar]
- 31.Gex-Fabry M, Balant-Gorgia AE, Balant LP. Therapeutic drug monitoring of olanzapine: the combined effect of age, gender, smoking, and comedication. Therapeutic drug monitoring. 2003;25:46–53. doi: 10.1097/00007691-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Wunder C, et al. Adherence to antihypertensive drug treatment in patients with apparently treatment-resistant hypertension in the INSPiRED pilot study. Blood pressure. 2019;28:168–172. doi: 10.1080/08037051.2019.1599814. [DOI] [PubMed] [Google Scholar]
- 33.Food and Drug Administration. International Conference on Harmonization (ICH) of Technical Requirements for the Registration of Pharmaceuticals for Human use, Validation of Analytical Procedures: Methodology, ICH Q2B (Geneva, 1996).
- 34.Fitzgerald JD, Ruffin R, Smedstad KG, Roberts R, McAinsh J. Studies on the pharmacokinetics and pharmacodynamics of atenolol in man. European journal of clinical pharmacology. 1978;13:81–89. doi: 10.1007/BF00609750. [DOI] [PubMed] [Google Scholar]
- 35.Mason WD, Winer N, Kochak G, Cohen I, Bell R. Kinetics and absolute bioavailability of atenolol. Clinical Pharmacology & Therapeutics. 1979;25:408–415. doi: 10.1002/cpt1979254408. [DOI] [PubMed] [Google Scholar]
- 36.Jovanović D, Cusić S, Rancić D, Srnić D, Perković-Vukcević N. A pharmacokinetic comparison of generic tablets containing bisoprolol with the innovator formulation in healthy volunteers. Journal of clinical pharmacology. 2006;46:1217–1222. doi: 10.1177/0091270006291626. [DOI] [PubMed] [Google Scholar]
- 37.Kirch W, et al. Pharmacokinetics of bisoprolol during repeated oral administration to healthy volunteers and patients with kidney or liver disease. Clinical pharmacokinetics. 1987;13:110–117. doi: 10.2165/00003088-198713020-00003. [DOI] [PubMed] [Google Scholar]
- 38.Jordö L, et al. Pharmacokinetic and pharmacodynamic properties of metoprolol in patients with impaired renal function. Clinical pharmacokinetics. 1980;5:169–180. doi: 10.2165/00003088-198005020-00004. [DOI] [PubMed] [Google Scholar]
- 39.Senthamil Selvan P, Pal TK. Chromatography-tandem mass spectrometry method for the simultaneous quantitation of metoprolol succinate and simvastatin in human plasma. Journal of pharmaceutical and biomedical analysis. 2009;49:780–785. doi: 10.1016/j.jpba.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 40.Sarkar AK, et al. Simultaneous determination of metoprolol succinate and amlodipine besylate in human plasma by liquid chromatography-tandem mass spectrometry method and its application in bioequivalence study. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2008;873:77–85. doi: 10.1016/j.jchromb.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 41.Selvan PS, Gowda KV, Mandal U, Solomon WDS, Pal TK. Simultaneous determination of fixed dose combination of nebivolol and valsartan in human plasma by liquid chromatographic-tandem mass spectrometry and its application to pharmacokinetic study. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2007;858:143–150. doi: 10.1016/j.jchromb.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Cheymol G, et al. Pharmacokinetic study and cardiovascular monitoring of nebivolol in normal and obese subjects. European journal of clinical pharmacology. 1997;51:493–498. doi: 10.1007/s002280050237. [DOI] [PubMed] [Google Scholar]
- 43.Abshagen U, Besenfelder E, Endele R, Koch K, Neubert B. Kinetics of canrenone after single and multiple doses of spironolactone. European journal of clinical pharmacology. 1979;16:255–262. doi: 10.1007/BF00608404. [DOI] [Google Scholar]
- 44.Krause W, Karras J, Seifert W. Pharmacokinetics of canrenone after oral administration of spironolactone and intravenous injection of canrenoate-K in healthy man. European journal of clinical pharmacology. 1983;25:449–453. doi: 10.1007/BF00542109. [DOI] [PubMed] [Google Scholar]
- 45.Haegeli L, et al. Sublingual administration of furosemide: new application of an old drug. British journal of clinical pharmacology. 2007;64:804–809. doi: 10.1111/j.1365-2125.2007.03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barr, W. H. et al Torasemide dose proportionality of pharmacokinetics and pharmacodynamics. Prog Pharmacol Clin Pharmacol, 29–37 (1990).
- 47.Neugebauer G, Besenfelder E, Möllendorff E. von. Pharmacokinetics and metabolism of torasemide in man. Arzneimittel-Forschung. 1988;38:164–166. [PubMed] [Google Scholar]
- 48.Barbanoj MJ, et al. Comparison of repeated-dose pharmacokinetics of prolonged-release and immediate-release torasemide formulations in healthy young volunteers. Fundamental & clinical pharmacology. 2009;23:115–125. doi: 10.1111/j.1472-8206.2008.00643.x. [DOI] [PubMed] [Google Scholar]