Abstract
Background:
We performed a systematic review with network meta-analysis to inform the comparative efficacy and tolerability of different therapies in the management of patients with left-sided or extensive mild to moderately active ulcerative colitis (UC).
Methods:
Through a systematic review up to March 1, 2018, we identified randomized controlled trials (RCTs) in (a) adults with left-sided or extensive mild-moderate UC, (b) treated with oral sulfasalazine, diazo-bonded 5-aminosalicylates (5-ASA), mesalamine (low- [<2 g/d], standard- [2–3 g/day] or high-dose [>3 g/day]), controlled ileal-release budesonide or budesonide MMX, alone or in combination with rectal 5-ASA therapy, and (c) compared to each other or placebo for (d) induction or maintenance of clinical remission. We performed pairwise and random-effects network meta-analysis using frequentist approach, and calculated odds ratio (OR) and 95% confidence intervals (CI); agents were ranked using surface under the cumulative ranking (SUCRA) probabilities. We used GRADE criteria to appraise quality of evidence.
Findings:
Based on 48 induction RCTs (8020 participants), combined oral and rectal 5-ASA (SUCRA, 0·99) and high-dose mesalamine (>3g/d) (SUCRA, 0·82) were ranked highest for induction of remission; both of these interventions were superior to standard-dose mesalamine (2–3g/d) (failure to induce remission with combined oral and rectal 5-ASA: OR, 0·41; 95% CI, 0·22–0·77; high-dose mesalamine: OR, 0·78; 95% CI, 0·66–0·93) with moderate confidence in estimates. Based on 28 maintenance RCTs (4218 participants), all interventions were superior to placebo for maintenance of remission; however, neither combined oral and rectal 5-ASA, nor high-dose mesalamine were superior to standard-dose mesalamine.
Interpretation:
In patients with mild-moderate extensive UC, combined oral and topical mesalamine therapy and high-dose mesalamine are superior to standard-dose mesalamine for induction of remission, but not maintenance of remission. Standard-dose mesalamine may be the preferred agent for maintenance in most patients.
Keywords: Positioning, Comparison, inflammatory bowel diseases, evidence-based medicine
INTRODUCTION
Ulcerative colitis (UC) is a chronic disabling inflammatory bowel disease that generally begins in young adulthood and lasts throughout life. Although the incidence and prevalence of UC has stabilized in Western Europe and North America (affecting >0.3% of the population), its incidence continues to rise in newly industrialized countries.1 Based on longitudinal population-based cohort studies, less than 20% of patients experience an aggressive disease course and the majority of patients with UC have a mild-moderate course, generally most active at diagnosis and then in varying periods of remission or mild activity.2–8 More than >90%patients receive 5-aminosalicylates (5-ASA) within 1 year of diagnosis for management of UC, and on long-term follow-up, 60–87% patients continue 5-ASA use. Only 50% of patients receive corticosteroids during the course of their disease, with even lower rates of use of immunosuppressive (20%) and biologic therapy (5–10%).2–8
Despite the majority of patients with UC having mild-moderate disease activity, there is considerable practice variability, between IBD specialists, gastroenterologists and primary care physicians.9 Some important area of variability include the dosing of mesalamine for induction and maintenance of remission, comparative efficacy of diazo-bonded 5-ASA and mesalamine, role of combined oral and topical 5-ASA, and positioning of recently approved budesonide MMX in the management of patients with extensive mild-moderate UC. Previous pair-wise meta-analyses focusing on head-to-head comparisons, while thorough and informative, have not adequately informed comparative efficacy of different approaches in the management of mild-moderate UC, due to paucity of head-to-head trials for some key comparisons (for example, role of high-dose 5-ASA (>3g/d) vs. standard-dose 5-ASA (2–3g/d, positioning of budesonide MMX, etc.).10–12 In contrast, network meta-analyses, which combine direct evidence (from head-to-head trials) and indirect evidence (comparisons of different interventions against a common comparator) may better inform comparative efficacy of different treatment approaches.13
Hence, we conducted a systematic review with pairwise and network meta-analyses to compare the efficacy and tolerability of candidate agents (sulfasalazine, diazo-bonded 5-ASA including olsalazine and balsalazide, mesalamine (low- [<2 g/d], standard- [2–3 g/day] or high-dose [>3 g/day]), controlled ileal release budesonide, budesonide MMX, alone or in combination with rectal 5-ASA therapy) in patients with extensive or left-sided mild-moderate UC. We used Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria for network meta-analysis to appraise quality of evidence.14
METHODS
We performed this systematic review according to the guidelines as prescribed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for systematic reviews incorporating network meta-analyses for health care interventions and also following an a priori established protocol (see appendix p2).15 For our research protocol, we followed good research practices as outlined in the Internal Society for Pharmacoeconomics and Outcomes Research report on interpreting indirect treatment comparisons and network meta-analysis for health-care decision making.16
Data Sources and Searches
A medical librarian (LJP) designed and performed a comprehensive literature search with input from study investigators utilizing various databases (included Ovid Epub, Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Scopus, and Web of Science) from inception to December 2015; the search was subsequently updated on Medline on March 1, 2018. The search strategy contained no language restrictions and used controlled vocabulary supplemented with keywords, expanded terminology, and varying algorithms to search for RCTs in adults with mild-moderate UC. The references of all identified relevant studies, as well as recent Cochrane reviews on the topic, were also manually reviewed to identify any potential relevant studies. Two study reviewers (NHN, MF) independently reviewed each study to exclude non-relevant studies with discrepancies discussed and resolved by a third reviewer (SS). Details of the search strategy are reported in the online supplement (see appendix p8). Figure 1 shows the schematic diagram of study selection.
Figure 1.
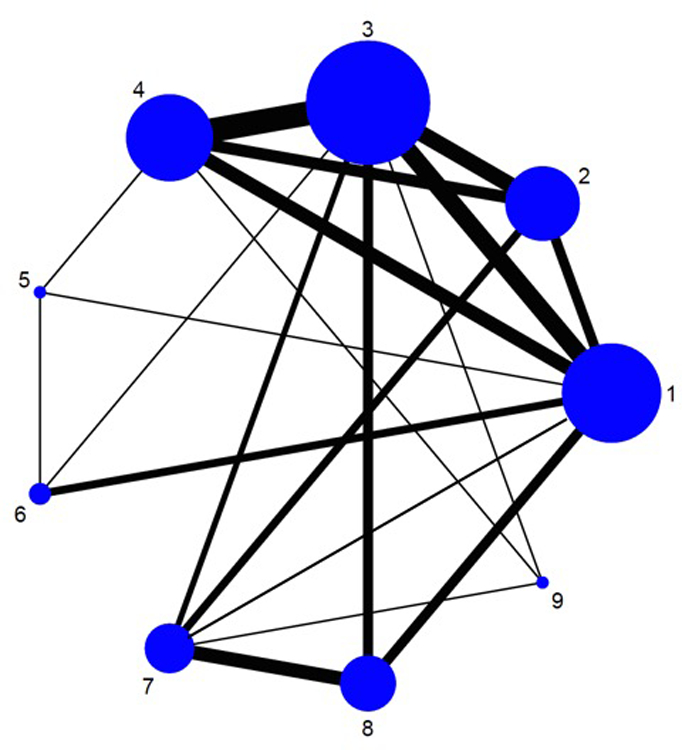
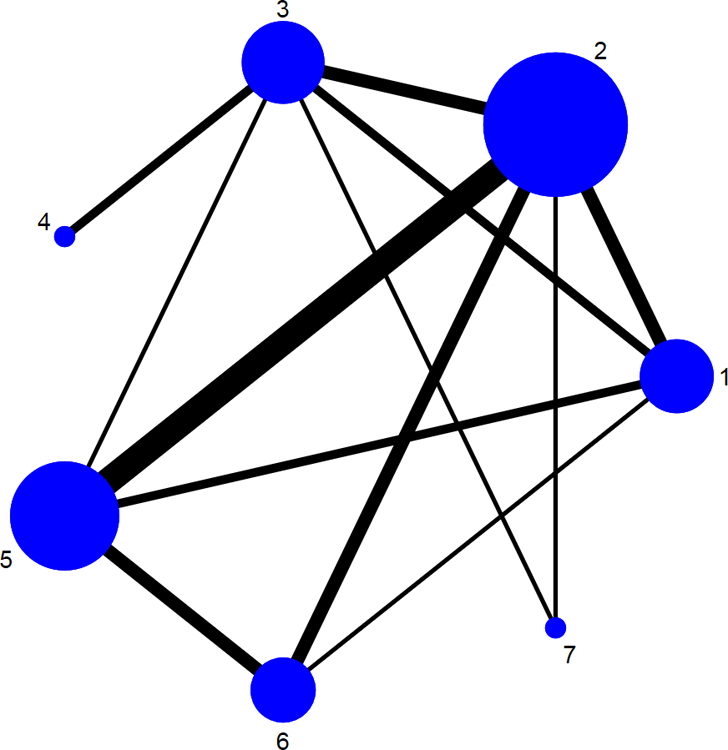
Network of included studies with the available direct comparisons for (A) induction and (B) maintenance of clinical remission in patients with mild-moderate ulcerative colitis. The size of the nodes and the thickness of the edges are weighted according to the number of studies evaluating each treatment and direct comparison, respectively. In panel A, number corresponds to 1: Placebo/Control, 2: Low-dose mesalamine (<2g), 3: Standard dose mesalamine (2–3g), 4: High-dose mesalamine (>3g), 5: Controlled ileal relelase budesonide, 6: Budesonide MMX, 7: Sulfsalazine, 8: Diazo-bonded 5-aminosalicylates (olsalazine/balsalazide), 9: Oral + Rectal 5-aminosalicylates. In panel B, number corresponds to 1: Placebo/Control, 2: Low-dose mesalamine (<2g), 3: Standard dose mesalamine (2–3g), 4: High-dose mesalamine (>3g), 5: Sulfsalazine, 6: Diazo-bonded 5-aminosalicylates (olsalazine/balsalazide), 7: Oral + Rectal 5-aminosalicylates.
Study Selection
Randomized controlled trials (RCTs) were included if they met the following inclusion criteria: (a) Adults (age ≥17 years) with extensive or left-sided mild-moderate UC; (b) Interventions: sulfasalazine, diazo-bonded 5-ASA including olsalazine and balsalazide, mesalamine (low- [<2 g/d], standard- [2–3 g/day] or high-dose [>3 g/day]), controlled ileal release budesonide, budesonide MMX, alone or in combination with rectal 5-ASA therapy; (c) comparator: any other active intervention or placebo; and (d) outcomes included induction and/or maintenance of clinical remission with or without endoscopic remission. Minimum duration of therapy for trials of induction and maintenance therapy was 4 and 24 weeks, respectively. We assumed comparability of different commercial preparations of mesalamine at equivalent doses based on an exhaustive systematic review that demonstrated that different mesalamine formulations have comparable efficacy and safety.17
We excluded the following studies: (a) observational studies, (b) short duration trials, (c) 5-ASA trials in patients with moderate-severe disease or steroid-dependent UC, trials in patients with proctitis, or trials of rectal therapy alone in patients with left-sided colitis and (d) trials of non-conventional agents which are not approved for UC.
Data Abstraction and Risk of Bias Assessment
Data abstraction was conducted with a standardized case report form to capture data on study-, participant- and treatment-related characteristics. Two reviewers independently reviewed and abstracted data with discrepancies resolved by consensus in consultation with a third reviewer. Risk of bias was assessed by two sets of authors independently, using the Cochrane Risk of Bias assessment tool, with disagreements addressed by re-evaluation, in conjunction with a third reviewer.18
Outcomes
Our primary outcome was overall and comparative efficacy of interventions reported as failure to achieve clinical remission in patients with active disease and failure to maintain clinical remission in patients with quiescent UC. Secondary outcomes of interest included failure to induce endoscopic remission (as defined by each study), and tolerability, defined as discontinuation of therapy due to adverse events.
Clinical remission was measured using various disease activity indices (DAI), most commonly, UCDAI, Mayo Clinic Score, simple clinical colitis activity index, Sutherland DAI, Rachmilewitz Clinical Activity Index, etc. These DAIs generally combined measures of stool frequency, rectal bleeding and/or physician global assessment, with a measure of endoscopic disease activity; in most indices, all patients who achieved clinical remission were also in endoscopic remission. There were differences in definition of remission across different DAIs; in some studies, if clinical and endoscopic outcomes were reported separately, then data on clinical remission was used for analysis. If clinical remission was not reported, then clinical response was abstracted as a surrogate outcome.
When outcomes were reported at multiple time points, then a hierarchical approach was followed. For assessment of induction trials, data abstraction hierarchy was at 6 weeks, then 4 weeks, and 8 weeks. For maintenance trials, last primary time point of individual trials was used for data abstraction. Mesalamine dosing was grouped as low-dose (<2g/d), standard-dose (2–3 g/d) and high-dose (>3g/d). For other interventions, where available, standard FDA-approved doses were used; however, trials of alternative doses of diazo-bonded 5-ASA and sulfasalazine were also included. All patients were analyzed in the group they were randomized to; patients lost to follow-up were considered as treatment failures.
Data Synthesis and Statistical Analysis
Direct pairwise meta-analysis was performed using a random effects model to estimate pooled OR and 95% CI.19 Since outcomes were reported as failure to achieve or maintain remission, ORs<1 indicate superior efficacy. We assessed statistical heterogeneity using I2 statistic, with values over 50% indicating substantial heterogeneity, and small study effect was assessed by examining funnel plot asymmetry, where >10 trials were available.20 Direct comparisons were performed using RevMan v5.3 (Cochrane Collaboration, Copenhagen, Denmark).
We conducted network meta-analysis using a multivariate random-effects meta-regression, using consistency model, as described by Ian White.21 We used a frequentist approach and provided a point estimate from the network along with 95% CI from the frequency distribution of the estimate. Decision to use frequentist approach was made a priori based on the statistical expertise of our team; results of frequentist and Bayesian approach is expected to be very similar. Network consistency was evaluated by comparing the direct estimates to the indirect estimates for each comparison, using a node-splitting technique. All network meta-analyses were performed using Stata v.14 (College Station, TX).
We calculated the relative ranking of interventions for induction and maintenance of clinical remission as their surface under the cumulative ranking (SUCRA), which represents the percentage of efficacy achieved by an approach compared to an imaginary approach that is always the best without uncertainty (i.e. SUCRA=100%).22 Higher SUCRA scores correspond to greater efficacy and superior tolerability. To demonstrate imprecision of ranking probabilities, we also estimated how each treatment would rank in 100 iterations.
Finally, we generated estimates of absolute event rates (or absolute risk) by calculating the estimated risk difference (RD, also known as absolute risk reduction) by combining the odds ratio (OR) for each intervention against placebo and the median placebo response rate across trials as the assumed control risk (ACR), by using the formula: Risk difference = (OR*ACR-ACR+ACR*ACR-OR*ACR*ACR)/(1-ACR+OR*ACR). The risk difference, which represents the difference between the event rates in the intervention and control group, was added back to the ACR to generate an estimate of the absolute risk for each intervention. 95% CI for the estimates were generated using the 95% CI of the odds ratios in the above calculations.
Quality of Evidence
The quality of evidence derived from the pairwise and network meta-analysis was judged using the GRADE framework. In this approach, direct evidence from RCTs starts at high quality and can be rated down based on risk of bias, indirectness, imprecision, inconsistency (or heterogeneity) and/or publication bias, to levels of moderate, low and very low quality. The rating of indirect estimates starts at the lowest rating of the two pairwise estimates that contribute as first-order loops to the indirect estimate but can be rated down further for imprecision or intransitivity (dissimilarity between studies in terms of clinical or methodological characteristics). If direct and indirect estimates were similar (i.e., coherent), then the higher of their rating was assigned to the network meta-analysis estimates.
RESULTS
From a total of 1316 unique citations identified through our comprehensive search strategy, we included 48 RCTs (8020 participants, including 1380 placebo-treated patients) comparing 8 active interventions for induction of remission,23–70 and 28 RCTs (4218 participants, including 575 placebo-treated patients) comparing 6 active interventions for maintenance of remission (see appendix p26).23,71–97 The available direct comparisons and network of trials for induction and maintenance of clinical remission are shown in Figures 1A and B.
Characteristics and Risk of Bias of the Included Trials
Summary characteristics of all included RCTs in the network meta-analysis are reported in the online supplement (see appendix p12). Induction trials were 4–12 weeks long, and trials of maintenance therapy were 24 to 72 weeks long. Overall, median rate of failure to induce clinical and endoscopic remission in placebo-arms of trials 87% (range, 45–96%), and 69% (range, 53–100%), respectively. In trials of maintenance therapy, median (range) of failure to maintain clinical remission in placebo-arms of trials was 56% (range, 33–75%). Most trials included patients with both left-sided and extensive UC; however, efficacy of oral therapy in these patients was not consistently stratified by disease location. Overall, most studies were deemed to be at low-risk of bias, except early trials of sulfasalazine which had high risk of bias.
Primary Outcomes
Failure to Induce Clinical Remission
Pairwise meta-analysis:
In placebo comparisons, low-, standard, and high-dose mesalamine, diazo-bonded 5-ASA, and budesonide MMX were all significantly superior to placebo (see appendix p27). No trials comparing sulfasalazine with placebo were identified in patients with mild-moderate UC. On active comparisons, standard- and high-dose mesalamine was superior to low-dose mesalamine for induction of clinical remission (see appendix p29). High-dose mesalamine was not significantly superior to standard-dose mesalamine (relative risk [RR] for failure to induce remission, 0·94; 95% CI, 0·88–1·01); however, on subgroup analysis only in patients with moderate UC (ASCEND II, ASCEND III, Hiwatashi et al),38,39,60 or reporting outcomes stratified by baseline UC disease severity,37,41,42 high-dose mesalamine was significantly superior to standard-dose mesalamine (6 trials, 1589 patients; RR, 0·92; 95% CI, 0·86–0·99). Sulfasalazine, but not standard-dose mesalamine was inferior to diazo-bonded 5-ASA for induction of clinical remission (see appendix p30); there was a trend towards superiority of balsalazide over standard-dose mesalamine (RR, 0·73; 95% CI, 0·52–1·02). There was no significant difference in efficacy of sulfasalazine and mesalamine (RR, 1·07 [0·91–1·26]) (see appendix p30); on restricting analyses to standard-dose mesalamine, there was trend favoring superiority of mesalamine over sulfasalazine (RR, 0·79; 95% CI, 0·58–1·06). Combined oral and rectal 5-ASA therapy was superior to oral 5-ASA alone (see appendix p30). Overall, for all pairwise comparisons, there was mild-moderate heterogeneity (I2<50%), except for comparison of high-dose mesalamine vs. placebo and mesalamine vs. diazo-bonded 5-ASA.
Network meta-analysis:
On network meta-analysis, low-, standard, and high-dose mesalamine, diazo-bonded 5-ASA, combined oral and rectal 5-ASA and budesonide MMX were superior to placebo for induction of clinical remission, with the largest effect size being observed with combined oral and rectal 5-ASA; sulfasalazine and controlled ileal-release budesonide were not superior to placebo (Figure 2A). On active comparisons, combined oral and rectal 5-ASA was significantly superior to all other interventions, except budesonide MMX where the results were not statistically significant (OR, 0·49; 95% CI, 0·24–1·02) (Figure 3). High-dose mesalamine was significantly superior to low- and standard-dose mesalamine, sulfasalazine, controlled ileal-release budesonide, but not more effective than diazo-bonded 5-ASA (OR, 0·85; 95% CI, 0·63–1·13) and budesonide MMX (OR, 0·94; 95% CI, 0·62–1·42). Standard-dose mesalamine was superior to low-dose mesalamine, sulfasalazine and controlled ileal-release budesonide, but not more effective than diazo-bonded 5-ASA or budesonide MMX (Figure 2B).
Figure 2.
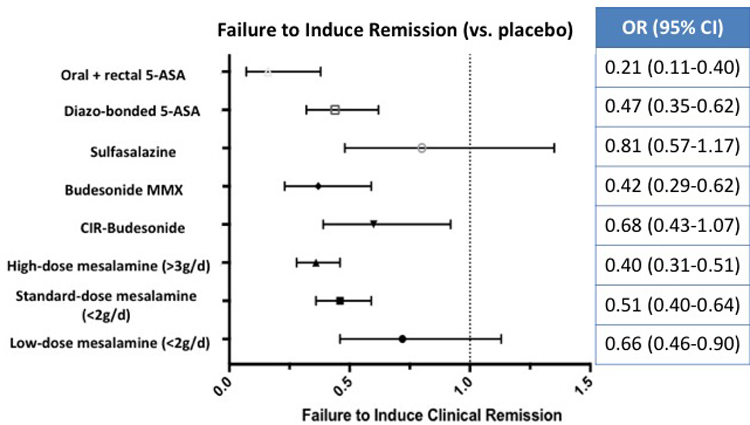
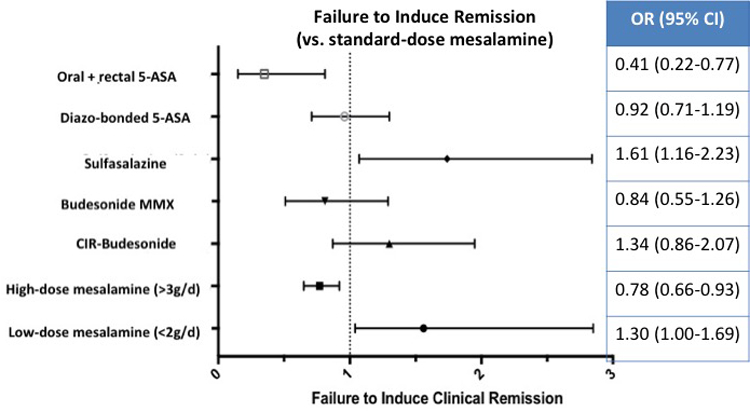
Comparative efficacy of different interventions for failing to induce remission in patients with mild-moderate ulcerative colitis against (A) placebo and (B) standard-dose (2–3g/d) mesalamine, based on network meta-analysis
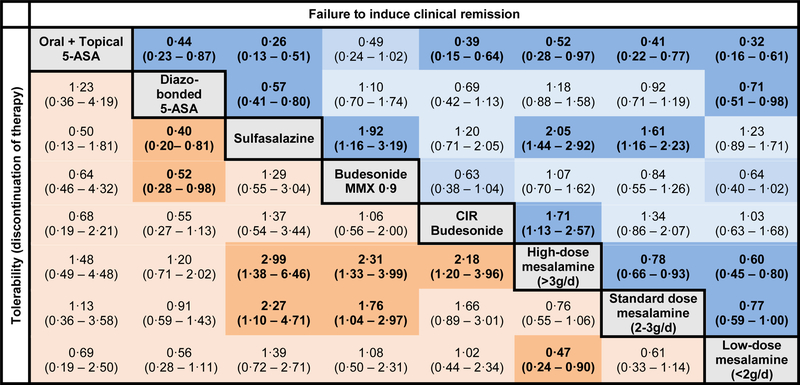
Figure 3. Comparative efficacy and tolerability of induction therapy for mild-to-moderate ulcerative colitis.
Comparisons should be read from left to right. Odds ratio for comparisons are in the cell in common between the column-defining and row-defining treatment. Bold numbers with darker background are statistically significant; values that were borderline significant are in a lighter shade. For risk of failing to achieve clinical remission, odds ratio <1 favors row-defining treatment. For risk of drug discontinuation (tolerability), odds ratio <1 favors column-defining treatment. The numbers in parentheses represents 95% confidence intervals
On SUCRA analysis, combined oral and rectal 5-ASA (SUCRA, 0·99) was ranked highest followed by high-dose mesalamine (SUCRA, 0.82); corresponding imprecision in ranking probabilities is shown in online supplement (see appendix p25). With a median placebo remission rate of 13% across trials, approximately 62·5%, 32·8% and 25·6% patients treated with combined oral and rectal 5-ASA, high-dose mesalamine and standard-dose mesalamine, respectively, may be expected to achieve induction of clinical remission. GRADE quality of evidence from direct and network-meta-analysis is summarized in Table 1. Overall, at least moderate quality evidence supports oral and rectal 5-ASA and high-dose 5-ASA for induction of clinical remission over standard-dose mesalamine and placebo.
Table 1.
GRADE Quality of Evidence of different interventions for induction and maintenance of clinical remission in patients with mild-moderate ulcerative colitis, based on direct meta-analysis and network meta-analysis
| Induction of Remission | Maintenance of Remission | |||
|---|---|---|---|---|
| Direct | Network | Direct | Network | |
| All interventions vs. Placebo | ||||
| Low-dose mesalamine | Moderate1 | Moderate | High | High |
| Standard-dose mesalamine | High | High | High | High |
| High-dose mesalamine | High | High | - | Moderate |
| CIR Budesonide | Low1,2 | Moderate | N/A | N/A |
| Budesonide MMX | Moderate1 | Moderate | N/A | N/A |
| Sulfasalazine | Moderate1 | Moderate | Low1,3 | Low |
| Diazo-bonded 5-ASA | Moderate1 | Moderate | Low1,3 | Moderate |
| Oral + rectal 5-ASA | - | Moderate | - | Low |
| All interventions vs. Low-dose mesalamine | ||||
| Standard-dose mesalamine | High | High | High | High |
| High-dose mesalamine | High | High | - | Moderate |
| CIR Budesonide | - | Low4 | N/A | N/A |
| Budesonide MMX | Moderate | N/A | N/A | |
| Sulfasalazine | Modserate1 | Low4 | Low4 | Moderate |
| Diazo-bonded 5-ASA | - | Low | Moderate1 | Low |
| Oral + rectal 5-ASA | - | Moderate | Low4 | Low |
| All interventions vs. Standard-dose mesalamine | ||||
| High-dose mesalamine | Moderate1 | High | Low4 | Low |
| CIR Budesonide | - | Low4 | N/A | N/A |
| Budesonide MMX | Low1,2 | Low | N/A | N/A |
| Sulfasalazine | Moderate | Moderate | Low4 | Low |
| Diazo-bonded 5-ASA | Low1,3 | Low4 | - | Low |
| Oral + rectal 5-ASA | Moderate1 | Low1,6 | Low | |
| All interventions vs. High-dose mesalamine | ||||
| CIR Budesonide | Moderate1 | Moderate | N/A | N/A |
| Budesonide MMX | - | Low | N/A | N/A |
| Sulfasalazine | - | Moderate | - | Low |
| Diazo-bonded 5-ASA | - | Low | - | - |
| Oral + rectal 5-ASA | Low4 | Moderate | - | Low |
| All interventions vs. CIR Budesonide | ||||
| Budesonide MMX | Low1,2 | Low | N/A | N/A |
| Sulfasalazine | - | Low | N/A | N/A |
| Diazo-bonded 5-ASA | - | Low | N/A | N/A |
| Oral + rectal 5-ASA | - | Moderate | N/A | N/A |
| All interventions vs. Budesonide MMX | ||||
| Sulfasalazine | - | Moderate | N/A | N/A |
| Diazo-bonded 5-ASA | - | Low4 | N/A | N/A |
| Oral + rectal 5-ASA | - | Low | N/A | N/A |
| All interventions vs. Sulfasalazine | ||||
| Diazo-bonded 5-ASA | Moderate1 | Moderate | Low1,5 | Low |
| Oral + rectal 5-ASA | Moderate1 | Moderate | - | Low |
| All interventions vs. Diazo-bonded 5-ASA | ||||
| Oral + rectal 5-ASA | - | Moderate | - | Low |
Rated down for imprecision
Risk of bias
Rated down for inconsistency
Rated down for very serious imprecision
Rated down for indirectness (very limited data on balsalazide which may be the preferred diazo-bonded 5-ASA given higher intolerability with olsalazine due to diarrhea)
Rated down for indirectness (comparator group received low-dose mesalamine, whereas combined mesalamine amount in intervention group exceeded 2g)
Failure to Maintain Clinical Remission
Pairwise meta-analysis:
In placebo comparisons, low- and standard-dose mesalamine and sulfasalazine, but not diazo-bonded 5-ASA, were significantly superior to placebo (see appendix p31); no placebo-controlled trials of high-dose 5-ASA or combination therapy of oral and rectal 5-ASA were identified. Of note, budesonide formulations are not approved or indicated for maintenance of remission. On active comparisons, standard-dose mesalamine was superior to low-dose mesalamine, and was not inferior to high-dose mesalamine for maintenance of clinical remission (see appendix p32). Diazo-bonded 5-ASA was significantly superior to mesalamine for maintenance of remission, though trials only used low-dose mesalamine (see appendix p33). There was a trend suggesting higher efficacy of diazo-bonded 5-ASA as compared to sulfasalazine (see appendix p33); all trials used sulfasalazine 2g/day, and 5 trials compared it with olsalazine. Sulfasalazine was numerically but not statistically inferior to mesalamine for maintenance of remission (see appendix p33); in 5/6 trials, low-dose mesalamine was used (0·75–1·5g/d). Based on 2 trials, combined oral and rectal 5-ASA was superior to oral 5-ASA for maintenance of remission (see appendix p33). Overall, for all pairwise comparisons, there was mild-moderate heterogeneity (I2<50%), except for comparison of diazo-bonded 5-ASA vs. placebo and sulfasalazine vs. placebo.
Network meta-analysis:
On network meta-analysis, low-, standard, and high-dose mesalamine, diazo-bonded 5-ASA, sulfasalazine and combined oral and rectal 5-ASA were superior to placebo for maintenance of clinical remission. On active comparisons, high- and standard-dose mesalamine were significantly superior to low-dose mesalamine (Figure 4). No other intervention was superior to other approaches, without any significant differences between standard- and high-dose mesalamine and combined oral and rectal 5-ASA.
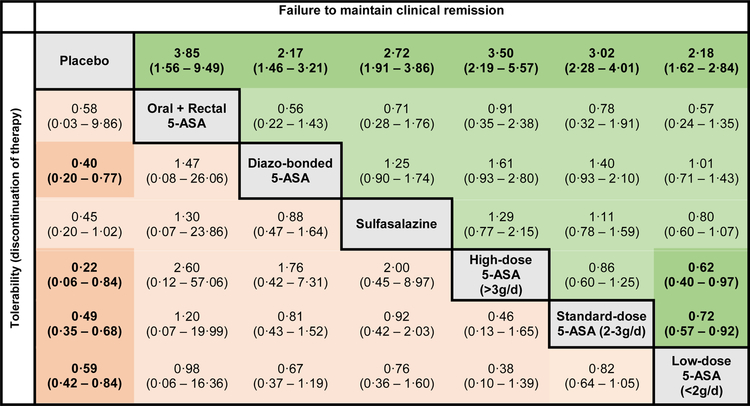
Figure 4. Comparative efficacy and tolerability of maintenance therapy for mild-to-moderate ulcerative colitis.
Comparisons should be read from left to right. Odds ratio for comparisons are in the cell in common between the column-defining and row-defining treatment. Bold numbers with darker background are statistically significant; values that were borderline significant are in a lighter shade. For risk of failing to achieve clinical remission, odds ratio <1 favors row-defining treatment. For risk of drug discontinuation (tolerability), odds ratio <1 favors column-defining treatment. The numbers in parentheses represents 95% confidence intervals
On SUCRA analysis, combined oral and rectal 5-ASA (SUCRA, 0·82) and high-dose mesalamine (SUCRA, 0·82) were ranked highest, followed by standard-dose mesalamine (SUCRA, 0·69); corresponding imprecision in ranking probabilities is shown in online supplement (see appendix p25). With a median placebo rate of clinical relapse of 56% across trials in patients with quiescent UC, approximately 14·6%, 16·2% and 18·5% patients treated with combined oral and rectal 5-ASA, high-dose mesalamine and standard-dose mesalamine, respectively, may relapse over the course of one year. GRADE quality of evidence is summarized in Table 1. Overall, high quality evidence supports standard-dose mesalamine over low-dose mesalamine or placebo for maintenance of clinical remission; only low quality evidence supported oral and rectal 5-ASA and high-dose mesalamine over standard-dose mesalamine for maintenance.
Secondary Outcomes
Failure to Induce Endoscopic Remission:
On network meta-analysis, overall and comparative efficacy of different interventions for induction of endoscopic remission was similar to findings on clinical remission (see appendix p34). All interventions, except low-dose mesalamine and controlled ileal-release budesonide were superior to placebo for induction of remission. On SUCRA analysis, combined therapy with oral and rectal 5-ASA (SUCRA, 0·95) and high-dose mesalamine (SUCRA, 0·81) were ranked highest.
Tolerability:
On network meta-analysis of trials of induction therapy, standard- (OR, 0·48; 95% CI, 0·34–0·69) and high-dose mesalamine (OR, 0·37; 95% CI, 0·25–0·53) and diazo-bonded 5-ASA (OR, 0·44; 95% CI, 0·27–0·71) were better tolerated than placebo (lower rates of treatment discontinuation). On active comparisons, standard- and high-dose mesalamine were better tolerated than budesonide MMX and sulfasalazine (Figure 3). There was no difference in the tolerability of diazo-bonded 5-ASA and mesalamine; diazo-bonded 5-ASA was better tolerated than sulfasalazine and budesonide MMX. On SUCRA analysis, standard- (SUCRA, 0·73) and high-dose mesalamine (SUCRA, 0·93) and diazo-bonded 5-ASA (SUCRA, 0·81) were best tolerated, whereas sulfasalazine (SUCRA, 0·15) and budesonide MMX (SUCRA, 0·33) were not well tolerated.
In contrast, in trials of maintenance therapy, all agents were well tolerated in patients with quiescent disease; in fact, all doses of mesalamine and diazo-bonded 5-ASA were better tolerated than placebo (Figure 4). Sulfasalazine was also numerically better tolerated than placebo (OR, 0·45; 95% CI, 0·20–1·02).
Small study effects and network coherence
Formal assessment of funnel plot asymmetry was performed for one comparison (high-dose mesalamine vs. standard-dose mesalamine for failure to induce clinical remission) with >10 trials (see appendix p35); for other direct comparisons, funnel plot asymmetry assessment is not recommended. There was no evidence of network incoherence in trials of induction therapy. However, in trials of maintenance therapy, network incoherence was observed for the comparison of low-dose mesalamine and sulfasalazine (p=0·003), diazo-bonded 5-ASA (p=0·04) and combination therapy of oral and rectal 5-ASA (p=0·007), and for the comparison of standard-dose mesalamine and combined therapy with oral and rectal 5-ASA (p=0·007).
DISCUSSION
The majority of patients with UC have mild-moderate disease activity and are at low risk of colectomy. These patients are frequently managed by primary care physicians and general gastroenterologists, with considerable practice variability.9 Optimal management of these may reduce the risk of disease progression and complications. Through a systematic review with network meta-analysis, including 48 RCTs (8020 participants) comparing 8 active interventions for induction of remission, and 28 RCTs (4218 participants, including 575 placebo-treated patients) comparing 6 active interventions for maintenance of remission in patients with left-sided or extensive UC, we are able to inform the treatment approach in these patients. For induction of remission, combined oral and rectal 5-ASA is the most effective treatment strategy, followed by high-dose mesalamine (moderate confidence in estimates); standard-dose mesalamine is comparable to diazo-bonded 5-ASA, but superior to sulfasalazine. For maintenance of remission, standard- and high-dose mesalamine are comparable (low confidence in estimates), and superior to low-dose mesalamine (high confidence in estimates). Budesonide MMX is not more effective than combined oral and rectal 5-ASA or high-dose mesalamine and has inferior tolerability. Due to paucity of data stratified by disease location (extensive colitis vs. left-sided colitis) and use of rectal therapy alone as an effective treatment strategy for left-sided colitis, but not extensive colitis, we opted not to evaluate extensive and left-sided colitis separately.
Optimization of 5-ASA is often an under-utilized approach when managing patients with mild-moderate UC;9 at the same time, there is occasional over-reliance of 5-ASA-based therapy with failure to recognize patients who may be at high-risk of colectomy, who may benefit from early escalation to immunosuppressive therapy. By informing comparative efficacy of different 5-ASA-based treatment strategies and a quantitative assessment of success of these therapies, we believe our findings can inform clinical practice and treatment guidelines.
Our study builds on findings from primary RCTs and prior meta-analyses, by systematically synthesizing available direct and indirect evidence on the efficacy and tolerability of treatment for the treatment of mild to moderately active extensive UC, and adding recent studies on budesonide MMX.10–12 In clinical practice in patients with extensive UC, addition of rectal 5-ASA for induction of remission is frequently under-utilized. This may be related to limited recognition of efficacy of this strategy, or physicians’ perception of what patients value and prefer. Decisions on preferred route of treatment administration are understandably patient-sensitive, with inter-individual variability. In survey studies and focused group-based qualitative studies in patients with mild-moderate UC, efficacy, speed of onset of action and avoidance of immunosuppressive agents are highly valued, as is route of administration.98–100 While patients generally prefer oral administration over rectal therapy, they are often open to rectal therapy as adjunct to be used during acute flares, especially if able to avoid immunosuppressive therapy. Appropriate dosing of mesalamine is another area of considerable variability. While several early trials used low-dose mesalamine (<2g/d) both for induction and maintenance of remission, our findings clearly demonstrate that this dose has significantly lower efficacy. While traditional meta-analyses had shown only a small benefit (6% lower odds of failing to induce remission) of high-dose mesalamine (>3g/d) over standard-dose (2–3g/d) mesalamine, especially in a subset of patients with moderate disease, our network meta-analysis suggests that the effect estimate may be larger (22% lower risk of failing to achieve remission). We estimate that, with median placebo rates of induction of remission of 13% in this population, approximately 33% and 26% patients treated with oral high- or standard-dose mesalamine may be expected to achieve remission, respectively. At the same time, our findings do not support superiority of high-dose mesalamine over standard-dose mesalamine for maintenance of remission. While RCTs on dose de-escalation from high- to standard-dose mesalamine have not been performed, our findings may support a cautious attempt at de-escalating patients with mild-moderate UC in clinical and endoscopic remission. This will help decrease healthcare costs associated with 5-ASA use in patients with UC.
Though traditional meta-analysis based on two trials comparing olsalazine vs. placebo failed to demonstrate superiority of diazo-bonded 5-ASA over placebo due to small sample size and low event rate, our network meta-analysis, by combining direct and indirect evidence, confirms superiority of diazo-bonded 5-ASA over placebo.
Our findings confirmed that budesonide MMX 9mg is a safe and effective alternative in inducing remission in patients with mild-moderate UC. However, it was not more effective than optimized 5-ASA, and given inferior tolerability and inability to use this for long-term maintenance, it may not be appropriate first-line medication. Studies have suggested a small benefit of budesonide MMX when added to 5-ASA in patients with persistent mild-moderate UC despite 5-ASA. Given superior tolerability to oral prednisone, it may be an appropriate alternative to prednisone in this instance.
Besides inherent limitations of individual trials, there are limitations to our analyses. First, for some comparisons, head-to-head trials were limited, especially for maintenance of remission. In some of these analyses, network incoherence was observed, especially for comparisons between low-dose mesalamine, diazo-bonded 5-ASA and sulfasalazine. Second, trials did not adequately stratify based on whether patients were 5-ASA-naïve or if they had previously been treated with 5-ASA; while in moderate-severe UC it is well-recognized that patients with non-response to tumor necrosis factor antagonists, or those being re-treated with the same agent after a drug holiday, have lower likelihood of response to a second biologic agent, it is unknown whether the same applies to mild-moderate UC, and how it may influence trial outcomes. Pooled analyses of individual participant data from clinical trials may help inform comparative efficacy of different interventions, stratified by prior exposure to 5-ASA therapy; similarly, individual participant data pooled analysis would also be able to inform efficacy of different interventions in patients with left-sided colitis vs. extensive colitis. Third, different trials utilized different disease activity indices and there were subtle differences in outcome definitions. However, most trials relied on a combination of improvement in clinical and endoscopic outcomes. Fourth, we did not specifically study comparative efficacy of interventions for left-sided colitis. In these patients, rectal 5-ASA therapy alone may be as effective, or even more effective than oral therapy.
Contextualizing findings on comparative efficacy and tolerability, coupled with patients’ values and preference as well cost and resource utilization considerations, we suggest a treatment algorithm for patients with mild-moderate UC. Patients with mild UC are likely to achieve remission with standard-dose mesalamine or diazo-bonded 5-ASA; a subset of patients, particularly those with more moderately active disease, or in area where high unit dose forms of mesalamine are available at a low cost, individual physicians may reasonably decide to use high-dose oral mesalamine for induction of remission. In patients with extensive colitis, rectal 5-ASA may be added either upfront to standard- or high-dose oral mesalamine or in 4–6 weeks in a subset of patients with suboptimal response to oral therapy. Likewise, budesonide MMX may be considered as monotherapy or in addition to 5-ASA therapy in patients with moderately active disease, either upfront or in case of suboptimal response to optimized 5-ASA therapy. Low-dose mesalamine is not recommended for induction of remission. While sulfasalazine has inferior tolerability and efficacy (in part due to limited ability to dose-escalate), it may be considered in a small subset of patients with mild extra-intestinal articular manifestations. For maintenance of remission, standard-dose mesalamine and diazo-bonded 5-ASA may be preferred options; high-dose mesalamine with or without rectal 5-ASA are no more effective than standard-dose mesalamine, and consideration should be made to cautiously de-escalate patients who required high-dose mesalamine for induction of remission to standard-dose, after 6–12 months.
In conclusion, based on a systematic review and network meta-analysis of 48 RCTs of induction therapy and 28 RCTs of maintenance therapy, we identified combined oral and rectal 5-ASA and high-dose mesalamine may be most likely to induce remission, and standard-dose mesalamine and diazo-bonded 5-ASA may be effective maintenance therapies for the majority of patients with mild-moderate extensive UC. Future studies on optimal risk stratification of patients with mild-moderate disease activity as having mild vs. severe disease (low vs. high risk of colectomy and adverse outcomes) is warranted to inform step therapy vs. early high-dose 5-ASA with or without rectal 5-ASA. Similarly, studies informing when to escalate patients with mild-moderate disease activity from optimized 5-ASA to immunosuppressive agents are warranted, especially for patients who require intermittent corticosteroids.
Supplementary Material
Research in Cosntext.
Evidence before this study
We searched PubMed from inception through May 2018 for previously published meta-analysis on pharmacological interventions for mild-moderate ulcerative colitis using the search terms ‘ulcerative colitis’ combined with ‘treatment’. Previous traditional pairwise meta-analyses provide a limited synthesis of comparative efficacy of different interventions, limited only to head-to-head trials, without incorporating indirect evidence. Additionally, the quality of this evidence has not been critically examined, which may inform clinical guidelines.
Added value of this study
Our study provides the first network meta-analysis of all pharmacological interventions for management of mild-moderate ulcerative colitis, with systematic assessment of quality of the evidence using GRADE methodology. Through a systematic review with network meta-analysis, including 48 RCTs (8020 participants) comparing 8 active interventions for induction of remission, and 28 RCTs (4218 participants) comparing 6 active interventions for maintenance of remission in patients with left-sided or extensive UC, we are able to inform the treatment approach in these patients. We found that for induction of remission, combined oral and rectal 5-ASA is the most effective treatment strategy, followed by high-dose mesalamine (moderate confidence in estimates); standard-dose mesalamine is comparable to diazo-bonded 5-ASA, but superior to sulfasalazine. For maintenance of remission, standard- and high-dose mesalamine are comparable (low confidence in estimates), and superior to low-dose mesalamine (high confidence in estimates). Budesonide MMX is not more effective than combined oral and rectal 5-ASA or high-dose mesalamine and has inferior tolerability.
Implications of all the available evidence
Contextualizing findings on comparative efficacy and tolerability, coupled with patients’ values and preference as well cost and resource utilization considerations, our findings can directly inform treatment guidelines. Patients with mild UC are likely to achieve remission with standard-dose mesalamine or diazo-bonded 5-ASA; a subset of patients, particularly those with more moderately active disease, or in area where high unit dose forms of mesalamine are available at a low cost, physicians may reasonably decide to use high-dose oral mesalamine for induction of remission. In patients with extensive colitis, rectal 5-ASA may be added either upfront to standard- or high-dose oral mesalamine or in 4–6 weeks in a subset of patients with suboptimal response to oral therapy. Likewise, budesonide MMX may be considered as monotherapy or in addition to 5-ASA therapy in patients with moderately active disease, either upfront or in case of suboptimal response to optimized 5-ASA therapy. For maintenance of remission, standard-dose mesalamine and diazo-bonded 5-ASA may be preferred options; high-dose mesalamine with or without rectal 5-ASA are no more effective than standard-dose mesalamine, and consideration should be made to cautiously de-escalate patients who required high-dose mesalamine for induction of remission to standard-dose, after 6–12 months.
Acknowledgments
Role of Funding Source
There was no funding for this study. NHN, MF, MHM and SS had access to raw data from individual studies. SS takes responsibility to submit for publication.
Funding:
None
Disclosures:
Dr. Fumery is supported by the French Society of Gastroenterology (SNFGE, bourse Robert Tournut), and reports lecture/consultant fees from Ferring, Abbvie, MSD, Takeda, Boehringer, Janssen, Pfizer.
Dr. Dulai has research support from Takeda and Pfizer, and has served as a consultant and received honorariums from Takeda.
Dr. Sandborn reports consulting fees from University of Western Ontario (owner of Robarts Clinical Trials, Inc), Abbvie, Akros Pharma, Allergan, Ambrx Inc., Amgen, Ardelyx, Arena Pharmaceuticals, Atlantic Pharmaceuticals, Avaxia, Biogen, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Conatus, Cosmo Technologies, Escalier Biosciences, Ferring, Ferring Research Institute, Forward Pharma, Galapagos, Genentech, Gilead Sciences, Immune Pharmaceuticals, Index Pharmaceuticals, Janssen, Kyowa Hakko Kirin Pharma, Lilly, Medimmune, Mesoblast, Miraca Life Sciences, Nivalis Therapeutics, Novartis, Nutrition Science Partners, Oppilan Pharma, Otsuka, Palatin, Paul Hastings, Pfizer, Precision IBD, Progenity, Prometheus Laboratories, Qu Biologics, Regeneron, Ritter Pharmaceuticals, Robarts Clinical Trials, Salix, Seattle Genetics, Seres Therapeutics, Shire, Sigmoid Biotechnologies, Takeda, Theradiag, Theravance, Tigenix, Tillotts Pharma, UCB Pharma, Vascular Biogenics, Vivelix; research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos; payments for lectures/speakers bureau from Abbvie, Janssen, Takeda; and holds stock/stock options in Escalier Biosciences, Oppilan Pharma, Precision IBD, Progenity, Ritter Pharmaceuticals.
Dr. Singh is supported by the American College of Gastroenterology Junior Faculty Development Award and Crohn’s and Colitis Foundation Career Development Award, and has received research grants from Pfizer and AbbVie.
None of the other authors have any relevant financial disclosures.
REFERENCES
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018; 390(10114): 2769–78. [DOI] [PubMed] [Google Scholar]
- 2.Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural History of Adult Ulcerative Colitis in Population-based Cohorts: A Systematic Review. Clin Gastroenterol Hepatol 2018; 16(3): 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng SC, Zeng Z, Niewiadomski O, et al. Early Course of Inflammatory Bowel Disease in a Population-Based Inception Cohort Study From 8 Countries in Asia and Australia. Gastroenterology 2016; 150(1): 86–95 e3. [DOI] [PubMed] [Google Scholar]
- 4.Niewiadomski O, Studd C, Hair C, et al. Prospective population-based cohort of inflammatory bowel disease in the biologics era: Disease course and predictors of severity. J Gastroenterol Hepatol 2015; 30(9): 1346–53. [DOI] [PubMed] [Google Scholar]
- 5.Vegh Z, Burisch J, Pedersen N, et al. Incidence and initial disease course of inflammatory bowel diseases in 2011 in Europe and Australia: results of the 2011 ECCO-EpiCom inception cohort. J Crohns Colitis 2014; 8(11): 1506–15. [DOI] [PubMed] [Google Scholar]
- 6.Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol 2009; 44(4): 431–40. [DOI] [PubMed] [Google Scholar]
- 7.Samuel S, Ingle SB, Dhillon S, et al. Cumulative incidence and risk factors for hospitalization and surgery in a population-based cohort of ulcerative colitis. Inflamm Bowel Dis 2013; 19(9): 1858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burisch J, Pedersen N, Cukovic-Cavka S, et al. Initial disease course and treatment in an inflammatory bowel disease inception cohort in Europe: the ECCO-EpiCom cohort. Inflamm Bowel Dis 2014; 20(1): 36–46. [DOI] [PubMed] [Google Scholar]
- 9.Gisbert JP, Gomollon F, Hinojosa J, Lopez San Roman A. Adherence of gastroenterologists to European Crohn’s and Colitis Organisation consensus on ulcerative colitis: a real-life survey in Spain. J Crohns Colitis 2010; 4(5): 567–74. [DOI] [PubMed] [Google Scholar]
- 10.Ford AC, Achkar JP, Khan KJ, et al. Efficacy of 5-aminosalicylates in ulcerative colitis: Systematic review and meta-analysis. Am J Gastroenterol 2011; 106(4): 601–16. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Parker CE, Bhanji T, Feagan BG, MacDonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2016; 4: CD000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Parker CE, Feagan BG, MacDonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2016; (5): CD000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013; 159(2): 130–7. [DOI] [PubMed] [Google Scholar]
- 14.Puhan MA, Schunemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014; 349: g5630. [DOI] [PubMed] [Google Scholar]
- 15.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162(11): 777–84. [DOI] [PubMed] [Google Scholar]
- 16.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 2011; 14(4): 417–28. [DOI] [PubMed] [Google Scholar]
- 17.Feagan BG, Chande N, MacDonald JK. Are there any differences in the efficacy and safety of different formulations of oral 5-ASA used for induction and maintenance of remission in ulcerative colitis? Evidence from cochrane reviews. Inflammatory Bowel Diseases 2013; 19(9): 2031–40. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions In: Higgins JPT, Green S, editor. http://www.cochrane-handbook.org; 2011.
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7(3): 177–88. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012; 3(2): 111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011; 64(2): 163–71. [DOI] [PubMed] [Google Scholar]
- 23.Andreoli ACR, Trotti R, Berri F, Prantera C. 5-aminosalicylic acid versus salazopirin (SASP) in the oral treatment of active ulcerative colitis (UC) and in remission. Clinical Controversies in Inflammatory Bowel Disease 1987: 170.
- 24.Bresci G, Carrai M, Venturini G, Gambardella L. Therapeutic effectiveness and tolerance of 5-aminosalicylic acid in short term treatment of patients with ulcerative colitis at a low or medium phase of activity. Int J Tissue React 1990; 12(4): 243–6. [PubMed] [Google Scholar]
- 25.Cai JT, Wu LF, Du Q, Qian KD. Olsalazine versus sulfasalazine in the treatment of ulcerative colitis: randomized controlled Clinical trial. Chinese journal of digestion, 2001; 21(10): 593–5 [Google Scholar]
- 26.D’Haens G, Hommes D, Engels L, et al. Once daily MMX mesalazine for the treatment of mild-to-moderate ulcerative colitis: A phase II, dose-ranging study. Aliment Pharmacol Ther 2006; 24(7): 1087–97. [DOI] [PubMed] [Google Scholar]
- 27.D’Haens GR, Kovacs A, Vergauwe P, et al. Clinical trial: Preliminary efficacy and safety study of a new Budesonide-MMX 9 mg extended-release tablets in patients with active left-sided ulcerative colitis. J Crohns Colitis 2010; 4(2): 153–60. [DOI] [PubMed] [Google Scholar]
- 28.Feagan B, Sandborn W, D’Haens G, et al. The value of a central image management system (CIMS) in the conduct of randomized controlled trials of therapy for ulcerative colitis (UC). J Crohns Colitis 2013; 7: S59–S60. [Google Scholar]
- 29.Feurle GE, Theuer D, Velasco S, et al. Olsalazine versus placebo in the treatment of mild to moderate ulcerative colitis: a randomised double blind trial. Gut 1989; 30(10): 1354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fruhmorgen P, Demling L. On the efficacy of ready-made-up commercially available salicylazosulphapyridine enemas in the treatment of proctitis, proctosigmoiditis and ulcerative colitis involving rectum, sigmoid and descending colon. Hepato-Gastroenterology 1980; 27(6): 473–6. [PubMed] [Google Scholar]
- 31.Good L, Nester T, Borgen L. A double-blind comparison of controlled release mesalamine tablets and sulfasalazine in the treatment of ulcerative colitis. Gastroenterology 1992; 102: A630 [Google Scholar]
- 32.Green JR, Lobo AJ, Holdsworth CD, et al. Balsalazide is more effective and better tolerated than mesalamine in the treatment of acute ulcerative colitis. The Abacus Investigator Group. Gastroenterology 1998; 114(1): 15–22. [DOI] [PubMed] [Google Scholar]
- 33.Green JR, Mansfield JC, Gibson JA, Kerr GD, Thornton PC. A double-blind comparison of balsalazide, 6.75 g daily, and sulfasalazine, 3 g daily, in patients with newly diagnosed or relapsed active ulcerative colitis. Aliment Pharmacol Ther 2002; 16(1): 61–8 [DOI] [PubMed] [Google Scholar]
- 34.Gross V, Bunganic I, Belousova EA, et al. 3g mesalazine granules are superior to 9mg budesonide for achieving remission in active ulcerative colitis: A double-blind, double-dummy, randomised trial. Journal of Crohn’s and Colitis 2011; 5(2): 129–38. [DOI] [PubMed] [Google Scholar]
- 35.Hanauer S, Schwartz J, Robinson M, et al. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. American Journal of Gastroenterology 1993; 88(8): 1188–97. [PubMed] [Google Scholar]
- 36.Hanauer SB, Barish C, Pambianco D, Sigmon R, Gannan R, Koval G. A multi-center, double-blind, placebo-controlled, dose-ranging trial of olsalazine for mild-moderately active ulcerative colitis. Gastroenterology 1996; 110: A921 [Google Scholar]
- 37.Hanauer SB, Sandborn WJ, Dallaire C, et al. Delayed-release oral mesalamine 4.8 g/day (800 mg tablets) compared with 2.4 g/day (400 mg tablets) for the treatment of mildly to moderately active ulcerative colitis: The ASCENDI trial. Can J Gastroenterol 2007; 21(12): 827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanauer SB, Sandborn WJ, Kornbluth A, et al. Delayed-release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: the ASCEND II trial. Am J Gastroenterol 2005; 100(11): 2478–85. [DOI] [PubMed] [Google Scholar]
- 39.Hiwatashi N, Suzuki Y, Mitsuyama K, Munakata A, Hibi T. Clinical trial: Effects of an oral preparation of mesalazine at 4 g/day on moderately active ulcerative colitis. A phase III parallel-dosing study. J Gastroenterol 2011; 46(1): 46–56. [DOI] [PubMed] [Google Scholar]
- 40.Ito H, Iida M, Matsumoto T, et al. Direct comparison of two different mesalamine formulations for the maintenance of remission in patients with ulcerative colitis: A double-blind, randomized study. Inflamm Bowel Dis 2010; 16(9): 1575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamm MA, Sandborn WJ, Gassull M, et al. Once-Daily, High-Concentration MMX Mesalamine in Active Ulcerative Colitis. Gastroenterology 2007; 132(1): 66–75. [DOI] [PubMed] [Google Scholar]
- 42.Kruis W, Bar-Meir S, Feher J, et al. The optimal dose of 5-aminosalicylic acid in active ulcerative colitis: A dose-finding study with newly developed mesalamine. Clin Gastroenterol Hepatol 2003; 1(1): 36–43. [DOI] [PubMed] [Google Scholar]
- 43.Kruis W, Brandes JW, Schreiber S, et al. Olsalazine versus mesalazine in the treatment of mild to moderate ulcerative colitis. Aliment Pharmacol Ther 1998; 12(8): 707–15. [DOI] [PubMed] [Google Scholar]
- 44.Levine DS, Riff DS, Pruitt R, et al. A randomized, double blind, dose-response comparison of balsalazide (6.75 g), balsalazide (2.25 g), and mesalamine (2.4 g) in the treatment of active, mild-to-moderate ulcerative colitis. Am J Gastroenterol 2002; 97(6): 1398–407. [DOI] [PubMed] [Google Scholar]
- 45.Lichtenstein GR, Kamm MA, Boddu P, et al. Effect of Once- or Twice-Daily MMX Mesalamine (SPD476) for the Induction of Remission of Mild to Moderately Active Ulcerative Colitis. Clin Gastroenterol Hepatol 2007; 5(1): 95–102. [DOI] [PubMed] [Google Scholar]
- 46.Mansfield JC, Giaffer MH, Cann PA, McKenna D, Thornton PC, Holdsworth CD. A double-blind comparison of balsalazide, 6.75 g, and sulfasalazine, 3 g, as sole therapy in the management of ulcerative colitis. Aliment Pharmacol Ther 2002; 16(1): 69–77. [DOI] [PubMed] [Google Scholar]
- 47.Miglioli M, Bianchi Porro G, Brunetti G, Sturniolo GC. Oral delayed-release mesalazine in the treatment of mild ulcerative colitis: a dose ranging study. Eur J Gastroenterol Hepatol 1990; 2: 229–234 [Google Scholar]
- 48.Mihas AA, Xynopoulos D, Mihas TA. A prospective trial of oral 5-aminosalicylic acid vs sulfasalazine in ulcerative colitis. Gastroenterology 1988; 94: A303 [Google Scholar]
- 49.Munakata A, Yoshida Y, Muto T, et al. Double-blind comparative study of sulfasalazine and controlled-release mesalazine tablets in the treatment of active ulcerative colitis. J Gastroenterol 1995; 30: 108–111 [PubMed] [Google Scholar]
- 50.Pontes C, Vives R, Torres F, Panes J. Safety and activity of dersalazine sodium in patients with mild-to-moderate active colitis: Double-blind randomized proof of concept study. Inflamm Bowel Dis 2014; 20(11): 2004–12. [DOI] [PubMed] [Google Scholar]
- 51.Pruitt R, Hanson J, Safdi M, et al. Balsalazide is superior to mesalamine in the time to improvement of signs and symptoms of acute mild-to -moderate ulcerative colitis. Am J Gastroenterol 2002; 97(12): 3078–86. [DOI] [PubMed] [Google Scholar]
- 52.Qian LP, Lin GJ, Xu SR, Ding WQ. Clinical effect of olsalazine sodium capsule in the treatment of ulcerative colitis. [Chinese]. Fudan University Journal of Medical Sciences 2004; 31(4): 421–4. [Google Scholar]
- 53.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 1989; 298(6666): 82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao SSC, Dundas SAC, Holdsworth CD, Cann PA, Palmer KR, Corbett CL. Olsalazine or sulphasalazine in first attacks of ulcerative colitis? A double blind study. Gut 1989; 30(5): 675–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riley SA, Mani V, Goodman MJ, Herd ME, Dutt S, Turnberg LA. Comparison of delayed release 5 aminosalicylic acid (mesalazine) and sulphasalazine in the treatment of mild to moderate ulcerative colitis relapse. Gut 1988; 29(5): 669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson M, Gitnick G, Balart L, Das K, Turkin D. Olsalazine in the treatment of mild to moderate ulcerative colitis. Gastroenterology 1988; 84: A381 [Google Scholar]
- 57.Rubin DT, Cohen RD, Sandborn WJ, et al. Budesonide MMX 9 mg for inducing remission in patients with Mild-to-Moderate ulcerative colitis not adequately controlled with oral 5-ASAs. J Crohns Colitis 2017; 11(7): 785–79128333362 [Google Scholar]
- 58.Rijk MCMTJ. The efficacy and safety of sulphasalazine and olsalazine in patients with active ulcerative colitis. Gastroeneterology 1991; 100: A243. [Google Scholar]
- 59.Safdi M, DeMicco M, Sninsky C, et al. A double-blind comparison of oral versus rectal mesalamine versus combination therapy in the treatment of distal ulcerative colitis. Am J Gastroenterol 1997; 92(10): 1867–71. [PubMed] [Google Scholar]
- 60.Sandborn WJ, Regula J, Feagan BG, et al. Delayed-Release Oral Mesalamine 4.8 g/day (800-mg Tablet) Is Effective for Patients With Moderately Active Ulcerative Colitis. Gastroenterology 2009; 137(6): 1934–43. [DOI] [PubMed] [Google Scholar]
- 61.Sandborn WJ, Travis S, Moro L, et al. Once-daily budesonide MMX extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: Results from the CORE i study. Gastroenterology 2012; 143(5): 1218–26.e [DOI] [PubMed] [Google Scholar]
- 62.Scherl EJ, Pruitt R, Gordon GL, et al. Safety and efficacy of a new 3.3g b.i.d. tablet formulation in patients with mild-to-moderately-active ulcerative colitis: A multicenter, randomized, double-blind, placebo-controlled study. Am J Gastroenterol 2009; 104(6): 1452–9. [DOI] [PubMed] [Google Scholar]
- 63.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. New Engl J Med 1987; 317(26): 1625–9. [DOI] [PubMed] [Google Scholar]
- 64.Sninsky CA, Cort DH, Shanahan F, et al. Oral mesalamine (Asacol) for mildly to moderately active ulcerative colitis: A multicenter study. Ann Intern Med 1991; 115(5): 350–5. [DOI] [PubMed] [Google Scholar]
- 65.Sutherland LR, Robinson M, Onstad G, et al. A double-blind, placebo controlled, multicentre study of the efficacy and safety of 5-aminosalicylic acid tablets in the treatment of ulcerative colitis. Can J Gastroenterol 1990; 4(7): 463–7. [Google Scholar]
- 66.Travis SPL, Danese S, Kupcinskas L, et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: Results from the randomised CORE II study. Gut 2014; 63(3): 433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tursi A, Brandimarte G, Giorgetti GM, Forti G, Modeo ME, Gigliobianco A. Low dose balsalazide plus a high potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Medical science monitor 2004; 10(11): 126–131 [PubMed] [Google Scholar]
- 68.Vecchi M, Meucci G, Gionchetti P, et al. Oral versus combination mesalazine therapy in active ulcerative colitis: A double-blind, double-dummy, randomized multicentre study. Aliment Pharmacol Ther 2001; 15(2): 251–6. [DOI] [PubMed] [Google Scholar]
- 69.Willoughby CP, Cowan RE, Gould SR, Machell RJ, Stewart JB. Double-blind comparison of olsalazine and sulphasalazine in active ulcerative colitis. Scand J Gastroenterol Supple 1988; 148: 40–4. [DOI] [PubMed] [Google Scholar]
- 70.Zinberg J, Molinas S, Das KM. Double-blind placebo-controlled study of olsalazine in the treatment of ulcerative colitis. Am J Gastroenterol 1990; 85(5): 562–6. [PubMed] [Google Scholar]
- 71.Ardizzone S, Petrillo M, Imbesi V, Cerutti R, Bollani S, Porro GB. Is maintenance therapy always necessary for patients with ulcerative colitis in remission? Aliment Pharmacol Ther 1999; 13(3): 373–9. [DOI] [PubMed] [Google Scholar]
- 72.Ardizzone S, Petrillo M, Molteni P, Desideri S, Porro GB. Coated oral 5-aminosalicylic acid (Claversal) is equivalent to sulfasalazine for remission maintenance in ulcerative colitis: A double- blind study. J Clin Gastroenterol 1995; 21(4): 287–9. [DOI] [PubMed] [Google Scholar]
- 73.D’Albasio G, Pacini F, Camarri E, et al. Combined therapy with 5-aminosalicylic acid tablets and enemas for maintaining remission in ulcerative colitis: A randomized double-blind study. Am J Gastroenterol 1997; 92(7): 1143–7. [PubMed] [Google Scholar]
- 74.Deventer SJHHD, Roskam-Mul MDM, Dekker W, Gasthuis K, Wetzels A, et al. Prospective randomized open label blinded endpoint (PROBE) trial of high versus low dose mesalazine for prevention of relapse in patients with UC in remission. Gastroeneterology 2001; 120: A454. [Google Scholar]
- 75.Dew MJ, Harries AD, Evans N, Evans BK, Rhodes J. Maintenance of remission in ulcerative colitis with 5-amino salicylic acid in high doses by mouth. BMJ 1983; 287(6384): 23–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dissanayake AS, Truelove SC. A controlled therapeutic trial of long term maintenance treatment of ulcerative colitis with sulphasalazine (Salazopyrin). Gut 1973; 14(12): 923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eliakim R, Wengrower D, Ligumsky M, Rachmilewitz D. Comparable efficacy of oral 5-aminosalicylic acid (Mesasal) and sulfasalazine in maintaining ulcerative colitis in remission. Isr J Med Sci 1990; 26(1): 47–9. [PubMed] [Google Scholar]
- 78.Fockens P, Mulder CJJ, Tytgat GNJ, et al. Comparison of the efficacy and safety of 1.5 compared with 3.0 g oral slow-release mesalazine (Pentasa) in the maintenance treatment of ulcerative colitis. Eur J Gastroenterol Hepatol 1995; 7(11): 1025–30. [DOI] [PubMed] [Google Scholar]
- 79.Gordon GL, Zakko S, Murthy U, et al. Once-daily mesalamine formulation for maintenance of remission in ulcerative colitis. J Clin Gastroenterol 2016; 50(4): 318–25. [DOI] [PubMed] [Google Scholar]
- 80.Green JR, Gibson JA, Kerr GD, et al. Maintenance of remission of ulcerative colitis: a comparison between balsalazide 3 g daily and mesalazine 1.2 g daily over 12 months. ABACUS Investigator group. Aliment Pharmacol Ther 1998; 12(12): 1207–16. [DOI] [PubMed] [Google Scholar]
- 81.Hawkey CJ, Dube LM, Rountree LV, Linnen PJ, Lancaster JF. A trial of zileuton versus mesalazine or placebo in the maintenance of remission of ulcerative colitis. Gastroenterology 1997; 112(3): 718–24. [DOI] [PubMed] [Google Scholar]
- 82.Ireland A, Mason CH, Jewell DP. Controlled trial comparing olsalazine and sulphasalazine for the maintenance treatment of ulcerative colitis. Gut 1988; 29(6): 835–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kiilerich S, Ladefoged K, Rannem T, Ranlov PJ. Prophylactic effects of olsalazine v sulphasalazine during 12 months maintenance treatment of ulcerative colitis. Gut 1992; 33(2): 252–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kruis W, Jonaitis L, Pokrotnieks J, et al. Randomised clinical trial: A comparative dose-finding study of three arms of dual release mesalazine for maintaining remission in ulcerative colitis. Aliment Pharmacol Ther 2011; 33(3): 313–22. [DOI] [PubMed] [Google Scholar]
- 85.Kruis W, Schreiber S, Theuer D, et al. Low dose balsalazide (1.5 g twice daily) and mesalazine (0.5 g three times daily) maintained remission of ulcerative colitis but high dose balsalazide (3.0 g twice daily) was superior in preventing relapses. Gut 2001; 49(6): 783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lichtenstein GR, Gordon GL, Zakko S, et al. Clinical trial: Once-daily mesalamine granules for maintenance of remission of ulcerative colitis - A 6-month placebo-controlled trial. Aliment Pharmacol Ther 2010; 32(8): 990–9. [DOI] [PubMed] [Google Scholar]
- 87.Mahmud N, O’Toole D, O’Hare N, Freyne PJ, Weir DG, Kelleher D. Evaluation of renal function following treatment with 5-aminosalicylic acid derivatives in patients with ulcerative colitis. Aliment Pharmacol Ther 2002; 16(2): 207–15. [DOI] [PubMed] [Google Scholar]
- 88.McIntyre PB, Rodrigues CA, Lennard-Jones JE, et al. Balsalazide in the maintenance treatment of patients with ulcerative colitis, a double-blind comparison with sulphasalazine. Aliment Pharmacol Ther 1988; 2(3): 237–43. [DOI] [PubMed] [Google Scholar]
- 89.Miner P Jr, Hanauer S, Robinson M, Schwartz J, Arora S. Safety and efficacy of controlled-release mesalamine for maintenance of remission in ulcerative colitis. Dig Dis Sci 1995; 40(2): 296–304. [DOI] [PubMed] [Google Scholar]
- 90.Misiewitz JJL-JJ, Connell AM, Baron JH, Jones FA. Controlled trial of sulfasalazine in maintenance therapy for ulcerative colitis. Lancet 1965; 1: 185–8. [Google Scholar]
- 91.Mulder CJ, Tytgat GN, Weterman IT, et al. Double-blind comparison of slow-release 5-aminosalicylate and sulfasalazine in remission maintenance in ulcerative colitis. Gastroenterology 1988; 95(6): 1449–53. [DOI] [PubMed] [Google Scholar]
- 92.Paoluzi OA, Iacopini F, Pica R, et al. Comparison of two different daily dosages (2.4 vs. 1.2 g) of oral mesalazine in maintenance of remission in ulcerative colitis patients: 1-Year follow-up study. Aliment Pharmacol Ther 2005; 21(9): 1111–9. [DOI] [PubMed] [Google Scholar]
- 93.Pica R, Cassieri C, Cocco A, et al. A randomized trial comparing 4.8 vs. 2.4g/day of oral mesalazine for maintenance of remission in ulcerative colitis. Dig Liver Dis 2015; 47(11): 933–7. [DOI] [PubMed] [Google Scholar]
- 94.Riley SAMV, Goodman MJ, Herd ME, Dutt S, Turnberg LA. Comparison of delayed-release 5-aminosalicylic acid (mesalazine) and sulfasalazine as maintenance treatment for patients with ulcerative colitis. Gastroeneterology 1988; 94: 1383–9. [DOI] [PubMed] [Google Scholar]
- 95.Rutgeerts P. Comparative efficacy of coated, oral 5-aminosalicylic acid (Claversal) and sulphasalazine for maintaining remission of ulcerative colitis. International Study Group. Aliment Pharmacol Ther 1989; 3(2): 183–91. [PubMed] [Google Scholar]
- 96.Wright JP, O’Keefe EA, Cumming L, Jaskiewicz K. Olsalazine in maintenance of clinical remission in patients with ulcerative colsitis. Dig Dis Sci 1993; 38(10): 1837–42. [DOI] [PubMed] [Google Scholar]
- 97.Yokoyama H, Takagi S, Kuriyama S, et al. Effect of weekend 5-aminosalicylic acid (mesalazine) enema as maintenance therapy for ulcerative colitis: Results from a randomized controlled study. Inflamm Bowel Dis 2007; 13(9): 1115–20. [DOI] [PubMed] [Google Scholar]
- 98.Gray JR, Leung E, Scales J. Treatment of ulcerative colitis from the patient’s perspective: a survey of preferences and satisfaction with therapy. Aliment Pharmacol Ther 2009; 29(10): 1114–20. [DOI] [PubMed] [Google Scholar]
- 99.Vaucher C, Maillard MH, Froehlich F, Burnand B, Michetti P, Pittet V. Patients and gastroenterologists’ perceptions of treatments for inflammatory bowel diseases: do their perspectives match? Scand J Gastroenterol 2016; 51(9): 1056–61. [DOI] [PubMed] [Google Scholar]
- 100.Dionne J, Marshall DA, Thabane L, Marshall JK. Conjoint analysis of patient preferences for 5ASA maintenance therapy in ulcerative colitis. Gastroenterology 2010; 138(5): S630. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.